Abstract
Over the last two decades polypharmacology has emerged as a new paradigm in drug discovery, even though developing drugs with high potency and selectivity toward a single biological target is still a major strategy. Often, targeting only a single enzyme or receptor shows lack of efficacy. High levels of inhibitor of a single target also can lead to adverse side effects. A second target may offer additive or synergistic effects to affecting the first target thereby reducing on- and off-target side effects. Therefore, drugs that inhibit multiple targets may offer a great potential for increased efficacy and reduced the adverse effects. In this review we summarize recent findings of rationally designed multitarget compounds that are aimed to improve efficacy and safety profiles compared to those that target a single enzyme or receptor. We focus on dual inhibitors/modulators that target the soluble epoxide hydrolase (sEH) as a common part of their design to take advantage of the beneficial effects of sEH inhibition.
Keywords: polypharmacology, multitarget agents, dual inhibitors/modulators, soluble epoxide hydrolase
1. Introduction
Drug discovery has been dominated by the approach of using a single protein target to treat a single disease with a single chemical entity. Such drugs possess high selectivity and potency against their specific targets and there is no doubt that this approach will continue to be a major strategy of drug development. Despite this, there are pitfalls of the single-target approach and a continued need for improvement to increase efficacy and/or reduce on- and off-target adverse events. Multitarget drugs [1] (also known as designed multiple ligands (DMLs) [2] or hybrid molecules [3]) emerged as a strategy to overcome these problems. Studies on FDA-approved new molecular entities from 2000 to 2015 [4] and from 2015 to 2017 [1] support the idea that polypharmacology is particularly useful for treating complex diseases such as inflammation, cancers and metabolic syndrome [5, 6]. Overall drug discovery based on small molecules is decreasing due in part to the rise and success of biologics such as immunotherapy for cancers [7]. However, the number of FDA-approved drugs that interact with multitargets is growing. One reason for this growth is the approval of pan-kinase inhibitors to treat some cancers which are refractory to monotherapy due to the nature of the disease. Multitarget drugs aim to improve efficacy by addressing more targets involved in the disease. The additive or synergistic effects of multitarget drugs also permit a reduced effective dose, which in turn, minimizes off-target effects. Some single-target drugs may have off-target side-effects or on-target side effects (mechanism-based toxicity) that limit their effective doses. These can potentially be alleviated with the help of including a secondary target that attenuates the side effect. The soluble epoxide hydrolase (sEH) is an enzyme in the arachidonic acid (ARA) cascade which participates in the degradation of epoxide metabolites formed from cytochrome P450 (CYP450) action on ARA and other unsaturated fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [8]. Inhibiting sEH and thus sustaining the titers of endogenous epoxy fatty acid (EpFAs) metabolites has been shown to be beneficial in various disease pathologies. Therefore, in this review, we focus on recent findings of dual inhibitors/modulators that are rationally designed to concurrently inhibit sEH as one of their multiple targets and the role of these compounds in various diseases.
2. Development and application of dual inhibitors of using sEH as a target
The soluble epoxide hydrolase (EC 3.3.2.10, sEH) is an alpha/beta-hydrolase fold enzyme which metabolizes various EpFAs into their corresponding diols [9]. The enzyme exists in mammals as a domain-swapped homodimer and is highly conserved among species [10]. sEH is well-expressed throughout the mammalian body with the highest levels found in the liver and kidney. The C-terminal domain of the enzyme houses the catalytic site for epoxide hydrolysis of EpFAs and the N-terminal has phosphatase activity with an uncertain biological role. Pharmacological inhibition of sEH has been used as a strategy with great success to investigate the biological role of the EpFAs in vivo.
2.1. Dual inhibition of sEH and enzymes within the ARA cascade
2.1.1. ARA metabolism and biological pathways
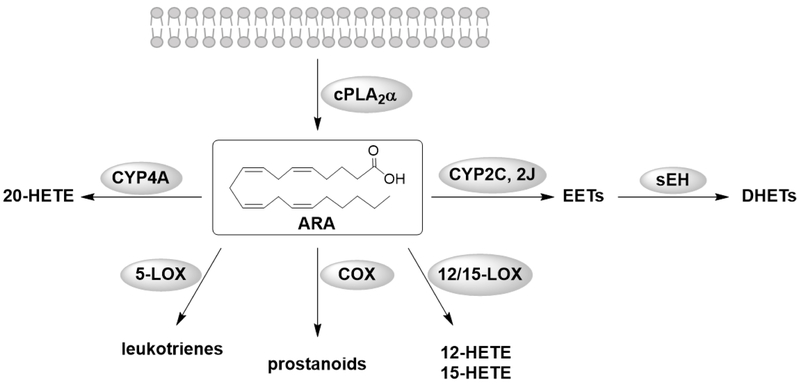
Several biological processes such as inflammation and allergies are mediated by ARA metabolites, named eicosanoids. The cascade starts with the release of ARA from cellular membranes by phospholipases, commonly type-IV cytosolic PLA2 α (cPLA2α) [11] and others such as diacylglycerol lipase. ARA is then transformed into eicosanoids by three main metabolic pathways; the cyclooxygenase (COX) pathway produces prostanoids, the lipoxygenases (LOXs) catalyze the formation of lipoxins (LXs), leukotrienes (LTs) and hydroxyeicosatetraenoic acid (HETEs), and the CYP450 pathway forms epoxyeicosatrienoic acids (EETs) and 20-hydroxyeicosatetraenoic acid (20-HETE) (Fig. 1). Although here the focus is on ARA, it is important to note that DHA and EPA and other unsaturated fatty acids are also metabolized by the same enzymes of this cascade and the EpFAs of all these lipid classes are also substrates of sEH [8]. Esters and amides of fatty acids and increasingly their oxidized metabolites described above could be considered another branch of the ARA cascade, but they usually are classified as endocannabinoids.
Figure 1.
Illustration of the different metabolism pathways of the ARA cascade.
The CYP450 pathway
The CYP450s are a large family of heme containing enzymes that are widely expressed in the body and across species [12]. They are primarily monooxygenases with some substrate selectivity. The reactions of these CYP450 enzymes are selective, but by no means specific. Also, these hydroxylation and epoxidation reactions are carried out by other CYP450 enzymes, often at high rate, and they can also be induced by xenobiotics [12]. CYP4A enzymes convert ARA into the vasoconstrictive 20-HETE [13], CYP2C and CYP2J are known to transform ARA into a variety of oxidized products but largely EETs in many tissues [12]. Four regioisomers of EETs, the 5,6-, 8,9-, 11,12-, and 14,15-EETs, are formed depending on where the substrate is oxidized [14]. EETs are autocrine and paracrine mediators in the renal and cardiovascular system and are vasodilatory and anti-inflammatory. They mediate hyperpolarization and vasorelaxation via activation of ion-channels and show mitogenic effects in the kidney [14]. They are sometimes considered as a single class of lipid leading to similar biological effect [15]. However, as studies become more sophisticated individual regioisomers appear to show different properties. Most of the described effects occur through activation of signal transduction pathways and modulation of gene expression [14]. Detailed biological effects mediated by EETs have been reviewed by Spector et al. [16], and distinct biological effects of individual regioisomers are currently under investigation.
The COX pathway
The COX enzymes exists as two isoforms, COX-1 and COX-2. COX-1 is constitutively expressed and produces prostanoids that function to maintain homeostasis and gastric epithelial cytoprotection [17]. In contrast, COX-2 is inducible by various stimuli such as inflammation, hormones and growth factors, and is therefore a more important source of prostanoid formation regarding inflammation and proliferative diseases such as cancers. However, COX-2 is also constitutively expressed in some tissues such as brain, kidney, and spinal cord [18]. COX isoforms exist as bifunctional homodimers and transform ARA into prostaglandin G2 (PGG2) via cyclooxygenase activity and then further into prostaglandin H2 (PGH2) via peroxidase activity. PGH2 is further metabolized into several prostanoids by various isomerases and synthases in a cell specific manner [17]. Prostanoids induce several biological effects through binding to G-protein-coupled prostanoid receptors. In the gastroduodenal tract, prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α) and prostacyclin (PGI2) maintain the normal mucosal integrity through mucus synthesis and secretion, hydrogen carbonate secretion, mucosal blood flow and cellular repair [19]. PGE2, PGI2, prostaglandin D2 (PGD2) and thromboxane A2 (TXA2) play important roles in controlling vascular tone. TXA2 causes platelet aggregation and vasoconstriction while PGI2 mediates vasodilatation [20]. In inflammation, the common symptoms rubor (redness), calor (heat), tumor (swelling) and dolor (pain) are mediated by PGE2 [17]. PGI2 and PGE2 also play important roles in pain sensation and hyperalgesia [21].
