Abstract
This paper forecasts the consequences for total Medicare expenditures between 2005 and 2030 of scientific progress in cancer. Because technological advance is uncertain, widely varying scenarios are modeled. A baseline scenario assumes year 2000 technology stays frozen. A second scenario incorporates recent cancer treatment advances and its attendant discomfort. Optimistic scenarios analyzed include the discovery of an inexpensive cure, a vaccine which prevents cancer, and vastly improved screening techniques. Applying the Future Elderly Model, we find that no scenario holds significant promise for guaranteeing the future financial health of Medicare.
INTRODUCTION
Cancer is largely a disease of old age. In 2000, over two-thirds of all cancer deaths were among people aged 65 and older. That year, cancer was the second leading cause of death among the elderly, accounting for 113 deaths per year per 10,000 elderly people.1 Looked at another way, of all cancer patients alive in 2001, 61% were aged 65 or older.2 Because cancer care is expensive, the treatment of elderly cancer patients places a substantial financing burden on Medicare. Our main purpose is to forecast how technological change in cancer treatment will affect future Medicare expenditures on elderly cancer patients over the next 25 years.
It would be foolhardy to claim clairvoyant knowledge of future technological advances in cancer treatment. Such predictions have failed before. In 1971, when President Nixon effectively declared the War on Cancer, there was great hope that a focused scientific effort could yield a cure within a reasonable amount of time. Back then, we knew much less than we do now about the basic biological mechanisms that cause cancer, so the grand strategy set out to win the War on Cancer involved substantial investment in basic laboratory research, alongside investments in clinical trials to test drugs arising out of the labs.3
Both public and private sector investments have been substantial. Today, the National Cancer Institute (NCI) is the single largest center funded by the National Institute on Health (NIH), with a 2004 spending exceeding $4.7 billion, or nearly 20% of the total NIH budget.4 Other government entities, such as the Department for Veterans Affairs, the Centers for Disease Control, and the National Institute for Environmental Health Services, contribute another $2 billion to cancer research and prevention efforts. This public investment, large though it is, is still less than pharmaceutical industry expenditures on cancer research--$7.4 billion in 2003.5
Though no cure has been forthcoming, the research effort has paid dividends. Between 1991 and 2000, the age-adjusted cancers death rate decreased by 7.2%.6 Prevention efforts reduced cigarette smoking, while clinical interventions such as early detection are the direct mechanisms responsible for much of the decline in age-specific death rates. In some cases, the detailed biological knowledge gained from laboratory research has yielded new treatments—for example, sophisticated chemotherapeutic agents that specifically block meiosis and division by cancer cells. In other cases, careful epidemiological research, even in the absence of causal biological knowledge, has been responsible.
Because there is no cure and because these gains have come incrementally, this has led some to argue that we are losing the War on Cancer.7 The strongest piece of evidence supporting this assessment is that, between 1991 and 2000, the annual cancer death rate has increased by 38,400 deaths or 7.4% per annum. If we are winning, then why are more people dying each year? The easy answer is that the increase is illusory—it is explained entirely by increases in the size and aging of the U.S. population over this period.8 So the gains are real, but given the substantial investment in cancer research, it is a disappointment to see raw death rates high and growing. One counterintuitive, but important, explanation is that cancer death rates are rising because of success in reducing heart disease mortality rates -- people who previously died from heart disease are living to die from cancer.9 Nevertheless, from a public finance perspective, it is a disappointment to see the rising social costs of cancer: one estimate places the 2003 direct medical and indirect costs (from lost productivity due to illness or death) at $189.5 billion.10
We examine one important potential public reward to cancer research—a reduction in Medicare spending arising from improved cancer care. As we have noted, reliable forecasts of new technologies is difficult (some would say impossible). Instead, we take another approach.
We look at five widely varying scenarios of technological change. The first assumes that cancer treatment technology extant in 2000 stay in place over the next 25 years. The second incorporates advances in cancer treatment between 2000 and 2004. The third envisions an inexpensive cure for cancer. The fourth envisions a vaccine that inoculates against the development of cancer until death. Unlike the cure, the vaccine requires caring (and paying medical care costs) for the existing cohort of cancer survivors. The fifth envisions substantial improvements in the early detection of cancer.
Our major finding is that, even if inexpensive and wildly effective new technologies will not necessarily lead to large declines in total medical expenditures. Demographic realities trump the ability of technological miracles to substantially address Medicare financing challenges.
METHODS
Data
Our primary data source for this study is the Medicare Current Beneficiary Survey (MCBS) for the years 1992–2000. The MCBS is a nationally representative sample of aged, disabled and institutionalized Medicare beneficiaries containing comprehensive data on health, health care use, and expenditures.
