Abstract
Vascular smooth muscle cells (VSMCs) play an important role in regulating blood flow and pressure by contracting and relaxing in response to a variety of mechanical stimuli. A fully differentiated and functional VSMC should have both the ability to contract and relax in response to environmental stimuli. In addition, it should have the proper mechanical properties to sustain the mechanically active vascular environment. Stem cells can differentiate towards VSMC lineages and so could be used as a potential treatment for vascular repair. However, few studies have assessed the time it takes for stems cells to acquire similar mechanical property to native VSMCs during differentiation.
In our study, changes in the mechanical properties of differentiating bone marrow and adipose-derived stem cells were determined by using atomic force microscopy indentation. Overall, bone marrow derived stem cells achieved higher elastic moduli than adipose tissue derived stem cell during differentiation. Immunofluorescence shows that both stem cell types have increasing VSMC-specific markers over differentiation. While adipose-derived stem cells were softer, they expressed slightly higher αSMA than the bone marrow cells as investigated by RT-PCR. Further investigations are required to better determine the appropriate mechanical environment for vascular smooth muscle differentiation.
Keywords: vascular smooth muscle cell differentiation, elastic modulus, bone marrow derived mesenchymal stem cell, adipose derived stem cell, immunofluorescence, AFM
Introduction
Stem cell therapy has been under intensive research in recent years due to its potential in regenerating tissue and treating injury. Vascular smooth muscle cell (VSMC), as one of the crucial constituents in human vascular regeneration, can be derived from a variety of stem cell sources including bone-marrow progenitor cells(1, 2), skin-derived precursors (3, 4) and adipose stem cells (5). Although it is known that VSMC and stem cells both can undergo significant phenotype shift in response to environment including mechanical stimuli (6, 7), no comparison of mechanical property change has been made between different stem cell sources during differentiation. A better understanding of the mechanical property change during VSMC differentiation will not only indicate the differentiation status the cell is undergoing but also guide us to better design cell-cultured scaffold based on the optimal environment it requires during differentiation.
Bone marrow-derived mesenchymal stem cells (BMSCs) have been shown to have the capacity to differentiate towards a variety of cell types, including vascular smooth muscle cell lineages (8), and thus, have shown great potential in tissue repair application (2). Previous studies showed a high similarity between native smooth muscle cell and mesenchymal stem cell differentiated towards a vascular smooth muscle lineage, which include expression of specific smooth muscle cell cytoskeleton protein during the differentiation of MSC following SMC pathway (9). Mesenchymal stem cells are also believed to stimulate angiogenesis in certain condition and are reported to stabilize vascular structure when co-cultured with other cells. These features together with their low immunogenicity make BMSCs a promising resource in vascular tissue repair (9–11).
Adipose tissue derived stem cells (ADSCs) have a mesenchymal-like morphology and are capable of differentiating to form different kinds of tissue-specific cells (12). Some groups have hypothesized that ADSCs may not be inherent to adipose tissue and that they are instead floating mesenchymal or blood peripheral stem cells passing into adipose tissue (13). However, there is no doubt that these cells isolated from adipose tissue, can be potentially useful stem cells for tissue repairing and disease treatment (14). ADSCs have been confirmed having the capability to differentiate to functional SM-like cells; many smooth muscle cell specific markers were observed upon differentiation (15, 16). Thus, ADSCs are also considered as a competitive candidate in repairing vascular tissue.
Vascular smooth muscle cell, as one of the main constituents of the media layer in blood vessel, is under consistently mechanical loading from changes in blood pressure. As cellular mechanical behavior is highly associated with tissue level function, it is very important to investigate and understand the mechanical behavior of VSMCs and VSMC-like cells before applying them to any tissue level application (17). Given the limited study of mechanical behavior of cells undergoing vascular smooth muscle cell differentiation, the overall goal of this study was to investigate the differences in cellular mechanical properties of BMSCs and ADSCs during VSMC differentiation using atomic force microscopy (AFM) nanoindentation. VSMC-related cell markers were further visualized by immunofluorescence and the gene expressions were assessed using PCR. People have suggested that ADSCs can be differentiated to contractile VSMCs induced by TGF-β1.(5) However, while ADSCs have been shown to be able to differentiate down VSMC lineages, prior studies have shown that it may take longer to differentiate these cells than cells from bone marrow. ADSCs can take up to 6 weeks to express the same level of differentiation markers seen in BMSCs within 7 days of induction with growth factors. (16, 18) Our hypothesis was that BMSCs can be more quickly differentiated to functional SMC-like cells compared to ADSCs with a better mechanical performance during differentiation. This study will help elucidate the potential of using different sources of stem cells to differentiate into functional VSMC with certain mechanical strength.
