Abstract
Exserohilum includes a number of plant pathogenic, saprobic and clinically relevant fungi. Some of these species are of great importance in human activities, but the genus has never been revised in a phylogenetic framework. In this study, we revise Exserohilum based on available ex-type cultures from worldwide collections, observation of the holotypes and/or protologues, and additional isolates from diverse substrates and geographical origins. Based on nine nuclear loci, i.e., ITS, LSU, act, tub2, cam, gapdh, his, tef1 and rpb2, as well as phenotypic data, the genus and species boundaries are assessed for Exserohilum. Three species, i.e., E. novae-zelandiae, E. paspali and E. sorghicola, are excluded from the genus and reallocated in Sporidesmiella and Curvularia, respectively, whereas E. heteropogonicola and E. inaequale are confirmed as members of Curvularia. Exserohilum rostratum is revealed as conspecific with species previously described in Exserohilum such as E. antillanum, E. gedarefense, E. leptochloae, E. longirostratum, E. macginnisii and E. prolatum. Additionally, E. curvatum is revealed as synonym of E. holmii, and E. fusiforme of E. oryzicola. A total of 11 Exserohilum phylogenetic species are described, illustrated and discussed, including one novel taxon, E. corniculatum. The placements of 15 other doubtful species are discussed, and E. elongatum is validated.
Keywords: Curvularia, Helminthosporium, human and plant pathogen, new species, Setosphaeria, systematics
INTRODUCTION
The genus Helminthosporium was erected by Link (1809). The type species, H. velutinum, is a saprobic dematiaceous fungus occurring on dead stems of Alnus, Cornus, Salix and numerous other plants (Voglmayr & Jaklitsch 2017). It has macronematous, rather straight conidiophores arising from stromata. Conidia are elongate, distoseptate, brown and are produced laterally from small, inconspicuous pores while the conidiophore is elongating. The production of terminal conidia usually determines the end of conidiophore growth (Luttrell 1963b, Hughes 1978, Alcorn 1988a). Originally, Helminthosporium was defined very vaguely and over the years it became a repository for numerous taxa of which only a few were congeneric with the type species. MycoBank (http://www.mycobank.org/, consulted in September 2017) lists over 760 names in Helminthosporium, but currently less than 50 are considered to represent true Helminthosporium species (Seifert et al. 2011, Tanaka et al. 2015, Voglmayr & Jaklitsch 2017). Refinements in the taxonomy of this genus resulted in the reallocation of many of its members to genera such as Alternaria, Corynespora, Dendryphion, Septonema and others (Bolle 1924, Wei 1950, Subramanian & Jain 1966, Ellis 1971, 1976, Simmons 1971, 2007, Sivanesan 1984, Seifert et al. 2011, Voglmayr & Jaklitsch 2017). Several grass parasites originally described in Helminthosporium differed from the type species in producing conidia from sympodial, often strongly geniculate conidiophores (Drechsler 1923). The conidiogenous cells of these fungi had pores which were surrounded by dark scars, in contrast to the inconspicuous small pores of H. velutinum (Alcorn 1988a). Nisikado ( 1928) classified the graminicolous Helminthosporium species into two subgenera, Cylindro-Helminthosporium and Eu-Helminthosporium. Cylindro-Helminthosporium included species with straight, cylindrical conidia that germinate from any cell, which later were accommodated in the segregate genus Drechslera by Ito ( 1930) and were often associated with the dictyosporous sexual morph Pyrenophora (Shoemaker 1961, Paul & Parbery 1968, Paul 1972). Eu-Helminthosporium grouped species with fusiform, often curved conidia which germinate from end cells. Later, Shoemaker ( 1959) erected Bipolaris for taxa previously accommodated in Eu-Helminthosporium. Bipolaris was a heterogeneous entity which included two subgroups based on differences of the hilum morphology and associated with two different sexual morphs. The group that has conidia with non- or slightly protruding hila was often associated with the sexual morph Cochliobolus, characterised by filiform ascospores that often appear more or less coiled in a helix within the ascus (Drechsler 1934, Nelson 1964, Alcorn 1983, 1996, Manamgoda et al. 2011). On the other hand, the group that has conidia with a protruding hilum had sexual morphs with fusoid ascospores enveloped in gelatinous sheaths, which was originally described in Trichometasphaeria (Luttrell 1958, 1963a, Nelson 1965). Later Leonard & Suggs ( 1974) erected Exserohilum to accommodate those Bipolaris s.lat. species with a distinctly protruding hilum, and Setosphaeria for the sexual morph. Setosphaeria differs from Trichometasphaeria by the production of non-clypeate ascomata which can be erumpent or superficial and produce larger ascospores (Leonard & Suggs 1974). Recently, Rossman et al. ( 2015) recommend to use the name Exserohilum over Setosphaeria according to Article 57.2 of the International Code of Nomenclature for algae, fungi and plants (McNeill et al. 2012). MycoBank currently lists 38 taxa in Exserohilum, most of which are associated with diseases of grasses (Sivanesan 1984, 1987), although a few have been described from other substrates such as river sediments (Sivanesan et al. 1993), soil (Guiraud et al. 1997, Steiman et al. 2000), grains (El Shafie 1980), the palm tree Borassus flabellifer (Subramanian 1956), plant debris (Castañeda-Ruiz et al. 1995), and humans (McGinnis et al. 1986, Padhye et al. 1986). Members of this genus are distinguished mainly on the basis of morphological features such as conidial shape and size, number of distosepta and the presence or absence of thick, dark distosepta (McGinnis et al. 1986, Sivanesan 1987). So far, eight Setosphaeria species have been described, most of which were obtained by mating of compatible isolates (Luttrell 1958, 1963a, Leonard & Suggs 1974, Leonard 1976, Alcorn 1978). However, homothallism has also been described in this genus (El Shafie & Webster 1981, Alcorn 1986). Successful mating is achieved by inoculating compatible strains onto culture media with sterilized fragments of natural substrates such as barley grains, maize leaf or wheat straw (Leonard & Suggs 1974, Alcorn 1978). A pre-incubation step near 5 °C, for a few months may be required before performing the mating tests in some species (Leonard 1976). Some Exserohilum s.lat. species show an atypical morphology and have been excluded from the genus by some authors. For instance, E. heteropogonicola and E. inaequale were reallocated to Curvularia as C. heteropogonicola and C. crassiseptum, respectively (Alcorn 1991, Zhang et al. 2004), and E. paspali was considered a synonym of Bipolaris micropus (Alcorn 1991). Although species of other genera might belong to Exserohilum, as previously noticed by other authors, like in the case of Helminthosporium leptochloae, which was considered similar to E. rostratum by Alcorn ( 1991), no synonymy or new combination in Exserohilum was proposed.
The type species of Exserohilum, E. turcicum, was originally described from Italy as Helminthosporium turcicum (Passerini 1876). This fungus causes northern leaf blight of corn, a widespread foliar disease characterised by oblong, straw-coloured to greyish necrotic lesions which can coalesce and cause significant death of foliar tissue. The reduction of photosynthetic leaf area can lead, in severe cases, to grain yield losses of 20–25 % (Smith et al. 1988). Exserohilum turcicum is also an important blight agent in Sorghum spp. (Bunker & Mathur 2006). Other Exserohilum species attacking economically relevant crops include E. pedicellatum (causing root rot of maize and brown lesions on wheat roots), E. prolatum (producing leaf spots on maize), and E. rostratum (associated with leaf spot and foot rot of wheat, damping off of sugarcane seedlings, leaf spot of banana, and blackening and seed germination failure in many cereals) (Sivanesan 1987, Lin et al. 2011). Many Exserohilum species attack weeds and some of them have been proposed as potential biocontrol agents, e.g., E. monoceras against Echinochloa spp. (Zhang & Watson 1997, Tosiah et al. 2011) and E. prolatum against Rottboellia cochinchinensis (Alloub et al. 2009). Presently, Exserohilum species have been reported from over 30 plant genera (Sivanesan 1987, 1992, Pachkhede 1989, Wu 1990, Sun et al. 1997, Chen et al. 2002, Lin et al. 2011, Sakoda & Tsukiboshi 2011).
Exserohilum spp. are emerging agents of opportunistic, sometimes life-threatening infections in humans. The most commonly reported species is E. rostratum, but some cases are attributed to E. longirostratum and E. macginnisii (McGinnis et al. 1986, De Hoog et al. 2000, Al-Attar et al. 2006). They have a broad clinical spectrum that includes skin infections (Hsu & Lee 1993, Lin et al. 2009), keratitis (Bouchon et al. 1994, Mathews & Maharajan 1999, Joseph et al. 2012), non-invasive allergies (Friedman et al. 1991, Torres et al. 1996) and invasive sinusitis (Lasala et al. 2005, Togitani et al. 2007, Derber et al. 2010) and disseminated infections (Bhigjee et al. 1993, Aquino et al. 1995, Levy et al. 2003). Predisposing factors include traumatisms, especially with plant material for keratitis, atopy for non-invasive sinusitis and immunosuppression for skin infections, invasive sinusitis and disseminated disease (Adler et al. 2006, Joseph et al. 2012). Recently, E. rostratum was reported as the main etiological agent in a dramatic outbreak of infections associated with contaminated glucocorticoid injections in the USA. The outbreak involved several states and there were over 749 reported cases of infection of which 31 % presented meningitis. Six percent of the infections were fatal (Kainer et al. 2012, Smith et al. 2013). A case of dermal granulomas caused by Exserohilum sp. in a bovine was reported by Whitford et al. ( 1989). Some authors have suggested that the three mentioned clinically-relevant Exserohilum species are conspecific based on analyses of sequences of the internal transcribed spacer region (ITS), the large subunit ribosomal rDNA (LSU), the actin (act) and the translation elongation factor 1-alpha (tef1) genes (Lin et al. 2011, Da Cunha et al. 2012).
Phylogenetic studies based on different loci indicated that Exserohilum belongs to the Pleosporaceae, Pleosporales (Berbee et al. 1999, Olivier et al. 2000, Zhang & Berbee 2001, Rossman et al. 2002, Kodsueb et al. 2006, Zhang et al. 2009, 2012, Amaradasa et al. 2014). However, none of these studies included more than four species of the genus, and so a reassessment of the genus is necessary to determine the evolutionary relationships of the remaining species. A revision of these fungi is also necessary to clarify the phylogenetic placement of species with atypical morphology, and to assess whether previously suggested synonymies (Alcorn 1991, Zhang et al. 2004) are correct. In this paper we present a monograph of the genus Exserohilum based on the analysis of multi-locus sequence data and the morphological study of numerous isolates and herbarium collections. A robust phylogenetic tree based on seven loci is provided, representing the main plant-pathogens and clinically-relevant species.
MATERIAL AND METHODS
Fungal isolates
The Exserohilum/Setosphaeria isolates included in this study were obtained from various substrates and countries and acquired from public culture collections, including the Westerdijk Fungal Biodiversity Institute (CBS; Utrecht, The Netherlands), the Faculty of Medicine of the Universitat Rovira i Virgili (FMR; Reus, Spain) and the Queensland Plant Pathology Herbarium (BRIP; Brisbane, Australia) as listed in Table 1. Herbarium specimens were loaned from the US National Fungus Collections (BPI; Maryland, USA), BRIP, Canadian National Mycological Herbarium (DAOM; Ottawa, Canada) and the Kew Royal Botanical Gardens (IMI; Kew, England).
Table 1.
Details of isolates included in phylogenetic analyses. GenBank accession numbers in bold were newly generated in this study. New species and new combinations are indicated in bold italic.
| Taxon | Old name/identified as1 | Strain no.2 | Other collections2 | Status of the strain1 , 3 | Geographical origin (country, province, locality) | Substrate | GenBank accession numbers4 |
||||||||
| ITS | LSU | act | cam | tef1 | gapdh | his | tub2 | rpb2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bipolaris chloridis | CBS 242.77B | ATCC 34706; IMI 208338 | Australia | Chloris gayana | HF934928 | HF934869 | – | – | – | HG779083 | – | – | HF934830 | ||
| B. cynodontis | CBS 285.51 | Kenya | Cynodon transvaalensis | HF934929 | HF934874 | – | – | – | HG779081 | – | – | HF934831 | |||
| CBS 305.64 | USA | Cynodon dactylon | HF934930 | HF934883 | – | – | – | HG779082 | – | – | HF934832 | ||||
| B. maydis | CBS 130.26 | ATCC 22246 | Unknown | Unknown | HF934923 | HF934873 | – | – | – | HG779084 | – | – | HF934825 | ||
| CBS 136.29 | PT | Japan | Zea mays | HF934926 | HF934879 | – | – | – | HG779086 | – | – | HF934828 | |||
| B. microlaenae | CBS 280.91 | BRIP 15613; IT: IMI 335218 | T | Australia | Microlaena stipoides | HF934933 | HF934877 | – | – | – | HG779092 | – | – | HF934835 | |
| B. oryzae | CBS 157.50 | Indonesia | Oryza sativa | HF934931 | HF934870 | – | – | – | HG779090 | – | – | HF934833 | |||
| CBS 199.54 | New Guinea | Oryza sativa | HF934932 | HF934884 | – | – | – | HG779091 | – | – | HF934834 | ||||
| B. sorghicola | CBS 249.49 | MUCL 9689 | Unknown | Sorghum vulgare var. sudanense | HF934927 | HF934868 | – | – | – | HG779087 | – | – | HF934829 | ||
| Curvularia aeria | CBS 294.61 | T | Brazil | Air | HF934910 | HF934902 | – | – | – | HF565450 | – | – | HF934812 | ||
| C. akaii | CBS 318.86 | Japan | Unknown | HF934921 | HF934897 | – | – | – | HG779118 | – | – | HF934823 | |||
| CBS 127728 | IMI 309517 | Japan | Themeda triandra | HF9349 20 | HF934898 | – | – | – | HG7791 19 | – | – | HF934822 | |||
| C. akaiiensis | CBS 127726 | T | India | Unknown | KJ415539 | KJ415494 | – | – | – | KJ415407 | – | – | LT852469 | ||
| C. andropogonis | CBS 186.49 | Indonesia | Andropogon nardus | LT631354 | LT715570 | – | – | – | LT715835 | – | – | LT852470 | |||
| C. borreriae | CBS 859.73 | Chile | Volcanic ash soil | HE861848 | LT715573 | – | – | – | HF565455 | – | – | LT852471 | |||
| C. caricapapayae | CBS 135941 | T | India | Carica papaya | HG778984 | HG779031 | – | – | – | HG779146 | – | – | HG779162 | ||
| C. comoriensis | CBS 110673 | Unknown | Unknown | LT631357 | LT715576 | – | – | – | LT715841 | – | – | LT852472 | |||
| C. crassiseptum | E. inaequale | CBS 503.90 | IT: BRIP 14583 | T | Nigeria | Plant material | LT631310 | LT715613 | – | – | – | LT715882 | – | – | LT852473 |
| C. cymbopogonis | CBS 4 19.78 | Netherlands | Yuccasp. | HG778985 | HG779032 | – | – | – | HG779129 | – | – | HG779163 | |||
| C. deightonii | CBS 537.70 | Denmark | Sorghum vulgare | LT631356 | LT715574 | – | – | – | LT715839 | – | – | LT852474 | |||
| C. gladioli | CBS 210.79 | Romania | Gladiolus sp. | HG778987 | HG779034 | – | – | – | HG779123 | – | – | HG779165 | |||
| C. hawaiiensis | CBS 173.57 | T | Hawaii | Oryza sativa | HG778988 | HG779035 | – | – | – | HG779140 | – | – | HG779166 | ||
| C. heteropogonicola | E. heteropogonicola | CBS 128052 | BRIP 14579 | T | India | Heteropogon contortus | KJ415548 | KJ415503 | – | – | – | KJ415398 | – | – | LT715769 |
| C. heteropogonis | CBS 284.91 | T | Australia | Heteropogon contortus | HF9349 19 | HF934893 | – | – | – | HF9349 19 | – | – | HF934821 | ||
| CBS 511.91 | Australia | Heteropogon contortus | HF934918 | HF934894 | – | – | – | HF934918 | – | – | HF9348 20 | ||||
| C. kusanoi | CBS 137.29 | ET | Japan | Eragrostis major | JN 192381 | JN600993 | – | – | – | LT715862 | – | – | LT715733 | ||
| C. lunata | CBS 730.96 | T | USA | Homo sapiens | HF934911 | HF934900 | – | – | – | JX256429 | – | – | HF934813 | ||
| C. micropus | B. micropus | BRIP 6516 | CBS 127234, IMI 31 2021 | USA | Paspalum notatum | HE792933 | LT715598 | – | – | – | LT715858 | – | – | LT715730 | |
| B. micropus | CBS 127235 | BRIP 65 20, IMI 31 2022 | ET | USA | Paspalum notatum | HE792934 | LT715599 | – | – | – | LT715859 | – | – | LT715731 | |
| B. micropus | BRIP 15689a | CBS 127236 | USA | Paspalum notatum | HE792935 | LT715600 | – | – | – | LT715860 | – | – | LT715732 | ||
| E. paspali | BRIP 16070 | CBS 128057 | A of E. paspali | Brazil | Paspalum conjugatum | LT837854 | LT715597 | – | – | – | LT715857 | – | – | LT715729 | |
| C. nicotiae | CBS 655.74 | IT | Algeria | Desert soil | KJ909772 | KM243291 | – | – | – | KM083614 | – | – | – | ||
| C. nodulosa | CBS 161.58 | A | Unknown | Eleusine indica | – | LT715603 | – | – | – | LT715863 | – | – | LT715734 | ||
| C. portulacae | CBS 239.48 | IT | USA | Portulaca oleracea | KJ909775 | LT715594 | – | – | – | LT715903 | – | – | – | ||
| CBS 127241 | Unknown | Unknown | LT715593 | – | – | – | – | LT715855 | – | – | – | ||||
| C. prasadii | CBS 143.64 | T | India | Jasminum sambac | HG778996 | HG779043 | – | – | – | HG779147 | – | – | HG779174 | ||
| C. spicifera | CBS 198.31 | Cyprus | Capsicum annuum | HF934916 | HF934905 | – | – | – | HG779136 | – | – | HF934818 | |||
| CBS 199.31 | Cyprus | Cucurbita maxima | HF934915 | HF934903 | – | – | – | HG779137 | – | – | HF934817 | ||||
| C. trifolii | CBS 173.55 | USA | Trifolium repens | HG779023 | HG779077 | – | – | – | HG779124 | – | – | HG779 208 | |||
| Exserohilum corniculatum | S. rostrata | BRIP 11426 | IT: IMI 167611 | T | Australia | Oryza sativa | LT837453 | LT883391 | LT837589 | LT838283 | LT883558 | LT883533 | LT860104 | LT896678 | LT852480 |
| E. holmii | S. holmii | BRIP 12679 | Australia | Dactyloctenium radulnas | LT837846 | LT883453 | LT837678 | LT852460 | LT896667 | LT882542 | LT860 190 | LT899370 | LT882525 | ||
| CBS 318.64 | ATCC 58 199 | Unknown | Dactyloctenium aegyptium | LT837457 | LT883395 | LT837596 | LT838290 | LT883565 | LT883537 | LT860111 | LT896686 | LT852487 | |||
| CBS 3 19.64 | Unknown | Dactyloctenium aegyptium | LT837458 | LT715622 | LT837597 | LT838291 | LT883566 | LT715891 | LT860112 | LT896685 | LT852488 | ||||
| H. holmii | CBS 413.65 | ATCC 15226 | IST of H. holmii | USA | Dactyloctenium aegyptium | LT837459 | LT715621 | LT837598 | LT838292 | LT883567 | LT715890 | LT860113 | LT896687 | LT852489 | |
| CBS 414.65 | ATCC 15225, IMI 103140 | A of T. holmii | USA | Dactyloctenium aegyptium | LT837460 | LT883396 | LT837599 | LT838293 | LT883568 | LT883538 | – | – | – | ||
| E. curvatum | CBS 505.90 | IT: IMI 281326, CBS 132712 | T of E. curvatum | Venezuela | Sorghum vulgare | KT265252 | LT7156 20 | LT837591 | LT838285 | LT883560 | LT715889 | LT860106 | LT896680 | LT852482 | |
| CBS 128053 | BRIP 12792 | Thailand | Dactyloctenium aegyptium | KT265253 | LT883441 | LT837663 | LT852447 | LT896652 | LT882555 | LT860175 | LT899383 | LT882513 | |||
| E. khartoumensis | S. khartoumensis | IMI 249 194 | CBS 132708 | IT | Sudan | Sorghum bicolor var. mayo | LT837461 | LT7156 19 | LT837600 | LT838294 | LT883569 | LT715888 | LT882489 | LT896688 | LT852490 |
| E. minor | S. minor | BRIP 14612 | Australia | Ascocarps formed by BRIP 13597 | LT837467 | LT715615 | LT837609 | LT838303 | LT883577 | LT715884 | LT860121 | LT896696 | LT852499 | ||
| S. minor | BRIP 14614 | Australia | Dactyloctenium aegyptium | LT837468 | LT715616 | LT837610 | LT838304 | LT883578 | LT715885 | LT860122 | LT896697 | LT852500 | |||
