Abstract
Coordination-driven self-assembly as a powerful bottom-up approach has been extensively used to construct multifarious supramolecular architectures with increasing complexity and functionality. Due to the unique cavity structures and precisely controllable dimensions, 3D supramolecules display unprecedented properties and functions in catalysis, sensing, gas storage, and smart materials. Herein, we have built two 3D nanocages with different sizes by changing the length of the organic ligand arms. The structures were characterized by 1D and 2D NMR spectroscopy, electrospray ionization-mass spectrometry (ESI-MS), traveling wave ion mobility-mass spectrometry (TWIM-MS), gradient tandem-mass spectrometry (gMS2), and transmission electron microscopy (TEM). Furthermore, the intermolecular dynamic exchange of two 3D nanocages was conducted to construct a series of hybrid 3D structures as evidenced by mass spectrometry.
Keywords: 3D nanocages, self-assembly, supramolecules, terpyridine
Self-assembly as a powerful bottom-up approach widely exists in the formation of complicated functional structures.[1] Synthetic supramolecular chemistry focused on the construction of specific and functionalized supramolecular architectures through self-assembly during the past few decades. Coordination-driven self-assembly is one of the most useful methods for preparing multifarious supra-molecular structures,[2] such as 1D helicates, 2D macrocycles, knots, links, 3D cages, and capsules.[1–13] 3D supramolecules with well-defined cavities are attractive due to their ability of encapsulating guest molecules. As a result, they have broad applications in catalysis,[14] drug delivery,[15] molecule recognition,[16] stabilization of (air)-sensitive molecules,[7,17] etc.
Terpyridine (tpy)-based building blocks that coordinate with transition metal ions (i.e., <tpy-M(II)-tpy>) have been applied for constructing supramolecules.[18] Numerous tpy-based metallo-supramolecules, including triangle,[19] hexagons,[20] fractals,[21] spoked wheels,[22] and nested concentric ring[23] have been reported. Despite several elegant reports,[24,25] the synthesis of tpy-based 3D nanocages is still challenging in terms of diversity and complexity.
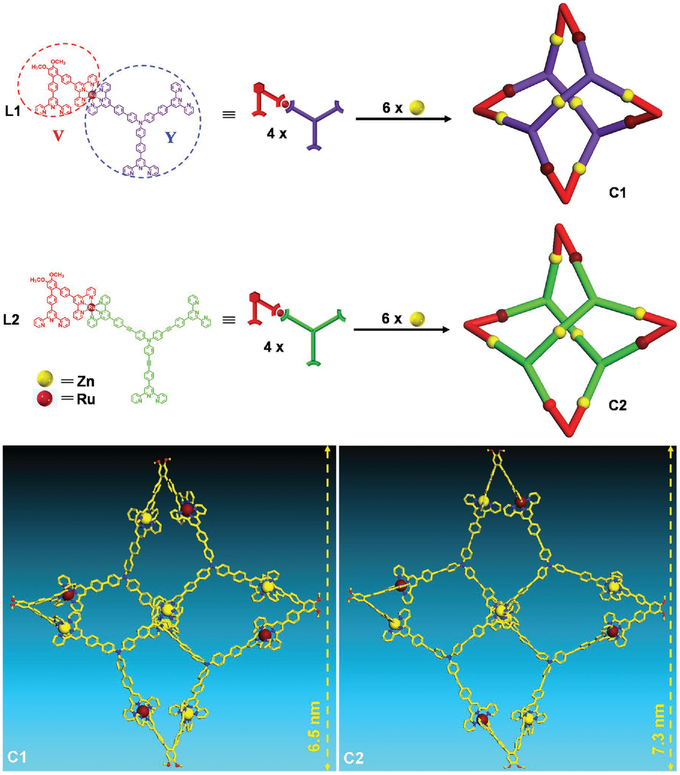
Recently, we reported two distinct 2D supramolecular snowflakes,[26] which were obtained by self-assembling metal–organic ligand containing a <tpy-Ru(II)-tpy> motif with a hexatopic ligand and Zn(II) ions. Interestingly, we found that the metal–organic ligand itself can coordinate with Zn(II) ions to form 3D nanocage, which is similar to Stang’s truncated tetrahedron[27] assembled by pyridine ligand. Herein, we have built two 3D nanocages, with different sizes by changing the length of the metal–organic ligand arms. The further dynamic ligand exchange between two preassembled nanocages generated as a series of hybrid supramolecular nanocages, suggesting the remarkable tolerance of such structures with different edge length.
