Abstract
Iron deficiency is closely associated with altered GABA metabolism and affective behavior. While mutation in the hemochromatosis (HFE) gene disrupts iron homeostasis and promotes oxidative stress that increases the risk of neurodegeneration, it is largely unknown whether HFE mutation modifies GABAergic homeostasis and emotional behavior. The goal of our study was to investigate the impact of HFE on GABAergic neurochemistry and redox–epigenetic regulation in the brain using H67D HFE-mutant mice that recapitulates the H63D-HFE mutation in humans. H67D mice displayed elevated redox-active iron levels in the brain by 32% compared to age-matched wild-type mice. Moreover, the H67D brain had increased isoprostane and decreased glutathione, indicating elevated oxidative stress. Additionally, the H67D brain had decreased global methylation and attenuated DNA methyltransferase (DNMT) activity. Direct addition of iron to purified DNMT in vitro decreased enzyme activity in a concentration-dependent manner. Last, H67D mice exhibited decreased anxiety-like behavior, which was associated with increased expression of the GABAA receptor α2 subunits by 93%, and these changes were also observed in H67D mice fed a low-iron diet. Taken together, our results suggest a putative role of HFE in regulating labile iron status in the brain, and mutation in H67D perturbs redox-methylation status, contributing to GABAergic dysfunction.—Ye, Q., Trivedi, M., Zhang, Y., Böhlke, M., Alsulimani, H., Chang, J., Maher, T., Deth, R., Kim, J. Brain iron loading impairs DNA methylation and alters GABAergic function in mice.
Keywords: epigenetics, hemochromatosis, HFE, anxiety, oxidative stress
Iron is an essential nutrient for proper brain development and function, including myelination, neurotransmitter synthesis, and electron transfer (1); however, excess iron is neurotoxic because of its ability to induce oxidative stress (2, 3). Worldwide, millions of people are at risk of high brain iron levels, resulting from genetic or environmental factors. For example, the hemochromatosis [hyperferremia (HFE)] protein is required for the regulation of iron transport (4), and mutations in the HFE gene are the primary cause of hereditary hemochromatosis, the most common genetic iron overload disorder, especially in the white population (5). Specifically, the H63D variant of the HFE gene [22.9% of gene variants worldwide (6)] has received increased attention because of a greater susceptibility to elevated iron and oxidative damage in the brain (7, 8). In addition to genetic factors, brain iron accumulation can also be caused by nongenetic factors, such as traumatic brain injury (21 million people per year worldwide) (9, 10), intracranial hemorrhage (1.75 million incidents per year worldwide) (11, 12), and natural aging (13).
Imbalance of brain iron status alters both neuroreceptor expression and neurotransmitter levels in the brain (14). Because iron metabolism is closely associated with monoamine turnover, extensive studies have investigated the effects of iron deficiency and overload on monoaminergic pathways. For example, activities of the dopamine (DA) transporter (DAT) and DA D2 receptor (D2DR) are modified by iron deficiency (15, 16). Specifically, iron deficiency reduces DAT expression and inhibits DAT function, resulting in decreased DA uptake and increased synaptic DA concentrations (15–18). D2DR expression has been consistently found to be reduced in response to iron deficiency (17, 18). Moreover, iron serves as a cofactor for tyrosine hydroxylase, the rate-limiting enzyme involved in DA biosynthesis (19). Consequently, iron deficiency is associated with decreased DA levels in brain tissues (20). However, we recently demonstrated that levels of DAT and D2DR expression are altered upon iron overload (21). In addition, excess iron causes DA autoxidation in the brain, in which DA is oxidized to DA o-quinone (22), which could also reduce intracellular DA levels.
Although the relationship between iron homeostasis and the dopaminergic pathway has been well characterized, information linking iron status and GABAergic neurochemistry is limited. GABA is one of the most abundant neurotransmitters in the brain, and dysregulation of GABA is involved in anxiety disorders (23), which are often observed in iron deficiency (14). For example, iron deficiency impairs GABA shunt enzymes, including glutamate decarboxylase (GAD) and GABA transaminase (GABA-T) (24–26), which is associated with increased anxiety-like behavior. The effects of iron overload on brain GABA status, however, are yet to be characterized.
Although oxidative stress plays a central role in iron-induced neurotoxicity (27), it is unclear how a perturbed cellular redox environment upon iron loading leads to altered expression of neuroreceptors. Mounting evidence has suggested that the methylation of DNA plays a fundamental role in regulating neuroreceptor expression; in the methylation process, a methyl group is added to the C5 position of cytosine residues, and this results in transcriptional inhibition (28). Dysregulation of DNA methylation is involved in the etiology of neurologic and psychiatric disorders (29, 30). Recent studies have suggested that the DNA methylation process is coupled with cellular redox status (31). Specifically, reduced glutathione (GSH) is the primary antioxidant in the brain (32), and previous research has demonstrated that the biosynthesis of GSH is coupled with the methylation process by maintaining the cycle of the methyl donor S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH) (33). In conditions of insufficient GSH, formation of SAM is decreased while SAH levels increase, and the resultant decrease in the SAM/SAH ratio impairs methylation capacity (33, 34). Hence, any changes in the redox state, represented by the GSH/oxidized glutathione disulfide (GSSG) ratio, can modify DNA methylation status, leading to epigenetic alterations and ultimately affecting protein expression in neurotransmission pathways (35, 36). Relevant to our investigation, several studies have previously demonstrated that redox-active metals, such as mercury and manganese, are capable of suppressing DNA methylation in the brain (37, 38).
It is known that the H63D variant of the HFE gene is associated with increased iron status, elevated oxidative stress in the brain, and increased risk of neurodegenerative disorders (4, 39), but it is yet to be determined whether HFE mutation alters DNA methylation in the brain and consequently contributes to GABAergic alterations and abnormal emotion. Hence, the goal of our study was to investigate the impact of HFE mutation on redox–epigenetic regulation and GABAergic neurochemistry using mice carrying the H67D-HFE knock-in mutation, which is homologous to the H63D-HFE mutation in humans (40). Our results demonstrate that the H67D mutation increases redox-active iron levels in the brain, which impairs DNA methylation capacity and increases expression of the GABAA receptor α2 subunit. Taken together, our results suggest a putative role of HFE in regulating labile iron status in the brain, and that the mutation in H67D perturbs redox-methylation status, contributing to GABAergic dysfunction.
MATERIALS AND METHODS
Ethics statement
This study was performed in strict accordance with recommendations in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA). The protocol was approved by the Northeastern University Animal Care and Use Committee.
Animals
H67D knock-in mutant mice and control wild-type (WT) mice were kindly provided by J. Connor (Pennsylvania State University, State College, PA, USA) (42). All mice used in these studies were of the mixed background of C57BL/6 and 129Sv/J strains. Male mice, 7 to 10 wk old, were fed facility chow (380 mg iron/kg diet, Prolab Isopro RMH 3000; LabDiet, St. Louis, MO, USA) and were given water ad libitum. Male mice were chosen because estrogen affects iron metabolism (43). To manipulate iron status in the brain, weanling WT or H67D mice (3–4 wk old) were fed an iron overload diet (10,000 mg iron/kg diet, as carbonyl iron, TD.09077; Harlan Teklad, Madison, WI, USA), an iron-deficient diet (5 mg iron/kg diet, TD.80396; Harlan Teklad), or a control diet (50 mg iron/kg diet, TD.07800; Harlan Teklad) for 4 wk.
Elevated plus maze test
The elevated plus maze (Med Associates, Fairfax, VT, USA) is composed of 2 open arms [35 (length) × 6 (width) cm] and 2 closed arms [35 (length) × 6 (width) cm with a wall 15 (height) cm], which extend from the central zone platform [6 (length) × 6 (width) cm]. The maze is 94 cm above the floor. Each mouse was placed in the center zone facing the open arm and allowed to explore the maze for 5 min. The number of entries, time spent, and distance traveled in the open arms and closed arms, as well as total distance traveled on the whole maze were recorded and analyzed by AnyMaze (Stoelting, Wood Dale, IL, USA). The maze was cleaned thoroughly with Quatricide TB (Pharmacal Research Laboratories, Naugatuck, CT, USA) after each test.
Tissue collection
After the last behavior test, mice were humanely killed by isoflurane overdose, followed by exsanguination and tissue collection, including blood, liver, and brain. Serum was collected from blood. All tissues were flash frozen in liquid nitrogen and stored at −80°C until analysis.
Real-time quantitative PCR
Total RNA in the whole brain was extracted using Trizol reagent (MilliporeSigma, Burlington, MA, USA). Real-time quantitative PCR (qPCR) was performed in the Bio-Rad Real-Time PCR systems (Bio-Rad, Hercules, CA, USA) using the iTaq Universal SYBR Green Supermix (Bio-Rad). Primer sequences were obtained from published studies as follows: GABA transporter (42), GAD65 and -67 (43, 44), GABA-T (45), GABAA receptor α1 (GABRA1) and α2 (GABRA2) subunits (46), and β-actin (47). Reactions were performed in a 10 µl mixture containing specific primers of each gene, cDNA, and iTaq Universal SYBR Green Supermix. Amplification conditions were as follows: 95°C for 2 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Relative mRNA expression level was calculated by the threshold cycle (Ct) value of each PCR product and normalized to that of β-actin by using the comparative 2−ΔΔCt method (48). Results are presented as percentage of values in WT mice.
