Summary
Nicotinamide adenine dinucleotide (NAD), the cell’s hydrogen carrier for redox enzymes, is well known for its role in redox reactions. More recently, it has emerged as a signaling molecule. By modulating NAD+ sensing enzymes, it controls hundreds of key processes from energy metabolism to cell survival, rising and falling depending on food intake, exercise and the time of day. NAD+ levels steadily decline with age, resulting in altered metabolism and increased disease susceptibility. Restoration of NAD+ levels in old or diseased animals can promote health and extend lifespan, prompting a search for safe and efficacious NAD-boosting molecules. Such molecules hold the promise of increasing the body’s resilience, not just to one disease, but to many, thereby extending healthy human lifespan.
Keywords: aging, epigenetics, sirtuins, chromatin, epigenetics, PARP1, cancer, cardiovascular disease, inflammation, nicotinamide riboside, nicotinamide mononucleotide, CD38, STAC
eTOC Blurb
Nicotinamide adenine nucleotide (NAD+) has emerged as a key regulator of cellular processes that control the body’s response to stress. Rajman et al. discuss NAD boosters, small molecules that raise NAD+ levels, which are now considered to be highly promising for the treatment of multiple diseases and the potential extension of human lifespan.
The rise, fall, and rise of NAD
Nicotinamide adenine dinucleotide (NAD) is one of the most important and interesting molecules in the body. It is required for over 500 enzymatic reactions and plays key roles in the regulation of almost all major biological processes (Ansari and Raghava, 2010). Above all, it may allow us to lead healthier and longer lives.
NAD was first described in 1906 by Harden and Young as a cell component that enhanced alcohol fermentation (Harden and Young, 1906). Then in 1936, Warburg showed that NAD is required for redox reactions (Warburg and Christian, 1936) and solidified the nomenclature: “NAD” refers to the chemical backbone irrespective of charge, “NAD+” and “NADH” refer to the oxidized and reduced forms respectively.
By 1960, it was assumed that all the exciting work on NAD had been done. In 1963, a breakthrough came with the discovery that NAD+ is a co-substrate for the addition of poly-ADP-ribose to proteins (Chambon et al., 1963). PARylation, as it is now called, is carried out by the poly(ADP-ribose) polymerases (PARPs), a family of 17 proteins involved in a wide variety of cellular functions. Some PARPs are mono-(ADP-ribose) transferases, so a better name for these proteins is MARTs (Bai, 2015; Gupte et al., 2017). PARPs control numerous cellular functions, from DNA repair to gene expression, though multiple members remain poorly characterized.
Much of the renewed interest in NAD over the last decade can be attributed to the sirtuins, a family of NAD+-dependent protein deacylases (SIRT1-7). In 1999 Frye discovered that mammalian sirtuins metabolized NAD+ (Frye, 1999), then the Guarente and Sternglanz labs revealed that yeast Sir2 is an NAD+-dependent histone deacetylase (Imai et al., 2000; Landry et al., 2000). Since then, sirtuins have been shown to play a major regulatory role in almost all cellular functions. At the physiological level, sirtuins impact inflammation, cell growth, circadian rhythms, energy metabolism, neuronal function, and stress resistance (Gertler and Cohen, 2013; Imai and Yoshino, 2013). In this article, we review the physiology, pharmacology and potential use of “NAD-boosting molecules” for the treatment of diverse diseases and potentially even aging.
NAD+ synthesis
NAD+ is one of the most abundant molecules in the human body, required for approximately 500 different enzymatic reactions and present at about three grams in the average person. Though it was once considered a relatively stable molecule, NAD+ is now known to be in a constant state of synthesis, degradation and recycling, not only in the cytoplasm but also within major organelles including the nucleus, Golgi and peroxisomes (Anderson et al., 2003). Recent advancements in high-resolution, high-sensitivity NAD+ metabolite tracing methods such as mitoPARP, PARAPLAY, and Apollo-NADP+ (Cambronne et al., 2016; Cameron et al., 2016; Dolle et al., 2010) have revealed that the concentration and distribution of NAD+ and its metabolites are different depending on the cell compartment and change in response to physiological stimuli and cellular stress. NAD+ has two main pools, the “free” pool and protein-associated, “bound” pool, and the ratio of these pools varies across different organelles, cell types, tissues and even the age of individuals. There is also evidence that there are rapid, local fluctuations of NAD+ (Zhang et al., 2012).
With the exception of neurons, mammalian cells cannot import NAD+, so they must synthesize it either de novo by the kynurenine pathway from tryptophan (trp), or forms of vitamin-B3 such as nicotinamide (NAM) or nicotinic acid (NA) (Figure 1). To maintain NAD+ levels, most NAD+ is recycled via salvage pathways rather than generated de novo. The majority of NAD+ is salvaged from NAM, the product of CD38 and the PARPs (Magni et al., 2004; Magni et al., 1999) or from the various forms of niacin taken up in the diet including NAM, NA, NR and nicotinamide mononucleotide (NMN) (Bogan and Brenner, 2008; Mills et al., 2016; Trammell et al., 2016b; Ummarino et al., 2017). The precursor NR is thought to be directed into the salvage pathway via equilibrative nucleoside transporters (ENTs) (Nikiforov et al., 2011) and converted to NMN by nicotinamide riboside kinases (NRK1/2) (Ratajczak et al., 2016). NR generates unexpectedly high levels of NAAD in mouse liver and heart, as well as in human PBMCs, though the actual mechanism remains to be determined (Trammell et al., 2016a).
Figure 1. Primary pathways of NAD metabolism.
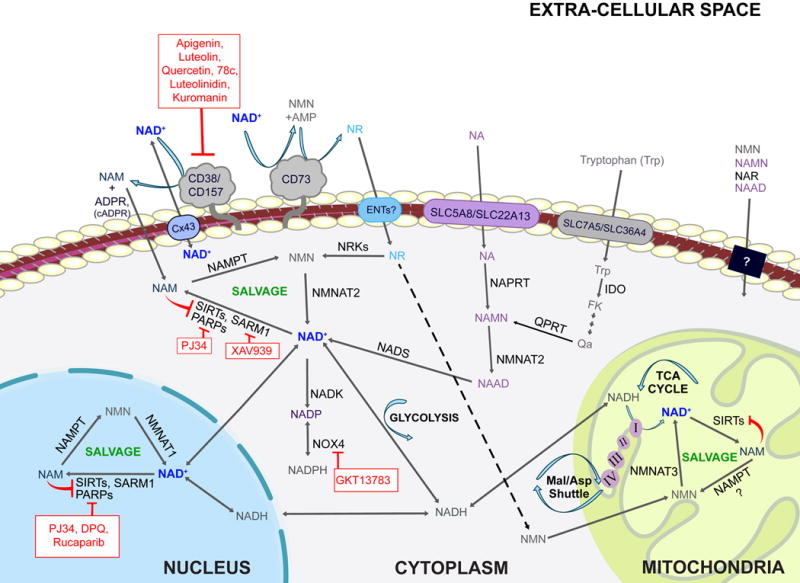
There are two major pathways contributing to NAD synthesis: de novo synthesis and salvage from precursors. The de novo pathway of NAD synthesis converts tryptophan to quinolinic acid (QA) via the kynurenine pathway. The salvage pathways recycle nicotinamide mononuclotide (NMN), nicotinamide riboside (NR), nicotinamide (NAM) and nicotinic acid (NA) in various cellular compartments including the nucleus and mitochondria. These precursors are present in the extracellular milieu and may be transported across the plasma membrane where they are utilized. Extracellular NAD is cleaved by nucleotide phosphatases (CD73) or glycohydrolases (CD38 and CD157). Cleavage by CD73 yields NMN which CD73 can then re-cleave to yield NR. Cleavage by CD38 or CD157 yields NAM. NAM is also produced within cells by NAD+-consuming enzymes such as sirtuins, PARPS and SARM1. NAM and NR are converted to NMN by NAMPT and NRKs respectively. NMN and NAMN then are converted to NAD and NAAD respectively and NAAD is amidated by NADS to yield NAD. Cellular NAD levels may be boosted by activators of the salvage pathway (green) or by inhibitors of enzymes that consume NAD+ such as CD38, PARPs, and SARM1 (red).
The rate of NAD+ synthesis in mammals is largely determined by the first step in the salvage pathway that converts NAM to NMN. In mammals, this is carried out by NAMPT, previously known as visfatin or PBEF (Yang et al., 2007). Levels of NAMPT are highly dynamic, responding to changing cellular demands for NAD+ and cell stresses such as DNA damage and starvation (Yang et al., 2007). NAMPT is an unusual enzyme in that it has both intracellular and extracellular forms known as iNAMPT and eNAMPT (Revollo et al., 2007b). In culture, NAMPT is released into supernatant by various cell types including HepG2 cells, primary hepatocytes, cardiomyocytes and leucocytes (Friebe et al., 2011; Pillai et al., 2013; Schuster et al., 2014) and in vivo, eNAMPT is present in cerebrospinal fluid (Hallschmid et al., 2009) and in serum of both mice and humans (Korner et al., 2007; Revollo et al., 2007a).
The levels of NAMPT expression are believed to control the body’s response to stress, exercise and nutrient status (Frydelund-Larsen et al., 2007; Rappou et al., 2016; Yang et al., 2007) and circadian rhythms (Asher et al., 2008; Masri et al., 2014; Ramsey et al., 2009). Indeed, obesity and high calorie diets reduce both NAMPT (Mercader et al., 2008) and NAD+ levels in various tissues including liver, white adipose (Yoshino et al., 2011), muscle and brown adipose (Canto et al., 2012). Additionally, increased levels of eNAMPT are seen in non-alcoholic fatty liver disease (NAFLD) (Garten et al., 2015), obesity, diabetes mellitus type 2 (T2DM), cardiovascular disease (Chang et al., 2011; Chen et al., 2006; Haider et al., 2006; Saddi-Rosa et al., 2013) and cancer (Audrito et al., 2015). Unlike mammals, invertebrates and yeast convert NAM to NA via Pnc1, a nutrient and stress-responsive NAM deamidase that serves a similar role to NAMPT (Anderson et al., 2003; Revollo et al., 2007a). Some microorganisms are incapable of synthesizing NAD+ de novo and therefore are exclusively reliant on external sources and salvage pathways (Ma et al., 2007).
NAD+ degradation
CD38 and CD157 (BST1) are glycohydrolases that cleave NAD+ to generate NAM and adenosine diphosphoribose (ADPR), while CD38 also hydrolyzes cyclic-ADPR (cADPR) to ADPR. To a lesser extent, both enzymes also act as ADP-ribosyl cyclases that catalyze the hydrolysis of NAD+ to generate NAM and cADPR, a Ca2+-mobilizing second messenger active in many cell types (Berthelier et al., 1998; Kirchberger and Guse, 2013; Malavasi et al., 2008). Both reactions generate NAM, which is rapidly recycled to NAD+ via the salvage pathway. CD38 is ubiquitously expressed while CD157 is expressed primarily in lymphoid tissue and gut (Hernandez-Campo et al., 2006). These enzymes have been implicated in energy metabolism, cell adhesion, various aspects of the immune response and have been linked to human diseases such as Parkinson’s, ovarian cancer, and leukemia (Quarona et al., 2013).
CD38 is a major consumer of NAD+ in mammals. Evidence for this includes the observations that mice lacking CD38 or treated with the CD38 inhibitor apigenin have about 50% more NAD+ (Escande et al., 2013) and that CD38 protein levels increase in various tissues during aging with a concurrent drop in NAD+ levels (Aksoy et al., 2006; Camacho-Pereira et al., 2016). By 32 months of age wildtype mice have about half the NAD+ levels of young mice, whereas CD38 knockout mice maintain their NAD+ levels, and are resistant to the negative effects of a high fat diet (HFD), including liver steatosis and glucose intolerance (Barbosa et al., 2007; Escande et al., 2013). Conversely, mice over-expressing CD38 have lower levels of NAD+, defective mitochondria, decreased oxygen consumption and increased lactate production (Barbosa et al., 2007; Camacho-Pereira et al., 2016). Inhibitors of CD38, such as apigenin and 78c (Haffner et al., 2015) are proof-of-concept molecules for the development of drugs to treat metabolic disorders and possibly other age-related diseases (Chillemi et al., 2013).
SARM1 is a newly discovered NAD+ cleavage enzyme in neurons and possibly other cell types (Conforti et al., 2014; Essuman et al., 2017; Gerdts et al., 2013; Osterloh et al., 2012). It is the first member of a new class of NAD+ consuming enzymes and unique among NADases in that its activity is dependent on SARM1’s Toll/interleukin-1 receptor (TIR)-domain. Moreover, it is the first example of a TIR domain, previously demonstrated to function as a protein interaction domain, possessing enzymatic activity. In response to neuronal injury, the catalytic (TIR)-domain of SARM1 initiates a cell destruction program by converting cytoplasmic NAD+ to ADPR, cADPR and NAM (Essuman et al., 2017). This depletes the cell of NAD+ and triggers axonal degeneration, which can be blocked by overexpression of NAMPT or NMNAT and by supplementation with NR (Gerdts et al., 2015). Knockout of SARM1 rescues the neuronal defects and embryonic lethality of NMNAT2-KO mice that are defective in their ability to salvage NAD+ (Gilley et al., 2015) indicating that SARM1 is a consumer of NAD+ even during embryonic development. Taken together, these results point to SARM1 as an attractive therapeutic target for the treatment of acute neuronal damage and possibly neurodegenerative diseases.
