The recent 2018 NIA-AA [National Institute on Aging, NIA; Alzheimer’s Association, AA] Research Framework “Towards a Biological Definition of Alzheimer’s Disease” (referred to below as the Research Framework) outlines a biomarker system to classify individuals in the Alzheimer’s disease (AD) continuum using imaging biomarkers and cerebrospinal fluid (CSF) biomarkers focused on amyloid-β (Aβ) [A], tau [T], and neurodegeneration [(N)] - the “AT(N)” biomarker system [1]. The AT(N) system has been proposed to define a biomarker-based approach to diagnose AD for research observational and interventional studies, but at the same time does not imply a specific order of events nor causality and acknowledges an uncertain relationship between the A and T biomarkers and disease symptoms [1]. The Research Framework defines an individual with biomarker evidence of both Aβ deposition and pathologic tau as having AD, yet acknowledges that amyloid and tau deposits may not be causal to AD [1]. The Research Framework distinguishes between AD which is reserved for the pathologic entity (defined by amyloid and tau biomarkers) and the Alzheimer’s clinical syndrome. As Alzheimer’s clinical syndrome has been shown to be a disease with mixed pathologies, and AD may also be multifactorial, other factors as illustrated in Figure 1 will likely contribute to and/or modify onset and progression of symptoms, as discussed more in sections below. Below we use the term AD (not strictly defined as amyloid+ and tau+ biomarkers), but rather more broadly inclusive of AD as a multifactorial and heterogenous disease.
Figure 1. Alzheimer’s disease is a multifactorial and heterogeneous disease.
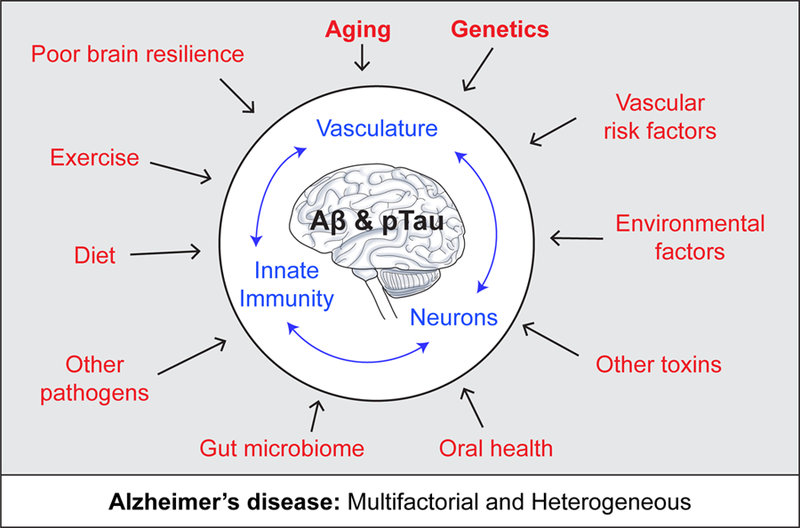
Alzheimer’s disease (AD) is defined as a unique neurodegenerative disease based on the presence of amyloid-β (Αβ) and tau deposits. Additional factors (red), however, contribute to the onset and progression of AD pathophysiological changes directly affecting brain vascular system (i.e., blood-brain barrier leakages, blood flow shortfalls) and innate immune system, and neuronal health and functioning independently and/or simultaneously with Aβ and tau pathologies. This includes, but is not limited to: genetic risk factors, vascular factors, environmental factors including socioeconomic stress, microbiome, and lifestyle. Aging still remains the key risk factor for AD, and also profoundly affects brain vasculature, innate immune responses and neuronal functions (blue).
Despite the substantial evidence indicating early vascular contributions to AD pathophysiology and dementia, vascular disease very commonly accompanies AD and may also be in causal pathway. Below, we first briefly discuss evidence that vascular dysfunction is a prominent and early feature in prodromal AD, and, without implying a causality, order of events, or specificity, suggest that adding vascular biomarkers to the proposed AT(N) biomarker system will help to better characterize and understand contributions of vascular dysfunction to cognitive impairment in patients suffering from AD. Next, we focus on 18F-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET), a molecular imaging biomarker for early preclinical AD and mild cognitive impairment (MCI) mentioned in the AT(N), and examine the evidence indicating that FDG-PET should also be considered a biomarker of vascular and/or blood-brain barrier (BBB) transport dysfunction rather than uniquely neuronal hypometabolism and neurodegeneration, as elaborated in recent reviews [2,3]. Recognizing these concepts will achieve a more balanced view of AD pathophysiology and its multifactorial origin and provide even better tools for early diagnosis of AD as well as pave the way for novel therapeutic approaches.
Vascular dysfunction and vascular biomarkers in AD.
Neuropathological studies have shown that cerebrovascular pathology is a major risk factor for clinically diagnosed AD-type dementia with clinical expression associated with low scores in most cognitive domains [4]. A large autopsy-based neuropathological study importantly revealed that 80% of patients diagnosed with AD and no evidence of mixed (vascular) dementia, had vascular pathology including cortical infarcts, lacunes, cerebral microbleeds and multiple microinfarcts indicative of small vessel disease (SVD), intracranial atherosclerosis, arteriolosclerosis, perivascular spacing and cerebral amyloid angiopathy (CAA) [5], supporting the concept that cerebrovascular dysfunction is prominent in AD and lowers the threshold for dementia for a given AD pathology burden. Further, mounting evidence shows that vascular risk factors (VRFs) are associated with lower FDG-PET [6], cerebrovascular disease as expected [7], higher cerebral Αβ burden [6,8], higher tau burden [9], and act synergistically with Aβ burden to promote cognitive decline [10]. Structural arterial changes leading to functional changes in cerebral blood flow (CBF) [11] are associated with the rate of accumulation of cerebral Αβ over time [12] and the overlap of cerebrovascular and cerebral Αβ pathologies in older adults [13]. The overlap of cerebrovascular and traditional AD pathologies is not exclusive to the late onset form of AD but also present in autosomal dominant AD (ADAD) [14]. It is important to extend epidemiology research beyond clinical VRFs to subclinical vascular measures that point to the mechanistic pathways linking vascular dysfunction to the various aspects of AD and dementia pathology in diverse cohorts.
