Abstract
Objective.
Paclitaxel, a microtubule inhibitor, is subject to tumor resistance while treating high-grade serous ovarian and uterine cancer. This study aims to directly compare the effects of SQ1274, a novel microtubule inhibitor that binds to the colchicine-binding site on tubulin, and paclitaxel in high-grade serous ovarian and uterine cancer cell lines both in vitro and in vivo.
Methods.
We assessed the sensitivity of ovarian (OVCAR8) and uterine (ARK1) cancer cell lines to SQ1274 and paclitaxel using XTT assays. We used western blot and quantitative real-time PCR to analyze changes in AXL RNA and protein expression by SQ1274 and paclitaxel. Differences in cell-cycle arrest and apoptosis were investigated using flow cytometry. Finally, we treated ovarian and uterine xenograft models with vehicle, paclitaxel, or SQ1274.
Results.
First, we demonstrate that SQ1274 has a much lower IC50 than paclitaxel in both ARK1 (1.26 nM vs. 15.34 nM, respectively) and OVCAR8 (1.34 nM vs. 10.29 nM, respectively) cancer cell lines. Second, we show SQ1274 decreases both RNA and protein expression of AXL. Third, we show that SQ1274 causes increased cell-cycle arrest and apoptosis compared to paclitaxel. Finally, we report that SQ1274 more effectively inhibits tumor growth in vivo compared to paclitaxel.
Conclusions.
SQ1274 presents as a viable alternative to paclitaxel for treating ovarian and uterine cancer. This study supports the development of SQ1274 as a chemotherapeutic to treat ovarian and uterine cancer.
1. Introduction
High-grade serous ovarian cancer presents at an advanced stage and is the deadliest of all gynecologic cancers; fewer than 50% of women with this disease survive 5 years [1,2]. Similarly, high-grade uterine serous cancer, which constitutes just 10% of all endometrial cancers, is responsible for 40% of endometrial-cancer-related deaths [3]. The standard of care for both of these cancers is surgical removal of tumors followed by combination chemotherapy with carboplatin and the microtubule stabilizer paclitaxel [4–6]. Although 60% to 85% of patients with high-grade serous ovarian cancer initially respond to this regimen, the majority eventually relapse with chemotherapy-resistant disease [7]. Additionally, most patients with ovarian cancer die because of the chemoresistance they develop [8,9]. Indeed, resistance to paclitaxel and related microtubule inhibitors is common in women with high-grade ovarian or uterine cancer. Thus, much research is focused on identifying new chemotherapy drugs.
One strong chemotherapeutic candidate is SQ1274, an optimized analogue of bifidenone [10,11]. Like paclitaxel, SQ1274 disrupts microtubule dynamics. However, rather than binding to the taxane binding site on microtubule polymers and stabilizing microtubules [12], SQ1274 binds to the colchicine-binding site on tubulin and destabilizes microtubules [10]. This is an attractive feature because many reports have shown that cancer cells are less susceptible to developing resistance to colchicine and colchicine derivatives than to other microtubule inhibitors [13–15]. One proposal is that, whereas cells develop resistance to paclitaxel and related compounds by upregulating expression of the drug efflux protein P-glycoprotein [16,17], this is less likely to occur in response to drugs that bind to the colchicine binding site on tubulin.
In initial testing, Williams et al. showed that many cancer cell types were sensitive to bifidenone including NCI-H460, SF-295, ACHN, M14, A375, UACC-62, and SK-Mel-2. Moreover, this compound caused cell cycle arrest in the G2/M phase of NCI-H460 human lung cancer cells [10]. In this study, we sought to directly compare the effects of SQ1274 and paclitaxel in high-grade serous and uterine cancer cell lines both in vitro and in vivo.
2. Materials and methods
2.1. Cell lines
The established human, immortalized, uterine serous cancer cell line ARK1 [18] was provided by Shi-Wen Jiang (Mercer University School of Medicine, Savannah, GA, USA). The human, immortalized, uterine serous cancer cell line ARK4 [19], was purchased from Dr. A. Santin (Yale University, New Haven, CT). The ovarian cancer cell line OVCAR8 was purchased from the National Cancer Institute-Frederick Division of Cancer Treatment and Diagnosis tumor cell line repository. The ovarian cancer cell line ES2 [20] was provided by Dr. Branimir Sikic (Stanford University). All cell lines were maintained in RPMI (Sigma, St. Louis, MO) supplemented with 10% heat-inactivated FBS (Sigma, St. Louis, MO) and 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA) at 37 °C in a 5% CO2 incubator. Cell lines were confirmed negative for mycoplasma, as indicated by the Mycoalert MycoPlasma Detection Kit (Lonza), before performing any experiments.
