Abstract
Bcl-2 inhibits cell death by at least two different mechanisms. On the one hand, its BH3 domain binds to pro-apoptotic proteins such as Bim and prevents apoptosis induction. On the other hand, the BH4 domain of Bcl-2 binds to the inositol 1,4,5-trisphosphate receptor (IP3R), preventing Ca2+ signals that mediate cell death. In normal T-cells, Bcl-2 levels increase during the immune response, protecting against cell death, and then decline as apoptosis ensues and the immune response dissipates. But in many cancers Bcl-2 is aberrantly expressed and exploited to prevent cell death by inhibiting IP3R-mediated Ca2+ elevation. This review summarizes what is known about the mechanism of Bcl-2’s control over IP3R-mediated Ca2+ release and cell death induction. Early insights into the role of Ca2+ elevation in corticosteroid-mediated cell death serves as a model for how targeting IP3R-mediated Ca2+ elevation can be a highly effective therapeutic approach for different types of cancer. Moreover, the successful development of ABT-199 (Venetoclax), a small molecule targeting the BH3 domain of Bcl-2 but without effects on Ca2+, serves as proof of principle that targeting Bcl-2 can be an effective therapeutic approach. BIRD-2, a synthetic peptide that inhibits Bcl-2-IP3R interaction, induces cell death induction in ABT-199 (Venetoclax)-resistant cancer models, attesting to the value of developing therapeutic agents that selectively target Bcl-2-IP3R interaction, inducing Ca2+-mediated cell death.
Keywords: Bcl-2; inositol 1,4,5-trisphosphate receptor (IP3R); calcium (Ca2+); endoplasmic reticulum; apoptosis; adrenal corticosteroid hormones; dexamethasone; prednisone
1. Introduction
The inositol 1,4,5-trisphosphate receptor (IP3R) is an intracellular Ca2+ channel located on the endoplasmic reticulum (ER). Its diverse roles in normal physiology, cell survival and death, and diseases including cancer have been the focus of excellent reviews published in just the last four years (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12). Here we concentrate on Bcl-2 regulation of IP3R function in normal development and how cancer cells exploit Bcl-2-IP3R interaction to promote their own survival. We start with an historical perspective summarizing how discoveries during the first half of the twentieth century led to cancer treatments mediated by corticosteroid hormones (prednisone, dexamethasone). We then review how Bcl-2 regulates Ca2+ to prevent cell death, and potential ways to target Bcl-2’s regulation of Ca2+ as a novel therapeutic approach for Bcl-2-positive malignancies.
Much of this review centers around normal T-cell development in the immune system, where Bcl-2 plays a critical role, and on B-cell malignancies which exploit Bcl-2 to remain alive despite adverse environmental circumstances and cancer treatments intended to kill them. The diagram in Figure 1 is directed at two audiences: (i) the basic scientist unfamiliar with Bcl-2-expressing hematologic malignancies and how Bcl-2-IP3R interaction plays a role; and (ii) the clinical scientists who are expert in testing and applying novel therapeutic agents but are not yet familiar with the potential value of treating cancer by targeting the Bcl-2-IP3R interaction.
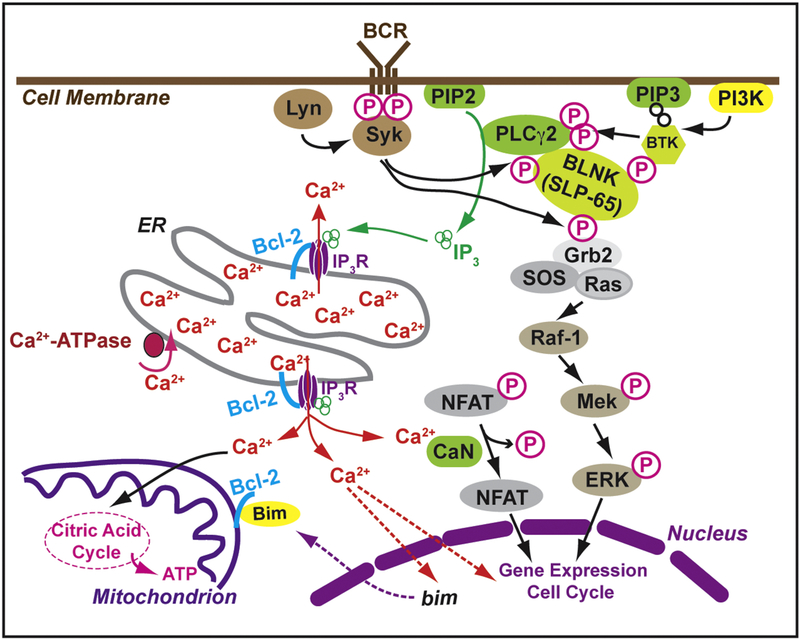
Figure 1. B-cell receptor (BCR) signaling pathways important to the pathophysiology and treatment options for chronic lymphocytic leukemia (CLL).
A critical step in BCR signaling is Bruton’s tyrosine kinase (BTK) which feeds forward into IP3 receptor-mediated Ca2+ release from the ER (left side of figure) and through the Ras-Raf-Mek-Erk pathway (right side of figure). The Bcl-2 protein is located on both the ER and mitochondria where it regulates Ca2+ signals important in generating a variety of light and death decisions.
The most common hematologic malignancies include chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), non-Hodgkin lymphoma, and multiple myeloma. The right half of Figure 1 is modified after several reviews by leading experts, summarizing recent advances in the treatment of CLL (13, 14, 15). In this cancer, chronic active B-cell receptor signaling promotes prolonged cell survival, aided by high levels of the anti-apoptotic protein Bcl-2. Exciting new therapeutic approaches have dramatically changed the therapeutic approach to CLL by targeting B-cell receptor signaling pathways, for example Bruton tyrosine kinase which is targeted by ibrutinib (16, 17) and the anti-apoptotic Bcl-2 protein which is targeted at one of its mechanisms of action by ABT-199/Venetoclax (18, 11). The left half of Figure 1 summarizes recent advances in targeting the interaction of Bcl-2 with IP3Rs, using peptide inhibitors or small molecules to induce IP3R-mediated Ca2+-elevation and cell death as a novel therapeutic approach (19, 20, 21, 22).
2. The path of discovery: From adrenal corticosteroids to IP3R-mediated Ca2+ elevation and apoptosis.
The Nobel Prize in Physiology or Medicine in 1950 was awarded jointly to Edward Kendall, Tadeus Reichstein and Philip Showalter Hench “for their discoveries relating to the hormones of the adrenal cortex, their structure and biological effects”. In the mid-1930s Kendall and Reichstein isolated and analyzed the composition of a number of similar hormones derived from the adrenal cortex. These became the basis for cortisone preparations that, with input from Kendall and Philip Hench, were first used at the end of the 1940s to treat rheumatoid arthritis and other inflammatory disorders. Also, in the 1940’s was the landmark discovery that cortisone preparations have a “lympholytic effect” (23, 24) and therefore have remarkable therapeutic activity in lymphoid malignancies (25, 26). Throughout the ensuing decades corticosteroid hormones were developed through clinical trials as extraordinarily effective anti-cancer agents, contributing to the cure of children with ALL (27, a type of cancer derived from an early developmental stage of B-lymphocytes. Corticosteroids (prednisone, dexamethasone) continue today to be essential components of treatment regimens for certain B-cell malignancies, including ALL (28, 29, 30) and multiple myeloma (31, 32, 33). Corticosteroids are still useful in treatment of refractory CLL (34, 35, 36).
Thirty years transpired between the initial use of corticosteroids in cancer treatment and recognition that Ca2+ plays an important role in cell death induction by these agents (37, 38, 39, 40), involving Ca2+-mediated endonuclease activation (39) and apoptotic DNA fragmentation (41, 42). Numerous refinements to the role of Ca2+ in endonuclease activation were to follow (43, 39, 44, 45, 46, 47) along with an expansion of knowledge regarding roles of Ca2+ in other pathways including interleukin-1b activation in response to corticosteroid treatment (48) and mechanisms of Ca2+ involvement in T-cell receptor (TCR)-mediated apoptosis (49, 50, 51, 52).
But the signaling pathways through which corticosteroids mediate effects on lymphocytes were poorly understood. One reason was that corticosteroid-induced cell death in rat lymph node lymphocytes was found to be independent of extracellular Ca2+ uptake (38). Further studies of corticosteroid-induced apoptosis were instrumental in revealing the crucial link between IP3R-mediated Ca2+ release from the ER and apoptosis induction (53, 54, 55, 56).
Now IP3R-mediated Ca2+ elevation is widely recognized as being a ‘double-edged sword’ that on the one hand promotes cell survival and on the other hand induces cell death (57, 58). Whereas physiological Ca2+ elevations are generally oscillatory in nature, Ca2+ elevations inducing cell death are the result of sustained transfer of high levels of Ca2+ from the ER to mitochondria, inducing Ca2+ mediated loss of mitochondrial membrane potential, cytochrome c release and apoptosis (59, 60, 61). Other mechanisms of Ca2+-mediated cell death include (i) elevation of the pro-apoptotic family member Bim (58); (ii) activation of Ca2+-sensitive proteases and endonucleases; and, activation of calcineurin (CaN), which in turn dephosphorylates and thus activates another pro-apoptotic Bcl-2 family member, Bad. Ca2+ mediated cell death mechanisms are extensively summarized in a recent review by two major contributors to understanding how Ca2+ fluxes mediate cell death (62, 2).
Because of the delicate balance between its functions in both cell survival and cell death, IP3R-mediated release of Ca2+ from the ER must be carefully balanced. This regulation is produced by a number of factors including kinases and phosphatases that bind to the IP3R, regulating channel opening and Ca2+ release (63, 64, 65, 66, 67, 68, 69, 70). In addition to their function as Ca2+ channels, IP3Rs serve as scaffolds and signal integrators, bringing proteins and protein complexes within close proximity to the ER and mitochondria (71, 72, 1). This is facilitated by the large tetrameric IP3R structure located on the cytoplasmic side of the ER (73, 67, 66, 72). The role of these regulatory mechanisms is particularly important in pathways involving phospholipase C activation and IP3 synthesis (74). These signaling hubs are important for control of pro-apoptotic and anti-apoptotic Bcl-2 family members, oncogenes and tumor suppressors in regulating cell death and cell survival (75, 76).
3. Prevention of excessive Ca2+ elevation and cell death by Bcl-2 during T-cell development
It has been over thirty years since the Bcl-2 protein was discovered and initially characterized (77, 78, 79, 80), twenty-five years since the first indication that Bcl-2 regulates intracellular Ca2+ dynamics (81, 82, 83, 84), and fourteen years since an interaction of Bcl-2 with the IP3R was reported (85). Bcl-2 is a 26 kDa integral membrane protein that normally resides on the outer mitochondrial membrane and endoplasmic reticulum (ER). It is anchored on these membranes by a C-terminal hydrophobic region and is mainly cytoplasmic in its location. Bcl-2 elicited widespread interest when it was found to promote cell survival by inhibiting apoptosis (86). From a functional standpoint, members of the Bcl-2 protein family generally fall into two opposing groups: anti-apoptotic proteins and pro-apoptotic proteins. Anti-apoptotic members such as Bcl-2 typically have four Bcl-2 homology (BH) domains (BH1–4). Pro-apoptotic members fall into two groups: those with three BH domains (BH1–3) and those with only a BH3 domain, the ‘BH3-only proteins’. These distinctions are useful from an operational standpoint but are undergoing revision and clarification based on recent findings (87).