With structural analysis and molecular modeling, we speculated that three-armed tpy-based organic ligand might assemble with metal components to build a truncated tetrahedron-shaped 3D nanocage structure (Scheme 1). The metal–organic ligand L1 containing three free terpyridines was designed with V and Y components connected by Ru(II). Such building block was synthesized by performing Suzuki coupling reaction on Ru(II)-tpy complex to avoid the by-products via self-sorting as shown in Scheme S1, Supporting Information. C1 was obtained in high yield (94.3%) by self-assembling L1 with Zn(NO3)2•6H2O.
Scheme 1.
Self-assembly of 3D supramolecular nanocages and representative energy-minimized structures of C1 and C2.
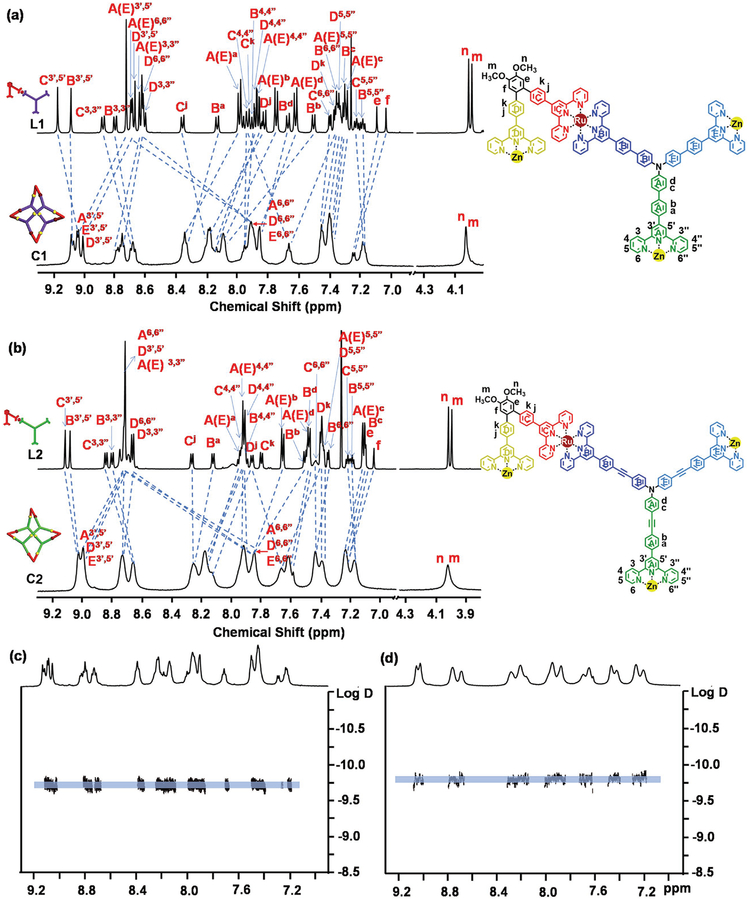
The 1H NMR spectrum of ligand L1 (Figure 1a) showed four distinguishable sets of tpy-phenyl resonances peaks (A–D, D, and E sets are chemical equivalent). However, after coordination with Zn(II), the 1H NMR spectrum of C1 became further complicated with five sets of tpy-phenyl signals (labeled as A–E). Two sets of peaks at 9.18 and ≈9.08 ppm for the tpyH3′,5′ protons of <tpy-Ru(II)-tpy> moieties showed negligible shifts. After coordinating with Zn(II) ion, all the free tpyH3′,5′ protons of L1 were significantly shifted to downfield due to the decrease of electron density upon complexation.[17] The expected upfield shift of doublet from 8.7 to 7.9 ppm (≈0.8 ppm) was attributed to the movement of tpyH6,6” protons after coordination. All of the assignments were readily confirmed by the 2D-COSY and 2D-NOESY spectra (Figures S23–S29, Supporting Information). 2D diffusion-ordered NMR spectroscopy (DOSY) (Figure 1c) showed a single band near a diffusion coefficient of 1.85 × 10−10 m2 s−1 (log D values as −9.73). Using the Stocks–Einstein equation, the experimental radius, that is, the semimajor axis radius, of C1 was 3.2 nm, was comparable with outer radius measured from the molecular modeling of C1 (≈3.3 nm, Scheme 1).
