Abstract
Obsessive compulsive disorder (OCD) is a common psychiatric illness and significant research has been ongoing to understand its neurobiological basis. Neuroimaging studies right from the 1980s have revealed significant differences between OCD patients and healthy controls. Initial imaging findings showing hyperactivity in the prefrontal cortex (mainly orbitofrontal cortex), anterior cingulate cortex and caudate nucleus led to the postulation of the cortico-striato-thalamo-cortical (CSTC) model for the neurobiology of OCD. However, in the last two decades emerging evidence suggests the involvement of widespread associative networks, including regions of the parietal cortex, limbic areas (including amygdala) and cerebellum. This narrative review discusses findings from structural [Magnetic Resonance Imaging (MRI), Diffusion Tensor Imaging(DTI)], functional [(functional MRI (fMRI), Single photon emission computed tomography (SPECT), Positron emission tomography (PET), functional near-infrared spectroscopy (fNIRS)], combined structural and functional imaging studies and meta-analyses. Subsequently, we collate these findings to describe the neurobiology of OCD including CSTC circuit, limbic system, parietal cortex, cerebellum, default mode network and salience network. In future, neuroimaging may emerge as a valuable tool for personalised medicine in OCD treatment.
Keywords: Neurobiology, neuroimaging, obsessive–compulsive disorder
INTRODUCTION
Obsessive–compulsive disorder (OCD) is a common psychiatric illness characterized by recurrent, intrusive thoughts, images, or impulses and repetitive acts or mental rituals. Besides schizophrenia, it is one of the main psychiatric disorders wherein significant differences between patients and healthy controls have been observed from neuroimaging studies dating back to the 1980s. Based on the hyperactivity in the prefrontal cortex (PFC) (mainly orbitofrontal cortex [OFC]), anterior cingulate cortex (ACC), and caudate nucleus demonstrated in the initial studies, the cortico-striato-thalamo-cortical (CSTC) model of OCD neurobiology was postulated.[1] Most of the early studies focused on the CSTC loop; however, in the previous two decades, emerging evidence suggests the involvement of widespread associative networks including regions of the parietal cortex, limbic areas, and cerebellum. In this narrative review, we discuss findings from structural (magnetic resonance imaging [MRI], diffusion tensor imaging [DTI]), functional (functional MRI [fMRI], single photon emission computed tomography [SPECT], positron emission tomography [PET], functional near-infrared spectroscopy [fNIRS]), and combined structural and functional imaging studies and subsequently collate these to describe the neurobiology of OCD. The study designs include (a) cross-sectional studies, (b) baseline and posttreatment imaging studies, and (c) symptom provocation paradigms comparing OCD patients and healthy controls. We have included data from only adult OCD patients for ease of understanding because child and adolescent imaging findings differ in a larger meta-analysis, suggesting neurodevelopment-related changes across age in OCD.[2]
STRUCTURAL IMAGING STUDIES (MAGNETIC RESONANCE IMAGING AND DIFFUSION TENSOR IMAGING)
MRI studies in OCD were initially region of interest based (investigate areas previously implicated in OCD), and later, voxel-based morphometry (VBM) was used (whole-brain analysis approach) for studying volumetric differences. Recent studies have used surface-based analysis to investigate cortical surface area and thickness. White matter (WM) changes have been studied using DTI. DTI is based on quantification of the diffusion of water molecules in the brain tissue and provides information about WM integrity and microstructure.[3] Fractional anisotropy (FA) is the main measure from DTI, and it depends on water molecule diffusivity which in turn depends on fiber density, axonal diameter, myelin sheath thickness, and fiber directionality. Decreased FA is suggestive of change in WM integrity.[3] A summary of MRI studies is shown in Table 1.
Table 1.
Magnetic resonance imaging studies in obsessive-compulsive disorder
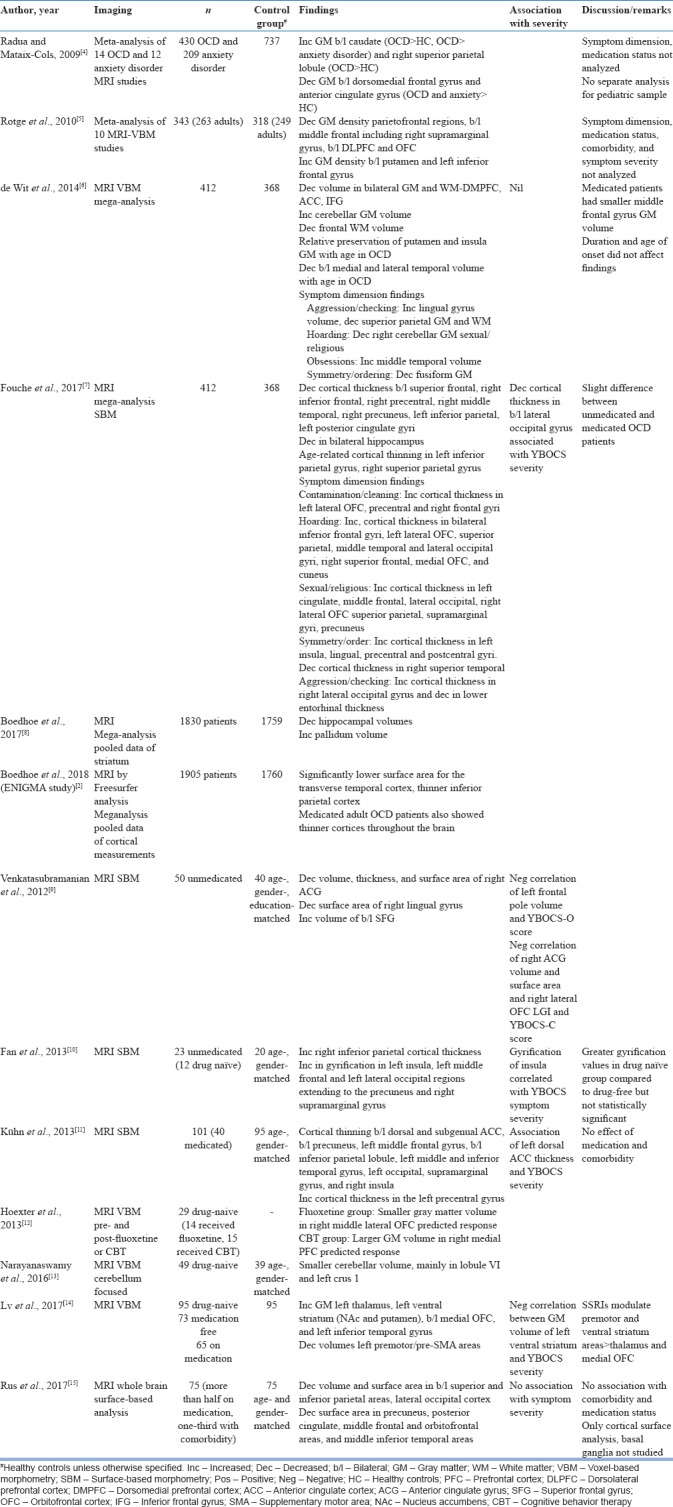
The largest mega-analytical study Enhancing NeuroImaging and Genetics by Meta-Analysis (ENIGMA) by pooling data of cortical thickness and surface area in 1905 patients and 1760 healthy controls showed lower surface area in the temporal cortex and thinner inferior parietal cortex, implicating areas other than those prevalent in the CSTC model.[2] Another mega-analysis comprising 412 OCD patients and 368 healthy controls showed similar findings of decreased parietal and temporal cortical thicknesses and cortical thinning in the right dorsolateral PFC (DLPFC), left posterior cingulate cortex (PCC), and bilateral hippocampi.[7] Other meta-analysis of VBM studies showed decreased gray matter (GM) in bilateral OFCs[4,5,6] and ACC[4,5,6] and increased GM in the basal ganglia (caudate,[4] putamen,[5] and pallidum[8]). Findings with regard to the parietal cortex were inconsistent with one meta-analysis showing increased volume[4] and the other showing decreased volume.[5] Increase in cerebellar GM was found in one meta-analysis.[6] In addition, investigation of age-related changes suggested relative preservation of putamen and insular GM and decrease in medial and lateral temporal cortical volume in OCD as compared to healthy controls with increasing age.[6] Differences with respect to symptom dimensions were reported in two mega-analyses.[6,7] Cortical thickness was increased in the left OFC[7] in contamination/cleaning, right OFC, left cingulate cortex, right parietal cortex,[7] and middle temporal cortex[6] in sexual/religious; right occipital[7] and lingual gyrus[6] in aggression/checking; and left insula, lingual, precentral, and postcentral gyrus[7] with decrease in fusiform GM[6] in symmetry/order symptom dimension. Most studies had recruited patients across heterogeneous symptom dimensions, contributory to the variability in imaging findings making comparisons difficult. In addition, other inclusion/exclusion criteria such as presence or absence of comorbidity, medication status (drug-naive, drug-free >1 month), and varied ages of onset of symptoms are additional confounding factors. Association of symptom severity with imaging has also been investigated; some studies have shown no relation[6,15] while others have shown that Y-BOCS (Yale Brown Obsessive Compulsive Scale) severity scores have a positive correlation with increase in left dorsal ACC thickness,[11] decrease in cortical thickness in bilateral occipital gyri,[7] and negative correlation with GM volume of left ventral striatum,[14] left frontal pole volume, right anterior cingulate gyrus volume and surface area, and right OFC gyrification.[9]
Findings from DTI studies in OCD are shown in Table 2.
