ABSTRACT
The activation of the small GTPase ARF6 has been implicated in promoting several pathological processes related to vascular instability and tumor formation, growth, and metastasis. ARF6 also plays a vital role during embryonic development. Recent studies have suggested that ARF6 carries out these disparate functions primarily by controlling protein trafficking within the cell. ARF6 helps direct proteins to intracellular or extracellular locations where they function in normal cellular responses during development and in pathological processes later in life. This transport of proteins is accomplished through a variety of mechanisms, including endocytosis and recycling, microvesicle release, and as yet uncharacterized processes. This Commentary will explore the functions of ARF6, while focusing on the role of this small GTPase in development and postnatal physiology, regulating barrier function and diseases associated with its loss, and tumor formation, growth, and metastasis.
KEYWORDS: adenosine diphosphate ribosylation factor 6; ARF6, barrier function; cancer; development; metastasis; postnatal physiology; protein trafficking; tumor growth; vascular stability
Introduction
ARF6 is a member of the adenosine diphosphate (ADP)-ribosylation factor (ARF) family of small GTPases and is part of the larger superfamily of RAS GTPases. ARFs were originally named for their ability to stimulate cholera toxin-mediated ADP ribosylation of the Gαs subunit of the heterotrimeric G proteins,1 which are utilized by many G-protein coupled receptors (GPCRs). However, ARFs are now known to regulate cell behavior and function by controlling protein and lipid trafficking in eukaryotic cells.
Mammals possess 6 different ARF isoforms that can be divided into 3 classes: class I (ARF1, ARF2, and ARF3); class II (ARF4 and ARF5); and class III (ARF6). However, unlike many other mammals, humans lack ARF2.2 Most research to date has focused on ARF1 and ARF6. ARF1 primarily regulates vesicular trafficking between the endoplasmic reticulum and Golgi and from the Golgi to the plasma membrane3 as well as phagocytosis.4 ARF1 also recruits CDC42 to Golgi vesicles where it activates N-WASP, which leads to Arp2/3 recruitment and actin polymerization.5 In contrast, ARF6 functions mostly at the plasma membrane where it is involved in endocytosis and recycling, cytokinetic abscission, and Rac-mediated cytoskeletal remodeling to promote cell migration, invasion, adhesion, and phagocytosis (Fig. 1).6-9 Recently, ARF6 also has been shown to control retromer trafficking between the Trans-Golgi network and late endosomes/lysosomes.10
Figure 1.
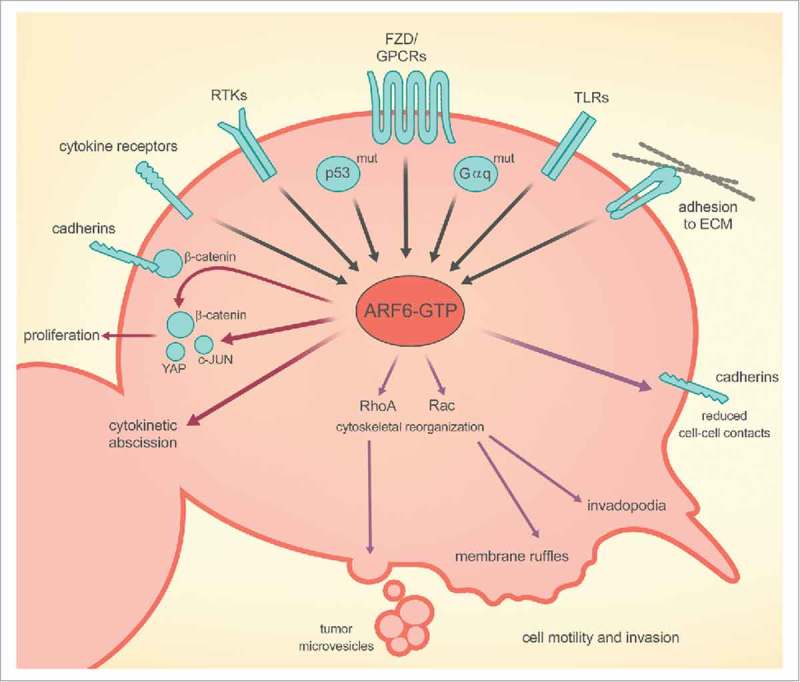
The diverse roles of ARF6 on cellular function and behavior. ARF6 can be activated (GTP-bound state) via several different receptor signaling pathways, including those controlled by cytokine receptors, receptor tyrosine kinases (RTKs), frizzled (FZD)(a type of G protein–coupled receptor or GPCR), Toll-like receptors (TLRs), and integrins. ARF6-GTP can induce cell proliferation (maroon arrows) and cell motility or invasion (purple arrows) by reducing cell-cell contacts, promoting invadopodia formation, membrane ruffling, and shedding of tumor microvesicles.
Like other GTPases, ARF6 adopts an active conformation when bound to GTP and in an inactive form when bound to GDP. Two different classes of proteins control the activation state of ARF6. Guanine nucleotide exchange factors (GEFs) activate ARF6 by promoting the exchange of GDP for GTP, while GTPase activating proteins (GAPs) inactivate ARF6 by stimulating the hydrolysis of GTP to GDP. Numerous ARF6-GEFs and ARF6-GAPs regulate ARF6 activity depending on cell type and the stimulus received by the cell.
The role of ARF6 in the endocytosis and recycling of receptor tyrosine kinases (RTKs), GPCRs, integrins, and cadherins is well established (Table 1, Fig. 2),6,11,12 More recently, it has been shown that ARF6 also directs the intracellular trafficking of key signaling proteins, such as VEGFR2, c-MET, β-catenin, and activated GNAQ, to intracellular signaling sites where signaling or transcription is enhanced (Table 1, Fig. 2).13-16 These recent findings illuminate the role of ARF6 in controlling the spatial and temporal localization of key signaling proteins.
Table 1.
ARF6 drives relocalization of cellular proteins to control cellular function.
