Abstract
The tumor microenvironment is closely associated with tumor malignancy, and includes tumor relapse and metastasis trigged by epithelial-mesenchymal transition (EMT), which leads to the expansion of cancer stem-like cells. Radiotherapy is known to acutely and persistently affect changes in this tumor microenvironment by altering the vascular functions of tumor endothelial cells. However, the precise role of endothelial cells in tumor malignancy following treatment with irradiation has not been completely elucidated. The present study investigated the differences in malignant behavior of liver cancer cells in response to irradiated endothelial cells. To achieve this, a co-cultivation system was established to identify the potential role of endothelial cells in malignant liver cancer cells using medium conditioned with endothelial cells. It was observed that the medium conditioned by endothelial cells when irradiated with a single dose (2 Gy), greatly increased the migratory and invasive properties of liver cancer cells, as well as inducing mesenchymal markers, and enhancing the sphere-forming ability of liver cancer cells, The mRNA levels of genes regulating the self-renewal of cancer stem cells were increased in liver cancer cells by treatment with medium conditioned with endothelial cells. However, neither the medium conditioned by endothelial cells irradiated with fractionated doses (2 Gy × 3; 2 Gy/day for 3 days) or with a single dose (6 Gy) greatly influenced the malignancy of liver cancer cells. In conclusion, the data obtained by the present study indicated that 2 Gy irradiation of endothelial cells influenced the increase in tumor malignancy in liver cancer cells. Furthermore, the distinct differences in the indirect effects of ionizing radiation on tumor malignancy may provide valuable information for the improvement in the efficacy of radiotherapy.
Keywords: ionizing radiation, endothelial cells, epithelial-mesenchymal transition, migration, invasion, cancer stem-like cells
Introduction
Radiotherapy is well known to be used for the treatment of about half of cancer patients (1–3). However, radioresistant cancer cells and the tumor recurrence after radiotherapy have been recognized as serious impediments to the long-term survival of cancer patients (4,5). Therefore, to overcome these problems, the biological effects of ionizing radiation on human tumors have been flourishingly and diversely investigated up to date. Furthermore, these problems are closely concerned with tumor microenvironments comprised of fibroblasts, a variety of inflammatory cells, perivascular cells and endothelial cells, as well as hypoxic conditions (3). In particular, tumor microenvironments have been not only shown to positively regulate the tumor malignancy, but also to greatly influence the radiosensitivity of cancer cells (3,6). In addition, under tumor microenvironment, the epithelial-mesenchymal transition (EMT) has been well shown to play a critical role in the tumor progression via triggering the motile and invasive activities of cancer cells to infiltrate into lymph or blood vascular systems (6,7). During the process of the EMT, the protein levels of epithelial markers are decreased, but that of mesenchymal markers are increased (8,9). The alternations in these marker proteins lead to the loss of cell-cell interaction, thereby allowing extravasation of cancer cells from the primary tumors (6,8,9). Thus, EMT can be easily found in the invasive regions of aggressive cancers (10,11). Furthermore, many reports indicate that EMT is regarded as a key mechanism to lead to the generation of cancer stem-like cells and the resistance of anti-cancer drugs (12–14).
Importantly, endothelial cells have been suggested to play a pivotal role in the tumor microenvironment as a major component of the tumor vascular system (15,16). Furthermore, these cells under tumor microenvironment have been known to affect cancer stem-like cells through the networks of growth factors and cytokines (15), thereby directly regulating the self-renewal of them (17,18). However, the effects of irradiated endothelial cells on tumor malignancy and cancer stem-like cells have not been fully clarified.
In this study, we investigated the differences in malignant behavior of liver cancer cells in response to irradiated endothelial cells. We found that 2 Gy irradiation of endothelial cells enhances EMT of liver cancer cells, and increases the self-renewal of cancer stem-like cells, whereas both 6 Gy- and fractionated irradiation (2 Gy × 3 days) do not greatly affect these events. Our observation also revealed that endothelial cells play a key role in modulating the malignancy of liver cancer cells in response to irradiation.
Materials and methods
Cell culture
The human liver cancer HepG2, Hep3B and Huh7 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). These liver cancer cell lines were maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with 10% (v/v) bovine calf serum, penicillin (50 U/ml), and streptomycin (50 µg/ml) (all from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Human umbilical vein endothelial cells (HUVECs) were purchased from ScienCell Research Laboratories, Inc. (Carlsbad, CA, USA). HUVECs plated on gelatin-coated 60-mm dishes were cultured in complete endothelial cell culture medium (ECM; ScienCell Research Laboratories, Inc.) supplemented with 5% fetal bovine serum, 1% antibiotics and 1% endothelial cell growth supplement in a humidified 5% CO2 incubator at 37°C. These cells from passages 2 to 5 were used for experiments.
Irradiation
HUVECs were exposed to γ-rays with a 137Cs irradiation source (Eckert & Ziegler, Berlin, Germany) at a dose rate of 2.6 Gy/min.
Preparation of conditioned medium
After the medium for endothelial cells was freshly replaced with new medium, HUVECs were irradiated with various doses of γ-rays, and then cultured for 24 h. Shortly afterward, the conditioned medium was harvested, filtered with 0.45-µm filter or centrifuged for 5 min at 700 × g to remove cells and debris and then transferred to tubes.
Cell growth curve
The HepG2 cells were plated onto 60-mm dishes at a density of 2×105 cells/dish, and then treated with the medium conditioned by HUVECs. At 24, 48 and 72 h after the treatment, the cells were trypsinized, washed with PBS, and then the number of HepG2 cells not stained with trypan blue were counted with a hemocytometer.
Clonogenic cell survival assay
Appropriate numbers of HepG2 cells were plated onto 60-mm dishes, incubated overnight, treated with the medium conditioned by HUVECs irradiated with various doses of γ-ray and then cultured for 14 days in a 5% CO2 incubator at 37°C. The colonies were fixed with 95% methanol and stained with 0.5% crystal violet. The colonies containing more than 60 cells were counted. The average of triplicate dishes was calculated for each sample. The results were normalized according to the plating efficiencies of the corresponding HepG2 cells treated with the medium conditioned by non-irradiated HUVECs, and then the surviving fractions were calculated.