Vascular dysfunction appears early in AD, as shown using different imaging biomarkers of BBB integrity [15–20], brain microbleeds [20–25], cerebrovascular reactivity [20,26,27], resting CBF [28–40,17,20,41], and increased cerebrovascular resistance [42]. BBB permeability to gadolinium, measured by dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI), is routinely used for clinical diagnosis of multiple sclerosis, stroke and brain tumors [43,44]. Only recently has the DCE-MRI technique been modified and advanced to detect subtle changes in BBB permeability in the living human brain with a sub-regional spatial resolution capable of detecting changes at the level of hippocampal subfields and different gray and white matter regions studied in parallel [15,19,20,45]. Early BBB breakdown has been shown in the hippocampus and its CA1 and dentate gyrus sub-regions in individuals with MCI [15], and in several gray and white matter regions in early stages of AD [16–18]. Additionally, BBB failure was found to be a core mechanism in cerebral SVD and dementia (see below) [45].
Widespread utilization of various imaging sequences could be easily implemented to evaluate different types of vascular dysfunction in AD pathophysiology. FLAIR [fluid-attenuated inversion recovery] is the most common sequence used in aging and AD studies to define macrostructural white matter hyperintensities (WMH). Microstructural changes at tissue level interstitial fluid (ISF) shifts are easily detected on diffusion tensor imaging (DTI) sequences and quantified using the mean diffusivity parameter, which several studies have shown is highly sensitive to white matter microstructural damage and correlates with BBB failure [46,47]. Another vascular biomarker, microbleeds, can be measured with short 5 min T2*-weighted sequences [20–25]; this would be easy to add to existing AD MRI protocols. Cerebral microbleeds are related to vascular wall damage by arteriosclerosis or CAA, and also reflects a marker of ischemic white matter disease [3]. Additionally, the DCE sequence to evaluate subtle, sub-regional BBB permeability lasts about 15 minutes, requires intravenous injection of a gadolinium contrast agent, and can be obtained in either coronal or transverse orientations for individual input function analysis. The DCE sequence has already been added to imaging protocols at several Alzheimer’s Disease Centers (ADC) including University of Southern California (USC), Washington University in St. Louis, and Banner Alzheimer’s Institute, and is also being used to study individuals with ADAD at USC in addition to its frequent use in patients with SVD (sporadic and genetic) and Binswanger’s type of dementia. Functional changes such as impaired cerebrovascular reactivity that reflects diminished vasodilation of cerebral vessels in response to a CO2 inhalation challenge can be measured using either blood oxygenation level dependent (BOLD) functional MRI [48,49] or arterial spin labeling (ASL) [26] at the tissue level, or transcranial Doppler (TCD) [27]. CBF reductions are detected by several different imaging methods, including pseudo-continuous ASL-MRI [17,28,33–37,50,41,51,52], 4-dimensional phase contrast angiography [53], dynamic susceptibility-contrast (DSC) MRI [38], single-photon emission-computed tomography (SPECT) [30–32,54], TCD [55], perfusion computed tomography (CT) [56], and [15O]-PET [29]. Recently, using advanced DSC methods it is now possible to specifically detect capillary dysfunction which is impaired in AD [57].
Beyond the recognized microvascular dysfunction, emerging evidence also indicates CBF reductions at large and medium sized arteries in adults at risk for AD [52] and in AD models [58], supporting that quantification of vascular changes at all levels of the intracranial vasculature may provide a more comprehensive and possibly more sensitive marker for detecting early AD changes. New methods of evaluating angiography of 3-dimensional vascular anatomy using time-of-flight (TOF) MRI sequences can provide several quantitative parameters such as number and order of branches, branch artery lengths and volumes, tortuosity, planarity, intensity etc. can be derived [59]. Already used clinically to evaluate vascular stenosis, detect aneurysms and vascular disease, TOF sequences could easily be added to MRI protocols and applied to cognitively normal older adults, MCI and AD for comprehensive analysis of angiographic data with the potential to provide new insights into vascular contributions to AD.
In addition to imaging biomarkers, CSF and blood-based biomarkers of vascular damage in the AD continuum are emerging such as, for example, CSF soluble platelet-derived growth factor receptor-β (sPDGFRb) reflecting mural cell injury [15,60] and CSF fibrinogen and standard albumin CSF/plasma quotient reflecting BBB breakdown [15,61,62]. Biofluid (CSF and blood) biomarkers of vascular damage should continue to be validated by multiple independent studies. Furthermore, the more conventional pattern of low Aβ42 in the CSF reflect a failure of drainage of Aβ from the ISF of the brain across blood vessels and by perivascular ISF flow [63,64].
Moreover, imaging biomarkers of SVD are already established, well-characterized and easy to recognize, including WMH, lacunes (subcortical infarcts of vascular origin), microbleeds, etc., as well as more subtle markers now emerging (such as microinfarcts and perivascular spaces, PVS) [19,63]. Beyond the vascular imaging biomarkers defined above, further inclusion of SVD features in the differential biological approach in sporadic AD, ADAD [65] and other dementias would be relatively easy to achieve and is highly relevant since SVD of the brain contributes to > 50% of all dementias worldwide including AD [19,66–69]. Neuroimaging techniques already used in SVD and vascular dementia should similarly be applied to AD and other dementias [70]. Acknowledging and further characterizing vascular contributions to the AD and association with biomarker-based AD pathology is important for ongoing observational studies in diverse cohorts and to target interventional strategies to prevent or slow cognitive decline and dementia. This may be particularly important in under-represented minority groups including African-Americans and Latinos at greater risk for cardiovascular disease (CVD), cerebrovascular disease and AD.
FDG-PET.