| Cellular Localization | Relocalization event | Cellular Outcome | References | |
|---|---|---|---|---|
| CELL SURFACE PROTEINS | ||||
| Cadherins | E-cadherin | internalization | increased β−catenin signaling, increased proliferation | Pellon-Cardenas et al.27 |
| internalization | increased cell motility | Palacios et al.35 Palacios et al.68 Tushir et al.69 |
||
| reduced at cell surface | increased invasion | Morishige et al.51 | ||
| VE-cadherin | internalization | reduced cell-cell adhesion, increased vascular permeability | Zhu et al.18 | |
| N-cadherin | stable in PM, β−catenin released - translocated to cytoplasm and nucleus | increased invasion and metastasis | Grossmann et al.13 | |
| RTKs | c-MET | Internalization (WNT3A Induced) |
hyperactive signaling, hyperproliferative, disorganized epithelium | Pellon-Cardenas et al.27 Tushir et al.69 |
| recycled to PM | increased cell migration | Parachoniak et al.12 | ||
| CSF1-R | internalization | hyperactive signaling, hyperproliferative, disorganized epithelium | Tushir et al.69 | |
| FGFR1 | internalization | facilitates nuclear accumulation of FGFR1 | Bryant et al.70 | |
| MuSK | internalization | not specified in study | Luiskandl et al.71 | |
| Integrin | β1 integrins | recycled to PM | cell spreading, focal adhesion formation, cell motility, angiogenesis | Hongu et al.22 |
| recycled to PM | increased cell motility | Powelka et al.56 | ||
| GPCR | LHCGR | internalization | not specified in study, possible desensitization, prolonged endosomal signaling, and/or resensitization | Kanamarlapudi et al.72 |
| β2AR | internalization and degradation | not specified in study, possible desensitization, prolonged endosomal signaling, and/or resensitization | Macia et al.73 | |
| TPβR | internalization | not specified in study, possible desensitization, prolonged endosomal signaling, and/or resensitization | Giguere et al.74 | |
| P2Y1 and P2Y12 | internalization, (recycling was not tested directly) | resensitization, increased platelet aggregation | Kanamarlapudi et al.75 | |
| Heparin-sulfate proteoglycan | Syndecan | recycling | cell spreading | Zimmermann et al.76 |
|
CYTOPLASMIC/ NUCLEAR PROTEINS |
||||
| G protein | GNAQQ209L | PM → cytoplasmic vesicles | increased oncogenic signaling and tumor growth | Yoo et al.16 |
| Rac1 | activation and recruitment to PM | increased cell motility | Tushir et al.54 Boshans et al., Marchesin et al., Palamidessi et al., Radhakrishna et al., Santy et al.,78-82 |
|
| Transcription factor | β−catenin | PM/AJs → cytoplasm→ nucleus | increased transcription, invasion, metastasis | Grossmann et al.13 |
| PM/AJs → cytoplasm→ nucleus | increased proliferation | Pellon-Cardenas et al.27 | ||
| YAP | cytoplasm→ nucleus | increased transcription, proliferation, tumor growth | Yoo et al.16 | |
| ENDOLYSOSOMAL & TGN PROTEINS | ||||
| Transmembrane glycoprotein | M6PR | retromer-mediated transport between TGN and endolysosomes | diffuse intracellular distribution of free cholesterol | Marquer et al.10 |
| Intralysosomal glycoprotein | NPC2 | retromer-mediated transport between TGN and endolysosomes | diffuse intracellular distribution of free cholesterol | Marquer et al.10 |
E-, epithelial; VE-, vascular endothelial; N-, neural; PM. Plasma membrane; CSFR-1, colony stimulating factor 1 receptor; FGFR1, fibroblast growth factor receptor 1; MuSK, muscle-specific kinase receptor; LHCGR, luteinizing hormone/choriogonadotropin receptor; GPCR, G protein-coupled receptor; β2-adrenergic receptor; TPβR, thromboxane A(2) receptor β; YAP. Yes-associated protein; AJ, adherens junctions; TGN, Trans-Golgi network; M6PR, mannose-6-phosphate receptor; NPC2, Niemann-Pick Disease, Type C2.
Figure 2.
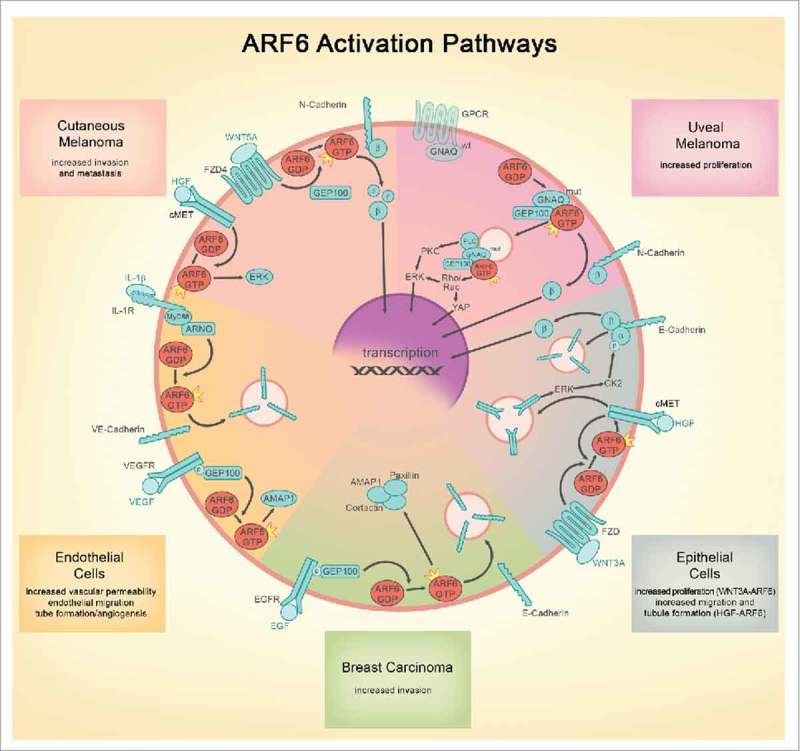
Similarities and differences of ARF6-mediated signaling in various model systems. The following common themes are illustrated: 1) Growth factors, inflammatory cytokines, and WNTs all activate ARF6 to control adherens junction integrity via endocytosis; 2) Both receptor tyrosine kinase and G-protein signaling are coordinated from endosomes by ARF6; and 3) β-catenin junctional and transcriptional function is controlled by WNT-activated ARF6 in non-neoplastic/normal epithelial cells as well as melanoma. ARF6 pathways appear to be divergent in the exact guanine exchange factors engaged to activate ARF6 and in the ultimate cellular phenotype (proliferation vs. barrier function/migration/invasion). Because engagement of ARF6 is a proximal event that is critical for endosomic signaling, ARF6 controls multiple downstream, parallel pathways. For this reason and because ARF6 activation leads to pathologic outcomes in both normal and neoplastic cells, ARF6 is an attractive target for development of therapeutic interventions. FZD = Frizzled, HGF = hepatocyte growth factor, VEGF = vascular endothelial growth factor, EGF = epithelial growth factor, GPCR = G protein coupled receptor, IL-1 = Interleukin 1, PLC = phospholipase C, PKC = protein kinase C, β = β catenin, α = α catenin, wt = wild type, mut = oncogenic mutant.
ARF6 function is vital during embryogenesis.17 Whether ARF6 plays an essential role in normal postnatal physiology has not yet been formally evaluated. However, the systemic inhibition of ARF6 by small molecule compounds does not produce observable pathology in adult animals when administered at therapeutic levels,13,18 suggesting that a reduction in ARF6 activity in adults does not have dire consequences to the organism. The aberrant activation of ARF6, however, does play an important role in many pathological states, including vascular leak, inflammatory processes, and cancer. Each of these roles for ARF6 will be discussed in the following sections.
ARF6 in development and postnatal physiology
ARF6 has been shown to be required for the normal development of several species from different phyla (Table 2), suggesting that it might be a universally important developmental gene. Transgenic expression of a dominant negative form of ARF6 (ARF6 T27N) in Drosophila led to a major neural commissure malformation, mimicking the phenotype of a deficiency in schizo/loner (the mammalian ortholog encodes the ARF-GEF IQSEC1, also known as GEP100 and BRAG2).19 Schizo/Loner was also shown to be important for myoblast fusion in Drosophila development, and blocking ARF6 activity inhibited myoblast fusion.20 In sea urchins, ARF6 also appears to be critical for early embryonic development.21 In mice, genetic deletion of Arf6 caused a mid-gestation in utero lethality, which is associated with an underdeveloped liver and malformation of the fetal hepatic cord.17 These studies indicate that ARF6 plays a vital role in development by supporting both the proliferation and the migration of embryonic cells in specific tissues. Although ARF6 is necessary for early embryonic development, genetic deletion of Arf6 in Tie2-positive cells (primarily endothelial cells) during mid-stage embryonic development does not trigger any noticeable developmental abnormality and normal physiology is observed, although neoangiogenesis is reduced upon tumor transplantation.22 This finding suggests that ARF6 is not essential for the development or normal function of all tissues.
Table 2.