Western blot analysis
Mouse monoclonal antibodies against Vimentin (cat. no. sc-6260; dilution 1:1,000; incubation time, 1 h at room temperature), E-cadherin (cat. no. sc-8426; dilution 1:1,000; incubation time, 1 h at room temperature) and Zeb1 (cat. no. sc-81428; dilution 1:1,000; incubation time, 1 h at room temperature) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). A mouse monoclonal antibody against N-cadherin (cat. no. 610920; dilution 1:1,000; incubation time, 1 h at room temperature) was purchased from BD Bioscience (San Jose, CA, USA). Rabbit monoclonal antibodies against Slug (cat. no. 9585; dilution 1:1,000; incubation time, 1 h at room temperature) and Snail (cat. no. 3879; dilution 1:1,000; incubation time, 1 h at room temperature) were purchased from Cell Signaling Technology, Inc. (Denver, MA, USA). Mouse monoclonal anti-β-actin (cat. no. A5441; dilution 1:1,000; incubation time, 1 h at room temperature), horseradish peroxidase-conjugated anti-mouse IgG (cat. no. A9044; dilution 1:10,000; incubation time, 1 h at room temperature) and horseradish peroxidase-conjugated anti-rabbit IgG (cat. no. A0545; dilution 1:10,000; incubation time, 1 h at room temperature) antibodies were purchased from Sigma-Aldrich; Merck KGaA. Cells were treated with lysis buffer [40 mM Tris-HCl (pH 8.0), 120 mM NaCl, and 0.1% (v/v) NP40] supplemented with protease inhibitor cocktail tablets (1 tablet/50 ml; Boehringer, Mannheim, Germany), and centrifuged for 15 min at 12,000 × g at 4°C. 30 µg of proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were blocked with 5% (w/v) nonfat dry milk in Tris-buffered saline and then incubated for 1 h with primary antibodies at the dilution of 1:1,000, at room temperature. Specific reaction bands were detected using peroxidase-conjugated secondary antibodies which were at the dilution of 1:10,000, and proteins were visualized using an enhanced chemiluminescence system (Amersham Biosciences, Piscataway, NJ, USA).
cDNA preparation and RT2 Profiler PCR Array
Total RNA was extracted from the cells using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and 1 µg of the isolated total RNAs was reverse transcribed to complementary DNA (cDNA) with the SuperScript First-Strand Synthesis System (Gibco; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. The reaction was performed at 25°C for 5 min and then at 42°C for 30 min, and was terminated at 85°C for 5 min. Next, the cDNA was diluted to 100 µl, and then was used as a template for RT2 Profiler PCR Array Human cancer stem cells (cat. no. 330231 PAHS-176ZA; Qiagen, Valencia, CA, USA). The information of transcripts is shown on the instruction manual of RT2 Profiler PCR Array supplied by the manufacturer. The transcripts were analyzed using a CFX96™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). PCR amplification was carried out using a thermal profile of beginning at 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec, and last extension at 60°C for 1 min. Analysis for the PCR array was performed with a web-based software (http://dataanalysis.sabiosciences.com/pcr/arrayanalysis.php) supplied by the manufacturer. The levels of gene expression were normalized to GAPDH, a housekeeping gene, and shown as the fold change compared with control.
Migration and invasion assays
Both migration and invasion assays were performed using the Transwell chamber (8-µm pore size; BD Biosciences). In total, 2×104 cells were resuspended in serum-free growth medium for these assays. For the invasion assay, the interior of the inserts was precoated with 10 mg/ml growth factor-reduced Matrigel (BD Biosciences). For both assays, the cells were added to the interior of the inserts. Growth medium supplemented with 10% (v/v) fetal bovine serum was added to the lower chamber. After incubation for 24 h, the cells attached on the upper surface of the filter were removed with a cotton swab. The cells on the lower surface of the filter were fixed and stained. The number of cells was determined by counting cells in five microscopic fields per well. In addition, the cells were imaged by phase contrast microscopy (Nikon Eclipse 80i; Nikon, Tokyo, Japan).
Sphere forming assay
Cells were grown in serum-free DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with B27 (Gibco; Thermo Fisher Scientific, Inc.), N2 (Gibco; Thermo Fisher Scientific, Inc.), 20 ng/ml basic fibroblast (Peprotech, London, UK) and 20 ng/ml epidermal growth factor (Peprotech) onto 24-well ultra-low attachment plates at 300 cells per well for 7 or 14 days, and then the size and number of spheres were determined using a phase-contrast Nikon microscope (TS100; Nikon). To measure the size of sphere, 12 spheres per group were randomly selected.
