Abstract
Hepatitis delta virus (HDV) infection of humans was first reported in 1977, and now it is now estimated that 15–20 million people are infected worldwide. Infection with HDV can be an acute or chronic process that occurs only in patients with an HBV infection. Chronic HDV infection commonly results in the most rapidly progressive form of viral hepatitis; it is the chronic viral infection that is most likely to lead to cirrhosis, and it is associated with an increased risk of hepatocellular carcinoma. HDV infection is the only chronic human hepatitis virus infection without a therapy approved by the Food and Drug Administration. Peginterferon alpha is the only recommended therapy, but it produces unsatisfactory results. We review therapeutic agents in development, designed to disrupt the HDV life cycle, that might benefit patients with this devastating disease.
Keywords: HCC risk, cancer, drug development, epidemiology
The hepatitis Delta virus (HDV) was first identified in humans in 1977, when a cohort of patients with hepatitis B virus (HBV) presented in Italy with severe hepatitis.1 Immunohistochemical analyses of liver biopsies and sera from these patients revealed a novel antigen pattern in the nuclei of liver cells, which researchers called the δ antigen.1, 2 Initially, this δ antigen was believed to be a new HBV antigen, but it was quickly found to be separate and associated with the HBV surface antigen (HBsAg).1 Transmission experiments in chimpanzees led to further characterization of the δ antigen (HDAg)—a structural component of a distinct infectious pathogen with a low molecular weight RNA genome that required HBV for its life cycle.2, 3 Subsequent cloning and sequencing of this genome confirmed that this was a unique RNA virus; its classification is still used today, as the only member of a separate genus, deltavirus.4
HDV is estimated to affect 15–20 million people worldwide from all age groups. Infection with this pathogen can lead to an acute or chronic disease, but only in individuals with HBV infection. Although HDV infection varies among geographic regions, it is believed that approximately 5% of the HBV-infected population worldwide is coinfected with HDV.5 Chronic infection with HDV is considered to be the most severe form of human viral hepatitis infection. Compared with other chronic viral hepatitis infections, HDV infection has been described to progress more rapidly, is more likely to lead to cirrhosis, and is associated with increased risk of hepatocellular carcinoma (HCC). HDV infection is the only chronic human hepatitis virus infection without a Food and Drug Administration-approved therapy.
Virology
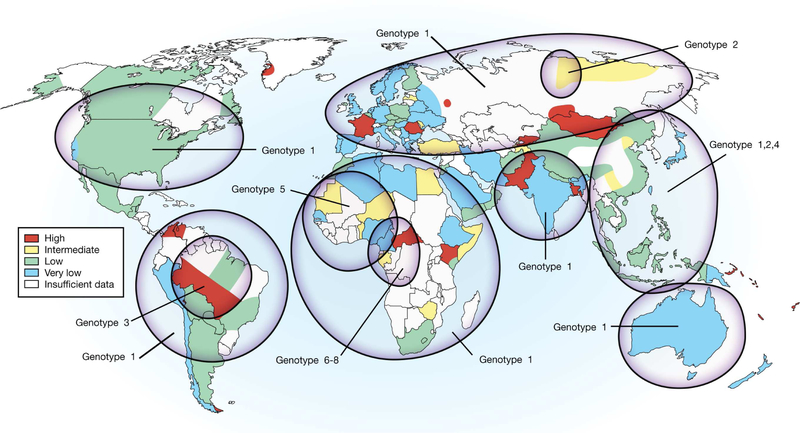
The HDV particle is approximately 36 nm in diameter and consists of a 1.7 kb single-stranded circular RNA genome of negative polarity.2, 6, 7 Eight genotypes of HDV have been identified (Figure 1); the separation in nucleotide sequence of the studied region is typically <16% among isolates of the same genotype and up to 40% over the full-length sequence among genotypes.8–10 Genotype 1 is found worldwide and is the predominant virus in North America, Europe, Australia, and the Middle East.9–11 Genotype 2 has been identified predominantly in Asia, Southeast Asia, and Russia.12–15 Genotype 3 has been identified in South America.16–18 Genotype 4 has been described in Japan and Taiwan.15, 19 Finally, genotypes 5–8 have been predominantly found in Africa.9, 10, 20
Figure 1. Map of the Prevalence of HDV Infection.
World map shows estimated prevalence of HDV infection and genotype distribution.
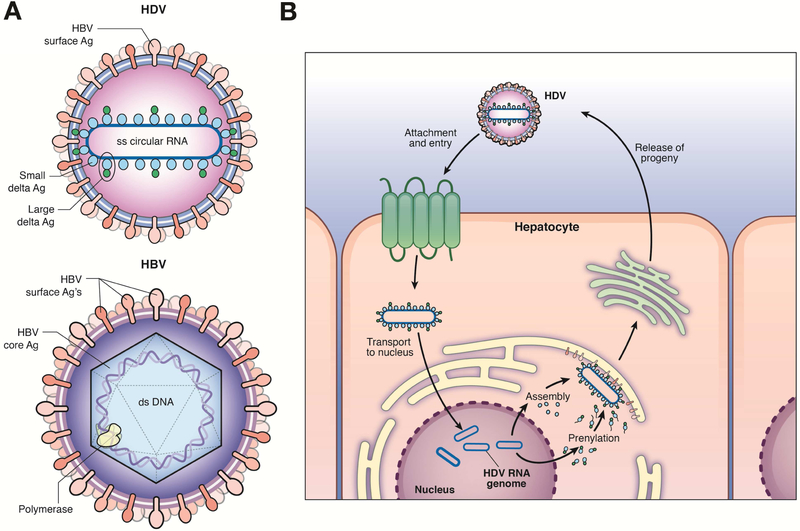
The HDV genome encodes 1 protein that exists in 2 forms: the small delta antigen (SHDAg) and the large delta antigen (LHDAg) (Figure 2a). The LHDAg is identical to the SHDAg except that it contains an extra 19 amino acids at its carboxyl terminus. This extension results from a specific RNA-editing event that occurs during genome replication.21 The addition of these 19 amino acids alters the carboxy terminus of the protein, which includes a CXXX-box motif (C=cysteine, X=one of the last 3 amino acids at the carboxyl terminus of the LHDAg). This CXXX-box motif is a substrate for farnesyltransferase, an enzyme that adds a farnesyl group to the cysteine of the CXXX-box.22 The farnesylation of the CXXX-box is essential for virion assembly.23 The complete HDV particle comprises a complex of the viral genome and both HDV antigen isoforms, all surrounded by a lipid envelope in which HBsAg proteins are embedded.24 The 3 isoforms of HBsAg (small, middle, and large [or pre-S1]) that are embedded are the same as those found on the HBV virion and are myristoylated at the N-terminus—a modification required for cell entry.25 HDV does not encode its own envelope proteins, but instead utilizes the HBV envelope proteins (Figure 2b) for HDV assembly and infection of new cells. The presence of HBV, either via coinfection or superinfection, is therefore essential for HDV propagation in humans.
Figure 2. HBV and HDV Structure.
(A) The small delta antigen (SHDAg) and the large delta antigen (LHDAg) are each derived from the single expressed reading frame in the HDV genome. The SHDAg and LHDAg differ based on a specific RNA editing event, occurring during genome replication—this results in the addition of 19 amino acids to the SHDAg carboxyl terminus. (B) HBV and HDV share envelope proteins. HDV uses the envelope proteins of HBV for assembly and infection of new hepatocytes. Therefore, the presence of HBV infection is required for HDV infection and propagation in humans.
HDV genome replication occurs solely through RNA-dependent RNA replication, without DNA intermediates or chromosomal integration events. Additionally, HDV does not encode its own RNA-dependent RNA polymerase but requires cell polymerases for its replication. Notably, HDV appears to recruit RNA polymerase II to replicate its RNA genome, in an RNA-dependent manner.26 It is believed that the double stranded-like structure of the HDV genomic RNA, created by the high intramolecular RNA folding into a collapsed circle, or rod-like structure, provides a template similar to double-stranded DNA, which enables recruitment of RNA polymerase II.27 Upon uncoating, the virus genome is transported to the liver cell nucleus where genome replication occurs.
