Abstract
Respiratory syncytial virus (RSV) is the leading viral cause of severe pediatric respiratory illness, and a safe and effective vaccine for use in infancy and early childhood is needed. We previously showed that deletion of the coding sequence for the viral M2–2 protein (ΔM2–2) down-regulated viral RNA replication and up-regulated gene transcription and antigen synthesis, raising the possibility of development of an attenuated vaccine with enhanced immunogenicity. RSV MEDI ΔM2–2 was therefore evaluated as a live intranasal vaccine in adults, RSV-seropositive children, and RSV-seronegative children. When results in RSV-seronegative children were compared to those achieved with the previous leading live attenuated RSV candidate vaccine, vaccine virus shedding was significantly more restricted, yet the post-vaccination RSV-neutralizing serum antibody achieved [geometric mean titer (GMT) = 1:97] was significantly greater. Surveillance during the subsequent RSV season showed that several seronegative RSV MEDI ΔM2–2 recipients had substantial antibody rises without reported illness, suggesting that the vaccine was protective yet primed for anamnestic responses to RSV. Rational design appears to have yielded a candidate RSV vaccine that is intrinsically superior at eliciting protective antibody in RSV-nai’ve children and highlights an approach for the development of live attenuated RSV vaccines.
INTRODUCTION
Respiratory syncytial virus (RSV) is the most important viral cause of severe acute lower respiratory illness (LRI) in infants and children worldwide (1,2). In the United States, RSV is the leading cause of hospitalization in children less than 1 year of age (3) and is associated with a considerable burden of emergency room and outpatient care, with 10% of children less than 5 years of age receiving medical attention for RSV-associated illness each year (1). Globally, RSV has been estimated to cause >3.5 million hospitalizations and 66,000 to 199,000 deaths annually (2). The relative importance of RSV as a pulmonary pathogen has also increased as the use of vaccines to prevent bacterial pneumonias has become widespread (4). Because of the substantial global impact of RSV, increased efforts are under way to develop RSV vaccines for use in infancy and early childhood (5, 6).
A live attenuated RSV vaccine would be an attractive strategy for immunization of children and infants beyond the neonatal period. Live attenuated vaccines mimic a mild natural infection and induce durable cellular and humoral immune responses. A live attenuated RSV vaccine would be administered intranasally, inducing local respiratory tract immunity in addition to systemic immunity. Furthermore, administration of candidate live attenuated RSV vaccines (7–11) is not associated with the vaccine-associated enhanced RSV disease that was observed in children who received formalin-inactivated RSV (12) and that also appeared to be associated with administration of RSV subunit vaccines in experimental animals (13, 14).
Efforts to develop live attenuated RSV vaccines have been under way since the 1970s. The process of attenuation has been challenging because conventional methods, such as passage of virus at suboptimal temperatures or in the presence of mutagens, are targeted imprecisely and are poorly controlled. In addition, clinical attenuation typically is based on restriction of replication, which decreases antigenic load and diminishes the immune response. In the past, achieving a balance between attenuation and immunogenicity has proved difficult: some live attenuated RSV vaccine candidates have been insufficiently attenuated (7,15), whereas others were highly attenuated but insufficiently immunogenic (9). In addition, some candidate vaccines have exhibited genetic instability, with a partial loss of attenuating mutations (8).
Progress in the elucidation of RSV gene function (5) and the use of reverse genetics systems (16) has led to the development of “rationally designed” engineered attenuated RSV strains that may improve upon previous conventional vaccine candidates. In one such virus, most of the open reading frame (ORF) of the RNA synthesis factor M2–2 was deleted (17). Compared to wild-type RSV, this RSV ΔM2–2 mutant exhibited a shift in the viral RNA synthesis program such that genome replication was decreased and gene transcription was increased (17). This resulted in a substantial increase in synthesis of the viral proteins, including the major neutralization and protective antigens, suggesting the possibility of an inherent increase in immunogenicity per infectious unit. In addition, because it is based on a large deletion that ablates expression of a viral protein, the attenuation phenotype of RSV ΔM2–2 would be expected to be very stable, obviating a complication of vaccines based on point mutations. In nonhuman primates, RSV ΔM2–2 was highly restricted in replication but induced substantial neutralizing serum antibody responses and protected against challenge, although it could not be determined whether RSV ΔM2–2 was inherently more immunogenic than wild-type RSV (18). On the basis of this preclinical profile of substantial attenuation and immunogenicity, we have now conducted a stepwise phase 1 evaluation of the RSV deletion mutant RSV MEDI ΔM2–2 vaccine candidate in adults and in RSV-seropositive and RSV-seronegative children. In RSV-seronegative children, RSV MEDI ΔM2–2 was highly restricted in replication yet more immunogenic than the previous lead live attenuated RSV vaccine candidate. Postvaccination surveillance provided evidence that some vaccinees experienced substantial increases in titers of serum RSV neutralizing antibodies in the absence of reported illness, which suggests that these vaccinees had been exposed to wild-type RSV but had been protected from significant disease. In addition, the increases in RSV-neutralizing serum antibody titers suggest that these vaccinees were primed for substantial anamnestic responses to RSV.
RESULTS
Study participants
RSV MEDI ΔM2–2 was sequentially evaluated in 15 adults, 15 RSV-seropositive children, and 30 RSV-seronegative infants and children. As noted in Materials and Methods, this vaccine was evaluated in an open-label trial in adults and in randomized, placebo-controlled, double-blind trials in the pediatric populations (Fig. 1). All adults received 106.0 plaque-forming units (PFU) of vaccine; 10 RSV-seropositive children received 106.0 PFU of vaccine and 5 received placebo; and 20 RSV-seronegative vaccinees received 105.0 PFU ofvaccine and 10 received placebo. The mean age of adult participants was 36 years (range, 20 to 49 years); of RSV-seropositive participants, 37 months (range, 18 to 59 months); and of RSV-seronegative participants, 13 months (range, 6 to 24 months). Of the 60 participants, 48% were female, 53% white, 33% black, and 14% described as of mixed race.
Fig. 1. Screening, enrollment, and follow-up of RSV-seropositive children and RSV-seronegative children in the phase 1 clinical trial of the RSV MEDI Δ M2–2 vaccine.