2.2. Preparation of chemotherapy agents
For in vitro use, aliquots of a stock solution of 0.01 M paclitaxel (Sigma-Aldrich, St. Louis, MO) in DMSO were stored at −20 °C. Aliquots of 13.8 mM SQ1274 (Sequoia Sciences, St. Louis, MO) in DMSO were stored at room temperature. For in vivo use, paclitaxel was diluted in supplemented PBS to 2.34 mM. SQ1274 was prepared by dissolving the compound to 19.6 mM in 38% PEG400 (Sequoia Sciences, St. Louis, MO), 22% ethanol, and 40% 20 mM citrate buffer.
2.3. Western blot analysis
Cells were lysed in 9 M Urea, 0.075 M Tris buffer (pH 7.6) 72 h after indicated treatment. Protein concentration was determined by using the Bradford assay, and proteins were subjected to reducing SDS/PAGE by standard methods. Western blots were incubated with primary antibodies against AXL (R&D Systems; 1:1000), phospho-Histone H3 (Millipore; 1:3000), Parp/cParp (Cell Signaling; 1:1000), Gas 6 (R&D Systems; 1:100) and β‑actin (Sigma Aldrich; 1:3000, St. Louis, MO). Blots were then incubated with appropriate horse-radish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch), and antibody complexes were detected with the Thermo Scientific SuperSignal West Pico Chemiluminescent kit. A ChemiDoc (Bio-Rad Laboratories) was used to detect the signal.
2.4. cDNA Preparation and qPCR
Total RNA was isolated from cells by using the RNeasy Mini Kit (Qiagen). cDNA was made from 1 μg of RNA by using the SuperScript IV system (Thermo Fisher Scientific) following the manufacturer’s directions. Applied Biosystems 7500 detection system and SYBR-green master mix (Thermo Fisher Scientific) were used to perform qPCR. mRNA expression was normalized with respect to 18S ribosomal RNA. Fold change was calculated using the 2−ΔΔCt method. Primer sequences for AXL were published previously [21–23].
2.5. Drug treatment and cell growth (XTT) assays
For cell growth assays, 4500 cells were plated in a 96 well plate in 10% RPMI with 1% penicillin and streptomycin. The cells were allowed to attach for 24 h and then either vehicle or drug was added to the wells. Cells were treated with either SQ 1274 or paclitaxel in increasing concentrations from 0.1 to 80 nM. After 72 h, an 2,3‑bis (2‑methoxy‑4‑nitro‑5‑sulfophenyl)‑2H‑tetrazolium‑5‑carboxanilide inner salt (XTT)-based assay (Sigma-Aldrich) was performed as described previously to measure cell viability [24]. The plates were placed back in the 37 °C incubator for 2 h. XTT metabolism was quantified by measuring the absorbance at 450 nm (Tecan Infinite M200 Pro).
2.6. Cell-cycle analysis
Cells were plated at a density of 4 × 105 cells per 10 cm dish and allowed adhering and growing for 24 h. Next, the cells were treated with vehicle (DMSO), paclitaxel, or SQ1274 and incubated for 24 h. Cells were collected, fixed with 70% ethanol for 30 min at 4 °C, pelleted, washed in PBS, and then stained with 100 μg/mL propidium iodide for 30 min at room temperature. Fluorescence was measured by flow cytometry on a FACSCalibur flow cytometer (BD Bioscience), and cell cycle was fit with FlowJo software.
2.7. Assessment of apoptotic nuclei
Cells were plated at a density of 1 × 105 cells per well in 6-well plates and allowed attaching overnight. DMSO, paclitaxel, or SQ1274 was added to the plate and incubated for 24 h. Then, cells were fixed in 4% paraformaldehyde (Thermo Fisher Scientific) and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in cold PBS. Mounting media containing 6‑diamiidino‑2‑phenylindole (DAPI; Vector Laboratories) was applied to fixed cells, and nuclear morphology was assessed by fluorescent microscopy.