18F-fluoro-2-deoxy-D-glucose (FDG), a radiolabeled form of 2-deoxy-D-glucose (2DG), which is an analog of glucose, is frequently used as a ligand for FDG-PET studies as an “surrogate” marker for glucose brain uptake [20]. Impaired FDG-PET uptake is often considered an exclusive biomarker of brain hypometabolism or neurodegeneration as proposed in the NIA-AA Research Framework [1]. However, below we examine evidence that FDG also tracks BBB transport of glucose, and therefore low FDG-PET uptake should also be considered as a biomarker of vascular dysfunction.
Glucose and its 2DG and FDG analogs are transported across the BBB via brain endothelial- specific glucose transporter-1 (GLUT1), and then taken up by different cell types (e.g., neurons) in the brain via their respective glucose transporters, which does not include GLUT1 [71–73]. The ubiquitous intracellular hexokinase then phosphorylates glucose, 2DG and FDG to their respective 6- phosphates (6P) [74–77]. However, after this initial phosphorylation step by hexokinase there are critical differences between glucose vs. 2DG/FDG metabolic fates in brain [71,74–77] as illustrated in Figure 2. After phosphorylation, glucose-6P is converted to fructose-6P that undergoes glycolysis followed by pyruvate entry into the Krebs cycle and oxidative phosphorylation. But, glucose analogs 2DG and FDG are not substrates for glucose-6P isomerase, and thus cannot be converted into fructose-6P, which is the necessary step to enter the glycolytic pathway as well as the subsequent Krebs cycle [74–77]. Instead, 2DG-6P and FDG-6P remain trapped in the brain in their 6P forms, and are only slowly eliminated from the brain [74–77], as has been shown by multiple independent studies. For example, 60–90 minutes following 2DG [75] or FDG [76,78] systemic administration, ~90–97% of 2DG or FDG was found in the mouse brain [75,76] or rat brain [76,78] in the form of 2DG-6P or FDG-6P, whereas <10% remains as pure 2DG or FDG with no other significant metabolites found in the brain. Due to very low brain glucose-6-phosphatase activity and poor 2DG- 6P membrane permeability [74,79,80], 2DG-6P remains trapped in brain cells [78,81] and is slowly eliminated from the brain.
Figure 2. Schematic illustrating key differences in brain metabolic fate of glucose and its non- metabolizable surrogate analog 2-deoxy-D-glucose (2DG) and its radiolabeled form 18F-fluoro- 2-deoxy-D-glucose (FDG).
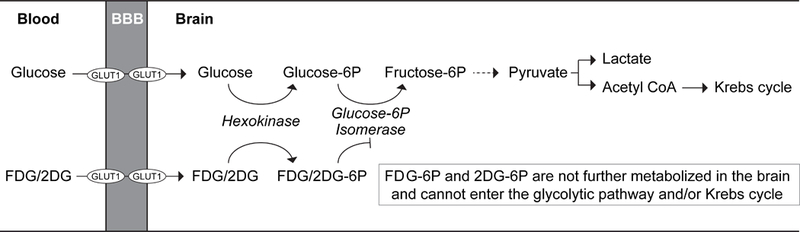
Glucose, a key energy metabolite in the brain, is transported across the blood-brain barrier (BBB) via endothelial-specific glucose transporter-1 (GLUT1) hexose transporter. After uptake by brain cells, glucose undergoes glycolysis followed by Krebs cycle and oxidative metabolism providing the fuel for physiological brain functions through the generation of high-energy adenosine-3 phosphate (ATP) molecules, the foundation for neuronal and non-neuronal cell maintenance and the generation of neurotransmitters. On the other hand, glucose surrogate analogs 2DG and FDG, although still transported across the BBB via GLUT1 hexose transporter, cannot enter the glycolytic pathway or Krebs cycle in brain. After the initial hexokinase step, 2DG- 6P and FDG-6P get trapped in the brain, because they are not substrates for glucose-6P isomerase, which is a necessary metabolic step in the glycolytic pathway. Therefore, 2DG and FDG are not metabolized by the glycolytic pathway or Krebs cycle, do not generate any ATP energy-donor molecules in brain, and their net metabolic rate in brain is zero joules.
Importantly, FDG-PET studies show diminished glucose uptake in several brain regions (e.g., precuneus, posterior cingulate, right angular gyrus, bilateral temporal cortices) prior to any detectable neurodegenerative changes, brain atrophy and/or conversion to AD [82]. Reduced regional FDG brain uptake in AD is not due to brain atrophy, as confirmed by studies in the posterior cingulate gyrus and parieto-temporal cortex [83]. Longitudinal FDG-PET findings have suggested that reductions in hippocampal glucose uptake during normal aging can predict cognitive decline years in advance of clinical AD diagnosis [84]. Diminished glucose uptake in the hippocampus, parietotemporal cortex and/or posterior cingulate cortex has been repeatedly shown by FDG-PET in early AD [85], and also in individuals at genetic risk for AD [86,87], with a positive family history of AD [88], and/or MCI or no cognitive impairment prior to progression to AD [89]. The patterns of FDG brain uptake can also discriminate individuals with normal cognition from MCI and AD patients [85], suggesting region-specific insufficiency in brain delivery and uptake of glucose to the brain. FDG-PET changes preceding neurodegeneration are not only found in humans [82–84,90], but also in transgenic AD models [91].
Although FDG-PET changes in AD are typically interpreted as the result of neuronal glucose hypometabolism, in vivo dynamic FDG-PET kinetic studies in humans consistently show significant reductions in glucose BBB transport in AD subjects compared to controls [92–95], consistent with post-mortem studies showing significantly reduced GLUT1 levels in brain capillaries, a site of the BBB in vivo [96–99]. On the other hand, a few studies that directly measured hexokinase activity levels in AD brains reported rather conflicting results showing a decrease [92,94], increase [100] or no change [93,101]. Additionally, in contrast to glucose, 2DG does not proceed beyond the initial phosphorylation step into glycolytic or Krebs metabolic pathways as shown by rodent [74–78] and human [92–95] studies, and does not generate a single high energy adenosine-3-phosphate (ATP) molecule to maintain functions of neurons and non-neuronal cells in brain. The lack of FDG contribution to brain energy metabolism supports the concept that FDG-PET tracks BBB transport of glucose and an initial phosphorylation step by hexokinase, but it does not dependably track all steps involved in energy metabolism of glucose in neurons and is not metabolized by neurons. New tracers such as 3-O-[11C]-methyl-glucose that exclusively track BBB transport and are not phosphorylated by hexokinase or metabolized should be used by future studies to specifically determine the role of glucose transport in AD as possibly an early biomarker [102].
