Abstract
Background and Aims
MicroRNAs (miRNAs) are small non-coding RNAs that act as post-transcriptional regulators of gene expression via sequence-specific cleavage or translational repression of target transcripts. They are transcribed as long single-stranded RNA precursors with unique stem–loop structures that are processed by a DICER-Like (DCL) ribonuclease, typically DCL1, to produce mature miRNAs. Although a plethora of miRNAs have been found to be regulated by pathogen infection in plants, the biological function of most miRNAs remains largely unknown. Here, the contribution of OsDCL1 to rice immunity was investigated.
Methods
Activation-tagged Osdcl1a (Osdcl1a-Ac) rice mutants were examined for resistance to pathogen infection. mRNA and small RNA deep sequencing, quantitative real-time PCR (RT-qPCR) and stem–loop reverse tanscripion–PCR (RT–PCR) were used to examine DCL1a-mediated alterations in the rice transcriptome. Rice diterpene phytoalexins were quantified by liquid chromatography–tandem mass spectrometry (LC-MSMS). Accumulation of O2·– was determined by nitroblue tetrazolium (NBT) staining.
Key Results
dcl1a-Ac mutants exhibit enhanced susceptibility to infection by fungal pathogens which was associated with a weaker induction of defence gene expression. Comparison of the mRNA and miRNA transcriptomes of dcl1a-Ac and wild-type plants revealed misregulation of genes involved in detoxification of reactive oxygen species. Consequently, dcl1a-Ac plants accumulated O2·– in their leaves and were more sensitive to methyl viologen-induced oxidative stress. Furthermore, dcl1a-Ac plants showed downregulation of diterpenoid phytoalexin biosynthetic genes, these genes also being weakly induced during pathogen infection. Upon pathogen challenge, dcl1a-Ac plants failed to accumulate major diterpenoid phytoalexins. OsDCL1a activation resulted in marked alterations in the rice miRNAome, including both upregulation and downregulation of miRNAs.
Conclusions
OsDCL1a activation enhances susceptibility to infection by fungal pathogens in rice. Activation of OsDCL1a represses the pathogen-inducible host defence response and negatively regulates diterpenoid phytoalexin production. These findings provide a basis to understand the molecular mechanisms through which OsDCL1a mediates rice immunity.
Keywords: DICER-Like (DCL), fungal pathogen, Fusarium fujikuroi, innate immunity, Magnaporthe oryzae, methyl viologen, microRNAs, Oryza sativa, oxidative stress, phytoalexins, rice
INTRODUCTION
MicroRNAs (miRNAs) are small non-coding RNAs that act as post-transcriptional regulators of gene expression via sequence-specific cleavage or translational repression of target transcripts in eukaryotes (Llave et al., 2002; Brodersen et al., 2008). MiRNA genes are transcribed by RNA polymerase II into long precursor transcripts with unique stem–loop structures (pri-miRNA) that are processed in a two-step process by a DICER-Like (DCL) ribonuclease, typically DCL1, to give rise to an miRNA-5p/miRNA-3p duplex (Kurihara and Watanabe, 2004). The miRNA duplex is exported to the cytoplasm, where one miRNA strand is selectively incorporated into an Argonaute 1 (AGO1)-containing RNA-induced silencing complex (RISC). This complex interacts with mRNA targets to direct cleavage or suppress translation.
Plant miRNAs have long been recognized as important regulators of gene expression in diverse developmental processes (Palatnik et al., 2003; Mallory et al., 2004; Rubio-Somoza and Weigel, 2011). They are also involved in hormone signal transduction and adaptation to abiotic and abiotic stress (Navarro et al., 2006; Jagadeeswaran et al., 2009; Li et al., 2010; Jeong and Green, 2013; Baldrich and San Segundo, 2016; Fei et al., 2016). Most of our knowledge of miRNAs involved in plant immune responses to pathogen infection is from studies of the interaction of arabidopsis plants with the bacterial pathogen Pseudomonas syringae.
Plants have evolved multiple defence mechanisms to defend themselves against pathogen infection, forming the innate immune system. Defence reactions are activated by the recognition of conserved pathogen-associated molecular patterns (PAMPs) by host membrane pattern recognition receptors (PRRs). This recognition triggers ‘PAMP-triggered immunity’ (PTI), which is effective against most pathogens (Jones and Dangl, 2006; Couto and Zipfel, 2016). PTI components include production of reactive oxygen species (ROS), reinforcement of the cell wall, activation of protein phosphorylation/dephosphorylation processes and accumulation of antimicrobial proteins, among others. The induction of pathogenesis-related (PR) genes is a ubiquitous response of plants to pathogen infection. Damage-associated molecular patterns (DAMPs) released from the plant cell wall after damage caused by the pathogen also induce plant defence responses. However, certain pathogens are able to suppress these basal resistance mechanisms by delivering effector proteins that can suppress PTI responses into the host cell. As a countermeasure, these microbial effectors are recognized by plant disease resistance proteins (R proteins), establishing ‘effector-triggered immunity’ (ETI). Plants also produce a variety of secondary metabolites as natural protection against microbial pathogens. Among them are phytoalexins, which are low molecular weight compounds with antimicrobial activity and structural diversity (e.g. flavonoids, terpenoids and indole phytoalexins) (Ahuja et al., 2012; Schmelz et al., 2014).
Rice is one of the most important crops worldwide and a primary source of food for more than half of the population. Rice blast caused by the fungus Magnaporthe oryzae is one of the most devastating fungal diseases of cultivated rice worldwide (Wilson and Talbot, 2009). Rice is also the model plant for research in monocotyledonous species with a sequenced genome (Goff et al., 2002; Yu et al., 2002). Evidence supports marked variations in the rice miRNA population during M. oryzae infection or treatment with M. oryzae elicitors (Campo et al., 2013; Li et al., 2014, 2016; Baldrich et al., 2015). Although an important fraction of the rice miRNA transcriptome has been found to respond to M. oryzae infection or treatment with M. oryzae elicitors, a role for these pathogen-regulated miRNAs has been demonstrated for only a few of them. They are miR7695, miR160 and miR398 which function as positive regulators for rice immunity against M. oryzae infection, and miR169 and miR319 which negatively regulate immunity against this fungus (Campo et al., 2013; Li et al., 2014, 2017; Zhang et al., 2018).
Regarding DCL1, a major miRNA processing component, three loci encoding DCL1 proteins are identified in the rice genome: OsDCL1a, OsDCL1b and OsDCL1c (Kapoor et al. 2008). Previous studies revealed that loss of function of OsDCL1a by RNA interference (RNAi; dcl1a-IR lines) results in abnormal shoot and root development with eventual growth arrest for the strongest RNAi lines (Liu et al., 2005). Later on, silencing of OsDCL1 was found to enhance resistance to rice blast fungus (Zhang et al., 2015). In contrast, a phenotype of susceptibility to pathogen infection was observed in arabidopsis dcl1 mutants, showing enhanced susceptibility to infection by bacterial (Pseudomonas syringae) and fungal (Botrytis cinerea) pathogens (Navarro et al., 2008; Seo et al., 2013; Weiberg et al., 2014). DCL1a silencing also results in abnormal growth and development in arabidopsis plants (Gasciolli et al., 2005). However, the DCL1-mediated mechanisms underlying these phenotypes of disease resistance or susceptibility in rice or arabidopsis remain unknown.
The goal of this research was to investigate the role of OsDCL1 in rice immunity against fungal pathogens. To rule out the disease phenotype of OsDCL1a knock-down mutants being an effect of its morphological phenotype, we searched for Osdcl1a activation mutants. Two OsDCL1a activation mutants were identified and characterized (named dcl1a-Ac mutants). Plant growth performance of Osdcl1a-Ac plants was not affected. OsDCL1a activation enhanced susceptibility to infection by the fungal pathogens M. oryzae (hemibiotroph) and Fusarium fujikuroi (necrotroph), the causal agents of the rice blast and bakanae disease, respectively. Susceptibility to pathogen infection in dcl1a-Ac plants was associated with weaker induction of defence gene expression. The mRNA transcriptome and miRNAome of dcl1a-Ac plants were obtained and compared with those of wild-type plants. OsDCL1a activation had an important impact on the expression of genes involved in two processes: ROS detoxification and synthesis of diterpene phytoalexins. dcl1a-Ac plants featured downregulation of genes involved in the biosynthesis of terpenoid phytoalexins. Upon pathogen infection, phytoalexin accumulation was compromised in dcl1a-Ac plants. Together, our results support that OsDCL1a plays an important role in rice immunity.
MATERIALS AND METHODS
Plant and fungal materials
Plants (Oryza sativa) were grown at 28 ºC/22 ºC day/night (16 h light/8 h dark cycle). Rice genotypes used were O. sativa japonica ‘Tainung 67’ (TN67), dcl1a-Ac mutants (M0066754, M0040827) from the Taiwan Rice Insertional Mutant collection (TRIM; http:/trim.sinica.edu.tw) and dcl1a-IR lines (Liu et al., 2005). Genotyping of dcl1a-Ac mutants was carried out by PCR on genomic DNA with DCL1a-specific primers combined with a T-DNA-specific primer located at the left border of the T-DNA (Supplementary Data Table S1).
The fungus M. oryzae (strain Guy 11) was grown as previously described (Campos-Soriano et al., 2013). The fungus F. fujikuroi (isolate 297) was grown for 15 d on potato dextrose agar (PDA) medium. Fungal spores were collected by adding sterile water to the surface of the mycelium and adjusted to the appropriate concentration.
Infection assays and elicitor treatment
Infection with M. oryzae involved spraying leaves of 3-week-old rice plants with a spore suspension (1 × 105 spores mL–1). In all experiments, mock inoculations were performed. Development of disease symptoms was followed over time. Lesion area was determined by using digital imaging software (Assess 2.0, American Phytopathological Society). For infection experiments with F. fujikuroi, seeds were sterilized with sodium hypochlorite (30 %, for 30 min), pre-germinated for 24 h on Murashige and Skoog (MS) medium without sucrose and then inoculated with F. fujikuroi spores (1 × 106 spores mL–1; 10 μL per seed). Fungal DNA on infected leaves was quantified by quantitative PCR (qPCR) with specific primers for the 28S DNA gene of the corresponding fungus (Qi and Yang, 2002; Jeon et al., 2013). Primers are given in Supplementary Data Table S1. A standard curve with fungal DNA was prepared for quantification of fungal DNA in infected leaf samples. For elicitor treatment, 3-week-old wild-type plants were sprayed with an elicitor suspension obtained by autoclaving and sonicating M. oryzae mycelium (300 μg mL–1) (Casacuberta et al., 1992).