2.8. Detection of apoptosis by annexin V staining
ARK1 and OVCAR8 cells were plated at a density of 1.2 × 105 cells per well in 6-well plates and allowed attaching overnight. Next, the cells were treated with vehicle (DMSO), paclitaxel (2 nM), or SQ1274 (2 nM) and incubated for 24 h. The percentage of apoptotic cells was determined by Annexin V allophycocyanin (BioLegend) staining according to manufacturer’s instructions and by flow cytometric analysis. The viability stain 7-amino-actinomycin D (BioLegend) was also included in the assay and used as indicator of membrane structural integrity. Data were collected on LSRFortessa X-20 (BD) flow cytometer and analyzed using FlowJo software.
2.9. Mouse studies
Experiments were conducted according to Institutional Animal Care and Use Committee Policy. Tumor xenograft models were established by injecting female NOD SCID (Jackson Laboratory) or NU/FOX (Charles River) mice age 6 to 8 weeks subcutaneously with ARK1 (10 × 106) or OVCAR8 (5 × 106) cells, respectively. The cells were resuspended in 1 mg/mL Matrigel (Corning) before subcutaneous injection. After tumors engrafted, they were measured every 3 days, and tumor volume (V) was calculated by using the equation: V = l × (w2/2) (l = longest diameter, w = shortest, perpendicular diameter).
After reaching an average tumor volume of 150 mm3 mice were either left untreated (n = 8 for both xenograft models), treated intraperitoneally with 50 mg/kg vehicle (Ark1 n = 8, OVCAR8 n = 10), treated intraperitoneally with 20 mg/kg of paclitaxel every 3 days for two weeks (ARK1 n = 10, OVCAR8 n = 9), or treated intraperitoneally with SQ 1274 treatment intraperitoneally with 50 mg/kg SQ 1274 for 3 days on and 2 days off for two weeks (ARK1 n = 10, OVCAR8 n = 9).
3. Results
3.1. SQ 1274 inhibits growth and decreases AXL expression in endometrial cancer and ovarian cancer cell lines
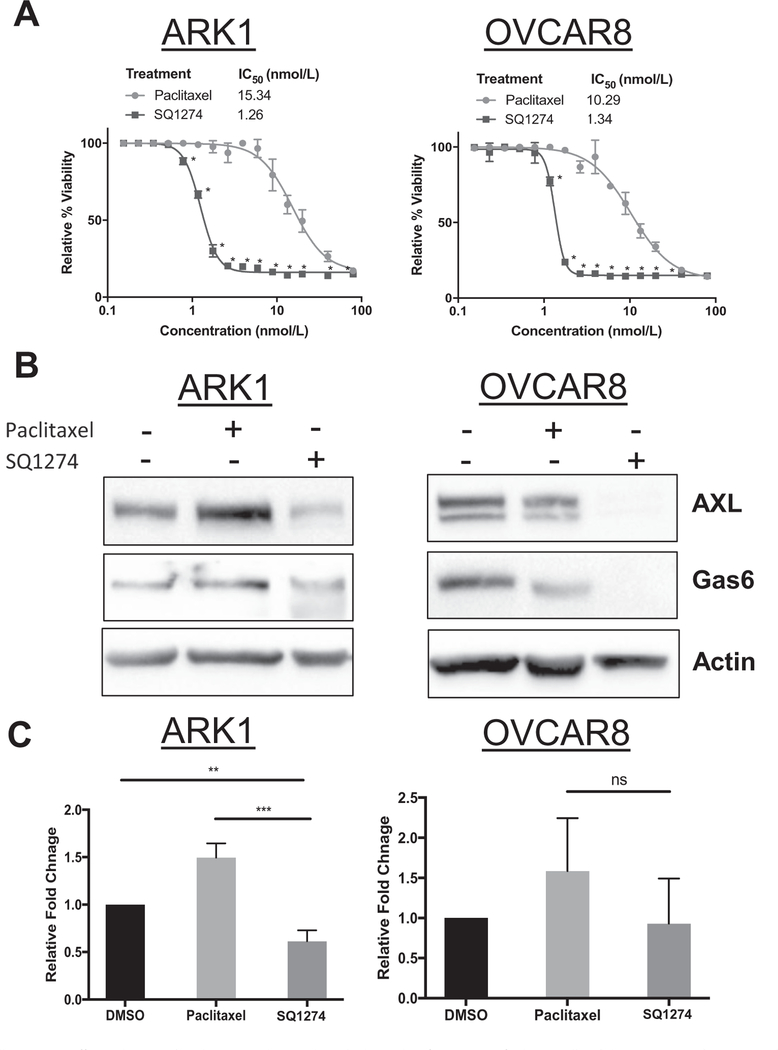
