Abstract
Background
Duodenal–jejunal bypass (DJB) is an important component of many types of current bariatric surgery including Roux-en-Y gastric bypass, mini-gastric bypass, biliopancreatic diversion, duodenal switch, and DJB plus sleeve gastrectomy. Surgery is often observed to ameliorate nonalcoholic steatohepatitis (NASH), but without a clearly delineated mechanism. In this study, we investigated the effects of DJB in diet-induced obese rats with NASH.
Materials and methods
Male Wistar rats were divided into four groups and fed the following diets over 6 months: A) normal chow (NC group, n=6); B) methionine–choline-deficient (MCD)–high-fat (HF) diet (HF group, n=6); C) MCD–HF diet for 3 months followed by DJB and MCD–HF diet for subsequent 3 months (DJB group, n=6); and D) MCD–HF diet for 3 months followed by treatment with pioglitazone (PGZ) with MCD–HF diet for subsequent 3 months (PGZ group, n=6). Body weight, glucose tolerance, the homeostatic model assessment-insulin resistance index, and lipid profiles were compared. Liver and visceral adipose tissue histology, inflammatory marker and hepatic stellate cell (HSC) activity, and hepatocyte autophagy were assessed.
Results
Compared with the HF group, the DJB group showed improved body weight, insulin sensitivity, lipid metabolism, and steatosis severity. The DJB group exhibited a significantly lower nonalcoholic fatty liver disease activity score than the HF and PGZ group (P<0.001 and P=0.003, respectively). Furthermore, DJB significantly reduced fat mass and adipocyte size. These effects were also observed in the PGZ group. Therefore, we speculated that the improvements induced by DJB are closely related to an alteration in insulin sensitivity. Moreover, DJB reduced HSC activity and TNF-α expression and enhanced hepatocyte autophagy.
Conclusion
DJB improves NASH through several mechanisms, particularly by altering insulin sensitivity, inflammatory responses, HSC activity, and hepatocyte autophagy.
Keywords: duodenal–jejunal bypass, nonalcoholic steatohepatitis, insulin sensitivity, hepatic stellate cell activity, hepatocyte autophagy
Introduction
Nonalcoholic fatty liver disease (NAFLD), a clinically important chronic liver disease, affects 20%–30% of the general population.1 Nonalcoholic steatohepatitis (NASH) is an advanced histological form of NAFLD, with a prevalence of 5.7%–17%.2 Nearly 20% of NAFLD patients with NASH may develop liver cirrhosis, and 30%–40% of these patients die because of liver-related complications, such as hepatocellular carcinoma. NASH has also been predicted to become the leading indication for liver transplantation in the USA within a decade.3 Based on these observations, understanding the pathophysiology of NASH and further development of effectively therapeutic interventions is becoming imperative. The current management of NAFLD and NASH typically includes a low-fat, low-calorie or Mediterranean diet, regular exercise, weight loss (if necessary), vitamin E, or pioglitazone (PGZ).4 Several novel therapeutic agents are undergoing clinical trials, such as the PPAR family PPARα/δ ligand (elafibranor), bile acid-derived ligand for the nuclear hormone receptor (farnesoid X-activated receptor), obeticholic acid, and the glucagon-like peptide-1 (GLP-1) pathway activating agents.5–7 However, the US Food and Drug Association has not approved any drugs for NASH to date.
Most patients undergoing Roux-en-Y gastric bypass (RYGB) for morbid obesity experience a rapid improvement in glucose tolerance within the first month before marked weight loss. Approximately 83.7% and 98.9% of patients with type 2 diabetes mellitus (T2DM) experienced diabetic resolution after RYGB and biliopancreatic diversion (BPD), respectively.8,9 Lassailly et al demonstrated that bariatric surgery successfully treated NASH in nearly 85% of patients in 1 year.10 Moreover, Furuya et al indicated that RYGB reduced hepatic steatosis and liver fibrosis by 84% and 75%, respectively, after 2 years of surgery.11 These findings illustrate the potential role of bypass surgery in ameliorating NASH in patients.
Duodenal–jejunal bypass (DJB) is an important component of not only RYGB and BPD but also other current bariatric surgery practices including mini-gastric bypass, duodenal switch, and DJB plus sleeve gastrectomy. Only a few studies with small sample sizes have indicated that DJB surgery or endoscopic DJB linear devices can improve glucose intolerance and NAFLD-related parameters.12–14 However, there is no study focusing on the effects and mechanism of DJB for resolution of NASH. This study hypothesized that the reduction of hepatic steatosis and NASH following DJB is associated with many benefits originating from a proximal intestinal diversion. We analyzed the alterations in insulin sensitivity, liver and visceral fat histology, inflammatory responses, hepatic stellate cell (HSC) activity, and hepatocyte autophagy in diet-induced obese rats with NASH undergoing DJB. Understanding the underlying metabolic and molecular changes from DJB may provide a useful therapeutic target for NASH resolution.
