Abstract
Olfactory mucosa contains neural stem cells, called olfactory stem cells (OSCs), which produce trophic support required for promoting axonal regeneration after nerve injury. However, the local tissue environment can reduce the viability/function of transplanted cells when placed directly on the injury. Although gelatin hydrogels have been shown to aid cell survival during transplantation, such OSC‐hydrogel combinations have not been extensively tested, particularly during recovery from facial nerve palsy. In this study, OSCs were isolated from the olfactory mucosae of newborn mice and were shown to express neural stem cell markers before differentiation, as well as cell‐type specific markers after differentiation, confirming their multipotency. The OSCs also secrete growth factors and various cytokines that promote nerve regeneration. To test the effects of OSC transplantation in vivo, Medgel, a biodegradable hydrogel sponge, was applied to retain OSCs around the injury site and to lessen the detrimental effects of the local environment in an established facial nerve palsy mouse model. When OSCs were transplanted into the injury site, accelerated recovery was observed for 1 week. When OSCs were transplanted with Medgel, a higher level and duration of accelerated recovery was observed. OSCs in Medgel also increased peripheral nerve function and increased the number of regenerated nerve fibers. These results suggest that OSCs implanted with Medgel accelerate and enhance recovery from facial palsy in mice. Because human OSCs can be easily obtained from olfactory mucosa biopsies with limited risk, this OSC‐Medgel combination is a candidate treatment option for accelerating recovery after facial nerve injury. stem cells translational medicine 2019;8:169&10
Keywords: Olfactory stem cells, Biodegradable hydrogel, Facial nerve palsy, Nerve regeneration
Significance Statement.
Olfactory stem cells (OSCs) have been shown to promote rubrospinal cord tract axonal regeneration when engrafted into the injured area. In this study, we investigated the effect of OSC transplantation in a mouse model of facial nerve injury. The OSCs were transplanted with and without biodegradable hydrogel, which is often used to strengthen the effect of transplanted cells by protecting them from the local environment and prolonging survival. OSCs accelerated recovery for one week. However, when OSCs were applied with the hydrogel, the accelerated recovery from facial nerve palsy was even more prominent and continued to be enhanced throughout the study.
Introduction
Peripheral facial nerve paralysis is one of the major complications of temporal bone trauma 1. When the facial nerve damage is mild, the damaged nerve can be restored with conservative medical treatment. However, when the nerve damage is severe, surgical intervention is often required to fully recover from palsy. These severe injuries to the facial nerve produce functional, esthetic, and psychological problems for the patient, and despite advances in the medical and surgical management of such injuries, functional recovery is not always satisfactory.
In many ways, facial nerve damage is similar to nerve damage in other areas of the body, including the spinal cord, suggesting that successful functional recovery treatments developed and tested in other neural environments could be applied to treat peripheral facial nerve paralysis. Lima et al. 2, for example, previously reported the results of their pilot clinical study of olfactory mucosa transplantation into injured spinal cords. Olfactory mucosa contains olfactory ensheathing cells as well as olfactory stem cells (OSCs), both of which support axonal outgrowth after spinal cord injury 3. OSCs are also self‐renewing and differentiate into diverse cell types 4. Human OSCs have been shown to secrete a broad range of neurotrophins (NTs), including brain‐derived neurotrophic factor (BDNF), glial cell‐derived neurotrophic factor (GDNF), nerve growth factor (NGF), NT‐3, NT‐4, vascular endothelial growth factor (VEGF), and ciliary neurotrophic factor (CNTF) 5, all of which promote recovery in peripheral nerves after injury 6. Furthermore, human OSCs exhibit high levels of peripherin 7, which has been suggested to play a role in axonal growth and regeneration 8. Taken together, these studies highlight OSCs as candidate stem cells for autologous transplantation.
Notably, the fate of transplanted cells is not only influenced by injury type, but also dependent on signals in the local microenvironment. A hostile local environment can reduce the viability and functionality of transplanted cells, a phenomenon often observed when the cells are injected into the lesion site alone. One technique used to avoid immediate host rejection of the transplanted cells is to inject the cells in a gelatin mixture. Gelatin, a denatured collagen, is a biodegradable polymer that has been extensively utilized for pharmaceutical and medical purposes without any adverse effect 9, 10. Various types of gelatin hydrogels have been transformed into sheets and used to accelerate tissue regeneration by holding and releasing various types of growth factors 11, 12. In fact, gelatin hydrogel has also been shown to act as a scaffold for mesenchymal stem cells, allowing their homogeneous growth 13. However, the use of gelatin hydrogels to mediate OSC transplantation has not been previously evaluated, particularly for treatment of facial nerve palsy.
In this study, we evaluated the in vivo application of OSC transplantation to treat facial nerve palsy in a mouse model. Notably, the OSCs were isolated from neonatal mice and impregnated in biodegradable gelatin hydrogel to enhance cell survival and function. To the best of our knowledge, this is the first time transplantation of this particular OSC‐gelatin hydrogel combination has been evaluated as a treatment for facial nerve palsy.
Materials and Methods
Animals
Specific‐pathogen‐free pregnant female ICR mice were obtained from Japan SLC, Inc. (Hamamatsu, Japan), and OSCs were prepared from the neonatal mice. Nonpregnant specific‐pathogen‐free female ICR mice (4‐weeks‐old) were used as facial nerve palsy models. Mice were housed 4 to 5 per cage in an animal housing facility maintained at 23°C with 55% humidity under a 12‐hours light/dark cycle. They had free access to food and drinking water. All of the animals were treated ethically in compliance with the regulations of the University Committee and in accordance with the Guidelines for Animal Experimentation at Nagoya City University (H22‐M33).