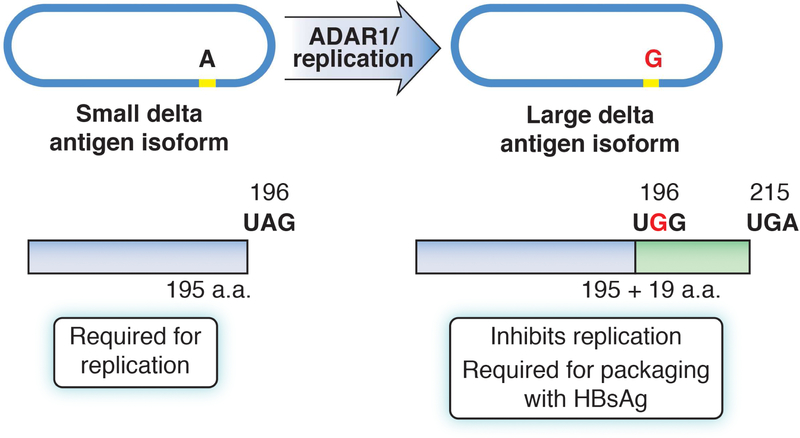
RNA replication occurs via a double-rolling circle mechanism28 by RNA polymerase II, which associates with the SHDAg to create linear multimeric copies of antigenomic RNA. The plus-sense antigenomic RNA is complementary to, and is of opposite polarity to, that of the incoming, negative-sense, genomic RNA. The linear multimeric copies of antigenomic RNA then undergo intramolecular cleavage by the antigenomic ribozyme to form linear monomers that are then ligated, forming closed circular antigenomes which serve as templates for production of genomic RNA via a similar mechanism.26 As replication continues, an ADAR1-mediated RNA editing event takes place on the antigenomic RNA, which modifies the amber stop codon of the reading frame encoding SHDAg. This results in translation proceeding to the next downstream stop codon, adding an extra 19 amino acids with resultant expression of the LHDAg.29 The addition of the extra 19 amino acids significantly changes the function of the delta antigens—the SHDAg is required for HDV RNA genomic replication whereas the LHDAg functions as a potent trans-dominant inhibitor of HDV RNA replication.30 Moreover, only the LHDAg mediates packaging with HBsAg into virions. Therefore, RNA editing event is an important part of the HDV life cycle that serves to turn off RNA replication and turn on packaging of newly created HDV genomes, virus assembly, and release.31
Epidemiology
Although an estimated 15–20 million people are infected with HDV worldwide, the global prevalence is not known. Certain areas of high prevalence have been described and include: Central Africa (15%–50%)32–34, West Africa (17%–30%)35, 36, the Mediterranean basin (27%)37, the Middle East (Iran, 7.8%)38, Northern Asia (Mongolia, 26%–60% in adults and >6% in school-aged children)39–41, Eastern Europe (Romania, 20%)42 (Parts of Russia, 22%)43, certain areas of Southeast Asia (Vietnam, 15%)44, and the Amazon basin of South America (13%–29%).45, 46
Initial reports of endemicity, with prevalence rates >20%, were described in Southern Europe after HDV’s initial discovery in Italy. However, rates of HDV infection in this region have decreased substantially since the 1990s as a result of disposable syringe practices, HBV vaccination programs, socioeconomic improvements, and deaths of individuals with chronic infection.47–50 However, despite these improvements, new patients with HDV continue to be identified in this region, likely due to migration from endemic areas.51–53
Reporting of HDV prevalence in the US continues to be incomplete. In a study in Northern California, the prevalence of HDV among HBV-infected patients was 8%. However, only 42% of the 1191 HBsAg-positive subjects in this study were tested for HDV.54 In a Midwestern population of HBV-infected patients, only 12% of 1007 HBsAg-positive patients were tested for an HDV antibody—among those tested, 3.3% were positive for the HDV antibody.55 In a Veterans administration study, where suboptimal testing for HDV was also identified, 3.4% of HBsAg-positive adults were reported to have HDV infection.56
Among injection-drug users (IDUs) in the US, the prevalence of HDV infection has increased. In an IDU cohort in Baltimore, Maryland, approximately 50% of HBV-infected individuals were found to be infected with HDV.57 In a cohort of IDUs assessed by the Urban Health Study in San Francisco, 36% of HBsAg-positive individuals were found to have HDV viremia.58 So, even in developed countries, subgroups of HBV-infected patients (such as IDUs) are at high risk for HDV infection. Also, immigration from endemic areas continues to be a significant risk factor for HDV infection in the developed world.
Pathogenesis
HBV infection is required for productive HDV infection in humans. HDV transmission in humans occurs either via co-infection with acute HBV or as a super-infection in patients with HBV infection. The exact mechanisms of interaction between these viruses have not been elucidated, but HDV reduces certain activities of HBV and viremia.59–62 Retrospective studies have found the presence of HDV infection to reduce HBV DNA levels in patients’ blood.62–64 In a study that followed patients with HBV and HDV coinfection for up to 8 years, researchers found 3 predominant patterns of HDV RNA and HBV DNA in blood samples from patients with active viral replication: high levels of HDV RNA and low levels of HBV DNA (54% of the patients), low levels of HDV RNA and high levels of HBV DNA (30% of patients), and equal levels of HDV RNA and HBV DNA (15% of patients).65
Little is known about the pathogenesis of HDV infection. Although HBV is not directly cytopathic to infected hepatocytes, HDV may have direct cytopathic effects specifically related to the SHDAg.66–68 Additionally, innate and adaptive immune responses could be involved in mediating liver damage. The LHDAg promotes an inflammatory response, possibly by activating STAT3 and NF-kB, although this might help clear HDV-infected cells.69, 70 The effects of this inflammatory response include endoplasmic reticulum stress and necroinflammation, along with possible increases in production of reactive oxygen species, which might ultimately promote development of HCC.70 HDV may also have the ability to interfere with interferon (IFN) alpha signaling, by blocking activation and translocation of STAT proteins, which may contribute to the persistence of HDV and impair IFN alpha-based therapies. The adaptive immune response to HDV infection has been described as weak. These features combined might allow chronic HDV infection to induce rapid, progressive liver disease.
Clinical Features of Acute Infection
Acute HDV infection can occur via either HBV co-infection (simultaneous infection with both viruses during the same exposure) or super-infection (infection with HDV after an established HBV infection, such as in an HBsAg-positive individual). The clinical course of an acute co-infection is similar to that of acute HBV infection. However, co-infection causes more severe disease, with an increased risk of acute hepatic failure.71 Additionally, acute co-infections cause a biphasic course in alanine aminotransferase (ALT) levels—2 peaks are often observed, several weeks apart, as HBV infection must first be established before HDV can begin to spread.71, 72 Acute superinfection of HDV has a more severe clinical course compared to acute co-infection, with an increased risk of acute liver failure.73 In patients with known HBV infection, acute superinfection may be mistaken for an HBV flare, whereas in patients with undiagnosed viral hepatitis, an acute superinfection may be mistaken for acute HBV infection. Similar to HBV mono-infections, HDV infection becomes chronic in fewer than 5% of patients who become co-infected as adults, but it becomes chronic in most people co-infected during the neonatal period, and in more than 90% of cases of super-infections.74, 75
Chronic Infection and Disease Progression
Once chronic HDV infection has been established (based on detection of the HDV antibody more than 6 months after infection, with detectable HDV RNA in serum or HDAg, detected by immunohistochemistry) levels of transaminases are usually increased to a greater extent than in patients with HBV mono-infection. Compared with chronic HBV or HCV infection, chronic HDV infection leads to more severe liver disease, with increased rates of fibrosis progression.76, 77 Chronic HDV infection progresses to cirrhosis within 2 years in 10%–15% of cases75, and within 5 to 10 years in up to 80% of cases.78 Despite the rapid progression to cirrhosis, markers that can be detected noninvasively that have been validated for monitoring fibrosis in patients with HBV or HCV infection, such as the aspartate aminotransferase to platelet ratio index or fibrosis-4 index, do not appear to be reliable for monitoring fibrosis in patients with chronic HDV infection.79 The performances of commercial noninvasive fibrosis assays (such as hepascore, fibroscore, or fibrosure) have not been evaluated in patients with HDV infection. Although transient elastography has been approved for the staging of liver disease, it has not been evaluated in patients with HDV infection. It is not known whether the extent of hepatic inflammation caused by HDV affects transient elastography measurements.
Outcomes related to chronic HDV infection have been reported to be more severe than those of chronic HBV infection. The risk of HCC, as a complication of cirrhosis, is up to 3-fold higher in co-infected patients compared to patients with HBV mono-infection (Table 1).78, 80–83 Patients with HDV infection are also 2-fold more likely to develop hepatic decompensation than patients with only HBV infection,84, 85 and are more likely to die from hepatic decompensation or HCC.84, 85 HDV genotype 1 appears to be more virulent than genotype 286, although additional factors, such as ethnicity and location, are likely to affect virulence.
Table 1.
Risk of HCC in Patients with HDV Infection
| Geographic area | Study type | Follow-up period | Number of patients with only HBV infection | Number of patients positive for anti-HDV | Results pertaining to HCC risk in patients with HDV compared to HBV mono-infection | Reference |
|---|---|---|---|---|---|---|
| Western Europe | Longitudinal study of patients with HBV infection and compensated cirrhosis | 6.6 years (median) | 161 | 39 | HCC risk increased 3.2-fold in patients positive for anti-HDV | ref78 |
| Jordan | cohort study of HBsAg-positive patients | 8 years | 195 | 20 | Higher prevalence of HDV in patients with HCC (10/15, 67%). Subjects positive for anti-HDV developed HCC an average 10 years earlier than anti-HDV negative patients | Ref83 |
| Greece | case-control | 9 years | 117 | 9 | 10% of patients with HCC (n=87) were HDV-positive compared to 0 patients without HCC | ref148 |
| Italy | cohort study | 2 years | 14 | 9 | Patients with HDV infection and cirrhosis developed HCC at a significantly younger age than patients with only HBV infection and cirrhosis | ref149 |
| Japan | cohort study | 121 months (mean) | 1058 | 69 | Patients positive for anti-HDV developed HCC at a rate of 7.84 per 1000 person-years compared to 2.73 in patients with only HBV infection | ref150 |
| England | retrospective, cross-sectional | 6 years | 880 | 82 | Risk of HCC was 9.7% in patients with HDV coinfection compared to 7.8% of patients with only HBV infection | Ref80 |
| Sweden | registry analysis | 12 years | 8510 | 327 | Compared to patients infected with only HBV (reference population) patients with HDV co-infection had an increased risk for HCC (standardized incidence ratio, −6.11) | Ref82 |
| United States | retrospective analysis of Veterans Health Administration population | 25,603 | 73 | Patients positive for anti-HDV developed HCC with a 2.9-fold higher incidence rate than patients negative for anti-HDV; HDV infection was independently associated with HCC (odds ratio, 2.1). | Ref56 |
Diagnosis and Tests
A moderate to high index of suspicion for HDV infection should be maintained in patients with specific risk factors. Screening for HDV infection continues to be inadequate in the US.54, 56, 58 Risk factors include a history of intravenous drug use, high-risk sexual behaviors, infection of a first-degree relative, and immigration from endemic regions. In patients with acute infections, HDV antigen can be measured by ELISA or radioimmunoassay. However, the virus can be detected only during the first 2 weeks of acute infection, and is only transiently detectable thereafter.87–89 Acute infection with HDV induces innate and adaptive immune response, so immunoglobulin M (IgM) and G (IgG) can also be detected, in immune-competent patients.90 Anti-HDV IgM typically appears 2–3 weeks after the onset of symptoms and disappears by 2 months after an acute infection (although it may persist for as long as 9 months in HDV superinfection).