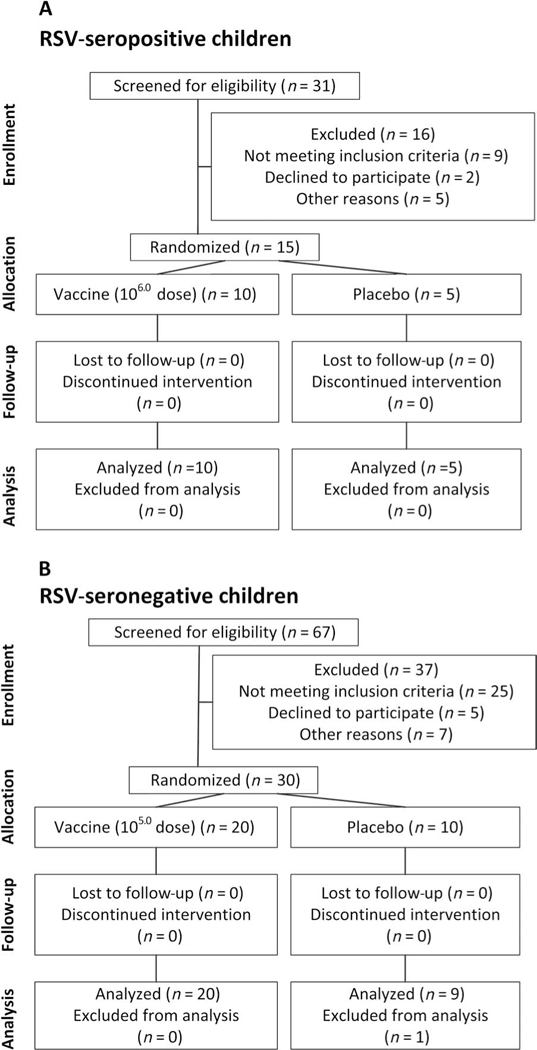
(A) RSV-seropositive children. (B) RSV-seronegative children. As described in the text, we initiated the study with administration of the RSV MEDI Δ M2–2 vaccine to 15 adults in an open-label evaluation. For this portion of the study, 31 adults were screened, 19 were eligible, 15 were enrolled, and none were lost to follow-up.
Infectivity and adverse events
During the 28-day postimmunization reporting period, 3 of 15 vaccinated adults developed respiratory illnesses [hoarseness (1), pharyngitis (1), and rhinorrhea and cough (1)], but none shed vaccine virus or had rises in serum RSV antibody titers. Upper respiratory illness [(URI); rhinorrhea] was observed in 4 of 10 vaccinated RSV-seropositive children during this 28-day period (Table 1); each symptomatic child had rhino-virus or enterovirus detected in nasal wash (NW) at the time of illness. As in the adults, none of the seropositive children shed vaccine virus, which is consistent with attenuation.
Table 1.
Replication of vaccine virus and clinical assessment in adults, RSV-seropositive children, and RSV-seronegative children. NT, not tested.
| Viral detection (culture) |
Viral detection (qPCR) |
% with indicated illness* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | Dose (log10 PFU/ml) |
No. of subjects |
% Infected |
% Shedding virus† |
Peak titer mean (SD) log10 PFU/ml |
Peak copy number log10 (SD) |
Fever | URI | LRI | Cough | OM | Respiratory or febrile illness |
Other |
| Adults | |||||||||||||
| Vaccinees | 6.0 | 15 | 0 | 0 | 0.6 (0.0) | NT | 0 | 20 | 0 | 7 | 0 | 20 | 27 |
| Seropositive children | |||||||||||||
| Vaccinees | 6.0 | 10 | 10 | 0 | 0.6 (0.0) | NT | 0 | 40 | 0 | 0 | 0 | 40 | 20 |
| Placebo recipients |
Placebo | 5 | 20 | 0 | 0.6 (0.0) | NT | 0 | 0 | 0 | 0 | 0 | 0 | 60 |
| Seronegative children | |||||||||||||
| Vaccinees | 5.0 | 20 | 95 | 85 | 1.5 (0.9) | 3.4 (0.9) | 20 | 85 | 0 | 35 | 5 | 85 | 45 |
| Infected placebo recipient‡ |
Placebo | 1 | 100 | 100 | 2.0 (0.0) | 3.3 (0.0) | 100 | 100 | 0 | 0 | 0 | 100 | 0 |
| Placebo recipients |
Placebo | 9 | 0 | 0 | 0.6 (0.0) | 1.7 (0.0) | 22 | 44 | 0 | 33 | 0 | 67 | 78 |
Illness abbreviations and definitions are as described in the text. URI was defined as rhinorrhea, pharyngitis, or hoarseness, and LRI was defined as wheezing, rhonchi, or rales, or having been diagnosed with pneumonia or laryngotracheobronchitis (croup).
% Shedding vaccine virus as detected by culture and/or rRT-qPCR. The limit of detection of vaccinevirus was 0.6 log^ PFU/ml by culture, and 1.7 log10™ copy numbers/ml by rRT-qPCR.
Data from the seronegative placebo recipient, as described in the text.
URI (rhinorrhea), cough, and febrile illnesses occurred frequently in RSV-seronegative vaccinees and placebo recipients during the 28 days after immunization (Table 1 and Fig. 2). The rates of fever (20% versus 22%) and cough (35% versus 33%) were comparable in vaccinees and placebo recipients (Table 1 and Fig. 2). URI occurred more often in seronegative vaccinees than in placebo recipients (85% versus 44%), but this difference was not significant and all episodes were of grade 1 (mild) severity (Table 1 and Fig. 2). None ofthe children experienced LRI, and one vaccinee had otitis media (OM). Other viruses were detected frequently in symptomatic children, including rhinovirus, enterovirus, parechovirus, adenovirus, coronavirus, bocavirus, and parainfluenza viruses types 1 and 4.
Fig. 2. Proportions of RSV-seronegative vaccinees and placebo recipients with indicated illnesses.
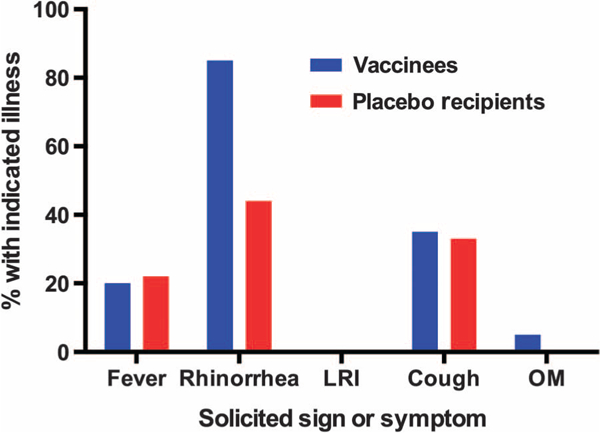
Vaccinees are shown in blue; placebo recipients are shown in red. The placebo recipient (twin B) infected with vaccine virus is not included. Fever occurred in 4 of 20 vaccinees (1 of grade 0 severity, 1 of grade 2 severity, and 2 of grade 3 severity) and in 2 of 9 placebo recipients (both of grade 2 severity): grade 0, <38°C; grade 1, ≥38°C but ≤38.6°C; grade 2, ≥38.7°C but ≤39.1°C; grade 3, ≥39.2°C but ≤40.5°C. The two episodes of grade 3 fever in vaccinees occurred on days 24 to 26 after vaccination, when shedding of vaccine virus was not detected. URI (rhinorrhea) occurred in 17 of 20 vaccinees and 4 of 9 placebo recipients; cough occurred in 7 of 20 vaccinees and 3 of 9 placebo recipients; all of these illnesses were of grade 1 severity (that is, not requiring medical attention). An episode of OM (grade 2 severity) occurred in a single vaccinee.
