Abstract
The alarmin family members S100A8 and S100A9 are acute phase inflammation proteins, but they also have been proposed as biomarkers in many malignant and non-malignant diseases. In this study, circulating S100A8 and S100A9 homodimers and S100A8/A9 heterodimers in plasma were systematically investigated by ELISA in aplastic anemia (AA) and myelodysplastic syndromes (MDS). Plasma was obtained from 58 severe AA (SAA) and 30 MDS patients, and from 47 age- and sex-matched healthy donors. In 40 out of the 58 AA subjects, S100A protein levels were measured before and 6 months after immunosuppressive therapy (IST). No differences were observed in AA patients at diagnosis compared to healthy controls for circulating S100A homodimers and heterodimers. After therapy, SAA-responders showed significantly increased circulating S100A8. Non-responding patients had significantly higher levels of circulating S100A8/A9 compared to responders and healthy controls, but without variations of S100A8 and S100A9 homodimers. In MDS patients, circulating S100A8 was significantly elevated compared to those of AA and/or healthy controls. By Pearson correlation analysis of protein levels and blood counts, multiple correlations were found. However, as S100A8 and S100A9 are abundantly present in white blood cells and platelets, correlations with blood counts likely mirror the higher number of cells in the blood of some patients. In conclusion, our findings indicate that circulating S100A8 is increased in MDS but not in AA, and that may be useful to distinguish these diseases in the differential diagnosis of bone marrow failure syndromes.
Keywords: S100A8, S100A9, Bone marrow failure, Aplastic anemia, Myelodysplastic syndromes
1. Introduction
Acquired aplastic anemia (AA) and myelodysplastic syndromes (MDS) are bone marrow (BM) failure syndromes characterized by peripheral blood (PB) cytopenias [1,2]. In AA, responsiveness to immunosuppressive therapies (IST) and several lines of indirect evidence, such as oligoclonal expansion of CD8+ lymphocytes, have linked autologous immune-mediated destruction of hematopoietic stem and progenitor cells (HSPCs) in AA [1]. MDS are a heterogeneous group of clonal premalignant diseases characterized by ineffective hematopoiesis, leading to progressive PB cytopenias, increased risk of developing acute myeloid leukemia (AML), and poor overall survival [3].
S100 proteins are a group of 21 calcium-binding proteins with different intra- and extra-cellular functions. There is evidence of dysregulated expression of S100 proteins in malignant and autoimmune diseases [4]. S100A8 and S100A9, two components of S100 family, preferentially exist as heterodimers or heterotetramers known as calprotectin or MRP8/14 [4]. Homodimers are calcium- and zinc-binding proteins, and they can modulate HSPC and leukemic cells differentiation through the Toll-like receptor 4 (TLR4) signaling pathway [5,6]. S100A8/A9 heterodimers, which are released by granulocytes and monocytes, interact with cytoskeleton components and induce apoptosis or survival via integrin and TLR4 downstream signaling [7]. In some autoimmune diseases, higher serum levels of calprotectin are associated with more aggressive clinical courses, and fecal levels are routinely monitored in inflammatory bowel diseases [7].
In the current work, we investigated circulating S100A8, S100A9, and S100A8/A9 protein levels in AA and MDS as potential biomarkers of disease severity and in the differential diagnosis of BM failure syndromes in general.
2. Materials and methods
2.1. Human plasma samples
Whole PB was collected in ethylenediaminetetraacetic acid (EDTA) tubes from AA (n = 58) and MDS (n = 30) patients and age- and sex-matched healthy subjects (n = 47) after informed consent was obtained in accordance with the Declaration of Helsinki and protocols approved by the National Heart, Lung, and Blood Institute Institutional Review Board [National Institutes of Health (NIH), Bethesda, MD, USA; Clinicaltrials.gov identifiers: NCT00260689, NCT00604201, NCT01328587, NCT01623167, NCT00001620, NCT00001397]. Severe AA (SAA) and MDS were diagnosed by standard criteria [8,9]. Healthy controls were recruited among donors of the NIH Clinical Center Department of Transfusion Medicine. Clinical characteristics are summarized in Table 1. In 40 out of the 58 screened AA patients, specimens were collected at the time of diagnosis and 6 months after starting IST. After centrifugation at 800g for 10 min, plasma was collected and stored at −80 °C until use.
Table 1.