NAD+-responsive signaling pathways
In mammals, the two main NAD+-responsive signaling protein families are the sirtuins and the PARPs (Frye, 1999; Grube and Burkle, 1992; Tanner et al., 2000). Both of these protein families use NAD+ as a co-substrate to modify target proteins, releasing NAM (Bai and Canto, 2012). Sirtuins, first discovered as yeast silencing and telomere protective proteins (Ivy et al., 1985; Rine and Herskowitz, 1987), regulate a wide variety of mammalian proteins involved in processes that include mitochondrial metabolism, inflammation, meiosis, autophagy, circadian rhythms, and apoptosis (Haigis and Sinclair, 2010) (Figure 2). The classic sirtuin reaction is the removal of an acetyl group from lysines on target proteins. The first step is the release of NAM from NAD+, which is followed by the formation of a peptidyl ADP-ribose intermediate covalently attached to the acetyl group of the lysine. The peptide chain with the targeted lysine is then liberated to yield o-acetyl-ADP ribose (OADPR). There are variants on this theme: SIRT5 and SIRT6 have desuccinylation and de-fatty-acylation activities respectively (Du et al., 2011; Jiang et al., 2013), while SIRT4 and SIRT6 can simply liberate NAM, leaving a covalently attached mono-ADP-ribose (Haigis et al., 2006; Liszt et al., 2005).
Figure 2. Hallmarks of NAD homeostasis.
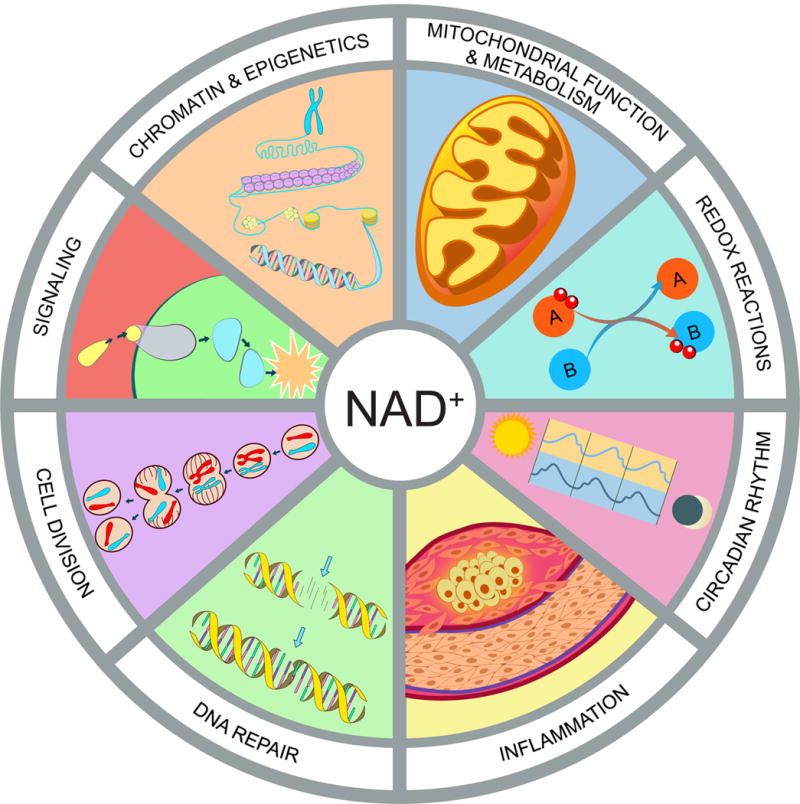
NAD+ is not merely a redox co-factor, it is also a key signaling molecule that controls cell function and survival in response to environmental changes such as nutrient intake and cellular damage. Fluctuations in NAD impact mitochondrial function and metabolism, redox reactions, circadian rhythm, immune response and inflammation, DNA repair, cell division, protein-protein signaling, chromatin and epigenetics.
The other major NAD+-responsive signaling proteins are the PARPs, which includes both Poly-ADP-ribose polymerases (PARP-1, 2, and 5) and mono-ADP-ribose transferases, which we will refer to as MARTs (PARP-3, 4 6, 10 and 14–16) (Liu and Yu, 2015). PARPs cleave NAD+ and transfer the ADP-ribose moiety to asparagine, aspartic acid, glutamic acid, arginine, lysine and cysteine residues on target proteins, forming branched poly-ADP-ribose polymers and releasing NAM in the process (Bai, 2015). PARP1 and PARP2 are required for numerous cellular processes including DNA repair and transcriptional regulation (Bai et al., 2007; Schreiber et al., 2002; Ziegler and Oei, 2001). PARP1 is best known for hyperactivation in response to DNA damage, depleting the cell of NAD+, most critically in the mitochondria, and thereby inducing apoptosis. The fact that the inhibition of PARP1 raises NAD+ levels in mice, improves mitochondrial function and protects against a HFD (Bai et al., 2011; Pirinen et al., 2014) implies that PARP1 also consumes NAD+ even under physiological conditions.
The functions of other PARPs (PARP3-16) are less well understood. Like PARP1 and 2, PARP3, 5a and 5b have been implicated in DNA-damage repair (Nagy et al., 2016; Rulten et al., 2011). Additionally, PARP5a and 5b, also known as tankyrases, are involved in mitosis and Wnt signaling (Chang et al., 2005; Chi and Lodish, 2000; Huang et al., 2009). PARP16 is a regulator of the unfolded protein response (Jwa and Chang, 2012), and PARP11 is involved in the organization of the nuclear envelope and nuclear pore complexes (Meyer-Ficca et al., 2015). As a component of the vault cytoplasmic ribonucleoprotein, PARP4 is also thought to associate with nuclear pore complexes but its function remains unclear (Chugani et al., 1993; Kickhoefer et al., 1999). PARP7, 10, 12, and 13 are believed to be involved in post-transcriptional regulation of mRNA by RNA-binding domains or ADP-ribosylation of RNA-binding proteins (Bock et al., 2015; Leung et al., 2011). PARP9, 10 and 14 regulate transcription of genes required for immune and inflammatory responses (Iwata et al., 2016; Verheugd et al., 2013). PARP6 regulates hippocampal dendritic development (Huang et al., 2016), and while its function is unknown, PARP15 has been associated with acute myeloid leukemia (Lee et al., 2016). In addition, several PARPs/MARTs may have a role in virus-host interactions (Daugherty et al., 2014).
Due to the role of both sirtuins and PARPs in the response to injury and stress, it is not surprising that there are increasing numbers of examples of the PARPs and sirtuins being co-regulated and regulating each other. For example, the deleted in breast cancer protein 1 (DBC1) binds to and inhibits both SIRT1 and PARP1, the latter of which is dependent on NAD+ binding to the “Nudix Homology Domain” (NHD) of DBC1 (Li et al., 2017). SIRT6 modifies PARP1 by mono-ADP-ribosylation, thereby increasing double strand DNA break (DSB) repair (Mao et al., 2011) whereas inhibition of PARP1 increases expression of SIRT1, SIRT4 and SIRT6 (Wencel et al., 2017).
Pharmacological NAD+ boosters
There are three main approaches that researchers and drug developers are exploring to increase NAD+ levels: supplementation with NAD+ precursors, activation of NAD biosynthetic enzymes, and inhibition of NAD+ degradation (Table 1).
Table 1.
Classes of NAD+ boosting molecules and known effects in humans
| Mechanism of Action | Pharmacological Agent | Health Outcomes Observ | References |
|---|---|---|---|
| NAD+ Precursors | Niacin (NA) | ↓ TC and LDL in dyslipidemia ↓ Serum phosphate after kidney injury ↑ GFR after kidney injury ↓ Risk of myocardial infarct ↓ Risk of stroke ↓ Risk of CVD mortality ↑ Recovery from schizophrenia ↓ Rate of cognitive decline in AD ↓ Rate of metastases/relapse in breast cancer ↑ HDL-C in sickle cell disease |
(Abram Hoffer, 2008; Garg et al., 2017; Morris et al., 2004; Phelan et al., 2017; Premkumar et al., 2007; Rennie et al., 2015; Scoffone et al., 2013) |
| NR | ↑ NAD+ in blood (dose-dependent) No Adverse effects |
(Trammell et al., 2016a) | |
| NAM | |||
| NaR | |||
| NaMN | |||
| NAAD | |||
| NMN | |||
| IHN | |||
| CD38 Inhibitors | Quercetin | ↓ LDL in CVD ↓ Blood pressure ↓ BMI ↓ Waist circumference ↓ Triglycerides |
(Dower et al., 2015; Katske et al., 2001; Lee et al., 2016a; Menezes et al., 2017; Pfeuffer et al., 2013) |
| Luteolin | ↓ Serum levels of IL-6 and TNF ↑ Adaptive functioning in autism |
(Tsilioni et al., 2015) | |
| Apigenin | |||
| 78c | |||
| Luteolinidin | |||
| Kuromanin | |||
| PARP Inhibitors | BGB-290, Olaparib, Rucaparib, Veliparib, CEP-9722, E7016, Talazoparib, Iniparib | ↓ Tumor size, metastases, and relapse in breast and ovarian cancer | (Brown et al., 2016; Evans and Matulonis, 2017) |
| Niraparib (MK – 4827) | |||
| PJ34 | |||
| DPQ | |||
| 3-aminobenzamide | |||
| SARM Inhibitors | XAV939 | ||
| NAMPT Activators | P7C3 |
NAD precursors
Homeostatic levels of NAD+ can be achieved by ingesting 15 mg of niacin daily. For most of the 20th century, this was considered optimal. It is now known that NAD+ levels decline with age and that raising levels back up to, or even above baseline, provides a surprising number of health benefits in a wide range or organisms, from yeast to rodents. The first evidence that NAD-boosting could increase lifespan came from studies our laboratory performed in yeast cells over 15 years ago. The yeast gene PNC1, encoding the enzyme that catalyzes the first step in the NAD+ salvage pathway from nicotinamide, is one of the most highly upregulated yeast genes in response to environmental stresses including heat, osmotic stress, and the restriction of amino acids and glucose (CR). We reasoned that the ability of these mild stresses to extend lifespan might be due to the upregulation of the PNC1 gene. Indeed, the constitutive overexpression of PNC1 was sufficient to increase the stress resistance and lifespan of yeast cells, whereas deletion of PNC1 rendered them unable to respond positively to caloric restriction and increased temperature (Anderson et al., 2003). In Drosophila, overexpression of the Pnc1 homolog D-NAAM extends mean lifespan by 30% (Balan et al., 2008), indicating that the upregulation of NAD synthesis might be a conserved longevity mechanism.
It has been challenging to constitutively upregulate NAD+ levels in mammals for reasons that are unclear (Frederick et al., 2015). The most common and effective approach has been a pharmacological one. NR and NMN are soluble and orally bioavailable endogenous molecules, making them the molecules of choice for animal experiments and human clinical trials (Conze et al., 2016; Trammell et al., 2016a). In rodents, NR is more efficient in boosting NAD+ than NA and NAM (Trammell et al., 2016a), possibly due to increased uptake (Imai, 2009; Imai and Guarente, 2014; Ratajczak et al., 2016; Revollo et al., 2007a).
The ability of NA to reduce cholesterol levels has been known for some time (Altschul et al., 1955) but only recently has this effect been ascribed to its ability to boost NAD+ levels (Canto et al., 2012). Though several studies have shown that NA can raise NAD+ levels (Canto et al., 2012; Kaneko et al., 2006; Santidrian et al., 2013), its use has been limited by unpleasant side effects that include flushing and itching of the skin caused by prostaglandin release (Birjmohun et al., 2005; Chen and Damian, 2014; Rolfe, 2014) and the fact that NAM inhibits PARPs and sirtuins (Bitterman et al., 2002).
Activation of NAD+ synthesis
An alternative approach to boost NAD+ levels is to directly activate NAD+ biosynthetic enzymes, in particular those that catalyze the rate-limiting steps of de novo synthesis and salvage pathways. Several enzymes are currently under investigation. The NAM salvage pathway is the predominant route in mammalian NAD+ biosynthesis (Magni et al., 1999) and NAMPT is the rate-limiting enzyme in the conversion of NAM to NAD+ (Revollo et al., 2007a). Under normal conditions NAMPT activity is sustained to maintain NAD+ homeostasis. NAMPT activity, however, declines with age and is exacerbated by acute lung injury, atherosclerosis, cancer, diabetes, rheumatoid arthritis and sepsis. Increasing systemic NAD+ biosynthesis with small chemical NAMPT activators is considered an attractive therapeutic approach. Several NAMPT activating compounds were apparently identified using a fluorometric NAMPT activity assay, but in that study only the inhibitors were characterized (Zhang et al., 2011). A neuroprotective agent, P7C3, may be a relatively weak NAMPT activator in vitro (Wang et al., 2014) although this result has not yet been replicated. NMNATs are also attractive targets for raising NAD+ in cells because they have dual substrate specificity for NMN and NaMN and contribute to both de novo and salvage pathways (Zhou et al., 2002). The green tea compound epigallocatechin gallate (EGCG) has been reported to activate NMNAT2 by more than 100% and NMNAT3 by 42% at 50 μM, although this needs to be confirmed, as no data was presented in the paper (Berger et al., 2005).
