Abstract
Anterograde cell surface transport of nascent G protein-coupled receptors (GPCRs) en route from the endoplasmic reticulum (ER) through the Golgi apparatus represents a crucial checkpoint to control the amount of the receptors at the functional destination and the strength of receptor activation-elicited cellular responses. However, as compared with extensively studied internalization and recycling processes, the molecular mechanisms of cell surface trafficking of GPCRs are relatively less defined. Here, we will review the current advances in understanding the ER-Golgi-cell surface transport of GPCRs and use angiotensin II type 1 receptor as a representative GPCR to discuss emerging roles of receptor-interacting proteins and specific motifs embedded within the receptors in controlling the forward traffic of GPCRs along the biosynthetic pathway.
Keywords: angiotensin II type 1 receptor, anterograde trafficking, biosynthesis, cell surface expression, C-terminus, endoplasmic reticulum, export, Golgi, G protein-coupled receptor, interacting protein, maturation, motif, signaling
Anterograde transport of GPCRs
G protein-coupled receptors (GPCRs) constitute the largest and most structurally diverse superfamily of membrane signaling proteins and modulate a wide variety of physiological and pathological functions. The functions of GPCRs are mediated through coupling to heterotrimeric G proteins, arrestins and other signaling molecules which in turn activate downstream effectors, such as protein kinases, adenylyl cyclases, phospholipases and ion channels. One important factor that crucially regulates the precise function of the receptors is their intracellular trafficking, including anterograde cell surface transport, endocytosis, recycling, and lysosomal degradation, which control the number of the receptors at the cell surface, the functional destination for most GPCRs, which in turn dictates the magnitude of receptor activation-elicited cellular responses at a given time. However, as compared with well-characterized internalization and recycling, the molecular mechanisms underlying the cell surface transport of nascent GPCRs are relatively less well understood.
Similar to many other plasma membrane proteins, the life of GPCRs begins at the endoplasmic reticulum (ER), where they are synthesized, folded and assembled. Correctly folded and properly assembled receptors are able to pass the ER quality-control system, move forward from the ER to the cell surface, en route pass through the ER-Golgi intermediate complex (ERGIC), Golgi cisternae, and trans-Golgi network (TGN), during which the receptors may undergo post-translational modifications to attain a fully mature status. Emerging evidence from the studies in recent years suggest that the cell surface transport of GPCRs is regulatable, mediated through multiple pathways, and in a cell type and receptor specific manner. The most significant progress towards the understanding of anterograde transport of GPCRs is the identification of a number of regulatory proteins (Table 1), which may function as chaperones, escort proteins, gatekeepers, transport machinery, sorting molecules or signaling proteins to regulate receptor correct folding, maturation, assembly, recruitment onto the transport vesicles, retention in and export from the ER and Golgi compartments, sorting from other plasma membrane proteins or other GPCRs, transportation along the microtubule network, and delivery to the plasma membrane. As demonstrated in different protein-protein interaction assays, many of these regulatory proteins are able to directly interact with the receptors they regulate and thus, by virtue of their ability to interact with selective GPCRs, some of these regulatory proteins may only regulate the cell surface transport of a specific GPCR, while others may influence the transport of a group of GPCRs. It is also of interest to note that the final impact of these regulatory proteins on the cell surface expression of the receptors could be beneficial or deleterious.
Table 1.
Some of the regulatory proteins involved in the anterograde cell surface transport of GPCRs
| Regulatory proteins | GPCRs | References |
|---|---|---|
| 14-3-3 | CaSR | 1 |
| ARF1 | α2B-AR, β2-AR, AT1R, CXCR4, M3-MR, PAR-2 | 2,3 |
| ARF3 | α2B-AR | 2 |
| ARF4 | Rhodopsin | 4,5 |
| ARF5 | α2B-AR | 2 |
| ARF6 | α2B-AR, V1aR, V2R, M2-MR | 2,6 |
| ATIP1/ATBP50 | AT2R | 7,8 |
| Caveolin | AT1R | 9,10 |
| CD4 | CCR5 | 11 |
| CD74 | AT1R | 12 |
| CLIC4 | H3R | 13 |
| CNIH4 | β2-AR, CCR5 | 14 |
| DRiP78 | D1R, M2-MR, AT1R, CCR5 | 9,15,16 |
| Filamin-2 | α2C-AR | 17 |
| Filamin A | D2R | 18 |
| gC1q-R | α1B-AR | 19 |
| GEC1 | KOR, EP3R | 20,21 |
| GGAs | α2B-AR | 22,23 |
| Golgin-160 | β1-AR | 24 |
| GABARAP | AT1R | 25 |
| Homer 1 | mGluR1a, mGluR5 | 26,27 |
| HSJ1b | Rhodopsin | 28 |
| Kif5B | 5-HT1AR | 29 |
| M10 | VN2R | 30 |
| MRAP/MRAP2 | MCR | 31 |
| Neurofilament-M | D1R | 32 |
| NinaA | Rhodopsin | 33 |
| ODR-4 | ODR-10, U131, STR112, STR113, | 34–36 |
| P11 | 5-HT1BR | 37 |
| P23/P24A | PAR-2, MOR, P2Y4R | 38 |
| PAPLA1 | Rhodopsin | 39 |
| PI3K C2A | DOR | 40 |
| Pontin | α2C-AR | 41 |
| PRAF2 | GABABR | 42 |
| Protachykinin | DOR | 43 |
| Protein 4.1G | A1AR, PTHR, mGluR1a | 44–46 |
| Protein 4.1N | D2R, D3R | 47 |
| PTEN | DOR | 48 |
| Rab1 | α1A-AR, α1B-AR, β1-AR, β2-AR, AT1R, AT2R, hCaR | 49–57 |
| Rab2 | α2B-AR | 58 |
| Rab6 | β2-AR, AT1R, rhodopsin | 58–60 |
| Rab8 | α2B-AR, β2-AR, rhodopsin | 61–63 |
| Rab26 | α2A-AR, α2B-AR | 64,65 |
| Rab43 | α1B-AR, α2A-AR, α2B-AR, α2C-AR, β2-AR, AT1R | 66 |
| RACK1 | TPβ, AT1R, β2-AR | 67 |
| RAMPs | CRLR, CaSR | 68,69 |
| RanBP2 | Opsin | 70 |
| Rap1A | α2C-AR | 71 |
| RGGTA | β2-AR | 72 |
| RTPs/REEPs | OR, T2R, α2C-AR | 73–75 |
| Sar1 | α2B-AR, β2-AR, AT1R, hCaR, LPA1 | 76–78 |
| Skb1Hs | SSTR1 | 79 |
| Syntenin-1 | GPR37 | 80 |
| Tamalin | mGluR1a | 81 |
| Tctex-1 | Rhodopsin | 82 |
| Tubulin | α2A-AR, α2B-AR, AT 1R | 83–85 |
| Usp4 | A2AR | 86 |
| Yif1B | 5-HT1AR | 29,87 |
Regulatory proteins: ARF, ADP-ribosylation factor; ATIP1/ATBP50, AT2R-interacting protein 1/AT2R binding protein of 50 kDa; CD74, antigens-associated invariant chain; CLIC4, chloride intracellular channel protein 4; CNIH4, protein cornichon homolog 4; DRiP78, dopamine receptor-interacting protein 78; gC1q-R, receptor for globular “Heads” of c1q; GEC1, glandular epithelial cell 1; GGAs, Golgi-localized, γ-adaptin ear domain homology, ADP ribosylation factor-binding proteins; ARF1-binding proteins; GABARAP, γ-aminobutyric acid receptor-associated protein; Kif5B, kinesin family 5B; MRAP, melanocortin 2 receptor accessory protein; PAPLA1, phosphatidic acid phospholipase A1; PI3K C2A, class II phosphoinositide 3-kinase α; PRAF2, prenylated Rab acceptor family 2; PTEN, phosphatase and tensin homolog; RACK1, receptor for activated C-kinase 1; RAMPs, receptor activity-modifying proteins;
RanBP2, Ran binding protein 2; RGGTA, Rab geranylgeranyltransferase α subunit; RTPs/REEPs, receptor transporting proteins/receptor expression enhancing proteins; Skb1Hs, human sequence of Shk1 kinase-binding protein; Usp4, ubiquitin specific protease 4, Yif1B, Yip1 interacting factor homolog B.
GPCRs: A1AR, A1 adenosine receptor; A2AR, A2 adenosine receptor; AR, adrenergic receptor; AT1R, angiotensin II type 1 receptor; AT2R, angiotensin II type 2 receptor; CaSR, calcium-sensing receptor CCR5, C-C chemokine receptor 5; CRLR, calcitonin receptor-like receptor; CXCR4, C-X-C chemokine receptor 4; DOR, δ-opioid receptor; D1R, dopamine D1 receptor; D2R, dopamine D2 receptor; D3R, dopamine D3 receptor; EP3R, prostaglandin EP3 receptor; GABABR, metabotropic γ-aminobutyric acid B receptor; H3R, histamine H3 receptor; hCaR, human calcium-sensing receptor; 5-HT1AR, 5-hydroxytryptamine receptor 1A; 5-HT1BR, 5-hydroxytryptamine receptor 1B; KOR, k-opioid receptor; LPA1, lysophosphatidic acid receptor 1; MCR, melanocortin receptor; mGluR, metabotropic glutamate receptor; MOR, μ-opioid receptor; MR, muscarinic receptor; OR, olfactory receptor; P2Y4R, pyrimidinergic receptor P2Y; PAR, protease-activated receptor; PTHR, parathyroid hormone receptor; SSTR, somatostatin receptor; STR, seven TM receptor; T2R, type 2 taste receptor; TPβ, thromboxane A2 receptor β isoform; V1aR, vasopressin 1a receptor; V2R, vasopressin 2 receptor; VN2R, vomeronasal 2 pheromone receptor.
