Abstract
Traumatic brain injury (TBI) affects 2.8 million people annually in the United States, with significant populations suffering from ongoing cognitive dysfunction. Impairments in decision-making can have major implications for patients and their caregivers, often enduring for years to decades, yet are rarely explored in experimental TBI. In the current study, the Rodent Gambling Task (RGT), an Iowa Gambling Task analog, was used to assess risk-based decision-making and motor impulsivity after TBI. During testing, rats chose between options associated with different probabilities of reinforcement (sucrose) or punishment (timeout). To determine effects of TBI on learned behaviors versus the learning process, rats were trained either before, or after, a bilateral frontal controlled cortical impact TBI, and then assessed for 12 weeks. To evaluate the degree to which monoamine systems, such as dopamine, were affected by TBI, rats were given an amphetamine challenge, and behavior recorded. Injury immediately and chronically decreased optimal decision-making, and biased rats towards both riskier, and safer (but suboptimal) choices, regardless of prior learning history. TBI also increased motor impulsivity across time, reflecting ongoing neural changes. Despite these similarities in trained and acquisition rats, those that learned the task after injury demonstrated reduced effects of amphetamine on optimal decision-making, suggesting a lesser role of monoamines in post-injury learning. Amphetamine also dose-dependently reduced motor impulsivity in injured rats. This study opens up the investigation of psychiatric-like dysfunction in animal models of TBI and tasks such as the RGT will be useful in identifying therapeutics for the chronic post-injury period.
Keywords: rodent gambling task, amphetamine, cFos, plasticity, behavior
1. Introduction
Traumatic brain injuries (TBIs) are a serious health problem in the United States with over 2.8 million resulting in hospital visits every year (Center for Disease Control, 2017). Brain injury is a leading contributor to life-long disabilities, and increases the risk of neurodegenerative disease (Plassman et al., 2000; Thurman et al., 1999; Zaloshnja et al., 2008). Despite the significance of this problem, there are no therapies approved specifically for the treatment of chronic TBI. Some of the most long-term, difficult to manage, and pervasive deficits associated with TBI are impairments revolving around cognition and executive function, including various memory deficits, poor impulse control, and reduced decision-making capacity. Often, impairments can result in symptoms resembling those found in psychiatric disorders such as gambling disorder or bipolar disorder (Kräplin et al., 2014; Zgaljardic et al., 2015). In particular, impairments in decision-making and impulsivity, are likely to contribute to poor quality of life, and may result in significant issues for both patients with TBI, and their caregivers (Marsh et al., 1998). While impulsive deficits are relatively common in the acute phase (e.g. impulsive aggression, 35% incidence; Dyer et al., 2006), they also extend into the chronic post-injury phase, and even occur in cases of milder brain injury (Bjork et al., 2016; Goswami et al., 2016). These impulsive deficits may also interact with decision-making capacity in TBI survivors.
Decision-making is not a unitary construct, and constitutes many different dimensions. Ultimately, selection of options comes down to the evaluation of various costs and benefits associated with each choice. While this is decidedly general, there are several types of decision-making that are substantially altered in both psychiatric and TBI populations. In particular, impulsive decision-making, in which the cost is often a time delay, and the benefit is a larger reinforcer (e.g., money, food), is frequently impaired after brain injury (Dixon et al., 2005; McHugh and Wood, 2008). Another form, risk-based decision-making, in which choices are made between different probabilistic outcomes (e.g., win or lose money), is also notable for its involvement in TBI-related deficits (Cotrena et al., 2014; Levine et al., 2005; Newcombe et al., 2011). Thus, the consequences of TBI strongly resemble psychiatric disease with regard to decision-making tendencies and may have common neural mechanisms. In particular, dopaminergic dysfunction has been identified following experimental and clinical TBI (Wagner et al., 2005; Wagner et al., 2014), and the monoamines, in general, are strongly involved in a number of decision-making processes, providing a potential mechanistic link (Ozga et al., 2018).
To enable causal study of psychiatric deficits after TBI and aid in the development of treatments, animal models are necessary. The experimental brain injury field has developed a number of models relevant for replicating the human sequelae of TBI in rodents and other species (O’Connor et al., 2011). However, the vast majority of studies in experimental TBI have largely focused on cognitive outcomes that are less relevant for chronic psychiatric-related dysfunction (e.g., spatial learning). Recently, our group has published studies demonstrating deficits in impulse control, attention, and impulsive decision-making lasting up to four months with continuous testing, which resemble reports in human patients (Vonder Haar et al., 2016; Vonder Haar et al., 2017). However, no animal studies have evaluated whether rodent models of TBI can successfully replicate the deficits in risky decisions that occur in human brain injury populations.
A common paradigm for studying risky decisions in patients is the Iowa Gambling Task (IGT) (Bechara et al., 1994). In this task, participants choose a card from four different decks, and either gain or lose money. Two choices are associated with large wins, but also significant losses (“risky”), while the other yield small gains, but relatively small losses (“safe”/optimal). To maximize monetary gain, participants must learn these contingencies. In psychiatric populations (e.g., substance dependence, gambling disorder), patients display increased preference for risky options, at the cost of maximizing return (Bechara, 2003; Brevers et al., 2013), and similar effects are observed after TBI (Cotrena et al., 2014; Levine et al., 2005; Sigurdardottir et al., 2010). To investigate these phenomena in animals, many different procedures are used (for reviews, see Bailey et al., 2016; de Visser et al., 2011b). One model that is particularly attractive due to its translational validity is the Rodent Gambling Task (RGT). The RGT is a relatively direct analog of the IGT, and presents rats with two low-risk options, and two high-risk options. However, it also layers on an aspect of impulsive action (requires withholding a response over a delay) that is not included in the IGT to enable concurrent investigations into motor impulsivity (Zeeb et al., 2009). A previous meta-analysis of over 200 animals demonstrated a significant correlation between impulsivity and poor decision-making in rats on this task (Barrus et al., 2015). Given that motor impulsivity is substantially increased after TBI in rats (Vonder Haar et al., 2016), it is likely that risky decisions would be affected in a similar fashion. In the current study, we evaluated effects of a bilateral frontal controlled cortical impact TBI on risk-based decision-making and motor impulsivity in the RGT, in the chronic post-injury period and assessed the role of monoaminergic systems by administering an amphetamine challenge.
2. Results
2. 1. Recovery from Surgery
Recovery was tracked with daily detailed post-surgical monitoring until rats were deemed “recovered” (stable weight, no overt motoric deficits or agitation to handling; minimum of three days monitoring). All sham rats (including craniotomy and intact) were considered recovered within one day. The weight of all sham rats only dropped to approximately 98% of pre-surgery baseline before recovering to 100% or more by the second day. TBI rats took between 1–5 days (mean: 1.28) to recover normal motor function, and between 1–3 days (mean: 1.38) to react normally to gentle handling. The weight of TBI rats dropped to 91% of baseline, and remained slightly below (93–97%) throughout the duration of testing.
2. 2. Effects of TBI on Rodent Gambling Task
2. 2. 1. Choice
In the RGT, optimal reinforcement rates may be obtained by choices preferring the 2-pellet option, and preference for the 3- and 4-pellet options are considered “risky” (see Methods & Fig 1). To determine if brain injury altered choice behavior, a linear mixed-effects regression with baseline choice as a covariate (Pct Choice ~ Group*Choice Option*Week + Baseline Choice; see Supplemental Table S1 for full statistics) was carried out for the Trained rats. The three-way interaction was significant (p < 0.001). The regression model was then examined for each choice option. For the all choice options, there was a significant effect of TBI (p’s < 0.001), and a significant TBI x week interaction for the 2-pellet and 4-pellet options (p’s < 0.009), such that for the 2-pellet option, the Sham group very slightly declined, while the TBI group remained at a low level of choice relative to baseline, while TBI animals increased choice of the 4-pellet option across time. The effects were large and persistent across the 12-week post-injury period (see Fig 2).