Materials and Methods
Cell Culture
For these studies, human bone marrow and adipose derived cells were purchased from commercial sources. Mesenchymal stromal cells isolated from human red bone marrow (BMSCs, Thermo Scientific HyClone, SV30110.01) were seeded at 1 × 104 cells/ cm2 in Φ 35-mm dishes (Fluorodish FD35–100) resuspended in Dulbecco’s modified low glucose Eagle medium (LG-DMEM, Gibco) supplemented with 20% FBS (Atlanta Biologicals, Lawrenceville, GA, USA) and 1% 100× penicillin-streptomycin (Fisher Scientific, Pittsburgh, PA, USA). After cells were adhered to plates (1 day culture in incubator), we added differentiation media containing 10ng/ml TGF-β1 to induce differentiation towards VSMC. BMSCs were cultured for 7 days and used for following study at different time points. Human adipose derived stem cells (ADSCs) isolated from human lipoaspirate tissue (Thermo Scientific HyClone, SV30102.01) were cultured in Φ 35-mm dishes with 1 × 104 cells/ cm2 in LG-DMEM (Gibco) supplemented with 10% FBS and 1% 100× penicillin-streptomycin. Similarly, culture media with 10ng/ml TGF-β1 was added to induce differentiation after cells adhere to plates. It should be noted that, according to the manufacturer, the bone marrow stem cell line (Thermo, SV30110.01) was derived from bone marrow aspirates of a single adult male donor while the adipose stem cell line (Thermo, SV30102.01) was derived from lipoaspirates pooled from multiple donors. Since many factors, including substrate stiffness and culture conditions, can affect cell mechanical properties (19), the cells were maintained in standard cell culture condition on tissue culture plastic dish to minimize potential confounding factors due to culture substrate selection. As a comparison positive control group, primary VSMCs were isolated from rat aortic tissue using standard methods. The Young’s modulus of human VSMC has previously been characterized to be in 10–100kPa which lies within the same range of rat VSMC (20, 21). Procedures were approved by the Clemson University Institutional Animal Use and Care Committee. The cells were cultured for 1 day in LG-DMEM with 10% FBS and 1% 100× penicillin-streptomycin.
AFM indentation
For AFM indentation, an Asylum Research MFP-3D AFM mounted on an Olympus IX-81 spinning disc confocal microscope placed on a vibration isolation table was used. Borosilicate spherical probes (radius = 2.5μm, Bruker Nano Inc) with a nominal spring constant of 0.06N/m were utilized to indent into cells. Cells were kept in 35-mm culture dishes with growth media throughout the experiment. Prior to loading with the AFM, a thermal fluctuation test is performed in air to calculate the actual spring constant of the cantilever (22). Single cells were randomly chosen by the optical microscope with a 20X objective lens while morphologically abnormal and overlapped cells were excluded. AFM tests were performed on one dish at a time while the rest of dishes were stored in an incubator until just prior to use. Each test takes less than 30mins for one dish. This is similar to the time cells are out of the incubator for other cell culture maintenance protocols (e.g., media changes, microscopy images). Similar to past studies using the same system (23), during this time outside the incubator, the temperature in the can decrease by ~5°C over 30mins. However, no noticeable changes in cell behavior and mechanical properties were observed over the 30mins time the cells were in the AFM. The AFM probe was positioned over the central part of the cell and was adjusted to avoid the edge of the cell. Each cell was indented 5 times to a depth of at least 1μm into the cell with a loading rate of 1μm/s in 5μm force distance. At each time point, 10 representative cells from each sample were chosen for evaluation and their elastic modulus were then analyzed based on at least 50 replicates per sample. [Figure1]
Figure 1.
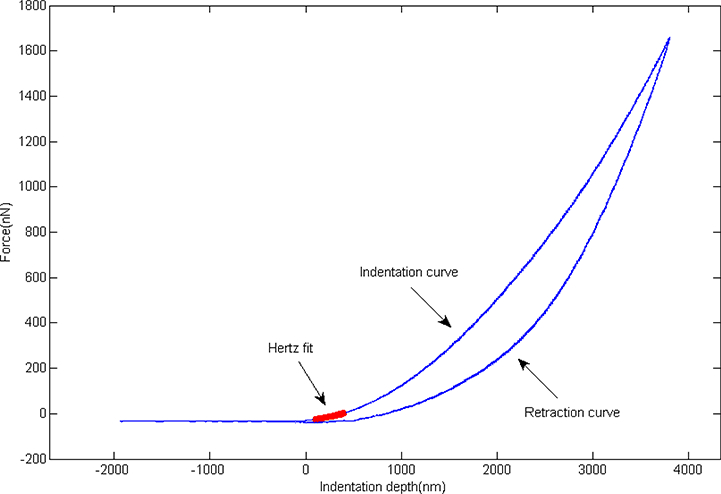
Representative AFM indentation consisting both an indent and retract curve. The contact point was determined by a certain degree of upwards shift while the Hertz model fit to the data over the indentation curve from 30nm to a 300nm indentation depth.
Indentation curve analysis
The Hertz contact model (24) was used to estimate the elastic modulus values from the indentation curves acquired from the contact between AFM spherical tip and cell. A MATLAB script was used to determine the contact point of the force curves by identifying the point at which force shifts upwards above the baseline noise level (25). The modulus was calculated using the Hertz model fit to the data over the indentation curve from 30nm to a 300nm indentation depth, a range over which the Hertz contact model still remain accurate (26). The Hertz contact model is used to simulate the elastic behavior between a spherical indenter (AFM probe) and an elastic half plane (cell) (27).
| (1) |
Where F represents the force, E is the elastic modulus of the cell, ν is Poisson’s ratio (ν=0.5 as the cell cytoplasm is assumed incompressible (28)), R is the radius of indenter, and δ is the indentation depth. The Hertz model has two major assumptions: linear elasticity and infinite sample thickness. These can result in significant errors if the experiments are far from these approximations. However, by using microsphere tips and the small indentation depth, the approximations of the Hertz model remain close to major assumptions and have reasonable accuracy in the calculation of elastic modulus for cells (29). The point-wise calculated elastic modulus was found to be mostly constant over the 30–300nm indentation range; this indicates that the conditions of the Hertz model are fairly well met in this indentation range (24).
