Abstract
Neuropathic pain represents a challenge to clinicians, because it is resistant to commonly prescribed analgesics due to its largely unknown mechanisms. Here, we investigated a descending dopaminergic pathway-mediated modulation of trigeminal neuropathic pain. We performed chronic constriction injury of the infraorbital nerve from the maxillary branch of trigeminal nerve to induce trigeminal neuropathic pain in mice. Our retrograde tracing showed that the descending dopaminergic projection from hypothalamic A11 nucleus to spinal trigeminal nucleus caudalis is bilateral. Optogenetic/chemogenetic manipulation of dopamine receptors D1 and D2 in the spinal trigeminal nucleus caudalis produced opposite effects on the nerve injury-induced trigeminal neuropathic pain. Specific excitation of dopaminergic neurons in the A11 nucleus attenuated the trigeminal neuropathic pain via the activation of D2 receptors in the spinal trigeminal nucleus caudalis. Conversely, specific ablation of the A11 dopaminergic neurons exacerbated such pain. Our results suggest that the descending A11–spinal trigeminal nucleus caudalis dopaminergic projection is critical for the modulation of trigeminal neuropathic pain and could be manipulated to treat such pain.
Keywords: Dopamine, Neuropathic pain, Descending modulation, Optogenetics, Chemogenetics
1. Introduction
Neuropathic pain is a physically and emotionally debilitating condition. However, its underlying mechanisms are still not fully understood. An animal model, chronic constriction injury of the infraorbital nerve (CCI-ION),7,28,40,54 has been established to study trigeminal neuropathic pain. The top-down pain modulation is largely mediated by descending monoaminergic signal pathways, which either inhibit or facilitate pain transmission at the level of spinal or medullary dorsal horn.3,6,22,36,38,46 Monoamines, including serotonin, norepinephrine, and dopamine (DA), act via their different receptors to exert a complex modulation of excitability of the dorsal horn neurons. In the CCI-ION model, previous studies28,40 have shown that descending serotonergic pathways are involved in the pathogenesis of trigeminal neuropathic pain; however, it is unclear whether descending dopaminergic signaling contributes to the CCI-ION-induced neuropathic pain. It was recently reported that descending dopaminergic pathways participate in pain transmission,15,16,27 but how different types of DA receptors are regulated to contribute to neuropathic pain development remains elusive. DA acts through five different metabotropic G protein-coupled receptors (D1-D5), including D1 subfamily (D1 and D5) and D2 subfamily (D2, D3, and D4). All DA receptors, except the D3 receptor, are present in the trigeminocervical complex;11 the D2 receptor is the most prominent in the spinal trigeminal nucleus caudalis (Sp5C).8,12 In the present study, we used D1-Cre and D2-Cre transgenic mice combined with optogenetic and chemogenetic manipulation to clarify the effects mediated by D1 and D2 receptors during trigeminal neuropathic pain in the CCI-ION model.
By using retrograde tracing, it has been revealed1 that five hypothalamic nuclei project to the Sp5C in the rat: the paraventricular nucleus, the lateral hypothalamic area, the perifornical hypothalamic area, the A11 nucleus, and the retrochiasmatic area. Specifically, the descending pathway from hypothalamic A11 to the Sp5C originates mainly from dopaminergic neurons.2 In the present study, we used dopamine transporter (DAT)-Cre transgenic mice combined with optogenetic stimulation or chemical lesion of dopaminergic neurons to determine the role of the descending dopaminergic projection from hypothalamic A11 to Sp5C in trigeminal neuropathic pain.
2. Methods
2.1. Animals
Male C57BL/6 wild-type (WT) mice (8–10 weeks) and D1-Cre, D2-Cre and DAT-Cre bacterial artificial chromosome transgenic mice (8–10 weeks) were used in this study. D1-Cre (line FK150) and D2-Cre (line ER44) transgenic mice23–25 were generated in GENSAT (www.gensat.org) and were provided by Dr. Mary Kay Lobo18,34 (University of Maryland School of Medicine). DAT-Cre mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All three types of Cre mice were on a C57BL/6 genetic background. The mice were housed under standard conditions with a 12 h light-dark cycle, and water and food pellets were available ad libitum. The mice were acclimated for 60 min before behavioral testing. All behavioral tests were carried out by an investigator blinded to the treatment groups. All experiments were carried out in accordance with the ethical guidelines of the National Institutes of Health and the International Association for the Study of Pain. All efforts were made to minimize animal suffering and to reduce the number of animals used. All experiments were approved by the Animal Care and Use Committee at the Texas A&M University College of Dentistry.
2.2. Trigeminal neuropathic pain model
We performed CCI-ION to induce trigeminal neuropathic pain in mice as described previously.28 The infraorbital nerve (ION) does not include any autonomic components, thus the CCI-ION has been considered as a classical neuropathic pain model.26 In brief, the mice were anesthetized with pentobarbital sodium (50 mg/kg, i.p.) and the ION was exposed through a 2 mm incision in the palatalbuccal mucosa using sterile technic. We loosely tied the ION with two 4-0 chromic gut ligatures. Tissue adhesive was used to close the incision. The control mice received only nerve exposure without ligation.28
2.3. Orofacial mechanical pain test with von Frey filaments
Orofacial mechanical pain was measured with a series of calibrated von Frey filaments (0.08, 0.15, 0.25, 0.41, 0.70, 1.2, and 2.0 g). Each mouse was placed into a 10-cm long plexiglass observation cylinder and allowed to poke out their heads and forepaws, but the mouse cannot turn around in the cylinder. After 60 min of acclimation, the von Frey filament was applied to the trigeminal nerve V2 branch-innervated facial skin. A positive response was defined as a sharp withdrawal of the head upon stimulation. Each filament was applied five times to the V2-innervated skin area for 1 s at 10 s intervals, starting from the lowest force of the filament (0.08 g) and continuing in ascending order. The head withdrawal threshold was calculated as the force at which the positive response occurred in three of five stimuli, which is an indicator for sensory component of pain in the CCI-ION model. Because the CCI-ION-induced neuropathic pain last at least 28 days and is at stable level on day 14 post-surgery, we chose that day to conduct all pain behavioral testing.
2.4. Conditioned place preference (CPP)
This test was conducted in a standard three-compartment apparatus including a dark room (normal mouse preferred location), a striped room and a buffer room. The movements of mice in each room were automatically recorded by a camera and analyzed with the ANY-maze software (Stoelting, IL). We performed the CPP test as described previously33 in the following order: preconditioning, conditioning (pairing), and testing. The preconditioning phase lasted for three days starting from day 8 after CCI-ION. During the preconditioning, all the mice were exposed to the environment with full access to all rooms for 30 min each day. On the third day, the movement of each mouse was recorded for 15 min as baseline. Next, the mice were paired with light stimulation in optogenetic manipulation or clozapine N-oxide (CNO) injection in chemogenetic activation. The normally preferred dark room was assigned as the pairing room. Following the three-day pairing, 15-min testing with free access to all the rooms was carried out. The time spent in each room was recorded to analyze the place preference, and altered time in paired dark room was used to indicate emotional component of trigeminal neuropathic pain in the CCI-ION model.
2.5. Optogenetic manipulation of neuronal activities in D1/D2-Cre mice
D1-Cre and D2-Cre mice were infused using sterile technic with the following Cre-inducible viruses into unilateral Sp5C according to predetermined coordinates (AP, −8.0 mm; ML, 1.5 mm; DV, 4.5 mm):44 1) a control virus (AAV5-EF1α-DIO-EYFP); 2) a virus expressing channelrhodopsin-2 (ChR2) for neuronal excitation (AAV5-EF1α-DIO-ChR2 (E123A)-EYFP-WPRE); 3) a virus expressing halorhodopsin from natronomonas pharaonis (NpHR) for neuronal inhibition (AAV5-EF1α-DIO-eNpHR3.0-EYFP). 0.6 μl of each virus (0.1 μl/min) was infused manually to express light-sensitive proteins in the Sp5C for optogenetic manipulation. Following the infusion, the needle (33 gauge, Hamilton) was remained in place for 5 min before being retracted from the Sp5C. Next, using sterile technic an optical fiber with a 1.25 mm ferruel cannula was implanted 0.3 mm above the virus infusion site through the same hole after syringe removal.31 Diode-pumped solid-state lasers (473 nm blue light for ChR2 and 532 nm green light for NpHR) produced from OEM Laser Systems (Midvale, Utah) were used for optogenetic manipulation of Sp5C D1/2-expressing neurons. The light stimulation was conducted at 20 Hz and the light intensity ranged from 3 to 5 mW. After experiments, the location of infusion and light stimulation site was confirmed histologically. To examine the effect of excitation of the A11 dopaminergic neurons on the CCI-ION-induced neuropathic pain, DAT-Cre mice were infused with AAV5-EF1α-DIO-ChR2 (E123A)-EYFP-WPRE and its control AAV5-EF1α-DIO-EYFP into the A11 nucleus (AP, −2.3 mm; ML, 0.2 mm; DV, 3.8 mm) as described previously29 with a minor modification, and blue light (473 nm) was used to excite A11 dopaminergic neurons.
