Abstract
Arthropod-borne viruses (arboviruses) have a long history of emerging to infect humans, but during recent decades, they have been spreading more widely and affecting larger populations. This is due to several factors, including increased air travel and uncontrolled mosquito vector populations. Emergence can involve simple spillover from enzootic (wildlife) cycles, as in the case of West Nile virus accompanying geographic expansion into the Americas; secondary amplification in domesticated animals, as seen with Japanese encephalitis, Venezuelan equine encephalitis, and Rift Valley fever viruses; and urbanization, in which humans become the amplification hosts and peridomestic mosquitoes, mainly Aedes aegypti, mediate human-to-human transmission. Dengue, yellow fever, chikungunya, and Zika viruses have undergone such urban emergence. We focus mainly on the latter two, which are recent arrivals in the Western Hemisphere. We also discuss a few other viruses with the potential to emerge through all of these mechanisms.
Keywords: arbovirus, chikungunya, Zika, yellow fever, dengue, mosquito
EVOLUTION AND EPIDEMIOLOGY OF EMERGING ARBOVIRUSES
Evolution
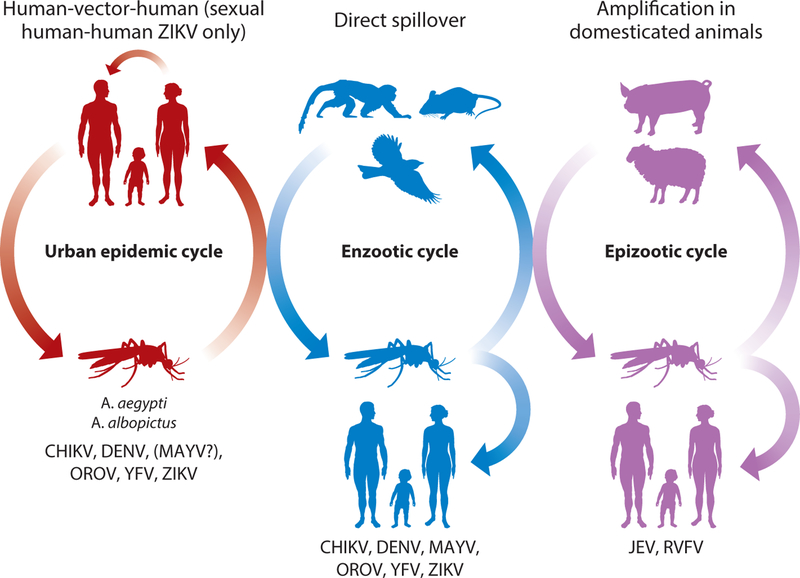
The emergence of arthropod-borne viruses (arboviruses) has been a threat to human health for centuries. Nearly all arboviruses are zoonotic, with ancestral transmission cycles in wildlife, and many of the most medically important arboviruses are transmitted by mosquito vectors (1). Among the mosquito-borne viruses, infection of humans occurs via three main mechanisms (Figure 1).
Figure 1.
Emergence mechanisms for arboviruses. Vectors are all mosquitoes except that urban transmission of OROV involves Culicoides spp. midges. Viruses with a history of urban emergence mostly use nonhuman primates as enzootic hosts and also infect people via direct spillover. Abbreviations: CHIKV, chikungunya virus; DENV, dengue virus; JEV, Japanese encephalitis virus; MAYV, Mayaro virus; OROV, Oropouche virus; RVFV, Rift Valley fever virus; YFV, yellow fever virus; ZIKV, Zika virus.
The first mechanism is direct spillover, where an enzootic or bridge vector transmits the virus from an enzootic host to a human. The second mechanism is amplification in domesticated animals, followed by spillover to humans; examples of this mechanism include Japanese encephalitis (JEV), an avian arbovirus that amplifies in swine, which often live in close proximity to people, and Rift Valley fever virus (RVFV), which amplifies in sheep, cattle, and other domesticated livestock. RVFV has never been detected outside of Africa. In 2016, JEV, historically found in Asia and Oceania, was detected for the first time in a human infection in Africa during a yellow fever epidemic (2). In the mechanisms of spillover and secondary livestock amplification, humans generally do not develop viremia sufficient in magnitude to contribute to ongoing transmission. The third mechanism is transition from the enzootic cycle to a human–mosquito– human cycle, where people serve as the amplification hosts and anthropophilic mosquitoes such as Aedes (Stegomyia) aegypti, A. (Steg.) albopictus, and others transmit the virus, often in urban settings. Arboviruses with the potential for urban spread are among the most important for public health.
We focus primarily on two of these viruses with a recent history of emergence into new geographic regions, especially in the New World: Zika virus (ZIKV) and chikungunya virus (CHIKV). We also discuss briefly two other viruses with a history of urbanization and spread, dengue virus (DENV) and yellow fever virus (YFV), as well as a few others with potential for interhuman transmission [Mayaro virus (MAYV) and Oropouche virus (OROV)] or for geographic spread ( JEV and RVFV).
The evolution of ZIKV (3) and CHIKV (4), like that of YFV (5), is believed to have occurred in sub-Saharan Africa in arboreal cycles involving nonhuman primates (NHPs) and sylvatic mosquito vectors in the genus Aedes (Figure 2). Although surveillance is limited, these cycles remain active in several parts of Africa with similar patterns of recent spread to other continents. ZIKV is believed to have spread to Asia many decades ago based on coalescent phylogenetic analyses as well as direct evidence of NHP seroprevalence in Asia as early as 1951 (6) and detection of ZIKV in A. aegypti collected in Malaysia in 1966 (7). In contrast, CHIKV is believed to have spread to Asia and the Americas centuries ago on sailing ships, where onboard transmission was probably mediated by A. aegypti and a susceptible crew and passengers (8). Outbreaks of YFV transmission in the Caribbean and continental Americas, as well as in Europe, are also well documented back to the sixteenth century, facilitated by the slave trade from Africa. However, among these three viruses plus DENV, only YFV is known to have initiated an enzootic NHP–mosquito cycle, transmitted within the Amazon, Araguaia, and Orinoco river basins of South America by Haemagogus and Sabethes spp. arboreal mosquitoes (5).