Recommendations.
We recommend the following extensions of the Research Framework. (1) Incorporate biomarkers of vascular dysfunction to assess vascular contributions to AD using imaging biomarkers such as FLAIR, DTI, T2*-weighted sequences, DCE, ASL, and DSC MRI sequences, TCD, BOLD-fMRI, and TOF, and molecular biomarkers of vascular damage in individuals with AD or dementia risk or with suspected dementia; whenever and whichever possible, vascular imaging biomarkers should be adopted in AD research studies, large epidemiological studies and interventional trials [103]. Integration of vascular dysfunction biomarkers into the diagnostic process may allow for earlier diagnosis of AD in some patient subsets. Recognizing and including the wealth of knowledge on how to prevent and treat vascular disease and on interventions to modify vascular dysfunction could significantly advance research in AD and dementia, thus ultimately helping patients. (2) Reclassify diminished FDG brain uptake by PET not as a unique biomarker of neuronal hypometabolism due to diminished hexokinase activity, but also as a biomarker tracking vascular, i.e. BBB transport, abnormality. This particularly, as a few direct studies determining hexokinase activity in AD subjects showed mixed results including a decrease [92,94], increase [100] or no change [93,101], suggesting that equating diminished FDG-PET uptake with cellular hypometabolism should not be made unless both transport and phosphorylation components are measured simultaneously by FDG-PET kinetic studies, which should show directly whether metabolism is affected or not, but unfortunately has not been done in most FDG-PET studies. This reclassification could have profound consequences for the diagnosis and treatment of AD patients because it would highlight the potential of FDG uptake to identify therapeutic windows of opportunity prior to the onset of irreversible neurodegeneration.
Recent evidence indicates that reducing stroke incidence also reduces dementia incidence [69,104]. Later this year (October 2018), a one day satellite meeting held by the World Health Summit will jointly discuss cerebrovascular and neurodegeneration diseases and the concept of dementia prevention by stroke prevention: https://www.worldhealthsummit.org/satellites/dementia-stroke-prevention.html. Similarly, managing and reducing VRFs may protect against cognitive decline, since VRFs act synergistically with Αβ to promote cognitive decline [10]. VRF reduction approaches may be particularly effective in ethnic minorities at greater risk for CVD, cerebrovascular disease and AD. Remarkably, a third of elderly individuals have considerable Alzheimer-type pathology (plaques and tangles) in brain but no cognitive impairment [105]. We are only beginning to understand some of the potential mechanisms of brain resistance and brain resilience [106]; just as biomarkers of disease are important, so are biomarkers of resilience. Finally, future longitudinal studies in individuals at genetic risk for AD should examine how changes in vascular biomarkers relate to amyloid and tau biomarker changes, structural and functional brain connectivity, and cognitive measures over time.
The 2018 Research Framework attempts to unify language of biomarker-based definition of AD, but it underrecognizes AD as a heterogeneous disease and does not clearly define AD in the context of multifactorial and functional systems contributing to disease pathophysiology. Many factors can influence onset and progression of cognitive dysfunction in AD, which besides aging, includes genetics, VRFs, environmental factors, microbiome, and lifestyle, to mention a few (see Figure 1). All these factors influence aging of the vascular system, innate immunity and neuronal health and function directly independent of amyloid and tau, as well as synergistically with Aβ and tau (see Figure 1). The Research Framework acknowledges vascular biomarkers could be added when they are defined, but unfortunately does not fully appreciate that several vascular biomarkers “ready-to-be-used” already exist and are well defined. Since amyloid and tau deposits may not be causal in AD pathogenesis, as recognized by the Research Framework [1], it is the right time to encourage inclusion of biomarkers of vascular dysfunction in observational and interventional research studies. Finally, rather than focusing only on amyloid and tau, broadening the perspective and study of contributing factors to AD will aid in patient-directed therapeutic efforts to apply the right drug(s) - at the right dose - at the right time - in the right study design - and with the right outcome measures for successful intervention to delay, prevent and/or reverse dementia and AD. Individualized, targeted therapies for AD patients will be successful when the complexity of AD pathophysiology is fully appreciated so that multidisciplinary team efforts can be mounted to successfully address one of the most challenging diseases in the 21st century.