Table 2.
Diffusion tensor imaging studies and combined magnetic resonance imaging+diffusion tensor imaging studies in obsessive-compulsive disorder
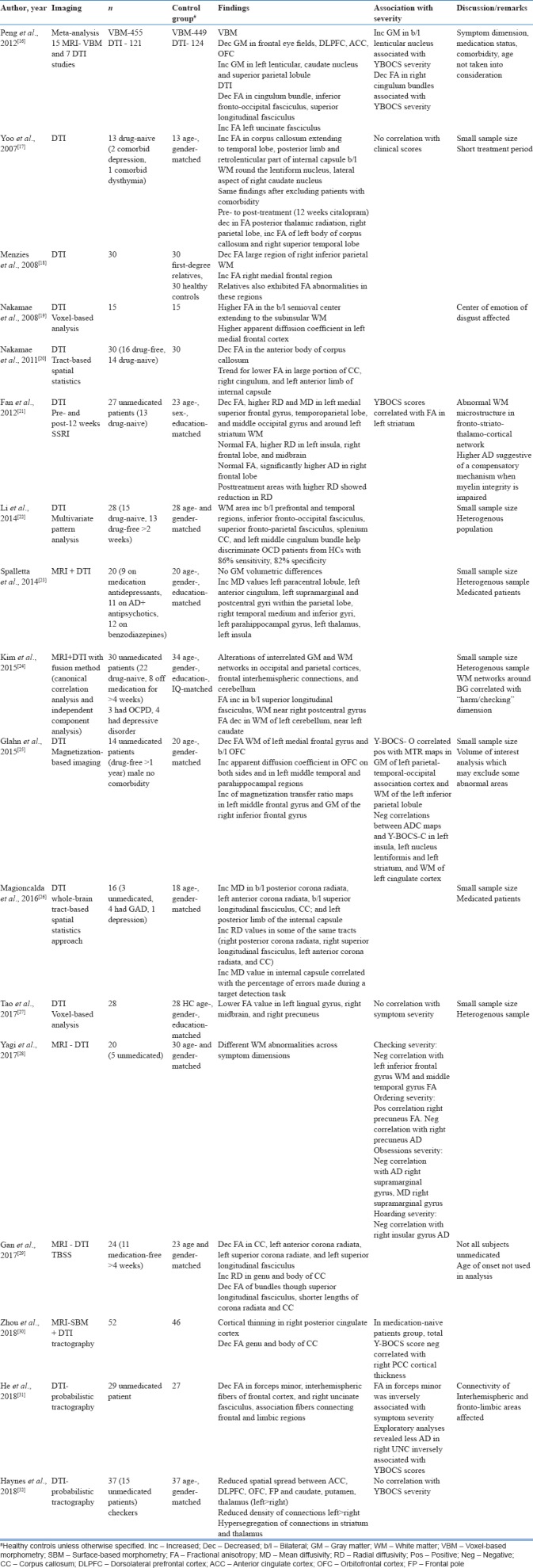
A meta-analysis of DTI studies in OCD revealed WM abnormalities in ACC and OFC,[33] similar to MRI studies. In DTI studies, positive correlations have been found between symptom severity, decreased FA in the right cingulum bundle[16] and forceps minor,[31] decreased axial diffusivity in the uncinate fasciculus, and increased FA in the left striatum[21] and left inferior parietal lobule;[25] whereas negative correlations with apparent diffusion coefficient of left insula, left lentiform nucleus, and left cingulate cortex.[25]
Among DTI studies, symptom dimension and symptom severity have been analyzed by Yagi et al., 2017;[28] checking severity showed negative correlation with left inferior frontal gyrus WM and middle temporal gyrus FA, and ordering severity showed positive correlation with the right precuneus FA and negative correlation with right precuneus axial diffusivity. Overall, obsessional severity showed negative correlation with axial diffusivity and mean diffusivity in the right supramarginal gyrus.[28] Kim et al., 2015[24] using a combined multimodal fusion analysis of structural MRI and DTI found that WM networks around the basal ganglia were associated with the symptom dimension of checking.
MAGNETIC RESONANCE SPECTROSCOPY
Proton magnetic resonance spectroscopy (1H-MRS) allows in vivo quantification of specific neurochemicals in different brain regions. Using magnetic field and a brief, tuned radiofrequency pulse, MRS generates resonance signals from hydrogen nuclei (protons) in neurochemical molecules, yielding a magnetic resonance spectrum; each molecule has a unique peak and the strength of each resonance reflects the molecule's concentration. N-acetyl aspartate (NAA), a marker of neuronal integrity, is the most widely reported neurochemical; others include creatine + phosphocreatine (total Cr), choline-containing compounds, myo-inositol, glutathione, lactate, and the amino acids – glutamate, glutamine, and γ-aminobutyric acid.[34]
MRS studies in OCD have targeted different parts of the CSTC circuit. Details of MRS studies in OCD are mentioned in Table 3.
Table 3.
Magnetic resonance spectroscopy and combined magnetic resonance spectroscopy+functional magnetic resonance imaging studies
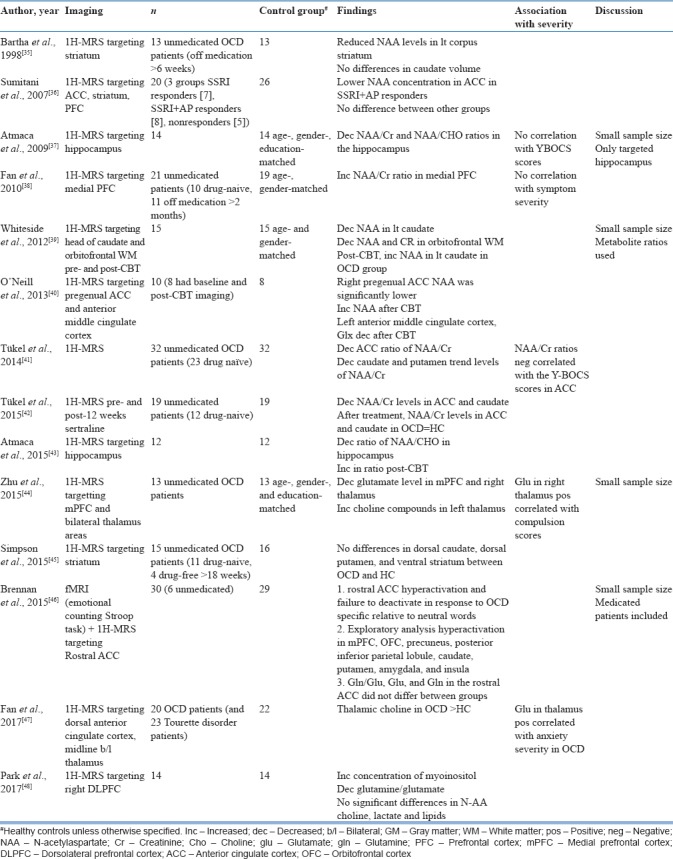
Studies targeting cortical regions have found increased NAA/Cr[38] and decreased glutamate[44] in the medial PFC, decreased glutamate and increased myoinositol in the DLPFC,[48] and decreased NAA in the ACC,[40,41] which increased after medication.[40] An older study had slightly different findings, with lower NAA concentration being found in the ACC in responders to selective serotonin reuptake inhibitors (SSRIs) with antipsychotic augmentation as compared to healthy controls, while no such differences were seen between SSRI responders and nonresponders.[36]
Studies targeting the striatum found decreased NAA levels.[35,41] A pre- and post-medication study found decreased NAA/Cr levels in the ACC and caudate in OCD patients at baseline, which normalized after 12 weeks of medication.[42] Similar results were seen in the caudate nucleus after 12 weeks cognitive behaviour therapy.[39] However, negative results with no difference in striatum between OCD patients and healthy controls were found in another study.[45]
A few studies targeting the thalamus[44,47] found decreased glutamate levels in the right thalamus[44] and increased choline levels in the midline in bilateral thalamic regions.[47] Both studies showed a positive association of glutamate with Y-BOCS symptom severity.[44,47] Among the other regions studied, Atmaca et al., 2009 found decreased NAA/Cr in the hippocampus.[37]
Brennan et al., 2015 reported increased activation in the rostral ACC on fMRI during emotional counting Stroop paradigm; however, no glutamate/metabolite abnormalities were noted in the ACC on MRS.[46] A meta-analysis of 17 MRS studies in OCD found decreased NAA levels in the frontal cortex, but no significant change in the basal ganglia. Meta-regression revealed that NAA reduction in the medial PFC positively correlated with Y-BOCS symptom severity.[49]
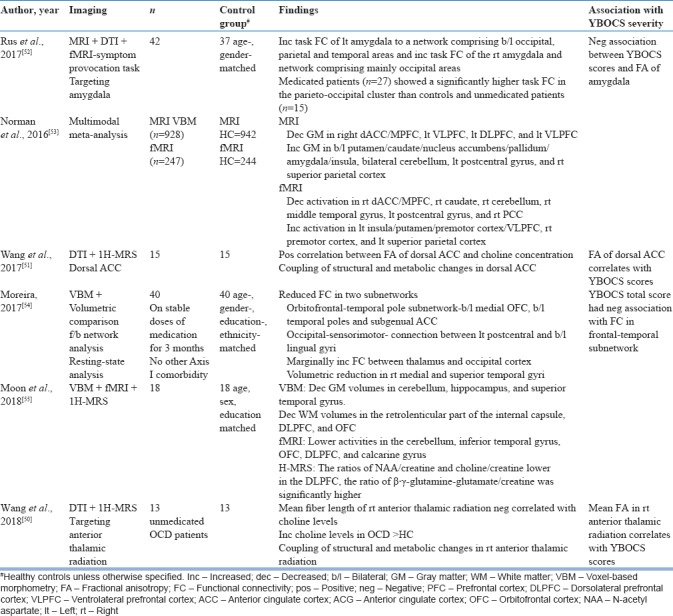
Two studies that combined DTI and MRS targeting the dorsal ACC and anterior thalamic radiation (ATR) found positive correlation between choline levels and FA in dorsal ACC and negative correlation between choline levels and fiber length in the right ATR[50,51] as shown in Table 4. In addition, FA in the ACC and right ATR correlated with Y-BOCS symptom severity.[50,51] The summary of combined structural and functional imaging studies (DTI + MRS) is shown in Table 4.