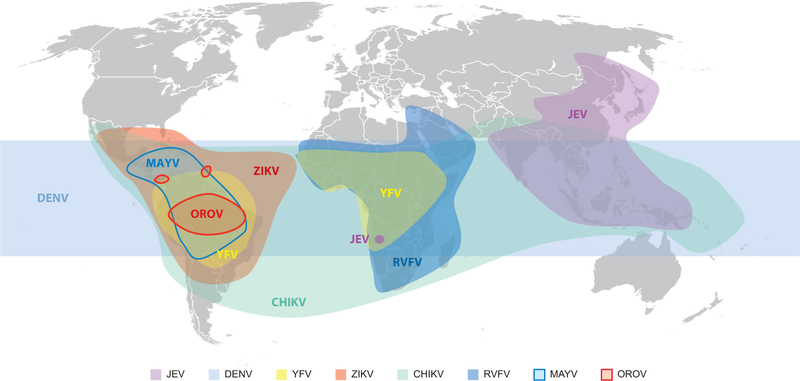
Figure 2.
Map showing the reported distributions of emerging arboviruses discussed in this review. Abbreviations: CHIKV, chikungunya virus; DENV, dengue virus; JEV, Japanese encephalitis virus; MAYV, Mayaro virus; OROV, Oropouche virus; RVFV, Rift Valley fever virus; YFV, yellow fever virus; ZIKV, Zika virus.
Epidemiology
Although direct spillover infections from enzootic ZIKV and CHIKV have been documented sporadically in Africa, and small urban outbreaks have also occurred within the enzootic range (9, 10), the largest outbreaks have been detected outside Africa. The first ZIKV outbreak detected outside Africa occurred on Yap Island of Micronesia, where more than half of the ∼7,000 inhabitants were infected in 2007 via transmission by A. (Steg.) hensilli (11). Only ∼20% of persons diagnosed retrospectively recalled any disease, which was characterized as a mild febrile illness. Rash, fever, arthralgia, and conjunctivitis were common signs and symptoms. The first major outbreak began in French Polynesia in 2013; more than half of the ∼200,000 residents were believed to have been infected, and transmission by A. aegypti and A. (Steg.) polynesiensis was suspected (12).
CHIKV emergence from Africa during the modern scientific era has been traced phylogenetically to the spread of the virus about one century ago from eastern Africa (East/Central/South African, or ECSA, lineage) to South and Southeast Asia. The Asian lineage continues to circulate in the latter region, as well as more recently in Oceania and now the Americas (13). A second, modern emergence of the Indian Ocean lineage (IOL), also from eastern Africa, affected islands in the Indian Ocean Basin, followed by India, Southeast Asia, and temporarily Europe. Finally, a distinct ECSA strain spread directly to Brazil in 2014 (14), resulting in the current co-circulation of both ECSA and Asian lineage strains in South America. These outbreaks have all involved transmission by A. aegypti, except for certain IOL strains that have adapted through a series of envelope glycoprotein substitutions for efficient transmission by A. albopictus. Epistatic interactions driven in some cases by founder effects have limited the ability of the Asian/American lineage to adapt for efficient transmission by A. albopictus (15).
Although transmission by mosquitoes is probably the most common mode of both ZIKV and CHIKV transmission, the exceptions that have been reported can lead to severe disease outcomes. Sexual ZIKV transmission was first suspected in a scientist infected in Senegal, followed by infection of his spouse in a region of Colorado not considered permissive for mosquito transmission (16). Sexual transmission, primarily male-to-female, has been detected in many travelers infected in epidemics who subsequently transmitted the virus to sexual partners in regions of North America and Europe not permissive for mosquito transmission. These findings have been accompanied by detection of ZIKV RNA in semen up to six months after infection (17). More importantly, congenital infections of both ZIKV and CHIKV have been reported during recent outbreaks. The former leads to congenital Zika syndrome (CZS), characterized by microcephaly and other neurologic disease as well as a wide range of auditory, ocular, cognitive, and anatomical defects in roughly 5–30% of babies born to women infected during pregnancy. Maternal infections with CHIKV can also lead to vertical transmission. This occurs most commonly during birth if there is maternal viremia (18), but in utero infections that can be fatal have also been described in prospective cohort studies of pregnant women in Brazil (19).
Both ZIKV (20) and CHIKV infections (21) are associated with an increased risk for Guillain Barré syndrome (GBS). The risk has not been well quantified owing to diagnostic and surveillance challenges in the affected regions of recent outbreaks.
Clinical Disease and Pathogenesis
Most arbovirus infections share some common clinical features. First, their incubation is short, typically 3–10 days. Second, most cases are asymptomatic, with the notable exception of chikungunya [<15% asymptomatic cases (18)] and Zika [about half of cases from the ongoing Latin American outbreak are asymptomatic (22)]. Typically, arbovirus infections manifest with the following signs and symptoms:
-
■
fever and flu-like symptoms, possibly associated with rash (e.g., ZIKV, CHIKV), with arthralgia (e.g., arthritogenic CHIKV, MAYV), or with icterus (e.g., RVFV, YFV);
-
■
encephalitis or menencephalitis (e.g., OROV, YFV, and less commonly CHIKV and ZIKV);
-
■
hemorrhagic fever (e.g., RVFV).
The type I interferon host response is the cornerstone of arbovirus infection control, and antibody-based immunity is usually considered long-lasting (23).
Zika.