Inhibition of NAD+ degradation
An alternative approach to raising NAD+ is to inhibit its degradation either by inhibiting PARPs or NADases, also known as glycohydrolases. The major NADase in mammals, CD38, is inhibited in vitro at low micromolar concentrations by flavonoids including luteolinidin, kuromanin, luteolin, quercetin and apigenin (IC50 <10 μM) (Escande et al., 2013; Kellenberger et al., 2011). These molecules also appear to target CD38 in vivo. Apigenin increases NAD+ levels in multiple tissues, decreases global proteome acetylation and improves glucose and lipid homeostasis in obese mice, ostensibly by increasing activity of SIRT1 and SIRT3 (Camacho-Pereira et al., 2016; Escande et al., 2013). Similarly, luteolinidin prevents the loss of NAD+ and preserves endothelial and myocardial function in the post-ischemic heart (Boslett et al., 2017). GlaxoSmithKline developed thiazoloquin(az)olinones such as the compound “78c”, which have greater potency than the flavonoids and can boost NAD+ levels in plasma, liver and muscle (Haffner et al., 2015). The target specificity of 78c and whether or not it enters cells rather than acting on extracellular CD38 remain unknown. PARP1 inhibitors are marketed as adjuncts to chemotherapy or monotherapies for cancer (Brown et al., 2016). SARM1 is another NADase, which initiates a local axonal degeneration program after nerve injury that involves the rapid breakdown of NAD+ to ADPR, cADPR and NAM (Essuman et al., 2017; Gerdts et al., 2015; Summers et al., 2016). XAV939, a putative SARM1 inhibitor that also inhibits PARP5a (TNKS) and 5b (TNKS2), was identified in a chemical genetic screen nearly a decade ago (Huang et al., 2009), though its potential to boost NAD+ has only recently become evident (Gerdts et al., 2015). XAV939 has excellent pharmacokinetic properties and is currently in clinical development to treat neurological disorders and axonal injury (Haikarainen et al., 2014). To date, it is unclear if XAV939 functions in vivo by inhibiting SARM1 or other targets.
Uptake and biodistribution of NAD+ precursors
How NAD+ and its precursors are taken up by cells and tissues is not well understood and is currently the subject of considerable research and some debate. It is generally agreed that tryptophan is taken up by SLC7A5 and SLC36A4 (Kanai et al., 1998) whereas NA is taken up by SLC5A8 and SLC22A13 (Bahn et al., 2008; Gopal et al., 2007) (see Figure 1). NAM, an uncharged molecule, rapidly diffuses across the plasma and mitochondrial membranes, consistent with the fact that NA and NAM have additive effects on raising NAD+ in cells (Hara et al., 2007). For larger charged molecules, specific transporters must exist. In general, cells are unable to take up NAD+, the one exception being neurons (Araki et al., 2004). How neurons take up NAD+ is not known. Bacteria transport NAD+ via ATP/ADP translocase (Haferkamp et al., 2004), though a mammalian equivalent NAD+ transporter has yet to be identified. In yeast, NR and NAR are transported by Nrt1 and an equilibrative nucleoside transporter (ENTs), FUN26 (Belenky et al., 2008). The same may be true in mammals based on a reduction in cellular NAD+ levels when the nucleoside transporter is inhibited by dipyridamole (Nikiforov et al., 2011), but there is no direct evidence of a specific transporter.
NAD+ is present in serum and the extracellular space and some data suggests that this is also the case for NMN (Revollo et al., 2007b). These data imply that circulating NAD+ precursors might be acting to coordinate NAD+ biosynthesis and mediate signals between organs. Indeed, the existence of the NMN generating enzyme eNAMPT in serum fits with this model, though some argue that NMN is not detectable in the extracellular milieu and that conditions do not favor catalytic activity (Audrito et al., 2015; Hara et al., 2011; Ratajczak et al., 2016; Revollo et al., 2007a; Revollo et al., 2007b). Under physiological conditions, the concentrations of NAD+ and NAM in extracellular fluids such as blood plasma are in the micromolar range and can be boosted by oral administration of NMN or NR (Trammell et al., 2016a).
Extracellularly, NAD+ is cleaved to NMN and AMP by nucleotide pyrophosphatases (NPPs) including CD73 (Aleo et al., 2001; Grozio et al., 2013) while the transmembrane glycohydrolases CD38 and CD157 cleave NAD+ to generate NAM and adenosine diphosphoribose (ADPR) and can also act as ADP-ribosyl cyclases that catalyze the hydrolysis of NAD+ to generate NAM, and cADPR, a cell-cell messenger that appears to mediate activated monocyte-induced Ca2+ signaling, ROS production, and apoptosis (Yang et al., 2011).
Whether or not NMN is taken up by a transporter is currently the subject of debate (Mills et al., 2016; Ratajczak et al., 2016). Brenner, Canto and colleagues argue that NMN is not taken up fast enough to invoke the presence of a transporter, and that both NAD+ and NMN undergo extracellular degradation to generate permeable precursors that can be taken up by cells (Ratajczak et al., 2016). On the other hand, Imai argues that this is likely a cell-type specific phenomenon and that some cell types can rapidly take up NMN (Mills et al., 2016). If so, the identification of the putative transporter will help resolve the debate and help identify which cell types and tissues are able to transport NMN across the plasma membrane. Additional studies with isotopically labeled NAD+ precursors to trace the uptake and metabolism of these molecules should help answer these questions.
The metabolism and biodistribution of NAD precursors in various tissues and within cells is poorly understood. The system is clearly complex, arising from variation in transporter expression, enzymatic machinery, extracellular degradation and resynthesis (Bogan and Brenner, 2008; Duarte-Pereira et al., 2016; Ratajczak et al., 2016; Zamporlini et al., 2014). NAMPT is expressed ubiquitously in the body but there are large differences in the levels of expression between tissues (Dietrich et al., 1966; Revollo et al., 2007b). Metabolic profiling of mouse tissues indicates that the activity of NMNAT isoforms, required for amidated NAD+ salvage pathways, which utilize NR or NAM, is much higher than NAMPT and is non-rate-limiting in most tissues, except blood. NADS activity, required for the deamidated pathway that utilizes NA, appears to be rate limiting in lung and skeletal muscle where levels are difficult to detect and the enzyme’s substrate, NAAD, accumulates (Mori et al., 2014). In brain and heart, the NAMPT-dependent pathway is the preferred route of salvage, whereas in skeletal muscle, NRK-dependent salvage pathways are the preferred mode for generating NAD+. Additionally, expression analysis of NRK subtypes shows that NRK1 is expressed ubiquitously while NRK2 is mainly present in skeletal muscle (Fletcher et al., 2017). Consistent with this, chronic NR supplementation raises NAD+ levels in muscle, but not in brain or white adipose tissue (Canto et al., 2012). Interestingly, the CD38 inhibitor 78c increases NAD+ by more than 5-fold in mouse liver but only >1.2-fold in muscle, arguing that CD38 activities are also tissue specific (Haffner et al., 2015). Whether or not the ability of CD38 to raise NAD+ levels in tissues is due to intra- or extracellular activity remains to be determined (Bonkowski and Sinclair, 2016; Gupte et al., 2017).
Effects of NAD+ boosters on physiology and health in mouse models
The initial discovery that genetically upregulating NAD+ biosynthesis can increase the stress resistance and lifespan of yeast cells and Drosophila (Anderson et al., 2002; Anderson et al., 2003; Balan et al., 2008), prompted investigation of NAD+ boosters in rodents, both wildtype and disease models, often with dramatic effects (Figure 3).
Figure 3. Physiological effects of NAD-boosting molecules.
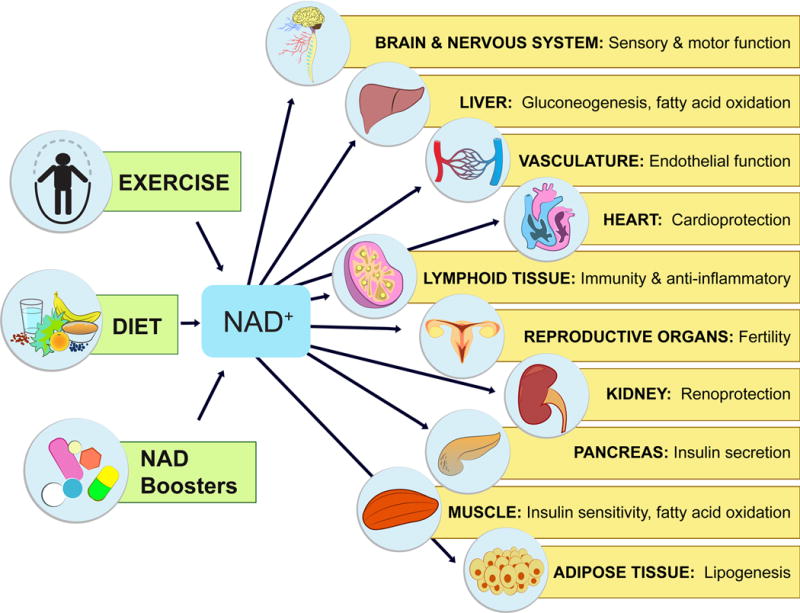
NAD+ levels steadily fall as we age, leading to a decline in the function of cells and organs. By raising NAD+, NAD+ boosters can have profound effects on the health and survival of mammals. Increases in NAD+ promote cognitive and sensory function, gluconeogenesis in liver, lipogenesis in adipose tissue, insulin secretion in pancreas, and insulin sensitivity in muscle. NAD+ also promotes endothelial cell proliferation and protects against cardio- and cerebrovascular disease. NAD regulates immune function and inflammation and, protects against acute injury in kidney. NAD promotes and extends fertility in both males and females, ostensibly by activation of sirtuins.
Liver Function
Key enzymes in NAD+ signaling pathways are known to protect the liver from fat accumulation, fibrosis and insulin resistance, which are related to the development of fatty liver diseases, such as NAFLD and NASH. SIRT1 and its downstream targets PGC-1α, PSK9, and SREBP1 maintain mitochondrial, cholesterol transport, and fatty acid homeostasis (Baur et al., 2006; Picard et al., 2004; Rodgers et al., 2005; Yang et al., 2006). SIRT2 controls gluconeogenesis by deacetylating phosphoenolpyruvate carboxykinase (PEPCK) (Jiang et al., 2011), SIRT3 regulates OXPHOS (Ahn et al., 2008), fatty acid oxidation (Hirschey et al., 2010), ketogenesis (Shimazu et al., 2010), and defense against oxidative stress (Finley et al., 2011; Someya et al., 2010), while SIRT6 controls gluconeogenesis (Dominy et al., 2012; Zhang et al., 2014).
Given the critical nature of these pathways in the liver, the maintenance of NAD+ levels is imperative for optimal organ function. As a result of obesity and aging, levels of NAMPT decline and CD38 levels increase, leading to a 2-fold decrease in steady state NAD+ levels by mid-age (Zhou et al., 2016). Raising NAD+ levels back to those of young or lean mice has been particularly effective at preventing and treating obesity, alcoholic steatohepatitis and NASH, while improving glucose homeostasis and mitochondrial dysfunction. Approaches that have worked well in rodents include the inhibition of NAD+ consuming enzymes such as PARPs (Bai et al., 2011; Gariani et al., 2017) and CD38 (Barbosa et al., 2007), the inhibition of nicotinamide N-methyltransferase (Nnmt) (Kraus et al., 2014) and supplementation with NAD+ precursors, NR (Canto et al., 2012) or NMN (Yoshino et al., 2011). NAD boosting appears to not only improve the health of the liver, but also increase its capacity for regeneration and protect it against hepatotoxicity. After partial hepatectomy (PHx), NR-treated mice have increased and more uniform liver regeneration, a shorter period of steatosis, increased DNA synthesis, and improved lipid metabolism as compared to untreated control mice (Mukherjee et al., 2017). Olaparib treatment raises NAD+ levels and prevents lipopolysaccharide (LPS)-induced acute hepatitis (Gariani et al., 2017). NAM and another PARP inhibitor, PJ34, are both effective in increasing NAD+ levels, and preventing hepatosteatosis and thymus atrophy, in a chick embryo model of dioxin-toxicity (Diani-Moore et al., 2017).
Kidney Function
Several lines of evidence indicate that reduced levels of NAD+ in aged kidneys and a corresponding decrease in sirtuin activity are largely responsible for reduced kidney function and resilience with age (Ugur et al., 2015). Consistent with this, activation of SIRT1 and SIRT3 by NAD+ supplementation protects against high-glucose-induced kidney mesangial cell hypertrophy (Zhuo et al., 2011) while treatment of mice with NMN protects from cisplatin-induced acute kidney injury (AKI) in a SIRT1-dependent manner (Guan et al., 2017). 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), a nucleoside converted to 5-amino-4-imidazolecarboxamide riboside 5′-monophosphate (ZMP) that stimulates AMPK activity, increases NAD+ levels and also protects against cisplatin-induced AKI in a SIRT3-dependent manner (Morigi et al., 2015). NAM supplementation in mice stimulates secretion of the kidney-protective prostaglandin PGE2, improves renal function after ischemia and similarly prevents cisplatin-induced AKI, ostensibly by stimulating NAD+ synthesis (Tran et al., 2016).
Skeletal Muscle Function
Old mice have increased markers of muscle atrophy and inflammation, as well as impaired insulin signaling and insulin-stimulated glucose uptake compared to young wild-type mice. Treatment of old mice with NAD+ precursors, such as NR and NMN, dramatically improves muscle function. For example, treatment of old mice with NMN (500 mg/kg/d i.p. for 7 days) reverses detrimental age-associated changes in muscle, by increasing mitochondrial function, increasing ATP production, reducing inflammation, and switching glycolytic type II muscle to a more oxidative fiber type (Gomes et al., 2013). Similarly, old and high fat diet (HFD)-fed mice treated with NR (400 mg/kg/d in chow) have improved oxidative metabolism in muscle as well as increased endurance and thermogenic capacity (Canto et al., 2012). NR also increases the number and quality of muscle stem cells (MuSC) and enhances muscle regeneration in old mice, in part, by improving mitochondrial function and preventing MuSC senescence (Zhang et al., 2016). In the context of muscular dystrophy models, NR improves mobility in dystrophic C. elegans mutants (Ryu et al., 2016), NAD+ supplementation of zebrafish reduces the percentage of detached muscle fibers and increases mobility (Goody et al., 2012), and in the MDX mouse, NR improves mitochondrial function, recovery from muscle injury, and running capacity (Ryu et al., 2016).
