SUMMARY
Recent identification of a mammary gland-specific microbiome led to studies investigating bacteria populations in breast cancer. Malignant breast tumors have lower Lactobacillus abundance compared with benign lesions, implicating Lactobacillus as a negative regulator of breast cancer. Diet is a main determinant of gut microbial diversity. Whether diet affects breast microbiome populations is unknown. In a non-human primate model, we found that consumption of a Western or Mediterranean diet modulated mammary gland microbiota and metabolite profiles. Mediterranean diet consumption led to increased mammary gland Lactobacillus abundance compared with Western diet-fed monkeys. Moreover, mammary glands from Mediterranean diet-fed monkeys had higher levels of bile acid metabolites and increased bacterial-processed bioactive compounds. These data suggest that diet directly influences microbiome populations outside the intestinal tract in distal sites such as the mammary gland. Our study demonstrates that diet affects the mammary gland microbiome, establishing an alternative mechanistic pathway for breast cancer prevention.
In Brief
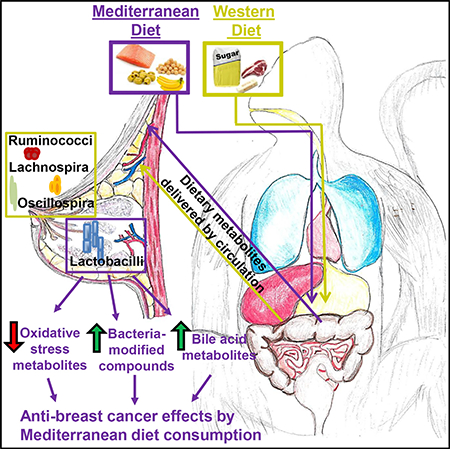
Using a non-human primate model of women’s health, Shively et al. demonstrate that diet plays a critical role in determining microbiota populations in tissues outside the gut, such as the mammary gland. These microbial populations modulate localized bile acid and bacterial-modified metabolites to potentially influence anticancer signaling pathways.
Graphical Abstract
INTRODUCTION
Diet has been extensively studied as a modifiable component of lifestyle that could influence breast cancer development. The Mediterranean diet is considered to be one of the healthiest dietary habits (Willett et al., 1995). Mediterranean dietary patterns are characterized by consumption of cereals (preferably as whole grains), legumes, nuts, vegetables and fruits, fish or seafood, white meat and eggs, and moderate to small amounts of poultry and dairy products. The principal source of dietary lipids in the Mediterranean diet is olive oil.
It has been suggested that a Mediterranean dietary pattern can protect against diabetes, cardiovascular disease, and cancer (Haegele et al., 1994; Pelucchi et al., 2009). Reported consumption of a Mediterranean diet pattern was associated with lower breast cancer risk for women with all subtypes of breast cancer, and a Western diet pattern was associated with greater risk (Castello´ et al., 2014). Protective effects of Mediterranean diet pattern on breast cancer risk were also observed in a recent prospective cohort study (Buckland et al., 2013) and a randomized, single-blind, controlled field trial in which participants were randomly allocated to a Mediterranean diet supplemented with either extra-virgin olive oil or mixed nuts or a control diet and advised to reduce dietary fat (Toledo et al., 2015). Associations between dietary intake and cancer may not be ascribed to a single nutrient but rather to multiple nutrients and foods and their synergistic effects. Thus, assessing diet as a whole, on the basis of overall dietary patterns, may provide more useful information on the role of diet in breast cancer risk than a single-nutrient approach. However, inference from population studies is difficult because of measurement error in dietary assessment that arises from dietary data being self-reported, often retrospective, and collected using food frequency questionnaires that require many assumptions (i.e., recipes, food composition tables) to derive nutrient and food intakes.
Diet is also recognized as a major component shaping the gut microbiome. Distinct patterns of gut microbiota composition are associated with the habitual consumption of animal fats, highfiber diets, and vegetable-based diets (Cotillard et al., 2013; David et al., 2014; Schnorr et al., 2014; Wu et al., 2011). Adherence to a Mediterranean diet decreased Ruminococcus and increased fecal Lachnospira and Prevotella in fecal samples, compared with samples from people consuming an omnivore diet (De Filippis et al., 2016). However, dietary influences on the microbiome in other tissue or organ sites other than the gut are undetermined.
A mammary gland-specific microbiome has been identified (Urbaniak et al., 2014). Mammary gland samples obtained from women undergoing lumpectomies, mastectomies, or breast reduction procedures living in Canada or Ireland showed distinct genus taxa differences: mammary gland samples from Canadian women displayed a high proportional abundance of Bacillus, Acinetobacter, Enterobacteriaceae, Pseudomonas, Staphylococcus, Propionibacterium, and Prevotella. Mammary gland samples from Irish women displayed a more than 3-fold higher abundance of Enterobacteriaceae, a 2-fold higher abundance of Staphylococcus, high Listeria welshimeri, a 2-fold higher abundance of Propionibacterium, and similar proportional abundance of Pseudomonas compared with Canadian women’s breast samples (Urbaniak et al., 2014). Whether the observed taxa differences or abundance differences were due to differences in BMI or obesity is unknown, because those data were unreported in the study. In another study investigating the microbiome of mammary gland tissue obtained from patients with benign tumors or malignant breast cancers, breast tissue from those with malignant breast cancer had decreased Lactobacillus (Hieken et al., 2016), suggesting mammary tissue dysbiosis as a possible driver of breast cancer.
To investigate the potential of diet pattern to modify breast tissue-specific microbiota populations, we obtained mammary gland samples from female Macaca fascicularis monkeys fed a Western diet or a Mediterranean diet for 31 months. This species is a well-established model of women’s health, with considerable use in the study of breast cancer risk (Dewi et al., 2013; Shively et al., 2004; Stute et al., 2006, 2012; Wood et al., 2006, 2007a, 2007b, 2007c). Our present study demonstrates that diet modulates mammary gland-specific microbiota. Consumption of a Mediterranean diet was associated with 10-fold higher breast tissue Lactobacillus abundance and altered breast tissue bile acid metabolite levels and bacterial-processed bioactive compounds, clearly demonstrating a dietary contribution to mammary gland health and homeostasis with implications for breast cancer prevention.
RESULTS
Female monkeys (M. fascicularis) were fed a Western diet or a Mediterranean diet for 31 months (see Table S1 for diet details). Mammary gland tissue sections (locations of tissue samples harvested for analyses are shown in Figure 1A) were used for 16S sequencing. We sequenced 16S (V4 region) genes on an Illumina MiSeq. We obtained 9.2096 × 104 high-quality bacterial reads. The final dataset had 1,470 operational taxonomic unit (OTUs) (including those occurring once with a count of 1) and a read range of 560 and 6,292. We excluded samples with fewer than 500 reads; a more typical cutoff is 1,000 reads. The number is arbitrary, but it is sufficient to reveal the most important gradients in microbial composition, even in high-diversity settings such as the gut. In breast tissue, where microbial diversity is lower, we used a lower cutoff to be sufficient.
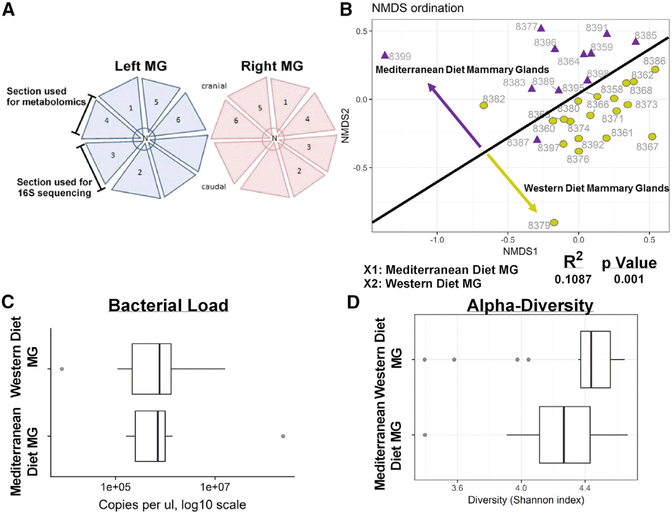
Figure 1. Habitual Diet Shifts Mammary Gland Bacterial Populations with No Effect on Quantity or Diversity.