Zika is generally a self-limited disease in children and adults. Macular or maculopapular rash is noted in 90–100% of cases. Arthritis and arthralgia are reported in 65% of cases and are typically much less prominent in Zika than in chikungunya. Conjunctivitis, enlarged lymph nodes, and a flu-like syndrome with headache and myalgia are also noted in 45–60% of symptomatic cases (11, 24). Fever is mild and inconstant (28–65%) in Zika, unlike dengue. Mucosal bleeding and digestive symptoms are uncommon (<10% of symptomatic cases) (24). Lymphopenia is frequently reported (24). Two major complications occur: GBS in adults and fetal developmental defects (CZS).
ZIKV-associated GBS is reported in 0.24/1,000 infections. It is a rare axonal or demyelinating polyneuropathy with symmetric muscle weakness and diminished or absent osteomuscular reflexes observable shortly after onset (median interval six days). About 30% of patients require respiratory assistance (25). Associated mortality is estimated at 0–4%.
ZIKV is also a major teratogenic arbovirus, the only example so far in humans for the Flaviviridae family. CZS includes microcephaly, macular atrophy, and craniofacial and musculoskeletal lesions. Intra-uterine growth restriction and fetal losses are also reported (19). CZS is reported in 1–13% of children born in most locations to ZIKV-infected mothers (26–31). Fetal susceptibility to severe manifestations is maximal during the first trimester of pregnancy (19, 29). Brain lesions include ventriculomegaly, lyssencephaly, and calcifications, as well neuronal and glial cell necrosis at the subcortical–cortical transition, with T and B cell infiltrates in the subcortical white matter (32–34). ZIKV’s cell and tissue tropism accounts for the spectrum of signs. ZIKV is able to infect a wide array of cells and organs (35), including the skin (dermal fibroblasts, epidermal keratinocytes, immature dendritic cells) (36) and the eye (ganglion cells, bipolar neurons, optic nerve, cornea, and aqueous humor) (35). Infection of the semen, sperm, and testicles (Leydig cells, Sertoli cells, and spermatogonia) is likely responsible for sexual transmission (17, 37).
Whether GBS occurs as a consequence of direct viral infection (suggested by the short interval between rash and GBS) or results from an autoimmune, cross-reactive mechanism targeting neurons and glial cells (suggested by the positivity of antiganglioside antibodies in ZIKV-associated GBS) remains to be determined (25, 38, 39).
Teratogenic effects appear related to a unique pathophysiologic sequence among arboviruses: placental barrier crossing; infection of the extravillous cytotrophoblasts and resident Hofbauer cells, but not of the mature syncytiotrophoblast separating them from the maternal blood; dissemination to the fetus; and fetal brain invasion and infection of cortical brain progenitors (along with mature neurons and glial cells), leading to microcephaly (40–42). Mechanisms associated with fetal brain invasion remain unknown. AXL, a ZIKV coreceptor expressed on cortical progenitor cells, could account for this specific tropism (43). Finally, as ZIKV is also able to infect adult neural stem cells, long-term effects on neural plasticity, learning, and memory could be expected (39).
Chikungunya.
CHIKV is responsible for an acute infection whose hallmarks are high fever for 3–5 days followed by severe, typically symmetric polyarthralgia (18). The disease is named after a Makonde word meaning “that which bends up,” describing the posture of patients with joint pains. Periarticular edema is reported in 32–95% of cases (44). Nonspecific macular or maculopapular rash is noted in 40–75% (45). Digestive tract symptoms occur in 47% (46) and peripheral lymphadenopathy in 41% (44). Hemorrhagic manifestations are uncommon (18). Lymphopenia, thrombocytopenia, and elevated transaminases are frequently noted (44). The main complications reported are chronic joint pain, severe organ dysfunction and encephalitis in the elderly, and severe neonatal infection. Approximately 35% of patients report persisting or relapsing polyarthralgia, with chronic polyarthritis in half (47). Incapacitating symptoms can last months to years and are more frequently reported in patients older than 35 years or when symptoms persist four months after onset (46, 48). Older patients with comorbidities also develop potentially fatal complications including encephalitis, myocarditis, and acute kidney or liver failure (18). The case-fatality rate is globally estimated at ∼1/1,000, with deaths mainly occurring in patients over 75 years of age (49).
Intrapartum transmission from a viremic mother to her child can have a dramatic impact on infant outcome. Vertical infection is rarely detected before delivery but occurs in about half of mothers with viremia at delivery (50). Neonatal infection can lead to encephalitis in 50% of cases and to acute respiratory failure in 8%. It can also impact postnatal neurologic development; a lower median Development Quotient at two years of age is reported in infants with perinatal infection when compared to uninfected controls (50, 51).
CHIKV infects a wide array of tissues, including liver, spleen, muscle, and lymph nodes (18). Fibroblasts located in the dermis, joint capsules, muscle fascia, and muscle tendinous insertions are major viral targets, and infection of these components, which are rich in nociceptive nerve endings, probably accounts for the severity of pain (52). Chronic joint symptoms may be related to the persistence of infected cells and/or of viral antigens, triggering prolonged proinflammatory responses driven by interleukin-6 and granulocyte-macrophage colony-stimulating factor (53). Infected macrophages could be a source of virus persistence in chronic lesions, although this is unproven (54). Neurologic symptoms reported in older patients likely reflect viral meningeal, choroid plexus, and ependymal cell infections suggested by animal models (18, 55). In contrast to most New World alphaviruses, CHIKV is not truly neurotropic, as it does not infect neurons or brain blood vessels and does not induce detectable brain destruction. However, it does infect the brain envelope and is detectable in the cerebrospinal fluid of patients who undergo a lumbar puncture (18). The restriction of vertical transmission to the intrapartum period reflects the non-permissiveness of the human placenta, as confirmed from the study of placental samples and in in vitro, ex vivo, and animal models. Mother-to-child transmission requires conditions that are met only during the delivery of viremic mothers, when physiologic placenta breaches allow the mixing of infected maternal blood and fetal blood (52).
