Abstract
OBJECTIVE
Adipose tissue insulin resistance is one of the pathophysiological components of type 2 diabetes. Herein we investigated: 1) adipose insulin resistance index (Adipose-IR) (calculated as fasting insulin × free fatty acids [FFAs]) in youth across the spectrum of adiposity from normal weight to obese and the spectrum from normal glucose tolerance (NGT) to impaired glucose tolerance (IGT) to type 2 diabetes, 2) the relationship of Adipose-IR with physical and metabolic characteristics, and 3) the predictive power of Adipose-IR for determining dysglycemia in youth.
RESEARCH DESIGN AND METHODS
A total of 205 youth had fasting glucose, insulin, FFA, Adipose-IR, body composition, visceral adipose tissue (VAT), leptin, and adiponectin evaluated.
RESULTS
Adipose-IR was 2.2-fold higher in obese NGT, 4.3-fold higher in IGT, and 4.6-fold higher in type 2 diabetes compared with that in normal-weight peers (all P < 0.05). Females with dysglycemia (IGT and type 2 diabetes) had higher Adipose-IR than their male counterparts (P < 0.001). Adipose-IR correlated positively with total body and visceral adiposity, fasting glucose, HOMA-IR, and leptin and negatively with adiponectin. Receiver operating characteristic curve analysis yielded an optimal cutoff for Adipose-IR of 9.3 μU/mL × mmol/L for determining dysglycemia with 80% predictive power.
CONCLUSIONS
Adipose-IR is a simple surrogate estimate that reflects pathophysiological alterations in adipose tissue insulin sensitivity in youth, with progressive deterioration from normal weight to obese and from NGT to IGT to type 2 diabetes. Adipose-IR can be applied in large-scale epidemiological/observational studies of the natural history of youth-onset type 2 diabetes and its progression or reversal with intervention strategies.
Introduction
Adipose tissue is an insulin-sensitive endocrine organ playing a pivotal role in glucose and lipid metabolism by buffering daily flux of fatty acids in the postprandial period and by secreting adipokines (1,2). The important role of insulin in adipose tissue is to suppress lipolysis and to promote glucose uptake and lipogenesis, thereby regulating secretion of free fatty acids (FFAs) into the bloodstream (3). Accordingly, adipose tissue insulin resistance is considered one of the pathophysiological components of type 2 diabetes (4). We recently demonstrated that obese youth with impaired glucose tolerance (IGT) compared with their normoglycemic peers of similar percentage body fat have diminished insulin suppression of lipolysis together with lower adipose tissue insulin sensitivity, measured with the hyperinsulinemic-euglycemic clamp combined with [2H5]glycerol tracer (5). Moreover, consistent with adult studies (6,7), we have demonstrated that experimentally induced increases in circulating FFA concentrations in normal-weight and obese youth rapidly induce peripheral insulin resistance and β-cell lipotoxicity, measured by the clamp methodology (8,9). Such observations in youth point to an important role of FFA in regulating insulin sensitivity and β-cell function and the critical function of adipose tissue insulin sensitivity in maintaining normal circulating FFA concentrations.
Although the clamp methodology combined with tracers (either glycerol or palmitate) is considered the most accurate in vivo measurement of adipocyte insulin resistance (10–12), it is not feasible in large epidemiological, observational, or interventional studies. Therefore, a surrogate index of adipose tissue insulin resistance (Adipose-IR), calculated as the product of fasting insulin and fasting FFA concentrations (3), has been used in adults to examine adipocyte dysfunction in obesity, prediabetes, and type 2 diabetes (13–15). Gastaldelli et al. (15) recently showed that Adipose-IR is increased twofold in obese adults with NGT and IGT and threefold in type 2 diabetes compared with lean NGT. The pediatric literature appears to be limited to two relevant studies with inconsistent findings (16,17). One study showed no differences in adipose insulin resistance measured by FFA suppression from fasting to the clamp steady-state hyperinsulinemia among lean youth versus obese youth versus youth with type 2 diabetes (16). The other study, in a large pediatric obesity clinic cohort of 962, obese youth age 7–20 years exhibited progressive increases in Adipose-IR from NGT to prediabetes to type 2 diabetes, correlating positively with BMI z score and adiposity (17). This observation would suggest that lean youth would have lower or better adipose insulin resistance than obese youth—contrary to what was reported in the former study (16). Against this background of conflicting findings, additional investigations of adipose tissue insulin resistance along the spectrum of adiposity from normal weight to obese and across glucose tolerance categories are needed to confirm or refute the sparse pediatric literature.
Therefore, the purpose of this study was 1) to evaluate the Adipose-IR index in youth from normal weight to obese and from NGT to IGT to type 2 diabetes categories, 2) to examine the relationship of Adipose-IR with physical and metabolic characteristics, and 3) to estimate, for the first time, the predictive power and the optimal cutoff value of Adipose-IR for determining dysglycemia in youth.