The LOX pathway
There are three major LOXs; 5-, 12- and 15-LOX named to reflect the major position of the oxygen atom inserted within the polyunsaturated fatty acids [22]. All LOX isoforms are dioxygenases and form hydroperoxides. 5-LOX inserts oxygen into ARA with the help of an iron atom in its catalytic site thereby generating 5-hydroperoxyeicosatetraenoic acid (5-HpETE). Either the 5-HpETE is released and metabolized by glutathione peroxidase to the corresponding 5-HETE or further metabolized by 5-LOX. A dehydrogenation produces leukotriene A4 (LTA4) in a second enzymatic step. Ultimately, unstable LTA4 is modified to generate leukotrienes and lipoxins. The LTA4 hydrolase (LTA4H) transforms the LTA4 into the proinflammatory leukotriene B4 (LTB4). The LTC4 synthase (LTC4S) conjugates glutathione to LTA4. The generated leukotriene C4 (LTC4) can be modified into several other cysteinyl-leukotrienes (LTD4 and LTE4). 12- and 15-LOX are involved in the synthesis of lipoxins (e.g. LXA4) which also play a role in the resolution inflammation [23]. Among the LOX enzymes, 5-LOX and the biosynthesis of the leukotrienes is the focus of this review. The 5-LOX accumulates either in the cytoplasm or in the nucleoplasm until activation [24]. Two cofactors, Ca2+ and ATP, are needed for the activation of 5-LOX. Ca2+ influx recruits 5-LOX to the nuclear membrane and calcium binds allosterically to the 5-LOX promoting the attachment to the membrane. The ATP-activation mechanism is not fully understood; however, a common hypothesis is that it extends enzyme stability [25]. Furthermore, the 5-lipoxygenase activating protein (FLAP) is needed to recruit ARA from the membrane to the 5-LOX. Leukotrienes are paracrine lipid mediators that take part in chronic inflammatory diseases as well as in the innate immune system. For example, LTB4 is an especially strong chemotactic substance for neutrophils, macrophages and eosinophils. LTB4 induces several functions such as adhesion to vascular endothelial cells, release of lysosomal proteins, production of reactive oxygen species and transendothelial migration [26].
Crosstalk within the ARA cascade
Complex diseases, such as inflammation, need an intrinsic communication among the different metabolites derived from ARA. Therefore, it is not surprising that evidence is building for crosstalk among the pathways of this cascade [27]. One of the first examples of crosstalk is the observation of aspirin-induced asthma [28], also known as Samter’s syndrome or aspirin-exacerbated respiratory disease (AERD) [29]. In 1975 it was theorized that the asthma results from the inhibition of COX instead of an allergic reaction and Knapp et al. described that unmetabolized ARA is shunted into the 5-LOX pathway producing LTE4 [30]. Paredes et al. [31] observed an increase of LTB4 levels in human osteoarthritic subchondral osteoblast of osteoarthritis patients treated with a COX-2 selective inhibitor NS-398, suggesting the shunting of ARA substrate from the COX to the LOX pathway via over-expression of FLAP [32]. Jung et al. treated mice with progressive renal disease with the sEH inhibitor (cis-4-[4-(3-adamantan-1-ylureido)cyclohexyloxy]benzoic acid, c-AUCB) and observed an augmentation of proteinuria [33]. The study revealed that inhibition of sEH increased not only EETs levels but also 5-HETE and 15-HETE levels. This result indicates a shift of ARA from the CYP450 pathway into the LOX pathway. Within this study, blocking CYP450s with fenbendazole produced the same effect. The results described above demonstrate that the crosstalk is bidirectional and likely multidirectional. However, the assumption that inhibiting one pathway leads only to a shift into another pathway is too simplistic. Thus, this theorized inter-pathway crosstalk is not fully elucidated and should be further investigated. Mazaleuskaya et al. developed an assay with an UPLC-MS/MS-method in which they evaluated the effects of drugs on the bioactive eicosanoid lipidome [34]. With this method they administered either a single drug or a combination of different drugs targeting the 5-LOX and COX pathways to patients. They then detected the redirections and shifts in the eicosanoid lipidome after 4 and 24 h. As an example, when both microsomal prostaglandin E synthase 1 (mPGES-1) and FLAP were inhibited, 5-HETE, LTs and PGE2 levels were reduced as expected, but this combination also led to shunting to other LOX pathway enzymes initially elevating 12-HETE (increase of 85%, p = 0.02) and 15-HETE (increase of 20%, p = 0.006). Interestingly, after 24 hours this increase vanished, but subsequent elevation of the COX metabolites PGF2α (53%, p = 0.002) and TXB2 (28%, p = 0.0004) was observed. This method enables the detection of complex interactions of drugs affecting the eicosanoid lipidome. With this approach the crosstalk in the ARA cascade can be further analyzed and better understood. The question of how the crosstalk is mediated, either by direct modulation of the enzyme activity or by transcriptional regulation of the enzymes, has not yet been determined in most cases [35]. However, it is known that the substrate preference of the enzymes can vary [36, 37]. For instance, EETs and 20-HETE can be metabolized by COX enzymes and allosteric binding of LOX changes the substrate specificity of these enzymes. These results indicate a regulation of the crosstalk via direct protein-substrate interaction which may extend to the metabolism of other fatty acids such as linoleic acid [35].
2.1.2. Dual inhibitors of sEH and COX
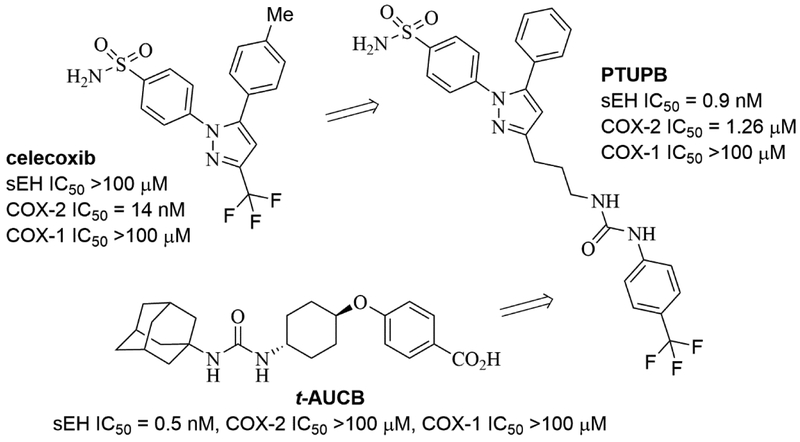
Dual inhibitors of sEH and COX enzymes have been reviewed [35]. Therefore, we will focus on recent reports about this topic. Earlier COX inhibitors (known as non-steroidal anti-inflammatory drugs, NSAIDs) have been long known to be highly effective drugs for reducing both pain and inflammation. However, high doses of these commonly-used compounds were found to cause gastrointestinal erosion due to reduction of prostanoids that maintain mucosal integrity [38]. Thus, COX-2 selective inhibitors (coxibs) such as rofecoxib and celecoxib were developed with the aim of limiting gastrointestinal side effects of non-selective COX inhibition by NSAIDs, but they are known to cause cardiovascular problems and have black box warnings [39, 40]. Although NSAIDs and coxibs continue to have a major beneficial effect in reducing suffering, it now appears that COX inhibitors in general have a variety of deleterious effects. In general, non-selective COX inhibitors have worse GI side effects than coxibs and coxibs worse cardiovascular effects than NSAIDs. This suggests that it may be impossible to avoid the mechanism-based adverse effects associated with COX inhibition simply by adjusting relative inhibition of COX-1 and COX-2, though COX inhibition is effective to attenuate the intended disease indications such as pain and inflammation. Simultaneous augmentation of CYP450-derived EETs along with COX inhibition exerts an additive response attenuating lipopolysaccharide (LPS)-induced pain and hypotension [41, 42]. In addition, CYP450-derived EETs, especially 8,9-EETs, are further metabolized by COX enzymes to angiogenic 11-hydroxy-8,9-EETs [37, 43]. Based on these findings, a single molecule that targets both sEH and COX-2, but not COX-1 has been developed, and it demonstrated analgesia in an animal model of pain [44]. The single molecule sEH/COX-2 dual inhibitor (4-(5-phenyl-3-{3-[3-(4-trifluoromethyl-phenyl)-ureido]-propyl}-pyrazol-1-yl)-benzenesulfonamide, PTUPB, Fig.2) was more efficacious than monotherapy (either a COX-2 selective inhibitor or an sEH inhibitor alone) and even surpassed the combination of both treatments in an LPS-induced inflammatory pain model.
Figure 2.
Chemical structure of sEH/COX-2 dual inhibitor PTUPB.
In addition to this example, beneficial effects of PTUPB have been shown in other pathological conditions such as kidney disease [45] and cancers [44-46]. PTUPB significantly suppressed tumor growth and metastasis in murine lung cancer model [46]. In this study there was no direct comparison between the dual sEH/COX-2 inhibitor and a combination of the treatments, however the multitargeted ligand approach was more effective than monotherapy. PTUPB also suppressed tumor growth of breast cancers [46] and glioblastoma [47]. As an adjuvant, it potentiated cisplatin and cisplatin/gemcitabine chemotherapy regimes in patient-derived xenograft (PDX) bladder cancer models [48]. In addition, PTUPB did not alter the ratio of PGI2 to TXA2 (an indication for a potential cardiovascular complication associated with COX-2 inhibition) [40], suggesting that the additional sEH inhibition is beneficial [46]. In a carbon tetrachloride-induced mouse model of liver injury, co-administration of celecoxib and an sEH inhibitor or PTUPB decreased fibrotic markers, while celecoxib alone showed no beneficial effect [49]. Finally, pharmacological or genetic ablation of sEH has been shown to be a potential treatment of Parkinson’s disease models [50]. PTUPB also prevented the reduction of dopamine and its metabolites against rotenone-induced neurodegeneration in a Drosophila model of Parkinson’s disease [51].