Kawasaki disease was reported as a serious adverse event (SAE) in a seronegative vaccinee. Vaccine virus was detected in this child’s NW by culture on study days 5,7, and 10 and by real-time reverse transcription quantitative polymerase chain reaction (rRT-qPCR) on study days 3 to 12, and the child had rhinorrhea on study days 8 to 11. The child was otherwise without symptoms until study day 32 when she became febrile. She subsequently developed a rash and oral lesions that were consistent with Kawasaki disease and was hospitalized on study day 35. The child was treated with aspirin and intravenous immunoglobulin (IGIV) and recovered without sequelae. The SAE was judged to be unrelated to the study product on the basis of the interval between infection with vaccine virus and onset of symptoms, as well as the absence of any known association between RSV and Kawasaki disease.
Transmission of vaccine virus occurred between a seronegative vaccinee and a placebo recipient who were 13-month-old twin siblings. Twin A (the vaccinee) had vaccine virus detected in NW on study days 5, 7, and 10 and rhinorrhea on study days 8 to 11. Twin B (the placebo recipient) had vaccine virus detected in NW on study days 14 and 17, accompanied by a temperature of 38.1°C on day 17 (infected placebo recipient, Table 1). The peak titer of vaccine virus shed by each child was 2.0 log10 PFU/ml. Sequence analysis revealed that the vaccine virus shed by both children retained the deletion of the M2–2 ORF. Illness, replication, and immunogenicity data from this infected placebo recipient are reported as a separate line item in Tables 1 and 2, and surveillance data from this placebo recipient were excluded from the analysis.
Table 2.
Replication of vaccine virus and antibody responses in adults, RSV-seropositive children, and RSV-seronegative children. ELISA, enzyme-linked immunosorbent assay.
| Serum RSV neutralizing antibody* [mean(SD)] |
Serum IgG ELISA RSV F*
[mean (SD)] |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | Dose (log10
PFU/ml) |
No. of subjects |
% Infected |
% Shedding virus† |
Pre (SD) |
Post (SD) |
≥4-Fold rise (%) |
Pre (SD) |
Post (SD) |
≥4-Fold rise (%) |
| Adults | ||||||||||
| Vaccinees | 6.0 | 15 | 0 | 0 | 9.3 (1.1) | 9.6 (0.9) | 0 | 15.1 (1.2) | 15.0 (1.2) | 0 |
| Seropositive children | ||||||||||
| Vaccinees | 6.0 | 10 | 10 | 0 | 7.5 (1.9) | 7.7 (1.8) | 0 | 14.2 (2.3) | 14.0 (2.1) | 10 |
| Placebo recipients | Placebo | 5 | 20 | 0 | 8.4 (2.7) | 9.0 (2.9) | 20 | 14.8 (1.1) | 15.6 (1.4) | 20 |
| Seronegative children | ||||||||||
| Vaccinees | 5.0 | 20 | 95 | 85 | 2.7 (0.9) | 6.6 (1.1) | 95 | 7.1 (2.7) | 13.6 (1.6) | 90 |
| Infected placebo recipient‡ |
Placebo | 1 | 100 | 100 | 2.3 (0.0) | 7.6 (0.0) | 100 | 5.6 (0.0) | 13.6 (0.0) | 100 |
| Placebo recipients | Placebo | 9 | 0 | 0 | 2.3 (0.0) | 2.3 (0.0) | 0 | 5.4 (1.5) | 5.1 (1.0) | 0 |
Antibody data are expressed as reciprocal mean log2 titers.
% Shedding vaccine virus as detected by culture and/or rRT-qPCR. The limit of detection of vaccine virus was 0.6 log10™ PFU/ml.
Data from the seronegative placebo recipient, as described in the text.
Replication and genetic stability of RSV MEDI ΔM2–2
As noted above, vaccine virus was not detected by viral culture in NW obtained from adults and RSV-seropositive children, consistent with the restriction in replication expected for live attenuated RSV vaccines in RSV-experienced populations. In contrast, vaccine virus was detected in NW by culture in 12 of 20 RSV-seronegative vaccinees and by RT-qPCR in 17 of 20 RSV-seronegative vaccinees (Table 1 and Fig. 3A; the 3 vaccinees who were negative by RT-qPCR also were negative by culture). The mean peak titer of virus detected by culture in these children was 1.5 log10 PFU/ml. This was significantly lower than that detected in NW from seronegative recipients of rA2 cp248/404/1030/DSH (2.5 log10 PFU/ml, P = 0.005; Fig. 3A), a previously studied vaccine candidate that was well tolerated and immunogenic in seronegative infants and children (8). Partial sequence analysis of NW isolates obtained at the peak of vaccine shedding from 15 RSV-seronegative vaccinees verified the presence of the M2–2 deletion. PCR products for sequence analysis could not be obtained from four seronegative vaccinees from whom infectious vaccine virus was not recovered and from one vaccinee with low peak titer (1.3 log10 PFU/ml).
Fig. 3. Vaccine virus shedding and serum RSV neutralizing antibody responses in seronegative recipients of RSV MEDI DM2–2 or rA2 cp248/404/1030/ΔSH.