2.6. Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)-based chemogenetic activation of D1/2-expressing neurons in the Sp5C
A Cre-inducible excitatory DREADD virus (hM3D) and its control were infused unilaterally (ipsilateral side of CCI-ION) into the Sp5C of D1-Cre and D2-Cre mice. For chemogenetic activation, D1-Cre and D2-Cre mice were infused using sterile technic with the following Cre-inducible viruses into unilateral Sp5C according to the above-mentioned coordinates: 1) a control virus (AAV5-hSyn-DIO-mCherry); 2) a virus expressing hM3D for neuronal activation (AAV5-hSyn-DIO-hM3D (Gq)-mCherry). Next, we injected CNO (1 mg/kg, i.p.) to specifically activate D1/2-expressing neurons in the Sp5C. The virus infusion was done as described in optogenetic manipulation and the infusion site was confirmed histologically after experiments.
2.7. Antegrade and retrograde tracing
Mice were anesthetized with 2% isoflurane and placed into a stereotaxic frame. 1 μl of the antegrade tracer 1,1′-dioctadecyl-3,3,3′3,3′ -tetramethyl-indocarbocyanine perchlorate (DiI) (100 mg/ml, Thermofisher) and the retrograde tracer fluorogold (FG) (3%, Thermofisher) were injected with a 30-gauge needle into A11 nucleus (AP, −2.3 mm; ML, 0.2 mm; DV, 3.8 mm) and Sp5C (AP, −8.0 mm; ML, 1.5 mm; DV, 4.5 mm)48, respectively, as described previously1. The needle was left in the corresponding area for a further 10 min before withdrawal from the brain. Only a single injection into the A11 nucleus or Sp5C was performed in each mouse. At 24 h after injection, the mice were deeply anesthetized with isoflurane and perfused with 4% paraformaldehyde for imaging or immunohistochemistry.
2.8. Immunohistochemistry
Following the perfusion, Sp5C- or A11-containing brain tissues were cut at 20 μm with a cryostat (CM1950, Leica, Chicago, IL). Free-floating slices were blocked in a 5% normal goat serum for 1 h followed by incubation with primary antibodies overnight at 4 °C. Next, the slices were washed and placed in a corresponding secondary antibody conjugated to Alexa Fluor 488 or Cy3 for 1 h at room temperature. Immunofluorescent imaging was observed and analyzed under a Leica fluorescence microscope (DMi8, Leica). The information for all the antibodies used in this study is listed below.
| Antibody | Dilution | Vendor | Catalog number |
|---|---|---|---|
| Anti-D1 receptor | 1:400 | Sigma | MAB5290 |
| Anti-D2 receptor | 1:400 | Millipore | AB5084P |
| Anti-Glutaminase | 1:500 | Abcam | Ab131554 |
| Anti-GABA | 1:500 | Sigma | A2052 |
| Anti-Cre | 1:500 | Sigma | C7988 |
| Anti-FG | 1:400 | Millipore | AB153-I |
| Anti-tyrosine hydroxylase (TH) | 1:800 | Abcam | Ab76442 |
| Donkey anti-mouse 488 | 1:600 | Jackson ImmunoResearch | 715-545-150 |
| Donkey anti-rabbit 488 | 1:500 | Jackson ImmunoResearch | 711-545-152 |
| Donkey anti-chicken 488 | 1:500 | Jackson ImmunoResearch | 703-545-155 |
| Mouse anti-rabbit Cy3 | 1:400 | Jackson ImmunoResearch | 211-165-109 |
| Donkey anti-mouse Cy3 | 1:500 | Jackson ImmunoResearch | 715-165-150 |
2.9. Chemical lesion of A11 dopaminergic neurons
Mice were first injected with desipramine (25 mg/kg, i.p., Sigma) to protect noradrenergic neurons.27 Next, the mice were anesthetized with 2% isoflurane and placed into a stereotaxic frame. Using sterile technic, a 30-gauge needle was used to inject 1 μl of 6-hydroxydopamine (6-OHDA) (2 μg/μl, Sigma) unilaterally into the A11 nucleus (AP, −2.3 mm; ML, 0.2 mm; DV, 3.8 mm). The needle was left in place for a further 10 min before withdrawal. The chemical lesion-produced ablation of A11 dopaminergic neuron was examined by immunohistochemical staining with the antibody against TH (a marker for dopaminergic neurons).
2.10. Drug administration
SCH23390 (D1 receptor antagonist, 1 μg/μl, Sigma) and spiperone (D2 receptor antagonist, 1 μg/μl, Sigma) were injected with a 30-gauge needle into the Sp5C (AP, −8.0 mm; ML, 1.5 mm; DV, 4.5 mm)48 to regulate the activities of the corresponding receptor. The injection volume was 1 μl.
2.11. Locomotor function determination
A Rotarod apparatus was used to measure locomotor function of mice. The rods in the apparatus were accelerated from 4 to 40 rpm over 5 min. The falling speed and the time to fall off the rod were recorded.
2.12. Statistics
Data are expressed as mean ± SEM. All statistical analyses were performed by SigmaStat software. Paired Student’s t-test and one-way analysis of variance (ANOVA) were performed, and Student-Newman-Keuls method was used for the post-hoc test of ANOVA. The level of significance was set at P < 0.05.
3. Results
3.1. Optogenetic manipulation of DA receptors D1 and D2 in the Sp5C differentially regulates trigeminal neuropathic pain
To reveal the role of DA receptors D1 and D2 in trigeminal neuropathic pain, we applied optogenetic stimulation in the Sp5C to specifically manipulate the function of these receptors. Immunohistochemical staining showed that the two DA receptors were expressed mostly on different neurons of the Sp5C (Fig. 1A). The percentages of D1- and D2-expressing cells were 42.33 ± 1.3% and 57.67 ± 1.3%, respectively. And only 10.23 ± 1.38% of cells expressed both D1 and D2 receptors. We also observed that D1 was predominantly expressed in the deep laminae of the Sp5C and D2 was mostly expressed in the superficial laminae of the Sp5C (Fig. 1A), which is consistent with the distribution of D1 and D2 receptors described in previous studies in rats.8,32 For optogenetic stimulation, we infused Cre-inducible viruses unilaterally into the Sp5C (ipsilateral side of CCI-ION) to express light-sensitive proteins (opsins) on D1/2-expressing neurons of D1-Cre or D2-Cre transgenic mice. And these viruses were strongly expressed in the Sp5C on day 14 after infusion (Fig. 1B). Because pain is not only an unpleasant sensation, but also related to emotional experience, we used von Frey filaments and CPP to measure sensory and emotional components of pain behaviors, respectively. On day 14 after CCI-ION, we observed that optogenetic inhibition of the Sp5C D1-expressing neurons in D1-Cre mice significantly attenuated the CCI-ION-induced trigeminal neuropathic pain, whereas optogenetic excitation of the Sp5C D1-expressing neurons significantly increased such pain in the CCI-ION model (Fig. 1C-E). The von Frey filament test showed that inhibiting the Sp5C D1-expressing neurons with light stimulation of inhibitory opsin NpHR robustly increased head withdrawal threshold, i.e., decreased pain; exciting the Sp5C D1-expressing neurons with light stimulation of excitatory opsin ChR2 significantly decreased the head withdrawal threshold, i.e., increased pain in the ipsilateral side of the CCI-ION (Fig. 1C). It should be noted that optogenetic manipulation had no effect on the pain thresholds in the contralateral side of the CCI-ION (Fig. 1D).
Figure 1. Optogenetic manipulation of DA receptors D1 and D2 in the Sp5C differentially regulates trigeminal neuropathic pain.