(A) Diagram of mammary gland sections aseptically obtained and used for analysis.
(B) Non-metric multidimensional scaling (NMDS) analysis to visualize microbiome similarities in ordination plots. Western diet 16S samples (n = 19) shown as mustard green circles and Mediterranean diet 16S samples (n = 11) shown as purple triangles. Variation in community structure was assessed with permutational multivariate ANOVA (PERMANOVA) with treatment group as the main fixed factor. Microbiota from Western diet-fed monkey mammary gland samples significantly varied from Mediterranean diet-fed monkey mammary gland microbiota (R2 = 0.1087, p = 0.001).
(C) Bacterial load was determined via qPCR targeting the V4 region of the 16S gene. Results are expressed in copy number per microliter of DNA extract. No significant differences were observed by diet groups.
(D) Alpha diversity was estimated with the Shannon index on raw OTU abundance tables after filtering out contaminants. No significant differences were observed by diet groups.
Error bars show the min to max distribution.
We summarized OTU abundances with the Bray-Curtis index and performed a non-metric multidimensional scaling (NMDS) analysis to visualize microbiome similarities in ordination plots (Figure 1B). Formal significance testing was achieved with permutational ANOVAs. Mediterranean diet consumers had markedly different mammary gland microbiota populations compared with mammary glands from monkeys consuming a Western diet, resulting in two distinct microbiota populations based on dietary consumption. Diet had no effect on microbial load (Figure 1C) or alpha diversity (Figure 1D).
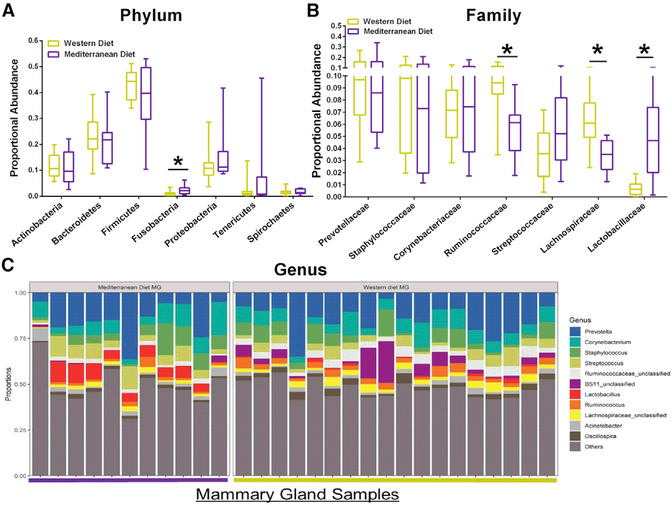
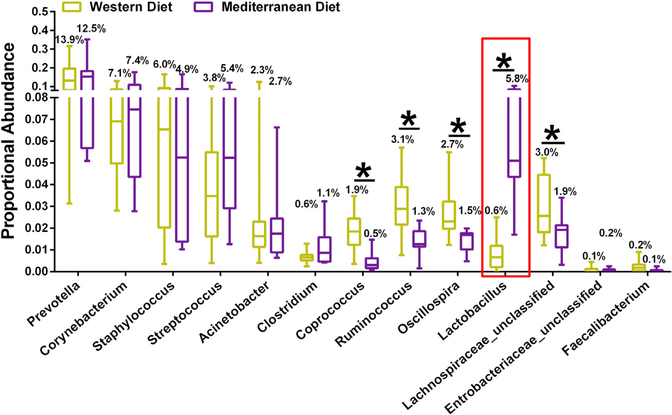
High-quality reads were classified using Greengenes as the reference database. We obtained consensus taxonomy for each OTU. We then aggregated OTU abundances into taxonomies and plotted the relative abundances. Consumption of Mediterranean diet was associated with significantly increased mammary gland Fusobacteria phylum populations, compared with a Western diet (Figure 2A). Consumption of Med-iterranean diet was associated with decreased mammary gland abundance of Ruminococcaceae and Lachnospiraceae bacteria and increased Lactobacillaceae populations compared with a Western diet (Figure 2B). After aggregating OTU abundances into genus-level groupings (Figures 2C and3), we observed an approximate 10-fold higher Lactobacillus abundance in the Mediterranean diet versus the Western diet group. Western diet-fed monkeys also had significantly higher abundance of Ruminococcus, Lachnospiraceae, Oscillospira, and Coprococcus genus bacteria in their mammary gland tissue.
Figure 2. Diet Modulates Mammary Gland Microbiota Clustering and Taxonomic Profiles.
(A) Taxonomic profiles of the mammary gland tissue from monkeys consuming a Western or Mediterranean diet at phylum level. *p < 0.05.
(B) Community composition between Western diet-fed and Mediterranean diet-fed monkey mammary gland samples at family level. *p < 0.05.
(C) Relative abundance of bacterial genera in different breast tissue samples is visualized by bar plots. Each bar represents a subject and each colored box a bacterial taxon. The height of a color box represents the relative abundance of that organism within the sample. “Other” represents lower abundance taxa. n = 11–18, *p < 0.05.
Error bars show the min to max distribution.
Figure 3. Differential Taxa between Western Diet-Fed and Mediterranean Diet-Fed Monkey Breast Tissue Microbiota.
Taxa with nominal p values < 0.05 at the genus level are shown by * and their mean percentage proportional abundances in each diet type. Consumption of Mediterranean diet led to an approximate 10-fold upregulation of Lactobacillus abundance in the mammary gland. n = 11–18, *p < 0.05.
Error bars show the min to max distribution.
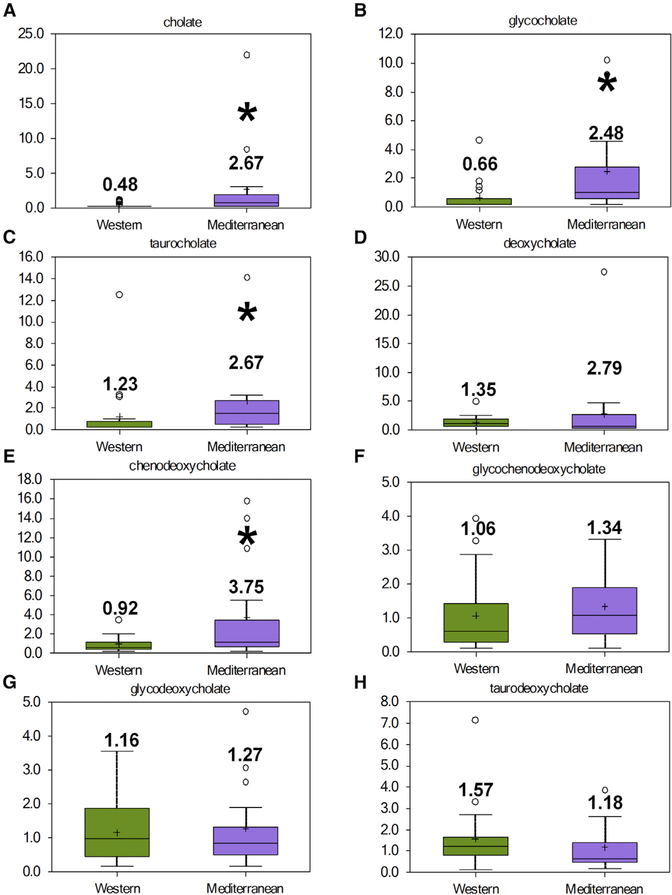
We used an untargeted global metabolomics approach to determine metabolite difference in mammary glands regulated by diet. Mediterranean diet consumers had significantly higher mammary gland levels of the bile acid metabolites cholate, gly-cocholate (GCA), taurocholate (TCA), and chenodeoxycholate (CDCA) compared with monkeys fed a Western diet (Figures 4A–4C and 4E). Although detectable (Figures 4D and 4F–4H), there were no significant differences in deoxycholate (DCA), glycochenodeoxycholate (GCDCA), glycodeoxycholate, or taur-odeoxycholate levels. Analysis of circulating plasma bile acid metabolites showed increased circulating cholate (Figure S1A) in blood from Mediterranean diet-fed monkeys compared with Western diet-fed animals. No other bile acid metabolites were significantly different between dietary groups (Figures S1B–S1H).