One of the most remarkable features of Bcl-2 is its lack of any obvious inherent function. Sequence analysis does not reveal any recognizable functional domains. Without any inherent activity of its own, enzymatic or otherwise, Bcl-2 and its anti-apoptotic relatives nevertheless exert widespread influence over various cell functions, ultimately influencing cell survival. Bcl-2 accomplishes this through its well documented interactions with other proteins, and through its localization on the outer mitochondrial membrane and the ER.
It is appealing to study the function of Bcl-2 in a cell type where this protein normally plays an important functional role as opposed to cancer cells where Bcl-2 levels are aberrantly expressed and elevated at abnormally high levels. For this reason, many investigators focused on the role of Bcl-2 in regulating IP3R-mediated Ca2+ signals in T-cells, because IP3R-mediated Ca2+ signals following TCR activation are of critical physiological importance in the immune system (88, 89, 90). Numerous studies highlight the importance of Bcl-2 in lymphocyte development and survival (Figure 2). The Bcl-2 knockout mouse, developed in the laboratory of the late Stanley Korsmeyer, demonstrates fulminant lymphoid apoptosis (91). Enforced expression of Bcl-2 in transgenic mice reduces negative selection, causing excessive accumulation of thymocytes (92, 93, 94). Transgenic Bcl-2 inhibits negative selection by a mechanism independent of its ability to antagonize Bax (95, 96). In addition, studies in hematopoietic cells and pre-lymphomatous B-cells suggest that Bcl-2 may regulate intracellular Ca2+ dynamics (82, 84), and findings in our laboratory suggest that positive versus negative selection decisions in the thymus are partly encoded by distinct Bcl-2-regulated Ca2+ signaling patterns (97).
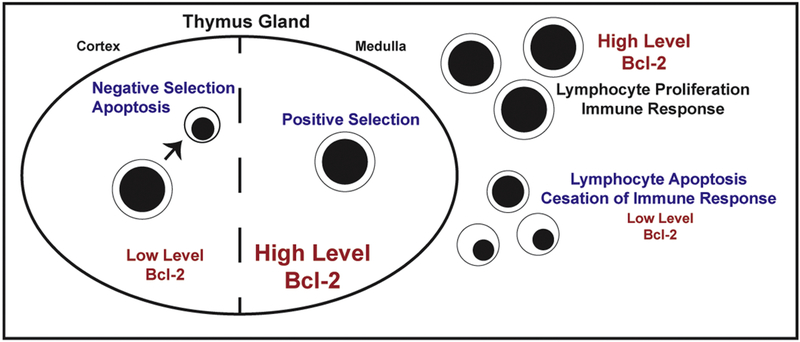
Figure 2. Bcl-2 and T-cell responses in the immune response.
T-cell levels of Bcl-2 vary during T-cell development, allowing for negative selection in the thymic cortex and facilitating positive selection in the thymic medulla. High levels of Bcl-2 insure cell survival during the immune response, then decline as apoptosis allows the immune response to decline.
The developing T-cell passes through successive maturational stages within the thymus (98) and Bcl-2 levels vary considerably throughout these different developmental stages (99, 81, 100, 101, 102) (Figure 2). The earliest precursors from the bone marrow or fetal liver do not express either the TCR or the CD4 and CD8 antigens (i.e., “double negative stage”), but do express Bcl-2: this provides an element of protection upon movement to the thymus gland. In the thymus these cells first express the TCR and both CD4 and CD8 antigens (i.e., “double positive stage”). Bcl-2 levels are down-regulated at this stage, rendering the cells very sensitive to Ca2+ induced apoptosis (81). This facilitates a stringent test of whether or not the T-cells respond to self-antigens, with strong responders undergoing apoptosis (“negative selection”) and weak responders avoiding apoptosis (“positive selection”) (103, 104). During negative selection, apoptosis is induced by Ca2+-dependent up-regulation of the pro-apoptotic Bcl-2 family member Bim (90). Positively selected cells advance to the “single positive stage” (CD4+/CD8-, CD4-/CD8+), where Bcl-2 levels are upregulated, and enter the circulation to mount immune responses to foreign antigens.
Antigen binding to the TCR triggers a signaling cascade that activates PLC-γ, which generates IP3. IP3 binds to IP3Rs, inducing channel opening and ER Ca2+ release, thus stimulating T cell proliferation (88, 105, 89, 106). Depending on the strength of TCR activation, a variety of Ca2+ response patterns are generated, including a transient Ca2+ elevation, sustained Ca2+ elevation, or Ca2+ oscillations (107). Ca2+ oscillations are the most important physiologically as they encode information by their frequency, amplitude and shape (108, 109). Consistent with earlier findings by Donnadieu et al (110), we find that strong TCR activation by a high concentration of anti-CD3 antibody induces a large transient elevation of Ca2+, whereas weak TCR activation by a low concentration of anti-CD3 antibody induces sustained Ca2+ oscillations (97).
Earlier in vitro studies demonstrated that TCR activation by a high concentration of anti-CD3 antibody induces thymocyte apoptosis, whereas lower concentrations of anti-CD3 antibody do not trigger apoptosis (40). TCR activation by physiologically-relevant antigenic peptides produce a similar effect (111): negatively-selecting antigenic peptides induce a strong Ca2+ flux in immature thymocytes, whereas positively-selecting peptides induce a smaller Ca2+ flux. We find that Bcl-2 selectively inhibits the pro-apoptotic high sustained Ca2+ elevations induced by strong TCR activation while enhancing the pro-survival Ca2+ oscillations induced by weak TCR activation (97). These Ca2+ signaling patterns differ in two important ways (97): high anti-CD3 induces a much longer Ca2+ elevation than low anti-CD3 (> 4 min versus < 1 min); and high antiCD3 triggers a much higher peak Ca2+ amplitude than low anti-CD3. High amplitude Ca2+ elevation, particularly if continuous and sustained, triggers cell death (57.
There was a thirty-year lapse between the initial clinical use of corticosteroids in cancer treatment and recognition that Ca2+ plays an important role in corticosteroid-induced lymphocyte cell death (37, 38, 39, 40), involving Ca2+-mediated endonuclease activation (39) and apoptotic DNA fragmentation (43, 42)., 58). The critical determinant of whether or not TCR stimulation induces apoptosis appears to lie in both the duration and amplitude of the Ca2+ elevation.
The positive effect of Bcl-2 on Ca2+ oscillations and its pro-survival effects are consistent with a number of other findings. For example, Ca2+ oscillations regulate thymocyte motility during positive selection, thereby modulating interactions with stromal cells (112). Ca2+ oscillations also lead to a sustained activation of CaN (104), which dephosphorylates and thereby activates nuclear factor of activated T-cells (NFAT). The continuous NFAT activation prevents NFAT nuclear dephosphorylation, allowing NFAT to remain in the nucleus and induce interleukin-2 production (107, 105, 113).
4. Bcl-2 promotion of normal cell survival through its regulation of IP3R-mediatd Ca2+ release.
Studies in which Bcl-2 was selectively targeted to the ER demonstrate that ER-localized Bcl-2 inhibits apoptosis (114, 115, 116). The anti-apoptotic activity of ER-localized Bcl-2 derives both from its binding to pro-apoptotic BH3-only proteins (e.g., Bim) (116) and to its interaction with IP3Rs to prevent excessive IP3R-mediated Ca2+ elevation. The concept that Bcl-2 regulates IP3R-mediated Ca2+ elevation evolved from evidence that Bcl-2 represses apoptosis by regulating ER-associated Ca2+ fluxes (83), ultimately leading to the discovery of an interaction between Bcl-2 and the IP3R (85), an interaction mediated by binding of the BH4 domain of Bcl-2 to a region located within the regulatory and coupling domain of the IP3R (117, 118, 119). Interactions between IP3Rs and other anti-apoptotic Bcl-2 family members, Bcl-xl and Mcl-2 are also reported (120, 121, 122, 123). The reader is referred to an excellent review discussing the full complexity of various Bcl-2 family member interactions with IP3Rs and their impact on cell survival and cell death (124). Moreover, Vervliet et al (125) discovered that Bcl-2 also binds to ryanodine receptors, dampening Ca2+ release from these intracellular channels.
Recent studies indicate that the BH4 domain of Bcl-2 is highly conserved in different classes of vertebrates and can act as a binding partner and inhibitor of IP3R channels (126). This same region is responsible for Bcl-2’s interaction with ryanodine receptors (125). In addition, a region in the BH4 domain of Bcl-2 (Ile14, Val15) has been found critical to stability and function as an inhibitor of Ca2+-mediated apoptosis (127). Furthermore, the significance of this region is further evidenced by findings indicating that the alpha helical nature of Ile14, Val15 region is essential to the function of the BH4 domain in inhibiting IP3R-mediated Ca2+ release (128).
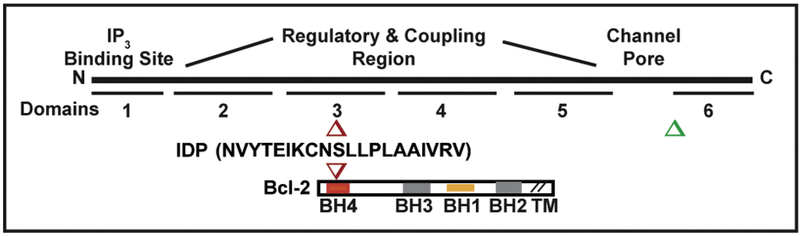
Early analysis of Bcl-2-IP3R interaction focused on what appeared to be a single interaction site involving BH4 domain interaction with IP3R domain 3, located in the regulatory and coupling region (Figure 3). Recent studies have expanded understanding of the Bcl-2 interaction to include a region in its C-terminal domain (C-term Dom, a.a. 2512–2749), which is in close proximity of the channel pore. This region was previously identified as critical for the Ca2+ regulatory functions of the Bcl-2 homologue Bcl-xl (129). This is particularly enlightening as earlier studies using synthetic peptides targeting the BH4 domain of Bcl-2 (BH4-Bcl-2), revealed this domain is necessary and sufficient to bind to the IP3R and to suppress its activity (117, 118, 119). Nevertheless, the relatively low affinity of inhibition by the BH4 domain (measured in vitro IC50=30μM) (118, Monaco, 2012 #5128) does not appear to explain the potent inhibitory effect of Bcl-2 full-length protein under physiological conditions. Using genetic and pharmacological approaches, Ivanova et al (22) implicate the C-terminal IP3R1 domain in Bcl-2 binding and cell death regulation. Furthermore, they demonstrated a direct interaction between a peptide corresponding to the transmembrane domain of Bcl-2 (TMD-Bcl-2) and the purified C-terminal fragment of IP3R1. This peptide was able to suppress IP3-induced Ca2+ release (IICR) when applied at high concentrations. These results suggest that the C-terminal region of Bcl-2 not only serves as an anchor for tethering Bcl-2 to membranes, but also an important functional regulator of IP3R activity.