Western blot analysis
Left brain hemispheres were homogenized, electrophoresed on 10% SDS-PAGE (40 µg protein), and transferred to nitrocellulose membranes. After blocking with 5% nonfat milk, the membranes were incubated with primary antibodies, including goat anti-GABRA1, goat anti-GABRA2 (1:200; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti–GABA-T (1:500; Abcam, Cambridge, MA, USA), or mouse anti-actin as a loading control (1:5000; Millipore, Bedford, MA, USA). Blots were incubated with secondary antibodies conjugated with horseradish peroxidase, including donkey anti-goat IgG (1:1000; Santa Cruz Biotechnology), goat anti-rabbit IgG (1:1000; Santa Cruz Biotechnology), or sheep anti-mouse IgG (1:1000 or 1:5000; GE Healthcare, Piscataway, NJ, USA). Immunoreactivity was detected using ECL West Dura substrate (Thermo Fisher Scientific, Waltham, MA, USA). Protein bands were visualized by ChemiDoc XRS (Bio-Rad), and intensities of protein bands were quantified using Image Lab v.4.1 (Bio-Rad).
Analysis of GABA and glutamate
Brain tissues were homogenized on ice (50 mg of tissue with 400 µl of 400 mM HClO4, 50 µM EDTA), then neutralized with 100 mM borate buffer (1:10). The homogenates were centrifuged at 14,000 g for 15 min at 4°C. Different methods were utilized to quantify GABA and glutamate. Both methods utilized an HPLC system with electrochemical detector consisting of an ESA 542 Autosampler (Thermo Fisher Scientific), ESA 582 dual-piston pump, and ESA Coulochem II Electrochemical Detector (Model 5020 Guard Cell and 5011 Analytical Cell). The column used for both methods was an ESA HR-80 RP C18 (80 × 4.6 mm, 3-µm particles, 120 Å pores).
For GABA measurement, samples were subjected to precolumn derivatization to an isoindole sulfonate derivative with an o-phthaldialdehyde (OPA)/sulfite reagent. A stock solution (100×) of derivatizing reagent was prepared by dissolving 22 mg OPA in 0.5 ml absolute ethanol, 0.5 ml of 1 M Na2SO3, and 9 ml of 0.1 M sodium tetraborate, and pH was adjusted to 10.4 with sodium hydroxide. Before injection onto the column, 6 µl of sample was added to 12 µl of the working solution and allowed to sit for 10 min. The isocratic mobile phase consisted of 0.122 M NaH2PO4/methanol/acetonitrile (v/v/v; 82:11:2.5). The flow rate was 0.8 ml/min. The detection cell potentials were EGuard Cell +650 mV, E1 +150 mV, and E2 +600 mV. Injections were 10 µl, and data were acquired from E2 with P/C Chrom software (H&A Scientific, Greenville, NC, USA).
For glutamate measurement, samples were subjected to precolumn derivatization to form an electrochemically active alkylthiolisoindole derivative. A stock derivatizing agent (4×) consisted of 5 µl 2-ME, 27 mg OPA, 1 ml methanol, and 9 ml of 0.1 M sodium tetraborate buffer (pH 9.3). The working 1× derivatizing solution was prepared by diluting stock derivatizing agent (1:3 with 0.1 M sodium tetraborate). Samples and working solution (1:2) were mixed on the autosampler and injected (10 µl) 2 min after initial mixing. The isocratic mobile phase consisted of 0.1 M Na2HPO4 adjusted to pH 6.75 with H3PO4/methanol (v/v; 72:28). Cell potentials were EGC +650 mV, E1 +150 mV, and E2 +600 mV. The flow rate was 1.2 ml/min, and data were acquired from E2 with P/C Chrom software.
Analysis of thiol species
Brain tissue was homogenized and centrifuged at 10,000 g, and the supernatant was collected for analysis. The supernatant (10 μl) was injected into an HPLC electrochemical detection system for the quantification of the following metabolites: cysteine, cystine, GSH, GSSG, homocysteine, homocystine, methionine, SAH, and SAM. HPLC columns and running conditions were the same as those previously described (34).
DNA isolation
DNA from brain tissue was isolated for analysis of DNA methylation as previously described (49) using the FitAmp Tissue DNA Extraction Kit from Epigentek (Farmingdale, NY, USA). The full protocol can be obtained from the manufacturer’s website. Isolated DNA was quantified using an ND-1000 NanoDrop Spectrophotometer (Thermo Fisher Scientific).
DNA methylation analysis
DNA was isolated as above, and any contaminating RNA was removed by RNase treatment. Assessment of global DNA methylation status was accomplished as previously described (49) using the MethylFlash Methylated DNA Quantification Kit (Epigentek) according to the manufacturer’s instructions. The methylated fraction of DNA was identified using 5-methylcytosine (5mC) mAb and quantified by an ELISA-like reaction. Levels of methylated DNA were calculated using the optical density on a microplate reader at 450 nm.
Analysis of DNA methyltransferase activity
The nuclear protein was isolated using the EpiQuik Nuclear Extraction Kit (Epigentek). The activity of DNA methyltransferase (DNMT) was analyzed using EpiQuik DNA Methyltransferase Activity/Inhibition Assay Kit, according to the manufacturer’s instructions (Epigentek). Briefly, primary antibody against 5mC was coated on the plate and coincubated with sample and cytosine for 1.5 h. During this process, DNMT contained in brain tissues converts cytosine into 5mC, which is subsequently bound to the primary antibody coated on the plate. Levels of bound 5mC were then analyzed by sandwich ELISA. The optical density measured represents the amount of 5mC produced over 1.5 h, and thus the activity of DNMT was expressed as optical density per milligram of nucleus protein per hour. For the in vitro assay of DNMT inhibition, 25 ng of purified human DNMT enzyme (Epigentek) was incubated with different concentrations of ferric chloride (FeCl3; MilliporeSigma) with or without apo-ferritin (from equine spleen; MilliporeSigma).
Isoprostane analysis
Isoprostane was chosen as a marker of oxidative stress associated with heavy metal exposure (50–52). The left brain hemisphere was homogenized to quantify levels of isoprostane using assay reagents (Cayman Chemicals, Ann Arbor, MI, USA) according to the manufacturer’s instructions.
Metal analysis
Brain tissues were digested using nitric acid (trace grade; Thermo Fisher Scientific), and metal concentrations were determined by inductively coupled plasma mass spectrometry (ICP-MS; Varian 810/820MS; Bruker, Billerica, MA, USA), as previously described (21, 52).
Determination of nonheme iron levels
Tissues were digested in an acid solution (10% trichloroacetic acid, 3 M HCl) at 65°C for 20 h, and nonheme iron concentrations were quantified by a colorimetric assay using bathophenanthroline disulfate as previously described (53).
Determination of labile iron levels
Brain tissues were homogenized in Tris-HCl buffer (0.1 mM, pH 7.4) and centrifuged at 10,000 g for 15 min at 4°C. Supernatant was collected and subjected to labile iron analysis using a dihydrorhodamine-based method as previously described (54). The concentration of labile iron in the brain was determined (μg/g tissue).
Ferritin analysis
Brain tissues were homogenized in RIPA buffer and centrifuged at 16,000 g for 7 min. Levels of ferritin in the supernatant were assessed using the mouse ferritin (light chain) ELISA kit (Abcam) according to the manufacturer’s instructions.
Statistical analysis
Values were expressed as means ± sem. Comparisons between H67D and WT mice were performed by the Student t test. Differences were considered significant at P < 0.05. For comparisons among multiple groups, 1- or 2-way ANOVA was performed by SigmaPlot 12.3 (Systat Software, San Jose, CA, USA). Differences were considered significant at P < 0.05.