Isolation, Culture, and Induced Differentiation of OSC
Newborn mice were deeply anesthetized by intraperitoneal administration of ketamine (250 mg/kg) and xylazine (25 mg/kg) and then killed by decapitation. The olfactory mucosae were immediately dissected in ice‐cold sterilized phosphate buffered solution (PBS). The isolated tissue was minced into fine pieces with a razor blade and filtered through a 70‐μm cell strainer (Becton, Dickinson and Company, Franklin Lakes, NJ). The filtrate was then centrifuged for 5 minutes, and the pellet was resuspended in 3 ml of Dulbecco's modified Eagle's medium (DMEM)/F‐12 (Life Technologies Corp., Carlsbad, CA) and dissociated mechanically by pipetting. The cell suspension was then filtered through a 40‐μm cell strainer (Becton, Dickinson and Company), and 1.0 × 105 cells/ml were cultured in medium consisting of DMEM/F‐12, 1% B‐27 Supplement Minus Vitamin A (Life Technologies Corp.), 1% penicillin–streptomycin (Life Technologies Corp.), 20 ng/ml human recombinant basic fibroblast growth factor (bFGF, R&D Systems, Inc., Minneapolis, MN), and 20 ng/ml human recombinant epidermal growth factor (EGF, R&D Systems, Inc.), in a 6‐well ultralow attachment plate (Corning Inc., Corning, NY). The cultures were maintained at 37°C in a humid atmosphere with 95% oxygen and 5% CO2. Fresh medium was added to the cultures every third day. After the formation of olfactory spheres (usually 7–14 days), OSCs were dissociated mechanically by pipetting. To examine the stemness or differentiation potential of the olfactory spheres, intact spheres were transferred onto poly‐d‐lysine/laminin‐coated 8‐chamber slides (Becton, Dickinson and Company) and cultured for an additional 7 days in fresh medium or differentiation medium consisting of the culture medium without growth factors 14.
Reverse Transcriptase‐Polymerase Chain Reaction
Total RNA was isolated from the olfactory spheres using an mirVana PARIS Kit (Life Technologies Corp.). RNA was reverse‐transcribed into cDNA using a high‐capacity cDNA reverse transcription kit (Life Technologies Corp.) according to the manufacturer's instructions. The cDNA was then amplified in a thermal cycler using the following program for each primer pair: denaturation at 94°C for 1 minute, annealing at a primer‐specific temperature (listed in Table 1) for 1 minute, and extension at 72°C for 1 minute. The products were amplified using 35 cycles for Nestin, Sox‐2, and Nanog and 25 cycles for Musashi‐1 and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH). The final Polymerase Chain Reaction (PCR) products were then separated by electrophoresis in 2.0% agarose gel. A DNA standard marker, ϕX174‐Hae III digest (Agilent Technologies Japan, Ltd. Shiga, Japan), was used to estimate the size of the amplified bands.
Table 1.
List of primers used for reverse transcriptase‐polymerase chain reaction
| Gene | Primer sequence (5′–3′) | Temperature (°C) | |
|---|---|---|---|
| Nestin | Forward: | AATGGGAGGATGGAGAATGGAC | 56 |
| Reverse: | TAGACAGGCAGGGCTAAGCAAG | ||
| Musashi‐1 | Forward: | GGCTTCGTCACTTTCATGGACC | 58 |
| Reverse: | GGGAACTGGTAGGTGTAACCAG | ||
| Sox‐2 | Forward: | TACCTCTTCCTCCCACTCC | 56 |
| Reverse: | GATTGCCATGTTTATCTCG | ||
| Nanog | Forward: | AGGGTCTGCTACTGAGATGCTCTG | 56 |
| Reverse: | CAACCACTGGTTTTTCTGCCACCG | ||
| Oct3/4 | Forward: | GCCAGGCTCCTGATCAACAGCATCA | 56 |
| Reverse: | ATGGCTGGACACCTGGCTTCAGACT | ||
| OMP | Forward: | CCCAGCAGATGCGGCTCCGA | 66 |
| Reverse: | GATCAGAAAGGCGCTCCCCA | ||
| GAPDH | Forward: | ACCACAGTCCATGCCATCAC | 58 |
| Reverse: | TCCACCACCCTGTTGCTGTA | ||
Immunocytochemistry
OSCs and differentiated OSCs were fixed in 4% paraformaldehyde (PFA, Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 10 minutes and rinsed 3 times with PBS. Nonspecific binding was blocked with 5% goat serum in PBS for 20 minutes, and spheres and cells were incubated overnight at 4°C with primary antibodies diluted in PBS containing 2% goat serum. The primary antibodies used in this study include mouse anti‐Nestin antibody (Abcam Plc., Cambridge, MA), mouse anti‐Musashi‐1 antibody (Abcam Plc.), mouse anti‐βIII‐tubulin antibody (Abcam Plc.), rabbit antigalactocerebroside (GalC) antibody (Millipore Corp., Billerica, MA), mouse antiglial fibrillary acidic protein (GFAP) antibody (Abcam Plc.), and mouse antiolfactory marker protein (OMP) antibody (Abcam Plc.). Alexa Fluor 488‐ or Alexa Fluor 546‐conjugated goat antirabbit IgG or antimouse IgG antibodies (Life Technologies Corp.) were used as the secondary antibodies. Nuclei were stained with Hoechst 33258 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) 15. Images were obtained by confocal laser scanning microscopy (Leica Microsystems GmbH, Wetzlar, Germany). Antibody specificity was confirmed using a universal negative control immunoglobulin (Dako A/S, Glostrup, Denmark).
