Abstract
Background
Floral nectar is an important determinant of plant–pollinator interactions and an integral component of pollination syndromes, suggesting it is under pollinator-mediated selection. However, compared to floral display traits, we know little about the evolutionary ecology of nectar. Combining a literature review with a meta-analysis approach, we summarize the evidence for heritable variation in nectar traits and link this variation to pollinator response and plant fitness. We further review associations between nectar traits and floral signals and discuss them in the context of honest signalling and targets of selection.
Scope
Although nectar is strongly influenced by environmental factors, heritable variation in nectar production rate has been documented in several populations (mean h2 = 0.31). Almost nothing is known about heritability of other nectar traits, such as sugar and amino acid concentrations. Only a handful of studies have quantified selection on nectar traits, and few find statistically significant selection. Pollinator responses to nectar traits indicate they may drive selection, but studies tying pollinator preferences to plant fitness are lacking. So far, only one study conclusively identified pollinators as selective agents on a nectar trait, and the role of microbes, herbivores, nectar robbers and abiotic factors in nectar evolution is largely hypothetical. Finally, there is a trend for positive correlations among floral cues and nectar traits, indicating honest signalling of rewards.
Conclusions
Important progress can be made by studies that quantify current selection on nectar in natural populations, as well as experimental approaches that identify the target traits and selective agents involved. Signal–reward associations suggest that correlational selection may shape evolution of nectar traits, and studies exploring these more complex forms of natural selection are needed. Many questions about nectar evolution remain unanswered, making this a field ripe for future research.
Keywords: Agents of selection, floral traits, heritability, honest signalling, meta-analysis, natural selection, nectar, signal–reward correlation
INTRODUCTION
Floral nectar is the primary reward offered to pollinators in the majority of angiosperms, and its amount, composition and placement is clearly an important determinant of plant–pollinator interactions. Nectar traits are thus probably formed by pollinator-mediated selection, yet the link from functional to adaptive significance is poorly known. Although a special issue on ‘Community and Evolutionary Ecology of Nectar’ was featured in Ecology over a decade ago (Irwin et al., 2004), few studies have specifically tackled these questions in the intervening time (see Supplementary Data Table S1 for citation history). This contrasts with the substantial number of studies that over the same period have used experimental approaches to identify pollinators as selective agents on floral traits involved in attracting visitors and maximizing pollen transfer per visit (e.g. Fishman and Willis, 2008; Caruso et al., 2010; Parachnowitsch and Kessler, 2010; Bartkowska and Johnston, 2012; Sletvold et al., 2012, 2016). This apparent neglect of the reward itself may be due to the unique challenges of studying nectar, including what aspects of nectar rewards are relevant and how to measure them (cf. Mitchell, 2004). Nectar is a notoriously plastic trait, and its expression is further modified by the interaction itself ‒ when visitors consume nectar, nectar characteristics change. The lack of attention may also reflect the perception that flower signal traits are the direct targets of selection, and nectar is expected to evolve via correlations with such honest signal traits (Mitchell and Waser, 1992; Fenster et al., 2004; Knauer and Schiestl, 2015). In this review, which limits its focus to floral nectar, we first discuss how nectar traits have been defined and measured. We then summarize the documented variation in nectar traits, how this variation influences pollinator behaviour, and the evidence that pollinator preferences translate into effects on plant fitness. Finally, we review links between the more commonly studied floral signals and nectar rewards to assess the hypothesis for honest signalling. We further highlight important knowledge gaps and future directions.
What is nectar?
At its simplest, floral nectar is a sweet aqueous solution that mediates interactions between plants and mutualists such as pollinators (De la Barrera and Nobel, 2004; Heil, 2011). However, nectar is usually far from such a simple solution, instead often containing proteins, amino acids (Baker and Baker, 1986; Nepi et al., 2012) and minerals (Afik et al., 2014), as well as secondary compounds (Adler, 2000), and other components such as colours (Hansen et al., 2007) and scents (Raguso, 2004). Floral nectaries themselves are diverse in form and have no single evolutionary origin (Pacini et al., 2003), hinting at the potential for complexity across the angiosperms. Floral nectar is clearly not one character but rather a trait class. In addition, nectar can play many roles beyond pollinator attraction, including mediating interactions with microbes (Álvarez-Pérez et al., 2012; Canto and Herrera, 2012), nectar robbers (Maloof and Inouye, 2000; Barlow et al., 2017) and herbivores (Adler and Bronstein, 2004). Even in the context of plant–pollinator interactions, nectar may not simply function as a reward but instead act to manipulate pollinator behaviour (Pyke, 2016). Therefore, while we often use ‘nectar’ to refer to the sweet aqueous reward for pollination, it is important to remember that nectar characteristics are rarely consistent across plant species.
Nectar measurements
The most common approaches for estimating nectar rewards in pollination studies are to measure volume and sugar concentrations, as these can be measured in the field. Fewer studies measure more complex nectar characters such as the types and proportions of sugars, amino acids or secondary metabolites. By far the easiest and most common measure is to assess volume using Drummond microcaps or filter paper (McKenna and Thomson, 1988). On the surface, measuring nectar volume is simple, but researchers must also decide whether to assess standing crop or nectar production rate (NPR). Standing crop refers to the nectar volume available at a given time and is the result of both nectar production by the plant and nectar removal by floral visitors. While standing crop represents the reality pollinators and other visitors face, a disadvantage of this measurement is that those visitors heavily influence the nectar present (Zimmerman, 1981). Unfortunately for researchers, NPR, which is under the plant’s control, is not necessarily strongly correlated to standing crop (Zimmerman, 1988; Wolff, 2006). Therefore, to assess nectar from the plant perspective, measurements are taken after excluding pollinators for some time, usually by covering flowers in mesh bags (Kearns and Inouye, 1993). To accurately assess NPR, flowers must first be drained of nectar and then resampled after a specified time to give the volume produced per time unit.
While nectar amounts are clearly important for visitors (e.g. Ott et al., 1985; Hodges, 1995; Leiss and Klinkhamer, 2005b; Dreisig, 2012), concentration and types of sugars can also affect behaviour (see below for detailed discussion). The adoption of hand-held refractometers has allowed researchers to assess the percentage of sugars in nectar in the field (as long as their species produces sufficient amounts of nectar). Therefore, sugar concentration is also a relatively easily measured and commonly reported nectar characteristic (Bolten et al., 1979). From NPR and concentration estimates it is straightforward to calculate sugar equivalents secreted in some known period of time (Kearns and Inouye, 1993), and this is also a commonly reported trait. Finally, analytical tools such as HPLC-MS, GC-MS and spectrophotometry have allowed for more detailed characterization of nectar components such as sugar composition (Perret et al., 2001; Witt et al., 2013), amino acids (Baker and Baker, 1986; Nepi et al., 2012), proteins (Nepi et al., 2012; Seo et al., 2013), colours (Hansen et al., 2007), secondary compounds (Manson et al., 2012; Egan et al., 2016) and scents (Raguso, 2004; Kessler and Baldwin, 2007).
The many aspects of, and approaches to, measuring nectar highlight the challenges for independent research programmes studying this complex trait and for us as a community to summarize our data and understanding. Methods often need to be adjusted to accommodate the size and shape of flowers, the presence of nectar spurs, or the problems of low nectar volumes. Some methods of nectar collection provide better estimates of specific nectar characteristics, such as total sugars (Morrant et al., 2009) or amino acids (Power et al., 2018), although these methods may preclude measurements of other characteristics, such as nectar volume. We suggest that these complexities should inspire researchers to define common protocols for measuring each characteristic of nectar to facilitate comparisons across studies and species. However, as protocols will vary between species with different phenotypes, our primary recommendation is that all methods for collecting and measuring nectar traits be reported in full detail. For field characterizations of nectar in sufficient sample sizes to address evolutionary ecology questions, combinations of microcapillary tubes or filter paper and refractometers or lab assays of sugars are likely to remain the most efficient and universally used.
LITERATURE REVIEW
Studies of nectar, and especially its evolutionary ecology, are strewn throughout floral biology research. Sometimes nectar is the focus of the paper and thus easy to find through a classic literature search, but more often it is measured along with a suite of floral characters, which can make it difficult to locate. To compile information on aspects of nectar evolution and ecology, we took multiple approaches, and therefore do not claim that our work represents a truly systematic review. Details of our literature review can be found in the online supplementary data, and the resulting database is used for all data summaries and analyses found below. In short, we collected information on natural selection, variation and heritability of nectar, as well as pollinator preferences and relationships between floral signals and nectar rewards, using our own collections, soliciting colleagues, and keyword searches in the databases ‘Web of Science’ and ‘Google Scholar’. The nectar ‘library’ resulting from our search can be accessed at https://www.zotero.org/groups/2196212/evolutionary_ecology_of_nectar.
BASICS OF EVOLUTION
Macroevolutionary associations between nectar traits and the main pollinator type indicate that nectar differentiation has evolved by pollinator-driven natural selection, and also suggests that we should be able to detect current selection on nectar traits paralleling these patterns. Natural selection requires covariation between traits and fitness, and a potential evolutionary response requires that the trait variation has a genetic basis. What is the current evidence of among-individual variation in nectar, the heritability of nectar traits and their link to variation in plant fitness?
Phenotypic variation of nectar
For natural selection to act, there must be phenotypic variation of the trait within a population. Before we examine the evidence for within-population phenotypic variation in nectar, first we explore sources of variability in nectar (Fig. 1A). Plants can be highly plastic in their nectar production, which can pose a challenge for assigning a value for the trait in an individual. For example, experimental manipulations show that nectar can respond to a range of environmental factors such as water availability (Carroll et al., 2001; Leiss and Klinkhamer, 2005a; Waser and Price, 2016), ambient humidity (Bertsch, 1983), temperature (Jakobsen and Kritjánsson, 1994; Petanidou and Smets, 1996), soil factors (Baude et al., 2011; Becklin et al., 2011), light (Pleasants, 1983; Devlin, 1988), elevated CO2 (Rusterholz and Erhardt, 1998; Dag and Eisikowitch, 2000), and interactions with herbivores (Aizen and Raffaele, 1996; Mu et al., 2016), nectar robbers (Lasso and Naranjo, 2003; Kaczorowski et al., 2014; Cuevas and Rosas-Guerrero, 2016) or nectar microbes (Vannette et al., 2013; Vannette and Fukami, 2018). And perhaps most obviously, recent visits by pollinators will also influence the current nectar available in flowers. Possibly even more alarming for evolutionary ecologists trying to understand nectar variation in their system, environmental factors can also have interactive effects on nectar traits (Hoover et al., 2012), suggesting that the environmental drivers of nectar variation can be complex (e.g. Enkegaard et al., 2016). These studies suggest that nectar might be particularly sensitive to microclimate variation across a population, affecting phenotypic measurements (Boose, 1997). However, not all plants respond to experimental treatments by altering nectar characteristics (Cane and Schiffhauer, 1997; Lehtilä and Strauss, 1999), suggesting that at least for some systems nectar may be less influenced by environmental factors.
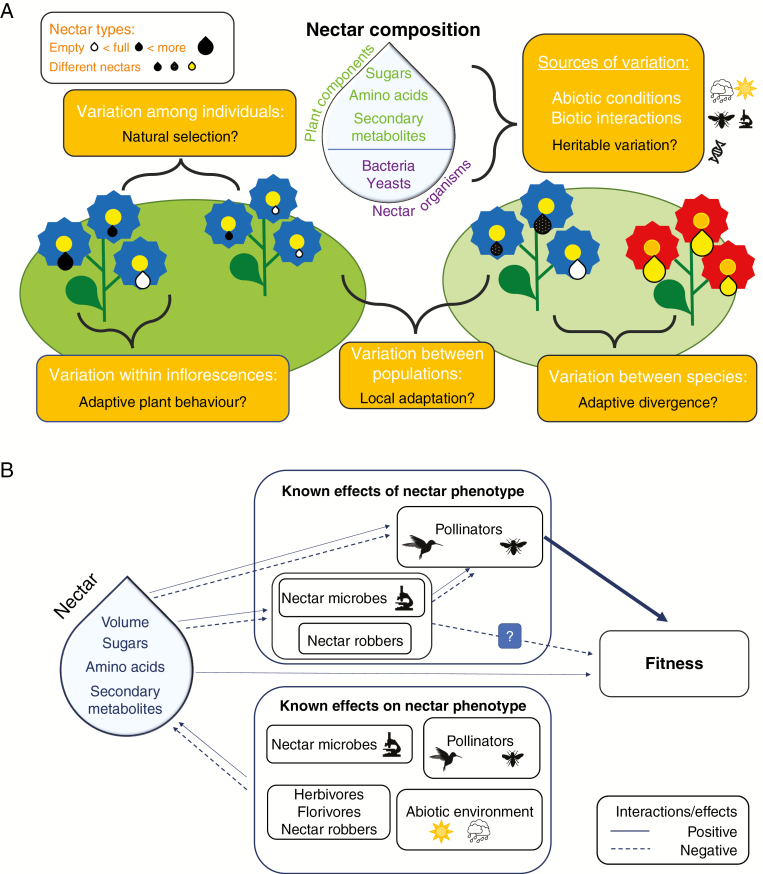
Fig. 1.