Mayaro fever.
MAYV, a close alphavirus relative of CHIKV, is responsible for a nonfatal acute fever with typically symmetric distal arthralgia that classifies it as an arthritogenic alphavirus. Joint edemas are evidenced in 20% of cases. Other symptoms include myalgia, reported in 74% of patients; skin rash in 67%; and peripheral adenomegaly in 53% (56). As with CHIKV, chronic joint pains are reported in ∼50% of patients (57). Hemorrhages are rarely reported (58).
Yellow fever.
Classical yellow fever is characterized by three clinical stages: nonspecific fever and flu-like symptoms; transient remission; and, in 15% of cases, the so-called period of intoxication that develops 3–6 days after onset, when viremia declines and the humoral immune response arises (59). This stage combines severe sepsis, liver dysfunction with icterus, renal dysfunction, hemorrhages, and myocarditis (60). Altered consciousness is frequently reported as a consequence of metabolic disorders rather than dissemination of the infection to the central nervous system, which is exceptional. Case-fatality rates in this stage range from 20% to 50% (61). Survivors report persisting fatigue for weeks. Hepatocytes are the primary site of viral replication, and viral infection directly accounts for liver, kidney, and myocardium dysfunction (59). Hemodynamic changes and hepatorenal syndrome also worsen renal failure. The hemorrhagic syndrome is multifactorial and is secondary to thrombopenia, altered liver synthesis of coagulation factors, and disseminated intravascular coagulation (60).
Dengue.
Dengue has been extensively described (62). Briefly, the infection follows three phases. The febrile phase features high-grade fever and flu-like signs and symptoms, including myalgia and inconstant macular rash, that last 3–7 days, after which most patients recover. The critical phase, mainly reported in children and young adults, is characterized by systemic vascular leakage with hemoconcentration, hypoproteinemia, ascites, and pleural effusion.The recovery phase can be associated with another rash.
The critical phase can lead to vascular collapse and shock. Skin and mucosal hemorrhages may also occur (62). Signs of impending deterioration must be carefully monitored by clinicians, especially in the time window from the febrile to critical phase. These signs are listed in the World Health Organization’s 2009 classification of “dengue with warning signs” (63). Host risk factors for severe dengue include young age, female sex, obesity, and genetic variants of the human major histocompatibility complex class I (64–66). Previous DENV infection increases the risk of severe infection with another DENV serotype, as preexisting, non-neutralizing antibodies facilitate infection of Fc receptor-bearing cells. This phenomenon is known as antibody-dependent enhancement (62).
Oropouche fever.
OROV, a bunyavirus, is responsible for Oropouche fever, an acute febrile illness that follows two phases: Fever and flu-like signs and symptoms, possibly associated with macular rash and conjunctivitis, are followed by milder recurrence in ∼60% of cases 1–10 days after recovery (67). Meningitis and skin and mucosal bleeding are rarely reported (68). Complete recovery is the rule, and fatal infections have not been reported.
Management and Development of Therapeutics
Because most complications attributed to arbovirus infections are directly virus associated, one could expect a major benefit from antiviral therapeutic development. However, there remains no specific, licensed anti–arbovirus agent. Current patient management is thus mostly supportive. Joint pain and myalgia often require analgesics and nonsteroidal drugs. GBS management requires careful monitoring of respiratory function, with ventilator support when appropriate, and intravenous immunoglobulins or plasma can be useful (69). Dengue fever management requires close monitoring and early identification of high-risk patients (62). Adequate fluid resuscitation is instrumental in the critical phase and should ideally be performed in experienced clinical facilities. Severe bleeding may require blood transfusion. Prophylactic platelet transfusions are not effective (70).
Passive immunotherapy is a promising approach for the management of neonates exposed to CHIKV. Human anti-CHIKV immunoglobulins purified from convalescent donors exhibit strong in vitro anti-CHIKV effects and strong protective effects in adult and neonatal mouse models (71). Such anti-CHIKV hyperimmune immunoglobulins are being evaluated in the prevention of mother-to-child CHIKV transmission in neonates born to viremic mothers (CHIKIVIG-01, clinical trial NCT02230163). Novel antiviral therapies are also being investigated. Drug repurposing strategies have identified potential inhibitors of Flaviviridae replication. Ivermectin is an antihelminthic drug that strongly inhibits YFV, DENV, and West Nile virus replication by targeting the NS3 helicase (72). A phase II/III clinical trial is now evaluating its efficacy and safety in Thai adults and children with dengue (NCT02045069). Daptomycin, mefloquine, and azithromycin inhibit in vitro ZIKV replication (73), and azithromycin also inhibits ZIKV-induced cytopathic effects in glial cell lines and human astrocytes (74). Azithromycin is considered safe for use during pregnancy but exhibits limited placental crossing, which will limit its antenatal use (75).
Drug screening strategies have identified BCX4430 as an adenosine nucleoside analog with antiviral effects against a wide array of emerging RNA viruses, including Ebola, Marburg, Middle East Respiratory syndrome-coronavirus (MERS-CoV), and severe acute respiratory syndrome-coronavirus (SARS-CoV) (76). Interestingly, BCX4430 also demonstrates strong in vitro efficacy against YFV, JEV, DENV-2, West Nile virus, and ZIKV, and inhibits ZIKV multiplication in a mouse model (77, 78). BCX4430 recently entered phase I human clinical studies in healthy volunteers with promising pharmacokinetics and tolerance (76, 77). Additional new approaches aim to identify host factors and pathways that are critical for viral replication and to identify putative inhibitors of these pathways as host-targeting antivirals. High-throughput screens using small interfering RNAs or CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9) have already identified new, druggable targets for arboviruses (79–81).