Cytokine, Growth Factor, and Neurotrophin Secretion
Media was collected from cultures with and without OSCs (1.0 × 105 cells/ml) after 4 days. Then, the secreted cytokines and growth factors were detected using an antibody array (Cytokine Antibody Array C Series 2000, RayBio, Norcross, GA) according to the manufacturer's instructions. In brief, membranes with immobilized antibodies were incubated with 1 ml of each media sample, followed by incubation with biotin‐conjugated antibodies and horseradish‐peroxidase‐conjugated streptavidin and detection by chemiluminescence. Integrated density values were calculated for each spot (area × relative intensity), and positive control signals on each membrane were used to normalize the cytokine signal intensities using ImageQuant LAS 4000 (GE Healthcare, Little Chalfont, UK). Next, the concentrations of BDNF, GDNF, and NGF were assayed by enzyme‐linked immunosorbent assay (ELISA) using a BDNF Emax ImmunoAssay System (Promega Corp., Madison, WI), a GDNF Emax ImmunoAssay System (Promega Corp.), and a Chemikine NGF Sandwich ELISA (Chemicon International Inc., Billerica, MA) following the manufacturer's instructions.
Preparation of OSCs for Transplantation
MedGel SP19 (MedGEL Corp, Tokyo, Japan), a biodegradable sponge composed of gelatin hydrogel 16, 17, was used as a scaffold for the transplanted OSCs. Neurospheres were collected during passages 3 to 6 for transplantation. The cells were rinsed in DMEM/F‐12, centrifuged for 5 minutes at 300 g, and resuspended in DMEM/F‐12. The single cells were then dissociated by pipetting, and then concentration was adjusted to 20,000 cells/μl before seeded into MedGel SP19.
Murine Facial Nerve Palsy Model and OSC Transplantation
Four‐week‐old mice were anesthetized by intraperitoneal administration of ketamine (100 mg/kg) and xylazine (10 mg/kg). The main trunk of the right facial nerve was exposed below the parotid gland, released from the surrounding connective tissue, and compressed for 30 seconds using mosquito hemostats as previously reported 18. After compression, the mice were randomly assigned to four experimental groups (n = 10 per group): the OSC/MedGel group, the OSC group, the MedGel group, and the mock group. The mice in the OSC/Medgel group received MedGel impregnated with OSCs (2.0 × 104 cells) in 10 μl of DMEM/F‐12. The mice in the OSC group received the same amount of OSCs (2.0 × 104 cells/10 μl) without MedGel. The mice in the Medgel group received MedGel with the same volume of DMEM/F‐12, but no OSCs. The mice in the mock group received the same volume of DMEM/F‐12 alone. After treatment, each animal was allowed to recover in an approved animal care facility until clinical evaluation. Notably, no deaths, significant weight loss, or other complications were observed in any of the animals during this study.
Clinical Evaluation of Facial Nerve Paralysis
The clinical signs of facial nerve paralysis were monitored every day following nerve compression. To evaluate facial nerve paralysis, eye blink, and whisker movement were scored separately as previously described 18. For each, a score of 0 was given for no detectable movement, 1 for detectable motion, 2 for significant but asymmetric voluntary motion, and 3 for symmetric voluntary motion. Facial nerve function was determined using the total scores for eye blink and whisker movement.
Electrophysiological Evaluation
The amplitude of the evoked compound muscle action potential (CMAP) of the buccinator muscle was examined for each mouse (n = 4 per group) using conventional procedures with an electromyography monitor (UA‐103U, Unique Medical Co., Ltd., Osaka, Japan) before surgery and 14 days postsurgery, as previously descried 18. Under anesthesia with ketamine (100 mg/kg) and xylazine (10 mg/kg), the facial nerve was exposed, bipolar needles were hooked to the main trunk, and the nerve was stimulated supermaximally by single 5‐ms electrical pulses with a frequency of 1 Hz. The response amplitude was measured from the peak to trough of the evoked response.
Histopathological Evaluation
Histopathological examination was performed on days 7 and 14 after surgery, as previously described 18. In short, mice were transcardially perfused with 4% PFA in PBS under deep anesthesia with ketamine (250 mg/kg) and xylazine (25 mg/kg). Following perfusion, the head was removed, decalcified in ethylenediaminetetraacetic acid (EDTA, Dojindo Molecular Technologies, Inc.), and embedded in paraffin. The heads were then sectioned (3‐μm thick) and stained using the Klüver–Barrera method. At 1 mm distal to the compression site, the structure of the facial nerve was evaluated via microscopy (Olympus Co., Ltd., Tokyo, Japan) with a CCD camera (Nikon, Co., Ltd., Tokyo, Japan). The number of myelinating fibers was counted.
Statistical Analyses
Data are presented as the means ± standard deviation. The facial nerve scoring data were evaluated with Kruskal–Wallis tests, whereas the other data were evaluated by one‐way factorial analysis of variance (ANOVA) followed by Dunnett's test for comparison with JMP Software (SAS Institute, Cary, NC). Differences with a probability value less than .05 were considered statistically significant.
Results
Olfactory Mucosa‐Derived OSCs Express Neural Stem Cell Markers and Differentiate into Neural Cells
On day 7 after plating olfactory mucosa cells, we detected floating, phase‐bright spheres of cells (Fig. 1A). Notably, these primary cultures continued to produce spheres for several months, suggesting that the olfactory spheres possessed high self‐renewal potential. To evaluate this characteristic, we investigated the expression of various neuronal markers by Reverse Transcriptase (RT)‐PCR and immunocytochemistry. Our results show that the neural stem cell markers Nestin and Musashi‐1 and the undifferentiated cell markers Sox‐2 and Nanog are expressed at the mRNA level in the olfactory spheres (Fig. 1B). Furthermore, Nestin and Musashi‐1 are also expressed at the protein level (Fig. 1C). The cells also express OMP (Fig. 1C), indicating that the cells can be classified as neural stem‐like cells from the olfactory mucosa. Interestingly, before inducing differentiation, GalC, an oligodendrocyte marker, was detected, but βIII‐tubulin and GFAP, markers of differentiated neurons and astrocytes, respectively, were not (Fig. 1C). However, after inducing differentiation, although Nestin, Musashi‐1, and OMP were still detected (Fig. 2), all of the differentiation markers (βIII‐tubulin, GalC, and GFAP) were also observed. Thus, the cells containing the olfactory spheres share similar characteristics with neural stem cells and can differentiate into multiple cell types.