Figure 1.
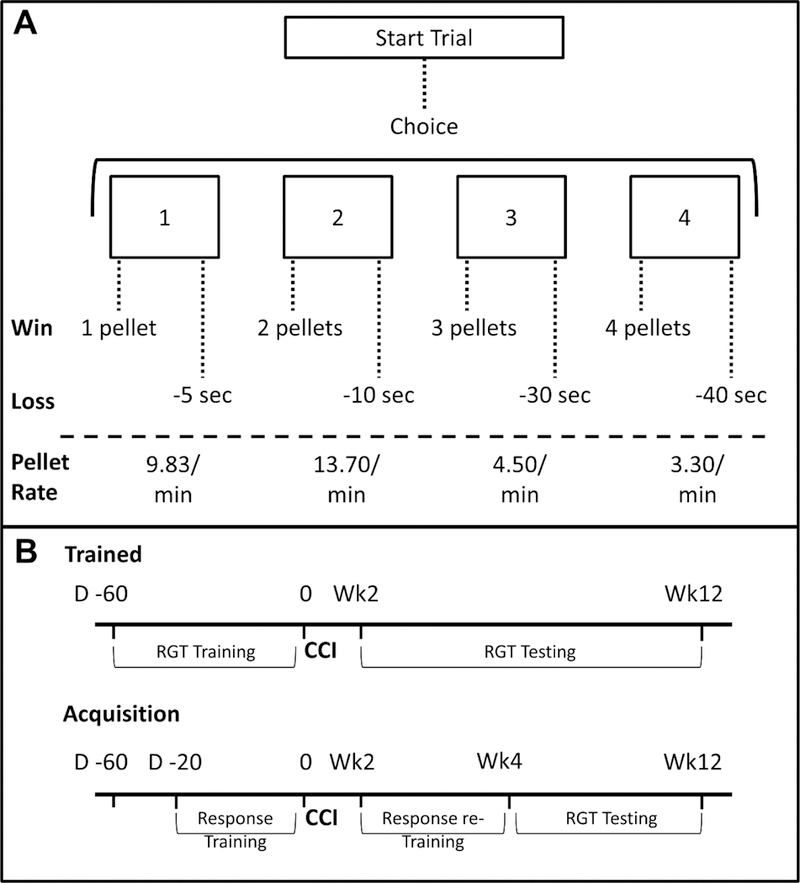
Task diagram and study design. A) On the rodent gambling task (RGT), rats have a choice between four options, each of which has different chances of “winning” pellets or “losing” time to earn pellets, resulting in set rates of reinforcement. B) Half of animals were trained on the RGT prior to TBI or sham surgery, while the other half learned the task after injury.
Figure 2.
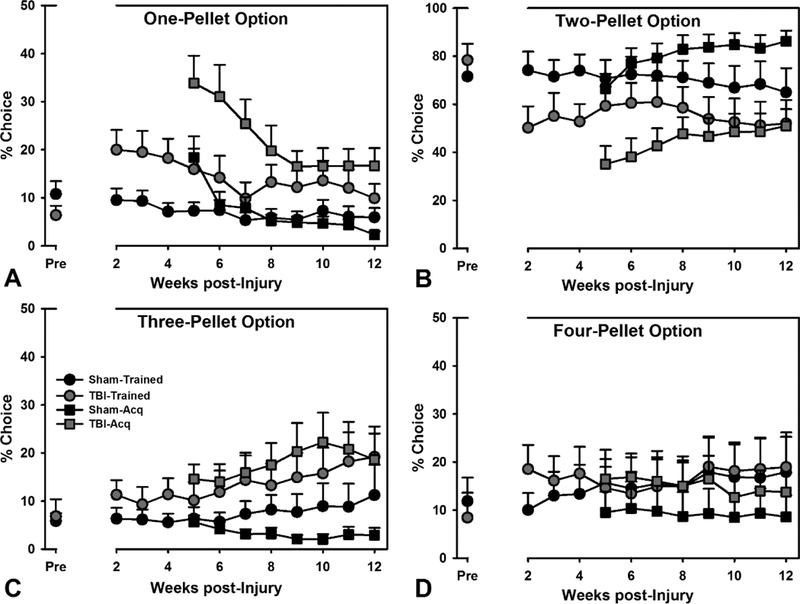
Choice on the RGT. A-D) Both Trained and Acquisition TBI animals were significantly different from their counterparts on all choice options (p’s < 0.009). Data are mean + SEM.
For rats tested in acquisition, effects of TBI were assessed in a linear mixed-effects regression (Pct Choice ~ Group*Choice Option*Week; see Table S1 for full statistics), and the three-way interaction was significant (p = 0.012). For these rats, there were significant effects of TBI across all choice options (p’s < 0.001), and a significant TBI x week interaction for the 3-pellet option (p = 0.002), such that the TBI group increased preference over time, while sham animals declined. All of these effects were similar in magnitude to those trained prior to injury, and choice profiles at 12 weeks ultimately resembled those trained before surgery (see Fig 2).
2. 2. 2. Other Variables
The RGT may also be used to measure several other variables of interest to gain insight into a host of other behavioral processes (see Methods): premature responses (motor impulsivity), omitted responses (motivation), pellets earned (overall efficiency), response latency (choice-specific slowing), and reinforcer collection latency (motor/motivational effects). A linear mixed-effects regression (Outcome ~ Group*Week [+ Baseline in Trained groups]) was performed for all other behavioral variables. For premature responses in the Trained groups, there was a significant TBI × week interaction (p < 0.001), such that TBI rats increased their premature responding across several weeks of testing. A similar interaction effect (p = 0.024) was also observed for the Acquisition rats with regard to impulsive responding. On omitted responses, Trained TBI rats started off quite high, but quickly reduced to sham levels, as indicated by a significant TBI × week interaction (p < 0.001). There was also a significant TBI × week interaction (p = 0.044) for the Acquisition rats, but this was due to a small, transient increase in omitted responding by sham rats during week 8. Brain-injured rats also collected fewer total pellets compared to sham rats over time (p’s < 0.001) across both Trained and Acquisition rats. With regard to response latency, for Trained rats there was a significant TBI × week interaction (p = 0.025), with a very slight decrease in the latency for the TBI rats immediately post-surgery. There was no significant group difference or interaction for the Acquisition rats on response latency (p’s > 0.427). The latency to collect the reinforcer in Trained rats was also affected by the injury (p = 0.002); TBI rats were slower to collect initially, but came down to sham levels over the testing period. A similar interaction was observed in the Acquisition rats (p < 0.001). Overall, the only behavioral differences that persisted across the testing period were impulsive responding and total pellets obtained (see Fig 3; see Table S1 for full statistical summary).
Figure 3.