Materials and methods
Animal model and experimental design
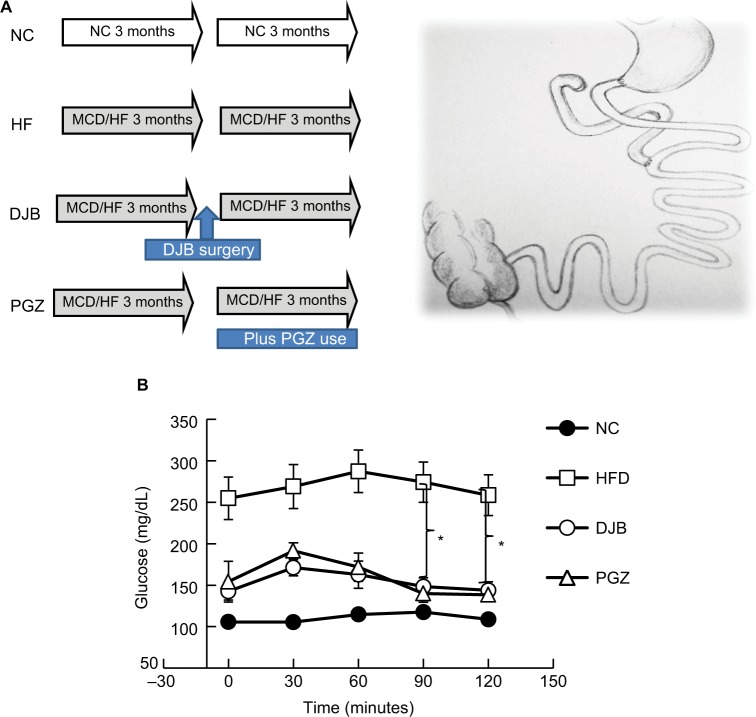
All experiments were performed in accordance with relevant guidelines and regulations of Laboratory Animal Center of National Cheng-Kung University, Taiwan. The research was also approved by the ethical committee of National Cheng-Kung University, Taiwan. A total of 24 male Wistar rats at 20 weeks of age were used. The rats were divided into four groups and fed the following diets plus specific intervention over a 6-month period: 1) normal chow for 6 months (NC group, n=6); 2) a methionine–choline-deficient (MCD)–high-fat (HF) diet (High Fat Diet Modified & Methionine/Choline Deficient Diet Pelleted; MP Biomedicals, Santa Ana, CA, USA) for 6 months (HF group, n=6); 3) a MCD–HF diet for 3 months followed by DJB (from the middle point of the study period onward), and MCD–HF diet for subsequent 3 months (DJB group, n=6); and 4) a MCD–HF diet for 3 months followed by treatment with PGZ at 5 mg/kg per day (Actos; Takeda Pharmaceutical Company Limited, Osaka, Japan) and MCD–HF diet for subsequent 3 months (PGZ group, n=6) (Figure 1A). The content of MCD–HF diet includes cotton seed oil, which contains 70% unsaturated fatty acids: 18% monounsaturated (oleic), 52% polyunsaturated (linoleic), and 26% saturated (primarily palmitic and stearic) fatty acids. The HF diet was used to induce obesity and insulin resistance in experimental rats. The MCD diet was used to develop an animal model of NASH through the induction of liver CYP2E1 and CYP4A, as described in previous studies.15–17 In the present study, we used both HF and MCD diets to mimic human dietary insulin resistance and NASH.
Figure 1.
Experimental design and oral glucose tolerance test.
Notes: (A) Animal experimental design and schematic of DJB. (B) Oral glucose tolerance test. DJB and PGZ reversed the glucose intolerance. *P<0.05.
Abbreviations: DJB, duodenal–jejunal bypass; DJB group, MCD–HF diet for 3 months followed by DJB and MCD–HF diet for subsequent 3 months; HF, high fat; HFD, high fat diet; HF group, MCD–HF diet; MCD, methionine–choline-deficient; NC group, normal chow; PGZ group, MCD–HF diet for 3 months followed by treatment with PGZ with MCD–HF diet for subsequent 3 months; PGZ, pioglitazone.
DJB was performed after 3 months of MCD–HF diet treatment. The DJB surgery included an end-to-end gastrojejunostomy and end-to-side jejunojejunostomy (Figure 1A). The biliopancreatic limb was 10 cm from the ligament of Treitz and the alimentary limb was 15 cm from the gastrojejunal anastomosis (Roux-en-Y reconstruction). The duodenal stump and two anastomoses were sutured in a one-layer manner by using microscopic surgical instruments and 6–0 Prolene under assistance from a surgical telescope. The rats were fed only glucose water in the first three postoperative days, and subsequently were allowed ad libitum access to water and the MCD–HF diet for further 3 months. PGZ, a PPAR-γ agonist, can ameliorate insulin resistance and improve glucose and lipid metabolism in patients with T2DM. It reverses the insulin resistance in the liver, adipose tissues, and muscles. PGZ was reported to improve liver histological findings by reducing steatosis, ballooning necrosis, and inflammation.18 The use of PGZ group was to emphasize the effects of improved insulin sensitivity on NASH reduction.
Metabolic parameters and blood sampling
After 6 months, an oral glucose tolerance test (OGTT) with 2 g/kg glucose administered orally was performed after a 12-hour fast. Blood was drawn from the tail vein at 0, 30, 60, 90, and 120 minutes. The glucose level was determined using OneTouch Ultra (Johnson & Johnson, New Brunswick, NJ, USA). Three days after the OGTT, the rats were sacrificed. The alanine aminotransferase (ALT), triglyceride (TG), total cholesterol, insulin, IL-6, and TNF-α levels were measured. Liver function was determined using an ALT assay kit (Sigma Diagnostics, Livonia, MI, USA). TG was measured using ELISA kit of glycerol-3-phosphate oxidase and a photometric system (DiaSys Diagnostic Systems GmgH, Holzheim, Germany). Serum cholesterol levels were determined using the ELISA kit and CHOD–PAP enzymatic test on the photometric system. Insulin, IL-6, and TNF-α levels were measured using enzyme immunoassay kits (R&D Systems, Inc., Minneapolis, MN, USA). Insulin resistance was calculated through the formula of homeostasis model assessment-insulin resistance (HOME-IR) index: insulin (μU/mL) × glucose (mg/dL)/405.