Another important progress achieved over the past years is that a number of specific motifs embedded within the receptors have been revealed to be required for the cell surface transport of GPCRs, such as the E(x)3LL motif in vasopressin receptor 2 (V2R)88, the F/Y(x)3F(x)3F motif in dopamine D1 receptor (D1R)15 and neuropeptide Y receptor type 289, the FN(x)2LL(x)3L motif in vasopressin V1b/V3 receptor90, the F(x)6LL motif in α2B-adrenergic receptor (AR), angiotensin II (Ang II) type 1 receptor (AT1R), α1B-AR, β2-AR and M1-muscarinic receptor (MR)91–93, the YS motif in α2A-AR and α2B-AR94 , the VxPx motif in rhodopsin4,5,82, the RRR and R(x)3R(x)4R motifs in α2B-AR23,83,95, di-acidic and di-basic motifs in AT1R, AT2R and GPR1584,96,97, and serine-rich motifs in melanocortin 5 receptor98. These motifs may regulate receptor correct folding, function as independent export signals to dictate receptor exit from the ER or the Golgi or as retention motifs to prevent receptor export from intracellular compartments, or provide docking sites for regulatory proteins.
In addition, the constitutive dimerization (including homo- and hetero-dimerization) in the ER and post-translational modifications of GPCRs also regulate their cell surface transport. For example, γ-aminobutyric acid B1 receptor (GABABR1) has an ER retention motif at its C-terminus and thus, when expressed alone it is retained in the ER, unable to transport to the cell surface. When co-expressed with GABABR2, it forms hetero-dimers with GABABR2 which masks the ER retention signal, leading to the ER export and cell surface transport99,100. Glycosylation at Asn residues and palmitoylation at Cys residues are the most common post-translational modifications of GPCRs which have been well demonstrated to play an important role in the maturation and cell surface transport of some GPCRs101–105. AT1R possesses three Asn-linked glycosylation sites at positions 4, 176 and 188 and glycosylation is absolutely required for its cell surface expression101,102. AT1R also has a Cys residue at position 355 in the distal C-terminal region which is in marked contrast to many other GPCRs containing Cys residues next to helix 8 which anchor the helix 8 to the plasma membrane. However, the effect of Cys355 on the cell surface transport of AT1R remains to be determined.
Next, we will use AT1R as a representative GPCR to discuss in more detail the function of specific motifs and regulatory proteins that may interact with these motifs in regulating AT1R transport to the cell surface. We will also discuss the relevance of transport mechanisms of AT1R to the anterograde trafficking of other GPCR members.
The role of the C-terminus in the trafficking and signaling of AT1R
Ang II is the major biologically active hormone produced by the renin-angiotensin system (RAS) and plays an important role in the maintenance of blood pressure and fluid homeostasis. The dysregulation of Ang II and the RAS directly contributes to a number of human diseases, such as hypertension, cardiac hypertrophy, congestive heart failure, stroke and diabetic nephropathy. The function of Ang II is mainly mediated through activating its cell surface receptors including AT1R and AT2R, both belong to the GPCR superfamily. There is only one AT1R subtype in human, whereas two AT1R subtypes, AT1aR and AT1bR which share 95% amino acid sequence identity, exist in rat and mouse106–108. As demonstrated in many studies, it is AT1R that mediates the most physiological actions of Ang II. Although AT2R has been suggested to counterbalance the actions of AT1R and genetic mutations of AT2R were associated with X-linked mental retardation109, the exact physiological functions of AT2R remain elusive.
AT1R couples to the Gq family G proteins and regulates a variety of signal transduction pathways, involving the activation of voltage-gated Ca2+ channels, phospholipases (PLC, PLD and PLA2), and mitogen-activated protein kinases (MAPK). In addition to G protein-dependent signaling pathways, AT1R is also able to activate several G protein-independent signaling pathways, such as those mediated via β-arrestins, which were initially identified to mediate GPCR internalization from the plasma membrane to the endosomal compartment and function as negative regulators of GPCR- and G protein-mediated signaling. More interestingly, recent studies have revealed that some AT1R ligands can preferentially activate one pathway and are therefore referred to as biased agonists. For example, the peptides TRV120023, TRV120027 and TRV120067 are β-arrestin-biased AT1R agonists which have been demonstrated to have beneficial cardiovascular effects in animal studies110,111.
AT1R has three short intracellular loops and a relatively large C-terminus. The C-terminus of AT1R possesses an amphipathic 8th α-helix in the membrane-proximal region (Fig. 1) and is the most important intracellular domain in the regulation of receptor functions, including G protein coupling, signaling, trafficking, phosphorylation and interaction with cytosolic proteins112–120 (Table 2). Based on the newly published high resolution crystal structures of truncated AT1R lacking the C-terminal forty residues after helix 8 in complex with an antagonist ZD7155121, the helix 8 of AT1R runs away from the membrane which is apparently different from other GPCRs in which the helix 8 is parallel to the membrane bilayer.
Fig. 1. Specific motifs identified in the C-terminus of AT1R involved in anterograde cell surface trafficking.

TM, transmembrane domain.
Table 2.
The interacting proteins of the C-terminus of AT1R
| Interacting proteins | Interaction sites | Function | References |
|---|---|---|---|
| β-Arrestin | 332–338aa | Internalization, signaling | 122 |
| ARAP1 | 319–359aa | Recycling | 123 |
| ATRAP | 339–359aa | Internalization | 124 |
| Ca2+/CaM | 307–320aa | Desensitization | 125 |
| Caveolin | YxFxxxxFxxY | ER-Golgi transport | 9,10 |
| CD74 | Membrane proximal region | ER retention, degradation | 12 |
| DRiP78 | YxFxxxxFxxY | ER-Golgi transport | 9 |
| eNOS | 306–325 aa | Signaling | 126 |
| GABARAP | Membrane proximal region F(x)6LL |
ER-Golgi transport | 25,127 |
| GASP | 296–359aa | Lysosomal sorting | 128 |
| GLP | 319–359aa | Signaling | 129 |
| G protein | Membrane proximal region | Signaling | 118,130 |
| Jak2 | YIPP | Signaling | 114 |
| PLCγ1 | YIPP | Signaling | 131 |
| Rab4 | Last 10aa | Recycling, phosphorylation | 132 |
| Rab5 | Last 10aa | Internalization | 133 |
| Rab7 | Last 10aa | Lysosome transport | 132 |
| Rab11 | Last 10aa | Recycling | 132 |
| Rab43 | Helix 8 | ER-Golgi transport, sorting | 66 |
| SHP-1 and 2 | YIPP | Signaling | 134 |
| Tubulin | KK | ER-Golgi transport | 84 |
ARAP1, AT1R-associated protein 1; ATRAP, AT1R-associated protein; CaM, calmodulin; eNOS, endothelial nitric oxide synthase; GABARAP, γ-aminobutyric acid receptor-associated protein; GASP, GPCR-associated sorting protein; GLP, GDP/GTP exchange-like protein; PLC, phospholipase C; SHP, Src homology domain 2-containing protein tyrosine phosphatase.
The crucial role of the C-termini in the ER export and cell surface transport has been known for a number of GPCRs, including AT1R15,90,91,135,136. The AT1R mutants lacking the C-terminus were arrested in the ER as indicated by extensive co-localization with the ER markers, unable to transport to the cell surface. Further mutagenesis of the C-terminus revealed several motifs essential for AT1R exit from the ER and subsequent transport to the Golgi and the cell surface91,137 (Fig. 1). Interestingly, these motifs may mediate AT1R interaction with specific regulatory proteins involved in the cell surface transport of the receptor.
The C-terminal hydrophobic motifs and their interacting proteins in the cell surface transport of AT1R
The F(x)6LL motif and GABARAP
The F(x)6LL motif (where x can be any residue and L is leucine or isoleucine) is highly conserved in the membrane-proximal C-termini of the family A GPCRs91. For AT1R, F309 and L316L317 in the C-terminus were identified to form an essential motif for the ER export and cell surface transport. Mutation of F309 and L316L317 individually or in combination abolished AT1R cell surface expression with intensive ER accumulation. Consistent with the loss of the ability to move to the cell surface, the mutated receptors were unable to initiate downstream signaling measured as the activation of the MAPK ERK1/2 in response to Ang II stimulation91.
In addition to AT1R, mutation of the F(x)6LL motif also markedly attenuated the ER export and cell surface transport of α2B-AR, β2-AR, α1B-AR and M1-MR91,92,138, implicating a general role of this motif in the anterograde traffic of family A GPCRs. Although the exact molecular mechanisms underlying the function of the F(x)6LL motif remain largely unknown, several studies suggest that it may modulate multiple events in the anterograde trafficking of GPCRs. Because the membrane-proximal C-termini of these GPCRs form structurally an α-helix, it has been postulated that these hydrophobic motifs are involved in the correct folding of GPCRs. It is also possible that the F and LL residues may regulate different aspects of receptor trafficking in which F is likely involved in folding of the receptor, possibly through interaction with other hydrophobic residues in neighboring domains92, whereas the LL sequence may function as an independent ER export signal, autonomously directing receptor export from the ER. Consistent with this possibility, the di-hydrophobic motifs, such as FF, have been demonstrated to function as ER export motifs139–143. Recent studies have also demonstrated that this motif may mediate receptor interaction with Rab1, Rab8 and a γ-aminobutyric acid receptor-associated protein (GABARAP)25,57,144, all of which have been shown to regulate the cell surface transport of GPCRs.