Acknowledgements
Supported by the National Institutes of Health (NIH) grants R01AG023084 (B.V.Z.), R01NS090904 (B.V.Z.), R01NS034467 (B.V.Z.), R01AG039452 (B.V.Z.), 1R01NS100459 (B.V.Z.), 5P01AG052350 (B.V.Z.), 5P50AG005142 (H.C.C.; University of Southern California Alzheimer’s Disease Research Center), EB015922 (A.W.T.; Laboratory of Neuro Imaging, LONI), R37- NS089323 (C.I.), R01-NS100447 (C.I.), R01-NS09544 (C.I.), R01-NS/HL037853 (C.I.), R01- NS034179 (C.I.), KL2-TR002002 (S.E.L.), R01-AG064248 (H.M.G.), RF1-AG054548 (H.M.G.), P30-AG005131 (H.M.G.), R21-AG056952 (H.M.G.), P30AG049638 (T.M.H.; S.N.L.), R01AG054069 (T.M.H.; S.N.L.), R01HL096814 (T.M.H.), R01AG053938 (T.M.H.) in addition to the Alzheimer’s Association, Cure Alzheimer’s Fund, and the Foundation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease reference no. 16 CVD 05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 2018;14:535–62. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Montagne A, Zhao Z, Zlokovic BV. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J Exp Med 2017. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 2018;14:133–50. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol 2016;15:934–43. doi: 10.1016/S1474-4422(16)30029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain J Neurol 2013;136:2697–706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Langbaum JBS, Chen K, Launer LJ, Fleisher AS, Lee W, Liu X, et al. Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiol Aging 2012;33:827.e11–19. doi: 10.1016/j.neurobiolaging.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bangen KJ, Nation DA, Delano-Wood L, Weissberger GH, Hansen LA, Galasko DR, et al. Aggregate effects of vascular risk factors on cerebrovascular changes in autopsy-confirmed Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 2015;11:394–403.e1. doi: 10.1016/j.jalz.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gottesman RF, Schneider ALC, Zhou Y, Coresh J, Green E, Gupta N, et al. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA 2017;317:1443–50. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Lowe VJ, Graff-Radford J, et al. Age, vascular health, and Alzheimer disease biomarkers in an elderly sample. Ann Neurol 2017;82:706–18. doi: 10.1002/ana.25071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rabin JS, Schultz AP, Hedden T, Viswanathan A, Marshall GA, Kilpatrick E, et al. Interactive Associations of Vascular Risk and β-Amyloid Burden With Cognitive Decline in Clinically Normal Elderly Individuals Findings From the Harvard Aging Brain Study. JAMA Neurol 2018. doi: 10.1001/jamaneurol.2018.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Avolio A, Kim MO, Adji A, Gangoda S, Avadhanam B, Tan I, et al. Cerebral Haemodynamics: Effects of Systemic Arterial Pulsatile Function and Hypertension. Curr Hypertens Rep 2018;20:20.. doi: 10.1007/s11906-018-0822-x. [DOI] [PubMed] [Google Scholar]
- [12].Hughes TM, Kuller LH, Barinas-Mitchell EJM, McDade EM, Klunk WE, Cohen AD, et al. Arterial stiffness and β-amyloid progression in nondemented elderly adults. JAMA Neurol 2014;71:562–8. doi: 10.1001/jamaneurol.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hughes TM, Wagenknecht LE, Craft S, Mintz A, Heiss G, Palta P, et al. Arterial stiffness and dementia pathology: Atherosclerosis Risk in Communities (ARIC)-PET Study. Neurology 2018;90:e1248–56. doi: 10.1212/WNL.0000000000005259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TLS, et al. White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Ann Neurol 2016;79:929–39. doi: 10.1002/ana.24647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].van de Haar HJ, Burgmans S, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, et al. Blood- Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology 2016;281:527–35. doi: 10.1148/radiol.2016152244. [DOI] [PubMed] [Google Scholar]
- [17].van de Haar HJ, Jansen JFA, van Osch MJP, van Buchem MA, Muller M, Wong SM, et al. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol Aging 2016;45:190–6. doi: 10.1016/j.neurobiolaging.2016.06.006. [DOI] [PubMed] [Google Scholar]
- [18].van de Haar HJ, Jansen JFA, Jeukens CRLPN, Burgmans S, van Buchem MA, Muller M, et al. Subtle blood-brain barrier leakage rate and spatial extent: considerations for dynamic contrast-enhanced MRI. Med Phys 2017. doi: 10.1002/mp.12328. [DOI] [PubMed] [Google Scholar]
- [19].Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Montagne A, Nation DA, Pa J, Sweeney MD, Toga AW, Zlokovic BV. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol (Berl) 2016;131:687–707. doi: 10.1007/s00401-016-1570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Poliakova T, Levin O, Arablinskiy A, Vasenina E, Zerr I. Cerebral microbleeds in early Alzheimer’s disease. J Neurol 2016;263:1961–8. doi: 10.1007/s00415-016-8220-2. [DOI] [PubMed] [Google Scholar]
- [22].Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 2008;70:1208–14. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- [23].Heringa SM, Reijmer YD, Leemans A, Koek HL, Kappelle LJ, Biessels GJ, et al. Multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer’s disease. J Alzheimers Dis JAD 2014;38:211–21. doi: 10.3233/JAD-130542. [DOI] [PubMed] [Google Scholar]
- [24].Shams S, Martola J, Granberg T, Li X, Shams M, Fereshtehnejad SM, et al. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis—the Karolinska Imaging Dementia Study. AJNR Am J Neuroradiol 2015;36:661–6. doi: 10.3174/ajnr.A4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shams S, Wahlund L-O. Cerebral microbleeds as a biomarker in Alzheimer’s disease? A review in the field. Biomark Med 2016;10:9–18. doi: 10.2217/bmm.15.101. [DOI] [PubMed] [Google Scholar]
- [26].Suri S, Mackay CE, Kelly ME, Germuska M, Tunbridge EM, Frisoni GB, et al. Reduced cerebrovascular reactivity in young adults carrying the APOE ε4 allele. Alzheimers Dement J Alzheimers Assoc 2015;11:648–657.e1. doi: 10.1016/j.jalz.2014.05.1755. [DOI] [PubMed] [Google Scholar]