Our Future Elderly Model (FEM), described below, requires extensive information on health status to account for potential competing risks. Based on MCBS self-reports, we develop indicators variables for the presence of major diseases and functional status limitations that have strong effects on both health care expenditures and survival. Among these include diabetes, cancer (excluding skin cancer), heart disease (myocardial infarction, heart attack, angina, coronary heart disease, congestive heart failure, or other heart condition), hypertension, stroke, lung disease (emphysema, asthma, or chronic obstructive pulmonary disease), Alzheimer’s disease, osteoarthritis, instrumental activities of daily living (IADL), activities of daily living (ADL) and nursing home residency.
For respondents who have or have had cancer, MCBS also has detailed information on parts of the body where the tumor was found, including lung, colon (colon, rectum, or bowel), breast, uterus, prostate, bladder, ovary, stomach, cervix, brain, kidney, throat, head, back, female organs, or other). To ensure a large enough sample size, we group cancer into 5 categories: lung cancer, breast cancer, prostate cancer, colon cancer, and other cancers.
One problem with using an annually collected dataset like the MCBS to examine cancer populations is that, because cancer is so deadly, long-term cancer survivors are oversampled. We address this issue by incorporating information on cancer populations from the Surveillance, Epidemiology, and End Results (SEER) database, which does not suffer from this bias. The SEER data contain important clinical information about cancer patients that the MCBS lacks, including stage of disease. We need both the MCBS and SEER, though, because the MCBS contains information on non-cancer health conditions that SEER lacks.
The Future Elderly Model
To simulate the impact of cancer treatment breakthroughs, we use a modified version of the Future Elderly Model (FEM).11 FEM is a micro-simulation model that tracks disease conditions, functional status, and health care expenditures of the Medicare population. The FEM has three components: a model of health status transitions, a model of health care expenditures, and a model of future entering Medicare cohorts. For this paper, we expand the basic FEM to include indicators for lung, breast, prostate, colon, and other cancers.
We adjust the FEM parameters to distinguish early from late stage cancers, and to account for biases caused by the MCBS sampling scheme. The MCBS does not ask participants with cancer to report stage at diagnosis. Furthermore, because mortality rates among cancer patients is high, the probability of selecting into the MCBS is lower for cancer patients, which makes MCBS cancer prevalence estimates lower than the true prevalence. For similar reasons, early stage patients are over-represented in the MCBS. To address these deficiencies, we use SEER to estimate the relative prevalence of early and late stage disease, and to adjust the cancer prevalence and mortality estimates to match the population numbers.
The FEM health status transition model consists of a series of flexibly modeled hazard functions, in which the hazard of contracting a disease, entering a new functional status, and dying are functions of demographic characteristics (gender, education, race, region, urban or rural), risk factors (obesity, underweight12, and having ever smoked), and other health conditions (diseases and functional status). We treat most health states as absorbing; that is, once someone contracts an illness, he has it forever. This assumption is consistent with the MCBS questionnaire (“Has a doctor ever told you …”) and with the course of most of the chronic diseases. However, we allow recoveries from functional limitations. For cancer patients, mortality estimates depend upon the stage of disease at diagnosis.
Expenditures estimates are based on pooled weighted least square regressions of total Medicare expenditures on demographic characteristics, risk factors, health conditions, and interactions of disease conditions and functional status. All expenditures are in 2000 dollars and adjusted using the Medical CPI.13
The mechanics of the FEM microsimulation work as follows. We draw, with replacement, a sample of 100,000 simulated individuals from the MCBS population in the year 2000. This simulated population inherits the joint distribution of health status and functional disability from the MCBS, and hence it reflects the prevalence of disease and disability seen in the 65+ population in 2000. Using prevalence estimates for breast, prostate, lung, colon and other cancers from the year 2000 SEER, we randomly assign late and early stage cancer to appropriate subgroups of this population so that it reflects the prevalence of cancer. We then apply the FEM expenditure model to estimate year 2000 Medicare costs for this population. Using Census Bureau age-specific population forecasts, we estimate total year 2000 Medicare costs.
We next apply the FEM disease transition model to the year 2000 simulated population to determine the characteristics of the 2001 simulated population. For each individual in the simulation population, we randomly draw mortality, disease, and disability information in 2001 that depends on the presence of disease and disability in 2000. As our initial sample ages, it becomes less representative of the entire 65+ population. We therefore replenish our sample yearly with a newly entering cohort of 65 years olds. We use the National Health Interview Survey (NHIS) to predict health status of these newly entering cohorts. With the newly refreshed 2001 microsimulation population in hand, we again apply the FEM expenditure model and Census Bureau age-specific population forecasts to estimate 2001 Medicare costs.
We repeat this microsimulation thirty times, progressively aging the simulated population, while refreshing it with incoming 65 year olds. This yields forecasts of Medicare costs for the years 2000 to 2030. Because the FEM disease transition and expenditure models reflect year 2000 technology, the cost forecasts reflect the effect of keeping 2000 medical technology unchanged until 2030. Henceforth, we will call these forecasts our “Baseline” estimates. The “Baseline” FEM does well in forecasting year 2000 Medicare expenditures, as well as Medicare expenditures on cancer.14
A major feature of the FEM is that it permits us to directly model the problem of competing mortality risks. In the FEM, people face an increased mortality risk from a wide and realistic variety of chronic conditions and disability states. This feature is especially important because the scenarios we consider envision radical changes, such as a cancer cure. Improvements in cancer treatment will keep some alive long enough to contract other diseases. If these diseases entail expensive treatments, it is quite possible that even inexpensive and effective cancer treatments could lead to increased Medicare expenditures. Whether this is indeed the case is an empirical issue.