Figure 1.
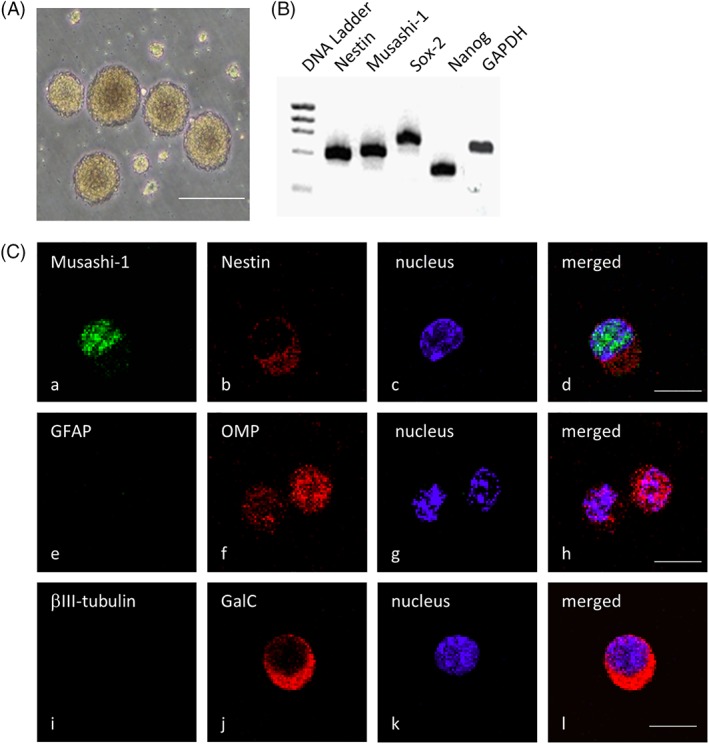
Olfactory spheres and olfactory stem cells express neural stem cell markers. (A): Representative image of the floating olfactory spheres observed under a phase‐contrast microscope. (B): Reverse Transcriptase‐Polymerase Chain Reaction analysis of nestin, Musashi‐1, Sox‐2, and Nanog mRNA expression in the olfactory spheres. (C): Representative immunostaining images showing the protein expression of the neural stem cell markers Musashi‐1 (Ca), nestin (Cb), and OMP (Cf) as well as the expression of GFAP (Ce), βIII‐tubulin (Ci), and GalC (Cj), which label differentiated astrocytes, neurons, and oligodendrocytes, respectively. The nuclei were counterstained with Hoechst 33258 (Cc, Cg, and Ck), and merged images are shown in (Cd), (Ch), and (Cl). The scale bars indicate 100 μm (Ca) and 50 μm (Cc).
Figure 2.
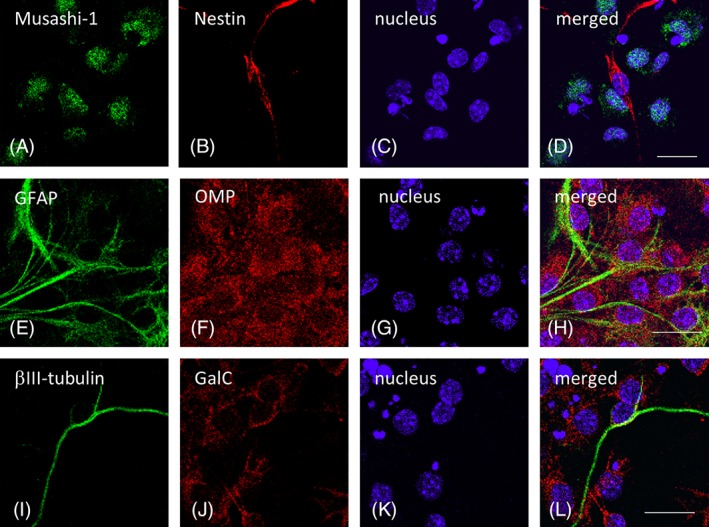
Neural stem cell markers and neuronal markers in differentiated cells from the olfactory spheres. Representative immunostaining images showing the expression of the neural stem cell markers Musashi‐1 (A), nestin (B), and OMP (F) as well as GFAP (E), βIII‐tubulin (I), and GalC (J), which label astrocytes, neurons, and oligodendrocytes, respectively, in the differentiated olfactory stem cells. The nuclei were counterstained with Hoechst 33258 (C, G, and K), and merged images are shown in (D), (H), and (L). The scale bars indicate 20 μm.
OSCs Secrete NGF and Several Types of Cytokines that Accelerate Nerve Regeneration
To evaluate the function and possible role of these murine OSCs in nerve regeneration, we analyzed the levels of secreted cytokines, growth factors, and NTs that are known to be involved in this process. As shown in Figure 3A, the secretion of Galectin‐1, growth arrest‐specific 1 (GAS1), insulin‐like growth factor‐binding proteins 2 and 3 (IGF‐BP2 and IGF‐BP3), soluble tumor necrosis factor receptor I (sTNF‐RI), and tumor necrosis factor‐related weak inducer of apoptosis receptor (TWEAKR) was significantly increased. However, the secretion of EGF, bFGF, or VEGF was largely unchanged (Fig. 3B). With reference to NT secretion into the culture media, only that of NGF was significantly increased, whereas the BDNF and GDNF levels were unchanged (Fig. 3C).
Figure 3.