Statistical analyses
All data presented as the mean ± standard error of the mean and are representative of at least three independent experimental repeats. Statistical analysis were performed with SPSS ver.18.0 (SPSS, Inc., Chicago, IL, USA). Differences between groups were analyzed using an unpaired Student's t-test and one-way analysis of variance (ANOVA) or two-way ANOVA, followed by Dunnett's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Endothelial cells enhance the malignancy of liver cancer cells
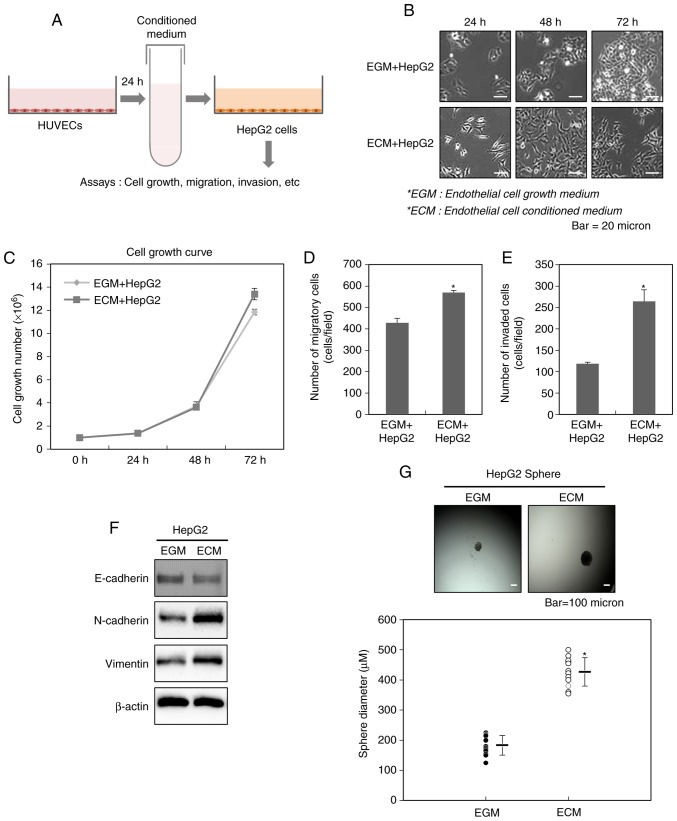
Endothelial cells under tumor microenvironments have been known to play a key role in the survival, growth and malignancy of cancer cells, as well as participation in the formation of tumor blood vessels (15,16). Therefore, we investigated whether endothelial cells affect the malignancy of tumor cells. To examine these effects in vitro, we used the endothelial cell conditioned medium (ECM) obtained from 24 h culture of HUVECs. At a ratio of 1:1, ECM was evenly mixed with DMEM, and then was subsequently treated to HepG2 cells (Fig. 1A).
Figure 1.
Endothelial cells increase the malignant potential of liver cancer cells. (A) A schematic illustration showing the procedure for the acquisition of the conditioned medium obtained from HUVECs and the applications in this study. (B) Morphological changes of HepG2 cells after the treatment with ECM. (C) Effect of ECM on the growth of HepG2 cells. After HepG2 cells were treated with ECM, the cell counting was performed at 24, 48 and 72 h. Increased (D) migration and (E) invasion of HepG2 cells after the treatment with ECM. The migratory and invasive properties of the cells were measured using the Transwell chamber. (F) Western blot analysis for the expressions of N-cadherin and vimentin after the treatment with ECM. Experiments were performed in triplicate, and the data shown are representative of a typical experiment. (G) Quantification of sphere-forming abilities of HepG2 cells after the treatment with ECM. The cells were grown in DMEM/F12 supplemented with B27, N2, basic fibroblast- and epidermal growth factor onto 24-well ultra-low attachment plates at 300 cells per well for 7 days, and the size of spheres were determined. To measure the size of sphere, 12 spheres per group (n=12/group) were randomly selected. The average size of each sphere is quantified in the standard deviation and shown in the representative graph. Results from three independent experiments are expressed as mean ± 1 SEM (*P<0.05). HUVECs, human umbilical vein endothelial cells; ECM, endothelial cell culture medium.
We first observed the changes in the cellular morphology of HepG2 cells after the treatment with ECM obtained from HUVECs. As shown in previous reports mentioning these changes observed during the process of EMT (19–21), Fig. 1B also showed that the treatment with ECM for 24, 48 and 72 h leads to the appearance of a fibroblast-like shape emerging as elongated and dispersed HepG2 cells, compared with control. This result indicates that endothelial cells may contribute to the acquisition of mesenchymal traits of liver cancer cells.
We next examined the effect of ECM on the cellular growth of HepG2 cells. As shown in Fig. 1C, the growth rate of HepG2 cells did not be greatly altered by the treatment with ECM.
The acquisition of mesenchymal traits of cancer cells leading to the malignant properties of them is closely related with the process of EMT (9,12–14). We therefore investigated whether ECM obtained from HUVECs affects EMT of HepG2 cells. Fig. 1D and E revealed that HepG2 cells treated with ECM show more migratory and invasive properties, compared with control. Consistent with these results, we also found an increase in the expression levels of the mesenchymal markers N-cadherin and vimentin in HepG2 cells treated with ECM, compared with control (Fig. 1F).
EMT has been well known to be closely associated to an increase in the cancer stem-like cell population (9,12–14). Thus, we examined whether ECM obtained from HUVECs can also change the numbers of cancer stem-like cells. To fulfill this purpose, we performed sphere forming assay after the treatment of HepG2 cells with ECM. As shown in Fig. 1G, the cells shows the larger spheres, compared with control, indicating that endothelial cells can assign the higher sphere-forming ability to liver cancer cells.
These data collectively suggest that endothelial cells play a key role in inducing EMT of liver cancer cells and expanding cancer stem-like cells.
Effect of irradiated endothelial cells on the cell survival of liver cancer cells
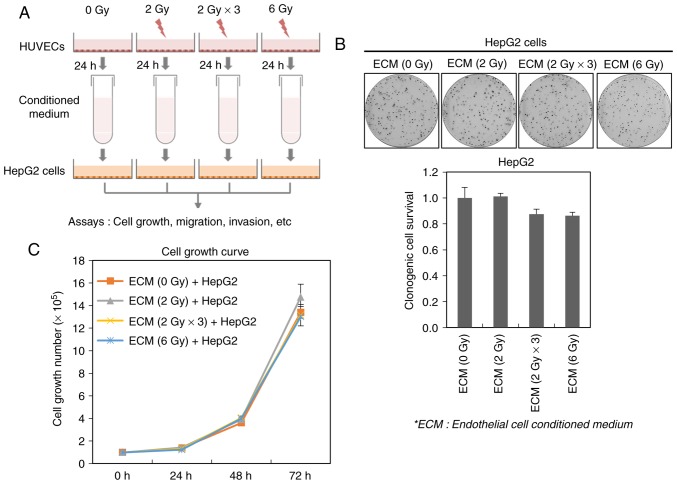
Ionizing radiation influence tumor microenvironment (22,23). Especially, the tumor vascular system composing tumor microenvironment has been reported to be highly sensitive to irradiation (23,24). Thus, we investigated whether irradiated endothelial cells can affect the viability of cancer cells. To fulfill this purpose, we acquired ECM obtained from HUVECs exposed to a single dose (2 or 6 Gy) or fractionated dose (2 Gy × 3; 2 Gy/day for 3 days) of ionizing radiation, and then treated it to HepG2 cells (Fig. 2A). Although either the fractionated dose-irradiated (2 Gy × 3)- or 6 Gy-irradiated ECM slightly suppressed the clonogenic survival of HepG2 cells, their survival was not greatly affected by all irradiated ECMs, indicating that irradiated endothelial cells do not seem to highly influence the survival of cancer cells (Fig. 2B). In addition, we further found that all irradiated ECMs do not modulate the growth rate of HepG2 cells (Fig. 2C).