Morphological examinations
Visceral epididymal fat pads were procured, and the weight was measured. H&E staining of liver and visceral fat was performed following standard procedures. The severity of fat accumulation, ballooning degeneration of hepatocytes, inflammation of acinar and portal tissues, and liver fibrosis were recorded. The NAFLD activity score (NAS) system was used to represent the quantitative severity of NAFLD and NASH.19 The score was calculated as the unweighted sum of the scores for steatosis (0–3; <5%, 5%–33%, 34%–66%, and >66%), lobular inflammation (0–3; none, <2, 2–4, and >4 foci), and ballooning (0–2; none, few, and many cells). Thus, the score ranged from 0 to 8. The NAS was interpreted by clinical pathologist who is familiar to liver disease. Furthermore, the size of adipocytes in visceral fat was compared under the same degree field for each study group. Liver tissues were immunostained with monoclonal mouse antihuman α-smooth muscle actin (α-SMA; United States Biological, Salem, MA, USA) and Masson’s trichrome following the manufacturer’s instructions.
Lipogenic and fibrogenic gene expression
Real-time quantitative polymerase chain reaction (RT-qPCR) of liver tissues was performed to determine the mRNA expression of the most important fibrogenic gene transforming growth factor (TGF)-β1 and the lipogenic gene sterol regulatory element-binding factor-1 (SREBF-1). The liver total RNA content was extracted using the Trizol method and RNeasy mini kit (Qiagen NV, Venlo, the Netherlands). The cDNA was collected after reaction in a PCR machine for 5 minutes at 25°C, 60 minutes at 50°C, and 15 minutes at 70°C, and it was subsequently stored at −20°C. The primers were TGF-β1, forward: 5′-gAgCAACATgTggAACTCTAC-CAg-3′ and reverse: 5′-AAgCCCTgTATTCCgTCTCCT-3′ and SREBF-1, forward: 5′-TTCCATTgACAAggCCATgC-3′ and reverse: 5′-gCCTCATgTAggAATACCCTCCTC-3′. The sample cDNA was diluted, and RT-qPCR was subsequently performed using a Lightcycler TaqMan master kit (Hoffman-La Roche Ltd., Basel, Switzerland) under the following PCR conditions: preincubation at 95°C for 10 minutes, one cycle; amplification at 95°C for 10 seconds, 60°C for 30 seconds, and 72°C for 5 seconds, 50 cycles; cooling at 40°C for 30 seconds, one cycle.
Hepatocyte autophagy and endoplasmic reticulum stress
Cell autophagy is an intracellular pathway that targets cellular components, including organelles and proteins, to lysosomes for degradation. Both macroautophagy and chaperone-mediated autophagy in hepatic lipid metabolism, insulin sensitivity, and cellular injury were reported to have potential mechanistic roles for autophagy in hepatic steatosis and NASH.20 Therefore, we examined the effects of DJB on the alterations in hepatocyte autophagy by determining the expression of microtubule-associated protein 1 light chain 3 (LC3), a cell macroautophagy marker, in a Western blot. We also measured the expression of glucose-regulated protein 78, the key chaperone protein mediating the endoplasmic reticulum stress response to prevent cell death.
Statistical analyses
Parametric data are expressed as means ± standard error of mean. The study groups were statistically analyzed using one-way ANOVA with Bonferroni multiple comparisons procedure among groups, such as for comparing NAS in each group. Significance was set at P<0.05. Statistical analyses were performed using SPSS, version 19.0 (IBM, Corporation, Armonk, NY, USA).
Results
DJB-ameliorated metabolic parameters in diet-induced obese rats with NASH
To define the metabolic changes resulted from DJB or PGZ under MCD–HF diet, we checked the weight of body, liver and visceral fat (net and adjusted to body weight), food intake, liver function, glucose level and insulin sensitivity, lipid profile, and inflammatory markers after 6 months (Table 1). We found that the postoperative food intake had no significant difference. DJB and PGZ both reduced body and liver weights (DJB and PGZ vs HF, body weight: P=0.003 and 0.005; liver weight: P=0.006 and <0.001), but there is no difference in body weight and liver weight between DJB and PGZ groups. Above data implicated that hepatic fat content could be reduced by both DJB and PGZ with comparable ability. DJB, but no PGZ, improved ALT value (DJB and PGZ vs HF, P=0.017 and 0.979). Compared with normal chow, the MCD–HF diet successfully induced glucose intolerance. This intolerance was improved both by DJB or PGZ (Figure 1B, DJB and PGZ vs HF, P=0.007 and 0.003 at 90 minutes; P=0.014 and 0.006 at 120 minutes). Insulin level was reduced by PGZ, but not by DJB (DJB and PGZ vs HF, P=0.830 and 0.002). It could be expected because GLP-1 is a well-known incretin hormone. HOMA-IR showed equivalent improvements in the DJB and PGZ groups (DJB and PGZ vs HF, P=0.035 and 0.002, group DJB vs PGZ P=0.990). The TG level was significantly reduced in the DJB and PGZ groups (groups C and D vs B, P=0.01 and 0.001; DJB vs PGZ, P=0.990). The progression of hepatic steatosis to inflammation is associated with proinflammatory cytokines. In this study, DJB and PGZ reduced the inflammatory cytokine TNF-α (DJB and PGZ vs HF, P=0.037 and 0.002; DJB vs PGZ, P=0.06).
Table 1.