Figure 4. Diet Differentially Modulates Bile Acid Metabolite Levels in the Mammary Glands.
Bile acid metabolites were determined by global untargeted metabolomics analysis of mammary gland samples from monkeys consuming a Western or Mediterranean diet for 31 months.
(A) Cholate (CA).
(B) Glycocholate (GCA).
(C) Taurocholate (TCA).
(D) Deoxycholate (DCA).
(E) Chenodeoxycholate (CDCA).
(F) Glycochenodeoxycholate (GCDCA).
(G) Glycodeoxycholate (GDCA).
(H) Taurodeoxycholate (TDCA).
n = 17–21, *p < 0.05. Error bars show the min to max distribution.
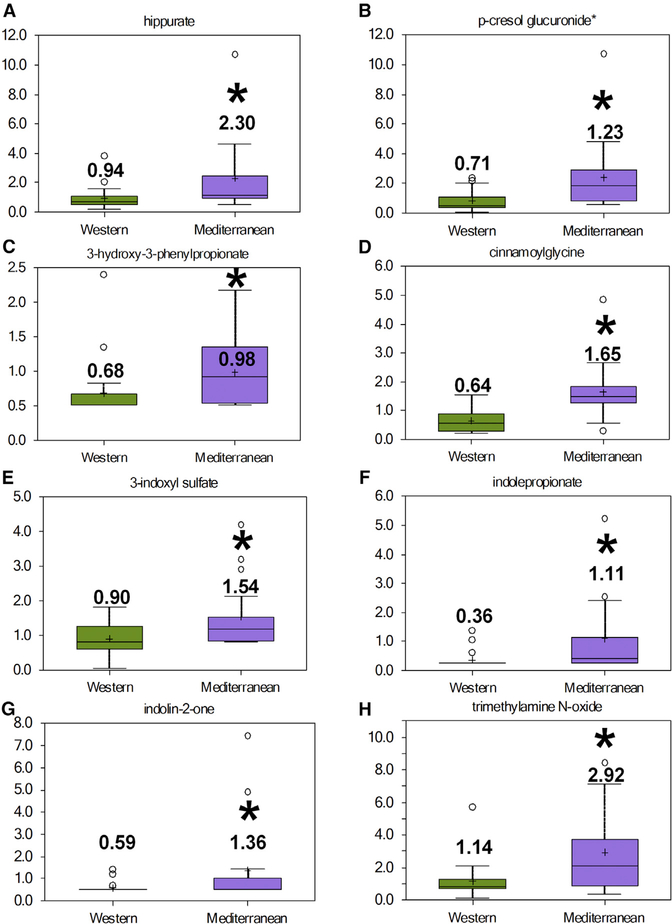
In the past decade, high levels of interest in gut microbiota effects on metabolite processing have led to the discovery of new bacterial-mediated transformation of dietary components and their impact on host health and the development of disease (Holmes et al., 2012; Zhang and Davies, 2016). Multiple metabolomic studies using germ-free and conventionally housed mice along with antibiotic-treated mice have identified metabolites regulated by host bacterial populations. We observed higher levels of many of these bacteria-processed metabolites in the mammary glands of Mediterranean diet-fed monkeys relative to metabolite levels in mammary glands of Western diet-fed monkeys (Figure 5). Consumption of Mediterranean diet was associated with significant elevations of hippurate (Figure 5A), p-cresol glucuronide (Figure 5B), 3-hydroxy-3-phenylpropionate (Figure 5C), cinnamoylglycine (Figure 5D), 3-indoxyl sulfate (Figure 5E), indole propionate (Figure 5F), indolin-2-one (Figure 5G), and trimethylamine N-oxide (Figure 5H) compared with Western diet. Mammary gland levels of the parent amino acids from which the bioactive microbial-derived compounds are derived (phenylalanine, tyrosine, and tryptophan) were not significantly altered between dietary groups in the mammary glands (Figure S2). Analyses of circulating plasma levels of bacterial-processed metabolites indicate differential regulation of bacterial-modified compounds when comparing regulated metabolites in the mammary gland with bacterial-modified metabolites in plasma samples. There were no diet-related differences in levels of phenylalanine-derived bacterial-modified metabolites (hippurate, 3-hydroxy-3- phenylpropionate, and cinnamoylglycine), but p-cresol-glucuronide, 3-indoxyl sulfate, indole propionate, and trimethylamine N-oxide were significantly elevated in the plasma of Mediterranean diet consumers (Figure S3). These data suggest that the elevated mammary gland phenylalanine-derived bacterial-modified metabolites may be site specific, possibly through modification by mammary gland localized microbiota, as plasma levels remained unchanged.
Figure 5. Diet Differentially Modulates Bacterial-Processed Bioactive Compounds.
(A) Hippurate.
(B) p-Cresol glucuronide.
(C) 3-Hydroxy-3-phenylpropionate.
(D) Cinnamoylglycine.
(E) 3-Indoxyl sulfate.
(F) Indole propionate.
(G) Indolin-2-one.
(H) Trimethylamine N-oxide.
n = 17–21, *p < 0.05. Error bars show the min to max distribution.
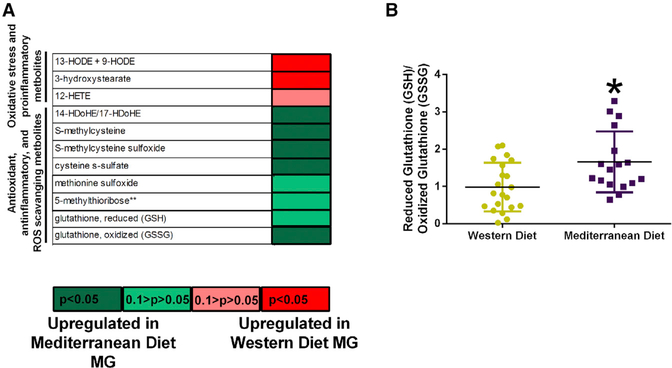
Diet along with microbial-mediated metabolites may regulate oxidative stress. We investigated the oxidative stress, inflammation, antioxidant, and reactive oxygen species-scavenging metabolite data to determine whether diet affected these processes in the mammary gland tissue (Figure 6). Consumption of the Mediterranean diet led to upregulated 14-HDoHE, S-methylcysteine, S-methylcysteine sulfoxide, cysteine s-sulfate, methionine sulfoxide, 5-methylribose, glutathione-reduced, and glutathione-oxidized metabolites compared with Western diet. Consumption of Western diet led to increased 13-HODE, 9-HODE, 3-hyrdoxystearate, and 12-HETE metabolites compared with Mediterranean diet (Figure 6A). Analysis of reduced glutathione (GSH)-to-oxidized glutathione (GSSG) ratio in the mammary gland tissues indicated elevated GSH-to-GSSG ratio in the Mediterranean diet-fed monkeys (Figure 6B).
Figure 6. Diet Shifts Oxidative Stress Metabolites in the Mammary Gland Tissue.
(A) Heatmap of oxidative stress, pro-inflammatory,antioxidant, anti-inflammatory, and reactive oxygen species (ROS)-scavenging metabolites in the mammary glands (MG).
(B) Reduced glutathione (GSH)-to-oxidized glutathione (GSSG) metabolite ratio in mammary glands of Western and Mediterranean diet-fed monkeys.
n = 17–21, *p < 0.05. Error bars show SD.