RT-qPCR and stem–loop RT–PCR
Total RNA was extracted using the TRIzol reagent (Invitrogen). The RNA concentrations were quantified by a NanoDrop ND-2000 spectrophotometer. First-strand cDNA was synthesized from DNase-treated total RNA (1 μg) with SuperScript III reverse transcriptase (Invitrogen GmbH) and oligo(dT). Quantitative real-time PCRs (RT-qPCRs) were performed in optical 96-well plates in a Light Cycler 480 (Roche) with SYBR Green. All reactions were performed in triplicate. The average cycle threshold (Ct) values were obtained by PCR from three independent biological replicates and normalized to the average Ct values for the cyclophilin 2 gene (Os02g02890) from the same RNA preparations to obtain the ΔCt value or normalized expression (relative expression). Primers used for RT-qPCR and stem–loop reverse transcription–PCR (RT–PCR) are listed in Supplementary Data Table S1. Analysis of variance (ANOVA) tests were used to evaluate differences in gene expression.
Quantification of rice diterpene phytoalexins
For quantification of rice phytoalexins, leaf segments were mixed with 40 vols of 80 % methanol, concentrated to dryness and resuspended in 0.5 mL of 80 % methanol.
Phytoalexins were quantified by liquid chromatography–tandem mass spectrometry (LC-MSMS) as previously described (Miyamoto et al., 2016). Three biological replicates with two technical replicates each were performed. ANOVA tests were used to evaluate differences in phytoalexin accumulation.
Treatment with methyl viologen, pigment quantification and determination of the superoxide ion
Leaf segments (approx. 2 cm in length) were treated with methyl viologen (MV) solution (10 μm) at room temperature in the dark for 12 h, then incubated at 28 ºC at a 16 h/8 h photoperiod cycle for 3 d. Chlorophylls and carotenoids were extracted and quantified spectrophotometrically (Lichtenthaler and Buschmann, 2001). For histochemical detection of the superoxide ion O2·–, leaf sections approx. 2 cm long were stained with nitroblue tetrazolium (NBT) (Campo et al., 2008).
RNA-Seq and small RNA-Seq
Libraries were prepared from leaves of 3-week-old wild-type (segregated azygous) and dcl1a-Ac plants (two biological replicates per genotype). Indexed libraries were prepared from 1 μg of purified RNA from each sample (TruSeq Stranded mRNA Sample Prep Kit, Illumina). RNAs were quantified using the Agilent 2100 Bioanalyzer (Agilent Technologies) and pooled such that each index-tagged sample was present in equimolar amounts, with a final concentration of the pooled samples of 2 nm. The pooled samples underwent cluster generation and sequencing with the Illumina HiSeq 2500 System (Genomics4life S.R.L., Baronissi, Salerno, Italy) in a 2 × 50 single-end format at a final concentration of 8 pmol. The raw sequence files generated underwent quality control analysis with FastQC v0.11.3 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Trimming and removal of adaptors involved use of Trimmomatic v0.33 (Bolger et al., 2014) (minimum quality score 35, minimum length 25). The obtained reads were then mapped against the O. sativa reference genome (MSU 7.0) with STAR (v2.4.0j) (Dobin et al., 2013) providing the reference gene annotation file with known transcripts (RGSP 7.0). Alignment files were filtered to remove reads with MAPQ <30. FeatureCounts v1.4.5-p1 (Liao et al., 2014) was used for read summarization at the gene level, with the strand-specific option ‘reversely stranded’. Statistical analysis of the read counts involved use of R 3.1.3 with the HTSFilter package to remove genes with low expression (Rau et al., 2013) and the edgeR package for differential expression analysis (McCarthy et al., 2012). Gene Ontology (GO) enrichment analysis of the differentially expressed genes involved use of the AgriGO webtool (http://bioinfo.cau.edu.cn/agriGO/;Du et al., 2010).
For small RNA sequencing (RNA-Seq) the minimum length established was 15 bp and the quality score was 35. The high quality reads were aligned against the O. sativa reference genome sequence (MSU 7.0) with Bowtie (version 1.1.1, parameters ‘v1’, ‘a’). FeatureCounts (version 1.4.5) was used together with miRBase v21 annotation to calculate gene expression values as raw read counts. Normalization was applied to the raw read counts by using the Trimmed Mean of M values (TMM) normalization. Data sets generated during the current study are available from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE109307).
RESULTS
Identification and characterization of activation-tagged DCL1a mutants
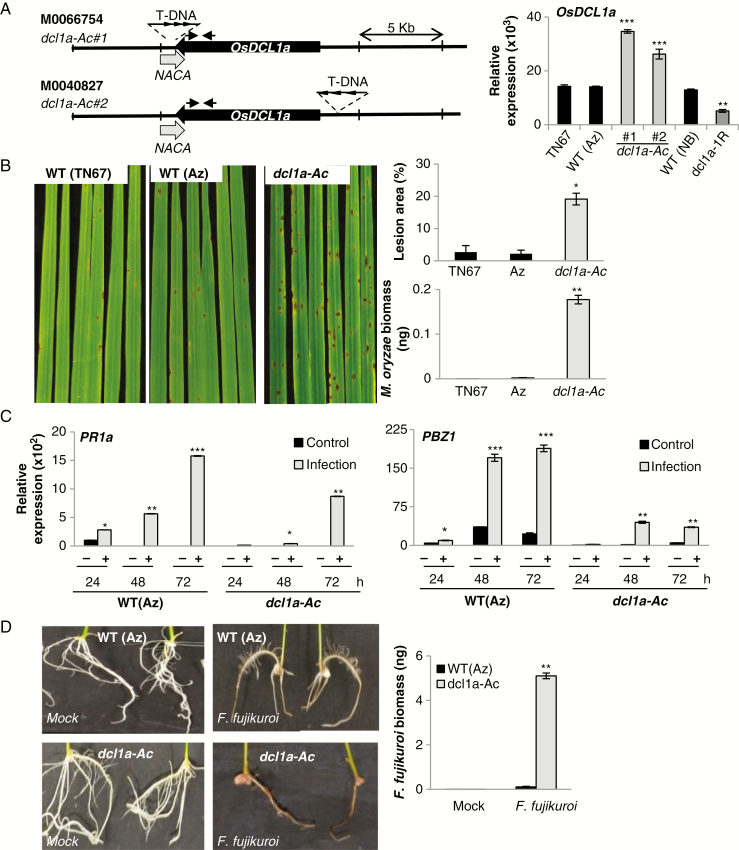
As previously mentioned, DCL1 silencing has a negative impact on plant growth in rice. To investigate the contribution of DCL1 in disease resistance with no influence of intrinsic developmental cues, we searched for DCL1 activation mutants in publicly available mutant collections. Two T-DNA tagged lines carrying the T-DNA insertion near OsDCL1a, lines M0066754 and M0040827, were identified in the activation/knockout TRIM collection generated in the Tainung 67 (TN67, japonica) background (Hsing et al., 2007; http://trim.sinica.edu.tw) (Fig. 1A). The T-DNA insertion site in each TRIM mutant line was confirmed by PCR followed by DNA sequencing. Homozygous, hemizygous and azygous plants were identified (Supplementary Data Fig. S1A, B). The T-DNA used for generating the TRIM library contains eight tandem repeats of the Cauliflower mosaic virus 35S promoter (CaMV35), which can activate the expression of genes located around the T-DNA insertion sites (Fig. 1A, left panel). OsDCL1a transcript levels were significantly higher in leaves from each mutant line as compared with azygous (segregated progeny) and wild-type TN67 plants (Fig. 1A, right panel), indicating that they are activation mutants for OsDCL1a (hereafter referred to as dcl1a-Ac#1 and dcl1a-Ac#2). As expected, OsDCL1a expression was lower in OsDCL1a RNAi (dcl1a-IR) plants than its parental genotype (O. sativa ‘Nipponbare’; Liu et al., 2005) (Fig. 1A, right panel). qPCR revealed that each of the dcl1a-Ac mutants has a single copy of the T-DNA inserted in its genome (Supplementary Data Table S2). Importantly, we found no obvious phenotype differences between dcl1a-Ac mutant and wild-type (azygous and TN67) plants grown under controlled greenhouse conditions (Supplementary Data Fig. S1C). An examination of the genomic regions flanking OsDCL1 identified one gene, nascent polypeptide-associated complex subunit alpha (NACA), that partially overlaps OsDCL1a (Fig. 1A). OsDCL1 and NACA locate in opposite strands of the DNA (MSU release 7). Thus, in the dcl1a-Ac#1 mutant, the T-DNA insertion site is found at the 3’-untranslated region (UTR) of both NACA and OsDCL1a. However, NACA transcripts accumulated at equivalent levels in mutant (dcl1a-Ac#1, dcl1a-Ac#2) and control plants (azygous, wild-type) (Supplementary Data Fig. S1D).
Fig. 1.