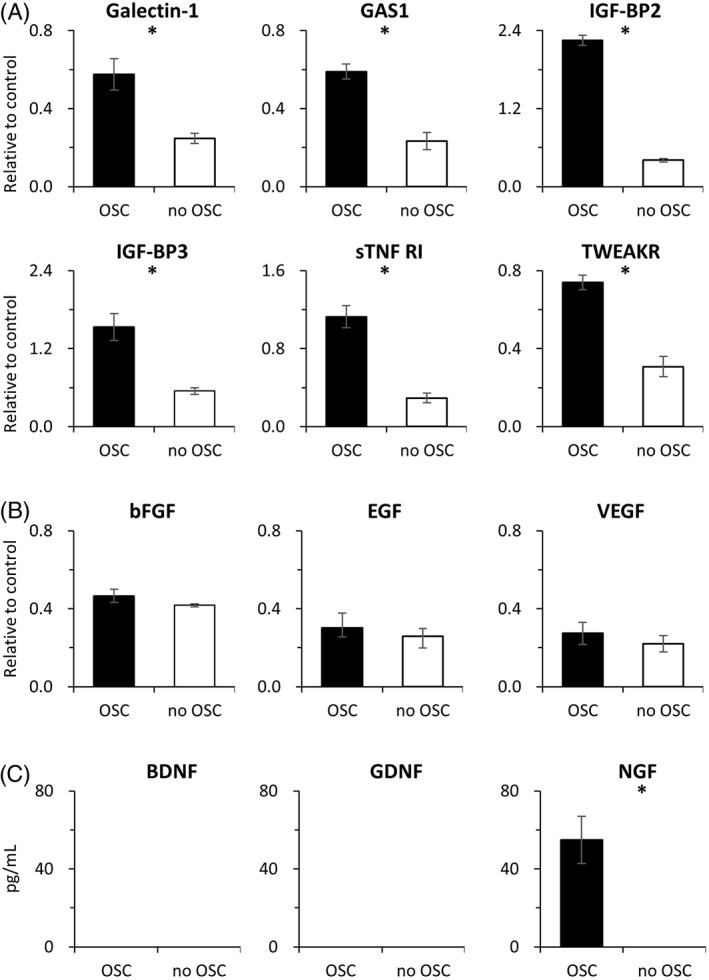
Murine cytokines, growth factors, and neurotrophins are secreted into the culture media by the olfactory stem cells (OSCs). (A): Relative expression of the murine cytokines Galectin‐1, GAS1, IGF‐BP2, IGF‐BP3, sTNF‐RI, and TWEAKR in media isolated from cultures with and without OSCs. (B): Relative expression of the growth factors bFGF, EGF, and VEGF in media isolated from cultures with and without OSCs. (C): Concentration of the murine neurotrophins NGF, BDNF, and GDNF in media isolated from cultures with or without OSCs. All assays were done in triplicate and are presented as the means ± standard deviation. * p < .05.
OSCs with MedGel Accelerate Recovery from Facial Nerve Paralysis
Although the isolated murine OSCs appear to have the potential to be effective during transplantation, it was essential to test their function in our previously established mouse model of facial nerve palsy 18. In this model, facial nerve paralysis develops immediately after main trunk compression, with complete recovery within 14 days. In this study, in addition to injecting the naked OSCs at the compression site, we also tested the effectiveness of the OSCs when impregnated in hydrogel. As shown in Figure 4A, there was no significant difference in final facial scores on day 14 among any of the treatment groups. However, the facial scores of the mice in the OSC/Medgel group were significantly higher on days 4–12 than those found for the mice in the other groups. Interestingly, mice in the OSC group exhibited superior recovery on days 4–7 compared with that of mice in the Medgel and mock groups, but all three of these groups had nearly identical recovery levels after day 8. The number of days required to reach a score of 6, which indicates complete recovery from facial paralysis, was 10.4 ± 0.5 days for the OSC/Medgel group, whereas the OSC, Medgel, and mock groups required 12.5 ± 0.7, 13.1 ± 0.5, and 13.5 ± 0.5 days, respectively (Fig. 4B). These data suggest that recovery from facial nerve paralysis was significantly faster in the OSC/Medgel group.
Figure 4.
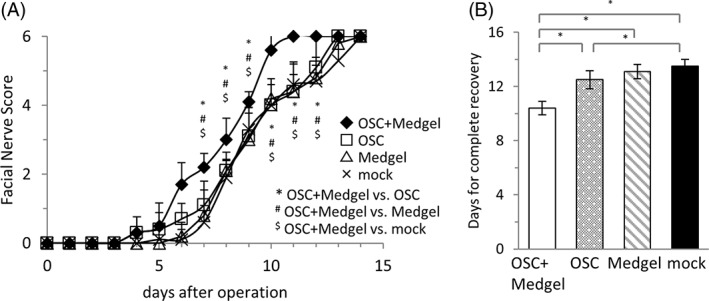
Acceleration of recovery by olfactory stem cells (OSCs) with Medgel after crush injury. (A): Time course of facial nerve scores in the OSC/Medgel, OSC, Medgel, and mock groups (n = 10 per group). The error bars indicate the standard errors of the means. *,#,$ p < .05. (B): The number of days required to recover after facial nerve compression was calculated for each group. * p < .05.
OSCs with MedGel Enhance Electrophysiological Recovery
To confirm the potential of OSC‐hydrogel transplantation and further evaluate the effect of this treatment on nerve function, we analyzed the electrophysiological activity (based on the evoked CMAP amplitude of the buccinator muscle during stimulation of the main trunk of the facial nerve) in the mice of each treatment group on day 14. Representative CMAP waves are shown in Figure 5A. Before nerve compression, the CMAP amplitude was 8.1 ± 0.1 mV for all mice. On day 14, the amplitude was 5.7 ± 0.6 mV in the OSC/Medgel group, 4.3 ± 1.0 mV in the OSC group, 3.3 ± 0.5 mV in the Medgel group, and 1.8 ± 0.3 mV in the mock group (Fig. 5B). These results show that the mean amplitude, and therefore the functional nerve recovery, was significantly higher in the OSC/Medgel group than it was in the other groups.
Figure 5.