We first compared the sensitivity of the ovarian cancer cell line OVCAR8 and the endometrial cancer cell line ARK1 to SQ1274 and paclitaxel. We found that the IC50 values for SQ1274 were 7.7− and 12.2− fold lower than the IC50 values for paclitaxel in OVCAR8 and ARK1 cells, respectively (Fig. 1A). Because we previously demonstrated that paclitaxel-resistant ovarian and uterine cancer cells overexpress the receptor tyrosine kinase AXL and that knockdown or inhibition of AXL restored paclitaxel sensitivity [21,25], we wondered whether SQ1274 affected AXL expression in these cells. Also, we questioned whether SQ 1274 treatment decreased Gas6 expression since we have shown that Gas6 levels are correlated to AXL expression in the past indicating that it is an important factor to consider when examining AXL protein expression [26]. Whereas paclitaxel-treated ARK1 cells had more AXL and Gas6 protein than untreated cells, SQ1274-treated cells had less. Similarly, SQ1274-treated OVCAR8 cells had less AXL and Gas6 protein than untreated cells (Fig. 1B). These same findings were also observed in the ARK4 and ES2 cell lines (S1A–B). However, at increasing concentrations of paclitaxel on the ARK1 cell line, we found that AXL expression also decreases (S2A). Additionally, whereas paclitaxel-treated cells had more AXL mRNA than DMSO-treated cells, this was not the case in SQ1274-treated cells (Fig. 1C). We conclude that SQ1274 more effectively inhibits OVCAR8 and ARK1 cell growth than paclitaxel and that it may do so by decreasing AXL expression.
Fig. 1.
OVCAR8 and ARK1 cells are more effectively treated with SQ1274 than paclitaxel. A) Results of XTT assay of relative cell viability in ARK1 and OVCAR8 cells treated with increasing concentrations of paclitaxel and SQ1274. Cell viability is reported relative to controls treated with vehicle. These assays were performed in triplicate and graphed as mean ± SD. *, P < 0.05 compared to controls. B) Western blot analysis of AXL and Gas6 expression in ARK1 and OVCAR8 cell lines treated with vehicle (DMSO), 2 nM paclitaxel, or 2 nM SQ1274. B-actin is shown as a loading control. C) qPCR was performed on cells treated with vehicle, 2 nM paclitaxel or 2 nM SQ1274 and AXL mRNA expression was assessed. Significance was calculated using SD or DDCT. Error bars indicate the range of fold change. **, P < 0.01; ***, P < 0.001.
