Abstract
BACKGROUND. Excessive insulin secretion may lead to glucose dysregulation. Our aim was to identify primary (independent of insulin resistance) insulin hypersecretion in subjects with normal glucose tolerance and its role in the progression of dysglycemia.
METHODS. In 1,168 adults, insulin secretion rate (ISR) and β cell function were estimated by C-peptide modeling during an oral glucose tolerance test (OGTT) and an i.v. glucose tolerance test. Whole-body insulin sensitivity was measured by a hyperinsulinemic-euglycemic clamp. After regressing ISR on insulin sensitivity, subjects in the upper tertile of the distribution of residuals were defined as primary hypersecretors. This approach was applied to a biethnic cohort of 182 obese adolescents, who received an OGTT, a hyperglycemic, and a euglycemic clamp.
RESULTS. Adult hypersecretors showed older age, more familial diabetes, sedentary lifestyle, increased fat mass, and worse lipid profile compared with the rest of the cohort, despite virtually identical BMI and insulin sensitivity. Insulin secretion was increased by 53% due to enhanced (+23%) β cell glucose sensitivity. Despite the resulting hyperinsulinemia, glucose tolerance was worse in hypersecretors among both adults and adolescents, coupled with higher indices of liver insulin resistance and increased availability of gluconeogenic substrates. At the 3-year follow-up, adult hypersecretors had increased incidence of impaired glucose tolerance/type 2 diabetes.
CONCLUSION. Primary insulin hypersecretion, independent of insulin resistance, is associated with a worse clinical and metabolic phenotype in adults and adolescents and predicts deterioration of glucose control over time.
FUNDING. The relationship between insulin sensitivity and cardiovascular disease (RISC) Study was partly supported by EU grant QLG1-CT-2001-01252.
Keywords: Endocrinology, Metabolism
Keywords: Beta cells, Diabetes, Insulin signaling
Primary insulin hypersecretion is associated with an adverse clinical and metabolic phenotype and predicts deterioration of glucose control over time in nondiabetic adults and adolescents.
Introduction
The prevailing view of the natural history of type 2 diabetes (T2D) is that an early decline of insulin sensitivity precedes and causes a progressive increase in insulin secretion, which initially compensates for insulin resistance (IR) to maintain glucose tolerance. At a later time, β cell function declines and hyperglycemia ensues (1). However, animal studies (2–8) and a few human studies (9–13) have suggested an alternative model, which postulates that, in some individuals, an inappropriate increase of insulin secretion, independent of IR, might be the first event triggering T2D progression (14–17). This paradigm, named primary insulin hypersecretion, hinges upon the demonstration that the hypersecretion is not just the compensatory response to IR. This requires that IR is directly and independently measured and that an unbiased criterion is then used to define the hypersecretory state, measured by an equally direct method.
Aims of the present study were (a) to identify primary insulin hypersecretion, (b) to describe the clinical and metabolic phenotype of subjects with primary insulin hypersecretion, (c) to assess its consequences for glucose homeostasis, and (d) to search for potential mechanisms. To this end, insulin secretion rate (ISR) and β cell function were estimated — by C-peptide/glucose modeling — in a large cohort of clinically healthy European Caucasians with normal glucose tolerance (NGT) during both an oral glucose tolerance test (OGTT) and an i.v. glucose tolerance test (IVGTT), while insulin sensitivity was measured by the hyperinsulinemic-euglycemic clamp technique. To validate the findings of this approach and to put them in the perspective of the natural history of T2D, we conducted a similar analysis on the euglycemic clamp data of an independent cohort of White American and African American adolescents in whom β cell function was estimated during both an OGTT and a hyperglycemic clamp.
Results
Adult cohort.
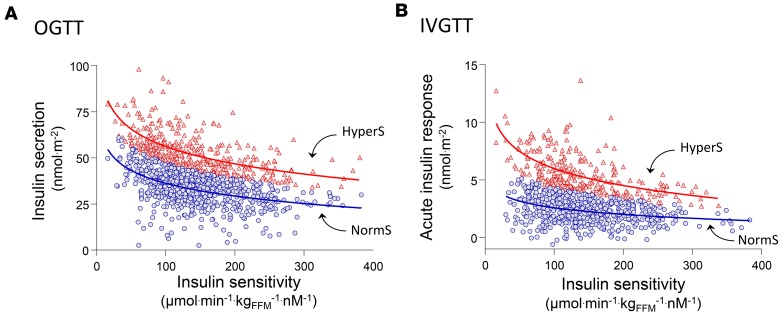
In participants of the relationship between insulin sensitivity and cardiovascular disease (RISC) study (n = 1,168) (Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.124912DS1), insulin sensitivity, expressed as the M value from the hyperinsulinemic-euglycemic clamp normalized by the steady-state plasma insulin concentrations (M/I), was 133 (interquartile range [IQR], 86) μmol.min–1.kgFFM–1.nM–1 (median [IQR]). In the same subjects, total insulin output over the 2 hours of the OGTT (ISROGTT) was 39 [IQR, 17] nmol/m2. As shown in Figure 1A, the relationship between M/I and ISROGTT was reciprocal and nonlinear; the best fit of the data was a log-linear relationship (ISROGTT = 99 – 12 ln[M/I], r = 0.43, P < 0.0001), which was statistically superior to a linear or log-log fit. From this regression, primary insulin hypersecretion was defined as the ISROGTT values in the upper tertile of the distribution of the regression residuals (HyperS, n = 389). In the comparison between HyperS subjects and the rest of the cohort (NormS, n = 779), insulin sensitivity (M/I) was virtually identical, as expected (Table 1). Using the acute insulin response to i.v. glucose (AIRIVGTT) as a measure of insulin secretion, HyperS can be similarly identified as the upper tertile of the distribution of the regression residuals against M/I (Figure 1B). Insulin hypersecretion identified by this approach was defined as primary to underscore its independence from insulin sensitivity, although it could be explained by other factors discussed below.
Figure 1. Identification of primary insulin hypersecretion.
(A) Relationship between total insulin secretion during a 75-g OGTT (ISROGTT) and insulin sensitivity (M/I) and in the RISC study cohort (n = 1,168). (B) Relationship between M/I and acute insulin response during an i.v. glucose tolerance test (AIRIVGTT) in the same cohort. Primary insulin hypersecretion was defined as either the ISROGTT or AIRIVGTT values in the upper tertile of the distribution of the residuals from the regression of log-linear data (ISROGTT = 99 – 12 ln[M/I], r = 0.43, P < 0.0001, and AIRIVGTT = 10 – 14 ln[M/I], r = 0.35, P < 0.0001, respectively). Using this cutoff, subjects were classified as hypersecretors (HyperS, red triangles; n = 389) or normosecretors (NormS, blue circles; n = 779).
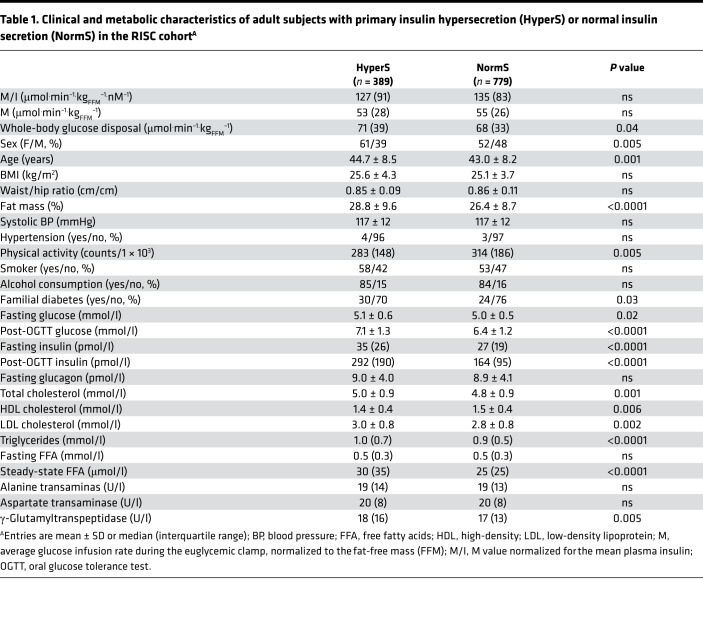
Table 1. Clinical and metabolic characteristics of adult subjects with primary insulin hypersecretion (HyperS) or normal insulin secretion (NormS) in the RISC cohortA.