GABARAP was originally identified through its binding to one subunit of the pentameric ionotropic γ-aminobutyric acid type A receptor (GABAAR) to regulate the plasma membrane transport via the microtubule tracks and to affect both the clustering and kinetic properties of the receptor. GABARAP was then identified as a binding partner for the C-terminus of AT1R in a yeast two-hybrid mouse brain library screening25. The interaction between GABARAP and AT1R was further confirmed by several protein-protein assays (GST fusion protein pull-down, co-immunoprecipitation and bioluminescence resonance energy transfer assays). Importantly, enhanced expression of GABARAP promoted AT1R cell surface expression whereas depletion of GABARAP by siRNA produced an opposing effect, indicating that GABARAP is involved in regulation of AT1R transport to the cell surface. The follow-up studies demonstrated that mutation of the F(x)6LL motif abolished the interaction of AT1R with GABARAP127, suggesting that GABARAP may bind to the F(x)6LL motif in the C-terminus of AT1R. However, it remains unknown if GABARAP is able to interact with other GPCRs which carry the F(x)6LL motif. Nevertheless, these data provide insights into the function of the F(x)6LL motif and the interacting protein GABARAP in regulating AT1R transport.
The caveolin-binding like motif YxFxxxxFxxY and the interacting proteins caveolin and DRiP78
Several studies suggest that the aromatic residues in the caveolin-binding-like motif YxFxxxxFxxY in the C-terminus are important for the optimal expression of AT1R at the plasma membrane9,10,137. Mutation of the YxFxxxxFxxY motif inhibited AT1R expression at the plasma membrane and the receptors were largely expressed in the perinuclear region. Caveolin-3 was shown to directly interact with AT1R through the caveolin scaffolding domain10. Most convincing data indicating that caveolin participated in the maturation of AT1R were generated from caveolin-1 knockout mice in which AT1R transport to the cell surface was significantly attenuated10. It was proposed that the interaction between AT1R and caveolin occurs likely during the export process between the ER and the Golgi and caveolin acts as a molecular chaperone to enhance AT1R transport to the cell surface.
Dopamine receptor-interacting protein 78 (DRiP78) is an ER membrane-associated chaperone protein belonging to the DnaJ/Hsp40 class. It was initially found as a dopamine D1 receptor (D1R)-associated protein by binding to the C-terminal FxxxFxxxF motif. Interestingly, either overexpression of DRiP78 or incubation with the C-terminal peptide to disrupt the interaction between DRiP78 and D1R induced the retention of D1R in the ER and slowed down receptor maturation, suggesting a strictly moderate level of endogenous DRiP78 available for association is important for efficient export of D1R from the ER15. In addition to D1R, the export trafficking of other GPCRs, including AT1R, was also regulated by DRiP789. In contrast to the inhibitory effect on D1 receptor, the expression of DRiP78 enhanced the plasma membrane expression of AT1R. However, the cell surface expression of the AT1R mutant in which the motif YxFxxxxFxxY was mutated was not affected by DRiP78, suggesting a functional interaction between DRiP78 and AT1R9.
The C-terminal charged motifs and their interacting proteins in the cell surface transport of AT1R
The di-basic motif KK and tubulin
There are four positively charged residues at positions 307, 308, 310 and 311 in the C-terminal membrane-proximal region of AT1R (Fig. 1). This positive cluster was shown to be required for the high affinity binding of the receptor to the negatively charged lipids of the plasma membrane145–147, to contain a nuclear localization signal which mediates AT1R translocation into the nucleus148,149, and to influence the total synthesis of the receptor150.
In GST fusion protein pulldown assays to search for interacting proteins using the C-terminus of α2B-AR as bait, tubulin was identified as an interacting protein of α2B-AR83. Further mutagenesis analysis of the C-terminus identified R437, R441 and R446 in the membrane-proximal region responsible for tubulin interaction. Subsequent studies revealed that tubulin also directly and strongly bound to AT1R (and α2A-AR, but not β2-AR)85 and the interaction domains were mapped to two consecutive Lys residues at positions 310 and 311 in the C-terminal membrane-proximal region of AT1R and the acidic C-terminus of tubulin84, suggesting that the interaction of AT1R and α2B-AR with tubulin is ionic in nature. Importantly, mutation of these Lys residues significantly inhibited receptor transport to the cell surface and the receptor mutants were extensively arrested in the ER84. These data suggest that AT1R (as well as other GPCRs such as α2-ARs) may directly contact with the microtubule network to coordinate its own ER-to-cell surface traffic.
The di-acidic ExE motif and ER export
Protein export from the ER is a selective process that is exclusively mediated through COPII-coated transport vesicles. In order to be efficiently exported in COPII vesicles, cargo proteins, particularly transmembrane proteins, may specifically interact with the components of COPII vesicles via ER export motifs which are short, linear sequences presented in the C-termini of cargo proteins. Of various ER export motifs identified, the di-acidic motifs have been found in the cytoplasmic C-termini of several membrane proteins, such as vesicular stomatitis virus glycoprotein (VSVG), cystic fibrosis transmembrane conductance regulator, and potassium channels to direct their export from the ER151–155.
The di-acidic ExD motif in the membrane-distal, nonstructural C-terminal portion of the rat AT2R and the ExE motif in the human AT2R were shown to play an obligatory role in receptor forward trafficking to the cell surface96. These motifs likely control receptor exit from the ER, as their mutants were accumulated in the ER. More interestingly, the export ability of each acidic residue in the di-acidic motifs cannot be fully substituted by other acidic residue, suggesting that distinct di-acidic motifs dictate optimal export trafficking of different AT2R. Moreover, the function of the di-acidic motifs in AT2R export is likely mediated through facilitating the recruitment of the cargo receptors onto the ER-derived COPII transport vesicles96.
Similar to AT2R, the cell surface expression of AT1R was attenuated by mutation of the motif ExE in the very end of the C-terminus (Fig. 1), indicating an important role of the di-acidic ExE motif in AT1R forward trafficking96. However, in contrast to AT1R and AT2R, the cell surface expression of β2-AR and α1B-AR was not altered by mutating the di-acidic motifs in their C-termini96. Therefore, the di-acidic motifs located in the membrane-distal C-termini may represent the first linear motifs which selectively recruit the Ang II receptors onto the COPII vesicles to control their export from the ER. However, it remains to be determined if these di-acidic motifs are able to mediate receptor interaction with any components of COPII vesicles.
The C-terminal helix 8 and small GTPases in the cell surface transport and sorting of AT1R
The helix 8 and Rab43
Consisting of more than 70 members, Rab GTPases form the largest subfamily of Ras-related small GTPases and are master regulators to coordinate almost every step of vesicle-mediated membrane transport, including cargo selection, vesicle formation, sorting, motility, tethering and fusion with the appropriate membranes. Several Rabs, including Rab4, Rab5, Rab7 and Rab11 have been shown to interact with the same domain in the C-terminus of AT1R to regulate receptor phosphorylation, internalization, recycling and lysosomal transport (Table 2).
As an initial approach to investigate the role of Rab GTPases in the anterograde transport of GPCRs, we determined the role of Rab1 in the cell surface transport of several GPCRs. Rab1 is the best characterized and well understood Rab GTPase, which localizes in the ER and the Golgi and regulates anterograde transport specifically from the ER to the Golgi and between the Golgi compartments. We found that transient expression of dominant-negative Rab1 mutants and siRNA-mediated depletion of Rab1 significantly reduced the cell surface expression of AT1R in HEK293 cells50, cardiac myocytes53 and vascular smooth muscle cells54. Consistently, inhibiting Rab1 function attenuates Ang II-mediated signaling, cardiomyocyte hypertrophic responses and smooth muscle cell phenotypic regulation50,53–55. In contrast to Rab1, Rab6 was shown to regulate the retrograde trafficking from the late to early Golgi cisternae and from the Golgi to the ER. Despite Rab1 and Rab6 control opposite transport in the early secretory pathway, the expression of Rab6 mutants also reduced the cell surface expression of AT1R58.
Rab43 was recently identified to control the cell surface transport of several GPCRs, including AT1R, but had no effect on the transport of non-GPCR transmembrane proteins epidermal growth factor receptor and VSVG66. Similar to Rab1, Rab43 is localized to the ER and Golgi, but its function is poorly studied. As expression of dominant negative Rab43 mutants and siRNA-mediated depletion of Rab43 significantly arrested AT1R in the ER and reduced the acquisition of complex N-linked glycosylation of AT1R, Rab43 specifically controls AT1R transport from the ER to the Golgi. More interestingly, Rab43 was shown to directly interact with AT1R and the interaction domain was mapped to the C-terminal 8th α-helix region. These data suggest that AT1R may physically associate with components of the transport machinery to control its transport to the cell surface en route from the ER.