Effects of DJB and PGZ on metabolic parameters in MCD and HF diet-induced rats of nonalcoholic steatohepatitis
| Metabolic parameters | NC group | HF group | DJB group | PGZ group | P-valuea |
|---|---|---|---|---|---|
|
| |||||
| Body weight (g) | 574.5±11.3* | 843.3±37.7 | 607.8±44.7* | 649.2±30.6* | 0.001 |
| Food intake (g/day) | 32.1±0.5 | 40.8±3.2 | 38.2±3.3 | 37.1±2.9 | 0.307 |
| Liver weight (g) | 12.3±0.6* | 23.3±1.4 | 15.6±0.3* | 13.1±1.5* | <0.001 |
| Liver weight normalized to body weight (g/kg) | 21.3±0.2 | 27.7±0.5 | 25.5±0.3 | 20.3±0.4 | <0.001 |
| ALT (U/L) | 52.5±2.7* | 79.3±2.7 | 57.0±4.2* | 73.2±5.7 | 0.018 |
| Insulin (μIU/mL) | 10.6±0.3* | 78.3±15.5 | 60.4±4.5 | 14.7±2.9* | 0.002 |
| Fasting glucose (mg/dL) | 105.7±4.2* | 254.8±25.5 | 142.5±10.4* | 154.3±24.5* | 0.004 |
| HOMA-IR | 2.73±0.02* | 51.00±14.09 | 21.59±3.11* | 5.95±1.80* | 0.023 |
| Triglycerides (mg/dL) | 55.0±6.5* | 168.3±25.8 | 75.0±2.6* | 55.3±5.3* | 0.001 |
| Cholesterol (mg/dL) | 72.2±4.8 | 92.0±11.0 | 103.3±10.3 | 78.2±6.3 | 0.226 |
| Epididymal fat (g) | 8.6±1.0* | 19.4±1.5 | 10.1±1.1* | 14.3±2.1* | 0.010 |
| Epididymal fat normalized to body weight (g/kg) | 15.0±0.9 | 32.7±1.2 | 17.0±0.8 | 23.1±1.4 | <0.001 |
| IL-6 (pg/mL) | 225.2±29.4* | 439.8±10.3 | 418.8±0.5 | 439.8±4.1 | 0.137 |
| TNF-α (pg/mL) | 140.0±13.9* | 540.1±49.2 | 395.8±66.2* | 280.2±53.1* | 0.004 |
Note:
P-value <0.05 vs HF group.
P-value indicated the significance among HF, DJB, and PGZ groups.
Abbreviations: ALT, alanine aminotransferase; DJB, duodenal–jejunal bypass; DJB group, MCD–HF diet for 3 months followed by DJB and MCD–HF diet for subsequent 3 months; HF, high fat; HF group, MCD–HF diet; HOMA-IR, homeostasis model assessment-insulin resistance; MCD, methionine–choline-deficient; NC group, normal chow; PGZ group, MCD–HF diet for 3 months followed by treatment with PGZ with MCD–HF diet for subsequent 3 months; PGZ, pioglitazone.
DJB reduced hepatic steatosis, inflammatory cell infiltration, and visceral fat mass
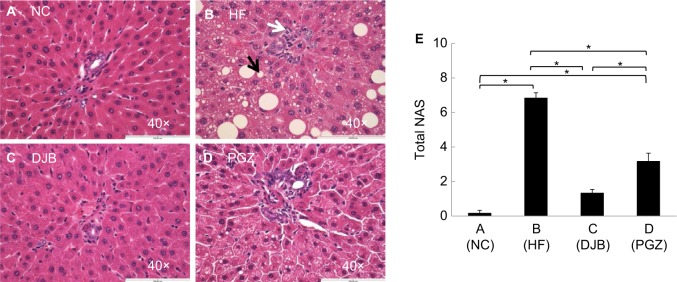
To define the hepatic morphological alteration caused by DJB and PGZ, we performed the H&E stain of liver and visceral tissue. In HF group, we observed a marked hepatic steatosis and lipid droplet accumulation (Figure 2A–D). Both DJB and PGZ attenuated the hepatic steatosis and inflammatory cell infiltration and the morphological improvement was more prominent in the DJB group. To quantify the severity of NAFLD, we calculated the NAS of each sample. The MCD–HF diet successfully induced NASH (NAS=6.83). DJB and PGZ groups exhibited lower NAS, and the effect was rendered more prominent by DJB (F(3,20) = 86.08, P<0.001; DJB and PGZ vs HF, P<0.001 and 0.003; DJB vs PGZ, P=0.003; Figure 2E) (Table 2). The NAS of the DJB and PGZ groups were 1.33 and 3.17, which indicated non-NASH and borderline NASH statuses, respectively.
Figure 2.
H&E staining of liver tissue after the 6-month protocol.
Notes: (A) NC group, normal liver histology; (B) HF group, development of hepatic steatosis with ballooning degeneration; (C, D) DJB and PGZ groups, reduced steatosis. The black arrow indicates a lipid droplet and white arrow indicates the infiltration of an inflammatory cell. Original magnification 40×. (E) Nonalcoholic fatty liver disease activity score (NAS). The DJB and PGZ groups had significantly lower NASs than HF group. *P<0.05.
Abbreviations: DJB, duodenal–jejunal bypass; DJB group, MCD–HF diet for 3 months followed by DJB and MCD–HF diet for subsequent 3 months; HF, high fat; HF group, MCD–HF diet; MCD, methionine–choline-deficient; NC group, normal chow; PGZ group, MCD–HF diet for 3 months followed by treatment with PGZ with MCD–HF diet for subsequent 3 months; PGZ, pioglitazone.
Table 2.
NAFLD activity score (NAS) in each group
| Group | NAS range | Average | SEM | P-value |
|---|---|---|---|---|
| NC (n=6) | 0–1 | 0.17 | 0.167 | <0.001 |
| HF (n=6) | 6–8 | 6.83 | 0.307 | <0.001 |
| DJB (n=6) | 1–2 | 1.33 | 0.211 | <0.001 |
| PGZ (n=6) | 2–5 | 3.17 | 0.497 | <0.001* |
Notes: P-value represents significance compared to HF group.
Significant P-value among groups.
Abbreviations: DJB, duodenal–jejunal bypass; DJB group, MCD–HF diet for 3 months followed by DJB and MCD–HF diet for subsequent 3 months; HF, high fat; HF group, MCD–HF diet; MCD, methionine–choline-deficient; NAFLD, nonalcoholic fatty liver disease; NC group, normal chow; PGZ group, MCD–HF diet for 3 months followed by treatment with PGZ with MCD–HF diet for subsequent 3 months; PGZ, pioglitazone.