The clinical and metabolic phenotype of HyperS (on the OGTT) showed prevalence of women, marginally higher age (by 1.7 years, on average), higher percent fat mass for a similar BMI, more familial diabetes, and a worse serum lipid profile. In addition, γ-glutamyltransferase levels (γ-GT) levels were higher, and estimated physical activity was lower. In multiple logistic analysis, positive predictors of HyperS were female sex (odds ratio [OR], 2.74 [95% CI, 2.02–3.63]), post-OGTT glucose (OR, 1.89 [95% CI, 1.64–2.19]), and fasting triglycerides (OR, 1.34 [95% CI, 1.16–1.57]), while HDL cholesterol (OR, 0.84 [95% CI, 0.75–1.00]) was a negative predictor.
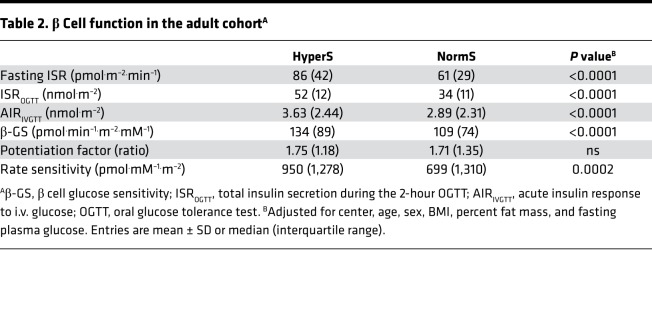
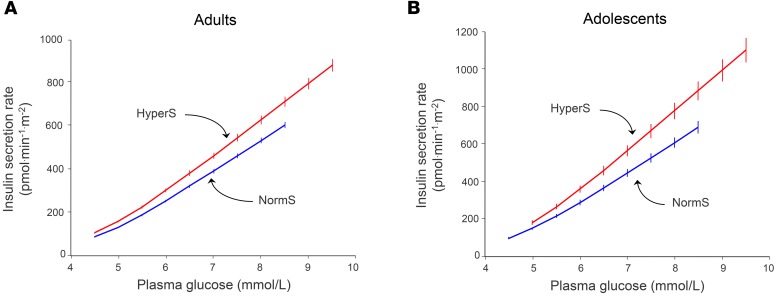
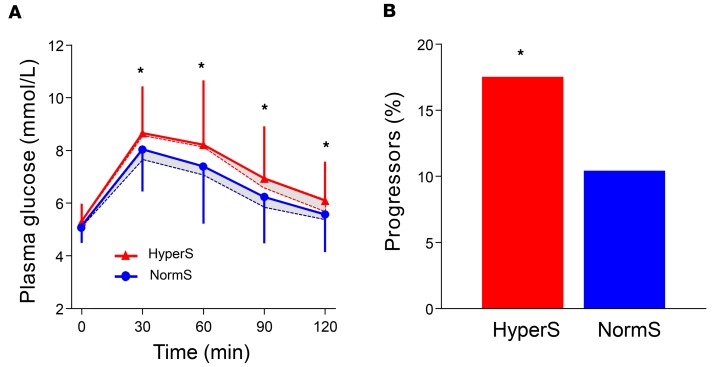
By design, all measures of insulin secretion — fasting as well as post-OGTT — were 40%–50% higher in HyperS than NormS and remained significantly higher after adjusting for anthropometric and metabolic differences (Table 2). Furthermore, the acute insulin response (AIR) to an i.v. glucose bolus (AIRIVGTT) also was 26% enhanced in HyperS vs. NormS. With regard to β cell function, insulin hypersecretion was associated with a 23% increase in β cell glucose sensitivity (i.e., the relation of insulin secretion to glucose levels; β-GS) (Figure 2A), which persisted after controlling for baseline differences (Table 2). Despite similar insulin sensitivity and higher β-GS and insulin concentrations, glucose tolerance was worse in HyperS than NormS (Figure 3).
Table 2. β Cell function in the adult cohortA.
Figure 2. Dose-response of insulin secretion in adults and adolescents.
(A) Dose-response of insulin secretion rate during a 75-g OGTT in hypersecretors (HyperS, red line, n = 389) and normosecretors (NormS, blue line, n = 779) in the RISC study cohort. (B) Dose-response of insulin secretion rate during a 75-g OGTT in HyperS (n = 61) and NormS (n = 121) in the adolescent group. Note the steeper slopes of adolescents vs. adults.
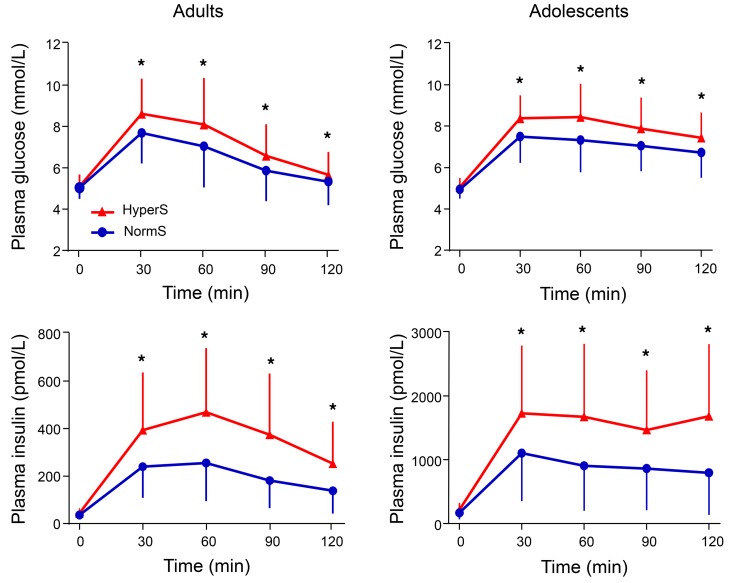
Figure 3. Plasma glucose and insulin concentrations during a 75-g OGTT in adults and adolescents.
Insulin hypersecretors (HyperS) are shown as red triangles (n = 389 in adults and n = 61 in adolescents), while normosecretors (NormS) are shown as blue circles (n = 779 in adults and n = 121 in adolescents). Repeated-measure ANOVA was performed including group (HyperS vs. NormS), time, and group × time interaction as factors, followed by post hoc pairwise comparisons using Tukey HSD tests. Plots are mean ± SD. Asterisks denote values that are significantly (*P ≤ 0.05) different between HyperS and NormS.
Adolescent cohort.