| S. minor | BRIP 14615 | IT: IMI 294530b, DAR 51591, ATCC 62323 | T of S. minor | Australia | Dactyloctenium aegyptium | LT837469 | LT883402 | LT837611 | LT838305 | LT883579 | LT883544 | LT860123 | LT896698 | LT852501 | |
| E. minor | BRIP 14616 | IT: IMI 294530a, DAR 51590 | T of E. minor | Australia | Dactyloctenium aegyptium | LT837470 | LT883403 | LT837612 | LT838306 | LT883580 | LT883545 | LT860124 | LT896699 | LT852502 | |
| E. monoceras | S. monoceras | BRIP 11542 | Australia | Setaria italica | LT837473 | LT883404 | LT837615 | LT838309 | LT896604 | LT883546 | LT860127 | LT896702 | LT852505 | ||
| S. monoceras | BRIP 12236 | Australia | Echinochloa colona | LT837472 | LT715637 | LT837614 | LT838308 | LT896603 | LT715876 | LT860126 | LT896701 | LT852504 | |||
| S. monoceras | BRIP 12271 | ATCC 36561, ATCC 36562 | A of S. monoceras | Australia | Echinochloa colona | LT837475 | LT883406 | LT837617 | LT838311 | LT896606 | LT883548 | LT860129 | LT898523 | LT852507 | |
| H. monoceras; D. monocera; H. crusgalli | CBS 198.29 | Japan | Echinochloa crusgalli | LT837853 | LT883460 | LT837686 | LT852468 | LT896674 | LT882534 | LT860 198 | LT899362 | LT882533 | |||
| S. monoceras | CBS 239.77 | Australia | Echinochloa colona | LT837474 | LT883405 | LT837616 | LT838310 | LT896605 | LT883547 | LT860128 | LT898522 | LT852506 | |||
| S. monoceras | CBS 209.78 | 77163-1 | Australia | Echinochloa colona | LT837471 | LT715638 | LT837613 | LT838307 | LT883581 | LT715875 | LT860125 | LT896700 | LT852503 | ||
| E. neoregeliae | CBS 132832 | IM 201-D | T | Japan | Neoregelia carolinae | LT837476 | LT715617 | LT837618 | LT838312 | LT896607 | LT715886 | LT860130 | LT898517 | LT852508 | |
| CBS 132833 | IT | Japan | Neoregelia carolinae | LT837477 | LT715618 | LT8376 19 | LT838313 | LT896608 | LT715887 | LT860131 | LT898518 | LT715765 | |||
| E. oryzicola | CBS 502.90 | T: IMI 273 194 | IT | Colombia | Oryza sativa | HF934949 | HF934886 | LT837640 | LT838323 | LT896629 | LT715878 | LT860152 | LT899345 | HF934851 | |
| E. fusiforme | CBS 376.76 | Turkey | Oryza sativa | LT837456 | LT883393 | LT837593 | LT838287 | LT883562 | LT883535 | LT860108 | LT896682 | LT852484 | |||
| E. fusiforme | BRIP 16229 | IT: CBS 132709, IMI 354683 | T of E. fusiforme | Australia | Echinochloa crusgalli | LT837455 | LT715636 | LT837592 | LT838286 | LT883561 | LT715877 | LT860107 | LT896681 | LT852483 | |
| E. pedicellatum | H. pedicellatum; D. pedicellata | CBS 322.64 | MUCL 9617 | ET | USA | Triticum aestivum | KT265258 | HF934889 | LT837641 | LT838324 | LT896630 | LT715902 | LT860153 | LT899382 | HF934854 |
| D. pedicellata | CBS 375.76 | Turkey | Oryza sativa | KT265259 | HF934890 | LT837642 | – | LT896631 | LT715879 | LT860154 | LT899381 | HF934855 | |||
| S. pedicellata | BRIP 1 2040 | Australia | Oryza sativa | LT837452 | LT883390 | LT837588 | LT838282 | LT883557 | LT883532 | LT860103 | LT896677 | LT852479 | |||
| E. protrudens | BRIP 14814 | IT: CBS 132710, IMI 316693 | T | Australia | Dactyloctenium aegyptium | LT631308 | LT715611 | LT837662 | LT852446 | LT896651 | LT715880 | LT860174 | LT899384 | LT715741 | |
| BRIP 14816 | Australia | Dactyloctenium aegyptium | LT631309 | LT715612 | LT837661 | LT852445 | LT896650 | LT715881 | LT860173 | LT899385 | LT715728 | ||||
| E. rostratum | S. holmii | BRIP 10724 | Australia | Eragrostis tenella | LT837828 | LT883436 | LT837654 | – | LT896643 | LT882560 | LT860166 | – | LT882508 | ||
| S. rostrata | BRIP 10995 | IMI 170 197 | Australia | Zea mays | LT837823 | LT883430 | LT837648 | LT852437 | LT896637 | LT882566 | LT860160 | LT899375 | LT882502 | ||
| S. rostrata | BRIP 11416 | IMI 170188 | Australia | Zea mays | LT837466 | LT883401 | LT837608 | LT838302 | LT883576 | LT883543 | LT8601 20 | LT896695 | LT852498 | ||
| S. rostrata | BRIP 11417 | IMI 167610 | Australia | Zea mays | LT837836 | LT883443 | LT837667 | LT852451 | LT896656 | LT882553 | LT860179 | LT899355 | LT882516 | ||
| S. rostrata | BRIP 11422 | Australia | Zea mays | LT837464 | LT883399 | LT837606 | LT838300 | LT883574 | LT883541 | LT860118 | LT896693 | LT852496 | |||
| S. rostrata | BRIP 11432 | Australia | Poaceae | LT837824 | LT883431 | LT837649 | LT852438 | LT896638 | LT882565 | LT860161 | LT899374 | LT882503 | |||
| S. rostrata | BRIP 1 2090 | Australia | Eragrostis brownii | LT837847 | LT883454 | LT837679 | LT852461 | LT896668 | LT882539 | LT860 191 | LT899369 | LT882526 | |||
| S. rostrata | BRIP 12147 | Australia | Dinebra retroflexa | LT837499 | LT883426 | LT837644 | LT838326 | LT896633 | LT882570 | LT860156 | LT899379 | LT882498 | |||
| S. rostrata | BRIP 12270 | Australia | Eragrostis pilosa | LT837851 | LT883458 | LT837684 | LT852466 | LT896672 | LT882536 | LT860 196 | LT899364 | LT882531 | |||
| S. rostrata | BRIP 13560 | Australia | Paspalidium distans | LT837822 | LT883429 | LT837647 | LT838329 | LT896636 | LT882567 | LT860159 | LT899376 | LT882501 | |||
| S. rostrata | BRIP 13592 | Australia | Ischaemum villosum | LT837849 | LT883456 | LT837681 | LT852463 | LT896670 | LT882541 | LT860 193 | LT899367 | LT882528 | |||
| S. holmii | BRIP 13599 | Australia | Dactyloctenium aegyptium | LT837844 | LT883451 | LT837676 | LT852458 | LT896665 | LT882544 | LT860188 | LT899348 | LT882523 | |||
| E. longirostratum | BRIP 14916 | Australia | Zea mays | LT837835 | LT883442 | LT837666 | LT852450 | LT896655 | LT882552 | LT860178 | LT899356 | LT882515 | |||
| E. longirostratum | BRIP 15274 | DNAP 1390 | Australia | Areca catechu | LT837840 | LT883447 | LT837672 | LT852454 | LT896661 | LT882548 | LT860184 | LT899352 | LT8825 19 | ||
| S. rostrata | BRIP 15403 | Australia | Chrysalidocarpus lutescens | LT837845 | LT883452 | LT837677 | LT852459 | LT896666 | LT882543 | LT860189 | LT899371 | LT882524 | |||
| S. rostrata | BRIP 15489 | Australia | Triticum aestivum | LT837821 | LT883428 | LT837646 | LT838328 | LT896635 | LT882568 | LT860158 | LT899377 | LT882500 | |||
| E. longirostratum | BRIP 16078 | Australia | Spinifex hirsutus | LT837826 | LT883433 | LT837651 | LT852440 | LT896640 | LT882563 | LT860163 | LT899372 | LT882505 | |||
| E. longirostratum | BRIP 16114 | Australia | Cymbopogon citratus | LT837843 | LT883450 | LT837675 | LT852457 | LT896664 | LT882545 | LT860187 | LT899349 | LT882522 | |||
| E. longirostratum | BRIP 20144b | Australia | Megathyrsus maximus | LT837500 | LT883427 | LT837645 | LT838327 | LT896634 | LT882569 | LT860157 | LT899378 | LT882499 | |||
| S. rostrata | BRIP 28001 | Australia | Sorghum sp. | LT837479 | LT883408 | LT837621 | LT838315 | LT896610 | LT883550 | LT860133 | LT8985 20 | LT852510 | |||
| S. rostrata | BRIP 29236c | Australia | Hordeum vulgare | LT837492 | LT8834 19 | LT837634 | LT838317 | LT896623 | LT882577 | LT860146 | LT899344 | LT882491 | |||
| Exserohilum sp. | BRIP 46107 | Australia | Vitis vinifera | LT837827 | LT883434 | LT837652 | LT852441 | LT896641 | LT882562 | LT860164 | LT899393 | LT882506 | |||
| S. rostrata | BRIP 52639 | Australia | Hordeum vulgare | LT837825 | LT883432 | LT837650 | LT852439 | LT896639 | LT882564 | LT860162 | LT899373 | LT882504 | |||
| Exserohilum sp. | BRIP 53634b | Australia | Croton sp. | LT837498 | LT883425 | LT837643 | LT838325 | LT896632 | LT882571 | LT860155 | LT899380 | LT882497 | |||
| CBS 188.68 | South Africa | Unknown | LT837839 | LT883446 | LT837671 | LT852453 | LT896660 | LT882549 | LT860183 | LT899354 | LT882518 | ||||
| H. leptochloae | CBS 196.29 | ATCC 6700, MUCL 18 207, MUCL 9609 | ST of H. leptochloae | Japan | Leptochloa chinensis | LT837462 | LT715627 | LT837601 | LT838295 | LT883570 | LT715896 | LT882488 | LT896689 | LT852491 | |
| H. halodes; D. halodes | CBS 229.39 | MUCL 18 205 | South Africa | Triticum aestivum | LT837829 | LT883437 | LT837655 | LT852443 | LT896644 | LT882559 | LT860167 | LT899391 | LT882509 | ||
| H. rostratum; D. rostrata | CBS 230.39 | MUCL 18214, MUCL 9692 | South Africa | Triticum aestivum | LT837852 | LT883459 | LT837685 | LT852467 | LT896673 | LT882535 | LT860 197 | LT899363 | LT882532 | ||
| CBS 273.52 | IMI 048842, MUCL 18221, MUCL 96 19 | Zambia | Pennisetum spicatum | LT837830 | LT883438 | LT837656 | LT852444 | LT896645 | LT882558 | LT860168 | LT899390 | LT882510 | |||
| E. gedarefense | CBS 297.80 | T of E. gedarefense | Sudan | Sorghum bicolor | KT265244 | LT715626 | LT837594 | LT838288 | LT883563 | LT715895 | LT860109 | LT896683 | LT852485 | ||
| S. rostrata; H. halodes; D. halodes | CBS 3 20.64 | USA | Bromus inermis | LT837490 | LT883417 | LT837632 | LT838316 | LT896621 | LT882579 | LT860144 | LT899343 | LT852517 | |||
| CBS 323.64 | USA | Zea mays | LT837833 | LT715632 | LT837664 | LT852448 | LT896653 | LT715901 | LT860176 | LT899358 | LT715751 | ||||
| E. macginnisii | CBS 325.87 | ATCC 60408, CDC B-4030, NCMH 2445 | T of E. macginnisii | USA | Homo sapiens | KT265237 | LT715629 | LT837602 | LT838296 | HE664082 | LT715898 | LT860114 | – | LT852492 | |
| E. antillanum | CBS 412.93 | FMR 4455, IMI 358615 | IT of E. antillanum | Cuba | Plant debris from forest soil | KT265246 | LT715625 | LT837587 | LT838281 | LT883556 | LT715894 | LT860102 | LT896676 | LT852478 | |
| S. rostrata | CBS 467.75 | ATCC 32 198, IMI 197560, SrA3 | Unknown | Unknown | LT837850 | HE664026 | LT837682 | LT852464 | HE664081 | LT882538 | LT860 194 | LT899366 | LT882529 | ||
| S. prolata | CBS 571.73 | ATCC 24775, IMI 175436 | A of S. prolata | USA | Zea mays | LT837831 | LT715623 | LT837657 | – | LT896646 | LT715892 | LT860169 | LT899389 | LT715760 | |
| CBS 572.73 | ATCC 24774, IMI 175435 | A of S. prolata | Guatemala | Zea mays | LT837832 | LT715624 | LT837658 | – | LT896647 | LT715893 | LT860170 | LT899388 | LT715759 | ||
| CBS 504.90 | IMI 276558 | Sudan | Sorghum bicolor | KT265243 | LT883394 | LT837595 | LT838289 | LT883564 | LT883536 | LT860110 | LT896684 | LT852486 | |||
| B. australiensis; D. australiensis | CBS 705.71 | India | Soil | LT837841 | LT883448 | LT837673 | LT852455 | LT896662 | LT882547 | LT860185 | LT899351 | LT8825 20 | |||
| E. rostratum | CBS 706.71 | India | Soil | LT837842 | LT883449 | LT837674 | LT852456 | LT896663 | LT882546 | LT860186 | LT899350 | LT882521 | |||
| S. rostrata | CBS 732.96 | AMMRL 106.9, PPCC 19686 | Unknown | Zea mays | KT265240 | LT715631 | LT837669 | LT852452 | LT896658 | LT715900 | LT860181 | LT899353 | LT715752 | ||
| E. macginnisii | CBS 1 20308 | Unknown | Homo sapiens | KT265236 | LT883435 | LT837653 | LT852442 | LT896642 | LT882561 | LT860165 | LT899392 | LT882507 | |||
| D. micropus | CBS 127233 | DAOM 71176 | USA | Leptochloa filiformis | LT837454 | LT883392 | LT837590 | LT838284 | LT883559 | LT883534 | LT860105 | LT896679 | LT852481 | ||
| E. longirostratum | CBS 128054 | BRIP 21343 R.11,1,OB40 | Namibia | Acacia mellifera subsp. detinens | LT837451 | LT715628 | LT837586 | LT838280 | LT883555 | LT715897 | LT860101 | LT896675 | LT852477 | ||
| E. longirostratum | CBS 128055 | BRIP 21347 | Namibia | Acacia mellifera subsp. detinens | LT837478 | LT883407 | LT8376 20 | LT838314 | LT896609 | LT883549 | LT860132 | LT8985 19 | LT852509 | ||
| S. rostrata, mating type A | CBS 128060 | BRIP 12214, Lutt. 8686 | USA | Zea mays | KT265245 | LT883397 | LT837604 | LT838298 | LT883572 | LT883539 | LT860116 | LT896691 | LT852494 | ||
| S. rostrata, mating type A | CBS 128061 | BRIP 12218, Lutt. 8868 | USA | Zea mays | KT265240 | LT715631 | LT837669 | LT852452 | LT896658 | LT715900 | LT860181 | LT899353 | LT715752 | ||
| S. rostrata | CBS 128062 | BRIP 12224 | Australia | On Barley seed on Sach's agar | KT265247 | LT883457 | LT837683 | LT852465 | LT896671 | LT882537 | LT860 195 | LT899365 | LT882530 | ||
| S. rostrata | CBS 128063 | BRIP 12223, SrA10 | USA | Ascospore isolate from Hay 3 × IMI 76563 | KT265239 | LT883398 | LT837605 | LT838299 | LT883573 | LT883540 | LT860117 | LT896692 | LT852495 | ||
| FMR 11028 | UTHSC 08-655 | USA | Homo sapiens | LT837837 | LT883444 | LT837668 | – | LT896657 | LT882551 | LT860180 | – | – | |||
| FMR 11271 | UTHSC 05-3456 | USA | Homo sapiens | LT837496 | LT883423 | LT837638 | LT838321 | LT896627 | LT882573 | LT860150 | – | LT882495 | |||
| FMR 11278 | UTHSC 06-2113 | USA | Homo sapiens | LT837493 | LT8834 20 | LT837635 | LT838318 | LT896624 | LT882576 | LT860147 | – | LT882492 | |||
| FMR 11280 | UTHSC 06-3237 | USA | Homo sapiens | LT837494 | LT883421 | LT837636 | LT8383 19 | LT896625 | LT882575 | LT860148 | – | LT882493 | |||
| FMR 11286 | UTHSC 07-1292 | USA | Homo sapiens | LT837848 | LT883455 | LT837680 | LT852462 | LT896669 | LT882540 | LT860 192 | LT899368 | LT882527 | |||
| FMR 11372 | UTHSC 07-1310 | USA | Homo sapiens | LT837497 | LT883424 | LT837639 | LT838322 | LT896628 | LT882572 | LT860151 | – | LT882496 | |||
| FMR 11390 | UTHSC 08-2940 | USA | Homo sapiens | LT837495 | LT883422 | LT837637 | LT8383 20 | LT896626 | LT882574 | LT860149 | – | LT882494 | |||
| FMR 11392 | UTHSC 08-3638 | USA | Homo sapiens | LT837489 | LT883416 | LT837631 | – | LT8966 20 | LT882580 | LT860143 | – | – | |||
| FMR 11395 | UTHSC 09-131 | USA | Homo sapiens | LT837491 | LT883418 | LT837633 | – | LT896622 | LT882578 | LT860145 | – | LT852518 | |||
| FMR 11399 | UTHSC 09-1259 | USA | Homo sapiens | LT837838 | LT883445 | LT837670 | – | LT896659 | LT882550 | LT860182 | – | LT882517 | |||
| D. longirostrata | FMR 11773 | IP 1229.80 | Martinique | Homo sapiens | LT837834 | HE664025 | LT837665 | LT852449 | LT896654 | LT882554 | LT860177 | LT899357 | LT882514 | ||
| E. turcicum | S. turcica | BRIP 12267 | Australia | Sorghum bicolor | LT837482 | LT883411 | LT837624 | – | LT896613 | LT883553 | LT860136 | LT899337 | LT852513 | ||
| S. turcica | BRIP 13326 | Australia | Sorghum sudanense | LT837480 | LT883409 | LT837622 | – | LT896611 | LT883551 | LT860134 | LT898521 | LT852511 | |||
| S. turcica | CBS 195.26 | Indonesia | Zea mays | LT837485 | LT883413 | LT837627 | – | LT896616 | LT882583 | LT860139 | LT899340 | – | |||
| S. turcica | CBS 384.58 | USA | Single ascospore isolate from holotype of S. turcica | LT837481 | LT883410 | LT837623 | – | LT896612 | LT883552 | LT860135 | LT899336 | LT852512 | |||
| S. turcica | CBS 330.64 | USA | Zea mays | LT837484 | LT715639 | LT837626 | – | LT896615 | LT715874 | LT860138 | LT899339 | LT852515 | |||
| S. turcica | CBS 385.58 | USA | Single ascospore isolate from holotype of S. turcica | LT837488 | LT715640 | LT837630 | – | LT8966 19 | LT715873 | LT860142 | – | LT852516 | |||
| S. turcica | CBS 386.58 | ATCC 13068, NRRL 5239 | USA | Sorghum halepense | LT837486 | LT883414 | LT837628 | – | LT896617 | LT882582 | LT860140 | LT899341 | – | ||
| S. turcica | CBS 387.58 | NRRL 5240 | USA | Zea mays | LT837483 | LT883412 | LT837625 | – | LT896614 | LT883554 | LT860137 | LT899338 | LT852514 | ||
| S. turcica | CBS 690.71 | ET | Germany | Zea mays | LT837487 | LT883415 | LT837629 | – | LT896618 | LT882581 | LT860141 | LT899342 | – | ||
| Porocercospora seminalis | Ce. seminalis | CBS 134906 | CPC 21305 | ET | USA | Bouteloua dactyloides | HF934942 | HF934865 | – | – | – | – | – | – | HF934847 |
| Ce. seminalis | CPC 21330 | USA | Bouteloua dactyloides | HF934948 | HF934863 | – | – | – | – | – | – | HF934849 | |||
| Ce. seminalis | CPC 21333 | USA | Bouteloua dactyloides | HF934946 | HF934859 | – | – | – | – | – | – | HF934850 | |||
| D. graminea; H. gramineum | CBS 280.31 | Hordeum vulgare | HF934954 | HF934857 | – | – | – | LT715872 | – | – | HF934856 | ||||
1 B: Bipolaris; C: Curvularia; Ce: Cercospora; D: Drechslera; E: Exserohilum; H: Helminthosporium; S: Setosphaeria; T: Trichometasphaeria.
2 ATCC: American Type Culture Collection, Bethesda, Maryland, USA; BRIP: Queensland Plant Pathology Herbarium, Brisbane, Australia; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; FMR: Faculty of Medicine collection, Reus, Spain; DAOM: Canadian National Mycological Herbarium, Ottawa, Canada; IMI: Kew Royal Botanical Gardens, Kew, England; MUCL: Mycothèque de L'Université Catholique de Louvain, Louvain-la-Neuve, Belgium; UTHSC: Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio Texas, USA.
3 ET: ex-epitype; IT: ex-isotype; IST: ex-isosyntype; NT: ex-neotype; PT: ex-paratype; ST: ex-syntype; T: ex-holotype; A: Authentic strain.
4 ITS: internal transcribed spacer region; LSU: large subunit ribosomal RNA gene; act: partial actin gene; tub2: partial β-tubulin gene; cam: partial calmodulin gene; gapdh: partial glyceraldehyde-3-phosphate dehydrogenase gene; his: partial histone H3 gene; tef1: partial translation elongation factor-1 alpha gene; rpb2: RNA polymerase II second largest subunit gene.
Phenotypic study and species descriptions
Colony morphology was studied mainly on synthetic nutrient-poor agar (SNA, Nirenberg 1976) supplemented with fragments of sterilized maize or banana leaves, after 7 d of incubation at 24 °C in the dark. Microscopic features were studied in clear lactic acid from colonies growing on the culture media mentioned above after 7–14 d of incubation at 24 °C under near UV light with a 12 h photoperiod. Fungal structures from herbarium material were also mounted in lactic acid, but these were gently heated with the flame of a Bunsen burner before observation if they appeared dehydrated. Size ranges of each structure in the species descriptions are derived from at least 30 measurements.
Molecular study
DNA extraction was carried out from colonies growing on MEA with the UltraClean® Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Solana Beach, CA, USA). Amplification and sequencing of nine nuclear loci, i.e., the internal transcribed spacer (ITS) region, large subunit ribosomal RNA gene (LSU), actin (act), β-tubulin (tub2), calmodulin (cam), glyceraldehyde-3-phosphate dehydrogenase (gapdh), histone H3 (his), translation elongation factor-1 alpha (tef1) and RNA polymerase II second largest subunit (rpb2) were performed with primers V9G (De Hoog & Gerrits van den Ende 1998) + ITS4 (White et al. 1990), LR0R + LR5 (Vilgalys & Hester 1990), Act1 + Act4 (Voigt & Wöstemeyer 2000), T1 (O’Donnell & Cigelnik 1997) + Bt2b (Glass & Donaldson 1995), CAL228F + CAL737R (Carbone & Kohn 1999), gpd1 + gpd2 (Berbee et al. 1999), CYLH3F + CYLH3R (Crous et al. 2004), 983F + 2218R (Rehner & Buckley 2005) and 5F2 + 7cR (O’Donnell et al. 2007), respectively. Sequencing was performed with the BigDye terminator sequencing kit v. 3.1 (Applied Biosystems) and an ABI PrismTM 3100 DNA sequencer (Applied Biosystems). The program SeqMan Pro (Lasergene, Madison, Wisconsin) was used to obtain consensus sequences from the complementary sequences of each isolate. BLAST queries (Altschul et al. 1990) were performed to compare sequences of the studied isolates with those of other fungi deposited in GenBank. Alignments were produced with MAFFT v. 7 (Katoh & Standley 2013), checked and refined using MEGA v. 6 (Tamura et al. 2013) and SequenceMatrix (Vaidya et al. 2011).
Two multi-locus phylogenies were analysed in order to evaluate the generic placement of the strains and to establish the phylogenetic relationship among species of Exserohilum s.str. The generic placement is based on a concatenated ITS, LSU, gapdh and rpb2 dataset including species of Exserohilum s.lat. and representatives of other helminthosporoid genera (i.e., Bipolaris, Curvularia, Johnalcornia and Porocercospora), and Pyrenophora used as outgroup. This phylogeny was constructed to assess if Exserohilum is a well-delimited genus, and to corroborate if the previous reallocation of E. heteropogonicola and E. inaequalis to Curvularia, and the synonymy of E. paspali with Bipolaris micropus are correct.