Immunofluorescence
We fixed cells in 24-well plates with 4% Paraformaldehyde (PFA, Sigma-Aldrich) at 37°C for 10 minutes and rinsed cells with phosphate buffered saline (PBS) two times for 30 minutes. PBS with 0.01 M Glycine and 0.1% Triton-X was placed on the cells for 30 minutes. Cells were then rinsed with 5% BSA/PBS and 1% BSA/PBS followed by incubation with primary antibodies against SMα-actin (αSMA) (Sigma-Aldrich), calponin (Abcam) and SM myosin heavy chain (SM-MHC) (Abcam) at 4°C. Fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibody (Millipore) was added the following day to detect the localization of anti-calponin antibodies while FITC-conjugated goat anti-mouse secondary antibody (Millipore) was used to detect the localization of antiαSMA and anti-SM-MHC antibodies. Images were visualized using a Nikon Eclipse TE2000-S fluorescence microscope (Nikon USA, Melville, New York, USA)
RNA Isolation and RT-PCR
We extracted total RNA using TRIzol (Invitrogen) from cells cultured during different differentiation time points to investigate if there is any correlation between the expression of αSMA and cellular mechanical property. The isolated RNA was further purified using RNeasy Kit (Qiagen, Valencia, CA) according to the instructions of the manufactures. RNA quantity was assessed using Take3 micro-volume plates (BioTek) and 1 μg was used for each reverse-transcription. DNA denaturation was performed in PCR by 3 minutes heating at 70 while complementary DNA (cDNA) was synthesized using QuantiTect reverse transcription kit (QiaGen) and equal volume of synthesized cDNA was then added to a master mix containing specifically designed primers for αSMA and β-actin. Reverse transcription was conducted consisting 1 hour heating at 44°C followed by a 10 minutes Rtease inactivation a 92°C. The amplification efficiency of primers was tested first to ensure the number of PCR cycles for amplification is in a linear range. The primer sequences for αSMA are designed as 5’- GGTGATGGTGGGAATGGG-3’ and 5’-GCAGGGTGGGATGCTCTT-3′to generate a 188 fragment size PCR product (30) and the primer sequences for β-actin are 5’- TGGGTCAGAAGGATTCCTATGT-3’ and 5’-CAGCCTGGATAGCAACGTACA-3′ to generate PCR product (31).
Quantitative Real-Time PCR analysis
For quantitative PCR analysis, the expression of αSMA and β-actin was assessed during the differentiation of both BMSCs and ADSC. We performed RT-PCR on Rotor-Gene (RG-3000, Corbett Research) using QuantiTect SYBR Green PCR Kit (QiaGen) to have a final reaction volume of 25ml. The expression of αSMA was normalized based on the transcript level of β-actin to determine the differentiated portion of total amount of stem cells.
Statistical analysis
ANOVA with Tukey multi comparison test using SAS software were performed to determine if there are significant differences among samples during differentiation. Student’s t-tests were used to examine significant differences between samples on a given day and across all time points. P value less than 0.05 were considered statistically significant based on the significant level of 5%. The statistical analysis was conducted on the elastic modulus of both two stem cells over differentiation.
Results
Elastic modulus of single cell during differentiation
The modulus values obtained from the 1-day cultured VSMCs were consistent with those from previously published studies of similar in vitro cultured VSMCs (32). Generally, the resulting elastic modulus of both BMSCs and ADSCs gradually increased during differentiation (Figure 2) and both stem cells reach 80% confluency at day 7. We found a significant increase in the mechanical property of day 3 BMSCs while observing a relatively steady raise in the Young’s modulus of ADSCs over 7 days differentiation. Force curves were then plotted according to each time point during differentiation (Figure 3). Both BMSCs and ADSCs exhibit an increasing force vs. indentation trend.
Figure 2.
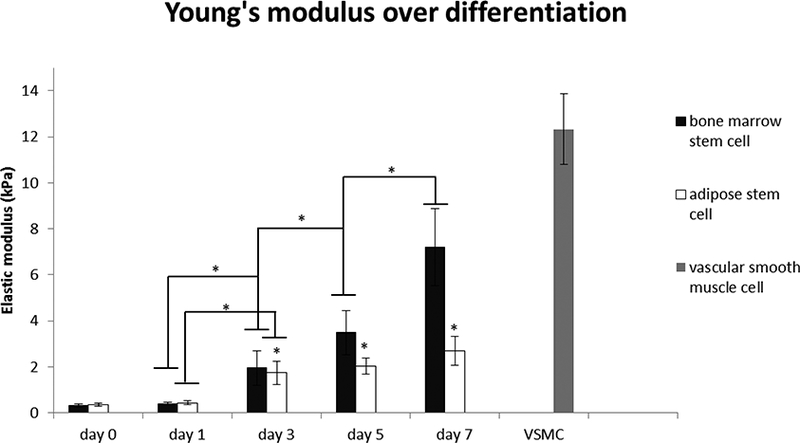
Apparent elastic modulus of BMSCs and ADSCs measured with a borosilicate spherical AFM probes of 5μm diameter at 1μm/s approaching speed with indentation depth from 30nm to 300nm for calculation (10cells per day). Data are presented as mean±standard error (*Significant difference between two groups (p<0.05)).