DISCUSSION
Consumption of a Mediterranean diet was associated with distinct mammary gland microbiota populations compared with Western diets, but no differences were observed in total bacteria content or diversity (Figure 1). Similar to human studies determining effects of Mediterranean diets on gut microbiota populations, Ruminococcus abundance was significantly decreased in mammary gland tissue of the Mediterranean diet group, suggesting some commonly regulated microbiota population between gut and mammary gland tissues (De Filippis et al., 2016). Unlike the human gut microbiome, consumption of Mediterranean diet was not associated with non-human primate mammary gland Prevotella abundance and was associated with reduced Lachnospiraceae abundance, suggesting that there may be differential regulation of microbiota by diet depending on tissue type (Figures 2 and 3). The fecal microbiome of a subset of these monkeys consuming either a Western (n = 10) or Mediterranean (n = 10) diet was also explored (Nagpal et al., 2018). Interestingly, the effect of diet on the fecal microbiome compared with the mammary gland microbiome displayed similarities as well as divergence. Increased Lactobacillus and decreased Ruminococcus and Coprococcus populations were observed in both mammary gland and feces of Mediterranean diet-fed monkeys. For Oscillospira, there were higher levels in feces and lower levels in mammary gland of Mediterranean diet-fed monkeys compared with the Western diet-consuming counterparts. Some microbiota populations differed by diet group in the feces but not in the mammary gland, as Faeca-libacterium and Prevotella were higher in the feces but not mammary gland of Mediterranean diet-fed monkeys.
Using a non-human primate model, we demonstrated that diet alone may modulate mammary gland microbiota population. Consumption of Mediterranean diet resulted in an approximate 10-fold increase in mammary gland Lactobacillus abundance compared with mammary tissue from Western diet-fed monkeys. Lactobacillus is often thought of as commensal bacteria and is commonly used in probiotic formulations. Oral consumption of Lactobacillus decreased triple-negative 4T1 breast cancer tumor growth in a syngeneic breast cancer model (Arago´ n et al., 2014; Maroof et al., 2012). This anti-tumor effect is thought to be mediated by immune cell modulation. For example, Lactobacillus administration in breast tumor-bearing mice altered cytokine production in favor of an anti-tumor Th1 profile (Maroof et al., 2012). Others showed that oral Lactobacillus increased immune cell stimulation in intestinal Peyer’s patches with subsequent increased migration to a host of other organ locations, including mammary gland tissue, implicating Lactobacillus supplementation as a possible breast cancer prevention agent (Brandtzaeg et al., 2004; de Moreno de LeBlanc et al., 2005). Intestinal immune stimulation followed by migration to distant organ sites represents one possible mechanism by which oral probiotics may affect tumorigenesis. However, lactating women given a daily oral dose of Lactobacillus displayed elevated Lactobacillus populations in their breast milk, suggesting that oral consumption of probiotics can directly affect the mammary gland microbiome (Jiménez et al., 2008). Moreover, orally administered Lactoba-cillus salivarius in late pregnancy significantly decreased the rate and severity of lactation mastitis, giving further evidence in support of orally administered bacteria modulating breast health (Fernández et al., 2016). Our study now provides strong evidence that dietary intake alone may modulate the mammary gland microbiome (Figures 2C and 3), especially because subjects were living side by side in the same building, representing a breast cancer prevention model.
We found elevated bile acid metabolites (Figure 4) in mammary glands of monkeys fed a Mediterranean diet compared with those fed a Western diet. The presence of bile acid in cystic breast tissue was identified decades ago in humans (Javitt et al., 1994). In women given oral labeled CDCA before breast cyst aspiration, CDCA levels were concentrated in the cyst fluid rather than circulating in the plasma. Moreover, farnesoid X receptor, a bile acid receptor, was identified both in benign and malignant breast tissue; it was associated with increased apoptosis and repressed aromatase expression (Swales et al., 2006).Therefore, elevated bile acid metabolites may increase far-nesoid X receptor (NR1H4) activation; this would then decrease localized estrogen synthesis and increase apoptosis, reducing breast neoplastic potential. Treatment of the estrogen receptor-positive breast cancer cell lineMCF7 with either CDCA or GCDCA resulted in differential effects on proliferation; GCDCA increased MCF7 proliferation, while treatment with CDCA reduced proliferation (Baker et al., 1992). We observed increased CDCA metabolite levels (anti-proliferative) in mammary glands of monkeys fed a Mediterranean diet (Figures 4E and 4F), with no significant differences in GCDCA (proliferative), possibly implicating bile acid metabolite regulation as a breast cancer prevention mechanism modulated by diet. TCA and GCA metabolites were enriched in mammary glands from Mediterranean diet-consuming monkeys compared with mammary gland tissue from Western diet-consuming monkeys (Figures 4B and 4C). These conjugated bile acids are modified by the presence of microbes (Ridlon et al., 2014, 2016a, 2016b; Swann et al., 2011). Interestingly, analysis of circulating plasma bile acid metabolite levels indicated no significant regulation of GCA, TCA, or CDCA by diet (Figure S1), most likely suggesting mammary gland-specific microbial modulation of bile acid metabolites (another possibility being the preferential accumulation of bile acids in the mammary gland tissue). Lactobacillus contains bile salt hydrolase activity, which was previously shown to be necessary for the production of secondary bile acid metabolites that serve as agonists for farnesoid X receptors (Li et al., 2013). The enrichment of Lactobacillus in the mammary glands of Mediterranean diet-fed monkeys (Figures 2C and 3) may then increase mammary gland-specific secondary bile acid metabolites to activate farnesoid X receptor (NR1H4) signaling for anticancer properties. Elevated plasma DCA was observed in postmenopausal women with newly diagnosed breast cancer compared with healthy age- and BMI-matched control plasma samples (Costarelli and Sanders, 2002a, 2002b). DCA is a mutagenic compound (Watabe and Bernstein, 1985). We observed no significant changes in DCA bile acid metabolite levels by diet, but there was a trend toward decreased DCA in the plasma of Mediterranean diet-fed monkeys (Figure S1).
On the other hand, estrogen metabolites are conjugated to bile acids for excretion. Bacteria with β-glucuronidase activity can deconjugate estrogen from bile acids to elevate estrogen metabolite bioavailability (Kwa et al., 2016). Lactobacillus has β-glucuronidase activity, suggesting that mammary glands from Mediterranean diet-fed monkeys with high Lactobacillus abundance (Figures 2 and 3) and higher bile acid metabolites (Figure 4) may have elevated localized estrogen bioavailability because of bacterial-mediated deconjugation independent of aromatase activity. We measured circulating serum estradiol levels at the time of necropsy in monkeys consuming Western or Mediterranean diet and observed comparable levels in the two groups (p = 0.29) (Table 1). Mammary tissue levels of estradiol were not measured and could differ depending on the tissue milieu of hormone converting enzymes and hormones that may be effected by microbiota. Tissue-level regulation of estradiol will be explored further in future studies. Although β-glucuronidase-positive bacteria populations in the gut are implicated in estrogen receptor-positive breast cancer (Kwa et al., 2016), the effect of enriched breast tissue-specific β-glucuronidase positive bacteria on bile acid deconjugation of estradiol metabolites needs further investigation.
Table 1.
Non-human Primate Subject Characteristics at Baseline and after 31 Months of Diet Administration
| Western Diet (n = 21) | Mediterranean Diet (n = 17) | |||
|---|---|---|---|---|
| Estimated age at study onset (years) | 8.8 ± 0.1 | 8.8 ± 0.2 | ||
| Body weight (% change) | 18.43 ± 4.70* | 2.60 ± 3.04 | ||
| BMI (% change) | 20.76 ± 4.15* | 5.90 ± 2.86 | ||
| Serum estradiol (pg/mL) | 87.1 ± 21.3 | 57.8 ± 15.5 | ||
| Baseline | Terminal | Baseline | Terminal | |
| Body weight (kg) | 3.08 ± 0.12 | 3.69 ± 0.25** | 3.07 ± 0.17 | 3.25 ± 0.23 |
| BMI (kg/m2) | 40.16 ± 1.08 | 48.73 ± 2.44** | 40.20 ± 1.22 | 42.61 ± 1.80 |
| Total plasma cholesterol (mg/dL) | 130.86 ± 5.00 | 157.19 ± 6.35** | 133.35 ± 4.42 | 148.65 ± 9.50 |
| HDL cholesterol (mg/dL) | 57.86 ± 3.29 | 72.43 ± 6.06** | 58.94 ± 2.75 | 57.24 ± 5.23 |
| Plasma cortisol (μg/dL) | 41.68 ± 2.12 | 36.80 ± 1.59* | 42.23 ± 1.78 | 31.64 ± 1.59*** |
p < 0.05, Western to Mediterranean diet comparison;
p < 0.05, Western diet baseline to terminal time point comparison;
p < 0.05, Mediterranean diet baseline to terminal time point comparison.