In addition to mediating GPCR forward transport, Rab43 may also play an important role in separating GPCRs from other plasma membrane proteins during maturation processing. Although GPCRs share a common structural topology and several proteins have been identified to control the sorting of GPCRs at the endosomal and lysosomal compartments after internalization156–165, how they are sorted from other plasma proteins at the ER level after their synthesis and then transported via specific routes are poorly understood. The Rab43-binding domain identified in the AT1R C-terminus was able to effectively convert the Rab43-independent transport of VSVG into Rab43-dependnet transport, specifically from the ER to the Golgi. These data provide strong evidence indicating that Rab43 controls not only ER-to-Golgi transport but also the ER sorting of AT1R and possibly other GPCR members by virtue of its ability to directly interact with the receptors.
ARF1/Sar1 family GTPases
Sar1 and six ARF GTPases belong to the same subfamily of Ras-like GTPases and are well characterized to play crucial roles in the formation and budding of different transport vesicles. In particular, Sar1 GTPase recruits the Sec23/24 and Sec13/31 complexes onto the ER membrane, leading to the formation of the COPII-coated vesicles. ARF1 is able to recruit different sets of coat proteins to form distinct transport vesicles that control protein transport at different intracellular organelles. In the early secretory pathway, ARF1 recruits a complex of cytosolic proteins, collectively known as coatomers, leading to the formation of COPI vesicles which mediate protein transport from the Golgi to the ER or from the ER-Golgi intermediate complex (ERGIC) to the Golgi and intra-Golgi traffic. In the post-Golgi transport, ARF1 interacts with adaptor proteins and Golgi-localized, γ-adaptin ear domain homology, ADP ribosylation factor-binding proteins (GGAs), both of which recruit clathrin onto the TGN, forming the clathrin-coated vesicles that mediate post-Golgi transport between the TGN, the plasma membrane and the endosomal compartment.
The role of Sar1 GTPase in the cell surface transport of GPCRs has been determined by transient expression of a GTP-restricted Sar1 mutant (Sar1H79G)76,77, which functions as a dominant negative mutant to block the release of the COPII vesicles from the ER membrane. Expression of Sar1H79G significantly attenuated the cell surface expression of AT1R76 and the receptors were extensively co-localized with GM130, a cis-Golgi marker which has been demonstrated to translocate from the cis-Golgi to ER exit sites in the presence of Sar1H79G. These data suggest that AT1R is able to export from the ER to ER exit sites in the cells expressing Sar1H79G. It is interesting to note that expression of Sar1H79G blocked the cell surface transport of several other GPCRs, but differentially regulates ER export of different GPCRs76,77.
The role of ARF1 in the cell surface transport of GPCRs including AT1R has also been studied2. Although the expression of GDP- and GTP-bound ARF1 mutants markedly reduced the cell surface transport of AT1R, they arrested the receptors in distinct intracellular compartments. Whereas expression of the GDP-bound mutant ARF1T31N arrested AT1R in the ER, the GTP-bound mutant ARF1Q71L induced an accumulation of the receptors in the post-ER compartments2. These data indicate that expression of different ARF1 mutants blocks the export of the cargo receptors from different subcellular compartments, consistent with multiple functions of ARF1 in the formation of different transport vesicles from distinct compartments. Altogether, the Ras-like small GTPases Rab1, Rab6, Rab43, Sar1 and ARF1 control the anterograde trafficking of AT1R, each may regulate the transport at distinct steps (e.g. recruitment onto COPII vesicles, ER export, ER-Golgi transport or Golgi-plasma membrane transport) (Fig. 2).
Fig. 2. Regulatory proteins involved in the ER-Golgi-plasma membrane (PM) transport of AT1R.
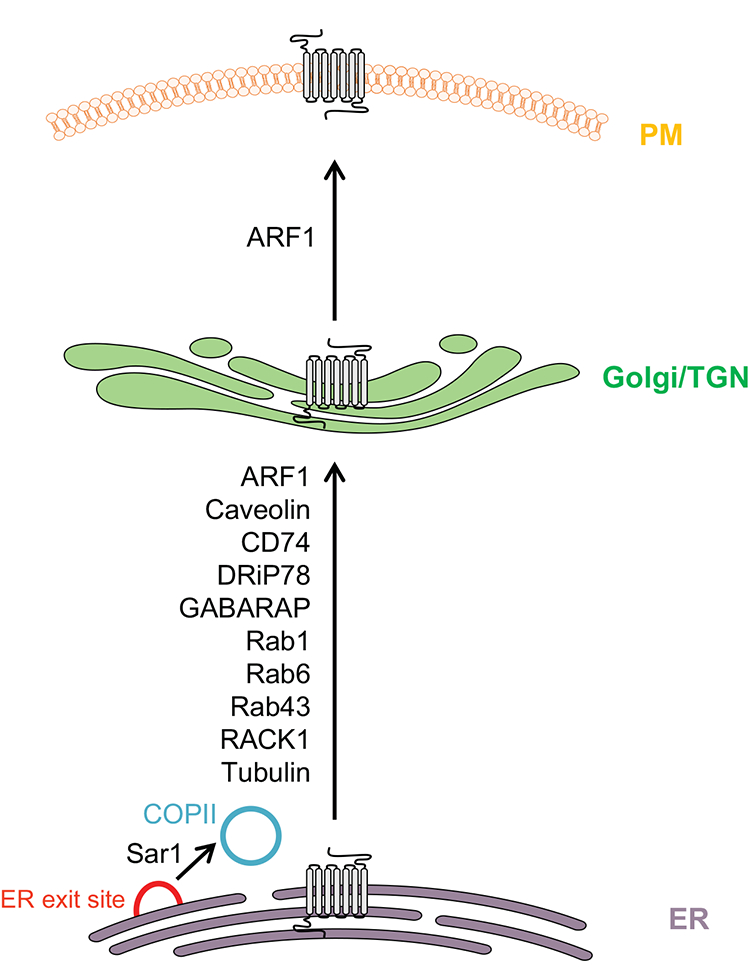
See text for detail.
Other AT1R-interacting proteins involved in the cell surface transport
The C-terminal interaction with CD74
CD74 is a type II transmembrane protein and acts as a chaperone for the trafficking of the major histocompatibility complex class II molecules. By using the yeast two-hybrid approach to screen a human kidney cDNA library, Szaszák et al. identified CD74 as an interacting protein for the AT1R C-terminus12. The interaction between AT1R and CD74 was verified by co-immunoprecipitation and co-localization assays. The CD74-binding site was mapped to the C-terminal membrane proximal region of AT1R. Interestingly, CD74 overexpression markedly reduced the cell surface expression of ATR and induced receptor accumulation in the ER and targeting to the proteasomal degradation pathway. These data suggest that, in contrast to other interacting proteins which promote the cell surface expression of AT1R, CD74 is likely a negative regulator of AT1R trafficking along the biosynthesis pathway.
AT1R interaction with chaperon proteins
In addition to DRiP78 as discussed above, several other chaperone proteins, including calnexin, Hsp70 and calreticulin, have also been shown to interact with AT1R and their interactions with the no-glycosylated AT1R mutant were stronger than with wild type AT1R166. Similarly, several ER-export deficient, misfolded GPCR mutants exhibited strong interactions with chaperone proteins167–169. These data suggest that ER chaperones may have a duel function in regulating GPCRs, not only promoting proper folding of the immature receptors but also preventing the export of terminally misfolded receptors from the ER to the Golgi.
AT1R interaction with RACK1
Receptor for activated C kinase 1 (RACK1) was identified to interact with the C-terminus and the first intracellular loop of the β isoform of thromboxane A2 receptor (TBβ) in yeast two-hybrid and GST fusion protein pulldown assays67. The fact that overexpression of RACK1 and its depletion by siRNA produced opposing effects on the cell surface expression of TBβ indicates an important role played by RACK1 in the cell surface traffic of TBβ. In addition to TBβ, the cell surface expression of CXCR4 and AT1R was also attenuated by siRNA-mediated knockdown of RACK1 and AT1R physically associated with AT1R as measured in coimmunoprecipitation assays67. As siRNA-mediated knockdown of RACK1 arrested the receptors in the ER, RACK1 is most likely involved in the ER-to-Golgi transport.
Concluding remarks
Over the past decade great progress has been made in elucidating molecular mechanisms underlying the anterograde transport of newly synthesized GPCRs en route from the ER through the Golgi body. It is increasingly apparent that, similar to the endocytic pathways, the cell surface transport of GPCRs is a complicate, highly coordinated process. In particular, multiple regulatory proteins have been identified to control receptor trafficking via direct interaction with the receptors at specific domains and these protein-protein interactions may lead to the formation of specialized transport machineries that drive the forward traffic of the receptors. However, the relationship between these interacting proteins and how they cooperate to ensure normal receptor export from the ER to the cell surface of GPCRs in general or AT1R in particular need further investigation. It is possible that multiple routes exist to mediate receptor cell surface transport, either from the ER to the Golgi or from the Golgi to the plasma membrane, and each route requires a group of distinct regulatory proteins. Since the abnormal plasma membrane expression, mistrafficking, and dysfunction of many GPCRs, including AT1R, are clearly implicated in the pathogenesis of a variety of human diseases, including neurological disorders, cardiovascular diseases, and cancer107, to further explore the regulatory mechanism of anterograde transport of GPCRs may provide an important foundation for developing new therapeutic means in treating human diseases involving abnormal trafficking and signaling of the receptors.
Synopsis:
As compared with internalization and recycling, anterograde cell surface transport of nascent GPCRs en route from the endoplasmic reticulum through the Golgi apparatus remains poorly understood. Here, we will review the current understanding of the cell surface trafficking of the GPCR superfamily and use AT1R as an example to discuss emerging roles of receptor-interacting proteins and specific motifs.