Conceptual diagram for (A) sources of nectar variation and evolutionary/ecological questions that can be addressed, and (B) paths of direct and indirect selective agents on nectar traits.
A further component to phenotypic variation in nectar is variation both within the flower and within the inflorescence (Fig. 1A). Nectar production is dynamic in many angiosperms, and can be stimulated by pollinator visitation (Castellanos et al., 2002) or change in composition in response to tissue damage (Adler et al., 2006; Kaczorowski et al., 2014), vary diurnally to match pollinator activity peak (Pandit and Choudhury, 2001), and change with flower age (Willson and Bertin, 1979; but see Pleasants, 1983) or sexual phase (Devlin et al., 1987; Castellanos et al., 2002). Furthermore, within inflorescences, not all flowers produce nectar similarly. At the extreme end, empty flowers can be common (i.e. nectar dimorphism; Anand et al., 2007) and this kind of variation in nectar amounts or other nectar components might be a strategy to manipulate pollinators (Bell, 1986; Biernaskie et al., 2002; Kessler et al., 2012). Predictable variation, such as declining nectar volume from lower to upper flowers, is also common, and such nectar gradients have been interpreted as adaptations to encourage pollinators to move upwards when visiting an inflorescence, decreasing geitonogamous self-pollination (Best and Bierzychudek, 1982; Fisogni et al., 2011; Schmid et al., 2016; Zhao et al., 2016). In sum, within-individual variance in nectar traits can be far greater than variance in floral morphology, which has implications for the measurement of nectar (Herrera et al., 2006; Canto et al., 2006, 2011). It is clear that, in many systems, not only average nectar production, but also the variation in nectar production itself may be a trait of interest.
The pronounced plasticity and variation within flowers and inflorescences suggest that measuring phenotypic values of nectar requires some caution. Ideally, nectar should be measured in all individuals at the same time and phenological stage, with a full characterization of variation among flowers. In reality, this will rarely be possible, especially for the samples sizes required for most evolutionary ecology questions. However, researchers should try to minimize the environmental variation that can cause measurement errors or collect sufficient samples across time and flowers to ensure the estimates accurately capture a plant’s nectar characteristics. Exactly how will depend on the system. Minimizing environmental influence has usually involved working in a controlled environment, which means that it does not really reflect the sources and consequences of phenotypic variation in a natural population. We instead encourage field studies that carefully plan their nectar measurements to account for the variation as far as possible. For example, in a study of phenotypic selection on floral traits, care should be taken to measure nectar traits at a similar phenological stage, enough flowers should be sampled to potentially include variation as a trait of interest, and all individuals should be sampled across a short time interval minimizing variation in ambient temperature, humidity, etc. This is challenging, because measurements of NPR require bagging flowers and nectar removal, and selection studies including multiple traits require substantial sample sizes. However, more field work is certainly needed to advance our understanding of nectar variation and its potential adaptive significance.
Despite these challenges, several studies have documented variation in nectar traits within (Real and Rathcke, 1988, 1991; Hodges, 1993) and among populations (Cruden, 1976; Brown et al., 2011; Gowda and Kress, 2013; Gijbels et al., 2014; Egan et al., 2016). As we will show in the following sections, there is evidence that phenotypic variance in nectar can have a heritable component and be sufficient to detect natural selection.
Heritable variation of nectar traits
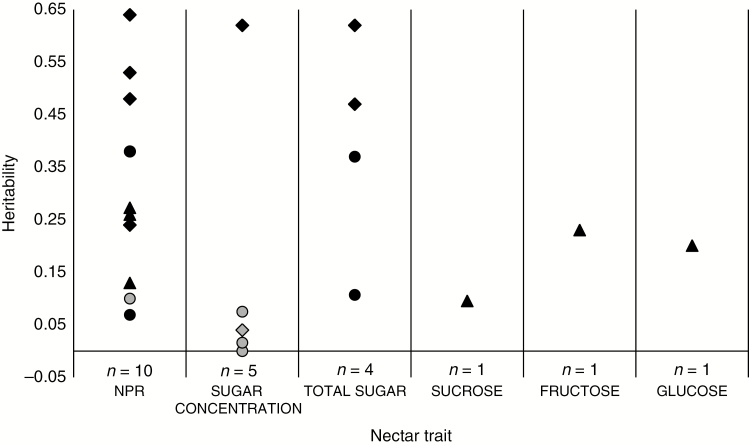
In the earlier mentioned special issue on ‘Community and Evolutionary Ecology of Nectar’, Mitchell (2004) raised the question of why we know so little about the heritability of nectar traits. At that time, a total of seven studies reported estimates of heritability for wild plants, mostly for NPR, with some additional estimates for sugar concentrations and secretion rates (cf. table 1 in Mitchell, 2004). Surprisingly few heritability estimates of nectar traits have been published after his call for attention, and Fig. 2 only includes data from three more recent studies. The limited number of studies available prevents any synthetic analysis of patterns of nectar heritability, but there is at least no doubt that NPR often is heritable and may harbour considerable genetic variation (Fig. 2). Nine of the ten published estimates are statistically significant, and the mean heritability of NPR is 0.31 (±0.19, SD). This is slightly lower than the mean of published heritability estimates on floral display traits (0.34–0.46, reviewed by Ashman and Majetic, 2006), which is not surprising given the high environmental variation commonly documented in nectar traits. Studies estimating the heritability of both nectar and morphological floral traits in the same system have mostly found a similar pattern, with lower heritability of nectar traits compared to other floral traits (Campbell, 1996; Vogler et al., 1999; Zu and Schiestl, 2017), although Klinkhamer and Wijk (1999) documented the opposite pattern in Echium vulgare. It should be noted that Fig. 2 includes estimates of both broad-sense heritability (clonal repeatability) and narrow-sense heritability (see online supplementary data for details). Broad-sense heritability may include non-additive genetic effects, and represents an upper bound on narrow-sense heritability (Falconer, 1989). Mean heritability of NPR is, as expected, lower if only narrow-sense estimates are considered (0.20 ± 0.12). Additive genetic variation in NPR is also supported by studies on crop species, where a few have documented high heritability for this trait (0.92 for cultivars of Lotus corniculatus, Murrell et al., 1982; 0.95 for commercial sunflower hybrids, Atlagić et al., 2003). In addition, crosses between closely related species that differ in pollination syndromes have been used to produce mapping populations for quantitative trait locus (QTL) analysis of traits associated with pollination. Large effect QTLs have been found to influence differences in nectar production between bee- and hummingbird-pollinated species of Mimulus (Schemske and Bradshaw, 1999) and Penstemon (Wessinger et al., 2014), whereas multiple small effect QTLs have been found to impact variation in nectar volume between bee- and hawkmoth-pollinated species of Petunia (Galliot et al., 2006), and between insect- and hummingbird-pollinated species of Ipomopsis (Nakazato et al., 2013). Such mapping studies clearly document a genetic basis of variation in NPR between species, but we are not aware of any study that has examined if the same QTL regions can explain variation within species.
Fig. 2.
Heritability of nectar traits estimated by crosses (circles, n = 8), clones (diamonds, n = 8) and response to selection (triangles, n = 6). Symbols in black are statistically significant at P < 0.05. NPR = nectar production rate. Data from a total of eight studies are included (Mitchell and Shaw, 1993; Campbell, 1996; Boose, 1997; Klinkhamer and Wijk, 1999; Vogler et al., 1999; Leiss et al., 2004; Kaczorowski et al., 2008; Zu and Schiestl, 2017).
The evidence for heritability of nectar traits besides NPR is scarce. Of the few studies that have estimated heritability of sugar concentrations, one each documented a significant heritability for total sugars (Klinkhamer and Wijk, 1999) and for sucrose, fructose and glucose concentrations (Zu and Schiestl, 2017) (Fig. 2). It should be noted that the former study estimated broad-sense heritability including possible non-additive genetic sources of variation, providing an upper limit of heritability (Falconer, 1989). In addition, a total of three studies (of which two estimated narrow-sense heritability) have combined nectar volume and sugar concentrations to calculate total sugar secretion per unit time, and all found significant heritability for this trait (Fig. 2). No study has reported heritability estimates for amino acid composition or concentrations, or for any other secondary compound found in nectar.
Only two of the included studies estimated heritability in the field (Campbell, 1996; Leiss et al., 2004). Because nectar traits are strongly affected by environmental conditions, estimates obtained in controlled environments are likely to be inflated compared to what would be observed in natural populations (Lynch and Walsh, 1998). In line with this, no significant heritability of NPR or sugar concentration was found in one of the field studies, due to high total phenotypic variance (VP) masking the substantial additive genetic variance (VA) detected (Campbell, 1996). However, in the second study, which estimated heritability of NPR both in the field and in a growth chamber, heritability was actually considerably higher when measured in the field (Leiss et al., 2004). Additional studies have found evidence of genotype by environment (G × E) interactions for NPR (Campbell, 1996; Boose, 1997; Vogler et al., 1999) and total sugar (Kaczorowski et al., 2008), showing that at least part of the large plasticity observed in nectar traits is heritable. This may impact the response to selection on nectar, and may be a mechanism that maintains additive genetic variation in nectar traits (Via and Lande, 1985; Mitchell, 2004).
The main insight emerging from the current summary of studies quantifying heritability of nectar traits is that the knowledge gaps previously identified by Mitchell (2004) largely persist. While the rise of molecular genetic studies has provided important knowledge on the genetic basis of differences in nectar traits between species, it seems that more classic quantitative genetic approaches to address intraspecific variation are becoming less common. The need to move studies into the field has also proven difficult, with only a single study quantifying the heritability of nectar production in both controlled and field conditions so far (Leiss et al., 2004).
Natural selection on nectar
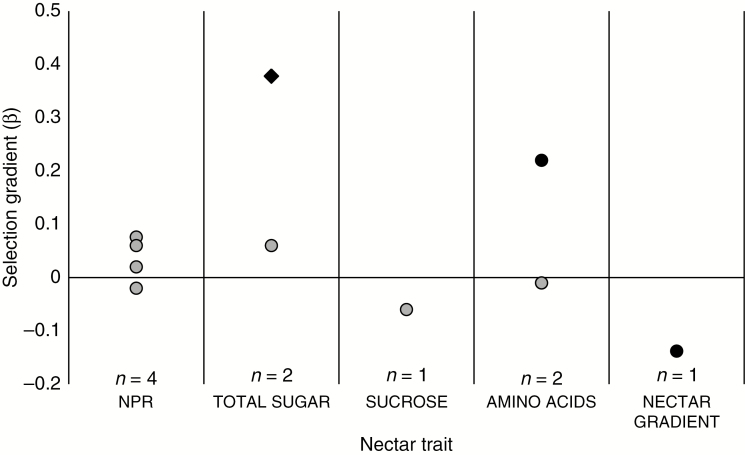
The evidence that nectar is under selection is at present much less convincing than the evidence that nectar is heritable. As far as we are aware, only six studies have published estimates of phenotypic selection gradients on nectar traits while controlling for plant size (see online supplementary data for details on how studies were selected), and only three of the ten estimates were statistically significant (Fig. 3). These three estimates document selection for increased sugar concentration via male fitness (Kulbaba and Worley, 2012), increasing amounts of specific amino acids via female fitness (Gijbels et al., 2014, 2015) and decreasing nectar volume in top flowers in the inflorescence (i.e. a nectar gradient, Zhao et al., 2016) via female fitness. Strikingly, we do not yet have a single estimate of statistically significant selection on NPR (Fig. 3), despite the very long-standing interest in the link between the amount of nectar, pollinator behaviour and pollination success. This would not change if we had been less strict in our criteria of study inclusion, because neither of the two studies that used univariate analysis to quantify selection differentials on NPR via seed production detected significant selection (cf. Mitchell et al., 1998; Caruso, 2001).