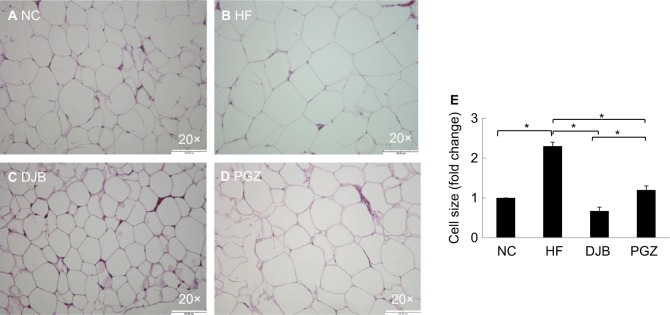
Excessive visceral fat is clinically an important risk factor for many metabolic syndrome-related disorders, such as obesity, diabetes, stroke, and coronary heart disease. In the present study, we measured the weight of epididymal fat pads and size of their adipocyte. Histologically, DJB significantly reduced epididymal fat mass and adipocyte size. The effects of DJB were also stronger than those of PGZ in microscopically observation (Figure 3).
Figure 3.
H&E staining of visceral epididymal fat tissue after 6 months.
Notes: (A) NC group, normal adipocyte size; (B) HF group, markedly increased size; (C) DJB group, reduced adipocyte size; (D) PGZ group, slightly reduced adipocyte size. Original magnification 20×. (E) Quantitative evaluation of adipocyte size by fold change. *P<0.05.
Abbreviations: DJB, duodenal–jejunal bypass; DJB group, MCD–HF diet for 3 months followed by DJB and MCD–HF diet for subsequent 3 months; HF, high fat; HF group, MCD–HF diet; MCD, methionine–choline-deficient; NC group, normal chow; PGZ group, MCD–HF diet for 3 months followed by treatment with PGZ with MCD–HF diet for subsequent 3 months; PGZ, pioglitazone.
DJB improved NASH and fibrotic reaction
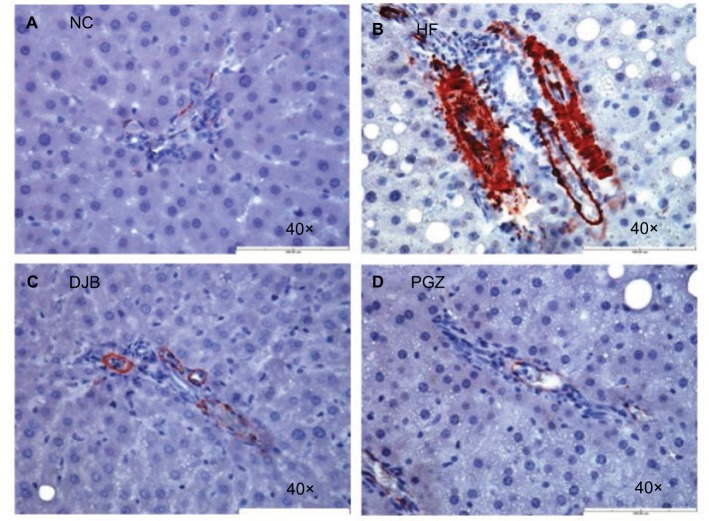
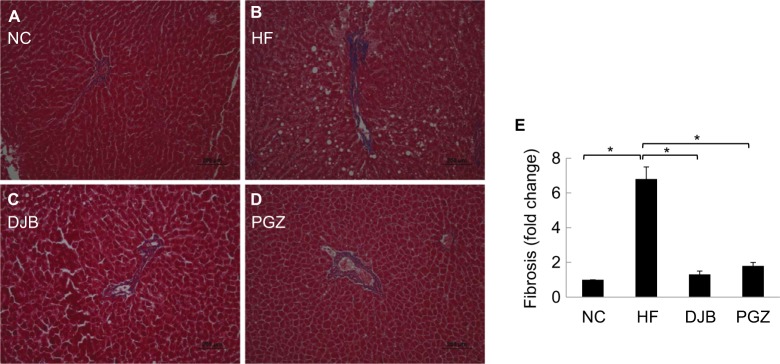
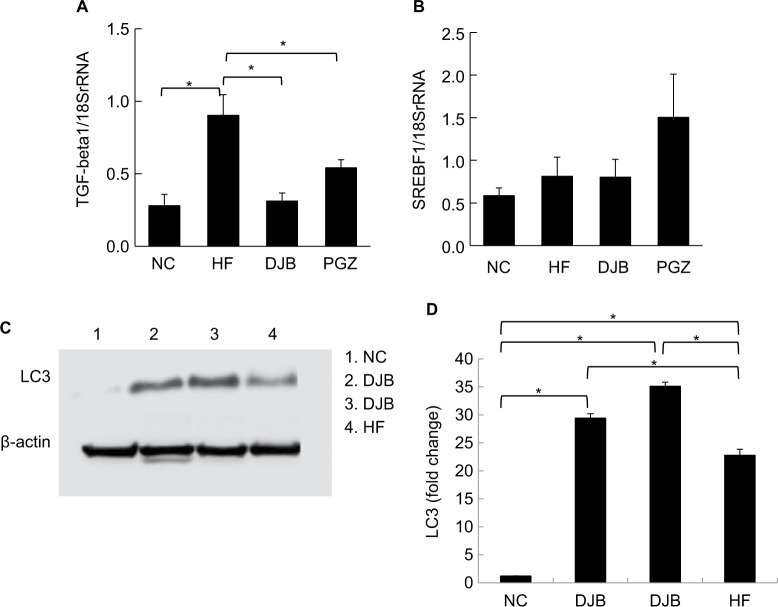
Advanced NASH may progress to a cirrhotic liver. Therefore, it is also worth noting whether DJB can prevent the progression of NASH to an irreversible fibrotic stage. Since we did not find the obvious fibrotic bundles in our liver tissues, probably due to insufficient time for the development of liver fibrosis, we observed the activity of HSCs by the expression of α-SMA and collagen bundle instead. The immunohistochemical staining revealed high α-SMA expression in HF group, but significantly lower in DJB and PGZ group (Figure 4). These results suggest that DJB might prevent liver fibrosis through reducing HSC activity. The Masson’s trichrome stain also showed increased collagen bundle in HF group, but DJB and PGZ attenuated its formation (Figure 5). In addition, the progression of hepatic steatosis to hepatic fibrosis is related to the upregulation of fibrogenic and lipogenic genes. In this study, the mRNA expression of fibrogenic gene, TGF-b1, and lipogenic gene, SREBF-1, was determined by RT-qPCR. The mRNA expression of TGF-β1 was significantly downregulated in the DJB and PGZ groups (Figure 6A). However, the expression of lipogenic gene, SREBF-1, seemed not to be influenced by DJB (Figure 6B).