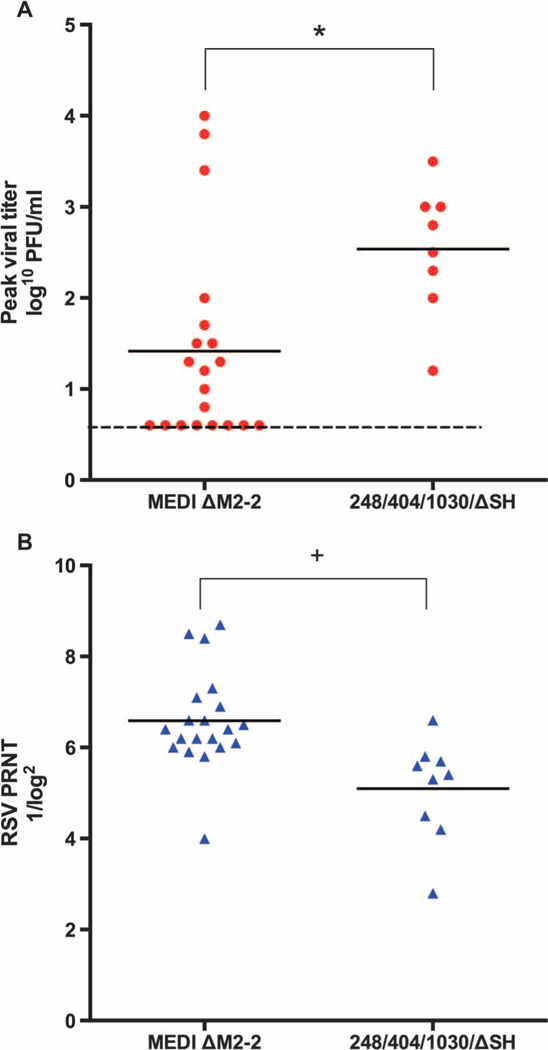
NW and serum specimens from the present study were evaluated for vaccine virus titer and serum neutralizing antibodies, respectively. Antibody testing was performed in parallel with specimens from the previous clinical evaluation of rA2 cp248/404/1030/DSH (8). (A) Peakviral titer (expressed as log10 PFU/ml). (B) RSV PRNTs, expressed as 1/log2. *P = 0.005; +P = 0.002 (Student’s t test).
Antibody responses to RSV MEDI ΔM2–2
A single RSV-seropositive vaccinee had a rise in RSV F serum immunoglobulin G (IgG) titer (Table 2). Thus, none of the adults and one RSV-seropositive vaccinee had evidence of infection with vaccine virus, which is consistent with attenuation. In addition, a seropositive placebo recipient had a rise in both RSV F serum IgG and RSV-neutralizin serum antibody titers [measured as plaque reduction neutralization titer (PRNT)], which likely indicated an infection with a circulating wild-type RSV that was not detected during the postinoculation follow-up period.
Nineteen of 20 RSV-seronegative children developed neutralizing serum antibody responses, and 18 of 20 developed RSV F serum IgG antibody responses after vaccination (Table 2 and Fig. 4). In seronegative children, the mean postvaccination PRNT was 6.6 log2, or 1:97 (Table 2); 85% achieved PRNT ≥6.0 log2, or 1:64 (Fig. 4). There was no apparent relationship between the magnitude of vaccine virus shedding and the magnitude of the antibody response. Mean PRNTs were significantly higher in seronegative recipients of MEDI ΔM2–2 than in recipients of rA2 cp248/404/1030/ΔSH (6.6 log2 versus 5.1 log2, or 1:97 versus 1:34; P = 0.002; Fig. 3B). With the exception of the twin to whom vaccine virus was transmitted, none of the RSV-seronegative placebo recipients developed a rise in RSV PRNT.
Fig. 4. Reverse cumulative distribution of RSV PRNT in seronegative vaccinees.
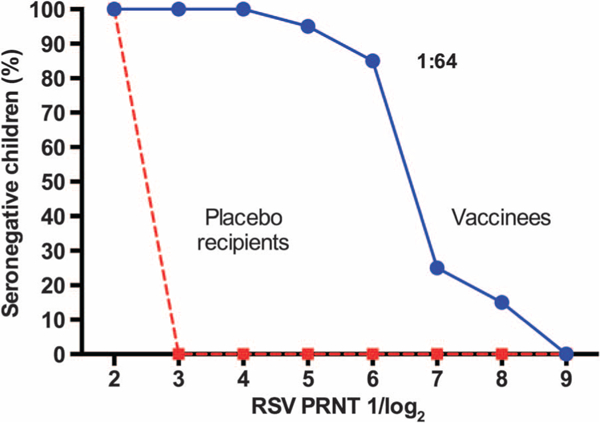
The blue solid line represents vaccinees, and the red dashed line represents placebo recipients. Antibody titers are expressed as 1/log2, but for ease of interpretation, the titer corresponding to the arithmetic value of 1:64, achieved by 85% of vaccinees, is also shown.
RSV surveillance in seronegative children: Illness and antibody response
Surveillance for medically attended acute respiratory illness (MAARI) was conducted for the seronegative cohort during the RSV season (1 November to 31 March) after vaccine administration, and pre-and postsurveillance sera were collected and tested for PRNT as described in Materials and Methods. MAARI was frequent, with 15 episodes occurring in seven vaccinees and 10 episodes occurring in six placebo recipients. However, only three instances of RSV-associated MAARI were detected: RSV subgroup A-associated illness occurred in one placebo recipient (rhinorrhea, cough, and wheezing), and RSV subgroup B-associated illness occurred in one vaccinee (rhinorrhea and cough) and one placebo recipient (rhinorrhea and fever).
Despite infrequent RSV MAARI, ≥4-fold increases in RSV PRNT were detected in postsurveillance sera from three placebo recipients and six vaccinees, including the three subjects in whom wild-type RSV was detected. The mean postsurveillance log2 PRNT in the three placebo recipients in whom rises were observed (6.6 log2) was identical to the mean log2 PRNT observed in the vaccine recipients after vaccination (Table 2). Thus, the mean RSV PRNT achieved after a single dose of RSV MEDI ΔM2–2 in RSV-seronegative children was the same as that achieved after a primary wild-type RSV infection. Notably, the postsur-veillance geometric mean PRNT in the six vaccinated children in whom rises were observed were significantly greater than those achieved in the placebo recipients after natural infection (9.8 log2 versus 6.6 log2, or 1:891 versus 1:97, P = 0.046; Fig. 5). One of the six vaccinees (subject 35, supplementary tables) did not shed vaccine virus and did not have a rise in antibody titer after vaccination, suggesting that he did not have a vaccine “take” and experienced a primary immune response to infection with wild-type RSV. The postsurveillance geometric mean PRNT among the remaining five vaccinees with an initial vaccine take was 10.3 log2 or 1:1261. Three of these five children achieved postsurveillance serum neutralizing antibody titers of >1:1000, indicating that potent anamnestic responses occurred after wild-type RSV infection in vaccine-primed individuals (Fig. 5). These data suggest that a number of vaccine recipients had clinically inapparent wild-type RSV infections during the surveillance period that boosted the RSV PRNT greater than ninefold (on average) over the titers achieved after immunization. For vaccine recipients without a significant rise in PRNT, the mean reciprocal log2 titer was 6.5 (1:91) after immunization and 6.3 (1:79) after the surveillance period, indicating that a neutralizing antibody response was sustained for at least 5 to 12 months after immunization.
Fig. 5. Rises in RSV PRNT during the surveillance period.