3.2. SQ 1274 induces cell cycle arrest and apoptosis
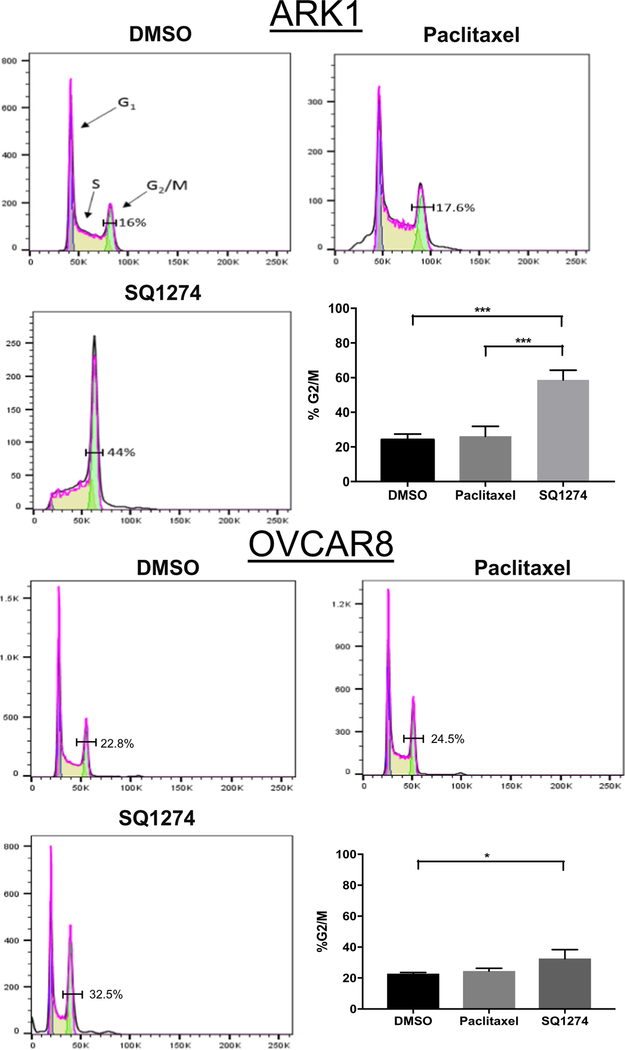
Previously, Williams et al. showed that SQ1274 causes human lung cancer cells to arrest in the G2/M phase [10,27]. To determine whether SQ1274 more effectively caused cell cycle arrest than paclitaxel, we treated ARK1 and OVCAR8 cells with equivalent concentrations of the two drugs. Flow cytometry analysis indicated that a higher percentage of SQ1274-treated cells were in the G2/M phase compared to DMSO-treated cells for uterine (44% vs. 16%) and ovarian (32.5% vs. 22.8%) cancer cell lines (Figs. 2A–B, S3A–B). Additionally, in the uterine cancer cell line there were significantly more SQ1274-treated cells in the G2/M phase compared to paclitaxel-treated cells (Fig. 2A). Consistent with findings by flow cytometry, Western blot analysis showed that SQ1274-treated cells had higher expression of the mitotic marker phosphor-Histone H3 compared to DMSO-treated cells in both cell lines (Fig. 3A).
Fig. 2.
SQ 1274 induces mitotic arrest. A & B) Representative image of flow cytometry analysis of cell-cycle distribution in ARK1 and OVCAR8 cells treated with DMSO, paclitaxel, or SQ1274. Numbers correspond to the percentage of cells found in the G2-M phase ± SD, *, P < 0.05; *** P < 0.001. Significance was calculated by one-way ANOVA.
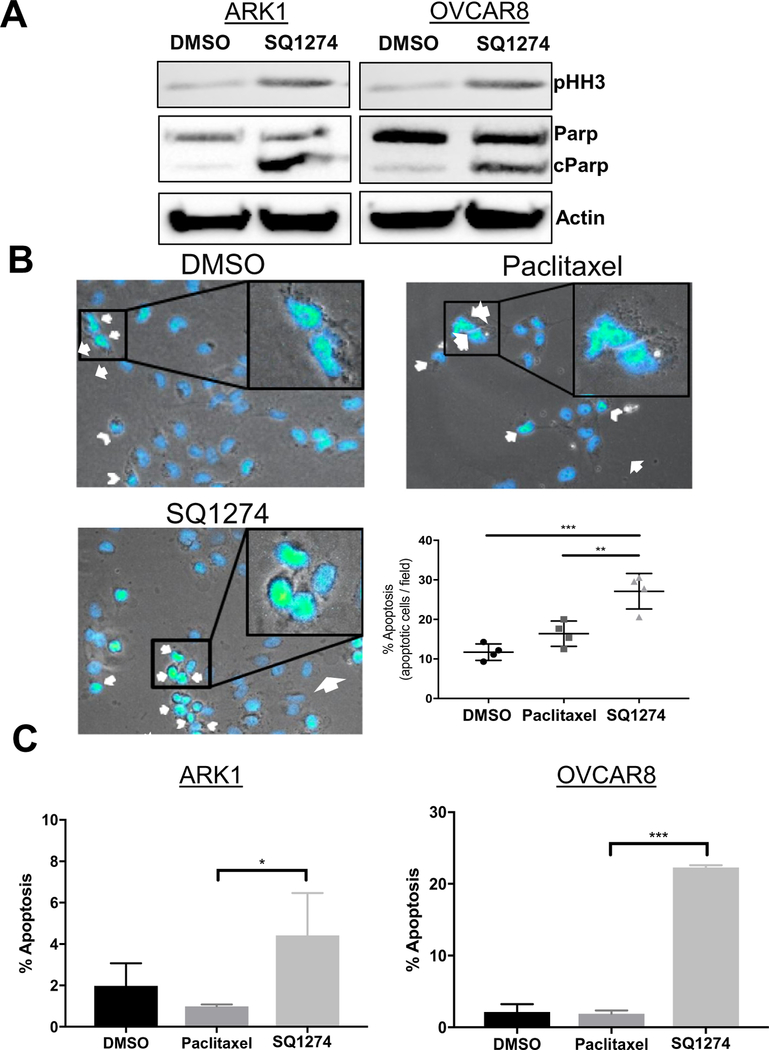
Fig. 3.