Figure 3. Bcl-2-IP3R interacting sites and derivation of BIRD-2.
The BH4 domain of Bcl-2 binds to IP3R domain 3 (red) and a region near domain 6 (green). BIRD-2 is a 20 amino acid synthetic peptide based on a coiled coil region in IP3R domain 3, with a DD/AA mutation introduced to block a protease cleavage site. BIRD-2 binds to the BH4 domain of Bcl-2 and functions as a decoy peptide, inhibiting Bcl-2-IP3R interaction and inducing cell death in Bcl-2-positive malignancies.
A recent report also indicates that Bcl-2 may not interact with IP3Rs across all circumstances or cell types (130), raising the important question of what regulates the Bcl-2-IP3R interaction in different types of cells. Also, it has been suggested that Bcl-2 may also regulate ER Ca2+ release through additional mechanisms besides its interaction with the IP3R. One proposed mechanism involves Bcl-2 interaction with Sarco/Endoplasmic Reticulum-associated Ca2+-ATPases (SERCA). These proteins pump Ca2+ ions from the cytoplasm into the ER lumen, maintaining large ER luminal Ca2+ stores. This steep Ca2+ concentration gradient from ER lumen to cytoplasm propels Ca2+ movement upon IP3R channel opening, leading to pro-apoptotic calcium spikes. Bcl-2’s interaction with SERCA attenuates ER Ca2+ filling, indirectly diminishing IP3R-mediated Ca2+ release and Ca2+-mediated apoptosis (131). Interestingly, Bcl-2 alone completely inhibits SERCA in vitro, which can trigger apoptosis by increasing cytosolic Ca2+ and inducing store-operated Ca2+ entry (132). Recent findings indicate that HSP70 regulates the Bcl-2-SERCA interaction, maintaining SERCA in an active state that may be essential for apoptosis regulation (132). Accordingly, an earlier report of the Bcl-2-SERCA interaction finds that Bcl-2 increases the ER Ca2+ pool, promoting the high luminal Ca2+ concentration required for normal cell function (133).
Recent work provides insight into how Bcl-2-IP3R interaction controls IP3R-mediated Ca2+ elevation, preventing Ca2+-induced cell death. Oakes et al (121) show that Bcl-2 regulates IP3R phosphorylation at serine 1755 within the regulatory and coupling domain of the IP3R. Protein kinase A (PKA) phosphorylates serine 1755 and serine 1589, increasing IP3-mediated channel opening and Ca2+ release (134, 135, 136). We previously reported that Bcl-2 decreases IP3R phosphorylation, although a specific phosphorylation site was not identified (85). In further work, we find that Bcl-2 inhibits IP3R phosphorylation at serine 1755, correlating with its inhibition of anti-CD3-induced Ca2+ elevation.
PKA-mediated protein phosphorylation is typically regulated by PP1α (137), and an IP3R-PP1α complex has been implicated in Bcl-2-mediated suppression of ER Ca2+ release in breast cancer cells (138). Bcl-2 also binds CaN (139) and increases the association of CaN with IP3Rs (140, 141, 120); this has a neuroprotective effect in primary neuronal cells (141). However, Bultynck et al predicted that CaN’s IP3R effects are indirect and may be secondary to PP1α acting with DARPP-32 (dopamine- and c-AMP-regulated phosphoprotein of 32 kDa) (142). DARPP-32 is a PKA-activated and CaN-deactivated PP1α inhibitor studied extensively in the brain (143). Tang et al (137) discovered a direct association between PP1α and IP3R-1 and established that the association with PP1α reverses PKA-mediated IP3R-1 phosphorylation. AKAP9, a multifunctional PKA anchoring protein, docks both PKA and PP1α to IP3R-1 (144). In experiments with medium spiny neurons from DARPP-32 knock-out mice, DARPP-32 was shown to regulate dopamine-induced Ca2+ oscillations (145). However, very little is known about the role of DARPP-32 in peripheral tissues, including lymphocytes, although DARPP-32 has been shown to increase the phosphorylation and activity of various ion channels (146). We report that Bcl-2 docks DARPP-32 and CaN in a complex on the IP3R, preventing exaggerated IP3R-mediated Ca2+ elevation in T-cells by decreasing PKA-mediated IP3R phosphorylation (147).
A very interesting report for the first time has implicated Bcl-2 in regulating store-operated Ca2+ channels, involving the BH1 domain of Bcl-2 (148). This work indicates that a triplicate amino acid substitution in the BH1 domain creates a mutant form of Bcl-2 that enhances thapsigargin-induced Ca2+ elevation, whereas these investigators find wild type Bcl-2 dampens thapsigargin-induced Ca2+ elevation. Their evidence implicates an effect of the mutant Bcl-2 on store-operated Ca2+, producing massive Ca2+ influx contributing to caspase activation and apoptosis.
5. Cancer cell exploitation of Bcl-2-IP3R interaction and the strategy of targeting this interaction to treat cancer.
Bcl-2-expression levels are elevated in many different malignancies (http://broadinstitute.org/ccle/home). Bcl-2 levels are invariably elevated in chronic lymphocytic leukemia (CLL) and follicular lymphoma (FL) (15, 13). Comparable levels of Bcl-2 are present in multiple myeloma (MM) (149). Bcl-2 levels are also elevated in acute myelogenous leukemia (AML) (150) and small cell lung cancer (SCLC) (151), in which novel therapeutic approaches are desperately needed. Cancer cells exploit Bcl-2 to stay alive in the stressful microenvironment and resist immunotherapy, chemotherapy and radiation therapy. Therefore, agents that inhibit Bcl-2 have the potential of dramatically improving outcomes in multiple types of cancer.
In support of targeting Bcl-2 for cancer treatment there is a distinct difference between normal cells and cancer cells in terms of Bcl-2 function. As summarized earlier in this review, Bcl-2 expression levels are carefully regulated in normal cells, such as in T-cells, where Bcl-2 levels increase during the immune response to prevent inadvertent cell death in response to proliferative Ca2+ signals, and then decline once the immune response wanes. The difference in Bcl-2 over-expressing cancer cells is that the Bcl-2 level does not decline and remains elevated over time, preventing normal, physiological cell death responses. Thus, Bcl-2 preserves Ca2+ homeostasis in normal cells by preventing excessive Ca2+ elevation, but cancer cells exploit Bcl-2 to stay alive under adverse growth conditions that would generally lead to cell death.
The Bcl-2 protein is expressed at abnormally high levels in a wide variety of cancers. The classic example of this is follicular lymphoma, where Bcl-2 levels are abnormally elevated by a t(14;18) chromosomal translocation (77, 78, 79). Bcl-2 elevation involves other mechanisms in a variety of cancers, and in many types of cancer the mechanism of Bcl-2 elevation may not be recognized (152). For example, Bcl-2 is elevated in CLL due to loss of microRNAs that normally repress Bcl-2 gene expression (153). Because of its role in preventing cell death, Bcl-2 has become a major therapeutic target in cancer.
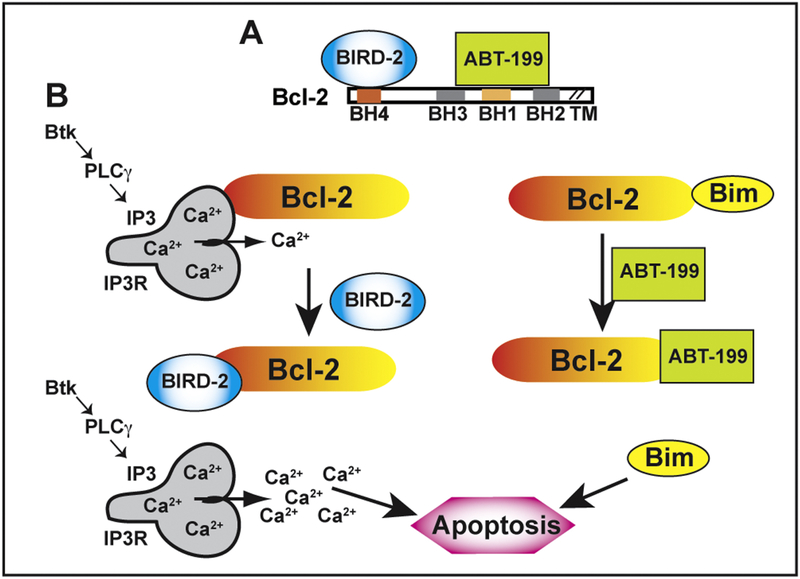
Bcl-2 promotes cell survival by two major mechanisms, illustrated in Figure 4. In mitochondria, Bcl-2 preserves cell survival by binding and inhibiting the function of pro-apoptotic proteins, illustrated by Bim in the figure. This function of Bcl-2 is mediated by its hydrophobic cleft, involving the BH3 domain which is responsible for binding pro-apoptotic proteins. On the ER, Bcl-2 promotes survival through its interaction with IP3Rs, mediated by its BH4 domain, preventing excessive Ca2+ elevation.
Figure 4. Targeting Bcl-2’s dual anti-apoptotic mechanisms.
(A) The decoy peptide BIRD-2 binds to the BH4 domain of Bcl-2, whereas the small molecule ABT-199/Venetoclax binds to the BH3 domain of Bcl-2. (B) Left: Bcl-2 binds via its BH4 domain to IP3Rs, preventing excessive IP3R-mediated Ca2+ elevation, thereby inhibiting Ca2+ induced apoptosis. BIRD-2 binds to the BH4 domain of Bcl-2, disrupting Bcl-2-IP3R interaction and thus inducing high amplitude Ca2+ elevation that triggers apoptosis. Right: The BH3 region of Bcl-2 binds and sequesters the pro-apoptotic protein Bim, preventing Bim-mediated apoptosis. ABT-199 (Venetoclax) displaces Bim, thus triggering Bim-mediated apoptosis.
Small molecules that bind to the hydrophobic cleft formed by the BH1–3 domains of Bcl-2 displace pro-apoptotic proteins from Bcl-2 and thus trigger apoptosis (154, 155, 156, 157, 158, 18). These molecules, including the Bcl-2 selective and platelet-sparing ABT-199 (Venetoclax), are already in clinical use to treat CLL, and undergoing clinical trials to test efficacy in other types of cancer (154, 155, 156, 157, 158, 18, 159, 3). For an in-depth understanding of how Bcl-2 interacts with its pro-apoptotic relatives and preserves outer mitochondrial membrane integrity, the reader is referred to a recent review by Llambi et al (160).