Vaccine Development
The first vaccine for an arboviral disease, the 17D yellow fever vaccine, was developed in 1937 and has been used extensively and successfully in Africa and the neotropics. However, despite its strong efficacy and low cost of manufacture, outbreaks continue to occur in these regions owing to inadequate vaccine coverage (82). Vaccines are also available to protect against JEV infection, but none has been licensed for MAYV, OROV, or RVFV.
Chikungunya vaccine development dates back to the 1970s, whereas development of Zika vaccines began only about 18 months ago but has advanced at an unprecedented pace. Nearly all platforms have been used for both vaccines, and two chikungunya vaccines recently completed phase I clinical trials: a virus-like particle vaccine (83) and a live-measles virus-vectored vaccine (84). Both were strongly immunogenic after 2–3 doses and are now in phase II trials. Among other recent chikungunya vaccines with extensive preclinical evaluation, a live-attenuated vaccine based on altered gene expression via a picornavirus internal ribosome entry site has been shown to protect mice and NHPs against all measures of disease, including fever and viremia, after a single dose (85). A vertebrate replication-defective chimera between CHIKV and the insect-specific alphavirus Eilat virus elicits similar protection in both models after a single vaccination (86). Among the >40 Zika vaccines published to date, DNA (87, 88), RNA (89), and inactivated virus versions (88) have begun clinical trials, and the first live-attenuated vaccine has been demonstrated safe and efficacious after a single dose in mice (90). However, vaccines for both viral diseases face uncertain futures owing to (a) potential limitations on the perceived markets after the peaks of the epidemics in the Americas, when many arboviral infections are typically misdiagnosed as dengue or other causes of acute febrile illness (91, 92), and (b) difficulties in planning for phase III efficacy trials during a postepidemic period when disease may become sporadic, as exemplified by CHIKV in Asia before 2007 (93). Also, with regard to ZIKV, concerns regarding the potential for interactions with immunity generated by other natural flavivirus infections as well as vaccines available for DENV, YFV, and JEV infections, and related fears of immune enhancement leading to more severe disease manifestations, will persist until these interactions are better understood. Furthermore, the association between ZIKV infection and GBS raises the concern that, if this manifestation results from autoimmune triggering, any ZIKV vaccine could carry a similar risk.
PROSPECTS FOR FUTURE OUTBREAKS AND CONTROL
The common thread of an enzootic cycle among these viruses renders control of future epidemics a Sisyphean task, for the simple reason that these cycles are remote, widespread, and not amenable to intervention. However, with effective vaccination and sustainable vector control programs, it is possible to control or even eliminate the urban (human) transmission cycles. For example, use of the live-attenuated YFV vaccine (17D strain), which has been administered since 1937, was instrumental in eliminating the urban transmission cycle throughout the Americas. However, as the current yellow fever outbreak in Brazil rages, it provides a reminder that when robust vaccination programs are not sustained and herd immunity wanes below a critical point, YFV emerges from the forest via spillover and causes severe human disease and mortality (94). Overall, the best current prospects for controlling most vector-borne diseases rely on reducing contact between the vector and susceptible humans, and in the case of Zika especially pregnant women and their partners, who represent the highest risk for severe disease.
Based on our experiences dating back to the mid-twentieth century, the most effective approach to reducing human–vector contact remains the elimination or reduction of mosquito populations. The campaign to eliminate A. aegypti in the Americas was spearheaded by the Pan American Health Organization in cooperation with Latin American governments, and relied heavily on the use of DDT (dichlorodiphenyltrichloroethane) and other persistent, highly toxic insecticides. Additionally, governments imposed major penalties on residents who failed to eliminate larval container habitats (standing water where A. aegypti could lay their eggs). Nowadays some of the insecticides used in that eradication campaign are considered environmentally unacceptable. Regrettably, over time government support and enforcement also waned and consequently the Americas have been fully reinfested with A. aegypti (95).
Several alternative approaches are considered environmentally acceptable, and if applied in a consistent and sustainable manner, these could in theory significantly contribute to the control and eventual elimination of mosquito populations. Such methods include the following:
Elimination of common household oviposition and larval sites. These include water containers ranging from large tanks used to store household water to used tires and other refuse that fill with rainwater and serve as larval habitats.
Community engagement, outreach, and personal responsibility supplemented by penalties for allowing larval development to occur on an individual’s property. Although this model has been applied in Singapore for years, it has achieved only modest success because one or a few properties in a given neighborhood can produce sufficient mosquitoes to sustain arbovirus transmission.
Application of larvicides and adulticide fumigation inside households to eliminate larval and adult mosquitoes, respectively. Fumigation may include application of residual insecticides with repellant activity (reviewed in 96).
Release of genetically modified male mosquitoes that express a dominant lethal gene, resulting in the death of all offspring from mating with wild females. This approach has been quite effective but has only been tested on relatively a small scale (97).
Release of A. aegypti harboring the endosymbiont bacterium Wolbachia. These mosquitoes can then either spread through natural populations and suppress arboviral transmission by interfering with replication in the mosquito, or males can mate with wild females to render the offspring sterile through a mechanism called cytoplasmic incompatibility. Controlled releases in Northern Australia (98), Vietnam (99), Brazil (100), and Colombia (101, 102) have been successful in introducing Wolbachia at high and stable rates of infection into natural populations. Potential limitations for approaches 4 and 5 are the need to release these mosquitoes over wide geographic ranges to overcome their limited flight range; the possibility that arboviruses will rapidly evolve resistance mechanisms; and the logistical, technical, and financial challenges of scaling up.
The use of lethal traps, which have been designed to be inexpensive, low-maintenance, and highly effective in reducing A. aegypti populations and transmission of CHIKV (103).
Footnotes
DISCLOSURE STATEMENT
S.C.W. holds patents for the attenuation of alphaviruses including chikungunya, and serves as a consultant for vaccine development for Sanofi-Pasteur, Moderna/Valera, Themis Biosciences, and GeoVax.