Fig. 3.
Selection gradients (β) on nectar traits estimated with multiple regression models (cf. Lande and Arnold, 1983) including at least one size-related trait (number of flowers, inflorescence height, etc.). Circles represent selection estimates via female fitness (total fruit or seed production per plant, n = 9) and diamonds represent selection estimates via male fitness (number of seeds sired per plant, n = 1). Symbols in black are statistically significant at P < 0.05. NPR = nectar production rate, nectar gradient = nectar volume in flowers decreases from the top to the bottom of the inflorescence (Zhao et al., 2016). Data from a total of six studies are included (Hodges, 1995; Mitchell et al., 1998; Benitez-Vieyra et al., 2010; Kulbaba and Worley, 2012; Gijbels et al., 2015; Zhao et al., 2016).
One reason why researchers tend to forget about nectar when measuring selection may be that they take pollinator-mediated selection on NPR for granted. However, the strong environmental effects on nectar trait expression combined with potential associations with antagonists and micro-organisms point to the need for experimental studies that actually test the prevalence and strength of pollinator-mediated selection on nectar traits (Fig. 1B). At present, this remains one of the most conspicuous knowledge gaps in our understanding of nectar evolution. A single exception is the recent study by Zhao et al. (2016), which manipulated the pollination environment to identify the selective agent on floral traits including nectar in the bumblebee-pollinated Aconitum gymnandrum. They found that the observed selection for a nectar gradient across the inflorescence disappeared in supplementally hand-pollinated plants, demonstrating that all selection was mediated by pollinators (∆β = −0.15). More surprisingly, they also documented significant non-pollinator mediated selection on NPR (β = 0.14 in hand-pollinated plants), suggesting selection via correlated traits or alternative selective agents. These contrasting findings reiterate the need for experimental approaches.
It is interesting that the only study to have documented significant phenotypic selection on nectar sugar concentration found selection acting via male fitness (Kulbaba and Worley, 2012; Fig. 3). In common with other traits that affect pollinator attraction, nectar production has been suggested to be sexually selected, because male function typically needs more pollinator visits to maximize reproductive success compared to female function (Bateman, 1948). Male-biased nectar production is common in hermaphrodites (Carlson and Harms, 2006), suggesting that the secretion pattern has evolved to ensure more visits in male-phase flowers. However, the prevalence of pollen limitation of female reproductive success (Ashman et al., 2004; Knight et al., 2005) indicates that reward production should also be important for female function, which is supported by the many studies that have documented pollinator-mediated selection on other attraction traits via female fitness (e.g. Caruso et al., 2010; Parachnowitsch and Kessler, 2010; Sletvold and Ågren, 2014; Trunschke et al., 2017).
AGENTS OF SELECTION
The amount, composition and timing of nectar production can influence the pollinators that a flower attracts and their behaviour when visiting, suggesting that populations and species have diverged in response to selection generated by the varying preferences of pollinators (e.g. Baker and Baker 1983a; Bruneau, 1997). Indeed, nectar is considered an integral part of pollination syndromes (Fenster et al., 2004), and can be used to predict a plant’s most common pollinators (Baker and Baker, 1990). Many studies have documented compelling correlations between pollination syndromes and nectar traits among species (e.g. Perret et al., 2001; Goldblatt, 2005; Marten-Rodriguez et al., 2009; Tavares et al., 2016; Tiedge and Lohaus, 2017), suggesting pollinator-mediated adaptation. However, similar patterns would also arise if pollinators simply track nectar variation that evolves via other selective agents. A more direct test of adaptive hypotheses is to associate nectar trait shifts with shifts in pollinator type (Bruneau, 1997; Nicolson, 2007). For example, evolution of hexose nectars from sucrose nectars was correlated with a shift from insect to bird pollination in the Canary Island flora (Dupont et al., 2004), indicating that sugar composition was an evolutionarily labile trait within lineages. Taken together, macroevolutionary patterns suggest that nectar characteristics may evolve readily in response to changes in the pollination regime, but the link from microevolutionary processes within populations to the observed species differences is poorly understood. In fact, at the microevolutionary scale, we have only a single conclusive example that pollinator preferences drive selection for nectar traits (Zhao et al., 2016). Here we review the documented relationships between nectar characteristics and pollinator preferences that are expected to lead to selection, as well as additional biotic interactions and abiotic factors that could influence selection on nectar traits (Fig. 1B).
Pollinators
Both observational and experimental data indicate that nectar, a critical food source for many flower visitors, mediates pollinator visitation. Variation in nectar traits leads to variation in the identity of visitors, as well as the frequency and length of visits (Thomson and Plowright, 1980; Galen and Plowright, 1985; Thomson, 1988; Hodges, 1995). The two most prominent nectar traits associated with animal pollination, nectar volume and sugar concentration, are also key components of pollination syndromes (Fenster et al., 2004), correlating with morphological traits that attract specific pollinator taxa. In general, bee-pollinated flowers have lower nectar volumes with higher sugar concentrations, while flowers pollinated by birds, bats and lepidopterans have higher nectar volumes with more dilute sugar solutions (Baker and Baker, 1983a). Studies comparing pollinator shifts within plant genera find that shifts from bee to bird or from bird to bat are associated with changes in nectar volume and sugar concentrations (Nicolson, 2007). However, the association of pollinators with particular nectar types does not necessarily demonstrate that they are the agents driving the evolution of nectar, highlighting an important missing link in the evolutionary ecology of nectar.
While pollination syndromes suggest macroevolutionary patterns of pollinator preference for nectar volume and sugar concentration, microevolutionary processes should be dictated by decisions that reflect a pollinator’s immediate metabolic requirements. We searched the literature on pollinator preferences for nectar to assess whether the general trends predicted from the pollination syndrome literature are born out in experimental tests of preferences. However, we found few studies comparing nectar sugars and volumes that met the search criteria for our meta-analysis (see online supplementary data), probably because this literature is diffuse and focuses on how nectar traits relate to floral signals, making interpretations of preferences for the nectar traits alone difficult to extract. Foraging economics predict pollinators should balance caloric value of nectar rewards with the cost of nectar collection (Heinrich, 1979), which implicitly suggests that, all else being equal, pollinators will prefer nectar with higher sugar concentrations, provided that the nectar’s increased viscosity does not exceed levels that inhibit its collection (Kim et al., 2011). Studies using artificial nectar indicate that bird pollinators generally prefer high sugar concentrations when other factors are held constant (e.g. Fleming et al., 2008; Leseigneur and Nicolson, 2009) and sugar concentration differences, specifically assigning flowers to high vs. low or no nectar rewards, have become a standard approach to testing bee preferences and biases for floral signals (e.g. Thomson, 1988; Cnaani et al., 2006; Biernaskie and Gegear, 2007). However, pollinators may also prefer greater nectar volumes (Schmid-Hempel and Schmid-Hempel, 1987; Mitchell, 1993; Jones and Reithel, 2001; Klinkhamer et al., 2001). Yet not all plants produce copious amounts of high-sugar nectar, suggesting that factors other than pollinator preference, such as the cost of producing nectar (Pyke, 1991), also contribute to nectar evolution.
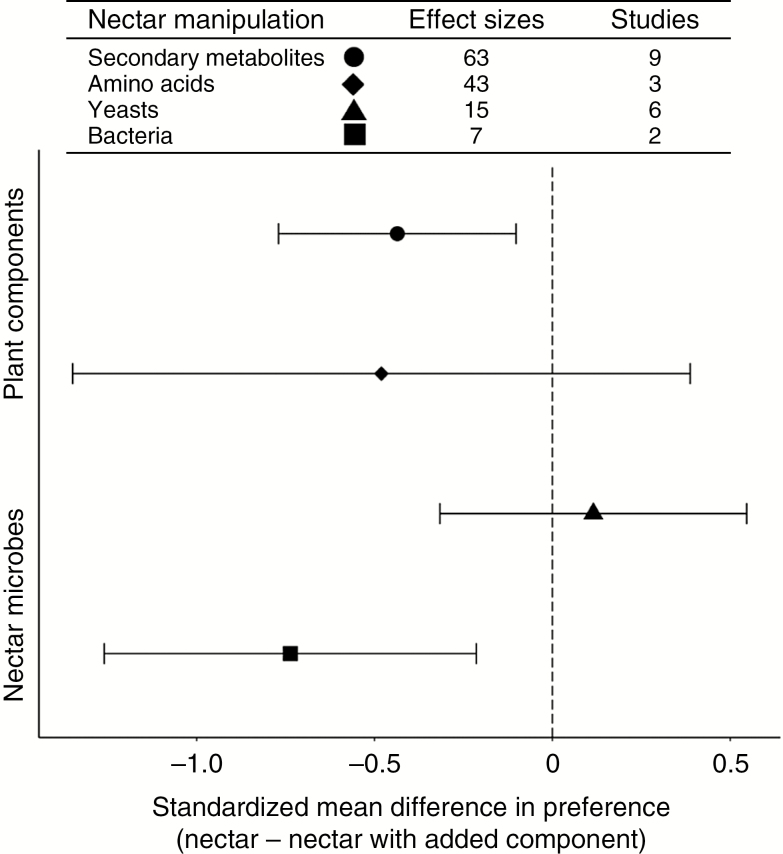
Other aspects of nectar composition, such as secondary metabolites (Adler, 2000; Raguso, 2004), the proportion of different sugars (Baker and Baker, 1983b) and amino acids (Baker and Baker, 1976; Nepi et al., 2012) can affect pollinator preferences and behaviour. As we show in our meta-analysis, a growing number of studies manipulating nectar secondary metabolites suggest these compounds generally reduce pollinator preferences (Fig. 4), which matches previous predictions on the putative costs of so-called ‘toxic nectar’ (Adler, 2000). However, responses to nectar secondary metabolites can be concentration-dependent; at low levels, pollinator responses may be equivocal, while higher concentrations lead to pollinator deterrence (Koehler et al., 2012; Wright et al., 2013; Manson et al., 2013). Some compounds, such as caffeine and nicotine, can even be attractive at low concentrations (Singaravelan et al., 2005; Wright et al., 2013) and may lead to higher rates of pollen transfer (Thomson et al., 2015). Furthermore, pollinator deterrence due to nectar secondary metabolites does not necessarily translate into reduced plant fitness (Kessler et al., 2008), although studies that measure plant reproduction are largely lacking (but see Adler and Irwin, 2005, 2012). Additionally, nectar scents may alter pollinator preferences (Raguso, 2004; Kessler and Baldwin, 2007; Galen et al., 2011), suggesting a broad range of secondary metabolites could be influencing pollinators. Preferences for sugar composition differ between pollinator taxa (Baker and Baker, 1983b), but may also depend on the total sugar concentration. For example, passerine birds prefer hexose over sucrose at low concentrations, but while some species may switch to a preference for sucrose at higher concentrations, others show no preference as concentrations increase (Brown et al., 2010; Odendaal et al., 2010). Interestingly, our meta-analysis showed the response to nectar amino acids to be somewhat neutral (Fig. 4), but we only found three studies, all with birds, so these data are also limited (method details in online supplementary data,Figs S1 and S2). Although amino acids can drive nectar preferences (Carter et al., 2006), affect the taste of nectar (Gardener and Gillman, 2002) and may function as a source of protein for some pollinators (Mevi-Schutz and Erhardt, 2005), more work is needed to assess their contribution to nectar evolution.
Fig. 4.
