ABSTRACT
Here we describe a novel bifunctional fusion protein, designated N-809. This molecule comprises the IL-15/IL15Rα superagonist complex containing the Fc-domain of IgG1 (N-803, formerly designated as ALT-803) fused to two single chain anti-PD-L1 domains. The fully human IgG1 portion of the N-809 molecule was designed to potentially mediate antibody dependent cellular cytotoxicity (ADCC). The studies reported here show that N-809 has the same ability to bind PD-L1 as an anti-PD-L1 monoclonal antibody. RNAseq studies show the ability of N-809 to alter the expression of an array of genes of both CD4+ and CD8+ human T cells, and to enhance their proliferation; CD8+ T cells exposed to N-809 also have enhanced ability to lyse human tumor cells. An array of genes was differentially expressed in human natural killer (NK) cells following N-809 treatment, and there was increased expression of several surface activating receptors; there was, however, no increase in the expression of inhibitory receptors known to be upregulated in exhausted NK cells. N-809 also increased the cytotoxic potential of NK cells, as shown by increased expression of granzyme B and perforin. The lysis of several tumor cell types was increased when either NK cells or tumor cells were exposed to N-809. Similarly, the highest level of ADCC was seen when both NK cells (from donors or cancer patients) and tumor cells were exposed to N-809. These studies thus demonstrate the multi-functionality of this novel agent.
KEYWORDS: ALT-803, N-803, IL-15, N-809, anti-PD-L1, immunotherapy, checkpoint inhibitor, cytokine, carcinoma, ADCC
Introduction
Cancer immunotherapy studies have now demonstrated promising clinical response rates in patients with melanoma and subsets of patients with other solid tumors. Those studies have involved monoclonal antibody (MAb) checkpoint inhibitors such as anti-CTLA4, anti‒programmed cell death-1 (PD-1), and anti‒programmed cell death ligand-1 (PD-L1), as well as cytokines such as IL-2 and IL-15.1–5 More recently, combination therapies employing two different checkpoint inhibitors or other immune stimulatory agents are being studied to enhance therapeutic outcomes.6–8
Most antibodies directed against PD-1/PD-L1 are of the IgG4 isotype, or of the IgG1 isotype engineered with an Fc domain mutation to impair antibody dependent cellular cytotoxicity (ADCC) activity. Multiple anti-cancer MAbs, such as anti-CTLA4 (ipilimumab), anti-CD20 (rituximab), anti-HER2 (trastuzumab, pertuzumab), and anti-EGFR (cetuximab), however, are of the IgG1 isotype, and thus have the potential to mediate ADCC. The ADCC mechanism has been implicated to contribute to clinical efficacy,9–11 although other studies have not supported this finding. Atezolizumab (Tecentriq®, Genentech) and avelumab (Bavencio®, EMD Serono) are fully human anti-PD-L1 therapies of the IgG1 isotype that have been FDA approved for the treatment of non-small cell lung cancer (NSCLC), bladder cancer, urothelial cancer, and metastatic Merkel cell carcinoma.12–15
Since PD-L1 is expressed on some immune cells, studies were conducted to evaluate avelumab-mediated ADCC using whole peripheral blood mononuclear cells (PBMC) as targets. Using natural killer (NK) cells from healthy donors and cancer patients, substantial lysis of a range of human tumor cell types was observed, with little or no lysis when human PBMC subsets were used as targets. Similar results were also seen in the analysis of 123 immune cell subsets from PBMC of patients treated with up to nine doses of avelumab.13,16 Moreover, while clinical benefit of using avelumab has been observed in a range of human tumors, adverse events beyond those seen with other anti-PD1/PD-L1 MAbs have not been observed.3,14,17,18
Despite the promising results described above, only 10–30% of patients with most carcinomas achieve objective responses when treated with anti-PD-1/PD-L1 monotherapies, even in trials that enrolled only those patients whose pre-treatment tumor specimens expressed PD-L1.19 One possible reason for this lack of response could be the absence of an inflammatory tumor microenvironment (TME), including activated anti-tumor CD8+ and CD4+ T cells as well as other effectors such as NK cells. The addition of an immune activating cytokine could potentially help to resolve this issue.
IL-15 is a pleiotropic cytokine that is essential for the regulation of many immune functions, most critically the development, proliferation, and activation of CD8+ memory T cells and NK cells. In contrast to IL-2, IL-15 does not support regulatory T cell (Treg) maintenance and proliferation.20–22 A first-in-human clinical trial found that patients administered recombinant human (rh)IL-15 showed significant increases in γδ T cells, CD8+ T cells, and NK cell frequencies, but the high doses resulted in toxicities and limited tumor responses.2 A relatively short half-life of the prokaryotic rhIL-15 was also observed.23
ALT-803, an IL-15 superagonist composed of an IL-15 N72D mutant and an IL-15RαSushi-Fc fusion complex, has recently been developed. ALT-803 has now been designated N-803; see Materials and Methods. This agent has been demonstrated to stimulate T cells and NK cells, induce memory CD8+ T cell proliferation, and have a longer half-life than rhIL-15.24–26 The combination of N-803 with various therapeutic antibodies has also previously been shown to increase ADCC by enhancing NK cell activity.27,28 In a murine glioblastoma model, administration of N-803 resulted in a durable anti-tumor immune response, including the generation of anti-tumor immune memory, and the combination of N-803 and anti-PD-L1 MAb resulted in prolonged survival.29 N-803 has also been shown to elicit anti-tumor activity in murine colon and breast carcinoma models along with enhanced CD8+ T and NK cell activities.30 In a Phase 1B trial of N-803 plus bacillus Calmette-Guerin (BCG) in patients with non-muscle-invasive bladder cancer (NMIBC), complete responses were observed in 100% of patients at 12 months,31 compared to BCG treatment alone, which typically results in disease recurrence in 50% of patients within 12 months.32 Furthermore, in an additional Phase 1B trial in patients with relapsed hematological malignancies after allogeneic stem cell transplantation, N-803 used as a single agent was shown to exhibit clinical efficacy without inducing severe graft vs. host disease.33 N-803 was shown to profoundly activate and promote proliferation of immune cells, particularly NK cells, in patients with cancer.33
Prior studies have shown the additive anti-tumor effects in preclinical models of the combined use of recombinant (r)IL-15 protein with an anti-PDL1 MAb when each agent was administered systemically.34,35 In a recent clinical study, Wrangle et al. employed N-803 in patients undergoing anti-PD1 therapy, or employed the combination in patients who had failed anti-PD1 therapy with evidence of clinical activity.36 Here, we describe a novel bifunctional fusion protein, designated N-809. This molecule comprises the IL-15 N72D/IL-15RαSushi complex containing the Fc-domain of IgG1 (formerly ALT-803, now designated N-803) fused to two single chain anti-PD-L1 variable region (ScFv) domains. The fully human IgG1 portion of the N-809 molecule was designed to potentially mediate ADCC. The rationale for designing this PD-L1 targeting molecule is three-fold: (a) to inhibit PD-1/PD-L1 interaction, and thus increase T-cell activity; (b)to mediate ADCC of PD-L1+ tumor cells; and (c) to bring the IL-15 agent to the TME, thereby stimulating the T cells and NK cells locally, while also potentially reducing IL-15 systemic toxicity. In addition, the clinical use of one agent vs. two agents would potentially reduce cost and efforts to both patients and health care professionals. The studies described here demonstrate the multi-functionality of the N-809 molecule.
Results
Effects of N-809 on CD4+ T-cell proliferation, gene expression, and cytokine secretion
The structure of N-809 is shown in Figure 1(a). It consists of an IL-15 N72D/IL-15RαSushi complex containing the Fc-domain of IgG1 (N-803) fused to two anti-PD-L1 ScFv domains. Binding of N-809 to PD-L1 on H441 human lung carcinoma cells, compared to the binding of an anti-PD-L1 MAb, is shown in Figure 1(b).
Figure 1.