Susceptibility of OsDCL1a activation mutants to infection by the pathogens M. oryzae and F. fujikuroi. (A) Representation of the T-DNA insertion mutants from the TRIM collection (lines M0066754 and M0040827) (left panel). Black and grey arrows represent OsDCL1a (Os03g02970) and the nearby genes (Nascent polypeptide-associated complex subunit alpha, NACA; Os03g02960) pointing in the direction of transcription. Arrowheads in the T-DNA represent the CaMV35S enhancer octamers. Arrows above the OsDCL1a gene indicate the position of primers used for RT-qPCR analysis. Right panel: OsDCL1a expression in leaves of 3-week-old dcl1a-Ac and dcl1a-IR plants determined by RT-qPCR. Tainung67 (TN67) and Nipponbare (NB) are the genetic backgrounds of the TRIM mutants and dcl1a-IR plants, respectively. (B) Susceptibility of the dcl1a-Ac#1 mutant to M. oryzae infection. Three-week-old rice plants were inoculated with M. oryzae spores (1 × 105 spores mL–1). Pictures were taken at 7 days post-inoculation (dpi). Right panels show the lesion area in infected leaves (measured by Assess 2.0) and quantification of M. oryzae DNA by qPCR at 7 dpi. (C) OsPR1a and OsPBZ1 expression in wild-type (segregated azygous) and dcl1a-Ac#1 plants determined by RT-qPCR at the indicated times after inoculation with M. oryzae spores (1 × 105 spores mL–1). (D) Susceptibility of dcl1a-Ac plants to infection by F. fujikuroi. Seeds of dcl1a-Ac#1 and wild-type (segregated azygous) plants were germinated for 24 h and inoculated with F. fujikuroi spores (106 spores mL–1). Roots from F. fujikuroi-inoculated seedlings at 7 dpi are shown. Right panel: F. fujikuroi DNA in roots of wild-type and dcl1a-Ac plants was quantified by qPCR at 7 dpi. Three independent infection experiments with each fungus were performed (at least 24 plants per genotype in each experiment). Data are the mean ± s.d. (n = 3 biological replicates) (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 by ANOVA).
Susceptibility to infection by fungal pathogens in dcl1a-Ac plants
To investigate the functional relevance of OsDCL1a activation in disease resistance, dcl1a-Ac plants were examined for resistance to infection by the rice blast fungus M. oryzae. The dcl1a-Ac plants consistently exhibited higher susceptibility to M. oryzae infection as compared with control plants (Fig. 1B). Susceptibility of dcl1a-Ac to blast was confirmed by measuring the average lesion area in the infected leaves and by qPCR measurement of fungal DNA, an indicator of fungal biomass in the infected leaves (Fig. 1B, right panels). The dcl1a-Ac#2 mutant also exhibited higher susceptibility to M. oryzae infection as compared with control plants (Supplementary Data Fig. S2). Increased susceptibility to blast infection in dcl1a-Ac plants is consistent with findings of resistance to M. oryzae in dcl1a-IR lines (Zhang et al., 2015). However, dcl1a-IR plants showed abnormal growth, whereas dcl1a-Ac plants grew and developed normally.
To obtain further insights into the mechanisms underlying susceptibility to M. oryzae infection in dcl1a-Ac plants, we examined the expression pattern of the defence genes OsPR1a and OsPBZ1 (a PR10 family member) in wild-type and dcl1a-Ac plants. These genes are widely used as indicators of induction of rice defence responses during pathogen infection, including M. oryzae infection (Midoh and Iwata, 1996; Agrawal et al., 2001). As expected, OsPR1a and OsPBZ1 expression was induced in wild-type plants during M. oryzae infection (Fig. 1C). Although OsPR1a and OsPBZ1 expression was also activated by fungal infection in dcl1a-Ac plants, their expression was induced at a much lower level in mutant plants than in wild-type plants at all times of infection. Reduced induction of defence gene expression agrees with the observed phenotype of susceptibility in dcl1a-Ac plants.
We also examined disease resistance of dcl1a-Ac plants against the necrotrophic fungus F. fujikuroi, the causal agent of bakanae, an important seed-borne disease of rice (Wulff et al., 2010). As compared with wild-type segregated azygous plants, dcl1a-Ac seedlings grew poorly and their roots turned necrotic on F. fujikuroi inoculation (Fig. 1D). Fungal biomass was greater in roots of dcl1a-Ac than of wild-type plants (Fig. 1D, right panel), thus confirming that dcl1a-Ac plants are more susceptible to infection by F. fujikuroi.
Together, the results demonstrate that OsDCL1a activation enhances susceptibility to infection by hemibiotrophic (M. oryzae) and necrotrophic (F. fujikuroi) fungal pathogens in rice and that disease susceptibility in dcl1a-Ac plants is associated with weaker induction of defence gene expression during pathogen infection.
Expression of DCL genes in dcl1a-Ac plants
In plants, the DCL gene family typically comprises four members, DCL1–DCL4, which have distinct functions in miRNA and small interfering RNA (siRNA) biogenesis (Arikit et al., 2013). A fifth DCL, DCL3b (also named DCL5), which is associated with the production of 24 nt siRNAs, appears to have evolved in monocots (Margis et al., 2006; Song et al., 2012; Wei et al., 2014). The rice genome contains three DCL1 genes (OsDCL1a, OsDCL1b and OsDCL1c). OsDCL1a is most closely related to AtDCL1a from a structural and functional point of view, and OsDCL1a silencing impairs miRNA biogenesis in rice (Liu et al., 2005). Additionally, the rice genome has two DCL2 paralogues with almost identical sequences (DCL2a/b) and unique DCL3 (OsDCL3a), DCL4 and DCL3b genes (Margis et al., 2006; Kapoor et al., 2008). OsDCL1a, OsDCL2a/b and OsDCL3a are ubiquitously expressed in vegetative tissues during development, but their expression is markedly reduced in the reproductive phase (Kapoor et al., 2008). DCL genes with low expression (OsDCL1b, OsDCL1c and OsDCL3b) feature inflorescence-, panicle- and/or early seed-specific expression (Kapoor et al., 2008). OsDCL1a was the most highly expressed OsDCL1 gene in leaves of 3-week-old wild-type rice plants (Supplementary Data Fig. S3). As expected, OsDCL1a expression was further increased in dcl1a-Ac plants, with no significant difference in the expression of any of the other DCL genes between dcl1a-Ac and wild-type plants (Supplementary Data Fig. S3).
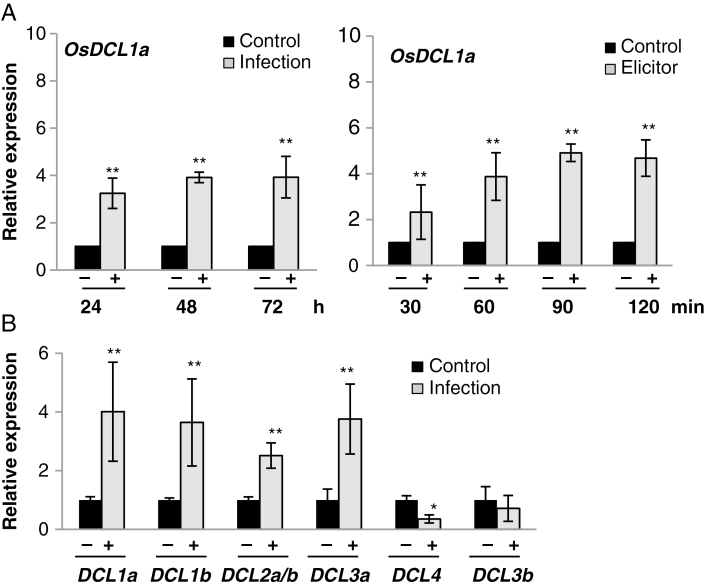
The apparently negative effect of OsDCL1a activation on resistance to fungal infection prompted us to investigate whether OsDCL1a expression itself is regulated as part of the host response to pathogen infection. This analysis revealed OsDCL1a expression induced in wild-type plants in response to M. oryzae infection [at 24, 48 and 72 h post-inoculation (hpi)] (Fig. 2A, left panel). A similar trend in OsDCL1a expression (i.e. upregulation) was observed after treatment with elicitors obtained by autoclaving and sonicating M. oryzae mycelium (Fig. 2A, right panel). Regarding other rice DCL genes, a different response to M. oryzae infection was observed depending on the family member. OsDCL1a, OsDCL1b, OsDCL2a/b and OsDCL3a expression was induced, whereas that of OsDCL4 was repressed by M. oryzae infection, and OsDCL3b was not affected (at least at the time examined, 72 hpi) (Fig. 2B).
Fig. 2.
Expression of OsDCL genes during infection with M. oryzae and treatment with fungal elicitors. (A) OsDCL1a expression at different times after inoculation with M. oryzae spores (1 × 105 spores mL–1) (left panel) or treatment with elicitors from this fungus (300 μg mL–1) (right panel) in wild-type plants. Black and red bars correspond to mock-inoculated and M. oryzae-inoculated (or elicitor-treated) plants, respectively. The expression level in mock-inoculated plants was set to 1.0. Three independent experiments (each with 24 plants per condition) were performed with similar results. Data are mean ± s.d. (*P ≤ 0.05; **P ≤ 0.01 by ANOVA). (B) Expression of OsDCL family members at 72 h post-inoculation (hpi) with M. oryzae spores.
From these results, we conclude that pathogen infection alters the expression of rice DCL genes, namely OsDCL1a, OsDCL1b, OsDCL2 and OsDCL3a. Knowing that these genes are involved in small RNA biogenesis pathways, this observation anticipates important small RNA-mediated transcriptional reprogramming of gene expression as part of the rice response to infection by the fungal pathogen M. oryzae.
Transcript profiling of dcl1a-Ac mutant plants
To investigate OsDCL1a-mediated alterations in the rice transcriptome, we used RNA-Seq analysis of dcl1a-Ac and wild-type (segregated azygous) plants. RNA was obtained from leaves of 3-week-old plants. Illumina Solexa sequencing produced 39.6 and 31.0 million reads in wild-type and dcl1a-Ac plants, respectively (Supplementary Data Table S3). The processed RNA-Seq reads were mapped to the rice genome (O. sativa ‘Nipponbare’ MSU 7.0). For calling differentially expressed genes (DEGs), a fold change of 2.0 was used as a cut-off, with the false discovery rate (FDR) set to 0.05.