Meta-analyses of pollinator response to nectar properties, either for plant-derived components such as nectar secondary metabolites and amino acids or microbe presence, separated into yeasts and bacteria. The number of effect sizes extracted from the studies available (n in column) for each category is included in the table above the figure. Raw data (as well as the individual effect size estimates included for each summary point here) and information on criteria for data extraction can be found in the online supplementary data.
Although pollinator behaviour studies involving nectar are common, only a minority (13 % of those included in our database) link pollinator preferences for nectar traits to estimates of plant reproduction. It is clear that some nectar is better than no nectar, as demonstrated by increased pollen transfer following nectar addition in non-rewarding plants (Johnson et al., 2004) and reduced reproductive success following spur-tip removal in nectar-producing plants (Ackerman et al., 1994). However, there is not much evidence that quantitative variation in nectar amount affects plant fitness. A convincing exception is the study of Brandenburg et al. (2012), which showed that Petunia axillaris introgression lines with reduced nectar volume experienced shorter visits by hawkmoth pollinators compared to the wild-type, resulting in reduced seed production in low-nectar plants. Changes in nectar composition may also alter pollinator behaviour, although this may not always lead to changes in plant reproduction, highlighting the danger in assuming preferences will drive selection. For example, enhanced nectar alkaloids in Gelsemium sempervirens reduced movement of a pollen analogue, yet there was no effect on fruit set despite reductions in visit length and visit number (Adler and Irwin, 2005). Many more studies are needed to establish how pollinator response translates into plant fitness variation and to what extent pollinators are current agents of selection on nectar traits.
Herbivores and nectar robbers
Although herbivores do not directly feed upon nectar, they can have a profound impact on nectar characteristics. For example, direct damage to flowers by florivores can significantly reduce nectar volume (Krupnick et al., 1999), but effects on other nectar traits have not been measured (McCall and Irwin, 2006). Foliar herbivory can indirectly affect nectar by reducing nectar production (Strauss et al., 1996; Chauta et al., 2017) and by increasing (Bruinsma et al., 2014; Chauta et al., 2017) or reducing (Bruinsma et al., 2008; Narbona and Dirzo, 2010) sugar concentrations. Foliar herbivory can also increase the concentration of nectar secondary metabolites (Adler et al., 2006; Halpern et al., 2010). These induced changes to nectar generally have a negative effect on pollinator visitation (reviewed by Kessler and Halitschke, 2009), although studies do not isolate changes in nectar characteristics from other impacts of herbivory, including changes in visual appearance, volatile profile, flower number and flowering phenology (Strauss and Whittall, 2006). In fact, although we identified several studies looking at pollinator response to herbivory, we were unable to tease apart whether these responses were due to changes in nectar or other factors. Although these data are not reported in our meta-analysis, the general trend was to reduce the length and number of pollinator visits. Herbivores may also influence nectar composition via constitutive nectar secondary metabolites. Constitutive concentrations of secondary metabolites can be correlated with concentrations of secondary metabolites in leaves, suggesting nectar secondary metabolites may be a systemic response to herbivore defence (Adler et al., 2006; Manson et al., 2012). Furthermore, a comparative phylogenetic study found that outcrossing Nicotiana species had significantly lower concentrations of nectar secondary metabolites than selfing species (Adler et al., 2012), which suggests that a trade-off between herbivore defence and pollinator attraction drives secondary metabolite levels. Herbivores may therefore influence selection on several nectar traits indirectly via effects on pollinators, and the need for defence may constrain pollinator-mediated selection on nectar secondary compounds.
Nectar robbers remove nectar without pollinating flowers and often damage floral tissue in the process. Major effects of nectar robbing include a reduction in volume (Newman and Thomson, 2005), altered sugar concentration (Pleasants, 1983), changes in subsequent nectar production (but see Lasso and Naranjo, 2003) and increases in nectar secondary metabolites (Kaczorowski et al., 2014). This variation in the quantity and quality of nectar due to robbing can translate into reduced pollinator visitation and the fitness consequences of nectar robbing for plants are generally negative (reviewed by Irwin et al., 2010). Plants may therefore be under selection to prevent nectar robbing, with potential strategies including defending nectar with secondary metabolites (Adler, 2000; Irwin et al., 2010); however, nectar secondary metabolites can deter both robbers and pollinators, creating conflicting selective pressure on nectar characteristics.
Nectar microbes
Nectar provides ideal habitat for many microbial species such as yeasts and bacteria, which thrive on the high sugar content. Yeasts living exclusively in floral nectar have been found in a wide range of plant species (Brysch-Herzberg, 2004; Herrera et al., 2009) and while some species have only been detected in particular plants (e.g. Manson et al., 2007), others are more widespread, such as Metschnikowia reukauffii, found in nectar samples from 20 out of 24 species screened for yeast in southern Spain (Pozo et al., 2011). Bacteria in nectar have received less attention than yeasts (but see Fridman et al., 2012; Vannette et al., 2013; Vannette and Fukami, 2018). Both taxa affect nectar characteristics; yeast metabolism reduces sugar concentrations but can also alter sugar ratios and increase nectar pH (Herrera et al., 2008; Álvarez-Pérez et al., 2012; Vannette et al., 2013), while bacteria can also reduce total sugar concentrations, alter sugar ratios, reduce nectar pH (Vannette et al., 2013), reduce nectar volume and alter amino acids (Vannette and Fukami, 2018). Yeasts and bacteria also produce volatiles that affect how a pollinator responds to nectar under laboratory conditions (Rering et al., 2017). While microbes can alter nectar properties, so too can nectar properties alter microbial growth and reproduction (e.g. Burdon et al., 2018) making microbe–nectar interactions complex and difficult to tease apart.
While nectar microbes may have direct costs for plants (Huang et al., 2011; Vannette and Fukami, 2018), both yeasts and bacteria are hypothesized to mainly impact plant fitness indirectly by modifying pollinator behaviour (Schaeffer and Irwin, 2014). We assessed the available data on the effects of nectar microbes on pollinator preferences using our meta-analysis framework (see online supplementary data). In general, yeasts had a neutral effect on pollinator preference (Fig. 4), although positive effects were seen in some studies (Good et al., 2014; Schaeffer and Irwin, 2014). For at least one example, the effect on pollinator preference appears to increase male plant fitness, as estimated by higher donation of fluorescent dye (Schaeffer and Irwin, 2014). In contrast, nectar bacteria show negative effects on pollinator behaviour (Fig. 4), although few bacterial species have been investigated (Vannette et al., 2013) and bacterial density may play an important role in determining the strength of this interaction (Junker et al., 2014). However, direct comparisons of yeasts and bacteria in nectar suggest that bacteria have a more negative impact on pollinator preference (Vannette et al., 2013; Good et al., 2014). These changes in pollinator behaviour due to nectar microbes may influence plant fitness, leading to selection for nectar conditions that support or deter microbe growth. For example, plants that are able to prevent bacterial colonization through the production of antimicrobial compounds such as nectar secondary metabolites may be favoured (Adler, 2000; Vannette and Fukami, 2016; Burdon et al., 2018), although pollinator response to these compounds could lead to antagonist selection on secondary metabolites.
Abiotic agents of selection
Biotic agents of selection are probably dominant in driving the evolution of nectar, but abiotic conditions should also play a role. As discussed in the context of phenotypic variation in nectar, plants can respond to a variety of abiotic conditions such as water availability, humidity, light and nutrients by altering nectar production and compounds (e.g. Pleasants, 1983; Devlin, 1988; Carroll et al., 2001; Burkle and Irwin, 2009). Nectar production is, for example, likely to increase with plant resource status and water availability, and selection on nectar is thus expected to partly reflect selection on resource acquisition. In addition to affecting trait expression, abiotic factors may also affect the response of biotic selective agents to variation in nectar traits. For example, the addition of nutrients to a natural population of Ipomopsis aggregata increased nectar volume by 38 % and pollen receipt, a proxy for pollinator visitation, by 26 % compared to control plants (Burkle and Irwin, 2009). Abiotic conditions may also constrain the types of nectar characteristics that evolve, even though costs of nectar production are rarely measured (but see Pyke, 1991; Ordano and Ornelas, 2005). Assessing abiotic drivers of selection on nectar should be relatively simple, requiring manipulation of abiotic conditions and measurement of selection in the different conditions, but to our knowledge this experimental approach has not been attempted with nectar.
TARGETS OF SELECTION: FLORAL SIGNALS VS. REWARDS
Floral signals are often assumed to be correlated with floral rewards and therefore provide ‘honest’ signals for pollinators (Wright and Schiestl, 2009; Schiestl and Johnson, 2013). If these signal–reward correlations are important for plant fitness, we would expect them to be targets of selection. However, the majority of selection studies on flowers focus on floral signals rather than nectar rewards (Harder and Johnson, 2009). This focus on signals could be for several reasons. First, researchers may hypothesize that signals are the targets of selection rather than nectar rewards, warranting a focus on the traits that can lead to adaptation (i.e. the targets of selection). Second, signals may be easier to measure in the field and thus the focus is more one of convenience than science. Third, the large temporal and environmental effects on nectar variation may be expected to preclude the detection of any selection on rewards. Fourth, researchers might assume a correlation between signals and rewards in nectar-producing plants, rather than directly measuring it.
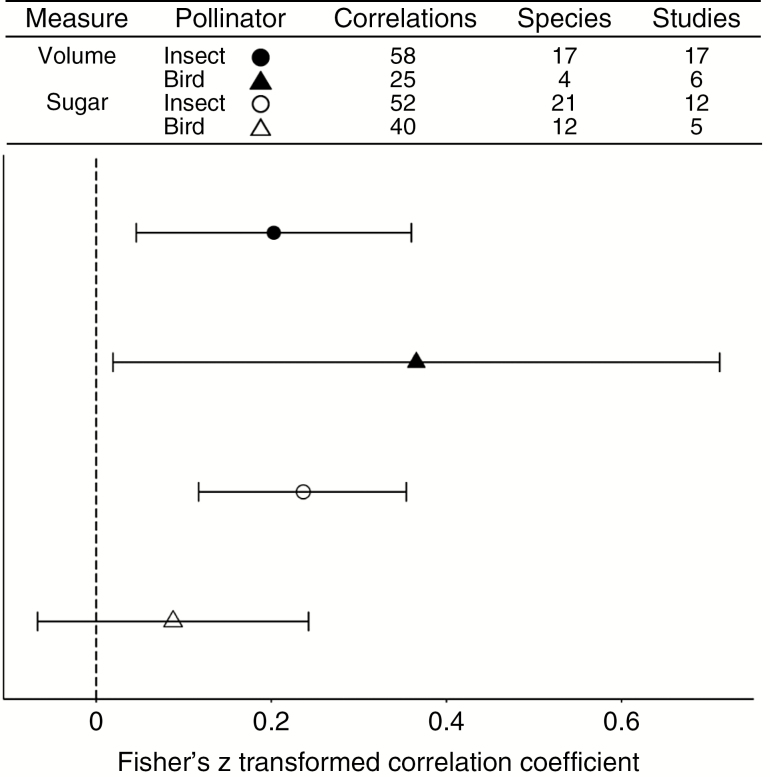
To address the final point, we gathered estimates of correlations between floral traits that can be used as signals (e.g. flower size, inflorescence size, flower colour, shape and floral scent) and nectar traits that can function as rewards. Using a meta-analysis approach, we asked whether these floral signals are correlated with nectar rewards (details in Figs S3–S5). In general, we found that signals were weakly but significantly correlated with nectar volumes and/or sugar amounts (Fig. 5). Both insect- and bird-pollinated plants tended to have positive correlations between signals and nectar, with the exception of nectar sugars in bird-pollinated species, although this category also had the fewest studies. Interestingly, we did not observe an overall stronger correlation with nectar sugar concentration than with nectar volume. If honest signals of reward are important to plant fitness, we might predict that nectar sugar concentrations would be easier to maintain in the face of visitation than nectar volumes and thus there would be a stronger correlation with sugars. However, we see no evidence for this pattern (Fig. 5).
Fig. 5.