Research Design and Methods
Participants
A total of 205 adolescents were examined (age 10 to <20 years, Tanner stages IV and V, 99 African American and 106 American and white, and 70 male and 135 female) from our National Institutes of Health–funded K24 grant investigating childhood insulin resistance (5,18–23). There were 49 who were normal weight and 89 who were overweight/obese (15 overweight with BMI ≥85th percentile for age and sex but <95th and 74 obese with BMI ≥95th percentile) with NGT, 38 who were obese with IGT, and 29 who were obese with type 2 diabetes. From here on we will refer to the overweight/obese group as “obese” for simplicity. Glucose tolerance status was determined with HbA1c and/or a 2-h oral glucose tolerance test (OGTT) (1.75 g glucose/kg [maximum 75 g]) (24). Healthy, normal-weight participants had normal fasting glucose and HbA1c and did not undergo a 2-h OGTT (23). Participants with isolated impaired fasting glucose (IFG) were excluded because IGT and combined IFG and IGT groups are characterized as having insulin resistance, whereas those with isolated IFG are not, while both have impaired β-cell function (25). All obese youth with type 2 diabetes were negative for GAD and insulinoma-associated protein 2 autoantibody. They all had adequate metabolic control, with a mean ± SE HbA1c of 6.6 ± 0.2% (range 4.7–8.3) and diabetes duration of 8.1 ± 1.8 months (0–39). They were on treatment consisting of lifestyle modification (n = 6), metformin alone (n = 13), metformin together with insulin (n = 7), and insulin alone (n = 3). None received degludec or insulin glargine 300 IU/mL. Participants were recruited through newspaper advertisements, flyers posted in the medical campus, city bus routes, and the outpatient clinics in the Children’s Hospital of Pittsburgh Weight Management and Wellness Center and the Division of Pediatric Endocrinology. The study was approved by the institutional review board of the University of Pittsburgh, and written informed parental consent and child assent were obtained from all participants before any research procedures in accordance with the ethics guidelines of Children’s Hospital of Pittsburgh.
Study Procedures and Fasting Blood Samples
All procedures were performed at the Pediatric Clinical and Translational Research Center (PCTRC) of Children’s Hospital of Pittsburgh. All participants underwent medical history, physical examination, and hematologic and biochemical tests. Height and weight were assessed to the nearest 0.1 cm and 0.1 kg, respectively, and used to calculate BMI. Pubertal development was assessed using Tanner criteria (26). Body composition was evaluated with DEXA for the measurement of total body fat mass and percent body fat. Abdominal total adipose tissue (TAT), subcutaneous adipose tissue (SAT), and visceral adipose tissue (VAT) were assessed by either computed tomography (CT) at L4-L5 intervertebral space or MRI (27,28). The switch from CT to MRI was imposed by the study section during the competitive grant renewal. However, there is a strong correlation (r = 0.89–0.95) and good agreement between CT and MRI for the measurement of abdominal adipose tissue (29).
Fasting blood samples were obtained after a 10- to 12-h overnight fast for the measurement of glucose, insulin, FFA, HbA1c, lipid profile, leptin, and adiponectin. In participants with type 2 diabetes, metformin and long-acting insulin were discontinued 48 h before study procedures. Some of the participants in the current study were included in previous publications reporting data unrelated to the current investigation (5,18–23).
Biochemical Measurements
Plasma glucose was determined by the glucose oxidase method using a glucose analyzer (Yellow Springs Instrument Co., Yellow Springs, OH) and insulin by a commercially available human-specific insulin radioimmunoassay kit (catalog no. HI-14 K; Linco/Millipore, St. Charles, MO). FFA concentration was determined using enzymatic colorimetric methods with a Wako (nonesterified fatty acids) NEFA-HR test kit (FUJIFILM Wako Diagnostics, Osaka, Japan). In our laboratory, intra- and interassay coefficients of variation were 2.1% and 6.5% for FFA and 6.3% and 7.4% for insulin. Plasma lipid concentrations were determined using the standards of the Centers for Disease Control and Prevention (18). Leptin and adiponectin was measured using a commercially available radioimmunoassay kit (LINCO Research, Inc.). HbA1c was measured by high-performance liquid chromatography (Tosoh Medics, Inc., San Francisco, CA).
Calculations
Adipose-IR, a surrogate index of adipose tissue insulin resistance, was calculated as fasting insulin × fasting FFA concentration. To use this index, we used our previously published data on whole-body lipolysis, using [2H5]glycerol tracer, and adipose tissue insulin sensitivity in obese youth with NGT (n = 97) versus IGT (n = 41) (5). The correlation between adipose tissue insulin sensitivity and Adipose-IR in the latter cohort was r = −0.605, P < 0.0001. The HOMA of insulin resistance (HOMA-IR) was calculated (HOMA Calculator at https://www.dtu.ox.ac.uk/homacalculator/download.php) as previously described (30).
Statistical Analysis
Univariate ANOVA using Bonferroni post hoc test and χ2 analyses was used to compare Adipose-IR and physical and metabolic characteristics across the four groups of youth (normal weight vs. obese with NGT, vs. IGT, and vs. type 2 diabetes). ANCOVA was used to adjust for sex, race, Tanner stage, BMI, and VAT (or percent body fat). Spearman correlation analysis as a nonparametric measure was used to examine bivariate relationships between Adipose-IR and physical and metabolic characteristics. Logistic regression analysis was performed to estimate the odds ratio of Adipose-IR for the determination of dysglycemia (IGT and type 2 diabetes combined), with adjustment for sex, race, Tanner stage, BMI, and VAT. Receiver operating characteristic (ROC) curve analysis was performed to assess the predictive power of Adipose-IR for determining dysglycemia and to identify an optimal cutoff value based on the balance between sensitivity and specificity. Data that did not meet normality assumptions (BMI, HbA1c, triglyceride, HDL, VLDL, total fat mass, percent body fat, VAT, SAT, TAT, leptin, adiponectin, fasting glucose, insulin and FFA, and Adipose-IR) were log10 transformed; untransformed data are presented for ease of interpretation. Data are means ± SEM with P ≤ 0.05.