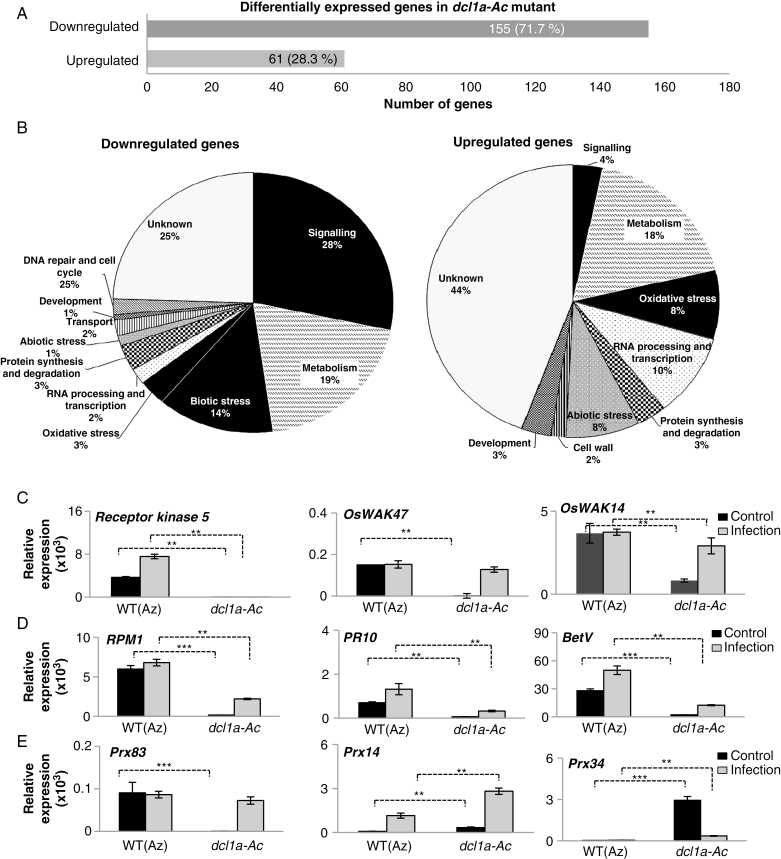
A total of 216 DEGs were found in dcl1a-Ac plants relative to wild-type plants, most downregulated in dcl1a-Ac plants (155 downregulated; 61 upregulated) (Fig. 3A; Supplementary Data Tables S4, S5). GO functional analysis revealed that many downregulated genes in dcl1a-Ac plants were in the categories ‘signalling’, ‘metabolism’, and ‘biotic stress’ (28, 19 and 14 %, respectively) (Fig. 3B, left panel).
Fig. 3.
Distribution and validation of differentially expressed genes in dcl1a-Ac plants. (A) Total number of differentially expressed genes in leaves of dcl1a-Ac plants compared with wild-type plants (downregulated and upregulated genes). (B) Functional categories of downregulated and upregulated genes in leaves of dcl1a-Ac plants. (C–E) Validation and fungal responsiveness of differentially expressed genes identified by RNA-Seq. Transcript levels were determined by RT-qPCR in leaves of control (non-infected) and M. oryzae-infected plants (at 72 hpi) (black and red bars, respectively). (C) Receptor kinase 5 (Os09g37880), OsWAK47 (Os04g30260) and OsWAK14 (Os10g39680). (D) Disease resistance RPM1 (Os11g12340), PR10 (Os12g36860) and Bet V (PR10 family; Os12g36850). (E) Prx83 (Os06g32990), Prx14 (Os07g48050) and Prx34 (Os03g02939). Four biological samples (including the same RNA samples used for RNA-Seq experiments for non-inoculated plants) and two technical replicates were examined (**P ≤ 0.01; ***P ≤ 0.001 by ANOVA).
The distribution of DEGs in functional categories differed greatly between upregulated and downregulated genes (i.e. genes associated with ‘biotic stress’ were not represented in the upregulated genes in dcl1a-Ac plants, whereas genes involved in oxidative stress were highly represented) (Fig. 3B, right panel). Genes involved in ‘signalling’ were less represented in upregulated than in downregulated genes (Fig. 3B; Supplementary Data Tables S4, S5). DEGs in dcl1a-Ac plants were classified according to their molecular functions by using the AgriGO tool (Du et al., 2010; http://bioinfo.cau.edu.cn/agriGO/) (Supplementary Data Fig. S4). Important differences were observed in the categories of protein kinase and oxidoreductase (monooxygenase) activities. For instance, the expression of many receptor-like kinases was downregulated in dcl1a-Ac vs. wild-type plants (Supplementary Data Table S4). The sub-family of cell wall-associated kinase (WAK) genes was the most highly represented of downregulated receptor kinase genes (up to 17 WAK genes were downregulated in dcl1a-Ac plants). WAKs are involved in perception of PAMPs and DAMPs for activation of defence-associated responses, and overexpression of WAK genes increases resistance to M. oryzae in rice (Li et al., 2009). Two brassinosteroid insensitive 1 receptor kinase (BRI1) genes were downregulated in dcl1a-Ac plants (Supplementary Data Table S4), these genes also being involved in recognition of PAMPs and activation of plant immune responses. Genes typically associated with disease resistance and defence mechanisms were also downregulated in dcl1a-Ac plants, such as several R genes and the OsWRKY47 transcription factor gene. Previous studies have shown overexpression of OsWRKY47 in rice accompanied by upregulation of PR10 and blast resistance (Wei et al., 2013). In agreement with this, dcl1a-Ac plants showed downregulation of both OsWRKY47 and PR10 expression (Supplementary Data Table S4). Among the genes downregulated in dcl1a-Ac were those involved in the biosynthesis of antifungal compounds, such as Agmantine hydroxycinnamoyltransferase1 (for producing antifungal hydroxycinnamoylagmantine derivatives) and strictosidine synthase (for producing alkaloids) (Supplementary Data Table S4).
Of note, genes encoding enzymes involved in oxidation–reduction reactions were highly represented among misregulated genes in dcl1a-Ac plants (up- and downregulated genes). They included several peroxidases and cytochrome P450 monooxygenase (CYP) genes (Supplementary Data Tables S4 and S5). CYPs catalyse the oxidation of many substrates for producing several metabolites, these enzymes being involved in the production of phytoalexins and phytohormones.
The expression of selected DEGs in dcl1a-Ac vs. wild-type plants was validated by RT-qPCR, including genes classified in the categories of ‘signalling’, ‘biotic stress’ and ‘oxidative stress’. We further extended this analysis by determining the expression of these genes under non-infection and infection conditions (e.g. 72 hpi with M. oryzae). In the absence of pathogen infection, the expression of receptor kinase genes (Receptor kinase 5, OsWAK47 and OsWAK14), disease resistance (RPM1) and defence genes (PR10 and BetV) was significantly lower in dcl1a-Ac than in wild-type plants (Fig. 3C, D). Prx83 was downregulated in dcl1a-Ac plants, but two other peroxidase genes (Prx14 and Prx34) were upregulated in the absence of pathogen infection (Fig. 3E). Together, these results indicate good correlation between RT-qPCR analysis and RNA-Seq data.
Upon pathogen challenge, the fungal responsiveness of Receptor kinase 5 was compromised in dcl1a-Ac plants, whereas WAK14, RPM1, PR10 and BetV reached a lower expression in dcl1a-Ac than in wild-type plants (Fig. 3C, D). Prx14 and Prx34 expression was more strongly induced by fungal infection in mutant than in wild-type plants (Fig. 3E). The lower induction of defence-related genes during pathogen infection (e.g. Receptor kinase, R and PR genes) and misregulation of genes involved in oxidative stress might well contribute to disease susceptibility in dcl1a-Ac plants.
DCL1 activation leads to reduced expression of diterpenoid phytoalexin biosynthesis genes and compromises phytoalexin accumulation during pathogen infection
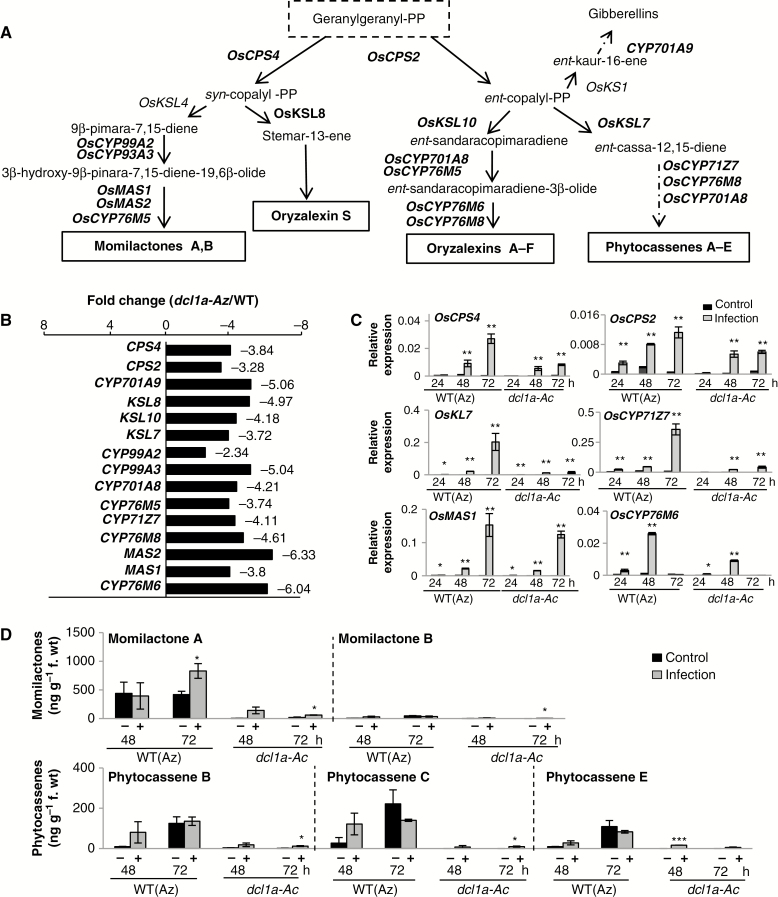
Diterpenoid phytoalexins are the major phytoalexins in rice and are classified into five groups by the carbon skeleton: momilactones (A and B), oryzalexins (A–F), oryzalexin S, phytocassenes (A–E) and ent-10-oxodepressin (Ahuja et al., 2012; Inoue et al., 2013; Yamane, 2013). Our RNA-Seq analysis revealed the downregulation of genes involved in the biosynthesis of momilactones, oryzalexins and phytocassenes in dcl1a-Ac vs. wild-type plants (Fig. 4A, B; Supplementary Data Table S4). Upon pathogen challenge, diterpenoid phytoalexin biosynthetic genes were induced to a lower extent in dcl1a-Ac than in wild-type plants (Fig. 4C).
Fig. 4.