Table 4.
Combined structural and functional studies
FUNCTIONAL IMAGING
Functional imaging studies measure neural activity at rest (PET, SPECT, and fMRI) and during cognitive tasks (fMRI and fNIRS).
Positron emission tomography and single photon emission computed tomography
PET studies measure regional glucose metabolic rates which correlate with brain activity. Since the early 1980s, studies using PET have shown areas of hypermetabolism in OCD in different regions of the PFC.
Increase in metabolism has been reported in the left OFC,[56,57,58] bilateral ACC,[57,59] bilateral caudate,[56,60] left premotor cortex,[61] right caudate,[62] putamen,[59,60] and thalamus[59] and decreased metabolism in the DLPFC.[58] Symptom severity associations have been reported with hypermetabolism in the left OFC, bilateral PFC, and ACC.[57]
Posttreatment reduction in metabolism has been reported in the right OFC,[63] cingulate cortex,[59] and right caudate.[60,63,64] However, another study has reported increase in caudate metabolism following successful treatment with paroxetine or CBT.[65] This difference in the finding could be due to improvement in depressive symptoms as assessed on Beck's Depression Inventory in the OCD patients rather than an isolated reflection of change in OC symptoms.[65]
SPECT studies focus on blood flow, receptor availability, and drug uptake. SPECT studies in OCD have found reduced uptake in the OFC, ACC,[66] PCC[67] and temporal, parietal, and occipital cortices[67] and increased uptake in the cerebellum.[66]
Receptor availability studies have shown decreased striatal dopamine transporter[68] and decreased thalamic, hypothalamic,[68,69] and midbrain serotonin transporter.[68,70] However, another study showed increased midbrain serotonin transporter which was more pronounced in early-onset OCD.[71]
With respect to Y-BOCS symptom severity, positive correlations have been found with OFC,[66,72] DLPFC, lateral and medial temporal cortex, and inferior parietal lobule uptakes[72] and negative correlations with posterior cingulate uptake[66] and serotonin transporter availability in hypothalamic and thalamic areas.[68,69]
A recent meta-analysis of eight PET and six SPECT studies, which included 188 OCD patients in a pre- and post-treatment design, found that decrease in metabolism in the caudate, OFC, and thalamus on PET and decrease in blood flow in the caudate on SPECT were associated with improvement corresponding to normalization of the CSTC circuit overactivity.[73] A summary of PET and SPECT studies is provided in Table 5.
Table 5.
Positron emission tomography and single photon emission computed tomography studies
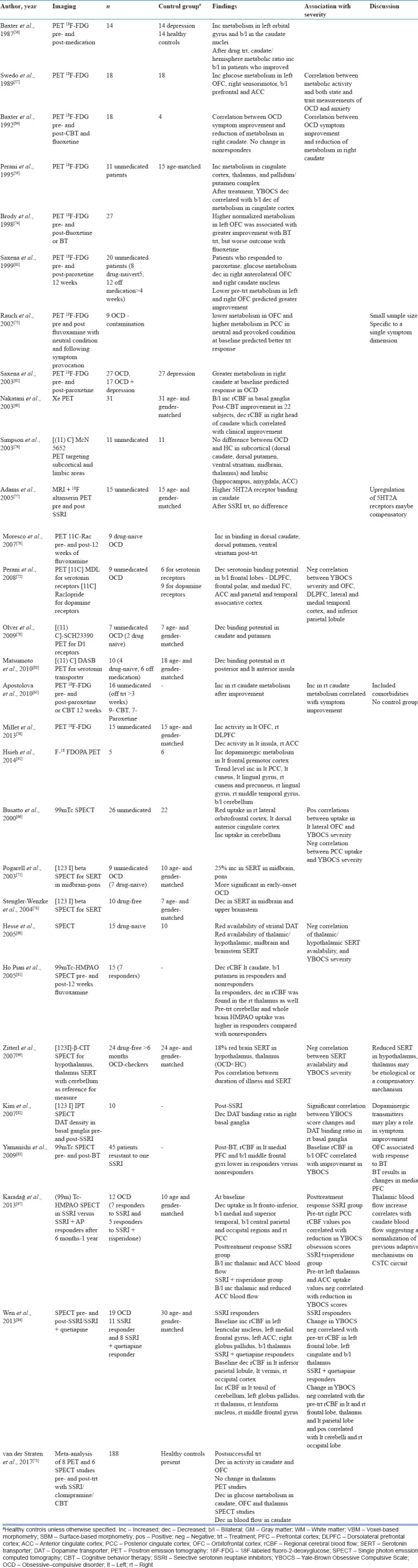
Despite a large number of PET and SPECT studies available, they had limitations such as small sample sizes and differences in inclusion/exclusion criteria with respect to comorbidity, heterogeneous symptom dimensions, and medication status. Moreover, the reference region for measurement varied in the SPECT studies, with some using cerebellum[69,70] and others using occipital cortex.[71] Due to these limitations, it is difficult to generalize the findings. However, many studies have investigated pre- and post-treatment changes, rendering them a useful tool to understand the neurobiology of OCD including identifying regions for treatment modulation.
Functional magnetic resonance imaging
fMRI is based on blood oxygen level dependence response in different brain regions and assessment of changes in activation and connectivity. fMRI acquisition is commonly done in the resting state, during symptom provocation, and along with cognitive tasks. There are two types of resting-state fMRI studies in OCD: (a) hypothesis-driven, seed-based analyses, based on local abnormalities mainly focusing on the frontostriatal circuit, and (b) hypothesis-free, data-driven analyses, based on global abnormalities. A summary of fMRI studies is shown in Table 6.
Table 6.