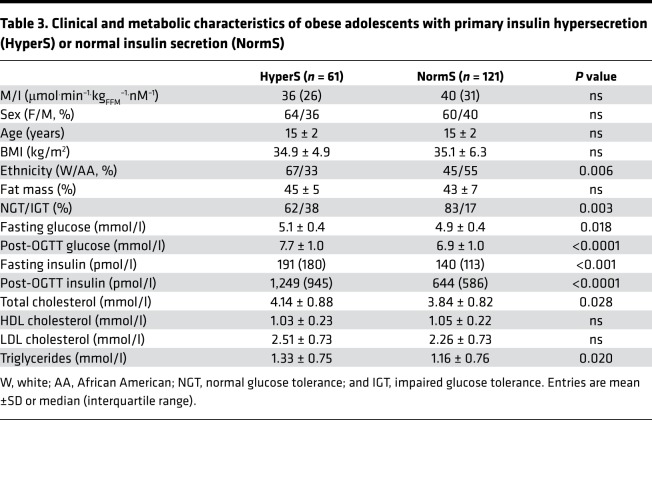
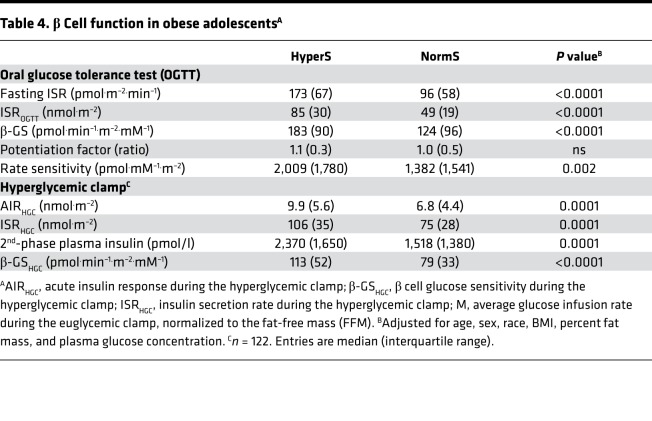
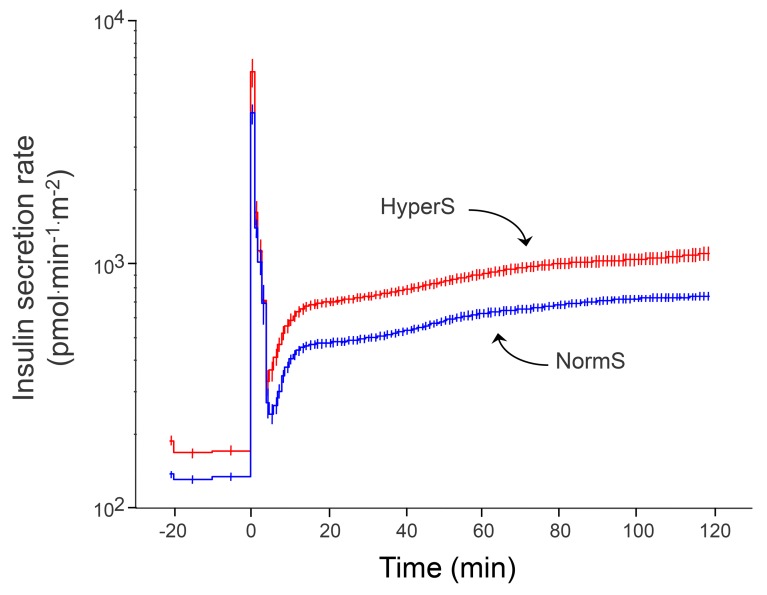
As a validation data set, we analyzed a cohort of obese nondiabetic adolescents (n = 182), in whom an OGTT, a hyperinsulinemic-euglycemic clamp, and a hyperglycemic clamp were performed in each subject. In this group, the definition of HyperS was the same as that of the adult cohort, i.e., the top tertile of the residuals from the regression of ISROGTT on M/I (ISROGTT = 144 – 23 ln[M/I], r = 0.52, P < 0.0001). In this group, there was a higher prevalence of hypersecretors among White Americans than African Americans (Table 3). As in adults, both fasting and post-OGTT insulin secretion were ~70% higher in HyperS than NormS (Table 4). β-GS was enhanced by ~50% (Figure 2B), and rate sensitivity was also higher, to a similar extent; nonetheless, glucose tolerance was significantly worse (Figure 3). In line with this pattern, during the hyperglycemic clamp both the AIR (AIRHGC) and the second phase insulin secretory response (ISRHGC) were higher in HyperS than NormS (Figure 4). Furthermore, β-GS calculated from the hyperglycemic clamp data was increased by 43%, similar to the value derived from the OGTT.
Table 3. Clinical and metabolic characteristics of obese adolescents with primary insulin hypersecretion (HyperS) or normal insulin secretion (NormS).
Table 4. β Cell function in obese adolescentsA.
Figure 4. Insulin secretion rates during a hyperglycemic clamp in obese adolescents.
Insulin hypersecretors (HyperS) are shown in red (n = 61), and normosecretors (NormS) are shown in blue (n = 121). Plots are mean ± SEM.
Mechanisms of altered glucose tolerance in HyperS.
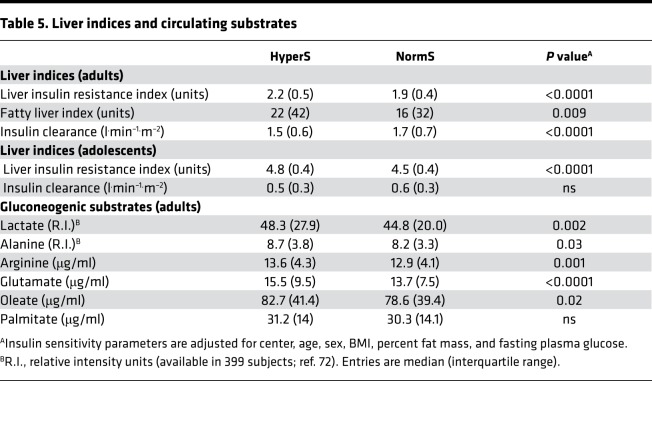
When the rate of appearance of ingested glucose is the same, the only determinant of glycemia other than peripheral tissue glucose utilization (which the clamp-derived M value measures) is endogenous glucose production (EGP). Though no direct measurements of EGP during the OGTT were available, the liver IR index (which has been shown to correlate with tracer-derived EGP measurements during fasting and clamp; ref. 18) was significantly higher in HyperS than NormS, a difference that persisted after multiple adjustments in both adults and adolescents (Table 5). In line with this was also a reduction in the metabolic clearance rate of insulin, which largely reflects hepatic insulin degradation (19). Finally, a proxy of hepatic steatosis, the fatty liver index (20) (available only in the adult cohort), was higher in HyperS than NormS, in agreement with their increased γ-GT levels (Table 1).
Table 5. Liver indices and circulating substrates.
The higher liver IR index in HyperS aligns with the finding of raised circulating concentrations of gluconeogenic substrates, including lactate, arginine, and glutamate. The increased plasma levels of a major free fatty acids (FFA) (i.e., oleate) suggest an increased rate of lipolysis in HyperS vs. NormS (Table 5).
Of a total of 1,168 subjects in the adult cohort, 185 (16%) had impaired fasting glycemia (IFG) diagnosed according to the American Diabetes Association (ADA) criteria (fasting plasma glucose between 100–125 mg/dl). The prevalence of IFG was well balanced between HyperS and NormS (17% vs. 15%, respectively, P = 0.31). In addition, when we analyzed NGT and IFG separately, in both groups the liver IR index was still higher in HyperS than NormS (NGT, 2.14 [IQR, 0.45] vs. 1.85 [IQR, 0.02], respectively, P < 0.0001; IFG, 2.28 [IQR, 0.44] vs. 2.00 [IQR, 0.41], respectively, P < 0.0001).
Follow-up.
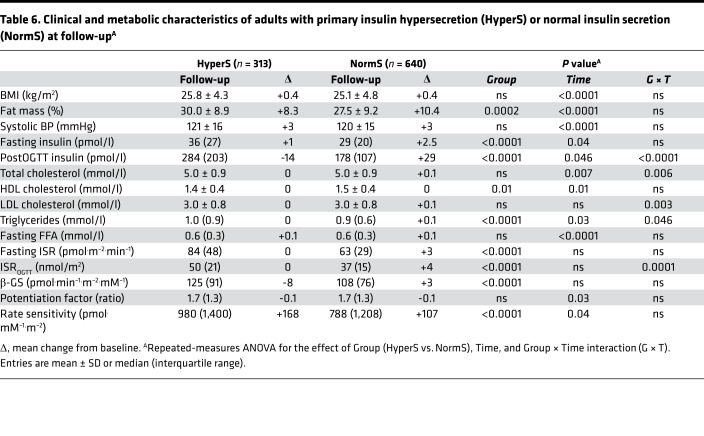
In the adult cohort at follow-up (n = 953), significantly more HyperS than NormS progressed to impaired glucose tolerance (IGT) or T2D (17.4% vs. 10.3%, P < 0.01) (Figure 5). HyperS were ~80% more likely to develop dysglycemia than NormS (OR, 1.82 [95% CI, 1.24–2.69], P = 0.003). This finding persisted after adjustment for sex, age, percent fat mass, BMI, familial diabetes, fasting glucose, and M/I (OR, 1.55 [95% CI, 1.03–2.33], P = 0.036). Baseline differences in percent fat mass, HDL cholesterol, and triglycerides between HyperS and NormS were maintained at follow-up (Table 6). With regard to β cell function, post-OGTT insulin release was still higher in HyperS vs. NormS, but it was reduced as compared with baseline only in HyperS (P = 0.0001 for the time × group interaction). This difference was also reflected in the post-OGTT plasma insulin concentrations (P < 0.0001 for the time x group interaction). Likewise, β-GS was still higher in HyperS, but it was reduced as compared with baseline (though the group x time interaction fell short of statistical significance).