The second multi-locus phylogeny was based on a concatenated alignment of ITS, act, tub2, gapdh, his, tef1 and rpb2 and included 98 isolates of Exserohilum/Setosphaeria, excluding E. paspali. This analysis was performed to evaluate species boundaries and species groupings within Exserohilum. Individual alignments of each locus and the concatenated four- and seven-locus datasets were analysed by maximum likelihood (ML) with gamma model of rate heterogeneity using the RAxML HPC BlackBox v. 8.2.8 (Stamatakis 2014) online server of the Cipres Science gateway portal (Miller et al. 2010). The maximum likelihood search option was used to search for the best-scoring tree after bootstrapping. By default, the RAxML BlackBox calculates statistical support for branches by rapid bootstrap analyses of 1 000 replicates (Stamatakis 2014). Bootstrap support (bs) values ≥ 70 % were considered significant. Incongruence among datasets was tested by a visual inspection of all groups with ≥ 70 % bs in partial trees of each locus to search for potentially conflicting groups. A Markov Chain Monte Carlo (MCMC) algorithm was used to generate phylogenetic trees with Bayesian probabilities from the concatenated four-locus and seven-locus datasets using MrBayes v. 3.2.6 (Ronquist et al. 2012). The best models of nucleotide substitution for each locus were determined using MrModeltest v. 2.3 (Nylander 2004) and the critical value for the topological convergence diagnostic set to 0.01. Two analyses of four MCMC chains were run from random trees, trees were sampled every 100 generations and 25 % of them were discarded as the burn-in phase. Posterior probabilities (pp) were determined from the remaining trees. The sequences generated during this study and the alignments used in the phylogenetic analyses were deposited in GenBank (Table 1) and TreeBASE (Submission 21627), respectively.
RESULTS
Molecular and phylogenetic analysis
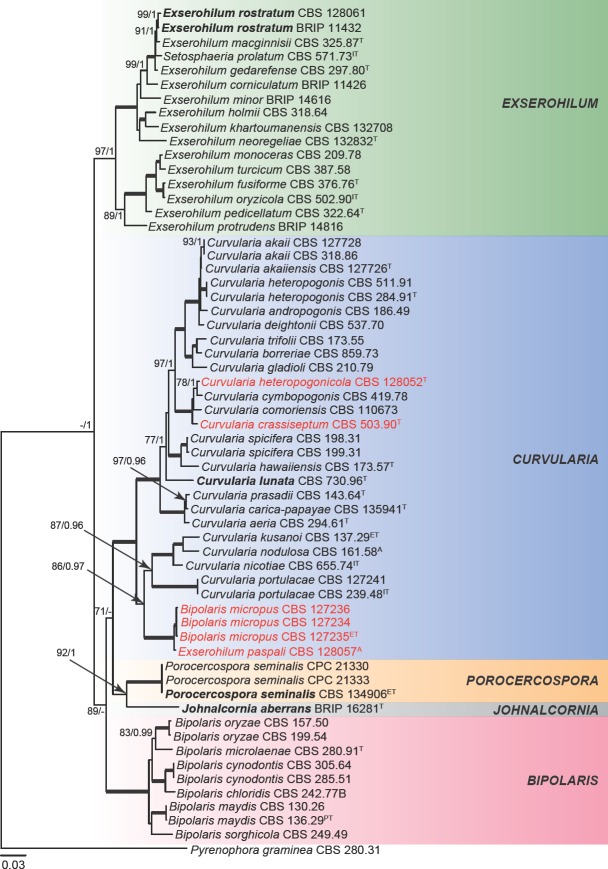
In Exserohilum, amplification success rate varied among the different loci tested, i.e., 100 % for ITS, LSU, act, gapdh and tef1, 99 % for his, 94 % for rpb2, 87 % for tub2 and 82 % for cam. BLAST searches with the ITS sequences revealed that E. heteropogonicola and E. inaequale are in fact members of Curvularia as suggested by Sivanesan ( 1984) and Zhang et al. ( 2004). The closest hit for the ITS sequence of CBS 128057, the authentic isolate of E. paspali, were three sequences of B. micropus, i.e., GenBank accession numbers HE792933, HE792934 and HE792935 (corresponding to CBS 127234, CBS 127235, CBS 127236, respectively), all of them 99 % identical. No close hits were found for the ITS sequence of E. novaezelandiae CBS 135842 (not type strain), but its LSU was 91 % identical to Conlarium duplumascospora (GenBank accession numbers JN936991, JN936992, JN936993), a member of Annulatascaceae, Sordariomycetes (Liu et al. 2012). This clearly indicates that E. novae-zelandiae is not a member of Exserohilum and should be excluded from this genus, thus a new combination is proposed in Sporidesmiella.
The first concatenated matrix contains 3 192 nucleotide characters, i.e., 616 from gapdh, 834 from ITS, 882 from LSU and 860 from rpb2. The second concatenated alignment contains 4 736 nucleotide characters, i.e., 653 from act, 597 from gapdh, 387 from his, 794 from ITS, 860 from rpb2, 896 from tef1 and 549 from tub2. For Bayesian analysis, MrModeltest proposed a GTR + I + G model for ITS, SYM + G for act, gapdh and rpb2, HKY + G for tub2, HKY + I + G for his and GTR + I + G for tef1. These models were incorporated in the analysis. During the generation of the Bayesian seven-locus tree, a total of 49 666 trees were sampled out of the 66 222 trees generated (75 %). The consensus tree obtained from the Bayesian analysis agreed with the topology of the best-scoring ML tree for the concatenated four-locus dataset (Fig. 1). Species of Exserohilum formed a well-supported clade (100 % bs / 1 pp) clearly separated from other graminicolous helminthosporoid genera, such as Bipolaris, Curvularia, Johnalcornia, Porocercospora and Pyrenophora. Interestingly, the authentic isolate of Exserohilum paspali CBS 128057 formed a clade with three isolates of Bipolaris micropus (CBS 127234, CBS 127235 and CBS 127236), which appeared more closely related to the genus Curvularia than to Exserohilum or Bipolaris. These results are in partial concordance with a previous proposal of E. paspali as a synonym of B. micropus by Sivanesan ( 1987) based on morphology. Nevertheless, they are phylogenetically closely related to Curvularia (Fig. 1) and a new combination is proposed in the taxonomy section.
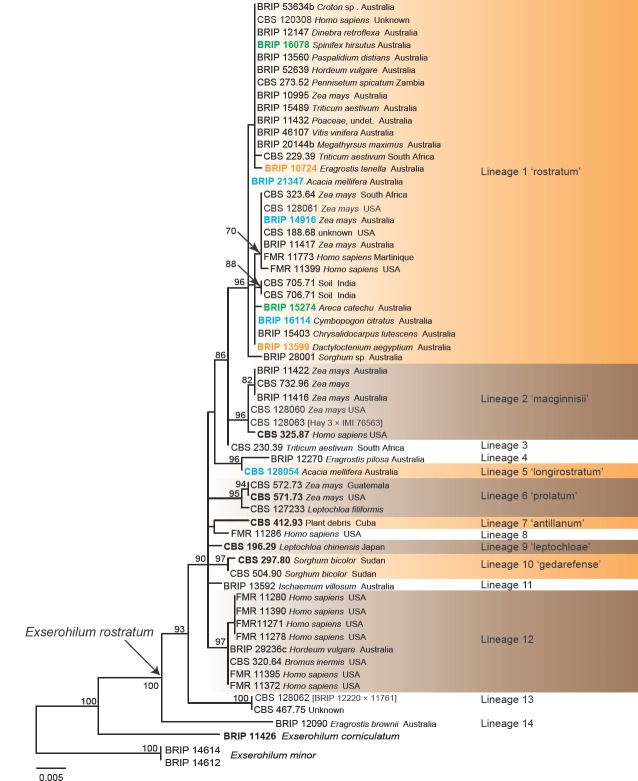
Fig. 1.
Phylogenetic tree inferred from a RAxML analysis based on a concatenated alignment of ITS, LSU, gapdh and rpb2 sequences of Exserohilum and related genera in Pleosporaceae. The bootstrap support values and Bayesian posterior probabilities are given at the nodes (MLBS/BPP). Clades with 100 % MLBS and 1 BPP are indicated by thick lines. In red font are indicated taxa previously known as Exserohilum. Ex-type, ex-isotype, ex-epitype and ex-paratype and authentic strains are indicated as T, IT, ET, PT, A, respectively. Generic types are indicated in bold. The tree was rooted to Pyrenophora graminea CBS 280.31.
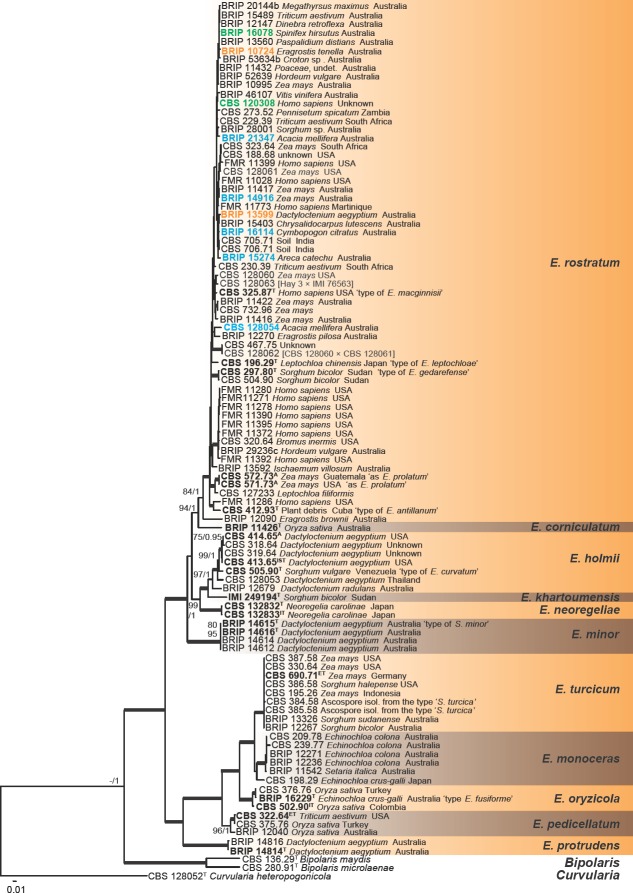
The combined phylogenetic tree inferred based on seven loci (Fig. 2) revealed the existence of 11 phylogenetic species in Exserohilum, including one novel taxon, E. corniculatum, which is described in the taxonomy section. Species in Exserohilum (Fig. 2) were distributed into two major fully supported subclades. The first clade includes isolates identified as E. corniculatum, E. holmii, E. khartoumensis, E. minor, E. neoregeliae and E. rostratum, whereas the second clade included isolates of E. monoceras, E. oryzicola, E. pedicellatum, E. protrudens and E. turcicum. Most species in the first subclade show one or more accentuated septa (dark and thick), especially in polar cells. The subclade comprising most isolates includes E. rostratum and E. corniculatum. Besides the numerous isolates of E. rostratum, it also includes several isolates identified as E. longirostratum and the ex-type strains of E. antillanum, E. gedarefense, E. macginnisii, E. prolatum and Helminthosporium leptochloae. Morphological and molecular analyses suggest that those species are conspecific with E. rostratum and are treated here as synonyms in the taxonomy section. All clinical isolates included in our study belong to E. rostratum. The second subclade includes isolates of E. curvatum, E. holmii, E. khartoumensis and E. neoregeliae. Morphological and molecular analyses suggest that E. curvatum is conspecific with E. holmii and is treated here as synonym in the taxonomy section. A clade formed by four isolates of E. minor revealed mostly fusiform conidia which lack accentuated septa, and they have a homothallic sexual behaviour.
Fig. 2.
Phylogenetic tree inferred from a RAxML analysis based on a concatenated alignment of ITS, act, gapdh, his, rpb2, tef1 and tub2 sequences of Exserohilum s.str. The bootstrap support values and Bayesian posterior probabilities are given at the nodes (MLBS/BPP). Clades with 100 % MLBS and 1 BPP are indicated by thickened lines. In the E. rostratum clade in green font are indicated taxa previously identified as E. macginnisii, in blue identified as E. longirostratum, in orange as E. holmii. Ex-type, ex-isotype, ex-neotype and ex-epitype strains are indicated in bold. The tree was rooted to Bipolaris maydis CBS 136.29, B. microlaenae CBS 280.91 and Curvularia heteropogonicola CBS 128052.
Another subclade included E. fusiforme, E. monoceras, E. oryzicola and E. turcicum. Morphological and molecular analyses suggest that E. fusiforme is conspecific with E. oryzicola and is treated here as synonym in the taxonomy section. All isolates in this subclade are characterised by mostly fusiform conidia which lack accentuated septa and show heterothallic sexuality. Two subclades grouped species showing conidia with a prominent subcylindrical basal extension in their conidia, at the base of which the hilum appears. One of these subclades includes two isolates of E. protrudens and the other one includes isolates of E. pedicellatum. In the former subclade, the basal extension is pale, while in the latter subclade it is strongly pigmented.
TAXONOMY
Dothideomycetes, Pleosporales, Pleosporaceae
Exserohilum K.J. Leonard & Suggs, Mycologia 66: 290. 1974
Synonyms. Setosphaeria K.J. Leonard & Suggs, Mycologia 66: 294. 1974.
Luttrellia Khokhr. & Gornostaĭ (as ‘Lutrellia’; non Luttrellia Shearer), Vodorosli, Griby i Mkhi Dal’nego Vostoka [Algae, Fungi and Mosses of the Soviet Far-East] (Vladivostok): 80. 1978.
Type species. Exserohilum turcicum (Pass.) K.J. Leonard & Suggs.
Adapted from Sivanesan ( 1987). Vegetative hyphae septate, branched, pale brown to dark brown, smooth to finely verruculose. Asexual morph. Conidiophores macronematous, mononematous, septate, cylindrical, olivaceous brown to brown, smooth to verruculose, often geniculate above. Conidiogenous cells integrated, terminal and intercalary, sympodial, mono- to polytretic, cicatrized; conidiogenous nodes smooth to rough. Conidia fusiform, cylindrical or obclavate, straight to curved, multi-distoseptate, with a protruding hilum. Sexual morph. Ascomata superficial, immersed or erumpent, globose to ellipsoid, unilocular, dark brown to black, with or without a beak, ostiolate, with simple rigid setae over the ostiolar apex and on the upper half of the ascoma where they are often mixed with hyaline, filiform, septate hyphae; peridium composed of pseudoparenchymatous cells, dark brown and thick-walled on the outside, but with more or less hyaline cells towards the inside forming a textura angularis. Pseudoparaphyses filiform, hyaline, septate, branched, anastomosing. Asci arising from a basal cushion of thin-walled pseudoparenchymatous cells, bitunicate, 1–8-spored, cylindrical to cylindrical-clavate, short or moderately long-stalked, thick-walled, with an apical nasse and fissitunicate dehiscence. Ascospores fusoid, hyaline to pale brown, smooth, 2–6 or rarely more transversely septate, constricted at the septa, surrounded by a hyaline mucilaginous sheath which often extends some distance beyond the ends of the spore.
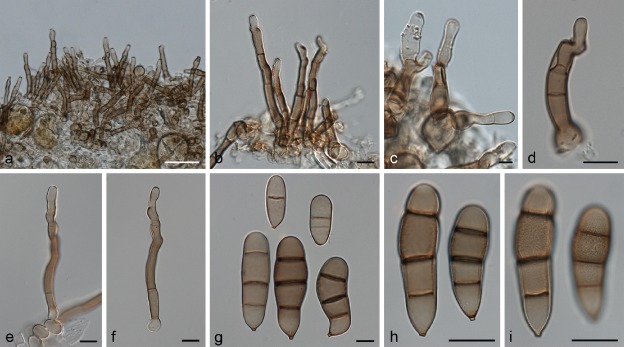
Exserohilum corniculatum Madrid, Hern.-Restr., Y.P. Tan & Crous, sp. nov. — MycoBank MB821483; Fig. 3
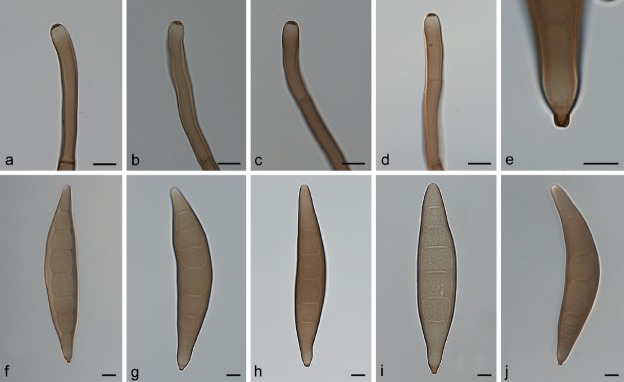
Fig. 3.
Exserohilum corniculatum (BRIP 11426 ex-type). a. Conidiophores; b–d. conidiogenous cells and conidia; e–h. conidia. — Scale bars: 10 μm.
Etymology. From the Latin corniculatum - horn-like, referring to the narrow apical extensions observed in rostrate conidia of this fungus, which resemble a horn.
Type material. Australia, Queensland, Home Hill, on leaf spot of Oryza sativa, 4 May 1972, W. Pont (BRIP 11426 holotype; BRIP 11426 culture ex-type; CBS H-21815, IMI 167611 isotypes).
On SNA + maize leaves. Vegetative hyphae septate, branched, pale olivaceous brown to dark olivaceous brown, smooth to finely verruculose, 2–7 μm wide. Conidiophores macronematous, mononematous, straight to flexuous, sometimes geniculate towards the apex, septate, unbranched, subcylindrical, brown, smooth-walled, but sometimes becoming finely verruculose near the conidiogenous loci, with cell walls usually thicker than those of the vegetative hyphae, 158–458 × 5–8 μm, with occasional subnodulose to nodulose swellings up to 9.5 μm wide. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- to polytretic, proliferating sympodially, 11–56.5 μm long, with scars up to 5.5 μm wide. Conidia mostly subcylindrical to fusiform, straight to slightly curved, pale olivaceous brown to dark olivaceous brown, smooth to irregularly verruculose, 4–10(–12)-septate, sometimes with accentuated septa delimiting the basal cell or both the basal and apical cells, often becoming rostrate by means of a narrow apical extension, 41–94.5(–104.5) × (11.5–)15–24 μm, with a strongly protruding hilum. Sexual morph not observed.
Culture characteristics — Colonies on SNA + sterilized maize leaves at 24 °C reaching 11 mm diam after 7 d, hairy, olivaceous black towards the periphery, greenish black on maize leaves, with a fimbriate margin; reverse concolorous with obverse.
Notes — Exserohilum corniculatum is unique in producing very narrow apical conidial extensions. The rostrate conidia of E. rostratum are usually much broader and do not resemble horns as in E. corniculatum.
Exserohilum holmii (Luttr.) K.J. Leonard & Suggs, Mycologia 66: 291. 1974 — Fig. 4
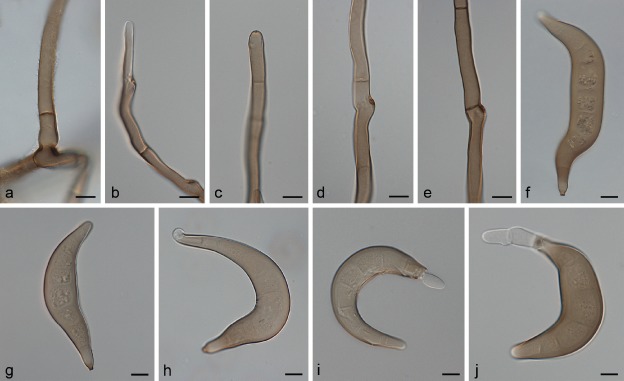
Fig. 4.
Exserohilum holmii (CBS 413.65 ex-isotype (a–d), CBS 128053 (e–h), CBS 505.90 (i–l), BRIP 12679 (m–p)). a–b, e–g, i–j, m–n, p. Conidiophores and conidia; c–d, h, k–l, o. conidia. — Scale bars: a = 50 μm; e, m–o = 20 μm; b–d, f–l, p = 10 μm.
Basionym. Trichometasphaeria holmii Luttr., Phytopathology 53: 285. 1963.
Synonyms. Helminthosporium holmii Luttr., Phytopathology 53: 285. 1963.
Drechslera holmii (Luttr.) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Keissleriella holmii (Luttr.) Arx, Gen. Fungi Sporul. Cult. (Lehr): 126. 1970.
Setosphaeria holmii (Luttr.) K.J. Leonard & Suggs, Mycologia 66: 295. 1974.
Exserohilum curvatum Sivan. & Muthaiyan, Trans. Brit. Mycol. Soc. 83: 3 19. 1984.
Type material. usa, Georgia, Griffin, on Hordeum vulgare, 15 Aug. 1961, E.S. Luttrell No. 7607 (BPI 623928 lectotype designated here (of Trichometasphaeria holmii, MBT3798 20)); on Dactyloctenium aegyptium, 15 Aug. 1961, E.S. Luttrell No. 1607-7 (CBS H-7027 isosyntype (of Helminthosporium holmii); CBS 413.65 culture ex-isosyntype); on Dactyloctenium aegyptium, 15 Aug. 1961, E.S. Luttrell No. 1607-5 (CBS 414.65 culture ex-isosyntype).
On SNA + maize leaves. Vegetative hyphae septate, branched, pale olivaceous to pale olivaceous brown, smooth-walled, 1–6.5 μm wide. Asexual morph. Conidiophores macronematous, mononematous, straight, curved or more or less flexuous, sometimes geniculate towards the apex, septate, unbranched, subcylindrical, pale to dark olivaceous brown, becoming paler at the apex, smooth-walled, but sometimes very finely verruculose around the conidiogenous loci, with cell walls usually thicker than those of the vegetative hyphae, 57–857.5 × 5–9 μm, occasionally with subnodulose to nodulose swellings up to 9.5 μm wide. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- to polytretic, proliferating sympodially, 11–36 μm long, with scars up to 4.5 μm wide. Conidia fusoid with obtuse ends, obovoid to clavate, obclavate rostrate, straight to moderately curved, mid olivaceous brown, with a small paler area at each pole, finely asperulate, but apical cell usually smooth, 3–9-distoseptate, 38.5–117.5 × 16.5–32 μm, with a strongly protruding hilum 2–4 μm wide. Sexual morph adapted from Luttrell ( 1963a) and Sivanesan ( 1987). Ascomata globose, 262–644 μm diam, unilocular, black, covered by rigid setae on the upper part, and a ostiole surrounded by short, rigid, dark brown setae; peridium composed of an outer layer of dark brown, thick-walled, pseudoparenchymatous polyhedral cells, which become thin-walled and hyaline towards the inner wall. Pseudoparaphyses numerous, hyaline, filiform, branched, sometimes anastomosing. Asci 1–8-spored, thick-walled when young, clavate, 174–232 × 28–36 μm. Ascospores fusoid, straight to curved, hyaline, (2–)3(–6)-septate, not or slightly constricted at the septa, surrounded by a mucilaginous sheath which may extend beyond either end after discharge, 47–78 × 12– 20 μm.
Culture characteristics — Colonies on SNA + sterilized maize leaves at 24 °C reaching 48–70 mm diam after 7 d, flat, translucent toward the periphery, hairy to powdery and greenish black at the centre and on the maize leaves, with a fimbriate margin; reverse concolorous with obverse.