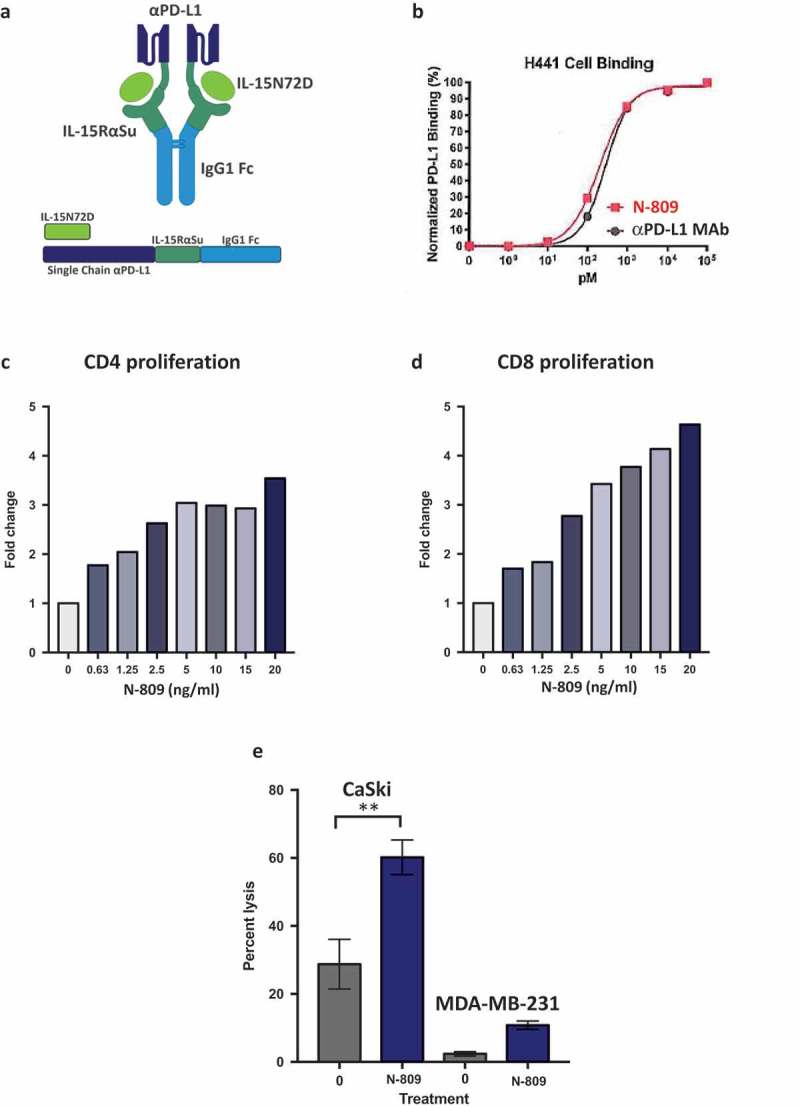
Schematic diagram of N-809, PD-L1 binding, and effects of N-809 on the proliferation and activity of CD4 and CD8 T cells. (a) Illustration of N-809, a bifunctional molecule created by fusing two single chain human anti-PD-L1 domains to an IL-15 superagonist scaffold with an IgG1 Fc portion. (b) Binding of N-809 to PD-L1+ H441 cells was analyzed by flow cytometry using APC labeled antibody specific for the Fc portion of hIgG. (c, d) Healthy donor CD4 and CD8 T cells were stimulated with plate-bound anti-CD3 and increasing concentrations of N-809. Fold change represents the change from untreated (0 ng/ml), anti-CD3 stimulated T cells for one representative donor. (e) Tumor cell lysis by an HPV E7-specific T cell line grown in the presence or absence of N-809 (37.5 ng/ml). Results of a lysis assay using CaSki (cervical carcinoma) cells as targets at a 10:1 E:T ratio is shown. MDA-MB-231 (breast carcinoma) cells were used as a control to determine specificity of the lysis. **P < 0.01.
One of the known functions of recombinant IL-15 is to stimulate the proliferation of activated human CD4+ and CD8+ T cells.22 To determine if N-809 maintains this effect, human CD4+ T cells were incubated with anti-CD3 antibody in the presence or absence of N-809 at different concentrations. As seen in Figure 1(c), N-809 increased the proliferation of CD4+ T cells at concentrations as low as 0.63 ng/ml, and at higher concentrations induced a 3.5-fold increase in proliferation compared to the anti-CD3‒only stimulated control. Similar results were observed employing PBMC from three additional healthy donors (Supplemental Table S1).
RNAseq analysis was used to determine the effects of N-809 treatment on gene expression of CD4+ T cells (Figure 2(a), (b) and (e)). Using the NantOmics panel of immune-related genes, 149 genes were up- or downregulated ≥2-fold, 59 genes changed ≥3-fold, and 6 genes changed ≥10-fold in CD4+ T cells from donor PBMC following N-809 treatment. A human 8-plex cytokine analysis was also performed to determine if CD4+ T-cell cytokine secretion changed when treated with N-809, compared to untreated cells. Cells from two healthy donors were analyzed, with significant increases in IFNγ, TNFα, IL-6 and IL-8 secretion observed for one donor following N-809 treatment (Supplemental Table S2). To evaluate if there were any major differences between the CD4 T cells from the two donors, NanoString analysis was performed. As can be seen in Supplemental Figure S1, many of the same genes were up- or downregulated in both donors.
Figure 2.
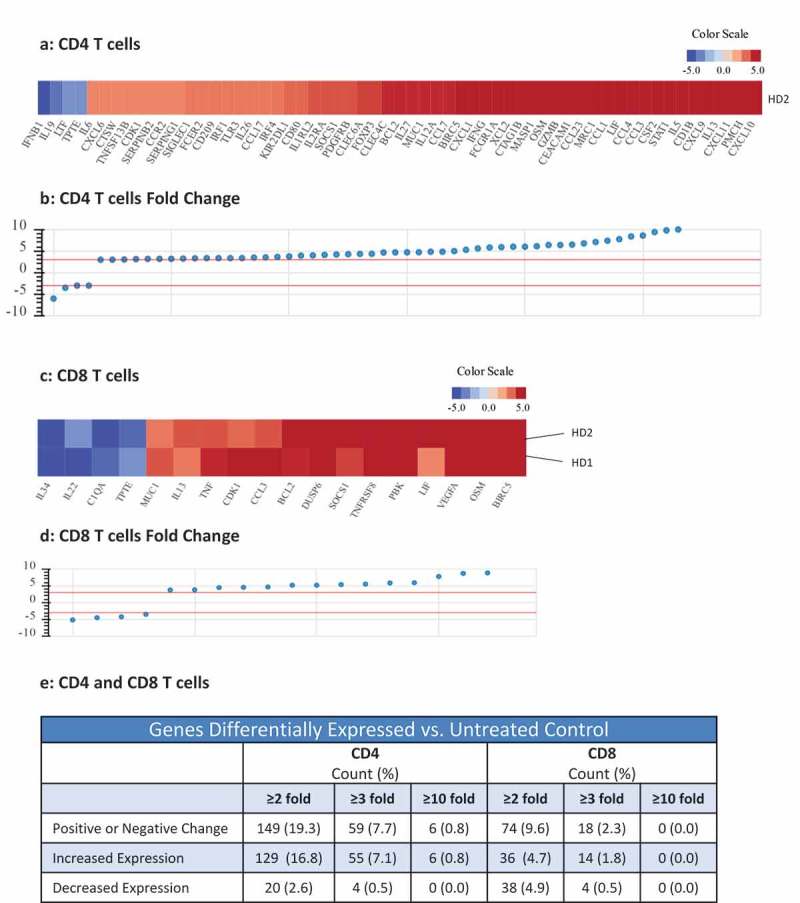
Effects of N-809 treatment on CD4 and CD8 T cell gene expression. Healthy donor (HD) CD4 and CD8 T cells were incubated ±N-809 (37.5 ng/ml) for 24h prior to RNA isolation for RNASeq analysis using the NantOmics panel of immune related genes. (a, c) Heat maps depicting gene expression analysis for genes that were up- or downregulated ≥3-fold after N-809 treatment compared to no treatment, for CD4 (a, one donor) or CD8 (c, two donors) T cells. (b, d) Graphs depicting the fold change for the genes in a and c, respectively. (e) Table showing the number of genes differentially expressed in N-809 treated cells vs. the untreated control.
Effects of N-809 on CD8+ T-cell proliferation, gene expression, cytokine secretion, and antigen-specific tumor cell lysis
The effects of N-809 treatment on human CD8+ T-cell proliferation were even more pronounced than that observed on CD4+ T cells. Increase in proliferation was directly proportional to the concentration of N-809 added, with as much as a 4.6-fold increase in CD8+ T-cell proliferation compared to the anti-CD3 only stimulated control (Figure 1(d)). Similar results were observed employing PBMC from three additional healthy donors (Supplemental Table S1). In addition, CD4 and CD8 T-cell proliferation assays comparing N-803 and N-809 were performed. The increased proliferation observed was of the same magnitude for both N-803‒ and N-809‒treated T cells (Supplemental Figure S2). RNAseq analysis showed differential effects of N-809 on human CD8+ T cells than seen with CD4+ T cells (Figure 2(c), (d) and (e)). Using the NantOmics panel of immune-related genes, 74 genes were up- or downregulated ≥2-fold, and 18 genes changed ≥3-fold in both donors following N-809 treatment. N-809 had little to no effect on the secretion of cytokines from CD8+ T cells isolated from two healthy donors (Supplemental Table S2). Treatment of an HLA-A2+ T-cell line specific for the HPV16 E711-19 epitope37 with N-809 increased the lysis of the HLA-A2+ HPV+ CaSki cervical cancer tumor cell line (Figure 1(e)). Lysis of the HLA-A2+, HPV‒ tumor cell line MDA-MB-231 was low, and increased slightly after treatment of the T cells with N-809.