2.1.3. Dual inhibitors of sEH and FLAP
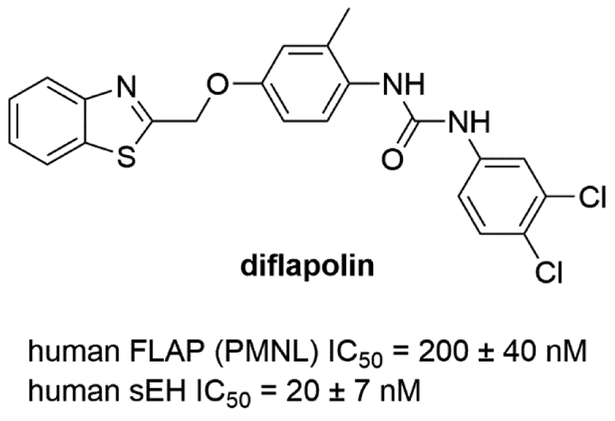
One possible approach to influence the 5-LOX pathway is modulating the activity of the FLAP enzyme. Without FLAP the recruitment of ARA to 5-LOX is reduced and consequently the production of leukotrienes. Liu et al. observed an enhanced anti-inflammatory effect combining an sEH inhibitor with a FLAP inhibitor in a murine model [42]. This suggests that a dual inhibitor may have similar or even enhanced efficacy compared to co-administration. To investigate this possibility, a dual sEH/FLAP inhibitor would be necessary, and this strategy has been pursued. Temmel et al. identified the first dual inhibitor of FLAP and sEH in a pharmacophore-based virtual screening by developing ligand-based pharmacophore models for FLAP and using known models for sEH [52]. By screening a commercial virtual library, 20 hit compounds were identified. Among them, a compound (diflapolin) showed dual inhibition against FLAP (IC50 = 200 nM in cell-based assay) and sEH (IC50 = 20 nM in cell-free assay) but did not inhibit isolated 5-LOX in the cell-free assay (Fig. 3). Diflapolin blocked leukotriene formation and suppressed neutrophil infiltration in a zymosan-induced mouse peritonitis model [53]. Diflapolin also showed high selectivity and demonstrated no interaction with other enzymes that metabolize ARA such as COX1/2, 12/15-LOX, LTA4H, LTC4S, mPGES-1 and CPLA2. In addition, diflapolin did not show cytotoxicity and was equally effective as the FLAP inhibitor MK886.
Figure 3.
Chemical structure of FLAP/sEH dual inhibitor.
2.1.4. Dual inhibitors of sEH and 5-LOX
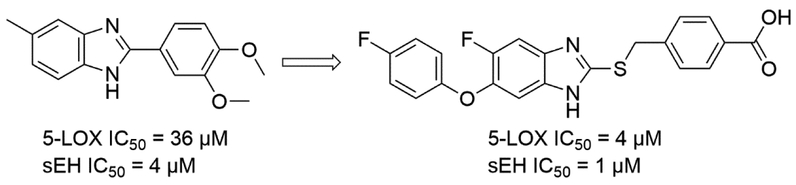
The first dual inhibitors of sEH and 5-LOX were discovered by an in silico approach. Moser et al. identified 80 hits by screening virtual library of 37,429 compounds by applying pharmacophore models for both targets [54]. Among them, only one compound was identified as a 5-LOX/sEH dual inhibitor with moderate inhibitory activities (5-LOX IC50 = 36 μM, sEH IC50 = 3.5 μM, respectively) by in vitro cell-free assays (Fig. 4). Nandha et al. further optimized this initial hit compound by incorporating several known pharmacophores of both 5-LOX and sEH [55]. Based on this structure-activity relationship (SAR) study, several potent 5-LOX/sEH dual inhibitors ranging from low micromolar to high nanomolar potencies against both targets have been obtained. The anti-inflammatory effects of these compounds were investigated in a rat paw edema model. Several compounds of this series showed a significant inhibition of the edema, which is comparable to the reference compound, ibuprofen (Fig. 4).
Figure 4.
An optimized 5-LOX/sEH dual inhibitor from an in silico hit.
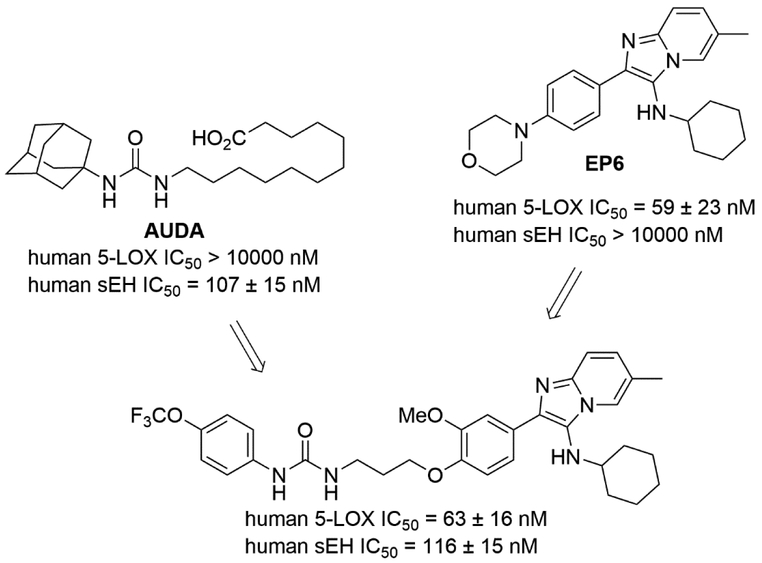
Meirer et al. developed a series of 5-LOX/sEH dual inhibitors by linking two pharmacophores [56]; an imidazo[1,2-a]pyridine motif from 5-LOX selective inhibitor EP6 [57] and a urea group from sEH selective inhibitors such as 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA) or 1-cyclohexyl-3-dodecyl urea (CDU) [58]. The SAR demonstrated that an n-propyl linker between two pharmacophores was required to maintain sEH inhibition, while not affecting 5-LOX inhibition (Fig. 5).
Figure 5.
A 5-LOX/sEH dual inhibitor that linked two pharmacophores for 5-LOX and sEH.
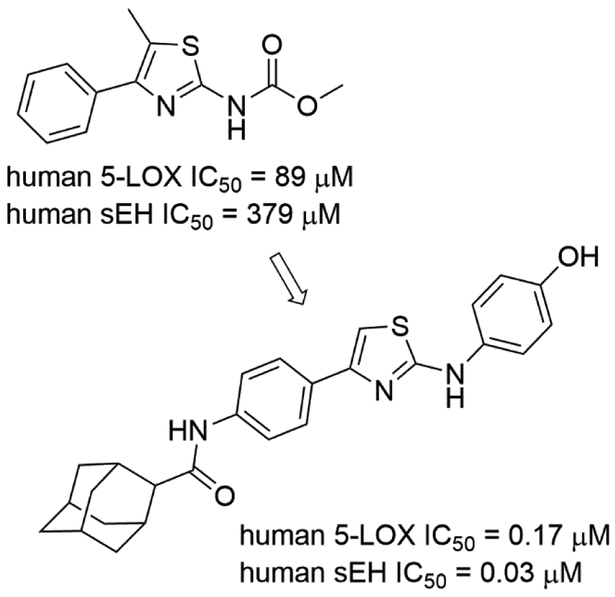
Achenbach et al. developed an in silico approach for fragment-based design of 5-LOX/sEH dual inhibitors [59]. The developed approach generated 274 hit fragments. Among them, 24 compounds were tested using STD-NMR and in vitro assays, finding five fragments that inhibited both targets. To demonstrate the feasibility of this approach, the authors screened their in-house library of compounds and found that a compound containing an aminothiazole core possessed better inhibitory activities against both targets (Fig. 6).
Figure 6.
A 5-LOX/sEH dual inhibitor that contain an aminothiazole fragment.
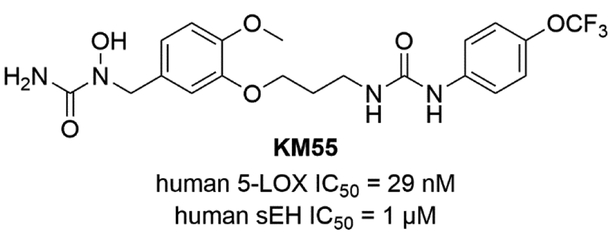
Meirer et al. developed 5-LOX/sEH dual inhibitors by linking two pharmacophores; an N-hydroxy urea moiety from a 5-LOX inhibitor Zileuton and a urea group from an sEH inhibitor TPAU with an n-propyl linker [60]. The 4-trifluormethoxyphenyl group which had been already used in earlier designs showed an excellent pharmacokinetic profile [61]. The 5-LOX/sEH dual inhibitor KM55 inhibited the adhesion of leukocytes onto endothelial cells by impairing leukocyte function. The adhesive properties of KM55-treated THP-1 cells were reduced back to control levels, which was more effective compared to either Zileuton or TPAU (Fig. 7).
Figure 7.
Chemical structure of 5-LOX/sEH dual inhibitor KM55.