Additional materials examined. Australia, Queensland, Goondiwindi, on leaf spot of Dactyloctenium radulans, 25 Apr. 1979, Y. Brouwer No. 7795b2 (BRIP 12679). – Thailand, Nakhon Pathom, on Dactyloctenium aegyptium, 19 Sept. 1990, J.L. Alcorn No. 9084b (CBS 128053). – Unknown country, on Dactyloctenium aegyptium, unknown date, R.R. Nelson (CBS 318.64); on Dactyloctenium aegyptium, unknown date, R.R. Nelson (CBS 3 19.64). – Venezuela, on seed of Sorghum vulgare, 24 Oct. 1983, M.C. Muthaiyan (culture ex-type of E. curvatum CBS 505.90).
Notes — Exserohilum holmii, originally described as Helminthosporium, was the cause of leaf blight of Dactyloctenium aegyptium in Georgia, USA (Luttrell 1963a). Luttrell ( 1963a) obtained the sexual morph in culture by mating compatible conidial strains on Sach's agar supporting sterilized barley grains. In the protologue, conidial size were longer and with more number of septa than those observed in this study (56–134 × 14–31 μm, 5–11-distoseptate vs 38.5–117.5 × 16.5–32 μm, 3–9-distoseptate). Exserohilum holmii has also been isolated from other grasses and other hosts including Coffea, Cymbopogon, Gossypium, Musa, Oryza, Psidium, Triticum, Solanum, etc. Besides the USA, E. holmii has been reported from Australia, India and Nigeria (Sivanesan 1987, Farr & Rossman 2017). Later, E. curvatum was introduced for a fungus growing on Sorghum vulgaris in Venezuela (Sivanesan 1984). It was distinguished from other species by the distinctively curved conidia (Sivanesan 1984). Nevertheless, E. curvatum appears to be a morphological variant. With the culture media and growth conditions used in our study, the conidia of this fungus were predominantly asymmetrical to slightly curved. Based on a culture on tap water agar (TWA) + wheat straw, conidia in the protologue of E. curvatum are longer (up to 1 20 μm vs 92.5 μm) and distinctly curved (Sivanesan 1984) than those observed in our study. In culture, E. curvatum is morphologically similar to E. holmii which also produces elongated conidia with end cells usually delimited by an accentuated distoseptum. Based on these morphological similarities and supported by the multi-locus sequence data analysis (Fig. 2), we consider E. curvatum as a synonym of E. holmii.
Exserohilum khartoumensis (El Shafie & J. Webster) P.M. Kirk, Index Fungorum 269: 1. 2015 — Fig. 5
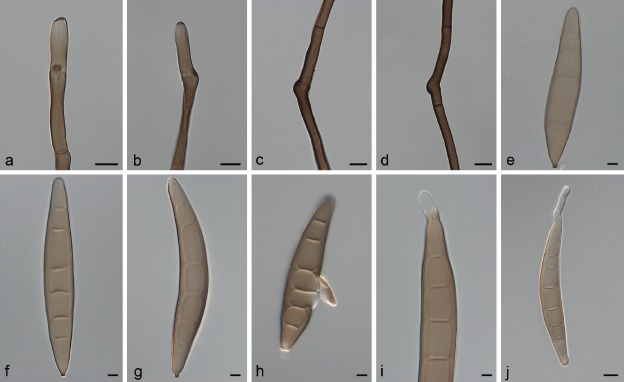
Fig. 5.
Exserohilum khartoumensis (CBS 132708 ex-isotype). a–e. Sexual morph: a. habit; b. ascoma; c, d. asci; e. ascospores; f–i. asexual morph:; f. conidiophore; g. conidiophore and conidia; h–i. conidia. — Scale bars: b = 50 μm; c–d = 20 μm; e–i = 10 μm.
Basionym. Setosphaeria khartoumensis El Shafie & J. Webster, Trans. Brit. Mycol. Soc. 77: 442. 1981.
Type material. Sudan, Khartoum, on seed of Sorghum bicolor var. mayo (HME 4006 holotype, not seen; IMI 249 194 (= CBS 132708) culture ex-isotype (of Setosphaeria khartoumensis)).
On maize meal agar with autoclaved Sorghum grains. Vegetative hyphae branched, septate, pale to mid-brown. Asexual morph. Conidiophores macronematous single or in small groups, straight to flexuous, geniculate above, septate, unbranched, brown to mid-brown, paler towards the apex, smooth, up to 240 × 5–7.5 μm thick. Conidia variable, broadly obclavate-rostrate, broadly ellipsoidal to cylindrical, straight sometimes slightly curved, end cells often rather pale and often cut off by a thick, dark septum, intermediate cells mid-dark golden brown, smooth, (6–)7–10(–12)-distoseptate, 55–160 × 15–25 μm, with a distinctly protuberant hilum. Sexual morph adapted from El Shafie & Webster ( 1981) and Sivanesan ( 1987). Ascomata unilocular, globose to ellipsoid, 200–300 × 190–300 μm, sometimes with a short cylindrical ostiolate beak, surrounded by rigid setae which also occur scattered over the upper surface of the ascomata. Setae dark brown, thick-walled, septate, bluntly rounded at the end, swollen at the base, 50–180 × 5–6 μm. Pseudoparaphyses filamentous, hyaline, septate, branched and anastomosing. Asci 1–8-spored, clavate to clavate-cylindrical, bitunicate, tapered at the base, thick-walled when young, 100–155 × 25–32.5 μm. Ascospores always 3-septate, constricted at the septa, fusoid, curved to straight, hyaline to pale brown, middle cells darker than the end cells, 42–44 × 10–15 μm, surrounded by hyaline, thin mucilaginous sheath which extends beyond the end of the spore after discharge.
Culture characteristics — Colonies on SNA + sterilized maize leaves at 24 °C reaching 52 mm diam after 7 d, hairy, with scarce aerial mycelium, translucent at the periphery, except for sparse strands of dark brown hyphae, cottony and pale mouse grey on maize leaves, with a fimbriate margin; reverse concolorous with obverse.
Notes — Exserohilum khartoumensis is a homothallic species isolated from Sorghum, and only known from the type locality, Khartoum, Sudan, (El Shafie & Webster 1981). In the protologue, both the sexual and asexual morphs were described under the name Setosphaeria khartoumensis. Recently, Kirk ( 2015) proposed the new combination in Exserohilum. In our phylogenetic tree, this species is represented by the isotype strain IMI 249 194 which forms a basal clade of E. holmii (Fig. 2).
Exserohilum minor Alcorn, Trans. Brit. Mycol. Soc. 86: 313. 1986 — Fig. 6
Fig. 6.
Exserohilum minor (IMI 294530 isotype). a–f. Sexual morph: a. ascoma; b–d. asci; e. ascospore; f. setae of the ascoma; g–i. asexual morph: g. conidiophore; h–i. conidia. — Scale bars: a = 50 μm; b–i = 10 μm.
Synonym. Setosphaeria minor Alcorn, Trans. Brit. Mycol. Soc. 86: 313. 1986.
Type material. Australia, Queensland, Saibai Island, on leaf spot of Dactyloctenium aegyptium, 1 June 1981, J.L. Alcorn (BRIP 14616 holotype; IMI 294530a isotype; BRIP 14616 culture ex-type).
Vegetative hyphae septate, branched, pale olivaceous to pale olivaceous brown, smooth to asperulate, 3–8.5 μm wide. Asexual morph based on IMI 294530b. Conidiophores macronematous, mononematous, rather straight, septate, unbranched, olivaceous brown, often paler at the apex, smooth to verruculose, length indeterminate, 3.5–7.5 μm wide, sometimes with a bulbous base up to 9.5 μm wide. Conidiogenous cells integrated, intercalary and terminal, mono- or polytretic, proliferating sympodially, mostly subcylindrical to slightly swollen, 13–48.5 × 3.5–7.5 μm, with scars up to 4.5 μm wide. Conidia fusiform, straight to slightly curved, pale olivaceous brown, smooth to verruculose, 6–8-distoseptate, 63.5–86 × 12– 19.5 μm, with a strongly protruding hilum 2–3 μm wide. Sexual morph based on IMI 294530a. Ascomata amphigenous, solitary to gregarious, erumpent, unilocular, subglobose to ovoid, black, often flattened at the base, ostiolate and sometimes papillate, 232.5–343 × 157.5–344 μm, covered by rigid setae on the upper part; peridium with outer wall layer of textura angularis. Setae dark brown, septate, unbranched, smooth to asperulate, thick-walled, 27.5–123.5 × 4.5–7.5 μm, with an obtuse, often paler apex, base sometimes swollen. Pseudoparaphyses filiform, hyaline, septate, branched, anastomosing, 2–4 μm wide. Asci 8-spored, subcylindrical to clavate, with a short stalk, 79.5–144.5 × 19.5–32.5 μm. Ascospores fusoid with obtuse ends, straight to slightly curved, pale olivaceous to pale olivaceous brown, 3(–5) septate, with central cells darker than the polar ones, constricted at the septa, 35.5–51.5 × 10–14.5 μm, enveloped by a mucilaginous sheath that extends from each end as a simple tubular appendage up to 47.5 μm long (in lactic acid mounts).
Culture characteristics — Colonies on SNA + sterilized maize leaves at 24 °C reaching 40–50 mm diam after 7 d, flat with scarce hairy aerial mycelium and whitish at the periphery, becoming cottony and pale olivaceous grey on the maize leaves, with a fimbriate to feathery margin; reverse concolorous with obverse.
Additional materials examined. Australia, Queensland, Saibai Island, ascocarps formed by BRIP 13597 in the laboratory (no culture), Mar. 1985, J.L. Alcorn (BRIP 14612); Queensland, Saibai Island, ascocarps formed by BRIP 13597 in the laboratory (no culture), Mar. 1985, J.L. Alcorn (BRIP 14614); Queensland, Saibai Island, on leaf spot of Dactyloctenium aegyptium, 1 June 1981, J.L. Alcorn (holotype of Setosphaeria minor BRIP 14615, isotype IMI 294530b, culture ex-type BRIP 14615).
Notes — Exserohilum minor is a homothallic species known only from Australia (Alcorn 1986). It is the cause of leaf spots on Dactyloctenium aegyptium. The isolates included in the present study appear to be degenerated since none of them produced the asexual morph in culture and the ascomata showed few short setae and ascospores with abnormal morphology. Therefore, in our study the description of microscopic features are based on the isotypes of E. minor IMI 294530a and S. minor IMI 294530b. However, in the protologue conidia were longer and with more distosepta (up to 135 μm long; 5–11-distoseptate) (Alcorn 1986). Exserohilum minor was phylogenetically placed in a separate basal clade to E. corniculatum, E. holmii, E. khartoumensis, E. neoregeliae and E. rostratum.
Exserohilum monoceras (Drechsler) K.J. Leonard & Suggs, Mycologia 66: 291. 1974 — Fig. 7, 8
Fig. 7.
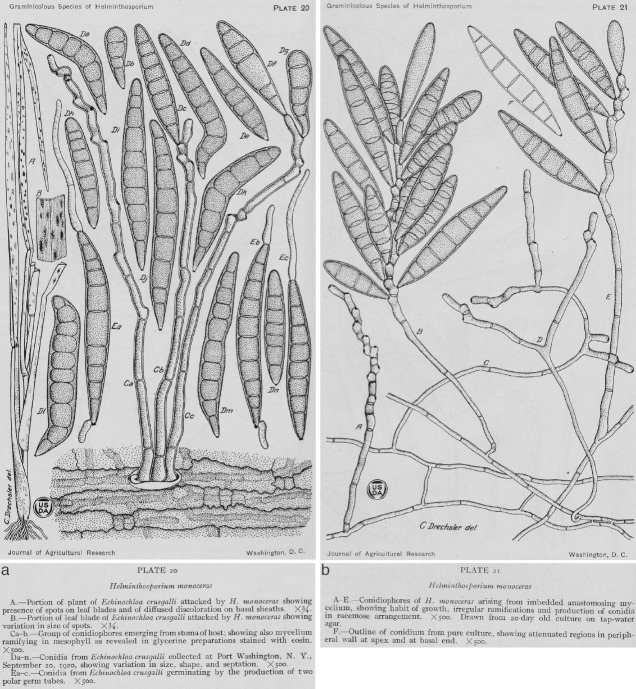
Original drawing of Helminthosporium monoceras (reproduced from Drechsler 1923). a. From natural substrate Echinochloa crus-galli; b. from culture.
Fig. 8.
Exserohilum monoceras CBS 198.29 (a–h), CBS 239.77 (i, j, l, m) and BRIP 11542 (k, n, o)). a, c–g, k–m. Conidiogenous cells with conidia; b, i–j. conidiogenous cells; h, n–o. conidia. — Scale bars: 10 μm.
Basionym. Helminthosporium monoceras Drechsler, J. Agric. Res. 24: 706. 1923.
Synonyms. Bipolaris monoceras (Drechsler) Shoemaker, Canad. J. Bot. 37: 883. 1959.
Drechslera monoceras (Drechsler) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Setosphaeria monoceras Alcorn, Mycotaxon 7: 411. 1978.
Luttrellia monoceras (Drechsler) Khokhr., as ‘Lutrellia’, Vodorosli, Griby i Mkhi Dal’nego Vostoka [Algae, Fungi and Mosses of the Soviet Far-East] (Vladivostok): 80. 1978.
Setomelanomma monoceras (Alcorn) S.A. Ahmed et al., Persoonia 33: 144. 2014 (nom. invalid Art. 41.1).
Helminthosporium crus-galli Y. Nisik. & C. Miyake, Ber. Ohara Inst. Landw. Forsch. Kurashiki 2: 597. 1925.
Type material. USA, New York, Long Island, Port Washington, on Echinochloa crus-galli, 20 Sept. 1922, C. Drechsler (BPI 429633 holotype, not seen (of Helminthosporium monoceras)).
Asexual morph adapted from Drechsler (1923). Conidiophores macronematous, single or in groups of 2–3, straight to flexuous, sometimes geniculate above, dark brown to olivaceous, paler at the apex, 1 20–325 × 6–9 μm. Conidiogenous cells integrated, terminal and intercalary, mono- to polytretic, proliferating sympodially, mostly subcylindrical. Conidia fusoid, mainly straight, yellowish when young, becoming dark olivaceous when fully matured, smooth, 3–10-septate, 40–150 × 15–22 μm, with a protruding hilum. On SNA + maize leaves (this study). Vegetative hyphae septate, branched, pale olivaceous to pale olivaceous brown, smooth, 3–6.5 μm wide. Conidiophores macronematous, mononematous, straight to flexuous, occasionally geniculate towards the apex, septate, mostly unbranched, pale to dark olivaceous brown, smooth, with cell walls often thicker than those of the vegetative hyphae, 181–743 × 4.5–10.5 μm, with occasional subnodulose intercalary swellings up to 11.5 μm wide. Conidiogenous cells terminal and intercalary, mostly subcylindrical, mono- to polytretic, proliferating sympodially, 33.5–103.5 μm long, conidiogenous loci with scars up to 6 μm wide. Conidia fusiform, straight to slightly curved, pale to dark olivaceous brown, smooth slightly verruculose near the hilum, 3–9-distoseptate, 76–1 19(–139.5) × 16–31 μm, hilum strongly protruding, 2.5–4.5 μm wide. Sexual morph adapted from Sivanesan ( 1987). Ascomata immersed, erumpent or superficial on the substrate, dark brown to black, globose to ellipsoid or ovoid, 300–500 × 260–400 μm, ostiolate, sometimes with a short broad beak, setose, especially on the upper half. Setae dark brown, unbranched, straight, paler towards the apex, septate, up to 450 μm long, 6–15 μm wide at the base which is sometimes swollen. Pseudoparaphyses filiform, hyaline, septate, branched and anastomosing. Asci 1–8-spored, cylindrical to clavate, sometimes with a short pedicel, 135–245 × 21–35 μm. Ascospores hyaline, fusoid to oblong, straight to mostly slightly curved, 2–5-(usually 3-)septate, constricted at the septa, 45–75 × 11– 20 μm, surrounded by a thin, hyaline mucilaginous sheath.
Culture characteristics — Colonies on SNA + sterilized maize leaves at 24 °C reaching 60–92 mm diam after 7 d, hairy to cottony or floccose, whitish to iron grey on maize leaves or olivaceous black, with a fimbriate margin; reverse concolorous with obverse.
Specimens examined. Australia, Queensland, Beerwah, on Echinochloa colona, Apr. 1972, J.L. Alcorn No. 19677 (BRIP 11418, CBS 239.77); Brisbane, on leaf spot of Setaria italica, 20 Mar. 1973, B. Campion No. 200 20a (BRIP 11542); from pairing single-spore cultures 77163-1 × 77163-5 on leaf sheaths of Triticum aestivum on modified Sach's agar, 23 Feb. 1978, J.L. Alcorn No. 7804b (holotype specimen of Setosphaeria monoceras BRIP 12567); Biloela, on leaf spot of Echinochloa colona, 13 Apr. 1977, M. Vincent No. 7792a (BRIP 12236); Biloela, on leaf spot of Echinochloa colona, 30 May 1977, J.L. Alcorn No. 77163 (paratype specimen of Setosphaeria monoceras BRIP 12271, culture ex-type BRIP 12271); Biloela, on leaf spot of Echinochloa colona, 30 May 1977, J.L. Alcorn No. 77163-1 (CBS 209.78). – Japan, on Echinochloa crus-galli, Nov. 1929, Y. Nisikado (CBS 198.29).
Notes — This species, formerly introduced as Helminthosporium monoceras, was isolated from a splotch in the grass Echinochloa crus-galli in Long Island, USA (Drechsler 1923) (Fig. 7). The holotype of H. monoceras (BPI 429633) is preserved in the US National Fungus Collection. The sexual morph, S. monoceras was obtained by Alcorn ( 1978) in Australia by pairing compatible single conidial isolates in modified Sach's agar media supporting sterilized wheat leaf sheaths. Unfortunately, none of the strains of E. monoceras serves as epitype, since they were collected in Australia and Japan, very distant geographically from the type locality in the USA. The type specimens of H. monoceras and S. monoceras are different and yet to be confirmed as the same using molecular phylogenetic studies. The correct phylogenetic position of E. monoceras is still unclear until molecular data from type material of H. monoceras becomes available.
It has been reported from Dichanthelium clandestinum, Echinochloa spp., Eragrostis spp., Panicum spp., Oryza sativa and Setaria viridis (Farr & Rossman 2017). In our phylogenetic tree, E. monoceras is represented by five Australian strains, which were isolated mainly from Echinochloa, but also from Setaria, and one strain isolated from Echinochloa crus-galli in Japan. Exserohilum monoceras formed a clade together with E. turcicum (Fig. 2). These two species are also similar in conidial morphology, dimensions and septation. Nevertheless, we consider them as different species based on substrate preferences; E. monoceras is mainly isolated from Echinochloa, Panicum and Setaria, while E. turcicum is mainly isolated from Zea mays and Sorghum.
Exserohilum neoregeliae Sakoda & Tsukib., Mycotaxon 118: 214. 2011 — Fig. 9
Fig. 9.
Exserohilum neoregeliae (CBS 132832 ex-type and CBS 132833). a–b. Conidiophores; c–g. conidiophores and conidia; h. upper part of conidium; i–k. conidia. — Scale bars: 10 μm.
Type material. Japan, Chiba, Narita, from living leaves of Neoregelia carolinae (imported from the Netherlands), 24 May 2006, T. Sakoda IM 201-D (NIAESH 20605 holotype, not seen; CBS 132832 culture ex-type; NIAESH 20606 isotype, not seen; CBS 132833 culture ex-isotype).
On SNA + maize leaves. Vegetative hyphae septate, branched, pale olivaceous to pale olivaceous brown, smooth to verruculose, 2.5–6.5 μm wide. Asexual morph. Conidiophores macronematous, mononematous, straight to flexuous, often strongly geniculate towards the apex, septate, almost always unbranched, pale olivaceous brown to dark brown, paler at the apex, smooth, with cell walls often thicker than those of the vegetative hyphae, 14–596 × 5.5–10 μm, often with a bulbous basal cell up to 22.5 μm wide. Conidiogenous cells integrated, terminal and intercalary, mono- to polytretic, proliferating sympodially, mostly subcylindrical, 9.5–52 μm long, conidiogenous loci with scars 2–4.5 μm wide. Conidia ellipsoidal, clavate, subcylindrical or fusiform, often appearing strongly rostrate at maturity, straight to more or less curved, pale olivaceous to dark brown, smooth to verruculose, 2–11-distoseptate, 22–161(– 191.5) × (10.5–)12–24.5(–33.5) μm, with the basal (and sometimes also the apical) cell delimited by a dark septum; hilum usually strongly protruding, 2.5–4.5 μm wide. Sexual morph not reported.
Culture characteristics — Colonies on SNA + sterilized maize leaves at 24 °C reaching 61–72 mm diam after 7 d, flat, with scarce aerial mycelium, hairy, becoming cottony on the maize leaves, grey olivaceous to olivaceous black, with a fimbriate margin; reverse concolorous with obverse.
Notes — Exserohilum neoregeliae caused leaf spots on Neoregelia carolinae plants imported from the Netherlands to Japan (Sakoda & Tsukiboshi 2011). It has not been reported from other countries or hosts since its original description. As in other members of Exserohilum, conidial size in this species can vary greatly depending on growth conditions. Based on colonies on V8 juice agar, the protologue describes conidia much longer (up to 285 μm in length) and with more septa (6–26-distosepta) than those obtained on SNA + maize leaves in our study.
Exserohilum oryzicola Sivan., Trans. Brit. Mycol. Soc. 83: 325. 1984 — Fig. 10
Fig. 10.
Exserohilum oryzicola CBS 502.90 ex-isotype (a–j) and BRIP 16229 (k–t)). a–h, k–o. Conidiophores, conidiogenous cells with conidia; i–j, q–t. conidia; p. chlamydospore. — Scale bars: a = 50 μm; b–t = 10 μm.
Synonym. Exserohilum fusiforme Alcorn, Mycotaxon 41: 337. 1991.
Type material. Colombia, Meta, Villavicencio, on leaves of Oryza sativa, 2 Nov. 1982, E.A. Urresta (IMI 273 194 holotype; CBS 502.90 culture ex-isotype).
On SNA + maize leaves. Vegetative hyphae septate, branched, pale olivaceous to pale olivaceous brown, smooth to verruculose, 2.5–7.5 μm wide. Conidiophores macronematous, mononematous, straight to flexuous, geniculate at the fertile part, septate, unbranched, pale olivaceous brown to dark brown, often paler at the apex, smooth to finely verruculose, with cell walls often thicker than those of the vegetative hyphae, 180–1436 × 4.5–8.5 μm, with subnodulose and nodulose intercalary swellings up to 11 μm wide, sometimes with a swollen basal cell up to 15.5 μm wide. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- to polytretic, proliferating sympodially, 14.5–86.5 μm long, conidiogenous loci with scars up to 5.5 μm wide. Conidia fusiform, straight to slightly curved, pale to dark olivaceous brown, smooth to finely verruculose near the hilum, 4–10-distoseptate, (41.5–) 67–179(–221) × (11–)16.5–22(–30) μm; hilum strongly protruding, 2.5–5 μm wide. Chlamydospores (only produced by isolate CBS 376.76) terminal and intercalary, ellipsoidal to subglobose, pale olivaceous brown, smooth, 7.5–22.5 μm wide. Sexual morph not reported.