Acknowledgements
This work was supported by National Institutes of Health Grant R01GM118915 (to G. Wu) and National Natural Science Foundation of China Grant 31771300 (to M. Zhang). We apologize to numerous colleagues whose work could not be cited in this article due to space limitation.
Footnotes
Conflict of interest The authors have no conflict of interest to declare.
References
- 1.Grant MP, Stepanchick A, Cavanaugh A, Breitwieser GE. Agonist-driven maturation and plasma membrane insertion of calcium-sensing receptors dynamically control signal amplitude. Sci Signal. 2011;4(200):ra78. [DOI] [PubMed] [Google Scholar]
- 2.Dong C, Zhang X, Zhou F, et al. ADP-ribosylation factors modulate the cell surface transport of G protein-coupled receptors. J Pharmacol Exp Ther. 2010;333(1):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo W, Wang Y, Reiser G. p24A, a type I transmembrane protein, controls ARF1-dependent resensitization of protease-activated receptor-2 by influence on receptor trafficking. J Biol Chem. 2007;282(41):30246–30255. [DOI] [PubMed] [Google Scholar]
- 4.Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc Natl Acad Sci U S A. 2005;102(9):3301–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazelova J, Astuto-Gribble L, Inoue H, et al. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28(3):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madziva MT, Birnbaumer M. A role for ADP-ribosylation factor 6 in the processing of G-protein-coupled receptors. J Biol Chem. 2006;281(17):12178–12186. [DOI] [PubMed] [Google Scholar]
- 7.Nouet S, Amzallag N, Li JM, et al. Trans-inactivation of receptor tyrosine kinases by novel angiotensin II AT2 receptor-interacting protein, ATIP. J Biol Chem. 2004;279(28):28989–28997. [DOI] [PubMed] [Google Scholar]
- 8.Wruck CJ, Funke-Kaiser H, Pufe T, et al. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler Thromb Vasc Biol. 2005;25(1):57–64. [DOI] [PubMed] [Google Scholar]
- 9.Leclerc PC, Auger-Messier M, Lanctot PM, Escher E, Leduc R, Guillemette G. A polyaromatic caveolin-binding-like motif in the cytoplasmic tail of the type 1 receptor for angiotensin II plays an important role in receptor trafficking and signaling. Endocrinology. 2002;143(12):4702–4710. [DOI] [PubMed] [Google Scholar]
- 10.Wyse BD, Prior IA, Qian H, et al. Caveolin interacts with the angiotensin II type 1 receptor during exocytic transport but not at the plasma membrane. J Biol Chem. 2003;278(26):23738–23746. [DOI] [PubMed] [Google Scholar]
- 11.Achour L, Scott MG, Shirvani H, et al. CD4-CCR5 interaction in intracellular compartments contributes to receptor expression at the cell surface. Blood. 2009;113(9):1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szaszak M, Chen HD, Chen HC, Baukal A, Hunyady L, Catt KJ. Identification of the invariant chain (CD74) as an angiotensin AGTR1-interacting protein. J Endocrinol. 2008;199(2):165–176. [DOI] [PubMed] [Google Scholar]
- 13.Maeda K, Haraguchi M, Kuramasu A, et al. CLIC4 interacts with histamine H3 receptor and enhances the receptor cell surface expression. Biochem Biophys Res Commun. 2008;369(2):603–608. [DOI] [PubMed] [Google Scholar]
- 14.Sauvageau E, Rochdi MD, Oueslati M, et al. CNIH4 interacts with newly synthesized GPCR and controls their export from the endoplasmic reticulum. Traffic. 2014;15(4):383–400. [DOI] [PubMed] [Google Scholar]
- 15.Bermak JC, Li M, Bullock C, Zhou QY. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat Cell Biol. 2001;3(5):492–498. [DOI] [PubMed] [Google Scholar]
- 16.Kuang YQ, Charette N, Frazer J, et al. Dopamine receptor-interacting protein 78 acts as a molecular chaperone for CCR5 chemokine receptor signaling complex organization. PLoS One. 2012;7(7):e40522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motawea HK, Jeyaraj SC, Eid AH, et al. Cyclic AMP-Rap1A signaling mediates cell surface translocation of microvascular smooth muscle alpha2C-adrenoceptors through the actin-binding protein filamin-2. Am J Physiol Cell Physiol. 2013;305(8):C829–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin R, Karpa K, Kabbani N, Goldman-Rakic P, Levenson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc Natl Acad Sci U S A. 2001;98(9):5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z, Hirasawa A, Shinoura H, Tsujimoto G. Interaction of the alpha(1B)-adrenergic receptor with gC1q-R, a multifunctional protein. J Biol Chem. 1999;274(30):21149–21154. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, Li JG, Chen Y, Huang P, Wang Y, Liu-Chen LY. GEC1 interacts with the kappa opioid receptor and enhances expression of the receptor. J Biol Chem. 2006;281(12):7983–7993. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Chen C, Kotsikorou E, Lynch DL, Reggio PH, Liu-Chen LY. GEC1-kappa opioid receptor binding involves hydrophobic interactions: GEC1 has chaperone-like effect. J Biol Chem. 2009;284(3):1673–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M, Huang W, Gao J, Terry AV, Wu G. Regulation of alpha2B-adrenergic receptor cell surface transport by GGA1 and GGA2. Sci Rep. 2016;6:37921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, Davis JE, Li C, et al. GGA3 Interacts with a G Protein-Coupled Receptor and Modulates Its Cell Surface Export. Mol Cell Biol. 2016;36(7):1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicks SW, Horn TA, McCaffery JM, Zuckerman DM, Machamer CE. Golgin-160 promotes cell surface expression of the beta-1 adrenergic receptor. Traffic. 2006;7(12):1666–1677. [DOI] [PubMed] [Google Scholar]
- 25.Cook JL, Re RN, deHaro DL, Abadie JM, Peters M, Alam J. The trafficking protein GABARAP binds to and enhances plasma membrane expression and function of the angiotensin II type 1 receptor. Circ Res. 2008;102(12):1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brakeman PR, Lanahan AA, O’Brien R, et al. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386(6622):284–288. [DOI] [PubMed] [Google Scholar]
- 27.Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, Worley PF. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem. 1999;274(36):25953–25957. [DOI] [PubMed] [Google Scholar]
- 28.Chapple JP, Cheetham ME. The chaperone environment at the cytoplasmic face of the endoplasmic reticulum can modulate rhodopsin processing and inclusion formation. J Biol Chem. 2003;278(21):19087–19094. [DOI] [PubMed] [Google Scholar]
- 29.Al Awabdh S, Miserey-Lenkei S, Bouceba T, et al. A new vesicular scaffolding complex mediates the G-protein-coupled 5-HT1A receptor targeting to neuronal dendrites. J Neurosci. 2012;32(41):14227–14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loconto J, Papes F, Chang E, et al. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112(5):607–618. [DOI] [PubMed] [Google Scholar]
- 31.Chan LF, Webb TR, Chung TT, et al. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci U S A. 2009;106(15):6146–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim OJ, Ariano MA, Lazzarini RA, Levine MS, Sibley DR. Neurofilament-M interacts with the D1 dopamine receptor to regulate cell surface expression and desensitization. J Neurosci. 2002;22(14):5920–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colley NJ, Baker EK, Stamnes MA, Zuker CS. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991;67(2):255–263. [DOI] [PubMed] [Google Scholar]
- 34.Dwyer ND, Troemel ER, Sengupta P, Bargmann CI. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell. 1998;93(3):455–466. [DOI] [PubMed] [Google Scholar]
- 35.Gimelbrant AA, Haley SL, McClintock TS. Olfactory receptor trafficking involves conserved regulatory steps. J Biol Chem. 2001;276(10):7285–7290. [DOI] [PubMed] [Google Scholar]
- 36.Chen C, Itakura E, Weber KP, Hegde RS, de Bono M. An ER complex of ODR-4 and ODR-8/Ufm1 specific protease 2 promotes GPCR maturation by a Ufm1-independent mechanism. PLoS Genet. 2014;10(3):e1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svenningsson P, Chergui K, Rachleff I, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311(5757):77–80. [DOI] [PubMed] [Google Scholar]
- 38.Luo W, Wang Y, Reiser G. Proteinase-activated receptors, nucleotide P2Y receptors, and mu-opioid receptor-1B are under the control of the type I transmembrane proteins p23 and p24A in post-Golgi trafficking. J Neurochem. 2011;117(1):71–81. [DOI] [PubMed] [Google Scholar]
- 39.Kunduri G, Yuan C, Parthibane V, et al. Phosphatidic acid phospholipase A1 mediates ER-Golgi transit of a family of G protein-coupled receptors. J Cell Biol. 2014;206(1):79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiwarski DJ, Darr M, Telmer CA, Bruchez MP, Puthenveedu MA. PI3K class II alpha regulates delta-opioid receptor export from the trans-Golgi network. Mol Biol Cell. 2017;28(16):2202–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filipeanu CM, Pullikuth AK, Guidry JJ. Molecular determinants of the human alpha2C-adrenergic receptor temperature-sensitive intracellular traffic. Mol Pharmacol. 2015;87(5):792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doly S, Shirvani H, Gata G, et al. GABAB receptor cell-surface export is controlled by an endoplasmic reticulum gatekeeper. Mol Psychiatry. 2016;21(4):480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan JS, Xu ZZ, Gao H, et al. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122(4):619–631. [DOI] [PubMed] [Google Scholar]