LITERATURE CITED
- 1.Weaver SC, Reisen WK. 2010. Present and future arboviral threats. Antivir. Res 85:328–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon-Loriere E, Faye O, Prot M, et al. 2017. Autochthonous Japanese encephalitis with yellow fever coinfection in Africa. N. Engl. J. Med 376:1483–85 [DOI] [PubMed] [Google Scholar]
- 3.Haddow AD, Schuh AJ, Yasuda CY, et al. 2012. Genetic characterization of zika virus strains: geographic expansion of the Asian lineage. PLOS Negl. Trop. Dis 6:e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers AM, Brault AC, Tesh RB, Weaver SC. 2000. Re-emergence of Chikungunya and O’nyongnyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J. Gene. Virol 81:471–79 [DOI] [PubMed] [Google Scholar]
- 5.Bryant JE, Holmes EC, Barrett AD. 2007. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLOS Pathog 3:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smithburn KC. 1954. Neutralizing antibodies against arthropod-borne viruses in the sera of long-time residents of Malaya and Borneo. Am. J. Hyg 59:157–63 [DOI] [PubMed] [Google Scholar]
- 7.Marchette NJ, Garcia R, Rudnick A. 1969. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg 18:411–15 [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB. 2015. Reappearance of chikungunya, formerly called dengue, in the Americas. Emerg. Infect. Dis 21(4):557–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grard G, Caron M, Mombo IM, et al. 2014. Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLOS Negl. Trop. Dis 8:e2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leroy EM, Nkoghe D, Ollomo B, et al. 2009. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg. Infect. Dis 15:591–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy MR, Chen TH, Hancock WT, et al. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med 360:2536–43 [DOI] [PubMed] [Google Scholar]
- 12.Musso D, Gubler DJ. 2016. Zika virus. Clin. Microbiol. Rev 29:487–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver SC, Forrester NL. 2015. Chikungunya: evolutionary history and recent epidemic spread. Antivir. Res 120:32–39 [DOI] [PubMed] [Google Scholar]
- 14.Nunes MR, Faria NR, de Vasconcelos JM, et al. 2015. Emergence and potential for spread of Chikungunya virus in Brazil. BMC Med 13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffey LL, Forrester N, Tsetsarkin K, et al. 2013. Factors shaping the adaptive landscape for arboviruses: implications for the emergence of disease. Future Microbiol 8:155–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foy BD, Kobylinski KC, Chilson Foy JL, et al. 2011. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis 17:880–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barzon L, Pacenti M, Franchin E, et al. 2016. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveill 21(32):1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver SC, Lecuit M. 2015. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med 372:1231–39 [DOI] [PubMed] [Google Scholar]
- 19.Brasil P, Pereira JP Jr., Raja Gabaglia C, et al. 2016. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report. N. Engl. J. Med 375(24):2321–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oehler E, Watrin L, Larre P, et al. 2014. Zika virus infection complicated by Guillain-Barre syndrome— case report, French Polynesia, December 2013. Euro Surveill 19(9):1–3 [DOI] [PubMed] [Google Scholar]
- 21.Wielanek AC, Monredon JD, Amrani ME, et al. 2007. Guillain-Barre syndrome complicating a Chikungunya virus infection. Neurology 69:2105–7 [DOI] [PubMed] [Google Scholar]
- 22.Gallian P, Cabie A, Richard P, et al. 2017. Zika virus in asymptomatic blood donors in Martinique. Blood 129:263–66 [DOI] [PubMed] [Google Scholar]
- 23.Nitatpattana N, Kanjanopas K, Yoksan S, et al. 2014. Long-term persistence of Chikungunya virus neutralizing antibodies in human populations of North Eastern Thailand. Virol. J 11:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brasil P, Calvet GA, Siqueira AM, et al. 2016. Zika virus outbreak in Rio de Janeiro, Brazil: clinical characterization, epidemiological and virological aspects. PLOS Negl. Trop. Dis 10:e0004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parra B, Lizarazo J, Jimenez-Arango JA, et al. 2016. Guillain-Barre syndrome associated with Zika virus infection in Colombia. N. Engl. J. Med 375:1513–23 [DOI] [PubMed] [Google Scholar]
- 26.Martines RB, Bhatnagar J, de Oliveira Ramos AM, et al. 2016. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet 388:898–904 [DOI] [PubMed] [Google Scholar]
- 27.Oliveira Melo AS, Malinger G, Ximenes R, et al. 2016. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet. Gynecol 47:6–7 [DOI] [PubMed] [Google Scholar]
- 28.Miranda-Filho Dde B, Martelli CM, Ximenes RA, et al. 2016. Initial description of the presumed congenital Zika syndrome. Am. J. Public Health 106:598–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cauchemez S, Besnard M, Bompard P, et al. 2016. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 387:2125–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, et al. 2016. Zika and the risk of microcephaly. N. Engl. J. Med 375:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honein MA, Dawson AL, Petersen EE, et al. 2017. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 317:59–68 [DOI] [PubMed] [Google Scholar]
- 32.Tang H, Hammack C, Ogden SC, et al. 2016. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18:587–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mlakar J, Korva M, Tul N, et al. 2016. Zika virus associated with microcephaly. N. Engl. J. Med 374(10):951–58 [DOI] [PubMed] [Google Scholar]
- 34.Culjat M, Darling SE, Nerurkar VR, et al. 2016. Clinical and imaging findings in an infant with Zika embryopathy. Clin. Infect. Dis 63:805–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miner JJ, Diamond MS. 2017. Zika virus pathogenesis and tissue tropism. Cell Host Microbe 21:134–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamel R, Dejarnac O, Wichit S, et al. 2015. Biology of Zika virus infection in human skin cells. J. Virol 89:8880–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansuy JM, Suberbielle E, Chapuy-Regaud S, et al. 2016. Zika virus in semen and spermatozoa. Lancet Infect. Dis 16:1106–7 [DOI] [PubMed] [Google Scholar]