Meta-analyses of correlations between floral signals (flower size, shape, colour, scent and inflorescence size) and nectar rewards (volume or sugar), separated by nectar reward type and the main pollinators (bird or insect). We pooled across signal type because the majority were flower size measures, which did not allow for comparisons across signal types. Number of correlations, plant species and studies for each category are included as columns in the table above the figure. Further details of the studies, analyses and raw data (as well as the individual Fisher’s z included for each summary point here) can be found in the online supplementary data.
While our meta-analyses suggest that signals and rewards may often be correlated, these results come with some important caveats. As Benitez-Vieyra et al. (2010, 2014) point out, while correlations between signals and rewards are often measured for populations (or species), evolution may also act on within-individual correlations, which are rarely examined. Moreover, even rewarding plants may benefit from not being completely honest (e.g. Bell, 1986; Gilbert et al., 1991), if this leads to reduced geitonogamy or lower costs of nectar production. Therefore, strong signal–reward correlations are unlikely, suggesting that we need to estimate selection on both signals and nectar rewards to predict their evolutionary trajectories.
FUTURE DIRECTIONS
Clearly, current understanding of the evolutionary ecology of nectar lags behind that of many other floral traits. Part of this is probably due to the complex nature of the trait, making researchers avoid something so hard to define and measure. However, this is probably not the whole explanation. First, nectar is very much a trait ‘in fashion’, with many recent studies addressing effects of nectar microbes and secondary compounds on aspects of pollinator behaviour in diverse contexts (e.g. Bartlewicz et al., 2016; Egan et al., 2016; Richardson et al., 2016; Rering et al., 2017; Stevenson et al., 2017). Second, floral scent is a similarly complicated trait, but several studies have now linked scent variation to plant fitness (Schiestl et al., 2011; Parachnowitsch et al., 2012; Gross et al., 2016). The current weak link from nectar via pollinator behaviour to plant fitness severely limits our understanding of nectar evolution and strengthening this link should constitute a major research direction in coming years. Here, we suggest some promising approaches.
We need to quantify current selection on nectar traits
It is clear that there is variation in nectar traits among individuals that affect pollinators and other plant–animal interactions. Furthermore, at least some of this variation is genetically based, but very little is known about selection on any nectar trait. This is striking, as the number of published selection estimates on other floral traits is steadily rising (Harder and Johnson, 2009; Sletvold and Ågren, 2014). How frequent is natural selection on nectar rewards? What nectar components (NPR, sugar concentration, secondary compounds) experience selection? Studies should take care to control for variation in other floral traits that are known to be under selection, and in particular plant size.
We need studies that examine whether the target of selection is nectar itself or the correlation between nectar and floral signals
Clearly, nectar rewards and floral signals do not function independently. However, disentangling how pollinators respond to these two floral components and to the correlation itself is necessary to accurately predict how selection will shape evolution within species. Studies that quantify correlational selection on floral signal and reward traits can tease apart whether the target of selection is the signal, the reward or their correlation. The single study to directly test this (Benitez-Vieyra et al., 2010) suggests that it was the correlation rather than the reward itself; this is clearly an area that needs further research.
We need studies that manipulate the putative selective agent(s)
A couple of studies have used experimental approaches to identify ants as selective agents on extrafloral nectar production (Rudgers and Strauss, 2004; Rutter and Rausher, 2004), but so far only one study has manipulated the pollination environment to quantify the role of pollinators as selective agents on floral nectar production (Zhao et al., 2016). This contrasts with the considerable number of experimental studies that in the last decade have focused on morphological floral traits (Chapurlat et al., 2015; Trunschke et al., 2017, and references therein). Are pollinators the dominant selective agent on nectar? Does the relative importance of different biotic interactions vary among nectar traits? As has been documented for many morphological traits, selection on floral nectar traits in Aconitum gymnandrum was found to be mediated by both pollinators and other factors (Zhao et al., 2016), reiterating the need for experiments to conclusively identify the main selective agents (Wade and Kalisz, 1990; Caruso et al., 2017).
We need estimates of heritability of nectar traits, in particular for nectar compounds
While a lack of heritable variation rarely seems to constrain evolution, knowledge on quantitative genetic variation and correlations among traits is essential to understand patterns of evolution (Lynch and Walsh, 1998). The current interest in mapping approaches to identify the genetic basis of traits has produced important insights into what governs nectar variation between species, but we also encourage classic quantitative genetic studies that can provide estimates of genetic correlations and additive genetic variation. In particular, few studies have quantified heritability in field conditions, and we can repeat the questions highlighted by Mitchell (2004): How common, strong or adaptive are G×E interactions? To what extent do nectar traits differ in G×E interactions, and why?
We need to link microevolutionary processes to species divergence and phylogenetic patterns
NPR and sugar ratios are traits that figure in current pollination syndrome classifications and where covariation with the dominating pollinators has been documented in many systems. Crosses between species with contrasting pollination syndromes for QTL mapping can be used to quantify current pollinator-mediated selection on these traits in the F2 generation planted in both parental environments (cf. Schemske and Bradshaw, 1999). Ideally, this would be combined with a manipulation of the pollination environment. It would also be informative to expand such studies to include intraspecific variation and determine whether the same chromosomal regions are involved in population differentiation.
We need to focus on within-individual variation in nectar as a trait of interest
Both predictable nectar gradients and more unpredictable variation across the inflorescence can govern pollinator behaviour, and this kind of variation is at least partly controlled by the plant. As already suggested by Zhao et al. (2016), who documented pollinator-mediated selection on a nectar gradient, but not on mean NPR, studies that focus on variation as a trait of interest are likely to yield new information.
CONCLUSIONS
Our review reveals a surprising number of gaps in our understanding of the evolutionary ecology of nectar. While macroevolutionary patterns of nectar traits indicate that they have been shaped by pollinator-mediated selection, we lack knowledge on the microevolutionary processes causing and maintaining these patterns. We suggest that heritable variation in nectar traits is unlikely to limit its evolution, but that we still need to link this variation to plant fitness. There is scarce evidence of natural selection on nectar traits by pollinators or other agents of selection, and we need studies in natural populations that quantify current selection on nectar, as well as experimental studies that identify the target traits and the selective agents involved. Despite the challenges associated with nectar, we cannot rely on studies that focus on floral morphology and signalling to fully address floral evolution in nectar-rewarding plants. It is time to push forward our understanding of nectar evolutionary ecology.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: Citation history of papers included in the Ecology Special Feature ‘Community and Evolutionary Ecology of Nectar’ as of February 2018. Fig. S1: Detailed effect sizes for all estimates used for (a) secondary metabolites, (b) amino acids and (c) microbes. Fig. S2: Funnel plots for effects of secondary metabolites, amino acids, yeasts and bacteria on pollinator preferences. Fig. S3: Meta-analyses of Fisher’s z-transformed correlations between floral signals and nectar traits for insect-pollinated species. Fig. S4: Meta-analyses of Fisher’s z-transformed correlations between floral signals and nectar traits for bird-pollinated species. Fig. S5: Funnel plots for correlations between flower signal traits and nectar (volume or sugar) for insect- and bird-pollinated species.
ACKNOWLEDGEMENTS
We would like to thank the participants at the Scandinavian Association for Pollination Ecologists (SCAPE) 2013 meeting for simulating discussion and providing the seed for our review, with special mention to Rocio Pérez-Barrales who contributed both to the initial formulation of our review and nectar reference database. Thanks also to Robert Junker, Jeff Ollerton and Judith Trunschke for providing additional correlations of floral traits and nectar from their study species.
LITERATURE CITED
- Ackerman JD, Rodríguez-Robles JA, Meléndez EJ. 1994. A meager nectar offering by an epiphytic orchid is better than nothing. Biotropica 26: 44. [Google Scholar]
- Adler LS. 2000. The ecological significance of toxic nectar. Oikos 91: 409–420. [Google Scholar]
- Adler LS, Bronstein JL. 2004. Attracting antagonists: does floral nectar increase leaf herbivory?Ecology 85: 1519–1526. [Google Scholar]
- Adler LS, Irwin RE. 2005. Ecological costs and benefits of defenses in nectar. Ecology 86: 2968–2978. [Google Scholar]
- Adler LS, Irwin RE. 2012. Nectar alkaloids decrease pollination and female reproduction in a native plant. Oecologia 168: 1033–1041. [DOI] [PubMed] [Google Scholar]
- Adler LS, Wink M, Distl M, Lentz AJ. 2006. Leaf herbivory and nutrients increase nectar alkaloids. Ecology Letters 9: 960–967. [DOI] [PubMed] [Google Scholar]
- Adler LS, Seifert MG, Wink M, Morse GE. 2012. Reliance on pollinators predicts defensive chemistry across tobacco species. Ecology Letters 15: 1140–1148. [DOI] [PubMed] [Google Scholar]
- Afik O, Delaplane KS, Shafir S, Moo-Valle H, Quezada-Euan JJG. 2014. Nectar minerals as regulators of flower visitation in stingless bees and nectar hoarding wasps. Journal of Chemical Ecology 40: 476–483. [DOI] [PubMed] [Google Scholar]
- Aizen MA, Raffaele E. 1996. Nectar production and pollination in Alstroemeria aurea: responses to level and pattern of flowering shoot defoliation. Oikos 76: 312. [Google Scholar]
- Álvarez-Pérez S, Herrera CM, de Vega C. 2012. Zooming-in on floral nectar: a first exploration of nectar-associated bacteria in wild plant communities. FEMS Microbiology Ecology 80: 591–602. [DOI] [PubMed] [Google Scholar]
- Anand C, Umranikar C, Shintre P, et al. 2007. Presence of two types of flowers with respect to nectar sugar in two gregariously flowering species. Journal of Biosciences 32: 769–774. [DOI] [PubMed] [Google Scholar]
- Ashman TL, Majetic CJ. 2006. Genetic constraints on floral evolution: a review and evaluation of patterns. Heredity 96: 343. [DOI] [PubMed] [Google Scholar]
- Ashman TL, Knight TM, Steets JA, et al. 2004. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85: 2408–2421. [Google Scholar]
- Atlagić J, Joksimović J, Sakač Z, Miklič V, Dušanić N. 2003. Mode of inheritance and heritability of disc flower corolla length and nectar content in sunflower. Genetika 35: 59–65. [Google Scholar]
- Baker I, Baker HG. 1976. Analyses of amino acids in flower nectars of hybrids and their parents, with phylogenetic implications. New Phytologist 76: 87–98. [Google Scholar]