ARF6 signaling pathways in development and disease.
| Biologic Process | Signaling Pathway | Outcome/Model System | References |
|---|---|---|---|
| Development | Schizo/Loner-Arf6 | neural commissure formation, Drosophila | Onel et al.19 |
| Schizo/Loner-Arf6 | myoblast fusion, Drosophila | Chen et al.20 | |
| ARF6 | early embryo formation, sea urchin | Dumas et al.21 | |
| HGF-ARF6 | liver development, mice | Suzuki et al.17 | |
| Barrier Structure & Function | |||
| Endothelial | IL1R-MYD88-ARNO-ARF6 | collagen-induced arthritis, mice | Zhu et al.18 |
| VEGF-ARNO-ARF6-RAC1 inhibited by SLIT2/ROBO4-GIT1-Paxillin | 1. retinal permeability, retinopathy, mice 2. retinal neovascularization, macular degeneration, mice |
Jones et al.26 | |
| LPS-TLR4-MYD88-ARNO-ARF6-VEcadherin | endotoxic shock/sepsis, mice | Davis et al.25 | |
| HGF-ARF6 GEFs*-β1integrin | tumor neoangiogenesis, mice | Hongu et al.22 | |
| Epithelial | HGF-ARF6-Ecadherin | epithelial cell migration, in vitro | Palacios et al.35,36 |
| HGF-ARF6-RAC1 | epithelial tube formation**, in vitro | Tushir and D'Souza-Schorey54 | |
| WNT3A-ARF6-cMET-ERK-CK2-Ecadherin/βcatenin | abnormal cyst filling/ductular proliferation, in vitro | Pellon-Cardenas et al.27 | |
| Cancer | |||
| glioma | HGF-ARF6-IQGAP1-RAC1 | invasion in vitro, invasion in mice | Hu et al.49 |
| cutaneous melanoma | HGF-ARF6-ERK | invasion, in vitro | Tague et al.52 |
| cutaneous melanoma | WNT5A/FZD4/LRP6-GEP100-ARF6-βcatenin | invasion in vitro, spontaneous lung metastasis in mice | Grossmann et al.13 |
| breast carcinoma | EGFR-GEP100-ARF6-AMAP1 | invasion in vitro, experimental metastasis+ in mice | Morishige et al.51, Onodera et al.61 |
| lung adenocarcinoma | HER2-GEP100-ARF6-AMAP1 | invasion, in vitro | Menju et al.50 |
| renal cell carcinoma | LPAR2-Gα12-EFA6-ARF6 | invasion in vitro, experimental metastasis2+ in mice | Hashimoto et al.63 |
| uveal melanoma | GNAQQ209L-GEP100-ARF6-PLC, ERK, YAP and βcatenin | proliferation and colony formation in vitro, tumor establishment and growth in mice | Yoo et al.16 |
ARF6 mediates a variety of biologic processes. In developing organisms, ARF6 is essential for embryogenesis. In mature organisms, ARF6 activation potentiates both inflammatory and neoplastic disease states. *Multiple ARF6 GEFs were implicated. **Also relevant to development of 3-dimensional epithelial structures in various organs. +Tail vein injection model. ++Tail vein injection model with bioluminescent scoring, no gross or microscopic lung masses reported.
The fundamental importance of ARF6 is also reflected by the fact that it is highly conserved across species. The amino acid sequence of ARF6 in Drosophila is 94% identical to the human sequence, and ARF6 in the mouse is 100% identical to human ARF6. Importantly, the protein structure and putative functions are also highly conserved from Drosophila to humans. Moreover, ARF6 is expressed at an appreciable level in almost all cell types throughout the life span, at least in humans and mice.17,23,24
Many groups, including our own, have demonstrated the detrimental effects of aberrant ARF6 activation in the pathogenesis of several human diseases (reviewed below), which encouraged the field to investigate potential therapeutic strategies of targeting ARF6-related signaling. By inhibiting ARF6-GEFs or ARF6 directly with systemic administration of small molecules such as SecinH3 or NAV-2729, several disease phenotypes were effectively ameliorated, and the treated animals showed no signs of toxic exposure or pathology related to ARF6 inhibition.13,18,22,25,26 These results suggest that reducing ARF6 activity by pharmacological inhibition will not produce detrimental effects in adults and can be used for therapeutic purposes.
Although pharmacologic inhibition of ARF6 does not seem to undermine the survival of mice and the normal function of healthy adult somatic cells, these studies do not fully address the role of ARF6 in adults. For example, ARF6 may be important for adult stem cell functions. ARF6 is activated by WNT3A in benign epithelial cells27 and WNT5A in cancer cells13 to control signaling specifically from the cadherin-pool of β-catenin (Table 1, Fig. 2). ARF6 also appears to be important for activating the WNT co-receptor LRP6.28 This link to WNT/β-catenin raises new questions about the role of ARF6 in adult stem cell function. WNT-β-catenin signaling maintains a transcriptional program that supports the stem cell state.29 SLIT-ROBO signaling opposes WNT signaling in cancer cells by inactivating ARF6.13 Likewise, in mammary stem cells SLIT-ROBO inhibits WNT signaling.30 A few reports indicate that SLIT-ROBO can regulate somatic stem cell and stem-like cell behavior.30-33 Thus, because ARF6 is downstream of WNT/β-catenin and SLIT-ROBO, it appears to be poised to integrate competing signals that modulate stem cell function. More work is needed to determine whether ARF6 has a role in stem cell biology.
In conclusion, the pharmacological inhibition of ARF6 at therapeutic dosages does not seem to have a detrimental effect on adult animal physiology. However, formal testing of the necessity of ARF6 in postnatal development and adulthood awaits Arf6 knockout studies in post-development mice.
The role of ARF6 in controlling barrier function and diseases related to the loss of barrier function
ARF6 signaling cascades are implicated in the control of vascular endothelial barrier stability (Table 2). In many inflammatory and infectious disease states, the presence of pathogen-associated molecular patterns (PAMPs) or the release of excessive proinflammatory cytokines (known as a cytokine storm) leads to a dramatic increase in vascular permeability, due at least in part to the internalization of VE-cadherin and the disassembly of adherens junctions.18,26 The destabilization of the endothelial barrier permits the influx of inflammatory cells into tissues. This extravasation is a hallmark of the innate immune response and can also increase fluid accumulation in tissues and lead to organ failure. Recent studies have elucidated an ARF6-dependent inflammatory signaling cascade in endothelial barrier destabilization that is independent of the well-described nuclear factor-κB (NF-κB)-dependent pathway.18,25 IL-1β and lipopolysaccharides (LPS) each activate ARF6 via the adaptor protein MYD88 and the ARF-GEF ARNO/CYTH2. MYD88-ARNO-ARF6 serves as a proximal signaling cascade that disrupts vascular stability and is functionally distinct from the inflammatory NF-κB signaling pathway. It is likely that other proinflammatory cytokines and PAMPs, as well as damage-associated molecular patterns (DAMPs) may also act through ARF6 to induce vascular permeability. For example, data suggest that TNFα may induce permeability through ARF6.18
Inhibition of ARF6 by the ARF-GEF inhibitor SecinH3 reduced inflammation in 2 different animal models—collagen-induced arthritis (CIA), a model of rheumatoid arthritis, and the carrageenan air-pouch model, a model of acute inflammation.18 Vascular leak was also reduced by ARF6 inhibition in the CIA model. These results suggest that limiting vascular leak by blocking ARF6 activation is beneficial in both slowing the progression of arthritis and in reducing acute inflammatory processes. However, such results might not be solely due to the stabilizing effect of such inhibitors on the vasculature. Other mechanisms, such as the effect of inhibiting ARF6 in inflammatory cells, could partially account for the beneficial effects of the inhibitors. Similar in vivo studies in mice following cell-specific loss of Arf6 will be required to determine the role of ARF6 in the endothelium, inflammatory cells, or other cell types in ameliorating pathology in these models or other models of inflammation.