- 38.Cao-Lormeau VM, Blake A, Mons S, et al. 2016. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387(10027):1531–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Saucedo-Cuevas L, Shresta S, Gleeson JG. 2016. The neurobiology of Zika virus. Neuron 92:949–58 [DOI] [PubMed] [Google Scholar]
- 40.Tabata T, Petitt M, Puerta-Guardo H, et al. 2016. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 20:155–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheridan MA, Yunusov D, Balaraman V, et al. 2017. Vulnerability of primitive human placental trophoblast to Zika virus. PNAS 114:E1587–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simoni MK, Jurado KA, Abrahams VM, et al. 2017. Zika virus infection of Hofbauer cells. Am. J. Reprod. Immunol 77(2):1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowakowski TJ, Pollen AA, Di Lullo E, et al. 2016. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell 18:591–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hochedez P, Jaureguiberry S, Debruyne M, et al. 2006. Chikungunya infection in travelers. Emerg. Infect. Dis 12:1565–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taubitz W, Cramer JP, Kapaun A, et al. 2007. Chikungunya fever in travelers: clinical presentation and course. Clin. Infect. Dis 45:e1–4 [DOI] [PubMed] [Google Scholar]
- 46.Borgherini G, Poubeau P, Staikowsky F, et al. 2007. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin. Infect. Dis 44:1401–7 [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Morales AJ, Cardona-Ospina JA, Fernanda Urbano-Garzon S, Sebastian Hurtado-Zapata J. 2016. Prevalence of post-Chikungunya infection chronic inflammatory arthritis: a systematic review and meta-analysis. Arthritis Care Res 68:1849–58 [DOI] [PubMed] [Google Scholar]
- 48.Schilte C, Staikowsky F, Couderc T, et al. 2013. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLOS Negl. Trop. Dis 7:e2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manimunda SP, Mavalankar D, Bandyopadhyay T, Sugunan AP. 2011. Chikungunya epidemic-related mortality. Epidemiol. Infect 139:1410–12 [DOI] [PubMed] [Google Scholar]
- 50.Torres JR, Falleiros-Arlant LH, Duenas L, et al. 2016. Congenital and perinatal complications of chikungunya fever: a Latin American experience. Int. J. Infect. Dis 51:85–88 [DOI] [PubMed] [Google Scholar]
- 51.Gerardin P, Samperiz S, Ramful D, et al. 2014. Neurocognitive outcome of children exposed to perinatal mother-to-child Chikungunya virus infection: the CHIMERE cohort study on Reunion Island. PLOS Negl. Trop. Dis 8:e2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Couderc T, Chretien F, Schilte C, et al. 2008. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLOS Pathog 4:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assuncao-Miranda I, Cruz-Oliveira C, Da Poian AT. 2013. Molecular mechanisms involved in the pathogenesis of alphavirus-induced arthritis. Biomed. Res. Int 2013:973516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva LA, Dermody TS. 2017. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J. Clin. Investig 127:737–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Couderc T, Lecuit M. 2009. Focus on Chikungunya pathophysiology in human and animal models. Microbes Infect. Inst. Pasteur 11:1197–205 [DOI] [PubMed] [Google Scholar]
- 56.Pinheiro FP, Freitas RB, Travassos da Rosa JF, et al. 1981. An outbreak of Mayaro virus disease in Belterra, Brazil. I. Clinical and virological findings. Am. J. Trop. Med. Hyg 30:674–81 [DOI] [PubMed] [Google Scholar]
- 57.Halsey ES, Siles C, Guevara C, et al. 2013. Mayaro virus infection, Amazon Basin region, Peru, 2010– 2013. Emerg. Infect. Dis 19:1839–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tasso de Oliveira Mota M, Ribeiro MR, Vedovello D, Lacerda Nogueira M. 2015. Mayaro virus: a neglected virus of the Americas. Future Virol 10:1109–22 [Google Scholar]
- 59.ter Meulen J, Sakho M, Koulemou K, et al. 2004. Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J. Infect. Dis 190:1821–27 [DOI] [PubMed] [Google Scholar]
- 60.Robertson SE, Hull BP, Tomori O, et al. 1996. Yellow fever: a decade of reemergence. JAMA 276:1157–62 [PubMed] [Google Scholar]
- 61.Jentes ES, Poumerol G, Gershman MD, et al. 2011. The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the informal WHO working group on geographic risk for yellow fever. Lancet Infect. Dis 11:622–32 [DOI] [PubMed] [Google Scholar]
- 62.Simmons CP, Farrar JJ, Nguyen VV, Wills B. 2012. Dengue. N. Engl. J. Med 366:1423–32 [DOI] [PubMed] [Google Scholar]
- 63.World Health Organization. 2009. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control, New Edition Geneva: World Health Organ. [PubMed] [Google Scholar]
- 64.Anders KL, Nguyet NM, Chau NV, et al. 2011. Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg 84:127–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen TH, Nguyen TL, Lei HY, et al. 2005. Association between sex, nutritional status, severity of dengue hemorrhagic fever, and immune status in infants with dengue hemorrhagic fever. Am. J. Trop. Med. Hyg 72:370–74 [PubMed] [Google Scholar]
- 66.Khor CC, Chau TN, Pang J, et al. 2011. Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat. Genet 43:1139–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinheiro FP, Travassos da Rosa AP, Travassos da Rosa JF, et al. 1981. Oropouche virus. I. A review of clinical, epidemiological, and ecological findings. Am. J. Trop. Med. Hyg 30:149–60 [PubMed] [Google Scholar]
- 68.Mouraao MP, Bastos MS, Gimaqu JB, et al. 2009. Oropouche fever outbreak, Manaus, Brazil, 2007–2008. Emerg. Infect. Dis 15:2063–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van den Berg B, Walgaard C, Drenthen J, et al. 2014. Guillain-Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol 10:469–82 [DOI] [PubMed] [Google Scholar]