Figure 5. Glucose tolerance at follow-up in the adult cohort.
(A) Plasma glucose concentrations during a 75-g OGTT at baseline (dotted lines) and follow-up (full lines) in insulin hypersecretors (HyperS, n = 313) and normosecretors (NormS, n = 640) in the RISC study cohort. Follow-up plasma glucose levels were analyzed by repeated-measure ANOVA including group (HyperS vs. NormS), time, and group × time interaction as factors, followed by post hoc pairwise comparisons using Tukey HSD tests. Plots are mean ± SD. (B) Incidence of dysglycemia at follow-up (progressors) in HyperS and NormS in the RISC study cohort. Asterisks denote values that are significantly (*P ≤ 0.05) different between HyperS and NormS.
Table 6. Clinical and metabolic characteristics of adults with primary insulin hypersecretion (HyperS) or normal insulin secretion (NormS) at follow-upA.
Discussion
In this study, we identified primary insulin hypersecretion in normotolerant adults by separately measuring insulin sensitivity and secretion. The attribute of primary, therefore, only indicates hypersecretion that is not compensatory to IR. Moreover, while using the regression residuals to adjust secretion for sensitivity is a rigorous statistical method, the quantile criterion (i.e., upper tertile represents hypersecretors) is arbitrary, as would be choosing any other threshold. Within these boundaries, we could clearly identify 2 groups of subjects with virtually identical whole-body insulin sensitivity in whom the size and impact of primary insulin hypersecretion could be examined. Incidentally, using fasting rather than post–glucose insulin secretion in the analysis yielded essentially similar results (data not shown). To further test its consistency, the approach was applied to a cohort of youths from a biethnic background.
In adults, individuals identified as primary hypersecretors shared a clinical phenotype of increased adiposity, a more sedentary lifestyle, and mild dyslipidemia. As expected from the analysis strategy, measures of both fasting and glucose-stimulated insulin secretion were higher in both adult and adolescent hypersecretors as compared with the respective normosecretors: a ~30%–40% higher estimated daily basal insulin output (40 [IQR, 25] U/24 hours vs. 29 [IQR, 18] U/24 hours, P < 0.0001) and a ~45%–55% higher insulin release in response to oral glucose (16 [IQR, 7] U/2 hours vs. 11 [IQR, 5] U/2 hours, P < 0.0001). Importantly, these differences were significant after adjustment for individual anthropometric and metabolic characteristics. Moreover, augmented insulin secretion was also detected in response to more specific β cell stimuli such as the IVGTT and the hyperglycemic clamp, confirming the intrinsic nature of the β cell hypersecretion. The potentially novel, and unexpected, finding was that β-GS was enhanced in hypersecretors of both study cohorts (by ~20% in adults and ~50% in adolescents) in the face of reduced glucose tolerance (Figure 3). β-GS quantitates the dependence of insulin release on glucose concentrations during stimulation (Figure 2), regardless of IR (21). While IGT is generally associated with reduced β cell glucose sensing (22, 23), the association was paradoxically inverted in hypersecretors. Glucose rate sensitivity, an index of early insulin release, also was increased in both adult and adolescent hypersecretors, thereby confirming the generalized hyperreactivity of the β cell in these individuals.
Previous clinical studies have argued for the occurrence of true insulin hypersecretion in prediabetes (10, 14–16) and for its primacy in the development of glucose intolerance (9–12, 14–17, 24, 25). However, in none of them has the effect of IR been adequately discounted. In fact, not only is IR strongly related to insulin secretion in a reciprocal manner (10), but the compensatory hyperinsulinemia feeds back negatively on insulin action. Prolonged hyperinsulinemia can lead to generalized IR by causing receptor and postreceptor defects (2, 3, 24, 26, 27). It also adversely affects body composition by increasing intracellular fat storage (24, 28). Furthermore, experimental euglycemic hyperinsulinemia applied for 72 hours does induce IR in human volunteers (29). More convincing evidence for primary hypersecretion has emerged from interventional clinical studies showing, for example, that diazoxide — an agonist of the K+/ATP channel (30) — lowers plasma glucose levels at the same time as it selectively curtails insulin secretion (30–33). Likewise, early after bariatric surgery, both plasma glucose and insulin concentrations fall markedly before there is any detectable change in insulin sensitivity (16, 34, 35). Conversely, short-term (5 days) high-fat feeding in healthy volunteers raises fasting glucose levels with no change in peripheral insulin action (M from euglycemic clamps), muscle mitochondrial function, and general and specific oxidative phosphorylation gene expression (9). However, detailed analysis of β cell function (glucose sensitivity, rate sensitivity, and potentiation) has not been previously attempted, to our knowledge.
β Cell glucose hypersensitivity may be explained by multiple mechanisms: excess circulating substrates (36), such as lactate (16, 37), lipids (38–42), and amino acids (16, 39, 43, 44); genetic and ethnic factors (45–51); environmental agents (14, 52, 53); gut hormones (54, 55); and neural signals (56, 57). In particular, autonomic nervous system activity affects insulin secretion by the direct action on the β cell of several neurotransmitters (56–59), which also exert control on food intake (60). Of note, in genetic models of congenital hyperinsulinism (e.g., glucokinase activating mutations; refs. 61, 62), there is associated hypoglycemia, both fasting and postcibal, along with suppressed plasma FFA and ketone levels.
The combination of hyperinsulinemia and normal insulin sensitivity increases glucose disposal (Table 1) and should, consequently, reduce glucose levels. Therefore, the higher plasma glucose levels of hypersecretors can only be explained by an increased rate of endogenous glucose release (Table 1 and Table 3) or reduced hepatic glucose uptake (or both) in the face of hyperinsulinemia, descriptively hepatic IR. In fact, we show that, in both adults and adolescents, indices of hepatic IR were significantly increased in hypersecretors as compared with normosecretors even after controlling for confounders. Moreover, circulating levels of gluconeogenic substrates — such as lactate, alanine, and glutamate — were all raised, along with lipid substrates, which provide the energy for gluconeogenesis (63). Under conditions of increased glucose utilization, peripheral tissues release more lactate (64), thereby completing an overactive Cori cycle (and glucose-alanine cycle; ref. 65) as well as an effective energy shuttle (66). Except for the preserved peripheral tissue insulin sensitivity, the resulting metabolic picture resembles that of early T2D (67), including mild increments in serum triglycerides and liver enzymes (Table 1) (68).