The angiogenic growth factor VEGF can also activate ARF6, leading to increased paracellular permeability of the endothelium.34 VEGF-mediated vascular leak is the core etiology of several human diseases, including vascular eye diseases such as diabetic retinopathy, retinopathy of prematurity, and age-related macular degeneration. Inhibition of ARF6 using small molecule inhibitors decreased vascular leak and associated pathology in several animal models of eye disease, including VEGF-induced permeability, oxygen-induced retinopathy, and laser-induced choroidal neovascularization.26
ARF6 appears to play a similar role in controlling epithelial barrier function (Table 2). E-Cadherin mediated cell-cell adhesion maintains the integrity and normal functions of epithelial cell layers. ARF6 activation remodels the membrane and actin cytoskeleton at the periphery of the cell and regulates recycling of plasma membrane components. Specifically, it has been shown that expression of the dominant negative ARF6 T27N mutant prevents hepatocyte growth factor (HGF)-induced internalization of E-cadherin-based junctional components in Madin-Darby canine kidney (MDCK) cells, whereas the constitutively active ARF6 Q67L mutant induces disassembly of adherens junctions (Fig. 2).35,36 ARF6 activation by one of its GEFs, GEP100, disrupts E-Cadherin-mediated cell-cell adhesion of human epidermoid carcinoma CaSki cells, thereby promoting their invasive phenotype.37 Taken together, these findings demonstrate that proper regulation of ARF6 activity is necessary for the maintenance of cell-cell adhesions in the epithelium.
A novel signaling pathway was described in MDCK cells by which ligand-activated erythropoietin-producing hepatocellular carcinoma (EPH) A2 increases cell compaction, increases E-cadherin-mediated contacts and suppresses ARF6 activity.38 EPH A2 ligation leads to activation of the NCK1 tyrosine kinase 1 followed by recruitment of G-protein coupled receptor kinase-interacting ArfGAP 1 (GIT1) to inactivate ARF6. Although it is not entirely clear if EPH signaling precedes E-cadherin-mediated adhesion or vice versa, these data provide additional evidence that ARF6 allows a cell to respond to environmental cues for epithelial barrier integrity and function, similar to the vascular system.
ARF6 in tumor formation, growth, invasion, and metastasis
A few studies have begun to address the role of ARF6 in tumor formation and growth (Table 2). One recent study16 showed that ARF6 acts as an effector of oncogenic GNAQ in uveal melanoma to control all known downstream signaling pathways, including PLC/PKC, Rho/Rac, YAP, and β-catenin (Fig. 2).39-42 Reducing ARF6 activity either by knocking down its expression or by pharmacological inhibition using the small molecule inhibitor NAV-2729 decreased cell proliferation and anchorage-independent colony growth in cell culture assays and tumor establishment and growth in an orthotopic xenograft mouse model of uveal melanoma (Table 2).16 At therapeutic levels, daily dosing of mice with NAV-2729 for 35 consecutive days produced no overt signs of toxicity. Red blood cell, white blood cell, and platelet counts and hematocrits were within the normal range, and histopathological examination of vital organs including the liver, lungs, heart, kidney, and brain revealed no significant lesions. However, toxicity was observed when mice were administered higher dosages of NAV-2729. These results indicate that prolonged inhibition of ARF6 in adult mice does not cause catastrophic physiological changes and suggest that ARF6 inhibition can be safely employed in adult animals for therapeutic purposes. When ARF6 was knocked down in uveal melanoma cells, GNAQ was relocated from cytoplasmic vesicles to the plasma membrane and oncogenic signaling was decreased. These results suggest that activation of ARF6 by oncogenic GNAQ causes the relocation of GNAQ to cytoplasmic vesicles where signaling is enhanced (Table 1, Fig. 2) and illustrate the important role of ARF6 in protein trafficking and signaling.16 Other reports have also suggested that ARF6 plays a role in the proliferation of various tumor cell types.43-45 Additionally, the SLIT-ROBO pathway is known to reduce ARF6 activation13,26 and has been shown to inhibit proliferation in several types of cancers.46
Cancer cells exhibit an aggressive phenotype that enables them to spread locally and to distant sites. Tumor cell movement through tissue is controlled by a series of coordinated events involving cell adhesion, cytoskeletal remodeling, changes in cell shape, and extracellular matrix proteolysis. ARF6 regulates all of these processes (Fig. 1, Table 2) by controlling the subcellular location of both membrane and cytoplasmic proteins (Table 1), and signaling mechanisms have been described at length elsewhere.6,47 More recently, it has been shown that ARF6 coordinates signaling and cell function from well-established oncogenes such as EGFR, HER2/ERBB2, c-MET, β-catenin, mutant p53, and mutant GNAQ (Fig. 1, Tables 1–2).13,16,35,48-52
Dissolution of adherens junctions is thought to allow cells to mobilize for migration or invasion. In MCF-7 breast cancer cells, overexpression of ARF6 along with its activating GEF GEP100 reduces E-cadherin-mediated cell-cell contacts and renders the cells more invasive (Fig. 2).51 Consistent with these findings, an independent study showed that epidermal growth factor (EGF)-induced ARF6 activation leads to E-cadherin internalization.53 In MDCK cells, ARF6 activation by hepatocyte growth factor (HGF) induces E-cadherin internalization and epithelial cell migration (Fig. 2).35,36 The link between HGF and ARF6 has been corroborated in additional, distinct systems such as melanoma, glioma and in embryonic development (Table 2).17,49,52 In cutaneous melanoma cells HGF activates ARF6 upstream of ERK to induce invadopodia activity.52 In glioma, HGF activates ARF6 leading to IQGAP1 recruitment of RAC1 and an invasive phenotype.49 During liver development, ARF6 has been reported to be essential for hepatic cord formation downstream of HGF signaling.17 Embryonic livers from Arf6 null mice showed defective elongation and branching into hepatic cords. This finding is consistent with the role described for HGF-induced ARF6 activation in the formation of epithelial structures by MDCK cells in vitro.54