Characteristics of patients and healthy controls.
| SAA | MDS | HC | |
|---|---|---|---|
| No. | 58 | 30 | 47 |
| Median age, years (range) | 34.4 (2–75) | 51.3 (23–71) | 37.4 (23–67) |
| Sex (M/F) | 28/30 | 16/14 | 22/25 |
| Disease status | - | ||
| SAA | 58 | ||
| WHO | |||
| RCUD | 10 | ||
| MDS-U | 7 | ||
| RCMD | 6 | ||
| RARS | 3 | ||
| RAEB-1 | 4 | ||
| IPSS | |||
| Low | 3 | ||
| Int-1 | 20 | ||
| Int-2 | 5 | ||
| BM cellularity | |||
| Hypocellular MDS | 11 | ||
| Normo cellular MDS | 11 | ||
| Hypercellular MDS | 7 | ||
| Not evaluable | 1 | ||
| Treatment | - | - | |
| hATG + CsA + Eltrombopag | 25 | ||
| rATG + CsA | 12 | ||
| hATG + CsA | 19 | ||
| Not evaluable | 3 | ||
| Transfusion | 55/58 | 15/17 | - |
| Not evaluable | 0 | 13 | |
| Median follow-up (months) | 7.5 (2.7–20.5) | - | - |
| Chromosome abnormalities | - | ||
| Del5q | 1 | ||
| Monosomy 7/Del of chr 7 | 4 | ||
| Del20q | 1 | ||
| Trisomy 8 | 1 | ||
| Negative | 55 | 20 | |
| Not evaluable | 10 |
SAA, severe acquired aplastic anemia; MDS, myelodysplastic syndromes; HC, healthy controls; WHO, World Health Organization; RCUD, refractory cytopenia with unilineage dysplasia; MDS-U, unclassifiable MDS; RCMD, refractory cytopenia with multilineage dysplasia; RARS, refractory anemia with ring sideroblasts; RAEB-1, refractory anemia with excess blasts; IPSS, International Prognostic Scoring System; BM, bone marrow; hATG, horse anti-thymocyte globulin; CsA, cyclosporine A; chr, chromosome; rATG, rabbit ATG.
2.2. ELISA for S100A proteins
Plasma levels of S100A proteins were measured by ELISA using the Human S100A8 ELISA kit (Thermo Fisher Scientific, Waltham, MA, USA) for S100A8 homodimers, the LEGEND MAX™ Human MRP14 ELISA kit with Pre-coated Plates (BioLegend, San Diego, CA, USA) for S100A9 homodimers, and the LEGEND MAX™ Human MRP8/14 (calprotectin) ELISA kit with Pre-coated Plates (BioLegend) for S100A8/A9 heterodimers. For these assays, samples were diluted as follows: 10-fold for S100A8 homodimers; 2-fold for S100A9 homodimers; and 40-fold for S100A8/A9 heterodimers. A standard curve was included in each assay, and samples and standards run in triplicate. Absorbance was read at 450 nm and 570 nm (for background subtraction) using the VICTOR3 1420 Multilabel Plates Counter (PerkinElmer, Waltham, MA, USA).
2.3. Data analysis and statistics
Data were analyzed using Prism (v.7.02; GraphPad software, La Jolla, CA, USA). Paired two-tailed t-tests for two group comparison and one-way analysis of variance (ANOVA) for three-group comparison were performed. Tukey’s multiple comparison test was used for multiple comparisons. Correlations between protein expression levels and clinical parameters were studied by Pearson correlation analysis and correlograms drawn employing a web tool developed by the Trans-NIH Center for Human Immunology, Autoimmunity, and Inflammation (NIH, Bethesda, MD, USA) based on corrplot R package (https://foocheung.shinyapps.io/corrplot/). Receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC) were performed to assess sensitivity and specificity for diagnosis using the healthy control (HC) group as reference. P < 0.05 was considered statistically significant.