Functional magnetic resonance imaging studies
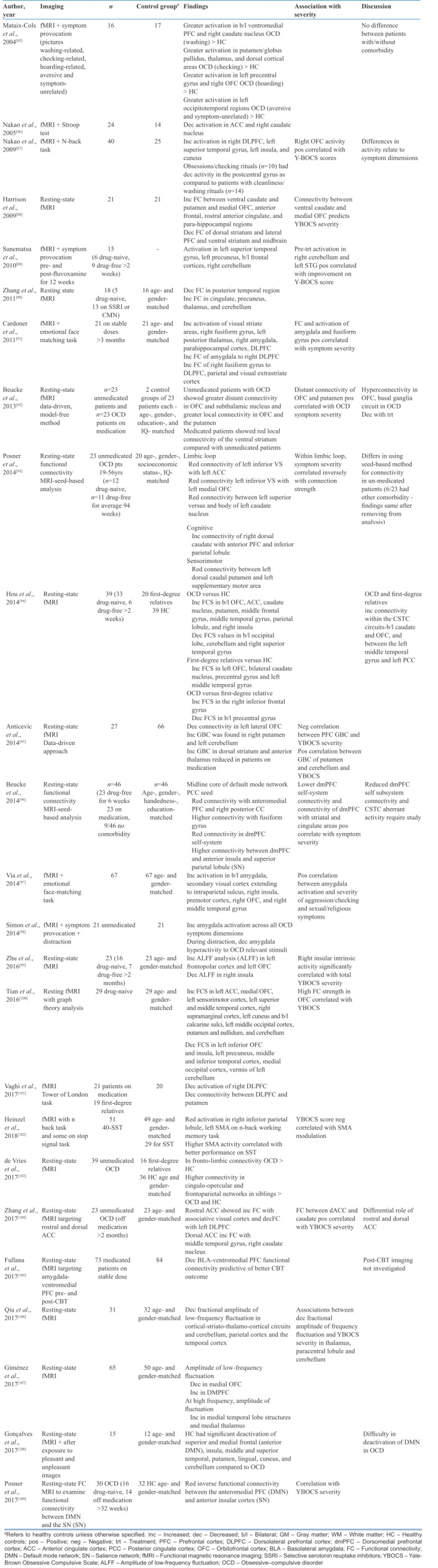
Resting-state fMRI studies have found increased functional connectivity between the caudate, putamen, and OFC, and ACC and parahippocampal areas.[88,90,92,93,100,103,104] In addition, the occipital cortex,[95,100] cerebellum,[95,100] and thalamic[90] connectivity with the striatum has been found to be increased. Decreased functional connectivity has been found within the OFC,[95] cerebellum,[94] and occipital areas.[94] Decreased functional connectivity has also been found between the dorsal striatum and lateral PFC,[88] ventral striatum and midbrain,[88] and posterior temporal region.[90,94] Symptom severity has been positively associated with connectivity in the OFC,[92,100] ACC,[104] putamen,[92,95] cerebellar region,[95] and lower dorsomedial PFC (DMPFC) intrasystem connectivity and the connectivity of DMPFC with striatal and cingulate areas.[96]
A symptom provocation fMRI study revealed greater activation in the ventromedial PFC (VMPFC) and caudate in washers; putamen, thalamus, and dorsal cortical areas in checkers; and left precentral gyrus and right OFC in hoarders.[85] Symptom provocation tasks and emotional processing tasks have found amygdala activation in OCD[91,97,98] with aggression/checking and sexual/religious symptom dimensions showing maximum correlation.[97]
Studies comparing activation on cognitive tasks in OCD patients and healthy controls have revealed differences in CSTC circuits as well as cerebellum and parietal areas. On the Stroop task, OCD patients showed decreased activation in the right ACC,[86,110] right caudate,[86] and right cerebellum.[110] On the n-back working memory task, increased activation in the right DLPFC, left superior temporal gyrus, left insula, and cuneus[87] and reduced activation in the right inferior parietal lobule and left supplementary motor area (SMA)[102] have been observed. On the Tower of London task, decreased activation of the right DLPFC and decreased connectivity between DLPFC and putamen were seen in OCD compared to healthy controls.[101] Symptom severity was found to be associated with right OFC activity[87] and SMA modulation.[102]
Newer fMRI studies have measured the amplitude of low-frequency brain fluctuations (aLFF) in various brain regions in OCD[30,106,107] and found the aLFF to be decreased in the OFC[107,111] and occipital and parietal regions[111] and increased in DMPFC[107] and temporal regions.[111]
A recent meta-analysis of 18 whole-brain resting-state fMRI studies with 541 patients and 572 healthy controls compared functional connectivity. In OCD, decreased connectivity within the frontoparietal and salience networks; between the salience, frontoparietal, and default-mode networks; and general dysconnectivity (no specific increase/decrease of connectivity) within the default-mode, frontoparietal, and salience networks were found.[112]
Pre- and post-medication-related fMRI studies have been summarized in Table 7.
Table 7.
Functional magnetic resonance imaging studies pre- and post-treatment
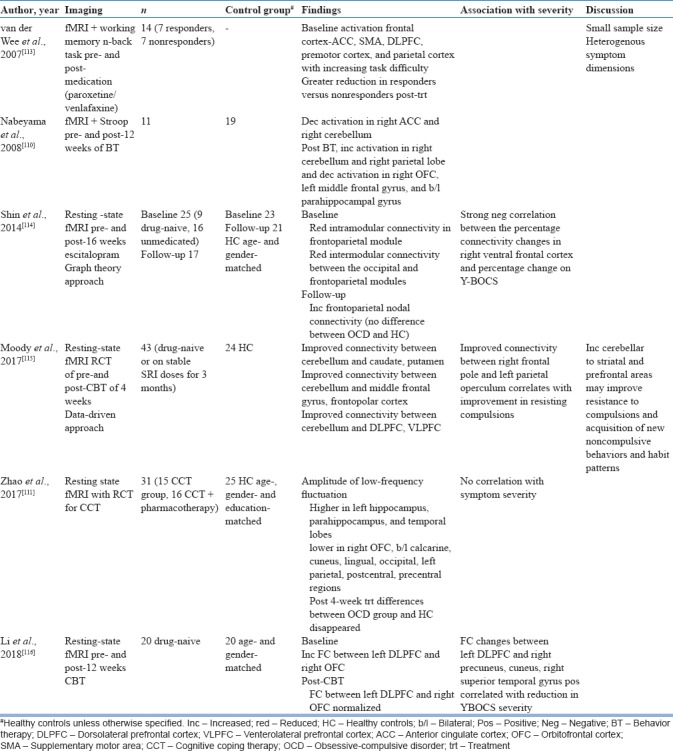
These have shown postmedication increase in frontoparietal connectivity;[114] decrease in connectivity of the ventral striatum;[92] and decrease in activation of the ACC, SMA, DLPFC, and parietal cortex on working memory task.[113] Predictors of improvement with medication included pretreatment activation of the left superior temporal cortex and right cerebellum on symptom provocation task.[89] Studies investigating fMRI changes post-CBT have found improved connectivity between the cerebellum and widespread areas in the caudate, putamen, frontopolar cortex, DLPFC, and VLPFC[115] and normalization of increased left DLPFC and right OFC connectivity.[116] Predictors of improvement following CBT included baseline decreased basolateral amygdala (BLA)-VMPFC functional connectivity.[105]
Functional near infra-red spectroscopy
NIRS is a new optical imaging modality which is portable and easy to use. It is based on the principle of neurovascular coupling and measures oxygenated and deoxygenated hemoglobin levels in brain areas to determine brain activity.[117] In fNIRS, NIRS is combined with cognitive/behavioral tasks. Two fNIRS-based studies in 20 and 12 adult OCD patients have been published.[118,119] These found decreased activation in the DLPFC during the verbal fluency task[118] and in the left lateral PFC on Stroop task[119] in OCD patients compared to healthy controls. A single case report has shown increased activation in the frontal and temporal areas on the verbal fluency task following improvement with escitalopram and CBT.[120] These studies are in line with studies in other modalities of imaging, implicating PFC areas in the neurobiology of OCD and their modulation, leading to improvement.
TOWARD UNDERSTANDING NEUROBIOLOGY FROM NEUROIMAGING
Neuroimaging findings dating back to the 1980s led to the initial understanding of the neurobiological basis of OCD. The CSTC model was proposed by Saxena et al., 1998.[1] It consists of an orbitofrontal loop, with projections from the OFC to the head of caudate nucleus and ventral striatum, connecting via the globus pallidus interna to the mediodorsal thalamus, and back from the thalamus to the OFC completing the loop. In addition, Saxena et al. proposed that OCD is mediated by an imbalance between the direct (excitatory, OFC-striatum-globus pallidus-thalamus-cortical) and indirect (inhibitory, DLPFC-striatum-globus pallidus-subthalamic nucleus-cortical) pathways within this circuit, which leads to hyperactivation across the OFC and thalamus.[1] Structural imaging studies supported the CSTC model with meta-analyses showing GM increase in the caudate and putamen[4,5,16] and decreased GM in the OFC.[4,5,16] Abnormalities in fractional anisotropy (reflecting white matter integrity) in the OFC,[25] ACC,[20,29] PFC,[22,24,25] and striatum[21] have been found in individual studies and meta-analyses.[33] In addition, functional studies have supported the CSTC model with a recent meta-analysis of PET and SPECT studies showing a decrease in metabolism in the caudate, OFC, and thalamus on PET and decrease in blood flow in the caudate on SPECT to be associated with improvement corresponding to normalization of the CSTC circuit overactivity.[73]
However, imaging findings have also implicated regions outside the CSTC loop, such as the parietal cortex, occipital cortex, and cerebellum in both structural[2,4,5,6,7,16] and functional studies; modified models of this circuit have been suggested postulated to include limbic regions (representative of the affective domain) connected to the OFC: hippocampus, anterior cingulate, and BLA.[58,66,88,91] Posterior brain regions, namely parietal, occipital, and cerebellar cortices (representative of the visuospatial domain) which are part of the posterior attention system involved in disengaging spatial attention, have also been implicated in OCD.[24,53]
The role of limbic system in OCD particularly the amygdala has been seen in neuroimaging studies.[46,52,53] Amygdala role in anxiety and negative affect has been well established and its association with compulsivity through connections with the corticostriatal system.[121] Recent studies show increased amygdala reactivity to emotional face stimuli and additionally increased activity in frontoparietal, OFCs, thalamus, and insula.[91,97,98] Findings from these studies suggest the limbic system activity is not altered during rest, but emotional stimuli lead to activation during exposure to OCD triggers.[122]
The other areas of limbic system overlap with default mode network (DMN) (comprising the posterior cingulate cortex, medial PFC, and lateral parietal lobule) and the salience network (SN) (comprising the dorsal ACC and anterior insular cortices). DMN and SN have been found to have reduced interconnectivity in OCD. These two networks have neural activity that is inversely correlated, and attentional processes can normally shift from internally focused introspective states mediated by the DMN to externally focused activity mediated by the SN.[123] In OCD, due to reduced inverse connectivity between the DMN and SN, there could be failure to de-active the DMN; subsequently, the introspective functioning may intrude upon the focused functioning required of the SN.[109] In addition, within the DMN, imaging studies have found increased PCC-VMPFC and decreased PCC-ACC and putamen functional connectivity in OCD.[96,124] PCC activity increases during attention to targets that are of high motivational value.[125] OCD patients may have increased responsiveness to symptom relevant stimuli due to a combination of increased activity in the direct excitatory CSTC pathway and decreased PCC activity in response to other rewarding stimuli, leading to increased activity with the DMN itself and subsequently greater focus on internal states and inability to suppress intrusive thoughts.[108] Dysfunction of posterior brain regions, especially the cerebellum, contributes to the pathogenesis of OCD through impaired inhibitory control; normalization of cerebellar function can occur with an improvement in OCD symptoms.