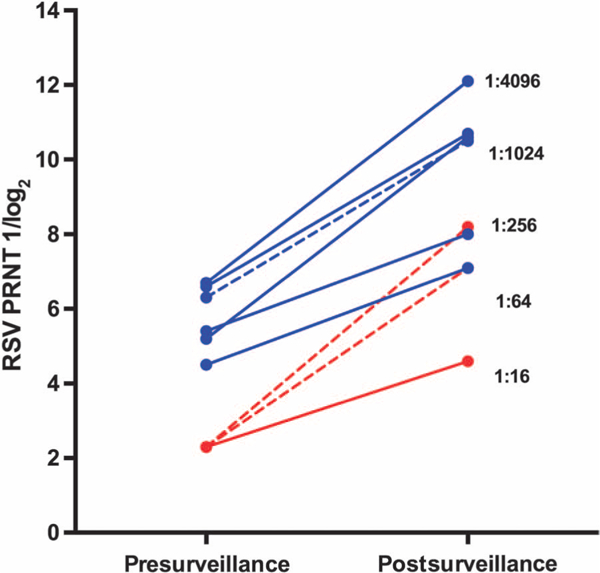
RSV PRNT in sera from six vaccinees and three placebo recipients in whom ≥4-fold rises in titer between the pre-and postsurveillance specimens were detected (see text for additional details). Blue solid lines, vaccinees with rises in RSV PRNT in whom no RSV MAARI was detected (n = 5; corresponding to subjects 33,35, 44,46, and 56 in the supplementary tables); blue dashed line, vaccinee with rise in RSV PRNT in whom RSV MAARI was detected (n = 1; subject 38 in the supplementary tables); red solid line, placebo recipient with rise in RSV PRNT in whom no RSV MAARI was detected (n = 1; subject 36 in the supplementary tables); red dashed lines, placebo recipients with rise in RSV PRNT in whom RSV MAARI was detected (n = 2; subjects 43 and 57 in the supplementary tables). Antibody titers are expressed as 1 /log2, but for ease of interpretation, titers corresponding to the arithmetic values of 1:16,1:64,1:256,1:1024, and 1:4096 are also shown.
DISCUSSION
RSV MEDI DM2–2 was created on the basis of knowledge of RSV gene function combined with the ability to make defined viral strains by reverse genetics (17). The vaccine was attenuated by deletion of most of the ORF encoding the M2–2 RNA synthesis regulatory factor (17, 19). To our knowledge, this is the first time that a viral vaccine candidate designed for increased gene transcription has been evaluated in humans. Previous live RSV vaccine candidates were attenuated by point mutations induced by serial passage at low temperature (cold-passaged) or growth in the presence of chemical mutagens and selection for reduced viral replication at elevated temperature (temperature-sensitive). These mutations were included in both biologically derived and reverse-engineered vaccine candidates (7–10,15). More recently, we used gene deletion as an additional attenuation strategy and created candidate vaccines in which the genes encoding either the small hydrophobic SH protein or the nonstructural NS2 protein were removed (8). For each of these previous vaccine candidates, restriction of viral replication was associated with clinical attenuation (8), but vaccine candidates that were highly restricted in replication did not induce substantial PRNT in RSV-naïve children. Thus, it was difficult to achieve an appropriate balance between attenuation and antibody response in the pediatric target population.
Previous analysis of ΔM2–2 mutants in cell culture showed that deletion of the M2–2 ORF results in a shift in the viral RNA synthesis program (17). During wild-type RSV infection, the intracellular accumulation of viral mRNAs increases rapidly until 12 to 15 hours after infection, followed by a shift in RNA synthesis to favor the accumulation of viral genomes and antigenomes. However, with the ΔM2–2 mutant, this shift is muted, and the accumulation of mRNAs continues to increase, whereas the accumulation of viral genomes and antigenomes is substantially delayed and decreased. The reduction in intracellular genomes available for packaging into progeny virions likely accounts for the attenuation phenotype. Consistent with this, the production of ΔM2–2 virions in vitro was substantially delayed compared to wild-type RSV; however, the final yield of ΔM2–2 was comparable to wild-type RSV, and therefore growth of ΔM2–2 should not impede manufacture. The increase in mRNA accumulation with the ΔM2–2 mutant in vitro results in a global increase in the synthesis of viral proteins, including RSV F and G, the viral neutralization and major protective antigens (17). We assume that this shift in RNA synthesis and increase in viral protein synthesis also occurred in vivo, leading to an enhanced neutralizing antibody response. RSV MEDI ΔM2–2 was significantly more restricted in replication yet induced significantly higher levels of neutralizing antibodies in seronegative children than rRSV cp248/404/1030/ ΔSH, the previous leading live RSV vaccine candidate. These data suggest that this attenuating mutation has produced a vaccine virus that induces a greater antibody response per infectious unit than previous attenuated strains via up-regulation of viral gene transcription and antigen synthesis. Also, though we were unable to measure cellular immune responses to the vaccine in these very young children because of limitations on specimen collection, we hypothesize that the M2–2 deletion might increase RSV-specific T cell responses as a result of increased expression of all viral genes and antigens.
We did not observe LRI after administration of this vaccine, and the rates of fever, cough, and OM were comparable in seronegative vaccinees and placebo recipients. Rhinorrhea occurred in 73% of seronegative children, and more often in seronegative vaccinees (85%) than placebo recipients (44%), although this difference was not statistically significant. Other respiratory viruses were detected frequently in both groups, consistent with previous studies in young children (8, 20). It is not possible to determine from a study of this size whether administration of RSV MEDI ΔM2–2 is associated with a true increased risk of rhinorrhea. This question could be addressed in expanded clinical trials. Even if administration of RSV MEDI ΔM2–2 were associated with rhinorrhea, this would likely be acceptable if the rhinorrhea were mild and transient and if efficacy against RSV-associated LRI is demonstrated. If the frequency or severity of rhinorrhea were unacceptable, it should also be possible to add additional attenuating mutations to a virus that contained the M2–2 deletion. A number of additional attenuating mutations have been developed and are available for inclusion with the M2–2 deletion (21).
Here, we also observed transmission of RSV MEDI ΔM2–2 between 13-month-old twin siblings. In both children, replication of vaccine virus was restricted, and illness was mild (rhinorrhea in one child and a single day of fever with maximum temperature of 38.1°C in the other child). With this type of intense child-to-child exposure, limited transmission of a live attenuated respiratory virus is not unexpected; for example, transmission of live attenuated influenza vaccine has been shown to occur in a daycare setting (22). Vaccine virus shed by both children retained the M2–2 deletion. This is consistent with the expectation that an attenuation phenotype based on a deletion mutation in a negative-stranded RNA virus should be very stable, which is an important advantage for the ΔM2–2 vaccine virus. Whereas the transmission potential for the vaccine candidate should be evaluated in additional studies, the stability of the attenuation phenotype should greatly mitigate concern. The potential for transmission of this highly attenuated stable virus should be weighed against the natural epidemiology of wild-type RSV, a ubiquitous seasonal pathogen that infects nearly all children by 2 years of age and that causes symptomatic primary infections (23).