Cardiac Function
NAD+ levels are critical for normal heart function and recovery from injury. Of all the NAD+−dependent signaling proteins, SIRT3 seems to be the most important in this context. SIRT3 knockout mice have hyperacetylated OXPHOS enzymes, reduced ATP (Sundaresan et al., 2009) and are hypersensitive to aortic constriction, ostensibly due to activation of CypD, a regulator of the mitochondrial permeability transition pore (Hafner et al., 2010; Sundaresan et al., 2009). These mice develop fibrosis and cardiac hypertrophy as early as 13 months of age, which is further exacerbated by the loss of SIRT3 in aged and hypertrophic hearts, a decline that can be reversed by treatment with NMN (Horton et al., 2016). Consistent with this, NAMPT overexpression and NMN treatment either 30 min before ischemia (500 mg/kg, i.p.) or repetitive administration just before and during reperfusion, provides marked protection against pressure over-load and ischemia/reperfusion (I/R) injury, reducing infarct size by as much as 44% (Hsu et al., 2009; Karamanlidis et al., 2013; Pillai et al., 2005; Yamamoto et al., 2014). Treatment with NAD+ precursors also improves heart function in old MDX mice with cardiomyopathy (Ryu et al., 2016), improves mitochondrial and cardiac function in a mouse model for iron deficiency-induced heart failure (Xu et al., 2015) and restores cardiac function to near normal levels in a mouse model of Friedreich’s Ataxia (FRDA) cardiomyopathy (Chan et al., 2013; Martin et al., 2017).
Endothelial and Vascular Function
Cardiovascular and cerebrovascular diseases contribute to the greatest decline in quality of life after 65 and are directly responsible for about one third of all deaths (Nichols et al., 2014; Ungvari et al., 2010). Understanding why this occurs and how to reverse it is key to future gains in human health. Treatment of old mice with NMN (~300mg/kg/d for 8 weeks) restores carotid artery endothelium-dependent dilation (EDD), a measure of endothelial function, while reducing aortic pulse wave velocity and elastic artery stiffness (de Picciotto et al., 2016). The performance of most organs and tissues is critically dependent on an abundant, fully functional microcapillary network that maintains a supply of oxygen, exchanges heat and various nutrients, and removes the waste products of metabolism. Yet one of the most profound changes to the body as it ages is a decline in the number and function of endothelial cells (ECs) that line the vasculature. Treatment of mice with NMN (500 mg/kg/d in water for 28 days) improves blood flow and increases endurance in elderly mice by promoting SIRT1-dependent increases in capillary density (Das et al., 2018 Das et al., in press at Cell, MS included in submission). Thus repleting NAD+ levels in the vascular endothelium is an attractive approach to increasing mobility in the elderly and treating conditions exacerbated by decreased blood flow, such as ischemia reperfusion injury, slow wound healing, liver dysfunction, and muscle myopathies.
DNA Repair and Cancer
Because NAD+ is involved in so many aspects of cancer biology, from mitochondrial activity to cell survival, there are a variety of ways it could be used in the clinic. The rationale for reducing NAD+ levels in tumors is that they will be less able to repair DNA damage, thereby increasing their sensitivity to chemotherapeutic agents. Natural inhibitors of PARP1 include the flavonoids myricetin, tricetin, quercetin, fisetin, rutin and naringin (Geraets et al., 2007). More potent PARP1 inhibitors, such as PJ34, DPQ, and the marketed drugs olaparib and rucaparib sensitize tumors to DNA damage, although the drugs have limited use because of toxicity (Alano et al., 2010; Almeida et al., 2017; Mathews and Berk, 2008). Inhibitors of NAMPT, such as FK866 and KPT-9274, selectively kills cancer cells, and are currently being tested for efficacy in patients with solid malignancies (Chini et al., 2014; Poljsak, 2016; Sharif et al., 2016). Inhibition of indoleamine 2,3-dioxygenase (IDO), which mediates the first step in the conversion of tryptophan in de novo NAD+ synthesis, shows promise in mice as an antitumor therapeutic target (Friberg et al., 2002; Muller et al., 2005; Uyttenhove et al., 2003).
The other approach to treating cancer has been to increase NAD+ levels, the rationale being that an excess of NAD+ will boost mitochondrial respiration and downregulate glycolysis, counteracting Warburg metabolism that cancer cells prefer (Wu et al., 2014). Increased NAD+ would also boost the activity of SIRT1 and SIRT6, both of which can inhibit tumors by downregulating beta-catenin signaling and glycolysis (Firestein et al., 2008; Sebastian et al., 2012). One concern is that raising NAD+ may promote DNA repair and angiogenesis, helping cancer cells thrive (Batra and Kislay, 2013; Fang et al., 2016; Li et al., 2017). Long-term studies of wildtype mice, however, failed to provide any evidence of increased tumor size or number (Shukla et al., 2014; Tummala et al., 2014). Interestingly, overexpression of NMNAT3 raises mitochondrial NAD+ and inhibits the growth of glioblastoma cells (Son et al., 2017) and supplementation with NA or NAM inhibits tumor growth and multi-organ metastasis in SCID mice (Santidrian et al., 2013). It will be important to test the effects of NAD+ boosters in gold-standard cancer models, either in the absence of, or combination with, standard chemotherapeutic agents.
Immunity and Inflammation
There is a growing body of evidence that NAD+ precursors can have anti-inflammatory effects. Treatment of 24-month-old mice with NMN for one week reduced the expression of inflammation markers such as TNFα and IL-6 in skeletal muscle (Gomes et al., 2013). Similarly, NR significantly reduced inflammation in a mouse model of AT autoimmunity (Fang et al., 2016) and in the muscular dystrophy MDX mouse model, though it is not clear if these effects are secondary to an improvement in muscle function (Ryu et al., 2016). NAM has been effective in the treatment of various inflammatory skin conditions (Niren, 2006), reduces the area of infiltration and demyelination in experimental autoimmune encephalomyelitis (EAE) mouse models (Kaneko et al., 2006) and prevents photo-immunosuppression and photo-carcinogenesis (Damian et al., 2008; Gensler, 1997; Yiasemides et al., 2009). However, it is not yet known if these effects are due to increaseing NAD+ levels or other physiological effects.
NAD+ metabolism also plays an important role in host-pathogen interactions. Several pathogenic organisms are auxotrophic and therefore dependent on the host for NAD+ or precursors. Targeting the uptake of these precursors in pathogens may be a fruitful avenue to pursue (Domergue et al., 2005; Ma et al., 2007; Moreira et al., 2015; Murray et al., 1995; Zerez et al., 1990). Other organisms have their own NAD+ salvage pathways that can be targeted by drugs to kill the pathogen. In fact, the antibiotic pyrazinamide, commonly used to treat tuberculosis, is converted to a toxic product by a pyrazinamide/nicotinamidase called PncA, which catalyzes the first step in the conversion of NAM back to NAD+. As with cancer, the biology of the host-pathogen interaction is complex and has produced conflicting results, depending on which downstream player, whether it be PARP1, CD38 or SIRT1, is being analyzed (Koedel et al., 2002; Moreira et al., 2015; Partida-Sanchez et al., 2007; Ren et al., 2014). Further investigation will be required to determine if NAD+ depletion or repletion in the host can be used as treatment for infections.
Neuronal Function
The neuroprotective effects of NAD+ precursors were first revealed by a study of middle cerebral artery (MCAO)-induced ischemia, where treatment with NAM reduced the extent of infarct in Wistar rats (Ayoub et al., 1999; Klaidman et al., 2003; Sadanaga-Akiyoshi et al., 2003), a finding recently replicated by treatment with NMN (Wei et al., 2017a). Though these early findings were indicative, it wasn’t until 2004 that NAD+ was brought into the forefront of neuroscience (Gerdts et al., 2015; Sasaki et al., 2016). The Wallerian degeneration slow (Wlds) mouse is so named for its neurons that are relatively resistant to axonal degeneration after nerve damage. The mutation was known to be due to an autosomal dominant mutation that fused the ubiquitination factor e4b (Ube4b) with NMNAT1 (Coleman et al., 1998), but the role of NAD+ was not appreciated until it was shown that increased NAD+ could mimic the Wlds mutation, ostensibly by activating SIRT1 (Araki et al., 2004). The same group recently identified neuronal SARM1 as the major cause of NAD+ depletion in injured neurons (Essuman et al., 2017).
Since then, numerous studies have reinforced the view that NAD+ levels are key to neuronal function and survival. This includes the dependence on NMNAT2 and its NAD synthesis activity for axonal survival (Yan et al., 2010). There is some evidence indicating that NMN treatment promotes axon degeneration, and that the dependence on NMNAT2 for axon survival is due to its consumption of NMN rather than its NAD synthesis activity (Di Stefano et al., 2015). This is supported by recent in vivo work in zebrafish larvae and mice showing that expression of a bacterial NMN-deamidase, which consumes NAD but does not have NAD synthesis activity, rescues axonal defects and promotes axon survival (Di Stefano et al., 2017). On the other hand, NMN preserves hippocampus-dependent spatial memory after forebrain ischemia (Park et al., 2016) and reduces edema, oxidative stress, inflammation, and neuronal death in a mouse collagenase-induced intracerebral hemorrhage (cICH) model (Wei et al., 2017b).
In addition to protecting damaged neurons, NAD+ precursors have shown promise in delaying the effects of several neurodegenerative diseases. In models of Alzheimer’s Disease (AD), NR and NMN treatment improve cognition and synaptic plasticity in mice and rats (Gong et al., 2013; Hou et al., 2018; Long et al., 2015; Sorrentino et al., 2017; Wang et al., 2016). NAM increases cell viability in a Drosophila model of PD (Jia et al., 2008) and several studies have also suggested that an NA-rich diet both reduces the risk of developing Parkinson’s Disease (PD) and improves the physical functioning of individuals with PD (Alisky, 2005; Fall et al., 1999; Hellenbrand et al., 1996), who typically have low kynurenine/tryptophan ratios (Widner et al., 2002). Both NR and NMN improve motor function and memory in worm and mouse models of ataxia telangiectasia (AT) (Fang et al., 2016). Furthermore, P7C3, a pro-neurogenic putative NAMPT activator (Pieper et al., 2010; Wang et al., 2014), is neuroprotective in animal models of Parkinson’s Disease and ALS (De Jesus-Cortes et al., 2012; Tesla et al., 2012). NAD-boosting regimens prevent and in some cases can reverse neuronal degeneration associated with hearing loss, prion toxicity, retinal damage, traumatic brain injury (TBI), and peripheral neuropathy (Brown et al., 2014; Dutca et al., 2014; Hamity et al., 2017; Lin et al., 2016; Vaur et al., 2017; Yin et al., 2014; Zhou et al., 2015).
Aging and Longevity
Total NAD+ levels were once considered extremely stable. Recently, however, it has become clear that a steady decline in total NAD+ levels over time is a natural part of life for all species, from yeast to humans (Balan et al., 2008; Belenky et al., 2007; Lin et al., 2004; Massudi et al., 2012; Mouchiroud et al., 2013; Zhang et al., 2016; Zhu et al., 2015). This decline, along with the decreased activity of NAD+ signaling proteins, is believed to be one of the major reasons organisms, including humans, age.
Given that budding yeast were first shown to live longer when the salvage pathway was upregulated, it was only appropriate that NAD+ precursors were first shown to extend lifespan in this species. Yeast grown on 10 μM NR have increased gene silencing, decreased rDNA recombination (a known cause of aging in yeast), and an increased lifespan of >4 generations (Belenky et al., 2007). Both NR and NMN improve neuronal and muscular function and extend the lifespan of C. elegans by >10% (Mouchiroud et al., 2013).
In mice, raising NAD+ levels has been effective in delaying progeroid (accelerated aging) phenotypes and extending lifespan in various models including BubR1 hypomorphs, xeroderma pigmentosum (XPA), ataxia telangiectasia (AT), and a Cockayne’s syndrome mouse model (Fang et al., 2016; Fang et al., 2014; North et al., 2014). In the case of the BubR1 mouse, median lifespan was extended by 58% percent and maximum by 21% (North et al., 2014), which is more than twice the effect of senolytics in this model (Baker et al., 2011). In part because of the time and cost, the number of studies testing NAD+ boosters in mouse lifespan are few. Treatment of old (20-month-old) mice with NR extended their lifespan by nearly 5%, even when started at an age where few treatments work well, with the exception of rapamycin. Increased lifespan was also associated with a variety of physiological benefits including improved mitochondrial function and preservation of stem cell function (Zhang et al., 2016). Though NMN or other NAD+ boosters have not yet been tested for their effects on murine lifespan, some mice have been dosed for long periods. For example, starting at 5 months, NMN was administered to mice for over a year. Treated mice had increased activity, improved insulin sensitivity and lipid profiles, improved vision, and greater bone-density (Mills et al., 2016). Taken together, these results along with the various health benefits and age-reversal activities listed above, support the possibility of using NAD+ boosters as therapeutics against a broad range of age-associated diseases and possibly as a way to delay aging and age-related physical decline.