Figure 2.
Effects of irradiated endothelial cells on the survival and growth of liver cancer cells. (A) A schematic illustration illustrating the procedure for the acquisition of the conditioned medium obtained from irradiated HUVECs and the applications in this study. (B) Effect of ECM obtained from irradiated HUVECs on the clonogenic survival of HepG2 cells. HUVECs were irradiated with a single dose (2 or 6 Gy) or fractionated dose (2 Gy × 3; 2 Gy/day for 3 days) of ionizing radiation. HepG2 cells were treated with ECM obtained from these irradiated HUVECs, cultured for 14 days, stained with 0.5% crystal violet, and the number of colonies was counted. (C) HUVECs were irradiated with a single dose (2 or 6 Gy) or fractionated dose (2 Gy × 3; 2 Gy/day for 3 days) of ionizing radiation. HepG2 cells were treated with ECM obtained from these irradiated HUVECs, cultured for 24, 48 and 72 h. The cell counting was performed at each time point. HUVECs, human umbilical vein endothelial cells; ECM, endothelial cell culture medium.
These data suggest that irradiated endothelial cells do not affect the cell survival and growth of liver cancer cells.
Irradiated endothelial cells modulates tumor malignancy of liver cancer cells
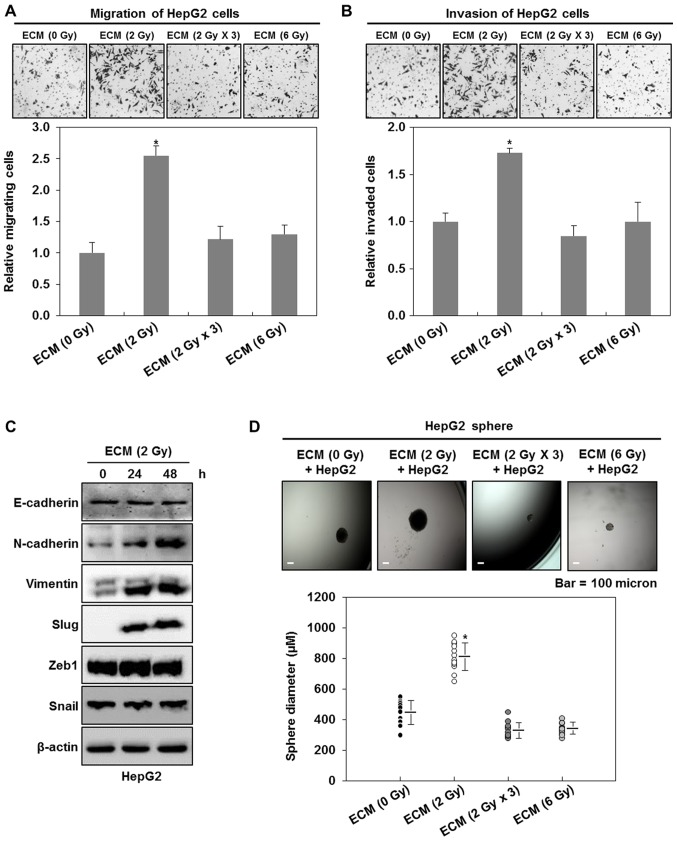
To further investigate the connection between irradiated endothelial cells and liver cancer cells in the tumor malignancy, we first measured the migratory and invasive abilities of HepG2 cells after the treatments with ECM obtained from irradiated HUVECs. As shown in Fig. 3A and B, we found that 2 Gy-irradiated ECM greatly increases the migratory and invasive traits of HepG2 cells, compared with the treatment with ECM obtained from non-irradiated endothelial cells, whereas 6 Gy- or 2 Gy × 3 (2 Gy/day for 3 days) irradiated ECM does hardly affect these events. Consistent with the above results, we further observed that 2 Gy-irradiated ECM greatly increase the expression levels of the mesenchymal cell markers N-cadherin and vimentin in HepG2 cells, whereas the expression of the epithelial cell marker, E-cadherin is not greatly modulated (Fig. 3C). In addition, we investigated whether 2 Gy-irradiated ECM affects the expression levels of EMT-regulating transcription factors, Slug, Zeb1 and Snail in HepG2 cells. Although the expression levels of Zeb1 and Snail did not be change, an increase in the expression levels of Slug was observed in HepG2 cells (Fig. 3C).
Figure 3.