N-809 induced phenotypic and gene expression changes in NK cells
The expression levels of cell surface and intracellular phenotypic markers were evaluated by flow cytometry in NK cells treated with N-809 (Figure 3(a)) using PBMC from four healthy donors. Of the 31 markers analyzed, increases in expression levels of several proteins were observed in all four donors. The results from one representative donor are shown in Figure 3(a). Increases were seen in activation receptors 4-1BB, DNAM-1, CD69, CD161, NKp30, NKp44, NKG2D and Tim-3; inhibitory receptor NKG2A; proliferation marker Ki-67; the IL-2Rα (CD25); cell death markers CD40L and CD95; as well as cytolytic molecules granzyme B and perforin. A decrease in expression was seen in the activation marker NKp46. Figure 3(b–e) show representative histograms of the expression levels of Tim-3 (b), 4-1BB (c), CD69 (d), and CD25 (e) in untreated (blue outline) and N-809‒treated (red shaded) NK cells. Supplemental Table S3 shows the results for the additional three donors. RNAseq studies were also conducted to evaluate the differences in gene expression between untreated and N-809‒treated NK cells. Using the NantOmics panel of immune-related genes, 98 genes were up- or downregulated ≥3-fold after N-809 treatment of NK cells from both healthy donors tested (Figure 4). Out of those 98 genes, 58 showed increased expression, many of which are involved in NK cell activation pathways; 40 showed decreased expression. In addition, 20 genes were upregulated ≥10-fold after N-809 treatment (Figure 4(c); Supplemental Table S4). N-809 also greatly affected the levels of cytokine secretion by NK cells. IFNγ, TNFα, and IL-8 levels substantially increased in NK cells from both donors, and IL-6 levels increased in one donor after N-809 treatment (Supplemental Table S2). The greatest increases were seen in IFNγ levels, from <1.6 to 4688 pg/ml for donor 1 and <1.6 to 528 pg/ml for donor 2 following N-809 treatment. To further validate the contribution of IL-15 to the changes observed in NK cell gene expression, NanoString analysis was conducted on untreated NK cells, and NK cells treated with N-803 or N-809. As can be seen in Supplemental Figure S3, treatment with either N-803 or N-809 resulted in modulation (upregulation or downregulation) of the same genes, suggesting little or no contribution of the anti-PDL1 moiety in this case.
Figure 3.
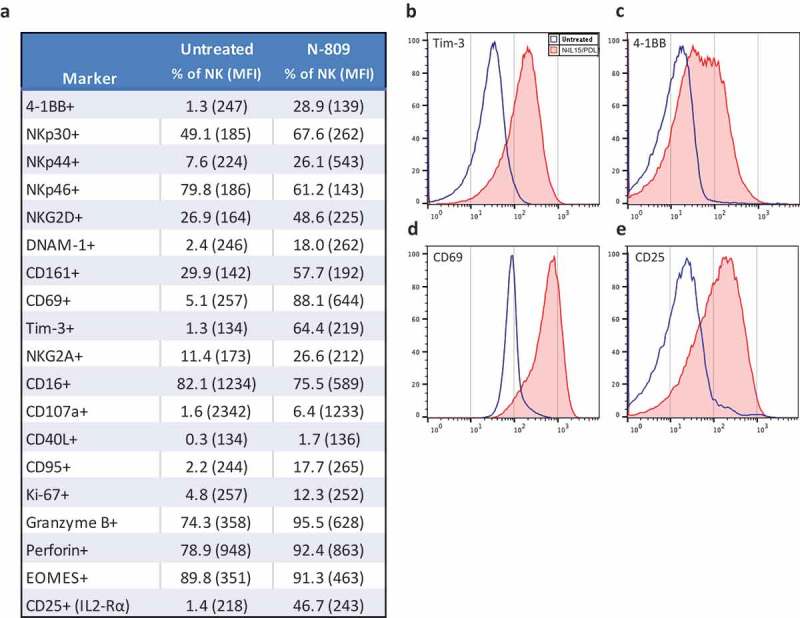
Effects of N-809 on NK cell phenotypic markers. Healthy donor NK cells were incubated ±N-809 (37.5 ng/ml) for 24h before being stained for multicolor flow cytometry. (a) Table of markers with increased or decreased expression after N-809 treatment. (b) Representative histograms of four phenotypic NK markers showing the change in expression between untreated cells (blue outline) and N-809‒treated cells (red shaded). Perforin expression was >90% for 3/4 donors, with a slight increase in percent expression and MFI after N-809 treatment, whereas the donor with the lowest expression levels (78.9%) had a greater increase in perforin with treatment. Markers that were stained for, but did not consistently change with treatment: CD11a, CD11c, CD158a, CD56, CD27, FasL, TRAIL, PD-L1, and 2B4. Similar results were seen for three additional donors (Supplemental Table S3).
Figure 4.
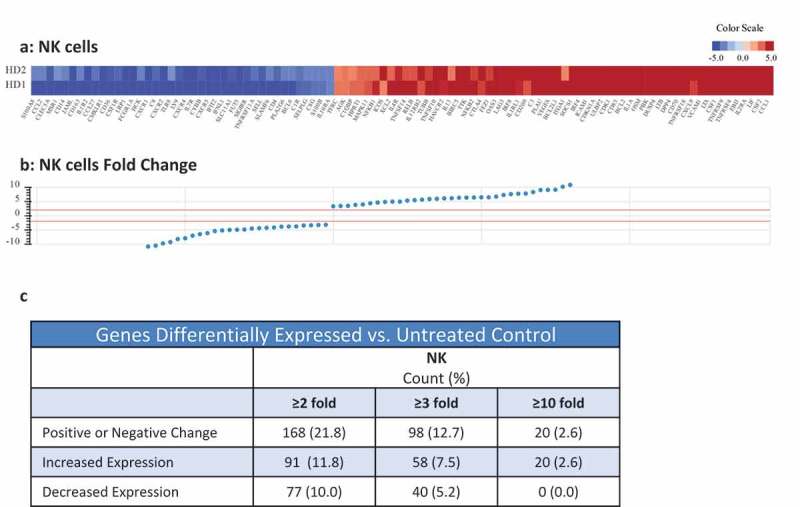
Effects of N-809 treatment on NK cell gene expression. Healthy donor NK cells were incubated ±N-809 (37.5 ng/ml) for 24h prior to RNA isolation for RNASeq analysis using the NantOmics panel of immune related genes. (a) Heat map of gene expression analysis for genes that were up- or downregulated ≥3-fold in both donors after N-809 treatment compared to no treatment. (b) Graph depicting the fold change for the genes in (a). (c) Table showing the number of genes differentially expressed in N-809 treated cells vs. the untreated control.
N-809‒treated human PBMC
We also analyzed the effect of N-809 on human PBMC in vitro employing the 123 immune cell subset assay as previously described.16 These immune cell subsets include maturation and activation markers on CD4 and CD8 T cells, B cells, dendritic cells, NK cells, and myeloid derived suppressor cells (MDSCs). No immune cell subsets were depleted by N-809 treatment. The subsets with the most significant changes include a decrease in monocytic MDSCs, an increase in Tregs, and an increase in Tim-3 expression on NK cells, mature (CD56dimCD16+) NK cells, and immature (CD56brCD16−) NK cells (Supplemental Figure S4). An increase in Tim-3 expression on these NK cell subsets marks an increase in highly functional NK cells with N-809 exposure.
The effect of N-809 on NK cell-mediated tumor cell lysis
To determine if N-809 treatment would increase NK cell lytic activity, human NK cells were treated for 24 hours with N-809 at different concentrations, washed to remove N-809, and then incubated with 111In-labeled human tumor cells (Figure 5(a)). Figure 5 shows representative results using NK cells from one healthy donor treated with various concentrations of N-809, using as targets human lung carcinoma cells (H441, Figure 5(b)), human cervical carcinoma cells (CaSki, Figure 5(c)), and human breast carcinoma cells (MDA-MB-231, Figure 5(d)). N-809 treatment of NK cells resulted in higher levels of tumor cell lysis than untreated control (0 ng/ml). There was no variability in NK-cell viability with increased doses, and up to 180 ng/ml was assayed. Similar results were observed using NK cells from three additional donors. One additional donor is shown in Supplemental Figure S5.
Figure 5.