2.2. Dual inhibition of sEH and targets outside the ARA cascade
2.2.1. Dual inhibitors of sEH and PPAR
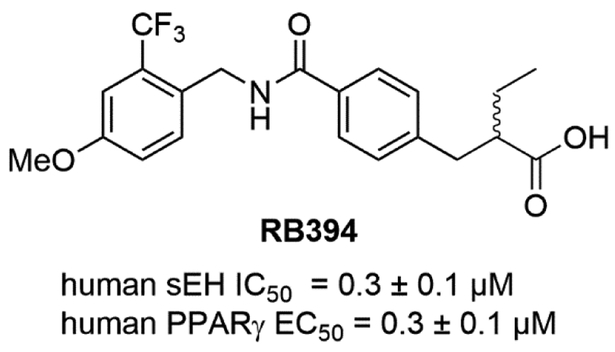
Several studies highlighted an extensive crosstalk between effects mediated by EETs and peroxisome proliferator-activated receptor (PPAR) signaling, which has been reviewed in detail by Spector and Norris [14]. PPARs belong to the family of nuclear receptors which are a group of three types of proteins (α, β/δ, γ) with differential tissue expression that act as transcription factors, regulate gene expression, and are activated by fatty acids and eicosanoids. Older PPARα agonists known collectively as fibrates, are potent hyperlipidic agents. These were the first potent inducers found for the sEH [62]. Both PPARα and PPARγ agonists induce levels of sEH. Thus, the beneficial clinical effects of PPARα agonists are diminished, in part, by their induction of sEH. PPARs play multiple roles in lipid and glucose homeostasis, however, among these effects, the anti-inflammatory and oxidative stress-reducing properties of EETs which are associated with PPARγ activation, are of special importance [63, 64]. Direct activation of PPARγ by synthetic agonists such as clinically approved thiazolidinediones (TZDs) leads to improved insulin sensitivity and lower blood glucose levels [65]. However, the major side effects of the TZD class are fluid retention and weight gain caused by PPARγ stimulation of epithelial sodium channel (ENaC)-mediated renal salt absorption [66], which limits the utility of these multimodal insulin sensitizers [67]. It was shown that sEH inhibition sustaining EETs levels led to reduced ENaC-mediated renal salt reabsorption [68-70]. Therefore, sEH inhibition might prevent the major undesired side effects of TZDs. Furthermore, TZDs are often associated with obesity-related hypertension, dyslipidemia, and heart disease within metabolic syndrome [71]. Similar to earlier observations in rodents [72], the adipose tissue of obese patients exhibits increased sEH levels [73], which leads to decreased PPAR transcriptional activity [74]. Thus, reminiscent of PPARα agonists, by inducing sEH, the TZDs antagonize some of their own clinically beneficial effects. These findings suggest that simultaneous inhibition of sEH and activation of PPARγ is a potential intervention for metabolic syndrome, leading to a concomitant improvement of blood pressure and blood glucose level. A study performed by Imig et al. tested this hypothesis with co-administration of the TZD rosiglitazone and the sEH inhibitor t-AUCB in spontaneously hypertensive obese rats [75]. The combined administration of both pharmacological agents led to synergistic improvement of vascular function and reduced fibrotic kidney damage. It should be noted that some early sEH inhibitors such as AUDA are not only EET mimics, but also PPARα agonists. The first dual PPAR/sEH modulators were developed by linking two known pharmacophores for both PPARα, γ, or δ, and sEH. and identified several moderate dual PPARγ/sEH and PPARα/sEH mediators[76]. The fusion of the N-benzyl piperidine-4-carboxamide to diverse PPAR pharmacophores was less successful yielding dual PPAR/sEH modulators which exhibited low binding affinity combined with high molecular weight and lipophilicity [77]. Finally, the identification of N-benzyl benzamides as a merged PPAR/sEH pharmacophore, led to the identification of lead compound RB394 (Fig. 8) [78]. RB394 is an equipotent PPARγ-selective full agonist and sEH inhibitor with a favorable pharmacokinetic and pharmacodynamic profile. Extensive in vivo profiling of RB394 in spontaneously hypertensive obese rats (SHROB), ZSF-1 (ZSF1-LeprfaLeprcp/Crl) obese rats, and unilateral ureteral obstruction rats (UUO) led to the validation that a simultaneous sEH inhibition and PPARγ activation are highly beneficial for metabolic syndrome. RB394 simultaneously and potently reduced blood pressure, blood glucose level, dyslipidemia and hypercholesteremia, as well as liver and kidney fibrosis in preventive and curative paradigms dosed at 10 mg/kg/day [79].
Figure 8.
Chemical structure of PPAR/sEH modulator.
Schierle et al. took advantage of an anti-inflammatory drug to define further multitarget drugs by selecting an anti-asthmatic CysLT1 receptor antagonist zafirlukast, which also possessed moderate modulating activities against PPARγ and sEH [80]. Minor modifications in the chemical structure of zafirlukast to improve its potential anti-inflammatory action led a potent modulator of PPARγ, sEH, and CysLT1R. This resulted in improved beneficial off-target activities (8.1-fold against PPARγ and 46.5-fold against sEH, respectively) and superior anti-inflammatory properties compared to the parent compound zafirlukast in the zymosan-induced paw edema model (Fig. 9).
Figure 9.
Minor chemical modification of zafirlukast to improve its beneficial off-targets.
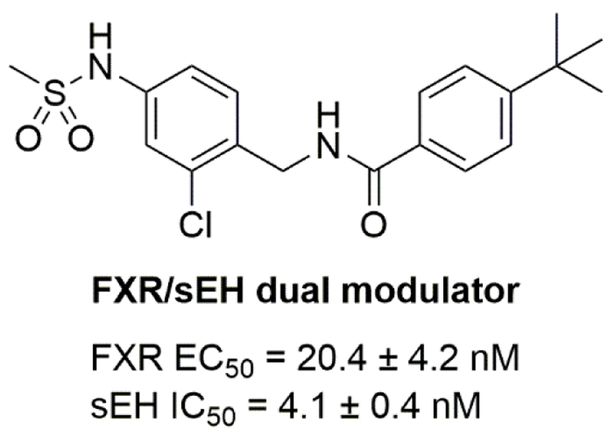
2.2.2. Dual inhibitors of sEH and FXR
Bile acids are physiological activators of the farnesoid X receptor (FXR), a ligand-activated nuclear receptor which regulates bile acid, lipid, and glucose homeostasis [81]. FXR agonists are currently under clinical investigation for treatment of nonalcoholic fatty liver disease (NAFDL) and nonalcoholic steatohepatitis (NASH) [82, 83]. Genetic deletion or pharmacological inhibition of sEH has been shown to promote anti-inflammatory effects in murine models of high-fat (HF)-diet-induced fatty liver [84, 85]. Thus, simultaneous modulation of FXR and sEH might have an additive or synergistic effect in the context of NAFDL and NASH. Schmidt et al. rationally designed a dual sEH/FXR modulator by combining previously published selective partial FXR agonists [86] and sEH inhibitors [87]. Subsequent optimization and a bioisosteric replacement strategy yielded a highly potent, orally available, and efficacious dual sEH/FXR modulator (Fig. 10) [88].
Figure 10.
Chemical structure of dual sEH/FXR modulator.
2.2.3. Dual inhibitors of sEH and FAAH
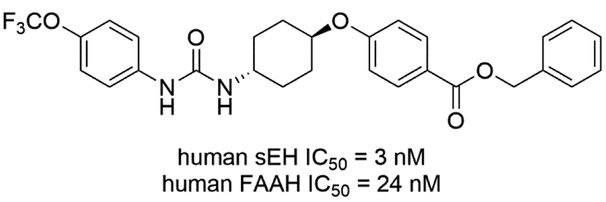
A significant percentage of NSAIDs and other anti-inflammatory drugs, both over-the-counter and prescription, target the modulation of lipids within the ARA cascade. However, there are lipids not related to this cascade that mediate both analgesic and anti-inflammatory effects. One such lipid signaling system is the endocannabinoid (EC) system. The main endocannabinoid lipids studied so far are N-arachidonoyl ethanolamide (AEA) and 2-arachidonylglycerol (2-AG), but N-(2-hydroxyethyl)hexadecanamide (palmitoylethanolamide, PEA) also contributes to these activities [89]. These lipids are agonists of the two isomeric G-protein coupled receptors of the EC system referred to as CB1 and CB2. Degradation of these lipid mediators occurs rapidly via the catalytic action of fatty acid amide hydrolase (FAAH). Thus, there have been several approaches targeting FAAH to elevate endocannabinoids and thereby elicit analgesic and anti-inflammatory effects. FAAH has been targeted with monotherapy and this inhibition shows no sign of CB1-mediated motor and psychotropic side effects that are associated with direct agonists of the cannabinoid receptor [90, 91]. Both EpFAs and endocannabinoids exert anti-inflammatory and analgesic effects but are known to act through independent mechanisms. Because the strategy is similar (elevating EpFAs and endocannabinoids by inhibiting each principal degrading enzyme) and they are not targeting the same pathway, concurrent inhibition of both enzymes was hypothesized to result in additive or synergistic responses. This approach was investigated in acute inflammatory and chronic pain using a peripherally restricted irreversible FAAH inhibitor [92] and an sEH inhibitor demonstrated to penetrate the central nerve system [93, 94]. The results indicated that coadministration of these monotherapy inhibitors elicited a synergistic (i.e. more than additive) analgesic response [95]. Therefore, Kodani et al. screened an existing sEH inhibitor library against FAAH and found a potent sEH inhibitor t-TUCB that also inhibits FAAH with nanomolar activity. Further structural optimization led to a FAAH/sEH dual inhibitor that possess 6-fold potent activity against human FAAH, while maintain sEH inhibition. Interestingly, the FAAH inhibition was limited to human enzyme as IC50s against FAAH enzymes derived from other species, such as mouse, rat, cat, dog, at least 10-fold lower (Fig. 11) [96].