Mitotic arrest induces apoptosis. A) Western blot analysis of mitotic and apoptotic markers in ARK1 and OVCAR8 cells treated with DMSO or 2 nM SQ1274 for 24 h. B) Representative images and quantitation of apoptotic OVCAR8 cells treated with DMSO, paclitaxel, or SQ1274 detected by staining with DAPI and microscopic analysis of nuclear morphology. Arrow, abnormal DNA condensation; arrowhead, nuclear blebbing. **, P < 0.01; ***, P < 0.001. C) Quantitation of apoptotic ARK1 and OVCAR8 cells treated with DMSO, paclitaxel, or SQ1274 detected by staining with Annexin V/7-AAD and flow cytometric analysis. The mean apoptosis percentage ± SD are plotted, *, P < 0.05; ***, P < 0.001.
Given that SQ1274 caused cell cycle arrest and reduced growth, we wondered whether it also caused apoptosis. Three findings indicate that this was the case. First, SQ1274-treated cells had higher levels of the apoptotic marker cleaved poly-ADP ribose polymerase than did DMSO-treated cells (Fig. 3A). Second, a significantly higher percentage of SQ1274-treated cells than DMSO-or paclitaxel-treated cells showed nuclear blebbing and abnormal DNA condensation (Figs. 3B, S4A), two common features of apoptosis. Finally, flow cytometry analysis of Annexin V stained cells showed a higher percentage of SQ1274-treated being in apoptosis compared to paclitaxel treated cells in both the OVCAR8 and ARK1 cell lines (Figs. 3C, S5A–B). Together, these results indicate that SQ1274 is able to initiate apoptosis in cells more effectively than paclitaxel (Fig. 4).
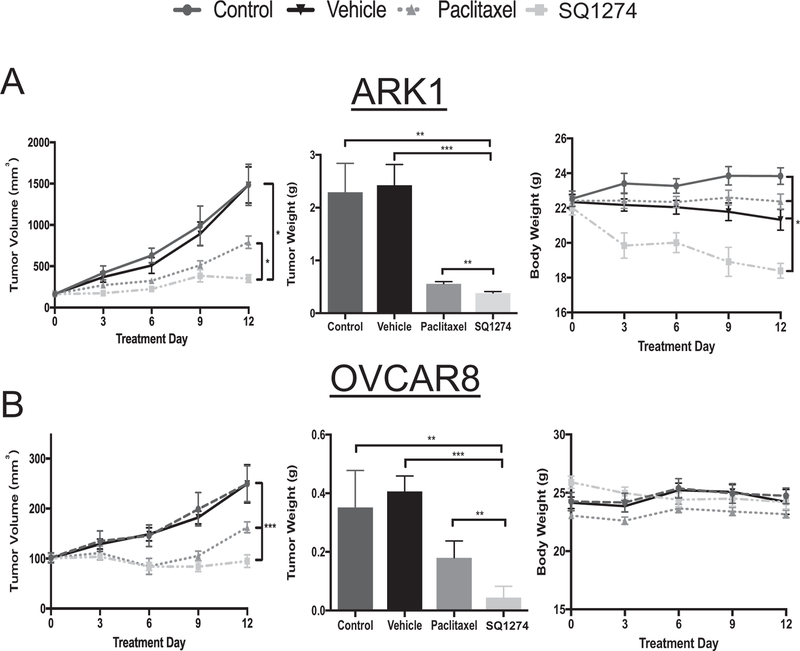
Fig. 4.