We conducted surveillance for RSV-associated MAARI in all RSV-seronegative children who participated in this study, with clinical assessment and NW for viral identification performed for each illness. There was no evidence of enhanced RSV disease in any vaccine recipient. This is consistent with studies in experimental animals, with many previous studies of live attenuated RSV vaccines in RSV-naïve children (10) and with the natural epidemiology of RSV infection and reinfection, which occur without disease enhancement.
We obtained serum specimens before and after the RSV surveillance period, which allowed us to compare the proportion of children with RSV MAARI to the proportion with a ≥ 4-fold rise in RSV PRNT between pre-and postseason specimens. RSV MAARI occurred in 1 of 20 vaccinees (RSV B virus) and 2 of 9 placebo recipients (1 RSV A and 1 RSV B virus). Although it is of interest that MEDI ΔM2–2 is a subgroup A RSV and the sole wild-type symptomatic infection that occurred in a vaccinee was a subgroup B RSV, additional work is needed to assess the level of protection against each subgroup. During the surveillance period, fourfold rises in neutralizing antibody occurred in 6 of 20 vaccinees and 3 of 9 placebo recipients; 1 of these 6 vaccinees and 2 of the 3 placebo recipients had RSV MAARI. Although the number of events is small, these data suggest that wild-type RSV infection without MAARI occurred more frequently in vaccinees (five of six) than in placebo recipients (one of three). Expanded studies are needed to further assess these findings.
When wild-type RSV infection occurred (either documented RSV-associated MAARI or inferred from rises in PRNT), the postsurveillance PRNT was substantial and significantly higher in vaccinees than in placebo recipients. This suggests that vaccinees were primed for strong anamnestic responses after infection with wild-type RSV. The mean PRNT in postsurveillance specimens was 9.8 log2 (1:891) in the six vaccinees with antibody rises and was 10.3 log2 (1:1261) in the five vaccinees with evidence of a vaccine take. This is well above the titers (~1:250) associated with protection of the lower respiratory tract against RSV infection in preclinical and clinical studies of RSV-IGIV (24, 25). Moreover, in contrast to RSV-IGIV, a live intranasal vaccine should also induce local and systemic cellular immunity and mucosal humoral immunity and therefore offer greater protection than would be suggested by PRNT alone.
In summary, the ΔM2–2 vaccine was significantly more restricted in replication yet induced higher PRNT than the previous lead live attenuated RSV vaccine candidate RSVcp248/404/1030/ΔSH. We postulate that these findings are the result of up-regulated gene transcription and antigen synthesis resulting in increased immunogenicity per infectious viral unit. The substantial rises in antibody titer observed in several vaccinees in the absence of MAARI during the RSV season are suggestive of protection and merit further investigation. Additionally, the M2–2 gene deletion was shown to be highly stable and is not susceptible to reversion. This mechanism of attenuation appears to “delink” viral replication and the immune response and may help overcome fundamental hurdles in live attenuated RSV vaccine development. Further evaluation of vaccine candidates containing the M2–2 deletion mutation is warranted.
MATERIALS AND METHODS
Vaccine
To generate a recombinant RSV vaccine with an M2–2 deletion, a complementary DNA (cDNA) fragment of 234 nucleotides containing the 78 C-terminal codons of the M2–2 ORF was removed from the antigenomic cDNA encoding the wild-type RSV A2 strain. The sequence encoding the first 12 codons of the M2–2 ORF, that is, the region that mostly overlaps the M2–1 ORF, was maintained (19). The resulting plasmid, designated pRSV A2 DM2–2, was used for the recovery of the drug substance at MedImmune. The MEDI ΔM2–2 virus was previously described in (19) and was provided under a Cooperative Research and Development Agreement between the National Institute of Allergy and Infectious Diseases (NIAID) and Med-Immune. Requests for this virus should be directed to MedImmune LLC.
Clinical trial material (CTM) was manufactured in qualified Vero cells at Meridian Life Science Inc. Sequence analysis confirmed that the seed virus and Final Drug Product, RSV Lot no. 002A, were of identical sequence, including the three polymorphisms. The RSV MEDI DM2–2 CTM was supplied to the clinical site as a frozen concentrate with a mean infectivity titer of 107.0 PFU/ml. CTM was stored at −70°C and diluted to dose on-site using Leibovitz L15 medium. L15 medium was also used as the placebo.
Study population, study design, and clinical trial oversight
This phase 1 trial was conducted at the Center for Immunization Research (CIR), Johns Hopkins Bloomberg School of Public Health, and Seattle Children’s Research Institute between September 2011 and March 2014 (ClinicalTrials.gov NCT01459198). The RSV MEDI ΔM2–2 vaccine was evaluated sequentially in (i) adults who were not screened for RSV serostatus (but who all proved to be RSV-seropositive), (ii) RSV-seropositive children ages 15 to 59 months, and (iii) RSV-seronegative children ages 6 to 24 months. Studies in adults were conducted as open-label trials, with all subjects receiving vaccine. Studies in children were conducted as randomized, double-blind, and placebocontrolled trials, with subjects randomized 2:1 to receive vaccine or placebo (Fig. 1). Randomization was performed in blocks of three (two vaccinees and one placebo recipient) to permit unblinding and analysis of adverse events throughout the study, which was done when each group of three subjects completed the initial follow-up period (about 28 days for RSV-seropositive children and about 56 days for RSV-seronegative children). To maintain the study blind, randomization and preparation of the study agent (vaccine or placebo) were performed by study personnel who were not involved in the clinical assessment of study subjects. Vaccine and placebo were each administered in a volume of 0.5 ml as nose drops (about 0.25 ml per nostril). Adults and RSV-seropositive vaccinees received 106.0 PFU of vaccine; RSV-seronegative vaccinees received 105.0 PFU of vaccine.
Written informed consent was obtained from study participants (adults) or from the parents or guardians of study participants (children) before enrollment. These studies were conducted in accordance with the principles of the Declaration of Helsinki and the Standards of Good Clinical Practice (as defined by the International Conference on Harmonization). The study was performed under NIAID-held Investigational New Drug applications (BB-IND no. 14763) and was reviewed by the U.S. Food and Drug Administration. The clinical protocol, consent forms, and Investigators’ Brochure were developed by CIR and NIAID investigators and were reviewed and approved by the Western Institutional Review Board, and the NIAID Office of Clinical Research Policy and Regulatory Operations (OCRPRO). Clinical data were reviewed by CIR and NIAID investigators and by the Data Safety Monitoring Board of the NIAID Division of Clinical Research.
Clinical assessment: Acute phase.