- [27].Hajjar I, Sorond F, Lipsitz LA. Apolipoprotein E, carbon dioxide vasoreactivity, and cognition in older adults: effect of hypertension. J Am Geriatr Soc 2015;63:276–81. doi: 10.1111/jgs.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC, Alzheimer’s Disease Neuroimaging Initiative. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 2016;7:11934.. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Thambisetty M, Beason-Held L, An Y, Kraut MA, Resnick SM. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol 2010;67:93–8. doi: 10.1001/archneurol.2009.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hirao K, Ohnishi T, Matsuda H, Nemoto K, Hirata Y, Yamashita F, et al. Functional interactions between entorhinal cortex and posterior cingulate cortex at the very early stage of Alzheimer’s disease using brain perfusion single-photon emission computed tomography. Nucl Med Commun 2006;27:151–6. [DOI] [PubMed] [Google Scholar]
- [31].Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med Off Publ Soc Nucl Med 2000;41:1155–62. [PubMed] [Google Scholar]
- [32].Matsuda H, Kitayama N, Ohnishi T, Asada T, Nakano S, Sakamoto S, et al. Longitudinal evaluation of both morphologic and functional changes in the same individuals with Alzheimer’s disease. J Nucl Med Off Publ Soc Nucl Med 2002;43:304–11. [PubMed] [Google Scholar]
- [33].Alexopoulos P, Sorg C, Forschler A, Grimmer T, Skokou M, Wohlschlager A, et al. Perfusion abnormalities in mild cognitive impairment and mild dementia in Alzheimer’s disease measured by pulsed arterial spin labeling MRI. Eur Arch Psychiatry Clin Neurosci 2012;262:69–77. doi: 10.1007/s00406-011-0226-2. [DOI] [PubMed] [Google Scholar]
- [34].Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 2009;250:856–66. doi: 10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hirao K, Ohnishi T, Hirata Y, Yamashita F, Mori T, Moriguchi Y, et al. The prediction of rapid conversion to Alzheimer’s disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage 2005;28:1014–21. doi: 10.1016/j.neuroimage.2005.06.066. [DOI] [PubMed] [Google Scholar]
- [36].Nation DA, Wierenga CE, Clark LR, Dev SI, Stricker NH, Jak AJ, et al. Cortical and subcortical cerebrovascular resistance index in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis JAD 2013;36:689–98. doi: 10.3233/JAD-130086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Michels L, Warnock G, Buck A, Macauda G, Leh SE, Kaelin AM, et al. Arterial spin labeling imaging reveals widespread and Aβ-independent reductions in cerebral blood flow in elderly apolipoprotein epsilon-4 carriers. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2016;36:581–95. doi: 10.1177/0271678X15605847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wirth M, Pichet Binette A, Brunecker P, Kobe T, Witte AV, Floel A. Divergent regional patterns of cerebral hypoperfusion and gray matter atrophy in mild cognitive impairment patients. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2017;37:814–24. doi: 10.1177/0271678X16641128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].de Eulate RG, Goni I, Galiano A, Vidorreta M, Recio M, Riverol M, et al. Reduced Cerebral Blood Flow in Mild Cognitive Impairment Assessed Using Phase-Contrast MRI. J Alzheimers Dis JAD 2017;58:585–95. doi: 10.3233/JAD-161222. [DOI] [PubMed] [Google Scholar]
- [40].Leijenaar JF, van Maurik IS, Kuijer JPA, van der Flier WM, Scheltens P, Barkhof F, et al. Lower cerebral blood flow in subjects with Alzheimer’s dementia, mild cognitive impairment, and subjective cognitive decline using two-dimensional phase-contrast magnetic resonance imaging. Alzheimers Dement Amst Neth 2017;9:76–83. doi: 10.1016/j.dadm.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer’s disease by spin- labeled magnetic resonance imaging. Ann Neurol 2000;47:93–100. [PubMed] [Google Scholar]
- [42].Yew B, Nation DA, Alzheimer’s Disease Neuroimaging Initiative. Cerebrovascular resistance: effects on cognitive decline, cortical atrophy, and progression to dementia. Brain J Neurol 2017;140:1987–2001. doi: 10.1093/brain/awx112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sourbron S, Ingrisch M, Siefert A, Reiser M, Herrmann K. Quantification of cerebral blood flow, cerebral blood volume, and blood-brain-barrier leakage with DCE-MRI. Magn Reson Med 2009;62:205–17. doi: 10.1002/mrm.22005. [DOI] [PubMed] [Google Scholar]
- [44].Larsson HBW, Courivaud F, Rostrup E, Hansen AE. Measurement of brain perfusion, blood volume, and blood-brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 tesla. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med 2009;62:1270–81. doi: 10.1002/mrm.22136. [DOI] [PubMed] [Google Scholar]
- [45].Wardlaw JM, Makin SJ, Valdes Hernandez MC, Armitage PA, Heye AK, Chappell FM, et al. Blood- brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimers Dement 2017;13:634–43. doi: 10.1016/j.jalz.2016.09.006. [DOI] [Google Scholar]
- [46].Munoz Maniega S, Chappell FM, Valdes Hernandez MC, Armitage PA, Makin SD, Heye AK, et al. Integrity of normal-appearing white matter: Influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2017;37:644–56. doi: 10.1177/0271678X16635657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Baykara E, Gesierich B, Adam R, Tuladhar AM, Biesbroek JM, Koek HL, et al. A Novel Imaging Marker for Small Vessel Disease Based on Skeletonization of White Matter Tracts and Diffusion Histograms. Ann Neurol 2016;80:581–92. doi: 10.1002/ana.24758. [DOI] [PubMed] [Google Scholar]
- [48].Sam K, Peltenburg B, Conklin J, Sobczyk O, Poublanc J, Crawley AP, et al. Cerebrovascular reactivity and white matter integrity. Neurology 2016;87:2333–9. doi: 10.1212/WNL.0000000000003373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pillai JJ, Mikulis DJ. Cerebrovascular reactivity mapping: an evolving standard for clinical functional imaging. AJNR Am J Neuroradiol 2015;36:7–13. doi: 10.3174/ajnr.A3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang T, Li Y, Guo X, Huang D, Ma L, Wang DJJ, et al. Reduced perfusion in normal-appearing white matter in mild to moderate hypertension as revealed by 3D pseudocontinuous arterial spin labeling. J Magn Reson Imaging JMRI 2016;43:635–43. doi: 10.1002/jmri.25023. [DOI] [PubMed] [Google Scholar]