Culture characteristics — Colonies on SNA + sterilized maize leaves at 24 °C reaching 44–94 mm diam after 7 d, hairy to cottony, grey olivaceous to olivaceous black, greenish black on maize leaves, with a fimbriate margin; reverse concolorous with obverse.
Additional materials examined. Australia, Queensland, Beaudesert, on leaf of Echinochloa crus-galli, 17 Mar. 1988, J.L. Alcorn (culture ex-holotype of E. fusiforme BRIP 16229 = CBS 132709). – Turkey, Ege Region, on Oryza sativa, July 1976, J. Oktar (CBS 376.76).
Notes — Exserohilum oryzicola was described growing on leaves of Oryza sativa from Colombia, characterized by long, tapered and fusiform conidia (Sivanesan 1984). Later, a morphologically similar fungus named E. fusiforme was introduced, but was distinguished from E. oryzicola by having smaller conidia (up to 141 μm) (Alcorn 1991). Exserohilum fusiforme is pathogenic to Echinochloa crus-galli causing numerous small leaf lesions and also can produce a few small linear spots on O. sativa (Alcorn 1991). In the phylogenetic tree, E. oryzicola represented by the ex-isotype strain CBS 502.90 and E. fusiforme represented by two isolates CBS 132709 (the ex-type strain) and CBS 376.76 were placed in the same clade, representing the same phylogenetic species (Fig. 2). Since both species are morphologically similar, they are known from the same substrate O. sativa, and their phylogenetic affinities, we consider E. fusiforme conspecific with E. oryzicola.
Exserohilum pedicellatum (A.W. Henry) K.J. Leonard & Suggs, Mycologia 66: 291. 1974 — Fig. 11
Fig. 11.
Exserohilum pedicellatum (CBS 375.76 (a–i) and BRIP 1 2040 (j–p)). a–c. Conidiophores; d–g, j–n. conidiophores and conidia; h–i, o–p. conidia. — Scale bars: 10 μm.
Basionym. Helminthosporium pedicellatum A.W. Henry, Tech. Bull. Minn. Agric. Exp. Stn. 22: 42. 1924.
Synonyms: Bipolaris pedicellata (A.W. Henry) Shoemaker, Canad. J. Bot. 37: 884. 1959.
Drechslera pedicellata (A.W. Henry) Subram. & B.L. Jain, Curr. Sci 35: 354. 1966.
Trichometasphaeria pedicellata R.R. Nelson, Mycologia 57: 665. 1965.
Setosphaeria pedicellata (R.R. Nelson) K.J. Leonard & Suggs, Mycologia 66: 295. 1974.
Type material. Usa, Minnesota, St. Paul, University Farm, substrate unknown, 23 Sept. 1925, A.W. Henry (BPI 429735 lectotype of Helminthosporium pedicellatum designated here (MBT379822)); on Triticum aestivum, Sept. 1964, R.R. Nelson (CBS H-12242 epitype designated here (MBT378850); CBS 322.64 culture ex-epitype).
On SNA + maize leaves. Vegetative hyphae septate, branched, subhyaline to pale olivaceous brown, smooth, 2.5–6 μm wide. Asexual morph. Conidiophores semi- to macronematous, mononematous, usually strongly geniculate towards the apex, unbranched to branched, septate, pale olivaceous brown to dark brown, smooth to asperulate, with cell walls usually thicker than those of the vegetative hyphae, 16– 196 × 4–8 μm, with subnodulose to nodulose intercalary swellings up to 9 μm wide, swellings with conidiogenous loci. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- and polytretic, proliferating sympodially, 10–43.5 μm long, with pores up to 1 μm wide, surrounded by scars 3.5–4.5 μm wide. Conidia mostly fusiform, straight to more or less curved, occasionally sigmoid, pale olivaceous brown to dark brown, paler in the area around the hilum, smooth, 4–8-distoseptate, (37–)44–88(–89) × (18–) 19.5–29(–32) μm, with a basal pedicel-like, subcylindrical extension, 6–15.5 × 4–6 μm, often delimited by a dark septum; hilum usually strongly protruding, 2.5–3.5 μm wide. Sexual morph adapted from Sivanesan ( 1987). Ascomata black, globose to ellipsoid, 250–625 × 210–600 μm, ostiolate, non-beaked, with rigid, dark brown setae surrounding the ostiole and also on the upper surface of the ascomata. Pseudoparaphyses filiform, hyaline, branched, septate and anastomosing. Asci 1–8-spored, cylindrical to cylindrical-clavate, short pedicellate, 125–210 × 21–32 μm. Ascospores fusoid, straight to curved, hyaline, (2–)3(–6)-septate, 40–60 × 11–18 μm, surrounded by a thin, hyaline mucilaginous sheath which may extend beyond the ends of the spore after discharge.
Culture characteristics — Colonies on SNA + sterilized maize leaves at 24 °C reaching 80 mm diam after 7 d, hairy to powdery, grey olivaceous to olivaceous black, with a fimbriate margin; reverse concolorous with obverse.
Additional materials examined. Australia, Queensland, Clare, on root of Oryza sativa, 11 Oct. 1976, M. Finlay (BRIP 1 2040). – Turkey, Ege region, on Oryza sativa, July 1976, J. Oktar (CBS 375.76).
Notes — This species was originally described as Helminthosporium pedicellatum from wheat roots in the USA (Henry 1924). It has been reported from root rots of various hosts, including Echinochloa, Oryza, Paspalum, Setaria, Sorghum, Triticum and Zea in Egypt, India, Pakistan, South Africa and the USA (Henry 1924, Sivanesan 1987, Gilbert 2002). It causes brown lesions on wheat roots and root rot of maize (Sivanesan 1987). The isolate CBS 322.64 was chosen as the epitype since it was collected in the same country and substrate as stated in the protologue. Unfortunately, CBS 322.64 no longer sporulate, so the morphology was described from CBS 375.76, which shows good sporulation, fits well with the description given in the protologue, and it is conspecific with CBS 322.64 (Fig. 2). In this species, some morphological variation among strains was observed; conidia in the strain BRIP 1 2040 were mostly clavate with rounded apex, while those of CBS 375.76 were mostly fusiform with acute apex. Exserohilum pedicellatum is easy to identify on account of its mostly broadly fusiform conidia with a basal, cylindrical, pedicel-like extension.
Exserohilum protrudens Alcorn, Trans. Brit. Mycol. Soc. 90: 146. 1988 — Fig. 12
Fig. 12.
Exserohilum protrudens (BRIP 14816). a. Conidiophore and conidia; b–i. conidia. — Scale bars: 10 μm.
Type material. Australia, Queensland, Torres Strait, Yorke Island, on leaf spot of Dactyloctenium aegyptium, 30 May 1985, R.A. Peterson (BRIP 14814 holotype; BRIP 14814 culture ex-type; IMI 316693 isotype).
Asexual morph adapted from Alcorn ( 1988b). Conidiophores macronematous, mononematous, straight to flexuous, geniculate, septate, unbranched, cylindrical, mid to dark olivaceous brown, paler towards the apex, smooth, basal cell commonly swollen, 11–21 μm wide, 6–10 μm wide near the base, 5–7.5 μm wide at the apex, up to 2 100 μm long. Conidiogenous cells terminal and intercalary, mono- and polytretic, cicatrized, verruculose and slightly swollen at the conidiogenous nodes. Conidia fusoid to obclavate-fusoid, sometimes shortly and broadly rostrate, straight or curved, (5–)7–8(–9)-distoseptate, 55–105 × 14–27 μm, smooth, olivaceous brown, concolorous except for the hilar protrusion which is paler; hilum is borne on a distinct truncate conical protrusion 2.5–5 μm long, 2.5–4 μm wide proximal to the body of the spore, and 2–3 μm wide at the hilar extremity. Sexual morph not reported.
Culture characteristics — Colonies on SNA + sterilized maize leaves at 24 °C reaching 40–61 mm diam after 7 d, mycelium mostly immersed, with scarce aerial mycelium, whitish, becoming pale mouse grey to mouse grey and cottony on the maize leaves, with a fimbriate margin; reverse concolorous with obverse.
Additional material examined. Australia, Queensland, Coconut Island, 1 June 1985, R.A. Peterson (BRIP 14816).
Notes — This species is only known from two specimens causing leaf spot on Dactyloctenium aegyptium from the Torres Strait Islands (Yorke Island and Coconut Island). Attempts to produce the sexual morph were not successful (Alcorn 1988b). Exserohilum protrudens is phylogenetically placed in a basal clade to E. monoceras, E. oryzicola, E. pedicellatum and E. turcicum.
Exserohilum rostratum (Drechsler) K.J. Leonard & Suggs, Mycologia 66: 290. 1974 — Fig. 13, 14, 15, 16, 17
Fig. 13.
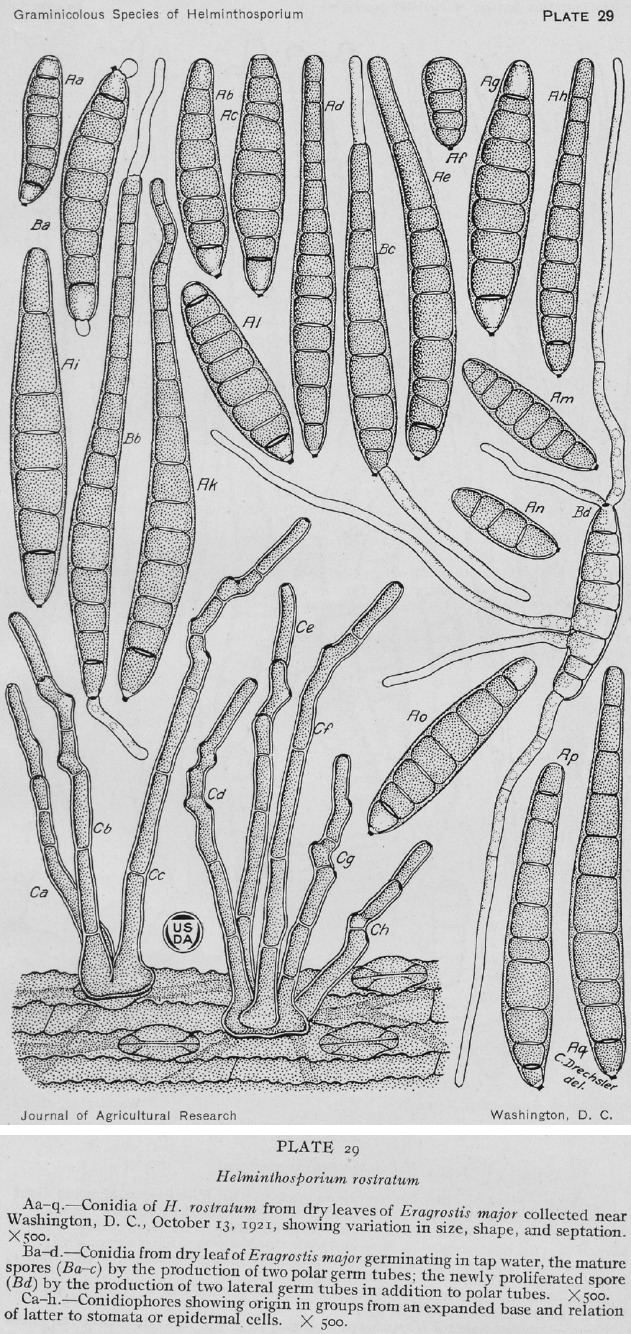
Original drawing of Helminthosporium rostratum from dry leaves of Eragrostis major collected near Washington (lectotype) (reproduced from Drechsler 1923).
Fig. 14.
Exserohilum rostratum (BPI 429032 (holotype of Helminthosporium halodes) (a–f), CBS 128061 (g–j), CBS 1 20380 (k–n)). a. Conidiophore; b–f, j, n. conidia; g–i, k–m. conidiophores and conidia. — Scale bars: a, k = 20 μm; b–j, l–n= 10 μm.
Fig. 15.
Exserohilum rostratum (CBS 325.87 (ex-type of E. macginnisii) (a–d), BRIP 11416 (e–h), CBS 128060 (i–k), BRIP 11422 (l–n), CBS 128054 (o–q), CBS 412.93 (ex-type of E. antillanum) (r–t)). a–b, r–t. Conidiophores and conidia; c. chlamydospore; d–q. conidia. — Scale bars: a–n, p–t = 10 μm; o = 40 μm.
Fig. 16.
Exserohilum rostratum (CBS 572.73 (as E. prolatum) (a–f), CBS 127233 (g–j), CBS 196.29 (ex-syntype of H. leptochloae) (k–n), CBS 3 20.64 (o–r)). a–d, g–i, k–m, o–p. Conidiophores and conidia; e–f, j, n, q–r. conidia. — Scale bars: a–j, l–r = 10 μm; k = 20 μm.
Fig. 17.
Phylogenetic tree inferred from a RAxML analysis based on rpb2 sequences of Exserohilum rostratum. The bootstrap support values are given at the nodes. In green font are indicated taxa previously identified as E. macginnisii, in blue identified as E. longirostratum, in orange as E. holmii. Ex-type, strains are indicated in bold. The tree was rooted to E. minor BRIP 14612 and BRIP 14614.
Basionym. Helminthosporium rostratum Drechsler, J. Agric. Res. 24: 724. 1923.
Synonyms: Bipolaris rostrata (Drechsler) Shoemaker, Canad. J. Bot. 37: 883. 1959.
Drechslera rostrata (Drechsler) M.J. Richardson & E.M. Fraser, Trans. Brit. Mycol. Soc. 51: 148. 1968.
Luttrellia rostrata (Drechsler) Gornostaĭ, as ‘Lutrellia’, Vodorosli, Griby i Mkhi Dal’nego Vostoka [Algae, Fungi and Mosses of the Soviet Far-East] (Vladivostok): 81. 1978.
Helminthosporium halodes Drechsler, J. Agric. Res. 24 (8): 709. 1923.
Helminthosporium halodes Drechsler var. tritici Mitra, Trans. Brit. Mycol. Soc. 15 (3-4): 287. 1931.
Helminthosporium halodes Drechsler var. elaeicola Kovachich, Trans. Brit. Mycol. Soc. 37 (4): 423. 1954.
Bipolaris halodes (Drechsler) Shoemaker, Canad. J. Bot. 37: 883. 1959.
Drechslera halodes (Drechsler) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Drechslera halodes (Drechsler) Subram. & B.L. Jain var. halodes (Drechsler) Subram. & B.L. Jain, Curr. Sci. 35: 354. 1966.
Drechslera halodes (Drechsler) Subram. & B.L. Jain var. elaeicola Kovachich, Trans. Brit. Mycol. Soc. 37: 423. 1954.
Exserohilum halodes (Drechsler) K.J. Leonard & Suggs, Mycologia 66: 290. 1974.
Helminthosporium leptochloae Y. Nisik. & C. Miyake, Ber. Ohara Inst. Landw. Forsch. Kurashiki 2: 483. 1924.
Helminthosporium longirostratum Subram., J. Indian Bot. Soc. 35: 463. 1957.
Exserohilum longirostratum (Subram.) Sivan., Trans. Brit. Mycol. Soc. 83 (2): 328. 1984.
Exserohilum prolatum K.J. Leonard & Suggs, Mycologia 66: 290. 1974.
Setosphaeria prolata K.J. Leonard & Suggs, Mycologia 66: 294. 1974.
Setosphaeria rostrata K.J. Leonard, Mycologia 68: 409. 1976.
Exserohilum gedarefense (El Shafie) Alcorn, as ‘gedarefensis’, Mycotaxon 17: 68. 1983.
Exserohilum macginnisii A.A. Padhye & Ajello, as ‘mcginnisii’, J. Clin. Microbiol. 24: 247. 1986.
Exserohilum antillanum R.F. Castañeda, Guarro & Cano, Mycol. Res. 99: 825. 1995.
Setomelanomma rostrata Green et al., Allergy, Asthma & Clinical Immunology 10: 3. 2014 (nom. invalid Art. 41.1).
Type material. USA, Washington DC, on dry leaves of Eragrostis major, Sept. 1921, C. Drechsler (BPI 430144 holotype (of Helminthosporium rostratum)).
Adapted from Leonard ( 1976). Vegetative hyphae septate, branched, pale olivaceous to pale olivaceous brown, smooth to verruculose, 2.5–8 μm wide. Asexual morph. Conidiophores macronematous, mononematous, straight to flexuous, geniculate towards the apex, septate, unbranched, subcylindrical, pale olivaceous brown to dark olivaceous brown, with cell walls usually thicker than those of the vegetative hyphae, smooth, becoming finely verruculose around the conidiogenous loci, 65–395.5 × 4–7.5 μm, base up to 9 μm wide. Conidiogenous cells integrated, terminal and intercalary, mono- to polytretic, proliferating sympodially, irregularly pigmented, mostly subcylindrical, 5.5–27.5 μm long, pores surrounded by scars up to 3–4.5 μm wide. Conidia ellipsoid, clavate, obclavate or subcylindrical, rostrate or not, straight to moderately curved, pale olivaceous brown to dark olivaceous brown, basal and apical cells often delimited by a dark septum, smooth to verruculose, 1–15-distoseptate, 15– 190 × 7–29 μm, hilum 3–4 μm wide. Sexual morph. Ascomata superficial, globose to ellipsoid, black, 340–600 × 330–580 μm, ostiolate, sometimes with a short, cylindrical, beak, with rigid, dark brown, septate setae surrounding the ostiole, and the upper surface of the ascomata. Pseudoparaphyses filiform, hyaline, septate, branched and anastomosing. Asci 1–8-spored, clavate to cylindrical-clavate, short stalked, 105–260 × 26–42 μm. Ascospores hyaline to pale brown, fusoid, straight to curved, (2–)3(–5)-septate, constricted at the septa, 29–85 × 9–21 μm, surrounded by a thin, hyaline mucilaginous sheath which may extend beyond the ends of the spore after discharge.
Culture characteristics — Colonies on SNA + sterilized maize leaves at 24 °C reaching 64–108 mm diam after 7 d, hairy, grey olivaceous to olivaceous black, with patches of cottony whitish to olivaceous grey mycelium on maize leaves and a fimbriate margin; reverse concolorous with obverse.
Additional materials examined. Australia, New South Wales, Broken Head, on leaf spot of Spinifex hirsutus, 6 Jan. 1988, J.L. Alcorn (BRIP 16078); Leadville, on Hordeum vulgare, 19 July 2002, G. Platz (BRIP 29236c); Sydney, on Sorghum sp., 1998, F. Benyon (BRIP 28001); Northern Territory, Howard Springs, on leaf spot of Areca catechu, 28 July 1986, M. Connelly (BRIP 15274); Queensland, Atherton, on stalk rot of Zea mays, 9 Apr. 1985, M.D. Ramsey (BRIP 14916); Bamaga, on leaf spot of Eragrostis tenella, 29 May 1981, J.L. Alcorn (BRIP 10724); Biloela, on leaf lesion of Zea mays, 12 Apr. 1972, N.T. Vock (BRIP 11416); Brisbane, on leaf blight of Cymbopogon citratus, 22 Feb. 1988, J.L. Alcorn (BRIP 16114); on leaf spot of Croton sp., 7 June 2010, L.I. Forsberg (BRIP 53634b); from pairing single-spore cultures BRIP 122 20 (USA, North Carolina, from Zea mays, 14 Jan. 1977, K.J. Leonard SrA3) × BRIP 11761 (Kingaroy, from stalk rot of Zea mays, 13 Apr. 1976, P.E. Mayers) on Barley seeds on Sach's agar, 22 Mar. 1997, J.L. Alcorn (BRIP 12224, culture CBS 128062); Gatton, on crown rot of Triticum aestivum, Oct. 1986, R.L. Dodman (BRIP 15489); Goomeri, on leaf spot of Zea mays, 26 Apr. 1972, C. Euler (BRIP 11422); Gympie, on leaf spot of Megathyrsus maximus, 6 July 1992, D.S. Loch (BRIP 20144b); Lawes, on leaf spot of Dinebra retroflexa, 4 Apr. 1977, J.L. Alcorn (BRIP 12147); Mundubbera, on leaf spot of Vitis vinifera, 11 Feb. 2005, C.M. Horlock and P. Jackson (BRIP 46107); Nebo, on undetermined Poaceae, 6 Sept. 1972, unknown collector (BRIP 11432); Norwin, on leaf spot of Zea mays, 28 Mar. 1972, K.M. Middleton (BRIP 10995); Parada, on ear rot of Zea mays, 13 Apr. 1972, W. Pont (BRIP 11417); Peregian Beach, on leaf spot of Paspalidium distans, 7 Mar. 1982, J.L. Alcorn No. 8230b (BRIP 13560); Rockhampton, on leaf spot of Chrysalidocarpus lutescens, 23 July 1986, unknown collector (BRIP 15403); Sabai Island, on leaf spot of Eragrostis brownii, 28 Feb. 1977, J.L. Alcorn No. 7728 (BRIP 1 2090); Sabai Island, on leaf spot of Ischaemum villosum, 1 June 1982, J.L. Alcorn No. 8 194a (BRIP 13592); Saibai Island, on leaf of Dactyloctenium aegyptium, 1 June 1981, J.L. Alcorn (BRIP 13599); Samford, on leaf spot of Eragrostis pilosa, 25 May 1977, J.L. Alcorn No. 77162 (BRIP 12270); Warwick, on Hordeum vulgare, 22 June 2009, K. Stephen (BRIP 52639). – Cuba, Ciudad de la Habana, Santiago de las Vegas, on plant debris from forest soil, 10 Feb. 1993, R.F. Castañeda-Ruiz (culture ex-isotype of E. antillanum CBS 412.93). – Guatemala, on seed of Zea mays, Feb. 1972, unknown collector, No. Ep1 (authentic mating type ‘minus’ of Setosphaeria prolata CBS 572.73). – India, Poona, from soil, unknown date, P.G. Patwardhan (CBS 705.71, CBS 706.71). – Japan, Okayama, Kurashiki, on Leptochloa chinensis, Oct. 19 19, Y. Nisikado (syntype of H. leptochloae CBS H-72 20; culture ex-syntype of H. leptochloae CBS 196.29). – Martinique, on heart valve prosthesis of Homo sapiens, 1980, unknown collector (FMR 11773). – Namibia, Windhoek, endophytic on 5-mo-old seedlings of Acacia mellifera subsp. detinens, Apr. 1993, G. Holz (CBS 128054); Windhoek, endophytic on 5-mo-old seedlings of Acacia mellifera subsp. detinens, Apr. 1993, G. Holz (CBS 128055). – South Africa, Gauteng Province, Johannesburg, unknown substrate, unknown date, P. Martin No. 1323 (CBS 188.68); Free State Province, Bethlehem, on Triticum aestivum, Apr. 1939, K. Putterill No 30456 (CBS 230.39); Gauteng Province, on Triticum aestivum, Apr. 1939, K. Putterill No. 30434 (CBS 229.39). – Sudan, on grains of Sorghum bicolor, Apr. 1979, A.E. El Shafie (culture ex-type of E. gedarefense CBS 297.80); 21 Mar. 1983, J.L. Alcorn (as E. gedarefense CBS 504.90). – Unknown country, on Zea mays, unknown date, J.L. Alcorn (CBS 732.96); from nasal mucosa of 45-yr-old male with acute myelogenous leukemia, unknown date, dep. I. Polacheck (as E. macginnisii CBS 1 20308); unknown substrate, unknown date, unknown collector (CBS 467.75 = ATCC 32 198). – Usa, Florida, Gainesville, on Zea mays, 27 May 1972, E.S. Luttrell 8868 ‘mating type A’ (CBS 128061); Georgia, Decatur County, on Zea mays, 8 June 1971, E.S. Luttrell 8686 ‘mating type A’ (CBS 128060); Georgia, from eye (wood splinter) of Homo sapiens, 2009, S.A. Sutton (FMR 11399); Mississippi, State College, on leaf of Zea mays, Oct. 1971, M.C. Futrell No. Ep3 (authentic mating type ‘plus’ of Setosphaeria prolata CBS 571.73); Montana, from maxillary sinus of Homo sapiens, 2009, S.A. Sutton (FMR 11395); New York, Douglaston, on Distichlis spicata, 26 Sept. 19 20, C. Drechsler (holotype of Helminthosporium halodes BPI 429032); North Carolina, ascospore isolate from Hay3 × IMI 76563, 14 Jan. 1977, K.J. Leonard (CBS 128063); North Carolina, on barley grains on Sach's agar by mating compatible isolates Ep1 × Ep3, 1974, K.J. Leonard (holotype specimen of Setosphaeria prolata BPI 622161); Oklahoma, Stillwater, on Leptochloa filiformis, 29 Aug. 1960, R.A. Shoemaker (CBS 127233); Texas, on elbow tissue of Homo sapiens, 2008, D.A. Sutton (FMR 11028); sinus, 2005, D.A. Sutton (FMR 11271); from cornea of Homo sapiens, 2006, D.A. Sutton (FMR 11278); great toe, 2006, D.A. Sutton (FMR 11280); shin skin of Homo sapiens, 2007, D.A. Sutton (FMR 11286); nasal inferior turbinate, 2008, D.A. Sutton (FMR 11390); unknown tissue of Homo sapiens, 2007, D.A. Sutton (FMR 11372); Tucson, Arizona, from nasal polyp from Homo sapiens, unknown date, A.A. Padhye (culture ex-type of E. macginnisii CBS 325.87); unknown state, on Bromus inermis, unknown date, R.R. Nelson (CBS 3 20.64); unknown state, on Zea mays, unknown date, R.R. Nelson No. 26 (CBS 323.64); Utah, maxillary sinus of Homo sapiens, 2008, D.A. Sutton (FMR 11392). – Zambia, on seed of Pennisetum spicatum, 1951, W.E. Kerr, No G. 32 (CBS 273.52).