For adults and RSV-seropositive children enrolled in this trial, clinical assessments and NWs were performed on study day 0 (the day of vaccination, with the NW performed before inoculation) and on days 3 to 7 and 10 after inoculation. After day 10, illness data (adverse events and reactogenicity events) were collected through day 28, with additional physical examinations performed and NWs obtained in the event of LRI. All LRIs were defined as SAEs, regardless of severity. For seronegative children, clinical assessments and NWs were performed on study days 0,3,5, and 7 and on days 10,12,14,17,19, 21, and 28 ±1 day. After the last scheduled NW, illness data were obtained for seronegative children through day 56, with physical examinations performed and additional NWs obtained in the event of LRI. Titers of vaccine virus in NW specimens were determined as described below. Fever, URI (rhinorrhea or pharyngitis), cough, LRI, and OM were defined as previously described (8). When illnesses occurred, NWs were tested for adventitious agents by rRT-PCR (Fast-track Diagnostics).
Clinical assessment: Surveillance.
RSV-seronegative children were monitored for symptomatic medically attended RSV-associated illness in this study as previously described (8). In brief, families were contacted weekly between 1 November and 31 March to determine whether MAARIs had occurred, which were defined as fever, URI, LRI, or OM. For each illness episode, a clinical assessment was performed, and a NW obtained for quantitative viral testing by rRT-qPCR and culture as described below.
Isolation, quantitation, and characterization of virus.
NWs were performed using a nasal bulb syringe and 15 to 20 ml of Lactated Ringer’s solution. NWs were snap-frozen on site and stored at −80°C. An aliquot of each NW was rapidly thawed and tested for infectivity by plaque assay in HEp-2 cells as previously described (15). Titers of vaccine virus are expressed as the number of PFU per milliliter of NW fluid. Specimens that were culture-negative were assigned a titer of 0.6 log10 PFU/ml.
Shedding of vaccine virus was also quantified by RT-qPCR amplification of the RSV matrix (M) protein. The following primers and probes were used: forward primer, 5’-gcaaatatggaaacatacgtgaacaa-3’, reverse primer, 5’-GGCACCCATATTGTAAGTGATGCA-3’, and probe, 5’-cttcacgaaggctccacata-3’. The assay was performed using the AgPath-ID One-Step RT-PCR kit and the 7300 Fast real-time PCR system (Applied Biosystems). Copy numbers of the gene of interest were calculated using a plasmid DNA standard curve for the RSV M gene. Analyses were performed using 7500 Fast System SDS software (Applied Biosystems). The limit of detection by RT-qPCR was 1.7 log10 copy numbers/ml; therefore, PCR-negative samples were assigned a titer of 1.7 log10 copy numbers/ml.
To determine whether the vaccine virus was genetically stable, the presence of the M2–2 deletion was verified by sequence analysis of NW isolates obtained from seronegative vaccinees at the time of peak viral shedding. Vaccine virus was isolated by one passage of NW fluid on Vero cells. RT-PCR was performed on total extracted RNA using RSV-specific primers, and consensus sequences of a region corre-spondingto nucleotides 7997 to 8817 of an RSV A2 reference sequence were generated (GenBank accession no. M74568, spanning the M2–2 deletion). In one case, only a shorter RT-PCR fragment could be obtained for sequence analysis of a region corresponding to nucleotides 8004 to 8695 of the reference sequence.
Immunologic assays
Serologic specimens: RSV MEDI ΔM2–2 study.
Sera were obtained to measure antibodies to RSV before inoculation, about 1 month after inoculation in adults and seropositive children and about 2 months after inoculation in seronegative children. To measure serum antibody responses during the surveillance period, sera were also obtained from seronegative children between 1 October and 31 October of the calendar year in which the child was enrolled and between 1 April and 30 April of the following calendar year. Thus, adults and seropositive children each had two serum specimens obtained, and seronegative children each had three or four serum specimens obtained, depending on the time of enrollment.
Serologic specimens: Previous study of RSVcp248/404/1030/ΔSH.
To compare antibody responses to RSV MEDI ΔM2–2 and the previously evaluated RSVcp248/404/1030/ΔSH in RSV-naïve children, paired pre-and postvaccination specimens from eight RSV-seronegative children who had received 105.3 PFU of RSVcp248/404/1030/ΔSH in an earlier study (8) were retested for neutralizing antibodies to RSV as described below.
Antibody assays.
Sera from all subjects were tested for antibodies to RSV by 60% complement-enhanced plaque reduction neutralization assay (26) and for IgG antibodies to the RSV F glycoprotein by ELISA. The ELISA was performed as previously described (15), except that the RSV F, provided by Novavax Inc., was a purified baculovirus-expressed protein (27); 20 ng per well was used in the assay. For the neutralizing antibody assay, the starting dilution was 1:10, and for the ELISA, the starting dilution was 1:200 for adult sera and 1:50 for pediatric sera. The PRNT and RSV F IgG titer are expressed as reciprocal log2. Antibody responses were defined as ≥4-fold increase in titer in paired specimens.
Data analysis
Infection was defined as either the detection of vaccine virus by culture or rRT-qPCR or a ≥4-fold rise in RSV serum neutralizing antibody or in RSV F serum antibody. The mean peak titer of vaccine virus shed (log10 PFU/ml) was calculated for infected vaccinees only. The neutralizing antibody and RSV F IgG reciprocal titers were transformed to log2 values for calculation of mean log2 titers, and Student’s t test was used to compare mean peak viral titers and antibody titers between groups. Rates ofillness among vaccinees and placebo recipients were compared by the two-tailed Fisher’s exact test.
Supplementary Material
Acknowledgments:
In loving memory of Alison Bermingham (1967–2015), who first characterized the M2–2 deletion mutation during her postdoctoral fellowship in P.L.C.’s laboratory. We thank H. Jin, R. Tang, and J. Schickli of MedImmune for providing a sample of RSV MEDI ΔM2–2 and information on its history; F. Dubovsky, S. Projan, T. Villafona, M. Esser, and J. Falloon of MedImmune for discussion and support; J. M. Reid, A. Cahill, and W. C. Gruber of Pfizer Inc. for providing serum samples from children enrolled in a previous study of the RSVcp248/404/ 1030/ΔSH vaccine candidate; A. Schmidt [Laboratory of Infectious Diseases (LID), NIAID] for assistance early in study development; L. Yang (LID, NIAID) for technical support; and the leadership and staff of the OCRPRO, Division of Clinical Research, NIAID, NIH, for regulatory sponsorship and support. We are grateful to E. Schappell, J. San Mateo, S. Woods, M. Gatto, M. Holmblad, N. Bylsma, C. Bull, and K. Wanionekfor expert clinical and laboratory assistance; to The Pediatric Group, Primary Pediatrics, Dundalk Pediatrics, Bright Oaks Pediatrics, and Johns Hopkins Community Physicians for allowing us to approach families in their practices; and to the families for their participation in this study.