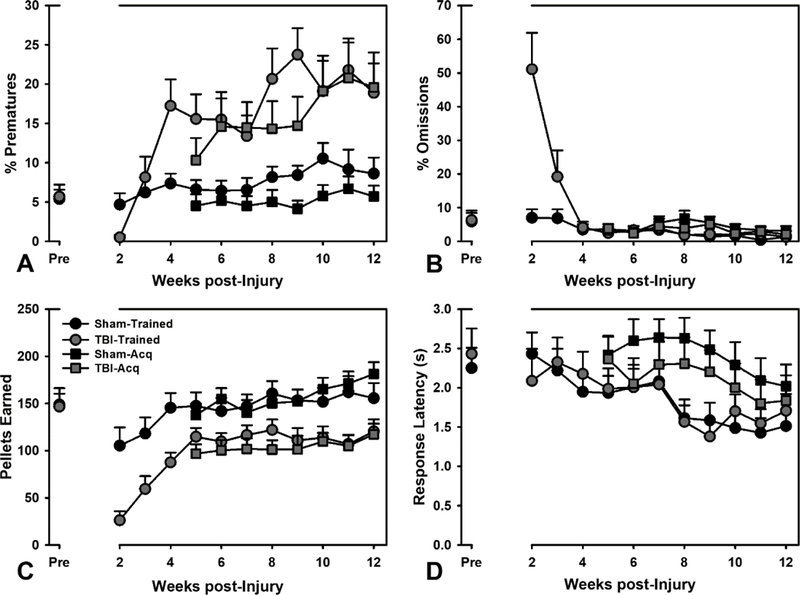
Performance on other RGT variables. A) Both Trained and Acquisition TBI animals increased their impulsive responding over the testing period (p’s < 0.024). B) Trained and Acquisition TBI groups also showed an interaction with regard to omissions (p’s < 0.044), however the Acquisition sham animals actually increased omissions during week 8. C) Both Trained and Acquisition TBI animals showed a decreased rate of pellet Acquisition across time (p’s < 0.001). D) With regard to response latency, Trained TBI animals had a significant interaction (p = 0.025) due to starting slightly lower, but ending up at approximately sham levels. Data are mean + SEM.
2. 3. Amphetamine Challenge
2. 3. 1. Choice
To determine the effects of a monoaminergic challenge on choice, amphetamine was administered in four doses. Choice data for the d-amphetamine challenge was examined in a linear mixed effects model with baseline choice on non-drug days as a covariate (Pct Choice ~ Group*Choice Option*Dose + Baseline Choice). For the Trained rats, there was no significant TBI × choice × dose interaction (p = 0.498), but there was a significant dose × choice effect (p < 0.001). Compared to saline, 1.0 and 1.5 mg/kg doses reduced choice of the 2-pellet option (p’s < 0.001), and increased choice of the 1-pellet option (p’s = 0.001).
The Acquisition rats displayed a significant three-way interaction of TBI × choice × dose (p = 0.049). Specifically, the TBI group did not change choice of the 2-pellet option with increasing d-amphetamine dose (p’s > 0.063), while the sham rats reduced 2-pellet choice at 1.0 and 1.5 mg/kg (p’s < 0.001). TBI rats also only increased choice of the 1-pellet option at the 1.0 mg/kg dose (p = 0.039), while the sham rats substantially increased choice at 1.0 and 1.5 mg/kg doses (p’s < 0.001). Sham rats also increased choice of the 3-pellet option at 1.5 mg/kg, and choice of the 4-pellet option at 1.0 mg/kg (p’s < 0.035; see Fig 4; see Table S2 for full statistical summary).
Figure 4.
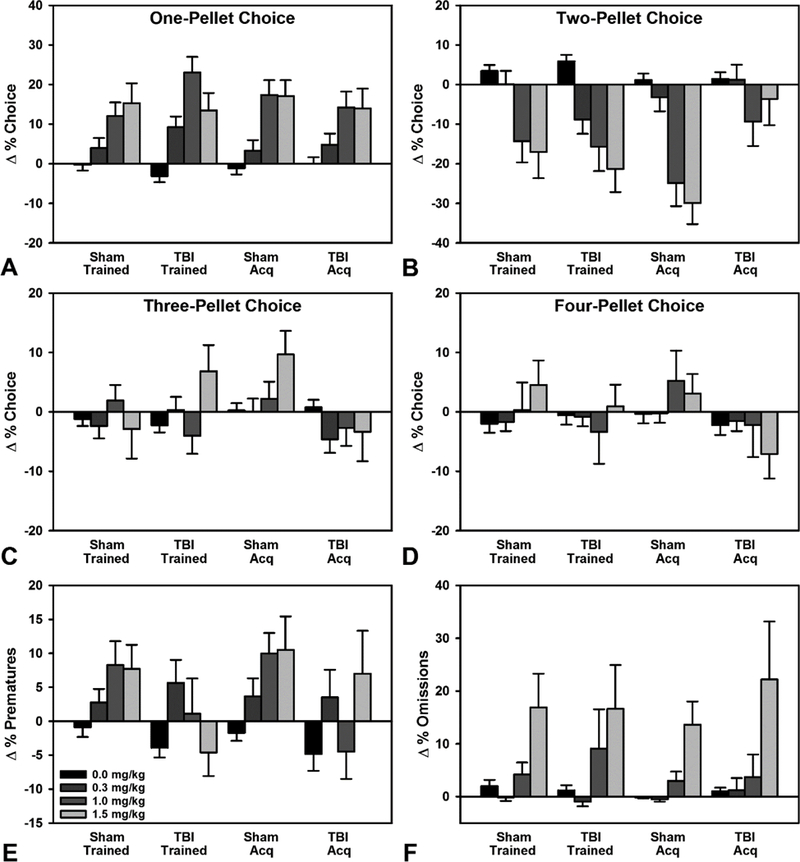
Effects of amphetamine on RGT performance. A) Trained animals, and Acquisition sham animals displayed a main effect of dose, with significant increases in choice of the 1-pellet option at 1.0 and 1.5 mg/kg (p’s = 0.001), however, Acquisition TBI animals were only significant at the 1.0 mg/kg dose (p = 0.039). B) Trained animals, and Acquisition sham animals significantly decreased choice of the 2-pellet option at 1.0 and 1.5 mg/kg (p’s < 0.001), while Acquisition TBI animals did not significantly change preference. C) Trained animals showed no effect of dose on 3-pellet choice. Acquisition sham animals increased preference at the 1.5 mg/kg dose (p = 0.035). D) Trained animals showed no effect of dose on 4-pellet choice. Acquisition sham animals increased preference at the 1.0 mg/kg dose (p = 0.021). E) Trained TBI animals significantly decreased premature responding compared to Trained shams at the 1.0 mg/kg dose (p = 0.007), while Acquisition animals showed no effect. F) There was an overall effect of dose on omissions for Acquisition animals, but no significant differences relative to saline. Data are mean + SEM.
2. 3. 2. Other Variables
Prematures and omissions were compared in a linear-mixed effects regression with baseline performance on non-drug days as a covariate (Outcome ~ Group*Dose + Baseline). For premature responses, there was a significant TBI × dose interaction in the Trained rats (p = 0.040), such that relative to the sham group, TBI rats displayed significantly reduced impulsive responding at the 1.0 mg/kg dose (p = 0.007). Rats in acquisition displayed considerable heterogeneity between the groups, leading to no significant TBI × dose interaction, or even main effect of dose (p’s > 0.228). On omissions, there were no TBI × dose interactions from either Trained or Acquisition rats (p’s > 0.581). The main effect of dose approached significance for Trained rats (p = 0.051), and was significant for Acquisition rats (p = 0.010). Despite a significant overall effect, no dose significantly altered omissions relative to vehicle (p’s > 0.064; see Fig 4; see Table S2 for full statistical summary).
2. 4. cFos Cell Counts
After an additional two weeks of washout from the amphetamine challenge, and at the conclusion of the study, to determine which brain regions were being used during the RGT task, animals were euthanized precisely 60 minutes after the conclusion of behavioral testing. Cells stained positive for cFos were compared in a two-factor ANOVA across four ROIs (Count~Training*Injury), and reduced to a one-way ANOVA if the interaction was not significant (Count~Injury). There was no significant interaction of task training and injury across any of the four ROIs (p’s > 0.264). Likewise, there was no significant effect of injury on any ROI (p’s > 0.114), likely due to high within-group variability (see Fig 5; see Table S3 for a full statistical summary).
Figure 5.