Figure 1.
1H NMR spectra. a) L1 and C1; b) L2 and C2; c) DOSY of C1; d) DOSY of C2 (CDCl3 for ligands and CD3CN for supramolecules).
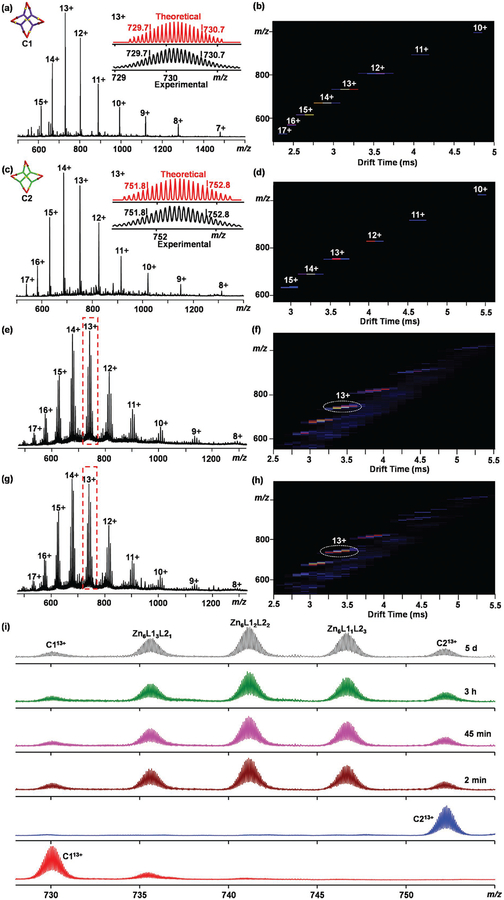
In addition, ESI-MS coupled with TWIMMS and gMS2 was applied to validate the proposed structure. ESI-MS showed a series of peaks with continuous charges from 7+ to 15+ due to successive loss of counterion. After deconvolution, the obtained molecular weight at 11365 Da agreed well with the proposed molecular composition [(C131H90N16O2)4Zn6Ru4(PF6−)20] (Figure 2a). The experimental isotope patterns of each charge state was consistent with the simulated isotopic distribution (Figure S1, Supporting Information). TWIM-MS showed a series of charge states with narrow drift time distribution ranging from 10+ to 17+, excluding the formation of any isomers or oligomers (Figure 2b). In order to examine the stability of the C1, gMS2 experiment was performed on the 13+ ions at m/z 730.1 by collision-induced dissociation with collision energies ranging from 5 to 20 V (Figure S3, Supporting Information). The complex ions were completely dissociated when the voltage reached 20 V, which was converted to a center-of-mass collision energy of 0.08 eV. This result was used in the comparison with C2.
Figure 2.
a,c) ESI-MS and b,d) TWIM-MS plot (m/z vs drift time) of C1 and C2. e–i) Dynamic ligand exchange of preassembled C1 and C2. e) ESI-MS and f) TWIM-MS plot of preassembled C1 and C2 at 2 min. g) ESI-MS and h) TWIM-MS plot of preassembled C1 and C2 at 5 d. i) Time-dependent spectra of dynamic exchange of C1 and C2 at 2 min, 45 min, 3 h, 5 d for expanded region of 13+ signals.