WNT3A also activates ARF6 in MDCK cells to induce E-cadherin internalization and accumulation of cytoplasmic and nuclear β-catenin.27 This is analogous to the WNT5A-ARF6-β-catenin pathway in cutaneous melanoma (Fig. 2).13 However, the precise mechanism of action of this pathway has been better characterized in epithelial cells. Specifically, WNT3A-mediated activation of ARF6 causes endocytosis of the receptor tyrosine kinase (RTK) c-MET and subsequent signaling from endosomes. Thus, similar to endosomal GNAQ signaling in uveal melanoma, ARF6 activation leads to endosomal signaling by RTKs in epithelial cells (Fig. 2). Endosomal c-MET activates ERK, leading to casein kinase 2-mediated phosphorylation of α-catenin and disassembly of adherens junctions. Corroborating the data showing that E-cadherin localization is controlled by ARF6, inactivation of ARF6, via overexpression of the ARF6-GAP SMAP1, causes E-cadherin to accumulate on the cell surface.55
In contrast to E-cadherin, N-cadherin in cutaneous melanoma persists at the cell surface upon ARF6 activation by WNT5A, while junctional β-catenin is released into the cytoplasm where it can be transported to the nucleus to induce transcription (Fig. 2).13 Likewise, in uveal melanoma, oncogenic GNAQ (Q209L) binds and recruits GEP100 to activate ARF6, which promotes the relocalization of β-catenin from the cell surface to the nucleus to induce transcription.16 At present the complete mechanism behind ARF6-dependent liberation of junctional β-catenin in cutaneous melanoma is unknown. Independence from cadherin internalization suggests that a novel mechanism remains to be discovered. However, for uveal melanoma, the process of β−catenin release from the membrane appears to require casein kinase 2 and possibly ERK activation,16 similar to ARF6-dependent, endosomal c-MET control of junctional β−catenin signaling in epithelial cells.27
Integrins attach cells to the surrounding extracellular matrix (ECM) by forming a link between the cellular cytoskeleton and the ECM by serving as matrix receptors. Integrin internalization and recycling back to the surface play integral roles in cell adhesion, establishing and maintaining cell polarity, controlling signaling pathways, and promoting cell migration and invasion. ARF6 facilitates β1 integrin recycling to the cell surface (Table 1) and colocalizes with β1 integrin at membrane ruffles in HeLa cells, while in MDA-MB-231 breast cancer cells, ARF6 controls β1 integrin-dependent cell migration.56
Degradation of the ECM by matrix metalloproteinases (MMPs) is required for tumor cell invasion. Both microvesicles that are shed from tumor cells and invadopodia release MMPs into the extracellular space, thereby promoting degradation of the ECM and invasion.57 Invadopodia likely act locally to degrade the pericellular ECM, while microvesicles can release MMPs at a more distant location thus creating a path for invasion. The nature of the ECM encountered by the tumor cells may also play a role in determining whether invasion occurs by microvesicle shedding or by invadopodia formation.58 ARF6 activation promotes both microvesicle shedding and invadopodia formation through the activation of Rho and Rac1 pathways (Fig. 1), while expression of a dominant negative ARF6 inhibits both microvesicle shedding and invadopodia formation.59,60
In MDA-MB-231 breast cancer cells, ARF6 is activated following the binding of GEP100 to phosphorylated EGFR, which leads to the formation of invadopodia through the recruitment of the ARF6 effector AMAP1, cortactin, and paxillin (Fig. 2).51,61 This receptor tyrosine kinase (RTK)-GEP100-ARF6-AMAP1 pathway has been implicated in lung cancer as well. In lung adenocarcinoma patient specimens, triple positive immunohistochemical staining for phosphorylated EGFR, ARF6, and AMAP1 was reported to correlate with reduced survival.62 Ectopic expression of HER2/ERBB2 in lung adenocarcinoma cells recruits and activates the GEP100-ARF6-AMAP1 axis and increases invasiveness in vitro in a similar fashion to breast cancer cells.50 New data suggest that ARF6 and AMAP1 may be important in other receptor pathways as well. In renal cell carcinoma, ARF6 is activated by the Gα12 G-protein via the GEF EFA6 following lysophosphatidic acid (LPA) engagement of the GPCR LPAR2 (Table 2).63 This GPCR-ARF6-AMAP1 pathway stimulates invadopodia activity, similar to the phenotype in breast cancer. Thus, RTK and GPCR signaling may employ similar ARF6-AMAP1 mechanisms to facilitate tumor invasion. The RTK-ARF6 data in breast and lung cancer model systems are provocative not only because these cancers are very common, but also because RTK signaling is a well-established driver in cancer and there are numerous FDA-approved therapies directed toward these signaling pathways. Nevertheless, unlike breast cancer, the clinical significance of HER2 abnormalities in lung adenocarcinoma is still uncertain (reviewed in Mar et al.64) and the clinical relevance of EGFR signaling in breast cancer is still under investigation.65 Regardless, these studies demonstrate that ARF6 is a signaling mediator for a variety of RTKs (Table 1, Table 2), suggesting that targeting ARF6 may dampen RTK-driven cancer progression.
ARF6 may also be activated in some tumor types by the loss of tumor suppressors. For example, reduced ROBO1 expression is one of 12 biomarkers used in clinical testing to accurately predict metastasis in uveal melanoma patients,66 and reduced SLIT-ROBO signaling appears to be a common feature of pancreatic ductal adenocarcinoma.67 SLIT2-ROBO4 signaling can suppress adhesion-induced Rac1 activation and endothelial membrane protrusion,26 while SLIT2-ROBO1 inhibits ARF6 activation, invadopodia formation, and invasion of melanoma cells.13
These studies illustrate the important role that ARF6 plays in tumor formation, growth, invasion, and metastasis and highlight how signaling from intracellular endosomes appear to be key to the effect of ARF6 in multiple pathways and cancer types. Furthermore, preclinical pharmacologic studies suggest that ARF6 might be an appropriate target for the development of drugs to treat many types of cancers.
Conclusions
ARF6 controls cellular functions and behaviors both during embryonic development and under certain pathological conditions by directing proteins to intracellular and extracellular locations where they can function efficiently. Although ARF6 is critical for embryonic development, its pharmacological inhibition does not appear to be detrimental in adult animals, suggesting that targeting ARF6 might be an efficacious approach for treating diseases that are driven, at least in part, by the activation of ARF6, including certain eye and inflammatory diseases and cancer.
Disclosure of potential conflicts of interest
DYL is co-founder and Chief Scientific Officer of Navigen, Inc.
Funding
This work was funded by grants to D.Y.L., W.Z., and A.H.G. from the NCI, NINDS, NHLBI, NIAMS, NEI, NCATS, Veterans Affairs, Huntsman Cancer Institute Cell Response and Regulation Program, George S. and Dolores Dore Eccles Foundation, the Ben B. and Iris M. Margolis Foundation, the H. A. and Edna Benning Fund for Medical Research, and the Harold J. Lloyd Charitable Trust (Career Development Melanoma Research Grant).