Assays to detect HDV IgM might be used to identify patients with acute infections, but IgM has been reported to increase during disease flares in patients with chronic HDV infection. HDV IgG and HDV total antibodies persist in serum after resolution of acute HDV infection and in patients with chronic coinfection. Today, these antibody tests are typically utilized as initial screening tests for the detection of HDV infection (HDV Total Antibody and HDV IgM Antibody). A quantitative microarray antibody capture (Q-MAC) assay that quantifies the amount of anti-HDV IgG in serum has been reported to accurately identify patients with HDV infection in Mongolia and the US.41, 58
After detection of HDV antibody, serum should be tested for HDV RNA, to confirm infection. Hybridization assays for HDV RNA have been largely replaced by qualitative or semiquantitative real-time PCR assays, due to their improved sensitivity and a lower limit of detection (as low as 10 genomes/ml).91 However, results obtained from different laboratories are often not comparable, due to the range in sensitivity of the assays.34, 92 Notably, a World Health Organization international RNA standard is now available, allowing for reporting of results in international units (IU). High-diversity HDV genotypes (such as genotypes 6–8) are often challenging to detect and quantify, although pan-genotypic assays have become available.91 New assays have been explored for HDV RNA quantitation41, 93, 94, and a quantitative HDV RNA assay has recently become commercially available in the US.
Another method of HDV detection, albeit invasive, is the intrahepatic detection of HDAg, in which liver tissue is collected and analyzed by immunohistochemistry. Although the reported sensitivities of this test for identification of HDV vary95, in cases for which a liver biopsy is obtained, these samples might be analyzed for HDV—the virus can be detected via immunohistochemistry in liver cell nuclei. However, with the availability of serologic assays, liver immunohistochemistry analyses are not often used.
IFN-based therapies
Although professional societal guidelines have recommended pegylated IFN (PegIFN) alpha for treatment of chronic HDV infection, there is no satisfactory or Food and Drug Administration-approved therapy for this potentially devastating disease.96–98 Although patients with HDV infection have been treated with PegIFN, its administration is limited and typically avoided in patients with cirrhosis, active autoimmune disease, or certain psychiatric disorders. A summary of the results from clinical trials of IFN-based therapies is presented in Supplementary Table 1.
Initially, the effects of IFN alpha-2a in patients with HDV infection were studied groups that received 9 million international units IFN 3 times per week (high-dose), 3 million international units IFN 3 times per week (low-dose), or no therapy for 1 year (control).99 A complete response was defined by normalization in level of ALT and negative results from PCR analyses of HDV RNA—this was achieved by 50% of patients in the high-dose IFN group. In the low-dose group, 21% of patients had a complete response and none of the patients in the no-therapy group had a complete response. During a follow-up period of up to 48 weeks after therapy, all patients had relapsed. Interestingly, in a subsequent analysis of the same cohort, with follow-up period of as long as 14 years after therapy, survival was significantly longer for patients who received high-dose IFN compared to patients who received low-dose IFN or patients who did not receive any therapy.100 Notably, achieving a 2 log10 decline in HDV RNA at end of treatment was associated with the significant increase in survival. There was no difference in long-term outcomes between the low-dose IFN group and the no therapy group, neither of which achieved the mean 2 log10 decrease in HDV RNA at end of treatment.
The effects of different durations of PegIFN alpha-2b therapy, and combinations with other drugs, were evaluated in patients with chronic HDV infection. Among patients given PegIFN alpha-2b (1.5 ug/kg/week) for 1 year, 43% were HDV-negative after a median post-therapy follow-up time of 16 months (range, 6–42 months).101 PegIFN is therefore at least as effective as standard IFN for the treatment of chronic HDV infection. However, extending therapy with PegIFN alpha-2b for 72 weeks102, or administering PegIFN alpha 2a with increasing doses up to 360 mcg/wk for up to 5 years103, 104, did not increased rates of sustained virologic response (<35%). Interestingly, 1 patient who received IFN alpha therapy for 12 years was reported to have a complete virologic response (negative results from PCR tests of HDV RNA with HBsAg seroconversion).105 Despite the variations of responses (ranging from 20% to 40%) to PegIFN alpha, levels of HDV RNA at 24 weeks of therapy can identify patients most likely to have a response to 48 weeks of therapy.106
Combination therapies with PegIFN alpha have been investigated, without much success. The combination of ribavirin with PegIFN alpha for 48 weeks followed by PegIFN monotherapy for an additional 24 weeks did not improve patient outcomes, compared to PegIFN monotherapy for 72 weeks.102 Nucleos(t)ide analogue therapy alone107, 108 has shown no benefit, and combination of nucleos(t)ide analogue with PegIFN did not provide any benefit compared with PegIFN monotherapy109–112.
New Therapeutic Approaches
Although PegIFN alpha can be effective in patients with HDV infection, this drug has significant side effects, because IFN receptors are expressed by many different cell types. In contrast, receptors for PegIFN lambda (a type III IFN) are restricted to specific cell types, including liver cells.113 IFN lambda was found to be expressed in response to viral infections and to have antiviral activity in mice.114 IFN lambda induces expression of IFN-stimulated genes to induce a broad-spectrum anti-virus immune response. IFN lambda binding to type III IFN receptors results in receptor dimerization, leading to activation of signal transduction pathways mediated by Janus kinase (JAK) and signal transducer and activator of transcription (STAT), similar to pathways stimulated by IFN alpha. In early-stage trials of patients with liver disease, PegIFN lambda reduced HBV and HCV virus levels in blood, similar to PegIFN alpha, but with significantly fewer side effects.115, 116 However, the limited utility of IFN-based therapies for patients with HBV infection and the efficacy of direct antiviral agents in patients with HCV infection has reduced the number of studies into the effects of PegIFN lambda.
IFN alpha and lambda were found to reduce markers of intrahepatic HDV infection in mice with humanized livers infected with HBV and HDV.117 In these mice, PegIFN lambda reduced HDV viremia by 1.2 log10 and serum levels of HBsAg by 0.4 log10. Given its tolerability profile and anti-HDV activity, PegIFN lambda is being tested in patients with chronic HDV infection (Table 2). In randomized, open-label, multi-center trial, 33 patients with chronic HDV infection are receiving PegIFN lambda (120 or 180 μg weekly) for 48 weeks.118 An interim analysis has revealed that tolerability was better than that of PegIFN alpha and that both doses of PegIFN lambda have activity against HDV—some patients were found to be negative for HDV RNA, by PCR analysis, at week 8 of therapy.118
Table 2.