Five Scenarios for Technological Advancement
We consider five scenarios on technological advance in cancer treatment. We relied on an expert panel on cancer and the biology of aging to guide us in scenario construction. This panel, which met in 2001, consisted of nationally known clinicians and laboratory researchers.15 In part, our motivation in picking these scenarios stems from fundamental uncertainty about the future of technological progress expressed by this panel. Hence, we consider scenarios that run the gamut from wide-eyed optimism to excessive pessimism.
Optimism comes in several varieties. One scenario envisions a cure for cancer. We assume that all cancer patients will be eligible for this cure, and will be restored to whatever health condition they had before the development of the cancer. Since we want this “Cure” scenario to be optimistic, we assume that treating a cancer patient will cost substantially less than treating a cancer patient today—$10,000 per cancer patient.16
Our second scenario envisions the development of a cancer vaccine.17 We assume that after vaccination, this vaccine prevents cancer from ever developing. However, to distinguish this scenario from the “Cure” scenario, this vaccine cannot cure patients with existing cancer -- such patients receive conventional cancer treatment modalities available in 2000. We further assume that Medicare pays for vaccination of the entire 65+ population (excluding those who have already had cancer) when the vaccine is first disseminated, and then subsequently pays each year to vaccinate incoming cohorts. We assume that this vaccine will cost about $500 per administration, which is similar to the cost of a Hepatitis B vaccine.
Our next scenarios both envision large improvements in the process of population screening for cancer. In these scenarios, the probability of developing cancer and the technology of cancer treatment remains unchanged. However, all cancers that develop are discovered and treated at an early stage. Of all the optimistic scenarios, the expert panel thought these “Early Detection” scenarios most likely to transpire within the next 30 years (though still unlikely). These scenarios share the same detection technology but are distinguished by cost assumptions. In one, “Early Detection (Zero Cost),” we assume that the costs of detection are free, or alternatively, discovered at no additional cost in the process of routine care. In the second, “Early Detection (Moderate Cost),” the costs of early detection roughly equal the annual costs of screening for colon cancer—$100 per elderly person per year.18 Though these scenarios are certainly optimistic, improved detection will increase the measured prevalence of cancer, as the set of undiagnosed cancers developing to late stage shrinks to null.
Our final scenario is more pessimistic. In the “Baseline” scenario, we assumed that the technology of cancer treatment that was extant in 2000 stays in place unchanged until 2030. This scenario is similar, except that we assume the technology of treatment extant in 2004 stays in place unchanged until 2030. We dub this our “Drug” scenario because it envisions the adoption of several new pharmaceutical agents into the standard treatment regimen, as well as a wider dissemination of some existing agents. In Exhibit 1, we make explicit our assumptions in this scenario. For example, we assume that 70% of all newly diagnosed early stage breast cancer patients will receive Tamoxifen. Our motivation for including this scenario is to estimate the cost-effectiveness of the technological developments that took place between 2000 and 2004.
Exhibit 1:
Description of Scenarios
| Scenarios | Costs | Impact |
|---|---|---|
| New Drug treatments | Rituximab to treat Non-Hodgkin lymphomas: $10,000 per patient; Trastuzumab (Herceptin) to treat metastatic breast cancer (30% of all breast cancer): $2,300 per patient; Tamoxifen to treat DCIS or stage 1 breast cancer (70% of all breast cancer): $8,210 per patient; Oxaliplatin to treat stage III and IV colorectal cancer (60% of all colorectal cancer): $11,826 per patient; Leuprolide acetate (Lupron) to treat prostate cancer: $700 per patient; and Temozolomide (Temodar) to treat brain cancer: $27,878; Costs for side effect medication: $10,000 per patient. |
Rituximab: relative risk reduction of 33%; Trastuzumab: relative risk reduction of 20%; Tamoxifen: relative risk reduction of 37%; Oxaliplatin: relative risk reduction of 23%; Leuprolide acetate: relative risk reduction of 52%; Temozolomide (Temodar): relative risk reduction of 28%. |