- 44.Lu D, Yan H, Othman T, Turner CP, Woolf T, Rivkees SA. Cytoskeletal protein 4.1G binds to the third intracellular loop of the A1 adenosine receptor and inhibits receptor action. Biochem J. 2004;377(Pt 1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito M, Sugai M, Katsushima Y, Yanagisawa T, Sukegawa J, Nakahata N. Increase in cell-surface localization of parathyroid hormone receptor by cytoskeletal protein 4.1G. Biochem J. 2005;392(Pt 1):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu D, Yan H, Othman T, Rivkees SA. Cytoskeletal protein 4.1G is a binding partner of the metabotropic glutamate receptor subtype 1 alpha. J Neurosci Res. 2004;78(1):49–55. [DOI] [PubMed] [Google Scholar]
- 47.Binda AV, Kabbani N, Lin R, Levenson R. D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N. Mol Pharmacol. 2002;62(3):507–513. [DOI] [PubMed] [Google Scholar]
- 48.Shiwarski DJ, Tipton A, Giraldo MD, et al. A PTEN-regulated checkpoint controls surface delivery of delta opioid receptors. J Neurosci. 2017;37(14):3741–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Filipeanu CM, Zhou F, Fugetta EK, Wu G. Differential regulation of the cell-surface targeting and function of beta- and alpha1-adrenergic receptors by Rab1 GTPase in cardiac myocytes. Mol Pharmacol. 2006;69(5):1571–1578. [DOI] [PubMed] [Google Scholar]
- 50.Wu G, Zhao G, He Y. Distinct pathways for the trafficking of angiotensin II and adrenergic receptors from the endoplasmic reticulum to the cell surface: Rab1-independent transport of a G protein-coupled receptor. J Biol Chem. 2003;278(47):47062–47069. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Wang G, Lin K, et al. Rab1 GTPase promotes expression of beta-adrenergic receptors in rat pulmonary microvascular endothelial cells. Int J Biochem Cell Biol. 2010;42(7):1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dupre DJ, Robitaille M, Ethier N, Villeneuve LR, Mamarbachi AM, Hebert TE. Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J Biol Chem. 2006;281(45):34561–34573. [DOI] [PubMed] [Google Scholar]
- 53.Filipeanu CM, Zhou F, Claycomb WC, Wu G. Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum-to-Golgi transport in cardiac myocytes. J Biol Chem. 2004;279(39):41077–41084. [DOI] [PubMed] [Google Scholar]
- 54.Yin H, Li Q, Qian G, et al. Rab1 GTPase regulates phenotypic modulation of pulmonary artery smooth muscle cells by mediating the transport of angiotensin II type 1 receptor under hypoxia. Int J Biochem Cell Biol. 2011;43(3):401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Wang G, Dupre DJ, et al. Rab1 GTPase and dimerization in the cell surface expression of angiotensin II type 2 receptor. J Pharmacol Exp Ther. 2009;330(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhuang X, Adipietro KA, Datta S, Northup JK, Ray K. Rab1 small GTP-binding protein regulates cell surface trafficking of the human calcium-sensing receptor. Endocrinology. 2010;151(11):5114–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammad MM, Kuang YQ, Morse A, Dupre DJ. Rab1 interacts directly with the beta2-adrenergic receptor to regulate receptor anterograde trafficking. Biol Chem. 2012;393(6):541–546. [DOI] [PubMed] [Google Scholar]
- 58.Dong C, Wu G. Regulation of anterograde transport of adrenergic and angiotensin II receptors by Rab2 and Rab6 GTPases. Cell Signal. 2007;19(11):2388–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deretic D, Papermaster DS. Rab6 is associated with a compartment that transports rhodopsin from the trans-Golgi to the site of rod outer segment disk formation in frog retinal photoreceptors. J Cell Sci. 1993;106 ( Pt 3):803–813. [DOI] [PubMed] [Google Scholar]
- 60.Shetty KM, Kurada P, O’Tousa JE. Rab6 regulation of rhodopsin transport in Drosophila. J Biol Chem. 1998;273(32):20425–20430. [DOI] [PubMed] [Google Scholar]
- 61.Dong C, Yang L, Zhang X, et al. Rab8 interacts with distinct motifs in {alpha}2B- and {beta}2-adrenergic receptors and differentially modulates their transport. J Biol Chem. 2010;285(26):20369–20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108 ( Pt 1):215–224. [DOI] [PubMed] [Google Scholar]
- 63.Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12(8):2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li C, Fan Y, Lan TH, Lambert NA, Wu G. Rab26 modulates the cell surface transport of alpha2-adrenergic receptors from the Golgi. J Biol Chem. 2012;287(51):42784–42794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang G, Wei Z, Wu G. Role of Rab GTPases in the export trafficking of G protein-coupled receptors. Small GTPases. 2018;9(1–2):130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li C, Wei Z, Fan Y, et al. The GTPase Rab43 controls the anterograde ER-Golgi trafficking and sorting of GPCRs. Cell Rep. 2017;21(4):1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parent A, Laroche G, Hamelin E, Parent JL. RACK1 regulates the cell surface expression of the G protein-coupled receptor for thromboxane A(2). Traffic. 2008;9(3):394–407. [DOI] [PubMed] [Google Scholar]
- 68.McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–339. [DOI] [PubMed] [Google Scholar]
- 69.Bouschet T, Martin S, Henley JM. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J Cell Sci. 2005;118(Pt 20):4709–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferreira PA, Nakayama TA, Pak WL, Travis GH. Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature. 1996;383(6601):637–640. [DOI] [PubMed] [Google Scholar]
- 71.Jeyaraj SC, Unger NT, Eid AH, et al. Cyclic AMP-Rap1A signaling activates RhoA to induce alpha(2c)-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells. Am J Physiol Cell Physiol. 2012;303(5):C499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lachance V, Cartier A, Genier S, et al. Regulation of beta2-adrenergic receptor maturation and anterograde trafficking by an interaction with Rab geranylgeranyltransferase: modulation of Rab geranylgeranylation by the receptor. J Biol Chem. 2011;286(47):40802–40813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119(5):679–691. [DOI] [PubMed] [Google Scholar]
- 74.Behrens M, Bartelt J, Reichling C, Winnig M, Kuhn C, Meyerhof W. Members of RTP and REEP gene families influence functional bitter taste receptor expression. J Biol Chem. 2006;281(29):20650–20659. [DOI] [PubMed] [Google Scholar]
- 75.Bjork S, Hurt CM, Ho VK, Angelotti T. REEPs are membrane shaping adapter proteins that modulate specific g protein-coupled receptor trafficking by affecting ER cargo capacity. PLoS One. 2013;8(10):e76366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong C, Zhou F, Fugetta EK, Filipeanu CM, Wu G. Endoplasmic reticulum export of adrenergic and angiotensin II receptors is differentially regulated by Sar1 GTPase. Cell Signal. 2008;20(6):1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhuang X, Chowdhury S, Northup JK, Ray K. Sar1-dependent trafficking of the human calcium receptor to the cell surface. Biochem Biophys Res Commun. 2010;396(4):874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao J, Wei J, Bowser RK, Dong S, Xiao S, Zhao Y. Molecular regulation of lysophosphatidic acid receptor 1 trafficking to the cell surface. Cell Signal. 2014;26(11):2406–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwarzler A, Kreienkamp HJ, Richter D. Interaction of the somatostatin receptor subtype 1 with the human homolog of the Shk1 kinase-binding protein from yeast. J Biol Chem. 2000;275(13):9557–9562. [DOI] [PubMed] [Google Scholar]
- 80.Dunham JH, Meyer RC, Garcia EL, Hall RA. GPR37 surface expression enhancement via N-terminal truncation or protein-protein interactions. Biochemistry. 2009;48(43):10286–10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kitano J, Kimura K, Yamazaki Y, et al. Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. J Neurosci. 2002;22(4):1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97(7):877–887. [DOI] [PubMed] [Google Scholar]
- 83.Duvernay MT, Wang H, Dong C, Guidry JJ, Sackett DL, Wu G. alpha2B-Adrenergic receptor interaction with tubulin controls its transport from the endoplasmic reticulum to the cell surface. J Biol Chem. 2011;286(16):14080–14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang X, Wang H, Duvernay MT, Zhu S, Wu G. The angiotensin II type 1 receptor C-terminal Lys residues interact with tubulin and modulate receptor export trafficking. PLoS One. 2013;8(2):e57805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu G, Davis JE, Zhang M. Regulation of alpha2B-adrenerigc receptor export trafficking by specific motifs. Prog Mol Biol Transl Sci. 2015;132:227–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milojevic T, Reiterer V, Stefan E, et al. The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol. 2006;69(4):1083–1094. [DOI] [PubMed] [Google Scholar]