Results from Clinical Trials of Therapeutic Agents for HDV Infection
| Therapeutic agent | Treatment duration | Baseline HDV load | Biochemical outcome measured | Virologic outcome measured | Serologic outcomes measured | Reference and number of patients |
|---|---|---|---|---|---|---|
| PegIFN lambda (120 or 180 μg/week) | 48 weeks | 4.5 log10 IU/mL* | NR | 6 of 10 (60%) with >/= 2 log10 decline at week 24 4 of 10 (40%) PCR negative by week 24 | NR | Ref148, n=33 |
| Daily, subcutaneous Myrcludex B (2 mg/day) for 24 weeks then PegIFN alpha monotherapy for 24 weeks | 48 weeks | 104,14 (copies/ml)* | normalized level of in 6/8 patients | 102.47 | No change in HBsAg | ref124 |
| Daily, subcutaneous Myrcludex B (2 mg/day) for 24 weeks then PegIFN alpha monotherapy for 24 weeks | 48 weeks | 104.14 (copies/ml)* | normalized level of ALT in 1/8 patients | 101.62 | NR | Ref124 |
| PegIFN alpha monotherapy | 48 weeks | 104.21 (copies/ml)* | normalized level of ALT in 1/8 patients | 102.11 | NR | ref 124 |
| Myrcludex B (2 mg, 5 mg, or 10 mg plus tenofovir (245 mg/day) vs tenofovir (245 mg/day) monotherapy | 24 weeks | NR | normalized levels of ALT in 42.8% of patients (2 mg), 50% (5 mg), 40%(10mg), or 6.6% (tenofovir only) | reduction of 1.75 log10 IU/ml^ (2 mg) reduction of 1.60 log10 IU/ml^ (5 mg) reduction of 2.70 log10 IU/ml^(10mg) 0.015 log 10 IU/ml^ (tenofovir) | No change in HBsAg | ref125 n=120 |
| Intravenous REP2139-Ca (500 mg/week) for 15 weeks, then intravenous REP2139-CA (500 mg/week plus PegIFN alpha (180 μg)for 15 weeks, then PegIFN alpha for 33 weeks | 63 weeks | 2.7×104to 2.3×107 IU/ml | normalized level of ALT in 7/12 patients | Undetectable in 7/12 patients after removal of all antiviral therapy | HBsAg reduction >5 log in 6 with REP monotherapy | Refs134–136 n=12 |
| Lonafarnib (100 mg twice daily) vs lonafarnib (200 mg twice daily) vs placebo | 4 weeks | median 9.27 ×105 IU/ml | no change | 0.73 log10 IU/mL* vs 1.54 log10 IU/ml* | No change | ref141 n=14 |
| Lonafarnib monotherapy (100–300 mg, 2 or 3 times per day) | 12 weeks | 5.19–5.90 log10 IU/ml* | mean decrease in level of ALT, from 107 to 56 U/L in all groups at week 4 | +0.03 log10 IU/ml* (200 mg twice daily) decrease of 1.78 log10 IU/ml* (300 mg twice daily) decrease of 1.31 log10 IU/ml* (100 mg 3 times per day) | No change | ref144 n=15 |
| lonafarnib (100–300 mg, twice daily) with PegIFN alpha 180 μg/w | 8 weeks | 5.36–6.53 log10 IU/ml* | NR | decrease of 2.97 log10 IU/ml* (100 mg twice daily) | No change | ref 144 |
| lonafarnib 100 mg twice daily plus ritonavir (100 mg/day) | 8 weeks | 6.56 log10 IU/ml* | normalized level of ALT in 3/3 patients by week 4 | decrease of 3.19 log10 IU/ml* | No change | ref 144 |
| lonafarnib 25 or 50 mg plus ritonavir (100 mg) twice daily, with or without PegIFN alpha or lonafarnib (75 mg or more) plus ritonavir (100 mg) twice daily | 12 or 24 weeks | NR | normalized level of ALT in 9/15 patients at week 24 | 1.74 log10 IU/ml* (lonafarnib 25 mg plus ritonavir) decrease of 5.57 log 10 IU/ml* (lonafarnib 25 mg plus ritonavir and PegIFN) | NR | ref145 n=55 |
| Lonafarnib (50 mg, 75 mg, or 100 mg) plus ritonavir (100 mg) daily vs placebo | 12 or 24 weeks | 4.58 log10 IU/ml^ | normalized level of ALT in 10 of 21 patients | 0.18 to-3.70 log10 IU/ml^ depending on virus kinetics | NR | ref146 n=21 |
| Dose escalation starting lonafarnib (50 mg twice daily) plus ritonavir (100 mg) twice daily vs lonafarnib (100 mg) twice daily plus ritonavir (100 mg) twice daily | 24 weeks | 4.60 log10 IU/ml* | normalized level of ALT in 8 of 15 patients | 1.58 log10 IU/ml* | NR | ref147 n=15 |
Symbols:
, Mean;
, Median
Abbreviations: IU, international units; NR, not reported
As previously observed, PegIFN lambda therapy was associated with transient increases in levels of transaminases and bilirubin in some patients. Although these increases might reflect a beneficial antiviral response, levels resolved with dose reduction and were not associated with hepatic decompensation. If the study finds that PegIFN lambda produces comparable rates of response to PegIFN alpha, PegIFN lambda increased tolerability could make it an attractive option for treatment of HDV infection—either as a monotherapy or in combination with other experimental therapies.
Inhibitors of virus entry
HBV and HDV bind to the solute carrier family 10 member 1 (SLC10A1 or NTCP) and enter hepatocytes, so inhibitors of this protein are being developed as therapeutics (Figure 4). The 48 N-terminal amino acids of the preS1 or L-HBsAg envelope protein are required for receptor binding119. Myrcludex B, a myristoylated lipopeptide derived from the pre-S1 domain of the HBV envelope, inhibits binding of HBV and HDV to NTCP and virus entry into hepatocytes in culture and in mice with humanized livers.61, 120,121.
Myrcludex B has been tested in clinical trials. A phase 1b/2a trial found a decrease of at least a 1 log10 in HDV RNA in patients who received Myrcludex B for 24 weeks123. Twenty-four patients with detectable HDV RNA in serum were given daily subcutaneous injections of Myrcludex B, alone or in combination with PegIFN alpha and compared to PegIFN alpha alone for 24 weeks. A reported interim analysis at the end of 24 weeks of treatment demonstrated no significant changes in HBsAg levels, the study’s primary endpoint. However, HDV RNA declines of 1.67 (Myrcludex B group), 2.6 (Myrcludex B plus PegIFN alpha group), and 2.2 log10 (PegIFN alpha group) were observed (Table 2). Two subjects in the Myrcludex B group, 5 in the Myrcludex B plus PegIFN group and 2 in the PegIFN group achieved serum HDV RNA levels below the lower limit of quantification during the reported interim analysis.124 Serum HBV DNA levels decreased to near or below-detectable levels in all patients in the Myrcludex B plus PegIFN group, whereas no changes were observed in patients from the other groups of this study.124 After 24 weeks of therapy, patients continued to receive PegIFN alpha monotherapy for a total of 48 weeks, which did not provide additional benefits, with response rates similar to those described for PegIFN alpha monotherapy, and with rebound following cessation of therapy. These findings indicate that Myrcludex B-mediated inhibition of viral entry can have inhibitory activity against HDV in humans. However, higher doses and longer treatment durations may be required, along with other drugs, for virus eradication. Additionally, pre-treatment levels of HDV RNA in participants were relatively low (1×103–1s105 copies/ml) and it is unclear whether similar results can be achieved in patients with higher serum levels of HDV RNA.
More recently, end of study results were reported from a multicenter, open-label phase 2 trial to assess the safety and efficacy of Myrcludex B in combination with tenofovir in patients with chronic HDV (Table 2).125 In this study, 120 patients received tenofovir (245 mg/day) and were randomly assigned to groups given tenofovir monotherapy or once-daily subcutaneous injections of Myrcludex B (2 mg, 5 mg, or 10 mg) for 24 weeks, followed by 24 weeks of tenofovir monotherapy. The primary endpoint was a reduction in HDV RNA by 2 logs, or negative results from assays for HDV RNA in serum. At the end of therapy, median decreases in levels of HDV in the groups that received Myrcludex B ranged from 1.6 log to 2.7 logs; the highest dose (10 mg) produced the greatest decrease in median serum level of HDV RNA. Levels of ALT normalized in as many as 50% of patients. At 12 weeks of follow up, HDV RNA relapse occurred in as many as 80% of the subjects who responded to Myrcludex B. Notably, levels of bile acids were reported to increase during therapy. This finding was reproduced in healthy volunteers—plasma bile acid exposure increased 19.2-fold, without signs of cholestasis.126 However, further studies of Myrcludex B are needed in patients with chronic HDV infection.
Inhibitors of HBsAg secretion
Nucleic acid polymers (NAPs) are phoshorothioated oligonucleotides with activities against a variety of infectious agents. Although their specific mechanisms are not understood, their activity appears to be dependent on polymer length and hydrophobicity.127 NAPs have inhibitory activity against herpes simplex virus128, 129, arenaviruses130, and HCV.131 In ducks infected with the duck HBV, 28 days administration of the NAP REP2055 reduced serum levels of duck HBsAg and duck HBV DNA and increased anti-duck HBs antibodies; levels of duck HBsAg and duck HBV DNA remained at low or undetectable levels in serum through 16 weeks after administration.132 (see Figure 3). REP 2055 was found to have tolerability issues early-phase trails, so REP2139 replaced REP2055 and was studied in patients with HBeAg-positive chronic HBV infection.133 In a phase 2 study, 3 of 12 patients given REP2139 had decreases in serum levels of HBsAg to below the limit of quantitation during 20–35 weeks of weekly intravenous infusion, resulting in a decrease in HBV DNA of 4–6 log10. 133
Figure 3. HDV Life Cycle and Therapeutic Targets.
Myrcludex-B attempts was designed to block entry of HDV into hepatocytes (A). REP2055 and REP2139-Ca were designed to inhibit HBsAg secretion (B). Lonafarnib inhibits HDV prenylation, required for virus packaging and secretion (C).
These encouraging results led to a small cohort study in patients with HBV and HDV coinfection. Patients were given weekly intravenous REP2139-Ca (500 mg) for 15 weeks, followed 15 weeks of REP2139-Ca (250 mg) combined with PegIFN alpha, and finally, PegIFN alpha monotherapy for 33 weeks (NCT02233075)(see Table 2).134 In this study, 4 of 12 patients had decreases in serum levels of HBsAg (as much as 5 log10 by week 15) and HDV RNA (below the limit of detection)135. After discontinuation of REP1239-Ca and continuation of pegIFN, levels of HbsAg rebounded in 6 patients. However, after discontinuation of therapy, 5 of 12 patients had increases in levels of HDV RNA, with 3 of the 5 returning to baseline levels.134. A long-term follow-up study of these patients is underway to assess their response to maintenance therapy.136
Although these results are promising, little is known about the safety of long-term administration of NAPs. Given the mechanism of action of NAPs against HBV, with inhibition of HBsAg secretion in animals, this raises the question of whether long-term administration would increase the risk of HCC.132, 137
Virus assembly and packaging inhibitors
The HDV lifecycle involves the covalent addition of prenyl lipids to proteins (prenylation).138 Prenylation promotes membrane association of proteins and also mediates interactions between proteins.138 Agents that inhibit the covalent addition of a farnesyl prenyl lipid group to the C-terminus of HDV large delta antigen disrupt its ability to interact with and form secreted particles with HBsAg (Figure 3).23, 139 Inhibition of farnesyltransferase inhibits secretion of virions and inhibit HDV in cultured hepatocytes and in mice.23, 140 The prenylation inhibitor lonafarnib was tested in patients with HDV infection at doses of 100 mg and 200 mg twice daily for 28 days, compared with placebo (Table 2).141 Both groups given lonafarnib had significant reductions in serum HDV RNA compared with patients given placebo. Patients in the 100 mg group had a mean decrease in HDV RNA of 0.73 log10 IU/mL whereas patients in the 200 mg group had a mean decrease of 1.54 log10 IU/mL. In aggregate, serum concentrations of lonafarnib correlated with change in HDV RNA (r2=0.78). However, patients given 200 mg had gastrointestinal side effects, including nausea, diarrhea, and weight loss. No patients were negative for HDV RNA during the 28-day study period, and HDV RNA levels returned to baseline levels by the end of the follow-up period.