- Baker HG, Baker I. 1983a A brief historical review of the chemistry of floral nectar. In: Bentley B, Elias T, eds. The biology of nectaries. New York: Columbia University Press, 126–152. [Google Scholar]
- Baker HG, Baker I. 1983b Floral nectar sugar constituents in relation to pollinator type. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Scientific and Academic Editions, 117–141. [Google Scholar]
- Baker HG, Baker I. 1986. The occurrence and significance of amino acids in floral nectar. Plant Systematics and Evolution 151: 175–186. [Google Scholar]
- Baker HG, Baker I. 1990. The predictive value of nectar chemistry to the recognition of pollinator types. Israel Journal of Botany 39: 157–166. [Google Scholar]
- Barlow SE, Wright GA, Ma C, et al. 2017. Distasteful nectar deters floral robbery. Current Biology 27: 2552–2558.e3. [DOI] [PubMed] [Google Scholar]
- Bartkowska MP, Johnston MO. 2012. Pollinators cause stronger selection than herbivores on floral traits in Lobelia cardinalis (Lobeliaceae). New Phytologist 193: 1039–1048. [DOI] [PubMed] [Google Scholar]
- Bartlewicz J, Lievens B, Honnay O, Jacquemyn H. 2016. Microbial diversity in the floral nectar of Linaria vulgaris along an urbanization gradient. BMC Ecology 16: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman AJ. 1948. Inter-sexual selection in Drosophila. Heredity 2: 349–368. [DOI] [PubMed] [Google Scholar]
- Baude M, Leloup J, Suchail S, et al. 2011. Litter inputs and plant interactions affect nectar sugar content: plant interactions and nectar content. Journal of Ecology 99: 828–837. [Google Scholar]
- Becklin KM, Gamez G, Uelk B, Raguso RA, Galen C. 2011. Soil fungal effects on floral signals, rewards, and aboveground interactions in an alpine pollination web. American Journal of Botany 98: 1299–1308. [DOI] [PubMed] [Google Scholar]
- Bell G. 1986. The evolution of empty flowers. Journal of Theoretical Biology 118: 253–258. [Google Scholar]
- Benitez-Vieyra S, Ordano M, Fornoni J, Boege K, Domínguez CA. 2010. Selection on signal-reward correlation: limits and opportunities to the evolution of deceit in Turnera ulmifolia L.: selection on signal-reward correlation. Journal of Evolutionary Biology 23: 2760–2767. [DOI] [PubMed] [Google Scholar]
- Benitez-Vieyra S, Fornoni J, Pérez-Alquicira J, Boege K, Domínguez CA. 2014. The evolution of signal-reward correlations in bee- and hummingbird-pollinated species of Salvia. Proceedings of the Royal Society B: Biological Sciences 281: 20132934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch A. 1983. Nectar production of Epilobium angustifolium L. at different air humidities; nectar sugar in individual flowers and the optimal foraging theory. Oecologia 59: 40–48. [DOI] [PubMed] [Google Scholar]
- Best LS, Bierzychudek P. 1982. Pollinator foraging on foxglove (Digitalis purpurea): a test of a new model. Evolution 36: 70–79. [DOI] [PubMed] [Google Scholar]
- Biernaskie JM, Gegear RJ. 2007. Habitat assessment ability of bumble-bees implies frequency-dependent selection on floral rewards and display size. Proceedings of the Royal Society B: Biological Sciences 274: 2595–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie JM, Cartar RV, Hurly TA. 2002. Risk-averse inflorescence departure in hummingbirds and bumble bees: could plants benefit from variable nectar volumes?Oikos 98: 98–104. [Google Scholar]
- Bolten AB, Feinsinger P, Baker HG, Baker I. 1979. On the calculation of sugar concentration in flower nectar. Oecologia 41: 301–304. [DOI] [PubMed] [Google Scholar]
- Boose DL. 1997. Sources of variation in floral nectar production rate in Epilobium canum (Onagraceae): implications for natural selection. Oecologia 110: 493–500. [DOI] [PubMed] [Google Scholar]
- Brandenburg A, Kuhlemeier C, Bshary R. 2012. Hawkmoth pollinators decrease seed set of a low-nectar Petunia axillaris line through reduced probing time. Current Biology 22: 1635–1639. [DOI] [PubMed] [Google Scholar]
- Brown M, Downs CT, Johnson SD. 2010. Sugar preferences of a generalist nonpasserine flower visitor, the African Speckled Mousebird (Colius striatus). Auk 127: 781–786. [Google Scholar]
- Brown M, Downs CT, Johnson SD. 2011. Covariation of flower traits and bird pollinator assemblages among populations of Kniphofia linearifolia (Asphodelaceae). Plant Systematics and Evolution 294: 199–206. [Google Scholar]
- Bruinsma M, IJdema H, van Loon JJA, Dicke M. 2008. Differential effects of jasmonic acid treatment of Brassica nigra on the attraction of pollinators, parasitoids, and butterflies. Entomologia Experimentalis et Applicata 128: 109–116. [Google Scholar]
- Bruinsma M, Lucas-Barbosa D, ten Broeke CJM, et al. 2014. Folivory affects composition of nectar, floral odor and modifies pollinator behavior. Journal of Chemical Ecology 40: 39–49. [DOI] [PubMed] [Google Scholar]
- Bruneau A. 1997. Evolution and homology of bird pollination syndromes in Erythrina (Leguminosae). American Journal of Botany 84: 54–71. [Google Scholar]
- Brysch-Herzberg M. 2004. Ecology of yeasts in plant–bumblebee mutualism in Central Europe. FEMS Microbiology Ecology 50: 87–100. [DOI] [PubMed] [Google Scholar]
- Burdon RCF, Junker RR, Scofield DG, Parachnowitsch AL. 2018. Bacteria colonising Penstemon digitalis show volatile and tissue-specific responses to a natural concentration range of the floral volatile linalool. Chemoecology 28: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkle LA, Irwin RE. 2009. The effects of nutrient addition on floral characters and pollination in two subalpine plants, Ipomopsis aggregata and Linum lewisii. Plant Ecology 203: 83–98. [Google Scholar]
- Campbell DR. 1996. Evolution of floral traits in a hermaphroditic plant: field measurements of heritabilities and genetic correlations. Evolution 50: 1442–1453. [DOI] [PubMed] [Google Scholar]
- Cane JH, Schiffhauer D. 1997. Nectar production of cranberries: Genotypic differences and insensitivity to soil fertility. Journal of the American Society for Horticultural Science 122: 665–667. [Google Scholar]
- Canto A, Herrera CM. 2012. Micro-organisms behind the pollination scenes: microbial imprint on floral nectar sugar variation in a tropical plant community. Annals of Botany 110: 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto A, Perez R, Medrano M, Castellanos MC, Herrera CM. 2006. Intra-plant variation in nectar sugar composition in two Aquilegia species (Ranunculaceae): contrasting patterns under field and glasshouse conditions. Annals of Botany 99: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto A, Herrera CM, García IM, Pérez R, Vaz M. 2011. Intraplant variation in nectar traits in Helleborus foetidus (Ranunculaceae) as related to floral phase, environmental conditions and pollinator exposure. Flora 206: 668–675. [Google Scholar]
- Carlson JE, Harms KE. 2006. The evolution of gender-biased nectar production in hermaphroditic plants. The Botanical Review 72: 179–205. [Google Scholar]
- Carroll AB, Pallardy SG, Galen C. 2001. Drought stress, plant water status, and floral trait expression in fireweed, Epilobium angustifolium (Onagraceae). American Journal of Botany 88: 438–446. [PubMed] [Google Scholar]
- Carter C, Shafir S, Yehonatan L, Palmer RG, Thornburg R. 2006. A novel role for proline in plant floral nectars. Naturwissenschaften 93: 72–79. [DOI] [PubMed] [Google Scholar]
- Caruso CM. 2001. Differential selection on floral traits of Ipomopsis aggregata growing in contrasting environments. Oikos 94: 295–302. [Google Scholar]
- Caruso CM, Scott SL, Wray JC, Walsh CA. 2010. Pollinators, herbivores, and the maintenance of flower colour variation: a case study with Lobelia siphilitica. International Journal of Plant Science 171: DOI: 10.1086/656511. [DOI] [Google Scholar]
- Caruso CM, Martin RA, Sletvold N, et al. 2017. What are the environmental determinants of phenotypic selection? A meta-analysis of experimental studies. The American Naturalist 190: 363–376. [DOI] [PubMed] [Google Scholar]
- Castellanos MC, Wilson P, Thomson JD. 2002. Dynamic nectar replenishment in flowers of Penstemon (Scrophulariaceae). American Journal of Botany 89: 111–118. [DOI] [PubMed] [Google Scholar]
- Chapurlat E, Ågren J, Sletvold N. 2015. Spatial variation in pollinator-mediated selection on phenology, floral display and spur length in the orchid Gymnadenia conopsea. New Phytologist 208: 1264–1275. [DOI] [PubMed] [Google Scholar]
- Chauta A, Whitehead S, Amaya-Marquez M, Poveda K. 2017. Leaf herbivory imposes fitness costs mediated by hummingbird and insect pollinators. PLoS One 12: e0188408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnaani J, Thomson JD, Papaj DR. 2006. Flower choice and learning in foraging bumblebees: effects of variation in nectar volume and concentration. Ethology 112: 278–285. [Google Scholar]
- Cruden RW. 1976. Intraspecific variation in pollen-ovule ratios and nectar secretion – Preliminary evidence of ecotypic adaptation. Annals of the Missouri Botanical Garden 63: 277. [Google Scholar]
- Cuevas E, Rosas-Guerrero V. 2016. Spatio-temporal variation of nectar robbing in Salvia gesneriflora and its effects on nectar production and legitimate visitors. Plant Biology 18: 9–14. [DOI] [PubMed] [Google Scholar]
- Dag A, Eisikowitch D. 2000. The effect of carbon dioxide enrichment on nectar production in melons under greenhouse conditions. Journal of Apicultural Research 39: 88–89. [Google Scholar]
- De la Barrera E, Nobel PS. 2004. Nectar: properties, floral aspects, and speculations on origin. Trends in Plant Science 9: 65–69. [DOI] [PubMed] [Google Scholar]
- Devlin B. 1988. The effects of stress on reproductive characters of Lobelia cardinalis. Ecology 69: 1716–1720. [Google Scholar]
- Devlin B, Horton JB, Stephenson AG. 1987. Patterns of nectar production of Lobelia cardinalis. American Midland Naturalist 117: 289–295. [Google Scholar]
- Dreisig H. 2012. How long to stay on a plant: the response of bumblebees to encountered nectar levels. Arthropod-Plant Interactions 6: 315–325. [Google Scholar]
- Dupont YL, Hansen DM, Rasmussen JT, Olesen JM. 2004. Evolutionary changes in nectar sugar composition associated with switches between bird and insect pollination: the Canarian bird-flower element revisited. Functional Ecology 18: 670–676. [Google Scholar]
- Egan PA, Stevenson PC, Tiedeken EJ, Wright GA, Boylan F, Stout JC. 2016. Plant toxin levels in nectar vary spatially across native and introduced populations. Journal of Ecology 104: 1106–1115. [Google Scholar]
- Enkegaard A, Kryger P, Boelt B. 2016. Determinants of nectar production in heather. Journal of Apicultural Research 55: 100–106. [Google Scholar]
- Falconer DS. 1989. Introduction to quantitative genetics. New York: Longman. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology Evolution and Systematics 35: 375–403. [Google Scholar]
- Fishman L, Willis JH. 2008. Pollen limitation and natural selection on floral characters in the yellow monkeyflower, Mimulus guttatus. New Phytologist 177: 802–810. [DOI] [PubMed] [Google Scholar]
- Fisogni A, Cristofolini G, Rossi M, Galloni M. 2011. Pollinator directionality as a response to nectar gradient: promoting outcrossing while avoiding geitonogamy: pollinator directionality as a response to nectar gradient. Plant Biology 13: 848–856. [DOI] [PubMed] [Google Scholar]
- Fleming PA, Xie S, Napier K, McWhorter TJ, Nicolson SW. 2008. Nectar concentration affects sugar preferences in two Australian honeyeaters and a lorikeet. Functional Ecology 22: 599–605. [Google Scholar]
- Fridman S, Izhaki I, Gerchman Y, Halpern M. 2012. Bacterial communities in floral nectar. Environmental Microbiology Reports 4: 97–104. [DOI] [PubMed] [Google Scholar]
- Galen C, Plowright RC. 1985. The effects of nectar level and flower development on pollen carry-over in inflorescences of fireweed (Epilobium angustifolium) (Onagraceae). Canadian Journal of Botany 63: 488–491. [Google Scholar]