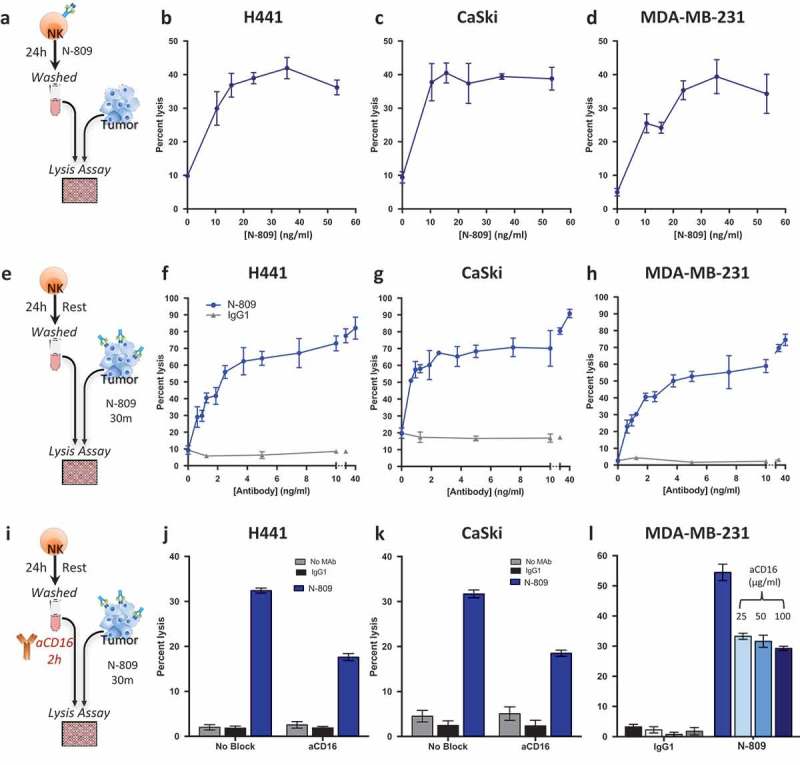
Treatment of NK cells with, or exposure of tumor cells to N-809 increased NK lysis. (a, e, i) Schematics of experimental procedures. All tumor lysis assays were performed using as targets: H441 (lung carcinoma), CaSki (cervical carcinoma), and MDA-MB-231 (breast carcinoma) at a 10:1 E:T ratio. Results from one representative donor are shown for each experiment. (b–d) NK cells were treated ±different concentrations of N-809 prior to being added to the tumor cells. (f-h): Tumor cells were exposed to IgG1 control or N-809 at concentrations up to 40 ng/ml before addition of untreated NK cells. (j, k) Tumor cells were exposed to no MAb, IgG1 control, or N-809 (3.75 ng/ml) before NK cells were added. NK cells had been pre-incubated ±anti-CD16 MAb (25 µg/ml). (l) MDA-MB-231 cells were exposed to N-809 (10 ng/ml). NK cells had been pre-incubated ±anti-CD16 MAb (25–100 µg/ml).
Effect of exposure of tumor cells to N-809 on NK cell lysis and ADCC
Since N-809 contains an IgG1 domain, studies were performed to determine whether the N-809 agent could also mediate ADCC using NK cells as effectors. Flow cytometry was performed to define the expression of PD-L1 on the H441, CaSki, and MDA-MB-231 tumor cell lines, and each expressed PD-L1 at varying levels (Supplemental Table S5). As shown in Figure 5(e–h), a 30-minute pre-incubation of tumor cells with extremely low levels of N-809 greatly increased NK cell‒mediated lysis of each of the three tumor cell lines. Tumor cells exposed to a non-tumor targeting IgG1 were used as controls, and no enhanced lysis was observed under these conditions. One additional donor is shown in Supplemental Figure S5. To determine how much of the tumor lysis could be attributed to the IgG1 portion of N-809, the ADCC mechanism was blocked by pretreating the NK cells with anti-CD16 MAb (Figure 5(i)). As Figure 5(j) shows, approximately 50% of the H441 tumor cell lysis could be blocked by anti-CD16 treatment. Similar results were seen using CaSki (Figure 5(k)) and MDA-MB-231 cells (Figure 5(l) and Supplemental Table S6). Similar results from an additional donor are shown in Supplemental Figure S5. Additional higher and lower concentrations of N-809 were also employed with similar results (Supplemental Table S6). To ensure that all Fc receptors were thoroughly blocked on the NK cells, high concentrations of anti-CD16 antibody (25, 50, and 100 µg/ml) were used to pretreat the NK cells. As shown in Figure 5(l), there was no additional blocking of tumor cell lysis with higher concentrations of anti-CD16, further indicating that the remaining tumor lysis enhancement was not due to ADCC, and most probably mediated by IL-15 stimulation of the NK cells. Similar results on the effect of high concentrations of anti-CD16 on NK cell lysis of tumor cells were observed with H441 and CaSki tumor cells as targets (data not shown).
Studies were also undertaken to determine effects on tumor lysis when both NK cells and tumor targets were exposed to N-809 (Figure 6(a)). As shown in Figure 6(b), lysis of H441 (lung cancer) cells was 3% with the use of untreated NK cells. Pretreating the NK cells with N-809 increased the tumor lysis to 35%, while only exposing the tumor cells to N-809 increased the lysis level to 60%. Combining N-809 treatment of NK cells with tumor cell exposure to N-809 resulted in the highest levels of tumor cell lysis, 78%. Similar results were seen with lysis of the tumor cell lines CaSki (cervical cancer, Figure 6(c)) and MDA-MB-231 (breast cancer, Figure 6(d)).
Figure 6.

Treating NK cells with N-809, combined with tumor cell exposure to N-809, resulted in the highest levels of tumor cell lysis. (a, e, i, m) Schematics of experimental procedures. Healthy donor (b–d) and cancer patient (j–l) NK cells were incubated ±N-809 (37.5 ng/ml), washed, and added to the lysis assay at a 10:1 E:T ratio. Tumor cells were exposed to IgG1 control or N-809 (20 ng/ml) before NK cells were added. Results from one representative healthy donor and one of two cancer patients are shown using three human tumor cell lines as targets: H441 (lung carcinoma, b and j), CaSki (cervical carcinoma, c and k), and MDA-MB-231 (breast carcinoma, d and l). (f–h and n–p) NK cells were treated as above. Tumor cells were exposed to IgG1 control or cetuximab (10 ng/ml) before NK cells were added. Similar results were observed in additional donors and an additional cancer patient. Tables display drug exposure of NK cells and tumor cells.
The tumor cell lines used in these studies express both PD-L1 and EGFR (Supplemental Table S5). EGFR is the target for cetuximab, a human anti-EGFR IgG1 MAb with the ability to mediate ADCC.38,39,40 Lysis of H441 targets exposed to cetuximab was also evaluated with untreated NK cells and N-809‒treated NK cells (Figure 6(e)). Cetuximab was used at a concentration of 10 ng/ml, which has been previously shown to elicit ADCC.41 As shown in Figure 6(f), untreated NK cells elicited only 3% lysis of H441 targets. Pretreating the NK cells with N-809 increased the tumor lysis to 35%, while exposing the tumor cells to cetuximab alone elicited 20% lysis. Combining N-809 treatment of NK cells with H441 tumor cell exposure to cetuximab resulted in the highest level of lysis (56%). Similar results were seen using CaSki and MDA-MB-231 tumor cells as targets (Figure 6(g, h)). Results from an additional healthy donor are shown in Supplemental Figure S6A-H. NK cells isolated from two prostate cancer patients as effectors showed similar results (Figure 6(i–p) and Supplemental Figure S6I-P).
Antitumor effects of N-809(mu) in a murine model of colon carcinoma
We have conducted studies employing fluorescently labeled N-803 and N-809 (Figure 7(a)) in athymic mice bearing human tumors. These studies demonstrate the accumulation of N-809 at the site of PDL1+ human HTB1 (bladder) tumor not seen with N-803. The human tumor CaSki, which does not express PDL1 in athymic mice, was employed as a control and did not show any accumulation of the agent. We next investigated the potential therapeutic efficacy of N-809(mu) in the MC38-CEA murine model of colon carcinoma in CEA-Tg mice where CEA is a self-antigen. N-809(mu) is a murine version of N-809, containing human N-IL15 and murine anti-PDL1. One subcutaneous administration of the agent showed evidence of anti-tumor activity. As shown in Figure 7, two subcutaneous administrations of N-809(mu) (Figure 7(c)) significantly reduced the growth of MC38-CEA tumors as compared to PBS treatment (Figure 7(b), P < 0.0001). Moreover, 60% of mice treated with N-809(mu) underwent complete tumor rejection. Thus, there was a clear anti-tumor effect of treatment with N-809(mu). In vivo studies evaluating the effects of N-809 on the TME are ongoing.
Figure 7.

(a) In vivo imaging of N-809. Athymic mice were implanted with 2 × 106 HTB1 or 4 × 106 CaSki tumor cells on the right flank. N-809 and N-803 were fluorescently labeled as described in Materials and Methods. When tumors reached 5–6 mm on one side, 100 µg of labeled N-809 or N-803 was injected ipsilaterally to the tumor, and imaged and analyzed using IVIS Imaging Living Image software. (b, c) MC38-CEA tumor cells (3 × 105) were implanted into the right flank of CEA transgenic female mice. On days 8 and 12 after tumor implant, mice were treated ipsilaterally on the trunk with (b) PBS (100 μl, s.c.) or (c) N-809(mu) (100 μg, s.c.). Graphs show tumor growth curves of individual mice (n = 10/group). Caption denotes number of cured animals in each treatment group.