Lonafarnib is metabolized by the cytochrome P450 family 3 subfamily A member 4 (CYP3A4), so researchers investigated whether addition of low-dose ritonavir (an inhibitor of CYP3A4) could increase the bioavailability and efficacy of lonafarnib. The addition of ritonavir to lonafarnib might allow for patients to be given lower doses of lonafarnib, which would reduce gastrointestinal side effects, and increase the amount of lonafarnib absorbed into the blood, increasing its pharmacokinetic and tolerability parameters. Ritonavir is given with HIV combination therapies for this purpose.142, 143 The combination of lonafarnib and ritonavir was tested in 4 phase 2 studies of patients with HDV infection (called the LOWR HDV studies).
In the open-label LOWR HDV-1 study, 15 patients were given lonafarnib monotherapy (100–300 mg) 2 or 3 times daily for up to 12 weeks, lonafarnib (100–300 mg) twice daily with PegIFN alpha (80 mcg, weekly) for 8 weeks, or lonafarnib (100 mg, twice daily) in combination with ritonavir (100 mg daily) for 8 weeks.144 This study found that lonafarnib was safe in patients with HDV infection, for up to 12 weeks, and that the addition of ritonavir increased serum levels of lonafarnib, even though patients were given lower doses. The combination of lonafarnib and ritonavir reduced the mean level of HDV RNA by 2.4 log10 at week 4 of administration and by 3.2 log10 at week 8. The effects of lonafarnib were dose dependent—patients with the highest levels of serum lonafarnib had the greatest log10 decreases in HDV RNA (r=0.68). The study also found the combination of lonafarnib (100 mg twice daily) with PegIFN alpha (180 mcg weekly) to be a feasible combination for future studies, whereas higher doses would have reduced tolerability. No subjects in this study tested negative for HDV RNA PCR, but 1 patient had a post-treatment transient increase in ALT, and during the follow-up period tested negative for HDV RNA in the PCR assay and had normalization of ALT. Sequencing analyses of HDV isolates found no evidence for resistance variants.
In the open-label, dose-optimization LOWR HDV-2 study, 55 patients were given combinations of lonafarnib with ritonavir, with or without PegIFN alpha, for 12, 24, or 48 weeks.145 Patients were assigned to high-dose groups (given doses of lonafarnib ranging from 75 mg, twice daily or more, plus ritonavir), low-dose groups (given 25 or 50 mg lonafarnib twice daily with ritonavir, 100 mg, twice daily), or low-dose combination groups (given 25 or 50 mg lonafarnib twice daily with ritonavir, 100 mg, twice daily and PegIFN alpha, 180 mcg, weekly). Because responses in the high-dose groups were comparable to those in the low-dose groups, the study was extended beyond 12 weeks for only the low-dose groups. At 24 weeks, 6/12 patients (50%) who received lonafarnib (50 mg) had levels of HDV RNA below limit of quantification or a decrease of 2 log10 or more (defined as responders). Patients given PegIFN alpha with lonafarnib (25 or 50 mg twice daily plus ritonavir) had the highest proportion of responders: 8 of 9 patients (89%) had levels of HDV RNA below limit of quantification or a decrease of 2 log10 or more week 24. Importantly, 60% and 88% of patients in the all-oral and PegIFN alpha combination groups, respectively, had normalized levels of ALT at week 24.5, of 5 patients (100%) with low baseline viral load (4 log10 IU/ml or lower) were responders to all-oral lonafarnib (50 mg and 100 mg ritonavir, twice daily). Adverse events for these lonafarnib (25 mg or 50 mg) regimens were predominantly mild to moderate. Again, as observed in LOWR HDV-1, several patients had post-treatment transient increases in levels of ALT, but later tested negative for HDV RNA in the PCR assays, and levels of ALT normalized.
The LOWR HDV-3 and 4 studies have been completed with end of study reports presented. The LOWR HDV-3 study was a randomized, double-blind study in which 21 patients received a daily all-oral combination of lonafarnib and ritonavir; some were assigned to groups given 24 weeks of lonafarnib and ritonavir and others to groups given 12 weeks of placebo and then 12 weeks of lonafarnib and ritonavir.146 Patients were randomly assigned to groups given lonafarnib (50, 75, or 100 mg) with ritonavir (100 mg) once daily for 24 weeks (n=12) or 12 weeks of placebo followed by lonafarnib (50, 75, or 100 mg) once daily for 12 weeks (n=9). After 12 weeks, the median decrease in HDV RNA from baseline was 1.6 log10 IU/mL in the lonafarnib (50 mg) group, 1.33 log10 in the lonafarnib (75 mg) group, and 0.83 log10 in the lonafarnib (100 mg) group. During this study, 6 patients achieved a decrease in serum level of HDV RNA of 2 log or more; HDV RNA levels became undetectable in 1 subject, below 14 IU/ml in 3 subjects, and <250 IU/ml in 2 subjects. Levels of ALT normalized in 4 of 6 subjects, and in 47% of the entire cohort. The combination was safe and tolerable for the 6 months of administration.
The LOWR HDV-4 study was an open-label, dose-escalation study in which 15 patients with chronic HDV infection were given lonafarnib with ritonavir for 24 weeks, to investigate the effects of rapid step-wise increases of lonafarnib to high doses.147 All patients received a baseline dose of lonafarnib (50 mg) twice daily with ritonavir (100 mg) twice daily. If this was tolerated, the dose of lonafarnib as increased to 75 mg twice daily after 4 weeks, followed by an increase to 100 mg twice daily after another 1 week, if tolerated. Based on the end of study reports, lonafarnib dose escalation to 100 mg twice daily was possible in 10 of 15 patients (66%), and maintenance doses of 100 mg until the end of dosing were possible for 5 of 15 patients (33%). Interestingly, of these 5 patients, 1 tested negative for HDV RNA in the PCR assay and another had a decrease in HDV RNA to the lower limit of quantification. At the end of treatment, the mean decrease in HDV RNA for all patients was 1.7 log10 IU/mL. Levels of ALT normalized in 53% of patients.
Future Directions
HDV infection is a worldwide problem in all age groups and results in the most rapidly progressive form of chronic viral hepatitis. There are knowledge gaps related to worldwide and US prevalence, as a result of suboptimal HDV screening. Although clinical outcomes, including HCC and hepatic decompensation, appear to be more severe in patients with HBV and HDV co-infection than in patients with HBV mono-infection, HDV is the only chronic hepatotropic viral infection without a satisfactory therapy. With advances in our understanding of the HDV life cycle, strategies to target hepatocyte entry, virus prenylation and assembly, and increase the anti-HDV immune system are being developed and tested. These agents might be successful as monotherapies or work in combination with other treatments for viral hepatitis. Nevertheless, there is great interest in eradicating a virus that was discovered more than 40 years ago and curing this devastating disease.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Abbreviations:
- HDV
hepatitis delta virus
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- RNA
ribonucleic acid
- HCC
hepatocellular carcinoma
- US
United States
- SHDAg
small hepatitis D antigen
- LHDAg
large hepatitis D antigen
- DNA
deoxyribonucleic acid
- VA
veterans administration
- IDU
injection drug user
- ALT
alanine aminotransferase
- EOT
end of treatment
- HCV
hepatitis C virus
- NAP
nucleic acid polymer
- HSV
herpes simplex virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Koh: None
Heller: None
Glenn: Board member and equity interest in, Eiger Biopharmaceuticals, Inc.