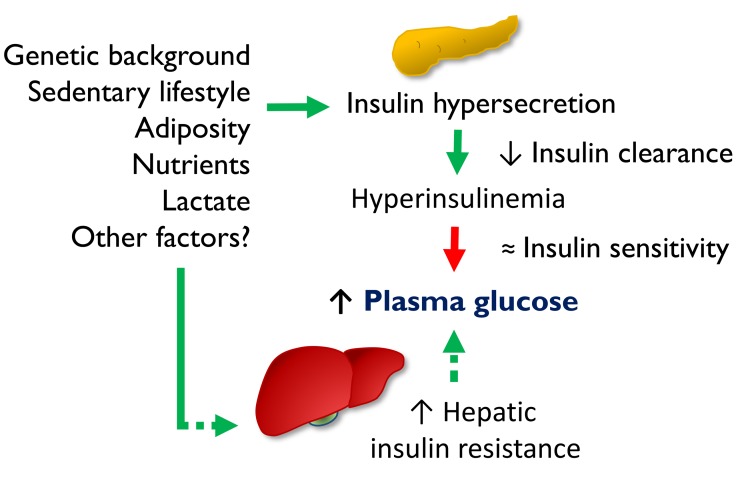
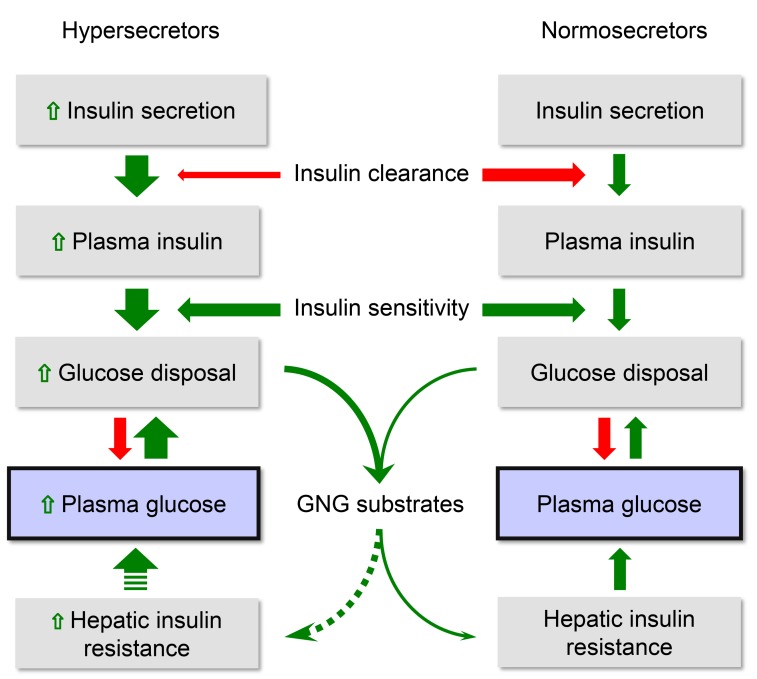
Overall, the current findings are compatible with our proposed sequence schematized in Figure 6. Multiple factors determine β cell hypersensitivity and insulin hypersecretion. The more corpulent phenotype of the hypersecretors does suggest that increased energy intake may be a prevalent etiology among a host of inherited and acquired influences (69). Hepatic IR may ensue before peripheral IR (9), leading to inappropriate EGP and mild hyperglycemia, coupled with increased cycling of substrates between peripheral tissues and the liver. The antecedence of hepatic over peripheral IR is physiologically compatible with the notion that the liver is about 3 times more sensitive to insulin than are peripheral tissues (70); the temporal dissociation is supported by studies showing more rapid development of hepatic than peripheral IR in response to short-term high-fat feeding in healthy volunteers (9).
Figure 6. Schematic summary of the insulin/glucose system in primary insulin hypersecretors and normosecretors.
Green arrows indicate stimulation, and red arrows denote inhibition. Dotted arrows indicate speculative mechanisms.
The follow-up data in the adult cohort demonstrate that primary insulin hypersecretion is a risk factor for progression of dysglycemia. In fact, over just 3 years, insulin output and β-GS had declined (Table 6), and more subjects had progressed to glucose intolerance (Figure 5) in hypersecretors as compared with normosecretors.
One limitation of the statistical approach is that the actual prevalence of primary insulin hypersecretion cannot be established precisely. By way of example, if glucose-stimulated insulin secretion is adjusted for all its potential confounders and measured determinants (center, age, sex, BMI, M/I, and mean post-OGTT plasma glucose levels), the distribution of the residuals shows that only about 1 in 10 adults has a value above 1 SD of the entire cohort (i.e., an excess insulin secretion ranging from 14–50 nmol.m–2 during the 2-hour OGTT). Whether different clinical phenotypes would become apparent by using different threshold criteria is unlikely but cannot be ruled out.
Another limitation is that EGP was not directly measured and liver IR could only be estimated. Of note in this regard is that hypersecretors had very similar fasting glucose but definitely higher stimulated glucose levels in comparison with normosecretors (Figure 3 and Figure 5). This pattern could result from a difference in oral glucose absorption. However, it is unlikely that gastric emptying would be uniformly faster in hypersecretors without distorting the OGTT glucose profile (as is the case, for example, after bariatric surgery; ref. 34). Thus, the higher glucose excursions in the hypersecretors must result from impaired suppression of endogenous glucose release during the OGTT. While this is a probable mechanism, as demonstrated in the small study by Brøns et al. (9), its direct measurement (using multiple glucose tracers) in large numbers of subjects would be technically formidable. The observed differences in suppressed FFA (Table 1) and circulating gluconeogenic precursors, if numerically small, go toward supporting this mechanism in our subjects.
Another limitation is that the HyperS youths in this cohort did not show the differences in clinical phenotype emerging in adults (Table 3). A potential explanation is that, by selection, these adolescents were all obese (by ~10 BMI units more than the adults); however, we cannot rule out an effect of age per se. Finally, in our adolescents, insulin hypersecretion showed a slightly higher prevalence in White Americans than African Americans. The latter observation is apparently in contradiction with previous reports, showing that African Americans have higher fasting and glucose-stimulated insulin levels than White Americans (45, 47–49). However, hyperinsulinemia in African Americans is driven mainly by an approximately 15%–30% lower hepatic insulin clearance (47, 49) and by a greater IR (48, 49), which would reduce the relative contribution of primary insulin hypersecretion to the overall insulin concentration.
In conclusion, we identified primary insulin hypersecretion in normotolerant adults and adolescents and described its adverse metabolic effects independent of insulin sensitivity, suggesting potential underlying mechanisms. Our data support a causal pathogenic role of the hypersecretory state in the early derangements of glucose homeostasis that characterize the natural history of T2D, and they warrant further studies to identify mechanisms leading to primary insulin hypersecretion and potential therapeutic approaches.
Methods
Study design and participants.
The RISC study is a multicenter, prospective, observational study with examinations at baseline (n = 1,566) and after a 3-year follow-up (n = 1,059). Details of study design and protocol have been reported (71). Participants were clinically healthy Europeans between 30 and 60 years of age. Exclusion criteria were chronic diseases (e.g., diabetes, dyslipidemia, hypertension, chronic lung, hepatic or kidney diseases, and neoplastic and inflammatory diseases), class III obesity, presence of carotid stenosis >40%, and treatment for hypertension, diabetes, dyslipidemia, obesity, and steroid therapy. A standardized examination protocol included anthropometry, blood pressure measurements, a fasting blood test, a hyperinsulinemic-euglycemic clamp, an OGTT, and an IVGTT. Brachial blood pressure was measured by a digital electronic tensiometer (model 705cp; Omron), with regular or large cuffs according to the arm circumference, in subjects seated for at least 10 minutes. Physical activity was measured objectively by a small single-axis accelerometer (Actigraph model AM7164-2.2; Computer Science and Applications). The acceleration signal was digitized with 10 samples per second, registered as counts over 1-minute intervals. Information regarding medical history, drug use, alcohol and cigarette consumption, and family history of diabetes (i.e., any first-degree family member with T2D) was collected using standardized self-reported questionnaires. For the purpose of this study, we excluded individuals with diabetes (n = 28) or IGT (n = 119) detected during the screening and those with missing information on the clamp-derived insulin sensitivity (n = 208) or OGTT-derived insulin secretion (n = 43). This resulted in a study population of 1,168 participants for the cross-sectional study. Among those, 215 individuals did not attend follow-up, resulting in a population of 953 participants for the prospective analysis (Supplemental Figure 1).