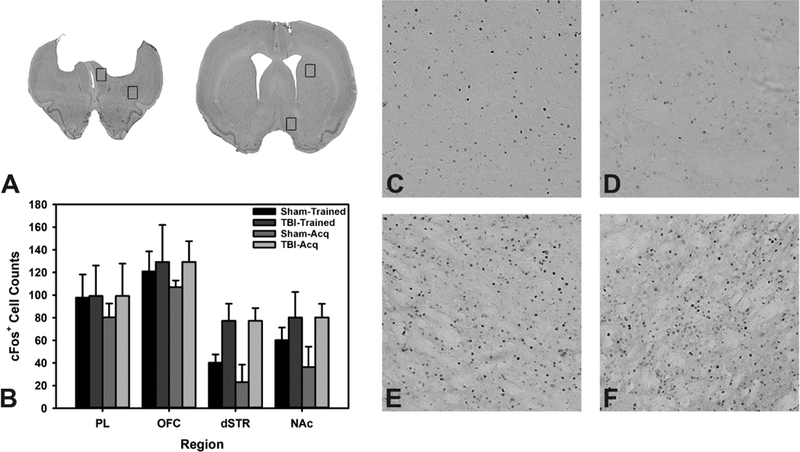
Analysis of cFos positive cells. A) Cresyl-violet of representative injured brain with boxes over regions of interest. B) Quantification of cFos positive cells. C) Exemplar sham dSTR. D) Exemplar sham NAc. E) Exemplar TBI dSTR. F) Exemplar TBI NAc. Data are mean + SEM.
2. 5. Sham Surgery Comparison
2. 5. 1. Choice
To examine effects of craniectomy, the Trained sham rats were compared in a mixed-effects regression with baseline as a covariate (Pct Choice ~ Group*Choice Option*Week + Baseline Choice; see Table S4 for full statistics). The three-way interaction was significant (p < 0.001). The regression model was then examined for each choice option. For the 2-, 3-, and 4-pellet choice options, there was a significant effect of craniectomy (p’s < 0.043), and a significant craniectomy x week interaction for the 3-pellet and 4-pellet options (p’s < 0.029). Despite significant effects, the magnitude of the difference was relatively small, largely within range of baseline differences, and driven by intact shams displaying riskier choice over time on the 3-pellet option (see Fig S1).
For the Acquisition sham rats, the same analysis (Pct Choice ~ Group*Choice Option*Week; see Table S4 for full statistics) was carried out, with a similarly significant three-way interaction (p < 0.001). The regression model was examined for each choice option. There was a significant effect of craniectomy and a significant craniectomy × week interaction on the 1-, 2-, and 4-pellet options (p’s < 0.019; p’s < 0.035). Effect sizes were quite small as above, and choice preferences largely collapsed on each other by the end of testing (see Fig S1).
2. 5. 2. Other Variables
For all other behavioral variables, a linear mixed-effects regression was performed (Outcome ~ Group*Week [+ Baseline in Trained groups]). On premature responses, there was no significant difference between Trained craniectomy and intact shams, or interaction with time (p = 0.258; p = 0.460), and a similar lack of effect was found for the Acquisition sham groups (p = 0.109; p = 0.383). For omissions, there was no significant difference between Trained craniectomy and intact shams, or interaction with time (p = 0.372; p = 0.122), but there was a significant interaction for the Acquisition sham rats (p < 0.001) such that intact shams slightly increased their omissions. For both Trained and Acquisition groups, the craniectomy groups earned more pellets over time (p’s < 0.05). With regard to response latency, there was no significant difference between Trained craniectomy and intact shams, or interaction with time (p = 0.982; p = 0.381), however, the Acquisition intact sham group demonstrated a significant interaction again, with slightly increased latencies (p < 0.001). On collection latency, there was no significant difference between Trained craniectomy and intact shams, or interaction with time (p = 0.487; p = 0.576), and similar findings were observed for the Acquisition sham groups (p = 0.855; p = 0.246). Overall, while significant, behavioral differences were minor between intact and craniectomy groups, with a tendency towards ‘impairment’ in the intact groups as opposed to those that received the full sham surgery (see Fig S2; see Table S4 for full statistical summary).
2. 6. Lesion Analysis
Total brain volumes were measured for all groups and subgroups and compared in a one-way ANOVA. There was a significant loss of tissue for both Trained and Acquisition injured groups (F1,21 = 8.22, p = 0.009; F1,19 = 5.06, p = 0.037). Lesions were similar in size to those reported in previous publications using the same injury model (Vonder Haar et al., 2016; Vonder Haar et al., 2017). There was no significant difference in total brain volume between craniotomy and intact shams (F1,19 = 0.07, p = 0.791). However, a visual inspection of craniotomy shams revealed slight damage to two subjects (drill mark; slight swelling due to torn dura).
3. Discussion
Decision-making deficits after TBI can have debilitating consequences in many facets of life for human patients, yet little research has examined these problems in animal models of brain injury. In the current study, we observed long-lasting deficits in decision-making and behavioral disinhibition that replicate what has been described in human populations (Cotrena et al., 2014; Levine et al., 2005; Sigurdardottir et al., 2010). On the RGT, injured rats displayed reduced preference for the most optimal (2-pellet) choice, and shifted towards suboptimal safer, and riskier options. This effect occurred regardless of learning history – TBI rats in acquisition, and those that were trained prior to injury, ultimately displayed similar choice profiles. Moreover, all injured rats escalated impulsive responding over the first several weeks post-injury, highlighting potential negative plasticity and re-organization in the post-acute phase. Finally, while Trained and Acquisition rats appeared similar in choice, they had a differential response to a d-amphetamine challenge such that injured rats in acquisition displayed a distinct pattern of choice relative to sham controls, or even compared to trained injured rats. These findings suggest that there may be significant interactions between learning history and monoaminergic systems, which could have major implications in rehabilitative settings where patients may be prescribed various psychotherapeutic drugs.
With rising concern over increased rates of various psychiatric diseases following TBI (Bhalerao et al., 2013; Carroll et al., 2014; Vaishnavi et al., 2009; Zgaljardic et al., 2015), more data are needed on the neurobiological changes permissive of increased impulsivity and poor decision-making. The RGT has been used to elucidate the substrates of neural control of risk-based decision-making for almost ten years (de Visser et al., 2011b; Zeeb et al., 2009; Zeeb and Winstanley, 2011). In that time, much has been learned about the neurochemistry of these types of behaviors. However, careful examination of many studies utilizing this assessment reveals that effects of many drugs, outside of major psychostimulants such as d-amphetamine, are relatively subtle in their effect on choice, particularly when administered after stable behavior has emerged (Barrus and Winstanley, 2016; Zeeb et al., 2009; Zeeb et al., 2015). Notably, and in contrast to many pharmacological studies, the magnitude of change after TBI on the RGT in the current study was both substantial and enduring, showing no signs of change at 12-weeks post-injury. This is not entirely surprising, given both the magnitude of the injury, and prior reports of chronic deficits in other psychiatric-related behaviors (Vonder Haar et al., 2016; Vonder Haar et al., 2017; Vonder Haar et al., 2018).
However, in the field of experimental brain injury, many assessments, even those in the cognitive domain, reveal relatively small deficits when subjected to repeat testing, which has been identified as a major problem (Fujimoto et al., 2004; Gold et al., 2013). The IGT, on which the RGT is modeled, has been suggested to identify trait-level differences in decision-making in a variety of psychiatric conditions (Brevers et al., 2013; Gansler et al., 2011; Sevy et al., 2007). Assessments of trait-like variables in animal models of TBI may provide a better understanding of the biological changes underlying long-term deficits, and indeed have proved successful, even after relatively mild TBI (Vonder Haar et al., 2017).