| Cancer Vaccines | $488.15 per person | Everyone who does not have cancer takes the vaccine which would prevent them from having cancer in the future. |
| Cure for Cancer | $10,000 per cancer patient | Assume that no one in the entering 65 years old cohort has cancer at the time. Cancer is treated immediately after its incidence and would be cured in the same year. |
| Early detection (Zero Cost) | No cost | All cancer diagnosed is at the early stage as a result of a better screening test. |
| Early detection (Moderate Cost) | $100 per person per year for beneficiaries without cancer | All cancer diagnosed is at the early stage as a result of a better screening test. |
We classify the major advances over this period into five categories: 1) monoclonal antibodies; 2) hormonal agents; 3) new cytotoxic agents or existing agents used in new situations; 4) oral agents that are in the same class as existing drugs; and 5) supportive drugs that decrease the side effects of existing therapies. In each of these categories, there are new drugs as well as existing drugs that disseminated more widely. For each drug, we assign cost, survival, and therapy targeting estimates based upon guidance from our expert panel, as well as a search of the clinical literature on cancer between 2000 and 2004.19
Exhibit 1 summarizes assumptions about cost and survival for each scenario. For the “Drug” scenario, the relative risk reduction compared with year 2000 treatment, as well as the patients for whom new treatments apply, are listed. Treatment costs listed in Exhibit 1 include the administration of these supportive drugs when appropriate.20
For each scenario, we adjust FEM cost and disease transition probabilities to correspond to scenario parameters. For example, for the “Vaccine” scenario, we set the probability of developing cancer to zero, and we increase Medicare expenditures by about $500 for everyone without cancer. We assume that the new breakthroughs start to apply in 2005. Therefore, before 2005, all scenarios have the same projections of disease conditions, functional status, and health care expenditures. In 2005, projections diverge as a large number of interdependent variables are affected by the different technological paths of the scenarios. Among the affected variables include cancer prevalence, cause-specific death rates, the prevalence of functional status limitations, health care expenditures, and the prevalence of other diseases (indirectly through changes in mortality). We compare outcomes in the “Baseline” scenario with outcomes in the “Drug,” “Cure,” “Vaccine,” and “Prevention” scenarios to estimate the marginal effect of technological breakthroughs on the prevalence of cancer and on health care expenditures over a 25-year period.
RESULTS
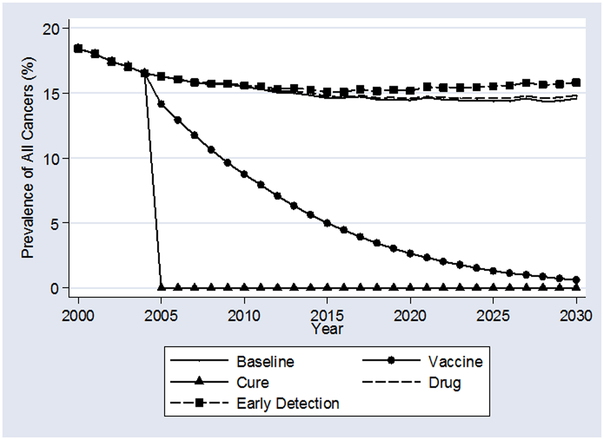
Exhibit 2 shows the evolution of cancer prevalence under the “Baseline” and five scenarios. In the “Baseline” scenario, cancer rates decline slowly from 20% of the elderly population to about 16% in 2015, and then stay there until 2030. The decline reflects the decline in age-adjusted cancer rates that occurred between 1991 and 2000 because of lower smoking rates among newer Medicare beneficiaries.21 That is, at any given age in the year 2000, there were fewer people with cancer than there were in 1991. Between 2000 and 2015, if technology were to remain fixed at year 2000 levels, the healthier cohorts would age into Medicare and replace existing, less healthy, cohorts. By 2015, this process reaches a steady state.
Exhibit 2:
Cancer Prevalence under Alternate Scenarios
The “Cure” and “Vaccine” scenarios yield intuitive time profiles of cancer prevalence. As the cure can be applied whether or not a cancer was developed before the 2005 discovery, cancer prevalence will drop sharply to zero. By contrast, our vaccine yields steadily declining cancer prevalence between 2005 and 2030. Under this scenario, there will be no new cancer cases contracted after 2005, so increasing numbers of the incoming Medicare cohorts will be free of cancer. As the existing stock of cancer survivors slowly die out, cancer prevalence rates decline toward zero.
Two scenarios yield higher cancer prevalence rates than “Baseline.” In the “Drug” scenario, since new therapies improve cancer survival relative to “Baseline,” the set of people alive with cancer at any given time will be greater. In the “Early Detection” scenarios, measured cancer prevalence rises over “Baseline” for two reasons: (1) all previously undetected cancers are found at an early stage, and (2) early stage cancer patients are more likely to survive than late stage patients.