Discussion
These studies were performed to determine the potential multi-functionality of this novel molecule. Studies have shown the ability of N-809 to alter the gene expression levels of human CD4+ and CD8+ T cells, increase their ability to proliferate, and increase the ability of CD8+ T cells to lyse human tumor cells. N-809 was also shown to alter the gene expression profile of human NK cells, enhance their ability to lyse human tumor cells, and further enhance that lytic ability via the ADCC mechanism. N-809 is thus unique in that it exhibits the properties of both an ADCC-mediating checkpoint inhibitor of the PD-1/PD-L1 axis and an IL-15 superagonist. One of the potential advantages of the bi-specific N-809 molecule is its ability to escort IL-15 to the tumor microenvironment, thereby stimulating cytolytic immune responses at the tumor site. The experiment shown in Figure 7(a) illustrates that indeed the N-809 agent is accumulated at the site of a PD-L1+ tumor, but not accumulated when the tumor is PD-L1NEG. In contrast, N-803 did not accumulate at the tumor site. The use of this fusion protein approach could also potentially reduce the toxicities associated with systemic IL-15 delivery.2
Prior clinical studies have shown the ability of rhIL-15 to enhance the levels of peripheral T cells and NK cells; some of the potential issues with the clinical use of rhIL-15 treatment are possible toxicity and relatively short half-life.2 Preclinical studies have shown that N-803 has greater bioactivity and potency than rhIL-15, although some studies have shown that it can also mediate some immune-related adverse effects via hyperproliferation and hyperactivation of NK cells, and induction of IFNγ.42 Prior preclinical studies have shown that N-803 promotes the expansion and tumor lysis capacity of NK cells and induces memory CD8+ T cells to proliferate and lyse tumor cells.24,26 The combination of N-803 with various therapeutic antibodies has been shown to increase the ADCC mechanism by enhancing NK cell activity.27,28 The first-in-human multi-center phase 1 trial (NCT01885897) of N-803 was in patients who relapsed after allogeneic hematopoietic cell transplantation (allo-HCT).33 N-803 stimulated activation, proliferation, and expansion of NK cells and CD8+ T cells without increasing regulatory T cells. Responses were observed in 19% of evaluable patients, including one complete remission lasting 7 months. Wrangle et al. reported that N-803 can re-induce objective responses to anti-PD-1 immunotherapy after treatment relapse or failure.36 Disease control was asserted in all 10 patients who had relapsed disease after previous anti-PD-1 immunotherapy. Additional clinical trials with N-803 in combination with anti-PD-1 MAbs are showing promising results (NCT01946789, NCT03054909). Favorable results have also been seen with another fusion protein, 2B8T2M, which is constructed with four single chain anti-CD20 domains fused to the N-803 scaffold.43
Studies showing a concentration dependent increase in T-cell proliferation with N-809 treatment illustrate that the N-803 domain of N-809 remained functional after fusion with the anti-PD-L1 domains. In addition, N-809 treatment of a human CD8+ T-cell line resulted in greatly enhanced tumor cell lysis. The importance of IL-15 stimulation of CD8+ T cells is supported by CD8+ T-cell depletion experiments in a murine model, which show that anti-tumor effects of N-803 are both NK and CD8+ T cell-dependent.30
The impact of N-809 on changes in the expression of immune related genes in CD4+ and CD8+ T cells is illustrated in Figure 2. The robust increase in the expression of the chemokines CXCL9, CXCL10, and CXCL11 stimulated by N-809 may support paracrine/autocrine stimulated anti-tumor immunity by CD4+ and CD8+ T cells.44 Heightened expression of the anti-apoptotic genes Bcl-2 and BIRC5 (survivin) in CD8+ T cells stimulated by N-809 may enhance their survival capability, as has been shown for 4-1BB costimulatory protein.45 Finally, as shown in Figure 4 and Supplemental Table S4, N-809 induced NK cells to express members of the TNFRSF family of costimulatory molecules such as 4-1BB (TNFRSF9), OX40 (TNFRSF4) and GITR (TNFRSF18), all commonly found to be influential to T cell activation. Recent studies have demonstrated upregulation of PD-L1 on tumor-exposed human NK cells, and that these NK cells may inhibit T cell proliferation.46 Future studies will evaluate the impact of N-809 on NK-cell phenotype and PD-L1 expression after co-culture with tumor cells.
Studies shown in Figures 5 and 6 demonstrate the ability of N-809 to enhance lysis of, and mediate ADCC in, multiple tumor types when either NK cells or tumor cells are pretreated with various concentrations of the molecule. Moreover, the highest levels of tumor cell lysis were observed when both NK cells and tumor cells were exposed to N-809. In vitro, addition of N-809 to the NK cells and to the tumor cells appears to have an additive effect; however, the concept is to bring the IL-15 superagonist to the tumor. Figure 6 also demonstrates the importance of having both portions of N-809 active. Bi-specificity of N-809 was further shown by the addition of the anti-CD16 antibody to NK cells, which blocks the NK cells’ ability to recognize antibody-coated targets and trigger ADCC; the remaining tumor cell lysis was most likely due to the IL-15 activation of NK cells. When tumor cells were exposed to cetuximab, a different MAb known to induce ADCC, the lysis was also increased when NK cells had been pre-treated with N-809.
N-809 treatment was shown to restore the dysregulated expression of NK cell receptors that can accompany NK cell exhaustion in cancer patients.47 NK cells treated with N-809 had increased expression of certain surface activating receptors, including 4-1BB, NKp30, NKp44, NKG2D, CD161, CD69, and DNAM-1 that are known to be downregulated in patients with various types of cancer due to NK cell exhaustion. N-809 treatment of NK cells, however, did not increase the expression of inhibitory receptors, such as CD158a, which is known to be upregulated in exhausted NK cells.47 N-809 also increased the cytotoxic potential of NK cells, as shown by increased expression of granzyme B and perforin. Furthermore, Tim-3 expression, which has been previously observed to mark highly functional NK cells, significantly increased with N-809 exposure. NK cells that had degranulated (as marked by CD107a) were predominantly Tim-3+.48 Similar increases in Tim-3 expression were observed when PBMC from cancer patients and healthy donors were treated in vitro with N-809, and 123 different immune cell subsets were evaluated. Furthermore, as previously observed in the peripheral blood of patients treated with anti-PD-L1 of the IgG1 isotype, no immune cell subsets were depleted by N-809 treatment.16 To further show the IL-15‒mediated capacity of the molecule, N-809 also increased the population of CD56dimTim-3+ NK cells 15-fold, as previously seen with rIL-15,48 suggesting the highly induced maturation of NK cells. The N-809‒induced increase in Ki-67 suggests an increase in NK cell proliferation. Thus, the combination of the reversion of NK cell exhaustion by IL-15 with a PD-1/PD-L1 checkpoint blockade could exhibit additional clinical benefits over the anti-PD-1/PD-L1 monoclonal antibodies. As shown in Supplemental Table S2, treatment with N-809 leads to high production of IFNγ from CD4 T cells and NK cells. IFNγ is an important pro-inflammatory cytokine, but also leads to FoxP3 expression and conversion of CD4+CD25neg T cells to Tregs.49 In addition, IFNγ is known to decrease survival of MDSC.50 These mechanisms may explain the increase in Tregs and decrease in MDSC observed after treating human PBMC with N-809.
Preclinical in vivo studies involving N-809 are ongoing in multiple tumor models. One question that will need to be addressed is the method of administration of N-809. Preclinical studies using N-803 comparing the i.v. and s.c. routes, and what effects the route of administration has on murine immune cells, pharmacokinetics, toxicities, and anti-tumor activity, have been recently reported.51 Notably, N-803, when administered either i.v. or s.c., induced comparable levels of proliferation and activation of CD8+ T cells and NK cells, and resulted in similar reduction of tumor burden in spite of differential pharmacokinetic profiles.51 Anti-PD-L1/PD-1 MAbs are administered i.v.; N-803 was initially administered i.v., but based on our preclinical findings and due to an increased frequency and severity of constitutional adverse events at high dose levels, recent clinical trials have employed s.c. administration, with fewer toxicity issues. Questions that need to be addressed are: (a) will the N-803 portion of the molecule enhance, inhibit, or have no effect on the anti-PD-L1 activity of the molecule, and, conversely, (b) what effect will the anti-PD-L1 portion of the molecule have on N-803 bioavailability, toxicity, and anti-tumor activity of the IL-15 (N-803) portion of the molecule? While the anti-tumor data shown in Figure 7 are promising, comprehensive studies have been initiated concerning the mode of action of each component of the N-809(mu) molecule, in syngeneic models bearing murine tumors. Preliminary studies have shown anti-tumor activity in a second syngeneic model. These studies are being undertaken to define the mechanisms involved in anti-tumor activity of the N-809 agent by analyses of immune cell subsets in the periphery and in the TME. While murine in vivo studies may shed some light on these questions, ultimate answers will come from rationally designed clinical studies. The in vitro studies described here demonstrate the multi-functionality of the N-809 fusion molecule via its ability to modulate, via the IL-15 effect, the gene expression profiles of human CD4+ T cells, CD8+ T cells, and NK cells, increase the proliferative capacity of human CD4+ and CD8+ T cells, increase the cytotoxic activity of antigen-specific CD8+ T cells, and enhance NK cell cytokine secretion and lysis of several human carcinoma cell types, which can also be further enhanced via the ADCC mechanism using NK cells from both cancer patients and healthy donors.