Our understanding of the neurobiology of OCD and its evolution over a neurodevelopmental perspective is expanding with ENIGMA consortium working group collecting data from 16 countries to generate neuroimaging meta-analyses of nearly 2000 patients across all age groups.[2,8] Such large-scale multicentric studies help in overcoming limitations of existing neuroimaging studies including small sample sizes and differences in inclusion/exclusion criteria with respect to comorbidity, heterogeneous symptom dimensions, and medication status and enable comparison of data. There is potential for neuroimaging evolve as a potential marker of response as well as illness phenotype.
Newer approaches involving machine-learning algorithms in neuroimaging may provide better insights into the future and develop personalized medicine. A recent study by Reggente et al., 2018 leveraged machine learning to assess pretreatment functional connectivity patterns within the default mode network and visual network and found these significantly predicted posttreatment OCD severity.[126] Further studies using machine learning in imaging are ongoing, and greater predictive power and understanding of neurobiology are likely in the near future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998;35:26–37. [PubMed] [Google Scholar]
- 2.Boedhoe PSW, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, et al. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: Findings from the ENIGMA obsessive-compulsive disorder working group. Am J Psychiatry. 2018;175:453–62. doi: 10.1176/appi.ajp.2017.17050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging. 2001;13:534–46. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 4.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 5.Rotge JY, Langbour N, Guehl D, Bioulac B, Jaafari N, Allard M, et al. Gray matter alterations in obsessive-compulsive disorder: An anatomic likelihood estimation meta-analysis. Neuropsychopharmacology. 2010;35:686–91. doi: 10.1038/npp.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchón JM, et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 2014;171:340–9. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- 7.Fouche JP, du Plessis S, Hattingh C, Roos A, Lochner C, Soriano-Mas C, et al. Cortical thickness in obsessive-compulsive disorder: Multisite mega-analysis of 780 brain scans from six centres. Br J Psychiatry. 2017;210:67–74. doi: 10.1192/bjp.bp.115.164020. [DOI] [PubMed] [Google Scholar]
- 8.Boedhoe PS, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, et al. Distinct subcortical volume alterations in pediatric and adult OCD: A Worldwide meta- and mega-analysis. Am J Psychiatry. 2017;174:60–9. doi: 10.1176/appi.ajp.2016.16020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatasubramanian G, Zutshi A, Jindal S, Srikanth SG, Kovoor JM, Kumar JK, et al. Comprehensive evaluation of cortical structure abnormalities in drug-naïve, adult patients with obsessive-compulsive disorder: A surface-based morphometry study. J Psychiatr Res. 2012;46:1161–8. doi: 10.1016/j.jpsychires.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Fan Q, Palaniyappan L, Tan L, Wang J, Wang X, Li C, et al. Surface anatomical profile of the cerebral cortex in obsessive-compulsive disorder: A study of cortical thickness, folding and surface area. Psychol Med. 2013;43:1081–91. doi: 10.1017/S0033291712001845. [DOI] [PubMed] [Google Scholar]
- 11.Kühn S, Kaufmann C, Simon D, Endrass T, Gallinat J, Kathmann N, et al. Reduced thickness of anterior cingulate cortex in obsessive-compulsive disorder. Cortex. 2013;49:2178–85. doi: 10.1016/j.cortex.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Hoexter MQ, Dougherty DD, Shavitt RG, D’Alcante CC, Duran FL, Lopes AC, et al. Differential prefrontal gray matter correlates of treatment response to fluoxetine or cognitive-behavioral therapy in obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2013;23:569–80. doi: 10.1016/j.euroneuro.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Narayanaswamy JC, Jose D, Kalmady SV, Agarwal SM, Venkatasubramanian G, Janardhan Reddy YC, et al. Cerebellar volume deficits in medication-naïve obsessive compulsive disorder. Psychiatry Res Neuroimaging. 2016;254:164–8. doi: 10.1016/j.pscychresns.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Lv Q, Wang Z, Zhang C, Fan Q, Zhao Q, Zeljic K, et al. Divergent structural responses to pharmacological interventions in orbitofronto-striato-thalamic and premotor circuits in obsessive-compulsive disorder. EBioMedicine. 2017;22:242–8. doi: 10.1016/j.ebiom.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rus OG, Reess TJ, Wagner G, Zaudig M, Zimmer C, Koch K, et al. Structural alterations in patients with obsessive-compulsive disorder: A surface-based analysis of cortical volume, surface area and thickness. J Psychiatry Neurosci. 2017;42:395–403. doi: 10.1503/jpn.170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng Z, Lui SS, Cheung EF, Jin Z, Miao G, Jing J, et al. Brain structural abnormalities in obsessive-compulsive disorder: Converging evidence from white matter and grey matter. Asian J Psychiatr. 2012;5:290–6. doi: 10.1016/j.ajp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Yoo SY, Jang JH, Shin YW, Kim DJ, Park HJ, Moon WJ, et al. White matter abnormalities in drug-naïve patients with obsessive-compulsive disorder: A diffusion tensor study before and after citalopram treatment. Acta Psychiatr Scand. 2007;116:211–9. doi: 10.1111/j.1600-0447.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 18.Menzies L, Williams GB, Chamberlain SR, Ooi C, Fineberg N, Suckling J, et al. White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. Am J Psychiatry. 2008;165:1308–15. doi: 10.1176/appi.ajp.2008.07101677. [DOI] [PubMed] [Google Scholar]
- 19.Nakamae T, Narumoto J, Shibata K, Matsumoto R, Kitabayashi Y, Yoshida T, et al. Alteration of fractional anisotropy and apparent diffusion coefficient in obsessive-compulsive disorder: A diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1221–6. doi: 10.1016/j.pnpbp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Nakamae T, Narumoto J, Sakai Y, Nishida S, Yamada K, Nishimura T, et al. Diffusion tensor imaging and tract-based spatial statistics in obsessive-compulsive disorder. J Psychiatr Res. 2011;45:687–90. doi: 10.1016/j.jpsychires.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Fan Q, Yan X, Wang J, Chen Y, Wang X, Li C, et al. Abnormalities of white matter microstructure in unmedicated obsessive-compulsive disorder and changes after medication. PLoS One. 2012;7:e35889. doi: 10.1371/journal.pone.0035889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li F, Huang X, Tang W, Yang Y, Li B, Kemp GJ, et al. Multivariate pattern analysis of DTI reveals differential white matter in individuals with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:2643–51. doi: 10.1002/hbm.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spalletta G, Piras F, Fagioli S, Caltagirone C, Piras F. Brain microstructural changes and cognitive correlates in patients with pure obsessive compulsive disorder. Brain Behav. 2014;4:261–77. doi: 10.1002/brb3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SG, Jung WH, Kim SN, Jang JH, Kwon JS. Alterations of gray and white matter networks in patients with obsessive-compulsive disorder: A Multimodal fusion analysis of structural MRI and DTI using mCCA+jICA. PLoS One. 2015;10:e0127118. doi: 10.1371/journal.pone.0127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glahn A, Prell T, Grosskreutz J, Peschel T, Müller-Vahl KR. Obsessive-compulsive disorder is a heterogeneous disorder: Evidence from diffusion tensor imaging and magnetization transfer imaging. BMC Psychiatry. 2015;15:135. doi: 10.1186/s12888-015-0535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magioncalda P, Martino M, Ely BA, Inglese M, Stern ER. Microstructural white-matter abnormalities and their relationship with cognitive dysfunction in obsessive-compulsive disorder. Brain Behav. 2016;6:e00442. doi: 10.1002/brb3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao J, Wang X, Zhong Z, Han H, Liu S, Wen S, et al. Alterations of white matter fractional anisotropy in unmedicated obsessive-compulsive disorder. Neuropsychiatr Dis Treat. 2017;13:69–76. doi: 10.2147/NDT.S123669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yagi M, Hirano Y, Nakazato M, Nemoto K, Ishikawa K, Sutoh C, et al. Relationship between symptom dimensions and white matter alterations in obsessive-compulsive disorder. Acta Neuropsychiatr. 2017;29:153–63. doi: 10.1017/neu.2016.45. [DOI] [PubMed] [Google Scholar]