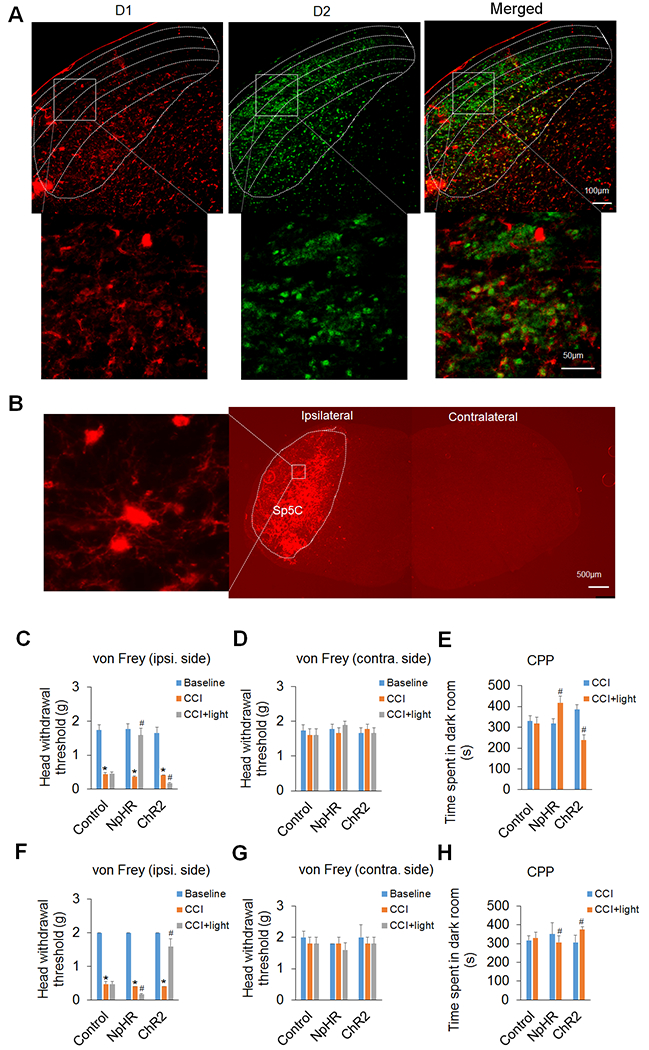
(A) Immunohistochemical staining showed that DA receptors D1 and D2 were expressed mostly in different neurons of the Sp5C. The upper panel displays the expression of D1 and/or D2 in the Sp5C at low magnification. Note that D1 was predominantly expressed in the deep laminae of the Sp5C and D2 was mostly expressed in the superficial laminae of the Sp5C. The lower panel displays the expression of D1 and/or D2 in the respective boxed areas of the upper panel at high magnification. (B) AAV5-mediated ChR2 expression in the Sp5C on day 14 after virus infusion into unilateral Sp5C represented that Cre-inducible viruses strongly expressed light-sensitive proteins on D1/2-expressing neurons in the ipsilateral Sp5C of D1-Cre or D2-Cre transgenic mice. The left image represents the boxed area in the ipsilateral Sp5C at high magnification. Note that there was no ChR2 expression in the contralateral Sp5C. (C–E) In D1-Cre mice, optogenetic inhibition of the Sp5C D1-expressing neurons significantly attenuated the CCI-ION-induced neuropathic pain on day 14 post-surgery and optogenetic excitation of the Sp5C D1-expressing neurons significantly increased such pain in the CCI-ION model. The von Frey filament test showed that inhibition of the Sp5C D1-expressing neurons with light stimulation of inhibitory opsin NpHR robustly increased head withdrawal threshold and excitation of the Sp5C D1-expressing neurons with light stimulation of excitatory opsin ChR2 significantly decreased the head withdrawal threshold in the ipsilateral side of the CCI-ION (C), though the optogenetic manipulation had no effect on the pain thresholds in the contralateral side of the CCI-ION (D). The CPP test showed that time spent in the dark room, which was paired with light stimulation, was significantly increased in the mice with the expression of NpHR in the Sp5C, but the time was markedly decreased in the mice with the expression of ChR2 in the Sp5C (E). Optogenetic stimulation did not significantly alter these behaviors in the D1-Cre mice with the expression of control virus in the Sp5C. (F–H) In D2-Cre mice, optogenetic inhibition of the Sp5C D2-expressing neurons significantly enhanced the CCI-ION-induced neuropathic pain on day 14 post-surgery and optogenetic excitation of the Sp5C D2-expressing neurons dramatically diminished such pain in both von Frey (F) and CPP (H) tests. Likewise, optogenetic stimulation had no effect on the pain thresholds in the contralateral side of the CCI-ION (G) and did not significantly alter these behaviors with the expression of control virus in the Sp5C. n = 6–7 mice per group. *P < 0.05 vs. the corresponding Baseline values; #P < 0.05 vs. the corresponding CCI group.
The CPP test in the D1-Cre mice showed that the time spent in the dark room (normally preferred location), which was paired with light stimulation, was significantly increased in the mice with the expression of NpHR in the Sp5C, whereas the time was markedly decreased in the mice with the ChR2 expression in the Sp5C (Fig. 1E). Optogenetic stimulation did not significantly alter these behaviors in the D1-Cre mice with the expression of control virus in the Sp5C (Fig. 1C-E).
Interestingly, we observed opposite responses to optogenetic manipulation in D2-Cre mice. Using both von Frey filaments and CPP tests on day 14 after CCI-ION, we showed that optogenetic inhibition of the Sp5C D2-expressing neurons significantly enhanced the CCI-ION-induced neuropathic pain and optogenetic excitation of the Sp5C D2-expressing neurons dramatically diminished such pain in the CCI-ION model (Fig. 1F and H). Likewise, optogenetic stimulation had no effect on the pain thresholds in the contralateral side of the CCI-ION (Fig. 1G) and did not significantly alter these behaviors in the D2-Cre mice with the expression of the control virus in the Sp5C (Fig. 1F-H).
3.2. Chemogenetic activation of DA receptors D1 and D2 in the Sp5C oppositely affects trigeminal neuropathic pain
To further verify the effects of manipulation of Sp5C D1/2-expressing neurons on neuropathic pain, we performed DREADDs-based chemogenetic activation in the Sp5C of D1-Cre and D2-Cre mice. With the unilateral infusion (ipsilateral side of CCI-ION) of a Cre-inducible excitatory DREADD virus (hM3D) or its control into the Sp5C of D1-Cre and D2-Cre mice, we found that chemogenetic activation of the Sp5C D1-expressing neurons with the injection of CNO significantly enhanced the CCI-ION-induced neuropathic pain on day 14 post-surgery, as measured by both decreased head withdrawal threshold in the von Frey filament test and decreased time spent in the paired dark room in the CPP test (Fig. 2A-C).
Figure 2. Chemogenetic activation of DA receptors D1 and D2 in the Sp5C oppositely affects trigeminal neuropathic pain.
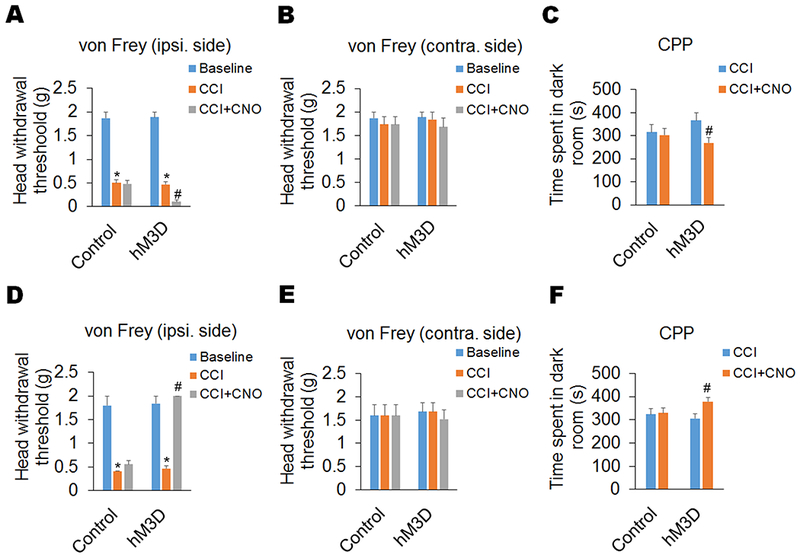
The Cre-inducible excitatory DREADD virus (hM3D) and its control were infused unilaterally (ipsilateral side of CCI-ION) into Sp5C of D1-Cre and D2-Cre mice. (A–C) In D1-Cre mice, chemogenetic activation of the Sp5C D1-expressing neurons with the injection of CNO (1 mg/kg, i.p) significantly enhanced the CCI-ION-induced neuropathic pain on day 14 post-surgery, including a decreased head withdrawal threshold in the ipsilateral side (A), but not contralateral side (B), in the von Frey filament test and decreased time spent in the paired dark room in the CPP test (C). (D–F) In D2-Cre mice, chemogenetic activation of the Sp5C D2-expressing neurons with the injection of CNO (1 mg/kg, i.p) significantly diminished neuropathic pain on day 14 post-surgery in the CCI-ION model, including an increased head withdrawal threshold in the ipsilateral side (D), but not contralateral side (E), in the von Frey filament test and increased time spent in the paired dark room in the CPP test (F). Chemogenetic activation did not significantly affect pain behaviors in the D1/2-Cre mice with the expression of control DREADD virus in the Sp5C. n = 6–7 mice per group. *P < 0.05 vs. the corresponding Baseline values; #P < 0.05 vs. the corresponding CCI group.