Figure 3.
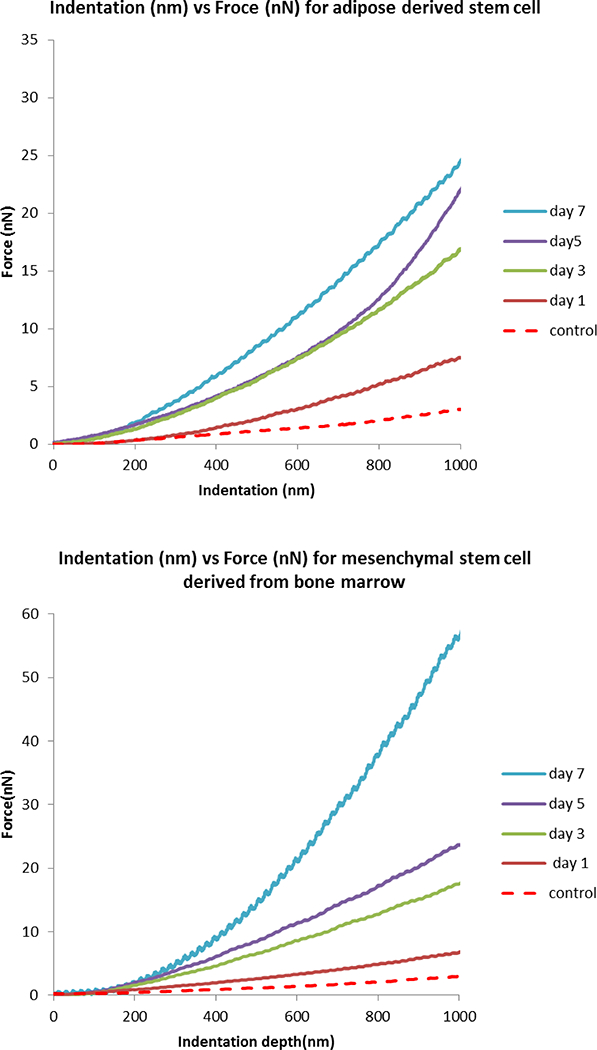
Averaged force vs. indentation curves of BMSCs and ADSCs. Curves shown in the figure are averaged at different time points over differentiation (control is undifferentiated cell at day 0). Generally BMSCs generate higher force in response to indentation compared to ADSCs.
Gene expression of SMC-specific markers
The main objective of this work is to determine whether the expression of specific SMC markers match the change in mechanical property during differentiation (Figure 4). Early and late SMC markers including αSMA, calponin and SM-MHC are detected by fluorescent staining and αSMA is further quantified by real time PCR. We found that for ADSCs, the expression of specific SMC markers gradually increased during differentiation while for BMSCs, the increase of the gene expression can be merely observed after three to five days differentiation. In addition, while all the BMSCs imaged stained positive for calponin and SM-MHC at day 3, ~25% of the ADSCs showed no discernable staining for calponin and SM-MHC at day 3 (Figure 4). At day 3, the ADSC cells did not seem as uniform as the BMSC cells; while many of the cells showed positive staining, there were some clusters of cells which showed no staining of calponin or SM-MHC. By day 7, all the cells imaged showed some positive staining for both markers. We found a baseline expression of αSMA in both undifferentiated stem cells. The expression of αSMA has a significant increase at day 3 for both stem cell types. Although the relative gene expression of αSMA for ADSCs is slightly higher than that of the BMSCs, the quantity of αSMA in terms of RNA level for ADSCs is still much lower than BMSCs based on the absolute number of their PCR cycles, which might be the reason why ADSCs exhibit lower elastic modulus than BMSCs (Figure 5). In addition, it should be noted that while both the BMSCs and the ADSCs showed positive staining for αSMA with immunofluorescence, the BMSCs had more elongated morphologies (Figure 5) than the ADSCs at day 7.
Figure 4.