RESULTS
H67D mutation increases redox-active iron and oxidative stress in brain
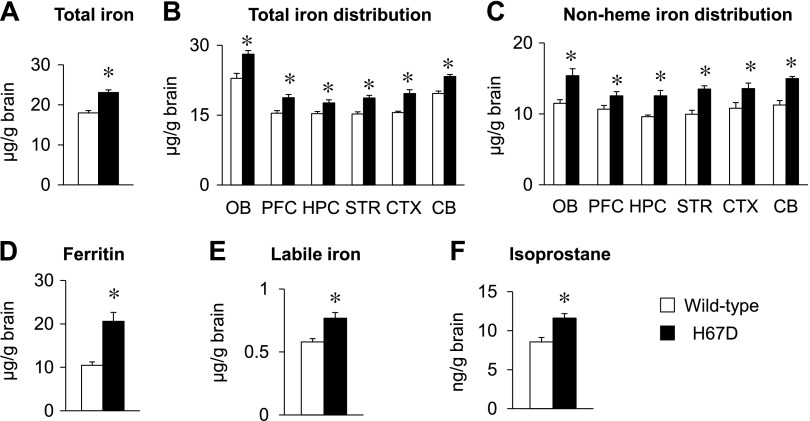
Compared to WT mice, total iron levels in serum, liver, and brain from mice with the H67D mutation were significantly increased (P < 0.001; Fig. 1A, and Supplemental Fig. S1), whereas levels of other essential metals, including zinc (the most abundant metal in the brain) and copper, were not altered in the brain (Supplemental Fig. S2A). To further investigate whether increased brain iron in H67D-mutant mice is region specific, we quantified the distribution of metals in different regions of the brain. Total iron levels were elevated across all brain regions, including the olfactory bulb, prefrontal cortex, hippocampus, striatum, cortex, and cerebellum (P < 0.05; Fig. 1B). Again, neither Zn nor Cu was altered (Supplemental Fig. S2B, C). Combined, these results indicate that H67D-mutant mice display iron accumulation in the whole brain without perturbations in homeostasis of other metals. Because total iron in tissues consists of heme and nonheme iron (Supplemental Fig. S3), and because nonheme iron serves as an indicator of cellular iron storage (53), we compared nonheme iron status in the brain in H67D mutant and WT mice by bathophenanthroline disulfate–based colorimetric assay. In parallel to elevated total iron, levels of nonheme iron were also increased across all brain regions in H67D mice (P < 0.05; Fig. 1C). Moreover, the primary iron storage protein ferritin was up-regulated by 97% in the H67D brain compared to age-matched WT mice (P = 0.004; Fig. 1D). Because cellular nonheme iron includes both protein-bound and free (labile) iron (Supplemental Fig. S3), and the latter is the primary form of iron that produces free radicals and causes oxidative damage (55), we determined levels of labile iron in brains of WT and H67D-mutant mice by dihydrorhodamine-based fluorescence assay. Upon H67D mutation, labile iron was elevated in the whole brain by 32% (P = 0.011; Fig. 1E). Last, levels of isoprostane, a well-established marker of lipid peroxidation and oxidative stress (56), were elevated in the brains of H67D mice compared to WT mice (P = 0.017; Fig. 1F). Collectively, our results demonstrate that the H67D mutation increases redox-active labile iron and oxidative stress in the brain.
Figure 1 .
H67D mutation increases redox-active iron and oxidative stress in brain. WT and H67D-mutant mice were humanely killed at age of 10 wk. Brain tissues were collected, and right brain hemisphere was dissected into different brain regions, including olfactory bulb (OB), prefrontal cortex (PFC), hippocampus (HPC), striatum (STR), cortex (CTX), and cerebellum (CB). A, B) Total iron levels in whole brain (A) and different brain regions (B) were quantified by ICP-MS. C) Levels of nonheme iron were measured by colorimetric assay using bathophenanthroline disulfate. D) Levels of ferritin in whole brain were quantified by ferritin ELISA. E) Labile iron in whole brain was determined using dihydrorhodamine-based fluorescence assay. F) Levels of isoprostane in whole brain were quantified by 8-isoprostane ELISA. Empty and solid bars represent WT and H67D-mutant mice, respectively. Data are presented as means ± sem (n = 4–7/group), and significant differences were analyzed by Student’s t test. *P < 0.05 vs. WT.
H67D mutation decreases thiol-antioxidant status in brain
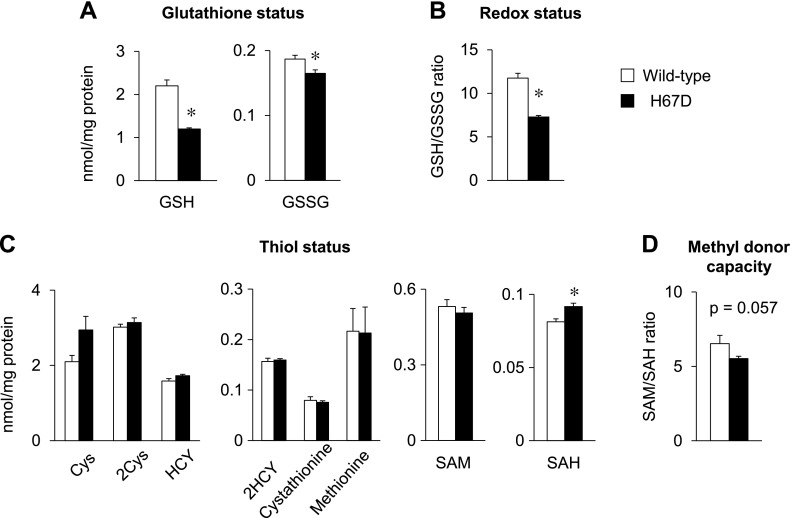
Increased labile iron and elevated lipid peroxidation in the H67D brain prompted us to examine the status of oxidative stress and to measure the levels of GSH, the major cellular antioxidant. Both GSH and GSSG levels were significantly reduced (P < 0.001 and P = 0.031, respectively) in the brains of H67D mice (Fig. 2A). Moreover, GSH/GSSG ratios in H67D mice were decreased by 38% compared to WT mice (Fig. 2B). Because the intracellular thiol status plays an important role in glutathione homeostasis, we separated thiol metabolite species and quantified their levels in the brains of H67D mice. Among all thiol species quantified, SAH was significantly increased in the H67D brains (P = 0.015; Fig. 2C), along with a trend of 15% decrease in the SAM/SAH ratio (P = 0.057; Fig. 2D). Together, these findings suggest that brain iron loading due to the H67D mutation decreases antioxidant reserves and alters cellular thiol metabolism.
Figure 2 .
H67D mutation decreases thiol status in brain. Brain tissue homogenates were used to determine levels of thiol species in brain by HPLC, including glutathione (A), redox status (B), thiol levels (C) and methyl donor capacity (D). Empty and solid bars represent WT and H67D-mutant mice, respectively. Data are presented as means ± sem (n = 4/group) and were analyzed by Student’s t test. *P < 0.05 vs. WT. 2Cys, cysteine disulfide; 2HCY, homocysteine disulfide; Cys, cysteine; HCY, homocysteine.
Global methylation is impaired in brain of H67D mice
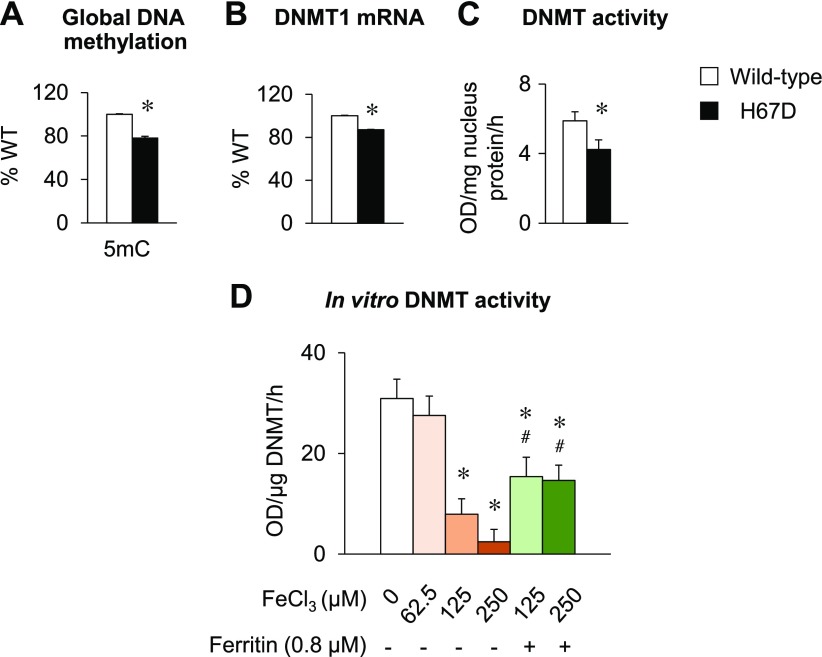
To assess whether altered thiol status in the H67D brain modifies DNA methylation capacity, we quantified levels of 5mC, the predominant form of methylated DNA in mammals (57–59) in the brains of WT and H67D mice. Levels of 5mC were significantly decreased in the brains of H67D mice (P < 0.001; Fig. 3A). We further quantified the expression and activity of DNMT, a key enzyme for DNA methylation (57), in the brains of H67D-mutant mice and their WT controls. While both DNMT1 and -3 are the most abundantly expressed DNMTs in the brain, DNMT1 is recognized as the main methyltransferase for maintenance in the adult brain, whereas DNMT3 is a de novo methyltransferase that promotes DNA methylation during embryo development (60). Because we used 8 to 10 wk old adult mice in our study, we quantified the expression of DNMT1. Interestingly, both mRNA levels (P = 0.001; Fig. 3B) and activity (P = 0.001; Fig. 3C) of DNMT were decreased in the H67D brain. To investigate whether iron loading directly affects DNMT activity, we performed an in vitro assay, in which purified DNMT enzyme was incubated with different concentrations of FeCl3. Exogenous iron decreased the activity of DNMT in a dose-dependent manner (P < 0.001), which was partially reversed when ferritin was added (P < 0.001, Fig. 3D). Combined, these results demonstrate that iron loading in the brain suppresses DNMT expression and activity, thereby impairing global DNA methylation status.