There is a growing body of evidence demonstrating an association between phenolic and phytochemical components in Mediterranean diets and important health benefits (Visioli and Bernardini, 2011). As shown in Figure 5, monkeys consuming a Mediterranean diet had significantly higher levels of several conjugated phenolic metabolites such as p-cresol sulfate (tyrosine metabolite), hippurate, cinnamoylglycine, and 3-hydroxy-3-phenylpropionate (phenylalanine metabolites) compared with the Western diet group. Other metabolites that accumulated in mammary tissue of the Mediterranean diet-fed monkeys included indole-containing substances (tryptophan metabolites) such as indolin-2-one, 3-indole sulfate, and indole propionate. These regulated polyphenol metabolites could have come from the olive oil, fruits, and vegetables enriched in the Mediterranean diet compared with the Western diet. There were no significant differences in tryptophan, phenylalanine, or tyrosine metabolite levels in the mammary glands (parental amino acid metabolite data shown in Figure S2), suggesting that these metabolite changes may reflect changes in microbial metabolism within the two diet regimens. Further supporting the role of microbial mediated bioactive compound generation, there were no changes in plasma metabolite levels of hippurate, cinnamoylglycine, 3-hydroxy-3-phenylpropionate, or phenylalanine (Figure S3) by diet, suggesting the microbial modulation of these metabolites may be localized to the mammary gland tissue. There were significant differences in circulating plasma metabolites p-cresol sulfate, tyrosine, tryptophan, 3-indole sulfate, and indole propionate (Figures S2 and S3), possibly indicating gut microbiome-dependent systematic effects.
Elevated hippurate is a phenylalanine metabolomic marker associated with increased gut microbiome diversity and elevated fruit and vegetable consumption and negatively correlated with metabolic syndrome risk in human patients (Pallister et al., 2017). Another metabolic benzoate-derivative marker, 3-hydroxy-3-phenylpropionate, also positively correlates with gut bacterial diversity and fruit and whole grain intake (Pallister et al., 2017). Comparison of plasma metabolite profiles between conventional and germ-free mice demonstrated the required presence of bacteria for the formation of hippurate, p-cresol sulfate, cinnamoylglycine, and 3-hydroxyl-3-phenylpropionate (Wikoff et al., 2009). We observed elevated hippurate (Figure 5A) and3-hydroxy-3-phenylpropionate (Figure 5C) in the mammary glands of Mediterranean diet-fed monkeys. These data suggest that the levels of benzoate-derived metabolites in those fed a Mediterranean diet correlated with increased fruit and vegetable intake but not mammary gland bacterial diversity (Figure 1E).
We also observed elevated bacterial-processed tryptophanderived metabolites 3-indoxyl sulfate (Figure 5E), indole propionate (Figure 5F), and indolin-2-one (Figure 5G). Mammary gland Lactobacillus (significantly higher in Mediterranean diet-fed monkeys) and Clostridium (trend toward higher levels in Mediterranean diet-fed monkeys) can convert tryptophan into indole-derived bioactive products (Chyan et al., 1999; Wikoff et al., 2009; Zelante et al., 2013; Zhang and Davies, 2016), which have diverse biological functions, including activation of aryl hydrocarbon receptor-mediated IL-22 production supporting Th17 response, stimulation of pregnane X receptor (NR1I2) to inhibit pro-tumorigenic NFκB signaling, and decreased pro-inflammatory TNF-α cytokine production. Some indole products also scavenge hydroxyl radicals, supporting an antioxidant environment (Chyan et al., 1999; Venkatesh et al., 2014; Zhou et al., 2006). Activation of Th17 immune responses may initially prevent establishment of tumor formation (Lim and Savan, 2014). Moreover, inhibition of inflammation and reduction of reactive oxygen species may prevent the development of breast cancer, highlighting possible molecular mechanisms by which dietary modulated bacterial-processed bioactive metabolites may inhibit breast cancer formation. In support of this mechanism, we show that Mediterranean diet consumers had reduced oxidative stress and pro-inflammatory metabolites in the mammary gland tissue (Figure 6). This was associated with an increase in reactive oxygen species-scavenging metabolites and decreased proinflammatory oxidized lipid products, suggesting that regulation of microbial-mediated metabolites by Mediterranean diet consumption may affect localized mammary gland oxidative stress.
In conclusion, we determined that diet significantly regulated mammary gland-specific microbiome populations in a well-established non-human primate model of women’s health. Mammary gland from Mediterranean diet consumers had a 10-fold higher Lactobacillus abundance compared with Western dietfed monkeys. Conversely, Western diet-fed monkeys had significantly higher abundance of Ruminococcus, Lachnospiraceae, Oscillospira, and Coprococcus genus bacteria in their mammary gland tissue. Mammary gland samples from Mediterranean dietfed monkeys had elevated bile acid metabolites and bacterial-processed bioactive compounds that may have anticancer properties. Taken together, these data highlight the impact of diet on mammary gland-specific microbiota and metabolites, which may represent a potential mechanism by which diet can affect breast inflammation and modulate breast cancer risk. Thus, changes in diet or supplementation with selected probiotics could represent a potential regimen for breast cancer prevention.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Katherine L. Cook (klcook@wakehealth.edu)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Subjects
All procedures involving primates were conducted in accordance with institutional, state, and federal laws for the use of nonhuman primates in laboratory settings and approved by the institutional animal care and use committee. Forty adult 8.8 year old research naive female cynomolgus macaques (M. fascicularis) were obtained (SNBL USA SRC, Alice, TX), quarantined in single cages for one month, and moved to social groups of four animals each which lived in pens (3 m X 3 m X 3 m) with daylight exposure, on a 12/12 light/dark cycle, with monkey chow and water available ad libitum.
The monkeys were habituated to their social groups, characterized during a 7 month baseline phase, and then assigned to receive either Western or Mediterranean diet for 31 months using stratified randomization, balanced on pretreatment characteristics that reflect overall health including body weight (BW), body mass index (BMI), basal cortisol, and plasma high density lipoprotein cholesterol concentrations. See Table 1 for baseline and terminal metabolic parameters. BMI was calculated as previously described (Jayo et al., 1993). In brief, BW and body length (suprasternal notch to pubic symphysis) were measured at the end of 7-month baseline metabolic normalization period and again after the 31 months of experimental diet administration. BMI was calculated as BW/ (body length (m))2. For cortisol measurements, a morning blood samples were taken within 5 min of capture and sedation with ketamine hydrochloride (10 mg/kg) and used with commercially available coated tube RIA kit from Diasource (Louvain-la-Neuve, Belgium). Total plasma cholesterol (TPC) and HDL were measured as previously described (Sophonsritsuk et al., 2013). In brief, TPC and HDL were determined in the Wake Forest Primate Center Clinical Chemistry Laboratory using ACE cholesterol and ACE HDL-C reagents and ACE ALERA autoanalyzer from Alfa Wasserman Diagnostic Technologies (West Caldwell, NJ). Estradiol was measured in serum samples obtained at necropsy using a commercially available competitive human targeted ELISA (Estradiol (Sensitive) ELISA kit # IB78239) obtained from IBL America (Minneapolis, MN, USA). In brief, the kit components had a standard range of 3.0 to 200.0 pg/mL and a reported sensitivity of < 1.4 pg/mL. In order to remove interfering substances, serum samples were extracted with ether, the extract was then dried and reconstituted with the zero standard calibrator from the kit prior to analysis. Values from human controls from outside manufacturers were within accepted ranges. Serum sample collection for estradiol measurement was not timed by the menstrual cycle.