However, these agents are only effective in types of cancer that have elevated levels of pro-apoptotic proteins such as Bim, rendering these cancer cells addicted to Bcl-2 interaction with pro-apoptotic proteins for their survival. Another limitation is that ABT-199/Venetoclax responsiveness varies among cancers (161). For example, CLL is highly responsive to ABT-199, although resistance is reported (162). On the other hand, ABT-199/Venetoclax response rates are 28% in Diffuse Large B-cell Lymphoma (DLBCL) and 31% in Follicular Lymphoma (FL) (163). Although Bcl-2 is commonly expressed in multiple myeloma at levels comparable to CLL and FL (149), responses to ABT-199/Venetoclax are limited to a small subset of myeloma lines (164) and patients with the CCND1/IGH translocation(164, 163). Also, AML is a Bcl-2-positive malignancy, but ABT-199 is effective in only a fraction of AML patients (165, 150). Main reasons for ABT-199/Venetoclax resistance include: (i) low expression levels pro-apoptotic proteins so the cancer cells are not primed to respond to ABT-199/Venetoclax (161); (ii) expression of Mcl-1 or Bcl-xl, which bind and inhibit pro-apoptotic proteins released from Bcl-2 by ABT-199 (166).
In collaboration with Jan Parys and Geert Bultynck in Belgium, we developed a synthetic peptide corresponding to the IP3R binding site for Bcl-2 (117, 118). This IP3RDerived Peptide (IDP), more recently termed BIRD2 (Bcl-2 IP3R Disruptor-2), inhibits the Bcl-2-IP3R interaction by binding to the BH4 domain of Bcl-2, destabilizing Bcl-2’s alpha-helical structure (117, 118, 119). By inhibiting Bcl-2-IP3R interaction, BIRD2 attenuates Bcl-2’s control over IP3R-mediated Ca2+ elevation, induces marked Ca2+ elevation and Ca2+-mediated apoptosis in primary human CLL cells, with minimal if any effect on the viability of normal human lymphocytes (19). BIRD-2 also induces apoptosis in diffuse large B-cell lymphoma lines (DLBCL) (119, 167) and in multiple myeloma cells, both in vitro and in an in vivo xenograft mouse model (20). BIRD-2 also induces apoptosis in small cell lung cancer, a Bcl-2 positive solid tumor (168), and in ovarian cancer (169).
In each of the known ABT-199/Venetoclax resistance mechanisms, agents that induce apoptosis by disrupting Bcl-2-IP3R interaction are expected to have value. First, these agents may increase the sensitivity of unprimed cancer cells to ABT-199/Venetoclax by increasing Bim expression levels. As proof of principle, we have reported that BIRD-2-induced Ca2+ elevation increases Bim levels in CLL and multiple myeloma cells (170, 20). Second, in cells resistant to ABT-199/Venetoclax due to increased expression of Mcl-1 and Bcl-xl, or decreased levels of Bax and Bak, agents that disrupt Bcl-2-IP3R interaction are still expected to induce Ca2+-mediated apoptosis. This is mainly because Ca2+ elevation induces apoptosis by multiple mechanisms not employed by ABT-199, including by activating Ca2+-sensitive proteases (calpains) that trigger caspase-independent apoptosis. As proof of principle, we have demonstrated that BIRD-2 induces this apoptotic mechanism in ABT compound-resistant multiple myeloma and SCLC cells (20, 168). Also, ABT-199 and BIRD-2 demonstrate synergy inducing cell death in cancer cells (20, 168). BIRD-2 synergizes with ABT-199 by inducing Ca2+-mediated elevation of the pro-apoptotic protein Bim (20). Thus, agents that target Bcl-2-IP3R interaction may not only be useful as single agents, but also be useful in combination with ABT- 199, perhaps facilitating ABT-199/Venetoclax use at lower, less toxic doses. Also, ABT199-/Venetoclax does not inhibit Bcl-2-IP3R interaction and does not trigger Ca2+ elevation, confirming that ABT-199 and BIRD-2 work by separate mechanisms (171, 22). Moreover, ABT199-/Venetoclax and BIRD-2 demonstrate a reciprocal relationship in terms of their ability to induce cell death (20, 172), confirming they work by independent mechanisms.
The utility of targeting the Bcl-2-IP3R interaction may be dependent upon a number of factors. Perhaps the most obvious of these factors is the level of Bcl-2 in different types of cancer cells and their reliance on Bcl-2 for survival (173). Although Bcl-2 is typically overexpressed in lymphoid malignancies, other Bcl-2 family members are expressed in non-lymphoid malignancies, including Mcl-1. Individual pro-apoptotic family members may also differ in their interaction with IP3Rs. For example, the BH4 domain of Bcl-2 interacts with IP3Rs, but the BH4 domain of Bcl-xL does not (119). As such, BIRD-2 is likely to be more effective in Bcl-2 positive malignancies than in Bcl-xl positive malignancies. Additionally, the anti-apoptotic Bcl-2 family member Mcl-1 contributes to apoptosis inhibition in lymphoid malignancies and is reported to interact with IP3Rs (129); we do not know where Mcl-1 interacts on the IP3R and if it would be inhibited by BIRD-2. Another factor is the IP3R isoform itself. There are three IP3R isoforms, which vary in both tissue distribution and in sensitivity to Ca2+ and IP3 regulation (174). A recent study discovered that the sensitivity of lymphoma cells to BIRD-2-induced apoptosis correlated best with IP3R isoform 2 (167), whereas numerous studies in other cell types demonstrated a stronger correlation with IP3R isoform 3 (54).
In B-cell malignancies like CLL (Figure 1), constitutive B-cell receptor signaling drives cell proliferation and survival via Bruton’s tyrosine kinase (BTK) (175, 176, 177). The immediate downstream target of BTK is phospholipase Cγ. (PLCγ), which catalyses the synthesis of IP3 from PIP2, provoking IP3R channel opening and Ca2+ release (17, 177). Recent findings indicate that BIRD-2-triggered Ca2+ rises and cell death are critically dependent on the increase in basal IP3 signaling that occurs downstream of the B-cell receptor in B-cell malignancies (178). As such, inhibition of PLC activity or buffering IP3 reduces BIRD-2-induced cell death in a variety of DLBCL and primary CLL cells, both in unsupported short-term cultures and in prolonged co-cultures with CD40L-expressing fibroblasts. Therefore, we postulate that the pro-apoptotic Ca2+ elevation we observe in BIRD-2-treated CLL cells is driven by constitutive signaling via BTK. Moreover, we find that BIRD-2 induces cell death in Ibrutinib-resistant cells, suggesting it may have therapeutic value in patients who relapse while taking ibrutinib (20).
Recently, a small molecule antagonist to the BH4 domain of Bcl-2, BDA-366, has been developed and demonstrated to have activity in lung cancer and multiple myeloma models (21, 179). BDA-366 binds the BH4 region of Bcl-2 with high affinity and selectivity. This development is very interesting as it suggests a different mode of action than BIRD-2, even though like BIRD-2 it was reported to elevate cytoplasmic Ca2+ levels. The proposed mechanism of action is that BDA-366 induces a conformational change in Bcl-2 that abrogates its anti-apoptotic function by converting Bcl-2 from a survival molecule to a cell death inducer. The authors find that BDA-366 suppresses growth of lung cancer xenografts derived from cell lines and patients without significant normal tissue toxicity at the effective doses (21, 179). Although the findings of this report are most intriguing, BDA-366 is toxic to a wide range of cell types, apparently regardless of Bcl-2 expression level, raising the possibility that Bcl-2 may not be the only target.
6. Summary and Future Directions
An inherent weakness in virtually any review is the potential exclusion of topics as important or even more important than the one covered. This certainly is the case with regard to the role of mitochondria in Ca2+ function, including metabolism and both cell survival and cell death. Indeed, one of the most important functions of IP3R-mediated Ca2+ signaling is in promoting cell survival by increasing mitochondrial Ca2+ uptake and metabolism. The close proximity of ER-localized IP3Rs to mitochondria facilitates Ca2+ transfer from the ER lumen into mitochondria (180, 181, 182). This promotes mitochondrial ATP production by activating multiple Ca2+-sensitive enzymes in the citric acid cycle and catalyzing the conversion of pyruvate to acetyl-CoA (183, 184). Insufficient ER-mitochondrial Ca2+ transfer results in autophagy, a survival mechanism through which cells digest intracellular components in order to produce ATP (185); conversely, excessive transfer of Ca2+ to mitochondria induces Ca2+ overload, resulting in loss of membrane potential, cytochrome c release and apoptosis (59, 60, 61).
Particularly exciting, novel concepts have been introduced in the field of Ca2+ signaling, deserving careful attention as these concepts are likely to guide future directions of research. One of these relates directly to cancer metabolism and is based on the important discovery that mitochondrial bioenergetics is positively regulated by constitutive, low level IP3R Ca2+ transfer from the ER to mitochondria (185, 186, 7). Another concept has to do with how we view anti-apoptotic proteins such as Bcl-2. The discerning reader with surely note that in this review we focus in a singular manner on the Bcl-2 protein, in isolation with only brief mention of other anti-apoptotic family members. A recent paper by Carrington et al (187) has analyzed the requirements of multiple immune cell subsets (e.g., naïve T cells require Bcl-2, regulatory T cells require Mcl-1), supporting a novel model in which survival is determined by participation of multiple anti-apoptotic proteins rather than a single anti-apoptotic protein. It will be important to determine if a similar model fits cancer cells. For example, does survival of a cancer cell depend just on Bcl-2, or does it depend on the participation of multiple anti-apoptotic family members. This may be important in terms of efforts to target Bcl-2 for cancer treatment. Perhaps it does and multiple anti-apoptotic proteins may need to be targeted to achieve optimal effect.
While the current review is focused on Bcl-2’s interaction with IP3R’s and its regulation of intracellular Ca2+ signaling, there are many other important dimensions of Bcl-2 protein family function, including its phosphorylation. Particularly interesting is the widespread distribution of Bcl-2 family members, beyond the ER and mitochondria. As recently reviewed elsewhere (188), these include the Golgi apparatus, nucleus and peroxisomes. The consequences broaden the Bcl-2 protein family impact to include not only Ca2+ homeostasis, but also cell cycle control and cell migration, topics of great relevance to the understanding and treatment of cancer.
Finally, the work summarized in the present review may provide a modest lesson for those interested in development of novel cancer treatments. Many in the current era seem to believe that understanding basic mechanism is an absolute pre-requisite for novel therapeutic development. It is therefore humbling to realize that the mechanism of corticosteroid-induced cell death is only partially understood, yet this hormone has produced many cancer cures over the past fifty years and still contributes to cures today.
Highlights.
Bcl-2 is an anti-apoptotic protein elevated in many different types of cancer.
Bcl-2 interacts with inositol 1,4,5-trisphosphate receptors, regulating calcium release from the endoplasmic reticulum and inhibiting apoptosis.
Bcl-2 interaction with inositol 1,4,5-trisphosphate receptors and control over calcium release can be blocked using synthetic peptides.