Figure 3 .
Global methylation is impaired in brain of H67D mice. Male WT and H67D-mutant mice were humanely killed at age of 10 wk. A) Left brain hemispheres were analyzed for 5mC by ELISA. B) Transcript levels of DNMT1 were determined by qPCR. C) Activity of DNMT was measured by ELISA. D) Purified human DNMT enzyme was incubated with different concentrations of FeCl3 with or without apo-ferritin, and activity was measured by ELISA. Empty and solid bars represent WT and H67D-mutant mice, respectively. Data are presented as means ± sem (n = 4–5/group for in vivo study, n = 3 for in vitro study), and significant differences were analyzed by Student’s t test or 1-way ANOVA. *P < 0.05 vs. WT (in vivo) or FeCl3 0 μM (in vitro). #P < 0.05 vs. no ferritin. OD, optical density.
H67D-mutant mice exhibit increased exploratory activity and reduced anxiety
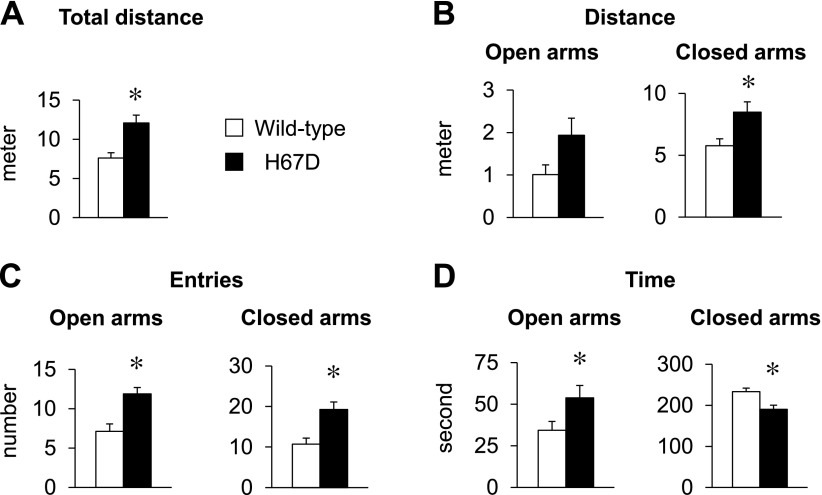
Because iron imbalance in the brain affects neurologic and behavioral function (14), we examined the emotion-related behavior in H67D mice by employing the elevated plus maze task. H67D mice traveled a greater distance in the whole maze (P = 0.004; Fig. 4A) and in the closed arms (P = 0.012; Fig. 4B) compared to WT mice. Moreover, H67D mice entered more frequently into both open arms (P = 0.002) and closed arms (P = 0.002; Fig. 4C). In addition, H67D mice spent more time in the open arms (P = 0.044) and less time in the closed arms (P = 0.004) (Fig. 4D). These results suggest that the H67D mutation promotes hyperactivity and anxiolytic behavior.
Figure 4 .
H67D-mutant mice exhibit increased exploratory activity and decreased anxiety. WT and H67D-mutant mice were subjected to elevated plus maze (EPM) task at age 7 or 8 wk for assessment of anxiety-like behavior, including total distance in whole maze (A) and distance traveled (B), entries (C), and time spent in open and closed arms (D). Empty and solid bars represent WT and H67D-mutant mice, respectively. Data are presented as means ± sem (n = 8–10/group), and significant differences were analyzed by Student’s t test. *P < 0.05 vs. WT.
GABA levels are decreased in brain of H67D mice
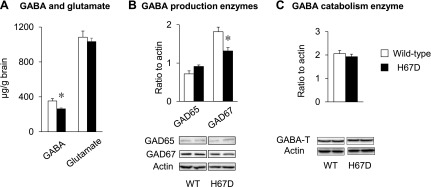
Because the GABAergic pathway plays a crucial role in anxiety behavior (61, 62), we characterized and compared the turnover of GABA in brains of H67D-mutant mice. GABA concentrations were significantly decreased by 25% in the whole brains of H67D mice compared to WT mice (P = 0.041; Fig. 5A). However, levels of glutamate (the endogenous precursor of GABA) did not differ between the two groups. Because GABA concentrations are maintained by the balance between production and catabolism, we quantified the expression and activity of the key enzymes responsible for GABA turnover. While the expression of GAD65 and -67, the enzymes that catalyze GABA production, was not altered at the transcript level (Supplemental Fig. S4), the protein expression of GAD67 was significantly decreased (P = 0.024, Fig. 5B). In contrast, GABA-T was up-regulated at the transcriptional level (P = 0.002, Supplemental Fig. S4), but its protein expression was not altered in the H67D brain (Fig. 5C). Combined, our data suggest that reduced GABA in the H67D brain is likely due to decreased synthesis.
Figure 5 .
GABA levels are decreased in brain of H67D mice. A) Right brain hemispheres collected from WT and H67D-mutant mice were subjected to HPLC-electrochemical detection of GABA and glutamate. B, C) Expression and activity of key enzymes involved in glutamate and GABA turnover, including production enzymes (B) and catabolism enzyme (C), were quantified by Western blot analysis. Empty and solid bars represent WT and H67D-mutant mice, respectively. Data are presented as means ± sem (n = 4 per group), and significant differences were analyzed by Student’s t test. *P < 0.05 vs. WT.
GABAA receptor α2 subunit is up-regulated in brain of H67D mice
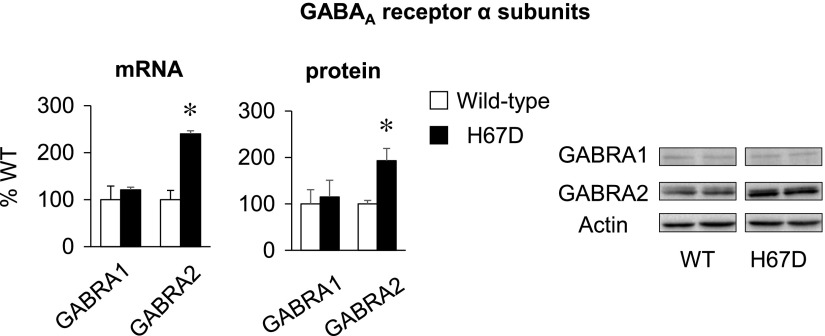
We also analyzed the expression of proteins involved in the GABAergic signaling pathway, including the GABA transporter and GABA receptors. mRNA expression of GABA transporter, a transporter regulating extracellular GABA levels (62), did not differ between H67D and WT mice (Supplemental Fig. S4). Interestingly, the H67D brain displayed a distinct difference in the expression of GABRA, which are closely associated with anxiety-like behavior (63); GABRA2 mRNA levels were significantly increased by 140% in the H67D brain compared to the WT controls (P < 0.001; Fig. 6), while GABRA1 mRNA levels were not altered in the H67D brain. Consistently, the protein expression of GABRA2 was increased by 93% in the H67D brain (P = 0.004) with no change in GABRA1 (Fig. 6). Together, these results suggest that the H67D mutation up-regulates GABRA2 in the brain, which contributes to anxiolytic behavior.
Figure 6 .
GABRA2 subunit is up-regulated in brain of H67D mice. Right brain hemispheres were collected from WT and H67D-mutant mice. mRNA and protein expression of GABAergic receptors was determined by qPCR and Western blot analysis, respectively. Empty and solid bars represent WT and H67D-mutant mice, respectively. Data are presented as means ± sem (n = 4/group), and significant differences were analyzed by Student’s t test. GABRA, GABAA receptor α subunit. *P < 0.05 vs. WT.
H67D mutation increases brain labile iron and alters GABAergic function and behavior
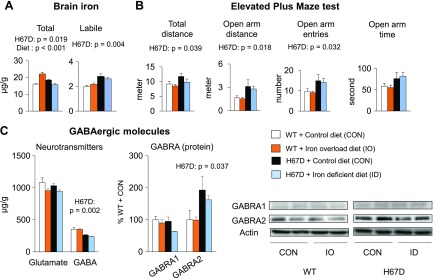
It has been shown that the H67D mutation exerts its own effects on neurologic function, which are independent of iron (64, 65), so it is therefore possible that the anxiolytic behavior and altered GABAergic function result from the intrinsic effect of the H67D mutation. First, to investigate whether brain iron loading alone induces anxiolytic behavior in the presence of native HFE, we fed WT mice an iron overload diet for 4 wk to induce brain iron overload. Although the iron overload diet increased total iron levels in the WT brain (diet effect, P < 0.001; Fig. 7A), levels of labile iron, anxiety-related behavior, and GABAergic neurochemistry were not altered (Fig. 7B, C). Next, to assess if the H67D mutation alone produces abnormal behavior and GABAergic dysfunction, we fed H67D mice a low-iron diet and compared the results to those from H67D and WT mice that were fed a control basal diet. Although the low-iron diet decreased total iron levels in the H67D brain, comparable to those in the WT brain, levels of labile iron remained high compared to WT mice fed the control diet (H67D effect, P = 0.004; Fig. 7A). In addition, H67D mice fed the low-iron diet still displayed reduced anxiety, decreased GABA levels, and increased GABRA2 expression compared to WT mice (H67D effect, P < 0.05; Fig. 7B, C). Combined, our results suggest that increased labile iron status in the H67D brain likely results from the intrinsic effects of H67D mutation, which consequently modifies GABAergic function and anxiety-like behavior.