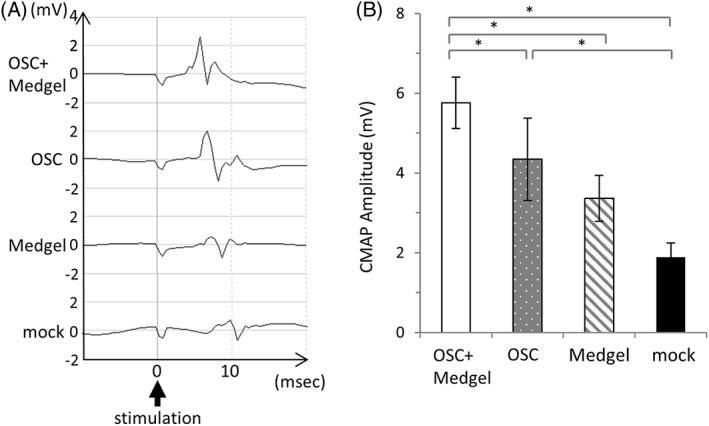
Enhancement of electrophysiological recovery by olfactory stem cells with Medgel. (A): Representative compound muscle action potential waves in the buccinator muscle of mice in each group after facial nerve trunk stimulation 14 days postsurgery. (B): The mean amplitude was calculated for each group (n = 4). The data are presented as the means ± standard deviation. * p < .05.
OSCs with MedGel Stimulate Nerve Fiber Regeneration
Finally, we investigated nerve regeneration using histological techniques. On day 7, most of the myelinated nerve fibers exhibited vacuous changes, but some regenerating fibers were also observed (Fig. 6A, upper panel). Notably, the number of regenerating nerve fibers per nerve at this time point was significantly higher in the OSC/Medgel (1,721 ± 191) and OSC (1,593 ± 243) groups than it was in the Medgel (874 ± 241) and mock (925 ± 112) groups (Fig. 6B). On day 14, more regenerating nerve fibers were observed in all of the groups (Fig. 6A, lower panel), being significantly more prevalent in the OSC/Medgel group (4,086 ± 695) compared with the OSC (2,273 ± 503), Medgel (1,951 ± 282), and mock (1,962 ± 222) groups (Fig. 6B).
Figure 6.
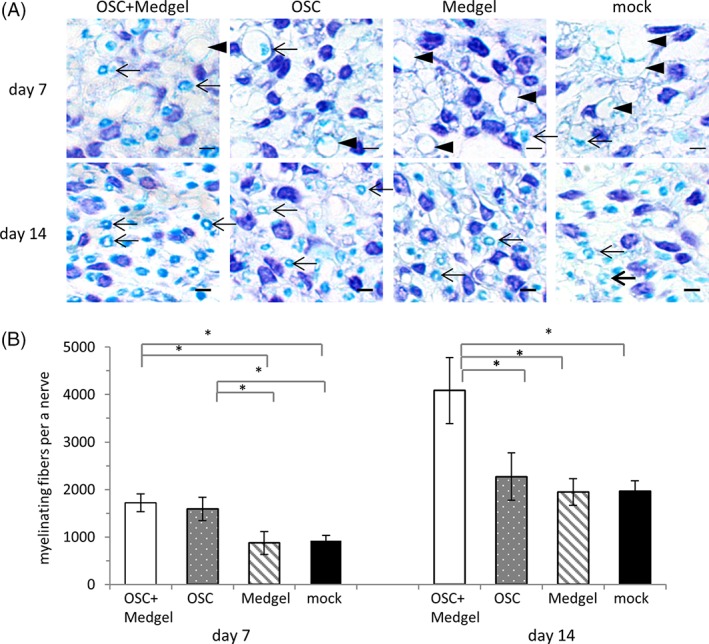
Stimulation of histopathological recovery by olfactory stem cells with Medgel. (A): Magnified images of sections of the right facial nerves 1 mm distal to the compression site 7 days (upper row) or 14 days (lower row) post‐treatment after staining with the Klüver–Barrera method. Arrowhead indicates nerve fibers with degraded myelin, whereas the arrows highlight regenerating nerve fibers with intact myelin. The scale bars indicate 100 μm. (B): The numbers of myelinated nerve fibers calculated for each group (n = 3). The data are presented as the means ± standard deviation. * p < .05.
Discussion
In a previous study investigating an effective treatment option for spinal cord injury, OSCs promoted rubrospinal cord tract axonal regeneration when engrafted into the injured area 19, 20. Notably, facial nerve injury requires the activation of similar processes as the spinal cord, suggesting that similar treatment option could be effective in this context. However, many treatments that are successful in other neural context have yet to be tested during recovery from facial nerve palsy. In this study, we investigated the effect of OSC transplantation in a mouse model of facial nerve injury. The OSCs were transplanted with and without biodegradable hydrogel, which is often used to strengthen the effect of transplanted cells by protecting them from the local environment and prolonging survival. Our results indicate that OSCs accelerate recovery after facial nerve injury. Unfortunately, this effect appears to be limited to 1 week in our model. However, when OSCs were applied with Medgel, the accelerated recover from facial nerve palsy was even more prominent and continued to be enhanced throughout the study.
Human OSCs can be isolated from olfactory mucosa biopsies, are self‐replicating, and form spheres in culture that express various neural stem cell and differentiation markers, including Nestin, GFAP, and βIII‐tubulin 4, 21. In the present study, we isolated OSCs from the olfactory mucosa of newborn mice. The murine OSCs also had the ability to grow as spheres and to self‐renew for several months. The OSCs and olfactory spheres also expressed Nestin but not GFAP or βIII‐tubulin. In addition to Nestin, these OSCs expressed the stem cell markers Musashi‐1, Nanog, and Sox‐2, all of which are not normally detected in OSCs. However, dental pulp stem cells do express Nestin, Musashi‐1, Nanog, and Sox‐2 22, indicating that the murine OSCs isolated here may be more similar to this subset of stem cells. Regardless of the stem cell population, these OSCs are the most similar to, they do appear to have stem cell characteristics with multipotential capacity, as indicated by the expression of βIII‐tubulin, GalC, and GFAP when cultured in differentiation medium.