As a validation cohort, we analyzed data from 182 obese nondiabetic adolescents recruited at the Pediatric Clinical and Translational Research Center of the Children’s Hospital of Pittsburgh. Participants were White American or African American adolescents between 10 and 20 years of age. All participants were in good health, as assessed by medical history, physical examination, and routine hematological and biochemical tests. Individuals with serious medical conditions or receiving treatments known to affect glucose or lipid metabolism were excluded.
GTTs and clamp procedures.
In all participants, an OGTT with 75 g glucose (1.75 g/kg in adolescents, up to 75 g) was performed after an overnight fast. Blood samples were collected at baseline and at 30, 60, 90, and 120 minutes after glucose ingestion for measurement of glucose, insulin, and C-peptide concentrations. Insulin sensitivity was evaluated during a hyperinsulinemic-euglycemic clamp. Exogenous insulin was administered as a primed-continuous infusion at a rate of 240 pmol.min−1.m−2 in adults and 480 pmol.min−1.m−2 in adolescents. Plasma glucose was clamped at 4.5–5.5 mmol/l with a variable 20% dextrose infusion adjusted every 5 minutes. During the clamp, blood was sampled at least every 20 minutes for determination of insulin concentrations. To evaluate the AIR to i.v. glucose, an IVGTT was performed in 828 adults at the end of the hyperinsulinemic-euglycemic clamp. A glucose bolus (0.3 mg/kg body weight) was administered over 1 minute, and plasma glucose and C-peptide concentrations were measured at 2, 4, 6, and 8 minutes after the bolus. In adolescents, first- and second-phase ISRs were assessed during a 2-hour hyperglycemic clamp in which plasma glucose was increased rapidly to 12.5 mmol/l by a bolus infusion of 25% dextrose and maintained at that level by a variable rate infusion of 20% dextrose.
Insulin sensitivity, insulin secretion, and insulin clearance parameters.
Whole-body insulin-mediated glucose disposal was calculated as the average glucose infusion rate (M value) during the final 40 minutes of the hyperinsulinemic-euglycemic clamp, normalized to the fat-free mass (FFM); insulin sensitivity was calculated as the ratio of M to the mean plasma insulin concentration measured during the same interval (M/I, in units of μmol.kgFFM−1.min−1.nM−1). Whole-body glucose disposal during the OGTT was estimated as the product of insulin-mediated glucose clearance (M/steady-state plasma glucose during the final 40 minutes of the euglycemic clamp) and the mean plasma glucose concentration during the OGTT. ISR (expressed in pmol.min−1.m−2) was estimated from C-peptide deconvolution (72), and total insulin output was calculated by integrating ISR over the duration of the test. β Cell function parameters were calculated by mathematical modeling of ISR and glucose concentrations, as previously reported (73). This model describes the relationship between ISR and glucose concentration as the sum of 2 components. The first component represents the dependence of ISR on absolute glucose concentration through a dose-response function relating the 2 variables. The slope of this dose-response function, named β-GS, is calculated. The dose-response is modulated by a potentiation factor, which accounts for the physiological processes that can acutely modify insulin secretion (e.g., protracted hyperglycemia, nonglucose substrates, gastrointestinal hormones, neural modulation). The potentiation factor, which is set to average 1 during the entire test, measures the relative potentiation or inhibition of ISR; its excursion is quantified by the ratio between the 2-hour and the baseline value (potentiation ratio). The second component of β cell function represents the dependence of ISR on the rate of change of glucose concentration and is determined by a single parameter (rate sensitivity), which is related to early insulin release. With the IVGTT, the AIR to i.v. glucose (8 minutes) was expressed as the incremental insulin secretion/glucose area ratio over the same time interval (in nmol.m−2.). During the hyperglycemic clamp, first-phase ISR (AIR) was calculated as the AUC between 2.5 and 12.5 min, while second-phase ISR was calculated from 15–120 minutes.
Insulin clearance, expressed in l.min–1.m–2, was calculated as the ratio of the average ISR during the OGTT and the average plasma insulin levels during the OGTT.
Definitions.
To identify primary insulin hypersecretion, the M/I value from the hyperinsulinemic-euglycemic clamp was regressed against the total insulin output over the 2-hour OGTT (ISROGTT) (Figure 1). Primary insulin hypersecretion was defined as the ISROGTT values in the upper tertile of the distribution of the residuals from the regression of log-linear data (ISROGTT = 99 – 12 ln[M/I], r = 0.43, P < 0.0001 for adults; ISROGTT = 144 – 23 ln[M/I], r = 0.52, P < 0.0001 for adolescents), which was statistically superior to a linear or log-log fit.
NGT (fasting glucose < 7.0 mmol/l and 2-hour glucose < 7.8 mmol/l), IFG (fasting glucose between 5.6–7.0 mmol/l and 2-hour glucose < 7.8 mmol/l), IGT (fasting glucose < 7.0 mmol/l and 2-hour glucose between 7.8 and 11.1 mmol/l), and T2D (fasting glucose ≥ 7.0 mmol/l and 2-hour glucose ≥ 11.1 mmol/l) were defined according to the 2018 ADA criteria (74). Based on the observed changes of glucose tolerance at follow-up, participants were classified as progressors if they stepped up from NGT to IGT or T2D.
Surrogate markers of hepatic IR and liver fat content.
The liver IR index was calculated in adults and adolescents with an algorithm based on OGTT insulin levels, percent fat mass, BMI, and HDL cholesterol (18). The liver IR index has been shown to correlate with the tracer-derived EGP relative to fasting plasma insulin (EGP × FPI) at baseline (r = 0.65, P < 0.001) and during clamp (r = 0.59, P < 0.001) in nondiabetic subjects (18).
The fatty liver index, available only in the adult cohort, was calculated from BMI, waist circumference, triglycerides, and γ-GT (20). The fatty liver index has been validated against liver ultrasound and has been proven an accurate predictor of hepatic steatosis in the general population (accuracy = 0.84 [95% CI, 0.81–0.87]) (20).
Body composition.
Body composition and FFM were assessed using a Body Composition Analyzer (model TB-300; Tanita) in adults and by dual-energy X-ray absorptiometry in adolescents. Waist circumference was measured as the narrowest circumference between the lower rib margin and anterior superior iliac crest. Hip circumference was measured around the widest portion of the buttocks, and the waist/hip circumference ratio was calculated.
Biochemical measurements.
Plasma glucose was measured by the glucose oxidase technique. Plasma samples were divided into aliquots and stored at –80°C until analysis. Plasma insulin and C-peptide were measured by fluoroimmunoassay in adults (AutoDELFIA Insulin kit; Wallac Oy) and by radioimmunoassay in adolescents (Linco, catalog 1011). Plasma FFA were measured in the fasting state and at timed intervals during the hyperinsulinemic-euglycemic clamp by a fluorimetric method (Wako); the values during the last 40 minutes of the clamp were averaged to express insulin inhibition of circulating FFA (steady-state FFA). Plasma concentrations of arginine, glutamate, oleate, and palmitate were measured by targeted metabolomics. Plasma lactate and alanine were measured in 399 adult participants using a mass spectrometry, nontargeted biochemical profiling approach, as previously reported (75).
Statistics.
Continuous variables are presented as mean ± SD, and nominal variables are expressed as percentages. Variables with a skewed distribution are presented as median (interquartile range; IQR) and were log-transformed in multivariate analyses. Differences between groups were tested by Mann-Whitney U test for continuous variables or χ2 for nominal variables. General linear models were used to test for differences while controlling for potential confounders. Multivariate logistic regression was used to identify factors associated with the hypersecretory state; OR and 95% CI were reported. Repeated measures were analyzed by 2-way repeated-measure ANOVA including group (HyperS vs. NormS), time (either “time during the OGTT” or “baseline vs. follow-up”), and group × time interaction as factors. Post hoc pairwise comparisons were performed by Tukey’s honest significant difference (HSD) tests. Statistical analyses were performed using JMP Pro 13.2.1 software (SAS Institute). A 2-sided P < 0.05 was considered statistically significant.