- Galen C, Kaczorowski R, Todd SL, Geib J, Raguso RA. 2011. Dosage-dependent impacts of a floral volatile compound on pollinators, larcenists, and the potential for floral evolution in the Alpine Skypilot Polemonium viscosum. The American Naturalist 177: 258–272. [DOI] [PubMed] [Google Scholar]
- Galliot C, Hoballah ME, Kuhlemeier C, Stuurman J. 2006. Genetics of flower size and nectar volume in Petunia pollination syndromes. Planta 225: 203–212. [DOI] [PubMed] [Google Scholar]
- Gardener MC, Gillman MP. 2002. The taste of nectar - a neglected area of pollination ecology. Oikos 98: 552–557. [Google Scholar]
- Gijbels P, Van den Ende W, Honnay O. 2014. Landscape scale variation in nectar amino acid and sugar composition in a Lepidoptera pollinated orchid species and its relation with fruit set. Journal of Ecology 102: 136–144. [Google Scholar]
- Gijbels P, Van den Ende W, Honnay O. 2015. Phenotypic selection on nectar amino acid composition in the Lepidoptera pollinated orchid species Gymnadenia conopsea. Oikos 1245: 421–427. [Google Scholar]
- Gilbert FS, Haines N, Dickson K. 1991. Empty flowers. Functional Ecology 5: 29–39. [Google Scholar]
- Goldblatt P. 2005. Radiation of pollination systems in the Iridaceae of sub-Saharan Africa. Annals of Botany 97: 317–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good AP, Gauthier M-PL, Vannette RL, Fukami T. 2014. Honey bees avoid nectar colonized by three bacterial species, but not by a yeast species, isolated from the bee gut. PLoS One 9: e86494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda V, Kress WJ. 2013. A geographic mosaic of plant–pollinator interactions in the Eastern Caribbean Islands. Biotropica 45: 224–235. [Google Scholar]
- Gross K, Sun M, Schiestl FP. 2016. Why do floral perfumes become different? Region-specific selection on floral scent in a terrestrial orchid. PLoS ONE 11: e0147975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern SL, Adler LS, Wink M. 2010. Leaf herbivory and drought stress affect floral attractive and defensive traits in Nicotiana quadrivalvis. Oecologia 163: 961–971. [DOI] [PubMed] [Google Scholar]
- Hansen DM, Olesen JM, Mione T, Johnson SD, Müller CB. 2007. Coloured nectar: distribution, ecology, and evolution of an enigmatic floral trait. Biological Reviews 82: 83–111. [DOI] [PubMed] [Google Scholar]
- Harder LD, Johnson SD. 2009. Darwin’s beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytologist 183: 530–545. [DOI] [PubMed] [Google Scholar]
- Heil M. 2011. Nectar: generation, regulation and ecological functions. Trends in Plant Science 16: 191–200. [DOI] [PubMed] [Google Scholar]
- Heinrich B. 1979. Bumblebee economics. Cambridge: Harvard University Press. [Google Scholar]
- Herrera CM, Pérez R, Alonso C. 2006. Extreme intraplant variation in nectar sugar composition in an insect-pollinated perennial herb. American Journal of Botany 93: 575–581. [DOI] [PubMed] [Google Scholar]
- Herrera CM, García IM, Pérez R. 2008. Invisible floral larcenies: microbial communities degrade floral nectar of bumble bee-pollinated plants. Ecology 89: 2369–2376. [DOI] [PubMed] [Google Scholar]
- Herrera CM, de Vega C, Canto A, Pozo MI. 2009. Yeasts in floral nectar: a quantitative survey. Annals of Botany 103: 1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges SA. 1993. Consistent interplant variation in nectar characteristics of Mirabilis multiflora. Ecology 74: 542–548. [Google Scholar]
- Hodges SA. 1995. The influence of nectar production on Hawkmoth behavior, self pollination, and seed production in Mirabilis multiflora (Nyctaginaceae). American Journal of Botany 82: 197–204. [Google Scholar]
- Hoover SER, Ladley JJ, Shchepetkina AA, Tisch M, Gieseg SP, Tylianakis JM. 2012. Warming, CO2, and nitrogen deposition interactively affect a plant–pollinator mutualism. Ecology Letters 15: 227–234. [DOI] [PubMed] [Google Scholar]
- Huang M, Sanchez-Moreiras AM, Abel C, et al. 2011. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytologist: DOI: 10.1111/j.1469-8137.2011.04001.x. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Adler LS, Agrawal AA. 2004. Community and evolutionary ecology of nectar. Ecology 85: 1477–1478. [Google Scholar]
- Irwin RE, Bronstein JL, Manson JS, Richardson L. 2010. Nectar robbing: ecological and evolutionary perspectives In: Futuyma DJ, Shafer HB, Simberloff D, eds. Annual review of ecology, evolution, and systematics, Vol. 41 Palo Alto: Annual Reviews, 271–292. [Google Scholar]
- Jakobsen HB, Kritjánsson K. 1994. Influence of temperature and floret age on nectar secretion in Trifolium repens L. Annals of Botany 74: 327–334. [Google Scholar]
- Johnson SD, Peter CI, Ågren J. 2004. The effects of nectar addition on pollen removal and geitonogamy in the non-rewarding orchid Anacamptis morio. Proceedings of the Royal Society. B 271: 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KN, Reithel JS. 2001. Pollinator-mediated selection on a flower color polymorphism in experimental populations of Antirrhinum (Scrophulariaceae). American Journal of Botany 88: 447–454. [PubMed] [Google Scholar]
- Junker RR, Romeike T, Keller A, Langen D. 2014. Density-dependent negative responses by bumblebees to bacteria isolated from flowers. Apidologie 45: 467–477. [Google Scholar]
- Kaczorowski RL, Juenger TE, Holtsford TP. 2008. Heritability and correlation structure of nectar and floral morphology traits in Nicotiana alata. Evolution 62: 1738–1750. [DOI] [PubMed] [Google Scholar]
- Kaczorowski RL, Koplovich A, Sporer F, Wink M, Markman S. 2014. Immediate effects of nectar robbing by Palestine Sunbirds (Nectarinia osea) on nectar alkaloid concentrations in Tree Tobacco (Nicotiana glauca). Journal of Chemical Ecology 40: 325–330. [DOI] [PubMed] [Google Scholar]
- Kearns CA, Inouye DW. 1993. Techniques for pollination biologists. Niwot: University Press of Colorado. [Google Scholar]
- Kessler A, Halitschke R. 2009. Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: predictions and case study. Functional Ecology 23: 901–912. [Google Scholar]
- Kessler D, Baldwin IT. 2007. Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. The Plant Journal 49: 840–854. [DOI] [PubMed] [Google Scholar]
- Kessler D, Bhattacharya S, Diezel C, et al. 2012. Unpredictability of nectar nicotine promotes outcrossing by hummingbirds in Nicotiana attenuata: variability in nectar nicotine promotes outcrossing. The Plant Journal 71: 529–538. [DOI] [PubMed] [Google Scholar]
- Kessler D, Gase K, Baldwin IT. 2008. Field experiments with transformed plants reveal the sense of floral scents. Science 321: 1200–1202. [DOI] [PubMed] [Google Scholar]
- Kim W, Gilet T, Bush JWM. 2011. Optimal concentrations in nectar feeding. Proceedings of the National Academy of Sciences of the United States of America 108: 16618–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkhamer PGL, Wijk CAM van der V. 1999. Genetic variation in floral traits of Echium vulgare. Oikos 85: 515–522. [Google Scholar]
- Klinkhamer PG, De Jong TJ, Linnebank LA. 2001. Small-scale spatial patterns determine ecological relationships: an experimental example using nectar production rates. Ecology Letters 4: 559–567. [Google Scholar]
- Knauer AC, Schiestl FP. 2015. Bees use honest floral signals as indicators of reward when visiting flowers. Ecology Letters 18: 135–143. [DOI] [PubMed] [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, et al. 2005. Pollen limitation of plant reproduction: Pattern and process. Annual Review of Ecology Evolution and Systematics 36: 467–497. [Google Scholar]
- Koehler A, Pirk CWW, Nicolson SW. 2012. Honeybees and nectar nicotine: deterrence and reduced survival versus potential health benefits. Journal of Insect Physiology 58: 286–292. [DOI] [PubMed] [Google Scholar]
- Krupnick GA, Weis AE, Campbell DR. 1999. The consequences of floral herbivory for pollinator service to Isomeris arborea. Ecology 80: 125–134. [Google Scholar]
- Kulbaba MW, Worley AC. 2012. Selection on floral design in Polemonium brandegeei (Polemoniaceae): Female and male fitness under hawkmoth pollination. Evolution 66: 1344–1359. [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37: 1210. [DOI] [PubMed] [Google Scholar]
- Lasso E, Naranjo ME. 2003. Effect of pollinators and nectar robbers on nectar production and pollen deposition in Hamelia patens (Rubiaceae). Biotropica 35: 57–66. [Google Scholar]
- Lehtilä K, Strauss SY. 1999. Effects of foliar herbivory on male and female reproductive traits of wild radish, Raphanus raphanistrum. Ecology 80: 116–124. [Google Scholar]
- Leiss KA, Klinkhamer PGL. 2005a Spatial distribution of nectar production in a natural Echium vulgare population: implications for pollinator behaviour. Basic and Applied Ecology 6: 317–324. [Google Scholar]
- Leiss KA, Klinkhamer PGL. 2005b Genotype by environment interactions in the nectar production of Echium vulgare. Functional Ecology 19: 454–459. [Google Scholar]
- Leiss KA, Vrieling K, Klinkhamer PGL. 2004. Heritability of nectar production in Echium vulgare. Heredity 92: 446–451. [DOI] [PubMed] [Google Scholar]
- Leseigneur CDC, Nicolson SW. 2009. Nectar concentration preferences and sugar intake in the white-bellied sunbird, Cinnyris talatala (Nectariniidae). Journal of Comparative Physiology B-Biochemical Systemic and Environmental Physiology 179: 673–679. [DOI] [PubMed] [Google Scholar]
- Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland: Sinauer Associates, Inc. [Google Scholar]
- Maloof JE, Inouye DW. 2000. Are nectar robbers cheaters or mutualists?Ecology 81: 2651–2661. [Google Scholar]
- Manson JS, Lachance M-A, Thomson JD. 2007. Candida gelsemii sp. nov., a yeast of the Metschnikowiaceae clade isolated from nectar of the poisonous Carolina jessamine. Antonie van Leeuwenhoek 92: 37–42. [DOI] [PubMed] [Google Scholar]
- Manson JS, Rasmann S, Halitschke R, Thomson JD, Agrawal AA. 2012. Cardenolides in nectar may be more than a consequence of allocation to other plant parts: a phylogenetic study of Asclepias. Functional Ecology 26: 1100–1110. [Google Scholar]
- Manson JS, Cook D, Gardner DR, Irwin RE. 2013. Dose-dependent effects of nectar alkaloids in a montane plant–pollinator community. Journal of Ecology 101: 1604–1612. [Google Scholar]
- Marten-Rodriguez S, Almarales-Castro A, Fenster CB. 2009. Evaluation of pollination syndromes in Antillean Gesneriaceae: evidence for bat, hummingbird and generalized flowers. Journal of Ecology 97: 348–359. [Google Scholar]
- McCall AC, Irwin RE. 2006. Florivory: the intersection of pollination and herbivory. Ecology Letters 9: 1351–1365. [DOI] [PubMed] [Google Scholar]
- McKenna MA, Thomson JD. 1988. A technique for sampling and measuring small amounts of floral nectar. Ecology 69: 1306–1307. [Google Scholar]
- Mevi-Schutz J, Erhardt A. 2005. Amino acids in nectar enhance butterfly fecundity: a long-awaited link. American Naturalist 165: 411–419. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ. 1993. Adaptive significance of Ipomopsis aggregata nectar production: observation and experiment in the field. Evolution 47: 25–35. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ. 2004. Heritability of nectar traits: why do we know so little?Ecology 85: 1527–1533. [Google Scholar]
- Mitchell RJ, Shaw RG. 1993. Heritability of floral traits for the perennial wild flower Penstemon centranthifolius (Scrophulariaceae): clones and crosses. Heredity 71: 185–192. [Google Scholar]
- Mitchell RJ, Waser NM. 1992. Adaptive significance of Ipomopsis aggregata nectar production: pollination success of single flowers. Ecology 73: 633–638. [Google Scholar]
- Mitchell RJ, Shaw RG, Waser NM. 1998. Pollinator selection, quantitative genetics, and predicted evolutionary responses of floral traits in Penstemon centranthifolius (Scrophulariaceae). International Journal of Plant Sciences 159: 331–337. [Google Scholar]
- Morrant DS, Schumann R, Petit S. 2009. Field methods for sampling and storing nectar from flowers with low nectar volumes. Annals of Botany 103: 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Chen Y, Yang Y, Fu R, Wang H, Compton SG. 2016. Seed predators can increase nectar volumes in an alpine daisy: but do the insects benefit?Plant Ecology 217: 1195–1205. [Google Scholar]