Blocking Bcl-2 interaction with inositol 1,4,5-trisphosphate receptors is proposed as a novel therapeutic target for Bcl-2-positive malignancies.
Acknowledgements
This work was supported in part by the August J. and Karen A. Coppola Charitable Trust, the Charles S. Britton II Endowed Chair in Hematology/Oncology and the Harrington Discovery Institute of University Hospitals Cleveland Medical Center and Case Western Reserve University. Also, the author was supported by National Institutes of Health grant 1R21CA186912–01A1 and American Society of Hematology Bridge Grant
Footnotes
Conflict of Interest
There is no conflict of interest, either financially or otherwise. There are not duplicate articles submitted or published elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mikoshiba K, Role of IP3 receptor signaling in cell functions and diseases, Advances in biological regulation, 57 (2015) 217–227. [DOI] [PubMed] [Google Scholar]
- 2.Orrenius S, Gogvadze V, Zhivotovsky B, Calcium and mitochondria in the regulation of cell death, Biochemical and biophysical research communications, 460 (2015) 72–81. [DOI] [PubMed] [Google Scholar]
- 3.Correia C, Lee SH, Meng XW, Vincelette ND, Knorr KL, Ding H, Nowakowski GS, Dai H, Kaufmann SH, Emerging understanding of Bcl-2 biology: Implications for neoplastic progression and treatment, Biochimica et biophysica acta, 1853 (2015) 1658–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak DO, Foskett JK, Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: A single-channel point of view, Cell calcium, 58 (2015) 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge MJ, The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease, Physiological reviews, 96 (2016) 1261–1296. [DOI] [PubMed] [Google Scholar]
- 6.Delbridge AR, Grabow S, Strasser A, Vaux DL, Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies, Nature reviews. Cancer, 16 (2016) 99–109. [DOI] [PubMed] [Google Scholar]
- 7.Lovy A, Foskett JK, Cardenas C, InsP3R, the calcium whisperer: Maintaining mitochondrial function in cancer, Mol Cell Oncol, 3 (2016) e1185563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raffaello A, Mammucari C, Gherardi G, Rizzuto R, Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes, Trends Biochem Sci, 41 (2016) 1035–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ando H, Kawaai K, Bonneau B, Mikoshiba K, Remodeling of Ca(2+) signaling in cancer: Regulation of inositol 1,4,5-trisphosphate receptors through oncogenes and tumor suppressors, Advances in biological regulation, (2017). [DOI] [PubMed] [Google Scholar]
- 10.Vervloessem T, Kerkhofs M, La Rovere RM, Sneyers F, Parys JB, Bultynck G, Bcl-2 inhibitors as anti-cancer therapeutics: The impact of and on calcium signaling, Cell calcium, 70 (2017) 102–116. [DOI] [PubMed] [Google Scholar]
- 11.Yap JL, Chen L, Lanning ME, Fletcher S, Expanding the Cancer Arsenal with Targeted Therapies: Disarmament of the Antiapoptotic Bcl-2 Proteins by Small Molecules, J Med Chem, 60 (2017) 821–838. [DOI] [PubMed] [Google Scholar]
- 12.Maji S, Panda S, Samal SK, Shriwas O, Rath R, Pellecchia M, Emdad L, Das SK, Fisher PB, Dash R, Bcl-2 Antiapoptotic Family Proteins and Chemoresistance in Cancer, Adv Cancer Res, 137 (2018) 37–75. [DOI] [PubMed] [Google Scholar]
- 13.Hallek M, Signaling the end of chronic lymphocytic leukemia: new frontline treatment strategies, Hematology Am Soc Hematol Educ Program, 2013 (2013) 138–150. [DOI] [PubMed] [Google Scholar]
- 14.Young RM, Staudt LM, Targeting pathological B cell receptor signalling in lymphoid malignancies, Nat Rev Drug Discov, 12 (2013) 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones JA, Byrd JC, How will B-cell receptor targeted therapies change future CLL therapy?, Blood, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy JJ, Hamdy A, Johnson AJ, Byrd JC, Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765, Blood, 117 (2011) 6287–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendriks RW, Yuvaraj S, Kil LP, Targeting Bruton’s tyrosine kinase in B cell malignancies, Nature reviews. Cancer, 14 (2014) 219–232. [DOI] [PubMed] [Google Scholar]
- 18.Vandenberg CJ, Cory S, ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia, Blood, 121 (2013) 2285–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong F, Harr MW, Bultynck G, Monaco G, Parys JB, De Smedt H, Rong YP, Molitoris JK, Lam M, Ryder C, Matsuyama S, Distelhorst CW, Induction of Ca(2+)-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction, Blood, 117 (2011) 2924–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavik AR, Zhong F, Chang MJ, Greenberg E, Choudhary Y, Smith MR, McColl KS, Pink J, Reu FJ, Matsuyama S, Distelhorst CW, A synthetic peptide targeting the BH4 domain of Bcl-2 induces apoptosis in multiple myeloma and follicular lymphoma cells alone or in combination with agents targeting the BH3-binding pocket of Bcl-2, Oncotarget, 6 (2015) 27388–27402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han B, Park D, Li R, Xie M, Owonikoko TK, Zhang G, Sica GL, Ding C, Zhou J, Magis AT, Chen ZG, Shin DM, Ramalingam SS, Khuri FR, Curran WJ, Deng X, Small-Molecule Bcl2 BH4 Antagonist for Lung Cancer Therapy, Cancer cell, 27 (2015) 852–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanova H, Ritaine A, Wagner L, Luyten T, Shapovalov G, Welkenhuyzen K, Seitaj B, Monaco G, De Smedt H, Prevarskaya N, Yule DI, Parys JB, Bultynck G, The trans-membrane domain of Bcl-2alpha, but not its hydrophobic cleft, is a critical determinant for efficient IP3 receptor inhibition, Oncotarget, 7 (2016) 55704–55720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougherty TF, White A, Effect of pituitary adrenotropic hormone on lymphoid tissue., Proc. Soc. Exp. Biol. Med, 53 (1943) 132–133. [Google Scholar]
- 24.Heilman RR, Kendall EC, The influence of 11-dehydro-17-hydroxy-corticosterone (compound E) on the growth of a malignant tumor in the mouse., Endocrinology, 34 (1944) 416–420. [Google Scholar]
- 25.Pearson OH, Eliel LP, Rawson RW, Dobriner K, Rhoads CP, ACTH- and cortisone-induced regression of lymphoid tumors in man: A preliminary report, Cancer, 2 (1949) 943–945. [DOI] [PubMed] [Google Scholar]
- 26.Pearson OH, Eliel LP, Use of pituitary adrenocorticotropic hormone (ACTH) and cortisone in lymphomas and leukemias, J. Am. Med. Assoc, 144 (1950) 1349–1353. [DOI] [PubMed] [Google Scholar]
- 27.Vietti TJ, Sullivan MP, Berry DH, Haddy TB, Haggard ME, Blattner RJ, The response of acute childhood leukemia to an initial and a second course of prednisone, Pediatrics, 66 (1965) 18–26. [DOI] [PubMed] [Google Scholar]
- 28.Gaynon PS, Carrel AL, Glucocorticosteroid therapy in childhood acute lymphoblastic leukemia, Adv Exp Med Biol, 457 (1999) 593–605. [DOI] [PubMed] [Google Scholar]
- 29.Inaba H, Pui CH, Glucocorticoid use in acute lymphoblastic leukaemia, Lancet Oncol, 11 (2010) 1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teuffel O, Kuster SP, Hunger SP, Conter V, Hitzler J, Ethier MC, Shah PS, Beyene J, Sung L, Dexamethasone versus prednisone for induction therapy in childhood acute lymphoblastic leukemia: a systematic review and meta-analysis, Leukemia, 25 (2011) 1232–1238. [DOI] [PubMed] [Google Scholar]
- 31.Alexanian R, Dimopoulos MA, Delasalle K, Barlogie B, Primary dexamethasone treatment of multiple myeloma, Blood, 80 (1992) 887–890. [PubMed] [Google Scholar]
- 32.Hideshima T, Raje N, Richardson PG, Anderson KC, A review of lenalidomide in combination with dexamethasone for the treatment of multiple myeloma, Ther Clin Risk Manag, 4 (2008) 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buyukkaramikli NC, de Groot S, Fayter D, Wolff R, Armstrong N, Stirk L, Worthy G, Albuquerque de Almeida F, Kleijnen J, Al MJ, Pomalidomide with Dexamethasone for Treating Relapsed and Refractory Multiple Myeloma Previously Treated with Lenalidomide and Bortezomib: An Evidence Review Group Perspective of an NICE Single Technology Appraisal, Pharmacoeconomics, 36 (2018) 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowen DA, Call TG, Jenkins GD, Zent CS, Schwager SM, Van Dyke DL, Jelinek DF, Kay NE, Shanafelt TD, Methylprednisolone-rituximab is an effective salvage therapy for patients with relapsed chronic lymphocytic leukemia including those with unfavorable cytogenetic features., Leukemia & lymphoma, 48 (2007) 2412–2417. [DOI] [PubMed] [Google Scholar]
- 35.Dungarwalla M, Evans SO, Riley U, Catovsky D, Dearden CE, Matutes E, High dose methylprednisolone and rituximab is an effective therapy in advanced refractory chronic lymphocytic leukemia resistant to fludarabine therapy., Haematologica, 93 (2008) 475–477. [DOI] [PubMed] [Google Scholar]
- 36.Castro JE, James DF, Sandoval-Sus JD, Jain S, Bole J, Rassenti L, Kipps TJ, Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia, Leukemia, 23 (2009) 1779–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiser N, Edelman IS, Calcium dependence of glucocorticoid-induced lymphocytolysis., Proc. Natl. Acad. Sci, 74 (1977) 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaiser N, Edelman IS, Further studies on the role of calcium in glucocorticoid-induced lymphocytolysis., Endocrinology, 103 (1978) 936–942. [DOI] [PubMed] [Google Scholar]
- 39.Cohen JJ, Duke RC, Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death., J. Immunol, 132 (1984) 38–42. [PubMed] [Google Scholar]
- 40.McConkey DJ, Nicotera P, Hartzell P, Bellomo G, Wyllie AH, Orrenius S, Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration, Arch Biochem Biophys, 269 (1989) 365–370. [DOI] [PubMed] [Google Scholar]
- 41.Wyllie AH, Kerr JF, Currie AR, Cell death: the significance of apoptosis, Int Rev Cytol, 68 (1980) 251–306. [DOI] [PubMed] [Google Scholar]
- 42.Wyllie AH, Morris RG, Smith AL, Dunlop D, Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis., J. Pathol, 142 (1984) 67–77. [DOI] [PubMed] [Google Scholar]
- 43.Wyllie AH, Glucocorticoid-induced thymocyte apoptosis is associated with endonuclease activation., Nature, 284 (1980) 555–556. [DOI] [PubMed] [Google Scholar]
- 44.Alnemri ES, Litwack G, Glucocorticoid-induced lymphocytolysis is not mediated by an induced endonuclease., J. Biol. Chem, 264 (1989) 4104–4111. [PubMed] [Google Scholar]
- 45.Vedeckis WV, Bradshaw HDJ, DNA fragmentation in S49 lymphoma cells killed with glucocorticoids and other agents, Mol. Cell. Endocrinol, 30 (1983) 215–227. [DOI] [PubMed] [Google Scholar]