- [51].Xekardaki A, Rodriguez C, Montandon M-L, Toma S, Tombeur E, Herrmann FR, et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology 2015;274:490–9. doi: 10.1148/radiol.14140680. [DOI] [PubMed] [Google Scholar]
- [52].Clark LR, Berman SE, Rivera-Rivera LA, Hoscheidt SM, Darst BF, Engelman CD, et al. Macrovascular and microvascular cerebral blood flow in adults at risk for Alzheimer’s disease. Alzheimers Dement Amst Neth 2017;7:48–55. doi: 10.1016/j.dadm.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rivera-Rivera LA, Turski P, Johnson KM, Hoffman C, Berman SE, Kilgas P, et al. 4D flow MRI for intracranial hemodynamics assessment in Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2016;36:1718–30. doi: 10.1177/0271678X15617171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Makedonov I, Black SE, MacIntosh BJ. Cerebral small vessel disease in aging and Alzheimer’s disease: a comparative study using MRI and SPECT. Eur J Neurol 2013;20:243–50. doi: 10.1111/j.1468-1331.2012.03785.x. [DOI] [PubMed] [Google Scholar]
- [55].Ruitenberg A, den Heijer T, Bakker SLM, van Swieten JC, Koudstaal PJ, Hofman A, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 2005;57:789–94. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- [56].Zhang B, Gu G-J, Jiang H, Guo Y, Shen X, Li B, et al. The value of whole-brain CT perfusion imaging and CT angiography using a 320-slice CT scanner in the diagnosis of MCI and AD patients. Eur Radiol 2017;27:4756–66. doi: 10.1007/s00330-017-4865-1. [DOI] [PubMed] [Google Scholar]
- [57].Nielsen RB, Egefjord L, Angleys H, Mouridsen K, Gejl M, M⌀ller A, et al. Capillary dysfunction is associated with symptom severity and neurodegeneration in Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 2017;13:1143–53. doi: 10.1016/j.jalz.2017.02.007. [DOI] [PubMed] [Google Scholar]
- [58].El Tayara NET, Delatour B, Volk A, Dhenain M. Detection of vascular alterations by in vivo magnetic resonance angiography and histology in APP/PS1 mouse model of Alzheimer’s disease. Magma N Y N 2010;23:53–64. doi: 10.1007/s10334-009-0194-y. [DOI] [PubMed] [Google Scholar]
- [59].Chen L, Mossa-Basha M, Balu N, Canton G, Sun J, Pimentel K, et al. Development of a quantitative intracranial vascular features extraction tool on 3D MRA using semiautomated open-curve active contour vessel tracing. Magn Reson Med 2018;79:3229–38. doi: 10.1002/mrm.26961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sagare AP, Sweeney MD, Makshanoff J, Zlokovic BV. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci Lett 2015;607:97–101. doi: 10.1016/j.neulet.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sweeney MD, Sagare AP, Zlokovic BV. Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2015. doi: 10.1038/jcbfm.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Craig-Schapiro R, Kuhn M, Xiong C, Pickering EH, Liu J, Misko TP, et al. Multiplexed immunoassay panel identifies novel CSF biomarkers for Alzheimer’s disease diagnosis and prognosis. PloS One 2011;6:e18850.. doi: 10.1371/journal.pone.0018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Weller RO, Hawkes CA, Kalaria RN, Werring DJ, Carare RO. White matter changes in dementia: role of impaired drainage of interstitial fluid. Brain Pathol Zurich Switz 2015;25:63–78. doi: 10.1111/bpa.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010;330:1774.. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lee S, Zimmerman ME, Narkhede A, Nasrabady SE, Tosto G, Meier IB, et al. White matter hyperintensities and the mediating role of cerebral amyloid angiopathy in dominantly-inherited Alzheimer’s disease. PloS One 2018;13:e0195838.. doi: 10.1371/journal.pone.0195838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].ladecola C The pathobiology of vascular dementia. Neuron 2013;80:844–66. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Montine TJ, Koroshetz WJ, Babcock D, Dickson DW, Galpern WR, Glymour MM, et al. Recommendations of the Alzheimer’s Disease-Related Dementias Conference. Neurology 2014;83:851–60. doi: 10.1212/WNL.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 2014. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hachinski V, World Stroke Organization. Stroke and Potentially Preventable Dementias Proclamation: Updated World Stroke Day Proclamation. Stroke 2015;46:3039–40. doi: 10.1161/STROKEAHA.115.011237. [DOI] [PubMed] [Google Scholar]
- [70].Filippi M, Agosta F, Barkhof F, Dubois B, Fox NC, Frisoni GB, et al. EFNS task force: the use of neuroimaging in the diagnosis of dementia. Eur J Neurol 2012;19:e131–140, 1487–501. doi: 10.1111/j.1468-1331.2012.03859.x. [DOI] [PubMed] [Google Scholar]
- [71].Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutr Burbank Los Angel Cty Calif 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 2015;18:521–30. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015;163:1064–78. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 1977;28:897–916. [DOI] [PubMed] [Google Scholar]
- [75].McDougal DB, Ferrendelli JA, Yip V, Pusateri ME, Carter JG, Chi MM, et al. Use of nonradioactive 2- deoxyglucose to study compartmentation of brain glucose metabolism and rapid regional changes in rate. Proc Natl Acad Sci U S A 1990;87:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Rokka J, Grӧnroos TJ, Viljanen T, Solin O, Haaparanta-Solin M. HPLC and TLC methods for analysis of [(18)F]FDG and its metabolites from biological samples. J Chromatogr B Analyt Technol Biomed Life Sci 2017;1048:140–9. doi: 10.1016/j.jchromb.2017.01.042. [DOI] [PubMed] [Google Scholar]
- [77].Sols A, Crane RK. Substrate specificity of brain hexokinase. J Biol Chem 1954;210:581–95. [PubMed] [Google Scholar]