METHOD DETAILS
Diets
Monkeys consumed either the Western or Mediterranean Diets (See Table S1. Ingredients in experimental diets. Related to Table 1) which were formulated to be isocaloric with respect to protein, fat and carbohydrates, and identical in cholesterol content (320mg / 2000 calories/day). The Western diet was formulated to be similar to that consumed by American women age 40–49 as reported by the USDA (U.S. Department of Agriculture, Agricultural Research Service, 2014). Specifically, protein and fat were derived mainly from animal sources; the diet was high in saturated fats and sodium, and low in monounsaturated (MUFA) and n-3 fatty acids (U.S. Department of Agriculture, Agricultural Research Service, 2014). The Mediterranean diet was formulated to mimic key aspects of the Mediterranean diet. Protein and fats are derived mainly from plant sources, with some lean protein from fish and dairy, and MUFAs are relatively high (Be´ dard et al., 2012; Kafatos et al., 2000). The Mediterranean diet n-6:n-3 fatty acid ratio was similar to a traditional hunter-gatherer type diet (Cordain et al., 2005), higher in complex carbohydrates and fiber, and lower in sodium and refined sugars (see Table S2. Experimental diet composition compared with human dietary patterns. Related to Table 1). Two animals were placed on a standard monkey chow due to Mediterranean diet intolerance. The data from these animals are not shown.
Breast tissue and plasma collection
Plasma was collected after 23 months of diet consumption. Breast tissue was collected after 31 months of diet consumption. Following ketamine (15 mg/kg) sedation sodium pentobarbital was administered to attain surgical anesthesia (approximately 13 mg/kg, i.v.). Tissues were collected according to the diagram shown in Figure 1A and snap frozen at –80°C or fixed in 4% paraformaldehyde. The final sample size was n = 21 for Western diet and n = 17 for Mediterranean diet.
DNA extraction, PCR, sequencing, and sequence processing
Mammary gland specimens were placed into a MoBio PowerMag Soil DNA Isolation Bead Plate. DNA was extracted following the manufacturer’s protocol. Bacterial 16S sequencing, data analysis, and interpretation were performed by Microbiome Insights, Vancouver, Canada. Bacterial 16S rRNA genes were PCR-amplified with dual-barcoded primers targeting the V4 region, following the methods of (Kozich et al., 2013). Amplicons were sequenced with an Illumina MiSeq using the 250-bp paired-end kit (v.2). Sequences were denoised, taxonomically classified using Greengenes (v. 13_8) as the reference database (https://www.mothur.org/wiki/ Greengenes-formatted_databases), and clustered into 97%-similarity operational taxonomic units (OTUs) with the mothur software package (v. 1.39.5) (Schloss et al., 2009), following the recommended procedure (https://www.mothur.org/wiki/MiSeq_SOP; accessed Nov 2017). The potential for contamination was addressed by co-sequencing DNA amplified from specimens and from four each of template-free controls and extraction kit reagents processed the same way as the specimens. Two positive controls, consisting of cloned SUP05 DNA, were also included (number of copies = 2*10^6). In brief, the SUP05 16S DNA sequence was taken from environmental data via. (NCBI Sequence ID: HQ673668.1). The sequence was then synthetically created using IDT gBlocks (https://www.idtdna.com/site/order/gblockentry [idtdna.com]), which was then PCR amplified and plasmid cloned using Invitrogen’s TOPO cloning kit (https://www.thermofisher.com/order/catalog/product/K457501 [thermofisher.com]). The cloned plasmid sequence was verified using our standard amplicon sequencing protocol. Operational taxonomic units were considered putative contaminants (and were removed) if their mean abundance in controls reached or exceeded 25% of their mean abundance in specimens.
Metabolomics analysis
Samples were processed and metabolomics was performed by Metabolon, Raleigh, NC. In brief, samples were prepared using the automated MicroLab STAR® system from Hamilton Company. Several recovery standards (see below) were added prior to the extraction process for quality control purposes. The resulting extract was divided into five fractions: two for analysis by two separate reverse phase (RP)/UPLC-MS/MS methods with positive ion mode electrospray ionization (ESI), one for analysis by RP/UPLC-MS/ MS with negative ion mode ESI, one for analysis by HILIC/UPLC-MS/MS with negative ion mode ESI, and one sample was reserved for backup. Samples were placed briefly on a TurboVap® (Zymark) to remove the organic solvent.
Authentic and Recovery standards used in non-targeted global metabolomics
Authentic standards of d7-glucose, d3-leucine, d8-phenylalanine and d5-tryptophan were purchased from Cambridge Isotope Laboratories (Andover, MA). D5-hippuric acid, d5-indole acetic acid and d9-progesterone were procured from C/D/N Isotopes, Inc. (Pointe-Claire, Quebec). Bromophenylalanine was provided by Sigma-Aldrich Co. LLC. (St. Louis, MO) and amitriptyline was from MP Biomedicals, LLC. (Aurora, OH). Recovery standards of DL-2-fluorophenylglycine and DL-4-chlorophenylalanine were from Aldrich Chemical Co. (Milwaukee, WI). Tridecanoic acid was purchased from Sigma-Aldrich (St. Louis, MO) and d6-cholesterol was from Cambridge Isotope Laboratories (Andover, MA). Standards for the HILIC dilution series of alpha-ketoglutarate, ATP, malic acid, NADH and oxaloacetic acid were purchased from Sigma-Aldrich Co. LLC. (St. Louis, MO) while succinic acid, pyruvic acid and NAD+ were purchased from MP Biomedicals, LLC. (Santa Ana, CA).
Ultrahigh performance liquid chromatography-tandem mass spectroscopy (UPLC-MS/MS)
All experiments used a Waters ACQUITY ultra-performance liquid chromatography (UPLC) and a Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. The scan range varied slighted between methods but covered 70–1000 m/z. Raw data were extracted, peak- identified and processed for quality control using Metabolon’s hardware and software. Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities. Peaks were quantified using area-under-the-curve.
QUANTIFICATION AND STATISTICAL ANALYSIS
16S statistical analysis
Alpha diversity was estimated with the Shannon index on raw OTU abundance tables after filtering out contaminants. The significance of diversity differences was tested with an ANOVA. To estimate beta diversity across samples, we excluded OTUs occurring in fewer than 10% of the samples with a count of less than three and computed Bray-Curtis indices. We visualized beta diversity, emphasizing differences across samples, using non-metric multidimensional (NMDS) ordination. No significant pen effect was observed on microbiota populations. Variation in community structure was assessed with permutational multivariate analyses of variance (PERMANOVA) with treatment group as the main fixed factor and using 4,999 permutations for significance testing. All analyses were conducted using R software. Proportional abundance of selected microbiota were graphed and multiple unpaired t tests performed (correcting for multiple comparisons using Holm-Sidak method) was used and a value of p ≤ 0.05 was considered statistically significant.
Metabolomics bioinformatics and statistics
The informatics system consisted of four major components, the Laboratory Information Management System (LIMS), the data extraction and peak-identification software, data processing tools for quality control and compound identification, and a collection of information interpretation and visualization tools for use by data analysts. The hardware and software foundations for these informatics components were the LAN backbone, and a database server running Oracle 10.2.1.1 Enterprise Edition, respectively. Following log transformation and imputation of missing values, if any, with the minimum observed value for each compound, Welch’s two-sample t test was used to identify biochemicals that differed significantly between Western diet and Mediterranean diet experimental groups. A p value of p ≤ 0.05 was considered statistically significant.