- 87.Carrel D, Masson J, Al Awabdh S, et al. Targeting of the 5-HT1A serotonin receptor to neuronal dendrites is mediated by Yif1B. J Neurosci. 2008;28(32):8063–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schulein R, Hermosilla R, Oksche A, et al. A dileucine sequence and an upstream glutamate residue in the intracellular carboxyl terminus of the vasopressin V2 receptor are essential for cell surface transport in COS.M6 cells. Mol Pharmacol. 1998;54(3):525–535. [DOI] [PubMed] [Google Scholar]
- 89.Walther C, Lotze J, Beck-Sickinger AG, Morl K. The anterograde transport of the human neuropeptide Y2 receptor is regulated by a subtype specific mechanism mediated by the C-terminus. Neuropeptides. 2012;46(6):335–343. [DOI] [PubMed] [Google Scholar]
- 90.Robert J, Clauser E, Petit PX, Ventura MA. A novel C-terminal motif is necessary for the export of the vasopressin V1b/V3 receptor to the plasma membrane. J Biol Chem. 2005;280(3):2300–2308. [DOI] [PubMed] [Google Scholar]
- 91.Duvernay MT, Zhou F, Wu G. A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. J Biol Chem. 2004;279(29):30741–30750. [DOI] [PubMed] [Google Scholar]
- 92.Duvernay MT, Dong C, Zhang X, Zhou F, Nichols CD, Wu G. Anterograde trafficking of G protein-coupled receptors: function of the C-terminal F(X)6LL motif in export from the endoplasmic reticulum. Mol Pharmacol. 2009;75(4):751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sawyer GW, Ehlert FJ, Shults CA. A conserved motif in the membrane proximal C-terminal tail of human muscarinic m1 acetylcholine receptors affects plasma membrane expression. J Pharmacol Exp Ther. 2010;332(1):76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dong C, Wu G. Regulation of anterograde transport of alpha2-adrenergic receptors by the N termini at multiple intracellular compartments. J Biol Chem. 2006;281(50):38543–38554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dong C, Nichols CD, Guo J, Huang W, Lambert NA, Wu G. A triple arg motif mediates alpha(2B)-adrenergic receptor interaction with Sec24C/D and export. Traffic. 2012;13(6):857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang X, Dong C, Wu QJ, Balch WE, Wu G. Di-acidic motifs in the membrane-distal C termini modulate the transport of angiotensin II receptors from the endoplasmic reticulum to the cell surface. J Biol Chem. 2011;286(23):20525–20535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okamoto Y, Bernstein JD, Shikano S. Role of C-terminal membrane-proximal basic residues in cell surface trafficking of HIV coreceptor GPR15 protein. J Biol Chem. 2013;288(13):9189–9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodrigues AR, Sousa D, Almeida H, Gouveia AM. Cell surface targeting of the Melanocortin 5 Receptor (MC5R) requires serine-rich terminal motifs. Biochim Biophys Acta Mol Cell Res. 2017;1864(7):1217–1226. [DOI] [PubMed] [Google Scholar]
- 99.Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27(1):97–106. [DOI] [PubMed] [Google Scholar]
- 100.Pagano A, Rovelli G, Mosbacher J, et al. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J Neurosci. 2001;21(4):1189–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jayadev S, Smith RD, Jagadeesh G, Baukal AJ, Hunyady L, Catt KJ. N-linked glycosylation is required for optimal AT1a angiotensin receptor expression in COS-7 cells. Endocrinology. 1999;140(5):2010–2017. [DOI] [PubMed] [Google Scholar]
- 102.Deslauriers B, Ponce C, Lombard C, Larguier R, Bonnafous JC, Marie J. N-glycosylation requirements for the AT1a angiotensin II receptor delivery to the plasma membrane. Biochem J. 1999;339 ( Pt 2):397–405. [PMC free article] [PubMed] [Google Scholar]
- 103.Rands E, Candelore MR, Cheung AH, Hill WS, Strader CD, Dixon RA. Mutational analysis of beta-adrenergic receptor glycosylation. J Biol Chem. 1990;265(18):10759–10764. [PubMed] [Google Scholar]
- 104.Petaja-Repo UE, Hogue M, Leskela TT, Markkanen PM, Tuusa JT, Bouvier M. Distinct subcellular localization for constitutive and agonist-modulated palmitoylation of the human delta opioid receptor. J Biol Chem. 2006;281(23):15780–15789. [DOI] [PubMed] [Google Scholar]
- 105.Blanpain C, Wittamer V, Vanderwinden JM, et al. Palmitoylation of CCR5 is critical for receptor trafficking and efficient activation of intracellular signaling pathways. J Biol Chem. 2001;276(26):23795–23804. [DOI] [PubMed] [Google Scholar]
- 106.Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351(6323):233–236. [DOI] [PubMed] [Google Scholar]
- 107.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292(1):C82–97. [DOI] [PubMed] [Google Scholar]
- 108.Forrester SJ, Booz GW, Sigmund CD, et al. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98(3):1627–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vervoort VS, Beachem MA, Edwards PS, et al. AGTR2 mutations in X-linked mental retardation. Science. 2002;296(5577):2401–2403. [DOI] [PubMed] [Google Scholar]
- 110.Boerrigter G, Lark MW, Whalen EJ, Soergel DG, Violin JD, Burnett JC Jr. Cardiorenal actions of TRV120027, a novel ss-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure. Circ Heart Fail. 2011;4(6):770–778. [DOI] [PubMed] [Google Scholar]
- 111.Ryba DM, Li J, Cowan CL, Russell B, Wolska BM, Solaro RJ. Long-term biased beta-arrestin signaling improves cardiac structure and function in dilated cardiomyopathy. Circulation. 2017;135(11):1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huynh J, Thomas WG, Aguilar MI, Pattenden LK. Role of helix 8 in G protein-coupled receptors based on structure-function studies on the type 1 angiotensin receptor. Mol Cell Endocrinol. 2009;302(2):118–127. [DOI] [PubMed] [Google Scholar]
- 113.Lee DK, Lanca AJ, Cheng R, et al. Agonist-independent nuclear localization of the apelin, angiotensin AT(1), and bradykinin B-2 receptors. J Biol Chem. 2004;279(9):7901–7908. [DOI] [PubMed] [Google Scholar]
- 114.Ali MS, Sayeski PP, Dirksen LB, Hayzer DJ, Marrero MB, Bernstein KE. Dependence on the motif YIPP for the physical association of Jak2 kinase with the intracellular carboxyl tail of the angiotensin II AT1 receptor. J Biol Chem. 1997;272(37):23382–23388. [DOI] [PubMed] [Google Scholar]
- 115.Morinelli TA, Raymond JR, Baldys A, et al. Identification of a putative nuclear localization sequence within ANG II AT(1A) receptor associated with nuclear activation. Am J Physiol-Cell Ph. 2007;292(4):C1398–C1408. [DOI] [PubMed] [Google Scholar]
- 116.Ju H, Venema VJ, Marrero MB, Venema RC. Inhibitory interactions of the bradykinin B2 receptor with endothelial nitric-oxide synthase. J Biol Chem. 1998;273(37):24025–24029. [DOI] [PubMed] [Google Scholar]
- 117.Thomas WG, Motel TJ, Kule CE, Karoor V, Baker KM. Phosphorylation of the angiotensin II (AT1A) receptor carboxyl terminus: a role in receptor endocytosis. Mol Endocrinol. 1998;12(10):1513–1524. [DOI] [PubMed] [Google Scholar]
- 118.Sano T, Ohyama K, Yamano Y, et al. A domain for G protein coupling in carboxyl-terminal tail of rat angiotensin II receptor type 1A. J Biol Chem. 1997;272(38):23631–23636. [DOI] [PubMed] [Google Scholar]
- 119.Becker BN, Cheng HF, Hammond TG, Harris RC. The type 1 angiotensin II receptor tail affects receptor targeting, internalization, and membrane fusion properties. Mol Pharmacol. 2004;65(2):362–369. [DOI] [PubMed] [Google Scholar]
- 120.Thomas WG, Baker KM, Motel TJ, Thekkumkara TJ. Angiotensin II receptor endocytosis involves two distinct regions of the cytoplasmic tail. A role for residues on the hydrophobic face of a putative amphipathic helix. J Biol Chem. 1995;270(38):22153–22159. [DOI] [PubMed] [Google Scholar]
- 121.Zhang H, Unal H, Gati C, et al. Structure of the Angiotensin receptor revealed by serial femtosecond crystallography. Cell. 2015;161(4):833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qian H, Pipolo L, Thomas WG. Association of beta-Arrestin 1 with the type 1A angiotensin II receptor involves phosphorylation of the receptor carboxyl terminus and correlates with receptor internalization. Mol Endocrinol. 2001;15(10):1706–1719. [DOI] [PubMed] [Google Scholar]
- 123.Guo DF, Chenier I, Tardif V, Orlov SN, Inagami T. Type 1 angiotensin II receptor-associated protein ARAP1 binds and recycles the receptor to the plasma membrane. Biochem Biophys Res Commun. 2003;310(4):1254–1265. [DOI] [PubMed] [Google Scholar]
- 124.Daviet L, Lehtonen JY, Tamura K, Griese DP, Horiuchi M, Dzau VJ. Cloning and characterization of ATRAP, a novel protein that interacts with the angiotensin II type 1 receptor. J Biol Chem. 1999;274(24):17058–17062. [DOI] [PubMed] [Google Scholar]
- 125.Thomas WG, Pipolo L, Qian H. Identification of a Ca2+/calmodulin-binding domain within the carboxyl-terminus of the angiotensin II (AT1A) receptor. FEBS Lett. 1999;455(3):367–371. [DOI] [PubMed] [Google Scholar]
- 126.Marrero MB, Venema VJ, Ju H, et al. Endothelial nitric oxide synthase interactions with G-protein-coupled receptors. Biochem J. 1999;343 Pt 2:335–340. [PMC free article] [PubMed] [Google Scholar]