SQ1274 inhibits tumor more effectively than paclitaxel. Tumor volume, tumor weight, and body weight measurements of mice injected with A) ARK1 no treatment n = 8, vehicle n = 8, paclitaxel n = 10, SQ1274 n = 10 or B) OVCAR8 cells no treatment n = 8, vehicle n = 10, paclitaxel n = 9, SQ1274 n = 9. The mean tumor volume, tumor weight, and body weight ± SD are plotted, *, P < 0.05; **, P < 0.01; *** P < 0.001. Significance was calculated by unpaired t-test.
3.3. SQ 1274 strongly inhibits tumor growth in vivo
Given the above in vitro data, we wondered whether SQ1274 would also be effective in vivo. We subcutaneously injected ARK1 and OVCAR8 cells into NOD/SCID and NU/FOX mice, respectively, allowed them to engraft, and then treated the mice with vehicle, paclitaxel, or SQ1274 for two weeks. We found in both xenograft models that SQ1274 more effectively inhibited tumor growth compared to paclitaxel. To begin to assess SQ1274 toxicity, we examined mouse weight throughout the experiment and found that SQ1274-treated mice maintained similar body weight to the control mice in the OVCAR8 xenograft model. In the ARK1 xenograft model there was a decrease in body for the SQ1274-treated mice compared to the control mice indicating the drug needs to be further optimized.
4. Discussion
Uterine and ovarian serous cancers are deadly diseases that present at an advanced stage because we lack effective screening tests [28–30]. Currently, the first-line adjuvant therapy for advanced gynecologic cancers is combination treatment with carboplatin and paclitaxel [29,31]. Although this treatment strategy is initially effective, the majority of tumors develop resistance to these drugs [16,31]. Colchicine-binding site microtubule inhibitors have recently been identified as an attractive improvement to taxane binding inhibitors [32–36]. Here, we present three lines of evidence that the novel colchicine-binding site inhibitor SQ1274 is a viable alternative to paclitaxel for treating ovarian and uterine cancer. First, we demonstrate that SQ 1274 has a much lower IC50 than paclitaxel in both uterine (1.26 vs. 15.34, respectively) and ovarian (1.34 vs. 10.29, respectively) cancer cell lines. Second, flow cytometric analysis indicates that SQ1274 causes cell-cycle arrest and apoptosis. Finally, we report that SQ1274 effectively prevents tumor growth in vivo.
Resistance of ovarian and uterine cancers to paclitaxel therapy is a major clinical issue. One mechanism contributing to this resistance is high expression of the receptor tyrosine kinase AXL [25,37–43]. For example, our lab and others have shown that high expression of AXL promotes invasion and migration in multiple cancers including ovarian and uterine serous cancer [26,44–47] and that AXL expression contributes to paclitaxel resistance in uterine serous cancer [25]. Thus, our finding that SQ1274-treated cells had lower levels of AXL protein than control or paclitaxel-treated cells indicates that this drug may act through inhibiting AXL to promote chemosensitivity.
We noted an important difference between our in vitro and in vivo results. In cell-based assays, SQ1274 was more effective than paclitaxel in reducing ovarian and uterine cancer cell growth, but the two drugs were equivalently effective in the in vivo model. However, we note that we used a subcutaneous xenograft model rather than an intraperitoneal model in which it is possible to assess metastasis. Thus, future experiments should compare the abilities of SQ1274 and paclitaxel to prevent metastasis in the intraperitoneal model. Nonetheless, our in vivo findings support SQ 1274 as a viable alternative to paclitaxel, especially in the case of cells that highly express AXL.
In summary, SQ1274 has many excellent qualities of a chemotherapy drug. SQ1274 targets the colchicine binding site to depolymerize microtubules, leading to cell-cycle arrest and apoptosis. SQ1274 also has the ability to suppress AXL expression at both the protein and RNA level. While paclitaxel is also able to cause cell-cycle arrest and apoptosis as well as decrease AXL expression in vitro [23], we show that SQ1274 can initiate these affects at a much lower dosage than paclitaxel. Finally, SQ1274 demonstrates strong tumor inhibition effects in vivo without causing significant loss of body weight. These pre-clinical observations, in combination with previous studies of SQ1274, strongly support its further development as a therapeutic alternative to paclitaxel for both ovarian and uterine serous cancers.
