Although the majority of prostate cancer (PCa) cases are now detected and treated at early stages, PCa remains the second leading cause of cancer death in American men (1). In the case of more advanced tumors, androgen deprivation can prevent cancer progression temporarily, but this inevitably leads to castration-resistant prostate cancer (CRPC). In recent years, several targeted therapies have entered the clinic that inhibit the androgen receptor (AR), a key driver of CRPC, but tumors often gain secondary resistance to these therapies (2). Additional studies that explore novel mechanisms of PCa progression could contribute to development of more effective therapies. In a paper published this year in Nature Cmmmmications, Laurent et al. identified a novel chemokine signaling pathway in PCa that links adipocyte-secreted CCL7 to PCa cell migration (3), suggesting that this signaling pathway is a potential therapeutic target.
Besides the predominant role of AR in PCa, other signaling pathways are also important for PCa progression, including chemokines such as CXCL12 (4). PCa cells express the chemokine receptor CXCR4 which is activated by CXCL12, a chemokine expressed by bone stromal cells (5). As a result, CXCR4-positive PCa cells frequently metastasize to the bone (6). Similarly, adipose tissue is recognized as an endocrine organ that can secrete a variety of factors (adipokines) and influence cell behavior (7). Not surprisingly a correlation exists between obesity and PCa aggressiveness (8–10), suggesting that secreted adipokines may influence PCa cell behavior.
Of particular interest to Laurent and colleagues was the interplay between periprostatic adipose tissue (PPAT) and PCa tumor cells, and how obesity affects this interplay. The authors first demonstrated that condition media from adipocytes can direct the migration of DU-145 and PC-3 PCa cell lines. The authors further identified that this migration is due to chemokine signaling between chemokine receptor CCR3 and ligand CCL7 secreted by mature adipocytes. Importantly, they found that CCR3 was expressed in these PCa cell lines as well as in patient tumors, and that their expression levels correlated with cancer aggressiveness. Perhaps most exciting, the authors demonstrated that the CCR3/CCL7 axis-mediated migration can be blocked by a CCR3 inhibitor or monoclonal antibodies for CCR3 and CCL7 (Figure 1).
Figure 1.
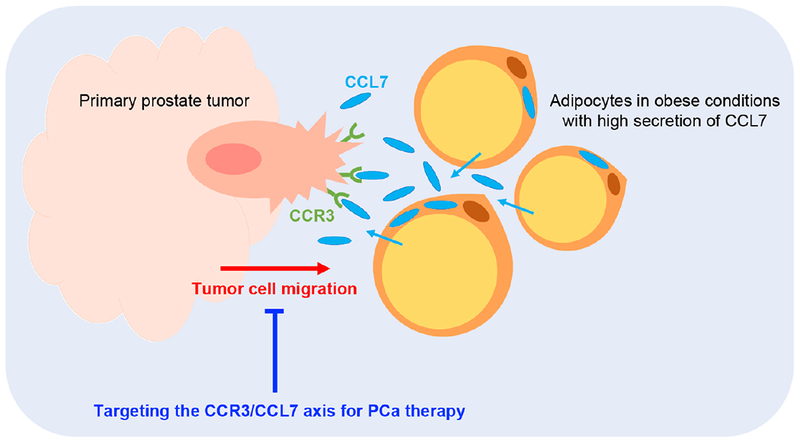
Targeting CCR3/CCL7-induced migration for PCa therapy. CCL7 secreted by mature adipocytes under obese conditions stimulates PCa cell migration. Laurent et al. demonstrate that this migration can be blocked by monoclonal antibodies for CCR3 or CCL7, or by chemical inhibition of CCR3. This chemokine signaling pathway represents a promising therapeutic target for PCa treatment.
Because obesity correlates with increased CCR3 expression that may reflect increased secretion of CCL7, Laurent et al. also assessed the effect of obesity on tumors in a murine orthotopic graft model of PCa. In this system, obese conditions led to an increase in tumor size that was dependent on CCR3 expression. Further investigation on metastases and animal survival could yield important insights into how the CCR3/CLL7 axis might affect PCa cell migration in vivo.
In their recent publication, Laurent et al. characterized a novel chemokine signaling pathway in PCa which links signaling molecules secreted by PPAT with tumor progression. The authors’ findings bring to light a few key questions about PCa progression and interplay between different components of the tumor microenvironment. Gaining a better understanding of the mechanism of increased CCR3 expression in PCa cells, the downstream targets of active CCR3 that trigger cell migration, and the long-term effects of obesity on CCR3/CCL7-mediated cell migration are essential. In addition, further studies to uncover relationships between CCR3/CCL7 signaling in PCa and other cancer-associated signaling pathways could reveal a complex, well-orchestrated network. One prediction could be that CCR3/CCL7 signaling induces PCa cells to disseminate from the primary tumor into adjacent tissue, while another chemokine signaling pathway like CXCR4/CXCL12 may encourage already freely circulating tumor cells to metastasize to distant secondary sites. Therefore, the findings by Laurent et al. have paved the way for these future studies, as well as the development of therapeutics that may have the potential to block tumor cell migration at a very early stage.
Acknowledgements
Funding: This work was supported in part by funding from the National Institutes of Health CA134514, CA130908, and CA193239 to H.H.).
Footnotes
Provenance: This is a Guest Commentary commissioned by Section Editor Peng Zhang (Department of Urology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
Comment on: Laurent V, Guérard A, Mazerolles C, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun 2016;7:10230.
References
- 1.A.C. Society. All Cancer Facts & Figures 2016. Available online: http://www.cancer.org/research/cancerfactsstatistics/allcancerfactsfigures/
- 2.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nature Reviews Cancer 2015;15:701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent V, Guérard A, Mazerolles C, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nature Communications 2016. Available online: http://www.nature.com/ncomms/2016/160112/ncomms10230/abs/ncomms10230.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taichman RS, Cooper C, Keller ET, et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 2002;62:1832–7. [PubMed] [Google Scholar]
- 5.Cai J, Kandagatla P, Singareddy R, et al. Androgens Induce Functional CXCR4 through ERG Factor Expression in TMPRSS2-ERG Fusion-Positive Prostate Cancer Cells. Transl Oncol 2010;3:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 2010;16:2927–31. [DOI] [PubMed] [Google Scholar]
- 7.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker AS, Thiel DD, Bergstralh E, et al. Obese men have more advanced and more aggressive prostate cancer at time of surgery than non-obese men after adjusting for screening PSA level and age: results from two independent nested case-control studies. Prostate Cancer Prostatic Dis 2013;16:352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol 2013;63:800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikesit D, Mochtar CA, Umbas R, et al. The impact of obesity towards prostate diseases. Prostate Int 2016;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