Expression of genes involved in the biosynthesis of diterpenoid phytoalexins in dcl1a-Ac plants. (A) Biosynthetic routes of diterpenoid phytoalexins in rice. Genes with expression downregulated in dcl1a-Ac compared with wild-type plants are indicated in red. Diterpenoid phytoalexins are synthesized from geranylgeranyl diphosphate (geranylgeranyl-PP), which is sequentially cyclized by the diterpene synthases CPSs (copalyl diphosphate synthases) and KSLs (termed kaurene synthase-like because of their similarity to the corresponding enzyme in gibberellic acid biosynthesis), then converted to each phytoalexin by P450 monooxygenases (CYPs) and dehydrogenases. OsCPS4 (syn-copalyl-diphosphate synthase 4, Os04g09900); OsCPS2 (ent-copalyl diphosphate synthase 2, Os02g36210); OsCYP93A3 (9β-pimara-7,15-diene oxidase, Os04g09920); OsCYP99A2 (cytochrome P450, Os04g10160); OsCYP76M5 (cytochrome P450, Os02g36030); OsCYP701A8 (ent-sandaracopimaradiene 3-hydrolase, Os06g37300); OsCYP71Z7 (ent-cassadiene C2-hydroxylase, Os02g36190); OsCYP76M8 (oryzalexin D synthase, Os02g36070); OsCYP76M6 (oryzalexin E synthase, Os02g36280); OsCYP701A9 (ent-kaurene oxidase, Os06g37224); OsKSL7 (ent-cassa-12-15-diene synthase, Os02g36140); OsKSL10 (ent-sandaracopiramadiene synthase, Os12g30824); OsKSL8 (stemar-13-ene synthase, Os11g28530); OsMAS (monilactone A synthase, OsMAS1, Os04g10000; and OsMAS2, Os04g10010). (B) Fold repression of expression (dcl1a-Ac vs. wild-type azygous plants) of genes involved in diterpenoid phytoalexin biosynthesis. (C) Expression of phytoalexin biosynthesis genes in wild-type (azygous) and dcl1a-Ac plants in response to M. oryzae infection (1 × 105 spores mL–1) or mock inoculation (red and black bars, respectively) (*P ≤ 0.05; **P ≤ 0.01 comparing the indicated genotypes or condition by ANOVA). (D) Accumulation of diterpenoid phytoalexins is compromised in leaves of dcl1a-Ac plants. Three biological samples for each genotype and condition were examined (*P ≤ 0.05; ***P ≤ 0.001 by ANOVA).
To investigate whether the downregulation of phytoalexin biosynthesis genes affects phytoalexin accumulation, we measured their levels in leaves of dcl1a-Ac and the wild type, under non-infection and infection conditions. Momilactone A and phytocassenes B, C and E accumulated at detectable levels in wild-type plants, but their accumulation was drastically reduced in dcl1a-Ac plants (Fig. 4D). It is of note that diterpenoid phytoalexins stayed almost at the basal level in M. oryzae-infected dcl1a-Ac plants (Fig. 4D), indicating that DCL1a activation, most probably, compromises diterpenoid phytoalexin production during pathogen infection. The antifungal activity of rice phytoalexins against M. oryzae has been described (Dillon et al., 1997; Umemura et al., 2003; Hasegawa et al., 2010). Failure to accumulate major rice phytoalexins in dcl1a-Ac plants would then facilitate pathogen growth in these plants.
Reduced tolerance to oxidative stress in dcl1a-Ac plants
Reactive oxygen species are constantly being generated during normal plant growth and development, and an imbalance between ROS generation and safe detoxification generates oxidative stress in plants. Knowing that a substantial number of genes encoding enzymes that function in oxidation–reduction reactions (e.g. peroxidase, glutathione S-transferase and CYP genes) were misregulated in dcl1a-Ac plants (Supplementary Data Tables S4 and S5), we hypothesized that DCL1 activation might affect ROS detoxification systems and/or ROS homeostasis. This, in turn, would affect redox-dependent cellular processes. ROS include superoxide anion (O2·–), hydroxyl radical (OH·) and hydrogen peroxide (H2O2), with OH· being the most reactive molecule. Furthermore, O2·− and H2O2 can react with each other in the presence of metal ions, such as iron, to form the more reactive hydroxyl radicals OH· and OH– via the Haber–Weiss and Fenton reactions. Hydroxyl radicals are highly reactive and interact with all biological molecules, leading to cellular damage.
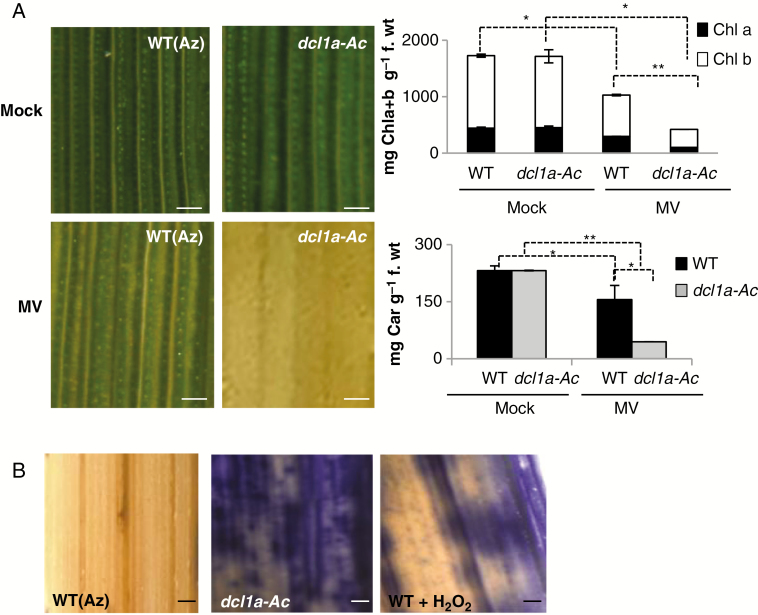
To investigate whether transcriptome alterations caused by DCL1 activation affect the host ROS detoxification system, we used the ROS-generating reagent, MV. This compound acts as an inhibitor of photosynthesis and promotes the formation of superoxide anion (O2·−), which results in reduced chlorophyll content and discoloration in MV-treated leaves. Leaves of dcl1a-Ac plants were greatly affected by treatment with MV, and the chlorophyll content was markedly reduced in leaves of MV-treated dcl1a-Ac vs. MV-treated leaves of wild-type plants (Fig. 5A). Carotenoids are also able to detoxify ROS, and treatment with MV resulted in a higher reduction of carotenoid content in leaves of dcl1a-Ac than in those of wild-type plants (Fig. 5A, right panel). The reduction in chlorophyll content in dcl1a-IR plants with MV treatment was similar to that of its wild-type parental genotype Nipponbare, whereas the carotenoid level appears to be lower in dcl1a-IR plants than in its parental genotype (although differences in carotenoid level between dcl1a-IR and wild-type plants were not significant) (Supplementary Data Fig. S5A).
Fig. 5.
Effect of treatment with methyl viologen (MV) and detection of superoxide ion in dcl1a-Ac plants. (A) Leaves of 3-week-old dcl1a-Ac (Osdcl1a-Ac#1) and wild-type (azygous) [WT(Az)] plants were treated with MV (10 μm). Right panels: quantification of chlorophylls (Chla+b) and carotenoids in mock-inoculated and MV-treated wild-type and dcl1a-Ac plants at 72 h after treatment. Data shown correspond to wild-type and dcl1a-Ac Plants. Scale bars = 250 μm. Data are the mean ± s.d. (*P ≤ 0.05; **P ≤ 0.01 by ANOVA). (B) Detection of superoxide ion radicals (O2·–) by nitroblue tetrazolium (NBT) staining. As a control, leaves were treated with H2O2 for 6 h. Three biological replicates with three technical replicates each were performed. Statistically significant differences were determined by one-way ANOVA.
Finally, we examined O2·– accumulation in dcl1a-Ac and wild-type plants grown under controlled conditions (i.e. in the absence of pathogen infection). For detecting O2·– in rice leaves, we used NBT staining. Of note, dcl1a-Ac plants accumulated high levels of O2·– in leaves (Fig. 5B). As a control, leaves of wild-type plants (TN67) were treated with the ROS-generating agent H2O2 and examined for O2·– accumulation. In contrast to dcl1a-Ac plants, dcl1a-IR plants showed no visible alterations in O2·– accumulation (Supplementary Data Fig. S5B).
Altogether, these results indicate that DCL1a activation renders the plant more sensitive to oxidative stress caused by MV treatment and induces O2·– accumulation in leaves. Disturbed ROS production and/or scavenging mechanisms might interfere with the normal functioning of host antioxidant systems, which might explain, at least in part, the phenotype of disease susceptibility in dcl1a-Ac plants. Further studies are needed to clarify the exact biochemical mechanisms by which DCL1a activation stimulates O2·– accumulation and possibly alters ROS homeostasis in rice.
Characterization of the miRNAome in the dcl1a-Ac mutant
Knowing that the activity of DCL1 is required for processing of miRNA precursors and production of mature miRNAs, we reasoned that DCL1a activation might affect the rice miRNAome. Accordingly, we used small RNA sequencing for characterizing the miRNA population in leaves of wild-type and dcl1a-Ac plants. Two small RNA libraries, representing independent biological replicates of each genotype, were prepared (the same biological samples as for mRNA transcript profiling). Illumina sequencing of small RNA libraries generated 36 million reads (15 and 21 million reads from wild-type and mutant plants, respectively) (Supplementary Data Table S6). After removing the adaptor sequences and sequences <15 bp, 32 million reads were obtained (14 and 18 million from wild-type and dcl1a-Ac plants, respectively). All unique sequences were aligned to the rice genome (Nipponbare reference genome MSU 7.0), and reads mapping to known non-coding RNA families (rRNAs, tRNAs, small nuclear RNAs and small nucleolar RNAs) were removed. The abundance of small RNAs was calculated as reads per kilobase million (RPKMs).