Notes — Exserohilum rostratum is by far the most commonly recorded and known species of the genus. This cosmopolitan species has been recorded from numerous hosts, especially Poaceae and other monocots, causing leaf spot and foot rot of wheat and other grasses, blight, damping-off, rots including leaf spot of banana (Sivanesan 1987, Farr & Rossman 2017). Clinical human reports of this species have also been increasing (Aquino et al. 1995, Adler et al. 2006, Al-Attar et al. 2006, Derber et al. 2010, Da Cunha et al. 2012, Kainer et al. 2012, Smith et al. 2013).
Exserohilum rostratum was first described as Helminthosporium rostratum, isolated from Eragrostis major in the USA (Drechsler 1923). The holotype, BPI 430144, is preserved at the US National Fungus Collection. Unfortunately, no culture is linked to the holotype. Among the specimens examined in our study, none of them were suitable to serve as an epitype, since there are no isolates from E. major from the USA. In the protologue, Drechsler ( 1923) described ellipsoidal conidia with 3–9 septa and rostrate conidia with 8–15 septa, measuring 32–184 × 14–22 μm (Fig. 13). Leonard ( 1976) noticed the wide morphological variability of this species when he introduced the synonymy of E. halodes (conidial size after emendation 15– 190 × 7–29, 1–15-septate). He pointed out that isolates of E. rostratum that originally produce strongly rostrate conidia may lose that characteristic in culture. Another factor that influences the conidial morphology was the light. Isolates that were exposed to light formed strongly rostrate conidia, but in the dark they formed only ellipsoidal conidia (Leonard 1976). During our study, conidial morphology in the specimens examined was also highly variable (Fig. 14, 15, 16). Furthermore, phylogenetic analysis based on multi-locus data show that E. antillanum, E. gedarefense, E. longirostratum, E. macginnisii, E. prolatum and Helminthosporium leptochloae are conspecific to E. rostratum, and therefore they are listed here as synonyms (Fig. 1).
In E. rostratum, 14 lineages were discerned with the individual analysis of the most informative gene, rpb2 (Fig. 17). The clinical isolates were mainly distributed in three lineages, i.e., ‘rostratum’, ‘macginnisii’ and lineage 12, except for the isolate FMR 11286 which formed an independent lineage. The lineage ‘rostratum’ includes 26 strains isolated mainly from monocotyledon plants (Fig. 17, Table 1) but also include four clinical isolates (FMR 11773, FMR 11028, FMR 11399 and CBS 1 20308) from Australia, USA, South Africa, India and Zambia. The lineage ‘macginnisii’ includes CBS 325.87 (the ex-type of E. macginnisii) isolated from a clinical sample, CBS 128060 (the mating type A used by Luttrell to produce the sexual morph), CBS 128063 (ascospore isolate from crossing: Hay 3 (R.R. Nelson) × IMI 76563), and three isolates from Zea mays CBS 732.96, BRIP 11422 and BRIP 11416 from Australia and the USA. The lineage 12 includes mostly clinical isolates from the USA (FMR 11390, FMR 11287, FMR 11271, FMR 11280, FMR 11372, FMR 11395, FMR 11392) and two isolates from plants BRIP 29236 (Hordeum vulgare, Australia) and CBS 3 20.64 (Bromus inernis, USA). The lineage ‘prolatum’ includes three isolates, two of them CBS 571.73 and CBS 572.73, isolated from Zea mays in the USA and Guatemala, respectively, and CBS 127233 deposited as ‘Drechslera micropus’ isolated from Leptochloa filiformis in the USA. The lineage ‘gedarefense’ includes two isolates identified as E. gedarefense including the type strain CBS 297.80 and CBS 504.90, both isolated from Sorghum bicolor in Sudan. The lineage ‘antillanum’ includes CBS 412.93 (ex-type strain of E. antillanum) isolated from plant debris in Cuba. The clade ‘leptochloae’ includes CBS 196.29 (ex-type strain of H. leptochloae) isolated from Leptochloae chinensis in Japan. Other lineages were formed by individual strains CBS 230.39, BRIP 13592, BRIP 1 2090, CBS 128054, and BRIP 12270. Interestingly, the isolate CBS 128062 (= BRIP 12224) which was the offspring of a cross among BRIP 122 20 (USA, North Carolina, from Zea mays, 14 Jan. 1977, K.J. Leonard SrA3) × BRIP 11761 (Kingaroy, from stalk rot of Zea mays, 13 Apr. 1976, P.E. Mayers) formed a basal lineage together with CBS 467.75 (= ATCC 32 198 = IMI 197560), which is labelled at the ATCC database as SrA3. However, with the information available in the CBS and IMI database we cannot corroborate this data.
Exserohilum turcicum (Pass.) K.J. Leonard & Suggs, Mycologia 66: 291. 1974 — Fig. 18
Fig. 18.
Exserohilum turcicum (BPI 431157 holotype (a–j), CBS 690.71 ex-epitype (k–s)). a–e. Conidiophores and conidiogenous cells; f–j. conidia; k–s. conidiophores and conidia. — Scale bars: a–j = 10 μm; k–s = 20 μm.
Basionym. Helminthosporium turcicum Pass., Boln Comiz. Agr. Parmense 10: 3. 1876.
Synonyms. Bipolaris turcica (Pass.) Shoemaker, as ‘turcicum’, Canad. J. Bot. 37: 884. 1959.
Drechslera turcica (Pass.) Subram. & B.L. Jain, Curr. Sci. 35: 355. 1966.
Luttrellia turcica (Pass.) Khokhr., as ‘Lutrellia’, Vodorosli, Griby i Mkhi Dal’nego Vostoka [Algae, Fungi and Mosses of the Soviet Far-East] (Vladivostok): 81. 1978.
Trichometasphaeria turcica Luttr., Phytopathology 48: 282. 1958.
Keissleriella turcica (Luttr.) Arx, Gen. Fungi Sporul. Cult. (Lehr): 126. 1970.
Setosphaeria turcica (Luttr.) K.J. Leonard & Suggs, Mycologia 66: 295. 1974.
Helminthosporium inconspicuum Cooke & Ellis, Grevillea 6 (no. 39): 88. 1878.
Type material. Italy, Parma, on Zea mays, date unknown, G. Passerini (BPI 431157 lectotype designated here (of Helminthosporium turcicum MBT379823)). – Germany, Lower Saxony, Einbeck, on Zea mays, unknown date, D. Heitmann No. W64A (CBS H-23323 epitype designated here, MBT378854; CBS 690.71 culture ex-epitype).
On Zea mays leaves (BPI 431157). Vegetative hyphae mostly immersed, septate, branched, pale olivaceous to pale olivaceous brown, smooth, 3–7.5 μm wide. Asexual morph. Conidiophores macronematous, single to caespitose, usually emerging from stomata, straight to flexuous, often geniculate above, septate, mostly unbranched, subcylindrical, septate, mostly simple, olivaceous brown, becoming paler towards the apex, finely verruculose, with cell walls thicker than those of the vegetative hyphae, length indeterminate, 5.5–10.5 μm wide, often with a bulbous base up to 19.5 μm wide, rarely with subnodulose and nodulose intercalary swellings up to 15 μm wide. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- and polytretic, proliferating sympodially, 10–86.5 μm long; pores up to 1 μm wide, surrounded by scars 4–5.5 μm wide. Conidia ellipsoidal, obclavate to fusiform, straight to slightly curved, pale olivaceous brown, smooth, sometimes asperulate at the base, (1–)3–9-distoseptate, (51–)60.5–126(–140) × (16.5–) 19.5–31(–33) μm, with a slightly to strongly protruding hilum 2–4.5 μm wide.
On SNA + maize leaves. Vegetative hyphae septate, branched, pale olivaceous to pale olivaceous brown, smooth to verruculose, with anastomoses, 2–9 μm wide. Asexual morph. Conidiophores semi-macronematous to macronematous, straight to flexuous, often geniculate at the fertile part, septate, often unbranched, subcylindrical, pale olivaceous brown to dark brown, paler at the apex, smooth to asperulate, with cell walls thicker than those of the vegetative hyphae, 169–1324.5 × 5.5–10 μm, often with a swollen, sometimes bulbous base up to 22 μm wide, with subnodulose to nodulose intercalary swellings up to 12.5 μm wide, swellings with conidiogenous loci. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- to polytretic, proliferating sympodially, 11–74 μm long, with scars up to 4.5 μm wide. Conidia fusoid, straight to more or less curved, pale to dark olivaceous brown, smooth to finely verruculose, (1–)4–7-distoseptate, (43–)76–135.5(–141) × (10–)16.5–22(–25) μm, with a strongly protruding hilum 2–4.5 μm wide. Microcyclic conidiation occasionally observed. Sexual morph (setosphaeria-like) adapted from Sivanesan ( 1987). Ascomata globose to ellipsoid, black, 350–725 × 345–500 μm, ostiolate, with rigid, dark brown, septate setae surrounding the ostiole, and the upper surface of the ascomata. Pseudoparaphyses filiform, hyaline, septate, branched and anastomosing. Asci 1–8-spored, cylindrical-clavate, short stalked, 175–250 × 24–31 μm. Ascospores hyaline, fusoid, straight to curved, (1–)3(–6)-septate, constricted at the septa, 40–78 × 12–18 μm, surrounded by a thin, hyaline mucilaginous sheath which may extend beyond the ends of the spore after discharge.
Culture characteristics — Colonies on SNA + sterilized maize leaves at 24 °C reaching 50–80 mm diam after 7 d, hairy to floccose, olivaceous grey to olivaceous black, cottony on maize leaves, with a fimbriate margin; reverse concolorous with obverse.
Additional materials examined. Australia, Queensland, Samford, on leaf blight of Sorghum bicolor, 25 May 1977, J.L. Alcorn No. 77159 (BRIP 12267); on leaf speckle of Sorghum sudanense, 6 Apr. 1981, R. Jones No. 22233 (BRIP 13326). – Indonesia, Lembang, on leaf of Zea mays, June 1926, M.B. Schwarz (CBS 195.26). – Usa, on leaf spot of Zea mays, Sept. 1964, R.R. Nelson (CBS 330.64); Georgia, dried culture of a crossing of compatible isolates on Sach's agar with Hordeum vulgare straw, 1954, E.S. Luttrell No. 6001 (holotype of Setosphaeria turcica BPI 623931); single ascospore isolate, Mar. 1958, E.S. Luttrell No. 1 198-9 (‘plus’ strain of S. turcica CBS 384.58); E.S. Luttrell No. 1 198-6 (‘minus’ strain of S. turcica CBS 385.58); on leaf of Sorghum halepense, Mar. 1958, E.S. Luttrell (CBS 386.58); Tifton, on leaf of Zea mays, Mar. 1958, E.S. Luttrell (CBS 387.58).
Notes — Helminthosporium turcicum was described on maize from Italy (Passerini 1876, Saccardo 1886). Luttrell ( 1958) obtained the ascomata by mating opposite compatible sexual strains, and introduce Trichometasphaeria turcica for the sexual morph. Later, Leonard & Suggs ( 1974) introduce Exserohilum with E. turcicum, based on H. turcicum, as the generic type and introduce the sexual morph as Setosphaeria. The herbarium material preserved at the US National Fungal Collection, BPI 431157 bears the label ‘Type?’. This material was collected by G. Passerini from the same locality and host as the type, and is therefore designated here as the lectotype. To stabilize the name, the isolate CBS 690.71 from Zea mays in Germany, is proposed as the ex-epitype, being from the same host and geographically close to the type locality, Italy, and its morphology fits well with the description of the species. This species causes the disease known as northern leaf blight of maize. It has also been reported on Euchlaena, Sorghum and other graminicolous plants. It is widespread in both tropical and subtropical areas. In the phylogenetic tree, E. turcicum is represented by eight strains isolated from Zea mays and Sorghum spp. collected from different geographical origins, i.e., Australia, Germany, Indonesia and the USA (Fig. 2).
DOUBTFUL OR EXCLUDED SPECIES
In this section are included species retained in Exserohilum based on morphology (without molecular data), and species transferred to other genera based on molecular and/or morphological data.
Exserohilum curvisporum Sivan., Abdullah & B.A. Abbas, Mycol. Res. 97: 1486. 1993 — Fig. 19
Fig. 19.
Exserohilum curvisporum (IMI 356632 holotype). a–e. Conidiophores and conidiogenous cells; f–j. conidia. — Scale bars: 10 μm.
Type material. Iraq, Basrah, isolated from sediments of Shatl-al-Arab River, 15 Dec. 1991, S.K. Abdullah & A. Abbas BSRA 10260 (IMI 356632 holotype).
Adapted from Sivanesan et al. ( 1993). Colonies effuse, pale brown. Vegetative hyphae pale brown, branched, septate, smooth, 4–5 μm wide. Conidiophores commonly unbranched, straight to flexuous, geniculate above, cicatrized, cylindrical, olivaceous brown, paler towards the apex, 125–450 × 6–8 μm. Conidia cylindrical to cylindrical-fusiform, mostly strongly curved, sometimes slightly curved or sigmoid, rarely straight, concolorous, pale brown, surface often granulose, 1–12-distoseptate, 65–165 × 12.5–22 μm, mostly 80–125 × 14–15 μm, with a basal distinctly protruding hilum up to 1 μm wide.
Notes — Exserohilum curvisporum is only known from the type locality, isolated from sediments of a river in Iraq. Although no molecular data exist for E. curvisporum, this species is retained in Exserohilum based on the characteristic hilum structure and conidial morphology.
Exserohilum echinochloae Sivan., Trans. Brit. Mycol. Soc. 83: 3 19. 1984 — Fig. 20
Fig. 20.
Exserohilum echinochloae (IMI 237838 holotype). a–d. Conidiogenous cells; e. lower part of conidium; f–j. conidia. — Scale bars: 10 μm.
Type material. Bangladesh, on leaves of Echinochloae colona, 10 Apr. 1979, M.A. Miah (IMI 237838 holotype).
Herbarium material. Colonies dark brown, effuse, sporulating abundantly. Vegetative hyphae septate, branched, pale olivaceous to pale olivaceous brown, 2.5–6 μm wide. Conidiophores macronematous, mononematous, straight, more or less bent or flexuous, septate, mostly unbranched, subcylindrical, brown, becoming paler and sometimes appearing geniculate toward the apex, smooth to asperulate, with cell walls thicker than those of the vegetative hyphae, 216–812.5 × 5.5–9.5 μm, and with a bulbous base up to 14.5 μm wide, often with subnodulose and nodulose intercalary swellings up to 13 μm wide, swellings with conidiogenous loci. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- and polytretic, proliferating sympodially, 26–88 μm long, pores 0.5–1 μm wide, surrounded by scars 4.5–5 μm wide. Conidia fusiform, straight to curved, olivaceous brown, smooth, asperulate at the base, 7–11-distoseptate, 111– 197.5 × 19–37 μm, basal cell often bulging a little, with a strongly protruding hilum 2.5–3.5 μm wide.
Notes — Exserohilum echinochloae is morphologically similar to E. monoceras and E. turcicum, but differs from them by having longer and wider conidia (Sivanesan 1984). Although no molecular data exist for E. echinochloae, this species is retained in Exserohilum based on the characteristic hilum structure and conidial morphology.
Exserohilum elongatum Hern.-Restr. & Crous, sp. nov. — MycoBank MB823162; Fig. 21
Fig. 21.
Exserohilum elongatum (IMI 321829 holotype). a–e. Conidiogenous cells; f–j. conidia. — Scale bars: 10 μm.
Synonym. Exserohilum elongatum Del Serrone et al., Phytopath. Mediterr. 30: 152. 1991 [nom. invalid Art. 40.1].
Type material. Italy, Piemonte, Cherasco (Cuneo), on leaves of Echinochloa crus-galli, 1979, Porta-Puhglia, IMI 321829 holotype designated here.
Herbarium material. Colonies brown, effuse, with abundant sporulation. Vegetative hyphae septate, branched, pale olivaceous to pale olivaceous brown, smooth to asperulate, 2–6 μm wide. Conidiophores macronematous, straight to flexuous, septate, mostly unbranched, subcylindrical, pale olivaceous brown to dark brown, often paler toward the apex, smooth to asperulate, with cell walls thicker than those of the vegetative hyphae, 168.5–1 190 × 7–9.5 μm, often with a bulbous base up to 18.5 μm wide, with subnodulose intercalary swellings up to 11.5 μm wide, swellings with conidiogenous loci. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- and polytretic, proliferating sympodially, 19.5–97 μm long, pores 0.5–1 μm wide, surrounded by scars 4–6 μm wide. Conidia clavate, cylindrical to fusiform, olivaceous brown, often somewhat paler at the apex or at the ends, smooth, asperulate at the base, 4–8-distoseptate, (42–)81–168 × 19–28.5 μm, with a strongly protruding hilum 2–3.5 μm wide.
Notes — Exserohilum elongatum is morphologically comparable with E. echinochloae and E. oryzicola. However, the protologue of the former (Del Serrone et al. 1991) describes longer conidia in respect to the other two species (i.e., 114–247 × 28–30 μm vs 150–210 × 25–35 μm and 170–210 × 20–28 μm, respectively). Nevertheless, we examined the material deposited at Kew Botanical Garden (IMI 321829) and conidia were shorter (42–168 × 19–28.5 μm) than those described in the original description (Del Serrone et al. 1991). The high morphological variation in species of Exserohilum, and the lack of molecular data in this species, make the correct placement of E. elongatum problematic.
Exserohilum frumentacei (Mitra) K.J. Leonard & Suggs, as ‘frumentaceum’, Mycologia 66: 291. 1974
Basionym. Helminthosporium frumentacei Mitra, Trans. Brit. Mycol. Soc. 15: 288. 1931.
Type material. India, Pusa, leaf-sheaths of Panicum frumentaceum, unknown date, Mitra.
Notes — This species was described from Panicum frumentaceum in India (Mitra 1931). Exserohilum frumentacei resembles E. monoceras in conidial size and morphology and probably represents the same species. However, culture of this species is not available for phylogenetic comparison.
Exserohilum glycinea (L.S. Srivast. et al.) P.M. Kirk, Index Fungorum 269: 1. 2015
Basionym. Setosphaeria glycinea L.S. Srivast. et al., Indian J. Mycol. Pl. Pathol. 12: 241. 1983.
Type material. India, Meghalaya, on leaves of Glycine max, 11 Aug. 1976 (IMI 209021 holotype, not seen).
Notes — The inclusion of this species in Exserohilum is doubtful. Originally, E. glycinea was described as the causal agent of leaf spot and blight of leaves of Glycine max (Srivastava et al. 1983), an uncommon substrate for Exserohilum species. It is only known by the sexual morph and was distinguished from other species of Setosphaeria by the presence of well-developed, long and septate setae on the ascomata, ascospores 5–6-septate, the enlargement of the third cell from the top of the ascospore and by the absence of asexual morph (Srivastava et al. 1983).
Exserohilum heteromorphum G.Y. Sun, Mycotaxon 92: 174. 2005
Type material. China, Shaanxi, on leaves of Echinochloa crus-galli var. mitis, unknown date, unknown collector (HMUABO 20579 holotype, not seen).
Notes — Exserohilum heteromorphum was differentiated from other species of the genus by the presence of strongly curved conidia (Sun et al. 2005). However, E. heteromorphum is morphologically similar to E. monoceras, which also has curved conidia (Drechsler 1923). Furthermore, E. heteromorphum was described from leaves of Echinochloa crus-galli, the same substrate as E. monoceras, and therefore likely represent the same species. Unfortunately, no cultures are available to confirm the phylogenetic relationship.
Exserohilum israeli Steiman et al., Antonie van Leeuwenhoek 78: 155. 2000
Type material. Israel, Timna Park, Negev desert, Arava valley, from soil, Aug. 1994 (CMPG 1339 holotype, not seen).
Notes — Exserohilum israeli was described from soil in Israel (Steiman et al. 2000). According to the protologue, this fungus is morphologically compatible with E. rostratum. No living culture of E. israeli is available for study.
Exserohilum lagenarioides Pachkhede, Geobios, New Rep. 8: 64. 1989
Notes — This name is currently considered as invalid in Index Fungorum following Art. 40.1, 40.4 and 8.4 of the International Code of Nomenclature for algae, fungi and plants (Melbourne Code).
Exserohilum longisporum G.Y. Sun, Mycol. Res. 101: 776. 1997
Type material. China, Hunan, Changsha, on Miscanthus sinensis, 12 Oct. 1992 (HMUABO 100133 holotype, not seen; HMAS 73782 isotype, not seen).