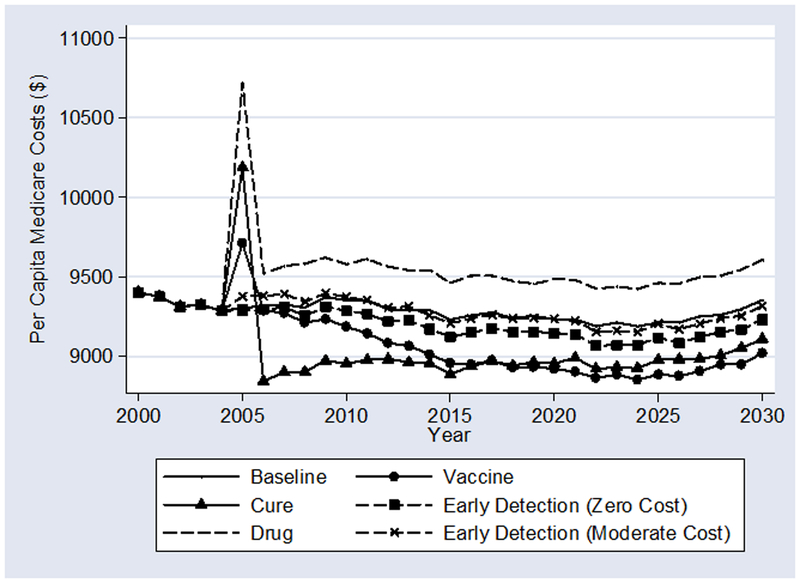
Exhibit 3 shows projected per-capita Medicare costs. Since we are interested in the tradeoffs due to greater longevity (with its attendant comorbidity), expenditures here include all Medicare spending. One-time adjustment costs of applying new technologies to the whole Medicare population explain the discontinuous jump in 2005 for the “Drug,” “Cure,” and “Vaccine” scenarios. There is no discontinuity in the “Baseline” and “Early Detection” scenarios because there is no pent up demand for treatments.
Exhibit 3:
Per-Capita Medicare Costs under Alternate Scenarios
Unsurprisingly, the “Cure” and “Vaccine” scenarios result in the lowest per-enrollee costs. At first, costs decline most relative to “Baseline” for the “Cure” scenario, but after several years, the cost decline under the “Vaccine” scenario dominates. The switch happens because the vaccine does not help existing cancer survivors, and cancer survivors spend more on medical care than others. Ultimately, vaccinating everyone for $100 is cheaper than treating every cancer case for $10,000.
In the short run, whether “Early Detection” raises or lowers per-capita costs relative to “Baseline” depends upon price. If early detection is free, per-capita costs are lowered. Unsurprisingly, the costs of treating previously undetected cancers at an early stage are less than the costs of treating later at a late stage. If early detection requires spending an additional $100 per enrollee, costs rise in the short run as all previously undetected cancers are found. In the long run, both scenarios reduce per-capita total costs.
Finally, Exhibit 3 shows that the 2000–2004 advances in cancer are expensive. The “Drug” scenario raises expenditures per enrollee by nearly $300 in the long run over “Baseline.” The denominator here is the number of Medicare enrollees, not just the number of cancer patients. $300 is thus a dramatic increase in Medicare expenditures for only a few years of new and more widely disseminated cancer technologies.
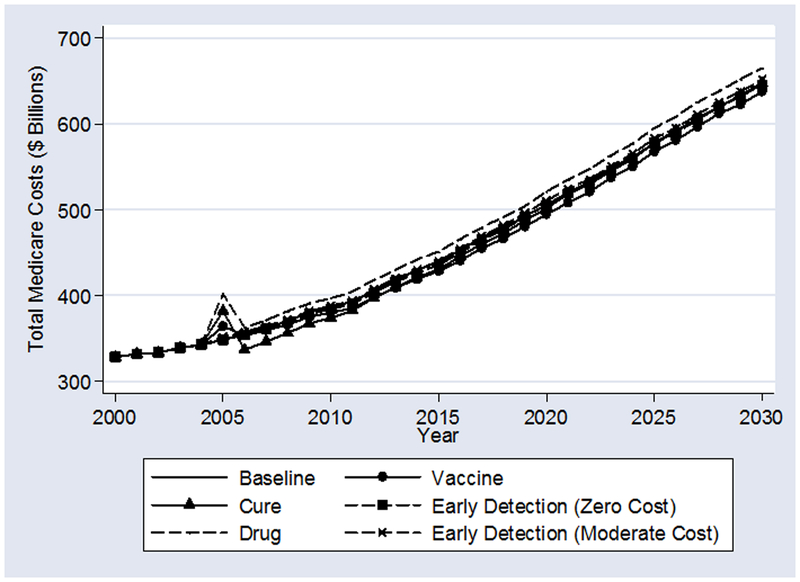
Exhibit 4 shows the FEM estimates of total Medicare expenditures. For all scenarios, because the population of elderly Americans will expand dramatically between 2005 and 2030, total medical expenditures also increase dramatically. One major lesson is that demographic facts can trump the ability of even the most optimistic technological developments to reduce future Medicare expenditures. Even miraculous technological developments lead to small declines in long-run total Medicare expenditures. Similarly, vastly improved early detection saves Medicare costs, but not very much.