Results
Physical and Metabolic Characteristics of Normal Weight Versus Obese With NGT Versus IGT Versus Type 2 Diabetes
There were no differences in age or race among the four groups. More advanced Tanner stages and more girls were among obese youth with NGT, IGT and type 2 diabetes compared with normal-weight peers (Table 1). There were no differences in BMI percentile, total fat mass, or percent body fat among obese subjects with NGT, IGT, and type 2 diabetes. However, abdominal VAT was progressively and significantly higher from normal weight to obese and from NGT to IGT (no further increase in type 2 diabetes). Obese youth with IGT had a worse lipid profile, with significantly higher total cholesterol, triglyceride, LDL, and VLDL and lower HDL compared with normal-weight and obese youth with NGT (Table 1). Youth with type 2 diabetes had significantly higher HbA1c and fasting glucose concentrations compared with all other groups. Obese youth with NGT, IGT, and type 2 diabetes had significantly higher leptin and lower adiponectin concentrations compared with normal-weight peers. HOMA-IR increased significantly and progressively from normal weight to obese and from NGT to IGT and type 2 diabetes, with no difference between the latter two groups (Table 1). All statistical differences in lipid and metabolic panel remained significant after adjustment for sex, race, Tanner stage, BMI, and VAT.
Table 1.
Physical and metabolic characteristics in normal-weight versus obese youth with NGT versus IGT versus type 2 diabetes
| NW, n = 49 | Ob-NGT, n = 89 | Ob-IGT, n = 38 | Ob-T2D, n = 29 | PANOVA | Ppost hoc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NW vs. Ob-NGT | NW vs. Ob-IGT | NW vs. Ob-T2D | Ob-NGT vs. Ob-IGT | Ob-NGT vs. Ob-T2D | Ob-IGT vs. Ob-T2D | ||||||
| Age (years) | 14.4 ± 0.3 | 14.6 ± 0.2 | 14.9 ± 0.4 | 15.2 ± 0.3 | NS | ||||||
| Race (% AA/AW) | 53/47 | 52/48 | 32 68 | 52/48 | NS | ||||||
| Sex (% M/F) | 49/51 | 32/68 | 18/82 | 38/62 | 0.024 | ||||||
| Tanner stage (% IV/V) | 55/45 | 29/71 | 21/79 | 28/72 | 0.003 | ||||||
| Adiposity parameters | |||||||||||
| BMI (kg/m2) | 20.6 ± 0.3 | 33.9 ± 0.7 | 36.8 ± 1.0 | 36.8 ± 1.0 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.058 | NS | NS |
| BMI percentile | 59.9 ± 3.0 | 97.0 ± 0.3 | 98.7 ± 0.2 | 99.0 ± 0.1 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | NS | NS |
| FM (kg) | 11.7 ± 0.8 | 37.1 ± 1.7 | 42.8 ± 1.8 | 41.2 ± 1.9 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | NS | NS |
| FFM (kg) | 41.3 ± 1.2 | 49.2 ± 1.0 | 51.4 ± 1.7 | 54.3 ± 2.0 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | NS | NS |
| % Body fat | 21.1 ± 1.2 | 40.4 ± 1.1 | 44.0 ± 0.8 | 42.3 ± 1.3 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | NS | NS |
| VAT (cm2) | 24.5 ± 2.2 | 60.8 ± 4.1 | 85.1 ± 5.1 | 83.6 ± 7.7 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.002 | 0.012 | NS |
| SAT (cm2) | 97.8 ± 8.4 | 469.2 ± 24.1 | 559.2 ± 26.4 | 542.9 ± 25.9 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.054 | NS | NS |
| TAT (cm2) | 122.3 ± 9.9 | 527.3 ± 26.4 | 644.3 ± 29.4 | 626.8 ± 30.7 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.014 | NS | NS |