References
- [1].Kahn RA, Gilman AG. Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J Biol Chem 1984; 259(10):6228-34; PMID:6327671 [PubMed] [Google Scholar]
- [2].Kahn RA, Cherfils J, Elias M, Lovering RC, Munro S, Schurmann A. Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J Cell Biol 2006; 172(5):645-50; PMID:16505163; http://dx.doi.org/ 10.1083/jcb.200512057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dong C, Zhang X, Zhou F, Dou H, Duvernay MT, Zhang P, Wu G. ADP-ribosylation factors modulate the cell surface transport of G protein-coupled receptors. J Pharmacol Exp Ther 2010; 333(1):174-83; PMID:20093398; http://dx.doi.org/ 10.1124/jpet.109.161489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beemiller P, Hoppe AD, Swanson JA. A phosphatidylinositol-3-kinase-dependent signal transition regulates ARF1 and ARF6 during Fcgamma receptor-mediated phagocytosis. PLoS biology 2006; 4(6):e162; PMID:16669702; http://dx.doi.org/ 10.1371/journal.pbio.0040162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen JL, Lacomis L, Erdjument-Bromage H, Tempst P, Stamnes M. Cytosol-derived proteins are sufficient for Arp2/3 recruitment and ARF/coatomer-dependent actin polymerization on Golgi membranes. FEBS Lett 2004; 566(1-3):281-6; PMID:15147909; http://dx.doi.org/ 10.1016/j.febslet.2004.04.061 [DOI] [PubMed] [Google Scholar]
- [6].D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 2006; 7(5):347-58; 2006/04/25; PMID:16633337; http://dx.doi.org/ 10.1038/nrm1910 [DOI] [PubMed] [Google Scholar]
- [7].Hongu T, Kanaho Y. Activation machinery of the small GTPase Arf6. Adv Biol Regul 2014; 54:59-66; PMID:24139303; http://dx.doi.org/ 10.1016/j.jbior.2013.09.014 [DOI] [PubMed] [Google Scholar]
- [8].Jackson CL, Bouvet S. Arfs at a glance. J Cell Sci 2014; 127(Pt 19):4103-9; PMID:25146395; http://dx.doi.org/ 10.1242/jcs.144899 [DOI] [PubMed] [Google Scholar]
- [9].Tang BL. Rab, Arf, and Arl-regulated membrane traffic in cortical neuron migration. J Cell Physiol 2016; 231(7):1417-23; PMID:26587959; http://dx.doi.org/ 10.1002/jcp.25261 [DOI] [PubMed] [Google Scholar]
- [10].Marquer C, Tian H, Yi J, Bastien J, Dall'Armi C, Yang-Klingler Y, Zhou B, Chan RB, Di Paolo G. Arf6 controls retromer traffic and intracellular cholesterol distribution via a phosphoinositide-based mechanism. Nat Commun 2016; 7:11919; PMID:27336679; http://dx.doi.org/ 10.1038/ncomms11919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Donaldson JG, Porat-Shliom N, Cohen LA. Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal 2009; 21(1):1-6. 2754696; 2008/07/24; PMID:18647649; http://dx.doi.org/ 10.1016/j.cellsig.2008.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Parachoniak CA, Luo Y, Abella JV, Keen JH, Park M. GGA3 functions as a switch to promote Met receptor recycling, essential for sustained ERK and cell migration. Dev Cell 2011; 20(6):751-63; PMID:21664574; http://dx.doi.org/ 10.1016/j.devcel.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Grossmann AH, Yoo JH, Clancy J, Sorensen LK, Sedgwick A, Tong Z, Ostanin K, Rogers A, Grossmann KF, Tripp SR, et al.. The small GTPase ARF6 stimulates beta-catenin transcriptional activity during WNT5A-mediated melanoma invasion and metastasis. Sci Signal 2013; 6(265):ra14 3961043; http://dx.doi.org/ 10.1126/scisignal.2003398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jing Z, Wei-Jie Y, Yi-Feng ZG, Jing H. Downregulation of Syndecan-1 induce glomerular endothelial cell dysfunction through modulating internalization of VEGFR-2. Cell Signal 2016; 28(8):826-37; PMID:27075925; http://dx.doi.org/ 10.1016/j.cellsig.2016.04.001 [DOI] [PubMed] [Google Scholar]
- [15].Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J 2011; 437(2):169-83; PMID:21711246; http://dx.doi.org/ 10.1042/BJ20110301 [DOI] [PubMed] [Google Scholar]
- [16].Yoo JH, Shi DS, Grossmann AH, Sorensen LK, Tong Z, Mleynek TM, Rogers A, Zhu W, Richards JR, Winter JM, et al.. ARF6 Is an Actionable Node that Orchestrates Oncogenic GNAQ Signaling in Uveal Melanoma. Cancer Cell 2016; 29(6):889-904; PMID:27265506; http://dx.doi.org/ 10.1016/j.ccell.2016.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Suzuki T, Kanai Y, Hara T, Sasaki J, Sasaki T, Kohara M, Maehama T, Taya C, Shitara H, Yonekawa H, et al.. Crucial role of the small GTPase ARF6 in hepatic cord formation during liver development. Mol Cell Biol 2006; 26(16):6149-56. 1592812; 2006/08/02; PMID:16880525; http://dx.doi.org/ 10.1128/MCB.00298-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhu W, London NR, Gibson CC, Davis CT, Tong Z, Sorensen LK, Shi DS, Guo J, Smith MC, Grossmann AH, et al.. Interleukin receptor activates a MYD88-ARNO-ARF6 cascade to disrupt vascular stability. Nature 2012; 492(7428):252-5; PMID:23143332; http://dx.doi.org/ 10.1038/nature11603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Onel S, Bolke L, Klambt C. The Drosophila ARF6-GEF Schizo controls commissure formation by regulating Slit. Development 2004; 131(11):2587-94; PMID:15148300; http://dx.doi.org/ 10.1242/dev.01147 [DOI] [PubMed] [Google Scholar]
- [20].Chen EH, Pryce BA, Tzeng JA, Gonzalez GA, Olson EN. Control of myoblast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell 2003; 114(6):751-62; PMID:14505574; http://dx.doi.org/ 10.1016/S0092-8674(03)00720-7 [DOI] [PubMed] [Google Scholar]
- [21].Dumas, M. The role of the small GTPase Arf6 during embryogenesis and its potential regulation by microRNA-31. Department of Biological Sciences, University of Delaware 2013. [Google Scholar]
- [22].Hongu T, Funakoshi Y, Fukuhara S, Suzuki T, Sakimoto S, Takakura N, Ema M, Takahashi S, Itoh S, Kato M, Hasegawa H, Mochizuki N, Kanaho Y. Arf6 regulates tumour angiogenesis and growth through HGF-induced endothelial beta1 integrin recycling. Nat Commun 2015; 6:7925; PMID:26239146; http://dx.doi.org/ 10.1038/ncomms8925 [DOI] [PubMed] [Google Scholar]
- [23].Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A 2004; 101(16):6062-7; PMID:15075390; http://dx.doi.org/ 10.1073/pnas.0400782101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res 2002; 12(6):996-1006; PMID:12045153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Davis CT, Zhu W, Gibson CC, Bowman-Kirigin JA, Sorensen L, Ling J, Sun H, Navankasattusas S, Li DY. ARF6 inhibition stabilizes the vasculature and enhances survival during endotoxic shock. J Immunol 2014; 192(12):6045-52; PMID:24835390; http://dx.doi.org/ 10.4049/jimmunol.1400309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jones CA, Nishiya N, London NR, Zhu W, Sorensen LK, Chan AC, Lim CJ, Chen H, Zhang Q, Schultz PG, et al.. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol 2009; 11(11):1325-31; PMID:19855388; http://dx.doi.org/ 10.1038/ncb1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pellon-Cardenas O, Clancy J, Uwimpuhwe H, D'Souza-Schorey C. ARF6-regulated endocytosis of growth factor receptors links cadherin-based adhesion to canonical Wnt signaling in epithelia. Mol Cell Biol 2013; 33(15):2963-75. 3719676; 2013/05/30; PMID:23716594; http://dx.doi.org/ 10.1128/MCB.01698-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim W, Kim SY, Kim T, Kim M, Bae DJ, Choi HI, Kim IS, Jho E. ADP-ribosylation factors 1 and 6 regulate Wnt/β-catenin signaling via control of LRP6 phosphorylation. Oncogene 2013; 32(28):3390-6; PMID:22907437; http://dx.doi.org/ 10.1038/onc.2012.373 [DOI] [PubMed] [Google Scholar]