Oppositely, chemogenetic activation of Sp5C D2-expressing neurons with the injection of CNO robustly diminished the Trigeminal neuropathic pain on day 14 post-surgery in the CCI-ION model, including an increased head withdrawal threshold in the von Frey filament test and increased time spent in the paired dark room in the CPP test (Fig. 2D-F). In the contralateral side of CCI-ION, chemogenetic activation of Sp5C D1/2-expressing neurons had no effect on mouse behaviors in both tests (Fig. 2B and E). In addition, chemogenetic activation did not significantly affect pain behaviors in the D1/2-Cre mice with the expression of control DREADD virus in the Sp5C (Fig. 2A-F).
3.3. DA receptors D1 and D2 are expressed in different types of neurons in the Sp5C
To understand the mechanisms underlying the opposite effects of manipulating D1R and D2R in the Sp5C, we examined neuronal types in which the two DA receptors are expressed using immunofluorescence double staining. Glutaminase and GABA are neuronal markers for glutamatergic excitatory neurons and GABAergic inhibitory neurons, respectively30,51. We observed that in the Sp5C of D1-Cre mice, Cre-positive D1 neurons co-labeled with glutaminase (Fig. 3A, 71.92 ± 11.37% of total D1 neurons) were more than those co-labeled with GABA (Fig. 3B, 23.36 ± 5.58% of total D1 neurons), and that in the Sp5C of D2-Cre mice, Cre-positive D2 neurons were primarily co-labeled with GABA (Fig. 3C, 78.89 ± 4.41% of total D2 neurons) and only a few of those were co-labeled with glutaminase (Fig. 3D, 11.89 ± 4.07% of total D2 neurons). Our data indicate that most D1 receptors are expressed in excitatory neurons of the Sp5C but D2 receptors are predominantly expressed in inhibitory neurons of the Sp5C.
Figure 3. DA receptors D1 and D2 are expressed in different types of neurons in the Sp5C.
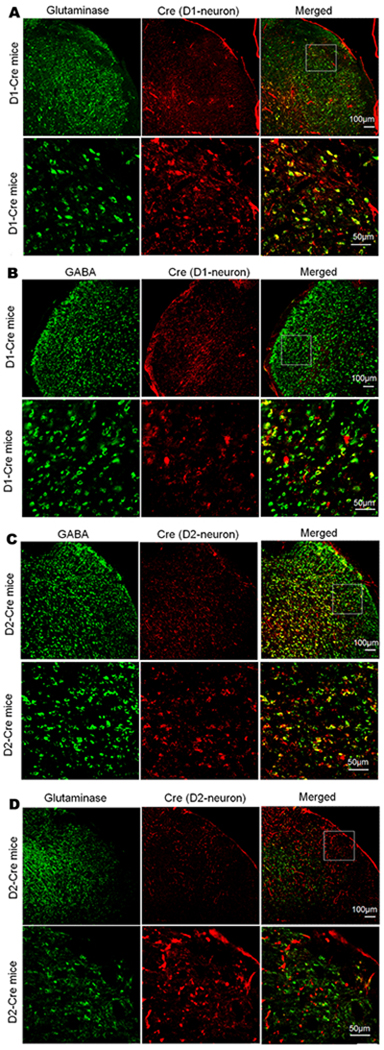
Immunofluorescence double staining was carried out to show co-localization of Cre with markers for different types of neurons in D1-Cre and D2-Cre mice. (A and B) In the Sp5C of D1-Cre mice, Cre-positive D1 neurons co-labeled with glutaminase (A) were more than those co-labeled with GABA (B). (C and D) In the Sp5C of D2-Cre mice, Cre-positive D2 neurons were primarily co-labeled with GABA (C) and only a few of those were co-labeled with glutaminase (D). In A–D, the upper panels display the expression of Cre and/or glutaminase/GABA in the Sp5C of D1-Cre mice (A and B) or D2-Cre mice (C and D) at low magnification. And the lower panels display the expression of Cre and/or glutaminase/GABA in the respective boxed areas of the upper panel at high magnification. The representative staining was repeated three times to confirm our results.
3.4. Specific excitation of dopaminergic neurons in the hypothalamic A11 attenuates trigeminal neuropathic pain via the activation of D2 receptor in the Sp5C
Our antegrade and retrograde tracing experiments showed that descending dopaminergic projection from hypothalamic A11 to Sp5C was bilateral. Following unilateral injections of the antegrade tracer DiI into the A11 and the retrograde tracer FG into the Sp5C, DiI and FG were expressed in both sides of Sp5C and A11, respectively (Fig. 4A and B). By double staining, we confirmed that the descending projection from hypothalamic A11 to Sp5C was dopaminergic. The retrograde tracer FG was co-expressed with TH, a specific marker for dopaminergic neurons, in both ipsilateral and contralateral sides of A11 nucleus (Fig. 4C and D). To explore the potential role of the descending dopaminergic projection in neuropathic pain, we used optogenetic stimulation in DAT-Cre mice after the excitatory virus AAV5-EF1α-DIO-ChR2 (E123A)-EYFP-WPRE and its control virus AAV5-EF1α-DIO-EYFP were injected into the A11 nucleus of the mice. We found that specific excitation of A11 dopaminergic neurons significantly increased head withdrawal thresholds in ipsilateral side (Fig. 4E), but not contralateral side (Fig. 4F), in the von Frey filament test and also increased the time spent in the paired dark room in the CPP test (Fig. 4G), suggesting that activation of A11-Sp5C descending dopaminergic pathway attenuates the CCI-ION-induced neuropathic pain.
Figure 4. Specific excitation of dopaminergic neurons in the hypothalamic A11 attenuates trigeminal neuropathic pain via the activation of D2 receptor in the Sp5C.
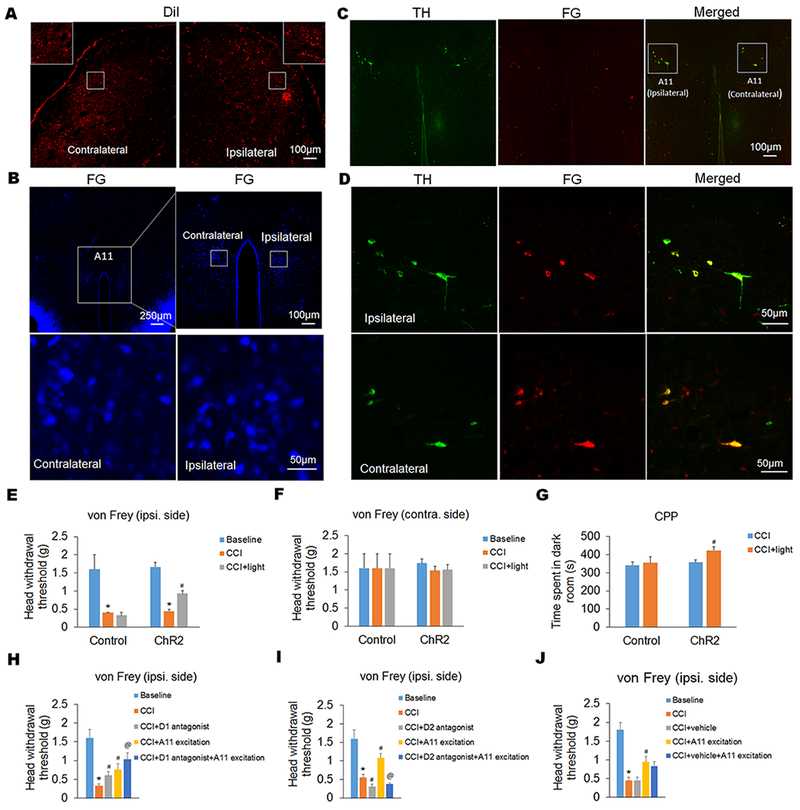
(A and B) By antegrade and retrograde tracing, we showed that descending projection from hypothalamic A11 to Sp5C was bilateral. Following unilateral injections of the antegrade tracer DiI into the A11 and the retrograde tracer FG into the Sp5C, DiI and FG were expressed in both sides of Sp5C (A) and A11 (B), respectively. The insets in (A) display higher magnification images of the respective boxed areas in the contralateral and ipsilateral Sp5C at low magnification. In the upper panel of (B), the right image represents the boxed A11 area in the left image at high magnification. And the lower panel of (B) displays the respective boxed areas in the contralateral and ipsilateral A11 of the upper right image at higher magnification. (C and D) By double staining, we showed that the descending projection from hypothalamic A11 to the Sp5C was dopaminergic (C). The retrograde tracer FG was co-expressed with TH, a specific marker for dopaminergic neurons, in both contralateral and ipsilateral sides of A11 nucleus (D). The expression of TH and/or FG in bilateral A11 was showed at low magnification in (C). And the expression of TH and/or FG in the ipsilateral and contralateral A11 within the respective boxed areas in (C) was showed at high magnification in (D). (E–G) By optogenetic stimulation of A11 dopaminergic neurons in DAT-Cre mice, we showed that specific excitation of A11 dopaminergic neurons significantly increased head withdrawal thresholds in ipsilateral side (E), but not contralateral side (F), in the von Frey filament test and also increased the time spent in the paired dark room in the CPP test (G). (H–J) By pharmacological intervention, we showed that intra-Sp5C injection of a D1 antagonist (SCH23390) inhibited the CCI-ION-induced neuropathic pain and enhanced the analgesic effect of A11 dopaminergic neuronal excitation (H); however, intra-Sp5C injection of a D2 antagonist (spiperone) exacerbated the CCI-ION-induced neuropathic pain and completely blocked the A11 dopaminergic neuronal excitation-produced analgesic effect in the CCI-ION model (I). As a control, intra-Sp5C injection of saline (vehicle) had no significant effect (J). n = 5–6 mice per group. *P < 0.05 vs. the corresponding Baseline values; #P < 0.05 vs. the corresponding CCI group; @P < 0.05 vs. the corresponding “CCI+A11 excitation” group.