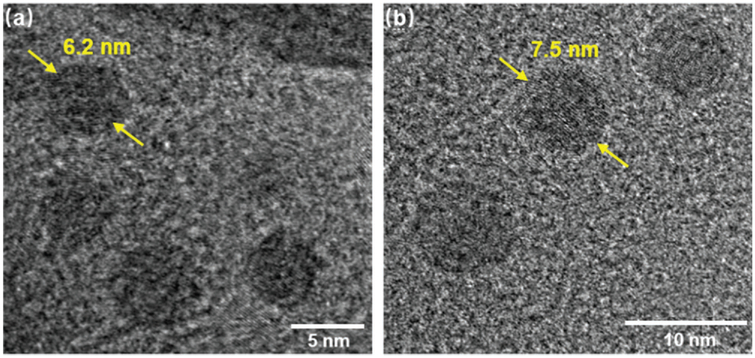
TEM was utilized to further confirm both shapes and sizes of individual C1 molecule by deposition of the dilute MeCN solution on carbon-coated grids. Individual circular structure with high contrast was observed for C1. The measured size was consistent with the theoretical diameter of 6.5 nm from molecular modeling (Figure 3a).
Figure 3.
TEM images of a) C1 and b) C2.
On the basis of C1, we stepped forward to construct larger version of supramolecular nanocage C2 by increasing the arm length of Y. L2 was prepared by introducing alkyne bond in a triphenylamine moiety, then assembled with Zn(II) to produce C2. We expected that this rigid and stable system with truncated tetrahedron shaped geometry was able to accommodate the variation of ligand arm length but without losing the entire structure integrity benefitting from the energy favorable features of the structure.
Figure 1b showed the 1H NMR spectra of ligand L2 and complex C2. After assembly characteristic downshift and upshift of (A, D, E)-tpyH3′,5′and tpyH6,6”protons together indicated the formation of the designed structure. DOSY showed a single band near a diffusion coefficient of 1.55 × 10−10 m2 s−1 (log D values as −9.81) with larger conducted semimajor axle radius (3.8 nm) (Figure 1d). TEM imaging of C2 also displayed clear images of circular structure with narrow dispersity of diameter around 7.5 nm (Figure 3b).
ESI-MS result of C2 (Figure 2c) displayed a series of peaks with continuous charge states from 8+ to 17+. The measured molecular weight of C2 was 11653 Da, supporting its composition [(C137H90N16O2)4Zn6Ru4(PF−6)20]. TWIM-MS displays one predominant series of signals with narrow distribution (Figure 2d), suggesting the absence of any kind of isomer. The 13+ ions of C2 were completely dissociated at 21 V in gMS2 (Figure S4, Supporting Information), which was converted to a center-of-mass collision energy of 0.08 eV, same as that of C1. Therefore, C1 and C2 possess similar stability.
Despite the difference of the ligands, C1 and C2 could be assembled with similar structure and stability. The next question we raised is whether we were able to assemble hybrid supramolecular nanocages containing both L1 and L2 or not. In our previous study,[26] ligand exchange between different snowflakes with various outer rims has been fully explored. In this work, we are curious about the intermolecular communication feature between C1 and C2, after ascending 2D snowflake structure into 3D nanocage.
We started the study by mixing preassembled C1 and C2 (CH3CN, 1 mg mL−1) solutions with 1:1 ratio at room temperature. All the experimental process was monitored by ESI-MS and TWIM-MS (Figure 2e–i). The dynamic ligand exchange started and almost finished within 2 min after the mixing. In addition to the original peaks of C1 and C2, for example C113+ and C213+, three new peaks appeared, corresponding to all the statistical hybrid 3D nanocages. No significant change was observed by extending the monitoring time (Figure 2i). The result showed that the dynamic ligand exchange between C1 and C2 was substantially faster than that between two snowflake supra-molecules, as the dynamic exchange between snowflakes reached equilibrium around 5 days. Such difference suggests that the energy barrier of the ligand communication between nanocages is much lower than that between two snowflakes. In the spectrum of TWIM-MS (Figure 3f,h), all the hybrid signals drifted out with very similar times, indicating that they should have the similar 3D nanocage structure as C1 and C2.