3. Results and discussion
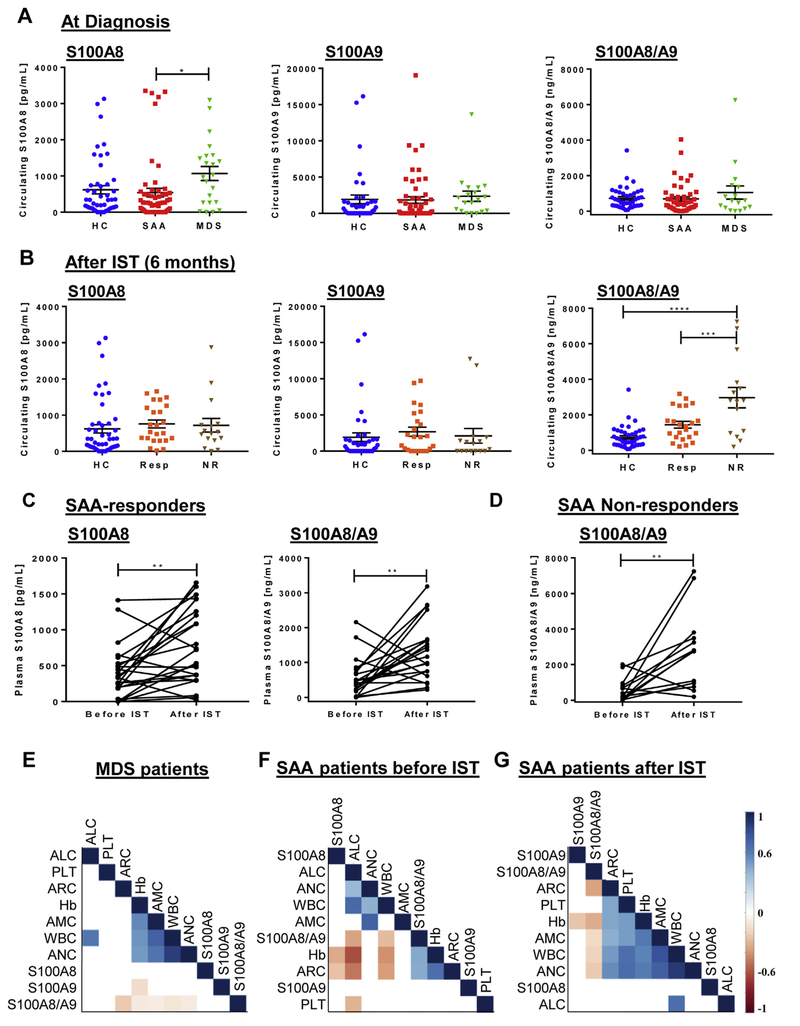
S100A8 and S100A9 are calcium- and zinc-binding proteins existing as homodimers or heterotetramer (S100A8/A9, also known as calprotectin) [7]. Higher S100A8/A9 serum levels have been described in many autoimmune, cancer, and non-malignant diseases, and related to disease progression [4–7,10]. To date, circulating expression of S100A8, S100A9, and S100A8/A9 have not been investigated in BM failure syndromes. In MDS, patients with deletion of the q arm of chromosome 5 had increased S100A8+ cells in BM, and Rps14 haploinsufficiency induced expression of S100A8 and S100A9 [5]. In our study, circulating S100A8, S100A9, and S100A8/A9 protein levels were measured in plasma of patients with SAA and MDS (Fig. 1). Circulating S100A8 was significantly elevated in MDS patients compared to SAA and healthy donors, but not in SAA at diagnosis compared to HC (Fig. 1A). Low-risk MDS patients had the highest levels of circulating S100A8. After therapy, no differences in S100A8 homodimer levels were found in SAA-responders (Fig. 1B) and SAA-non responders compared to HC. S100A8/A9 levels were significantly elevated in SAA-non responders compared to SAA-responders and HC. Significantly increased circulating S100A8 and S100A8/A9 levels were observed in SAA-responders after IST. SAA-non responders showed significantly elevated S100A8/A9 levels after IST compared to before IST, but there were no significant differences in S100A8 levels before and after IST (Fig. 1C and D). To assess the specificity and sensitivity of circulating S100A8, S100A9, and S100A8/A9 in the diagnosis of SAA and MDS, a receiver operating characteristic (ROC) curve analysis was employed. Circulating S100A8 and S100A9 proteins did not show any association with SAA. Further, circulating S100A8 (area under the curve [AUC], 0.65; P = 0.043) displayed significant association to MDS. Higher levels of S100A8 in our MDS patients compared to AA may reflect the different pathophysiology of these diseases [1–3,11]. The innate immune system has been implicated in the development of MDS, and S100A8/S100A9 might modulate expansion of myeloid-derived suppressor cells and pyroptosis through TLR signaling [11]. In addition, dysplastic bone marrow cells overexpress inflammasome proteins, such as NLRP3 and S100A9 which may trigger the activation of the inflammasome-mediated pyroptosis [12]. However, in our cohort of MDS patients, circulating S100A9 levels were not different from healthy controls or between patients with different risk (P = 0.803). Our findings suggest that circulating S100A8 or S100A8/A9 may play different biological roles, as they can act as extra- or intra-cellular stimuli on several types of immune cells, such as macrophages, inducing the production of proinflammatory cytokines and sustaining immune responses [4,13,14]. Thus, TNF-α, IL-6, CCL-3, IL-1Ra, and HGF are already reported to be increased in MDS patients using a Luminex-based assay [15]. Therefore, circulating S100A8 may contribute to increase the secretion of these cytokines, while circulating S100A9 may have a dispensable role. Our data suggest S100A8 as a biomarker in the differential diagnosis of BM failure syndromes, which requires further study in larger and more homogeneous populations supported by additional in vitro experiments.