Figure 7 .
Brain labile iron status determines abnormal behavior and GABAergic dysfunction. Weanling mice were fed basal control (50 mg iron/kg diet), iron-overload (10,000 mg iron/kg), or iron-deficient (5 mg iron/kg) diet for 4 wk. A) Levels of total iron and labile iron were analyzed by ICP-MS and dihydrorhodamine-based fluorescence assay, respectively. B) Anxiety-like behavior was measured by elevated plus maze test. C) GABA levels in brain were quantified by HPLC–electrochemical detection, and expression of GABAergic receptors was determined by Western blot analysis. Data are presented as means ± sem (n = 4–7/group), and significant differences were analyzed by 2-way ANOVA.
DISCUSSION
It has been consistently demonstrated that iron deficiency impairs GABA shunt enzymes, including GAD and GABA-T (24–26), suggesting that GABA homeostasis can be altered by iron status. However, less information is available about the relationship between iron overload and GABAergic neurochemistry. Because excess iron promotes the generation of reactive oxygen species (27), and because increased oxidative stress is associated with GABAergic dysfunction (66), we postulated that brain iron loading modifies GABA homeostasis and affective behavior. We used H67D-mutant mice, a mouse model of brain iron accumulation, to define the role of redox-active iron in GABAergic neurochemistry and behavior.
H67D-mutant mice exhibited increased total and nonheme iron across all brain regions. More importantly, levels of redox-active labile iron, the major determinant of iron-induced oxidative stress (55), were elevated in the H67D brain. In contrast, an iron overload diet did not alter the status of labile iron in the WT brain, although total iron levels in the brain were elevated. These results suggest that normal function of the HFE protein is required to maintain homeostasis of the labile iron pool in the brain, especially when total iron is increased. A previous in vitro study provided a plausible mechanism whereby HFE protein reduces the iron uptake mediated by transferrin, thereby reducing intracellular levels of labile iron (67). Therefore, dysfunctional HFE protein due to the H67D mutation could increase dissociation of iron from transferrin and elevate cellular labile iron. We note that there was an almost 2-fold increase in ferritin levels in the H67D brain, but up-regulation of ferritin did not decrease labile iron to normal levels (i.e., in WT brain). This could be due to differential capacities for iron uptake and storage among different cell types in the brain (68). While both glial cells and neurons are capable of taking up iron (69), glial cells have the greater capacity to take up and store iron (69) and are more resistant to iron-induced toxicity compared to neurons (70). A previous study showed that increased ferritin levels in the H67D brain are mostly confined to glial cells (40). Combined with our results, these findings suggest that increased labile iron in the H67D brain also likely occurs in neurons, which are poor in ferritin expression (40), and thereby modifies neuronal function. Future studies are warranted to evaluate the effects of H67D mutation on labile iron homeostasis in different cell types in the brain and iron-induced toxicity. To our knowledge, this is the first study to evaluate the essential role of HFE protein in regulating redox-active iron in the brain.
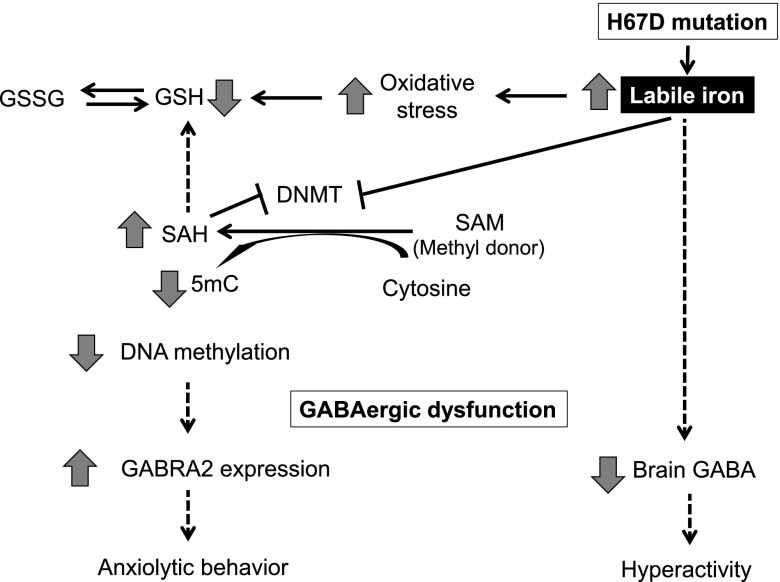
We further found that increased levels of labile iron in the H67D brain perturb the cellular redox environment and impair global DNA methylation. Our data offer 2 possible mechanisms (Fig. 8). First, toxic hydroperoxyl radicals produced by excess iron react with tissue hydrogen peroxide, and the resultant oxidative stress (71) depletes GSH, the major nonenzymatic intracellular antioxidant. This could alter cysteine and methionine metabolism, resulting in increased SAH production (72). Excess SAH inhibits DNMT activity and causes DNA hypomethylation (73). Second, it was previously reported that the iron chelator deferoxamine increases expression of DNMT and induces DNA hypermethylation in certain breast cancer cells (74). In corroboration with these findings, we demonstrated a direct inhibitory effect of excess iron on DNMT activity in vitro, which was partially restored by the addition of ferritin. Evidence suggests that labile iron enters the cell nucleus and is incorporated into the iron-sulfur cluster of several DNA processing enzymes (75). Thus, it is possible that increased labile iron by H67D mutation enters the nucleus and affects the activity of DNMT. Future studies characterizing subcellular distribution of iron and the mechanism of iron transport into the nucleus upon H67D mutation would test this idea.
Figure 8 .
Proposed mechanism of iron–redox–methylation hypothesis. Brain iron loading by H67D mutation induces emotional dysfunction by impairing GABAergic signaling. On the one hand, iron could directly alter enzyme activity (i.e., GAD) in GABA turnover, resulting in reduced GABA levels; on the other hand, excess iron perturbs DNA methylation by at least two possible mechanisms: first, oxidative stress depletes GSH and drives SAM/SAH cycle to produce more SAH, which inhibits DNMT activity; and second, iron directly inhibits DNMT activity. Both pathways impair DNA methylation capacity and up-regulate GABRA α2 receptor. Collectively, these changes in GABAergic signaling reduce anxiety and increase explorative activity in mice.
Our finding of decreased anxiety in H67D-mutant mice is consistent with the idea that imbalanced iron metabolism produces unstable emotional status (14). Moreover, H67D-mutant mice exhibited hyperactive behavior. Similarly, some hemochromatosis patients display schizophrenia-like or bipolar symptoms (76, 77). In rodents, Heidari et al. (78) found that Hfe−/−xTfr2mut mice with brain iron overload are hyperactive. However, mixed results also exist; iron overload is associated with hypoactivity, increased anxiety, or no change in emotional behavior. For example, Archer and Fredriksson (79) demonstrated that iron overload induced by oral iron succinate in mice results in elevated iron in the basal ganglia along with a short period of hypoactivity, followed by hyperactivity. In another study, rats loaded with iron by diet do not exhibit altered anxiety-related behavior or general activity despite elevated iron in the prefrontal cortex (80). These differences could be attributed to at least two possibilities. First, prolonged brain iron accumulation resulting from genetic mutations (e.g., H67D or Hfe−/−xTfr2mut) contributes to abnormal affective behavior, particularly hyperactivity and reduced anxiety, whereas subchronic exposure to exogeneous iron could trigger temporal changes in affective behavior, which could also depend on the form of iron used. Second, the H67D mutation could exert its intrinsic effects on emotional behavior, perhaps by the gain of mutant function rather than by the loss of HFE function. In support of this idea, we previously demonstrated that HFE-knockout mice display increased anxiety compared to WT mice (81), which is the opposite of the results in H67D-mutant mice. Also, it has been shown that different variants of the HFE gene result in cellular iron overload, but exhibit distinct or opposite effects on physiologic processes, including cholesterol production, lipid raft, apoptosis, and risks for Alzheimer disease (64). At the cellular level, endoplasmic reticulum stress is increased in human neuronal cells by the H63D mutation, but not by iron exposure (65). Together, these results indicate that the H67D mutation in the presence of increased brain labile iron contributes to abnormal affective behavior early in life.