- 29.Gan J, Zhong M, Fan J, Liu W, Niu C, Cai S, et al. Abnormal white matter structural connectivity in adults with obsessive-compulsive disorder. Transl Psychiatry. 2017;7:e1062. doi: 10.1038/tp.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou C, Xu J, Ping L, Zhang F, Chen W, Shen Z, et al. Cortical thickness and white matter integrity abnormalities in obsessive-compulsive disorder: A combined multimodal surface-based morphometry and tract-based spatial statistics study. Depress Anxiety. 2018;35:742–51. doi: 10.1002/da.22758. [DOI] [PubMed] [Google Scholar]
- 31.He X, Steinberg E, Stefan M, Fontaine M, Simpson HB, Marsh R, et al. Altered frontal interhemispheric and fronto-limbic structural connectivity in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2018;39:803–10. doi: 10.1002/hbm.23883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haynes WIA, Clair AH, Fernandez-Vidal S, Gholipour B, Morgiève M, Mallet L, et al. Altered anatomical connections of associative and limbic cortico-basal-ganglia circuits in obsessive-compulsive disorder. Eur Psychiatry. 2018;51:1–8. doi: 10.1016/j.eurpsy.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Piras F, Piras F, Caltagirone C, Spalletta G. Brain circuitries of obsessive compulsive disorder: A systematic review and meta-analysis of diffusion tensor imaging studies. Neurosci Biobehav Rev. 2013;37:2856–77. doi: 10.1016/j.neubiorev.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartha R, Stein MB, Williamson PC, Drost DJ, Neufeld RW, Carr TJ, et al. A short echo 1H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. Am J Psychiatry. 1998;155:1584–91. doi: 10.1176/ajp.155.11.1584. [DOI] [PubMed] [Google Scholar]
- 36.Sumitani S, Harada M, Kubo H, Ohmori T. Proton magnetic resonance spectroscopy reveals an abnormality in the anterior cingulate of a subgroup of obsessive-compulsive disorder patients. Psychiatry Res. 2007;154:85–92. doi: 10.1016/j.pscychresns.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Atmaca M, Yildirim H, Ozdemir H, Koc M, Ozler S, Tezcan E, et al. Neurochemistry of the hippocampus in patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2009;63:486–90. doi: 10.1111/j.1440-1819.2009.01993.x. [DOI] [PubMed] [Google Scholar]
- 38.Fan Q, Tan L, You C, Wang J, Ross CA, Wang X, et al. Increased N-acetylaspartate/creatine ratio in the medial prefrontal cortex among unmedicated obsessive-compulsive disorder patients. Psychiatry Clin Neurosci. 2010;64:483–90. doi: 10.1111/j.1440-1819.2010.02128.x. [DOI] [PubMed] [Google Scholar]
- 39.Whiteside SP, Abramowitz JS, Port JD. The effect of behavior therapy on caudate N-acetyl-l-aspartic acid in adults with obsessive-compulsive disorder. Psychiatry Res. 2012;201:10–6. doi: 10.1016/j.pscychresns.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 40.O’Neill J, Gorbis E, Feusner JD, Yip JC, Chang S, Maidment KM, et al. Effects of intensive cognitive-behavioral therapy on cingulate neurochemistry in obsessive-compulsive disorder. J Psychiatr Res. 2013;47:494–504. doi: 10.1016/j.jpsychires.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tükel R, Aydın K, Ertekin E, Özyıldırım SŞ, Taravari V. Proton magnetic resonance spectroscopy in obsessive-compulsive disorder: Evidence for reduced neuronal integrity in the anterior cingulate. Psychiatry Res. 2014;224:275–80. doi: 10.1016/j.pscychresns.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Tükel R, Aydın K, Ertekin E, Özyıldırım SŞ, Barburoğlu M. 1H-magnetic resonance spectroscopy in obsessive-compulsive disorder: Effects of 12 weeks of sertraline treatment on brain metabolites. Eur Arch Psychiatry Clin Neurosci. 2015;265:219–26. doi: 10.1007/s00406-014-0545-1. [DOI] [PubMed] [Google Scholar]
- 43.Atmaca M, Yildirim H, Yilmaz S, Caglar N, Mermi O, Gurok MG, et al. 1HMRS results of hippocampus in the patients with obsessive-compulsive disorder before and after cognitive behavioral therapy. Int J Psychiatry Clin Pract. 2015;19:285–9. doi: 10.3109/13651501.2015.1072220. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, Fan Q, Han X, Zhang H, Chen J, Wang Z, et al. Decreased thalamic glutamate level in unmedicated adult obsessive-compulsive disorder patients detected by proton magnetic resonance spectroscopy. J Affect Disord. 2015;178:193–200. doi: 10.1016/j.jad.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Simpson HB, Kegeles LS, Hunter L, Mao X, Van Meter P, Xu X, et al. Assessment of glutamate in striatal subregions in obsessive-compulsive disorder with proton magnetic resonance spectroscopy. Psychiatry Res. 2015;232:65–70. doi: 10.1016/j.pscychresns.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brennan BP, Tkachenko O, Schwab ZJ, Juelich RJ, Ryan EM, Athey AJ, et al. An examination of rostral anterior cingulate cortex function and neurochemistry in obsessive-compulsive disorder. Neuropsychopharmacology. 2015;40:1866–76. doi: 10.1038/npp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan S, Cath DC, van den Heuvel OA, van der Werf YD, Schöls C, Veltman DJ, et al. Abnormalities in metabolite concentrations in tourette's disorder and obsessive-compulsive disorder-A proton magnetic resonance spectroscopy study. Psychoneuroendocrinology. 2017;77:211–7. doi: 10.1016/j.psyneuen.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Park SE, Choi NG, Jeong GW. Metabolic abnormality in the right dorsolateral prefrontal cortex in patients with obsessive-compulsive disorder: Proton magnetic resonance spectroscopy. Acta Neuropsychiatr. 2017;29:164–9. doi: 10.1017/neu.2016.48. [DOI] [PubMed] [Google Scholar]
- 49.Aoki Y, Aoki A, Suwa H. Reduction of N-acetylaspartate in the medial prefrontal cortex correlated with symptom severity in obsessive-compulsive disorder: Meta-analyses of (1)H-MRS studies. Transl Psychiatry. 2012;2:e153. doi: 10.1038/tp.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang R, Fan Q, Zhang Z, Chen Y, Zhu Y, Li Y, et al. Anterior thalamic radiation structural and metabolic changes in obsessive-compulsive disorder: A combined DTI-MRS study. Psychiatry Res Neuroimaging. 2018;277:39–44. doi: 10.1016/j.pscychresns.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Wang R, Fan Q, Zhang Z, Chen Y, Tong S, Li Y, et al. White matter integrity correlates with choline level in dorsal anterior cingulate cortex of obsessive compulsive disorder patients: A combined DTI-MRS study. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:3521–4. doi: 10.1109/EMBC.2017.8037616. [DOI] [PubMed] [Google Scholar]
- 52.Rus OG, Reess TJ, Wagner G, Zimmer C, Zaudig M, Koch K, et al. Functional and structural connectivity of the amygdala in obsessive-compulsive disorder. Neuroimage Clin. 2017;13:246–55. doi: 10.1016/j.nicl.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, et al. Structural and functional brain abnormalities in attention-deficit/Hyperactivity disorder and obsessive-compulsive disorder: A comparative meta-analysis. JAMA Psychiatry. 2016;73:815–25. doi: 10.1001/jamapsychiatry.2016.0700. [DOI] [PubMed] [Google Scholar]
- 54.Moreira PS, Marques P, Soriano-Mas C, Magalhães R, Sousa N, Soares JM, et al. The neural correlates of obsessive-compulsive disorder: A multimodal perspective. Transl Psychiatry. 2017;7:e1224. doi: 10.1038/tp.2017.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moon CM, Kim BC, Jeong GW. Associations of neurofunctional, morphometric and metabolic abnormalities with clinical symptom severity and recognition deficit in obsessive-compulsive disorder. J Affect Disord. 2018;227:603–12. doi: 10.1016/j.jad.2017.11.059. [DOI] [PubMed] [Google Scholar]
- 56.Baxter LR, Jr, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE, et al. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. A comparison with rates in unipolar depression and in normal controls. Arch Gen Psychiatry. 1987;44:211–8. doi: 10.1001/archpsyc.1987.01800150017003. [DOI] [PubMed] [Google Scholar]
- 57.Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, et al. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 1989;46:518–23. doi: 10.1001/archpsyc.1989.01810060038007. [DOI] [PubMed] [Google Scholar]
- 58.Millet B, Dondaine T, Reymann JM, Bourguignon A, Naudet F, Jaafari N, et al. Obsessive compulsive disorder networks: Positron emission tomography and neuropsychology provide new insights. PLoS One. 2013;8:e53241. doi: 10.1371/journal.pone.0053241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perani D, Colombo C, Bressi S, Bonfanti A, Grassi F, Scarone S, et al. [18F] FDG PET study in obsessive-compulsive disorder. A clinical/metabolic correlation study after treatment. Br J Psychiatry. 1995;166:244–50. doi: 10.1192/bjp.166.2.244. [DOI] [PubMed] [Google Scholar]
- 60.Nakatani E, Nakgawa A, Ohara Y, Goto S, Uozumi N, Iwakiri M, et al. Effects of behavior therapy on regional cerebral blood flow in obsessive-compulsive disorder. Psychiatry Res. 2003;124:113–20. doi: 10.1016/s0925-4927(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 61.Hsieh HJ, Lue KH, Tsai HC, Lee CC, Chen SY, Kao PF, et al. L-3,4-dihydroxy-6-[F-18]fluorophenylalanine positron emission tomography demonstrating dopaminergic system abnormality in the brains of obsessive-compulsive disorder patients. Psychiatry Clin Neurosci. 2014;68:292–8. doi: 10.1111/pcn.12139. [DOI] [PubMed] [Google Scholar]