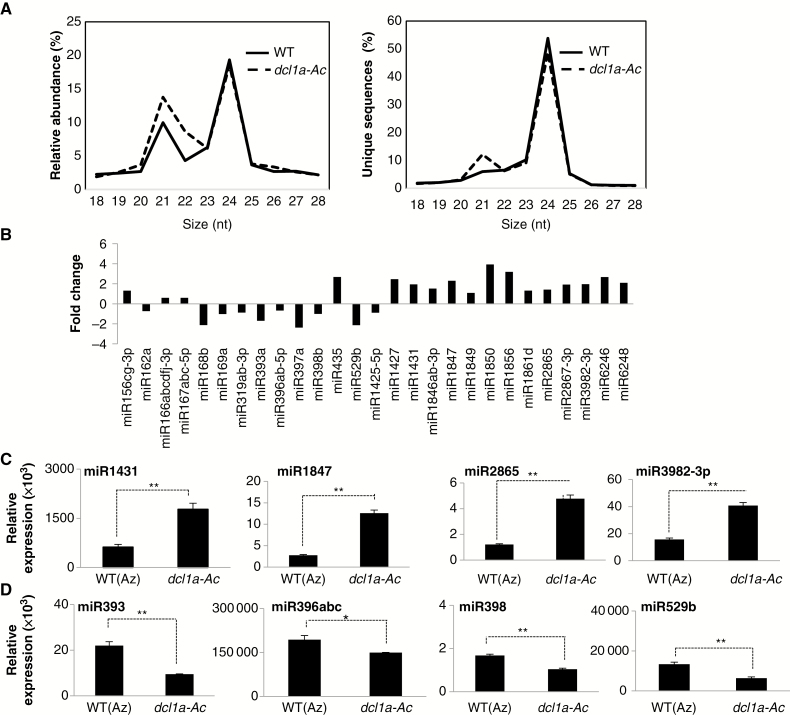
Consistent with the distribution of small RNA sizes typically observed in plants, the 24 nt small RNA class was the most abundant size class in both genotypes, with the 21 nt small RNAs forming a secondary peak (Fig. 6A). However, in dcl1a-Ac plants, the small RNA size distribution showed a substantial increase in the 21 nt small RNA class when considering both relative abundance and distinct reads (Fig. 6A). The observed increase in the 21 nt small RNA population might be due to DCL1 being involved in the production of almost all canonical 21 nt miRNAs.
Fig. 6.
Impact of DCL1a activation on the rice leaf miRNAome. (A) Abundance and unique small RNA sequences for each size class in leaves of wild-type (WT) and dcl1a-Ac plants. (B) Expression profiling of known miRNAs in dcl1a-Ac plants relative to wild-type plants. Representative examples are shown. Reads retrieved from the Illumina sequencing data sets for each family member were normalized to the total count of reads obtained in the corresponding library. Fold change was calculated on the basis of normalized reads (RPKM) (dcl1a-Ac vs. the wild type). (C, D) Stem–loop RT–PCR of miRNAs upregulated (C) and downregulated (D) in dcl1a-Ac plants (*P ≤ 0.05; **P ≤ 0.01 by ANOVA).
A blast search against the miRNA database (miRBase release 21) allowed us to identify known miRNAs present in our small RNA sequencing data. Differentially expressed miRNAs were defined as those with changes in expression ≥1.5-fold (upregulated) or ≤0.5-fold (downregulated), and a P-value ≤0.05. By using these criteria, 90 miRNAs corresponding to 61 miRNA families were found to be differentially expressed in dcl1a-Ac plants (Supplementary Data Table S7). Although the most obvious trend that could be expected from transcriptional activation of OsDCL1a was an enrichment of miRNAs (which are likely to involve DCL1 in their biogenesis), differentially expressed miRNAs in dcl1a-Ac plants included both upregulated and downregulated miRNAs. Representative examples of differentially expressed miRNAs in dcl1a-Ac plants are shown in Fig. 6B. The expression of selected miRNAs was validated by stem–loop RT–PCR, including upregulated miRNAs (miR1431, miR1847, miR2865 and miR3982-3p) and downregulated miRNAs (miR393, miR396abc, miR398 and miR529b) in dcl1a-Ac plants (Fig. 6C, D). A concordance between the sequencing-based profiling and stem–loop RT–PCR was observed, which supports upregulation and downregulation of miRNAs in dcl1a-Ac plants.
According to the small RNA-Seq data and stem–loop RT–qPCR analysis, miR398 accumulation was lower in dcl1a-Ac than in wild-type plants (Fig. 6D), which agreed with a reduced level of miR398 precursor transcripts and increased accumulation of miR398-targeted superoxide dismutase 2 (SOD2) transcripts in dcl1a-Ac plants (Supplementary Data Fig. S6). Previous studies reported that transgenic rice lines overexpressing MIR398 exhibit enhanced resistance to M. oryzae infection (Li et al., 2014), which agrees with the observed phenotype of susceptibility to M. oryzae infection in dcl1a-Ac plants (with reduced miR398b accumulation as compared with the wild type).
Collectively, our results demonstrate that DCL1a activation results in important perturbations in the rice miRNAome. Presumably, perturbations in miRNA expression patterns might lead to altered expression of the corresponding target genes, which might contribute to susceptibility to M. oryzae infection in dcl1a-Ac plants.
DISCUSSION
In this work, we provide evidence that OsDCL1a, a component of the miRNA biogenesis pathway, functions as a negative regulator of the rice defence response. Several lines of evidence support this conclusion. First, mutant plants in which OsDCL1a expression is activated by T-DNA tagging were susceptible to infection by hemibiotrophic and necrotrophic fungal pathogens (M. oryzae and F. fujikuroi, respectively). Secondly, susceptibility to pathogen infection was accompanied by a weaker induction of defence-related marker genes (e.g. OsPR1a and OsPBZ1) during M. oryzae infection. Thirdly, genes involved in the production of diterpenoid phytoalexins were downregulated in dcl1a-Ac mutant plants. The finding that OsDCL1a expression itself is regulated, not only by M. oryzae infection but also by treatment with M. oryzae elicitors in wild-type plants, supports that OsDCL1 is a component of PTI responses in rice. Also, the observation that OsDCL1a-Ac plants are susceptible to M. oryzae infection agrees with previous results of resistance to M. oryzae infection in rice plants silenced for Osdcl1a expression by RNAi (dcl1a-IR plants; Zhang et al., 2015). In contrast to dcl1a-IR plants showing developmental abnormalities (Liu et al., 2005), the Osdcl1a-Ac mutant plants grew and developed normally.
It is of note that, whereas DCL1a appears to function as a negative regulator in rice immunity, this gene was reported to act as a positive regulator of immune responses in arabidopsis. Thus, arabidopsis dcl1 mutants (dcl1-7 and dcl1-9 mutants) showed hypersusceptibility to infection by bacterial (P. syringae) and fungal (B. cinerea) pathogens (Navarro et al., 2008; Seo et al., 2013; Weiberg et al., 2014). The regulatory activity of DCL1a in rice probably differs from its arabidopsis counterpart in determining the outcome of the plant–pathogen interaction. Alternatively, DCL1a might execute its regulatory role via different pathways depending on the type (fungal or bacterial pathogens) or lifestyle of the pathogen (biotrophs, hemibiotrophs or necrotrophs). Further investigation is needed to understand why altered DCL1a expression has a different impact on susceptibility/resistance to pathogen infection in rice and arabidopsis.
The comparison of the dcl1a-Ac and wild-type transcriptomes allowed us to identify OsDCL1a-mediated processes related to blast resistance. Under normal growth conditions, R genes (RPM1 and Verticillium wilt disease resistance genes), and receptor kinase genes, including many WAK receptor kinase genes, were downregulated in dcl1a-Ac vs. wild-type plants. The involvement of these genes in resistance to pathogen infection is well documented in several plant species (Boyes et al., 1998; Fradin et al., 2009). In particular, WAK receptor kinases are known to regulate resistance to M. oryzae in rice (Li et al., 2009). Downregulation of defence-related receptor kinases suggests that pathogen perception might be extensively affected in these plants, which might result in no detection of the pathogen, suppression of PAMP/DAMP-elicited defence responses or production of ineffective defence responses in dcl1a-Ac plants.
We also show that protective antioxidant systems do not function properly in dcl1a-Ac plants under normal growth conditions, as revealed by failure to alleviate MV-mediated oxidative stress. In line with this, dcl1a-Ac plants accumulate high levels of the superoxide ion O2·– in their leaves. Although O2·– is moderately reactive and does not cause extensive damage by itself, this radical undergoes transformation into the more reactive and toxic OH·, which is highly reactive and causes cellular damage. In the absence of infection, O2·– accumulation appears not to cause negative effects in plant growth. However, ROS production is also a typical response of plant tissues to pathogen attack (so-called oxidative burst). If ROS are not effectively detoxified in dcl1a-Ac, their overproduction during pathogen infection would facilitate oxidative damage in the host plant, which, in turn, would render the host plant more susceptible to pathogen infection. How DCL1a activation compromises ROS detoxification mechanisms deserve further investigation.
Even more interesting is the fact that genes involved in diterpenoid phytoalexin biosynthesis were the most predominant group of downregulated genes in dcl1a-Ac plants. The accumulation of momilactones and oryzalexins has been found critical to counteract M. oryzae infection in rice (Dillon et al., 1997; Umemura et al., 2003). Also, diterpenoid phytoalexin genes show faster and/or stronger induction in resistant than in susceptible rice cultivars (Hasegawa et al., 2010; Bagnaresi et al., 2012). The observed phenotype of disease susceptibility in dcl1a-Ac plants might then be attributed, at least in part, to downregulation of phytoalexin biosynthesis genes which is consistent with the observation that dcl1a-Ac plants fail to accumulate major diterpenoid phytoalexins also during pathogen infection. Together, these findings reinforce the notion that OsDCL1a is a negative regulator of immune responses in rice and also support a DCL1-mediated regulation of secondary metabolic defence pathways with relevance to pathogen resistance, most probably via regulation of miRNA accumulation.
Characterization of the miRNAome in leaves of dcl1a-Ac plants allowed us to identify alterations in the accumulation of specific miRNA families caused by DCL1a activation. The observed increase in the accumulation of miRNAs in dcl1a-Ac plants is consistent with targeted activation of OsDCL1 in this mutant. Furthermore, we observed downregulation of different MIR genes in dcl1a-Ac plants, pointing to factors other than processing of miRNA precursors by DCL1a for the control of miRNA accumulation in rice. Several reasons can explain the otherwise paradoxical decrease in accumulation of miRNAs in dcl1a-Ac mutant plants. In addition to OsDCL1a, miRNA accumulation might be affected by the spatio-temporal expression pattern of other components of the miRNA biogenesis pathway. The abundance of mature miRNAs might be affected by precursor processing by DCL1 and also by miRNA stability (which also depends on miRNA modifications such as 3’ end methylation or nucleotide addition), binding of miRNAs to AGO (which protects miRNAs from degradation) or sequestration by target mimic RNAs. As an additional complexity, evidence exists of autoregulatory feedback loops between miRNA and target genes, whereby target genes can control the level of an miRNA in addition to being regulated by it. The best known example is the transcriptional/translational interlocked feedback loop governing the miR168–AGO1 pair function, featuring miR168-guided cleavage of AGO1 and post-transcriptional stabilization of miR168 by AGO1 (Vaucheret et al., 2006).