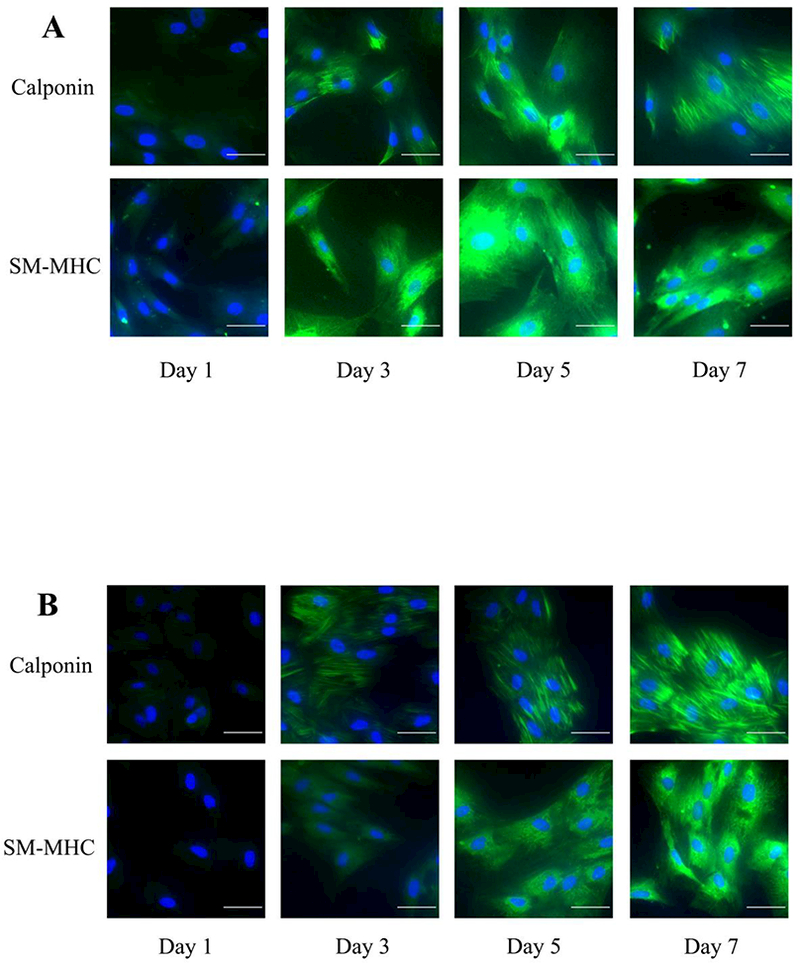
The immunofluorescence staining of SMC-specific marker calponin and SM-MHC over 7 day differentiation from (A) BMSCs and (B) ADSCs. Nucleus were stained with DAPI while antibodies against calponin and SM-MHC were used for immunostaining (scale bar=50μm). Note that some of the ADSC cells in the day 3 images do not show calponin staining.
Figure 5.
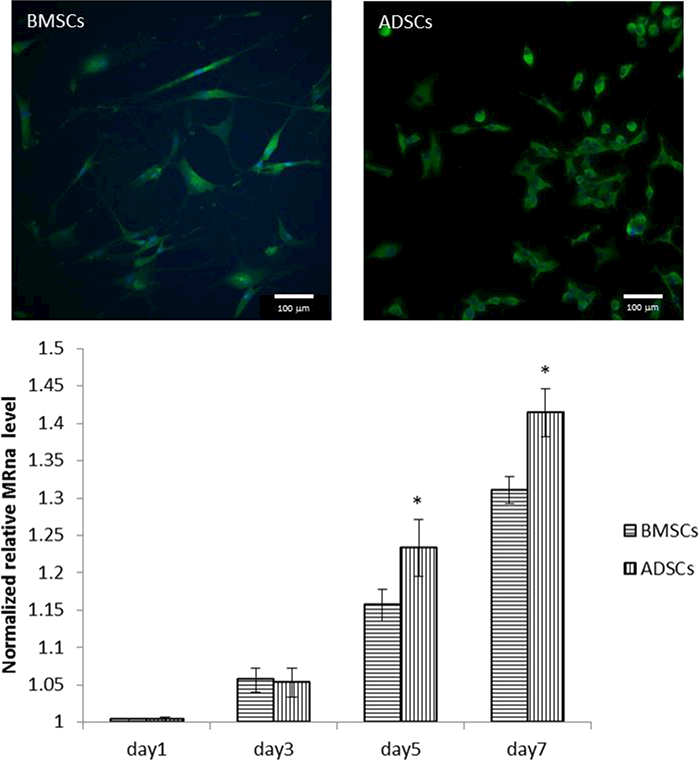
Immunofluorescence staining of αSMA at day 7 for BMSCs (left) and ADSCs (right). Nucleus were stained with DAPI (blue) and an antibody against αSMA was used for immunostaining (scale bar = 100μm). The graph shows the expression of αSMA normalized to control gene β-actin during SMC differentiation for 7 days using real-time PCR (*Significant difference between two groups (p<0.05)).
Discussion
One of the major challenges in the development of cell therapy for vascular disease is to validate a reliable cell source in order to translate the progress from cell study to clinical application. Since mature SMCs cultured in vitro can easily lose their ability to proliferate and contract if used as seeded cells for blood vessel construction (33). Recent research has been focused on exploring alternative cell source which can be served as donor for cardiovascular cells. It has been shown that BMSCs have the ability to differentiate into cells characteristic of blood vessels (34, 35) and are believed to help stabilize vascular structure when co-cultured with other type of cells (36). On the other hand, ADSCs have been shown to be capable of differentiating into smooth muscle like cells expressing both early and late SMC markers (30) and are believed to improve vasculogenesis for cell therapy in adult (37). Thus, in our study, we analyzed the progression of the mechanical properties of these two types of stem cells during differentiation towards a vascular smooth muscle cell lineage. This data can help to determine the cells suitability as a source for vascular smooth muscle repair.
The mechanical behavior of vascular smooth muscle cell not only suggests its ability to contract and relax but also indicates tissue level function. However, limited research has been done to analyze the mechanical property change during vascular smooth muscle cell differentiation, let alone any comparison between different stem cell sources. In the present study, we investigated if the process of vascular smooth muscle cell differentiation varies given different sources of stem cells. The result shows the mechanical property of differentiating stem cells changes significantly depending on the cell source. The Hertz model was utilized in our study to evaluate cell’s elastic modulus. Although Hertz model makes several simplifying assumptions, it can still provide good qualitatively comparison of the elastic modulus between different cells during differentiation. Previous research shows the result of cell’s modulus remains reasonable if the indentation depth used in Hertz fit is small before significant non-linearity occurs with the increase of indentation depth (38).