| Lipid parameters | |||||||||||
| TC (mg/dL) | 146.1 ± 4.3 | 154.5 ± 3.2 | 172.7 ± 5.4 | 155.3 ± 6.0 | <0.0001# | NS | <0.0001 | NS | 0.016 | NS | NS |
| TG (mg/dL) | 69.0 ± 4.5 | 105.7 ± 5.3 | 142.0 ± 14.4 | 133.1 ± 13.2 | <0.0001# | 0.004 | <0.0001 | <0.0001 | 0.011 | NS | NS |
| HDL (mg/dL) | 49.2 ± 1.4 | 42.0 ± 0.9 | 37.7 ± 1.4 | 38.0 ± 1.4 | <0.0001# | <0.0001 | <0.0001 | <0.0001 | 0.080 | NS | NS |
| LDL (mg/dL) | 83.2 ± 3.6 | 91.4 ± 2.8 | 107.4 ± 4.9 | 91.0 ± 5.5 | 0.001# | NS | <0.0001 | NS | 0.017 | NS | NS |
| VLDL (mg/dL) | 13.8 ± 0.9 | 21.2 ± 1.1 | 26.3 ± 2.5 | 26.6 ± 2.7 | <0.0001# | 0.002 | <0.0001 | <0.0001 | NS | NS | NS |
| Glycemic parameters | |||||||||||
| HbA1c, % [mmol/mol] | 5.24 ± 0.06 [33.83 ± 0.62] | 5.27 ± 0.04 [34.07 ± 0.50] | 5.33 ± 0.07 [34.68 ± 0.82] | 6.57 ± 0.15 [48.31 ± 1.67] | <0.0001# | NS | NS | <0.0001 | NS | <0.0001 | <0.0001 |
| FPG (mg/dL) | 95.1 ± 0.8 | 95.4 ± 0.6 | 96.8 ± 1.2 | 121.5 ± 4.8 | <0.0001# | NS | NS | <0.0001 | NS | <0.0001 | <0.0001 |
| HOMA-IR | 1.8 ± 0.1 | 3.4 ± 0.1 | 5.3 ± 0.5 | 5.5 ± 0.2 | <0.0001# | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS |
| Adipokines | |||||||||||
| Leptin (ng/mL) | 8.1 ± 0.9 | 32.8 ± 2.0 | 36.4 ± 2.8 | 32.7 ± 2.7 | <0.0001# | <0.0001 | <0.0001 | <0.0001 | NS | NS | NS |
| Adiponectin (µg/mL) | 12.9 ± 0.8 | 7.5 ± 0.5 | 6.0 ± 0.5 | 5.1 ± 0.6 | <0.0001# | <0.0001 | <0.0001 | <0.0001 | NS | 0.052 | NS |
Data are means ± SEM. VAT, SAT, and TAT data were available for 195 subjects (47 NW, 85 Ob-NGT, 36 Ob-IGT, and 27 Ob-T2D). HbA1c is missing in seven participants (three in lean and four in obese NGT). Total n for leptin is 188 (48 NW, 77 Ob-NGT, 35 Ob-IGT, and 28 Ob-T2D) and for adiponectin 182 (37 NW, 82 Ob-NGT, 36 Ob-IGT, and 27 Ob-T2D). AA, African American; AW, American white; FFM, fat-free mass; FM, fat mass; FPG, fasting plasma glucose; NW, normal weight; Ob, obese; T2D, type 2 diabetes; TC, total cholesterol; TG, triglyceride.
#P < 0.05 after adjustment for sex, race, Tanner stage, BMI, and VAT.
Adipose-IR in Youth
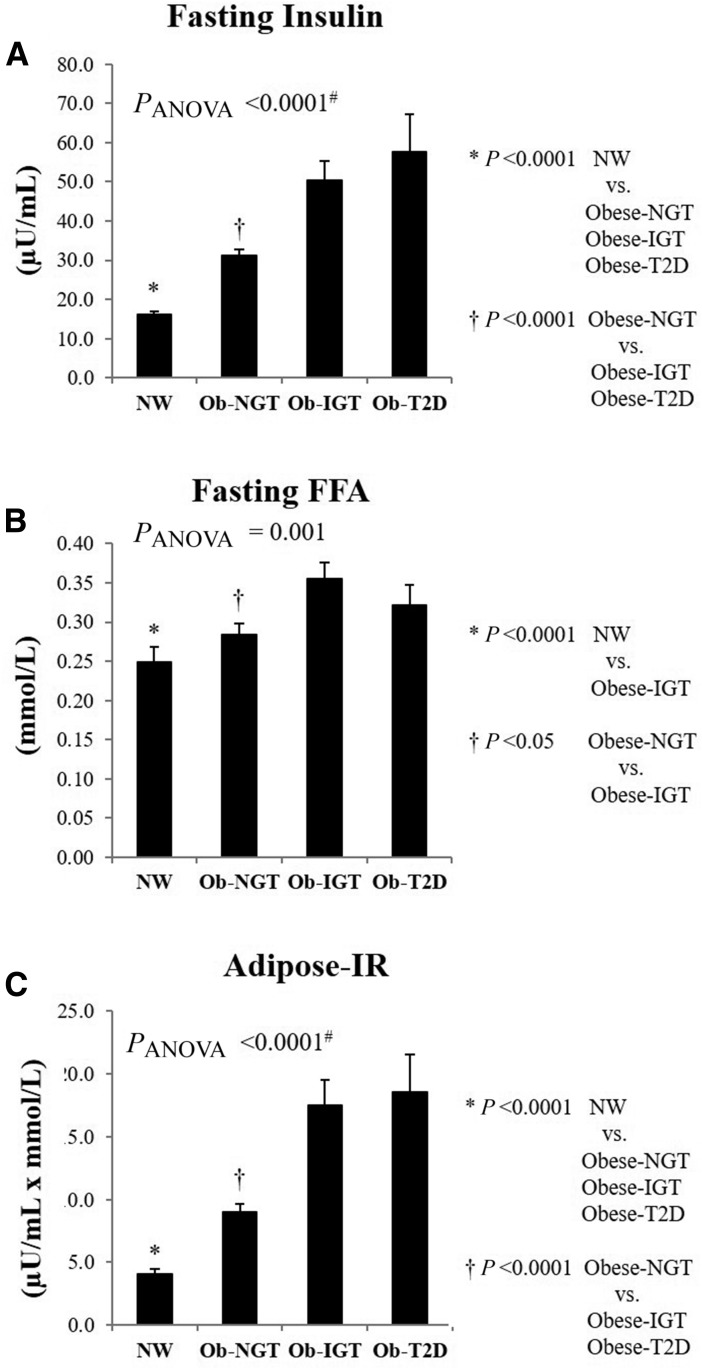
There was progressive increase in fasting insulin from normal weight to obese and from NGT to IGT and type 2 diabetes (Fig. 1A). Despite ∼3.2-fold higher fasting insulin in obese youth with IGT, fasting FFA was higher compared with normal-weight and obese youth with NGT (Fig. 1B). Consequently, Adipose-IR increased from lean to obese and from NGT to IGT to type 2 diabetes, being 2.2-fold higher in obese youth with NGT, 4.3-fold higher in IGT, and 4.6-fold higher in type 2 diabetes compared with normal weight (Fig. 1C). These differences remained significant after adjustment for VAT, sex, race, Tanner stage, and BMI. Replacement of VAT with percent body fat in the ANCOVA model did not change the significant difference in Adipose-IR across the groups. There was no difference in Adipose-IR between IGT versus type 2 diabetes before or after adjustment for the covariates. When the analyses were performed separately in each sex, there were similar patterns of progressive increases in Adipose-IR in both males and females (Supplementary Fig. 1).