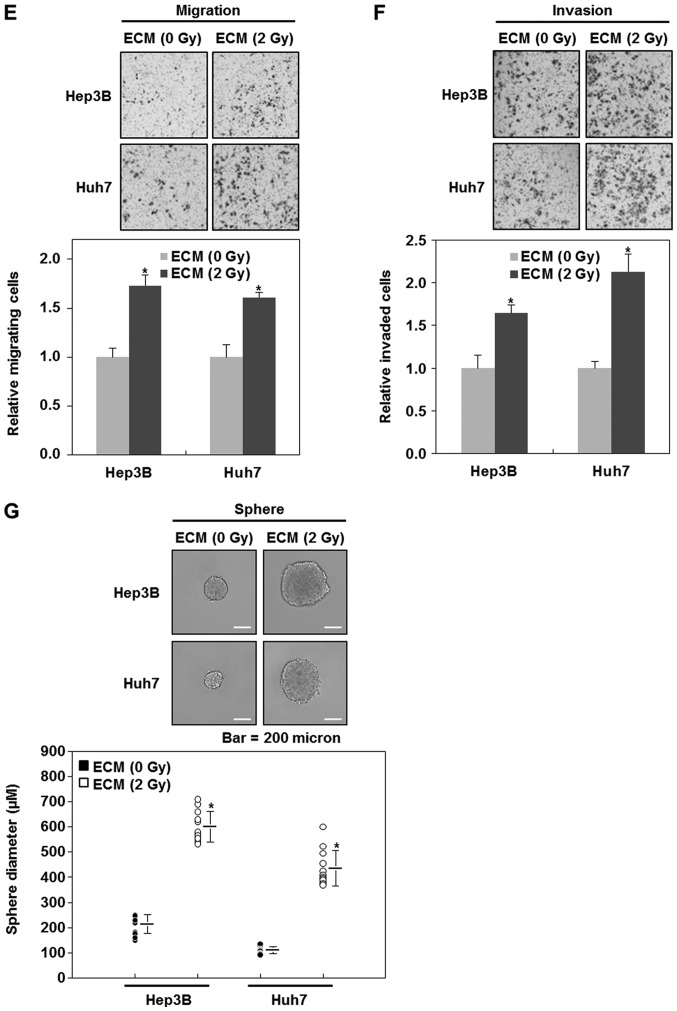
2 Gy-irradiated endothelial cells enhance the malignant potential of liver cancer cells. (A) Migratory and (B) invasive properties of HepG2 cells after the treatment with ECM obtained from irradiated HUVECs. These properties of the cells were measured using the Transwell chamber (×200 magnification). (C) Western blot analysis for the expressions of E-cadherin, N-cadherin, vimentin, slug, zeb1 and snail after the treatment with ECM. Experiments were performed in triplicate, and the data shown are representative of a typical experiment. (D) Quantification of sphere-forming abilities of HepG2 cells after the treatment with ECM obtained from irradiated HUVECs. The cells were grown in DMEM/F12 supplemented with B27, N2, basic fibroblast- and epidermal growth factor onto 24-well ultra-low attachment plates at 300 cells per well for 7 days, and the size of spheres were determined. To measure the size of sphere, 12 spheres per group (n=12/group) were randomly selected. The average size of each sphere is quantified in the standard deviation and shown in the representative graph. *P<0.05 vs. control. HUVECs, human umbilical vein endothelial cells; ECM, endothelial cell culture medium. (E) Migratory and (F) invasive properties of Hep3B or Huh7 cells after the treatment with ECM obtained from 2 Gy-irradiated HUVECs. These properties of the cells were measured using the Transwell chamber (×200 magnification). (G) Quantification of sphere-forming abilities of Hep3B or Huh7 cells after the treatment with ECM obtained from 2 Gy-irradiated HUVECs. The cells were grown in DMEM/F12 supplemented with B27, N2, basic fibroblast- and epidermal growth factor onto 24-well ultra-low attachment plates at 300 cells per well for 7 days, and the size of spheres were determined. To measure the size of sphere, 12 spheres per group (n=12/group) were randomly selected. The average size of each sphere is quantified in the standard deviation and shown in the representative graph. Results from three independent experiments are expressed as mean ± 1 SEM *P<0.05 vs. control. HUVECs, human umbilical vein endothelial cells; ECM, endothelial cell culture medium.
EMT has been well known to be an event leading to an increase in the population of cancer stem-like cells (9,12–14). Thus, we next investigated whether the treatments with ECM obtained from irradiated HUVECs also affect the size of cancer stem-like cells in HepG2 cells. As shown in Fig. 3D, 2 Gy-irradiated ECM induces larger spheres of HepG2 cells, whereas 6 Gy- or 2 Gy × 3 (2 Gy/day for 3 days)-irradiated ECM does slightly decrease the size of HepG2 spheres, indicating that 2 Gy-irradiated endothelial cells contribute to the higher sphere-forming ability of liver cancer cells. When we confirmed these events using the other liver cancer cell lines, Hep3B and Huh7 cells, we had similar results to those obtained from HepG2 cells (Fig. 3E-G).
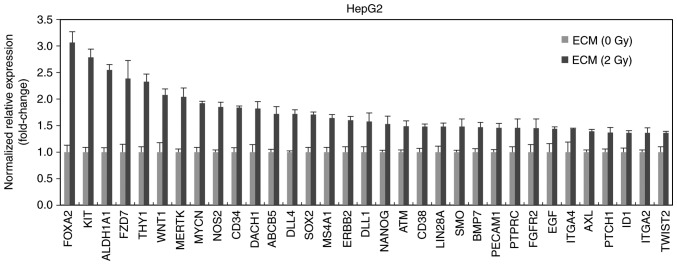
In addition, we determined the level of cancer stem-like cell-related factors after the treatment with 2 Gy-irradiated ECM in HepG2 cells. 2 Gy-irradiated ECM increased the mRNA levels of Foxa2, c-Kit, ALDH1A1, FZD7 ((Frizzled 7) and Thy1 (CD90) more than 2 times (Fig. 4). These factors are already known to contribute to stemness of cancer cells (25–29).
Figure 4.
RT2 Profiler PCR Array for cancer stem cell-related factors in liver cancer cells treated with ECM obtained from 2 Gy-irradiated endothelial cells. cDNAs obtained from HepG2 cells were subjected to RT2 Profiler PCR Array designed for cancer stem cell-related factors. Experiments were conducted in triplicate, and the data shown are representative of a typical experiment. ECM, endothelial cell culture medium.