Human trials
NAD+ boosters have shown efficacy in a variety of mouse models of human disease (Figure 4), prompting numerous clinical trials of NAD+ boosters in humans (Table 1). The most studied of the NAD+ precursors in humans is NA (niacin). In large doses greater that a gram, NA is an effective way to treat hypercholesterolemia as it lowers LDL,and is one of the few drugs that significantly raises HDL (Altschul et al., 1955; Garg et al., 2017; Rivin, 1962). It is commercially available either as compressed NA (Niacor®) or extended-release to prevent flushing caused by prostaglandin release (Niaspan®, Advicor®, Simcor®). Niaspan® was first approved for several indications by the FDA in 1997 and later used in combination with statins for the treatment of primary hyperlipidemia and mixed dyslipidemia. NA and NAM have been or are currently being evaluated for other health benefits including treatments for acne, kidney diseases, lupus, Alzheimer’s disease, schizophrenia, Diabetes Mellitus, non-small-cell lung carcinoma, obesity, HIV induced dyslipidemia, non-alcoholic fatty liver disease (NAFLD), sickle cell disease and others. Though NA has been shown to raise NAD+ levels in rodents (Canto et al., 2012; Santidrian et al., 2013), the possibility that NA improves cholesterol profiles in humans, at least in part, by raising NAD+ levels (Gariani et al., 2016) is generally not discussed in the literature.
Figure 4. Potential impact of NAD+ boosters on human health via NAD+ signaling pathways.
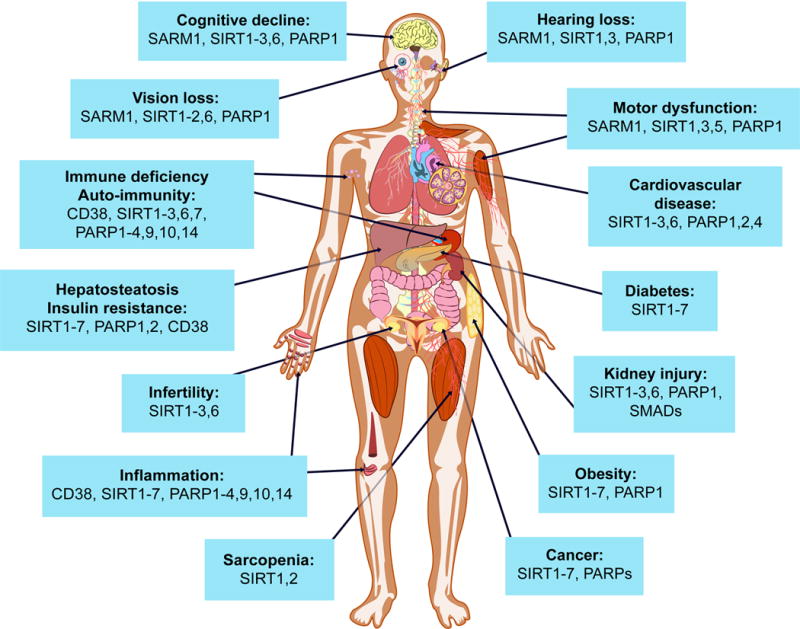
A decline in NAD+ during aging is believed to be a major cause of disease and disability, such as hearing and vision loss, as well as cognitive and motor dysfunction, immune deficiencies, auto-immunity and dysregulation of the inflammatory response leading to arthritis, metabolic dysfunction and cardiovascular disease. In mouse models, NAD+ boosters prevent or treat a variety of different diseases, prompting a search for NAD+ boosters that are safe and effective as drugs to treat both rare and common diseases, and potentially aging itself.
In rodents, NAD+ boosters such as NR and NMN appear to raise NAD+ to greater levels than NA and NAM, though no head-to-head study has yet been performed. NR is currently sold as a supplement either alone or in combination with methylated resveratrol by companies such as Elysium Health and HPN that typically contain “NIAGEN®”. Research into the effects of NR and NMN in humans is gaining considerable traction, with a number of registered clinical trials being recently completed or currently underway (see Clinicaltrials.gov). For example, a randomized, double-blind, three-arm crossover pharmacokinetic study in 12 human subjects showed that NR raises NAD+ by as much as 2.7-fold in human blood with a single oral dose of 1000 mg, with NAAD emerging as a sensitive biomarker (Trammell et al., 2016a). Researchers at the University of Washington completed a clinical trial with 140 participants showing that orally administered NR gives a dose dependent increase in NAD+ from 250–1000 mg/d plateauing at a 2-fold increase in NAD+ at day 9 (Airhart et al., 2017). Another study reported positive effects of NR on vascular endothelial function in healthy middle-aged and older adults, with further investigations of motor and cognitive changes to come (Heilbronn, 2017). At least seven other studies are now underway assessing the effects of NR on such parameters as muscle mitochondrial function, cognition, immune function, kidney function, traumatic brain injury, brown fat activity, lipid accumulation, energy metabolism, cardiovascular risk, body composition, and acetylcarnitine levels.
An international collaborative team between Keio University in Tokyo and Washington University School of Medicine in St Louis is running a Phase I human clinical study of NMN in Japan (Tsubota, 2016). Clinical trials examining the safety and efficacy of NMN are also currently being run at Washington University, investigating the effect on insulin senstivity, endothelial function, lipids, body and liver fat and markers of cardiovascular and metabolic health. Metrobiotech, a Boston-based company, has generated a pipeline of novel NAD+ precursors, the first of which, MIB-626, is being tested in clinical trials in Boston. Calico has a program to develop NAMPT activators but the program is on hold. Luteolin, a CD38 inhibitor, had positive neuroprotective effects on children with autism but whether NAD+ is responsible for the benefit was not investigated (Tsilioni et al., 2015). SARM1, a promising therapeutic target for axonopathies (Essuman et al., 2017) is in preclinical development at Disarm Therapeutics. PARP1/2 inhibitors improve the health of mice on a HFD (Cerutti et al., 2014). Although, their toxicity may preclude them from use in chronic diseases, there are others with fewer side effects in development (Malyuchenko et al., 2015).
Perspective.
Given that NAD+ was discovered over 100 years ago, it is surprising how little we know about its biology, pharmacology, and role in human disease. Though there is much to learn, we know this much: NAD+ boosters seem relatively safe and have a remarkable ability to prevent and treat diseases. Whether these findings will translate to humans is the next big question. Preliminary results in small human clinical trials look promising but there is a long way to go. For NAD+ boosters to be used widely as drugs, it will be important to know their safety profile and understand fundamental aspects of NAD+ biology and physiology, such as the tissue distribution of NAD+ precursors and other NAD boosters, their transport into the cell and into organelles, and the metabolism of NAD+ intermediates in the gut, the bloodstream, on the plasma membrane, and within organelles. There are also practical issues to overcome, such as how to stabilize NAD+ boosters, how to best deliver them and at what dose, what are the best biomarkers and analytical methods, and whether it is best to modulate the degradation or synthesis of NAD+ to achieve the desired efficacy in specific diseases. It is exciting to imagine an NAD+ booster being tested in humans for the ability to increase vitality, reduce all causes of mortality, and extend healthy lifespan. If that happens, more than the discoverers of NAD+ could ever have imagined, NAD+ would truly be the molecule of life.
Acknowledgments
We would like to thank members of the Sinclair lab who helped with writing and editing, especially Michael Shultz, Michael Bonkowski, Alice Kane, and Ian Lippincott for graphic design. DAS is a consultant to and inventor on patent applications licensed to MetroInternational Biotech, Arc Bio, Life Biosciences, Liberty Biosecurity and Jumpstart Fertility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airhart SE, Shireman LM, Risler LJ, Anderson GD, Naganda Gowda GA, Raftery D, Tian R, Shen DD, O’Brien KD. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLOS ONE. 2017 doi: 10.1371/journal.pone.0186459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy P, Escande C, White TA, Thompson M, Soares S, Benech JC, Chini EN. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem Biophys Res Commun. 2006;349:353–359. doi: 10.1016/j.bbrc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleo MF, Giudici ML, Sestini S, Danesi P, Pompucci G, Preti A. Metabolic fate of extracellular NAD in human skin fibroblasts. J Cell Biochem. 2001;80:360–366. doi: 10.1002/1097-4644(20010301)80:3<360::aid-jcb90>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Alisky JM. Niacin improved rigidity and bradykinesia in a Parkinson’s disease patient but also caused unacceptable nightmares and skin rash–a case report. Nutr Neurosci. 2005;8:327–329. doi: 10.1080/10284150500484638. [DOI] [PubMed] [Google Scholar]
- Almeida GS, Bawn CM, Galler M, Wilson I, Thomas HD, Kyle S, Curtin NJ, Newell DR, Maxwell RJ. PARP inhibitor rucaparib induces changes in NAD levels in cells and liver tissues as assessed by MRS. NMR Biomed. 2017;30 doi: 10.1002/nbm.3736. [DOI] [PubMed] [Google Scholar]
- Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem Biophys. 1955;54:558–559. doi: 10.1016/0003-9861(55)90070-9. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Cohen H, Lin SS, Manchester JK, Gordon JI, Sinclair DA. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J Biol Chem. 2002;277:18881–18890. doi: 10.1074/jbc.M111773200. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari HR, Raghava GP. Identification of NAD interacting residues in proteins. BMC Bioinformatics. 2010;11:160. doi: 10.1186/1471-2105-11-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Audrito V, Serra S, Brusa D, Mazzola F, Arruga F, Vaisitti T, Coscia M, Maffei R, Rossi D, Wang T, et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 2015;125:111–123. doi: 10.1182/blood-2014-07-589069. [DOI] [PubMed] [Google Scholar]
- Ayoub IA, Lee EJ, Ogilvy CS, Beal MF, Maynard KI. Nicotinamide reduces infarction up to two hours after the onset of permanent focal cerebral ischemia in Wistar rats. Neurosci Lett. 1999;259:21–24. doi: 10.1016/s0304-3940(98)00881-7. [DOI] [PubMed] [Google Scholar]
- Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, Krick W, Burckhardt BC, Sabolic I, Burckhardt G. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13) J Biol Chem. 2008;283:16332–16341. doi: 10.1074/jbc.M800737200. [DOI] [PubMed] [Google Scholar]
- Bai P. Biology of Poly(ADP-Ribose) Polymerases: The Factotums of Cell Maintenance. Mol Cell. 2015;58:947–958. doi: 10.1016/j.molcel.2015.01.034. [DOI] [PubMed] [Google Scholar]
- Bai P, Canto C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 2012;16:290–295. doi: 10.1016/j.cmet.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai P, Houten SM, Huber A, Schreiber V, Watanabe M, Kiss B, de Murcia G, Auwerx J, Menissier-de Murcia J. Poly(ADP-ribose) polymerase-2 [corrected] controls adipocyte differentiation and adipose tissue function through the regulation of the activity of the retinoid X receptor/peroxisome proliferator-activated receptor-gamma [corrected] heterodimer. J Biol Chem. 2007;282:37738–37746. doi: 10.1074/jbc.M701021200. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, Kaplun A, VanBerkum MF, Arking R, Freeman DC, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa MT, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, Chini EN. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- Batra V, Kislay B. Mitigation of gamma-radiation induced abasic sites in genomic DNA by dietary nicotinamide supplementation: metabolic up-regulation of NAD(+) biosynthesis. Mutat Res. 2013;749:28–38. doi: 10.1016/j.mrfmmm.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+ Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Belenky PA, Moga TG, Brenner C. Saccharomyces cerevisiae YOR071C encodes the high affinity nicotinamide riboside transporter Nrt1. J Biol Chem. 2008;283:8075–8079. doi: 10.1074/jbc.C800021200. [DOI] [PubMed] [Google Scholar]
- Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- Berthelier V, Tixier JM, Muller-Steffner H, Schuber F, Deterre P. Human CD38 is an authentic NAD(P)+ glycohydrolase. Biochem J. 1998;330(Pt 3):1383–1390. doi: 10.1042/bj3301383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birjmohun RS, Hutten BA, Kastelein JJ, Stroes ES. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2005;45:185–197. doi: 10.1016/j.jacc.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Bock FJ, Todorova TT, Chang P. RNA Regulation by Poly(ADP-Ribose) Polymerases. Mol Cell. 2015;58:959–969. doi: 10.1016/j.molcel.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boslett J, Hemann C, Zhao YJ, Lee HC, Zweier JL. Luteolinidin Protects the Postischemic Heart through CD38 Inhibition with Preservation of NAD(P)(H) J Pharmacol Exp Ther. 2017;361:99–108. doi: 10.1124/jpet.116.239459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JS, Kaye SB, Yap TA. PARP inhibitors: the race is on. Br J Cancer. 2016;114:713–715. doi: 10.1038/bjc.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, Jaffrey SR. Activation of SIRT3 by the NAD(+) precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014;20:1059–1068. doi: 10.1016/j.cmet.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Pereira J, Tarrago MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016;23:1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, Goodman RH. Biosensor reveals multiple sources for mitochondrial NAD(+) Science. 2016;352:1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron WD, Bui CV, Hutchinson A, Loppnau P, Graslund S, Rocheleau JV. Apollo-NADP(+): a spectrally tunable family of genetically encoded sensors for NADP(+) Nat Methods. 2016;13:352–358. doi: 10.1038/nmeth.3764. [DOI] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, Leoni V, Schon EA, Dantzer F, Auwerx J, et al. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014;19:1042–1049. doi: 10.1016/j.cmet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P, Weill JD, Mandel P. Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res Commun. 1963;11:39–43. doi: 10.1016/0006-291x(63)90024-x. [DOI] [PubMed] [Google Scholar]
- Chan PK, Torres R, Yandim C, Law PP, Khadayate S, Mauri M, Grosan C, Chapman-Rothe N, Giunti P, Pook M, et al. Heterochromatinization induced by GAA-repeat hyperexpansion in Friedreich’s ataxia can be reduced upon HDAC inhibition by vitamin B3. Hum Mol Genet. 2013;22:2662–2675. doi: 10.1093/hmg/ddt115. [DOI] [PubMed] [Google Scholar]