Among the miRNAs accumulating at a lower level in dcl1a-Ac than in wild-type plants were miR398 and miR393. A role for miR398 in protecting the plant against oxidative stress has been reported in arabidopsis and rice, and transgenic rice lines overexpressing miR398 exhibit resistance to M. oryzae (Jagadeeswaran et al., 2009; Li et al., 2014). Downregulation of miR398 in dcl1a-Ac plants is then consistent with a phenotype of susceptibility to M. oryzae in these plants. However, other studies in arabidopsis demonstrated that miR398 negatively regulates immune responses against bacterial pathogens (Li et al., 2010). Regarding miR393, its overexpression in arabidopsis plants renders the host plant more resistant to biotrophic pathogens but more susceptible to the necrotrophic pathogens (Robert-Seilaniantz et al., 2011). These findings indicate that certain miRNAs (e.g. miR393 and miR398) might function as positive or negative regulators of immune responses depending on the host plant and/or the pathogen lifestyle. Further investigation will reveal whether dcl1a-Ac plants respond in a different manner (e.g. susceptibility or resistance) to infection by pathogens other than M. oryzae and F. fujikuroi.
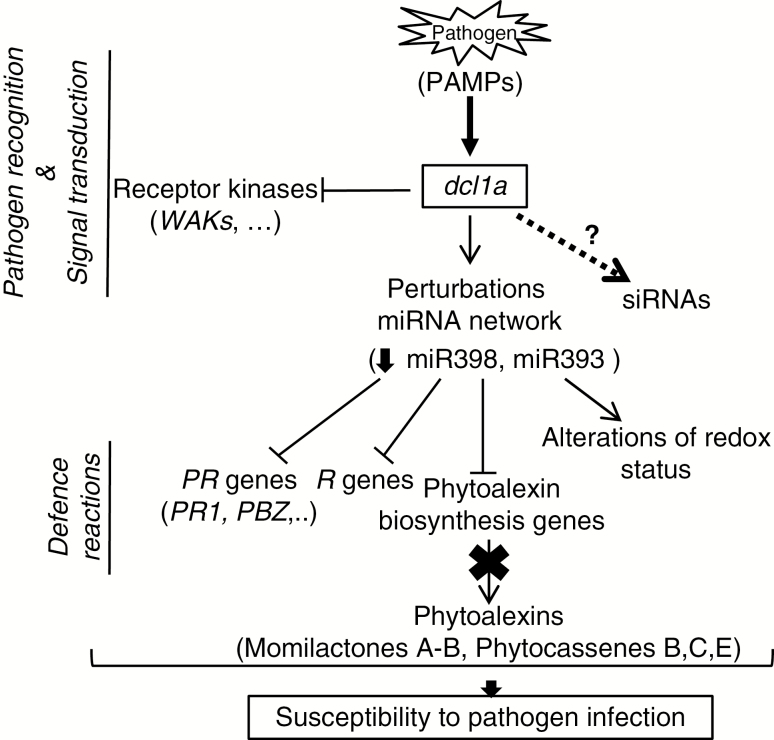
All these findings allowed us propose a working model for the regulation of defence responses to M. oryzae infection in rice plants by which OsDCL1a would mediate pathogen recognition processes and defence reactions (Fig. 7). According to this model, pathogen perception would trigger OsDCL1a activation which in turn would have pleiotropic effects on the rice defence response. On the one hand, OsDCL1a activation would negatively affect PAMP recognition and signal transduction itself and, on the other hand, perturbations in the rice miRNAome caused by Osdcl1a activation would repress the pathogen-inducible host defence responses, such as PR expression and diterpene phytoalexin biosynthesis and accumulation, while altering the cellular redox status. All these factors would decrease the ability of the host plant to detect the invading pathogen and respond in a timely and appropriate manner. Moreover, even though DCL1 is predominantly involved in the production of miRNAs, we have examples of endogenous siRNAs that are generated by DCL1 activity in arabidopsis, such as certain natural antisense transcript-derived siRNAs and long siRNAs (Borsani et al., 2005; Katiyar-Agarwal et al., 2006). Therefore, the possibility that overexpression of DCL1a affects the production of other types of small RNAs which, in turn, might regulate rice defence responses should not be ruled out.
Fig. 7.
Model for the role of OsDCL1a in disease susceptibility. In response to M. oryzae infection, OsDCL1a is activated. Pathogen-induced OsDCL1a expression, as well as OsDCL1a activation in dcl1a-Ac plants, would cause perturbations in the host miRNAome, which, in turn, would negatively affect pathogen recognition processes and expression of stress-responsive genes (such as PR genes). Additionally, OsDCL1a activation would negatively affect diterpenoid biosynthesis and alter ROS homeostasis, thereby compromising the ability of the host plant to mount a timely, targeted defence response.
Collectively, results presented here expand our knowledge of the molecular mechanisms involved in blast resistance while providing evidence on the important role of DCL1a (and miRNAs) in rice immunity. In this respect, although a plethora of rice miRNAs have been found to be regulated by pathogen infection in rice, the biological function of most pathogen-regulated miRNAs remains largely unknown. Changes induced by OsDCL1a activation in the miRNAome are expected to cause altered expression of their corresponding target genes. To understand the impact of alterations in the miRNAome caused by DCL1a overexpression, and how these alterations might contribute to disease resistance in rice, a better knowledge of target genes for rice miRNAs is needed. Clearly, altered OsDCL1a expression and accompanying alterations in miRNA levels might affect diverse biological processes that are under miRNA regulation, which might then decrease the plant’s ability to cope with pathogen infection. As DCL1 is responsible for the majority of the miRNA processing in plants, a better understanding of the biological processes that are regulated by DCL1a will open up promising new avenues for the control of the rice blast disease. This is of paramount importance when considering that over half of the world’s population relies on rice as the main source of calories and because the rice blast fungus M. oryzae has developed into a model system for the study of plant–pathogen interactions. The main challenge now is to elucidate how miRNAs function in regulating mechanisms involved in disease resistance in rice. Understanding these mechanisms will provide powerful tools for developing novel strategies to improve disease resistance in plants.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: analysis of OsDCL1a mutant plants. Figure S2: susceptibility of dcl1a-Ac♯2 plants to infection by the fungal pathogen M. oryzae. Figure S3: expression of OsDCL genes in wild-type and dcl1a-Ac plants under normal conditions (non-infection). Figure S4: distribution of differentially expressed genes in dcl1a-Ac plants. Figure S5: effect of methyl viologen on chlorophylls and carotenoids, and detection of O2· in dcl1a-IR plants. Figure S6: accumulation of miR398 and OsSOD2 transcripts in dcl1a-Ac plants. Table S1: sequences of oligonucleotides used. Table S2: T-DNA copy number in dcl1a-Ac mutants. Table S3: statistics of RNA-Seq in dcl1a-Ac and wild-type plants. Table S4: downregulated genes in dcl1a-Ac plants relative to wild-type plants sorted by functional category. Table S5: upregulated genes in dcl1a-Ac plants relative to wild-type plants sorted by functional category. Table S6: summary of small RNA sequencing data sets from wild-type and dcl1a-Ac plants. Table S7: list of miRNAs differentially accumulating in dcl1a-Ac plants.
ACKNOWLEDGEMENTS
We thank Drs M. Aragona for providing isolate 297 of F. fujikuroi, and X. Cao (CAS, Beijing) for the dcl1a-IR lines. This work was supported by the Spanish Ministry of Economy and Competitiveness (MINECO, grants BIO2012-32838, BIO2015-67212-R), the CSIC/NSC (Spanish Research Council/National Science Council of Taiwan)-Formosa Program (2009TW0041) and the Japan Society for the Promotion of Science (JSPS KAKENHI, grant no. 17H03811 to K.O.). We also acknowledge support from the CERCA Programme (‘Generalitat de Catalunya’) and MINECO (‘Severo Ochoa Programme for Centres of Excellence in R&D’ 2016–2019, SEV-2015-0533). R.S.-G. was the recipient of a PhD grant from MINECO (BES-2013–065521).