- Murrell DC, Tomes DT, Shuel RW. 1982. Inheritance of nectar production in birdsfoot trefoil. Canadian Journal of Plant Science 62: 101–105. [Google Scholar]
- Nakazato T, Rieseberg LH, Wood TE. 2013. The genetic basis of speciation in the Giliopsis lineage of Ipomopsis (Polemoniaceae). Heredity 111: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbona E, Dirzo R. 2010. Experimental defoliation affects male but not female reproductive performance of the tropical monoecious plant Croton suberosus (Euphorbiaceae). Annals of Botany 106: 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepi M, Soligo C, Nocentini D, et al. 2012. Amino acids and protein profile in floral nectar: much more than a simple reward. Flora 207: 475–481. [Google Scholar]
- Newman DA, Thomson JD. 2005. Effects of nectar robbing on nectar dynamics and bumblebee foraging strategies in Linaria vulgaris (Scrophulariaceae). Oikos 110: 309–320. [Google Scholar]
- Nicolson S. 2007. Nectar consumers In: Nicolson S, Nepi M, Pacini E, eds. Nectaries and nectar. Dordrecht: Springer Netherlands, 289–342. [Google Scholar]
- Odendaal TC, Brown M, Downs CT, Johnson SD. 2010. Sugar preferences and digestive efficiency of the village weaver: a generalist avian pollinator of African plants. Journal of Experimental Biology 213: 2531–2535. [DOI] [PubMed] [Google Scholar]
- Ordano M, Ornelas JF. 2005. The cost of nectar replenishment in two epiphytic bromeliads. Journal of Tropical Ecology 21: 541–547. [Google Scholar]
- Ott JR, Real LA, Silverfine EM. 1985. The effect of nectar variance on bumblebee patterns of movement and potential gene dispersal. Oikos 45: 333. [Google Scholar]
- Pacini E, Nepi M, Vesprini JL. 2003. Nectar biodiversity: a short review. Plant Systematics and Evolution 238: 7–21. [Google Scholar]
- Pandit S, Choudhury BC. 2001. Factors affecting pollinator visitation and reproductive success in Sonneratia caseolaris and Aegiceras corniculatum in a mangrove forest in India. Journal of Tropical Ecology 17: 431–447. [Google Scholar]
- Parachnowitsch AL, Kessler A. 2010. Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytologist 188: 393–402. [DOI] [PubMed] [Google Scholar]
- Parachnowitsch AL, Raguso RA, Kessler A. 2012. Phenotypic selection to increase floral scent emission, but not flower size or colour in bee-pollinated Penstemon digitalis. New Phytologist 195: 667–675. [DOI] [PubMed] [Google Scholar]
- Perret M, Chautems A, Spichiger R, Peixoto M, Savolainen V. 2001. Nectar sugar composition in relation to pollination syndromes in Sinningieae (Gesneriaceae). Annals of Botany 87: 267–273. [DOI] [PubMed] [Google Scholar]
- Petanidou T, Smets E. 1996. Does temperature stress induce nectar secretion in Mediterranean plants?New Phytologist 133: 513–518. [Google Scholar]
- Pleasants JM. 1983. Nectar production patterns in Ipomopsis aggregata (Polemoniaceae). American Journal of Botany 70: 1468–1475. [Google Scholar]
- Power EF, Stabler D, Borland AM, Barnes J, Wright GA. 2018. Analysis of nectar from low-volume flowers: A comparison of collection methods for free amino acids. Methods in Ecology and Evolution 9: 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo MI, Herrera CM, Bazaga P. 2011. Species richness of yeast communities in floral nectar of Southern Spanish plants. Microbial Ecology 61: 82–91. [DOI] [PubMed] [Google Scholar]
- Pyke GH. 1991. What does it cost a plant to produce floral nectar?Nature 350: 58–59. [Google Scholar]
- Pyke GH. 2016. Floral nectar: Pollinator attraction or manipulation?Trends in Ecology & Evolution 31: 339–341. [DOI] [PubMed] [Google Scholar]
- Raguso RA. 2004. Why are some floral nectars scented?Ecology 85: 1486–1494. [Google Scholar]
- Real L, Rathcke BJ. 1988. Patterns of individual variability in floral resources. Ecology 69: 728–735. [Google Scholar]
- Real LA, Rathcke BJ. 1991. Individual variation in nectar production and its effect on fitness in Kalmia latifolia. Ecology 72: 149–155. [Google Scholar]
- Rering C, Beck JJ, Hall GW, McCartney MM, Vannette R. 2017. Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytologist. [DOI] [PubMed] [Google Scholar]
- Richardson LL, Bowers MD, Irwin RE. 2016. Nectar chemistry mediates the behavior of parasitized bees: consequences for plant fitness. Ecology 97: 325–337. [DOI] [PubMed] [Google Scholar]
- Rudgers JA, Strauss SY. 2004. A selection mosaic in the facultative mutualism between ants and wild cotton. Proceedings of the Royal Society of London. Series B: Biological Sciences 271: 2481–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusterholz HP, Erhardt A. 1998. Effects of elevated CO2 on flowering phenology and nectar production of nectar plants important for butterflies of calcareous grasslands. Oecologia 113: 341–349. [DOI] [PubMed] [Google Scholar]
- Rutter MT, Rausher MD. 2004. Natural selection on extrafloral nectar production in Chamaecrista fasciculata: the costs and benefits of a mutualism trait. Evolution 58: 2657–2668. [DOI] [PubMed] [Google Scholar]
- Schaeffer RN, Irwin RE. 2014. Yeasts in nectar enhance male fitness in a montane perennial herb. Ecology 95: 1792–1798. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Bradshaw HD. 1999. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proceedings of the National Academy of Sciences USA 96: 11910–11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl FP, Johnson SD. 2013. Pollinator-mediated evolution of floral signals. Trends in Ecology & Evolution 28: 307–315. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Huber FK, Gomez JM. 2011. Phenotypic selection on floral scent: trade-off between attraction and deterrence?Evolutionary Ecology 25: 237–248. [Google Scholar]
- Schmid-Hempel P, Schmid-Hempel R. 1987. Efficient nectar-collecting by honeybees II. Response to factors determining nectar availability. The Journal of Animal Ecology: 219–227. [Google Scholar]
- Schmid B, Nottebrock H, Esler KJ, et al. 2016. Responses of nectar-feeding birds to floral resources at multiple spatial scales. Ecography 39: 619–629. [Google Scholar]
- Seo PJ, Wielsch N, Kessler D, et al. 2013. Natural variation in floral nectar proteins of two Nicotiana attenuata accessions. BMC Plant Biology 13: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravelan N, Nee’man G, Inbar M, Izhaki I. 2005. Feeding responses of free-flying honeybees to secondary compounds mimicking floral nectars. Journal of Chemical Ecology 31: 2791–2804. [DOI] [PubMed] [Google Scholar]
- Sletvold N, Ågren J. 2014. There is more to pollinator-mediated selection than pollen limitation: Interaction intensity and selection strength. Evolution 68: 1907–1918. [DOI] [PubMed] [Google Scholar]
- Sletvold N, Trunschke J, Wimmergren C, Ågren J. 2012. Separating selection by diurnal and nocturnal pollinators on floral display and spur length in Gymnadenia conopsea. Ecology 93: 1880–1891. [DOI] [PubMed] [Google Scholar]
- Sletvold N, Trunschke J, Smit M, Verbeek J, Ågren J. 2016. Strong pollinator-mediated selection for increased flower brightness and contrast in a deceptive orchid. Evolution 70: 716–724. [DOI] [PubMed] [Google Scholar]
- Stevenson PC, Nicolson SW, Wright GA. 2017. Plant secondary metabolites in nectar: impacts on pollinators and ecological functions. Functional Ecology 31: 65–75. [Google Scholar]
- Strauss SY, Whittall JB. 2006. Non-pollinator agents of selection on floral traits. In: Ecology and evolution of flowers. Oxford: Oxford University Press, 120–138. [Google Scholar]
- Strauss SY, Conner JK, Rush SL. 1996. Foliar herbivory affects floral characters and plant attractiveness to pollinators: Implications for male and female plant fitness. American Naturalist 147: 1098–1107. [Google Scholar]
- Tavares DC, Freitas L, Gaglianone MC. 2016. Nectar volume is positively correlated with flower size in hummingbird-visited flowers in the Brazilian Atlantic Forest. Journal of Tropical Ecology 32: 335–339. [Google Scholar]
- Thomson JD. 1988. Effects of variation in inflorescence size and floral rewards on the visitation rates of traplining pollinators of Aralia hispida. Evolutionary Ecology 2: 65–76. [Google Scholar]
- Thomson JD, Draguleasa MA, Tan MG. 2015. Flowers with caffeinated nectar receive more pollination. Arthropod-Plant Interactions 9: 1–7. [Google Scholar]
- Thomson JD, Plowright RC. 1980. Pollen carryover, nectar rewards, and pollinator behavior with special reference to Diervilla lonicera. Oecologia 46: 68–74. [DOI] [PubMed] [Google Scholar]
- Tiedge K, Lohaus G. 2017. Nectar sugars and amino acids in day- and night-flowering Nicotiana species are more strongly shaped by pollinators’ preferences than organic acids and inorganic ions. PLoS ONE 12: e0176865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunschke J, Sletvold N, Ågren J. 2017. Interaction intensity and pollinator-mediated selection. New Phytologist 214: 1381–1389. [DOI] [PubMed] [Google Scholar]
- Vannette RL, Fukami T. 2016. Nectar microbes can reduce secondary metabolites in nectar and alter effects on nectar consumption by pollinators. Ecology 97: 1410–1419. [DOI] [PubMed] [Google Scholar]
- Vannette RL, Fukami T. 2018. Contrasting effects of yeasts and bacteria on floral nectar traits. Annals of Botany 121: 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannette RL, Gauthier M-PL, Fukami T. 2013. Nectar bacteria, but not yeast, weaken a plant–pollinator mutualism. Proceedings of the Royal Society B: Biological Sciences 280: 20122601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via S, Lande R. 1985. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39: 505–522. [DOI] [PubMed] [Google Scholar]
- Vogler DW, Peretz S, Stephenson AG. 1999. Floral plasticity in an iteroparous plant: the interactive effects of genotype, environment, and ontogeny in Campanula rapunculoides (Campanulaceae). American Journal of Botany 86: 482–494. [PubMed] [Google Scholar]
- Wade MJ, Kalisz S. 1990. The causes of natural selection. Evolution 44: 1947–1955. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV. 2016. Drought, pollen and nectar availability, and pollination success. Ecology 97: 1400–1409. [DOI] [PubMed] [Google Scholar]
- Wessinger CA, Hileman LC, Rausher MD. 2014. Identification of major quantitative trait loci underlying floral pollination syndrome divergence in Penstemon. Philosophical Transactions of the Royal Society B: Biological Sciences 369: 20130349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson MF, Bertin RI. 1979. Flower-visitors, nectar production, and inflorescence size of Asclepias syriaca. Canadian Journal of Botany 57: 1380–1388. [Google Scholar]
- Witt T, Juergens A, Gottsberger G. 2013. Nectar sugar composition of European Caryophylloideae (Caryophyllaceae) in relation to flower length, pollination biology and phylogeny. Journal of Evolutionary Biology 26: 2244–2259. [DOI] [PubMed] [Google Scholar]
- Wolff D. 2006. Nectar sugar composition and volumes of 47 species of Gentianales from a Southern Ecuadorian montane forest. Annals of Botany 97: 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GA, Schiestl FP. 2009. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Functional Ecology 23: 841–851. [Google Scholar]
- Wright GA, Baker DD, Palmer MJ, et al. 2013. Caffeine in floral nectar enhances a pollinator’s memory of reward. Science 339: 1202–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Lu N, Conner JK. 2016. Adaptive pattern of nectar volume within inflorescences: bumblebee foraging behavior and pollinator-mediated natural selection. Scientific Reports 6: 34499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M. 1981. Patchiness in the dispersion of nectar resources: probable causes. Oecologia 49: 154–157. [DOI] [PubMed] [Google Scholar]
- Zimmerman M. 1988. Pollination biology of montane plants: relationship between rate of nectar production and standing crop. American Midland Naturalist 120: 50–57. [Google Scholar]
- Zu P, Schiestl FP. 2017. The effects of becoming taller: direct and pleiotropic effects of artificial selection on plant height in Brassica rapa. The Plant Journal 89: 1009–1019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.