Figure 1.
Fasting insulin (A), fasting FFA (B), and Adipose-IR (C) (fasting insulin × fasting FFA) in normal-weight (NW) vs. obese (Ob) subjects with NGT vs. IGT vs. type 2 diabetes (T2D). #P < 0.05 after adjustment for sex, race, Tanner stage, BMI, and VAT.
Sex-Specific Differences in Adipose-IR
In addition to the ANCOVA model with adjustment for sex among the covariates (VAT, race, Tanner stage, and BMI), we compared differences in Adipose-IR in males and females separately (Supplementary Table 1). For the purposes of this analysis, we combined youth with IGT and type 2 diabetes as a single group of “dysglycemia” because IGT and type 2 diabetes represent a spectrum of dysglycemia, with IGT being a “virgin” state of type 2 diabetes, and because there was no difference in Adipose-IR between obese youth with IGT versus type 2 diabetes (Fig. 1C). Adipose-IR progressively increased from normal weight to obese and from NGT to dysglycemia in both males and females (Supplementary Table 1). Adipose-IR did not differ significantly in normal-weight and obese NGT males and females before or after adjustment for the covariates. However, obese females versus males with dysglycemia had higher Adipose-IR before and after adjustment for VAT, race, Tanner stage, and BMI (20.28 ± 2.04 vs. 11.71 ± 2.63 μU/mL × mmol/L, PANCOVA = 0.011) (Supplementary Table 1). Replacement of VAT with percent body fat in the ANCOVA model did not change the significant difference in Adipose-IR between males and females with dysglycemia. When males and females were compared in the IGT and type 2 diabetes groups separately, Adipose-IR was 2.0-fold higher in females versus males with IGT (19.31 ± 2.28 vs. 9.51 ± 2.01 μU/mL × mmol/L, P = 0.011) and 1.7-fold higher in females versus males with type 2 diabetes (21.94 ± 3.99 vs. 13.10 ± 4.15 μU/mL × mmol/L, P = 0.103). There was no difference in treatment modalities between males and females, and adjustment for diabetes treatment between females and males did not change the results.
Relationship of Adipose-IR With Physical and Metabolic Characteristics
Adipose-IR correlated significantly and positively with BMI, total fat mass, percent body fat, abdominal adipose tissue (visceral, subcutaneous, and total), total cholesterol, triglyceride, LDL, VLDL, leptin, fasting glucose, and HOMA-IR and negatively with HDL and adiponectin (Table 2). Males and females showed similar associations between total and abdominal adiposity measures and Adipose-IR when analyzed separately (Supplementary Table 2).
Table 2.
Associations between Adipose-IR and physical and metabolic characteristics
| Variable | r | P |
|---|---|---|
| BMI | 0.559 | <0.0001 |
| Total fat mass | 0.529 | <0.0001 |
| % body fat | 0.510 | <0.0001 |
| VAT | 0.557 | <0.0001 |
| SAT | 0.576 | <0.0001 |
| TAT | 0.583 | <0.0001 |
| Total cholesterol | 0.296 | <0.0001 |
| Triglyceride | 0.461 | <0.0001 |
| HDL | −0.279 | <0.0001 |
| LDL | 0.242 | 0.001 |
| VLDL | 0.449 | <0.0001 |
| Leptin | 0.526 | <0.0001 |
| Adiponectin | −0.410 | <0.0001 |
| Fasting glucose | 0.193 | 0.006 |
| HOMA-IR | 0.812 | <0.0001 |
Logistic Regression and ROC Curve Analyses of Adipose-IR for the Prediction of Dysglycemia
For the logistic regression analysis, Adipose-IR was a significant predictor of dysglycemia (IGT and type 2 diabetes combined) independent of sex, race, Tanner stage, BMI, and VAT, with an odds ratio of 1.114 (B = 0.108 [95% CI 1.052–1.180], P < 0.0001). For a 1-unit increase in Adipose-IR, the odds of having dysglycemia increased by 11%. Additionally, the ROC curve analysis of Adipose-IR revealed an optimal cutoff value of 9.3 μU/mL × mmol/L for predicting dysglycemia (71.6% sensitivity and 71.7% specificity), with ROC area under the curve (AUC) of 0.800 (predictive power 80%). The predictive power of Adipose-IR was similar to that of fasting insulin (ROC AUC 80%) and HOMA-IR (80%) but superior to that of HbA1c (69%) and FFA (66%).