Materials and methods
Reagents
The ALT-803 agent, originally developed by Altor, has been acquired by NantCell and has now been designated N-803. The N-809 molecule (Figure 1(a)) consists of two anti-PD-L1 ScFv domains linked to N-803 (the IL-15 N72D/IL-15RαSushi complex).24,30,52 For the murine studies, a murine version of N-809, containing human N-803 and murine anti-PDL1 was utilized, and designated N-809(mu). Both the N-809 and N-809(mu) molecules were obtained from Altor BioScience (Nant affiliated company) (Miramar, FL) as part of a Cooperative Research and Development Agreement (CRADA) with the National Cancer Institute, NIH. The cetuximab (Bristol-Myers Squibb, Princeton, NJ) and the isotype (rituximab, Genentech) antibodies were purchased from the National Institutes of Health Pharmacy.
Generation and characterization of the N-809 fusion protein
To create a single-chain two-domain anti-PD-L1 scFv gene fragment, the V-gene segments of the light and heavy chains were synthesized by Genewiz, LLC using amino acid sequences obtained from NantWorks, LLC. The VL gene segment was fused to the 5′ end of the VH gene segment via a linker (Gly4Ser)3. The anti-PD-L1 scFv gene was directly linked to the 5′ end of IL-15RαSuFc as described previously.43,53 The anti-PD-L1 scFv/IL-15RαSuFc was co-transfected with another plasmid containing IL-15 with an N72D mutation into CHO cells to create the N-809 fusion protein. The fusion proteins were purified as previously described.43 The purified fusion proteins were analyzed by reducing SDS-PAGE (12% BisTris gel) followed by SimplyBlueTM Safe Stain (Invitrogen). The homogeneity of N-809 molecules was characterized by size exclusion chromatography. To confirm that N-809 retains binding to PD-L1, we utilized PD-L1+ H441 human lung papillary adenocarcinoma cells. Briefly, 1 × 105 cells were incubated with logarithmic dilutions N-809 at 2 × 106 cells/ml for 30 minutes at 4°C. Binding was assessed and analyzed by flow cytometry using a BD FACSVerse. Binding was graphed as the geometric mean fluorescence intensity (MFI). IL-15 biological activity was determined by measuring proliferation of IL-15 dependent 32Dβ cells. 32Dβ cells (1x104 cells/well) were incubated with N-809 or N-803 for 72 hours at 37°C. Cell proliferation was assessed using Presto Blue (Thermo Fisher Scientific). The EC50 was similar to that of N-803 (20.6 pM; data not shown).
Cell lines and cultures
Peripheral blood mononuclear cells (PBMCs) from healthy donors were obtained from the NIH Clinical Center Blood Bank (NCT00001846). Cancer patient PBMCs were obtained from prostate cancer patients before treatment on a trial at the NCI (NCT00113984).7 All subjects reviewed and signed an informed consent form approved by the NCI Institutional Review Board. PBMCs were isolated using the LSM Lymphocyte Separation Medium (MP Biomedicals, Santa Ana, CA), washed three times, and frozen at 5 × 107 cells/ml in 10% DMSO and 90% FBS at −80°C for 24 hours, then moved to liquid nitrogen for storage. Cell counts were performed on a Nexcelom Cellometer Auto 2000 (Nexcelom Bioscience, Lawrence, MA) with AO/PI staining. PBMCs had >95% viability before and after freezing. NK cells, CD4+ T cells, or CD8+ T cells from healthy donors or cancer patients were isolated from PBMCs using the Human NK Cell Isolation (negative selection, Kit 130-092-657), the Human CD4+ T Cell Isolation Kit (negative selection, Kit 130-096-533), or the Human CD8+ T Cell Isolation Kit (negative selection, Kit 130-096-495) (Miltenyi Biotech, San Diego, CA) following the manufacturer’s instructions, resulting in >90% purity. NK cells and T cells were rested or treated with experimental agents in RPMI-1640 supplemented with 10% HsAB (Omega Scientific, Tarzana, CA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (Mediatech, Herndon, VA) at 1 × 106/ml before use in tumor lysis assays. For treatment of NK cells and T cells, N-809 was used at 37.5 ng/ml. Before being used in Indium-release tumor lysis assays, NK cells were washed. IL-15-dependent 32Dβ cells were cultured in complete IMDM supplemented with 10 ng/ml IL-15 (kindly provided by Dr. J. Yovandich, NCI, Frederick, MD).
Human tumor cell lines (CaSki: cervical carcinoma; H441: lung carcinoma; MDA-MB-231: breast carcinoma) were purchased from American Type Culture Collection (Manassas, VA). All cultures were free of mycoplasma and maintained in RPMI-1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (Mediatech). An HPV E7 antigen-specific T-cell line was obtained from Dr. Christian Hinrichs of the Experimental Transplantation and Immunology Branch at the Center for Cancer Research, National Cancer Institute, Bethesda, Maryland. MC38 murine colon carcinoma cells expressing human CEA (MC38-CEA)54 were generated by retroviral transduction with CEA cDNA, and were used at low passage numbers.
T-cell proliferation assay
For proliferation assays, 96-well round bottom plates were coated with 100 µl of anti-CD3 (Clone: OKT3; eBiosciences, San Diego, CA) at 0.5 µg/ml in PBS for 2 hours. Coated plates were washed three times with 100 µl of PBS before the addition of T cells (1 × 105/well). Cells were cultured in RPMI 1640 complete medium with 10% HsAB alone or in the presence of N-809 at 37°C and 5% CO2. T-cell proliferation was measured by 3H-thymidine (PerkinElmer, Waltham, MA) incorporation pulsed on day 4, at 1 µCi (0.037 MBq)/well and quantified 20 hours later using a liquid scintillation counter (PerkinElmer). All experiments were done in triplicate. Proliferation of anti-CD3 stimulated, untreated T cells was defined as 1, and the fold change was calculated.
NK cell lysis, ADCC, and blocking experiments
NK cells were incubated with or without N-809 for 24 hours, and were washed once with media before being used as effectors in 20-hour 111In-release lysis assays. Target cells were labeled with 20 μCi of 111In-oxyquinoline (GE Healthcare, Chicago, IL) at 37°C for 20 minutes and used as targets at 2,000 cells/well in a 96-well round-bottom culture plate. For ADCC experiments, the targets were incubated for 30 minutes with the test MAb or the isotype control MAb before the addition of NK cells at a 10:1 E:T ratio. The plates were incubated for 20 hours at 37°C in a humidified atmosphere containing 5% CO2, then harvested and counted on a Wizard2 gamma counter (PerkinElmer, Shelton, CT). All samples were tested in triplicate, and specific lysis was calculated from the average. Spontaneous release was determined by incubating targets with medium alone; complete lysis was determined by adding Triton X-100 (Sigma-Aldrich, St. Louis, MO) to a final concentration of 0.05 percent. Specific lysis was determined using the following equation: Percent lysis = (experimental – spontaneous)/(complete – spontaneous) × 100. For the blocking experiments, NK cells were incubated for 2 hours with anti-CD16 antibody (25 µg/ml, clone B73.1, eBioscience, San Diego, CA) prior to their being added to the lysis assays at a 10:1 E:T ratio.
Flow cytometric analysis
NK cells isolated from four healthy donors were incubated for 24 hours with either no treatment or treated with N-809 (37.5 ng/ml) prior to staining. Flow cytometry of NK cells was performed on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed in FlowJo 10 (TreeStar Inc., Ashland, OR). Staining of NK cells was performed with three panels, and the antibodies used were: anti-2B4-FITC, anti-GranzymeB-FITC, anti-NKp44-PE, anti-FASL-PE, anti-CD122-PerCP-Cy5.5, anti-DNAM1-PerCP-Cy5.5, anti-CD11c-PE-CD594, anti-CD40L-PE-CD594, anti-NKp46-PE-Cy7, anti-CD16-PE-Cy7, anti-CD95-BV421, anti-Tim3-BV421, anti-Ki67-BV510, anti-CD27-BV510, anti-CD69-BV510, anti-CD56-BV605, anti-CD11b-BV711, anti-Perforin-BV711, anti-CD161-BV785, anti-NKp30-APC, anti-NKG2D-APC, anti-TRAIL-APC, anti-CD16-AF700, anti-41BB-AF700, anti-CD107a-APC-Cy7, and anti-CD25-APC-Fire750 were all obtained from BioLegend (San Diego, CA). Anti-PD-L1-PE-Cy7, anti-CD158a-BV421, and anti-CD11a-FITC, were obtained from BD Biosciences (San Jose, CA). Anti-NKG2A-PE was from R&D Systems (Minneapolis, MN). Anti-EOMES-PerCP-Cy5.5 was from eBioscience (ThermoFisher Scientific, Grand Island, NY). Live/Dead fixable blue dead cell stain was obtained from Molecular Probes (ThermoFisher Scientific, Grand Island, NY).
Multicolor flow cytometry was also performed on frozen PBMC from three cancer patients and three healthy donors as previously described.16 A total of 123 peripheral immune cell subsets were analyzed, including nine classic immune cell subsets, 25 PD-L1+ subsets, and 89 refined subsets related to maturation and function.