LITERATURE CITED
- Agrawal GK, Rakwal R, Jwa NS, Agrawal VP. 2001. Signalling molecules and blast pathogen attack activates rice OsPR1a and OsPR1b genes: a model illustrating components participating during defence/stress response. Plant Physiology and Biochemistry 39: 1095–1103. [Google Scholar]
- Ahuja I, Kissen R, Bones AM. 2012. Phytoalexins in defense against pathogens. Trends in Plant Science 17: 73–90. [DOI] [PubMed] [Google Scholar]
- Arikit S, Zhai J, Meyers BC. 2013. Biogenesis and function of rice small RNAs from non-coding RNA precursors. Current Opinion in Plant Biology 16: 170–179. [DOI] [PubMed] [Google Scholar]
- Bagnaresi P, Biselli C, Orrù L, et al. 2012. Comparative transcriptome profiling of the early response to Magnaporthe oryzae in durable resistant vs susceptible rice (Oryza sativa L.) genotypes. PLoS One 7: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrich P, San Segundo B. 2016. MicroRNAs in rice innate immunity. Rice 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrich P, Campo S, Wu M-T, Liu T-T, Hsing Y-IC, San Segundo B. 2015. MicroRNA-mediated regulation of gene expression in the response of rice plants to fungal elicitors. RNA Biology 12: 847–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsani O, Zhu J, Verslues PE, Sunkar R, Zhu JK. 2005. Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell 123: 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Nam J, Dangl JL. 1998. The Arabidopsis thaliana RPM1 disease resistance gene product is a peripheral plasma membrane protein that is degraded coincident with the hypersensitive response. Proceedings of the National Academy of Sciences, USA 95: 15849–15854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, et al. 2008. Widespread translational inhibition by plant miRNAs and siRNAs. Science 320: 1185–1190. [DOI] [PubMed] [Google Scholar]
- Campo S, Manrique S, García-Martínez J, San Segundo B. 2008. Production of cecropin A in transgenic rice plants has an impact on host gene expression. Plant Biotechnology Journal 6: 585–608. [DOI] [PubMed] [Google Scholar]
- Campo S, Peris-Peris C, Siré C, et al. 2013. Identification of a novel microRNA (miRNA) from rice that targets an alternatively spliced transcript of the Nramp6 (Natural resistance-associated macrophage protein 6) gene involved in pathogen resistance. New Phytologist 199: 212–227. [DOI] [PubMed] [Google Scholar]
- Campos-Soriano L, Valè G, Lupotto E, San Segundo B. 2013. Investigation of rice blast development in susceptible and resistant rice cultivars using a gfp-expressing Magnaporthe oryzae isolate. Plant Pathology 62: 1030–1037. [Google Scholar]
- Casacuberta JM, Raventós D, Puigdoménech P, San Segundo B. 1992. Expression of the gene encoding the PR-like protein PRms in germinating maize embryos. Molecular & General Genetics 234: 97–104. [DOI] [PubMed] [Google Scholar]
- Couto D, Zipfel C. 2016. Regulation of pattern recognition receptor signalling in plants. Nature Reviews. Immunology 16: 537–552. [DOI] [PubMed] [Google Scholar]
- Dillon VM, Overton J, Grayer RJ, Harbone JB. 1997. Differences in phytoalexin response among rice cultivars of different resistance to blast. Phytochemistry 44: 599–603. [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, et al. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z. 2010. AgriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Research 38: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Q, Zhang Y, Xia R, Meyers BC. 2016. Small RNAs add zing to the zig–zag–zig model of plant defenses. Molecular Plant-Microbe Interactions 29: 165–1659. [DOI] [PubMed] [Google Scholar]
- Fradin EF, Zhang Z, Juarez A yala JC, et al. 2009. Genetic dissection of verticillium wilt resistance mediated by tomato Ve1. Plant Physiology 150: 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasciolli V, Mallory AC, Bartel DP, Vaucheret H. 2005. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting siRNAs. Current Biology 15: 1494–1500. [DOI] [PubMed] [Google Scholar]
- Goff SA, Ricke D, Lan T, et al. 2002. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296: 92–100. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Mitsuhara I, Seo S, et al. 2010. Phytoalexin accumulation in the interaction between rice and the blast fungus. Molecular Plant-Microbe Interactions 23: 1000–1011. [DOI] [PubMed] [Google Scholar]
- Hsing YI, Chern CG, Fan MJ, et al. 2007. A rice gene activation/knockout mutant resource for high throughput functional genomics. Plant Molecular Biology 63: 351–364. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Sakai M, Yao Q, Tanimoto Y, Toshima H, Hasegawa M. 2013. Identification of a novel casbane-type diterpene phytoalexin, ent-10-oxodepressin, from rice leaves. Bioscience, Biotechnology, and Biochemistry 77: 760–765. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran G, Saini A, Sunkar R. 2009. Biotic and abiotic stress down-regulate miR398 expression in Arabidopsis. Planta 229: 1009–1014. [DOI] [PubMed] [Google Scholar]
- Jeon Y-A, Yu S-H, Lee YY, et al. 2013. Incidence, molecular characteristics and pathogenicity of Gibberella fujikuroi species complex associated with rice seeds from Asian countries. Mycobiology 41: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong DH, Green PJ. 2013. The role of rice microRNAs in abiotic stress responses. Journal of Plant Biology 56: 187–197. [Google Scholar]
- Jones JDG, Dangl JL. 2006. The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Arora R, Lama T, et al. 2008. Genome-wide identification, organization and phylogenetic analysis of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families and their expression analysis during reproductive development and stress in rice. BMC Genomics 9: 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar-Agarwal S, Morgan R, Dahlbeck D, et al. 2006. A pathogen-inducible endogenous siRNA in plant immunity. Proceedings of the National Academy of Sciences, USA 103: 18002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y, Watanabe Y. 2004. Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proceedings of the National Academy of Sciences, USA 101: 12753–12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhou SY, Zhao WS, Su SC, Peng YL. 2009. A novel wall-associated receptor-like protein kinase gene, OsWAK1, plays important roles in rice blast disease resistance. Plant Molecular Biology 69: 337–346. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang Q, Zhang J, Wu L, Qi Y, Zhou J-M. 2010. Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiology 152: 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lu Y-G, Shi Y, et al. 2014. Multiple rice microRNAs are involved in immunity against the blast fungus Magnaporthe oryzae. Plant Physiology 164: 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhao S-L, Li J-L, et al. 2017. Osa-miR169 negatively regulates rice immunity against the blast fungus Magnaporthe oryzae.Frontiers in Plant Science 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZY, Xia J, Chen Z, et al. 2016. Large-scale rewiring of innate immunity circuitry and microRNA regulation during initial rice blast infection. Scientific Reports 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Buschmann C. 2001. Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Current Protocols in Food Analytical Chemistry 2–2: 171–178. [Google Scholar]
- Liu B, Li P, Li X, et al. 2005. Loss of function of OsDCL1 affects microRNA accumulation and causes developmental defects in rice. Plant Physiology 139: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llave C, Xie Z, Kasschau KD, Carrington JC. 2002. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297: 2053–2056. [DOI] [PubMed] [Google Scholar]
- Mallory AC, Reinhart BJ, Jones-Rhoades MW, et al. 2004. MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5’ region. EMBO Journal 23: 3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margis R, Fusaro AF, Smith NA, et al. 2006. The evolution and diversification of Dicers in plants. FEBS Letters 580: 2442–2450. [DOI] [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, Smyth GK. 2012. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Research 40: 4288–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midoh N, Iwata M. 1996. Cloning and characterization of a probenazole-inducible gene for an intracellular pathogenesis-related protein in rice. Plant & Cell Physiology 37: 9–18. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Fujita M, Shenton MR, et al. 2016. Evolutionary trajectory of phytoalexin biosynthetic gene clusters in rice. The Plant Journal 87: 293–304. [DOI] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, et al. 2006. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312: 436–439. [DOI] [PubMed] [Google Scholar]
- Navarro L, Jay F, Nomura K, He SY, Voinnet O. 2008. Suppression of the microRNA pathway by bacterial effector proteins. Science 321: 964–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik JF, Allen E, Wu X, et al. 2003. Control of leaf morphogenesis by microRNAs. Nature 425: 257–263. [DOI] [PubMed] [Google Scholar]
- Qi M, Yang Y. 2002. Quantification of Magnaporthe grisea during infection of rice plants using real-time polymerase chain reaction and northern blot/phosphoimaging analyses. Phytopathology 92: 870–876. [DOI] [PubMed] [Google Scholar]
- Rau A, Gallopin M, Celeux G, Jaffrézic F. 2013. Data-based filtering for replicated high-throughput transcriptome sequencing experiments. Bioinformatics 29: 2146–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Grant M, Jones JDG. 2011. Hormone crosstalk in plant disease and defense: more than just jasmonate–salicylate antagonism. Annual Review of Phytopathology 49: 317–343. [DOI] [PubMed] [Google Scholar]
- Rubio-Somoza I, Weigel D. 2011. MicroRNA networks and developmental plasticity in plants. Trends in Plant Science 16: 258–264. [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Huffaker A, Sims JW, et al. 2014. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Journal of Plant Journal 79: 659–678. [DOI] [PubMed] [Google Scholar]
- Seo J, Wu J, Lii Y, Li Y, Jin H. 2013. Contribution of small RNA pathway components in plant immunity. Molecular Plant-Microbe Interactions 26: 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Li P, Zhai J, et al. 2012. Roles of DCL4 and DCL3b in rice phased small RNA biogenesis. The Plant Journal 69: 462–474. [DOI] [PubMed] [Google Scholar]
- Umemura K, Ogawa N, Shimura M, Koga J, Usami H, Kono T. 2003. Possible role of phytocassane, rice phytoalexin, in disease resistance of rice against the blast fungus Magnaporthe grisea. Bioscience, Biotechnology, and Biochemistry 67: 899–902. [DOI] [PubMed] [Google Scholar]
- Vaucheret H, Mallory AC, Bartel DP. 2006. AGO1 homeostasis entails coexpression of MIR168 and AGO1 and preferential stabilization of miR168 by AGO1. Molecular Cell 22: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Gu L, Song X, et al. 2014. Dicer-like 3 produces transposable element-associated 24-nt siRNAs that control agricultural traits in rice. Proceedings of the National Academy of Sciences, USA 111: 3877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Ou B, Li J, et al. 2013. Transcriptional profiling of rice early response to Magnaporthe oryzae identified OsWRKYs as important regulators in rice blast resistance. PLoS One 8: e59720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Bellinger M, Jin H. 2014. Small RNAs: a new paradigm in plant–microbe interactions. Annual Review of Phytopathology 52: 495–516. [DOI] [PubMed] [Google Scholar]
- Wilson RA, Talbot NJ. 2009. Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nature Reviews. Microbiology 7: 185–195. [DOI] [PubMed] [Google Scholar]
- Wulff EG, Sørensen JL, Lübeck M, Nielsen KF, Thrane U, Torp J. 2010. Fusarium spp. associated with rice Bakanae: ecology, genetic diversity, pathogenicity and toxigenicity. Environmental Microbiology 12: 649–657. [DOI] [PubMed] [Google Scholar]
- Yamane H. 2013. Biosynthesis of phytoalexins and regulatory mechanisms of it in rice. Bioscience, Biotechnology, and Biochemistry 77: 1141–1148. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu S, Wang J, et al. 2002. A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296: 79–92. [DOI] [PubMed] [Google Scholar]
- Zhang D, Liu M, Tang M, et al. 2015. Repression of microRNA biogenesis by silencing of OsDCL1 activates the basal resistance to Magnaporthe oryzae in rice. Plant Science 237: 24–32. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao Y, Shan D, et al. 2018. Magnaporthe oryzae defeats rice defense by inducing miR319b and suppressing jasmonic acid signaling. Plant Physiology: pp.01665.2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.