Furthermore, we found that intra-Sp5C injection of D1 and D2 antagonists showed opposite effects on pain behaviors in the CCI-ION model (Fig. 4H and I). Notably, the administration of D1 antagonist synergistically enhanced the analgesic effect of A11 excitation (Fig. 4H), and the administration of D2 antagonist blocked the analgesic effect of A11 excitation (Fig. 4I), suggesting that Sp5C D2 (not D1) receptor activation mediates the effect of A11 dopaminergic neuron excitation on the CCI-ION-induced neuropathic pain. As a control, intra-Sp5C injection of vehicle had no effect on the A11 excitation-produced analgesic effect (Fig. 4J).
3.5. Specific ablation of the A11 dopaminergic neurons exacerbates trigeminal neuropathic pain
To further demonstrate the involvement of A11-Sp5C descending dopaminergic pathway in neuropathic pain, we conducted chemical lesion of the A11 dopaminergic neurons by injecting 6-OHDA unilaterally into the A11 nucleus. We also injected systemically desipramine (i.p.) to protect noradrenergic neurons27. Double staining of harvested A11-containing brain slices with antibodies against TH and the retrograde tracer FG (injected into Sp5C) showed that the chemical lesion specifically ablated A11 dopaminergic neurons, which projected to Sp5C (Fig. 5A and B). The chemical lesion-produced specific ablation of A11 dopaminergic neurons further reduced the CCI-decreased head withdrawal threshold in ipsilateral side of the mice, but the A11 lesion alone had no effect on the head withdrawal threshold (Fig. 5C and D).
Figure 5. Chemical lesion-produced specific ablation of A11 dopaminergic neurons exacerbates trigeminal neuropathic pain.
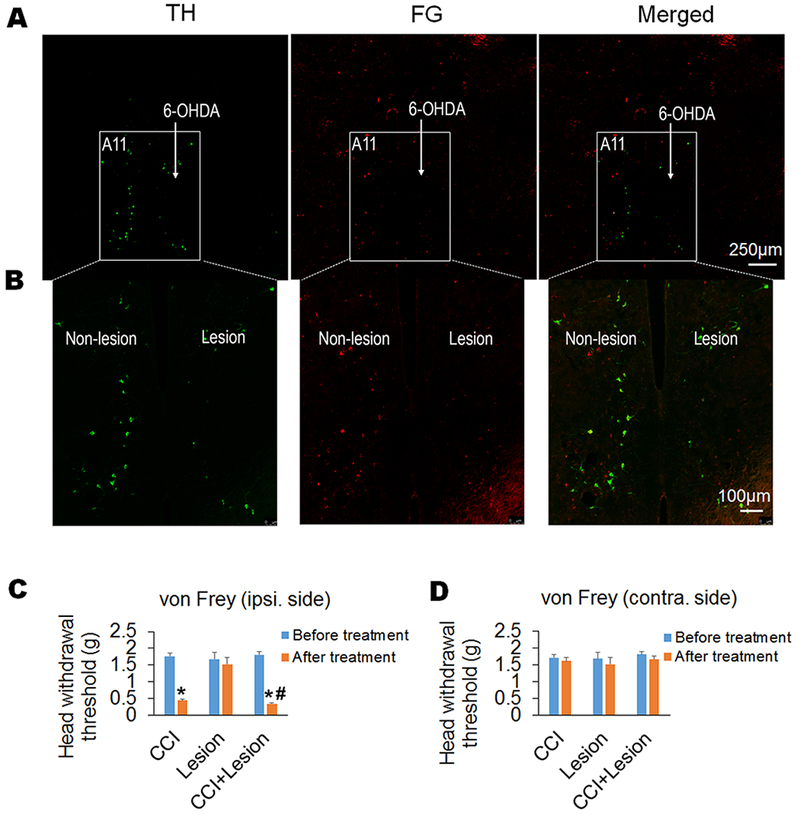
(A and B) Chemical lesion of A11 nucleus was conducted by injecting 6-OHDA into unilateral A11 nucleus of mice after systemic injection of desipramine (i.p.) to protect noradrenergic neurons. Double staining of harvested A11-containing brain slices with antibodies against TH and the retrograde tracer FG (injected into Sp5C) showed that the chemical lesion specifically ablated A11 dopaminergic neurons, which projected to Sp5C. The expression of TH and/or FG in bilateral A11 was showed at low magnification in (A). And the expression of TH and/or FG in the non-lesion and lesion sides of A11 within the respective boxed areas in (A) was showed at high magnification in (B). (C and D) The chemical lesion-produced specific ablation of A11 dopaminergic neurons further reduced CCI-decreased head withdrawal threshold in the ipsilateral side compared to the CCI-ION alone group (C), but the A11 lesion had no effect on the head withdrawal threshold in the contralateral side of the mice with CCI-ION (D). As a control, the unilateral A11 lesion alone did not affect the head withdrawal thresholds in both ipsilateral and contralateral sides of the mice without CCI (C and D). n = 7–10 mice per group. *P < 0.05 vs. the corresponding Baseline values; #P < 0.05 vs. the CCI alone group.
3.6. Both optogenetic and chemogenetic manipulation do not affect locomotor function of mice
To exclude the effect of optogenetic/chemogenetic manipulation of A11 dopaminergic neurons or Sp5C D1/2-expressing neurons on locomotor function of mice, we tested mouse movement using a Rotarod apparatus after optogenetic stimulation or chemogenetic activation. We observed that optogenetic manipulation (both NpHR-mediated neuronal inhibition and ChR2-mediated neuronal excitation) of Sp5C D1-expressing neurons in D1-Cre mice had no significant effects on falling speed and time to fall in the Rotarod testing (Fig. 6A and B). We also observed that chemogenetic activation of Sp5C D1-expressing neurons in D1-Cre mice had no significant effects on falling speed and time to fall in the Rotarod testing (Fig. 6C and D). In addition, optogenetic/chemogenetic manipulation of A11 dopaminergic neurons in DAT-Cre mice or Sp5C D2-expressing neurons in D2-Cre mice had also no significant effects on locomotor function of the mice (data not shown).
Figure 6. Both optogenetic and chemogenetic manipulation do not affect locomotor function of mice.
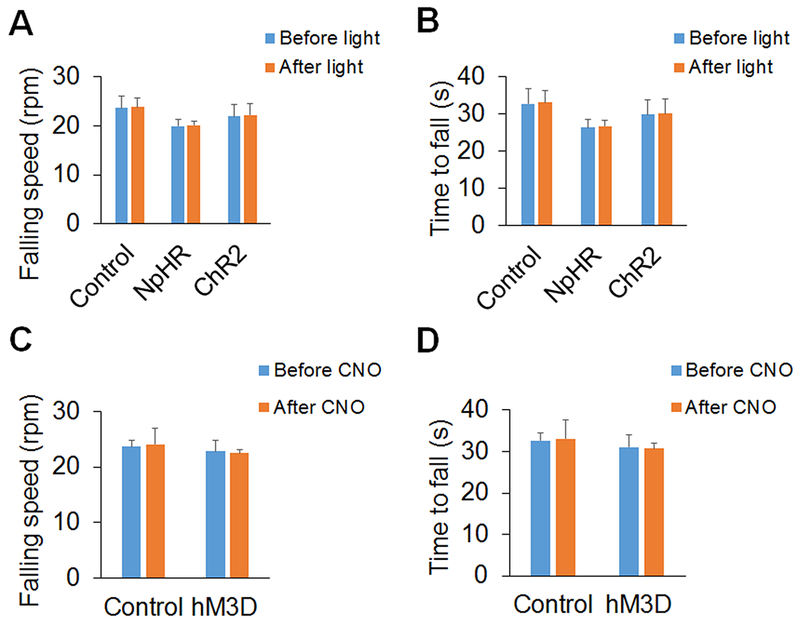
(A and B) Optogenetic manipulation (both NpHR-mediated neuronal inhibition and ChR2-mediated neuronal excitation) of Sp5C D1-expressing neurons in D1-Cre mice had no significant effects on falling speed (A) and time to fall (B) in the Rotarod testing. (C and D) Chemogenetic activation of Sp5C D1-expressing neurons in D1-Cre mice had no significant effects on falling speed (C) and time to fall (D) in the Rotarod testing. n = 6 mice per group.