- 127.Alam J, Deharo D, Redding KM, Re RN, Cook JL. C-terminal processing of GABARAP is not required for trafficking of the angiotensin II type 1A receptor. Regul Pept. 2010;159(1–3):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heydorn A, Sondergaard BP, Ersboll B, et al. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP). J Biol Chem. 2004;279(52):54291–54303. [DOI] [PubMed] [Google Scholar]
- 129.Guo DF, Tardif V, Ghelima K, et al. A novel angiotensin II type 1 receptor-associated protein induces cellular hypertrophy in rat vascular smooth muscle and renal proximal tubular cells. J Biol Chem. 2004;279(20):21109–21120. [DOI] [PubMed] [Google Scholar]
- 130.Kai H, Alexander RW, Ushio-Fukai M, Lyons PR, Akers M, Griendling KK. G-Protein binding domains of the angiotensin II AT1A receptors mapped with synthetic peptides selected from the receptor sequence. Biochem J. 1998;332 ( Pt 3):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Venema RC, Ju H, Venema VJ, et al. Angiotensin II-induced association of phospholipase Cgamma1 with the G-protein-coupled AT1 receptor. J Biol Chem. 1998;273(13):7703–7708. [DOI] [PubMed] [Google Scholar]
- 132.Esseltine JL, Dale LB, Ferguson SS. Rab GTPases bind at a common site within the angiotensin II type I receptor carboxyl-terminal tail: evidence that Rab4 regulates receptor phosphorylation, desensitization, and resensitization. Mol Pharmacol. 2011;79(1):175–184. [DOI] [PubMed] [Google Scholar]
- 133.Seachrist JL, Laporte SA, Dale LB, et al. Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J Biol Chem. 2002;277(1):679–685. [DOI] [PubMed] [Google Scholar]
- 134.Marrero MB, Venema VJ, Ju H, Eaton DC, Venema RC. Regulation of angiotensin II-induced JAK2 tyrosine phosphorylation: roles of SHP-1 and SHP-2. Am J Physiol. 1998;275(5 Pt 1):C1216–1223. [DOI] [PubMed] [Google Scholar]
- 135.Heymann JA, Subramaniam S. Expression, stability, and membrane integration of truncation mutants of bovine rhodopsin. Proc Natl Acad Sci U S A. 1997;94(10):4966–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pankevych H, Korkhov V, Freissmuth M, Nanoff C. Truncation of the A1 adenosine receptor reveals distinct roles of the membrane-proximal carboxyl terminus in receptor folding and G protein coupling. J Biol Chem. 2003;278(32):30283–30293. [DOI] [PubMed] [Google Scholar]
- 137.Gaborik Z, Mihalik B, Jayadev S, Jagadeesh G, Catt KJ, Hunyady L. Requirement of membrane-proximal amino acids in the carboxyl-terminal tail for expression of the rat AT1a angiotensin receptor. FEBS Lett. 1998;428(3):147–151. [DOI] [PubMed] [Google Scholar]
- 138.Zhou F, Filipeanu CM, Duvernay MT, Wu G. Cell-surface targeting of alpha2-adrenergic receptors -- inhibition by a transport deficient mutant through dimerization. Cell Signal. 2006;18(3):318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nufer O, Kappeler F, Guldbrandsen S, Hauri HP. ER export of ERGIC-53 is controlled by cooperation of targeting determinants in all three of its domains. J Cell Sci. 2003;116(Pt 21):4429–4440. [DOI] [PubMed] [Google Scholar]
- 140.Nufer O, Guldbrandsen S, Degen M, et al. Role of cytoplasmic C-terminal amino acids of membrane proteins in ER export. J Cell Sci. 2002;115(Pt 3):619–628. [DOI] [PubMed] [Google Scholar]
- 141.Otte S, Barlowe C. The Erv41p-Erv46p complex: multiple export signals are required in trans for COPII-dependent transport from the ER. EMBO J. 2002;21(22):6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dominguez M, Dejgaard K, Fullekrug J, et al. gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J Cell Biol. 1998;140(4):751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273(5280):1396–1399. [DOI] [PubMed] [Google Scholar]
- 144.Dong C, Yang L, Zhang X, et al. Rab8 interacts with distinct motifs in alpha2B- and beta2-adrenergic receptors and differentially modulates their transport. J Biol Chem. 2010;285(26):20369–20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mozsolits H, Unabia S, Ahmad A, Morton CJ, Thomas WG, Aguilar MI. Electrostatic and hydrophobic forces tether the proximal region of the angiotensin II receptor (AT1A) carboxyl terminus to anionic lipids. Biochemistry. 2002;41(24):7830–7840. [DOI] [PubMed] [Google Scholar]
- 146.Kamimori H, Unabia S, Thomas WG, Aguilar MI. Evaluation of the membrane-binding properties of the proximal region of the angiotensin II receptor (AT1A) carboxyl terminus by surface plasmon resonance. Anal Sci. 2005;21(2):171–174. [DOI] [PubMed] [Google Scholar]
- 147.Hirst DJ, Lee TH, Pattenden LK, Thomas WG, Aguilar MI. Helix 8 of the angiotensin- II type 1A receptor interacts with phosphatidylinositol phosphates and modulates membrane insertion. Sci Rep. 2015;5:9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee DK, Lanca AJ, Cheng R, et al. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem. 2004;279(9):7901–7908. [DOI] [PubMed] [Google Scholar]
- 149.Lu D, Yang H, Shaw G, Raizada MK. Angiotensin II-induced nuclear targeting of the angiotensin type 1 (AT1) receptor in brain neurons. Endocrinology. 1998;139(1):365–375. [DOI] [PubMed] [Google Scholar]
- 150.Zhu S, Zhang MX, Davis JE, et al. A single mutation in helix 8 enhances the angiotensin II type 1a receptor transport and signaling. Cellular Signalling. 2015;27(12):2371–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sevier CS, Weisz OA, Davis M, Machamer CE. Efficient export of the vesicular stomatitis virus G protein from the endoplasmic reticulum requires a signal in the cytoplasmic tail that includes both tyrosine-based and di-acidic motifs. Mol Biol Cell. 2000;11(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nishimura N, Balch WE. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277(5325):556–558. [DOI] [PubMed] [Google Scholar]
- 153.Nishimura N, Bannykh S, Slabough S, et al. A di-acidic (DXE) code directs concentration of cargo during export from the endoplasmic reticulum. J Biol Chem. 1999;274(22):15937–15946. [DOI] [PubMed] [Google Scholar]
- 154.Zuzarte M, Rinne S, Schlichthorl G, Schubert A, Daut J, Preisig-Muller R. A di-acidic sequence motif enhances the surface expression of the potassium channel TASK-3. Traffic. 2007;8(8):1093–1100. [DOI] [PubMed] [Google Scholar]
- 155.Wang X, Matteson J, An Y, et al. COPII-dependent export of cystic fibrosis transmembrane conductance regulator from the ER uses a di-acidic exit code. J Cell Biol. 2004;167(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Arakaki AKS, Pan WA, Lin H, Trejo J. The alpha-arrestin ARRDC3 suppresses breast carcinoma invasion by regulating G protein-coupled receptor lysosomal sorting and signaling. J Biol Chem. 2018;293(9):3350–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sposini S, Jean-Alphonse FG, Ayoub MA, et al. Integration of GPCR Signaling and Sorting from Very Early Endosomes via Opposing APPL1 Mechanisms. Cell Rep. 2017;21(10):2855–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dores MR, Lin H, N JG, Mendez F, Trejo J. The alpha-arrestin ARRDC3 mediates ALIX ubiquitination and G protein-coupled receptor lysosomal sorting. Mol Biol Cell. 2015;26(25):4660–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dores MR, Paing MM, Lin H, Montagne WA, Marchese A, Trejo J. AP-3 regulates PAR1 ubiquitin-independent MVB/lysosomal sorting via an ALIX-mediated pathway. Mol Biol Cell. 2012;23(18):3612–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276(49):45509–45512. [DOI] [PubMed] [Google Scholar]
- 162.Verma R, Marchese A. The endosomal sorting complex required for transport pathway mediates chemokine receptor CXCR4-promoted lysosomal degradation of the mammalian target of rapamycin antagonist DEPTOR. J Biol Chem. 2015;290(11):6810–6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lauffer BE, Melero C, Temkin P, et al. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol. 2010;190(4):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Puthenveedu MA, Lauffer B, Temkin P, et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143(5):761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bowman SL, Shiwarski DJ, Puthenveedu MA. Distinct G protein-coupled receptor recycling pathways allow spatial control of downstream G protein signaling. J Cell Biol. 2016;214(7):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lanctot PM, Leclerc PC, Escher E, Guillemette G, Leduc R. Role of N-glycan-dependent quality control in the cell-surface expression of the AT1 receptor. Biochem Biophys Res Commun. 2006;340(2):395–402. [DOI] [PubMed] [Google Scholar]
- 167.Mizrachi D, Segaloff DL. Intracellularly located misfolded glycoprotein hormone receptors associate with different chaperone proteins than their cognate wild-type receptors. Mol Endocrinol. 2004;18(7):1768–1777. [DOI] [PubMed] [Google Scholar]
- 168.Morello JP, Salahpour A, Petaja-Repo UE, et al. Association of calnexin with wild type and mutant AVPR2 that causes nephrogenic diabetes insipidus. Biochemistry. 2001;40(23):6766–6775. [DOI] [PubMed] [Google Scholar]
- 169.Fan J, Perry SJ, Gao Y, Schwarz DA, Maki RA. A point mutation in the human melanin concentrating hormone receptor 1 reveals an important domain for cellular trafficking. Mol Endocrinol. 2005;19(10):2579–2590. [DOI] [PubMed] [Google Scholar]