Figure 11.
Chemical structure of FAAH/sEH dual inhibitor.
2.2.4. Dual inhibitors of sEH and c-RAF
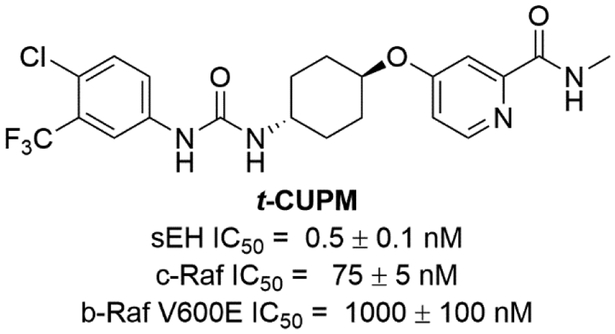
RAF proto-oncogene serine/threonine-protein kinase known as c-RAF (originally known as Raf1) was discovered in 1985 [97]. In the early 1990s, Bayer and Onyx cooperated to screen a combinatorial library of about 200,000 compounds against c-RAF to develop a drug targeting the RAS-RAF-MEK-ERK pathway. c-RAF was selected based on the finding that disrupting the RAF1 gene inhibited the tumor growth in lung, breast, and ovarian cancers in athymic mice validating it as an anticancer target [98]. Importantly sorafenib has been identified as a pan-kinase inhibitor inhibiting several kinases in addition to c-RAF. Sorafenib is the first FDA-approved drug developed from the screening of combinatorial libraries, which also validates combinatorial chemistry as a valuable tool for drug development. Pan-kinase inhibition helps to treat cancers due to the complex nature of the diseases. However, untargeted (and even on-target) kinase inhibition also causes unwanted adverse effects [99, 100]. BRAF is a human gene that encodes for the b-RAF kinase and its mutations, especially V600E, have been targets of several cancer therapies [101]. However, in some cases b-RAF inhibitors can induce tumor growth [102]. It has been demonstrated that inhibition of b-RAF in RAS mutant cancer cells leads to MEK hyperactivation through c-RAF (Fig. 12) [103]. In addition, it has been known that c-RAF, not b-RAF, is a major player in pancreatic cancer. Furthermore, pancreatic cancer is developed from pancreatitis, which is a chronic inflammation of this organ. Stabilizing the anti-inflammatory lipid mediator EETs with sEH inhibitors blocks inflammation by reducing cytokine-induced endothelial cell adhesion molecule (VCAM) and NF-κB and Iκκ kinase activities [104, 105]. Thus, dual inhibition of c-RAF and sEH is expected to be beneficial to treat this disease. Liu et al. reported sorafenib was able to inhibit sEH using in vitro assays and thus to increase the EETs/DHETs ratio in plasma of lipopolysaccharide-induced rodent [106]. Therefore, Hwang et al. developed a series of compounds aiming to refine the kinase inhibition but also maintain their ability to inhibit sEH [107]. A SAR study showed that minor changes to the chemical structures of either known sEH inhibitors or sorafenib (sEH IC50 = 12 ± 2 nM, c-RAF IC50 = 45 ± 5 nM, and b-RAF V600E IC50 = 13 ± 2 nM) dramatically affected their kinase but not their sEH inhibition based on in vitro enzyme assays. Among them, trans-5-{4-[3-(4-chloro-3-trifluoromethyl-phenyl)-ureido]-cyclohexyloxy}-pyridine-2-carboxylic acid methylamide (t-CUPM, sEH IC50 = 0.5 ± 0.1 nM, c-RAF IC50 = 75 ± 5 nM, and b-RAF V600E IC50 = 570 ± 30 nM) showed improved sEH inhibition and similar inhibitory activities against c-RAF, but not b-RAF [108].
Figure 12.
Chemical structures of the sEH/c-RAF dual inhibitor t-CUPM.
Then Liao et al. demonstrated an anti-cancer effect of the sEH/ c-RAF dual inhibitor t-CUPM [109]. The compound was used to inhibit mutant KrasG12D-initiated murine pancreatic cancer cell growth using PK03 cells derived from LSL-KrasG12D/Pdx1-Cre mice [110], a well-established genetically engineered murine pancreatic cancer model. Both t-CUPM and sorafenib, but not the sEH inhibitor t-AUCB, showed similar dose-dependent inhibition of cell growth using a colony formation assay. Both compounds significantly inhibited the phosphorylation of MEK1/2 and ERK1/2, but only t-CUPM inhibited the phosphorylation of c-RAF (mutant Kras-activated signal) in a dose-dependent manner. In a mutant Kras-initiated and caerulein-induced models of pancreatitis-carcinogenesis in LSL-KrasG12D/Pdx1-Cre mice, t-CUPM reduced the severity of chronic pancreatitis and significantly suppressed the formation and progression of pancreatic intraepithelial neoplasia [111]. This suggests that co-inhibition of sEH and c-RAF, not b-RAF, is critical to prevent chronic pancreatitis and carcinogenesis.
3. Summary and Perspectives
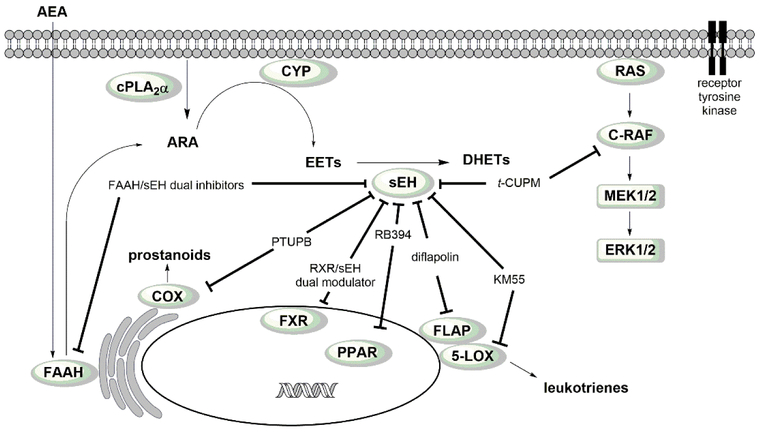
Polypharmacology has emerged as a new paradigm in drug discovery over the last two decades. Several approved drugs have been identified retrospectively as having beneficial polypharmacological profiles which provides strong support for the concepts of polypharmacology and multitarget drug design. The next challenge will be to rationally design compounds with desired polypharmacological profiles and transform them into drug candidates. Molecules with optimal multitarget activities have the potential of improving the efficacy and safety of drugs. Targeting sEH as a single target with a single compound has demonstrated great potential as an efficacious and safe strategy for several indications. In fact, sEH inhibitors as single target agents have previously been evaluated in human clinical trials and continue to be pursued for various diseases. Results from several human phase 1 clinical trials [112, 113] have demonstrated their safety in humans, which underscores benefit of sEH inhibition. The outcomes from the later phases of human clinical trials will confirm sEH as a valid single target to treat such diseases. By combining the benefits of inhibiting sEH with other targets (Table 1 and Fig. 13), multitarget compounds are safer and, as demonstrated, more efficacious than monotherapy.
Table 1.
Efficacy results in live cells or in vivo models of dual inhibitors/modulators.
| Target | Agent | Disease | species | Major outcomes | Reference |
|---|---|---|---|---|---|
| sEH/COX-2 (section 2.1.2) | PTUPB | Inflammatory Pain | Rat | Oral PTUPB reduced intraplantar LPS pain in von Frey assay | Hwang et al. 2011 |
| Kidney Disease | Rat | Dual COX-2/sEH inhibitor reduced renal damage and inflammation in ZDF Rats | Hye Khan et al. 2016 | ||
| Cancer | Mouse | Dual Inhibitor PTUPB inhibits Primary Tumor Growth and Metastasis. | Zhang et al. 2014 | ||
| Cancer | Glioblastoma cells, Mouse | PTUPB suppresses glioblastoma growth in vitro and in vivo | Li et al. 2017 | ||
| Mouse | PTUPB decreased fibrotic markers in liver injury | Harris et al. 2018 | |||
| Parkinson’s disease | Drosophila | Prevent the reduction of dopamine and its metabolites | Lakkappa et al. 2018 | ||
| sEH/FLAP (section 2.1.3) | diflapolin | Peritonitis | Mouse | Diflapolin blocked leukotriene formation and suppressed neutrophil infiltration | Garscha et al. 2017 |
| sEH/5-LOX (section 2.1.4) | Inflammatory Edema | Rat | Significant inhibition of the edema | Nandha et al. 2018 | |
| KM55 | LPS | THP-1, HUVEC cells | Significantly inhibited the LPS-induced adhesion of leukocytes to endothelial cells | Meirer et al. 2008 | |
| sEH/PPAR(section 2.2.1) | RB394 | ZSF1 Rat | Reduced blood pressure, blood glucose level, dyslipidemia and hypercholesteremia, as well as liver and kidney fibrosis | Hye Khan et al. 2018 | |
| Inflammatory Edema | Mouse | Superior anti-inflammatory properties in the zymosan-induced paw edema model | Schierle et al. 2018 | ||
| sEH/FXR (section 2.2.2) | FXR/sEH dual modulator | Inflammation | HEPG2, HuT-78 cells | Robustly repressed NF-κB in hepatocarcinoma cells and reduced the Pam3CSK4 stimulated release of TNFα from the T-cell line HuT-78 | Schmidt et al. 2017 |
| sEH/FAAH | FAAH/sEH dual inhibitor | A hit compound, t-TUCB, has been optimized through SAR study. The optimized compounds showed improved cross-species potencies against both FAAH and sEH. | Kodani et al. 2018 | ||
| sEH/C-RAF (section 2.2.4) | t-CUPM | pancreatic carcinoma | Mouse, PK03 cells | Inhibition of murine Pancreatic carcinoma growth in vitro and in vivo by t-CUPM | Liao et al. 2016 |
Figure 13.