Fig. 1.
Circulating levels of S100A proteins in myelodysplastic syndromes (MDS) and severe aplastic anemia (SAA) patients before and after immunosuppressive therapy (IST). Subjects were divided based on hematological improvement in SAA-responders (Resp) or SAA-non responders (NR). (A) Circulating S100A8, S100A9, and S100A8/A9 levels in SAA at diagnosis, MDS patients, and healthy controls (HC). (B) Circulating S100A8, S100A9, and S100A8/A9 levels in SAA patients after 6 months of IST. Plasma levels of S100A8, S100A9, and S100A8/A9 were compared before and after therapy by paired t-test in responders (C) and non-responders (D). (E–G) Correlograms using S100A protein levels and blood counts. Circulating S100A8, S100A9, and S100A8/A9 levels were correlated with hemoglobin level (Hb), white blood cell (WBC), platelet count (Plt), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), absolute monocyte count (AMC), and absolute reticulocyte count (ARC) in MDS patients (E), and SAA before (F) and after (G) therapy. Values range between −1 (red) and +1 (blue). P < 0.05 was considered statistically significant (*). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
S100A8 may be involved in the block of erythroid differentiation that can occur in MDS, as inactivation of S100A8 alone rescued erythroid cells to normal hematopoiesis [5], and higher levels of S100A9 can induce differentiation of leukemic cells [6]. S100A9 activity is regulated by S100A8 and higher ratios of S100A9 over S100A8 are required to induce leukemic cell differentiation [6]. In our cohort, no differences in circulating S100A9 levels were seen in MDS and SAA patients, and SAA-responders after 6 months of therapy.
Neutrophils and monocytes are sources of circulating calprotectin, representing 40% or 5% of total cytoplasmic proteins in granulocytes or monocytes, respectively [6]. In addition, platelets abundantly express S100A8/A9 [10]. Pearson analysis was performed to investigate the correlation of circulating S100A proteins with clinical parameters of hemoglobin (Hb) level, white blood cell (WBC) count, platelet (Plt) count, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), absolute monocyte count (AMC), and absolute reticulocyte count (ARC) in MDS patients, and before and after IST in SAA patients. When SAA and MDS patients were combined, no correlations were found between circulating S100A proteins and blood counts. When considered as MDS patients and SAA before and after IST, multiple correlations were observed (Fig. 1E–G). Plt counts positively correlated with circulating S100A9 levels in SAA patients before IST, while S100A8/A9 levels after IST negatively related to Hb levels and ARC. However, these correlations may reflect higher number of peripheral blood cells in some patients, as neutrophils and platelets are the physiological sources of S100A8 and S100A9.
Although genomic and molecular testing has improved the definition of BM failure syndromes, distinguishing MDS from other diseases and prognostic definition are still challenging [1–3]. Our findings indicated that elevated levels of circulating S100A8 in MDS, but not in AA, might be useful as an additional tool in differential diagnosis. Further, higher S100A protein levels in AA patients after therapy may reflect the improvement of blood count.
Acknowledgments
The authors would like to thank Xingmin Feng, Lemlem Alemu, and Diego Quinones Raffo for technical assistance, and Kinneret Broder and Therese Intrater for assistance in obtaining samples from healthy volunteers.
Role of funding source
This research was supported by the Intramural Research Program of the NIH, National Heart, Lung, and Blood Institute.
Clinicaltrials.gov identifiers: NCT00260689, NCT00604201, NCT01328587, NCT01623167, NCT00001620, NCT00001397.