References
- 1.Rizzetto M, Canese MG, Arico S, et al. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977;18:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizzetto M, Hoyer B, Canese MG, et al. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci U S A 1980;77:6124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzetto M, Canese MG, Gerin JL, et al. Transmission of the hepatitis B virus-associated delta antigen to chimpanzees. J Infect Dis 1980;141:590–602. [DOI] [PubMed] [Google Scholar]
- 4.Wang KS, Choo QL, Weiner AJ, et al. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature 1986;323:508–14. [DOI] [PubMed] [Google Scholar]
- 5.Noureddin M, Gish R. Hepatitis delta: epidemiology, diagnosis and management 36 years after discovery. Curr Gastroenterol Rep 2014;16:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonino F, Hoyer B, Shih JW, et al. Delta hepatitis agent: structural and antigenic properties of the delta-associated particle. Infect Immun 1984;43:1000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gudima S, Chang J, Moraleda G, et al. Parameters of human hepatitis delta virus genome replication: the quantity, quality, and intracellular distribution of viral proteins and RNA. J Virol 2002;76:3709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deny P Hepatitis delta virus genetic variability: from genotypes I, II, III to eight major clades? Curr Top Microbiol Immunol 2006;307:151–71. [DOI] [PubMed] [Google Scholar]
- 9.Le Gal F, Brichler S, Drugan T, et al. Genetic diversity and worldwide distribution of the deltavirus genus: A study of 2,152 clinical strains. Hepatology 2017;66:1826–1841. [DOI] [PubMed] [Google Scholar]
- 10.Radjef N, Gordien E, Ivaniushina V, et al. Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades. J Virol 2004;78:2537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shakil AO, Hadziyannis S, Hoofnagle JH, et al. Geographic distribution and genetic variability of hepatitis delta virus genotype I. Virology 1997;234:160–7. [DOI] [PubMed] [Google Scholar]
- 12.Imazeki F, Omata M, Ohto M. Complete nucleotide sequence of hepatitis delta virus RNA in Japan. Nucleic Acids Res 1991;19:5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivaniushina V, Radjef N, Alexeeva M, et al. Hepatitis delta virus genotypes I and II cocirculate in an endemic area of Yakutia, Russia. J Gen Virol 2001;82:2709–18. [DOI] [PubMed] [Google Scholar]
- 14.Lee CM, Changchien CS, Chung JC, et al. Characterization of a new genotype II hepatitis delta virus from Taiwan. J Med Virol 1996;49:145–54. [DOI] [PubMed] [Google Scholar]
- 15.Wu JC, Chiang TY, Sheen IJ. Characterization and phylogenetic analysis of a novel hepatitis D virus strain discovered by restriction fragment length polymorphism analysis. J Gen Virol 1998;79 (Pt 5):1105–13. [DOI] [PubMed] [Google Scholar]
- 16.Nakano T, Shapiro CN, Hadler SC, et al. Characterization of hepatitis D virus genotype III among Yucpa Indians in Venezuela. J Gen Virol 2001;82:2183–9. [DOI] [PubMed] [Google Scholar]
- 17.Casey JL, Brown TL, Colan EJ, et al. A genotype of hepatitis D virus that occurs in northern South America. Proc Natl Acad Sci U S A 1993;90:9016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parana R, Kay A, Molinet F, et al. HDV genotypes in the Western Brazilian Amazon region: A preliminary report. Am J Trop Med Hyg 2006;75:475–9. [PubMed] [Google Scholar]
- 19.Sakugawa H, Nakasone H, Nakayoshi T, et al. Hepatitis delta virus genotype IIb predominates in an endemic area, Okinawa, Japan. J Med Virol 1999;58:366–72. [DOI] [PubMed] [Google Scholar]
- 20.Le Gal F, Gault E, Ripault MP, et al. Eighth major clade for hepatitis delta virus. Emerg Infect Dis 2006;12:1447–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casey JL. Control of ADAR1 editing of hepatitis delta virus RNAs. Curr Top Microbiol Immunol 2012;353:123–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glenn JS, Watson JA, Havel CM, et al. Identification of a prenylation site in delta virus large antigen. Science 1992;256:1331–3. [DOI] [PubMed] [Google Scholar]
- 23.Bordier BB, Marion PL, Ohashi K, et al. A prenylation inhibitor prevents production of infectious hepatitis delta virus particles. J Virol 2002;76:10465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonino F, Heermann KH, Rizzetto M, et al. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol 1986;58:945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sureau C The role of the HBV envelope proteins in the HDV replication cycle. Curr Top Microbiol Immunol 2006;307:113–31. [DOI] [PubMed] [Google Scholar]
- 26.Sureau C, Negro F. The hepatitis delta virus: Replication and pathogenesis. J Hepatol 2016;64:S102–S116. [DOI] [PubMed] [Google Scholar]
- 27.Chang J, Nie X, Chang HE, et al. Transcription of hepatitis delta virus RNA by RNA polymerase II. J Virol 2008;82:1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor JM. Hepatitis delta virus: cis and trans functions required for replication. Cell 1990;61:371–3. [DOI] [PubMed] [Google Scholar]
- 29.Luo GX, Chao M, Hsieh SY, et al. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol 1990;64:1021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glenn JS, White JM. trans-dominant inhibition of human hepatitis delta virus genome replication. J Virol 1991;65:2357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao M, Hsieh SY, Taylor J. Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol 1990;64:5066–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makuwa M, Caron M, Souquiere S, et al. Prevalence and genetic diversity of hepatitis B and delta viruses in pregnant women in Gabon: molecular evidence that hepatitis delta virus clade 8 originates from and is endemic in central Africa. J Clin Microbiol 2008;46:754–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foupouapouognigni Y, Noah DN, Sartre MT, et al. High prevalence and predominance of hepatitis delta virus genotype 1 infection in Cameroon. J Clin Microbiol 2011;49:1162–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andernach IE, Leiss LV, Tarnagda ZS, et al. Characterization of hepatitis delta virus in sub-Saharan Africa. J Clin Microbiol 2014;52:1629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lunel-Fabiani F, Mansour W, Amar AO, et al. Impact of hepatitis B and delta virus co-infection on liver disease in Mauritania: a cross sectional study. J Infect 2013;67:448–57. [DOI] [PubMed] [Google Scholar]
- 36.Mansour W, Malick FZ, Sidiya A, et al. Prevalence, risk factors, and molecular epidemiology of hepatitis B and hepatitis delta virus in pregnant women and in patients in Mauritania. J Med Virol 2012;84:1186–98. [DOI] [PubMed] [Google Scholar]
- 37.Amini N, Alavian SM, Kabir A, et al. Prevalence of hepatitis d in the eastern mediterranean region: systematic review and meta analysis. Hepat Mon 2013;13:e8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amini N, Alavian SM, Kabir A, et al. Clinical Features and Seroepidemiology of Anti-HDV Antibody in patients With Chronic Hepatitis B Virus Infection in Iran: A Meta-Analysis. Hepat Mon 2011;11:960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davaalkham D, Ojima T, Uehara R, et al. Hepatitis delta virus infection in mongolia: analyses of geographic distribution, risk factors, and disease severity. Am J Trop Med Hyg 2006;75:365–9. [PubMed] [Google Scholar]
- 40.Tsatsralt-Od B, Takahashi M, Nishizawa T, et al. High prevalence of dual or triple infection of hepatitis B, C, and delta viruses among patients with chronic liver disease in Mongolia. J Med Virol 2005;77:491–9. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Oidovsambuu O, Liu P, et al. A novel quantitative microarray antibody capture assay identifies an extremely high hepatitis delta virus prevalence among hepatitis B virus-infected mongolians. Hepatology 2017;66:1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popescu GA, Otelea D, Gavriliu LC, et al. Epidemiology of hepatitis D in patients infected with hepatitis B virus in bucharest: a cross-sectional study. J Med Virol 2013;85:769–74. [DOI] [PubMed] [Google Scholar]
- 43.Semenov AV. [Prevalence of seronegative hepatitis D among patients with chronic viral hepatitis B]. Zh Mikrobiol Epidemiol Immunobiol 2012:106–9. [PubMed] [Google Scholar]
- 44.Sy BT, Ratsch BA, Toan NL, et al. High prevalence and significance of hepatitis D virus infection among treatment-naive HBsAg-positive patients in Northern Vietnam. PLoS One 2013;8:e78094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braga WS, Castilho Mda C, Borges FG, et al. Hepatitis D virus infection in the Western Brazilian Amazon - far from a vanishing disease. Rev Soc Bras Med Trop 2012;45:691–5. [DOI] [PubMed] [Google Scholar]
- 46.Crispim MA, Fraiji NA, Campello SC, et al. Molecular epidemiology of hepatitis B and hepatitis delta viruses circulating in the Western Amazon region, North Brazil. BMC Infect Dis 2014;14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stroffolini T, Ferrigno L, Cialdea L, et al. Incidence and risk factors of acute Delta hepatitis in Italy: results from a national surveillance system. SEIEVA Collaborating Group. J Hepatol 1994;21:1123–6. [DOI] [PubMed] [Google Scholar]
- 48.Hadziyannis SJ. Review: hepatitis delta. J Gastroenterol Hepatol 1997;12:289–98. [DOI] [PubMed] [Google Scholar]
- 49.Hadziyannis SJ. Decreasing prevalence of hepatitis D virus infection. J Gastroenterol Hepatol 1997;12:745–6. [DOI] [PubMed] [Google Scholar]
- 50.Gaeta GB, Stroffolini T, Chiaramonte M, et al. Chronic hepatitis D: a vanishing Disease? An Italian multicenter study. Hepatology 2000;32:824–7. [DOI] [PubMed] [Google Scholar]