Conclusions
The current study, employing a simple surrogate estimate of Adipose-IR using fasting insulin and FFA concentrations, reveals that 1) Adipose-IR increases progressively across the span of adiposity from normal weight to overweight/obese and along the spectrum of glucose tolerance from NGT to IGT to type 2 diabetes; 2) Adipose-IR correlates directly with adiposity indices, including total body and visceral adiposity, and leptin; 3) the higher the Adipose-IR, the worse the lipid profile; 4) Adipose-IR correlates directly with fasting glucose concentration and HOMA-IR and inversely with adiponectin; 5) there is a significant sex difference in Adipose-IR in youth with dysglycemia, being worse in females; and 6) Adipose-IR is a significant predictor of dysglycemia with an odds ratio of 1.114 and an optimal cutoff value of 9.3 μU/mL × mmol/L. Such observations would justify the use of Adipose-IR to assess adipose tissue insulin sensitivity (a pathophysiological component of type 2 diabetes) in large-scale epidemiological/observational studies of the natural history of youth-onset type 2 diabetes and in prevention/intervention studies of the effectiveness of any intervention in modifying the course of adipose tissue insulin sensitivity in high-risk youth.
Our cross-sectional observation of significant increases in Adipose-IR in obese youth with NGT, IGT, and type 2 diabetes compared with that in normal-weight youth supplements the adult literature (13–15), in addition to confirming and advancing the very limited pediatric literature (16,17). Hershkop et al. (17) described in their multiethnic cohort of 962 obese children and adolescents that Adipose-IR increased from NGT to prediabetes to type 2 diabetes (17) and was positively associated with adiposity indices and negatively with adiponectin—consistent with our findings. Unlike ours, their study included prepubertal youth in whom Adipose-IR was lower compared with pubertal youth. Further, their OGTT data demonstrated that among the participants with NGT there was an increase in Adipose-IR related to an increase in 2-h glucose levels. This may have been a result of the inverse relationship of Adipose-IR with the oral disposition index, implying that with declining β-cell function and increasing 2-h glucose concentrations even in the normal range, Adipose-IR deteriorates (17). They also showed that Adipose-IR is a strong determinant of FFA AUC during the OGTT. Even though the two studies show a similar trend of an increase in Adipose-IR from NGT to prediabetes to type 2 diabetes, the magnitude of the increase is different between the two studies: ∼50% in the former and ∼94% in ours. This could be due to inclusion of only IGT in our cohort versus IFG and IGT in the former, especially given that it is not known whether the alteration in Adipose-IR is as severe in IFG as it is in IGT. However, the observation by Hershkop et al. (17) that, in participants with NGT, the increase in Adipose-IR related to an increase in 2-h glucose concentration would suggest that Adipose-IR may be worse in IGT than IFG. The same would be construed from their finding of lower FFA suppression during the OGTT with increasing 2-h glucose concentrations (17). Our study did not include OGTT. Pediatric as well as adult studies are needed to examine adipose tissue insulin sensitivity in IFG separate from IGT to demonstrate whether adipose tissue insulin resistance is worse in IGT compared with IFG.
Besides confirming the sparse pediatric literature, the current study advances it in a few relevant ways, including the examination of Adipose-IR in normal-weight youth, demonstration of sex differences in Adipose-IR, and the ROC curve analyses of Adipose-IR for prediction of dysglycemia. Our finding that Adipose-IR is 2.2-fold higher in obese youth with NGT versus normal-weight peers suggests that an impairment of adipose tissue insulin sensitivity is already present in obese youth with normoglycemia. This observation is concordant with adult data of twofold higher Adipose-IR in obese NGT versus lean NGT (15) but inconsistent with a previous pediatric study showing no difference in baseline FFA concentrations and no difference in % FFA suppression during a hyperinsulinemic-euglycemic clamp between lean versus obese youth (16). This discrepancy might be due to different sample sizes for lean and obese groups between the two studies, potential heterogeneity of the obese group with respect to glucose tolerance in the latter study, or different measurements of adipose insulin resistance. The presence of impaired adipose tissue insulin sensitivity in obese youth with NGT, before any alteration in glucose homeostasis, is concordant with our previous research demonstrating that abnormalities in insulin sensitivity of glucose metabolism and β-cell dysfunction are also present in obese youth with NGT (19,20).
Contrary to the significant increase in Adipose-IR from NGT to IGT in the current study and that reported previously in pediatrics (17), obese adults with NGT versus IGT had similar Adipose-IR (NGT 8.0 ± 1.1 μU/mL × mmol/L vs. IGT 9.2 ± 0.7 μU/mL × mmol/L) (15). Such differing findings in youth and adults suggest a distinct youth-adult contrast with respect to insulin resistance associated with lipid metabolism. This youth-adult contrast could be interpreted from the observation that obese youth with IGT have ∼75–80% higher or worse Adipose-IR compared with that reported in adults (15). Although this is not a head-to-head comparison of Adipose-IR between youth and adults, our present finding of severe adipocyte insulin resistance in obese youth with IGT is consistent with our recent findings of 50% lower hepatic and peripheral insulin sensitivity with respect to glucose metabolism in obese adolescents versus equally obese adults with IGT (31). Similar observations were made in the Restoring Insulin Secretion (RISE) study demonstrating remarkable insulin resistance of glucose metabolism in obese youth with IGT and recent-onset type 2 diabetes in comparison with equally obese adults with IGT or recent-onset type 2 diabetes (32,33). Analysis of RISE banked plasma samples for FFA concentrations may allow for direct comparison between youth and adults with respect to adipose tissue insulin sensitivity, shedding light on the severity of adipose tissue insulin resistance in youth and potentially providing an explanation for why the disease appears decades earlier in youth.