Of particular interest from the current data, is the large scale increase in motor impulsivity following the injury (Fig 3). This is notable because, while the RGT measures behavioral disinhibition, it does not engender a strong prepotent response as choice options remain illuminated for 10 s, providing a large amount of time for rats to respond. Interestingly, this impulsive responding increased across time, peaking at 8–10 weeks post-injury, and approximately four-fold above sham levels. These data map strongly onto prior TBI findings in the five-choice serial reaction time task, with both large-scale increases in impulsivity, and the tendency to increase across time (Vonder Haar et al., 2016). Importantly, these data suggest ongoing neural changes in the post-acute period that ultimately result in high levels of impulsivity and may be treatable. One potential explanation for this may be the neuronal death that occurs at locations functionally connected, but distal to the site of injury, a process known as diaschisis (Carrera and Tononi, 2014). Notably, major dopaminergic changes have been identified in the striatum following CCI injuries (Chen et al., 2015; Wagner et al., 2005), and this increased disinhibition may be reflective of cell death occurring in the dorsal and ventral striatum. Theoretically, these changes should be moderately preventable or reversible through psychostimulant treatment, and/or other therapies to prevent cell death.
In the current study, we administered a d-amphetamine challenge as a means of assaying monoaminergic function. Notably, previous data have shown that larger doses of d-amphetamine can actually reduce impulsivity in severely-injured rats (Vonder Haar et al., 2016). This finding was replicated in the Trained TBI rats, while the Acquisition TBI rats had an extremely variable response such that there was not even a main effect of dose (Fig 4). This further supports the potential of dopaminergic therapies, although additional study is clearly warranted. The effects of d-amphetamine on choice behavior were also altered in a surprising fashion for TBI rats. In both sham groups, as well as the Trained TBI group, we replicated the common effect of amphetamine shifting preference from the 2-pellet (optimal) option to the 1-pellet (safest, but suboptimal) option (Baarendse et al., 2013; Silveira et al., 2016; Zeeb et al., 2009; Zeeb et al., 2013). However, in the Acquisition TBI group, the 2-pellet reduction was significantly attenuated, yet preference for the 1-pellet option was still increased. This suggests that the shift in decision-making came from the riskiest 3- and 4-pellet options instead, highlighting potential benefits of d-amphetamine for these rats. Although amphetamines are used clinically to treat the impulsive symptoms of ADHD, recent evidence suggests that they may not be particularly effective in adults (Castells et al., 2018). This, however, does not preclude their use in individuals with TBI, and indeed, amphetamine has been suggested to help improve recovery acutely after experimental lesion or brain injury (Feeney et al., 1982; Ramic et al., 2006). Whether this would translate to treatment instigated in the chronic post-injury period remains to be seen. Moreover, the unique response of the Acquisition TBI rats to amphetamine with regard to decision-making suggests an interesting interaction between learning history and pharmacology which may need to be considered for the pharmaceutical treatment of TBI patients.
The differences in post-injury choice, combined with the interesting difference in TBI rats under d-amphetamine challenge suggests that brain-injured rats may be utilizing different neural circuits in order to meet the demands of this behavioral test. Given the size of the lesion cavity that formed due to TBI, large portions of the medial prefrontal cortex (mPFC), including anterior cingulate and prelimbic cortices, were severely damaged and likely nonfunctional (Fig 5). In addition, it is likely that indirect damage occurred in surrounding and connected regions such as the OFC, dorsal striatum, and ventral striatum. While the mPFC is essential for mediating impulse control (Dalley et al., 2004), and likely contributed to deficits in motor impulsivity, previous studies have indicated a relatively small role in its control over choice on the RGT (de Visser et al., 2011a; Paine et al., 2013; Zeeb et al., 2015). The circuit connecting the basolateral amygdala (BLA) to the OFC has previously been demonstrated to be critical to RGT performance, with the BLA and OFC contributing to stable risky decisions, and acquisition of behavior, respectively (Zeeb and Winstanley, 2011; Zeeb and Winstanley, 2013). Unfortunately, in the current study, tissue samples were not collected rostral enough to provide information over the role of the BLA. Given the differences between Trained and Acquisition TBI groups that emerged when challenged with amphetamine, there is potential that these circuits may be differentially affected by the learning process after injury. While differences were revealed by amphetamine challenge, no significant regional changes in neural activation under normal testing were observed when brains were examined with cFos (Fig 5). It should be noted that this does not preclude functional reorganization of circuits that occurred over time as cFos measurements were only made on stable behavior at the very end of the study. Despite obvious deficits, injured rats are still capable of performing the RGT to a degree. At the core of this behavioral measure is the ability to detect environmental contingencies with maximal reinforcement rates and accurately choose among these options. Notably, the explicit detection and identification of environmental contingencies is an area in which patients with brain injuries struggle (Schlund and Pace, 2000; Schlund, 2002), potentially explaining the impairments on this task. What is not clear from existing literature is whether TBI alters sensitivity to reinforcement, to punishment, or both. Some evidence has been given for reduced sensitivity to reinforcement in TBI patients (Larson et al., 2007), while another group has suggested reduced fear processing, affecting sensitivity to loss in the IGT (Visser-Keizer et al., 2016), both of which would certainly map onto deficits in dopamine neurotransmission discussed above. This would be consistent with a transition to striatal control of behavior, yet we failed to find any significant differences in activation patterns within the striatum of injured animals, likely due to large heterogeneity within the groups (Fig 5). Further studies will be required to conclusively establish whether reduced reinforcement/punishment sensitivity is sufficient to drive the observed deficits, and whether this might be a viable behavioral treatment target.
The data shown here demonstrate a long-lasting decision-making phenotype in TBI rats that directly mimics what is observed in human patients (Cotrena et al., 2014; Levine et al., 2005; Sigurdardottir et al., 2010). Moreover, similar deficits emerge in brain-injured animals, regardless of the timing of learning (Trained vs. Acquisition TBI groups), suggesting that these chronic deficits are not explicitly tied to learning (Fig 2 & 3). By focusing animal assessment on trait-like variables, rather than assessments of rapid learning, we can increase the relevance of our animal models to the human condition, and likely improve the translational potential of therapeutics assessed in this way. There are some limitations to operant measures of this type, most notably the longer training period relative to common behavioral neuroscience tasks. However, this is mitigated by the high-throughput nature, and resolution of data obtained from these behaviors. There may be some justifiable concern about the high resolution of the data, especially when using sensitive data analyses such mixed-effects modeling. Notably, we detected statistically significant differences in craniectomy versus intact shams. These types of differences have also been shown previously on motor and pain-related behaviors (Cole et al., 2011; Elliott et al., 2012), but similar to what was found in this study, effects tend to be transitory or small in nature. In the current study, craniotomy effects were quite small (see Table S4), and did not appear to be meaningful differences, and further were in the direction of craniectomy improving function relative to intact. This could lead to concern over similar issues in injured versus sham comparisons, however by attending to the large effects (Fig 2 & 3), it can be determined that the differences in sham versus TBI were not merely significant, but also meaningful. Ultimately, this behavioral approach to modeling chronic deficits due to TBI has high utility, and will likely be very useful in assessing therapeutics directed towards the chronic post-injury period. However, more data are still needed. In particular, sex differences will likely be crucial to understand going forward as there tend to be differences in recovery due to TBI (Ley et al., 2013; Styrke et al., 2013), baseline impulsive tendencies (Weafer and de Wit, 2014), and even subtle, but meaningful differences in processing probabilistic information (Singh, 2016). In addition, further studies will be needed to definitively evaluate whether brain regions outside of the injured area drive observed deficits, and whether these problems may be rescued by pharmacological or rehabilitative therapies, which could eventually help the millions of individuals suffering from TBI-related dysfunction.