Considering the tpy and triphenylamine have a wide range of applications in optoelectronics, we studied the photophysical and electrochemical properties of the complexes. In the absorption spectra (Figure S5, Supporting Information), C1 and C2 showed characteristic absorption bands peaked at 380 and 500 nm, which corresponds to the intramolecular charge transfer (ICT) transition from triphenylamine moiety to the tpy-metal complex moieties and metal-to-ligand charge transfer (MLCT) transitions, respectively.[28] Oxidation and reduction properties of these two complexes were determined through cyclic voltammetry (CV) with results displayed in Figure S6, Supporting Information. Two tpy-ligand-centered redox couples were observed between −2 and −0.5 V.
In summary, by taking advantage of the diversity of the coordination ability between terpyridine with transition metal ions, two supramolecular nanocages were assembled successfully by stepwise approach. The structures were characterized by 1D and 2D NMR, ESI-MS, TWIM-MS, gMS2, TEM, along with molecular modeling. Furthermore, the dynamic intermolecular exchange behavior between two 3D nanocages was investigated by ESI-MS and TWIM-MS. It showed that such versatile supramolecular structures were able to tolerate different types of ligands with various combinations and form hybrid supramolecular nanocages. The rapid communication between supramolecules via ligand exchange further increased the complexity of the system. Electrochemical and photophysical properties of C1 and C2 were also investigated for future potential applications, such as host–guest, supramolecular catalysis, gas storage, etc.
Supplementary Material
Acknowledgements
This work was supported by the National Science Foundation (CHE-1506722 to X.L.), National Institutes of Health (1R01GM128037 to X.L.), Program for JLU Science and Technology Innovative Research Team (JLUSTIRT to M.W.), the Aid Project for the Leading Young Teachers in Henan Provincial Institutions (2015GGJS-157 to X-Q.H.). The support of Program of Introducing Talents of Discipline to Universities of China (111 Program, B17019) is also appreciated. The authors thank Prof. Shengqian Ma at the University of South Florida for CV test.
Footnotes
The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/marc.201800404.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Zhe Zhang, Key Laboratory for Water Quality and Conservation of the Pearl, River Delta, Ministry of Education, Environmental Research at Great Bay, Guangzhou University, Guangzhou 510006, P. R. China; Department of Chemistry, University of South Florida, Tampa 33620, USA; Key Laboratory of Pesticide and Chemical Biology, Ministry of Education, School of Chemistry, Central China Normal University, Wuhan 430079, P. R. China.
Heng Wang, Department of Chemistry, University of South Florida, Tampa 33620, USA.
Junjuan Shi, State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, P. R. China.
Yaping Xu, State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, P. R. China.
Lei Wang, Department of Chemistry, University of South Florida, Tampa 33620, USA.
Sammy Shihadeh, Department of Chemistry, University of South Florida, Tampa 33620, USA.
Fu-Jie Zhao, College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450001, P. R. China.
Xin-Qi Hao, College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450001, P. R. China.
Pingshan Wang, Key Laboratory for Water Quality and Conservation of the Pearl, River Delta, Ministry of Education, Environmental Research at Great Bay, Guangzhou University, Guangzhou 510006, P. R. China.
Changlin Liu, Key Laboratory of Pesticide and Chemical Biology, Ministry of Education, School of Chemistry, Central China Normal University, Wuhan 430079, P. R. China.
Ming Wang, State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, P. R. China.
Xiaopeng Li, Department of Chemistry, University of South Florida, Tampa 33620, USA.