In our current study, a significant increase in elastic modulus was observed from day 3 BMSCs during differentiation. In the following days, the value reaches its peak at day 7 which is very close to the elastic modulus of vascular smooth muscle cell in control group (Figure 2). Cells in both groups reached 80% confluency at day 7. Since cells are known to stiffen when in confluent cultures (39), it is not clear whether any additional increases in modulus that can be observed after day 7 are due to the cells differentiating further or from the additional cell-cell contacts that occur in very confluent cell cultures. Compared to BMSCs, the overall elastic modulus of ADSCs was much lower and underwent a smoother increase during differentiation. As has previously been established (1), there is a high similarity between the expression of certain genes in BMSCs and smooth muscle cells; therefore, we are not surprised to discover that BMSCs eventually have the similar elastic modulus as in vitro 1-day cultured vascular smooth muscle cell after differentiation. However, the differentiation potential of BMSCs into different vascular lineages is still not fully understood (40). Due to the variable nature and incomplete characterization of isolated mesenchymal stem cell, both the culture condition and isolation method are highly variable (41). In our research, we found that BMSCs from a commercial source have the potential to differentiate into smooth muscle-like cells with mechanical properties similar to native VSMCs, which is crucial in clinical use. However, our current experiments are not sufficient to characterize the whole mechanical behavior of cells and thus further investigation is required to determine if the differentiated stem cells are functional given a physiological loading condition. Considering the difficulty in obtaining large number of mesenchymal stem cells from bone marrow for clinical use, ADSCs provide an attractive alternative cell source, as they can be harvested in relatively large quantities with minimal morbidity. While the elastic modulus of ADSCs increased more slowly over time during differentiation compared to BMSCs, the results show ADSCs still have the potential to gradually acquire higher elastic modulus during differentiation induced by TGF-beta 1. Thus, these cells could be a promising cell source for tissue repair. However, further study is required to determine if the elastic modulus of ADSCs can get even closer to natural vascular smooth muscle cell if given different conditions including the combination of growth factor, co-culture with different cells, and cyclic mechanical loading.
To further establish that cells differentiated are functional smooth muscle cell, the expression of SMC-specific markers were examined by PCR and fluorescent staining. Fluorescent microscopic images of FITC-labeled αSMA reveals that cells differentiated from both stem cell sources have a baseline level of αSMA with minor but constant increase over differentiation. After 7 days culture, most cells appear to be elongated with highly expressed αSMA throughout the cells (Figure 5). Previous research on suggests that αSMA levels in the cells is correlated to higher cellular contractility in differentiating cells.(32, 42) In addition, VSMC in contractile phenotypes that express increased levels of αSMA are stiffer than synthetic VSMCs with less αSMA. (32, 42) We believe that the increase of αSMA along with morphological and adhesive alterations, result in the change of elastic modulus measured in our AFM experiments. Our work shows that there is a correlation between the level of αSMA in the cell and the cellular mechanical properties. The gene expression of αSMA was analyzed by PCR. Consistent with fluorescent staining, αSMA has a baseline expression for both undifferentiated BMSCs and ADSCs along with the increase in baseline expression over differentiation. It is interesting to note that while ADSCs had slight but statistically significant higher expression of αSMA at day 7, they had lower elastic moduli than the BMSCs. In the fluorescence immunostaining images, while both cell types showed clear positive staining for αSMA and spindle like morphologies, BMSCs did appear to have more elongated “contractile-like” morphologies than the ADSCs (Figure 5). It may be that the BMSCs at day 7 had more organized (and thus stiffer (32)) cytoskeletal arrangements than the ADSCs at the same time points.
However, the expression of αSMA alone does not definitely suggest the cells are fully differentiated, other groups have found that the mid-differentiation marker calponin and late stage differentiation marker SM-MHC may have more SMC lineage correlativity than αSMA (43). In our study, both of the two markers were found to be significantly enhanced after 7 days culture compared to the merely detection at day 1 culture. There is also an increase in the levels of both markers over time during differentiation which correlates with the change in elastic modulus. All the results suggest that when induced by TGF-beta 1, both of the two stem cell types differentiate down a SMC lineage while acquiring basic SMC functionality reflected by the increase of elastic modulus. Although TGF-beta 1 activation is not completely understood (44), it has been well documented to be responsible for the induction of mural cell (pericytes or smooth muscle cell) differentiation which are crucial in modulating blood flow and vasculogensis.
Functional VSMC plays an important role in blood circulation by contracting and relaxing. Previous studies showed that there is a difference in the mechanical properties of vascular smooth muscle cells as they shift between synthetic/proliferative and contractile/quiescent phenotypes VSMC (32, 45, 46). While cells are naturally viscoelastic, the elastic property measured previously still provides some insight into the mechanical property of VSMC. In our study, we utilized AFM to analyze the mechanical properties of live cells undergoing differentiation down a smooth muscle cell lineage. Based on that, we demonstrate both stem cells used in our study stiffen when differentiating down VSMC lineages as induced by TGF-beta 1. This change in mechanical properties is also correlated to the change in αSMA expression. Taken together, these results help to analyze the functionality of differentiated cells that may have potential use in clinical application. In addition, while it is possible to assess individual cell mechanical properties in 2D cultures, it will be very difficult to do so when the cells are cultured inside 3D matrices for potential tissue engineering or regenerative medicine applications. The results from this study, which show the correlation between αSMA expression and cellular mechanical properties, could help researchers to assess, at least qualitatively, the mechanical properties of differentiating cells in setups where direct cell probing cannot be performed.