Study approval.
The studies were conducted according to the principles expressed in the Declaration of Helsinki and approved by the local ethics committee of each center. Written informed consent was obtained prior to recruitment from all adult participants, as well as from parents of adolescent participants, who also gave their written informed assent.
Author contributions
DT contributed study design, data analysis and interpretation, and manuscript write-up; AN contributed study design, data collection, analysis and interpretation, and manuscript editing; SA contributed data collection and manuscript editing; AM contributed mathematical modeling of insulin secretion and β cell function parameters, data analysis and interpretation, and manuscript editing; EF contributed study design and supervision, funding, data collection, analysis and interpretation, manuscript write-up, and critical revision. All authors read and approved the final submitted version of the manuscript. DT and EF are the guarantors of this work and, as such, have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Material
Acknowledgments
The RISC Study was partly supported by EU grant QLG1-CT-2001-01252. See Supplemental Acknowledgments for consortium details. DT is funded through the EFSD Mentorship Programme.
Version 1. 12/20/2018
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
License: Copyright 2018, American Society for Clinical Investigation.
Reference information: JCI Insight. 2018;3(24):e124912. https://doi.org/10.1172/jci.insight.124912.
Contributor Information
Domenico Tricò, Email: domenico.trico@for.unipi.it.
Andrea Natali, Email: andrea.natali@med.unipi.it.
Silva Arslanian, Email: Silva.Arslanian@chp.edu.
Andrea Mari, Email: andrea.mari@cnr.it.
References
- 1.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin C, Desai KS, Steiner G. Receptor and postreceptor insulin resistance induced by in vivo hyperinsulinemia. Can J Physiol Pharmacol. 1983;61(8):802–807. doi: 10.1139/y83-123. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi M, Olefsky JM. Effect of experimental hyperinsulinemia on insulin binding and glucose transport in isolated rat adipocytes. Am J Physiol. 1978;235(1):E53–E62. doi: 10.1152/ajpendo.1978.235.1.E53. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen DJ, et al. A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol Metab. 2015;4(7):507–518. doi: 10.1016/j.molmet.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth Flach RJ, et al. Protein Kinase Mitogen-activated Protein Kinase Kinase Kinase Kinase 4 (MAP4K4) Promotes Obesity-induced Hyperinsulinemia. J Biol Chem. 2016;291(31):16221–16230. doi: 10.1074/jbc.M116.718932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Templeman NM, Clee SM, Johnson JD. Suppression of hyperinsulinaemia in growing female mice provides long-term protection against obesity. Diabetologia. 2015;58(10):2392–2402. doi: 10.1007/s00125-015-3676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehran AE, et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012;16(6):723–737. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Alemzadeh R, Jacobs W, Pitukcheewanont P. Antiobesity effect of diazoxide in obese Zucker rats. Metab Clin Exp. 1996;45(3):334–341. doi: 10.1016/S0026-0495(96)90287-5. [DOI] [PubMed] [Google Scholar]
- 9.Brøns C, et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol (Lond) 2009;587(Pt 10):2387–2397. doi: 10.1113/jphysiol.2009.169078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100(5):1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loves S, et al. Effects of Diazoxide-Mediated Insulin Suppression on Glucose and Lipid Metabolism in Nondiabetic Obese Men. J Clin Endocrinol Metab. 2018;103(6):2346–2353. doi: 10.1210/jc.2018-00161. [DOI] [PubMed] [Google Scholar]
- 12.Dankner R, Chetrit A, Shanik MH, Raz I, Roth J. Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over a 24-year follow-up: a preliminary report. Diabetes Care. 2009;32(8):1464–1466. doi: 10.2337/dc09-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizza RA, Mandarino LJ, Genest J, Baker BA, Gerich JE. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia. 1985;28(2):70–75. doi: 10.1007/BF00279918. [DOI] [PubMed] [Google Scholar]
- 14.Corkey BE. Diabetes: have we got it all wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes? Diabetes Care. 2012;35(12):2432–2437. doi: 10.2337/dc12-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23(7):804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pories WJ, Dohm GL. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care. 2012;35(12):2438–2442. doi: 10.2337/dc12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31 Suppl 2:S262–S268. doi: 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- 18.Vangipurapu J, et al. A novel surrogate index for hepatic insulin resistance. Diabetologia. 2011;54(3):540–543. doi: 10.1007/s00125-010-1966-7. [DOI] [PubMed] [Google Scholar]
- 19.Ferrannini E, Wahren J, Faber OK, Felig P, Binder C, DeFronzo RA. Splanchnic and renal metabolism of insulin in human subjects: a dose-response study. Am J Physiol. 1983;244(6):E517–E527. doi: 10.1152/ajpendo.1983.244.6.E517. [DOI] [PubMed] [Google Scholar]
- 20.Bedogni G, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mari A, et al. Impaired beta cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia. 2010;53(4):749–756. doi: 10.1007/s00125-009-1647-6. [DOI] [PubMed] [Google Scholar]
- 22.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90(1):493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- 23.Michaliszyn SF, et al. β-cell function, incretin effect, and incretin hormones in obese youth along the span of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes. 2014;63(11):3846–3855. doi: 10.2337/db13-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavin JR, Roth J, Neville DM, de Meyts P, Buell DN. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci USA. 1974;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258(5083):766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 26.Sbraccia P, et al. Chronic primary hyperinsulinaemia is associated with altered insulin receptor mRNA splicing in muscle of patients with insulinoma. Diabetologia. 1996;39(2):220–225. doi: 10.1007/BF00403966. [DOI] [PubMed] [Google Scholar]
- 27.Pontiroli AE, Alberetto M, Capra F, Pozza G. The glucose clamp technique for the study of patients with hypoglycemia: insulin resistance as a feature of insulinoma. J Endocrinol Invest. 1990;13(3):241–245. doi: 10.1007/BF03349549. [DOI] [PubMed] [Google Scholar]
- 28.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126(1):12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Prato S, Leonetti F, Simonson DC, Sheehan P, Matsuda M, DeFronzo RA. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia. 1994;37(10):1025–1035. doi: 10.1007/BF00400466. [DOI] [PubMed] [Google Scholar]
- 30.Alemzadeh R, Langley G, Upchurch L, Smith P, Slonim AE. Beneficial effect of diazoxide in obese hyperinsulinemic adults. J Clin Endocrinol Metab. 1998;83(6):1911–1915. doi: 10.1210/jcem.83.6.4852. [DOI] [PubMed] [Google Scholar]
- 31.Schreuder T, Karreman M, Rennings A, Ruinemans-Koerts J, Jansen M, de Boer H. Diazoxide-mediated insulin suppression in obese men: a dose-response study. Diabetes Obes Metab. 2005;7(3):239–245. doi: 10.1111/j.1463-1326.2004.00449.x. [DOI] [PubMed] [Google Scholar]
- 32.van Boekel G, Loves S, van Sorge A, Ruinemans-Koerts J, Rijnders T, de Boer H. Weight loss in obese men by caloric restriction and high-dose diazoxide-mediated insulin suppression. Diabetes Obes Metab. 2008;10(12):1195–1203. doi: 10.1111/j.1463-1326.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- 33.Loves S, et al. Effects of Diazoxide-Mediated Insulin Suppression on Glucose and Lipid Metabolism in Nondiabetic Obese Men. J Clin Endocrinol Metab. 2018;103(6):2346–2353. doi: 10.1210/jc.2018-00161. [DOI] [PubMed] [Google Scholar]
- 34.Camastra S, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54(8):2093–2102. doi: 10.1007/s00125-011-2193-6. [DOI] [PubMed] [Google Scholar]