Exhibit 4:
Total Medicare Costs under Alternate Scenarios
In addition to demographic trends, these results are driven by competing risks of death. Those saved from cancer by technological miracles will die of other things in expensive ways. Substantial progress in extending the lives of cancer patients will inevitably lead to an increased prevalence of other diseases. For example, in the “Cure” scenario, the FEM estimates imply that the prevalence of heart disease in 2030 will increase by 69 cases per 10,000 elderly over the “Baseline” scenario. Stroke prevalence will increase by 7 cases per 10,000; hypertension by 45 cases per 10,000; osteoarthritis by 46 cases; and so on. The other scenarios display qualitatively similar, if less dramatic, declines in the average health of the population resulting from improved cancer care.
The “Drug” scenario is the highest cost scenario. In 2030 alone, forecasted Medicare expenditure are $20 billion higher than “Baseline.” Further analysis suggests that the increase in costs is due largely to the increased use of expensive supportive therapies, such as erythropoietin, the new anti-emetics, and the bisphosponates (which prevent some adverse bone side effects).
DISCUSSION
In one sense, the “Drug” scenario cost estimates are a lower bound. If the incremental progress of the past few years continues into the future, elderly medical care costs are likely to increase even beyond levels projected here. Exhibit 5 provides rough cost-effectiveness estimates. In the “Drug” scenario, there will be an increase over “Baseline” of 2.79 million life-years at a cost to Medicare of $349 billion between 2005 and 2030. This amounts to a cost per life year saved of nearly $143,000, which is not conventionally seen as cost-effective. However, since much of these costs are due to supportive therapies, much of the benefit comes from quality of life improvements for cancer patients, which are not measured here.
Exhibit 5:
Estimated Effects of Medical Breakthroughs on Marginal Costs and Life Expectancy 2005–2030
| Technological Scenario |
Marginal Screening and Treatment Costs (in billions) |
Marginal Total Costs (in billions) |
Marginal Total Life Years Gained (in millions) |
Marginal Costs Per Year of Life Gained |
|---|---|---|---|---|
| New Drug treatments | $349 | $399 | 2.79 | $142,890 |
| Cancer Vaccines | $59 | −$210 | 15.44 | −$13,573 |
| Cure for cancer | $178 | −$120 | 27.05 | −$4,420 |
| Early detection (zero cost) | $0 | −$43 | 9.46 | −$4,529 |
| Early detection (moderate cost) | $118 | $75 | 9.46 | $7,904 |
Note: Cumulative real, discounted costs over the period 2005–2030 are reported. All the costs are in Year 2000 dollars.
Exhibit 5 shows that a cure would increase cancer care and screening costs by about $178 billion, saving nearly 27 million life-years over the 25 year period. Total medical expenditures would decline by about $120 billion -- a decline of almost $5 billion per year.22 As we have seen, the cost savings from an inexpensive cure are mitigated by competing risks of other diseases.
A cancer vaccine would be an even better bargain: an investment of $59 billion would save $210 billion -- about $8.4 billion per year -- and over 15 million life-years over the 25 year period.23
For our “Early Detection” scenarios, the estimates suggest that nearly 9.5 million life-years would be saved over the next 25 years. This is over one-third the life-years saved by a cure. Whether early detection would save or cost Medicare money depends upon the price of detection. If screening costs nothing over existing care, Medicare would save $43 billion over 25 years. If screening costs an additional annual $100 per enrollee, total Medicare expenditures would increase by $75 billion over 25 years -- about $3 billion per year.
While these savings in the optimistic scenarios may seem large, they are small relative to projected Medicare budgets. For example, in 2030 alone, Medicare is projected to spend over $650 billion in the “Baseline” scenario.
CONCLUSION
In a 1999 essay in Science on the future of medical technology, Pardes, Manton, et al. argue that conventional forecasts of Medicare expenditures are fundamentally blinkered.24 Among the reasons they cite is their expectation that the nature of future medical technological advance is changing to favor “high technology” over more costly “half-way” and “palliative” technologies.25 They write:
Conventional economic thinking suggests that new medical technology for improving the health of seniors often increases costs and absorbs savings as a result of better health. But technological advances are defining new paradigms for medicine to which traditional economic theory may not apply. Improved understanding of human biology at the molecular level may make [costly] invasive surgery, intensive care units, and long-term nursing home care far less necessary.
Our findings suggest that “conventional economic thinking” cannot be dismissed so easily. We consider a broad range of scenarios in this paper because we cannot predict, with any precision, the future of cancer treatment. We can be fairly certain, however, that no technological advance in the treatment of cancer alone, no matter how “high,” will fundamentally alter the financial future of Medicare.
Supplementary Material
ACKNOWLEDGEMENTS
Principal funding for this study came from the Centers for Medicare and Medicaid Services (CMS Contract no. 500–95-0056, with additional funding from the National Institute on Aging through its support of the RAND Roybal Center for Health Policy Simulation (P30AG024968). The authors are solely responsible for the paper’s contents. No statement in this paper should be construed as being an official position of the CMS.