DATA AND SOFTWARE AVAILABILITY
The metabolomics (Table S3. Monkey mammary gland metabolite profiles. Related to Figures 4, 5, and 6) and genus level 16S sequencing OTU proportional abundance (Table S4. Monkey mammary gland genus OTU proportional abundance. Related to Figures 2 and 3) are deposited in the supplemental data section.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| d7-glucose, d3-leucine, d8-phenylalanine and d5-tryptophan | Cambridge Isotope Laboratories | (Evans et al., 2014) |
| D5-hippuric acid, d5-indole acetic acid and d9-progesterone | C/D/N Isotopes, INC | (Evans et al., 2014) |
| Bromophenylalanine, tridecanoic acid, alpha-ketoglutarate, ATP, malic acid, NADH and oxaloacetic acid | Sigma-Aldrich | (Evans et al., 2014) |
| Amitriptyline, succinic acid, pyruvic acid and NAD+ | MP Biomedicals, LLC. | (Evans et al., 2014) |
| DL-2-fluorophenylglycine and DL-4-chlorophenylalanine | Aldrich Chemical | (Evans et al., 2014) |
| Critical Commercial Assays | ||
| Estradiol (Sensitive) ELISA kit | IBL America Minneapolis, MN, USA | # IB78239 |
| PowerMag Soil DNA Isolation kit | Mo Bio Laboratories- QIAGEN | 27000-4-KF |
| MiSeq Reagent Kits v2 | Illumina | MS-102-2003 |
| Total cholesterol | Alfa Wasserman | http://www.alfawassermannus.com/dt-aceaxcel.asp |
| HDL cholesterol | Alfa Wasserman | http://www.alfawassermannus.com/dt-aceaxcel.asp |
| Cortisol RIA | Diasource (Louvain-la-Neuve, Belgium) | Cortisol RIA CT KIPI-28000 |
| Deposited Data | ||
| Metabolomics | Metabolon Raleigh, NC, USA | Uploaded in Supplemental data (Table S3) |
| 16S sequencing OTU proportional abundance | Microbiome Insights Vancouver, Canada | Uploaded in supplemental data (Table S4) |
| Experimental Models: Organisms/Strains | ||
| Cynomolgus macaques (M. fascicularis) | SNBL USA SRC, Alice, TX | https://snbl.com/ |
| Oligonucleotides | ||
| Dual-barcoded primers | IDT | Supplemental Data, Appendix D in Kozich et al., 2013 |
| Software and Algorithms | ||
| Greengenes (v. 13_8) | (Schloss et al., 2009) | https://www.mothur.org/wiki/Greengenes-formatted_databases |
| mothur software package (v. 1.39.5) | (Kozich et al., 2013) | (http://www.mothur.org/wiki/MiSeq_SOP) |
| Metabolon peak identification software | (Evans et al., 2014) | https://www.metabolon.com/what-we-do/our-technology |
Highlights.
Diet modulates mammary gland microbiota populations in a non-human primate model
Consumption of Mediterranean diet elevates mammary gland Lactobacillus abundance
Mediterranean diet increases breast bile acid and bacterial-modified metabolites
Consumption of Mediterranean diet decreases reactive oxygen species metabolites
ACKNOWLEDGMENTS
This research was supported by the Chronic Disease Research Fund (to K.L.C.), American Cancer Society Research Scholar Grant RSG-16–204-01NEC (to K.L.C.), Career Catalyst Grant from the Susan G. Komen foundation CCR18547795 (to K.L.C.), a grant from the Prevent Cancer Foundation (to K.L.C.), and NIH grant HL-087103 (to C.A.S.). Shared resource services were provided by the Wake Forest Baptist Comprehensive Cancer Center’s NCI Cancer Center Support Grant (P30CA012197). We also acknowledge the editorial assistance of Karen Klein, MA, at the Wake Forest Clinical and Translational Science Institute (UL1 TR001420; McClain, principal investigator [PI]).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing financial interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes three figures and four tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.08.078.
REFERENCES
- Aragón F, Carino S, Perdigón G, and de Moreno de LeBlanc A (2014). The administration of milk fermented by the probiotic Lactobacillus casei CRL 431 exerts an immunomodulatory effect against a breast tumour in a mouse model. Immunobiology 219, 457–464. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture, Agricultural Research Service (2014). Energy intakes: percentages of energy from protein, carbohydrate, fat, and alcohol, by gender and age What We Eat in America, National Health and Nutrition Examination Survey 2011–2012 (Washington: U.S. Department of Agriculture; ). [Google Scholar]
- Baker PR, Wilton JC, Jones CE, Stenzel DJ, Watson N, and Smith GJ (1992). Bile acids influence the growth, oestro-gen receptor and oestrogen-regulated proteins of MCF-7 human breast cancer cells. Br. J. Cancer 65, 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Be´ dard A, Riverin M, Dodin S, Corneau L, and Lemieux S (2012). Sex differences in the impact of the Mediterranean diet on cardiovascular risk profile. Br. J. Nutr 108, 1428–1434. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Johansen FE, Baekkevold ES, Carlsen HS, and Farstad IN (2004). The traffic of mucosal lymphocytes to extraintestinal sites.J. Pediatr. Gastroenterol. Nutr 39 (Suppl 3), S725–S726. [DOI] [PubMed] [Google Scholar]
- Buckland G, Travier N, Cottet V, González CA, Luján-Barroso L, Agudo A, Trichopoulou A, Lagiou P, Trichopoulos D, Peeters PH, et al. (2013). Adherence to the Mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int. J. Cancer 132, 2918–2927. [DOI] [PubMed] [Google Scholar]
- Castelló A, Pollán M, Buijsse B, Ruiz A, Casas AM, Baena-Cañada JM, Lope V, Antolín S, Ramos M, Muñoz M, et al. ; GEICAM researchers (2014). Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study. Br. J. Cancer 111, 1454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, and Pappolla MA (1999). Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J. Biol. Chem 274, 21937–21942. [DOI] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, and Brand-Miller J (2005). Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr 81, 341–354. [DOI] [PubMed] [Google Scholar]
- Costarelli V, and Sanders TA (2002a). Plasma bile acids and risk of breast cancer. IARC Sci. Publ 156, 305–306. [PubMed] [Google Scholar]
- Costarelli V, and Sanders TA (2002b). Plasma deoxycholic acid concentration is elevated in postmenopausal women with newly diagnosed breast cancer. Eur. J. Clin. Nutr 56, 925–927. [DOI] [PubMed] [Google Scholar]
- Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, et al. ; ANR MicroObes consortium (2013). Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, et al. (2016). High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65, 1812–1821. [DOI] [PubMed] [Google Scholar]
- de Moreno de LeBlanc A, Matar C, Thé riault C, and Perdigón G (2005). Effects of milk fermented by Lactobacillus helveticus R389 on immune cells associated to mammary glands in normal and a breast cancer model. Immunobiology 210, 349–358. [DOI] [PubMed] [Google Scholar]
- Dewi FN, Wood CE, Lees CJ, Willson CJ, Register TC, Tooze JA, Franke AA, and Cline JM (2013). Dietary soy effects on mammary gland development during the pubertal transition in nonhuman primates. Cancer Prev. Res. (Phila.) 6, 832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AM, Bridgewater BB, Liu Q, Mitchell MW, Robinson RJ, Dai H, Stewart SJ, DeHaven CD, and Miller LAD (2014). High Resolution Mass Spectrometry Improves Data Quantity and Quality as Compared to Unit Mass Resolution Mass Spectrometry in High-Throughput Profiling Metabolomics. Metabolomics 4, 132. [Google Scholar]
- Fernández L, Cárdenas N, Arroyo R, Manzano S, Jiménez E, Martin V, and Rodríguez JM (2016). Prevention of infectious mastitis by oral administration of Lactobacillus salivarius PS2 during late pregnancy. Clin. Infect. Dis 62, 568–573. [DOI] [PubMed] [Google Scholar]
- Haegele AD, Briggs SP, and Thompson HJ (1994). Antioxidant status and dietary lipid unsaturation modulate oxidative DNA damage. Free Radic. Biol. Med 16, 111–115. [DOI] [PubMed] [Google Scholar]
- Hieken TJ, Chen J, Hoskin TL, Walther-Antonio M, Johnson S, Ramaker S, Xiao J, Radisky DC, Knutson KL, Kalari KR, et al. (2016). The Microbiome of Aseptically Collected Human Breast Tissue in Benign and Malignant Disease. Sci. Rep 6, 30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E, Kinross J, Gibson GR, Burcelin R, Jia W, Pettersson S, and Nicholson JK (2012). Therapeutic modulation of microbiota-host metabolic interactions. Sci. Transl. Med 4, 137rv6. [DOI] [PubMed] [Google Scholar]