- 62.Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LR, Jr, et al. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatry. 2003;160:522–32. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- 63.Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S, et al. Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology. 1999;21:683–93. doi: 10.1016/S0893-133X(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 64.Baxter LR, Jr, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49:681–9. doi: 10.1001/archpsyc.1992.01820090009002. [DOI] [PubMed] [Google Scholar]
- 65.Apostolova I, Block S, Buchert R, Osen B, Conradi M, Tabrizian S, et al. Effects of behavioral therapy or pharmacotherapy on brain glucose metabolism in subjects with obsessive-compulsive disorder as assessed by brain FDG PET. Psychiatry Res. 2010;184:105–16. doi: 10.1016/j.pscychresns.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Busatto GF, Zamignani DR, Buchpiguel CA, Garrido GE, Glabus MF, Rocha ET, et al. A voxel-based investigation of regional cerebral blood flow abnormalities in obsessive-compulsive disorder using single photon emission computed tomography (SPECT) Psychiatry Res. 2000;99:15–27. doi: 10.1016/s0925-4927(00)00050-0. [DOI] [PubMed] [Google Scholar]
- 67.Karadağ F, Kalkan Oğuzhanoğlu N, Yüksel D, Kıraç S, Cura C, Ozdel O, et al. The comparison of pre- and post-treatment (99m) Tc HMPAO brain SPECT images in patients with obsessive-compulsive disorder. Psychiatry Res. 2013;213:169–77. doi: 10.1016/j.pscychresns.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Hesse S, Müller U, Lincke T, Barthel H, Villmann T, Angermeyer MC, et al. Serotonin and dopamine transporter imaging in patients with obsessive-compulsive disorder. Psychiatry Res. 2005;140:63–72. doi: 10.1016/j.pscychresns.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Zitterl W, Aigner M, Stompe T, Zitterl-Eglseer K, Gutierrez-Lobos K, Schmidl-Mohl B, et al. [123I]-beta-CIT SPECT imaging shows reduced thalamus-hypothalamus serotonin transporter availability in 24 drug-free obsessive-compulsive checkers. Neuropsychopharmacology. 2007;32:1661–8. doi: 10.1038/sj.npp.1301290. [DOI] [PubMed] [Google Scholar]
- 70.Stengler-Wenzke K, Müller U, Angermeyer MC, Sabri O, Hesse S. Reduced serotonin transporter-availability in obsessive-compulsive disorder (OCD) Eur Arch Psychiatry Clin Neurosci. 2004;254:252–5. doi: 10.1007/s00406-004-0489-y. [DOI] [PubMed] [Google Scholar]
- 71.Pogarell O, Hamann C, Pöpperl G, Juckel G, Choukèr M, Zaudig M, et al. Elevated brain serotonin transporter availability in patients with obsessive-compulsive disorder. Biol Psychiatry. 2003;54:1406–13. doi: 10.1016/s0006-3223(03)00183-5. [DOI] [PubMed] [Google Scholar]
- 72.Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, et al. In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42:306–14. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- 73.van der Straten AL, Denys D, van Wingen GA. Impact of treatment on resting cerebral blood flow and metabolism in obsessive compulsive disorder: A meta-analysis. Sci Rep. 2017;7:17464. doi: 10.1038/s41598-017-17593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brody AL, Saxena S, Schwartz JM, Stoessel PW, Maidment K, Phelps ME, et al. FDG-PET predictors of response to behavioral therapy and pharmacotherapy in obsessive compulsive disorder. Psychiatry Res. 1998;84:1–6. doi: 10.1016/s0925-4927(98)00041-9. [DOI] [PubMed] [Google Scholar]
- 75.Rauch SL, Shin LM, Dougherty DD, Alpert NM, Fischman AJ, Jenike MA, et al. Predictors of fluvoxamine response in contamination-related obsessive compulsive disorder: A PET symptom provocation study. Neuropsychopharmacology. 2002;27:782–91. doi: 10.1016/S0893-133X(02)00351-2. [DOI] [PubMed] [Google Scholar]
- 76.Simpson HB, Lombardo I, Slifstein M, Huang HY, Hwang DR, Abi-Dargham A, et al. Serotonin transporters in obsessive-compulsive disorder: A positron emission tomography study with [(11)C] McN 5652. Biol Psychiatry. 2003;54:1414–21. doi: 10.1016/s0006-3223(03)00544-4. [DOI] [PubMed] [Google Scholar]
- 77.Adams KH, Hansen ES, Pinborg LH, Hasselbalch SG, Svarer C, Holm S, et al. Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int J Neuropsychopharmacol. 2005;8:391–401. doi: 10.1017/S1461145705005055. [DOI] [PubMed] [Google Scholar]
- 78.Moresco RM, Pietra L, Henin M, Panzacchi A, Locatelli M, Bonaldi L, et al. Fluvoxamine treatment and D2 receptors: A pet study on OCD drug-naïve patients. Neuropsychopharmacology. 2007;32:197–205. doi: 10.1038/sj.npp.1301199. [DOI] [PubMed] [Google Scholar]
- 79.Olver JS, O’Keefe G, Jones GR, Burrows GD, Tochon-Danguy HJ, Ackermann U, et al. Dopamine D1 receptor binding in the striatum of patients with obsessive-compulsive disorder. J Affect Disord. 2009;114:321–6. doi: 10.1016/j.jad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 80.Matsumoto R, Ichise M, Ito H, Ando T, Takahashi H, Ikoma Y, et al. Reduced serotonin transporter binding in the insular cortex in patients with obsessive-compulsive disorder: A [11C] DASB PET study. Neuroimage. 2010;49:121–6. doi: 10.1016/j.neuroimage.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 81.Ho Pian KL, van Megen HJ, Ramsey NF, Mandl R, van Rijk PP, Wynne HJ, et al. Decreased thalamic blood flow in obsessive-compulsive disorder patients responding to fluvoxamine. Psychiatry Res. 2005;138:89–97. doi: 10.1016/j.pscychresns.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 82.Kim CH, Cheon KA, Koo MS, Ryu YH, Lee JD, Chang JW, et al. Dopamine transporter density in the basal ganglia in obsessive-compulsive disorder, measured with [123I]IPT SPECT before and after treatment with serotonin reuptake inhibitors. Neuropsychobiology. 2007;55:156–62. doi: 10.1159/000106474. [DOI] [PubMed] [Google Scholar]
- 83.Yamanishi T, Nakaaki S, Omori IM, Hashimoto N, Shinagawa Y, Hongo J, et al. Changes after behavior therapy among responsive and nonresponsive patients with obsessive-compulsive disorder. Psychiatry Res. 2009;172:242–50. doi: 10.1016/j.pscychresns.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 84.Wen SL, Cheng MH, Cheng MF, Yue JH, Wang H. Pharmacotherapy response and regional cerebral blood flow characteristics in patients with obsessive-compulsive disorder. Behav Brain Funct. 2013;9:31. doi: 10.1186/1744-9081-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML, et al. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–76. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 86.Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. A functional MRI comparison of patients with obsessive-compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Res. 2005;139:101–14. doi: 10.1016/j.pscychresns.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 87.Nakao T, Nakagawa A, Nakatani E, Nabeyama M, Sanematsu H, Yoshiura T, et al. Working memory dysfunction in obsessive-compulsive disorder: A neuropsychological and functional MRI study. J Psychiatr Res. 2009;43:784–91. doi: 10.1016/j.jpsychires.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 88.Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, López-Solà M, Hernández-Ribas R, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 89.Sanematsu H, Nakao T, Yoshiura T, Nabeyama M, Togao O, Tomita M, et al. Predictors of treatment response to fluvoxamine in obsessive-compulsive disorder: An fMRI study. J Psychiatr Res. 2010;44:193–200. doi: 10.1016/j.jpsychires.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 90.Zhang T, Wang J, Yang Y, Wu Q, Li B, Chen L, et al. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J Psychiatry Neurosci. 2011;36:23–31. doi: 10.1503/jpn.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cardoner N, Harrison BJ, Pujol J, Soriano-Mas C, Hernández-Ribas R, López-Solá M, et al. Enhanced brain responsiveness during active emotional face processing in obsessive compulsive disorder. World J Biol Psychiatry. 2011;12:349–63. doi: 10.3109/15622975.2011.559268. [DOI] [PubMed] [Google Scholar]
- 92.Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:619–29. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- 93.Posner J, Marsh R, Maia TV, Peterson BS, Gruber A, Simpson HB, et al. Reduced functional connectivity within the limbic Cortico-Striato-Thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:2852–60. doi: 10.1002/hbm.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hou JM, Zhao M, Zhang W, Song LH, Wu WJ, Wang J, et al. Resting-state functional connectivity abnormalities in patients with obsessive-compulsive disorder and their healthy first-degree relatives. J Psychiatry Neurosci. 2014;39:304–11. doi: 10.1503/jpn.130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:595–605. doi: 10.1016/j.biopsych.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beucke JC, Sepulcre J, Eldaief MC, Sebold M, Kathmann N, Kaufmann C, et al. Default mode network subsystem alterations in obsessive-compulsive disorder. Br J Psychiatry. 2014;205:376–82. doi: 10.1192/bjp.bp.113.137380. [DOI] [PubMed] [Google Scholar]