RNA analysis
CD4, CD8, and NK cells isolated from two healthy donors were incubated for 24 hours with either no treatment or treated with N-809 (37.5 ng/ml) prior to RNA extraction. Total RNA was extracted from 1 × 106 cells per sample, using an RNeasy Mini kit, from Qiagen (Hilden, Germany). RNAseq was performed by NantOmics on the Illumina sequencing platform. The resulting reads were aligned to refseq build 73 using BowTie2 v2.2.6, then processed by RSEM v.1.2.25 (using default parameters) to estimate transcripts per million (TPM) for each isoform. Gene-level TPM estimates are made using a weighted-average of the isoform estimates, weighted by the percentage that RSEM estimates each isoform is expressed among all isoforms in the sample. Genes within putative immune-specific processes that were consistently upregulated or downregulated in both donors by more than 3-fold in T cells and NK cells treated with N-809 compared to the untreated control were included in further analyses. NanoString analysis was also performed on CD4 T cells and NK cells after treatment with N-809 (37.5 ng/ml) or N-803 (25 ng/ml), using the nCounter PanCancer Immune Profiling Panel (NanoString Technologies, Inc., Seattle, WA), run by the Genomics Laboratory, Frederick National Laboratory for Cancer Research, Frederick, MD. Raw data files (RCC) and reporter library files (RLF) were uploaded into nSolver analysis software, version 3.0.22 (NanoString). The nCounter Advanced Analysis-Quick Analysis (nSolver, NanoString) was used to analyze the raw data. Normalization of raw data was calculated through the geNorm algorithm, which chose the most consistently expressed housekeeping genes for dataset normalization. Genes that were consistently upregulated or downregulated in both donors by more than 3-fold in NK cells treated with N-803 compared to the untreated control were included in further analyses.
Cytokine analysis
NK cells, CD4+ T cells, and CD8+ T cells were isolated from two healthy donors. Isolated cells were incubated for 24 hours with either no treatment or treated with N-809 (37.5 ng/ml). All cell concentrations were maintained at 1 × 106 cells/ml. Supernatants were collected and analyzed by Multi-Array technology (Meso Scale Diagnostics, Gaithersburg, MD) for detection of cytokines.
In vivo imaging and murine anti-tumor studies
All mice were housed in microisolator cages under pathogen-free conditions, in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines. All animal studies were conducted under approval of the NIH Intramural Animal Care and Use Committee. Athymic mice (generously provided by Mary Custer, NCI-Frederick) were instilled with 2 × 106 HTB1 or 4 × 106 CaSki tumor cells on the right flank. Tumors were allowed to grow until they reached ~5–6 mm on one side. N-809 and N-803 were modified using the SiteClick Antibody Azido Modification Kit (Invitrogen, Carlsbad, CA) and the Click-iT Alexa Fluor 647 sDIBO Alkyne for Antibody Labeling kit (Invitrogen), according to the manufacturer’s protocol. 100 µg of labeled N-809 or N-803 was injected ipsilaterally to the tumor, and imaged and analyzed using IVIS Imaging (Perkin Elmer, Waltham, MA) Living Image software at 20 min post-injection and 24-hour post-injection (16 F-stop, 1 sec. exposure).
Six- to 8-week-old female C57BL/6 mice transgenic for CEA (designated CEA-Tg)55,56 were obtained from the NCI Frederick Cancer Research Facility (Frederick, MD). Mice were in microisolator cages and housed under pathogen-free conditions, in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines. All animal studies were conducted under approval of the NIH Intramural Animal Care and Use Committee. MC38-CEA (3 × 105) tumor cells were implanted subcutaneously (s.c.) into the right flank of mice. On day 8 after tumor implant, mice were randomized into treatment groups to receive PBS (100 μl, s.c.) or N-809(mu) (100 μg, s.c.). Mice received a second treatment on day 12. Tumor volumes of individual animals were determined twice weekly using digital calipers and using the formula (length2 × width)/2.
Statistical analyses
Statistical analyses were performed in GraphPad Prism 7 (GraphPad Software, La Jolla, CA), using multiple T-tests, with a desired false discovery rate of 1.00%. RM ANOVA was used to analyze tumor growth curves.
Funding Statement
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health, and by a Cooperative Research and Development Agreement (CRADA) between the NCI and NantBioScience.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- CRADA
Cooperative Research and Development Agreement
- E:T
effector to target cell
- HsAB
human AB serum
- MAb
monoclonal antibody
- MDSC
myeloid derived suppressor cell
- MFI
mean fluorescence intensity
- NK
natural killer
- NCI
National Cancer Institute
- NIH
National Institutes of Health
- NSCLC
non-small cell lung cancer
- PBMC
peripheral blood mononuclear cell
- PD-1
programmed cell death-1
- PD-L1
programmed cell death ligand-1
- TME
tumor microenvironment
- Treg
regulatory T cells
Acknowledgments
The authors thank Curtis Randolph for excellent technical assistance, and Debra Weingarten for her assistance in the preparation of this manuscript.
Disclosure of potential conflicts of interest
Laboratory of Tumor Immunology and Biology, National Cancer Institute: The authors have no potential conflicts of interest to disclose. The Laboratory of Tumor Immunology and Biology, National Cancer Institute, has a Cooperative Research and Development Agreement (CRADA) with NantBioScience.
NantBioScience, Inc., Affiliates: Christopher Szeto and Shahrooz Rabizadeh are employees of NantOmics. Robby Newman, Warren Marcus, and Hing C. Wong are employees of NantWorks. Patrick Soon-Shiong is a founder and an executive at NantOmics and NantBioScience.
Supplementery Material
Supplemental data for this article can be accessed here.
References
- 1.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28(19):3167–3175. doi: 10.1200/jco.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human Interleukin-15 in patients with cancer. J Clin Oncol 2015;33(1):74–82. doi: 10.1200/JCO.2014.57.3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, Lamping E, Oyelakin I, Marte JL, Lepone LM, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol 2017;18(5):587–598. doi: 10.1016/s1470-2045(17)30239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SAIL-2. the first effective immunotherapy for human cancer. J Immunol 2014;192(12):5451–5458. doi: 10.4049/jimmunol.1490019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callahan MK, Kluger H, Postow MA, Segal NH, Lesokhin A, Atkins MB, Kirkwood JM, Krishnan S, Bhore R, Horak C, et al. Nivolumab plus ipilimumab in patients with advanced melanoma: updated survival, response, and safety data in a phase I dose-escalation study. J Clin Oncol 2018;36(4):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012;13(5):501–508. doi: 10.1016/S1470-2045(12)70006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, Yao Z, Dubois S, Ju W, Muller JR, Waldmann TA.. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci U S A 2009;106(18):7513–7518. doi: 10.1073/pnas.0902637106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, Lamy A, Penault-Llorca F, Frebourg T, Michel P, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol 2009;27(7):1122–1129. doi: 10.1200/jco.2008.18.0463 [DOI] [PubMed] [Google Scholar]
- 10.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002;99(3):754–758. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, Chang HM, Borucka E, Lurje G, Sherrod AE, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol 2007;25(24):3712–3718. doi: 10.1200/jco.2006.08.8021 [DOI] [PubMed] [Google Scholar]
- 12.Apolo AB, Infante JR, Hamid O, Patel MR, Wang D, Kelly K, Mega AE, Britten CD, Ravaud A, Mita AC, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase 1b trial: analysis of safety, clinical activity, and PD-L1 expression. J Clin Oncol 2016;34(15_suppl):4514. doi: 10.1200/JCO.2016.34.15_suppl.4514 [DOI] [Google Scholar]
- 13.Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, Schlom J. Antibody-dependent cellular cytotoxicity (ADCC) activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 2015;3(10):1148–1157. doi: 10.1158/2326-6066.CIR-15-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016;17(10):1374–1385. doi: 10.1016/S1470-2045(16)30364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khanna S, Thomas A, Abate-Daga D, Zhang J, Morrow B, Steinberg SM, Orlandi A, Ferroni P, Schlom J, Guadagni F, et al. Malignant mesothelioma effusions are infiltrated by CD3(+) T cells highly expressing PD-L1 and the PD-L1(+) tumor cells within these effusions are susceptible to ADCC by the anti-PD-L1 antibody avelumab. J Thorac Oncol 2016;11(11):1993–2005. doi: 10.1016/j.jtho.2016.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donahue RN, Lepone LM, Grenga I, Jochems C, Fantini M, Madan RA, Heery CR, Gulley JL, Schlom J. Analyses of the peripheral immunome following multiple administrations of avelumab, a human IgG1 anti-PD-L1 monoclonal antibody. J Immunother Cancer 2017;5(1):20. doi: 10.1186/s40425-017-0220-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, Mega AE, Britten CD, Ravaud A, Mita AC, et al. Avelumab, an anti–programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase ib study. J Clin Oncol 2017;35(19):2117–2124. doi: 10.1200/jco.2016.71.6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJL, Leach J, Edenfield WJ, Wang D, Grote HJ, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017;18(5):599–610. doi: 10.1016/s1470-2045(17)30240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipson EJ, Forde PM, Hammers H-J, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol 2015;42(4):587–600. doi: 10.1053/j.seminoncol.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A 2000;97(21):11445–11450. doi: 10.1073/pnas.200363097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 1996;4(4):329–336. [DOI] [PubMed] [Google Scholar]