- Chang P, Coughlin M, Mitchison TJ. Tankyrase-1 polymerization of poly(ADP-ribose) is required for spindle structure and function. Nat Cell Biol. 2005;7:1133–1139. doi: 10.1038/ncb1322. [DOI] [PubMed] [Google Scholar]
- Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27:515–527. doi: 10.1002/dmrr.1201. [DOI] [PubMed] [Google Scholar]
- Chen AC, Damian DL. Nicotinamide and the skin. Australas J Dermatol. 2014;55:169–175. doi: 10.1111/ajd.12163. [DOI] [PubMed] [Google Scholar]
- Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, Lee YJ. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- Chi NW, Lodish HF. Tankyrase is a golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. J Biol Chem. 2000;275:38437–38444. doi: 10.1074/jbc.M007635200. [DOI] [PubMed] [Google Scholar]
- Chillemi A, Zaccarello G, Quarona V, Ferracin M, Ghimenti C, Massaia M, Horenstein AL, Malavasi F. Anti-CD38 antibody therapy: windows of opportunity yielded by the functional characteristics of the target molecule. Mol Med. 2013;19:99–108. doi: 10.2119/molmed.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini CC, Guerrico AM, Nin V, Camacho-Pereira J, Escande C, Barbosa MT, Chini EN. Targeting of NAD metabolism in pancreatic cancer cells: potential novel therapy for pancreatic tumors. Clin Cancer Res. 2014;20:120–130. doi: 10.1158/1078-0432.CCR-13-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani DC, Rome LH, Kedersha NL. Evidence that vault ribonucleoprotein particles localize to the nuclear pore complex. J Cell Sci. 1993;106(Pt 1):23–29. doi: 10.1242/jcs.106.1.23. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Conforti L, Buckmaster EA, Tarlton A, Ewing RM, Brown MC, Lyon MF, Perry VH. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc Natl Acad Sci U S A. 1998;95:9985–9990. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Gilley J, Coleman MP. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014;15:394–409. doi: 10.1038/nrn3680. [DOI] [PubMed] [Google Scholar]
- Conze DB, Crespo-Barreto J, Kruger CL. Safety assessment of nicotinamide riboside, a form of vitamin B3. Hum Exp Toxicol. 2016 doi: 10.1177/0960327115626254. [DOI] [PubMed] [Google Scholar]
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol. 2008;128:447–454. doi: 10.1038/sj.jid.5701058. [DOI] [PubMed] [Google Scholar]
- Daugherty MD, Young JM, Kerns JA, Malik HS. Rapid evolution of PARP genes suggests a broad role for ADP-ribosylation in host-virus conflicts. PLoS Genet. 2014;10:e1004403. doi: 10.1371/journal.pgen.1004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus-Cortes H, Xu P, Drawbridge J, Estill SJ, Huntington P, Tran S, Britt J, Tesla R, Morlock L, Naidoo J, et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc Natl Acad Sci U S A. 2012;109:17010–17015. doi: 10.1073/pnas.1213956109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano M, Loreto A, Orsomando G, Mori V, Zamporlini F, Hulse RP, Webster J, Donaldson LF, Gering M, Raffaelli N, et al. NMN Deamidase Delays Wallerian Degeneration and Rescues Axonal Defects Caused by NMNAT2 Deficiency In Vivo. Curr Biol. 2017;27:784–794. doi: 10.1016/j.cub.2017.01.070. [DOI] [PubMed] [Google Scholar]
- Di Stefano M, Nascimento-Ferreira I, Orsomando G, Mori V, Gilley J, Brown R, Janeckova L, Vargas ME, Worrell LA, Loreto A, et al. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ. 2015;22:731–742. doi: 10.1038/cdd.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diani-Moore S, Shoots J, Singh R, Zuk JB, Rifkind AB. NAD+ loss, a new player in AhR biology: prevention of thymus atrophy and hepatosteatosis by NAD+ repletion. Sci Rep. 2017;7:2268. doi: 10.1038/s41598-017-02332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich LS, Fuller L, Yero IL, Martinez L. Nicotinamide mononucleotide pyrophosphorylase activity in animal tissues. J Biol Chem. 1966;241:188–191. [PubMed] [Google Scholar]
- Dolle C, Niere M, Lohndal E, Ziegler M. Visualization of subcellular NAD pools and intra-organellar protein localization by poly-ADP-ribose formation. Cell Mol Life Sci. 2010;67:433–443. doi: 10.1007/s00018-009-0190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domergue R, Castano I, De Las Penas A, Zupancic M, Lockatell V, Hebel JR, Johnson D, Cormack BP. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science. 2005;308:866–870. doi: 10.1126/science.1108640. [DOI] [PubMed] [Google Scholar]
- Dominy JE, Jr, Lee Y, Jedrychowski MP, Chim H, Jurczak MJ, Camporez JP, Ruan HB, Feldman J, Pierce K, Mostoslavsky R, et al. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol Cell. 2012;48:900–913. doi: 10.1016/j.molcel.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Pereira S, Pereira-Castro I, Silva SS, Correia MG, Neto C, da Costa LT, Amorim A, Silva RM. Extensive regulation of nicotinate phosphoribosyltransferase (NAPRT) expression in human tissues and tumors. Oncotarget. 2016;7:1973–1983. doi: 10.18632/oncotarget.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutca LM, Stasheff SF, Hedberg-Buenz A, Rudd DS, Batra N, Blodi FR, Yorek MS, Yin T, Shankar M, Herlein JA, et al. Early detection of subclinical visual damage after blast-mediated TBI enables prevention of chronic visual deficit by treatment with P7C3-S243. Invest Ophthalmol Vis Sci. 2014;55:8330–8341. doi: 10.1167/iovs.14-15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O’Neil L, White TA, Sinclair DA, Chini EN. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 2013;62:1084–1093. doi: 10.2337/db12-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD+ Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron. 2017;93:1334–1343 e1335. doi: 10.1016/j.neuron.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall PA, Fredrikson M, Axelson O, Granerus AK. Nutritional and occupational factors influencing the risk of Parkinson’s disease: a case-control study in southeastern Sweden. Mov Disord. 1999;14:28–37. doi: 10.1002/1531-8257(199901)14:1<28::aid-mds1007>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Fang EF, Kassahun H, Croteau DL, Scheibye-Knudsen M, Marosi K, Lu H, Shamanna RA, Kalyanasundaram S, Bollineni RC, Wilson MA, et al. NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016;24:566–581. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, Mitchell JR, Croteau DL, Bohr VA. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 2014;157:882–896. doi: 10.1016/j.cell.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011;19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RS, Ratajczak J, Doig CL, Oakey LA, Callingham R, Da Silva Xavier G, Garten A, Elhassan YS, Redpath P, Migaud ME, et al. Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol Metab. 2017;6:819–832. doi: 10.1016/j.molmet.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DW, Davis JG, Davila A, Jr, Agarwal B, Michan S, Puchowicz MA, Nakamaru-Ogiso E, Baur JA. Increasing NAD synthesis in muscle via nicotinamide phosphoribosyltransferase is not sufficient to promote oxidative metabolism. J Biol Chem. 2015;290:1546–1558. doi: 10.1074/jbc.M114.579565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, Mellor AL, Munn DH, Antonia SJ. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002;101:151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- Friebe D, Neef M, Kratzsch J, Erbs S, Dittrich K, Garten A, Petzold-Quinque S, Bluher S, Reinehr T, Stumvoll M, et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia. 2011;54:1200–1211. doi: 10.1007/s00125-010-2042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydelund-Larsen L, Akerstrom T, Nielsen S, Keller P, Keller C, Pedersen BK. Visfatin mRNA expression in human subcutaneous adipose tissue is regulated by exercise. Am J Physiol Endocrinol Metab. 2007;292:E24–31. doi: 10.1152/ajpendo.00113.2006. [DOI] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Garg A, Sharma A, Krishnamoorthy P, Garg J, Virmani D, Sharma T, Stefanini G, Kostis JB, Mukherjee D, Sikorskaya E. Role of Niacin in Current Clinical Practice: A Systematic Review. Am J Med. 2017;130:173–187. doi: 10.1016/j.amjmed.2016.07.038. [DOI] [PubMed] [Google Scholar]
- Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, Moullan N, Zhang H, Perino A, Lemos V, et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190–1204. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariani K, Ryu D, Menzies KJ, Yi HS, Stein S, Zhang H, Perino A, Lemos V, Katsyuba E, Jha P, et al. Inhibiting poly ADP-ribosylation increases fatty acid oxidation and protects against fatty liver disease. J Hepatol. 2017;66:132–141. doi: 10.1016/j.jhep.2016.08.024. [DOI] [PubMed] [Google Scholar]
- Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11:535–546. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- Gensler HL. Prevention of photoimmunosuppression and photocarcinogenesis by topical nicotinamide. Nutr Cancer. 1997;29:157–162. doi: 10.1080/01635589709514618. [DOI] [PubMed] [Google Scholar]
- Geraets L, Moonen HJ, Brauers K, Wouters EF, Bast A, Hageman GJ. Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. J Nutr. 2007;137:2190–2195. doi: 10.1093/jn/137.10.2190. [DOI] [PubMed] [Google Scholar]
- Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science. 2015;348:453–457. doi: 10.1126/science.1258366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Sasaki Y, DiAntonio A, Milbrandt J. Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J Neurosci. 2013;33:13569–13580. doi: 10.1523/JNEUROSCI.1197-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler AA, Cohen HY. SIRT6, a protein with many faces. Biogerontology. 2013;14:629–639. doi: 10.1007/s10522-013-9478-8. [DOI] [PubMed] [Google Scholar]
- Gilley J, Orsomando G, Nascimento-Ferreira I, Coleman MP. Absence of SARM1 rescues development and survival of NMNAT2-deficient axons. Cell Rep. 2015;10:1974–1981. doi: 10.1016/j.celrep.2015.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging. 2013;34:1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody MF, Kelly MW, Reynolds CJ, Khalil A, Crawford BD, Henry CA. NAD+ biosynthesis ameliorates a zebrafish model of muscular dystrophy. PLoS Biol. 2012;10:e1001409. doi: 10.1371/journal.pbio.1001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal E, Miyauchi S, Martin PM, Ananth S, Roon P, Smith SB, Ganapathy V. Transport of nicotinate and structurally related compounds by human SMCT1 (SLC5A8) and its relevance to drug transport in the mammalian intestinal tract. Pharm Res. 2007;24:575–584. doi: 10.1007/s11095-006-9176-1. [DOI] [PubMed] [Google Scholar]
- Grozio A, Sociali G, Sturla L, Caffa I, Soncini D, Salis A, Raffaelli N, De Flora A, Nencioni A, Bruzzone S. CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J Biol Chem. 2013;288:25938–25949. doi: 10.1074/jbc.M113.470435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube K, Burkle A. Poly(ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span. Proc Natl Acad Sci U S A. 1992;89:11759–11763. doi: 10.1073/pnas.89.24.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Wang SR, Huang XZ, Xie QH, Xu YY, Shang D, Hao CM. Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1-Dependent Manner. J Am Soc Nephrol. 2017;28:2337–2352. doi: 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte R, Liu Z, Kraus WL. PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes Dev. 2017;31:101–126. doi: 10.1101/gad.291518.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haferkamp I, Schmitz-Esser S, Linka N, Urbany C, Collingro A, Wagner M, Horn M, Neuhaus HE. A candidate NAD+ transporter in an intracellular bacterial symbiont related to Chlamydiae. Nature. 2004;432:622–625. doi: 10.1038/nature03131. [DOI] [PubMed] [Google Scholar]
- Haffner CD, Becherer JD, Boros EE, Cadilla R, Carpenter T, Cowan D, Deaton DN, Guo Y, Harrington W, Henke BR, et al. Discovery, Synthesis, and Biological Evaluation of Thiazoloquin(az)olin(on)es as Potent CD38 Inhibitors. J Med Chem. 2015;58:3548–3571. doi: 10.1021/jm502009h. [DOI] [PubMed] [Google Scholar]
- Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider DG, Holzer G, Schaller G, Weghuber D, Widhalm K, Wagner O, Kapiotis S, Wolzt M. The adipokine visfatin is markedly elevated in obese children. J Pediatr Gastroenterol Nutr. 2006;43:548–549. doi: 10.1097/01.mpg.0000235749.50820.b3. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haikarainen T, Krauss S, Lehtio L. Tankyrases: structure, function and therapeutic implications in cancer. Curr Pharm Des. 2014;20:6472–6488. doi: 10.2174/1381612820666140630101525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallschmid M, Randeva H, Tan BK, Kern W, Lehnert H. Relationship between cerebrospinal fluid visfatin (PBEF/Nampt) levels and adiposity in humans. Diabetes. 2009;58:637–640. doi: 10.2337/db08-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamity MV, White SR, Walder RY, Schmidt MS, Brenner C, Hammond DL. Nicotinamide riboside, a form of vitamin B3 and NAD+ precursor, relieves the nociceptive and aversive dimensions of paclitaxel-induced peripheral neuropathy in female rats. Pain. 2017;158:962–972. doi: 10.1097/j.pain.0000000000000862. [DOI] [PubMed] [Google Scholar]
- Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007;282:24574–24582. doi: 10.1074/jbc.M610357200. [DOI] [PubMed] [Google Scholar]