These results collectively demonstrated that 2 Gy-irradiated endothelial cells can trigger the generation of cancer stem-like cells which are thought to be closely connected with the process of EMT.
Discussion
Although radiotherapy is widely known as a highly efficient treatment for a variety of cancer patients, tumor relapse and metastasis occurring after radiotherapy still remain as a major problem to be solved (4,5). In addition, they have been shown to be the main causes leading to poor survival rates of cancer patients (4,5). Tumor malignancy is characterized by them, which are closely related with the expansion of cancer stem-like cells (12,30). EMT is considered as a primary mechanism responsible for the expansion of cancer stem-like cells (9,12,30).
Tumor microenvironment is known to contribute to the induction of EMT, and the maintenance and survival of cancer stem-like cells (31,32). Furthermore, the induction of EMT, and the maintenance and survival of cancer stem-like cells can be regulated by tumor microenvironment comprised of cancer cells and diverse stromal cells containing immune cells, fibroblasts and endothelial cells (12,30–32). Especially, endothelial cells are suggested to be a key determinant of tumor microenvironment as they are a main component of vascular system responsible for delivering oxygen and nutrients to tumor cells (15,16).
In this study, we preferentially established a co-cultivation system to find a potential role of endothelial cells in the malignancy of liver cancer cells using ECM conditioned by endothelial cells, and then observed that the treatment with ECM increases the migratory and invasive properties of liver cancer cells, and leads to the expansion of cancer stem-like cells, as well as the appearance of a fibroblast-like shape and the dispersal, indicating that endothelial cells are responsible for the acquisition of mesenchymal traits of liver cancer cells. This observation is in good agreement with previous reports that endothelial cells are involved in both the induction of EMT and the generation of cancer stem-like phenotype in various tumor types including glioblastomas, squamous cell carcinomas, colorectal and breast cancers (18,33–36).
There are many investigations indicating the functional and morphological changes in irradiated vasculatures in normal tissues (37–39), but is relatively little known about the effects of ionizing radiation on tumor vasculatures. However, it has been already suggested that endothelial cells in tumor microenvironments are rather sensitive to ionizing radiation so that irradiation-induced apoptotic cell death of endothelial cells in tumor vasculatures plays a key role in the reduction of tumor size caused by irradiation (39–41). Controversially, it has been reported that ionizing radiation does not only lead to the capillary-like tube formation of endothelial cells, but also promotes the metastatic properties of cancer cells (42,43).
Most of previous studies concerned with tumor microenvironments have largely focused on the direct influences of ionizing radiation on the cells, but not the indirect influences of it (44,45). Thus, additional studies are needed to clarify the indirect effects of ionizing radiation on the tumor microenvironments. As part of these studies, we investigated whether irradiated endothelial cells affect the tumor malignancy of liver cancer cells through the treatment with ECM conditioned by endothelial cells irradiated with 2 Gy, fractionated dose (2 Gy × 3; 2 Gy/day for 3 days) or 6 Gy.
Direct exposure of the cells to ionizing radiation has been well known to efficiently suppress the clonogenic cell survival and the cell proliferation (44,45). However, although the clonogenic cell survival assay showed a slight decline in the cell survival of liver cancer cells treated with ECM conditioned by endothelial cells irradiated with fractionated dose (2 Gy × 3; 2 Gy/day for 3 days) or 6 Gy, we did not observe the efficient suppression of cell survival in liver cancer cells after the treatments with all irradiated ECMs. Previously, many evidences indicated that ionizing radiation can not only affect the directly irradiated cells, but also influence the non-irradiated cells surrounding them (3). This biological phenomenon is termed as the radiation-induced bystander effect (44). In addition, the bystander effect has been reported to emerge as cellular and molecular events including DNA damage response, cell cycle arrest, cell growth delay, cell transformation and cell death in the non-irradiated cells (3,44,45). Unlike these reports, we did not find the decrease in the survival and proliferation of non-irradiated liver cancer cells after the treatment with all irradiated ECMs. These results may be due to the difference in cellular responses of non-irradiated cells in accordance with cell types, the genetic and functional traits of the cells.
Many evidences showed that EMT greatly contributes to tumor malignancy via inducing metastasis and triggering tumor relapse (6–9). Furthermore, it is significantly concerned with the generation of cancer stem-like cells (12–14). Thus, we examined whether irradiated endothelial cells affect the induction of EMT in liver cancer cells. Interestingly, we found that ECM conditioned by endothelial cells irradiated with 2 Gy greatly increases the migratory and invasive properties of liver cancer cells, as well as inducing mesenchymal markers. Furthermore, it also efficiently enhanced the sphere-forming ability of liver cancer cells, and increased the mRNA levels of Foxa2, c-Kit, ALDH1A1, FZD7 and Thy1 (CD90) known to regulate self-renewal of cancer stem cells. However, our results showed that either ECM conditioned by endothelial cells irradiated with fractionated dose (2 Gy × 3; 2 Gy/day for 3 days) or that with 6 Gy does not greatly influence the malignancy of liver cancer cells. Particularly, c-Kit and FZD7 are well known to be the receptors for Stem Cell Factor (SCF), a cytokine and secreted WNT proteins, respectively (26,28). Therefore, it can be reasonably assumed that these increased receptors of liver cancer cells caused by irradiated endothelial cells may participate in enhancing the induction of malignant liver cancer cells via interacting with their soluble ligands secreted from a variety of stromal cells in tumor microenvironment in vivo. In a good agreement with our assumption, it has been commonly indicated that soluble factors secreted from endothelial cells play a key role in tumor malignancy (18,33–36). Especially, the communications between one cell and the others can be mediated by these soluble signaling molecules including cytokines and growth factors (15,17,18). These factors such as interleukin-6 (IL-6), IL-8, transforming growth factor-β1 (TGF-β1) and tumor necrosis factor-alpha (TNF-α), have been reported to play a pivotal role in the cell-to-cell communications and the bystander effects (46). Moreover, they do not only contribute to tumor malignancy, but also are induced by ionizing radiation (46). The principal reason why the malignant potential of liver cancer cells is raised only by ECM conditioned by endothelial cells irradiated with 2 Gy but not by the others may be due to these secreted factors. Thus, it is necessary to further identify that what kind of them is highly secreted from endothelial cells in response to 2 Gy irradiation, and greatly contributes to the malignancy of liver cancer cells, compared with them secreted from endothelial cells irradiated with fractionated dose (2 Gy × 3; 2 Gy/day for 3 days) or 6 Gy. In addition, although we performed the investigation only under three conditions (2 Gy, fractionated dose (2 Gy/day for 3 days) and 6 Gy), endothelial cells irradiated with various doses of ionizing radiation are also likely to diversely affect the biological events of tumor cells. However, there have been no comparative studies investigating these effects as yet. Thus, further studies are also needed to precisely define the pivotal role of these irradiated endothelial cells in the tumor malignancy.