- 22.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006;6(8):595–601. doi: 10.1038/nri1901 [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi H, Carrasquillo JA, Paik CH, Waldmann TA, Tagaya Y. Differences of biodistribution, pharmacokinetics, and tumor targeting between interleukins 2 and 15. Cancer Res 2000;60(13):3577–3583. [PubMed] [Google Scholar]
- 24.Alter S, Rhode PR, Jeng EK, Wong HC. Targeted IL-15-based protein fusion complexes as cancer immunotherapy approaches. J Immunol Sci 2018;2(1):15–18. doi: 10.29245/2578-3009/2018/1.1111 [DOI] [Google Scholar]
- 25.Rhode PR, Egan JO, Xu W, Hong H, Webb GM, Chen X, Liu B, Zhu X, Wen J, You L, et al. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res 2016;4(1):49–60. doi: 10.1158/2326-6066.cir-15-0093-t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong HC, Jeng EK, Rhode PR. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8(+) T cells into innate-like effector cells with antitumor activity. OncoImmunology 2013;2(11):e26442. doi: 10.4161/onci.26442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jochems C, Tritsch SR, Pellom ST, Su Z, Soon-Shiong P, Wong HC, Gulley JL, Schlom J. Analyses of functions of an anti-PD-L1/TGFβR2 bispecific fusion protein (M7824). Oncotarget 2017;8(43):75217–75231. doi: 10.18632/oncotarget.20680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosario M, Liu B, Kong L, Collins LI, Schneider SE, Chen X, Han K, Jeng EK, Rhode PR, Leong JW, et al. The IL-15-based ALT-803 complex enhances FcgammaRIIIa-triggered NK cell responses and in vivo clearance of B cell lymphomas. Clin Cancer Res 2016;22(3):596–608. doi: 10.1158/1078-0432.ccr-15-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathios D, Park CK, Marcus WD, Alter S, Rhode PR, Jeng EK, Wong HC, Pardoll DM, Lim M. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int J Cancer 2016;138(1):187–194. doi: 10.1002/ijc.29686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim PS, Kwilas AR, Xu W, Alter S, Jeng EK, Wong HC, Schlom J, Hodge JW. IL-15 superagonist/IL-15RalphaSushi-Fc fusion complex (IL-15SA/IL-15RalphaSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget 2016;7(13):16130–16145. doi: 10.18632/oncotarget.7470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosser CJ, Nix J, Ferguson L, Wong HC. Mp15-12 phase Ib trial of Alt-803, an Il-15 superagonist, plus Bacillus Calmette Guerin (Bcg) for the treatment of Bcg-naive patients with non-muscle-invasive bladder cancer (Nmibc). J Urol 197(4):e175. doi: 10.1016/j.juro.2017.02.497 [DOI] [Google Scholar]
- 32.Sfakianos JP, Kim PH, Hakimi AA, Herr HW. The effect of restaging transurethral resection on recurrence and progression rates in patients with nonmuscle invasive bladder cancer treated with intravesical bacillus Calmette-Guerin. J Urol 2014;191(2):341–345. doi: 10.1016/j.juro.2013.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, Weisdorf DJ, Blazar BR, Ustun C, DeFor TE, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018;131(23):2515–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu P, Steel JC, Zhang M, Morris JC, Waitz R, Fasso M, Allison JP, Waldmann TA. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci U S A 2012;109(16):6187–6192. doi: 10.1073/pnas.1203479109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res 2010;16(24):6019–6028. doi: 10.1158/1078-0432.CCR-10-1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, Miller JS, Farhad M, Anderton K, Lindsey K, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol 2018;19(5):694–704. doi: 10.1016/S1470-2045(18)30148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin BY, Campbell TE, Draper LM, Stevanovic S, Weissbrich B, Yu Z, Restifo NP, Rosenberg SA, Trimble CL, Hinrichs CS. Engineered T cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. JCI Insight 2018;3:8. doi: 10.1172/jci.insight.99488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Foncillas J, Diaz-Rubio E. Progress in metastatic colorectal cancer: growing role of cetuximab to optimize clinical outcome. Clin Transl Oncol 2010;12(8):533–542. doi: 10.1007/s12094-010-0551-3 [DOI] [PubMed] [Google Scholar]
- 39.Kies MS, Holsinger FC, Lee JJ, William WN Jr., Glisson BS, Lin HY, Lewin JS, Ginsberg LE, Gillaspy KA, Massarelli E, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol 2010;28(1):8–14. doi: 10.1200/JCO.2009.23.0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kol A, Terwisscha van Scheltinga A, Pool M, Gerdes C, de Vries E, de Jong S. ADCC responses and blocking of EGFR-mediated signaling and cell growth by combining the anti-EGFR antibodies imgatuzumab and cetuximab in NSCLC cells. Oncotarget 2017;8(28):45432–45446. doi: 10.18632/oncotarget.17139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jochems C, Hodge JW, Fantini M, Fujii R, Morillon YM 2nd, Greiner JW, Padget MR, Tritsch SR, Tsang KY, Campbell KS, et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 2016;7(52):86359–86373. doi: 10.18632/oncotarget.13411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y, Luan L, Rabacal W, Bohannon JK, Fensterheim BA, Hernandez A, Sherwood ER. IL-15 superagonist-mediated immunotoxicity: role of NK Cells and IFN-gamma. J Immunol 2015;195(5):2353–2364. doi: 10.4049/jimmunol.1500300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu B, Kong L, Han K, Hong H, Marcus WD, Chen X, Jeng EK, Alter S, Zhu X, Rubinstein MP, et al. A novel fusion of ALT-803 (interleukin (IL)-15 superagonist) with an antibody demonstrates antigen-specific antitumor responses. J Biol Chem 2016;291(46):23869–23881. doi: 10.1074/jbc.M116.733600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karin N. Chemokines and cancer: new immune checkpoints for cancer therapy. Curr Opin Immunol 2018;51:140–145. doi: 10.1016/j.coi.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 45.Chacon JA, Wu RC, Sukhumalchandra P, Molldrem JJ, Sarnaik A, Pilon-Thomas S, Weber J, Hwu P, Radvanyi L. Co-stimulation through 4-1BB/CD137 improves the expansion and function of CD8(+) melanoma tumor-infiltrating lymphocytes for adoptive T-cell therapy. PLoS One 2013;8(4):e60031. doi: 10.1371/journal.pone.0060031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sierra JM, Raffo Iraolagoitia XL, Secchiari F, Ziblat A, Nuñez SY, Torres NI, Regge MV, Domaica CI, Zwirner NW, Fuertes MB. Tumor-experienced NK cells inhibit T cell proliferation and activation through PD-L1 (abstr 704). AACR Annual Meeting, April 14–18, 2018; Chicago, IL. Cancer Res 2018;78(Suppl 13):704. doi: 10.1158/1538-7445.AM2018-704 [DOI] [Google Scholar]
- 47.Bi J, Tian Z. NK cell exhaustion. Front Immunol 2017;8:760. doi: 10.3389/fimmu.2017.00760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012;119(16):3734–3743. doi: 10.1182/blood-2011-11-392951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, Liu A, Xu L, Sun B, Zhang JZ. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25- T cells to CD4+ Tregs. J Clin Invest 2006;116(9):2434–2441. doi: 10.1172/JCI25826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medina-Echeverz J, Haile LA, Zhao F, Gamrekelashvili J, Ma C, Metais JY, Dunbar CE, Kapoor V, Manns MP, Korangy F, et al. IFN-gamma regulates survival and function of tumor-induced CD11b+ Gr-1high myeloid derived suppressor cells by modulating the anti-apoptotic molecule Bcl2a1. Eur J Immunol 2014;44(8):2457–2467. doi: 10.1002/eji.201444497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu B, Jones M, Kong L, Noel T, Jeng EK, Shi S, England CG, Alter S, Miller JS, Cai W, et al. Evaluation of the biological activities of the IL-15 superagonist complex, ALT-803, following intravenous versus subcutaneous administration in murine models. Cytokine 2018;107:105–112. doi: 10.1016/j.cyto.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Felices M, Chu S, Kodal B, Bendzick L, Ryan C, Lenvik AJ, Boylan KLM, Wong HC, Skubitz APN, Miller JS, et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol 2017;145(3):453–461. doi: 10.1016/j.ygyno.2017.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu X, Marcus WD, Xu W, Lee HI, Han K, Egan JO, Yovandich JL, Rhode PR, Wong HC. Novel human interleukin-15 agonists. J Immunol 2009;183(6):3598–3607. doi: 10.4049/jimmunol.0901244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fallon JK, Vandeveer AJ, Schlom J, Greiner JW. Enhanced antitumor effects by combining an IL-12/anti-DNA fusion protein with avelumab, an anti-PD-L1 antibody. Oncotarget 2017;8(13):20558–20571. doi: 10.18632/oncotarget.16137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clarke P, Mann J, Simpson JF, Rickard-Dickson K, Primus FJ. Mice transgenic for human carcinoembryonic antigen as a model for immunotherapy. Cancer Res 1998;58(7):1469–1477. [PubMed] [Google Scholar]
- 56.Kass E, Schlom J, Thompson J, Guadagni F, Graziano P, Greiner JW. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res 1999;59(3):676–683. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