Figure 4.
α-SMA expression after 6 months.
Notes: (A) NC group, no enhancement; (B) HF group, enhanced expression; (C, D) DJB or PGZ group, returned to normal expression. Original magnification 40×.
Abbreviations: DJB, duodenal–jejunal bypass; DJB group, MCD–HF diet for 3 months followed by DJB and MCD–HF diet for subsequent 3 months; HF, high fat; HF group, MCD–HF diet; MCD, methionine–choline-deficient; NC group, normal chow; PGZ group, MCD–HF diet for 3 months followed by treatment with PGZ with MCD–HF diet for subsequent 3 months; PGZ, pioglitazone.
Figure 5.
Masson’s trichrome stain for collagen bundle.
Notes: (A, B) NC and HF groups, increase of collagen bundle. (C, D) DJB or PGZ group, returned to normal condition. Original magnification 20×. (E) Quantitative evaluation of collagen bundle by fold change. *P<0.05.
Abbreviations: DJB, duodenal–jejunal bypass; DJB group, MCD–HF diet for 3 months followed by DJB and MCD–HF diet for subsequent 3 months; HF, high fat; HF group, MCD–HF diet; MCD, methionine–choline-deficient; NC group, normal chow; PGZ group, MCD–HF diet for 3 months followed by treatment with PGZ with MCD-HF diet for subsequent 3 months; PGZ, pioglitazone.
Figure 6.
(A) mRNA expression of fibrogenesis gene. TGF-β1 was significantly downregulated by DJB and PGZ. (B) mRNA expression of lipogenesis gene. SREBF-1 was not improved by DJB or PGZ. Each value is expressed as mean ± standard error of the mean (n=6). *P<0.05. (C) DJB increased LC3 expression (lane 1: NC group; lanes 2 and 3: DJB group; lane 4: HF group). The presenting grouping blots [LC3 or β-actin] were cropped from the same field of the same gel. (D) Quantitative evaluation of LC3 expression by fold change. *P<0.05.
Abbreviations: DJB, duodenal–jejunal bypass; DJB group, MCD–HF diet for 3 months followed by DJB and MCD–HF diet for subsequent 3 months; HF, high fat; HF group, MCD–HF diet; MCD, methionine–choline-deficient; NC group, normal chow; PGZ group, MCD–HF diet for 3 months followed by treatment with PGZ with MCD–HF diet for subsequent 3 months; PGZ, pioglitazone.
DJB enhanced hepatocyte autophagy
Recently, the role of autophagy in hepatic lipid metabolism has been noticed and the NAFLD may be related to the defects of hepatic cell autophagy.20 It has not yet known whether DJB can modify the function of hepatic cell autophagy. We then observe the expression of LC3 in different groups. During the insult of the MCD–HF diet, hepatocyte autophagy was activated as a protective reaction to increase lipophagy (Figure 6C, lane 4). DJB increased the LC3 activity; that is, DJB can consolidate or strengthen the hepatic cell autophagy (Figure 6C, lanes 2 and 3). DJB significantly enhanced the LC3 expression compared to PGZ (P=0.02 and<0.001) (Figure 6D).
Discussion
The initial “two-hit hypothesis” was proposed for NASH pathogenesis by Day and James in 1998.21 Accumulating evidence demonstrates that NASH is a multifactorial disease comprising genetic predisposition, gut-derived endotoxin and ethanol, intestinal microbiota, environmental factors, cell apoptosis, and hepatic autophagy.22 In this study, we discovered multiple benefits from DJB for resolution of NASH.
Though PGZ sometimes induce weight gain due to water retention of body composition in human use, our rat model did not show this effect. Other rat models also demonstrated an equal or even a decrease in body weight while using PGZ.23,24 Based on our results and these reports, whether PGZ can also induce weight gain in different species needs further investigation. We also observed the decrease in body weight in DJB group. Therefore, body weight loss may be a confounding factor when explaining the metabolic improvement from DJB or just body weight loss. We could not identify the time point at which NASH completely developed before the occurrence of the significant body weight difference in the experimental period; instead, we emphasized the clinical observation of early improvements before large body weight loss in a short period after RYGB. In real world, body weight loss after bypass bariatric surgery is an expected beneficial effect in clinical practice. In future, additional studies with checkups at different intervals during the experimental period should be considered.
Currently, many methods and indices are available for the estimation of insulin resistance. Gutch et al have tested and validated the reliability of these methods and indices, including HOMA-IR, the quantitative insulin sensitivity check index, the hyperinsulinemic euglycemic clamp, and intravenous glucose tolerance test.25 Although HOMA-IR has been proposed as a more simple method of evaluating insulin sensitivity, it is less accurate than the hyperinsulinemic euglycemic clamp test. This was a limitation of the present study. Furthermore, it is worth to mention that insulin level was reduced by PGZ with improvement of insulin sensitivity, but not in DJB. In many studies of human subjects after bariatric surgery, the secretion of GLP-1 after meals was enhanced. As we know, GLP-1 is an incretin factor, and it function is to promote the secretion of insulin from pancreatic β cells.