Signaling pathways of fatty acids and modes of action of multitarget agents.
Highlights.
Multitarget agents can improve efficacy or adverse effects of single target drugs
Dual inhibitors/modulators that target a soluble epoxide hydrolase are reviewed
Benefits by the dual inhibitors/modulators to treat various diseases are reviewed
Acknowledgments
We would like to thank Dr. Christophe Morisseau for his help during the manuscript preparation. This work was supported by Deutsche Forschungsgemeinschaft (DFG; Sachbeihilfe PR1405/2-2; SFB 1039 Teilprojekt A07; Heisenberg-Professur PR1405/4-1) and by research funding programe Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz (LOEWE) of the State of Hessen, Research Center for Translational Medicine and Pharmakology TMP) (to E.P.), by the Else-Kröner-Fresenius Foundation graduate college for Translational Research Innovation–Pharma (TRIP) (to K.H.),by R01 NCI/CA172431 (to B.D.H.); by NIEHS/R01 ES002710 (to B.D.H.); and by NIDDK R01 DK103616 (to B.D.H.).
Abbreviations:
- ARA
arachidonic acid
- sEH
soluble epoxide hydrolase
- COX
cyclooxygenase
- FLAP
5-lipoxygenase-activating protein
- LOX
lipoxygenase
- PPAR
peroxisome proliferator-activated receptor
- FXR
farnesoid X receptor
- FAAH
fatty acid amide hydrolase
- RAF
rapidly accelerated fibrosarcoma
- DML
designed multiple ligand
- CYP450
cytochrome P450
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- LX
lipoxins
- LT
leukotriene
- HETE
hydroxyeicosatetraenoic acid
- EET
epoxyeicosatrienoic acids
- DHET
dihydroxyeicosatrienoic acids
- 20-HETE
20-hydroxyeicosatetraenoic acid
- PGG2
prostaglandin G2
- PGH2
prostaglandin H2
- PGE2
prostaglandin E2
- PGF2α
prostaglandin F2α
- PGI2
prostacyclin
- PGD2
prostaglandin D2
- TXA2
thromboxane A2
- 5-HpETE
5-hydroperoxyeicosatetraenoic acid
- LTA4H
leukotriene A4 hydrolase
- LTA4
leukotriene A4
- LTC4S
LTC4 synthase
- LTB4
leukotriene B4
- LTC4
leukotriene C4
- LTD4
leukotriene D4
- LTE4
leukotriene E4
- AIA
aspirin induced asthma
- AERD
aspirin-exacerbated respiratory disease
- c-AUCB
cis-4-[4-(3-adamantan-1-ylureido)cyclohexyloxy]benzoic acid
- NSAID
non-steroidal anti-inflammatory drug
- LPS
lipopolysaccharide
- PTUPB
4-(5-phenyl-3-{3-[3-(4-trifluoromethyl-phenyl)-ureido]-propyl}-pyrazol-1-yl)-benzenesulfonamide
- CDU
1-cyclohexyl-3-dodecyl urea
- SAR
structure activity relationship
- CIU
N-cyclohexyl- N'-iodophenyl urea
- TZD
thiazolidinedione
- t-AUCB
trans-4-[4-(3-Adamantan-1-ylureido)cyclohexyloxy]benzoic acid
- AUDA
12-(3-adamantan-1-yl-ureido) dodecanoic acid
- NAFDL
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- EC
endocannabinoid
- AEA
N-arachidonoyl ethanolamide
- 2-AG
2-arachidonylglycerol
- PEA
palmitoylethanolamide
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- EpFAs
epoxy fatty acids
- t-TUCB
trans-4-{4-[3-(4-trifluoromethoxyphenyl)ureido]cyclohexyloxy}benzoic acid
- t-CUPM
trans-4-{4-[3-(4-chloro-3-trifluoromethyl-phenyl)-ureido]-cyclohexyloxy}-pyridine-2-carboxylic acid methylamide
- MEK
mitogen-activated protein kinase kinase
- ERK
extracellular signal-regulated kinase
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
B.D.H., K.M.W. and S.H.H are inventors of University of California patents on the synthesis and use of sEH/COX-2, sEH/FAAH and sEH/PDE dual inhibitors. The other authors declare that they have no competing of interests. E.P. is a co-inventor on Goethe University of Frankfurt patents on synthesis and use of dual sEH/PPARγ and sEH/FXR modulators.
References
- [1].Ramsay RR, et al. , A perspective on multi-target drug discovery and design for complex diseases. Clin Transl Med, 2018. 7(1): p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Morphy R and Rankovic Z, Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem, 2005. 48(21): p. 6523–6543. [DOI] [PubMed] [Google Scholar]
- [3].Meunier B, Hybrid molecules with a dual mode of action: dream or reality? Acc Chem Res, 2008. 41(1): p. 69–77. [DOI] [PubMed] [Google Scholar]
- [4].Lin HH, et al. , Network Analysis of Drug-target Interactions: A Study on FDA-approved New Molecular Entities Between 2000 to 2015. Sci Rep, 2017. 7(1): p. 12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rask Larsen J, et al. , The pharmacological management of metabolic syndrome. Expert Rev Clin Pharmacol, 2018. 11(4): p. 397–410. [DOI] [PubMed] [Google Scholar]
- [6].Binesh Marvasti T and Adeli K, Pharmacological management of metabolic syndrome and its lipid complications. Daru, 2010. 18(3): p. 146–154. [PMC free article] [PubMed] [Google Scholar]
- [7].Li W, et al. , Ligandomics: a paradigm shift in biological drug discovery. Drug Discov Today, 2018. 23(3): p. 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morisseau C and Hammock BD, Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol, 2013. 53: p. 37–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Morisseau C and Hammock BD, Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol, 2005. 45: p. 311–333. [DOI] [PubMed] [Google Scholar]
- [10].Tran KL, et al. , Lipid sulfates and sulfonates are allosteric competitive inhibitors of the N-terminal phosphatase activity of the mammalian soluble epoxide hydrolase. Biochemistry, 2005. 44(36): p. 12179–12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dubois RN, et al. , Cyclooxygenase in biology and disease. FASEB J, 1998. 12(12): p. 1063–1073. [PubMed] [Google Scholar]
- [12].Roman RJ, P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol rev, 2002. 82(1): p. 131–185. [DOI] [PubMed] [Google Scholar]
- [13].Elshenawy OH, et al. , Clinical Implications of 20-Hydroxyeicosatetraenoic Acid in the Kidney, Liver, Lung and Brain: An Emerging Therapeutic Target. Pharmaceutics, 2017. 9(1): p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Spector AA and Norris AW, Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol, 2007. 292(3): p. C996–1012. [DOI] [PubMed] [Google Scholar]
- [15].Spector AA, Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res, 2009. 50 Supp1: p. S52–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Spector AA and Kim H-Y, Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. BBA-Mol Cell Biol L, 2015. 1851(4): p. 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ricciotti E and FitzGerald GA, Prostaglandins and Inflammation. Arterioscl Throm Vas, 2011. 31(5): p. 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hetu PO and Riendeau D, Cyclo-oxygenase-2 contributes to constitutive prostanoid production in rat kidney and brain. Biochem J, 2005. 391(Pt 3): p. 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wilson DE, Role of prostaglandins in gastroduodenal mucosal protection. J Clin Gastroenterol, 1991. 13: p. S65–71. [DOI] [PubMed] [Google Scholar]
- [20].Sandoo A, et al. , The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J, 2010. 4: p. 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moriyama T, et al. , Sensitization of TRPV1 by EP(1)and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain, 2005. 1: p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Haeggström JZ and Funk CD, Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease. Chem Rev, 2011. 111(10): p. 5866–5898. [DOI] [PubMed] [Google Scholar]
- [23].Brady HR and Serhan CN Lipoxins: putative braking signals in host defense, inflammation and hypersensitivity. Curr. Opin. Nephrol. Hypertens. 1996, 5(1), p. 20–27. [PubMed] [Google Scholar]
- [24].Luo M, et al. , Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. P Natl Acad Sci USA, 2003. 100(21): p. 12165–12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smyrniotis CJ, et al. , ATP allosterically activates the human 5-lipoxygenase molecular mechanism of arachidonic acid and 5(S)-hydroperoxy-6(E),8(Z),11(Z),14(Z)-eicosatetraenoic acid. Biochemistry, 2014. 53(27): p. 4407–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Toda A, Yokomizo T, and Shimizu T, Leukotriene B4 receptors. Prostag Oth Lipid M, 2002. 68–69: p. 575–585. [DOI] [PubMed] [Google Scholar]
- [27].Meirer K, Steinhilber D, and Proschak E, Inhibitors of the arachidonic acid cascade: interfering with multiple pathways. Basic Clin Pharmacol Toxicol, 2014. 114(1): p. 83–91. [DOI] [PubMed] [Google Scholar]
- [28].Szczeklik A, The cyclooxygenase theory of aspirin-induced asthma. Eur Respir J, 1990. 3(5): p. 588–593. [PubMed] [Google Scholar]
- [29].Chen BS, et al. , Aspirin sensitivity syndrome (Samter's Triad): an unrecognized disorder in children with nasal polyposis. Int J Pediatr Otorhinolaryngol, 2013. 77(2): p. 281–283. [DOI] [PubMed] [Google Scholar]
- [30].Knapp HR, Sladek K, and FitzGerald GA, Increased excretion of leukotriene E4 during aspirin-induced asthma. Transl Res, 1992. 119(1): p. 48–51. [PubMed] [Google Scholar]