Funding: R.A.K., K.M.L., B.T., and J.A.E. were funded by NIAID contract HHS 272200900010C. C.L., P.L.C., and UJ.B. were funded by the Intramural Program of the NIAID. This work was also supported in part by a Cooperative Research and Development Agreement between NIAID, NIH, and MedImmune. Clinical research support was also provided to J.A.E. by UL1TR000423.
Footnotes
Competing interests: P.L.C. is an inventor on U.S. patents 6,713,066 and 7,485,440, entitled “Production of attenuated RSV vaccines involving modification of M2 ORF2.”
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/7/312/312ra175/DC1 Source data
REFERENCES AND NOTES
- 1.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P, The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 360,588–598 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simöes EAF, Rudan I, Weber MW, Campbell H, Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 375, 1545–1555 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ, Bronchiolitis- associated hospitalizations among US children, 1980–1996. JAMA 282, 1440–1446 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Levine OS, Knoll MD, Jones A, Walker DG, Risko N, Gilani Z, Global status of Haemophilus influenzae type b and pneumococcal conjugate vaccines: Evidence, policies, and introductions. Curr. Opin. Infect. Dis. 23, 236–241 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Collins PL, Melero JA, Progress in understanding and controlling respiratory syncytial virus: Still crazy after all these years. Virus Res. 162, 80–99 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham BS, Anderson LJ, Challenges and opportunities for respiratory syncytial virus vaccines. Curr. Top. Microbiol. Immunol. 372, 391–404 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE Jr., Boyce TG, Halburnt LL, Reed GW, Whitehead SS, Anderson EL, Wittek AE, Casey R, Eichelberger M, Thumar B, Randolph VB, Udem SA, Chanock RM, Murphy BR, Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J. Infect. Dis. 182, 1331–1342 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, Polack FP, Randolph VB, Deatly A, Hackell J, Gruber W, Murphy BR, Collins PL, Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J. Infect. Dis. 191, 1093–1104 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Wright PF, Karron RA, Madhi SA, Treanor JJ, King JC, O’Shea A, Ikizler MR, Zhu Y, Collins PL, Cutland C, Randolph VB, Deatly AM, Hackell JG, Gruber WC, Murphy BR, The interferon antagonist NS2 protein of respiratory syncytial virus is an important virulence determinant for humans. J. Infect. Dis. 193, 573–581 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Wright PF, Karron RA, Belshe RB, Shi JR, Randolph VB, Collins PL, O’Shea AF, Gruber WC, Murphy BR, The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine 25, 7372–7378 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malkin E, Yogev R, Abughali N, Sliman J, Wang CK, Zuo F, Yang C-F, Eickhoff M, Esser MT, Tang RS, Dubovsky F, Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLOS One 8, e77104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH, Respiratory syncytial virus disease in infants despite prior administration of antigenic in-activated vaccine. Am. J. Epidemiol. 89, 422–434 (1969). [DOI] [PubMed] [Google Scholar]
- 13.Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA, Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine 8, 497–502 (1990). [DOI] [PubMed] [Google Scholar]
- 14.Connors M, Collins PL, Firestone C-Y, Sotnikov AV, Waitze A, Davis AR, Hung PP, Chanock RM, Murphy BR, Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia—RSV recombinants or RSV. Vaccine 10, 475–484 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Karron RA, Wright PF, Crowe JE Jr., Clements Mann ML, Thompson J, Makhene M, Casey R, Murphy BR, Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants and children. J. Infect. Dis. 176, 1428–1436 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Collins PL, Hill MG, Camargo E, Grosfeld H, Chanock RM, Murphy BR, Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5’ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc. Natl. Acad. Sci. U.S.A. 92, 11563–11567 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermingham A, Collins PL, The M2–2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc. Natl. Acad. Sci. U.S.A. 96, 11259–11264 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng MN, Whitehead SS, Bermingham A, Claire M St., Elkins WR, Murphy BR, Collins PL, Recombinant respiratory syncytial virus that does not express the NS1 or M2–2 protein is highly attenuated and immunogenic in chimpanzees. J. Virol. 74, 9317–9321 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin H, Zhou H, Cheng X, Tang R, Munoz M, Nguyen N, Recombinant respiratory syncytial viruses with deletions in the NS1, NS2, SH, and M2–2 genes are attenuated in vitro and in vivo. Virology 273, 210–218 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Englund JA, Karron RA, Cunningham CK, LaRussa P, Melvin A, Yogev R, Handelsman E, Siberry GK, Thumar B, Schappell E, Bull CV, Chu HY, Schaap-Nutt A, Buchholz U, Collins PL, Schmidt AC; International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1096 Study Group, Safety and infectivity of two doses of live-attenuated recombinant cold-passaged human parainfluenza type 3 virus vaccine rHPIV3cp45 in HPIV3-seronegative young children. Vaccine 31, 5706–5712 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karron RA, Buchholz UJ, Collins PL, Live-attenuated respiratory syncytial virus vaccines. Curr. Top. Microbiol. Immunol. 372, 259–284 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vesikari T, Karvonen A, Korhonen T, Edelman K, Vainionpää R, Salmi A, Saville MK, Cho I, Razmpour A, Rappaport R, O’Neill R, Georgiu A, Gruber W, Mendelman PM, Forrest B; CAIV-T Transmission Study Group, A randomized, double-blind study of the safety, transmis-sibility and phenotypic and genotypic stability of cold-adapted influenza virusvaccine. Pediatr. Infect. Dis. J. 25, 590–595 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Glezen WP, Taber LH, Frank AL, Kasel JA, Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 140, 543–546 (1986). [DOI] [PubMed] [Google Scholar]
- 24.Siber GR, Leombruno D, Leszczynski J, McIver J, Bodkin D, Gonin R, Thompson CM, Walsh EE, Piedra PA, Hemming VG, Prince GA, Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J. Infect. Dis. 169, 1368–1373 (1994). [DOI] [PubMed] [Google Scholar]
- 25.Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ, Arrobio J, Meissner HC, Fulton DR, Welliver RC, Tristram DA, Siber GR, Prince GA, Van Raden M, Hemming VG; Respiratory Syncytial Virus Immune Globulin Study Group, Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N. Engl. J. Med. 329, 1524–1530 (1993). [DOI] [PubMed] [Google Scholar]
- 26.Coates HV, Alling DW, Chanock RM, An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am. J. Epidemiol. 83, 299–313 (1966). [DOI] [PubMed] [Google Scholar]
- 27.Smith G, Raghunandan R, Wu Y, Liu Y, Massare M, Nathan M, Zhou B, Lu H, Boddapati S, Li J, Flyer D, Glenn G, Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLOS One 7, e50852 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