- 46.Gaido ML, Cidlowski JA, Identification, purification, and characterization of a calcium-dependent endonuclease (NUC18) from apoptotic rat thymocytes, J. Biol. Chem, 266 (1991) 18580–18585. [PubMed] [Google Scholar]
- 47.Ellis RE, Yuan J, Horvitz HR, Mechanisms and functions of cell death., Annu. Rev. Cell Biol, 7 (1991) 663–698. [DOI] [PubMed] [Google Scholar]
- 48.Henkart PA, ICE family proteases: Mediators of all apoptotic cell death?, Cell, 4 (1996) 195–201. [DOI] [PubMed] [Google Scholar]
- 49.Liu Z-G, Smith SW, McLaughlin KA, Schwartz LM, Osborne BA, Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77, Nature, 367 (1994) 281–284. [DOI] [PubMed] [Google Scholar]
- 50.Zacharchuk CM, Mercep M, Chakraborti PK, Simons SS, Ashwell JD, Programmed T lymphocyte death: Cell activation- and steroid-induced pathways are mutually antagonistic., J. Immunol, 145 (1990) 4037–4045. [PubMed] [Google Scholar]
- 51.Zhao Y, Tozawa Y, Iseki R, Mukai M, Iwata M, Calcineurin activation protects T cells from glucocorticoid-induced apoptosis, J. Immunol, 154 (1995) 6346–6354. [PubMed] [Google Scholar]
- 52.Vito P, Lacana E, D’Adamio LD, Interfering with apoptosis: Ca2+-binding protein ALG-2 and alzheimer’s disease gene ALG-3., Science, 271 (1996) 521–525. [DOI] [PubMed] [Google Scholar]
- 53.Lam M, Dubyak G, Distelhorst CW, Effect of glucocorticosteroid treatment on intracellular calcium homeostasis in mouse lymphoma cells., Mol. Endocrinol, 7 (1993) 686–693. [DOI] [PubMed] [Google Scholar]
- 54.Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li S-H, Ross CA, Snyder SH, Lymphocyte apoptosis: Mediation by increased type 3 inositol 1,4,5,-trisphosphate receptor., Science, 273 (1996) 503–507. [DOI] [PubMed] [Google Scholar]
- 55.Jayaraman T, Marks AR, T cells deficient in inositol 1,4,5-trisphosphate receptor are resistant to apoptosis, Mol. Cell Biol, 17 (1997) 3005–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jayaraman T, Marks AR, Calcineurin is downstream of the inositol 1,4,5-trisphosphate receptor in the apoptotic and cell growth pathways, The Journal of biological chemistry, 275 (2000) 6417–6420. [DOI] [PubMed] [Google Scholar]
- 57.Kaufman RJ, Malhotra JD, Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics, Biochimica et biophysica acta, 1843 (2014) 2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joseph SK, Hajnoczky G, IP3 receptors in cell survival and apoptosis: Ca2+ release and beyond., Apoptosis : an international journal on programmed cell death, 12 (2007) 951–968. [DOI] [PubMed] [Google Scholar]
- 59.Szalai G, Krishnamurthy R, Hajnoczky G, Apoptosis driven by IP(3)-linked mitochondrial calcium signals, The EMBO journal, 18 (1999) 6349–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hajnoczky G, Davies E, Madesh M, Calcium signaling and apoptosis, Biochemical and biophysical research communications, 304 (2003) 445–454. [DOI] [PubMed] [Google Scholar]
- 61.Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha RS, Yi M, Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis., Cell calcium, 40 (2006) 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhivotovsky B, Orrenius S, Calcium and cell death mechanisms: a perspective from the cell death community, Cell calcium, 50 (2011) 211–221. [DOI] [PubMed] [Google Scholar]
- 63.Mackrill J, Protein-protein interactions in intracellular Ca2+ release channel function., The Biochemical journal, 337 (1999) 345–361. [PMC free article] [PubMed] [Google Scholar]
- 64.Roderick HL, Bootman MD, Bi-directional signalling from the InsP3 receptor: regulation by calcium and accessory factors., Biochem Soc Transactions, 31 (2003) 950–953. [DOI] [PubMed] [Google Scholar]
- 65.Choe CU, Ehrlich BE, The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork, Sci STKE, 2006 (2006) re15. [DOI] [PubMed] [Google Scholar]
- 66.Foskett JK, White C, Cheung KH, Mak DO, Inositol trisphosphate receptor Ca2+ release channels, Physiological reviews, 87 (2007) 593–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mikoshiba K, IP3 receptor/Ca2+ channel: from discovery to new signaling concepts., Journal of neurochemistry, 102 (2007) 1426–1446. [DOI] [PubMed] [Google Scholar]
- 68.Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G, Parys JB, Regulation of inositol 1,4,5-trisphosphate-induced Ca(2+) release by reversible phosphorylation and dephosphorylation., Biochimica et biophysica acta, 1793 (2009) 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parys JB, The IP3 receptor as a hub for Bcl-2 family proteins in cell death control and beyond, Sci Signal, 7 (2014) pe4. [DOI] [PubMed] [Google Scholar]
- 70.Prole DL, Taylor CW, Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs, The Journal of physiology, 594 (2016) 2849–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patterson RL, Boehning D, Snyder SH, Inositol 1,4,5-trisphosphate receptors as signal integrators, Annual review of biochemistry, 73 (2004) 437–465. [DOI] [PubMed] [Google Scholar]
- 72.Parys JB, De Smedt H, Inositol 1,4,5-trisphosphate and its receptors, Adv Exp Med Biol, 740 (2012) 255–279. [DOI] [PubMed] [Google Scholar]
- 73.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K, Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400, Nature, 342 (1989) 32–38. [DOI] [PubMed] [Google Scholar]
- 74.Konieczny V, Keebler MV, Taylor CW, Spatial organization of intracellular Ca2+ signals, Semin Cell Dev Biol, 23 (2012) 172–180. [DOI] [PubMed] [Google Scholar]
- 75.Akl H, Bultynck G, Altered Ca(2+) signaling in cancer cells: proto-oncogenes and tumor suppressors targeting IP3 receptors, Biochimica et biophysica acta, 1835 (2013) 180–193. [DOI] [PubMed] [Google Scholar]
- 76.Bittremieux M, Parys JB, Pinton P, Bultynck G, ER functions of oncogenes and tumor suppressors: Modulators of intracellular Ca(2+) signaling, Biochimica et biophysica acta, 1863 (2016) 1364–1378. [DOI] [PubMed] [Google Scholar]
- 77.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM, Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation., Science, 226 (1984) 1097–1099. [DOI] [PubMed] [Google Scholar]
- 78.Bakshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, Korsmeyer SJ, Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18., Cell, 41 (1985) 899–906. [DOI] [PubMed] [Google Scholar]
- 79.Cleary ML, Smith SD, Sklar J, Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation., Cell, 47 (1986) 19–28. [DOI] [PubMed] [Google Scholar]
- 80.Tsujimoto Y, Croce CM, Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma., Proc. Natl. Acad. Sci. USA, 83 (1986) 5214–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andjelic S, Jain N, Nikolic-Zugic J, Immature thymocytes become sensitive to calcium-mediated apoptosis with the onset of CD8, CD4, and the T cell receptor expression: a role for bcl-2?, J. Exp. Med, 178 (1993) 1745–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baffy G, Miyashita T, Williamson JR, Reed JC, Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production., J. Biol. Chem, 268 (1993) 6511–6519. [PubMed] [Google Scholar]
- 83.Lam M, Dubyak G, Chen L, Nuñez G, Miesfeld RL, Distelhorst CW, Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum -associated Ca2+ fluxes., Proc. Natl. Acad. Sci. USA, 91 (1994) 6569–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zornig M, Busch G, Beneke R, Gulbins E, Lang F, Ma A, Korsmeyer S, Moroy T, Survival and death of prelymphomatous B-cells from N-myc/bcl-2 double transgenic mice correlates with the regulation of intracellular Ca2+ fluxes, Oncogene, 11 (1995) 2165–2174. [PubMed] [Google Scholar]
- 85.Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, Velez P, Distelhorst CW, Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER., J Cell Biol, 166 (2004) 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaux DL, Cory S, Adams J, Bcl-2 gene promotes haemopoietic cell survival and co-operates with c-myc to immortalize pre-B cells., Nature, 335 (1988) 440–442. [DOI] [PubMed] [Google Scholar]
- 87.Aouacheria A, Rech de Laval V, Combet C, Hardwick JM, Evolution of Bcl-2 homology motifs: homology versus homoplasy, Trends in cell biology, 23 (2013) 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berridge MJ, Lymphocyte activation in health and disease., Critical reviews in immunology, 17 (1997) 155–178. [DOI] [PubMed] [Google Scholar]
- 89.Gallo EM, Cante-Barrett K, Crabtree GR, Lymphocyte calcium signaling from membrane to nucleus., Nat Immunol, 7 (2006) 25–32. [DOI] [PubMed] [Google Scholar]
- 90.Cante-Barrett K, Gallo EM, Winslow MM, Crabtree GR, Thymocyte negative selection is mediated by protein kinase C- and Ca2+-dependent transcriptional induction of Bim of cell death, Journal of immunology, 176 (2006) 2299–2306. [DOI] [PubMed] [Google Scholar]
- 91.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ, Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair, Cell, 75 (1993) 229–240. [DOI] [PubMed] [Google Scholar]
- 92.Strasser A, Harris AW, Cory S, bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship., Cell, 67 (1991) 889–899. [DOI] [PubMed] [Google Scholar]
- 93.Siegel RM, Katsumata M, Miyashita T, Louie DC, Greene MI, Reed JC, Inhibition of thymocyte apoptosis and negative antigenic selection in bcl-2 transgenic mice., Proceedings of the National Academy of Sciences of the United States of America, 89 (1992) 7003–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Strasser A, Harris AW, von Boehmer H, Cory S, Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene., Proceedings of the National Academy of Sciences of the United States of America, 91 (1994) 1376–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.St. Clair EG, Anderson SJ, Oltvai ZN, Bcl-2 counters apoptosis by bax heterodimerization-dependent and -independent mechanisms in the T-cell lineage., The Journal of biological chemistry, 272 (1997) 29347–29355. [DOI] [PubMed] [Google Scholar]
- 96.Williams O, N. T., M. Halligey, D. Kioussis, H.J.M. Brady, The action of Bax and Bcl-2 on T cell selection., J Exp Med, 188 (1998) 1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhong F, Davis MC, McColl KS, Distelhorst CW, Bcl-2 differentially regulates Ca2+ signals according to the strength of T cell receptor activation., J Cell Biol, 172 (2006) 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takahama Y, Journey through the thymus: stromal guides for T-cell development and selection., Nature reviews. Immunology, 6 (2006) 127–135. [DOI] [PubMed] [Google Scholar]