- 51.William Tong CY, Asher R, Toby M, et al. A re-assessment of the epidemiology and patient characteristics of hepatitis D virus infection in inner city London. J Infect 2013;66:521–7. [DOI] [PubMed] [Google Scholar]
- 52.Servant-Delmas A, Le Gal F, Gallian P, et al. Increasing prevalence of HDV/HBV infection over 15 years in France. J Clin Virol 2014;59:126–8. [DOI] [PubMed] [Google Scholar]
- 53.Wedemeyer H, Heidrich B, Manns MP. Hepatitis D virus infection--not a vanishing disease in Europe! Hepatology 2007;45:1331–2; author reply 1332–3. [DOI] [PubMed] [Google Scholar]
- 54.Gish RG, Yi DH, Kane S, et al. Coinfection with hepatitis B and D: epidemiology, prevalence and disease in patients in Northern California. J Gastroenterol Hepatol 2013;28:1521–5. [DOI] [PubMed] [Google Scholar]
- 55.Safaie P, Razeghi S, Rouster SD, et al. Hepatitis D diagnostics:utilization and testing in the united states. Virus Res 2018. [DOI] [PubMed] [Google Scholar]
- 56.Kushner T, Serper M, Kaplan DE. Delta Hepatitis within the Veterans Affairs Medical System in the United States: Prevalence, Risk Factors, and Outcomes. J Hepatol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kucirka LM, Farzadegan H, Feld JJ, et al. Prevalence, correlates, and viral dynamics of hepatitis delta among injection drug users. J Infect Dis 2010;202:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahale P, Aka PV, Chen X, et al. Hepatitis D viremia among injection drug users in San Francisco. J Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sureau C, Taylor J, Chao M, et al. Cloned hepatitis delta virus cDNA is infectious in the chimpanzee. J Virol 1989;63:4292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu JC, Chen PJ, Kuo MY, et al. Production of hepatitis delta virus and suppression of helper hepatitis B virus in a human hepatoma cell line. J Virol 1991;65:1099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lutgehetmann M, Mancke LV, Volz T, et al. Humanized chimeric uPA mouse model for the study of hepatitis B and D virus interactions and preclinical drug evaluation. Hepatology 2012;55:685–94. [DOI] [PubMed] [Google Scholar]
- 62.Colombo P, Di Blasi F, Magrin S, et al. Smouldering hepatitis B virus replication in patients with chronic liver disease and hepatitis delta virus superinfection. J Hepatol 1991;12:64–9. [DOI] [PubMed] [Google Scholar]
- 63.Lee SD, Wang JY, Wu JC, et al. Hepatitis D virus (delta agent) superinfection in an endemic area of hepatitis B infection: immunopathologic and serologic findings. Scand J Infect Dis 1987;19:173–7. [DOI] [PubMed] [Google Scholar]
- 64.Sagnelli E, Felaco FM, Rapicetta M, et al. Interaction between HDV and HBV infection in HBsAg-chronic carriers. Infection 1991;19:155–8. [DOI] [PubMed] [Google Scholar]
- 65.Schaper M, Rodriguez-Frias F, Jardi R, et al. Quantitative longitudinal evaluations of hepatitis delta virus RNA and hepatitis B virus DNA shows a dynamic, complex replicative profile in chronic hepatitis B and D. J Hepatol 2010;52:658–64. [DOI] [PubMed] [Google Scholar]
- 66.Cole SM, Gowans EJ, Macnaughton TB, et al. Direct evidence for cytotoxicity associated with expression of hepatitis delta virus antigen. Hepatology 1991;13:845–51. [PubMed] [Google Scholar]
- 67.Govindarajan S, Fields HA, Humphrey CD, et al. Pathologic and ultrastructural changes of acute and chronic delta hepatitis in an experimentally infected chimpanzee. Am J Pathol 1986;122:315–22. [PMC free article] [PubMed] [Google Scholar]
- 68.Popper H, Thung SN, Gerber MA, et al. Histologic studies of severe delta agent infection in Venezuelan Indians. Hepatology 1983;3:906–12. [DOI] [PubMed] [Google Scholar]
- 69.Williams V, Brichler S, Radjef N, et al. Hepatitis delta virus proteins repress hepatitis B virus enhancers and activate the alpha/beta interferon-inducible MxA gene. J Gen Virol 2009;90:2759–67. [DOI] [PubMed] [Google Scholar]
- 70.Abbas Z, Afzal R. Life cycle and pathogenesis of hepatitis D virus: A review. World J Hepatol 2013;5:666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pasetti G, Calzetti C, Degli Antoni A, et al. Clinical features of hepatitis delta virus infection in a northern Italian area. Infection 1988;16:345–8. [DOI] [PubMed] [Google Scholar]
- 72.Raimondo G, Smedile A, Gallo L, et al. Multicentre study of prevalence of HBV-associated delta infection and liver disease in drug-addicts. Lancet 1982;1:249–51. [DOI] [PubMed] [Google Scholar]
- 73.Farci P, Niro GA. Clinical features of hepatitis D. Semin Liver Dis 2012;32:228–36. [DOI] [PubMed] [Google Scholar]
- 74.Rizzetto M Hepatitis D: virology, clinical and epidemiological aspects. Acta Gastroenterol Belg 2000;63:221–4. [PubMed] [Google Scholar]
- 75.Yurdaydin C, Idilman R, Bozkaya H, et al. Natural history and treatment of chronic delta hepatitis. J Viral Hepat 2010;17:749–56. [DOI] [PubMed] [Google Scholar]
- 76.Mathurin P, Thibault V, Kadidja K, et al. Replication status and histological features of patients with triple (B, C, D) and dual (B, C) hepatic infections. J Viral Hepat 2000;7:15–22. [DOI] [PubMed] [Google Scholar]
- 77.Sagnelli E, Felaco FM, Filippini P, et al. Influence of HDV infection on clinical, biochemical and histological presentation of HBsAg positive chronic hepatitis. Liver 1989;9:229–34. [DOI] [PubMed] [Google Scholar]
- 78.Fattovich G, Boscaro S, Noventa F, et al. Influence of hepatitis delta virus infection on progression to cirrhosis in chronic hepatitis type B. J Infect Dis 1987;155:931–5. [DOI] [PubMed] [Google Scholar]
- 79.Takyar V, Surana P, Kleiner DE, et al. Noninvasive markers for staging fibrosis in chronic delta hepatitis. Aliment Pharmacol Ther 2017;45:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cross TJ, Rizzi P, Horner M, et al. The increasing prevalence of hepatitis delta virus (HDV) infection in South London. J Med Virol 2008;80:277–82. [DOI] [PubMed] [Google Scholar]
- 81.Abbas Z, Abbas M, Abbas S, et al. Hepatitis D and hepatocellular carcinoma. World J Hepatol 2015;7:777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ji J, Sundquist K, Sundquist J. A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J Natl Cancer Inst 2012;104:790–2. [DOI] [PubMed] [Google Scholar]
- 83.Toukan AU, Abu-el-Rub OA, Abu-Laban SA, et al. The epidemiology and clinical outcome of hepatitis D virus (delta) infection in Jordan. Hepatology 1987;7:1340–5. [DOI] [PubMed] [Google Scholar]
- 84.Romeo R, Del Ninno E, Rumi M, et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 2009;136:1629–38. [DOI] [PubMed] [Google Scholar]
- 85.Niro GA, Smedile A, Ippolito AM, et al. Outcome of chronic delta hepatitis in Italy: a long-term cohort study. J Hepatol 2010;53:834–40. [DOI] [PubMed] [Google Scholar]
- 86.Su CW, Huang YH, Huo TI, et al. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology 2006;130:1625–35. [DOI] [PubMed] [Google Scholar]
- 87.Ponzetto A, Negro F, Popper H, et al. Serial passage of hepatitis delta virus in chronic hepatitis B virus carrier chimpanzees. Hepatology 1988;8:1655–61. [DOI] [PubMed] [Google Scholar]
- 88.Shattock AG, Morgan BM. Sensitive enzyme immunoassay for the detection of delta antigen and anti-delta, using serum as the delta antigen source. J Med Virol 1984;13:73–82. [DOI] [PubMed] [Google Scholar]
- 89.Shattock AG, Morris MC. Evaluation of commercial enzyme immunoassays for detection of hepatitis delta antigen and anti-hepatitis delta virus (HDV) and immunoglobulin M anti-HDV antibodies. J Clin Microbiol 1991;29:1873–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aragona M, Macagno S, Caredda F, et al. Serological response to the hepatitis delta virus in hepatitis D. Lancet 1987;1:478–80. [DOI] [PubMed] [Google Scholar]
- 91.Olivero A, Smedile A. Hepatitis delta virus diagnosis. Semin Liver Dis 2012;32:220–7. [DOI] [PubMed] [Google Scholar]
- 92.Le Gal F, Brichler S, Sahli R, et al. First international external quality assessment for hepatitis delta virus RNA quantification in plasma. Hepatology 2016;64:1483–1494. [DOI] [PubMed] [Google Scholar]
- 93.Le Gal F, Dziri S, Gerber A, et al. Performance Characteristics of a New Consensus Commercial Kit for Hepatitis D Virus RNA Viral Load Quantification. J Clin Microbiol 2017;55:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mahale P, Aka PV, Chen X, et al. Hepatitis D Viremia Among Injection Drug Users in San Francisco. J Infect Dis 2018;217:1902–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu JC, Chen TZ, Huang YS, et al. Natural history of hepatitis D viral superinfection: significance of viremia detected by polymerase chain reaction. Gastroenterology 1995;108:796–802. [DOI] [PubMed] [Google Scholar]
- 96.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–398. [DOI] [PubMed] [Google Scholar]
- 98.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Farci P, Mandas A, Coiana A, et al. Treatment of chronic hepatitis D with interferon alfa-2a. N Engl J Med 1994;330:88–94. [DOI] [PubMed] [Google Scholar]
- 100.Farci P, Roskams T, Chessa L, et al. Long-term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology 2004;126:1740–9. [DOI] [PubMed] [Google Scholar]
- 101.Castelnau C, Le Gal F, Ripault MP, et al. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology 2006;44:728–35. [DOI] [PubMed] [Google Scholar]
- 102.Niro GA, Ciancio A, Gaeta GB, et al. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology 2006;44:713–20. [DOI] [PubMed] [Google Scholar]