It have been already shown that direct exposure of a variety of cancer cells to ionizing radiation can trigger tumor malignancy via increasing the migratory and invasive properties of them, and expanding the population of cancer stem-like cells (47–50). Similarly to these reports, we found a possibility that the indirect exposure to ionizing radiation mediated by irradiated endothelial cells can also elicit the highly malignant potential from cancer cells.
In summary, we found that 2 Gy irradiation of endothelial cells influences the increase in the tumor malignancy of liver cancer cells. Our observations indicate that the distinct differences in the indirect effects of ionizing radiation on tumor malignancy may provide a valuable clue to the improvement in the efficacy of radiotherapy.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Research Foundation of Korea grant funded by the Korea government (MSIP) (grant no. 50596-2018).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SDK performed all functional assays, and contributed to conception, design, collection and assembly of data. JMY contributed to data analysis and interpretation, and wrote the manuscript. MTP contributed to data analysis and interpretation, and wrote the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Baskar R, Itahana K. Radiation therapy and cancer control in developing countries: Can we save more lives? Int J Med Sci. 2017;14:13–17. doi: 10.7150/ijms.17288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Datta NR, Samiei M, Bodis S. Radiotherapy infrastructure and human resources in Europe-present status and its implications for 2020. Eur J Cancer. 2014;50:2735–2743. doi: 10.1016/j.ejca.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton S, Jaffray D, Milosevic M. Radiation effects on the tumor microenvironment: Implications for nanomedicine delivery. Adv Drug Deliv Rev. 2017;109:119–130. doi: 10.1016/j.addr.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa K, Yoshioka Y, Isohashi F, Seo Y, Yoshida K, Yamazaki H. Radiotherapy targeting cancer stem cells: Current views and future perspectives. Anticancer Res. 2013;33:747–754. [PubMed] [Google Scholar]
- 6.Marie-Egyptienne DT, Lohse I, Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: Potential role of hypoxia. Cancer Lett. 2013;341:63–72. doi: 10.1016/j.canlet.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–180. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Shim JW, Eum DY, Kim SD, Choi SH, Yang K, Heo K, Park MT. Downregulation of UHRF1 increases tumor malignancy by activating the CXCR4/AKT-JNK/IL-6/Snail signaling axis in hepatocellular carcinoma cells. Sci Rep. 2017;7:2798. doi: 10.1038/s41598-017-02935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 11.Vasko V, Espinosa AV, Scouten W, He H, Auer H, Liyanarachchi S, Larin A, Savchenko V, Francis GL, de la Chapelle A, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci USA. 2007;104:2803–2808. doi: 10.1073/pnas.0610733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asiedu MK, Beauchamp-Perez FD, Ingle JN, Behrens MD, Radisky DC, Knutson KL. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene. 2014;33:1316–1324. doi: 10.1038/onc.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang-Verslues WW, Chang PH, Wei PC, Yang CY, Huang CK, Kuo WH, Shew JY, Chang KJ, Lee EY, Lee WH. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene. 2011;30:2463–2474. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- 15.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 17.Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, He X, Hervey-Jumper SL, Heth JA, Muraszko KM, et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011;71:6061–6072. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, Tozzi F, Sceusi E, Zhou Y, Tachibana I, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171–185. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai JK, Wu HC, Shen YC, Hsieh HY, Yang SY, Chang CC. Krüppel-like factor 4 is involved in cell scattering induced by hepatocyte growth factor. J Cell Sci. 2012;125:4853–4864. doi: 10.1242/jcs.108910. [DOI] [PubMed] [Google Scholar]
- 20.Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174:175–183. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura-Tsuchiya R, Ishikawa T, Kokura S, Mizushima K, Adachi S, Okajima M, Matsuyama T, Okayama T, Sakamoto N, Katada K, et al. The inhibitory effect of heat treatment against epithelial-mesenchymal transition (EMT) in human pancreatic adenocarcinoma cell lines. J Clin Biochem Nutr. 2014;55:56–61. doi: 10.3164/jcbn.14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen DH, Oketch-Rabah HA, Illa-Bochaca I, Geyer FC, Reis-Filho JS, Mao JH, Ravani SA, Zavadil J, Borowsky AD, Jerry DJ, et al. Radiation acts on the microenvironment to affect breast carcinogenesis by distinct mechanisms that decrease cancer latency and affect tumor type. Cancer Cell. 2011;19:640–651. doi: 10.1016/j.ccr.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karar J, Maity A. Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol Ther. 2009;8:1994–2001. doi: 10.4161/cbt.8.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuonen F, Secondini C, Rüegg C. Molecular pathways: Emerging pathways mediating growth, invasion, and metastasis of tumors progressing in an irradiated microenvironment. Clin Cancer Res. 2012;18:5196–5202. doi: 10.1158/1078-0432.CCR-11-1758. [DOI] [PubMed] [Google Scholar]