- [31].Paredes Y, et al. , Study of the role of leukotriene B()4 in abnormal function of human subchondral osteoarthritis osteoblasts: effects of cyclooxygenase and/or 5-lipoxygenase inhibition. Arthritis Rheum, 2002. 46(7): p. 1804–1812. [DOI] [PubMed] [Google Scholar]
- [32].Maxis K, et al. , The shunt from the cyclooxygenase to lipoxygenase pathway in human osteoarthritic subchondral osteoblasts is linked with a variable expression of the 5-lipoxygenase-activating protein. Arthritis Res Ther, 2006. 8(6): p. R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jung O, et al. , Inhibition of the soluble epoxide hydrolase promotes albuminuria in mice with progressive renal disease. PLoS One, 2010. 5(8): p. e11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mazaleuskaya LL, et al. , A broad-spectrum lipidomics screen of antiinflammatory drug combinations in human blood. JCI Insight, 2016. 1(12): e87031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hwang SH, et al. , Rationally designed multitarget agents against inflammation and pain. Curr Med Chem, 2013. 20(13): p. 1783–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wecksler AT, Garcia NK, and Holman TR, substrate specificity effects of lipoxygenase products and inhibitors on soybean lipoxygenase-1. Bioorgan Med Chem, 2009. 17(18): p. 6534–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang JY, et al. , Regiospecific and enantioselective metabolism of 8,9-epoxyeicosatrienoic acid by cyclooxygenase. Biochem Biophys Res Commun, 1992. 183(1): p. 138–143. [DOI] [PubMed] [Google Scholar]
- [38].Funk CD and FitzGerald GA COX-2 inhibitors and cardiovascular risk. J. Cardiovasc. Pharmacol. 2007, 50(5), p. 470–479. [DOI] [PubMed] [Google Scholar]
- [39].Ritter JM, Harding I, and Warren JB, Precaution, cyclooxygenase inhibition, and cardiovascular risk. Trends Pharmacol Sci, 2009. 30(10): p. 503–508. [DOI] [PubMed] [Google Scholar]
- [40].Antman EM, DeMets D, and Loscalzo J, Cyclooxygenase inhibition and cardiovascular risk. Circulation, 2005. 112(5): p. 759–770. [DOI] [PubMed] [Google Scholar]
- [41].Schmelzer KR, et al. , Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA, 2006. 103(37): p. 13646–13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu JY, et al. , Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol, 2010. 79(6): p. 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rand AA, et al. , Cyclooxygenase-derived proangiogenic metabolites of epoxyeicosatrienoic acids. Proc Natl Acad Sci USA, 2017. 114(17): p. 4370–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hwang SH, et al. , Synthesis and structure-activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase. J Med Chem, 2011. 54(8): p. 3037–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hye Khan MA, et al. , A dual COX-2/sEH inhibitor improves the metabolic profile and reduces kidney injury in Zucker diabetic fatty rat. Prostaglandins Other Lipid Mediat, 2016. 125: p. 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang G, et al. , Dual inhibition of cyclooxygenase-2 and soluble epoxide hydrolase synergistically suppresses primary tumor growth and metastasis. Proc Natl Acad Sci USA, 2014. 111(30): p. 11127–11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li J, et al. , COX-2/sEH dual inhibitor PTUPB suppresses glioblastoma growth by targeting epidermal growth factor receptor and hyaluronan mediated motility receptor. Oncotarget, 2017. 8(50): p. 87353–87363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang F, et al. , COX-2/sEH Dual Inhibitor PTUPB Potentiates the Antitumor Efficacy of Cisplatin. Mol Cancer Ther, 2018. 17(2): p. 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Harris TR, et al. , Celecoxib does not protect against fibrosis and inflammation in a carbon tetrachloride-induced model of liver injury. Mol Pharmacol, 2018. 94(2): p. 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ren Q, et al. , Soluble epoxide hydrolase plays a key role in the pathogenesis of Parkinson's disease. Proc Natl Acad Sci USA, 2018. 775(20): p. 5283–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lakkappa N, et al. , Evaluation of antiparkinson activity of PTUPB by measuring dopamine and its metabolites in Drosophila melanogaster: LC-MS/MS method development. J Pharm Biomed Anal, 2018. 149: p. 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Temml V, et al. , Discovery of the first dual inhibitor of the 5-lipoxygenase-activating protein and soluble epoxide hydrolase using pharmacophore-based virtual screening. Sci Rep-UK, 2017. 7: p. 42751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Garscha U, et al. , Pharmacological profile and efficiency in vivo of diflapolin, the first dual inhibitor of 5-lipoxygenase-activating protein and soluble epoxide hydrolase. Sci Rep-UK, 2017. 7(1): p. 9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Moser D, et al. , Dual-target virtual screening by pharmacophore elucidation and molecular shape filtering. ACS Med Chem Lett, 2012. 3(2): p. 155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nandha B, Ramareddy SA, and Kuntal H, Synthesis of substituted fluorobenzimidazoles as inhibitors of 5-lipoxygenase and soluble epoxide hydrolase for anti-inflammatory activity. Arch Pharm Chem Life Sci, 2018. 351:e1800030. [DOI] [PubMed] [Google Scholar]
- [56].Meirer K, et al. , Synthesis and structure-activity relationship studies of novel dual inhibitors of soluble epoxide hydrolase and 5-lipoxygenase. J Med Chem, 2013. 56(4): p. 1777–1781. [DOI] [PubMed] [Google Scholar]
- [57].Wisniewska JM, et al. , Molecular characterization of EP6--a novel imidazo[1,2-a]pyridine based direct 5-lipoxygenase inhibitor. Biochem Pharmacol, 2012. 55(2): p. 228–240. [DOI] [PubMed] [Google Scholar]
- [58].Shen HC, Soluble epoxide hydrolase inhibitors: A patent review. Expert opin Ther Pat, 2010. 20(1): p. 941–956. [DOI] [PubMed] [Google Scholar]
- [59].Achenbach J, et al. , Exploring the chemical space of multitarget ligands using aligned self-organizing maps. ACS Med Chem Lett, 2013. 4(12): p. 1169–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Meirer K, et al. , Design, Synthesis and Cellular Characterization of a Dual Inhibitor of 5-Lipoxygenase and Soluble Epoxide Hydrolase. Molecules, 2016. 22(1): p. 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rose TE, et al. , 1-Aryl-3-(1-acylpiperidin-4-yl)urea Inhibitors of Human and Murine Soluble Epoxide Hydrolase: Structure–Activity Relationships, Pharmacokinetics, and Reduction of Inflammatory Pain. J Med Chem, 2010. 55(19): p. 7067–7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].(a) Prestwich GD and Hammock BD Rapid purification of cytosolic epoxide hydrolase from normal and clofibrate-treated animals by affinity chromatography. Proc. Natl. Acad. Sci. U.S.A 1985, 82(6), p. 1663–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Moody DE, et al. ; Epoxide metabolism in the liver of mice treated with clofibrate (ethyl-alpha-(p-chlorophenoxyisobutyrate)), a peroxisome proliferator. Toxicol. Appl. Pharmacol 1985, 75(3), p. 351–362. [DOI] [PubMed] [Google Scholar]
- [63].Idris-Khodja N, et al. , Vascular smooth muscle cell peroxisome proliferator-activated receptor gamma protects against endothelin-1-induced oxidative stress and inflammation. J Hypertens, 2017. 55(7): p. 1390–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Samokhvalov V, et al. , PPARgamma signaling is required for mediating EETs protective effects in neonatal cardiomyocytes exposed to LPS. Front Pharmacol, 2014. 5: p. 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Staels B and Fruchart JC, Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes, 2005. 54(8): p. 2460–2470. [DOI] [PubMed] [Google Scholar]
- [66].Guan Y, et al. , Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med, 2005. 11(8): p. 861–866. [DOI] [PubMed] [Google Scholar]
- [67].Eldor R, DeFronzo RA, and Abdul-Ghani M, In vivo actions of peroxisome proliferator-activated receptors: glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care, 2013. 36 Suppl 2: p. S162–S174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jung O, et al. , Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension, 2005. 45(4): p. 759–765. [DOI] [PubMed] [Google Scholar]