- 35.Reed MA, et al. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab. 2011;96(8):2525–2531. doi: 10.1210/jc.2011-0165. [DOI] [PubMed] [Google Scholar]
- 36.Erion KA, Berdan CA, Burritt NE, Corkey BE, Deeney JT. Chronic Exposure to Excess Nutrients Left-shifts the Concentration Dependence of Glucose-stimulated Insulin Secretion in Pancreatic β-Cells. J Biol Chem. 2015;290(26):16191–16201. doi: 10.1074/jbc.M114.620351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Federspil G, Zaccaria M, Pedrazzoli S, Zago E, DePalo C, Scandellari C. Effects of sodium DL-lactate on insulin secretion in anesthetized dogs. Diabetes. 1980;29(1):33–36. doi: 10.2337/diab.29.1.33. [DOI] [PubMed] [Google Scholar]
- 38.Crespin SR, Greenough WB, Steinberg D. Stimulation of insulin secretion by long-chain free fatty acids. A direct pancreatic effect. J Clin Invest. 1973;52(8):1979–1984. doi: 10.1172/JCI107382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newsholme P, Cruzat V, Arfuso F, Keane K. Nutrient regulation of insulin secretion and action. J Endocrinol. 2014;221(3):R105–R120. doi: 10.1530/JOE-13-0616. [DOI] [PubMed] [Google Scholar]
- 40.Rebelos E, et al. Influence of endogenous NEFA on beta cell function in humans. Diabetologia. 2015;58(10):2344–2351. doi: 10.1007/s00125-015-3685-6. [DOI] [PubMed] [Google Scholar]
- 41.Natali A, et al. Plasma HDL-cholesterol and triglycerides, but not LDL-cholesterol, are associated with insulin secretion in non-diabetic subjects. Metab Clin Exp. 2017;69:33–42. doi: 10.1016/j.metabol.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Tricò D, Natali A, Mari A, Ferrannini E, Santoro N, Caprio S. Triglyceride-rich very low-density lipoproteins (VLDL) are independently associated with insulin secretion in a multiethnic cohort of adolescents. Diabetes Obes Metab. 2018;20(12):2905–2910. doi: 10.1111/dom.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Floyd JC, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45(9):1487–1502. doi: 10.1172/JCI105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gannon MC, Nuttall FQ. Amino acid ingestion and glucose metabolism--a review. IUBMB Life. 2010;62(9):660–668. doi: 10.1002/iub.375. [DOI] [PubMed] [Google Scholar]
- 45.Arslanian S, Suprasongsin C. Differences in the in vivo insulin secretion and sensitivity of healthy black versus white adolescents. J Pediatr. 1996;129(3):440–443. doi: 10.1016/S0022-3476(96)70078-1. [DOI] [PubMed] [Google Scholar]
- 46.Haffner SM, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45(6):742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 47.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in african-american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51(10):3014–3019. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- 48.Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated beta-cell function? Diabetes Care. 2008;31(7):1445–1447. doi: 10.2337/dc08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michaliszyn SF, Bonadonna RC, Sjaarda LA, Lee S, Farchoukh L, Arslanian SA. β-Cell lipotoxicity in response to free fatty acid elevation in prepubertal youth: African American versus Caucasian contrast. Diabetes. 2013;62(8):2917–2922. doi: 10.2337/db12-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansson LE, Lindblad U, Larsson CA, Råstam L, Ridderstråle M. Polymorphisms in the adiponutrin gene are associated with increased insulin secretion and obesity. Eur J Endocrinol. 2008;159(5):577–583. doi: 10.1530/EJE-08-0426. [DOI] [PubMed] [Google Scholar]
- 51.Stumvoll M, Fritsche A, Häring HU. Clinical characterization of insulin secretion as the basis for genetic analyses. Diabetes. 2002;51 Suppl 1:S122–S129. doi: 10.2337/diabetes.51.2007.s122. [DOI] [PubMed] [Google Scholar]
- 52.Hectors TL, et al. Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function. Diabetologia. 2011;54(6):1273–1290. doi: 10.1007/s00125-011-2109-5. [DOI] [PubMed] [Google Scholar]
- 53.Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7(6):346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- 54.Vilsbøll T, Holst JJ. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia. 2004;47(3):357–366. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 55.Aulinger BA, Vahl TP, Wilson-Pérez HE, Prigeon RL, D’Alessio DA. β-Cell Sensitivity to GLP-1 in Healthy Humans Is Variable and Proportional to Insulin Sensitivity. J Clin Endocrinol Metab. 2015;100(6):2489–2496. doi: 10.1210/jc.2014-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campfield LA, Smith FJ. Neural control of insulin secretion: interaction of norepinephrine and acetylcholine. Am J Physiol. 1983;244(5):R629–R634. doi: 10.1152/ajpregu.1983.244.5.R629. [DOI] [PubMed] [Google Scholar]
- 57.Roy MW, Lee KC, Jones MS, Miller RE. Neural control of pancreatic insulin and somatostatin secretion. Endocrinology. 1984;115(2):770–775. doi: 10.1210/endo-115-2-770. [DOI] [PubMed] [Google Scholar]
- 58.Campfield LA, Smith FJ. Alteration of islet neurotransmitter sensitivity following ventromedial hypothalamic lesion. Am J Physiol. 1983;244(5):R635–R640. doi: 10.1152/ajpregu.1983.244.5.R635. [DOI] [PubMed] [Google Scholar]
- 59.Ahrén B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43(4):393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 60.Cota D, Proulx K, Seeley RJ. The role of CNS fuel sensing in energy and glucose regulation. Gastroenterology. 2007;132(6):2158–2168. doi: 10.1053/j.gastro.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 61.Dunne MJ, Cosgrove KE, Shepherd RM, Aynsley-Green A, Lindley KJ. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev. 2004;84(1):239–275. doi: 10.1152/physrev.00022.2003. [DOI] [PubMed] [Google Scholar]
- 62.De León DD, Stanley CA. Mechanisms of Disease: advances in diagnosis and treatment of hyperinsulinism in neonates. Nat Clin Pract Endocrinol Metab. 2007;3(1):57–68. doi: 10.1038/ncpendmet0368. [DOI] [PubMed] [Google Scholar]
- 63.Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13(10):572–587. doi: 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Natali A, et al. Effects of insulin on hemodynamics and metabolism in human forearm. Diabetes. 1990;39(4):490–500. doi: 10.2337/diab.39.4.490. [DOI] [PubMed] [Google Scholar]
- 65.Felig P. The glucose-alanine cycle. Metab Clin Exp. 1973;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- 66.Brooks GA. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018;27(4):757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 67.Gastaldelli A, et al. Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: a quantitative study. Diabetes. 2000;49(8):1367–1373. doi: 10.2337/diabetes.49.8.1367. [DOI] [PubMed] [Google Scholar]
- 68.Bonnet F, et al. Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes. 2011;60(6):1660–1667. doi: 10.2337/db10-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berthoud HR, Münzberg H, Morrison CD. Blaming the Brain for Obesity: Integration of Hedonic and Homeostatic Mechanisms. Gastroenterology. 2017;152(7):1728–1738. doi: 10.1053/j.gastro.2016.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferrannini E. Physiology of glucose homeostasis and insulin therapy in type 1 and type 2 diabetes. Endocrinol Metab Clin North Am. 2012;41(1):25–39. doi: 10.1016/j.ecl.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 71.Hills SA, et al. The EGIR-RISC STUDY (The European group for the study of insulin resistance: relationship between insulin sensitivity and cardiovascular disease risk): I. Methodology and objectives. Diabetologia. 2004;47(3):566–570. doi: 10.1007/s00125-004-1335-5. [DOI] [PubMed] [Google Scholar]
- 72.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41(3):368–377. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 73.Mari A, Ferrannini E. Beta-cell function assessment from modelling of oral tests: an effective approach. Diabetes Obes Metab. 2008;10 Suppl 4:77–87. doi: 10.1111/j.1463-1326.2008.00946.x. [DOI] [PubMed] [Google Scholar]
- 74.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 75.Gall WE, et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5(5):e10883. doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.