4. Methods
4. 1. Subjects
Subjects were 47 male Long-Evans rats (Charles River, Wilmington, MA). Subjects were approximately 2.5 months old at the start of training, 4 months at injury, and 7 months at euthanasia. Each rat was food-restricted and maintained at 12–14 g/day to increase motivation for food reinforcement. Rats were pair-housed on a reverse light cycle prior to surgery and single-housed after surgery. Rats were randomly assigned to either Acquisition (n = 23) or Trained (n = 24) groups for the duration of the study.
4. 2. Apparatus
Testing took place in a bank of 16 standard five-hole operant conditioning chambers with a stimulus light at the back of each hole, and an infrared beam to measure nose pokes (Med Associates, St. Albans, VT). A food tray with a light was placed at the opposite wall and a pellet dispenser above it. Chambers were controlled by custom software written in Med-PC IV. Sucrose pellets (45 mg, BioServ, Fleming, NJ) were used as reinforcers.
4. 3. Behavioral Training
4. 3.1. Initial Training
RGT training was performed as described in previous publications (see Figure 1) (Adams et al., 2017; Zeeb et al., 2009). In brief, training began with two 20-min habituation sessions in which sucrose pellets were placed in all five nose-poke holes and the food hopper. Rats then began training based on the five-choice serial reaction time task (Carli et al., 1983) in order to shape responses to the presentation of a cue light in one of the five choice holes. Rats nose-poked in the food hopper to start a trial. After a 5-s delay, a cue light appeared in one of the five-choice holes and was left on for 30 s, then gradually reduced to 20 and 10 s as rats began to respond more rapidly across sessions. Premature responses before the light, incorrect responses, or omitted responses were punished with a 5-s timeout with the houselight illuminated, while correct responses delivered a single sucrose pellet. This allowed for assessment of premature motor responses within the RGT as well. Sessions lasted 30 min or until 100 reinforcers were obtained. Rats were trained until they completed at least 50 trials with an 80% accuracy at the 10-s stimulus setting. Rats in the Acquisition group were advanced to surgery at this point.
4. 3. 2. RGT Training
In the RGT rats were allowed 30 min to earn as many sucrose pellets as possible. Four choice options were presented, each associated with a different probability and magnitude of reward and punishment. Choice P1 had a 90:10% chance of one sucrose pellet or a 5-s timeout. Choice P2 had an 80:20% chance of two pellets or a 10-s timeout. Choice P3 had a 60:40% chance of three pellets or a 30-s timeout. Choice P4 had a 40:60% chance of four pellets or a 40-s timeout.
Rats were initially exposed to seven sessions of ‘forced-choice,’ in which only one option was available, to familiarize them with the different P1-P4 contingencies. After, they were allowed to choose freely for the duration of the study. On each trial, a nose-poke response into the lit food hopper began a 5-s delay, after which the choice holes associated with options P1-P4 became available. Premature responses made before the choice holes were illuminated were punished with a 5-s timeout in which no reinforcement could be earned. Upon choice, either the associated number of sucrose pellets would be delivered, and the food hopper lit (‘wins’), or no pellets would be delivered (‘losses’) and the choice hole would slowly (1 hz) flash for the duration of the timeout. Hole location for each contingency (P1-P4) was kept consistent through the study (counterbalanced between subjects with two versions of the program). Rats in the Trained group reached a stable baseline as assessed statistically (no effect of session over a three-session period) and confirmed with visual analysis of individual subjects within 23 free-choice sessions, and then were advanced to surgery.
4. 4. TBI Surgery
Rats were pair-matched for performance (Trained: RGT performance, Acquisition: sessions to initial training criteria), and then randomly assigned to TBI (n = 24) or Sham (n = 23) group. Controlled cortical impact (CCI) procedures were carried out aseptically, as previously described (Vonder Haar et al., 2016; Vonder Haar et al., 2017). In brief, rats were anesthetized with isoflurane (5% induction, 2–4% maintenance) in 0.5 L/min oxygen. Local analgesic (bupivicane, 0.25%) was given at the incision site and general analgesic (ketoprofen, 5 mg/kg) was given subcutaneously. Rats were placed in a stereotaxic frame, surgical site sterilized, and a midline incision performed. After retracting the periosteum, a 6 mm circular craniectomy was measured out, centered at +3.0, +0.0 mm from bregma and performed using a surgical drill. A severe bilateral, frontal CCI (5 mm in diameter, −2.5 mm depth, 3 m/s velocity, 500 ms dwell time) was then induced using a Leica Impact One CCI device (Leica Biosystems, Buffalo Grove, IL). Bleeding was stopped, the incision site sutured closed, and triple-antibiotic applied to the site. Half of sham rats received either craniectomy, which included all of the above with the exception of impact and half received intact procedures, which did not include craniectomy or impact, but did include anesthesia and analgesia. TBI and craniotomy surgeries took approximately 30 minutes (range: 20–55), while intact sham surgeries took approximately 15 minutes (range: 12–20). Rats were placed on free-feeding for five days following the surgery, after which, they were leaned back down to 14 g/day.
4. 5. Behavioral Assessment
After seven days of recovery, assessment began on the RGT. Rats in the Trained condition were placed back on the full RGT program. Rats in the Acquisition group were put back in the last stage of initial training (see above) to verify they could respond. After two weeks of this, they were put through the RGT training as described above. Rats were assessed until 12-weeks post-injury.
4. 6. Pharmacological Challenge
In weeks 8–10 post-injury, an d-amphetamine challenge was conducted as previously described (Zeeb et al., 2009). D-amphetamine doses (0.0, 0.3, 1.0, and 1.5 mg/kg; Sigma-Aldrich, St. Louis, MO) were administered according to a balanced Latin square design with one day of washout (no behavioral testing) and one day of baseline performance between each dose. Data from challenge days only are presented in Figure 4, and data from baseline days in Figures 2 and 3.
4. 7. Immunohistochemistry and Lesion Analysis
Following behavioral assessment (12-weeks post-injury), and at post-behavior intervals of 60 minutes (exactly 90 minutes after start of behavior), rats were transcardially perfused with 0.9% phosphate buffered saline, followed by 3.7% phosphate buffered formaldehyde. After perfusions, the brains were post-fixed in 3.7% formaldehyde for 24 h. Brains were then embedded in a gel matrix (15% gelatin) with five brains per gel block, and sliced, frozen, on a sliding microtome at 30 μm.
To detect cells that were active during behavior, staining for the early-immediate gene cFos was performed. Slices were blocked in normal goat serum overnight, then incubated with rabbit anti-Fos primary antibody (Abcam AB190289, 1:20,000) for 72 h, rinsed, and then incubated in biotinylated goat anti-rabbit IgG secondary antibody (Vector, BA-1000; 1:2,000) for 2 h, rinsed, and reacted with an avidin-biotin complex kit (Vectastain PK-6100) and catalyzed with 0.05% diaminobenzadine and 0.15% hydrogen peroxide.
Four regions of interest (ROIs) were selected: prelimbic cortex (PL), orbitofrontal cortex (OFC), dorsal striatum (dSTR), and nucleus accumbens shell (NAc). Images from each ROI were taken at 20× magnification on an Olympus BX-43 microscope with DP-80 13.5 megapixel camera in CellSens software. Cell counts were then performed automatically in ImageJ (NIH, Bethesda, MD) by thresholding the brightness of the image and setting minimum and maximum pixel size criteria to define cells. Automated counts were verified by hand.
For lesion analysis, sections were mounted to slides and stained for cresyl violet to visualize the extent of the lesion. Images were captured on a Konica Minolta copier at 600 DPI, and remaining brain size estimated in ImageJ (NIH, Bethesda, MD) by measuring 4 sections transversing the lesion cavity (+4.5, +3.5, +2.5, +1.5 from bregma), averaging their area, and multiplying by the thickness (Coggeshall, 1992).