- Hara N, Yamada K, Shibata T, Osago H, Tsuchiya M. Nicotinamide phosphoribosyltransferase/visfatin does not catalyze nicotinamide mononucleotide formation in blood plasma. PLoS One. 2011;6:e22781. doi: 10.1371/journal.pone.0022781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden A, Young WJ. The Alcoholic Ferment of Yeast-Juice Part II.–The coferment of yeast-juice. Proceedings of the Royal Society of London Series B, Containing Papers of a Biological Character. 1906;78:369–375. [Google Scholar]
- Heilbronn LK. Clinical Trials Corner. Nutr Healthy Aging. 2017;4:193–194. doi: 10.3233/NHA-170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellenbrand W, Boeing H, Robra BP, Seidler A, Vieregge P, Nischan P, Joerg J, Oertel WH, Schneider E, Ulm G. Diet and Parkinson’s disease. II: A possible role for the past intake of specific nutrients. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology. 1996;47:644–650. doi: 10.1212/wnl.47.3.644. [DOI] [PubMed] [Google Scholar]
- Hernandez-Campo PM, Almeida J, Sanchez ML, Malvezzi M, Orfao A. Normal patterns of expression of glycosylphosphatidylinositol-anchored proteins on different subsets of peripheral blood cells: a frame of reference for the diagnosis of paroxysmal nocturnal hemoglobinuria. Cytometry B Clin Cytom. 2006;70:71–81. doi: 10.1002/cyto.b.20087. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, Leone TC, Pagliarini DJ, Muoio DM, Bedi KC, Jr, et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;2 doi: 10.1172/jci.insight.84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, Zhang Y, Moritoh K, O’Connell JF, Baptiste BA, et al. NAD(+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci U S A. 2018 doi: 10.1073/pnas.1718819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JY, Wang K, Vermehren-Schmaedick A, Adelman JP, Cohen MS. PARP6 is a Regulator of Hippocampal Dendritic Morphogenesis. Sci Rep. 2016;6:18512. doi: 10.1038/srep18512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Imai S. Nicotinamide phosphoribosyltransferase (Nampt): a link between NAD biology, metabolism, and diseases. Curr Pharm Des. 2009;15:20–28. doi: 10.2174/138161209787185814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes Metab. 2013;15(Suppl 3):26–33. doi: 10.1111/dom.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy JM, Hicks JB, Klar AJ. Map positions of yeast genes SIR1, SIR3 and SIR4. Genetics. 1985;111:735–744. doi: 10.1093/genetics/111.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H, Goettsch C, Sharma A, Ricchiuto P, Goh WW, Halu A, Yamada I, Yoshida H, Hara T, Wei M, et al. PARP9 and PARP14 cross-regulate macrophage activation via STAT1 ADP-ribosylation. Nat Commun. 2016;7:12849. doi: 10.1038/ncomms12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Li X, Gao H, Feng Z, Li X, Zhao L, Jia X, Zhang H, Liu J. High doses of nicotinamide prevent oxidative mitochondrial dysfunction in a cellular model and improve motor deficit in a Drosophila model of Parkinson’s disease. J Neurosci Res. 2008;86:2083–2090. doi: 10.1002/jnr.21650. [DOI] [PubMed] [Google Scholar]
- Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, et al. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature. 2013;496:110–113. doi: 10.1038/nature12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Wang S, Xiao M, Lin Y, Zhou L, Lei Q, Xiong Y, Guan KL, Zhao S. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol Cell. 2011;43:33–44. doi: 10.1016/j.molcel.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jwa M, Chang P. PARP16 is a tail-anchored endoplasmic reticulum protein required for the PERK- and IRE1alpha-mediated unfolded protein response. Nat Cell Biol. 2012;14:1223–1230. doi: 10.1038/ncb2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Wang J, Kaneko M, Yiu G, Hurrell JM, Chitnis T, Khoury SJ, He Z. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J Neurosci. 2006;26:9794–9804. doi: 10.1523/JNEUROSCI.2116-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC, Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18:239–250. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger E, Kuhn I, Schuber F, Muller-Steffner H. Flavonoids as inhibitors of human CD38. Bioorg Med Chem Lett. 2011;21:3939–3942. doi: 10.1016/j.bmcl.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Kickhoefer VA, Siva AC, Kedersha NL, Inman EM, Ruland C, Streuli M, Rome LH. The 193-kD vault protein, VPARP, is a novel poly(ADP-ribose) polymerase. J Cell Biol. 1999;146:917–928. doi: 10.1083/jcb.146.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchberger T, Guse AH. Measuring CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) activity by reverse-phase HPLC. Cold Spring Harb Protoc. 2013;2013:569–573. doi: 10.1101/pdb.prot073007. [DOI] [PubMed] [Google Scholar]
- Klaidman L, Morales M, Kem S, Yang J, Chang ML, Adams JD., Jr Nicotinamide Offers Multiple Protective Mechanisms in Stroke as a Precursor for NAD<sup>+</sup>, as a PARP Inhibitor and by Partial Restoration of Mitochondrial Function. Pharmacology. 2003;69:150–157. doi: 10.1159/000072668. [DOI] [PubMed] [Google Scholar]
- Koedel U, Winkler F, Angele B, Fontana A, Pfister HW. Meningitis-associated central nervous system complications are mediated by the activation of poly(ADP-ribose) polymerase. J Cereb Blood Flow Metab. 2002;22:39–49. doi: 10.1097/00004647-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Korner A, Garten A, Bluher M, Tauscher R, Kratzsch J, Kiess W. Molecular characteristics of serum visfatin and differential detection by immunoassays. J Clin Endocrinol Metab. 2007;92:4783–4791. doi: 10.1210/jc.2007-1304. [DOI] [PubMed] [Google Scholar]
- Kraus D, Yang Q, Kong D, Banks AS, Zhang L, Rodgers JT, Pirinen E, Pulinilkunnil TC, Gong F, Wang YC, et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508:258–262. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Cheong HS, Koh Y, Ahn KS, Yoon SS, Shin HD. Genetic Association of PARP15 Polymorphisms with Clinical Outcome of Acute Myeloid Leukemia in a Korean Population. Genet Test Mol Biomarkers. 2016;20:696–701. doi: 10.1089/gtmb.2016.0007. [DOI] [PubMed] [Google Scholar]
- Leung AK, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol Cell. 2011;42:489–499. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bonkowski MS, Moniot S, Zhang D, Hubbard BP, Ling AJ, Rajman LA, Qin B, Lou Z, Gorbunova V, et al. A conserved NAD+ binding pocket that regulates protein-protein interactions during aging. Science. 2017;355:1312–1317. doi: 10.1126/science.aad8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JB, Kubota S, Ban N, Yoshida M, Santeford A, Sene A, Nakamura R, Zapata N, Kubota M, Tsubota K, et al. NAMPT-Mediated NAD(+) Biosynthesis Is Essential for Vision In Mice. Cell Rep. 2016;17:69–85. doi: 10.1016/j.celrep.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280:21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- Liu C, Yu X. ADP-ribosyltransferases and poly ADP-ribosylation. Curr Protein Pept Sci. 2015;16:491–501. doi: 10.2174/1389203716666150504122435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AN, Owens K, Schlappal AE, Kristian T, Fishman PS, Schuh RA. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol. 2015;15:19. doi: 10.1186/s12883-015-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Pan SJ, Zupancic ML, Cormack BP. Assimilation of NAD(+) precursors in Candida glabrata. Mol Microbiol. 2007;66:14–25. doi: 10.1111/j.1365-2958.2007.05886.x. [DOI] [PubMed] [Google Scholar]
- Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci. 2004;61:19–34. doi: 10.1007/s00018-003-3161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni G, Amici A, Emanuelli M, Raffaelli N, Ruggieri S. Enzymology of NAD+ synthesis. Adv Enzymol Relat Areas Mol Biol. 1999;73:135–182, xi. doi: 10.1002/9780470123195.ch5. [DOI] [PubMed] [Google Scholar]
- Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- Malyuchenko NV, Kotova EY, Kulaeva OI, Kirpichnikov MP, Studitskiy VM. PARP1 Inhibitors: antitumor drug design. Acta Naturae. 2015;7:27–37. [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443–1446. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AS, Abraham DM, Hershberger KA, Bhatt DP, Mao L, Cui H, Liu J, Liu X, Muehlbauer MJ, Grimsrud PA, et al. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri S, Rigor P, Cervantes M, Ceglia N, Sebastian C, Xiao C, Roqueta-Rivera M, Deng C, Osborne TF, Mostoslavsky R, et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell. 2014;158:659–672. doi: 10.1016/j.cell.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews MT, Berk BC. PARP-1 inhibition prevents oxidative and nitrosative stress-induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler Thromb Vasc Biol. 2008;28:711–717. doi: 10.1161/ATVBAHA.107.156406. [DOI] [PubMed] [Google Scholar]
- Mercader J, Granados N, Caimari A, Oliver P, Bonet ML, Palou A. Retinol-binding protein 4 and nicotinamide phosphoribosyltransferase/visfatin in rat obesity models. Horm Metab Res. 2008;40:467–472. doi: 10.1055/s-2008-1065324. [DOI] [PubMed] [Google Scholar]
- Meyer-Ficca ML, Ihara M, Bader JJ, Leu NA, Beneke S, Meyer RG. Spermatid head elongation with normal nuclear shaping requires ADP-ribosyltransferase PARP11 (ARTD11) in mice. Biol Reprod. 2015;92:80. doi: 10.1095/biolreprod.114.123661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira D, Rodrigues V, Abengozar M, Rivas L, Rial E, Laforge M, Li X, Foretz M, Viollet B, Estaquier J, et al. Leishmania infantum modulates host macrophage mitochondrial metabolism by hijacking the SIRT1-AMPK axis. PLoS Pathog. 2015;11:e1004684. doi: 10.1371/journal.ppat.1004684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori V, Amici A, Mazzola F, Di Stefano M, Conforti L, Magni G, Ruggieri S, Raffaelli N, Orsomando G. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLoS One. 2014;9:e113939. doi: 10.1371/journal.pone.0113939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, Novelli R, Remuzzi G, Benigni A. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest. 2015;125:715–726. doi: 10.1172/JCI77632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Chellappa K, Moffitt A, Ndungu J, Dellinger RW, Davis JG, Agarwal B, Baur JA. Nicotinamide adenine dinucleotide biosynthesis promotes liver regeneration. Hepatology. 2017;65:616–630. doi: 10.1002/hep.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- Murray MF, Nghiem M, Srinivasan A. HIV infection decreases intracellular nicotinamide adenine dinucleotide [NAD] Biochem Biophys Res Commun. 1995;212:126–131. doi: 10.1006/bbrc.1995.1945. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Kalousi A, Furst A, Koch M, Fischer B, Soutoglou E. Tankyrases Promote Homologous Recombination and Check Point Activation in Response to DSBs. PLoS Genet. 2016;12:e1005791. doi: 10.1371/journal.pgen.1005791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J. 2014;35:2929. doi: 10.1093/eurheartj/ehu378. [DOI] [PubMed] [Google Scholar]
- Nikiforov A, Dolle C, Niere M, Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286:21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niren NM. Pharmacologic doses of nicotinamide in the treatment of inflammatory skin conditions: a review. Cutis. 2006;77:11–16. [PubMed] [Google Scholar]
- North BJ, Rosenberg MA, Jeganathan KB, Hafner AV, Michan S, Dai J, Baker DJ, Cen Y, Wu LE, Sauve AA, et al. SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J. 2014;33:1438–1453. doi: 10.15252/embj.201386907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Long A, Owens K, Kristian T. Nicotinamide mononucleotide inhibits post-ischemic NAD(+) degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiol Dis. 2016;95:102–110. doi: 10.1016/j.nbd.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida-Sanchez S, Rivero-Nava L, Shi G, Lund FE. CD38: an ecto-enzyme at the crossroads of innate and adaptive immune responses. Adv Exp Med Biol. 2007;590:171–183. doi: 10.1007/978-0-387-34814-8_12. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, et al. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142:39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- Pillai VB, Sundaresan NR, Kim G, Samant S, Moreno-Vinasco L, Garcia JG, Gupta MP. Nampt secreted from cardiomyocytes promotes development of cardiac hypertrophy and adverse ventricular remodeling. Am J Physiol Heart Circ Physiol. 2013;304:H415–426. doi: 10.1152/ajpheart.00468.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirinen E, Canto C, Jo YS, Morato L, Zhang H, Menzies KJ, Williams EG, Mouchiroud L, Moullan N, Hagberg C, et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljsak B. NAD+ in Cancer Prevention and Treatment: Pros and Cons. Journal of Clinical & Experimental Oncology. 2016;5 [Google Scholar]
- Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, Funaro A, Horenstein AL, Malavasi F. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom. 2013;84:207–217. doi: 10.1002/cyto.b.21092. [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappou E, Jukarainen S, Rinnankoski-Tuikka R, Kaye S, Heinonen S, Hakkarainen A, Lundbom J, Lundbom N, Saunavaara V, Rissanen A, et al. Weight Loss Is Associated With Increased NAD(+)/SIRT1 Expression But Reduced PARP Activity in White Adipose Tissue. J Clin Endocrinol Metab. 2016;101:1263–1273. doi: 10.1210/jc.2015-3054. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Joffraud M, Trammell SA, Ras R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud ME, et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat Commun. 2016;7:13103. doi: 10.1038/ncomms13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren JH, Tao Y, Zhang ZZ, Chen WX, Cai XF, Chen K, Ko BC, Song CL, Ran LK, Li WY, et al. Sirtuin 1 regulates hepatitis B virus transcription and replication by targeting transcription factor AP-1. J Virol. 2014;88:2442–2451. doi: 10.1128/JVI.02861-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol. 2007a;23:164–170. doi: 10.1097/MOG.0b013e32801b3c8f. [DOI] [PubMed] [Google Scholar]