NOTES
- 1.National Center for Health Statistics, “Health, United States 2002.” Hyattsville, MD: Centers for Disease Control and Prevention, 2002. (Available at http://www.cdc.gov/nchs/hus.htm, last accessed May 5, 2005). [Google Scholar]
- 2.National Cancer Institute “Estimated U.S. Cancer Prevalence,” 2004. (Available at http://cancercontrol.cancer.gov/ocs/prevalence/prevalence.html#age, last accessed May 5, 2005).
- 3.Leaf C, “Why We’re Losing the War on Cancer [and How to Win It]” Fortune v. 149 #6 (2004) p. 76–91, referring to estimates from the Tufts Center for the Study of Drug Development. [PubMed] [Google Scholar]
- 4.National Institutes of Health, “Summary of the FY 2004 President’s Budget.” Washington D.C.: Department of Health and Human Services, 2004: (Available at http://www.nih.gov/news/budgetfy2004/fy2004presidentsbudget.pdf, last accessed May 5, 2005) [Google Scholar]
- 5.Leaf C, “Losing the War on Cancer,” 2004. Ibid. [PubMed] [Google Scholar]
- 6.Ries L, Eisner M, Kosary C, et al. (eds.) “SEER Cancer Statistics Review, 1975–2000.” Bethesda, MD: National Cancer Institute, 2003. [Google Scholar]
- 7.See Bailar JC and Gornik HL “Cancer Undefeated,” New England Journal of Medicine, v. 336 (1997); [DOI] [PubMed] [Google Scholar]; Bailar JC and Smith BM “Progress against cancer?,” New England Journal of Medicine, v. 314 (1986) p. 1226–1232.; [DOI] [PubMed] [Google Scholar]; Leaf C “Losing the War on Cancer,” 2004, Ibid. While the National Cancer Institute no longer calls the research and clinical efforts aimed at finding a solution for cancer the “War on Cancer,” this phrase is still commonly used in the popular press, and even in the scientific literature. [Google Scholar]
- 8.Edwards BK, et al. “Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on the U.S. cancer burden.” Cancer v. 94 (2002) p. 2766–2792. [DOI] [PubMed] [Google Scholar]
- 9.See Llorca J and Delgado-Rodriguez M, “Competing Risks using Markov Chains: Impact of Cerebrovascular Disease and Ischemic Heart Disease in Cancer Mortality” International Journal of Epidemiology. v 30 (2001) p. 99–101. This problem of competing mortality risks is a major theme of our paper as well. [DOI] [PubMed] [Google Scholar]
- 10.National Heart, Lung, and Blood Institute “NHLBI Year 2003 Fact Book.” Bethesda, MD: National Institutes of Health, 2003. [Google Scholar]
- 11.The accompanying technical appendix provides substantial detail on the Future Elderly Model (FEM). See content.healthaffairs.org/cgi/content/full/hlthaff.w5.r53/DC2.
- 12.Respondents are if their body mass index (BMI) is greater than 30. Respondents are underweight if their BMI is less than 20.
- 13.We dropped Medicare HMO enrollees. By doing so, we may overestimate the health care expenditures if HMOs actually reduce expenditures, but we expect the bias is introduced to be small. We derive stage-specific cancer expenditure estimates from the results reported in Bhattacharya J, Garber A, and Miller M “Trends in Medical Expenditures and Survival from Cancer Among Medicare Beneficiaries, 1986–2000” mimeo, Stanford University, 2005. They use SEER data linked to Medicare administrative records to generate their estimates. [Google Scholar]
- 14.For details on the performance of the FEM in predicting year 2000 Medicare expenditures, see Goldman DP et al. “Health Status and Medical Treatment of the Future Elderly: Final Report” TR-169-CMS Santa Monica, CA: RAND Corporation, 2004. [Google Scholar]; Since we use SEER data, linked to Medicare administrative records, to scale FEM estimates of Medicare expenditures on cancer, it is not surprising that the modified version of the FEM that we use in this paper predicts year 2000 Medicare expenditures on cancer well, such as those reported by Brown ML, Rile GF, Schussler N, and Etzioni R “Estimating Health Care Costs Related to Cancer Treatment From SEER-Medicare Data” Medical Care v. 40 #8 (2002) p. 104–117. [DOI] [PubMed] [Google Scholar]
- 15.Our expert panel on cancer and the biology of aging was convened at RAND in 2001. This panel was conducted by Dr. Paul Shekelle using a modified version of the RAND/UCLA Appropriateness method. Among the experts on the cancer and biology of aging panel included Drs. Bergman Richard N., Campisi Judith, Ershler William, Finch Caleb E., and Miller Richard A.. A complete report on the conduct and content of these expert panels can be found in “Health Status and Medical Treatment of the Future Elderly: Final Report” TR-169-CMS Santa Monica, CA: RAND Corporation, 2004. [Google Scholar]
- 16.This $10,000 figure is an underestimate of the costs of chemotherapy. The “Cure” results should thus be seen as a lower bound on the total expenditures associated with a cure, and an upper bound on cost-effectiveness.