- 97.Via E, Cardoner N, Pujol J, Alonso P, López-Solà M, Real E, et al. Amygdala activation and symptom dimensions in obsessive-compulsive disorder. Br J Psychiatry. 2014;204:61–8. doi: 10.1192/bjp.bp.112.123364. [DOI] [PubMed] [Google Scholar]
- 98.Simon D, Adler N, Kaufmann C, Kathmann N. Amygdala hyperactivation during symptom provocation in obsessive-compulsive disorder and its modulation by distraction. Neuroimage Clin. 2014;4:549–57. doi: 10.1016/j.nicl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu Y, Fan Q, Zhang H, Qiu J, Tan L, Xiao Z, et al. Altered intrinsic insular activity predicts symptom severity in unmedicated obsessive-compulsive disorder patients: A resting state functional magnetic resonance imaging study. BMC Psychiatry. 2016;16:104. doi: 10.1186/s12888-016-0806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian L, Meng C, Jiang Y, Tang Q, Wang S, Xie X, et al. Abnormal functional connectivity of brain network hubs associated with symptom severity in treatment-naive patients with obsessive-compulsive disorder: A resting-state functional MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2016;66:104–11. doi: 10.1016/j.pnpbp.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 101.Vaghi MM, Hampshire A, Fineberg NA, Kaser M, Brühl AB, Sahakian BJ, et al. Hypoactivation and dysconnectivity of a frontostriatal circuit during goal-directed planning as an endophenotype for obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:655–63. doi: 10.1016/j.bpsc.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heinzel S, Kaufmann C, Grützmann R, Hummel R, Klawohn J, Riesel A, et al. Neural correlates of working memory deficits and associations to response inhibition in obsessive compulsive disorder. Neuroimage Clin. 2018;17:426–34. doi: 10.1016/j.nicl.2017.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Vries FE, de Wit SJ, van den Heuvel OA, Veltman DJ, Cath DC, van Balkom AJ, et al. Cognitive control networks in OCD: A resting-state connectivity study in unmedicated patients with obsessive-compulsive disorder and their unaffected relatives. World J Biol Psychiatry. 2017:1–13. doi: 10.1080/15622975.2017.1353132. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Z, Fan Q, Zhu Y, Tan L, Chen Y, Gao R, et al. Intrinsic functional connectivity alteration of dorsal and rostral anterior cingulate cortex in obsessive-compulsive disorder: A resting fMRI study. Neurosci Lett. 2017;654:86–92. doi: 10.1016/j.neulet.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 105.Fullana MA, Zhu X, Alonso P, Cardoner N, Real E, López-Solà C, et al. Basolateral amygdala-ventromedial prefrontal cortex connectivity predicts cognitive behavioural therapy outcome in adults with obsessive-compulsive disorder. J Psychiatry Neurosci. 2017;42:378–85. doi: 10.1503/jpn.160215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qiu L, Fu X, Wang S, Tang Q, Chen X, Cheng L, et al. Abnormal regional spontaneous neuronal activity associated with symptom severity in treatment-naive patients with obsessive-compulsive disorder revealed by resting-state functional MRI. Neurosci Lett. 2017;640:99–104. doi: 10.1016/j.neulet.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 107.Giménez M, Guinea-Izquierdo A, Villalta-Gil V, Martínez-Zalacaín I, Segalàs C, Subirà M, et al. Brain alterations in low-frequency fluctuations across multiple bands in obsessive compulsive disorder. Brain Imaging Behav. 2017;11:1690–706. doi: 10.1007/s11682-016-9601-y. [DOI] [PubMed] [Google Scholar]
- 108.Gonçalves ÓF, Soares JM, Carvalho S, Leite J, Ganho-Ávila A, Fernandes-Gonçalves A, et al. Patterns of default mode network deactivation in obsessive compulsive disorder. Sci Rep. 2017;7:44468. doi: 10.1038/srep44468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Posner J, Song I, Lee S, Rodriguez CI, Moore H, Marsh R, et al. Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2017;38:678–87. doi: 10.1002/hbm.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nabeyama M, Nakagawa A, Yoshiura T, Nakao T, Nakatani E, Togao O, et al. Functional MRI study of brain activation alterations in patients with obsessive-compulsive disorder after symptom improvement. Psychiatry Res. 2008;163:236–47. doi: 10.1016/j.pscychresns.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 111.Zhao HZ, Wang CH, Gao ZZ, Ma JD, Huang P, Li HF, et al. Effectiveness of cognitive-coping therapy and alteration of resting-state brain function in obsessive-compulsive disorder. J Affect Disord. 2017;208:184–90. doi: 10.1016/j.jad.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 112.Gürsel DA, Avram M, Sorg C, Brandl F, Koch K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: A meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev. 2018;87:151–60. doi: 10.1016/j.neubiorev.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 113.van der Wee NJ, Ramsey NF, van Megen HJ, Denys D, Westenberg HG, Kahn RS, et al. Spatial working memory in obsessive-compulsive disorder improves with clinical response: A functional MRI study. Eur Neuropsychopharmacol. 2007;17:16–23. doi: 10.1016/j.euroneuro.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 114.Shin DJ, Jung WH, He Y, Wang J, Shim G, Byun MS, et al. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:606–14. doi: 10.1016/j.biopsych.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 115.Moody TD, Morfini F, Cheng G, Sheen C, Tadayonnejad R, Reggente N, et al. Mechanisms of cognitive-behavioral therapy for obsessive-compulsive disorder involve robust and extensive increases in brain network connectivity. Transl Psychiatry. 2017;7:e1230. doi: 10.1038/tp.2017.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li P, Yang X, Greenshaw AJ, Li S, Luo J, Han H, et al. The effects of cognitive behavioral therapy on resting-state functional brain network in drug-naive patients with obsessive-compulsive disorder. Brain Behav. 2018;8:e00963. doi: 10.1002/brb3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: Basis of functional neuroimaging. Cerebrovasc Brain Metab Rev. 1995;7:240–76. [PubMed] [Google Scholar]
- 118.Hirosawa R, Narumoto J, Sakai Y, Nishida S, Ishida T, Nakamae T, et al. Reduced dorsolateral prefrontal cortical hemodynamic response in adult obsessive-compulsive disorder as measured by near-infrared spectroscopy during the verbal fluency task. Neuropsychiatr Dis Treat. 2013;9:955–62. doi: 10.2147/NDT.S45402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okada K, Ota T, Iida J, Kishimoto N, Kishimoto T. Lower prefrontal activity in adults with obsessive-compulsive disorder as measured by near-infrared spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:7–13. doi: 10.1016/j.pnpbp.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 120.Nakanishi M, Oshita H, Tanaka Y, Inoue A, Kawashima C, Okamoto K, et al. Near-infrared spectroscopy during the verbal fluency task before and after treatment with image exposure and SSRI therapy in patients with obsessive-compulsive disorder. Case Rep Psychiatry 2014. 2014 doi: 10.1155/2014/591023. 591023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cho YT, Ernst M, Fudge JL. Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J Neurosci. 2013;33:14017–30. doi: 10.1523/JNEUROSCI.0170-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van den Heuvel OA, van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, et al. Brain circuitry of compulsivity. Eur Neuropsychopharmacol. 2016;26:810–27. doi: 10.1016/j.euroneuro.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 123.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jang JH, Kim JH, Jung WH, Choi JS, Jung MH, Lee JM, et al. Functional connectivity in fronto-subcortical circuitry during the resting state in obsessive-compulsive disorder. Neurosci Lett. 2010;474:158–62. doi: 10.1016/j.neulet.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 125.Leech R, Scott G, Carhart-Harris R, Turkheimer F, Taylor-Robinson SD, Sharp DJ, et al. Spatial dependencies between large-scale brain networks. PLoS One. 2014;9:e98500. doi: 10.1371/journal.pone.0098500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reggente N, Moody TD, Morfini F, Sheen C, Rissman J, O’Neill J, et al. Multivariate resting-state functional connectivity predicts response to cognitive behavioral therapy in obsessive-compulsive disorder. Proc Natl Acad Sci U S A. 2018;115:2222–7. doi: 10.1073/pnas.1716686115. [DOI] [PMC free article] [PubMed] [Google Scholar]