- 103.Heller T, Rotman Y, Koh C, et al. Long-term therapy of chronic delta hepatitis with peginterferon alfa. Aliment Pharmacol Ther 2014;40:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guedj J, Rotman Y, Cotler SJ, et al. Understanding early serum hepatitis D virus and hepatitis B surface antigen kinetics during pegylated interferon-alpha therapy via mathematical modeling. Hepatology 2014;60:1902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lau DT, Kleiner DE, Park Y, et al. Resolution of chronic delta hepatitis after 12 years of interferon alfa therapy. Gastroenterology 1999;117:1229–33. [DOI] [PubMed] [Google Scholar]
- 106.Keskin O, Wedemeyer H, Tuzun A, et al. Association Between Level of Hepatitis D Virus RNA at Week 24 of Pegylated Interferon Therapy and Outcome. Clin Gastroenterol Hepatol 2015;13:2342–49e1-2. [DOI] [PubMed] [Google Scholar]
- 107.Kabacam G, Onder FO, Yakut M, et al. Entecavir treatment of chronic hepatitis D. Clin Infect Dis 2012;55:645–50. [DOI] [PubMed] [Google Scholar]
- 108.Lau DT, Doo E, Park Y, et al. Lamivudine for chronic delta hepatitis. Hepatology 1999;30:546–9. [DOI] [PubMed] [Google Scholar]
- 109.Yurdaydin C, Bozkaya H, Onder FO, et al. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat 2008;15:314–21. [DOI] [PubMed] [Google Scholar]
- 110.Wedemeyer H, Yurdaydin C, Dalekos GN, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 2011;364:322–31. [DOI] [PubMed] [Google Scholar]
- 111.Wedemeyer H, Yurdaydin C, Ernst S, et al. 96 weeks of pegylated-interferon-alpha-2a plus tenofovir or placebo for the treatment of hepatitis delta: the HIDIT-2 study. Hepatology 2013;58:222A–223A. [Google Scholar]
- 112.Wranke A, Heidrich B, Ernst S, et al. Anti-HDV IgM as a marker of disease activity in hepatitis delta. PLoS One 2014;9:e101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lasfar A, Abushahba W, Balan M, et al. Interferon lambda: a new sword in cancer immunotherapy. Clin Dev Immunol 2011;2011:349575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ank N, West H, Bartholdy C, et al. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol 2006;80:4501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chan HLY, Ahn SH, Chang TT, et al. Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: A randomized phase 2b study (LIRA-B). J Hepatol 2016;64:1011–1019. [DOI] [PubMed] [Google Scholar]
- 116.Foster GR, Chayama K, Chuang WL, et al. A randomized, controlled study of peginterferon lambda-1a/ribavirin +/− daclatasvir for hepatitis C virus genotype 2 or 3. Springerplus 2016;5:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Giersch K, Homs M, Volz T, et al. Both interferon alpha and lambda can reduce all intrahepatic HDV infection markers in HBV/HDV infected humanized mice. Sci Rep 2017;7:3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hamid SS, Etzion O, Lurie Y, et al. A phase 2 randomized clinical trial to evaluate the safety and efficacy of pegylated intereron lambda monotherapy in patients with chronic hepatitis delta virus infection. Interim results from the LIMT HDV study. Hepatology 2017;66:496A. [Google Scholar]
- 119.Engelke M, Mills K, Seitz S, et al. Characterization of a hepatitis B and hepatitis delta virus receptor binding site. Hepatology 2006;43:750–60. [DOI] [PubMed] [Google Scholar]
- 120.Gripon P, Cannie I, Urban S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J Virol 2005;79:1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barrera A, Guerra B, Notvall L, et al. Mapping of the hepatitis B virus pre-S1 domain involved in receptor recognition. J Virol 2005;79:9786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Volz T, Giersch K, Allweiss L, et al. Myrcludex-B Inhibits Establishment of HDV Super-Infection in HBV Infected Mice and Reduces HDV Viremia in Stabily HBV/HDV Co-infected Mice. J Hepatol 2015;62:514. [Google Scholar]
- 123.Bogomolov P, Alexandrov A, Voronkova N, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B - first results of a Phase Ib/IIa study. J Hepatol 2016. [DOI] [PubMed] [Google Scholar]
- 124.Bogomolov P, Alexandrov A, Voronkova N, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. J Hepatol 2016;65:490–8. [DOI] [PubMed] [Google Scholar]
- 125.Wedemeyer H, Bogomolov P, Blank A, et al. Final results of a multicenter, open-label phase 2b clinical trial to assess safety and efficacy of Myrcludex B in combination with Tenofovir in patients with chronic HBV/HDV co-infection. J Hepatol 2018;68:S3. [Google Scholar]
- 126.Blank A, Eidam A, Haag M, et al. The NTCP-inhibitor Myrcludex B: Effects on Bile Acid Disposition and Tenofovir Pharmacokinetics. Clin Pharmacol Ther 2018;103:341–348. [DOI] [PubMed] [Google Scholar]
- 127.Vaillant A Nucleic acid polymers: Broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antiviral Res 2016;133:32–40. [DOI] [PubMed] [Google Scholar]
- 128.Bernstein DI, Goyette N, Cardin R, et al. Amphipathic DNA polymers exhibit antiherpetic activity in vitro and in vivo. Antimicrob Agents Chemother 2008;52:2727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guzman EM, Cheshenko N, Shende V, et al. Amphipathic DNA polymers are candidate vaginal microbicides and block herpes simplex virus binding, entry and viral gene expression. Antivir Ther 2007;12:1147–56. [PubMed] [Google Scholar]
- 130.Lee AM, Rojek JM, Gundersen A, et al. Inhibition of cellular entry of lymphocytic choriomeningitis virus by amphipathic DNA polymers. Virology 2008;372:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Matsumura T, Hu Z, Kato T, et al. Amphipathic DNA polymers inhibit hepatitis C virus infection by blocking viral entry. Gastroenterology 2009;137:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Noordeen F, Scougall CA, Grosse A, et al. Therapeutic Antiviral Effect of the Nucleic Acid Polymer REP 2055 against Persistent Duck Hepatitis B Virus Infection. PLoS One 2015;10:e0140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Al-Mahtab M, Bazinet M, Vaillant A. Safety and Efficacy of Nucleic Acid Polymers in Monotherapy and Combined with Immunotherapy in Treatment-Naive Bangladeshi Patients with HBeAg+ Chronic Hepatitis B Infection. PLoS One 2016;11:e0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bazinet M, Pantea V, Cebotarescu V, et al. Initial follow-up results from the REP 301 trial: Safety and efficacy of REP2139-Ca and pegylated interferon alpha-2a in caucasian patients with chronic HBV/HDV co-infection. Hepatology 2016;64:912A. [Google Scholar]
- 135.Bazinet M, Pantea V, Cebotarescu V, et al. Significant reduction of HBsAg and HDV RNA by the nucleic acid polymer REP 2139 in caucasian patients with chronic HBV/HDV co-infection. J Hepatol 2015;62:s257–s258. [Google Scholar]
- 136.Bazinet M, Pantea V, Cebotarescu V, et al. Establishment of persistent functional remission of HBV and HDV infection following REP 2139 and pegylated interferon alpha 2a therapy in patients with chronic HBV/HDV coinfection: 18 month follow-up results from the REP 301-LTF study. J Hepatol 2018;68:S365–s504. [Google Scholar]
- 137.Wang Y, Cui F, Lv Y, et al. HBsAg and HBx knocked into the p21 locus causes hepatocellular carcinoma in mice. Hepatology 2004;39:318–24. [DOI] [PubMed] [Google Scholar]
- 138.Glenn JS, Marsters JC Jr., Greenberg HB. Use of a prenylation inhibitor as a novel antiviral agent. J Virol 1998;72:9303–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Einav S, Glenn JS. Prenylation inhibitors: a novel class of antiviral agents. J Antimicrob Chemother 2003;52:883–6. [DOI] [PubMed] [Google Scholar]
- 140.Bordier BB, Ohkanda J, Liu P, et al. In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus. J Clin Invest 2003;112:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koh C, Canini L, Dahari H, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tomaru A, Takeda-Morishita M, Banba H, et al. Analysis of the pharmacokinetic boosting effects of ritonavir on oral bioavailability of drugs in mice. Drug Metab Pharmacokinet 2013;28:144–52. [DOI] [PubMed] [Google Scholar]
- 143.Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J Antimicrob Chemother 2004;53:4–9. [DOI] [PubMed] [Google Scholar]
- 144.Yurdaydin C, Keskin O, Kalkan C, et al. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The LOWR HDV-1 study. Hepatology 2018;67:1224–1236. [DOI] [PubMed] [Google Scholar]
- 145.Yurdaydin C, Idilman R, Keskin O, et al. A phase 2 dose-optimization study of lonafarnib with ritonavir for the treatment of chronic delta hepatitis-end of treatment results from the LOWR HDV-2 study. J Hepatol 2017;66:S33–34. [Google Scholar]
- 146.Koh C, Surana P, Han MAT, et al. A phase 2 study exploring once daily dosing of ritonavir boosted lonafarnib for the treatment of chronic delta hepatitis - end of study results from the LOWR HDV-3 study. J Hepatol 2017;66:S101–S102. [Google Scholar]
- 147.Wedemeyer H, Port K, Deterding K, et al. A phase 2 dose-escalation study of lonafarnib plus ritonavir in patients with chronic hepatitis D: final results from the lonafarnib with ritonavir in HDV-4 (LOWR HDV-4) study. J Hepatol 2017;66:S24. [Google Scholar]
- 148.Trichopoulos D, Day NE, Tzonou A, et al. Delta agent and the etiology of hepatocellular carcinoma. Int J Cancer 1987;39:283–6. [DOI] [PubMed] [Google Scholar]
- 149.Verme G, Brunetto MR, Oliveri F, et al. Role of hepatitis delta virus infection in hepatocellular carcinoma. Dig Dis Sci 1991;36:1134–6. [DOI] [PubMed] [Google Scholar]
- 150.Tamura I, Kurimura O, Koda T, et al. Risk of liver cirrhosis and hepatocellular carcinoma in subjects with hepatitis B and delta virus infection: a study from Kure, Japan. J Gastroenterol Hepatol 1993;8:433–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.