DJB improved liver function and ALT level, but PGZ did not. This finding was compatible with clinical reported temporary hepatotoxicity caused by use of thiazolidinediones such as troglitazone. These results seem to contradict the benefits of PGZ reported in a clinical placebo-controlled study conducted by Belfort et al18 ALT is often included as an outcome in NASH trials; however, its clinical implications are limited, because the changes in ALT reflect acute changes in necroinflammatory activity but are not necessarily parallel to histological disease activity and stage.26 In addition, we observed the lower expression of IL-6 in the DJB group than in the MCD–HF and PGZ groups; however, the results did not reach statistical significance, possibly because of the small sample size (n=6) and wide variation among the individual animals. Nevertheless, we cannot neglect the crucial role of inflammatory cytokines in insulin resistance and NASH.
In this study, no severe fibrotic bundles were found in liver tissues although Masson’s trichrome staining was performed, probably because of the lack of adequate time for the development of liver fibrosis. Xu et al reported a significant development of liver fibrosis from NASH only after 6 months of an HF diet;27 however, we observed the activity of HSCs instead. We observed that DJB inhibited the activity of HSCs to prevent the progression of diet-induced NASH. An MCD diet was reported to upregulate the mRNA expression of TGF-b1 and its downstream molecules, α1 (I) procollagen (collagen I) and plasminogen activator inhibitor-1, in rat livers.17 In this study, the mRNA expression of TGF-b1 was decreased by both DJB and PGZ. About the lipogenic pathway, no differences were observed in SREBF-1 expression regardless of DJB. The influence of DJB on the lipogenesis pathway still needs to be clarified.
Autophagy, the autodigestion of cellular proteins and organelles within a cell, has recently been shown to play an important role in the pathogenesis of insulin resistance, obesity, and NAFLD. Autophagy is also involved in hepatic lipid homeostasis and innate immune response to harmful oxidants and cytokines.28,29 A study reported that autophagic flux was impaired in the livers of mice fed with an MCD diet. Activation of autophagy using rapamycin can attenuate steatosis, fibrosis, inflammation, mitochondrial dysfunction, and ER stress.30 Thus, the pharmacological agents of promoting cell autophagy may provide a novel therapeutic strategy for NASH treatment. By observing the expression of LC3, we found that DJB may improve hepatocyte autophagy. GLP-1 was ever reported to ameliorate NAFLD by enhancing the mitochondrial structure and promoting autophagy via the sirtuin 1 (SIRT1)–SIRT3–forkhead box O3A pathway.31 Liu Y et al have also demonstrated the role of GLP-1 on resolution of NAFLD by promoting hepatic autophagy, fatty acids oxidation or reducing fatty acids synthesis.32 Therefore, the benefit from DJB may be related to the increased secretion of incretin factors following bypass.
Therapeutic options for NAFLD and NASH include diet modification, increased physical activity, weight loss, vitamin E, or PGZ. Several novel therapeutic agents are emerging in clinical trials. It is worth noting that a bile acid-derived ligand for farnesoid X-activated receptor, obeticholic acid, has shown effective results. Bile acids are no longer considered solely for lipid absorption but have diverse effects on regulating host immunity and inflammation. Recently, the role of microbiota in NASH progression and liver carcinogenesis has also been noticed. PK Jena and YJ Wan have further investigated the interaction of microbiota and FXR in Western diet-induced liver injury through FXR knockout (KO) mice. They found that male Western diet-fed FXR KO mice had the most severe steatohepatitis. Hepatic inflammation can be reduced by antibiotics. The improvement was accompanied by decreased free and conjugated secondary bile acids as well as changes in gut microbiota. The results revealed that Lactococcus, Lactobacillus, and Coprococcus protect the liver from inflammation.33 Not only for therapeutic medicine or probiotics, PK Jena and YJ Wan also examined the application of prebiotics in treating NASH. They found that both synbiotics Bifidobacterium infantis and milk oligosaccharides are effective in reversing cancer-prone NASH using Western diet-fed FXR KO mice.34
In this study, we did not assess the effects of DJB on the alterations of incretin hormones and genetic expression. Clinically, there is strong evidence stating that bariatric surgery can cure T2DM in most morbidly obese patients by improving incretin factors. Another critical point is the possible discrepancies between humans and rats in relation to DJB surgery. Whether this concept from the rat model can be applied to humans is questionable. Regarding NASH induction in rats and naturally developed NASH in humans, previous studies have shown a different distribution of bile acid metabolomics and amino acid metabolomics related to entering the citrate cycle and undergoing glycolysis or gluconeogenesis in hepa-tocytes.35 Evidence has also indicated that metabolic alterations after RYGB in rodents might be different from those in humans. The total energy expenditure increase in rodents after RYGB is not observed in humans. In rodent RYGB models, incretins increase not only postprandially but also in the fasting state; however, in humans, an incretin increase occurs only in response to meals. In RYGB rats, the wet weight of the small intestine increases markedly, indicating the hypertrophy of the intestinal mucosa with the increased release of GLP-1 and PYY from L-cells.36 Arora et al observed decreases in rat-specific Lactobacillus animalis and L. reuteri in the small intestine of diabetic fa/fa rats after RYGB and found that the decrease was related to glucose tolerance.37 These findings are critical because the mechanisms have a secondary beneficial effect on liver histology. Therefore, not all results of rodent NASH models can be incorporated into the discussion of the mechanisms in humans. Despite these limitations, we revealed the various mechanisms underlying the potential therapeutic role of DJB for NASH. DJB can be used as an alternative therapeutic option for NASH patients who fail to respond to life modifications and medical interventions.