4. Discussion
In the present study, we reveal that 1) specific manipulation of DA receptors D1 and D2 in the Sp5C oppositely regulates nerve injury-induced trigeminal neuropathic pain; 2) specific excitation of dopaminergic neurons in the hypothalamic A11 attenuates the trigeminal neuropathic pain via the activation of D2 receptors in the Sp5C; 3) specific ablation of the A11 dopaminergic neurons exacerbates such pain. Taken together, our results suggest that the descending dopaminergic projection from hypothalamic A11 nucleus to the Sp5C can control trigeminal neuropathic pain.
Previous studies have demonstrated that descending dopaminergic pathways from hypothalamic A11 nucleus contribute to pain regulation.2,27,32,37,55 The A11-originated descending pain modulation pathway plays an important role in spinal dorsal horn neurons-mediated pathological pain plasticity,27 and also regulates formalin-induced trigeminal pain behaviors.2 However, it is unknown whether the A11 descending dopaminergic pathways contribute to neuropathic pain. Using antegrade and retrograde tracing, we observed that the A11 dopaminergic neurons project bilaterally to Sp5C in mice, which is consistent with a recent report in rats.1 As an important neurotransmitter in the top-down pain modulation system, DA exerts its function by binding to two types of DA receptors named D1-like and D2-like receptors.5 And these receptors are expressed in trigeminal nucleus and maybe exert different modulation on pain.2,8,27,39,55 By immunohistochemistry, we showed that DA receptors D1 and D2 in the Sp5C are mostly expressed in different neurons. Our optogenetic and chemogenetic manipulation experiments further revealed that D1- and D2-expressing neurons in the Sp5C mediate opposite effects on neuropathic pain in the CCI-ION model. Previous studies have shown that the activation of D1-like receptors produces excitatory effects on both sensory and motor neurons,20,50 whereas the activation of D2-like receptors produces inhibitory actions on synaptic transmission of spinal and trigeminal neurons.8,9,14,19,21,52 Thus, we reasoned that exciting D1- and D2-expressing neurons may cause a pain increase and decrease, respectively. Our optogenetic/chemogenetic manipulation data exactly prove this assumption. We also showed that D1 and D2 receptors in the Sp5C display different neuronal localization: most D1 receptors are expressed in excitatory neurons of the Sp5C but D2 receptors are predominantly expressed in inhibitory neurons of the Sp5C. The difference of expression patterns for Sp5C D1 and D2 receptors could be a possible mechanism underlying their distinct effects.
It has been reported that D2-like receptors have higher affinities and are activated by lower concentrations of DA than D1-like receptors.43 Therefore, it is conceivable that the effect of activation of descending dopaminergic pathway from A11 nucleus to Sp5C on pain transmission may be dependent on local DA concentration: low levels of DA will activate D2-like receptors to inhibit pain; high levels of DA will activate D1-like receptors to enhance pain. This receptor binding priority could be shifted toward a preferential activation of D2-like receptors while even higher levels of DA are produced,13,43 such as stimulation of A11 dopaminergic neurons. Our experiments using DAT-Cre mice provide essential evidence to support this hypothesis. We found that optogenetic excitation of A11 dopaminergic neurons markedly attenuates the CCI-ION-induced neuropathic pain and this effect is completely inhibited by intra-Sp5C injection of a D2 receptor antagonist.
Our results suggest that the specific activation of A11 dopaminergic neurons by optogenetic stimulation may release plenty of DA into the Sp5C, and the increased synaptic concentration of DA diminishes the neuropathic pain upon binding to D2 receptors in the Sp5C. Previous studies have also shown that local electrical and chemical excitation of the A11 dopaminergic neurons reduces noxious stimulation-produced responses in both spinal dorsal horn and Sp5C via a D2 receptor-mediated pathway.11,17,53 Therefore, specific excitation of the A11 dopaminergic neurons followed by Sp5C D2 receptor activation could be developed as a new approach for the treatment of trigeminal neuropathic pain. A previous study reported strong D2 receptor staining in fibers of laminae I/IIo of trigeminocervical complex.8 Moreover, activation of D2 receptors in the superficial medullary dorsal horn might specifically contribute to descending dopaminergic pathway-mediated tonic inhibition of trigeminal nociceptive inputs.32 Therefore, presynaptic D2 receptors likely play an important role in the anti-nociceptive effect produced by excitation of A11 dopaminergic projecting neurons, which has been suggested by the pioneering study of Fleetwood-Walker et al.17 using electrical stimulation of the A11.
We also employed chemical lesion of dopaminergic neurons to observe the effect of the reduction of A11 dopaminergic neurons on the CCI-ION-induced neuropathic pain. We found that specific ablation of dopaminergic neurons in the hypothalamic A11 exacerbates trigeminal neuropathic pain. A previous study using the facial formalin pain model in rats reported that DA denervation of the A11 nucleus induces trigeminal analgesia.2 And the number of TH-immunoreactive neurons in the A11 is reduced by approximately 40% in that study.2 Besides the differences in animals and pain models, the disparity between our investigation and the above-mentioned study may also relate to the degree of A11 lesion, because 6-OHDA-produced chemical lesion in our experiments caused a much greater decrease of DA, i.e., approximately 70% reduction of dopaminergic neurons in the ipsilateral A11 nucleus. This explanation is supported by previous electrophysiological studies10,11 using electrical lesion, which almost fully removed dopaminergic neurons in the A11 nucleus. They found that the electrical lesion facilitates the responses of trigeminocervical neurons to both noxious and innocuous mechanical stimuli.10,11
In the CPP test, we measured pain facilitation or inhibition produced by optogenetic/chemogenetic manipulations based on the time in the paired dark room. This method has been used in previous studies4,33,35,47,56 to assess pain in acute and chronic pain models. We observed that mice spent more time in the dark room when they were paired with optogenetic/chemogenetic inhibition of pain; oppositely, mice spent less time in the dark room when they were paired with optogenetic/chemogenetic facilitation of pain. Although the dark room is the normally preferred location of mice, the mice still spend their time in different rooms during the CPP test. Increased time in the dark room indicates pain inhibition produced by optogenetic/chemogenetic manipulations, because mice prefer to stay in the room where they experience less pain. However, we do not know whether such inhibition can produce pleasure. It will be interesting to investigate the relationship between pain inhibition and pleasure induction when we analyze pain behaviors with the CPP test.
Although the majority of neurons in the A11 nucleus are dopaminergic, some neurons containing calcitonin gene-related peptide (CGRP) and GABA are also included in this area with different densities and distributions.2,11,41,42,45,49 It has been suggested that GABAergic neurons in the A11 might contribute to the regulation of dopaminergic neurons.1 Facial noxious stimulation can activate A11 GABAergic neurons which then inhibits dopaminergic neurons in the A11 nucleus through local GABA circuits.2 In addition, TH- and CGRP-dually immunoreactive neurons are distributed throughout the entire rostrocaudal A11 nucleus.11,41 Thus, A11 is a heterogenous nucleus including DA-, GABA-, and CGRP-containing neurons. However, how GABA- and CGRP-containing neurons in the A11 nucleus are involved in the descending dopaminergic pathway to control neuropathic pain is still unclear. Unravelling the interactions of DA-, GABA-, and CGRP-containing neurons in the A11 nucleus will provide more information about the top-down control of pain transmission. Nevertheless, while other neurotransmitters may play a role in trigeminal neuropathic pain, our present study provides strong evidence to show that the descending dopaminergic pathway from A11 to Sp5C contributes to trigeminal neuropathic pain. Our results suggest that this descending dopaminergic pathway could be manipulated to treat such pain.