The sex-specific analysis of Adipose-IR in the current data reveals that obese females versus males with dysglycemia (IGT and type 2 diabetes) have ∼73% higher Adipose-IR, which is in line with the lower insulin sensitivity of glucose metabolism (measured by hyperinsulinemic-euglycemic clamp) in girls compared with boys of similar BMI (34). It would be critical to examine whether the more severe Adipose-IR in females has any pathophysiological implications with respect to sex-related therapeutic response. In the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) trial, metformin plus rosiglitazone was significantly more effective in girls than boys in maintaining glycemic control (HbA1c <8%) (35). Moreover, among girls, metformin plus rosiglitazone was significantly more effective than metformin alone and metformin plus lifestyle, whereas in boys, metformin plus rosiglitazone was not more effective than either metformin alone or metformin plus lifestyle. It could be speculated that obese girls with type 2 diabetes and severe Adipose-IR require more potent insulin sensitizers, such as rosiglitazone, to improve their global insulin resistance and improve both glucose and lipid metabolism compared with boys.
Data are very limited with respect to the predictive ability of Adipose-IR (14) in contrast to the widely used diabetes risk indicators, such as HbA1c, fasting and 2-h OGTT glucose concentrations, and mathematical indices of insulin sensitivity, for determining prediabetes and type 2 diabetes in adults and youth (36,37). Our ROC curve analysis together with logistic regression provides novel data in the pediatric literature and reveals a cutoff value of 9.3 μU/mL × mmol/L for Adipose-IR with a dysglycemia predictive power of 80%. In our cohort, HOMA-IR is similarly predictive of dysglycemia (ROC AUC 80%). This could be due to using fasting insulin in calculating both HOMA-IR and Adipose-IR. On the other hand, the predictive power of HbA1c (ROC AUC 69%) or FFA (ROC AUC 66%) is much less than that of either Adipose-IR or HOMA-IR. Our logistic analysis further confirms that Adipose-IR is a significant predictor of dysglycemia independent of sex, race, Tanner stage, BMI, and VAT. Longitudinal studies of large cohorts are needed to examine the validity of the predictive power of Adipose-IR in progression to IGT or type 2 diabetes in high-risk youth with NGT.
The strengths of the present investigation include 1) the use of a simple surrogate index of Adipose-IR, using fasting insulin and FFA concentrations, having verified it against adipose tissue insulin sensitivity measured with [2H5]glycerol tracer in our subcohort (5); 2) a thorough characterization of Adipose-IR on a wide spectrum of weight status and glucose tolerance, from normal weight to obese and from NGT to IGT to type 2 diabetes, in a pediatric population; 3) a first-time evaluation of the predictive power of Adipose-IR for determining dysglycemia in youth; 4) the balanced representation of white and black youth; and 5) the novel observation of sex-related differences in Adipose-IR. Potential perceived limitations would be that we included youth with type 2 diabetes who were on different treatment modalities (i.e., metformin, insulin, metformin plus insulin, or lifestyle modification). This could potentially act as a significant confounder modulating lipolysis (38). However, this diversity in therapy is inevitable because once a youth is diagnosed with type 2 diabetes, treatment is initiated, and in a clinical setting therapeutic approaches are dictated by the severity of hyperglycemia and provider choices. As to the relatively modest correlation (r = −0.605, P < 0.0001) between Adipose-IR and adipose tissue insulin sensitivity calculated from whole-body lipolysis measured by glycerol tracer (5), the correlation may have been stronger if glycerol turnover data were available in all 205 participants of the present cohort instead of the 138 subjects in the prior publication. Furthermore, glycerol turnover measurement with stable isotope methodology is a much more sensitive measure of whole-body lipolysis than FFA concentrations because FFA is reesterified within adipose tissue while glycerol is not and FFA oxidation and clearance can vary depending on sex, obesity, and glycemic status (10). It would also be important to examine alterations in FFA dynamics/suppression during the OGTT besides a fasting-based index. Lastly, our suggested cutoff value of Adipose-IR for defining dysglycemia may not apply to all children and adolescents. Large pediatric cohorts with a variety of weight status and with diverse ethnic backgrounds should be investigated to examine the validity of Adipose-IR for predicting prediabetes and type 2 diabetes. Finally, the cross-sectional nature of our evaluation does not allow us to examine causal relationships between adipose tissue insulin resistance and other significant pathogenic factors of type 2 diabetes.
In summary, Adipose-IR is a simple surrogate index that can be used in large-scale pediatric epidemiological studies where the applicability of tracer studies is limited owing to feasibility, cost, and labor intensiveness. It provides an easy approach to investigate adipose tissue insulin sensitivity, a key pathophysiological component of type 2 diabetes, and it can be used repeatedly in longitudinal studies of the natural history of type 2 diabetes or in therapeutic intervention/prevention trials targeting reversing the trajectory of youth-onset type 2 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the children who participated in this study and their parents, Nancy Guerra for assistance and Resa Stauffer for laboratory expertise at the UPMC Children’s Hospital of Pittsburgh, and the nursing staff of the Pediatric Clinical and Translational Research Center for outstanding care of the participants and meticulous attention to the research.
Funding. This study was supported by National Institute of Child Health and Human Development grants K24-HD01357 and R01-HD27503 (to S.A.), National Center for Advancing Translational Sciences Clinical and Translational Science Award UL1TR000005, and National Center for Research Resources grant UL1RR024153 to the General Clinical Research Center.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.Y.K. and S.A. designed the study, analyzed data, and wrote the manuscript. F.B. and H.T. contributed data and reviewed the manuscript. S.F.M. collected and maintained the database and reviewed the manuscript. S.Y. helped with data analysis and reviewed the manuscript. J.Y.K., F.B., H.T., S.F.M., S.Y., and S.A. approved the manuscript in its final version. S.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1178/-/DC1.