- [29].Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol 2013; 25(2):254-64; PMID:23347562; http://dx.doi.org/ 10.1016/j.ceb.2013.01.004 [DOI] [PubMed] [Google Scholar]
- [30].Harburg G, Compton J, Liu W, Iwai N, Zada S, Marlow R, Strickland P, Zeng YA, Hinck L. SLIT/ROBO2 signaling promotes mammary stem cell senescence by inhibiting Wnt signaling. Stem Cell Reports 2014; 3(3):385-93; PMID:25241737; http://dx.doi.org/ 10.1016/j.stemcr.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Biteau B, Jasper H. Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep 2014; 7(6):1867-75; PMID:24931602; http://dx.doi.org/ 10.1016/j.celrep.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marlow R, Binnewies M, Sorensen LK, Monica SD, Strickland P, Forsberg EC, Li DY, Hinck L. Vascular Robo4 restricts proangiogenic VEGF signaling in breast. Proc Natl Acad Sci U S A 2010; 107(23):10520-5. 2890778; 2010/05/26; PMID:20498081; http://dx.doi.org/ 10.1073/pnas.1001896107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhou WJ, Geng ZH, Spence JR, Geng JG. Induction of intestinal stem cells by R-spondin 1 and Slit2 augments chemoradioprotection. Nature 2013; 501(7465):107-11; PMID:23903657; http://dx.doi.org/ 10.1038/nature12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hashimoto A, Hashimoto S, Ando R, Noda K, Ogawa E, Kotani H, Hirose M, Menju T, Morishige M, Manabe T, et al.. GEP100-Arf6-AMAP1-cortactin pathway frequently used in cancer invasion is activated by VEGFR2 to promote angiogenesis. PLoS One 2011; 6(8):e23359; PMID:21858086; http://dx.doi.org/ 10.1371/journal.pone.0023359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Palacios F, Price L, Schweitzer J, Collard JG, D'Souza-Schorey C. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J 2001; 20(17):4973-86. 125602; 2001/09/05; PMID:11532961; http://dx.doi.org/ 10.1093/emboj/20.17.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Palacios F, Schweitzer JK, Boshans RL, D'Souza-Schorey C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol 2002; 4(12):929-36; PMID:12447393; http://dx.doi.org/ 10.1038/ncb881 [DOI] [PubMed] [Google Scholar]
- [37].Hiroi T, Someya A, Thompson W, Moss J, Vaughan M. GEP100/BRAG2: activator of ADP-ribosylation factor 6 for regulation of cell adhesion and actin cytoskeleton via E-cadherin and alpha-catenin. Proc Natl Acad Sci U S A 2006; 103(28):10672-7; PMID:16807291; http://dx.doi.org/ 10.1073/pnas.0604091103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Miura K, Nam JM, Kojima C, Mochizuki N, Sabe H. EphA2 engages Git1 to suppress Arf6 activity modulating epithelial cell-cell contacts. Mol Biol Cell 2009; 20(7):1949-59; PMID:19193766; http://dx.doi.org/ 10.1091/mbc.E08-06-0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, et al.. Hippo-Independent Activation of YAP by the GNAQ Uveal Melanoma Oncogene through a Trio-regulated Rho GTPase signaling circuitry. Cancer Cell 2014; 25(6):831-45. 4074519; PMID:24882515; http://dx.doi.org/ 10.1016/j.ccr.2014.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, Simpson EM, Barsh GS, Bastian BC. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009; 457(7229):599-602. 2696133; PMID:19078957; http://dx.doi.org/ 10.1038/nature07586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, et al.. Mutations in GNA11 in uveal melanoma. N Engl J Med 2010; 363(23):2191-9. 3107972; PMID:21083380; http://dx.doi.org/ 10.1056/NEJMoa1000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, et al.. Mutant Gq/11 Promote Uveal Melanoma Tumorigenesis by Activating YAP. Cancer cell 2014; 25(6):822-30. 4075337; PMID:24882516; http://dx.doi.org/ 10.1016/j.ccr.2014.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bill A, Schmitz A, Albertoni B, Song JN, Heukamp LC, Walrafen D, Thorwirth F, Verveer PJ, Zimmer S, Meffert L, et al.. Cytohesins are cytoplasmic ErbB receptor activators. Cell 2010; 143(2):201-11; 2010/10/16; PMID:20946980; http://dx.doi.org/ 10.1016/j.cell.2010.09.011 [DOI] [PubMed] [Google Scholar]
- [44].Knizhnik AV, Kovaleva OV, Komelkov AV, Trukhanova LS, Rybko VA, Zborovskaya IB, Tchevkina EM. Arf6 promotes cell proliferation via the PLD-mTORC1 and p38MAPK pathways. J Cell Biochem 2012; 113(1):360-71; PMID:21928324; http://dx.doi.org/ 10.1002/jcb.23362 [DOI] [PubMed] [Google Scholar]
- [45].Li M, Wang J, Ng SS, Chan CY, He ML, Yu F, Lai L, Shi C, Chen Y, Yew DT, Kung HF, Lin MC. Adenosine diphosphate-ribosylation factor 6 is required for epidermal growth factor-induced glioblastoma cell proliferation. Cancer 2009; 115(21):4959-72; 2009/07/31; PMID:19642173; http://dx.doi.org/ 10.1002/cncr.24550 [DOI] [PubMed] [Google Scholar]
- [46].Dickinson RE, Duncan WC. The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction 2010; 139(4):697-704; PMID:20100881; http://dx.doi.org/ 10.1530/REP-10-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schweitzer JK, Sedgwick AE, D'Souza-Schorey C. ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis. Semin Cell Dev Biol 2011; 22(1):39-47; 2010/09/15; PMID:20837153; http://dx.doi.org/ 10.1016/j.semcdb.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hashimoto A, Oikawa T, Hashimoto S, Sugino H, Yoshikawa A, Otsuka Y, Handa H, Onodera Y, Nam JM, Oneyama C, et al.. P53- and mevalonate pathway-driven malignancies require Arf6 for metastasis and drug resistance. J Cell Biol 2016; 213(1):81-95; PMID:27044891; http://dx.doi.org/ 10.1083/jcb.201510002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hu B, Shi B, Jarzynka MJ, Yiin JJ, D'Souza-Schorey C, Cheng SY. ADP-ribosylation factor 6 regulates glioma cell invasion through the IQ-domain GTPase-activating protein 1-Rac1-mediated pathway. Cancer Res 2009; 69(3):794-801. 2633432; 2009/01/22; PMID:19155310; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Menju T, Hashimoto S, Hashimoto A, Otsuka Y, Handa H, Ogawa E, Toda Y, Wada H, Date H, Sabe H. Engagement of overexpressed Her2 with GEP100 induces autonomous invasive activities and provides a biomarker for metastases of lung adenocarcinoma. PLoS One 2011; 6(9):e25301 3178645; http://dx.doi.org/ 10.1371/journal.pone.0025301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Morishige M, Hashimoto S, Ogawa E, Toda Y, Kotani H, Hirose M, Wei S, Hashimoto A, Yamada A, Yano H, et al.. GEP100 links epidermal growth factor receptor signalling to Arf6 activation to induce breast cancer invasion. Nat Cell Biol 2008; 10(1):85-92; 2007/12/18; PMID:18084281; http://dx.doi.org/ 10.1038/ncb1672 [DOI] [PubMed] [Google Scholar]
- [52].Tague SE, Muralidharan V, D'Souza-Schorey C. ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc Natl Acad Sci U S A 2004; 101(26):9671-6. 470733; 2004/06/24; PMID:15210957; http://dx.doi.org/ 10.1073/pnas.0403531101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xu R, Zhang Y, Gu L, Zheng J, Cui J, Dong J, Du J. Arf6 regulates EGF-induced internalization of E-cadherin in breast cancer cells. Cancer Cell Int 2015; 15(1):11; PMID:25678857; http://dx.doi.org/ 10.1186/s12935-015-0159-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tushir JS, D'Souza-Schorey C. ARF6-dependent activation of ERK and Rac1 modulates epithelial tubule development. EMBO J 2007; 26(7):1806-19; PMID:17363898; http://dx.doi.org/ 10.1038/sj.emboj.7601644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kon S, Tanabe K, Watanabe T, Sabe H, Satake M. Clathrin dependent endocytosis of E-cadherin is regulated by the Arf6GAP isoform SMAP1. Exp Cell Res 2008; 314(7):1415-28; PMID:18331728; http://dx.doi.org/ 10.1016/j.yexcr.2007.11.006 [DOI] [PubMed] [Google Scholar]