Notes — Exserohilum longisporum was described from Miscanthus sinensis in China (Sun et al. 1997). According to the protologue, this fungus is morphologically compatible with E. rostratum. No living culture of E. longisporum is available for study.
Exserohilum oryzae Sivan., Mycol. Pap. 158: 231. 1987 — Fig. 22
Fig. 22.
Exserohilum oryzae (IMI 214168 holotype). a–c. Conidiophores and conidiogenous cells; d–f. conidiogenous cells and conidia; g–i. conidia; j. lower part of conidium. — Scale bars: 10 μm.
Type material. Yugoslavia (currently Macedonia), Kočani, on Oryzae sativa, June 1977, Karov Ilija 5 (IMI 214168 holotype).
Herbarium material. Vegetative hyphae septate, branched, pale olivaceous to pale brown, smooth-walled, 3.5–5.5 μm wide, anastomosing. Conidiophores macronematous, mononematous, straight, curved or geniculate, mostly unbranched, subcylindrical, pale olivaceous brown to dark brown, becoming paler towards the apex, smooth to asperulate, with cell walls usually thicker than those of the vegetative hyphae, 21–7 20 × 3.5–7.5 μm, sometimes slightly swollen at the base up to 10 μm wide, often with subnodulose and nodulose intercalary swellings up to 10 μm wide, swellings with conidiogenous loci. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- and polytretic, proliferating sympodially, 10–54 μm long, with pores up to 1 μm wide, surrounded by scars 3–5 μm wide. Conidia fusiform with an obtuse apex and a truncate obconic base, rarely clavate, straight to more or less curved, pale olivaceous brown to dark brown, often paler at both poles, smooth to asperulate with the ornamentation more evident at the base, (3–)7–9-distoseptate, (34–)61–150 × 10–24 μm, with a protruding hilum, 2.5–5.5 μm wide. Germination unipolar or bipolar. Microcyclic conidiation frequent.
Notes — The protologue describes narrower hyphae (up to 4.5 μm wide), conidiophores slightly shorter and narrower (up to 600 μm long, 5–6 μm wide) and conidia slightly longer and wider, 96–160 × 18–25 μm (Sivanesan 1987) than those observed here (34–150 × 10–24 μm). Sivanesan possibly excluded short conidia from his description because he might have considered them immature. They were included here because even these short conidia were able to germinate. Exserohilum oryzae and E. oryzicola are very similar species isolated from the same substrate. According to Sivanesan ( 1987), they differ in conidial size. Nevertheless, the high morphological variation in species of Exserohilum, and the lack of molecular data in this species, makes the correct placement of E. oryzae difficult to assess.
Exserohilum oryzinum Sivan., Trans. Brit. Mycol. Soc. 83: 325. 1984 — Fig. 23
Fig. 23.
Exserohilum oryzinum (IMI 152682 holotype). a. Conidiophore with a swelling base; b–e. conidiogenous cells; f–j. conidia. — Scale bars: 10 μm.
Type material. Egypt, Alexandria, from leaves of Oryza sp., 8 Nov. 1970, M.K. El-Kazaz (IMI 152682 holotype).
Herbarium material. Vegetative hyphae septate, branched, pale olivaceous to medium brown, smooth to asperulate 3–7.5 μm wide, anastomosing. Conidiophores macronematous, straight to flexuous, septate, mostly unbranched, subcylindrical, pale olivaceous brown to dark brown, becoming paler towards the apex, smooth to asperulate, with cell walls thicker than the vegetative hyphae, up to 810 μm long, 4–8 μm wide, sometimes swollen at the base up to 9 μm wide, with subnodulose and nodulose intercalary swellings up to 11 μm wide, swellings with conidiogenous loci. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- and polytretic, proliferating sympodially, 9–61.5 μm long, pores up to 1 μm wide, surrounded by scars 3.5–5.5 μm wide. Conidia mostly falcate or sigmoid, rarely fusiform or clavate, pale to medium olivaceous brown, smooth to asperulate with the ornamentation more evident at the base, (4–)6–10-distoseptate, (56–)80–155 × 14–27 μm, with a protruding hilum, 2.5–4 μm wide. Germination uni- or bipolar. Microcyclic conidiation frequent.
Notes — Exserohilum oryzinum is only known from Egypt growing on Oryza sp. This species is distinguished from other Exserohilum spp. by the distinctively curved to sigmoid, pale to mid-brown conidia. Although no molecular data exist for E. oryzinum, this species is retained in Exserohilum based on the characteristic hilum structure and conidial morphology (Sivanesan 1984).
Exserohilum parlierense W.Q. Chen & Michailides, as ‘parlierensis’, Mycotaxon 83: 153. 2002
Type material. USA, California, on leaves of Pistacia vera, 16 Aug. 2001, Q.W. Chen (ATCC MYA-2456 holotype, not seen; CH-26 culture ex-type).
Notes — Exserohilum parlierense was described from Pistacia vera in the USA (Chen et al. 2002). According to the protologue, this fungus is morphologically compatible with E. rostratum.
Exserohilum phragmitis W.P. Wu, as ‘phragmatis’, J. Hebei Acad. Sci., Selected papers: 60. 1990
Type material. China, Hebei, on leaves of Phragmites (IBHAS 4150 holotype, not seen).
Notes — This name we considered here as invalid following Art. 8 and 40 of the International Code of Nomenclature for algae, fungi and plants (Melbourne Code). The data of the type material listed here is accordingly to information in Index Fungorum. However, the publication linked to the protologue did not describe a new species.
Exserohilum psidii Sivan., Mycol. Res. 96: 489. 1992 — Fig. 24
Fig. 24.
Exserohilum psidii (IMI 299549 holotype). a–d. Conidiogenous cells; e–j. conidia. — Scale bars: a–i = 10 μm; j = 20 μm.
Type material. India, Warangal, on Psidium sp., 15 Oct. 1985, Madhukar D2 (IMI 299549 holotype, not seen).
Herbarium material. Colonies on TWA + wheat straw effuse floccose, dark brown. Vegetative hyphae septate, branched, subhyaline to mid olivaceous brown, smooth to asperulate, 3–7 μm wide. Conidiophores macronematous, straight to flexuous, septate, unbranched, subcylindrical, pale olivaceous brown to dark brown, smooth to asperulate, with cell walls thicker than those of the vegetative hyphae, up to 1 086 μm long, 5.5–11 μm wide, often swollen at the base up to 18 μm wide, with subnodulose and nodulose intercalary swellings up to 12 μm wide, swellings with conidiogenous loci. Conidiogenous cells integrated, terminal and intercalary, mostly subcylindrical, mono- and polytretic, proliferating sympodially, 16.5–118.5 μm long, with pores up to 1 μm wide, surrounded by scars up to 4.5–5.5 μm wide. Conidia mostly fusiform, rarely clavate, with an obtuse apex and a truncate obconic base, straight to slightly curved, pale to mid olivaceous brown, smooth, asperulate at the base, (5–)6(–7)-distoseptate, (53–)112.5–148 × 16–23 μm, with a protruding hilum 2.5–3.5 μm wide. Germination uni- or bi-polar.
Notes — The protologue describes narrower conidiophores (up to 6 μm wide) and conidia much longer and wider 150–175 (– 190) × 27–30(–35) μm (Sivanesan 1992) than those observed here (53–148 × 16–23 μm). The morphology of this species resembles E. monoceras and E. turcicum. Nevertheless, the high morphological variation in species of Exserohilum and the lack of molecular data in this species, make the correct placement of E. psidii difficult.
Exserohilum sodomii Guiraud et al., Antonie van Leeuwenhoek 72: 318. 1997
Type material. Israel, Neguev desert, Dead Sea (road of Sodom), from soil, Aug. 1994, unknown collector (CMPG1340 holotype, not seen).
Notes — Exserohilum sodomii was described from soil in Israel, as well as E. israeli by the same authors (Guiraud et al. 1997, Steiman et al. 2000). According to the protologue, this fungus is morphological compatible with E. rostratum. No living culture of E. sodomii is available for study.
Curvularia micropus (Drechsler) Hern.-Restr., Y.P. Tan & Crous, comb. nov. — MycoBank MB822994; Fig. 25
Fig. 25.
Curvularia micropus (CBS 127235 ex-epitype (a–d), CBS 127236 (e–g), CBS 128057 (h–k)). a–c, e, h. Conidiophores and conidia; d, f–g, i–k. conidia. — Scale bars: 10 μm.
Basionym. Helminthosporium micropus Drechsler, J. Agric. Res. 24: 722. 1923.
Synonyms. Bipolaris micropus (Drechsler) Shoemaker, Canad. J. Bot. 37: 884. 1959.
Drechslera micropus (Drechsler) Subram. & B.L. Jain, as ‘micropa’, Curr. Sci. 35: 354. 1966.
Exserohilum paspali J.J. Muchovej & Nesio, Trans. Brit. Mycol. Soc. 89: 126. 1987.
Type material. USA, Florida, Wauchula, on Paspalum boscianum (?), 2 May 1921, C. Drechsler (IMI 296605, not seen (holotype of Helminthosporium micropus); BPI 429621, syntype (of Helminthosporium micropus)); Georgia, Tifton, on Paspalum notatum, 17 July 1970, E.S. Luttrell No. Lutt. 8530 (BRIP 65 20 epitype designated here (MBT378847); CBS 127235 = BRIP 65 20 culture ex-epitype.
On SNA + banana leaf. Vegetative hyphae pale brown, septate. Conidiophores macronematous, single or in small groups, erect, septate, unbranched, cylindrical, brown, smooth, 80–335 × 4–8 μm. Conidiogenous cells terminal and intercalary, geniculate, mono- and polytretic, 8–37.5 × 3.5–6.5 μm. Conidia cylindrical (longer ones) to ellipsoid (shorter ones) or sigmoid, straight or slightly curved, subhyaline to pale brown, verruculose, 3–9-distoseptate, 30–70 × 10–18.5 μm, protruding hilum 1–3.5 μm long, 1.5–3 μm wide. Sexual morph not observed.
Culture characteristics — On SNA + sterilized maize leaf after 7 d at 24 °C in the dark, hairy to floccose, olivaceous grey to olivaceous black, cottony on maize leaves, with a fimbriate margin; reverse concolorous with obverse.
Additional materials examined. Brazil, Minas Gerais, Viçosa, on Paspalum conjugatum, 10 May 1986, J.J. Muchovej (authentic culture of E. paspali BRIP 16070 = CBS 128057). – Usa, Florida, Lakeland, on Paspalum notatum, 3 Apr. 1970, E.S. Luttrell, Lutt. 8452 (BRIP 6516 = CBS 127234); Georgia, Tifton, on Paspalum notatum, 3 Apr. 1987, A.Y. Rossman (BRIP 15689a = CBS 127236).
Notes — Curvularia micropus attacks leaf blades of young plants of Paspalum, killing the foliar tissues (Drechsler 1923, Muchovej & Ribeiro Nesio 1987, Sivanesan 1987). Curvularia micropus, originally described as Helminthosporium (Drechsler 1923), was transferred to Bipolaris (Shoemaker 1959) and later to Drechslera (Subramanian & Jain 1966). Other species listed as synonyms of B. micropus are Helminthosporium leptochloae (Sivanesan 1987) and E. paspali (Alcorn 1991). However, molecular data generated in this study revealed that H. leptochloae is conspecific with E. rostratum (Fig. 2), whereas E. paspali and B. micropus are conspecific (Fig. 1). Nevertheless, this species is better placed in Curvularia. In the multi-gene tree, three strains isolated from Paspalum in the USA and one from Brazil, are placed in a basal clade of Curvularia (Fig. 1). Type material of H. micropus is preserved at the IMI (holotype IMI 296605) and at the US National Fungal Collection (BPI 4296 20, BPI 429621, BPI 429615, as syntype), unfortunately there is no culture available. The strain CBS 127234 was collected from the same state as the type material (Florida) but this isolate was sterile under the culture conditions tested. It is, however, genetically identical to the strain CBS 127235. In order to stabilize the use of the name, we propose CBS 127235 as the ex-epitype strain of C. micropus, since it fits well with the description given in the protologue and it was collected in a neighbouring state.
Curvularia sorghicola (Sivan.) Madrid & Crous, comb. nov. — MycoBank MB822997; Fig. 26
Fig. 26.
Curvularia sorghicola (IMI 225559 holotype). a–f. Conidiophores; g–i. conidia. — Scale bars: 10 μm.
Basionym. Exserohilum sorghicola Sivan., Mycol. Pap. 158: 237. 1987.
Type material. Ethiopia, on leaves of Sorghum sp., 15 Sept. 1976, unknown collector (IMI 225559 holotype).
Adapted from Sivanesan ( 1987). Leaf spots irregularly elongate, running parallel to the midrib, surrounded by a thick dark purple border. Vegetative hyphae mostly immersed, septate, branched, pale olivaceous to pale olivaceous brown, sometimes with purplish tinges, smooth, 2.5–7.5 μm wide, giving rise to chains and clumps of swollen subcylindrical, globose to irregularly shaped cells up to 24 μm wide. Conidiophores macronematous, single to fasciculate, straight to flexuous, often geniculate above, septate, mostly unbranched, subcylindrical, olivaceous brown, becoming paler towards the apex and sometimes also toward the base, smooth to asperulate, with cell walls thicker than those of the vegetative hyphae, 28.5–112 × 3–7.5 μm, often with a bulbous base up to 14 μm wide. Conidiogenous cells integrated, intercalary and terminal, mostly subcylindrical, mono- and polytretic, proliferating sympodially, 7–46 × 3–7.5 μm, with pores up to 1 μm wide, surrounded by scars 3–4.5 μm wide. Conidia mostly clavate, straight to curved, pale olivaceous brown, often paler towards both ends, smooth to asperulate, 3(–5)-distoseptate, often constricted at the septa, 30–57 × 11– 19 μm, with a protruding hilum, 1.5–3 μm wide.
Notes — This fungus was originally described as an atypical species of Exserohilum (Sivanesan 1987), and examination of the holotype revealed that its conidia produce hila which are delimited from the basal cell by a septum, different from other Exserohilum species. This kind of hilum is produced by many Curvularia species (Alcorn 1991, Zhang et al. 2004, Madrid et al. 2014), and therefore this is the correct genus for E. sorghicola. Morphologically, this fungus resembles members of the trifolii-clade of Curvularia in producing predominantly 4-celled conidia with a strongly protruding hilum (Madrid et al. 2014). However, there is no culture available for DNA sequence analyses, and its exact phylogenetic placement in Curvularia still has to be assessed.
Sporidesmiella novae-zelandiae (S. Hughes) Madrid, Hern.-Restr. & Crous, comb. nov. — MycoBank MB822998; Fig. 27
Fig. 27.
Sporidesmiella novae-zelandiae (DAOM 159962 isotype). a–h. Conidiophores and conidia; i–l. conidia. — Scale bars: a = 20 μm; b–h = 10 μm.
Basionym. Sporidesmium hyalospermum var. novae-zelandiae S. Hughes, New Zealand J. Bot 16: 349. 1978.
Synonym. Sporidesmiella hyalosperma var. novae-zelandiae (S. Hughes) P.M. Kirk, Trans. Brit. Mycol. Soc. 79: 479. 1982.
Exserohilum novae-zelandiae (S. Hughes) H.P. Upadhyay & Mankau, Mycologia 83: 373. 1991.
Type material. New Zealand, Canterbury Province, Okuti Valley, near Little River, on rotten wood, 17 May 1963, S.J. Hughes (PDD 304 20 holotype (of Sporidesmium hyalospermum var. novae-zealandiae), not seen; DAOM 159962 isotype).
Vegetative hyphae septate, branched, subhyaline to pale brown, smooth and thin-walled, 1–2.5 μm wide. Conidiophores macronematous, mononematous, septate, unbranched, straight or flexuous, showing either percurrent or sympodial proliferations and sometimes both, dark brown, becoming paler towards the apex, smooth to asperulate, thick-walled, length undetermined, 4.5–9 μm wide, often with a bulbous base up to 13 μm wide. Conidiogenous cells integrated, terminal, mostly subcylindrical, often flexuous, holoblastic, mono- and polyblastic, 7.5–38 μm long, with flat or slightly convex, non-darkened scars 3.5–5 μm wide. Conidia solitary, mostly clavate with an obconically truncate base, pale olivaceous to pale golden brown, smooth, 3–4-distoseptate, 20.5–28 × 11.5–15 μm, 3–5.5 μm wide at the base, basal cell cut-off by a dark-brown septum.
Additional material examined. Mexico, Laguna de Zempoala, Morelos, plant debris (of grass), 22 Feb. 1989, R. Mankau (CBS 135842).
Notes — This species was originally described as Sporidesmium hyalospermum var. novae-zelandiae by Hughes ( 1978). This and the type variety were reallocated to Sporidesmiella by Kirk ( 1982) and var. novae-zelandiae was later transferred to Exserohilum by Upadhyay & Mankau ( 1991) as E. novae-zelandiae. The combination proposed by the latter authors is not appropriate since the conidiogenous cells in the holotype lack the darkened scars and conspicuous pores typical of Exserohilum spp. and produce conidia with an obconically truncate base, not with a truly protruding hilum. Although DNA sequence data are lacking for the type specimens of the varieties of S. hyalosperma, morphological differences indicate that S. hyalosperma var. novae-zelandiae and S. hyalosperma var. hyalosperma are different fungi. The latter differs from the variety novae-zelandiae in having conidiophores that proliferate almost always percurrently, with numerous conspicuous annellations and narrower (9–12 μm wide) conidia (Morgan-Jones & Cole 1964, Kirk 1982, Wu & Zhuang 2005). Furthermore, the isolate CBS 135842, identified as Exserohilum novae-zelandiae by Upadhyay & Mankau ( 1991), proved to be related to Annulatascaceae, Sordariomycetes (unpubl. data), revealing that the fungus is phylogenetically distant from Exserohilum (Pleosporaceae, Dothideomycetes). Based on morphological and molecular evidence we consider that ‘Exserohilum’ novae-zelandiae should be, at least provisionally, retained in Sporidesmiella. Currently, Sporidesmiella comprises 24 species (Seifert et al. 2011). However, Sporidesmiella fusiformis is the only species with molecular data available in this genus, and it belongs to Didymosphaeriaceae (Dothideomycetes) (Shenoy et al. 2006) rather than Annulatascaceae, like S. novae-zelandiae. Further studies are needed in order to establish generic boundaries in Sporidesmiella.
DISCUSSION
In this study, we have reviewed the taxonomic circumscription of Exserohilum (= Setosphaeria) using molecular and morphological data. This is the first study that presents a robust phylogeny using a broad distribution of Exserohilum isolates from different hosts and geographic origins. Furthermore, we also studied the holotype material of several species and protologues of species listed in the genus to provide a statement of their placement in the genus. The phylogenetic analysis based on four genes gapdh, rpb2, LSU and ITS (Fig. 1), shows that the morphologically atypical species E. paspali is conspecific with B. micropus, as previously noticed by Alcorn ( 1991), but belongs in Curvularia rather than in Exserohilum or Bipolaris. This phylogenetic analysis also confirms that C. crassiseptum and C. heteropogonicola, previously known as Exserohilum species, are correctly placed in Curvularia (Alcorn 1991, Zhang et al. 2004). Furthermore, excluding those species or others such as E. novae-zelandiae, Exserohilum is highly supported as a monophyletic clade, which is clearly different from other helminthosporoid genera such as Bipolaris, Curvularia, Johnalcornia, Porocercospora and Pyrenophora.
Exserohilum species are defined as pathogenic fungi to humans and plants, mainly grasses, as well as, saprobic, endophytic and soil-borne fungi. Species of this genus are frequently found as asexual morphs in nature, although the sexual morph was often obtained by combining compatible strains, except for E. minor and E. khartoumensis (Drechsler 1923, Luttrell 1963a, Nelson 1965, Alcorn 1978, 1986, El Shafie & Webster 1981). The setosphaeria-like sexual morph of different Exserohilum species can be morphologically very similar and therefore morphology of the asexual morph is considered more reliable for identification purposes (Leonard 1976, Alcorn 1986, Sivanesan 1987). The traditional morphological attributes of conidia that have been used as taxonomical criteria at the generic rank for Bipolaris, Curvularia, Exserohilum and Pyrenophora are mainly the germination patterns of the conidia, septum ontogeny and hilum morphology (Leonard & Suggs 1974, Alcorn 1983, 1990, 1991, Sivanesan 1987). Among those features, the hilum morphology is the most valuable criteria to delineate Exserohilum species. The hilum is characterised by an enveloping structure around the hilar protrusion which is often thickened basally or laterally (Alcorn 1983). However, in some Curvularia species, a structure resembling that of the hilum in Exserohilum can be present, but in Curvularia, the hilum is separated from the conidial body by a septum.
By combining morphological data with multi-locus analysis from ITS, act, gapdh, his, rpb2, tef1 and tub2 sequences, we were able to delimit 11 species in Exserohilum, one of which is formally proposed as a new species, E. corniculatum, in addition to other previously described species, i.e., E. holmii, E. khartoumensis, E. minor, E. monoceras, E. neoregeliae, E. oryzicola, E. pedicellatum, E. protrudens, E. rostratum and E. turcicum. The phylogenetic position of E. monoceras is still unresolved, since molecular data from type material was not available. The phylogenetic tree shows a clade of E. turcicum, closely related to strains identified as E. monoceras; however, more study is needed on members of these clades in order to resolve their relationship (Fig. 2). This study demonstrated that some species can be morphologically highly variable, but molecularly they are very closely related and they are treated here as synonyms, E. curvatum with E. holmii and E. fusiforme with E. oryzicola. Exserohilum rostratum was an exceptional case, where this species is shown as conspecific to E. antillanum, E. gedarefense, E. longirostratum, E. macginnisii, E. prolatum and H. leptochloae. Morphological variability in conidial shape, size and pigmentation of E. rostratum has been already noticed in natural substrate (Drechsler 1923), as well as in culture, which is influenced by external factors like carbon source, glucose concentration, type of culture media, light exposure and pH, among others (Mitra 1931, Kafi & Tarr 1966, Tarr & Kafi 1968, Leonard 1976, Anahosur & Sivanesan 1978, Honda & Aragaki 1978). Previously, Leonard & Suggs ( 1974) observed morphological similarities among E. rostratum, H. leptochloae and E. longirostratum. More recently, Da Cunha et al. ( 2012) demonstrated that E. rostratum, E. longirostratum and E. macginnisii are very closely related based on multigene sequence analysis of clinical isolates, but did not propose any synonymy. Here we propose them as synonyms of E. rostratum.
For an accurate species identification of Exserohilum species, a molecular analysis is required. Among the nine loci used in this study, ITS, act and rpb2 were able to resolve 11, 13 and 12 OTUs, respectively, with varying statistical support. Although each of these loci proved to be suitable barcoding markers for species identification, a combined analysis is highly recommended. The gapdh gene is recommended for species resolution in Bipolaris and Curvularia (Da Cunha et al. 2012, Manamgoda et al. 2012,) but in Exserohilum this region showed E. rostratum (including E. corniculatum) as a polyphyletic group. However, the other nine species were well-supported.