We also identified that changes in the GABAergic pathway at least in part contribute to hyperactivity and anxiolytic behavior in H67D-mutant mice. GABA deficiency, also seen in attention-deficit/hyperactivity disorder (82), could account for hyperactivity in H67D-mutant mice. While both GAD65 and GAD67 synthesize GABA, the latter contributes to more than 90% of basal GABA production in mice (83, 84). Moreover, a knockdown of GAD67, but not GAD65, leads to GABA deficiency in the brain (85), suggesting a primary role of GAD67 for GABA synthesis in the brain. In addition, the finding that decreased brain GABA is correlated with decreased protein expression of GAD67, but not with its mRNA levels, suggests a posttranscriptional regulation of GAD67 upon brain iron loading induced by H67D mutation.
In contrast to GABA turnover enzymes, we observed an up-regulation of the GABRA2 receptor at both transcript and protein levels. This could result from impaired methylation capacity in the H67D brain because DNA methylation at promoter regions generally inhibits gene expression (86), and therefore overall hypomethylation in the iron-loaded H67D brain could promote GABRA2 up-regulation. However, there was no change in GABRA1 expression, suggesting that other epigenetic mechanisms are also involved, such as histone modification. Similarly, there could be epigenetic changes in the promoter of DNMT1 that could lead to altered DNMT1 mRNA and activity. In addition, a recent study reported that global m6A in the brain is increased upon stress (87). This finding suggests that m6A, although low in vertebrates (88), could play an important role in the development of neuropsychiatric disorders (87). On the basis of our finding that 5mC is decreased in the H67D brain, it is interesting to further investigate whether H67D mutation/iron overload alters noncanonical methylation of DNA, such as m6A and m4C, and if changes in these epigenetic modifications contribute to altered GABAergic neurochemistry. In addition, genome-wide epigenomic studies are warranted in the future to identify specific sites that are altered under the influence of elevated brain and/or H67D mutation, which may eventually lead to neurodevelopmental or neuropsychiatric diseases. Taken together, our findings suggest that H67D mutation could be an additional risk for brain iron accumulation and epigenetic dysregulation in affective disorders and thereby provide a pathophysiological basis for the influence of brain iron loading on redox/epigenetic control of mental health.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors are grateful to M. Han (Northeastern University) for help during animal experiments, and P. Paydary and P. Larese-Casanova (Northeastern University) for help with ICP-MS analysis. This work was supported, in part, by U.S. National Institutes of Health, National Institute of Environmental Health Sciences Grant K99/R00 ES017781 (to J.K.) and a Northeastern University TIER1 Interdisciplinary grant (to J.K.). The authors declare no conflicts of interest.
Glossary
- 5mC
5-methylcytosine
- D2DR
dopamine D2 receptor
- DA
dopamine
- DAT
dopamine transporter
- DNMT
DNA methyltransferase
- GABA-T
GABA transaminase
- GABRA
GABAA receptor α subunit
- GAD
glutamate decarboxylase
- GSH
reduced glutathione
- GSSG
oxidized glutathione disulfide
- HFE
hyperferremia
- ICP-MS
inductively coupled plasma mass spectrometry
- OPA
o-phthaldialdehyde
- qPCR
quantitative PCR
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Q. Ye, M. Trivedi, R. Deth, and J. Kim designed research; Q. Ye, M. Trivedi, Y. Zhang, M. Böhlke, H. Alsulimani, and J. Chang performed research; Q. Ye, M. Trivedi, M. Böhlke, and J. Kim analyzed data; and T. Maher, R. Deth, and J. Kim provided necessary laboratory space and reagents for research.
REFERENCES
- 1.Beard, J. L., Connor, J. R., Jones, B. C. (1993) Iron in the brain. Nutr. Rev. 51, 157–170 10.1111/j.1753-4887.1993.tb03096.x [DOI] [PubMed] [Google Scholar]
- 2.Salvador, G. A., Uranga, R. M., Giusto, N. M. (2010) Iron and mechanisms of neurotoxicity. Int. J. Alzheimers Dis. 2011, 720658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schipper, H. M. (2004) Brain iron deposition and the free radical–mitochondrial theory of ageing. Ageing Res. Rev. 3, 265–301 10.1016/j.arr.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 4.Nandar, W., Connor, J. R. (2011) HFE gene variants affect iron in the brain. J. Nutr. 141, 729S–739S 10.3945/jn.110.130351 [DOI] [PubMed] [Google Scholar]
- 5.Bradley, L. A., Johnson, D. D., Palomaki, G. E., Haddow, J. E., Robertson, N. H., Ferrie, R. M. (1998) Hereditary haemochromatosis mutation frequencies in the general population. J. Med. Screen. 5, 34–36 10.1136/jms.5.1.34 [DOI] [PubMed] [Google Scholar]
- 6.Hanson, E. H., Imperatore, G., Burke, W. (2001) HFE gene and hereditary hemochromatosis: a HuGE review. Human Genome Epidemiology. Am. J. Epidemiol. 154, 193–206 [DOI] [PubMed] [Google Scholar]
- 7.Bartzokis, G., Lu, P. H., Tishler, T. A., Peters, D. G., Kosenko, A., Barrall, K. A., Finn, J. P., Villablanca, P., Laub, G., Altshuler, L. L., Geshwind, D. H., Mintz, J., Neely, E., Connor, J. R. (2010) Prevalent iron metabolism gene variants associated with increased brain ferritin iron in healthy older men. J. Alzheimers Dis. 20, 333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nandar, W. Connor, J. R. (2011) HFE gene cariants affect iron in the brain. J. Nutr. 141, 729S–739S [DOI] [PubMed] [Google Scholar]
- 9.Raz, E., Jensen, J. H., Ge, Y., Babb, J. S., Miles, L., Reaume, J., Grossman, R. I., Inglese, M. (2011) Brain iron quantification in mild traumatic brain injury: a magnetic field correlation study. AJNR Am. J. Neuroradiol. 32, 1851–1856 10.3174/ajnr.A2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan, N. B. (2014) Chronic neurodegenerative consequences of traumatic brain injury. Restor. Neurol. Neurosci. 32, 337–365 [DOI] [PubMed] [Google Scholar]
- 11.Garton, T., Keep, R. F., Hua, Y., Xi, G. (2016) Brain iron overload following intracranial haemorrhage. Stroke Vasc. Neurol. 1, 172–184 10.1136/svn-2016-000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caceres, J. A., Goldstein, J. N. (2012) Intracranial hemorrhage. Emerg. Med. Clin. North Am. 30, 771–794 10.1016/j.emc.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta-Cabronero, J., Betts, M. J., Cardenas-Blanco, A., Yang, S., Nestor, P. J. (2016) In vivo MRI mapping of brain iron deposition across the adult lifespan. J. Neurosci. 36, 364–374 10.1523/JNEUROSCI.1907-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, J., Wessling-Resnick, M. (2014) Iron and mechanisms of emotional behavior. J. Nutr. Biochem. 25, 1101–1107 10.1016/j.jnutbio.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erikson, K. M., Jones, B. C., Beard, J. L. (2000) Iron deficiency alters dopamine transporter functioning in rat striatum. J. Nutr. 130, 2831–2837 10.1093/jn/130.11.2831 [DOI] [PubMed] [Google Scholar]
- 16.Bianco, L. E., Wiesinger, J., Earley, C. J., Jones, B. C., Beard, J. L. (2008) Iron deficiency alters dopamine uptake and response to l-DOPA injection in Sprague-Dawley rats. J. Neurochem. 106, 205–215 10.1111/j.1471-4159.2008.05358.x [DOI] [PubMed] [Google Scholar]
- 17.Erikson, K. M., Jones, B. C., Hess, E. J., Zhang, Q., Beard, J. L. (2001) Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol. Biochem. Behav. 69, 409–418 10.1016/S0091-3057(01)00563-9 [DOI] [PubMed] [Google Scholar]
- 18.Unger, E. L., Wiesinger, J. A., Hao, L., Beard, J. L. (2008) Dopamine D2 receptor expression is altered by changes in cellular iron levels in PC12 cells and rat brain tissue. J. Nutr. 138, 2487–2494 10.3945/jn.108.095224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsey, A. J., Hillas, P. J., Fitzpatrick, P. F. (1996) Characterization of the active site iron in tyrosine hydroxylase. Redox states of the iron. J. Biol. Chem. 271, 24395–24400 10.1074/jbc.271.40.24395 [DOI] [PubMed] [Google Scholar]
- 20.Beard, J. L., Unger, E. L., Bianco, L. E., Paul, T., Rundle, S. E., Jones, B. C. (2007) Early postnatal iron repletion overcomes lasting effects of gestational iron deficiency in rats. J. Nutr. 137, 1176–1182 10.1093/jn/137.5.1176 [DOI] [PubMed] [Google Scholar]
- 21.Chang, J., Kueon, C., Kim, J. (2014) Influence of lead on repetitive behavior and dopamine metabolism in a mouse model of iron overload. Toxicol. Res. 30, 267–276 10.5487/TR.2014.30.4.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen, X. M., Dryhurst, G. (1998) Iron- and manganese-catalyzed autoxidation of dopamine in the presence of l-cysteine: possible insights into iron- and manganese-mediated dopaminergic neurotoxicity. Chem. Res. Toxicol. 11, 824–837 10.1021/tx980036t [DOI] [PubMed] [Google Scholar]