- 25.Peng Q, Qin J, Zhang Y, Cheng X, Wang X, Lu W, Xie X, Zhang S. Autophagy maintains the stemness of ovarian cancer stem cells by FOXA2. J Exp Clin Cancer Res. 2017;36:171. doi: 10.1186/s13046-017-0644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan HT. c-Kit expression in human normal and malignant stem cells prognostic and therapeutic implications. Leuk Res. 2009;33:5–10. doi: 10.1016/j.leukres.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Condello S, Morgan CA, Nagdas S, Cao L, Turek J, Hurley TD, Matei D. β-Catenin-regulated ALDH1A1 is a target in ovarian cancer spheroids. Oncogene. 2015;34:2297–2308. doi: 10.1038/onc.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakrabarti R, Wei Y, Hwang J, Hang X, Andres Blanco M, Choudhury A, Tiede B, Romano RA, DeCoste C, Mercatali L, et al. ΔNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat Cell Biol. 2014;16:1004–1015. doi: 10.1038/ncb3040. 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: Concepts and molecular links. Semin Cancer Biol. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carnero A, Lleonart M. The hypoxic microenvironment: A determinant of cancer stem cell evolution. Bioessays. 2016;38(Suppl 1):S65–S74. doi: 10.1002/bies.201670911. [DOI] [PubMed] [Google Scholar]
- 32.van der Horst G, Bos L, van der Pluijm G. Epithelial plasticity, cancer stem cells, and the tumor-supportive stroma in bladder carcinoma. Mol Cancer Res. 2012;10:995–1009. doi: 10.1158/1541-7786.MCR-12-0274. [DOI] [PubMed] [Google Scholar]
- 33.Sharma A, Shiras A. Cancer stem cell-vascular endothelial cell interactions in glioblastoma. Biochem Biophys Res Commun. 2016;473:688–692. doi: 10.1016/j.bbrc.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Dong Z, Lauxen IS, Filho MS, Nör JE. Endothelial cell-secreted EGF induces epithelial to mesenchymal transition and endows head and neck cancer cells with stem-like phenotype. Cancer Res. 2014;74:2869–2881. doi: 10.1158/0008-5472.CAN-13-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sigurdsson V, Hilmarsdottir B, Sigmundsdottir H, Fridriksdottir AJ, Ringnér M, Villadsen R, Borg A, Agnarsson BA, Petersen OW, Magnusson MK, Gudjonsson T. Endothelial induced EMT in breast epithelial cells with stem cell properties. PLoS One. 2011;6:e23833. doi: 10.1371/journal.pone.0023833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li YQ, Ballinger JR, Nordal RA, Su ZF, Wong CS. Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res. 2001;61:3348–3354. [PubMed] [Google Scholar]
- 38.Wilson CM, Gaber MW, Sabek OM, Zawaski JA, Merchant TE. Radiation-induced astrogliosis and blood-brain barrier damage can be abrogated using anti-TNF treatment. Int J Radiat Oncol Biol Phys. 2009;74:934–941. doi: 10.1016/j.ijrobp.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 39.Park MT, Oh ET, Song MJ, Kim WJ, Cho YU, Kim SJ, Han JY, Suh JK, Choi EK, Lim BU, et al. The radiosensitivity of endothelial cells isolated from human breast cancer and normal tissue in vitro. Microvasc Res. 2012;84:140–148. doi: 10.1016/j.mvr.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 42.Asuthkar S, Velpula KK, Nalla AK, Gogineni VR, Gondi CS, Rao JS. Irradiation-induced angiogenesis is associated with an MMP-9-miR-494-syndecan-1 regulatory loop in medulloblastoma cells. Oncogene. 2014;33:1922–1933. doi: 10.1038/onc.2013.151. [DOI] [PubMed] [Google Scholar]
- 43.Zhou YC, Liu JY, Li J, Zhang J, Xu YQ, Zhang HW, Qiu LB, Ding GR, Su XM, Mei-Shi, Guo GZ. Ionizing radiation promotes migration and invasion of cancer cells through transforming growth factor-beta-mediated epithelial-mesenchymal transition. Int J Radiat Oncol Biol Phys. 2011;81:1530–1537. doi: 10.1016/j.ijrobp.2011.06.1956. [DOI] [PubMed] [Google Scholar]
- 44.Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powathil GG, Munro AJ, Chaplain MA, Swat M. Bystander effects and their implications for clinical radiation therapy: Insights from multiscale in silico experiments. J Theor Biol. 2016;401:1–14. doi: 10.1016/j.jtbi.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Najafi M, Fardid R, Hadadi G, Fardid M. The mechanisms of radiation-induced bystander effect. J Biomed Phys Eng. 2014;4:163–172. [PMC free article] [PubMed] [Google Scholar]
- 47.Kim RK, Cui YH, Yoo KC, Kim IG, Lee M, Choi YH, Suh Y, Lee SJ. Radiation promotes malignant phenotypes through SRC in breast cancer cells. Cancer Sci. 2015;106:78–85. doi: 10.1111/cas.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomez-Casal R, Bhattacharya C, Ganesh N, Bailey L, Basse P, Gibson M, Epperly M, Levina V. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol Cancer. 2013;12:94. doi: 10.1186/1476-4598-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim RK, Suh Y, Cui YH, Hwang E, Lim EJ, Yoo KC, Lee GH, Yi JM, Kang SG, Lee SJ. Fractionated radiation-induced nitric oxide promotes expansion of glioma stem-like cells. Cancer Sci. 2013;104:1172–1177. doi: 10.1111/cas.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su Z, Li G, Liu C, Ren S, Tian Y, Liu Y, Qiu Y. Ionizing radiation promotes advanced malignant traits in nasopharyngeal carcinoma via activation of epithelial-mesenchymal transition and the cancer stem cell phenotype. Oncol Rep. 2016;36:72–78. doi: 10.3892/or.2016.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.