Conclusion
In vitro 7-day culture of BMSCs induced by TGF-beta 1 exhibit greater elastic modulus than ADSCs. We also found that BMSCs can acquire the mechanical property close to VSMC cultured in vitro. This result indicates BMSCs may be a better candidate in differentiating to functional VSMC in terms of mechanical strength. However, further research is required to determine if the differentiation can be affected by environmental change including cyclic loading and combination of growth factor. Optimization of such differentiation can have potential use in clinical application in future.
Acknowledgements
This work was supported by NIH K25 HL092228 and NSF RII-EPS 0903795.
Footnotes
Conflict of interest
The authors have no competing financial interests to disclose.
Reference
- 1.Han JH, Liu JY, Swartz DD, & Andreadis ST (2010) Molecular and functional effects of organismal ageing on smooth muscle cells derived from bone marrow mesenchymal stem cells. Cardiovasc. Res 87(1):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rezai N, Podor TJ, & McManus BM (2004) Bone marrow cells in the repair and modulation of heart and blood vessels: Emerging opportunities in native and engineered tissue and biomechanical materials. Artif. Organs 28(2):142–151. [DOI] [PubMed] [Google Scholar]
- 3.Ji H, Kim HS, Kim HW, & Leong KW (2017) Application of induced pluripotent stem cells to model smooth muscle cell function in vascular diseases. Curr Opin Biomed Eng 1:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinbach SK & Husain M (2016) Vascular smooth muscle cell differentiation from human stem/progenitor cells. Methods 101:85–92. [DOI] [PubMed] [Google Scholar]
- 5.Park WS, et al. (2013) Functional expression of smooth muscle-specific ion channels in TGF-beta(1)-treated human adipose-derived mesenchymal stem cells. American Journal of Physiology-Cell Physiology 305(4):C377–C391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beamish JA, He P, Kottke-Marchant K, & Marchant RE (2010) Molecular Regulation of Contractile Smooth Muscle Cell Phenotype: Implications for Vascular Tissue Engineering. Tissue Engineering Part B-Reviews 16(5):467–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanthilal M & Darling EM (2014) Characterization of mechanical and regenerative properties of human, adipose stromal cells. Cell Mol Bioeng 7(4):585–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaveh K, Ibrahim R, Abu Bakar MZ, & Ibrahim TA (2011) Mesenchymal Stem Cells, Osteogenic Lineage and Bone Tissue Engineering: A Review. Journal of Animal and Veterinary Advances 10(17):2317–2330. [Google Scholar]
- 9.Galmiche MC, Koteliansky VE, Briere J, Herve P, & Charbord P (1993) Stromal Cells from Human Long-Term Marrow Cultures Are Mesenchymal Cells That Differentiate Following a Vascular Smooth-Muscle Differentiation Pathway. Blood 82(1):66–76. [PubMed] [Google Scholar]
- 10.Au P, Tam J, Fukumura D, & Jain RK (2008) Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 111(9):4551–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choong CSN, Hutmacher DW, & Triffitt JT (2006) Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolactone substrates for enhanced potentials in bone tissue engineering. Tissue Eng. 12(9):2521–2531. [DOI] [PubMed] [Google Scholar]
- 12.Zuk PA, et al. (2001) Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 7(2):211–228. [DOI] [PubMed] [Google Scholar]
- 13.Chong PP, Selvaratnam L, Abbas AA, & Kamarul T (2012) Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J. Orthop. Res 30(4):634–642. [DOI] [PubMed] [Google Scholar]
- 14.Mizuno H, Tobita M, & Uysal AC (2012) Concise Review: Adipose-Derived Stem Cells as a Novel Tool for Future Regenerative Medicine. Stem Cells 30(5):804–810. [DOI] [PubMed] [Google Scholar]
- 15.Marra KG, Brayfield CA, & Rubin JP (2011) Adipose Stem Cell Differentiation into Smooth Muscle Cells. Adipose-Derived Stem Cells: Methods and Protocols 702:261–268. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez LV, et al. (2006) Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc. Natl. Acad. Sci. U. S. A 103(32):12167–12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janmey PA (1998) The cytoskeleton and cell signaling: Component localization and mechanical coupling. Physiol. Rev 78(3):763–781. [DOI] [PubMed] [Google Scholar]
- 18.Narita Y, Yamawaki A, Kagami H, Ueda M, & Ueda Y (2008) Effects of transforming growth factor-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res 333(3):449–459. [DOI] [PubMed] [Google Scholar]
- 19.Byfield FJ, Reen RK, Shentu T-P, Levitan I, & Gooch KJ (2009) Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J. Biomech 42(8):1114–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynn Ray J, Leach R, Herbert J-M, & Benson M (2001) Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci 23(4):185–188. [DOI] [PubMed] [Google Scholar]
- 21.Wuyts FL, et al. (1995) Elastic properties of human aortas in relation to age and atherosclerosis - a structural model. Phys. Med. Biol 40(10):1577–1597. [DOI] [PubMed] [Google Scholar]