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007b;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivin AU. Hypercholesterolemia. Use of niacin and niacin combinations in therapy. Calif Med. 1962;96:267–269. [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rolfe HM. A review of nicotinamide: treatment of skin diseases and potential side effects. J Cosmet Dermatol. 2014;13:324–328. doi: 10.1111/jocd.12119. [DOI] [PubMed] [Google Scholar]
- Rulten SL, Fisher AE, Robert I, Zuma MC, Rouleau M, Ju L, Poirier G, Reina-San-Martin B, Caldecott KW. PARP-3 and APLF function together to accelerate nonhomologous end-joining. Mol Cell. 2011;41:33–45. doi: 10.1016/j.molcel.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Ryu D, Zhang H, Ropelle ER, Sorrentino V, Mazala DA, Mouchiroud L, Marshall PL, Campbell MD, Ali AS, Knowels GM, et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med. 2016;8:361ra139. doi: 10.1126/scitranslmed.aaf5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanaga-Akiyoshi F, Yao H, Tanuma S, Nakahara T, Hong JS, Ibayashi S, Uchimura H, Fujishima M. Nicotinamide attenuates focal ischemic brain injury in rats: with special reference to changes in nicotinamide and NAD+ levels in ischemic core and penumbra. Neurochem Res. 2003;28:1227–1234. doi: 10.1023/a:1024236614015. [DOI] [PubMed] [Google Scholar]
- Saddi-Rosa P, Oliveira C, Crispim F, Giuffrida FM, de Lima V, Vieira J, Doria A, Velho G, Reis A. Association of circulating levels of nicotinamide phosphoribosyltransferase (NAMPT/Visfatin) and of a frequent polymorphism in the promoter of the NAMPT gene with coronary artery disease in diabetic and non-diabetic subjects. Cardiovasc Diabetol. 2013;12:119. doi: 10.1186/1475-2840-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santidrian AF, Matsuno-Yagi A, Ritland M, Seo BB, LeBoeuf SE, Gay LJ, Yagi T, Felding-Habermann B. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J Clin Invest. 2013;123:1068–1081. doi: 10.1172/JCI64264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Nakagawa T, Mao X, DiAntonio A, Milbrandt J. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD+ depletion. Elife. 2016;5 doi: 10.7554/eLife.19749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber V, Ame JC, Dolle P, Schultz I, Rinaldi B, Fraulob V, Menissier-de Murcia J, de Murcia G. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem. 2002;277:23028–23036. doi: 10.1074/jbc.M202390200. [DOI] [PubMed] [Google Scholar]
- Schuster S, Penke M, Gorski T, Petzold-Quinque S, Damm G, Gebhardt R, Kiess W, Garten A. Resveratrol differentially regulates NAMPT and SIRT1 in Hepatocarcinoma cells and primary human hepatocytes. PLoS One. 2014;9:e91045. doi: 10.1371/journal.pone.0091045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151:1185–1199. doi: 10.1016/j.cell.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif T, Ahn DG, Liu RZ, Pringle E, Martell E, Dai C, Nunokawa A, Kwak M, Clements D, Murphy JP, et al. The NAD(+) salvage pathway modulates cancer cell viability via p73. Cell Death Differ. 2016;23:669–680. doi: 10.1038/cdd.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt FW, Denu JM, et al. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010;12:654–661. doi: 10.1016/j.cmet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Bhaskaran N, Babcook MA, Fu P, Maclennan GT, Gupta S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis. 2014;35:452–460. doi: 10.1093/carcin/bgt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son MJ, Ryu JS, Kim JY, Kwon Y, Chung KS, Mun SJ, Cho YS. Upregulation of mitochondrial NAD+ levels impairs the clonogenicity of SSEA1+ glioblastoma tumor-initiating cells. Exp Mol Med. 2017;49:e344. doi: 10.1038/emm.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, Moullan N, Potenza F, Schmid AW, Rietsch S, et al. Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature. 2017;552:187–193. doi: 10.1038/nature25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers DW, Gibson DA, DiAntonio A, Milbrandt J. SARM1-specific motifs in the TIR domain enable NAD+ loss and regulate injury-induced SARM1 activation. Proc Natl Acad Sci U S A. 2016;113:E6271–E6280. doi: 10.1073/pnas.1601506113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner KG, Landry J, Sternglanz R, Denu JM. Silent information regulator 2 family of NAD- dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc Natl Acad Sci U S A. 2000;97:14178–14182. doi: 10.1073/pnas.250422697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesla R, Wolf HP, Xu P, Drawbridge J, Estill SJ, Huntington P, McDaniel L, Knobbe W, Burket A, Tran S, et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2012;109:17016–17021. doi: 10.1073/pnas.1213960109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016a;7:12948. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammell SA, Yu L, Redpath P, Migaud ME, Brenner C. Nicotinamide Riboside Is a Major NAD+ Precursor Vitamin in Cow Milk. J Nutr. 2016b;146:957–963. doi: 10.3945/jn.116.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, et al. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilioni I, Taliou A, Francis K, Theoharides TC. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl Psychiatry. 2015;5:e647. doi: 10.1038/tp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota K. The first human clinical study for NMN has started in Japan. NPJ Aging Mech Dis. 2016;2:16021. doi: 10.1038/npjamd.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummala KS, Gomes AL, Yilmaz M, Grana O, Bakiri L, Ruppen I, Ximenez-Embun P, Sheshappanavar V, Rodriguez-Justo M, Pisano DG, et al. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell. 2014;26:826–839. doi: 10.1016/j.ccell.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Ugur S, Ulu R, Dogukan A, Gurel A, Yigit IP, Gozel N, Aygen B, Ilhan N. The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity. Ren Fail. 2015;37:332–336. doi: 10.3109/0886022X.2014.986005. [DOI] [PubMed] [Google Scholar]
- Ummarino S, Mozzon M, Zamporlini F, Amici A, Mazzola F, Orsomando G, Ruggieri S, Raffaelli N. Simultaneous quantitation of nicotinamide riboside, nicotinamide mononucleotide and nicotinamide adenine dinucleotide in milk by a novel enzyme-coupled assay. Food Chem. 2017;221:161–168. doi: 10.1016/j.foodchem.2016.10.032. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- Vaur P, Brugg B, Mericskay M, Li Z, Schmidt MS, Vivien D, Orset C, Jacotot E, Brenner C, Duplus E. Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration. FASEB J. 2017 doi: 10.1096/fj.201700221RR. [DOI] [PubMed] [Google Scholar]
- Verheugd P, Forst AH, Milke L, Herzog N, Feijs KL, Kremmer E, Kleine H, Luscher B. Regulation of NF-kappaB signalling by the mono-ADP-ribosyltransferase ARTD10. Nat Commun. 2013;4:1683. doi: 10.1038/ncomms2672. [DOI] [PubMed] [Google Scholar]
- Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper AA, Ready JM, McKnight SL. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell. 2014;158:1324–1334. doi: 10.1016/j.cell.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu X, Yang Y, Takata T, Sakurai T. Nicotinamide mononucleotide protects against beta-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016;1643:1–9. doi: 10.1016/j.brainres.2016.04.060. [DOI] [PubMed] [Google Scholar]
- Warburg O, Christian W. Pyridin, the hydrogen-transferring component of the fermentation enzymes (pyridine nucleotide) Biochemische Zeitschrift (in German) 1936;287:291. [Google Scholar]
- Wei CC, Kong YY, Hua X, Li GQ, Zheng SL, Cheng MH, Wang P, Miao CY. NAD replenishment with nicotinamide mononucleotide protects blood-brain barrier integrity and attenuates delayed tissue plasminogen activator-induced haemorrhagic transformation after cerebral ischaemia. Br J Pharmacol. 2017a doi: 10.1111/bph.13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CC, Kong YY, Li GQ, Guan YF, Wang P, Miao CY. Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway. Sci Rep. 2017b;7:717. doi: 10.1038/s41598-017-00851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wencel PL, Lukiw WJ, Strosznajder JB, Strosznajder RP. Inhibition of Poly(ADP-ribose) Polymerase-1 Enhances Gene Expression of Selected Sirtuins and APP Cleaving Enzymes in Amyloid Beta Cytotoxicity. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner B, Leblhuber F, Fuchs D. Increased neopterin production and tryptophan degradation in advanced Parkinson’s disease. J Neural Transm (Vienna) 2002;109:181–189. doi: 10.1007/s007020200014. [DOI] [PubMed] [Google Scholar]
- Wu LE, Gomes AP, Sinclair DA. Geroncogenesis: metabolic changes during aging as a driver of tumorigenesis. Cancer Cell. 2014;25:12–19. doi: 10.1016/j.ccr.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Barrientos T, Mao L, Rockman HA, Sauve AA, Andrews NC. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep. 2015;13:533–545. doi: 10.1016/j.celrep.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9:e98972. doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Feng Y, Zheng J, Ge X, Zhang Y, Wu D, Zhao J, Zhai Q. Nmnat2 delays axon degeneration in superior cervical ganglia dependent on its NAD synthesis activity. Neurochem Int. 2010;56:101–106. doi: 10.1016/j.neuint.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Yang D, Elner SG, Chen X, Field MG, Petty HR, Elner VM. MCP-1-activated monocytes induce apoptosis in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:6026–6034. doi: 10.1167/iovs.10-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Vought BW, Satterlee JS, Walker AK, Jim Sun ZY, Watts JL, DeBeaumont R, Saito RM, Hyberts SG, Yang S, et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature. 2006;442:700–704. doi: 10.1038/nature04942. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiasemides E, Sivapirabu G, Halliday GM, Park J, Damian DL. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101–105. doi: 10.1093/carcin/bgn248. [DOI] [PubMed] [Google Scholar]
- Yin TC, Britt JK, De Jesus-Cortes H, Lu Y, Genova RM, Khan MZ, Voorhees JR, Shao J, Katzman AC, Huntington PJ, et al. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Rep. 2014;8:1731–1740. doi: 10.1016/j.celrep.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamporlini F, Ruggieri S, Mazzola F, Amici A, Orsomando G, Raffaelli N. Novel assay for simultaneous measurement of pyridine mononucleotides synthesizing activities allows dissection of the NAD(+) biosynthetic machinery in mammalian cells. FEBS J. 2014;281:5104–5119. doi: 10.1111/febs.13050. [DOI] [PubMed] [Google Scholar]
- Zerez CR, Roth EF, Jr, Schulman S, Tanaka KR. Increased nicotinamide adenine dinucleotide content and synthesis in Plasmodium falciparum-infected human erythrocytes. Blood. 1990;75:1705–1710. [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- Zhang P, Tu B, Wang H, Cao Z, Tang M, Zhang C, Gu B, Li Z, Wang L, Yang Y, et al. Tumor suppressor p53 cooperates with SIRT6 to regulate gluconeogenesis by promoting FoxO1 nuclear exclusion. Proc Natl Acad Sci U S A. 2014;111:10684–10689. doi: 10.1073/pnas.1411026111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RY, Qin Y, Lv XQ, Wang P, Xu TY, Zhang L, Miao CY. A fluorometric assay for high-throughput screening targeting nicotinamide phosphoribosyltransferase. Anal Biochem. 2011;412:18–25. doi: 10.1016/j.ab.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Zhang T, Berrocal JG, Yao J, DuMond ME, Krishnakumar R, Ruhl DD, Ryu KW, Gamble MJ, Kraus WL. Regulation of poly(ADP-ribose) polymerase-1-dependent gene expression through promoter-directed recruitment of a nuclear NAD+ synthase. J Biol Chem. 2012;287:12405–12416. doi: 10.1074/jbc.M111.304469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CC, Yang X, Hua X, Liu J, Fan MB, Li GQ, Song J, Xu TY, Li ZY, Guan YF, et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol. 2016;173:2352–2368. doi: 10.1111/bph.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Ottenberg G, Sferrazza GF, Hubbs C, Fallahi M, Rumbaugh G, Brantley AF, Lasmezas CI. Neuronal death induced by misfolded prion protein is due to NAD+ depletion and can be relieved in vitro and in vivo by NAD+ replenishment. Brain. 2015;138:992–1008. doi: 10.1093/brain/awv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Kurnasov O, Tomchick DR, Binns DD, Grishin NV, Marquez VE, Osterman AL, Zhang H. Structure of human nicotinamide/nicotinic acid mononucleotide adenylyltransferase. Basis for the dual substrate specificity and activation of the oncolytic agent tiazofurin. J Biol Chem. 2002;277:13148–13154. doi: 10.1074/jbc.M111469200. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A. 2015;112:2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Fu B, Bai X, Zhang B, Wu L, Cui J, Cui S, Wei R, Chen X, Cai G. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cell Physiol Biochem. 2011;27:681–690. doi: 10.1159/000330077. [DOI] [PubMed] [Google Scholar]
- Ziegler M, Oei SL. A cellular survival switch: poly(ADP-ribosyl)ation stimulates DNA repair and silences transcription. Bioessays. 2001;23:543–548. doi: 10.1002/bies.1074. [DOI] [PubMed] [Google Scholar]