- 17.According to our expert panel, given the state of current technology, a cancer preventing vaccine is less likely to arise than an immunogenic agent to treat existing cancer. Confusingly, this latter type of treatment is also often called a cancer vaccine. Typically the target population for these immunogenic agents is cancer patients, rather than the population at large. Because this more latter kind of cancer vaccine does not differ in any qualitatively important way from our “Cure” scenario, we focus our “Vaccine” scenario on a treatment that prevents cancer altogether in the population at large.
- 18.We derive this annual screening cost from Pignone M, Saha S, Hoerger T, and Mandelblatt J, “Cost Effectiveness Analysis of Colorectal Cancer Screening: A Systematic Review.” Annals of Internal Medicin. v 137 #2 (2002), p. 96–104. These costs encompass an annual stool guaiac exam and a colonoscopy every five years. [DOI] [PubMed] [Google Scholar]
- 19.We updated the work of our expert panels on cancer breakthroughs up to 2004 with a detailed review of the clinical literature to construct the “Drug” scenario. Some sources we consulted include: Body JJ, “Effectiveness and cost of bisphosphonate therapy in tumor bone disease.” Cancer v. 97 #3(suppl) (2003), p. 59–65; [DOI] [PubMed] [Google Scholar]; Chen J, et al. “Efficacy of ondansetron and prochlorperazine for the prevention of postoperative nausea and vomiting after total hip replacement or total knew replacement procedures: A randomized, double-blind, comparative trial.” Archives of Internal Medicine. v. 158 #13 (1998); [DOI] [PubMed] [Google Scholar]; Cremieux P, et al. “Cost-minimization analysis of once-weekly versus thrice-weekly epoetin alfa for chemotherapy-related anemia.” Journal of Managed Care Pharmacy. v. 10 #6 (2004) p. 531–537; [DOI] [PMC free article] [PubMed] [Google Scholar]; Elkin E, et al. “HER-2 Testing and Trastuzumab Therapy for Metastatic Breast Cancer: A Cost-Effectiveness Analysis.” Journal of Clinical Oncology. v. 22, #5 (2004); [DOI] [PubMed] [Google Scholar]; Halbert RJ “Outpatient Cancer drug costs: Changes, drivers, and the future.” Cancer v. 94 #4 (2002) p. 1142–1150; [PubMed] [Google Scholar]; Parsons SK, et al. “Impact of Pharmacy Practices on the Cost of Colony-Stimulating Factor Use in Pediatric Stem Cell Transplantation: An Institution-Based Analysis.” Journal of Pediatric Hematology/Oncology v. 23 #1 (2001); [DOI] [PubMed] [Google Scholar]; Andre T, Boni C, Mounedji-Boudiaf L, et al. “Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer.” New England Journal of Medicine v. 350 (2004) p. 2343–51; [DOI] [PubMed] [Google Scholar]; Schrag D “The price tag on progress – chemotherapy for colorectal cancer.” New England Journal of Medicine. v. 351 #4 (2004) p. 317–319; TIME Magazine staff “Cancer Drugs: Information on what cancer drugs are available now and what drugs are in the pipeline,” Web Exclusive (May 20, 2001). http://www.time.com/time/health/article/0,8599,127226,00.html (last accessed May 5, 2005); [DOI] [PubMed] [Google Scholar]; U.S. Department of Health and Human Services “Medicare to Extend Access to Certain Drugs for Beneficiaries with Serious and Chronic Illnesses.” (2004) http://www.hhs.gov/news/press/2004pres/20040624.html (last accessed May 5, 2005).
- 20.Body JJ (2003), Ibid; [Google Scholar]; Chen J et al. (1998), Ibid; [Google Scholar]; Cremieux P, et al. (2004), Ibid; [Google Scholar]; Parsons SK et al. (2001), Ibid. [Google Scholar]
- 21.Ries L, et al. (2003), Ibid. [Google Scholar]
- 22.We adjust future expenditures for inflation using the medical care consumer price index. However, we do not adjust for expected annual growth in real medical care expenditures, nor do we apply a discount rate to these calculations. Real health care spending -- adjusted for demographic changes -- grew by about 4% annually during the period spanned by our data. Cost-effectiveness studies often discount future spending by 3%. If we discount, then ideally, we should also include a trend for real spending growth. The net effect of including both real growth and discounting in these calculations is not appreciably different from the estimates we present.
- 23.Compared with the “Cure” scenario, fewer lives are saved under than the “Vaccine” scenario because those with cancer receive care using existing technologies in the latter case.
- 24.Pardes H, Manton KG, Lander ES, Tolley HD, Ullian AD, Palmer H “Effects of Medical Research on Health Care and the Economy.” Science. v. 283 #5398 (2004) p. 36–7. [DOI] [PubMed] [Google Scholar]
- 25.This categorization of technological advance derives from a famous essay by Louis Thomas -- Thomas L “The Lives of a Cell,” New York, NY: Bantam Books; (1975). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.