In conclusion, we revealed in this study that the descending dopaminergic pathway from A11 to Sp5C is critical for controlling trigeminal neuropathic pain. DA receptors D1 and D2 in the Sp5C differentially contribute to the modulation of the trigeminal neuropathic pain. Specific excitation of the A11 dopaminergic neurons attenuates the trigeminal neuropathic pain via a D2 receptor-mediated signaling in the Sp5C, which could be developed as a new therapy for such pain.
Supplementary Material
Figure S1. Localization of microinjection within the Sp5C. (A) 1 μl of methylene blue (0.05%, w/v) was injected into one side of Sp5C. We observed that the dye was displayed in the ipsilateral Sp5C and the average diameter of diffusing area (circled area in the Sp5C) for the microinjection is 1.23 ± 0.11 mm within the ipsilateral Sp5C. (B) The coronal section showing Sp5C in the mouse brain atlas at Bregma −8.00 mm. The slide with dye diffusion in (A) displays a similar section with the one in (B).
Figure S2. The specificity of the Cre antibody. (A) We harvested Sp5C-containing brainstem tissues from WT mice and did immunostaining with the Cre antibody. And we did not observed any positive signals. (B) Using Western blotting, we found that the Cre antibody specifically detected Cre in the brainstem tissues of D1-Cre mice and only one single band was shown on the blot. These results indicate that the Cre antibody we used is specific.
Figure S3. The specificity of the Glutaminase antibody. We harvested Sp5C-containing brainstem tissues from D1-Cre mice and did immunostaining and Western blotting. The Glutaminase antibody was pre-incubated with its blocking peptide (0.5 mg/ml, dissolved in PBS) or PBS (as a control) overnight at 4°C. (A) Pre-incubation of the Glutaminase antibody with PBS had no effect on the signals in the double immunostaining with Glutaminase and Cre antibodies. (B) Pre-incubation of the Glutaminase antibody with the blocking peptide almost completely blocked the signals detected by the Glutaminase antibody, but did not affect the signals detected by the Cre antibody. (C) Using Western blotting, we found that the Glutaminase antibody specifically detected Glutaminase in the brainstem tissues of D1-Cre mice and only one single band was shown on the blot. And we further showed that pre-incubation of the Glutaminase antibody with the blocking peptide almost completely blocked the band on the blot. The blocking peptide-mediated specific blocking indicates that the Glutaminase antibody we used is specific.
Figure S4. The specificity of the GABA antibody. We harvested Sp5C-containing brainstem tissues from D2-Cre mice and did immunostaining. The GABA antibody was pre-incubated with its immunogen GABA-BSA (40μM, dissolved in PBS) as a blocking agent or PBS (as a control) overnight at 4°C. (A) Pre-incubation of the GABA antibody with PBS had no effect on the signals in the double immunostaining with GABA and Cre antibodies. (B) Pre-incubation of the GABA antibody with the blocking agent GABA-BSA completely blocked the signals detected by the GABA antibody, but did not affect the signals detected by the Cre antibody. The blocking agent GABA-BSA-mediated specific blocking indicates that the GABA antibody we used is specific.
Acknowledgments:
This work was supported by National Institutes of Health Grants R01 DE022880 (F.T.) and K02 DE023551 (F.T.). The authors thank Jeanne Santa Cruz (Texas A&M University College of Dentistry) for her excellent scientific editing.
Footnotes
Conflict of interest statement: The authors have no conflict of interest to declare.
References
- [1].Abdallah K, Artola A, Monconduit L, Dallel R, Luccarini P. Bilateral descending hypothalamic projections to the spinal trigeminal nucleus caudalis in rats. PLoS One 2013;8:e73022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abdallah K, Monconduit L, Artola A, Luccarini P, Dallel R. GABAAergic inhibition or dopamine denervation of the A11 hypothalamic nucleus induces trigeminal analgesia. Pain 2015;156:644–55. [DOI] [PubMed] [Google Scholar]
- [3].Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 1984;7:309–38. [DOI] [PubMed] [Google Scholar]
- [4].Beaudry H, Daou I, Ase AR, Ribeiro-da-Silva A, Seguela P. Distinct behavioral responses evoked by selective optogenetic stimulation of the major TRPV1+ and MrgD+ subsets of C-fibers. Pain 2017;158:2329–39. [DOI] [PubMed] [Google Scholar]
- [5].Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 2011;63:182–217. [DOI] [PubMed] [Google Scholar]
- [6].Benarroch EE. Descending monoaminergic pain modulation: bidirectional control and clinical relevance. Neurology 2008;71:217–21. [DOI] [PubMed] [Google Scholar]
- [7].Benoist JM, Gautron M, Guilbaud G. Experimental model of trigeminal pain in the rat by constriction of one infraorbital nerve: changes in neuronal activities in the somatosensory cortices corresponding to the infraorbital nerve. Exp Brain Res 1999;126:383–98. [DOI] [PubMed] [Google Scholar]
- [8].Bergerot A, Storer RJ, Goadsby PJ. Dopamine inhibits trigeminovascular transmission in the rat. Ann Neurol 2007;61:251–62. [DOI] [PubMed] [Google Scholar]
- [9].Carp JS, Anderson RJ. Dopamine receptor-mediated depression of spinal monosynaptic transmission. Brain Res 1982;242:247–54. [DOI] [PubMed] [Google Scholar]
- [10].Charbit AR, Akerman S, Goadsby PJ. Trigeminocervical complex responses after lesioning dopaminergic A11 nucleus are modified by dopamine and serotonin mechanisms. Pain 2011;152:2365–76. [DOI] [PubMed] [Google Scholar]
- [11].Charbit AR, Akerman S, Holland PR, Goadsby PJ. Neurons of the dopaminergic/calcitonin gene-related peptide A11 cell group modulate neuronal firing in the trigeminocervical complex: an electrophysiological and immunohistochemical study. J Neurosci 2009;29:12532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen JF, Qin ZH, Szele F, Bai G, Weiss B. Neuronal localization and modulation of the D2 dopamine receptor mRNA in brain of normal mice and mice lesioned with 6-hydroxydopamine. Neuropharmacology 1991;30:927–41. [DOI] [PubMed] [Google Scholar]
- [13].Clemens S, Belin-Rauscent A, Simmers J, Combes D. Opposing modulatory effects of D1- and D2-like receptor activation on a spinal central pattern generator. J Neurophysiol 2012;107:2250–9. [DOI] [PubMed] [Google Scholar]
- [14].Clemens S, Hochman S. Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J Neurosci 2004;24:11337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dias EV, Sartori CR, Mariao PR, Vieira AS, Camargo LC, Athie MC, Pagliusi MO, Tambeli CH, Parada CA. Nucleus accumbens dopaminergic neurotransmission switches its modulatory action in chronification of inflammatory hyperalgesia. Eur J Neurosci 2015;42:2380–9. [DOI] [PubMed] [Google Scholar]
- [16].Dieb W, Ouachikh O, Durif F, Hafidi A. Nigrostriatal dopaminergic depletion produces orofacial static mechanical allodynia. Eur J Pain 2016;20:196–205. [DOI] [PubMed] [Google Scholar]
- [17].Fleetwood-Walker SM, Hope PJ, Mitchell R. Antinociceptive actions of descending dopaminergic tracts on cat and rat dorsal horn somatosensory neurones. J Physiol 1988;399:335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iniguez SD, O’Donnell P, Kravitz A, Lobo MK. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry 2015;77:212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gajendiran M, Seth P, Ganguly DK. Involvement of the presynaptic dopamine D2 receptor in the depression of spinal reflex by apomorphine. Neuroreport 1996;7:513–6. [DOI] [PubMed] [Google Scholar]
- [20].Gallagher JP, Inokuchi H, Shinnick-Gallagher P. Dopamine depolarisation of mammalian primary afferent neurones. Nature 1980;283:770–2. [DOI] [PubMed] [Google Scholar]
- [21].Garraway SM, Hochman S. Modulatory actions of serotonin, norepinephrine, dopamine, and acetylcholine in spinal cord deep dorsal horn neurons. J Neurophysiol 2001;86:2183–94. [DOI] [PubMed] [Google Scholar]
- [22].Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev 2004;27:729–37. [DOI] [PubMed] [Google Scholar]
- [23].Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 2013;80:1368–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci 2007;27:9817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 2003;425:917–25. [DOI] [PubMed] [Google Scholar]