- 99.Hockenberry DM, Zutter M, Hickey W, Nahm M, Korsmeyer SJ, BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death., Proc. Natl. Acad. Sci. USA, 88 (1991) 6961–6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gratiot-Deans J, Ding L, Turka LA, Nunez G, bcl-2 proto-oncogene expression during human T cell development, J. Immunol, 151 (1993) 83–91. [PubMed] [Google Scholar]
- 101.Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ, Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes., Journal of immunology, 151 (1993) 2546–2554. [PubMed] [Google Scholar]
- 102.Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ, Bcl-2 is upregulated at the CD4+ CD8+ stage during positive selection and promotes thymocyte differentiation at serveral control points., Immunity, 1 (1994) 197–205. [DOI] [PubMed] [Google Scholar]
- 103.Hogquist KA, Signal strength in thymic selection and lineage commitment, Curr Opinion Immunol, 13 (2001) 225–231. [DOI] [PubMed] [Google Scholar]
- 104.Neilson JR, Winslow MM, Hur EM, Crabtree GR, Calcineurin B1 is essential for positive but not negative selection during thymocyte development, Immunity, 20 (2004) 255–266. [DOI] [PubMed] [Google Scholar]
- 105.Lewis RS, Calcium oscillations in T-cells: mechanisms and consequences for gene expression., Biochem Soc Transactions, 31 (2003) 925–929. [DOI] [PubMed] [Google Scholar]
- 106.Fracchia KM, Pai CY, Walsh CM, Modulation of T Cell Metabolism and Function through Calcium Signaling, Front Immunol, 4 (2013) 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Randriamampita C, Trautmann A, Ca2+ signals and T lymphocytes “New mechanisms and functions in Ca2+ signaling”, Biology of the Cell, 96 (2003) 69–78. [DOI] [PubMed] [Google Scholar]
- 108.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI, Differential activation of transcription factors induced by Ca2+ response amplitude and duration., Nature, 386 (1997) 855–308. [DOI] [PubMed] [Google Scholar]
- 109.Dolmetsch RE, Xu K, Lewis RS, Calcium oscillations increase the efficiency and specificity of gene expression., Nature, 392 (1998) 933–936. [DOI] [PubMed] [Google Scholar]
- 110.Donnadieu E, Bismuth G, Trautmann A, Calcium fluxes in T lymphocytes., The Journal of biological chemistry, 267 (1992) 25864–25872. [PubMed] [Google Scholar]
- 111.Mariathasan S, Bachmann MF, Bouchard D, Ohteki T, Ohashi PS, Degree of TCR internalization and Ca2+ flux correlates with thymocyte selection., Journal of immunology, 161 (1998) 6030–6037. [PubMed] [Google Scholar]
- 112.Bhakta NR, Oh DY, Lewis RS, Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment., Nat Immunol, 6 (2005) 143–151. [DOI] [PubMed] [Google Scholar]
- 113.Tomida T, Hirose K, Takizawa A, f. Shibasaki, M. Lino, NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillations., The EMBO journal, 22 (2003) 3825–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu W, Cowie A, Wasfy GW, Penn LZ, Leber B, Andrews DW, Bcl-2 mutants with restricted subcellular location reveal spatially distinct pathways for apoptosis in different cell types., EMBO J, 15 (1996) 4130–4141. [PMC free article] [PubMed] [Google Scholar]
- 115.Wang NS, Unkila M, Reineks EZ, Distelhorst CW, Transient expression of wild-type or mitochondrially targeted Bcl-2 induced apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death., The Journal of biological chemistry, 276 (2001) 44117–44128. [DOI] [PubMed] [Google Scholar]
- 116.Thomenius MJ, Wang NS, Reineks EZ, Wang Z, Distelhorst CW, Bcl-2 on the endoplasmic reticulum regulates Bax activity by binding to BH3-only proteins., The Journal of biological chemistry, 278 (2003) 6243–6250. [DOI] [PubMed] [Google Scholar]
- 117.Rong Y, Aromolaran AS, Bultynck G, Zhong F, Li X, McColl KS, Herlitze S, Matsuyama S, Roderick HL, Bootman MD, Mignery GA, Parys JB, DeSmedt H, Distelhorst CW, Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals., Molecular cell, 31 (2008) 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rong Y, Bultynck G, Aromolaran AS, Zhong F, Parys JB, De Smedt H, Mignery GA, Roderick HL, Bootman MD, Distelhorst CW, The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor., Proc Natl Acad Sci, 106 (2009) 14397–14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Monaco G, Decrock E, Akl H, Ponsaerts R, Vervliet T, Luyten T, De Maeyer M, Missiaen L, Distelhorst CW, De Smedt H, Parys JB, Leybaert L, Bultynck G, Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl, Cell death and differentiation, 19 (2012) 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Erin N, Billingsley ML, Domoic acid enhances Bcl-2-calcineurin-inositol-1,4,5-trisphosphate receptor interactions and delayed neuronal death in rat brain slices., Brain Res, 1014 (2004) 45–52. [DOI] [PubMed] [Google Scholar]
- 121.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ, Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum., Proceedings of the National Academy of Sciences of the United States of America, 102 (2005) 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK, The endoplasmic reticulum gateway to apoptosis by Bcl-Xl modulation of the InsP3R., Nature cell biology, 7 (2005) 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C, Apoptosis regulation by Bcl-XL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating., Proceedings of the National Academy of Sciences of the United States of America, 104 (2007) 12565–12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vervliet T, Parys JB, Bultynck G, Bcl-2 proteins and calcium signaling: complexity beneath the surface, Oncogene, 35 (2016) 5079–5092. [DOI] [PubMed] [Google Scholar]
- 125.Vervliet T, Decrock E, Molgo J, Sorrentino V, Missiaen L, Leybaert L, De Smedt H, Kasri NN, Parys JB, Bultynck G, Bcl-2 binds to and inhibits ryanodine receptors, Journal of cell science, 127 (2014) 2782–2792. [DOI] [PubMed] [Google Scholar]
- 126.Ivanova H, Luyten T, Decrock E, Vervliet T, Leybaert L, Parys JB, Bultynck G, The BH4 domain of Bcl-2 orthologues from different classes of vertebrates can act as an evolutionary conserved inhibitor of IP3 receptor channels, Cell calcium, 62 (2017) 41–46. [DOI] [PubMed] [Google Scholar]
- 127.Monaco G, La Rovere R, Karamanou S, Welkenhuyzen K, Ivanova H, Vandermarliere E, Di Martile M, Del Bufalo D, De Smedt H, Parys JB, Economou A, Bultynck G, A double point mutation at residues Ile14 and Val15 of Bcl-2 uncovers a role for the BH4 domain in both protein stability and function, The FEBS journal, 285 (2018) 127–145. [DOI] [PubMed] [Google Scholar]
- 128.Monaco G, Decrock E, Nuyts K, Wagner LE 2nd, Luyten T, Strelkov SV, Missiaen L, De Borggraeve WM, Leybaert L, Yule DI, De Smedt H, Parys JB, Bultynck G, Alpha-helical destabilization of the Bcl-2-BH4-domain peptide abolishes its ability to inhibit the IP3 receptor, PloS one, 8 (2013) e73386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eckenrode EF, Yang J, Velmurugan GV, Foskett JK, White C, Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dpendent Ca2+ signaling., The Journal of biological chemistry, 285 (2010) 13678–13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schulman JJ, Wright FA, Kaufmann T, Wojcikiewicz RJ, The Bcl-2 protein family member Bok binds to the coupling domain of inositol 1,4,5-trisphosphate receptors and protects them from proteolytic cleavage, The Journal of biological chemistry, 288 (2013) 25340–25349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schoneich C, Antia-poptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA). The Biochemical journal, 383 (2004) 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dremina ES, Sharov VS, Schoneich C, Heat-shock proteins attenuate SERCA inactivation by the anti-apoptotic protein Bcl-2: possible implications for the ER Ca2+-mediated apoptosis, The Biochemical journal, 444 (2012) 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kuo TH, Kim H-RC, Zhu L, Yu Y, Lin H-M, Tsang W, Modulation of endoplasmic reticulum calcium pump by Bcl-2., Oncogene, 17 (1998) 1903–1910. [DOI] [PubMed] [Google Scholar]
- 134.Volpe P, Alderson-Lang BH, Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release. II. Effect of cAMP-dependent protein kinase, Am J Physiol, 258 (1990) C1086–1091. [DOI] [PubMed] [Google Scholar]
- 135.Wagner LE, Li W-H, Yule DI, Phosphorylation of Type-1 inositol 1,4,5-trisphosphate receptors by cyclic nucleotide-dependent protein kinases., The Journal of biological chemistry, 278 (2003) 45811–45817. [DOI] [PubMed] [Google Scholar]
- 136.Wagner LE, Joseph SK, Yule DI, Regulation of single inositol 1,4,5-trisphosphate receptor channel activity by protein kinase A phosphorylation., The Journal of physiology, 586 (2008) 357–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tang TS, Tu H, Wang Z, Bezprozvanny I, Modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by protein kinase a and protein phosphatase 1alpha, J Neurosci, 23 (2003) 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu L, Kong D, Zhu L, Zhu W, Andrews DW, Kuo TH, Suppression of IP3-mediated calcium release and apoptosis by Bcl-2 involves the participation of protein phosphatase 1, Mol Cell Biochem, 295 (2007) 153–165. [DOI] [PubMed] [Google Scholar]
- 139.Shibasaki F, Kondo E, Akagi T, McKeon F, Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2., Nature, 386 (1997) 728–731. [DOI] [PubMed] [Google Scholar]
- 140.Erin N, Bronson SK, Billingsley ML, Calcium-dependent interaction of calcineurin with Bcl-2 in neuronal tissue., Neuroscience, 117 (2003) 541–555. [DOI] [PubMed] [Google Scholar]
- 141.Erin N, Lehman RAW, Boyer PJ, Billingsley ML, In vitro hypoxia and excitotoxicity in human brain induce calcineurin-Bcl-2 interactions., Neuroscience, 117 (2003) 557–565. [DOI] [PubMed] [Google Scholar]
- 142.Bultynck G, Vermassen E, Szlufcik K, De Smet P, Fissore RA, Callewaert G, Missiaen L, De Smedt H, Parys JB, Calcineurin and intracellular Ca2+-release channels: regulation or association?, Biochemical and biophysical research communications, 311 (2003) 1181–1193. [DOI] [PubMed] [Google Scholar]
- 143.Walaas SI, Hemmings HC Jr., Greengard P, Nairn AC, Beyond the dopamine receptor: regulation and roles of serine/threonine protein phosphatases, Front Neuroanat, 5 (2011) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tu H, Tang T.-s., Wang Z, Bezprozvanny I, Association of Type 1 inositol 1,4,5-trisphosphate receptor with AKAP9 (Yotiao) and protein kinase A., The Journal of biological chemistry, 279 (2004) 19375–19382. [DOI] [PubMed] [Google Scholar]