References
- [1].a) Lehn J-M, Science 2002, 295, 2400; [DOI] [PubMed] [Google Scholar]; b) Davis AV, Yeh RM, Raymond KN, Proc. Nat. Acad. Sci. U. S. A 2002, 99, 4793; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wurthner F, You C-C, Saha-Moller CR, Chem. Soc. Rev 2004, 33, 133. [DOI] [PubMed] [Google Scholar]
- [2].a) Cook TR, Zheng Y-R, Stang PJ, Chem. Rev 2013, 113, 734; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chakrabarty R, Mukherjee PS, Stang PJ, Chem. Rev 2011, 111, 6810; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mukherjee S, Mukherjee PS, Chem. Commun 2014, 50, 2239. [DOI] [PubMed] [Google Scholar]
- [3].a) Seidel SR, Stang PJ, Acc. Chem. Res 2002, 35, 972; [DOI] [PubMed] [Google Scholar]; b) Fujita M, Tominaga M, Hori A, Therrien B, Acc. Chem. Res 2005, 38, 369. [DOI] [PubMed] [Google Scholar]
- [4].Olenyuk B, Whiteford JA, Fechtenkötter A, Stang PJ, Nature 1999, 398, 796. [DOI] [PubMed] [Google Scholar]
- [5].Fujita D, Ueda Y, Sato S, Mizuno N, Kumasaka T, Fujita M, Nature 2016, 540, 563. [DOI] [PubMed] [Google Scholar]
- [6].Zhao C, Sun Q-F, Hart-Cooper WM, DiPasquale AG, Toste FD, Bergman RG, Raymond KN, J. Am. Chem. Soc 2013, 135, 18802. [DOI] [PubMed] [Google Scholar]
- [7].a) Mal P, Breiner B, Rissanen K, Nitschke JR, Science 2009, 324, 1697; [DOI] [PubMed] [Google Scholar]; b) Riddell IA, Hristova YR, Clegg JK, Wood CS, Breiner B, Nitschke JR, J. Am. Chem. Soc 2013, 135, 2723. [DOI] [PubMed] [Google Scholar]
- [8].Danon JJ, Krüger A, Leigh DA, Lemonnier J-F, Stephens AJ, Vitorica-Yrezabal IJ, Woltering SL, Science 2017, 355, 159. [DOI] [PubMed] [Google Scholar]
- [9].Fowler DA, Rathnayake AS, Kennedy S, Kumari H, Beavers CM, Teat SJ, Atwood JL, J. Am. Chem. Soc 2013, 135, 12184. [DOI] [PubMed] [Google Scholar]
- [10].Hiraoka S, Yamauchi Y, Arakane R, Shionoya M, J. Am. Chem. Soc 2009, 131, 11646. [DOI] [PubMed] [Google Scholar]
- [11].H. CG, Wataru K, Shohei T, Motoo S, Mitsuhiko, Angew. Chem. Int. Ed 2012, 51, 2606. [Google Scholar]
- [12].Li J-R, Yakovenko AA, Lu W, Timmons DJ, Zhuang W, Yuan D, Zhou H-C, J. Am. Chem. Soc 2010, 132, 17599. [DOI] [PubMed] [Google Scholar]
- [13].a) Bhat IA, Samanta D, Mukherjee PS, J. Am. Chem. Soc 2015, 137, 9497; [DOI] [PubMed] [Google Scholar]; b) Song B, Zhang Z, Wang K, Hsu C-H, Bolarinwa O, Wang J, Li Y, Yin G-Q, Rivera E, Yang H-B, Liu C, Xu B, Li X, Angew. Chem. Int. Ed 2017, 56, 5258. [DOI] [PubMed] [Google Scholar]
- [14].Marcos V, Stephens AJ, Jaramillo-Garcia J, Nussbaumer AL, Woltering SL, Valero A, Lemonnier J-F, Vitorica-Yrezabal IJ, Leigh DA, Science 2016, 352, 1555. [DOI] [PubMed] [Google Scholar]
- [15].Samanta SK, Moncelet D, Briken V, Isaacs L, J. Am. Chem. Soc 2016, 138, 14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barrow SJ, Kasera S, Rowland MJ, del Barrio J, Scherman OA, Chem. Rev 2015, 115, 12320. [DOI] [PubMed] [Google Scholar]