Conclusion
DJB has a potential role of reducing NAFLD severity and preventing NASH progression. The multifactorial mechanism might be related to the modulation of insulin sensitivity, reduction of TNF-α expression, activity of HSCs, and autophagy of hepatocytes.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of nonalcoholic fatty liver disease. QJM. 2010;103(2):71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, Mccullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 3.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150(5):1147–1159. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. The Lancet. 2016;387(10019):679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 8.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 9.Rubino F, R’bibo SL, del Genio F, Mazumdar M, McGraw TE. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 2010;6(2):102–109. doi: 10.1038/nrendo.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379–388. doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Furuya CK, de Oliveira CP, de Mello ES, et al. Effects of bariatric surgery on nonalcoholic fatty liver disease: preliminary findings after 2 years. J Gastroenterol Hepatol. 2007;22(4):510–514. doi: 10.1111/j.1440-1746.2007.04833.x. [DOI] [PubMed] [Google Scholar]
- 12.Petry TZ, Fabbrini E, Otoch JP, et al. Effect of duodenal-jejunal bypass surgery on glycemic control in Type 2 diabetes: a randomized controlled trial. Obesity. 2015;23(10):1973–1979. doi: 10.1002/oby.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koehestanie P, de Jonge C, Berends FJ, Janssen IM, Bouvy ND, Greve JW. The effect of the endoscopic duodenal-jejunal bypass liner on obesity and type 2 diabetes mellitus, a multicenter randomized controlled trial. Ann Surg. 2014;260(6):984–992. doi: 10.1097/SLA.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 14.de Jonge C, Rensen SS, Koek GH, et al. Endoscopic duodenal-jejunal bypass liner rapidly improves plasma parameters of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11(11):1517–1520. doi: 10.1016/j.cgh.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105(8):1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George J, Pera N, Phung N, Leclercq I, Yun Hou J, Farrell G. Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis. J Hepatol. 2003;39(5):756–764. doi: 10.1016/s0168-8278(03)00376-3. [DOI] [PubMed] [Google Scholar]
- 17.Ota T, Takamura T, Kurita S, et al. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology. 2007;132(1):282–293. doi: 10.1053/j.gastro.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 20.Amir M, Czaja MJ. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5(2):159–166. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 22.Liu W, Baker RD, Bhatia T, Zhu L, Baker SS. Pathogenesis of nonalcoholic steatohepatitis. Cell Mol Life Sci. 2016;73(10):1969–1987. doi: 10.1007/s00018-016-2161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding SY, Shen ZF, Chen YT, Sun SJ, Liu Q, Xie MZ. Pioglitazone can ameliorate insulin resistance in low-dose streptozotocin and high sucrose-fat diet induced obese rats. Acta Pharmacol Sin. 2005;26(5):575–580. doi: 10.1111/j.1745-7254.2005.00090.x. [DOI] [PubMed] [Google Scholar]
- 24.Gad MZ, Ehssan NA, Ghiet MH, Wahman LF. Pioglitazone versus metformin in two rat models of glucose intolerance and diabetes. Pak J Pharm Sci. 2010;23(3):305–312. [PubMed] [Google Scholar]
- 25.Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. 2015;19(1):160. doi: 10.4103/2230-8210.146874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahady SE, Webster AC, Walker S, Sanyal A, George J. The role of thiazolidinediones in non-alcoholic steatohepatitis – a systematic review and meta analysis. J Hepatol. 2011;55(6):1383–1390. doi: 10.1016/j.jhep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Xu ZJ, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55(4):931–940. doi: 10.1007/s10620-009-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavallard VJ, Gual P. Autophagy and non-alcoholic fatty liver disease. BioMed Res Int. 2014;2014(4):1–13. doi: 10.1155/2014/120179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10(8):837–858. doi: 10.1016/j.cgh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Chen R, Wang Q, Song S, Liu F, He B, Gao X. Protective role of autoph-agy in methionine-choline deficient diet-induced advanced nonalcoholic steatohepatitis in mice. Eur J Pharmacol. 2016;770(C):126–133. doi: 10.1016/j.ejphar.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Tong W, Ju L, Qiu M, et al. Liraglutide ameliorates non-alcoholic fatty liver disease by enhancing mitochondrial architecture and promoting autophagy through the SIRT1/SIRT3-FOXO3a pathway. Hepatol Res. 2016;46(9):933–943. doi: 10.1111/hepr.12634. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Wei R, Hong TP. Potential roles of glucagon-like peptide-1-based therapies in treating non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20(27):9090–9097. doi: 10.3748/wjg.v20.i27.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jena PK, Sheng L, Liu HX, et al. Western diet-induced dysbiosis in farnesoid X receptor knockout mice causes persistent hepatic inflammation after antibiotic treatment. Am J Pathol. 2017;187(8):1800–1813. doi: 10.1016/j.ajpath.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jena PK, Sheng L, Nagar N, et al. Synbiotics Bifidobacterium infantis and milk oligosaccharides are effective in reversing cancer-prone nonalcoholic steatohepatitis using Western diet-fed FXR knockout mouse models. J Nutr Biochem. 2018;57:246–254. doi: 10.1016/j.jnutbio.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han J, Dzierlenga AL, Lu Z, et al. Metabolomic profiling distinction of human nonalcoholic fatty liver disease progression from a common rat model. Obesity. 2017;25(6):1069–1076. doi: 10.1002/oby.21855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutz TA, Bueter M. The physiology underlying Roux-en-Y gastric bypass: a status report. Am J Physiol Regul Integr Comp Physiol. 2014;307(11):R1275–R1291. doi: 10.1152/ajpregu.00185.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arora T, Seyfried F, Docherty NG, et al. Diabetes-associated micro-biota in fa/fa rats is modified by Roux-en-Y gastric bypass. ISME J. 2017;11(9):2035–2046. doi: 10.1038/ismej.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]