- [78].Southworth R, Parry CR, Parkes HG, Medina RA, Garlick PB. Tissue-specific differences in 2-fluoro- 2-deoxyglucose metabolism beyond FDG-6-P: a 19F NMR spectroscopy study in the rat. NMR Biomed 2003;16:494–502. doi: 10.1002/nbm.856. [DOI] [PubMed] [Google Scholar]
- [79].Hers HG, De Duve C. [The hexosephosphatase system; partition of activity of glucose-6- phosphatase in the tissues]. Bull Soc Chim Biol (Paris) 1950;32:20–9. [PubMed] [Google Scholar]
- [80].Sokoloff L Measurement of local cerebral glucose utilization and its relation to local functional activity in the brain. Adv Exp Med Biol 1991;291:21–42. [DOI] [PubMed] [Google Scholar]
- [81].Huang MT, Veech RL. Metabolic fluxes between [14C]2-deoxy-D-glucose and [14C]2-deoxy-D- glucose-6-phosphate in brain in vivo. J Neurochem 1985;44:567–73. [DOI] [PubMed] [Google Scholar]
- [82].Hunt A, Schonknecht P, Henze M, Seidl U, Haberkorn U, Schroder J. Reduced cerebral glucose metabolism in patients at risk for Alzheimer’s disease. Psychiatry Res 2007;155:147–54. doi: 10.1016/j.pscychresns.2006.12.003. [DOI] [PubMed] [Google Scholar]
- [83].Samuraki M, Matsunari I, Chen W-P, Yajima K, Yanase D, Fujikawa A, et al. Partial volume effect- corrected FDG PET and grey matter volume loss in patients with mild Alzheimer’s disease. Eur J Nucl Med Mol Imaging 2007;34:1658–69. doi: 10.1007/s00259-007-0454-x. [DOI] [PubMed] [Google Scholar]
- [84].Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, et al. Multicenter standardized 18F- FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med Off Publ Soc Nucl Med 2008;49:390–8. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 2011;32:1207–18. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ossenkoppele R, van der Flier WM, Zwan MD, Adriaanse SF, Boellaard R, Windhorst AD, et al. Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology 2013;80:359–65. doi: 10.1212/WNL.0b013e31827f0889. [DOI] [PubMed] [Google Scholar]
- [87].Protas HD, Chen K, Langbaum JBS, Fleisher AS, Alexander GE, Lee W, et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol 2013;70:320–5. doi: 10.1001/2013.jamaneurol.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mosconi L, Rinne JO, Tsui WH, Murray J, Li Y, Glodzik L, et al. Amyloid and metabolic positron emission tomography imaging of cognitively normal adults with Alzheimer’s parents. Neurobiol Aging 2013;34:22–34. doi: 10.1016/j.neurobiolaging.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 2010;75:230–8. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Myoung PS, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med Off Publ Soc Nucl Med 2006;47:1778–86. [PubMed] [Google Scholar]
- [91].Niwa K, Kazama K, Younkin SG, Carlson GA, ladecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis 2002;9:61–8. doi: 10.1006/nbdi.2001.0460. [DOI] [PubMed] [Google Scholar]
- [92].Piert M, Koeppe RA, Giordani B, Berent S, Kuhl DE. Diminished glucose transport and phosphorylation in Alzheimer’s disease determined by dynamic FDG-PET. J Nucl Med Off Publ Soc Nucl Med 1996;37:201–8. [PubMed] [Google Scholar]
- [93].Jagust WJ, Seab JP, Huesman RH, Valk PE, Mathis CA, Reed BR, et al. Diminished glucose transport in Alzheimer’s disease: dynamic PET studies. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 1991;11:323–30. doi: 10.1038/jcbfm.1991.65. [DOI] [PubMed] [Google Scholar]
- [94].Fukuyama H, Kameyama M, Harada K, Nishizawa S, Senda M, Mukai T, et al. Glucose metabolism and rate constants in Alzheimer’s disease examined with dynamic positron emission tomography scan. Acta Neurol Scand 1989;80:307–13. [DOI] [PubMed] [Google Scholar]
- [95].Gejl M, Brock B, Egefjord L, Vang K, Rungby J, Gjedde A. Blood-Brain Glucose Transfer in Alzheimer’s disease: Effect of GLP-1 Analog Treatment. Sci Rep 2017;7:17490.. doi: 10.1038/s41598-017-17718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Ann Neurol 1994;35:546–51. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- [97].Mooradian AD, Chung HC, Shah GN. GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol Aging 1997;18:469–74. [DOI] [PubMed] [Google Scholar]
- [98].Kalaria RN, Harik SI. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J Neurochem 1989;53:1083–8. [DOI] [PubMed] [Google Scholar]
- [99].Horwood N, Davies DC. Immunolabelling of hippocampal microvessel glucose transporter protein is reduced in Alzheimer’s disease. Virchows Arch Int J Pathol 1994;425:69–72. [DOI] [PubMed] [Google Scholar]
- [100].Iwangoff P, Armbruster R, Enz A, Meier-Ruge W. Glycolytic enzymes from human autoptic brain cortex: normal aged and demented cases. Mech Ageing Dev 1980;14:203–9. [DOI] [PubMed] [Google Scholar]
- [101].Bigl M, Bmckner MK, Arendt T, Bigl V, Eschrich K. Activities of key glycolytic enzymes in the brains of patients with Alzheimer’s disease. J Neural Transm Vienna Austria 1996 1999;106:499–511. doi: 10.1007/s007020050174. [DOI] [PubMed] [Google Scholar]
- [102].Kilbourn MR. Small Molecule PET Tracers for Transporter Imaging. Semin Nucl Med 2017;47:536–52. doi: 10.1053/j.semnuclmed.2017.05.005. [DOI] [PubMed] [Google Scholar]
- [103].Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–97. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sposato LA, Kapral MK, Fang J, Gill SS, Hackam DG, Cipriano LE, et al. Declining Incidence of Stroke and Dementia: Coincidence or Prevention Opportunity? JAMA Neurol 2015;72:1529–31. doi: 10.1001/jamaneurol.2015.2816. [DOI] [PubMed] [Google Scholar]
- [105].Azarpazhooh MR, Avan A, Cipriano LE, Munoz DG, Sposato LA, Hachinski V. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimers Dement J Alzheimers Assoc 2018;14:148–56. doi: 10.1016/j.jalz.2017.07.755. [DOI] [PubMed] [Google Scholar]
- [106].Arenaza-Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: Clarifying terminology for preclinical studies. Neurology 2018;90:695–703. doi: 10.1212/WNL.0000000000005303. [DOI] [PMC free article] [PubMed] [Google Scholar]