- 23.Lydiard, R. B. (2003) The role of GABA in anxiety disorders. J. Clin. Psychiatry 64(Suppl 3), 21–27 [PubMed] [Google Scholar]
- 24.Li, D. (1998) Effects of iron deficiency on iron distribution and gamma-aminobutyric acid (GABA) metabolism in young rat brain tissues. Hokkaido Igaku Zasshi 73, 215–225 [PubMed] [Google Scholar]
- 25.Batra, J., Seth, P. K. (2002) Effect of iron deficiency on developing rat brain. Indian J. Clin. Biochem. 17, 108–114 10.1007/BF02867982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittal, R. D., Pandey, A., Mittal, B., Agarwal, K. N. (2003) Effect of latent iron deficiency on GABA and glutamate neuroreceptors in rat brain. Indian J. Clin. Biochem. 18, 111–116 10.1007/BF02867677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farina, M., Avila, D. S., da Rocha, J. B., Aschner, M. (2013) Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem. Int. 62, 575–594 10.1016/j.neuint.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu, H., Zhang, Y. (2014) Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell 156, 45–68 10.1016/j.cell.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klengel, T., Pape, J., Binder, E. B., Mehta, D. (2014) The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology 80, 115–132 10.1016/j.neuropharm.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 30.Grayson, D. R., Guidotti, A. (2013) The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology 38, 138–166 10.1038/npp.2012.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García-Giménez, J. L., Romá-Mateo, C., Pérez-Machado, G., Peiró-Chova, L., Pallardó, F. V. (2017) Role of glutathione in the regulation of epigenetic mechanisms in disease. Free Radic. Biol. Med. 112, 36–48 10.1016/j.freeradbiomed.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 32.Dringen, R. (2000) Metabolism and functions of glutathione in brain. Prog. Neurobiol. 62, 649–671 10.1016/S0301-0082(99)00060-X [DOI] [PubMed] [Google Scholar]
- 33.Lertratanangkoon, K., Wu, C. J., Savaraj, N., Thomas, M. L. (1997) Alterations of DNA methylation by glutathione depletion. Cancer Lett. 120, 149–156 10.1016/S0304-3835(97)00300-5 [DOI] [PubMed] [Google Scholar]
- 34.Hodgson, N., Trivedi, M., Muratore, C., Li, S., Deth, R. (2013) Soluble oligomers of amyloid-β cause changes in redox state, DNA methylation, and gene transcription by inhibiting EAAT3 mediated cysteine uptake. J. Alzheimers Dis. 36, 197–209 10.3233/JAD-130101 [DOI] [PubMed] [Google Scholar]
- 35.Abdolmaleky, H. M., Cheng, K. H., Faraone, S. V., Wilcox, M., Glatt, S. J., Gao, F., Smith, C. L., Shafa, R., Aeali, B., Carnevale, J., Pan, H., Papageorgis, P., Ponte, J. F., Sivaraman, V., Tsuang, M. T., Thiagalingam, S. (2006) Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum. Mol. Genet. 15, 3132–3145 10.1093/hmg/ddl253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marutha Ravindran, C. R., Ticku, M. K. (2005) Role of CpG islands in the up-regulation of NMDA receptor NR2B gene expression following chronic ethanol treatment of cultured cortical neurons of mice. Neurochem. Int. 46, 313–327 10.1016/j.neuint.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 37.Pilsner, J. R., Lazarus, A. L., Nam, D. H., Letcher, R. J., Sonne, C., Dietz, R., Basu, N. (2010) Mercury-associated DNA hypomethylation in polar bear brains via the LUminometric Methylation Assay: a sensitive method to study epigenetics in wildlife. Mol. Ecol. 19, 307–314 10.1111/j.1365-294X.2009.04452.x [DOI] [PubMed] [Google Scholar]
- 38.Tarale, P., Chakrabarti, T., Sivanesan, S., Naoghare, P., Bafana, A., Krishnamurthi, K. (2016) Potential role of epigenetic mechanism in manganese induced neurotoxicity. BioMed Res. Int. 2016, 2548792 10.1155/2016/2548792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pulliam, J. F., Jennings, C. D., Kryscio, R. J., Davis, D. G., Wilson, D., Montine, T. J., Schmitt, F. A., Markesbery, W. R. (2003) Association of HFE mutations with neurodegeneration and oxidative stress in Alzheimer’s disease and correlation with APOE. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 119B, 48–53 10.1002/ajmg.b.10069 [DOI] [PubMed] [Google Scholar]
- 40.Nandar, W., Neely, E. B., Unger, E., Connor, J. R. (2013) A mutation in the HFE gene is associated with altered brain iron profiles and increased oxidative stress in mice. Biochim. Biophys. Acta 1832, 729–741 10.1016/j.bbadis.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 41.Qian, Y., Yin, C., Chen, Y., Zhang, S., Jiang, L., Wang, F., Zhao, M., Liu, S. (2015) Estrogen contributes to regulating iron metabolism through governing ferroportin signaling via an estrogen response element. Cell. Signal. 27, 934–942 10.1016/j.cellsig.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 42.Cai, Y. Q., Cai, G. Q., Liu, G. X., Cai, Q., Shi, J. H., Shi, J., Ma, S. K., Sun, X., Sheng, Z. J., Mei, Z. T., Cui, D., Guo, L., Wang, Z., Fei, J. (2006) Mice with genetically altered GABA transporter subtype I (GAT1) expression show altered behavioral responses to ethanol. J. Neurosci. Res. 84, 255–267 10.1002/jnr.20884 [DOI] [PubMed] [Google Scholar]
- 43.Pléau, J. M., Esling, A., Geutkens, S., Dardenne, M., Homo-Delarche, F. (2001) Pancreatic hormone and glutamic acid decarboxylase expression in the mouse thymus: a real-time PCR study. Biochem. Biophys. Res. Commun. 283, 843–848 10.1006/bbrc.2001.4884 [DOI] [PubMed] [Google Scholar]
- 44.Liu, W., Liu, Z., Liu, L., Xiao, Z., Cao, X., Cao, Z., Xue, L., Miao, L., He, X., Li, W. (2008) A novel human foamy virus mediated gene transfer of GAD67 reduces neuropathic pain following spinal cord injury. Neurosci. Lett. 432, 13–18 10.1016/j.neulet.2007.11.054 [DOI] [PubMed] [Google Scholar]
- 45.Veeraiah, P., Noronha, J. M., Maitra, S., Bagga, P., Khandelwal, N., Chakravarty, S., Kumar, A., Patel, A. B. (2014) Dysfunctional glutamatergic and γ-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol. Psychiatry 76, 231–238 10.1016/j.biopsych.2013.09.024 [DOI] [PubMed] [Google Scholar]
- 46.Nakai, T., Nagai, T., Wang, R., Yamada, S., Kuroda, K., Kaibuchi, K., Yamada, K. (2014) Alterations of GABAergic and dopaminergic systems in mutant mice with disruption of exons 2 and 3 of the Disc1 gene. Neurochem. Int. 74, 74–83 10.1016/j.neuint.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 47.Aziz, A., Vanhille, L., Mohideen, P., Kelly, L. M., Otto, C., Bakri, Y., Mossadegh, N., Sarrazin, S., Sieweke, M. H. (2006) Development of macrophages with altered actin organization in the absence of MafB. Mol. Cell. Biol. 26, 6808–6818 10.1128/MCB.00245-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livak, K. J., Schmittgen, T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(t)) method. Methods 25, 402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 49.Trivedi, M., Shah, J., Hodgson, N., Byun, H. M., Deth, R. (2014) Morphine induces redox-based changes in global DNA methylation and retrotransposon transcription by inhibition of excitatory amino acid transporter type 3–mediated cysteine uptake. Mol. Pharmacol. 85, 747–757 10.1124/mol.114.091728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitsanakis, V. A., Thompson, K. N., Deery, S. E., Milatovic, D., Shihabi, Z. K., Erikson, K. M., Brown, R. W., Aschner, M. (2009) A chronic iron-deficient/high-manganese diet in rodents results in increased brain oxidative stress and behavioral deficits in the morris water maze. Neurotox. Res. 15, 167–178 10.1007/s12640-009-9017-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santos, D., Milatovic, D., Andrade, V., Batoreu, M. C., Aschner, M., Marreilha dos Santos, A. P. (2012) The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology 292, 90–98 10.1016/j.tox.2011.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye, Q., Kim, J. (2016) Mutation in HFE gene decreases manganese accumulation and oxidative stress in the brain after olfactory manganese exposure. Metallomics 8, 618–627 10.1039/C6MT00080K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torrance, J. D., Bothwell, T. H. (1968) A simple technique for measuring storage iron concentrations in formalinised liver samples. S. Afr. J. Med. Sci. 33, 9–11 [PubMed] [Google Scholar]