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- [1].Young NS, Calado RT, Scheinberg P, Current concepts in the pathophysiology and treatment of aplastic anemia, Blood 108 (8) (2006) 2509–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fenaux P, Adès L, How we treat lower-risk myelodysplastic syndromes, Blood 121 (21) (2013) 4280–4286. [DOI] [PubMed] [Google Scholar]
- [3].Zeidan AM, Al Ali N, Barnard J, Padron E, Lancet JE, Sekeres MA, Steensma DP, DeZern A, Roboz G, Jabbour E, Garcia-Manero G, List A, Komrokji R, Comparison of clinical outcomes and prognostic utility of risk stratification tools in patients with therapy-related vs. de novo myelodysplastic syndromes: a report on behalf of the MDS clinical research consortium, Leukemia 31 (6) (2017) 1391–1397. [DOI] [PubMed] [Google Scholar]
- [4].Bresnick AR, Weber DJ, Zimmer DB, S100 proteins in cancer, Nat. Rev. Cancer 15 (2) (2015) 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schneider RK, Schenone M, Ferreira MV, Kramann R, Joyce CE, Hartigan C, Beier F, Brümmendorf TH, Germing U, Platzbecker U, Büsche G, Knüchel R, Chen MC, Waters CS, Chen E, Chu LP, Novina CD, Lindsley RC, Carr SA, Ebert BL, Rps14 haploinsufficiency causes a block in erythroid differentiation mediated by S100A8 and S100A9, Nat. Med 22 (3) (2016) 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Laouedj M, Tardif MR, Gil L, Raquil MA, Lachhab A, Pelletier M, Tessier PA, Barabé F, S100A9 induces differentiation of acute myeloid leukemia cells through TLR4, Blood 129 (14) (2017) 1980–1990. [DOI] [PubMed] [Google Scholar]
- [7].Pruenster M, Vogl T, Roth J, Sperandio M, S100A8/A9: from basic science to clinical application, Pharmacol. Ther 167 (2016) 120–131. [DOI] [PubMed] [Google Scholar]
- [8].Camitta BM, Thomas ED, Nathan DG, et al. , Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality, Blood 48 (1) (1976) 63–70. [PubMed] [Google Scholar]
- [9].Greenberg PL, Stone RM, Al-Kali A, Barta SK, Bejar R, Bennett JM, Carraway H, De Castro CM, Deeg HJ, DeZern AE, Fathi AT, Frankfurt O, Gaensler K, Garcia-Manero G, Griffiths EA, Head D, Horsfall R, Johnson RA, Juckett M, Klimek VM, Komrokji R, Kujawski LA, Maness LJ, O’Donnell MR, Pollyea DA, Shami PJ, Stein BL, Walker AR, Westervelt P, Zeidan A, Shead DA, Smith C, Myelodysplastic Syndromes, Version 2.2017, NCCN clinical practice guidelines in oncology, J. Natl. Compr. Canc. Netw 15 (1) (2017) 60–87. [DOI] [PubMed] [Google Scholar]
- [10].Sakuma M, Tanaka A, Kotooka N, Hikichi Y, Toyoda S, Abe S, Taguchi I, Node K, Simon DI, Inoue T, Myeloid-related protein-8/14 in acute coronary syndrome, Int. J. Cardiol 249 (2017) 25–31. [DOI] [PubMed] [Google Scholar]
- [11].David A, Sallman DA, Cluzeau T, Basiorka AA, List A, Unraveling the pathogenesis of MDS: the NLRP3 inflammasome and pyroptosis drive the MDS phenotype, Front. Oncol 6 (2016) 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Basiorka AA, McGraw KL, Eksioglu EA, Chen X, Johnson J, Zhang L, Zhang Q, Irvine BA, Cluzeau T, Sallman DA, Padron E, Komrokji R, Sokol L, Coll RC, Robertson AA, Cooper MA, Cleveland JL, O’Neill LA, Wei S, List AF, The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype, Blood 128 (25) (2016) 2960–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, Chazin WJ, Nakatani Y, Yui S, Makino H, The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis, Arthritis Res. Ther 8 (3) (2006) R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA, Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion, J. Immunol 170 (6) (2003) 3233–3242. [DOI] [PubMed] [Google Scholar]
- [15].Feng X, Scheinberg P, Wu CO, Samsel L, Nunez O, Prince C, Ganetzky RD, McCoy JP Jr, Maciejewski JP, Young NS, Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes, Haematologica 96 (4) (2011) 602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]