4. 8. Data Analysis
The primary variable obtained from the RGT was percent choice among the four options. However, premature responses, omitted responses, number of pellets earned, response latency, and reinforcer collection latency were also recorded and analyzed. Repeated-measures data (RGT variables, pharmacological challenges) were analyzed with linear mixed-effects regression. Counts of cFos positive cells for each ROI and brain volumes were analyzed in a one-way ANOVA. Transformations were applied as appropriate to normalize data for each recorded session. The arcsin-squareroot transformation was applied to percent data (choice), log transformation for data bounded on the lower end (prematures, omissions, latencies), and the square-root transformation for count data (cell counts). All data analyses were performed with R statistical software (http://www.r-project.org/) in the lme4, lmerTest, and stats libraries. A p-value equal to or less than 0.05 was considered significant.
Supplementary Material
Highlights.
TBI chronically decreases optimal decision-making, independent of learning history
Motor impulsivity increases across time for 10 weeks post-injury
Amphetamine did not strongly affect acquisition TBI rats’ decision-making
Amphetamine reduced motor impulsivity in TBI rats
Acknowledgements
We would like to thank Robelle Dalida, Taylor Scott, Noah Berdar, and Lindsay Veltri for their assistance with behavioral testing. Funding for this project was provided by grants from the National Center for Responsible Gaming, National Institute of General Medical Sciences (NIGMS; 5P20GM109098–04), and West Virginia University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no financial or commercial relationships based on the research reported in this paper.
References
- Adams WK, Vonder Haar C, Tremblay M, Cocker PJ, Silveira MM, Kaur S, Baunez C, Winstanley CA, 2017. Deep-brain stimulation of the subthalamic nucleus selectively decreases risky choice in risk-preferring rats. eNeuro 4, ENEURO. 0094-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJJ, Winstanley CA, Vanderschuren LJMJ, 2013. Simultaneous blockade of dopamine and noradrenaline reuptake promotes disadvantageous decision making in a rat gambling task. Psychopharmacology 225, 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MR, Simpson EH, Balsam PD, 2016. Neural substrates underlying effort, time, and risk-based decision making in motivated behavior. Neurobiol. Learn. Mem 133, 233–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrus MM, Hosking JG, Zeeb FD, Tremblay M, Winstanley CA, 2015. Disadvantageous decision-making on a rodent gambling task is associated with increased motor impulsivity in a population of male rats. J. Psychiatry Neurosci 40, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrus MM, Winstanley CA, 2016. Dopamine D3 receptors modulate the ability of win-paired cues to increase risky choice in a rat gambling task. J. Neurosci 36, 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW, 1994. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50, 7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A, 2003. Risky business: Emotion, decision-making, and addiction. J. Gambl. Stud 19, 23–51. [DOI] [PubMed] [Google Scholar]
- Bhalerao SU, Geurtjens C, Thomas GR, Kitamura CR, Zhou C, Marlborough M, 2013. Understanding the neuropsychiatric consequences associated with significant traumatic brain injury. Brain Inj 27, 767–774. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Burroughs TK, Franke LM, Pickett TC, Johns SE, Moeller FG, Walker WC, 2016. Laboratory impulsivity and depression in blast-exposed military personnel with post-concussion syndrome. Psychiatry Res 246, 321–325. [DOI] [PubMed] [Google Scholar]
- Brevers D, Bechara A, Cleeremans A, Noël X, 2013. Iowa Gambling Task (IGT): Twenty years after–gambling disorder and IGT. Front. Psychol 4, 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ, 1983. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav. Brain Res 9, 361–380. [DOI] [PubMed] [Google Scholar]
- Carrera E, Tononi G, 2014. Diaschisis: Past, present, future. Brain 137, 2408–2422. [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Cancelliere C, Côté P, Hincapié CA, Kristman VL, Holm LW, Borg J, Nygren-de Boussard C, Hartvigsen J, 2014. Systematic review of the prognosis after mild traumatic brain injury in adults: Cognitive, psychiatric, and mortality outcomes: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil 95, S152–S173. [DOI] [PubMed] [Google Scholar]
- Castells X, Blanco‐Silvente L, Cunill R, 2018. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst. Rev ePub, ahead of print. [DOI] [PMC free article] [PubMed]
- Center for Disease Control, 2017. Injury prevention and control: Traumatic brain injury Vol. 2018, ed.^eds. [Google Scholar]
- Chen Y-H, Huang EY-K, Kuo T-T, Ma H-I, Hoffer BJ, Tsui P-F, Tsai J Jr, Chou Y-C, Chiang Y-H, 2015. Dopamine release impairment in striatum after different levels of cerebral cortical fluid percussion injury. Cell Transplant 24, 2113–2128. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, 1992. A consideration of neural counting methods. Trends Neurosci 15, 9–13. [DOI] [PubMed] [Google Scholar]
- Cole JT, Yarnell A, Kean WS, Gold E, Lewis B, Ren M, McMullen DC, Jacobowitz DM, Pollard HB, O’Neil JT, Grunberg NE, Dalgard CL, Frank JA, Watson WD, 2011. Craniotomy: True sham for traumatic brain injury, or a sham of a sham? J. Neurotrauma 28, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrena C, Brancoa LD, Zimmermanna N, Cardosoa CO, Grassi-Oliveiraa R, Fonseca RP, 2014. Impaired decision-making after traumatic brain injury: The Iowa Gambling Task. Brain Inj 28, 1070–1075. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW, 2004. Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neurosci. Biobehav. Rev 28, 771–784. [DOI] [PubMed] [Google Scholar]
- de Visser L, Baars A, van’t Klooster J, van den Bos R, 2011a. Transient inactivation of the medial prefrontal cortex affects both anxiety and decision-making in male wistar rats. Front. Neurosci 5, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser L, Homberg J, Mitsogiannis M, Zeeb F, Rivalan M, Fitoussi A, Galhardo V, van den Bos R, Winstanley C, Dellu-Hagedorn F, 2011b. Rodent versions of the iowa gambling task: Opportunities and challenges for the understanding of decision-making. Front. Neurosci 5, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MR, Jacobs EA, Sanders S, Guercio JM, Soldner J, Parker-Singler S, Robinson A, Small S, Dillen JE, 2005. Impulsivity, self-control, and delay discounting in persons with acquired brain injury. Behavioral Interventions 20, 101–120. [Google Scholar]
- Dyer KF, Bell R, McCann J, Rauch R, 2006. Aggression after traumatic brain injury: Analysing socially desirable responses and the nature of aggressive traits. Brain Inj 20, 1163–1173. [DOI] [PubMed] [Google Scholar]
- Elliott MB, Oshinsky ML, Amenta PS, Awe OO, Jallo JI, 2012. Nociceptive neuropeptide increases and periorbital allodynia in a model of traumatic brain injury. Headache: The Journal of Head and Face Pain 52, 966–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney DM, Gonzalez A, Law WA, 1982. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217, 855–857. [DOI] [PubMed] [Google Scholar]
- Fujimoto ST, Longhi L, Saatman KE, McIntosh TK, 2004. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev 28, 365–378. [DOI] [PubMed] [Google Scholar]
- Gansler DA, Jerram MW, Vannorsdall TD, Schretlen DJ, 2011. Does the Iowa Gambling Task measure executive function? Arch. Clin. Neuropsychol 26, 706–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EM, Su D, López-Velázquez L, Haus DL, Perez H, Lacuesta GA, Anderson AJ, Cummings BJ, 2013. Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen. Med 8, 483–516. [DOI] [PubMed] [Google Scholar]