- [69].Capdevila JH, et al. , The Cyp2c44 epoxygenase regulates epithelial sodium channel activity and the blood pressure responses to increased dietary salt. J Biol Chem, 2014. 289(1): p. 4377–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hye Khan MA, et al. , Epoxyeicosatrienoic acid analogue lowers blood pressure through vasodilation and sodium channel inhibition. Clin Sci, 2014. 127(7): p. 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Grundy SM, Drug therapy of the metabolic syndrome: minimizing the emerging crisis in polypharmacy. Nat Rev Drug Discov, 2006. 5(4): p. 295–309. [DOI] [PubMed] [Google Scholar]
- [72].De Taeye BM, et al. , Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity 2010, 18(3), p. 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fleming I, The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease. Pharmacol Rev, 2014. 66(4): p. 1106–1140. [DOI] [PubMed] [Google Scholar]
- [74].Kim J, et al. , Inhibition of soluble epoxide hydrolase prevents renal interstitial fibrosis and inflammation. Am J Physiol Renal Physiol, 2014. 307(8): p. F971–F980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Imig JD, et al. , Soluble epoxide hydrolase inhibition and peroxisome proliferator activated receptor gamma agonist improve vascular function and decrease renal injury in hypertensive obese rats. Exp Biol Med, 2012. 237(12): p. 1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].la Buscato E, et al. , Design and synthesis of dual modulators of soluble epoxide hydrolase and peroxisome proliferator-activated receptors. J Med Chem, 2012. 55(23): p. 10771–10775. [DOI] [PubMed] [Google Scholar]
- [77].Blöcher R, et al. , Design and synthesis of fused soluble epoxide hydrolase/peroxisome proliferator-activated receptor modulators. MedChemComm, 2016. 7(6): p. 1209–1216. [Google Scholar]
- [78].Blöcher R, et al. , N-Benzylbenzamides: A Novel Merged Scaffold for Orally Available Dual Soluble Epoxide Hydrolase/Peroxisome Proliferator-Activated Receptor gamma Modulators. J Med Chem, 2016. 59(1): p. 61–81. [DOI] [PubMed] [Google Scholar]
- [79].Hye Khan MA, Kolb L, Skibba M, Hartmann M, Blöcher R, Proschak E, Imig JD, A novel dual PPARγ agonist/sEH inhibitor treats diabetic complications in a rat model of type 2, Diabetologia. 2018, DOI: 10.1007/s00125-018-4685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Schierle S, et al. , Boosting Anti-Inflammatory Potency of Zafirlukast by Designed Polypharmacology. J Med Chem, 2018. 67(13): p. 5758–5764. [DOI] [PubMed] [Google Scholar]
- [81].Makishima M, et al. , Identification of a nuclear receptor for bile acids. Science, 1999. 284(5418): p. 1362–1365. [DOI] [PubMed] [Google Scholar]
- [82].Neuschwander-Tetri BA, et al. , Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet, 2015. 385(9972): p. 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Mudaliar S, et al. , Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology, 2013. 145(3): p. 574–582. [DOI] [PubMed] [Google Scholar]
- [84].Mangels N, et al. , The soluble epoxide hydrolase determines cholesterol homeostasis by regulating AMPK and SREBP activity. Prostaglandins Other Lipid Mediat, 2016. 125: p. 30–39. [DOI] [PubMed] [Google Scholar]
- [85].Liu Y, et al. , Inhibition of soluble epoxide hydrolase attenuates high-fat-diet-induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS One, 2012. 7(6): p. e39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Merk D, et al. , Extending the structure-activity relationship of anthranilic acid derivatives as farnesoid X receptor modulators: development of a highly potent partial farnesoid X receptor agonist. J Med Chem, 2014. 57(19): p. 8035–8055. [DOI] [PubMed] [Google Scholar]
- [87].Kompa AR, et al. , Soluble epoxide hydrolase inhibition exerts beneficial anti-remodeling actions post-myocardial infarction. Int J Cardiol, 2013. 167(1): p. 210–219. [DOI] [PubMed] [Google Scholar]
- [88].Schmidt J, et al. , A Dual Modulator of Farnesoid X Receptor and Soluble Epoxide Hydrolase To Counter Nonalcoholic Steatohepatitis. J Med Chem, 2017. 60(18): p. 7703–7724. [DOI] [PubMed] [Google Scholar]
- [89].Ezzili C, Otrubova K, and Boger DL, Fatty acid amide signaling molecules. Bioorg Med Chem Lett, 2010. 20(20): p. 5959–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Fegley D, et al. , Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther, 2005. 313(1): p. 352–358. [DOI] [PubMed] [Google Scholar]
- [91].Jayamanne A, et al. , Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol, 2006. 147(3): p. 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sasso O, et al. , Peripheral FAAH inhibition causes profound antinociception and protects against indomethacin-induced gastric lesions. Pharmacol Res, 2012. 65(5): p. 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ulu A, et al. , Inhibition of soluble epoxide hydrolase as a novel approach to high dose diazepam induced hypotension. J Clin Toxicol, 2016. 6(3). pii: 1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ostermann AI, et al. , Oral treatment of rodents with soluble epoxide hydrolase inhibitor 1-(1-propanoylpiperidin-4-yl)-3-[4-(trifluoromethoxy)phenyl]urea (TPPU): Resulting drug levels and modulation of oxylipin pattern. Prostaglandins Other Lipid Mediat, 2015. 121: p. 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sasso O, et al. , Peripheral FAAH and soluble epoxide hydrolase inhibitors are synergistically antinociceptive. Pharmacol Res, 2015. 97: p. 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kodani SD, et al. , Identification and optimization of soluble epoxide hydrolase inhibitors with dual potency towards fatty acid amide hydrolase. Bioorg Med Chem Lett, 2018. 28(4): p. 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Roskoski R Jr., RAF protein-serine/threonine kinases: structure and regulation. Biochem Biophys Res Commun, 2010. 399(3): p. 313–317. [DOI] [PubMed] [Google Scholar]
- [98].Kasid U and Dritschilo A, RAF antisense oligonucleotide as a tumor radiosensitizer. Oncogene, 2003. 22(37): p. 5876–5884. [DOI] [PubMed] [Google Scholar]
- [99].Shah DR, Shah RR, and Morganroth J, Tyrosine Kinase Inhibitors: Their On-Target Toxicities as Potential Indicators of Efficacy. Drug Safety, 2013. 36(6): p. 413–426. [DOI] [PubMed] [Google Scholar]
- [100].Orphanos GS, Ioannidis GN, and Ardavanis AG, Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol, 2009. 48(7): p. 964–970. [DOI] [PubMed] [Google Scholar]
- [101].Asati V, Bharti SK, and Mahapatra DK, Mutant B-Raf Kinase Inhibitors as Anticancer Agents. Anticancer Agents Med Chem, 2016. 16(12): p. 1558–1575. [DOI] [PubMed] [Google Scholar]
- [102].Bhargava A, et al. , Registered report: Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. eLife, 2016. 5:e11999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Heidorn SJ, et al. , Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell, 2010. 140(2): p. 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Node K, et al. , Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science, 1999. 285(5431): p. 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bettaieb A, et al. , Effects of soluble epoxide hydrolase deficiency on acute pancreatitis in mice. PLoS One, 2014. 9(11): p. e113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Liu JY, et al. , Sorafenib has soluble epoxide hydrolase inhibitory activity, which contributes to its effect profile in vivo. Mol Cancer Ther, 2009. 8(8): p. 2193–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hwang SH, et al. , Synthesis and biological evaluation of sorafenib- and regorafenib-like sEH inhibitors. Bioorg Med Chem Lett, 2013. 23(13): p. 3732–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wecksler AT, et al. , Biological evaluation of a novel sorafenib analogue, t-CUPM. Cancer Chemother Pharmacol, 2015. 75(1): p. 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Liao J, et al. , Inhibition of mutant KrasG12D-initiated murine pancreatic carcinoma growth by a dual c-Raf and soluble epoxide hydrolase inhibitor t-CUPM. Cancer Lett, 2016. 371(2): p. 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Guerra C and Barbacid M, Genetically engineered mouse models of pancreatic adenocarcinoma. Mol Oncol, 2013. 7(2): p. 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Liao J, et al. , Inhibition of Chronic Pancreatitis and Murine Pancreatic Intraepithelial Neoplasia by a Dual Inhibitor of c-RAF and Soluble Epoxide Hydrolase in LSL-KrasG(1)(2)D/Pdx-1-Cre Mice. Anticancer Res, 2016. 36(1): p. 27–37. [PMC free article] [PubMed] [Google Scholar]
- [112].Lazaar AL, et al. , Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor. Br J Clin Pharmacol, 2016. 81(5): p. 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Chen D, et al. , Pharmacokinetics and pharmacodynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. J Clin Pharmacol, 2012. 52(3): p. 319–328. [DOI] [PubMed] [Google Scholar]