References
- 1.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia 2002;45:1201–1210 [DOI] [PubMed] [Google Scholar]
- 2.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548–2556 [DOI] [PubMed] [Google Scholar]
- 3.Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 1989;84:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeFronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JY, Nasr A, Tfayli H, Bacha F, Michaliszyn SF, Arslanian S. Increased lipolysis, diminished adipose tissue insulin sensitivity, and impaired β-cell function relative to adipose tissue insulin sensitivity in obese youth with impaired glucose tolerance. Diabetes 2017;66:3085–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefan N, Wahl HG, Fritsche A, Häring H, Stumvoll M. Effect of the pattern of elevated free fatty acids on insulin sensitivity and insulin secretion in healthy humans. Horm Metab Res 2001;33:432–438 [DOI] [PubMed] [Google Scholar]
- 7.Giacca A, Xiao C, Oprescu AI, Carpentier AC, Lewis GF. Lipid-induced pancreatic β-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab 2011;300:E255–E262 [DOI] [PubMed] [Google Scholar]
- 8.Hughan KS, Bonadonna RC, Lee S, Michaliszyn SF, Arslanian SA. β-Cell lipotoxicity after an overnight intravenous lipid challenge and free fatty acid elevation in African American versus American white overweight/obese adolescents. J Clin Endocrinol Metab 2013;98:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michaliszyn SF, Bonadonna RC, Sjaarda LA, Lee S, Farchoukh L, Arslanian SA. β-Cell lipotoxicity in response to free fatty acid elevation in prepubertal youth: African American versus Caucasian contrast. Diabetes 2013;62:2917–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Søndergaard E, Jensen MD. Quantification of adipose tissue insulin sensitivity. J Investig Med 2016;64:989–991 [DOI] [PubMed] [Google Scholar]
- 11.Søndergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab 2017;102:1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ter Horst KW, van Galen KA, Gilijamse PW, et al. Methods for quantifying adipose tissue insulin resistance in overweight/obese humans. Int J Obes 2017;41:1288–1294 [DOI] [PubMed] [Google Scholar]
- 13.Abdul-Ghani MA, Molina-Carrion M, Jani R, Jenkinson C, Defronzo RA. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the anti-lipolytic effect of insulin. Acta Diabetol 2008;45:147–150 [DOI] [PubMed] [Google Scholar]
- 14.Barchetta I, Angelico F, Del Ben M, et al. Phenotypical heterogeneity linked to adipose tissue dysfunction in patients with Type 2 diabetes. Clin Sci (Lond) 2016;130:1753–1762 [DOI] [PubMed] [Google Scholar]
- 15.Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the San Antonio Metabolism Study. Diabetes 2017;66:815–822 [DOI] [PubMed] [Google Scholar]
- 16.Kelsey MM, Forster JE, Van Pelt RE, Reusch JE, Nadeau KJ. Adipose tissue insulin resistance in adolescents with and without type 2 diabetes. Pediatr Obes 2014;9:373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hershkop K, Besor O, Santoro N, Pierpont B, Caprio S, Weiss R. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J Clin Endocrinol Metab 2016;101:2423–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care 2009;32:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tfayli H, Lee S, Arslanian S. Declining β-cell function relative to insulin sensitivity with increasing fasting glucose levels in the nondiabetic range in children. Diabetes Care 2010;33:2024–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. Declining β-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care 2011;34:2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tfayli H, Bacha F, Gungor N, Arslanian S. Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet cell antibody-negative versus -positive patients. Diabetes 2009;58:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tfayli H, Bacha F, Gungor N, Arslanian S. Islet cell antibody-positive versus -negative phenotypic type 2 diabetes in youth: does the oral glucose tolerance test distinguish between the two? Diabetes Care 2010;33:632–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjaarda L, Lee S, Tfayli H, Bacha F, Bertolet M, Arslanian S. Measuring β-cell function relative to insulin sensitivity in youth: does the hyperglycemic clamp suffice? Diabetes Care 2013;36:1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 26.Tanner JM. Growth and maturation during adolescence. Nutr Rev 1981;39:43–55 [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity (Silver Spring) 2008;16:1066–1071 [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Kuk JL, Kim Y, Arslanian SA. Measurement site of visceral adipose tissue and prediction of metabolic syndrome in youth. Pediatr Diabetes 2011;12:250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klopfenstein BJ, Kim MS, Krisky CM, Szumowski J, Rooney WD, Purnell JQ. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br J Radiol 2012;85:e826–e830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab 2011;96:2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arslanian S, Kim JY, Nasr A, et al. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: who is worse off? Pediatr Diabetes 2018;19:205–211 [DOI] [PubMed] [Google Scholar]
- 32.RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care 2018;41:1707–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran A, Jacobs DR Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 1999;48:2039–2044 [DOI] [PubMed] [Google Scholar]
- 35.Zeitler P, Hirst K, Pyle L, et al.; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care 2007;30:1544–1548 [DOI] [PubMed] [Google Scholar]
- 37.Kim JY, Goran MI, Toledo-Corral CM, Weigensberg MJ, Shaibi GQ. Comparing glycemic indicators of prediabetes: a prospective study of obese Latino youth. Pediatr Diabetes 2015;16:640–643 [DOI] [PubMed] [Google Scholar]
- 38.McTernan PG, Harte AL, Anderson LA, et al. Insulin and rosiglitazone regulation of lipolysis and lipogenesis in human adipose tissue in vitro. Diabetes 2002;51:1493–1498 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.