- [56].Powelka AM, Sun J, Li J, Gao M, Shaw LM, Sonnenberg A, Hsu VW. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic 2004; 5(1):20-36; PMID:14675422; http://dx.doi.org/ 10.1111/j.1600-0854.2004.00150.x [DOI] [PubMed] [Google Scholar]
- [57].Muralidharan-Chari V, Clancy JW, Sedgwick A, D'Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 2010; 123(Pt 10):1603-11; PMID:20445011; http://dx.doi.org/ 10.1242/jcs.064386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sedgwick AE, Clancy JW, Olivia Balmert M, D'Souza-Schorey C. Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion. Sci Rep 2015; 5:14748; PMID:26458510; http://dx.doi.org/ 10.1038/srep14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D'Souza-Schorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 2009; 19(22):1875-85; 2009/11/10; PMID:19896381; http://dx.doi.org/ 10.1016/j.cub.2009.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Muralidharan-Chari V, Hoover H, Clancy J, Schweitzer J, Suckow MA, Schroeder V, Castellino FJ, Schorey JS, D'Souza-Schorey C. ADP-ribosylation factor 6 regulates tumorigenic and invasive properties in vivo. Cancer Res 2009; 69(6):2201-9; 2009/03/12; PMID:19276388; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Onodera Y, Hashimoto S, Hashimoto A, Morishige M, Mazaki Y, Yamada A, Ogawa E, Adachi M, Sakurai T, Manabe T, et al.. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J 2005; 24(5):963-73. 554134; PMID:15719014; http://dx.doi.org/ 10.1038/sj.emboj.7600588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Oka S, Uramoto H, Shimokawa H, Yamada S, Tanaka F. Epidermal growth factor receptor-GEP100-Arf6 axis affects the prognosis of lung adenocarcinoma. Oncology 2014; 86(5-6):263-70; PMID:24902879; http://dx.doi.org/ 10.1159/000360089 [DOI] [PubMed] [Google Scholar]
- [63].Hashimoto S, Mikami S, Sugino H, Yoshikawa A, Hashimoto A, Onodera Y, Furukawa S, Handa H, Oikawa T, Okada Y, et al.. Lysophosphatidic acid activates Arf6 to promote the mesenchymal malignancy of renal cancer. Nat Commun 2016; 7:10656; PMID:26854204; http://dx.doi.org/ 10.1038/ncomms10656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mar N, Vredenburgh JJ, Wasser JS. Targeting HER2 in the treatment of non-small cell lung cancer. Lung Cancer 2015; 87(3):220-5; PMID:25601485; http://dx.doi.org/ 10.1016/j.lungcan.2014.12.018 [DOI] [PubMed] [Google Scholar]
- [65].Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, Ueno NT. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat 2012; 136(2):331-45; PMID:23073759; http://dx.doi.org/ 10.1007/s10549-012-2289-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn 2010; 12(4):461-8. 2893630; 2010/04/24; PMID:20413675; http://dx.doi.org/ 10.2353/jmoldx.2010.090220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, et al.. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012; 491(7424):399-405; PMID:23103869; http://dx.doi.org/ 10.1038/nature11547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Palacios F, D'Souza-Schorey C. Modulation of Rac1 and ARF6 activation during epithelial cell scattering. J Biol Chem 2003; 278(19):17395-400; PMID:12609992; http://dx.doi.org/ 10.1074/jbc.M300998200 [DOI] [PubMed] [Google Scholar]
- [69].Tushir JS, Clancy J, Warren A, Wrobel C, Brugge JS, D'Souza-Schorey C. Unregulated ARF6 activation in epithelial cysts generates hyperactive signaling endosomes and disrupts morphogenesis. Mol Biol Cell 2010; 21(13):2355-66; PMID:20462959; http://dx.doi.org/ 10.1091/mbc.E09-09-0824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bryant DM, Wylie FG, Stow JL. Regulation of endocytosis, nuclear translocation, and signaling of fibroblast growth factor receptor 1 by E-cadherin. Mol Biol Cell 2005; 16(1):14-23; PMID:15509650; http://dx.doi.org/ 10.1091/mbc.E04-09-0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Luiskandl S, Woller B, Schlauf M, Schmid JA, Herbst R. Endosomal trafficking of the receptor tyrosine kinase MuSK proceeds via clathrin-dependent pathways, Arf6 and actin. FEBS J 2013; 280(14):3281-97; PMID:23621612; http://dx.doi.org/ 10.1111/febs.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kanamarlapudi V, Thompson A, Kelly E, Lopez Bernal A. ARF6 activated by the LHCG receptor through the cytohesin family of guanine nucleotide exchange factors mediates the receptor internalization and signaling. J Biol Chem 2012; 287(24):20443-55; PMID:22523074; http://dx.doi.org/ 10.1074/jbc.M112.362087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Macia E, Partisani M, Paleotti O, Luton F, Franco M. Arf6 negatively controls the rapid recycling of the beta2 adrenergic receptor. J Cell Sci 2012; 125(Pt 17):4026-35; PMID:22611259; http://dx.doi.org/ 10.1242/jcs.102343 [DOI] [PubMed] [Google Scholar]
- [74].Giguere P, Rochdi MD, Laroche G, Dupre E, Whorton MR, Sunahara RK, Claing A, Dupuis G, Parent JL. ARF6 activation by Galpha q signaling: Galpha q forms molecular complexes with ARNO and ARF6. Cell Signal 2006; 18(11):1988-94; 2006/05/03; PMID:16650966; http://dx.doi.org/ 10.1016/j.cellsig.2006.03.003 [DOI] [PubMed] [Google Scholar]
- [75].Kanamarlapudi V, Owens SE, Saha K, Pope RJ, Mundell SJ. ARF6-dependent regulation of P2Y receptor traffic and function in human platelets. PLoS One 2012; 7(8):e43532 3420901; http://dx.doi.org/ 10.1371/journal.pone.0043532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zimmermann P, Zhang Z, Degeest G, Mortier E, Leenaerts I, Coomans C, Schulz J, N'Kuli F, Courtoy PJ, David G. Syndecan recycling corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell 2005; 9(3):377-88; PMID:16139226; http://dx.doi.org/ 10.1016/j.devcel.2005.07.011 [DOI] [PubMed] [Google Scholar]
- [77].D'Souza-Schorey C, Li G, Colombo MI, Stahl PD. A regulatory role for ARF6 in receptor-mediated endocytosis. Science 1995; 267(5201):1175-8; PMID:7855600; http://dx.doi.org/ 10.1126/science.7855600 [DOI] [PubMed] [Google Scholar]
- [78].Boshans RL, Szanto S, van Aelst L, D'Souza-Schorey C. ADP-ribosylation factor 6 regulates actin cytoskeleton remodeling in coordination with Rac1 and RhoA. Mol Cell Biol 2000; 20(10):3685-94; PMID:10779358; http://dx.doi.org/ 10.1128/MCB.20.10.3685-3694.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Marchesin V, Montagnac G, Chavrier P. ARF6 promotes the formation of Rac1 and WAVE-dependent ventral F-actin rosettes in breast cancer cells in response to epidermal growth factor. PLoS One 2015; 10(3):e0121747; PMID:25799492; http://dx.doi.org/ 10.1371/journal.pone.0121747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 2008; 134(1):135-47; PMID:18614017; http://dx.doi.org/ 10.1016/j.cell.2008.05.034 [DOI] [PubMed] [Google Scholar]
- [81].Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci 1999; 112 ( Pt 6):855-66; PMID:10036235 [DOI] [PubMed] [Google Scholar]
- [82].Santy LC, Ravichandran KS, Casanova JE. The DOCK180/Elmo complex couples ARNO-mediated Arf6 activation to the downstream activation of Rac1. Curr Biol 2005; 15(19):1749-54; 2005/10/11; PMID:16213822; http://dx.doi.org/ 10.1016/j.cub.2005.08.052 [DOI] [PubMed] [Google Scholar]