- [17].Mal P, Breiner B, Rissanen K, Nitschke JR, Science 2009, 324, 1697. [DOI] [PubMed] [Google Scholar]
- [18].Chakraborty S, Newkome GR, Chem. Soc. Rev 2018, 10.1039/c8cs00030a. [DOI] [PubMed] [Google Scholar]
- [19].Schultz A, Cao Y, Huang M, Cheng SZD, Li X, Moorefield CN, Wesdemiotis C, Newkome GR, Dalton Trans 2012, 41, 11573. [DOI] [PubMed] [Google Scholar]
- [20].Newkome GR, Cho TJ, Moorefield CN, Baker GR, Cush R, Russo PS, Angew. Chem. Int. Ed 1999, 38, 3717. [PubMed] [Google Scholar]
- [21].a) Newkome GR, Wang P, Moorefield CN, Cho TJ, Mohapatra PP, Li S, Hwang S-H, Lukoyanova O, Echegoyen L, Palagallo JA, Iancu V, Hla S-W, Science 2006, 312, 1782; [DOI] [PubMed] [Google Scholar]; b) Jiang Z, Li Y, Wang M, Liu D, Yuan J, Chen M, Wang J, Newkome GR, Sun W, Li X, Wang P, Angew. Chem. Int. Ed 2017, 56, 11450; [DOI] [PubMed] [Google Scholar]
- [22].Lu X, Li X, Cao Y, Schultz A, Wang JL, Moorefield CN, Wesdemiotis C, Cheng SZ, Newkome GR, Angew. Chem. Int. Ed 2013, 52, 7728. [DOI] [PubMed] [Google Scholar]
- [23].a) Wang M, Wang C, Hao X-Q, Liu J, Li X, Xu C, Lopez A, Sun L, Song M-P, Yang H-B, Li X, J. Am. Chem. Soc 2014, 136, 6664; [DOI] [PubMed] [Google Scholar]; b) Wang H, Qian X, Wang K, Su M, Haoyang W-W, Jiang X, Brzozowski R, Wang M, Gao X, Li Y, Xu B, Eswara P, Hao X-Q, Gong W, Hou J-L, Cai J, Li X, Nat. Comm 2018, 9, 1815; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yin G-Q, Wang H, Wang X-Q, Song B, Chen L-J, Wang L, Hao X-Q, Yang H-B, Li X, Nat. Comm 2018, 9, 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].a) Xie T-Z, Endres KJ, Guo Z, Ludlow JM, Moorefield CN, Saunders MJ, Wesdemiotis C, Newkome GR, J. Am. Chem. Soc 2016, 138, 12344; [DOI] [PubMed] [Google Scholar]; b) Chakraborty S, Hong W, Endres KJ, Xie T-Z, Wojtas L, Moorefield CN, Wesdemiotis C, Newkome GR, J. Am. Chem. Soc 2017, 139, 3012. [DOI] [PubMed] [Google Scholar]
- [25].a) Wang M, Wang C, Hao X-Q, Li X, Vaughn TJ, Zhang Y-Y, Yu Y, Li Z-Y, Song M-P, Yang H-B, Li X, J. Am. Chem. Soc 2014, 136, 10499; [DOI] [PubMed] [Google Scholar]; b) Chen M, Wang J, Liu D, Jiang Z, Liu Q, Wu T, Liu H, Yu W, Yan J, Wang P, J. Am. Chem. Soc 2018, 140, 2555. [DOI] [PubMed] [Google Scholar]
- [26].Zhang Z, Wang H, Wang X, Li Y, Song B, Bolarinwa O, Reese RA, Zhang T, Wang X-Q, Cai J, Xu B, Wang M, Liu C, Yang H-B, Li X, J. Am. Chem. Soc 2017, 139, 8174. [DOI] [PubMed] [Google Scholar]
- [27].Leininger S, Fan J, Schmitz M, Stang PJ, Proc. Natl. Acad. Sci 2000, 97, 1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].a) Robson KCD, Koivisto BD, Gordon TJ, Baumgartner T, Berlinguette CP, Inorg. Chem 2010, 49, 5335; [DOI] [PubMed] [Google Scholar]; b) Baitalik S, Wang X-Y, Schmehl RH, J. Am. Chem. Soc 2004, 126, 16304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.