- 22.Butt HJ & Jaschke M (1995) Calculation of Thermal Noise in Atomic-Force Microscopy. Nanotechnology 6(1):1–7. [Google Scholar]
- 23.Deitch S, Gao BZ, & Dean D (2012) Effect of matrix on cardiomyocyte viscoelastic properties in 2D culture. Molecular & cellular biomechanics : MCB 9(3):227–249. [PMC free article] [PubMed] [Google Scholar]
- 24.Kuznetsova TG, Starodubtseva MN, Yegorenkov NI, Chizhik SA, & Zhdanov RI (2007) Atomic force microscopy probing of cell elasticity. Micron 38(8):824–833. [DOI] [PubMed] [Google Scholar]
- 25.Crick SL & Yin FCP (2007) Assessing micromechanical properties of cells with atomic force microscopy: importance of the contact point. Biomech. Model. Mechanobiol 6(3):199–210. [DOI] [PubMed] [Google Scholar]
- 26.Dimitriadis EK, Horkay F, Maresca J, Kachar B, & Chadwick RS (2002) Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys. J 82(5):2798–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rico F, et al. (2005) Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Physical Review E 72(2):10. [DOI] [PubMed] [Google Scholar]
- 28.Radmacher M (2002) Measuring the elastic properties of living cells by the atomic force microscope. Atomic Force Microscopy in Cell Biology 68:67–90. [DOI] [PubMed] [Google Scholar]
- 29.Mahaffy RE, Shih CK, MacKintosh FC, & Kas J (2000) Scanning probe-based frequency-dependent microrheology of polymer gels and biological cells. Phys. Rev. Lett 85(4):880–883. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, et al. (2010) Differentiation of Adipose-Derived Stem Cells into Contractile Smooth Muscle Cells Induced by Transforming Growth Factor-beta 1 and Bone Morphogenetic Protein-4. Tissue Engineering Part A 16(4):1201–1213. [DOI] [PubMed] [Google Scholar]
- 31.Heydarkhan-Hagvall S, et al. (2003) Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J. Cell. Biochem 89(6):1250–1259. [DOI] [PubMed] [Google Scholar]
- 32.Hemmer JD, Dean D, Vertegel A, Langan E, & LaBerge M (2008) Effects of serum deprivation on the mechanical properties of adherent vascular smooth muscle cells. Proceedings of the Institution of Mechanical Engineers Part H-Journal of Engineering in Medicine 222(H5):761–772. [DOI] [PubMed] [Google Scholar]
- 33.Lagna G, et al. (2007) Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. J. Biol. Chem 282(51):37244–37255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kashiwakura Y, et al. (2003) Isolation of bone marrow stromal cell-derived smooth muscle cells by a human SM22 alpha promoter - In vitro differentiation of putative smooth muscle progenitor cells of bone marrow. Circulation 107(16):2078–2081. [DOI] [PubMed] [Google Scholar]
- 35.Minguell JJ, Erices A, & Conget P (2001) Mesenchymal stem cells. Exp. Biol. Med 226(6):507–520. [DOI] [PubMed] [Google Scholar]
- 36.Sorrell JM, Baber MA, & Caplan AI (2009) Influence of Adult Mesenchymal Stem Cells on In Vitro Vascular Formation. Tissue Engineering Part A 15(7):1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miranville A, et al. (2004) Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110(3):349–355. [DOI] [PubMed] [Google Scholar]
- 38.Chizhik SA, Huang Z, Gorbunov VV, Myshkin NK, & Tsukruk VV (1998) Micromechanical properties of elastic polymeric materials as probed by scanning force microscopy. Langmuir 14(10):2606–2609. [Google Scholar]
- 39.Efremov YM, et al. (2013) The effects of confluency on cell mechanical properties. J. Biomech 46(6):1081–1087. [DOI] [PubMed] [Google Scholar]
- 40.Ramkisoensing AA, et al. (2011) Human Embryonic and Fetal Mesenchymal Stem Cells Differentiate toward Three Different Cardiac Lineages in Contrast to Their Adult Counterparts. PLoS ONE 6(9):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roobrouck VD, Ulloa-Montoya F, & Verfaillie CM (2008) Self-renewal and differentiation capacity of young and aged stem cells. Exp. Cell Res 314(9):1937–1944. [DOI] [PubMed] [Google Scholar]
- 42.Kinner B, Zaleskas JM, & Spector M (2002) Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp. Cell Res 278(1):72–83. [DOI] [PubMed] [Google Scholar]
- 43.Lee SH, Hungerford JE, Little CD, & IruelaArispe ML (1997) Proliferation and differentiation of smooth muscle cell precursors occurs simultaneously during the development of the vessel wall. Dev. Dyn 209(4):342–352. [DOI] [PubMed] [Google Scholar]
- 44.Abe M, et al. (1994) An Assay for Transforming Growth-Factor-Beta Using Cells Transfected with a Plasminogen-Activator Inhibitor-1 Promoter Luciferase Construct. Anal. Biochem 216(2):276–284. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto T, Sato J, Yamamoto M, & Sato M (2000) Smooth muscle cells freshly isolated from rat thoracic aortas are much stiffer than cultured bovine cells: Possible effect of phenotype. Jsme International Journal Series C-Mechanical Systems Machine Elements and Manufacturing 43(4):867–874. [Google Scholar]
- 46.Miyazaki H, Hasegawa Y, & Hayashi K (2002) Tensile properties of contractile and synthetic vascular smooth muscle cells. Jsme International Journal Series C-Mechanical Systems Machine Elements and Manufacturing 45(4):870–879. [Google Scholar]