Human OSCs have been shown to secrete NTs, including BDNF, GDNF, NGF, NT‐3, NT‐4, VEGF, and CNTF 5. In the current study, the secretion of NGF, which enhances axonal regeneration 23, was increased in media isolated from OSC cultures. Moreover, several types of cytokines were increased, many of which can accelerate nerve regeneration. Galectin‐1, for example, interferes with hydrogen peroxide production that prevents axonal regeneration 24, and GAS1 is a coreceptor for the GDNF ligand family 25. IGFBP‐2 and IGFBP‐3 bind to IGF‐II and IGF‐I, respectively, and prolong the half‐lives of the IGFs 26. Furthermore, soluble TNF‐RI inhibits TNF‐α, which induces optic nerve degeneration 27, and the TWEAK receptor is encoded by fibroblast growth factor‐inducible‐14, which promotes neurite outgrowth 28. Taken together, the known functions of these cytokines indicate that any one of them alone or in combination may be candidates for accelerating regeneration after facial nerve injury. Future work is required to elucidate the full network and relationships between these cytokines, growth factors, and NTs during recovery.
Although the implanted stem cells influence their environment via the secretion of various factors, the fate of the cells is also dictated by that same microenvironment 29. If the cells are not protected, then their effects are often muted or the cells are destroyed before inducing any beneficial function. One technique used to protect the cells is to incorporate them into gelatin hydrogel. Medgel is a biodegradable hydrogel sponge that has been shown to preserve various proteins for more than 2 weeks 9. The same type of hydrogel containing bFGF has also been clinically used to treat facial nerve paralysis without any aberrant side‐effects 30. Medgel has also been used to encase mesenchymal stem cells, allowing them to grow homogeneously 13. For these reasons, we chose to test Medgel in our facial nerve palsy mouse model, both alone and in association with the murine OSCs. After compression of the facial nerve, facial movements were absent until day 3 and then recovered gradually until fully recovered at day 14, consistent with our previous study 31. OSCs impregnated in Medgel accelerated recovery on days 4–12, whereas OSCs alone only accelerated recovery on days 4–7. When used alone, Medgel did not accelerate recovery. The recovery curves based on this data indicate that although OSCs accelerate recovery from facial nerve injury, the effect was observed for only a week. In contrast, the effect of OSCs was prolonged when applied with Medgel. We speculate that the Medgel helped retain the OSCs around the injury site and increased their survival and function, thus allowing them to secrete the cytokines and various factors necessary to mediate an enhanced and accelerated recovery response.
The effects of OSCs, Medgel, and their combination were further evaluated with reference to nerve function and regeneration. CMAP amplitude is a quantitative indicator of Wallerian degeneration of the nerve after injury 32. The CMAP amplitudes from the OSC/Medgel group on day 14 were significantly greater than they were for the other groups, reflecting the best recovery curve. The CMAP amplitude was also greater in the OSC group than it was in the mock group, again demonstrating the effect, albeit modest, of OSCs on recovery. Histopathologically, nearly all of the myelin surrounding the nerves was degraded by day 7 regardless of treatment. However, greater myelin regeneration was observed in the OSC/Medgel group on days 7 and 14 than in the other groups, again supporting our other data indicating that this treatment results in the best recovery. However, significant myelin regeneration was also observed in the OSC group compared with the Medgel only and mock groups on day 7. These results support the effect of naked OSCs on facial nerve scores and also reflect their limited period.
Summary
Collectively, our data show that the most prominent effect on facial nerve recovery was observed when OSCs were impregnated in the biodegradable hydrogel. Because human OSCs are located in the olfactory epithelium, they can be easily harvested from the nasal cavity with limited risk 4. In a clinical setting, OSC‐impregnated hydrogel could be used to treat patients with severe facial palsy caused by temporal bone fracture, which requires decompression surgery. Decompression surgery is performed by exposing the facial nerve after opening the bony fallopian canal. At this stage of the surgery, OSCs impregnated in hydrogel could be placed directly onto the exposed injured nerve to accelerate and enhance recovery. Similarly, other traumas or diseases resulting in facial nerve palsy that are treated with decompression surgery, such as Bell's palsy or Ramsay Hunt syndrome, could also be treated with OSCs in Medgel in this manner. Clinical studies are, therefore, required to determine the effects of this treatment.
Author Contributions
S.E.: conception and design, financial support, collection and assembly of data, data analysis and interpretation, article writing; S.K.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; Y.H.: financial support, conception and design, data analysis and interpretation; Y.N.: financial support, conception and design, manuscript writing; S.M.: administrative support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgment
This work was supported by grants‐in‐aid for scientific research from the Ministry of Education, Culture, Sports, Sciences, and Technology of Japan (2446266 and 25462650).