Besides the 11 phylogenetic species recognised here, the taxonomic placement at species and generic level of other 15 species listed as ‘doubtful’ (i.e., E. curvisporum, E. echinochloae, E. elongatum, E. frumentacei, E. glycinea, E. heteromorphum, E. israeli, E. lagenarioides, E. longisporum, E. oryzae, E. oryzinum, E. parlierense, E. phragmitis, E. psidii and E. sodomii) have to be confirmed as members of Exserohilum by molecular data.
The present investigation significantly extends the knowledge of the taxonomy of Exserohilum. Nevertheless, extensive sampling of uncommon species and inclusion of additional data like chemical compounds (i.e., secondary metabolites), ecology and host range for species are needed in order to resolve some taxonomic and phylogenetic aspects.
Acknowledgments
We thank the collections director Lisa A. Castlebury at the US National Fungus Collections, the MycoBank curator Konstanze Bench, the CBS collection curator Gerard Verkleij, and the technical staff at the Westerdijk Institute, Arien van Iperen (cultures and deposit of herbarium samples), Janneke Bloem (DNA isolation and sequencing) and Trix Merkx (deposit of isolates) for their invaluable assistance. We also thank editors and reviewers for critically reviewing the manuscript. Hugo Madrid was partially funded by Comisión Nacional de Investigación Científica y Tecnológica (CONICYT), Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), Chile, project no. 11140562.
REFERENCES
- Adler A, Yaniv I, Samra Z, et al. 2006. Exserohilum: an emerging human pathogen. European Journal of Clinical Microbiology and Infectious Diseases 25: 247–253. [DOI] [PubMed] [Google Scholar]
- Al-Attar A, Williams CG, Redett RJ. 2006. Rare lower extremity invasive fungal infection in an immunosupressed patient: Exserohilum longirostratum. Plastic and Reconstructive Surgery 117: 44e–47e. [DOI] [PubMed] [Google Scholar]
- Alcorn JL. 1978. Setosphaeria monoceras sp. nov., ascigerous state of Exserohilum monoceras. Mycotaxon 7: 411–414. [Google Scholar]
- Alcorn JL. 1983. Cochliobolus ellisii sp. nov. Transactions of the British Mycological Society 81: 172–174. [Google Scholar]
- Alcorn JL. 1986. A new homothallic Setosphaeria species and its Exserohilum anamorph. Transactions of the British Mycological Society 86: 313–317. [Google Scholar]
- Alcorn JL. 1988a. The taxonomy of Helminthosporium species. Annual Review of Phytopathology 26: 37–56. [Google Scholar]
- Alcorn JL. 1988b. A new species of Exserohilum. Transactions of the British Mycological Society 90: 146–148. [Google Scholar]
- Alcorn JL. 1990. Additions to Bipolaris, Cochliobolus and Curvularia. Mycotaxon 39: 361–392. [Google Scholar]
- Alcorn JL. 1991. New combinations and synonymy in Bipolaris and Curvularia, and a new species of Exserohilum. Mycotaxon 41: 329–343. [Google Scholar]
- Alcorn JL. 1996. Cochliobolus heliconiae sp. nov. Australian Systematic Botany 9: 813–817. [Google Scholar]
- Alloub H, Juraimi AS, Kadir J, et al. 2009. Field efficacy of Exserohilum prolatum-A potential mycoherbicide for biological control of itchgrass (Rottboelia cochinchinensis). Journal of Biological Sciences 9: 1 19–127. [Google Scholar]
- Altschul SF, Gish W, Miller W, et al. 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Amaradasa BS, Madrid H, Groenewald JZ, et al. 2014. Porocercospora seminalis gen. et comb. nov. the causal organism of buffalograss false smut. Mycologia 106: 77–85. [DOI] [PubMed] [Google Scholar]
- Anahosur KH, Sivanesan A. 1978. Setosphaeria rostrata. CMI Descriptions of Pathogenic Fungi and Bacteria 587. [Google Scholar]
- Aquino VM, Norvell JM, Krisher K, et al. 1995. Fatal disseminated infection due to Exserohilum rostratum in a patient with aplastic anemia: case report and review. Clinical Infectious Diseases 20: 176–178. [DOI] [PubMed] [Google Scholar]
- Berbee ML, Pirseyedi M, Hubbard S. 1999. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91: 964–977. [Google Scholar]
- Bhigjee AI, Parmanand V, Hoosen AA, et al. 1993. Disseminated Exserohilum infection. Journal of Infection 26: 336–337. [DOI] [PubMed] [Google Scholar]
- Bolle PC. 1924. Die durch Schwärzepilze (Phaeodyctiae) erzeugten Pflanzenkrankheiten. Mededelingen Phytopathologisch Laboratorium ‘Willie Commelin Scholten’ 7: 1–77. [Google Scholar]
- Bouchon CL, Greer DL, Genre CF. 1994. Corneal ulcer due to Exserohilum longirostratum. American Journal of Clinical Pathology 101: 452–455. [DOI] [PubMed] [Google Scholar]
- Bunker RN, Mathur K. 2006. Host range of leaf blight pathogen (Exserohilum turcicum) of Sorghum. Indian Phytopathology 59: 370–372. [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castañeda-Ruiz RF, Guarro J, Cano J. 1995. A new species of Exserohilum from Cuba. Mycological Research 99: 825–826. [Google Scholar]
- Chen W-Q, Ntahimpera N, Morgan DP, et al. 2002. Mycoflora of Pistacia vera in the Central Valley, California. Mycotaxon 83: 147–158. [Google Scholar]
- Crous PW, Groenewald JZ, Risede J-M, et al. 2004. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50: 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cunha KC, Sutton DA, Gené J, et al. 2012. Molecular identification and in vitro response to antifungal drugs of clinical isolates of Exserohilum. Antimicrobial Agents and Chemotherapy 56: 4951–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog GS, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189. [DOI] [PubMed] [Google Scholar]
- De Hoog GS, Guarro J, Gené J, et al. 2000. Atlas of Clinical Fungi. 2nd ed Centraalbureau voor Schimmelcultures, Utrecht. [Google Scholar]
- Del Serrone P, Porta-Puglia A, Sivanesan A. 1991. A new species of Exserohilum on barnyardgrass. Phytopathologia Mediterranea 30: 151–154. [Google Scholar]
- Derber C, Elam K, Bearman G. 2010. Invasive sinonasal disease due to dematiaceous fungi in immunocompromised individuals: case report and review of the literature. International Journal of Infectious Diseases 14, supplement 3: e329–e332. [DOI] [PubMed] [Google Scholar]
- Drechsler C. 1923. Some graminicolous species of Helminthosporium: I. Journal of Agricultural Research 24: 641–739. [Google Scholar]
- Drechsler C. 1934. Phytopathological and taxonomic aspects of Ophiobolus, Pyrenophora and Helminthosporium and a new genus, Cochliobolus. Phytopathology 24: 953–983. [Google Scholar]
- El Shafie AE. 1980. Drechslera gedarefensis sp. nov. from Sorghum grains. Transactions of the British Mycological Society 74: 437–438. [Google Scholar]
- El Shafie AE, Webster J. 1981. Setosphaeria khartoumensis sp. nov. and its Exserohilum conidial state. Transactions of the British Mycological Society 77: 442–446. [Google Scholar]
- Ellis MB. 1971. Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew. [Google Scholar]
- Ellis MB. 1976. More dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew. [Google Scholar]
- Farr DF, Rossman AY. 2017. Fungal databases, U.S. National Fungus Collections, ARS, USDA: https://nt.ars-grin.gov/fungaldatabases/. Accessed October 2017. [Google Scholar]
- Friedman GC, Hartwick RW, Ro JY, et al. 1991. Allergic fungal sinusitis. Report of three cases associated with dematiaceous fungi. American Journal of Clinical Pathology 96: 368–372. [DOI] [PubMed] [Google Scholar]
- Gilbert RL. 2002. First report of Exserohilum pedicellatum on Zea mays in Australia. New Disease Reports 6: 3 [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraud P, Steiman R, Seigle-Murandi F, et al. 1997. Exserohilum sodomii, a new species isolated from soil near the Dead Sea (Israel). Antonie van Leeuwenhoek 72: 317–325. [DOI] [PubMed] [Google Scholar]
- Henry AW. 1924. Root-rots of wheat. University of Minnesota Agricultural Experiment Station Technical Bulletin 22: 1–71. [Google Scholar]
- Honda Y, Aragaki M. 1978. Photosporogenesis in Exserohilum rostratum. Temperature effects on sporulation and spore morphology. Mycologia 70: 343–354. [Google Scholar]
- Hsu MM, Lee JY. 1993. Cutaneous and subcutaneous phaeohyphomycosis caused by Exserohilum rostratum. Journal of the American Academy of Dermatology 28: 340–344. [DOI] [PubMed] [Google Scholar]
- Hughes SJ. 1978. New Zealand fungi 25. Miscellaneous species. New Zealand Journal of Botany 16: 311–370. [Google Scholar]
- Ito S. 1930. On some ascigerous stages of the species of Helminthosporium parasitic on cereals. Proceedings of the Imperial Academy of Tokyo 6: 352–355. [Google Scholar]
- Joseph NM, Kumar MA, Stephen S, et al. 2012. Keratomycosis caused by Exserohilum rostratum. Indian Journal of Pathology and Microbiology 55: 248–249. [DOI] [PubMed] [Google Scholar]
- Kafi A, Tarr SAJ. 1966. Growth, sporulation and conidial characteristics of five graminicolous species of Helminthosporium: I. Effect of nutrients. Transactions British Mycological Society 49: 327–337. [Google Scholar]
- Kainer MA, Reagan DR, Nguyen DB, et al. 2012. Fungal infections associated with contaminated methylprednisolone in Tennessee. New England Journal of Medicine 367: 2 194–2 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM. 1982. New or interesting microfungi VI. Sporidesmiella gen. nov. (Hyphomycetes). Transactions of the British Mycological Society 79: 479–489. [Google Scholar]
- Kirk PM. 2015. Index Fungorum no. 269. www.indexfungorum.org. [Google Scholar]
- Kodsueb R, Dhanasekaran V, Aptroot A, et al. 2006. The family Pleosporaceae intergeneric relationships and phylogenetic perspectives based on analyses of partial 28S rDNA. Mycologia 98: 571–583. [DOI] [PubMed] [Google Scholar]
- Lasala PR, Smith MB, McGinnis MR, et al. 2005. Invasive Exserohilum sinusitis in a patient with aplastic anemia. The Pediatric Infectious Disease Journal 24: 939–941. [DOI] [PubMed] [Google Scholar]
- Leonard KJ. 1976. Synonymy of Exserohilum halodes with E. rostratum, and induction of the ascigerous state, Setosphaeria rostrata. Mycologia 68: 402–411. [Google Scholar]
- Leonard KJ, Suggs EG. 1974. Setosphaeria prolata, the ascigerous state of Exserohilum prolatum. Mycologia 66: 281–297. [Google Scholar]
- Levy I, Stein J, Ashkenazi S, et al. 2003. Ecthyma gangrenosum caused by disseminated Exserohilum in a child with leukemia: a case report and review of the literature. Pediatric Dermatology 20: 495–497. [DOI] [PubMed] [Google Scholar]
- Lin SC, Sun PL, Ju YM, et al. 2009. Cutaneous phaeohyphomycosis caused by Exserohilum rostratum in a patient with cutaneous T-cell lymphoma. International Journal of Dermatology 48: 295–298. [DOI] [PubMed] [Google Scholar]
- Lin S-H, Huang S-L, Li Q-Q, et al. 2011. Characterization of Exserohilum rostratum, a new causal agent of banana leaf spot disease in China. Australasian Plant Pathology 40: 246–259. [Google Scholar]
- Link HF. 1809. Observationes in ordines plantarum naturales. Dissertatio I. Magazin der Gesellschaft Naturforschenden Freunde Berlin 3: 3–42. [Google Scholar]
- Liu F, Dian-Ming H, Cai L. 2012. Conlarium duplumascospora gen. et sp. nov. and Jobellisia guangdongensis sp. nov. from freshwater habitats in China. Mycologia 104: 1178–1186. [DOI] [PubMed] [Google Scholar]
- Luttrell ES. 1958. The perfect stage of Helminthosporium turcicum. Phytopathology 48: 281–287. [Google Scholar]
- Luttrell ES. 1963a. A Trichometasphaeria perfect stage of a Helminthosporium causing leaf blight of Dactyloctenium. Phytopathology 53: 281–285. [Google Scholar]
- Luttrell ES. 1963b. Taxonomic criteria in Helminthosporium. Mycologia 55: 643–674. [Google Scholar]
- Madrid H, Da Cunha KC, Gené J, et al. 2014. Novel Curvularia species from clinical specimens. Persoonia 33: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manamgoda DS, Cai L, Bahkali AH, et al. 2011. Cochliobolus: an overview and current status of species. Fungal Diversity 51: 3–42. [Google Scholar]
- Manamgoda DS, Cai L, McKenzie EHC, et al. 2012. A phylogenetic and taxonomic re-evaluation of the Bipolaris-Cochliobolus-Curvularia complex. Fungal Diversity 56: 131–144. [Google Scholar]
- Mathews MS, Maharajan SV. 1999. Exserohilum rostratum causing keratitis in India. Medical Mycology 37: 131–132. [PubMed] [Google Scholar]
- McGinnis MR, Rinaldi MG, Winn RE. 1986. Emerging agents of phaeohyphomycosis: pathogenic species of Bipolaris and Exserohilum. Journal of Clinical Microbiology 24: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill J, Barrie FF, Buck WR, et al. 2012. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). Koeltz Scientific Books, Königstein: (Regnum Vegetabile no. 154). [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), November 14, 2010, New Orleans, Louisiana: 1–8. [Google Scholar]
- Mitra M. 1931. A comparative study of species and strains of Helminthosporium on certain Indian cultivated crops. Transactions of the British Mycological Society 15: 254–293. [Google Scholar]
- Morgan-Jones G, Cole ALJ. 1964. Concerning Endophragmia hyalosperma (Corda) comb. nov. Transactions of the British Mycological Society 47: 489–495. [Google Scholar]
- Muchovej JJ, Ribeiro Nesio ML. 1987. A new Exserohilum from Brazil. Transactions of the British Mycological Society 89: 126–128. [Google Scholar]
- Nelson RR. 1964. The perfect stage of Helminthosporium cynodontis. Mycologia 56: 64–69. [Google Scholar]
- Nelson RR. 1965. The perfect stage of Helminthosporium pedicellatum. Mycologia 57: 665–668. [Google Scholar]
- Nirenberg HI. 1976. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: 1–117. [Google Scholar]
- Nisikado Y. 1928. Studies on Helminthosporium diseases of Gramineae in Japan. Special Report of the Ohara Institute for Agricultural Research 4: 111–126. [Google Scholar]
- Nylander JAA. 2004. MrModeltest v. 2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- O'Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are non-orthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- O'Donnell K, Sarver BAJ, Brandt M, et al. 2007. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic fusaria including isolates from the 2005–06 multistate contact lens-associated U.S. keratitis outbreaks. Journal of Clinical Microbiology 45: 2235–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier C, Berbee ML, Shoemaker RA, et al. 2000. Molecular phylogenetic support from ribosomal DNA sequences for origin of Helminthosporium from Leptosphaeria-like ancestors Mycologia 92: 736–746. [Google Scholar]
- Pachkhede AU. 1989. A new species of Exserohilum from India. Geobios New Reports 8: 64–65. [Google Scholar]
- Padhye AA, Ajello L, Wieden MA, et al. 1986. Phaeohyphomycosis of the nasal sinuses caused by a new species of Exserohilum. Journal of Clinical Microbiology 24: 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerini G. 1876. La nebbia del grano turco. Bolletino del Comizio Agrario Parmense 10: 1–3. [Google Scholar]
- Paul AR. 1972. Pyrenophora erythrospila sp. nov., the perfect state of Drechslera erythrospila. Transactions of the British Mycological Society 59: 97–102. [Google Scholar]
- Paul AR, Parbery DG. 1968. Pyrenophora dictyoides sp. nov. the perfect state of Helminthosporium dictyoides. Transactions of the British Mycological Society 51: 707–710. [Google Scholar]
- Rehner SA, Buckley E. 2005. A Beauveria phylogeny inferred from nuclear ITS and EF-1a sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Crous PW, Hyde KD, et al. 2015. Recommended names for pleomorphic genera in Dothideomycetes. IMA Fungus 6: 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Farr DF, Castlebury LA, et al. 2002. Setomelanomma holmii (Pleosporales, Phaeosphaeriaceae) on living spruce twigs in Europe and North America. Canadian Journal of Botany 80: 1 209–1215. [Google Scholar]
- Saccardo PA. 1886. Sylloge Fungorum IV: 420–421. [Google Scholar]
- Sakoda T, Tsukiboshi T. 2011. Exserohilum neoregeliae sp. nov., a new pathogen of Neoregelia carolinae. Mycotaxon 118: 213–218. [Google Scholar]
- Seifert K, Morgan-Jones G, Gams W, et al. 2011. The genera of hyphomycetes. CBS Biodiversity Series 9. CBS-KNAW Fungal Biodiversity Centre, Utrecht. [Google Scholar]
- Shenoy BD, Jeewon R, Wu WP, et al. 2006. Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycological Research 110: 916–928. [DOI] [PubMed] [Google Scholar]
- Shoemaker RA. 1959. Nomenclature of Drechslera and Bipolaris, grass parasites segregated from 'Helminthosporium'. Canadian Journal of Botany 37: 879–887. [Google Scholar]
- Shoemaker RA. 1961. Pyrenophora phaeocomes (Reb. ex Fr.) Fr. Canadian Journal of Botany 39: 901–908. [Google Scholar]
- Simmons EG. 1971. Helminthosporium allii as type of a new genus. Mycologia 63: 380–386. [Google Scholar]
- Simmons EG. 2007. Alternaria. An identification manual CBS Biodiversity Series 6. CBS Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Sivanesan A. 1984. New species of Exserohilum. Transactions of the British Mycological Society 83: 319–329. [Google Scholar]
- Sivanesan A. 1987. Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycological Papers 158: 1–261. [Google Scholar]
- Sivanesan A. 1992. New Bipolaris, Curvularia and Exserohilum species. Mycological Research 96: 485–489. [Google Scholar]
- Sivanesan A, Abdullah SK, Abbas BA. 1993. Exserohilum curvisporum sp. nov., a new hyphomycete from Iraq. Mycological Research 97: 1486–1488. [Google Scholar]
- Smith IM, Dunez J, Lelliott RA, et al. 1988. European Handbook of Plant Diseases. Blackwell Scientific Publications, Oxford. [Google Scholar]
- Smith RM, Schaefer MK, Kainer MA, et al. 2013. Fungal Infections associated with contaminated methylprednisolone injections. New England Journal of Medicine 369: 1598–1609. [DOI] [PubMed] [Google Scholar]
- Srivastava LS, Dhar V, Shambi HS, et al. 1983. A new leaf spot disease of soybean in India. Indian Journal of Mycology and Plant Pathology 12: 240–242. [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiman R, Guiraud P, Seigle-Murandi F, et al. 2000. Exserohilum israeli, a new species isolated from soil near Timna Park (Israel), and its physiological properties. Antonie van Leeuwenhoek 78: 153–161. [DOI] [PubMed] [Google Scholar]
- Subramanian CV. 1956. Hyphomycetes – II. Journal of the Indian Botanical Society 35: 446–494. [Google Scholar]
- Subramanian CV, Jain BL. 1966. A revision of some graminicolous Helminthosporia. Current Science 35: 352–355. [Google Scholar]
- Sun GY, Zhang R, Zhou W, et al. 2005. Exserohilum heteromorphum sp. nov., a new helminthosporioid fungus from Echinochloa in China. Mycotaxon 92: 173–176. [Google Scholar]
- Sun GY, Zhang R, Zhu MQ, et al. 1997. A new species of Exserohilum from China. Mycological Research 101: 776–779. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, et al. 2015. Revision of the Massarineae (Pleosporales, Dothideomycetes). Studies in Mycology 82: 75–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr SAJ, Kafi A. 1968. Growth, sporulation and conidial characteristics of five graminicolous species of Helminthosporium. Transactions of the British Mycological Society 51: 771–777. [Google Scholar]
- Togitani K, Kobayashi M, Sakai M, et al. 2007. Ethmoidal sinusitis caused by Exserohilum rostratum in a patient with malignant lymphoma after non-myeloablative allogeneic peripheral blood stem cell transplantation. Transplant Infectious Disease 9: 137–141. [DOI] [PubMed] [Google Scholar]
- Torres C, Ro JY, el-Naggar AK, et al. 1996. Allergic fungal sinusitis: a clinicopathologic study of 16 cases. Human Pathology 27: 793–799. [DOI] [PubMed] [Google Scholar]
- Tosiah S, Kadir J, Sariah M, et al. 2011. Efficacy of Exserohilum monoceras, a potential fungus for biocontrol of Echinochloa species. Journal of Tropical Agriculture and Food Science 39: 117–124. [Google Scholar]
- Upadhyay HP, Mankau R. 1991. Dactylaria nervicola sp. nov. and Exserohilum novae-zelandiae comb. nov. from Mexico. Mycologia 83: 371–376. [Google Scholar]
- Vaidya G, Lohman DJ, Meier R. 2011. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27: 171–180. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several species of Cryptococcus. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. 2017. Corynespora, Exserohilum and Helminthosporium revisited – New species and generic reclassification. Studies in Mycology 87: 43–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt K, Wöstemeyer J. 2000. Reliable amplification of actin genes facilitates deep-level phylogeny. Microbiological Research 155: 179– 195. [DOI] [PubMed] [Google Scholar]
- Wei CT. 1950. Notes on Corynespora. Mycological Papers 34: 1–10. [Google Scholar]
- White TJ, Bruns TD, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal genes for phylogenetics. In: Gelfand M, Sninsky JI, White TJ. (eds), PCR protocols: a guide to methods and applications: 315–322. New York, Academic Press. [Google Scholar]
- Whitford HW, Schwartz WL, Richards H. 1989. Exserohilum dermal granulomas in a bovine. Journal of Veterinary Diagnostic Investigation 1: 78–81. [DOI] [PubMed] [Google Scholar]
- Wu W. 1990. Contribution to the knowledge of graminicolous 'Helminthosporium' species in Hebei Province (II). Journal of the Hebei Academy of Sciences Selected Papers 60: 49–64. [Google Scholar]
- Wu W, Zhuang W. 2005. Sporidesmium, Endophragmiella and related genera from China. Fungal Diversity Press, University of Hong Kong, Hong Kong. [Google Scholar]
- Zhang G, Berbee ML. 2001. Pyrenophora phylogenetics inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 93: 1048–1063. [Google Scholar]
- Zhang M, Zhang TY, Wu YM. 2004. A new name and a new variety in Curvularia. Mycosystema 23: 177–178. [Google Scholar]
- Zhang W, Watson AK. 1997. Efficacy of Exserohilum monoceras for the control of Echinochloa species in rice (Oryza sativa). Weed Science 45: 144–150. [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, et al. 2012. Pleosporales. Fungal Diversity 53: 1–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, et al. 2009. Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology 64: 85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]