- 54.Esposito, B. P., Breuer, W., Sirankapracha, P., Pootrakul, P., Hershko, C., Cabantchik, Z. I. (2003) Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood 102, 2670–2677 10.1182/blood-2003-03-0807 [DOI] [PubMed] [Google Scholar]
- 55.Kruszewski, M. (2003) Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat. Res. 531, 81–92 10.1016/j.mrfmmm.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 56.Montuschi, P., Barnes, P. J., Roberts II, L. J. (2004) Isoprostanes: markers and mediators of oxidative stress. FASEB J. 18, 1791–1800 10.1096/fj.04-2330rev [DOI] [PubMed] [Google Scholar]
- 57.Cyr, A. R., Domann, F. E. (2011) The redox basis of epigenetic modifications: from mechanisms to functional consequences. Antioxid. Redox Signal. 15, 551–589 10.1089/ars.2010.3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, Y., Hodgson, N., Trivedi, M., Deth, R. (2016) Neuregulin 1 promotes glutathione-dependent neuronal cobalamin metabolism by stimulating cysteine uptake. Oxid. Med. Cell. Longev. 2016, 3849087 10.1155/2016/3849087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rasmussen, K. D., Helin, K. (2016) Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 30, 733–750 10.1101/gad.276568.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okano, M., Bell, D. W., Haber, D. A., Li, E. (1999) DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 99, 247–257 [DOI] [PubMed] [Google Scholar]
- 61.Nuss, P. (2015) Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr. Dis. Treat. 11, 165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brambilla, P., Perez, J., Barale, F., Schettini, G., Soares, J. C. (2003) GABAergic dysfunction in mood disorders. Mol. Psychiatry 8, 721–737, 715 [DOI] [PubMed] [Google Scholar]
- 63.Raud, S., Sütt, S., Luuk, H., Plaas, M., Innos, J., Kõks, S., Vasar, E. (2009) Relation between increased anxiety and reduced expression of alpha1 and alpha2 subunits of GABA(A) receptors in Wfs1-deficient mice. Neurosci. Lett. 460, 138–142 10.1016/j.neulet.2009.05.054 [DOI] [PubMed] [Google Scholar]
- 64.Ali-Rahmani, F., Schengrund, C. L., Connor, J. R. (2014) HFE gene variants, iron, and lipids: a novel connection in Alzheimer’s disease. Front. Pharmacol. 5, 165 10.3389/fphar.2014.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu, Y., Lee, S. Y., Neely, E., Nandar, W., Moyo, M., Simmons, Z., Connor, J. R. (2011) Mutant HFE H63D protein is associated with prolonged endoplasmic reticulum stress and increased neuronal vulnerability. J. Biol. Chem. 286, 13161–13170 10.1074/jbc.M110.170944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han, M., Chang, J., Kim, J. (2016) Loss of divalent metal transporter 1 function promotes brain copper accumulation and increases impulsivity. J. Neurochem. 138, 918–928 10.1111/jnc.13717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roy, C. N., Blemings, K. P., Deck, K. M., Davies, P. S., Anderson, E. L., Eisenstein, R. S., Enns, C. A. (2002) Increased IRP1 and IRP2 RNA binding activity accompanies a reduction of the labile iron pool in HFE-expressing cells. J. Cell. Physiol. 190, 218–226 10.1002/jcp.10056 [DOI] [PubMed] [Google Scholar]
- 68.Urrutia, P., Aguirre, P., Esparza, A., Tapia, V., Mena, N. P., Arredondo, M., González-Billault, C., Núñez, M. T. (2013) Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem. 126, 541–549 10.1111/jnc.12244 [DOI] [PubMed] [Google Scholar]
- 69.Bishop, G. M., Dang, T. N., Dringen, R., Robinson, S. R. (2011) Accumulation of non–transferrin-bound iron by neurons, astrocytes, and microglia. Neurotox. Res. 19, 443–451 10.1007/s12640-010-9195-x [DOI] [PubMed] [Google Scholar]
- 70.Oshiro, S., Kawahara, M., Kuroda, Y., Zhang, C., Cai, Y., Kitajima, S., Shirao, M. (2000) Glial cells contribute more to iron and aluminum accumulation but are more resistant to oxidative stress than neuronal cells. Biochim. Biophys. Acta 1502, 405–414 10.1016/S0925-4439(00)00065-X [DOI] [PubMed] [Google Scholar]
- 71.Thomas, C., Mackey, M. M., Diaz, A. A., Cox, D. P. (2009) Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: implications for diseases associated with iron accumulation. Redox Rep. 14, 102–108 10.1179/135100009X392566 [DOI] [PubMed] [Google Scholar]
- 72.James, S. J., Cutler, P., Melnyk, S., Jernigan, S., Janak, L., Gaylor, D. W., Neubrander, J. A. (2004) Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 80, 1611–1617 10.1093/ajcn/80.6.1611 [DOI] [PubMed] [Google Scholar]
- 73.Lee, W. J., Shim, J. Y., Zhu, B. T. (2005) Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 68, 1018–1030 10.1124/mol.104.008367 [DOI] [PubMed] [Google Scholar]
- 74.Pogribny, I. P., Tryndyak, V. P., Pogribna, M., Shpyleva, S., Surratt, G., Gamboa da Costa, G., Beland, F. A. (2013) Modulation of intracellular iron metabolism by iron chelation affects chromatin remodeling proteins and corresponding epigenetic modifications in breast cancer cells and increases their sensitivity to chemotherapeutic agents. Int. J. Oncol. 42, 1822–1832 10.3892/ijo.2013.1855 [DOI] [PubMed] [Google Scholar]
- 75.Zhang, C. (2014) Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein Cell 5, 750–760 10.1007/s13238-014-0083-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milman, N. (1991) Hereditary haemochromatosis in Denmark, 1950–1985. Clinical, biochemical and histological features in 179 patients and 13 preclinical cases. Dan. Med. Bull. 38, 385–393 [PubMed] [Google Scholar]
- 77.Serata, D., Del Casale, A., Rapinesi, C., Mancinelli, I., Pompili, P., Kotzalidis, G. D., Aimati, L., Savoja, V., Sani, G., Simmaco, M., Tatarelli, R., Girardi, P. (2012) Hemochromatosis-induced bipolar disorder: a case report. Gen. Hosp. Psychiatry 34, 101.e101–103 [DOI] [PubMed] [Google Scholar]
- 78.Heidari, M., Johnstone, D. M., Bassett, B., Graham, R. M., Chua, A. C., House, M. J., Collingwood, J. F., Bettencourt, C., Houlden, H., Ryten, M., Olynyk, J. K., Trinder, D., Milward, E. A. (2016) Brain iron accumulation affects myelin-related molecular systems implicated in a rare neurogenetic disease family with neuropsychiatric features. Mol. Psychiatry 21, 1599–1607 10.1038/mp.2015.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Archer, T., Fredriksson, A. (2007) Functional consequences of iron overload in catecholaminergic interactions: the Youdim factor. Neurochem. Res. 32, 1625–1639 10.1007/s11064-007-9358-1 [DOI] [PubMed] [Google Scholar]
- 80.Han, M., Kim, J. (2015) Effect of dietary iron loading on recognition memory in growing rats. PLoS One 10, e0120609 10.1371/journal.pone.0120609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye, Q., Kim, J. (2015) Effect of olfactory manganese exposure on anxiety-related behavior in a mouse model of iron overload hemochromatosis. Environ. Toxicol. Pharmacol. 40, 333–341 10.1016/j.etap.2015.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edden, R. A., Crocetti, D., Zhu, H., Gilbert, D. L., Mostofsky, S. H. (2012) Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 69, 750–753 10.1001/archgenpsychiatry.2011.2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Asada, H., Kawamura, Y., Maruyama, K., Kume, H., Ding, R. G., Kanbara, N., Kuzume, H., Sanbo, M., Yagi, T., Obata, K. (1997) Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. USA 94, 6496–6499 10.1073/pnas.94.12.6496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kash, S. F., Johnson, R. S., Tecott, L. H., Noebels, J. L., Mayfield, R. D., Hanahan, D., Baekkeskov, S. (1997) Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. USA 94, 14060–14065 10.1073/pnas.94.25.14060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chattopadhyaya, B., Di Cristo, G., Wu, C. Z., Knott, G., Kuhlman, S., Fu, Y., Palmiter, R. D., Huang, Z. J. (2007) GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron 54, 889–903 10.1016/j.neuron.2007.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dahl, C., Grønbæk, K., Guldberg, P. (2011) Advances in DNA methylation: 5-hydroxymethylcytosine revisited. Clin. Chim. Acta 412, 831–836 [DOI] [PubMed] [Google Scholar]
- 87.Yao, B., Cheng, Y., Wang, Z., Li, Y., Chen, L., Huang, L., Zhang, W., Chen, D., Wu, H., Tang, B., Jin, P. (2017) DNA N6-methyladenine is dynamically regulated in the mouse brain following environmental stress. Nat. Commun. 8, 1122 10.1038/s41467-017-01195-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koziol, M. J., Bradshaw, C. R., Allen, G. E., Costa, A. S. H., Frezza, C., Gurdon, J. B. (2016) Identification of methylated deoxyadenosines in vertebrates reveals diversity in DNA modifications. Nat. Struct. Mol. Biol. 23, 24–30 10.1038/nsmb.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.