References
- 1. Chang CY, Cass SP. Management of facial nerve injury due to temporal bone trauma. Am J Otol 1999;20:96–114. [PubMed] [Google Scholar]
- 2. Lima C, Escada P, Pratas‐Vital J et al. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair 2010;24:10–22. [DOI] [PubMed] [Google Scholar]
- 3. Ishihara M, Mochizuki‐Oda N, Iwatsuki K et al. Primary olfactory mucosal cells promote axonal outgrowth in a three‐dimensional assay. J Neurosci Res 2014;92:847–855. [DOI] [PubMed] [Google Scholar]
- 4. Murrell W, Feron F, Wetzig A et al. Multipotent stem cells from adult olfactory mucosa. Dev Dyn 2005;233:496–515. [DOI] [PubMed] [Google Scholar]
- 5. Marshall CT, Lu C, Winstead W et al. The therapeutic potential of human olfactory‐derived stem cells. Histol Histopathol 2006;21:633–643. [DOI] [PubMed] [Google Scholar]
- 6. Abrams M, Widenfalk J. Emerging strategies to promote improved functional outcome after peripheral nerve injury. Restor Neurol Neurosci 2005;23:367–382. [PubMed] [Google Scholar]
- 7. Roisen FJ, Klueber KM, Lu CL et al. Adult human olfactory stem cells. Brain Res 2001;890:11–22. [DOI] [PubMed] [Google Scholar]
- 8. Chadan S, Moya KL, Portier MM et al. Identification of a peripherin dimer: changes during axonal development and regeneration of the rat sciatic nerve. J Neurochem 1994;62:1894–1905. [DOI] [PubMed] [Google Scholar]
- 9. Tabata Y. Significance of release technology in tissue engineering. Drug Discov Today 2005;10:1639–1646. [DOI] [PubMed] [Google Scholar]
- 10. Zekorn D. Intravascular retention, dispersal, excretion and break‐down of gelatin plasma substitutes. Bibl Haematol 1969;33:131–140. [DOI] [PubMed] [Google Scholar]
- 11. Ishida K, Kuroda R, Miwa M et al. The regenerative effects of platelet‐rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng 2007;13:1103–1112. [DOI] [PubMed] [Google Scholar]
- 12. Sakakibara Y, Nishimura K, Tambara K et al. Prevascularization with gelatin microspheres containing basic fibroblast growth factor enhances the benefits of cardiomyocyte transplantation. J Thorac Cardiovasc Surg 2002;124:50–56. [DOI] [PubMed] [Google Scholar]
- 13. Umehara K, Iimura T, Sakamoto K et al. Canine oral mucosal fibroblasts differentiate into osteoblastic cells in response to BMP‐2. Anat Rec 2012;295:1327–1335. [DOI] [PubMed] [Google Scholar]
- 14. Lin J, Feng L, Hamajima Y et al. Directed differentiation of mouse cochlear neural progenitors in vitro. Am J Physiol Cell Physiol 2009;296:C441–C452. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Goshima F, Esaki S, Luo C et al. Oncolytic viral therapy with a combination of HF10, a herpes simplex virus type 1 variant and granulocyte‐macrophage colony‐stimulating factor for murine ovarian cancer. Int J Cancer 2014;134:2865–2877. [DOI] [PubMed] [Google Scholar]
- 16. Yamamoto M, Takahashi Y, Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials 2003;24:4375–4383. [DOI] [PubMed] [Google Scholar]
- 17. Tabata Y, Nagano A, Ikada Y. Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng 1999;5:127–138. [DOI] [PubMed] [Google Scholar]
- 18. Esaki S, Kitoh J, Katsumi S et al. Hepatocyte growth factor incorporated into herpes simplex virus vector accelerates facial nerve regeneration after crush injury. Gene Ther 2011;18:1063–1069. [DOI] [PubMed] [Google Scholar]
- 19. Xiao M, Klueber KM, Guo Z et al. Human adult olfactory neural progenitors promote axotomized rubrospinal tract axonal reinnervation and locomotor recovery. Neurobiol Dis 2007;26:363–374. [DOI] [PubMed] [Google Scholar]
- 20. Xiao M, Klueber KM, Lu C et al. Human adult olfactory neural progenitors rescue axotomized rodent rubrospinal neurons and promote functional recovery. Exp Neurol 2005;194:12–30. [DOI] [PubMed] [Google Scholar]
- 21. Buiakova OI, Baker H, Scott JW et al. Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proc Natl Acad Sci U S A 1996;93:9858–9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karbanova J, Soukup T, Suchanek J et al. Characterization of dental pulp stem cells from impacted third molars cultured in low serum‐containing medium. Cells Tissues Organs 2011;193:344–365. [DOI] [PubMed] [Google Scholar]
- 23. Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci 1988;8:2394–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quinta HR, Wilson C, Blidner AG et al. Ligand‐mediated Galectin‐1 endocytosis prevents intraneural H2O2 production promoting F‐actin dynamics reactivation and axonal re‐growth. Exp Neurol 2016;283:165–178. [DOI] [PubMed] [Google Scholar]
- 25. Schueler‐Furman O, Glick E, Segovia J et al. Is GAS1 a co‐receptor for the GDNF family of ligands? Trends Pharmacol Sci 2006;27:72–77. [DOI] [PubMed] [Google Scholar]
- 26. Duan C, Xu Q. Roles of insulin‐like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol 2005;142:44–52. [DOI] [PubMed] [Google Scholar]
- 27. Kitaoka Y, Kitaoka Y, Kwong JM et al. TNF‐alpha‐induced optic nerve degeneration and nuclear factor‐kappaB p65. Invest Ophthalmol Vis Sci 2006;47:1448–1457. [DOI] [PubMed] [Google Scholar]
- 28. Tanabe K, Bonilla I, Winkles JA et al. Fibroblast growth factor‐inducible‐14 is induced in axotomized neurons and promotes neurite outgrowth. J Neurosci 2003;23:9675–9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelly S, Bliss TM, Shah AK et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A 2004;101:11839–11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hato N, Nota J, Komobuchi H et al. Facial nerve decompression surgery using bFGF‐impregnated biodegradable gelatin hydrogel in patients with Bell palsy. Otolaryngol Head Neck Surg 2012;146:641–646. [DOI] [PubMed] [Google Scholar]
- 31. Most SP. Facial nerve recovery in bcl2 overexpression mice after crush injury. Arch Facial Plast Surg 2004;6:82–87. [DOI] [PubMed] [Google Scholar]
- 32. Esslen E. A method of demonstrating and measuring disturbances of central innervation: electrotonomyography. Electroencephalogr Clin Neurophysiol 1967;23:387. [PubMed] [Google Scholar]