- 145.Tang TS, Bezprozvanny I, Dopamine receptor-mediated Ca(2+) signaling in striatal medium spiny neurons, The Journal of biological chemistry, 279 (2004) 42082–42094. [DOI] [PubMed] [Google Scholar]
- 146.Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P, DARPP-32: an integrator of neurotransmission, Annu Rev Pharmacol Toxicol, 44 (2004) 269–296. [DOI] [PubMed] [Google Scholar]
- 147.Chang MJ, Zhong F, Lavik AR, Parys JB, Berridge MJ, Distelhorst CW, Feedback regulation mediated by Bcl-2 and DARPP-32 regulates inositol 1,4,5-trisphosphate receptor phosphorylation and promotes cell survival, Proceedings of the National Academy of Sciences of the United States of America, 111 (2014) 1186–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chiu WT, Chang HA, Lin YH, Lin YS, Chang HT, Lin HH, Huang SC, Tang MJ, Shen MR, Bcl(−)2 regulates store-operated Ca(2+) entry to modulate ER stress-induced apoptosis, Cell Death Discov, 4 (2018) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pettersson M, Jernberg-Wiklund H, Larsson LG, Sundstrom C, Givol I, Tsujimoto Y, Nilsson K, Expression of the bcl-2 gene in human multiple myeloma cell lines and normal plasma cells, Blood, 79 (1992) 495–502. [PubMed] [Google Scholar]
- 150.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, Cortes J, DeAngelo DJ, Debose L, Mu H, Dohner H, Gaidzik VI, Galinsky I, Golfman LS, Haferlach T, Harutyunyan KG, Hu J, Leverson JD, Marcucci G, Muschen M, Newman R, Park E, Ruvolo PP, Ruvolo V, Ryan J, Schindela S, Zweidler-McKay P, Stone RM, Kantarjian H, Andreeff M, Konopleva M, Letai AG, Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia, Cancer discovery, 4 (2014) 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van Meerbeeck JP, Fennell DA, De Ruysscher DK, Small-cell lung cancer, Lancet, 378 (2011) 1741–1755. [DOI] [PubMed] [Google Scholar]
- 152.Yip KW, Reed JC, Bcl-2 family proteins and cancer, Oncogene, 27 (2008) 6398–6406. [DOI] [PubMed] [Google Scholar]
- 153.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu C, Kipps TJ, Negrini M, Croce C, miR-15 and miR-16 induce apoptosis by targeting BCL2., Proc Natl Acad Sci USA, 102 (2005) 13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Billard C, BH3 mimetics: status of the field and new developments, Mol Cancer Ther, 12 (2013) 1691–1700. [DOI] [PubMed] [Google Scholar]
- 155.Davids MS, Letai A, ABT-199: taking dead aim at BCL-2, Cancer cell, 23 (2013) 139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hogdal LJ, Letai A, BCL-2 inhibition: stemming the tide of myeloid malignancies, Cell Stem Cell, 12 (2013) 269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Martin LA, Dowsett M, BCL-2: a new therapeutic target in estrogen receptor-positive breast cancer?, Cancer cell, 24 (2013) 7–9. [DOI] [PubMed] [Google Scholar]
- 158.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW, ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets, Nature medicine, 19 (2013) 202–208. [DOI] [PubMed] [Google Scholar]
- 159.Juin P, Geneste O, Gautier F, Depil S, Campone M, Decoding and unlocking the BCL-2 dependency of cancer cells, Nature reviews. Cancer, 13 (2013) 455–465. [DOI] [PubMed] [Google Scholar]
- 160.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR, A unified model of mammalian BCL-2 protein family interactions at the mitochondria, Molecular cell, 44 (2011) 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A, BH3 profiling identifies three distinct classes of apoptotic blocks to predict the response to ABT-737 and conventional chemotherapeutic agents., Cancer cell, 12 (2007) 171–185. [DOI] [PubMed] [Google Scholar]
- 162.Davids MS, Deng J, Wiestner A, Lannutti BJ, Wang L, Wu CJ, Wilson WH, Brown JR, Letai A, Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia, Blood, 120 (2012) 3501–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gibson CJ, Davids MS, BCL-2 Antagonism to Target the Intrinsic Mitochondrial Pathway of Apoptosis, Clinical cancer research : an official journal of the American Association for Cancer Research, 21 (2015) 5021–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Touzeau C, Dousset C, Le Gouill S, Sampath D, Leverson JD, Souers AJ, Maiga S, Bene MC, Moreau P, Pellat-Deceunynck C, Amiot M, The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma, Leukemia, 28 (2014) 210–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M, Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia, Cancer cell, 10 (2006) 375–388. [DOI] [PubMed] [Google Scholar]
- 166.Choudhary GS, Al-Harbi S, Mazumder S, Hill BT, Smith MR, Bodo J, Hsi ED, Almasan A, MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies, Cell death & disease, 6 (2015) e1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Akl H, Monaco G, La Rovere R, Welkenhuyzen K, Kiviluoto S, Vervliet T, Molgo J, Distelhorst CW, Missiaen L, Mikoshiba K, Parys JB, De Smedt H, Bultynck G, IP3R2 levels dictate the apoptotic sensitivity of diffuse large B-cell lymphoma cells to an IP3R-derived peptide targeting the BH4 domain of Bcl-2, Cell death & disease, 4 (2013) e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Greenberg EF, McColl KS, Zhong F, Wildey G, Dowlati A, Distelhorst CW, Synergistic killing of human small cell lung cancer cells by the Bcl-2-inositol 1,4,5-trisphosphate receptor disruptor BIRD-2 and the BH3-mimetic ABT-263, Cell death & disease, 6 (2015) e2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xie Q, Xu Y, Gao W, Zhang Y, Su J, Liu Y, Guo Y, Dou M, Hu K, Sun L, TATfused IP3Rderived peptide enhances cisplatin sensitivity of ovarian cancer cells by increasing ER Ca2+ release, Int J Mol Med, 41 (2018) 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rong Y, Barr P, Yee VC, Distelhorst CW, Targeting Bcl-2 based on the interaction of its BH4 domain with the inositol 1,4,5-trisphosphate receptor., Biochimica et biophysica acta, 1793 (2009) 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vervloessem T, Ivanova H, Luyten T, Parys JB, Bultynck G, The selective Bcl-2 inhibitor venetoclax, a BH3 mimetic, does not dysregulate intracellular Ca(2+) signaling, Biochimica et biophysica acta, 1864 (2017) 968–976. [DOI] [PubMed] [Google Scholar]
- 172.Vervloessem T, Akl H, Tousseyn T, De Smedt H, Parys JB, Bultynck G, Reciprocal sensitivity of diffuse large B-cell lymphoma cells to Bcl-2 inhibitors BIRD-2 versus venetoclax, Oncotarget, 8 (2017) 111656–111671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Placzek WJ, Wei J, Kitada S, Zhai D, Reed JC, Pellecchia M, A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy, Cell death & disease, 1 (2010) e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thrower EC, Hagar RE, Ehrlich BE, Regulation of Ins(1,4,5)P3 receptor isoforms by endogenous modulators., TRENDS in Pharmacological Sciences, 22 (2001) 580–586. [DOI] [PubMed] [Google Scholar]
- 175.Rushworth SA, Murray MY, Zaitseva L, Bowles KM, MacEwan DJ, Identification of Bruton’s tyrosine kinase as a therapeutic target in acute myeloid leukemia, Blood, 123 (2014) 1229–1238. [DOI] [PubMed] [Google Scholar]
- 176.Tai YT, Chang BY, Kong SY, Fulciniti M, Yang G, Calle Y, Hu Y, Lin J, Zhao JJ, Cagnetta A, Cea M, Sellitto MA, Zhong MY, Wang Q, Acharya C, Carrasco DR, Buggy JJ, Elias L, Treon SP, Matsui W, Richardson P, Munshi NC, Anderson KC, Bruton tyrosine kinase inhibition is a novel therapeutic strategy targeting tumor in the bone marrow microenvironment in multiple myeloma, Blood, 120 (2012) 1877–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Woyach JA, Johnson AJ, Byrd JC, The B-cell receptor signaling pathway as a therapeutic target in CLL, Blood, 120 (2012) 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bittremieux M, La Rovere RM, Akl H, Martines C, Welkenhuyzen K, Dubron K, Baes M, Janssens A, Vandenberghe P, Laurenti L, Rietdorf K, Morciano G, Pinton P, Mikoshiba K, Bootman MD, Efremov DG, De Smedt H, Parys JB, Bultynck G, Constitutive IP3 signaling underlies the sensitivity of B-cell cancers to the Bcl-2/IP3 receptor disruptor BIRD-2, Cell death and differentiation, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Deng J, Park D, Wang M, Nooka A, Deng Q, Matulis S, Kaufman J, Lonial S, Boise LH, Galipeau J, Deng X, BCL2-BH4 antagonist BDA-366 suppresses human myeloma growth, Oncotarget, 7 (2016) 27753–27763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T, Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses., Science, 280 (1998) 1763–1766. [DOI] [PubMed] [Google Scholar]
- 181.Pizzo P, Pozzan T, Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics., Trends in cell biology, 17 (2007) 511–517. [DOI] [PubMed] [Google Scholar]
- 182.Drago I, Pizzo P, Pozzan T, After half a century mitochondrial calcium in- and efflux machineries reveal themselves, The EMBO journal, 30 (2011) 4119–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rutter GA, Rizzuto R, Regulation of mitochondrial metabolism by ER Ca2+ release: an intimate connection, Trends Biochem Sci, 25 (2000) 215–221. [DOI] [PubMed] [Google Scholar]
- 184.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R, Calcium and apoptosis: ER-mitochondrial Ca2+ transfer in the control of apoptosis., Oncogene, 27 (2008) 6407–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cardenas C, Miller RA, Simth I, Bui T, Molgo J, Muller M, Vais H, Cheung K-H, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK, Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria, Cell, 142 (2010) 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cardenas C, Muller M, McNeal A, Lovy A, Jana F, Bustos G, Urra F, Smith N, Molgo J, Diehl JA, Ridky TW, Foskett JK, Selective Vulnerability of Cancer Cells by Inhibition of Ca(2+) Transfer from Endoplasmic Reticulum to Mitochondria, Cell Rep, 15 (2016) 219–220. [DOI] [PubMed] [Google Scholar]
- 187.Carrington EM, Zhan Y, Brady JL, Zhang JG, Sutherland RM, Anstee NS, Schenk RL, Vikstrom IB, Delconte RB, Segal D, Huntington ND, Bouillet P, Tarlinton DM, Huang DC, Strasser A, Cory S, Herold MJ, Lew AM, Anti-apoptotic proteins BCL-2, MCL-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo, Cell death and differentiation, 24 (2017) 878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Popgeorgiev N, Jabbour L, Gillet G, Subcellular Localization and Dynamics of the Bcl-2 Family of Proteins, Front Cell Dev Biol, 6 (2018) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]