- [26].Kernisant M, Gear RW, Jasmin L, Vit JP, Ohara PT. Chronic constriction injury of the infraorbital nerve in the rat using modified syringe needle. J Neurosci Methods 2008;172:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim JY, Tillu DV, Quinn TL, Mejia GL, Shy A, Asiedu MN, Murad E, Schumann AP, Totsch SK, Sorge RE, Mantyh PW, Dussor G, Price TJ. Spinal dopaminergic projections control the transition to pathological pain plasticity via a D1/D5-mediated mechanism. J Neurosci 2015;35:6307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim YS, Chu Y, Han L, Li M, Li Z, Lavinka PC, Sun S, Tang Z, Park K, Caterina MJ, Ren K, Dubner R, Wei F, Dong X. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 2014;81:873–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Koblinger K, Fuzesi T, Ejdrygiewicz J, Krajacic A, Bains JS, Whelan PJ. Characterization of A11 neurons projecting to the spinal cord of mice. PLoS One 2014;9:e109636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koizumi H, Mosher B, Tariq MF, Zhang R, Koshiya N, Smith JC. Voltage-Dependent Rhythmogenic Property of Respiratory Pre-Botzinger Complex Glutamatergic, Dbx1-Derived, and Somatostatin-Expressing Neuron Populations Revealed by Graded Optogenetic Inhibition. eNeuro 2016;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kusumoto-Yoshida I, Liu H, Chen BT, Fontanini A, Bonci A. Central role for the insular cortex in mediating conditioned responses to anticipatory cues. Proc Natl Acad Sci U S A 2015;112:1190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lapirot O, Melin C, Modolo A, Nicolas C, Messaoudi Y, Monconduit L, Artola A, Luccarini P, Dallel R. Tonic and phasic descending dopaminergic controls of nociceptive transmission in the medullary dorsal horn. Pain 2011;152:1821–31. [DOI] [PubMed] [Google Scholar]
- [33].Lee M, Manders TR, Eberle SE, Su C, D’Amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, Wang J. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci 2015;35:5247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 2010;330:385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Martinez E, Lin HH, Zhou H, Dale J, Liu K, Wang J. Corticostriatal Regulation of Acute Pain. Front Cell Neurosci 2017;11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mason P Deconstructing endogenous pain modulations. J Neurophysiol 2005;94:1659–63. [DOI] [PubMed] [Google Scholar]
- [37].Megat S, Shiers S, Moy JK, Barragan-Iglesias P, Pradhan G, Seal RP, Dussor G, Price TJ. A Critical Role for Dopamine D5 Receptors in Pain Chronicity in Male Mice. J Neurosci 2018;38:379–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Millan MJ. Descending control of pain. Prog Neurobiol 2002;66:355–474. [DOI] [PubMed] [Google Scholar]
- [39].Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 1998;78:189–225. [DOI] [PubMed] [Google Scholar]
- [40].Okubo M, Castro A, Guo W, Zou S, Ren K, Wei F, Keller A, Dubner R. Transition to persistent orofacial pain after nerve injury involves supraspinal serotonin mechanisms. J Neurosci 2013;33:5152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Orazzo C, Pieribone VA, Ceccatelli S, Terenius L, Hokfelt T. CGRP-like immunoreactivity in A11 dopamine neurons projecting to the spinal cord and a note on CGRP-CCK cross-reactivity. Brain Res 1993;600:39–48. [DOI] [PubMed] [Google Scholar]
- [42].Pappas SS, Tiernan CT, Behrouz B, Jordan CL, Breedlove SM, Goudreau JL, Lookingland KJ. Neonatal androgen-dependent sex differences in lumbar spinal cord dopamine concentrations and the number of A11 diencephalospinal dopamine neurons. J Comp Neurol 2010;518:2423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Paulus W, Trenkwalder C. Less is more: pathophysiology of dopaminergic-therapy-related augmentation in restless legs syndrome. Lancet Neurol 2006;5:878–86. [DOI] [PubMed] [Google Scholar]
- [44].Paxinos G FK. The mouse brain in stereotaxic coordinates. London: Academic Press, 2003. [Google Scholar]
- [45].Qu S, Ondo WG, Zhang X, Xie WJ, Pan TH, Le WD. Projections of diencephalic dopamine neurons into the spinal cord in mice. Exp Brain Res 2006;168:152–6. [DOI] [PubMed] [Google Scholar]
- [46].Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain 2002;100:1–6. [DOI] [PubMed] [Google Scholar]
- [47].Rodriguez E, Sakurai K, Xu J, Chen Y, Toda K, Zhao S, Han BX, Ryu D, Yin H, Liedtke W, Wang F. A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat Neurosci 2017;20:1734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Romero-Reyes M, Akerman S, Nguyen E, Vijjeswarapu A, Hom B, Dong HW, Charles AC. Spontaneous behavioral responses in the orofacial region: a model of trigeminal pain in mouse. Headache 2013;53:137–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Skagerberg G, Lindvall O. Organization of diencephalic dopamine neurones projecting to the spinal cord in the rat. Brain Res 1985;342:340–51. [DOI] [PubMed] [Google Scholar]
- [50].Smith DO, Lowe D, Temkin R, Jensen P, Hatt H. Dopamine enhances glutamate-activated currents in spinal motoneurons. J Neurosci 1995;15:3905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Takazawa T, Choudhury P, Tong CK, Conway CM, Scherrer G, Flood PD, Mukai J, MacDermott AB. Inhibition Mediated by Glycinergic and GABAergic Receptors on Excitatory Neurons in Mouse Superficial Dorsal Horn Is Location-Specific but Modified by Inflammation. J Neurosci 2017;37:2336–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tamae A, Nakatsuka T, Koga K, Kato G, Furue H, Katafuchi T, Yoshimura M. Direct inhibition of substantia gelatinosa neurones in the rat spinal cord by activation of dopamine D2-like receptors. J Physiol 2005;568:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Taniguchi W, Nakatsuka T, Miyazaki N, Yamada H, Takeda D, Fujita T, Kumamoto E, Yoshida M. In vivo patch-clamp analysis of dopaminergic antinociceptive actions on substantia gelatinosa neurons in the spinal cord. Pain 2011;152:95–105. [DOI] [PubMed] [Google Scholar]
- [54].Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci 2008;28:10482–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wei H, Viisanen H, Pertovaara A. Descending modulation of neuropathic hypersensitivity by dopamine D2 receptors in or adjacent to the hypothalamic A11 cell group. Pharmacol Res 2009;59:355–63. [DOI] [PubMed] [Google Scholar]
- [56].Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, Zamponi GW. Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell Rep 2015;12:752–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Localization of microinjection within the Sp5C. (A) 1 μl of methylene blue (0.05%, w/v) was injected into one side of Sp5C. We observed that the dye was displayed in the ipsilateral Sp5C and the average diameter of diffusing area (circled area in the Sp5C) for the microinjection is 1.23 ± 0.11 mm within the ipsilateral Sp5C. (B) The coronal section showing Sp5C in the mouse brain atlas at Bregma −8.00 mm. The slide with dye diffusion in (A) displays a similar section with the one in (B).
Figure S2. The specificity of the Cre antibody. (A) We harvested Sp5C-containing brainstem tissues from WT mice and did immunostaining with the Cre antibody. And we did not observed any positive signals. (B) Using Western blotting, we found that the Cre antibody specifically detected Cre in the brainstem tissues of D1-Cre mice and only one single band was shown on the blot. These results indicate that the Cre antibody we used is specific.
Figure S3. The specificity of the Glutaminase antibody. We harvested Sp5C-containing brainstem tissues from D1-Cre mice and did immunostaining and Western blotting. The Glutaminase antibody was pre-incubated with its blocking peptide (0.5 mg/ml, dissolved in PBS) or PBS (as a control) overnight at 4°C. (A) Pre-incubation of the Glutaminase antibody with PBS had no effect on the signals in the double immunostaining with Glutaminase and Cre antibodies. (B) Pre-incubation of the Glutaminase antibody with the blocking peptide almost completely blocked the signals detected by the Glutaminase antibody, but did not affect the signals detected by the Cre antibody. (C) Using Western blotting, we found that the Glutaminase antibody specifically detected Glutaminase in the brainstem tissues of D1-Cre mice and only one single band was shown on the blot. And we further showed that pre-incubation of the Glutaminase antibody with the blocking peptide almost completely blocked the band on the blot. The blocking peptide-mediated specific blocking indicates that the Glutaminase antibody we used is specific.
Figure S4. The specificity of the GABA antibody. We harvested Sp5C-containing brainstem tissues from D2-Cre mice and did immunostaining. The GABA antibody was pre-incubated with its immunogen GABA-BSA (40μM, dissolved in PBS) as a blocking agent or PBS (as a control) overnight at 4°C. (A) Pre-incubation of the GABA antibody with PBS had no effect on the signals in the double immunostaining with GABA and Cre antibodies. (B) Pre-incubation of the GABA antibody with the blocking agent GABA-BSA completely blocked the signals detected by the GABA antibody, but did not affect the signals detected by the Cre antibody. The blocking agent GABA-BSA-mediated specific blocking indicates that the GABA antibody we used is specific.