- 70.Lee TH, Wong JG, Leo YS, et al. 2016. Potential harm of prophylactic platelet transfusion in adult dengue patients. PLOS Negl. Trop. Dis 10:e0004576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Couderc T, Khandoudi N, Grandadam M, et al. 2009. Prophylaxis and therapy for Chikungunya virus infection. J. Infect. Dis 200:516–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mastrangelo E, Pezzullo M, De Burghgraeve T, et al. 2012. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J. Antimicrob. Chemother 67:1884–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barrows NJ, Campos RK, Powell ST, et al. 2016. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe 20(2):259–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Retallack H, Di Lullo E, Arias C, et al. 2016. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. PNAS 113:14408–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Briggs GG, Freeman RK, Yaffe SJ. 2008. Drugs in Pregnancy and Lactation New York: Lippincott Williams & Wilkins. 2144 pp. [Google Scholar]
- 76.Taylor R, Kotian P, Warren T, et al. 2016. BCX4430—a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J. Infect. Public Health 9:220–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Julander JG, Siddharthan V, Evans J, et al. 2017. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antivir. Res 137:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eyer L, Zouharova D, Sirmarova J, et al. 2017. Antiviral activity of the adenosine analogue BCX4430 against West Nile virus and tick-borne flaviviruses. Antivir. Res 142:63–67 [DOI] [PubMed] [Google Scholar]
- 79.Karlas A, Berre S, Couderc T, et al. 2016. A human genome-wide loss-of-function screen identifies effective chikungunya antiviral drugs. Nat. Commun 7:11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Savidis G, McDougall WM, Meraner P, et al. 2016. Identification of Zika virus and dengue virus dependency factors using functional genomics. Cell Rep 16:232–46 [DOI] [PubMed] [Google Scholar]
- 81.Zhang R, Miner JJ, Gorman MJ, et al. 2016. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 535:164–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barrett AD. 2017. Yellow fever live attenuated vaccine: a very successful live attenuated vaccine but still we have problems controlling the disease. Vaccine 35:5951–55 [DOI] [PubMed] [Google Scholar]
- 83.Chang LJ, Dowd KA, Mendoza FH, et al. 2014. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. Lancet 384:2046–52 [DOI] [PubMed] [Google Scholar]
- 84.Ramsauer K, Schwameis M, Firbas C, et al. 2015. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect. Dis 15:519–27 [DOI] [PubMed] [Google Scholar]
- 85.Roy CJ, Adams AP, Wang E, et al. 2014. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J. Infect. Dis 209:1891–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erasmus JH, Auguste AJ, Kaelber JT, et al. 2017. A chikungunya fever vaccine utilizing an insect-specific virus platform. Nat. Med 23:192–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dowd KA, Ko SY, Morabito KM, et al. 2016. Rapid development of a DNA vaccine for Zika virus. Science 354:237–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abbink P, Larocca RA, De La Barrera RA, et al. 2016. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 353:1129–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Richner JM, Himansu S, Dowd KA, et al. 2017. Modified mRNA vaccines protect against Zika virus infection. Cell 168:1114–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shan C, Muruato AE, Nunes BT, et al. 2017. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat. Med 23(6):763–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forshey BM, Guevara C, Laguna-Torres VA, et al. 2010. Arboviral etiologies of acute febrile illnesses in Western South America, 2000–2007. PLOS Negl. Trop. Dis 4:e787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Capeding MR, Chua MN, Hadinegoro SR, et al. 2013. Dengue and other common causes of acute febrile illness in Asia: an active surveillance study in children. PLOS Negl. Trop. Dis 7:e2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weaver SC, Osorio JE, Livengood JA, et al. 2012. Chikungunya virus and prospects for a vaccine. Expert Rev. Vaccines 11:1087–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilder-Smith A, Monath TP. 2017. Responding to the threat of urban yellow fever outbreaks. Lancet Infect. Dis 17:248–50 [DOI] [PubMed] [Google Scholar]
- 95.Gubler D, Vasilakis N. 2016. The arboviruses—quo vadis? In Arboviruses: Molecular Biology, Evolution and Control, ed. Gubler D, Vasilakis N, pp. 1–6. Norwich, UK: Caister Acad. [Google Scholar]
- 96.Vasilakis N, Weaver SC. 2017. Flavivirus transmission focusing on Zika. Curr. Opin. Virol 22:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wise de Valdez MR, Nimmo D, Betz J, et al. 2011. Genetic elimination of dengue vector mosquitoes. PNAS 108:4772–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ritchie SA, Townsend M, Paton CJ, et al. 2015. Application of wMelPop Wolbachia strain to crash local populations of Aedes aegypti. PLOS Negl. Trop. Dis 9:e0003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nguyen TH, Nguyen HL, Nguyen TY, et al. 2015. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasites Vectors 8:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Callaway E 2016. Rio fights Zika with biggest release yet of bacteria-infected mosquitoes. Nature 539:17–18 [DOI] [PubMed] [Google Scholar]
- 101.Aliota MT, Peinado SA, Velez ID, Osorio JE. 2016. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep 6:28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aliota MT, Walker EC, Uribe Yepes A, et al. 2016. The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLOS Negl. Trop. Dis 10:e0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barrera R, Acevedo V, Felix GE, et al. 2016. Impact of autocidal gravid ovitraps on chikungunya virus incidence in Aedes aegypti (Diptera: Culicidae) in areas with and without traps. J. Med. Entomol 54:387–95 [DOI] [PMC free article] [PubMed] [Google Scholar]