- Javitt NB, Budai K, Miller DG, Cahan AC, Raju U, and Levitz M (1994). Breast-gut connection: origin of chenodeoxycholic acid in breast cyst fluid. Lancet 343, 633–635. [DOI] [PubMed] [Google Scholar]
- Jayo JM, Shively CA, Kaplan JR, and Manuck SB (1993). Effects of exercise and stress on body fat distribution in male cynomolgus monkeys. Int. J. Obes. Relat. Metab. Disord 17, 597–604. [PubMed] [Google Scholar]
- Jiménez E, Fernández L, Maldonado A, Martin R, Olivares M, Xaus J, and Rodríguez JM (2008). Oral administration of Lactobacillus strains isolated from breast milk as an alternative for the treatment of infectious mastitis during lactation. Appl. Environ. Microbiol 74, 4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos A, Verhagen H, Moschandreas J, Apostolaki I, and Van Westerop JJ (2000). Mediterranean diet of Crete: foods and nutrient content. J. Am. Diet. Assoc 100, 1487–1493. [DOI] [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, and Schloss PD (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol 79, 5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwa M, Plottel CS, Blaser MJ, and Adams S (2016). The intestinal microbiome and estrogen receptor-positive female breast cancer. J. Natl. Cancer Inst 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, and Gonzalez FJ (2013). Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun 4, 2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C, and Savan R (2014). The role of the IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev. 25, 257–271. [DOI] [PubMed] [Google Scholar]
- Maroof H, Hassan ZM, Mobarez AM, and Mohamadabadi MA (2012). Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model. J. Clin. Immunol 32, 1353–1359. [DOI] [PubMed] [Google Scholar]
- Nagpal R, Shively CA, Appt SA, Register TC, Michalson KT, Vitolins MZ, and Yadav H (2018). Gut microbiome composition in non-human primates consuming a Western or Mediterranean diet. Front. Nutr 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallister T, Jackson MA, Martin TC, Zierer J, Jennings A, Mohney RP, MacGregor A, Steves CJ, Cassidy A, Spector TD, and Menni C (2017). Hippurate as a metabolomic marker of gut microbiome diversity: modulation by diet and relationship to metabolic syndrome. Sci. Rep. 7, 13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelucchi C, Bosetti C, Rossi M, Negri E, and La Vecchia C (2009). Selected aspects of Mediterranean diet and cancer risk. Nutr. Cancer 61, 756–766. [DOI] [PubMed] [Google Scholar]
- Ridlon JM, Kang DJ, Hylemon PB, and Bajaj JS (2014). Bile acids and the gut microbiome. Curr. Opin. Gastroenterol 30, 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridlon JM, Harris SC, Bhowmik S, Kang DJ, and Hylemon PB (2016a). Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7, 22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridlon JM, Wolf PG, and Gaskins HR (2016b). Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes 7, 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. (2009). Introducing mothur: open-source, platform-independent, communitysupported software for describing and comparing microbial communities. Appl. Environ. Microbiol 75, 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, Turroni S, Biagi E, Peano C, Severgnini M, et al. (2014). Gut microbiome of the Hadza hunter-gatherers. Nat. Commun 5, 3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Grant KA, Johnson JL, and Cline JM (2004). Effects of social status and moderate alcohol consumption on mammary gland and endometrium of surgically postmenopausal monkeys. Menopause 11, 389–399. [DOI] [PubMed] [Google Scholar]
- Sophonsritsuk A, Appt SE, Clarkson TB, Shively CA, Espeland MA, and Register TC (2013). Differential effects of estradiol on carotid artery inflammation when administered early versus late after surgical menopause. Menopause 20, 540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stute P, Register TC, Blair RM, and Cline JM (2006). Effects of tibolone on estrogen biosynthesis in the mammary tissue of postmenopausal monkeys. Menopause 13, 232–240. [DOI] [PubMed] [Google Scholar]
- Stute P, Sielker S, Wood CE, Register TC, Lees CJ, Dewi FN, Williams JK, Wagner JD, Stefenelli U, and Cline JM (2012). Life stage differences in mammary gland gene expression profile in non-human primates. Breast Cancer Res. Treat 133, 617–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, and Bishop-Bailey D (2006). The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res. 66, 10120–10126. [DOI] [PubMed] [Google Scholar]
- Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, and Holmes E (2011). Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl. Acad. Sci. U S A 108 (Suppl 1), 4523–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo E, Salas-Salvadó J, Donat-Vargas C, Buil-Cosiales P, Estruch R, Ros E, Corella D, Fito M, Hu FB, Arós F, et al. (2015). Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern. Med. 175, 1752–1760. [DOI] [PubMed] [Google Scholar]
- Urbaniak C, Cummins J, Brackstone M, Macklaim JM, Gloor GB, Baban CK, Scott L, O’Hanlon DM, Burton JP, Francis KP, et al. (2014). Microbiota of human breast tissue. Appl. Environ. Microbiol 80, 3007–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, et al. (2014). Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41, 296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visioli F, and Bernardini E (2011). Extra virgin olive oil’s polyphenols: biological activities. Curr. Pharm. Des 17, 786–804. [DOI] [PubMed] [Google Scholar]
- Watabe J, and Bernstein H (1985). The mutagenicity of bile acids using a fluctuation test. Mutat. Res 158, 45–51. [DOI] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, and Siuzdak G (2009). Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U S A 106, 3698–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, and Trichopoulos D (1995). Mediterranean diet pyramid: a cultural model for healthy eating. Am. J. Clin. Nutr 61 (6, Suppl), 1402S–1406S. [DOI] [PubMed] [Google Scholar]
- Wood CE, Register TC, Franke AA, Anthony MS, and Cline JM (2006). Dietary soy isoflavones inhibit estrogen effects in the postmenopausal breast. Cancer Res. 66, 1241–1249. [DOI] [PubMed] [Google Scholar]
- Wood CE, Register TC, and Cline JM (2007a). Soy isoflavonoid effects on endogenous estrogen metabolism in postmenopausal female monkeys. Carcinogenesis 28, 801–808. [DOI] [PubMed] [Google Scholar]
- Wood CE, Register TC, Lees CJ, Chen H, Kimrey S, and Cline JM (2007b). Effects of estradiol with micronized progesterone or medroxyprogesterone acetate on risk markers for breast cancer in postmenopausal monkeys. Breast Cancer Res. Treat 101, 125–134. [DOI] [PubMed] [Google Scholar]
- Wood CE, Sitruk-Ware RL, Tsong YY, Register TC, Lees CJ, and Cline JM (2007c). Effects of estradiol with oral or intravaginal progesterone on risk markers for breast cancer in a postmenopausal monkey model. Menopause 14, 639–647. [DOI] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. (2011). Linking longterm dietary patterns with gut microbial enterotypes. Science 334, 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, et al. (2013). Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385. [DOI] [PubMed] [Google Scholar]
- Zhang LS, and Davies SS (2016). Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 8, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Nelson EL, Gru€n F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, and Blumberg B (2006). Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J. Clin. Invest 116, 2280–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The metabolomics (Table S3. Monkey mammary gland metabolite profiles. Related to Figures 4, 5, and 6) and genus level 16S sequencing OTU proportional abundance (Table S4. Monkey mammary gland genus OTU proportional abundance. Related to Figures 2 and 3) are deposited in the supplemental data section.