- Goswami R, Dufort P, Tartaglia M, Green R, Crawley A, Tator C, Wennberg R, Mikulis D, Keightley M, Davis KD, 2016. Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Structure and Function 221, 1911–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräplin A, Dshemuchadse M, Behrendt S, Scherbaum S, Goschke T, Bühringer G, 2014. Dysfunctional decision-making in pathological gambling: pattern specificity and the role of impulsivity. Psychiatry Res 215, 675–682. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kelly KG, Stigge-Kaufman DA, Schmalfuss IM, Perlstein WM, 2007. Reward context sensitivity impairment following severe TBI: An event-related potential investigation. J. Int. Neuropsychol. Soc 13, 615–625. [DOI] [PubMed] [Google Scholar]
- Levine B, Black SE, Cheung G, Campbell A, O’Toole C, Schwartz ML, 2005. Gambling task performance in traumatic brain injury: Relationships to injury severity, atrophy, lesion location, and cognitive and psychosocial outcome. Cogn. Behav. Neurol 18, 45–54. [PubMed] [Google Scholar]
- Ley EJ, Short SS, Liou DZ, Singer MB, Mirocha J, Melo N, Bukur M, Salim A, 2013. Gender impacts mortality after traumatic brain injury in teenagers. Journal of Trauma and Acute Care Surgery 75, 682–686. [DOI] [PubMed] [Google Scholar]
- Marsh NV, Kersel DA, Havill JH, Sleigh JW, 1998. Caregiver burden at 1 year following severe traumatic brain injury. Brain Inj 12, 1045–1059. [DOI] [PubMed] [Google Scholar]
- McHugh L, Wood RL, 2008. Using a temporal discounting paradigm to measure decision-making and impulsivity following traumatic brain injury: A pilot study. Brain Inj 22, 715–721. [DOI] [PubMed] [Google Scholar]
- Newcombe VF, Outtrim JG, Chatfield DA, Manktelow A, Hutchinson PJ, Coles JP, Williams GB, Sahakian BJ, Menon DK, 2011. Parcellating the neuroanatomical basis of impaired decision-making in traumatic brain injury. Brain 134, 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor WT, Smyth A, Gilchrist MD, 2011. Animal models of traumatic brain injury: A critical evaluation. Pharmacol. Ther 130, 106–113. [DOI] [PubMed] [Google Scholar]
- Ozga JE, Povroznik JM, Engler-Chiurazzi EB, Vonder Haar C, 2018. Executive (dys)function after traumatic brain injury: Special considerations for behavioral pharmacology. Behav. Pharmacol in press. [DOI] [PMC free article] [PubMed]
- Paine TA, Asinof SK, Diehl GW, Frackman A, Leffler J, 2013. Medial prefrontal cortex lesions impair decision-making on a rodent gambling task: Reversal by D1 receptor antagonist administration. Behav. Brain Res 243, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JCS, 2000. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 55, 1158–1166. [DOI] [PubMed] [Google Scholar]
- Ramic M, Emerick AJ, Bollnow MR, O’Brien TE, Tsai S-Y, Kartje GL, 2006. Axonal plasticity is associated with motor recovery following amphetamine treatment combined with rehabilitation after brain injury in the adult rat. Brain Res 1111, 176–186. [DOI] [PubMed] [Google Scholar]
- Schlund MW, Pace G, 2000. The effects of traumatic brain injury on reporting and responding to causal relations: An investigation of sensitivity to reinforcement contingencies. Brain Inj 14, 573–583. [DOI] [PubMed] [Google Scholar]
- Schlund MW, 2002. The effects of brain injury on choice and sensitivity to remote consequences: Deficits in discriminating response-consequence relations. Brain Inj 16, 347–357. [DOI] [PubMed] [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, Bechara A, 2007. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr. Res 92, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdardottir S, Jerstad T, Andelic N, Roe C, Schanke A-K, 2010. Olfactory dysfunction, gambling task performance and intracranial lesions after traumatic brain injury. Neuropsychology 24, 504. [DOI] [PubMed] [Google Scholar]
- Silveira MM, Murch WS, Clark L, Winstanley CA, 2016. Chronic atomoxetine treatment during adolescence does not influence decision-making on a rodent gambling task, but does modulate amphetamine’s effect on impulsive action in adulthood. Behav. Pharmacol 27, 350–363. [DOI] [PubMed] [Google Scholar]
- Singh V, 2016. Sex-differences, handedness, and lateralization in the Iowa gambling task. Front. Psychol 7, 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrke J, Sojka P, Björnstig U, Bylund P-O, Stålnacke B-M, 2013. Sex differences in symptoms, disability, and life satisfaction three years after mild traumatic brain injury: A population-based cohort study. J. Rehabil. Med 45, 749–757. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE, 1999. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil 14, 602–615. [DOI] [PubMed] [Google Scholar]
- Vaishnavi S, Rao V, Fann JR, 2009. Neuropsychiatric problems after traumatic brain injury: Unraveling the silent epidemic. Psychosomatics 50, 198–205. [DOI] [PubMed] [Google Scholar]
- Visser-Keizer AC, Westerhof-Evers HJ, Gerritsen MJ, van der Naalt J, Spikman JM, 2016. To fear is to gain? The role of fear recognition in risky decision making in TBI patients and healthy controls. PLoS One 11, e0166995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonder Haar C, Lam FCW, Adams WA, Riparip L-K, Kaur S, Muthukrishna M, Rosi S, Winstanley CA, 2016. Frontal traumatic brain injury in rats causes long-lasting impairments in impulse control that are differentially sensitive to pharmacotherapeutics and associated with chronic neuroinflammation. ACS Chem. Neurosci 7, 1531–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonder Haar C, Martens KM, Riparip L-K, Rosi S, Wellington CL, Winstanley CA, 2017. Frontal traumatic brain injury increases impulsive decision making in rats: A potential role for the inflammatory cytokine interleukin-12. J. Neurotrauma 34, 2790–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonder Haar C, Ferland JMN, Kaur S, Riparip L-K, Rosi S, Winstanley CA, 2018. Cocaine self-administration is increased after frontal traumatic brain injury and associated with neuroinflammation. Eur. J. Neurosci ePub, ahead of print. [DOI] [PMC free article] [PubMed]
- Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE, 2005. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J. Neurochem 95, 457–465. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Scanion JM, Becker CR, Ritter AC, Niyonkuru C, Dixon CE, Conley YP, Price JC, 2014. The influence of genetic variants on striatal dopamine transporter and D2 receptor binding after TBI. J. Cereb. Blood Flow Metab 34, 1328–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, de Wit H, 2014. Sex differences in impulsive action and impulsive choice. Addict. Behav 39, 1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaloshnja E, Miller T, Langlois JA, Selassie AW, 2008. Prevalence of Long-term disability from traumatic brain injury in the civilian population of the United States, 2005. The Journal of Head Trauma Rehabilitation 23, 394–400. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA, 2009. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology 34, 2329–2343. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA, 2011. Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. J. Neurosci 31, 2197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA, 2013. Functional disconnection of the orbitofrontal cortex and basolateral amygdala impairs acquisition of a rat gambling task and disrupts animals’ ability to alter decision-making behavior after reinforcer devaluation. J. Neurosci 33, 6434–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Wong AC, Winstanley CA, 2013. Differential effects of environmental enrichment, social-housing, and isolation-rearing on a rat gambling task: Dissociations between impulsive action and risky decision-making. Psychopharmacology 225, 381–395. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Baarendse P, Vanderschuren L, Winstanley CA, 2015. Inactivation of the prelimbic or infralimbic cortex impairs decision-making in the rat gambling task. Psychopharmacology 232, 4481–4491. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Seale GS, Schaefer LA, Temple RO, Foreman J, Elliott TR, 2015. Psychiatric Disease and Post-Acute Traumatic Brain Injury. J. Neurotrauma 32, 1911–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