Supplementary Material
HIGHLIGHTS.
SQ1274, a colchicine-binding site inhibitor, is a viable alternative to paclitaxel in treating ovarian and uterine cancer.
SQ1274 has a much lower IC50 than paclitaxel in both ovarian and uterine cancer.
SQ1274 decreases both RNA and protein expression of AXL.
SQ1274 causes increased cell-cycle arrest and apoptosis compared to paclitaxel.
SQ1274 effectively prevents tumor growth in vivo.
Acknowledgements
The authors thank Deborah Frank, PhD, for the scientific editing of this manuscript. CMS and JAL provided SQ1274. Research reported in this publication was supported by the Reproductive Scientist Development Program (RSDP) NIH grant 2K12HD000849-28 (with co-funding from the American College of Obstetrics and Gynecologists and the March of Dimes) and Barnes Jewish Hospital Fund.
Footnotes
Conflict of interest and disclosure statements
KAM, STR, JMQ, LG, CMS, JAL, ARI, ARH, and KCF have no conflicts of interest. MAP participates as a consultant/speaker for Roche, Genentech, Tesaro, Clovis Oncology, and AstraZeneca. DGM serves as a speaker for Clovis Oncology and AstraZeneca. CM serves on the speaker bureau and advisory board for Genentech. PHT participates as a speaker/consultant for Celsion, Tesaro, and Merck and is on the advisory board for Tesaro and Aravive.
References
- [1].Bowtell DD, et al. , Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer, Nat. Rev. Cancer 15 (11) (2015) 668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hendricks DT, et al. , FHIT gene expression in human ovarian, endometrial, and cervical cancer cell lines, Cancer Res 57 (11) (1997) 2112. [PubMed] [Google Scholar]
- [3].Hamilton CA, et al. , Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers, Br. J. Cancer 94 (5) (2006) 642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sagae S, et al. , Gynecologic Cancer InterGroup (GCIG) consensus review for uterine serous carcinoma, Int. J. Gynecol. Cancer 24 (9) (2014) S83–S89. [DOI] [PubMed] [Google Scholar]
- [5].Uterine Neoplasms. NCCN Clinical Practice Guidelines in Oncology 2017.
- [6].Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. NCCN Clinical Practice Guidelines in Oncology 2017.
- [7].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2017, CA Cancer J. Clin 67 (1) (2017) 7–30. [DOI] [PubMed] [Google Scholar]
- [8].Stockler MR, et al. , Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer, J. Clin. Oncol 32 (13) (2014) 1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Coleman RL, et al. , Latest research and clinical treatment of advanced-stage epithelial ovarian cancer. Nature reviews, Clin. Oncol 10 (4) (2013) 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Williams RB, et al. , Isolation and identification of the novel tubulin polymerization inhibitor bifidenone, J. Nat. Prod 80 (3) (2017) 616–624. [DOI] [PubMed] [Google Scholar]
- [11].Huang Z, et al. , A total synthesis of bifidenone, J. Org. Chem 82 (8) (2017) 4235–4241. [DOI] [PubMed] [Google Scholar]
- [12].Field Jessica J., Díaz José F., Miller John H., The binding sites of microtubule-stabilizing agents, Chem. Biol 20 (3) (2013) 301–315. [DOI] [PubMed] [Google Scholar]
- [13].Arnst KE, et al. , A potent, metabolically stable tubulin inhibitor targets the colchicine binding site and overcomes taxane resistance, Cancer Res 78 (1) (2018) 265. [DOI] [PubMed] [Google Scholar]
- [14].Lu Y, et al. , An overview of tubulin inhibitors that interact with the colchicine binding site, Pharm. Res 29 (11) (2012) 2943–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bai Z, et al. , BZML, a novel colchicine binding site inhibitor, overcomes multidrug resistance in A549/Taxol cells by inhibiting P-gp function and inducing mitotic catastrophe, Cancer Lett 402 (2007) 81–92. [DOI] [PubMed] [Google Scholar]
- [16].Fojo T, Menefee M, Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents, Ann. Oncol 18 (suppl_5) (2007) v3–v8. [DOI] [PubMed] [Google Scholar]
- [17].Nguyen TL, et al. , Evading Pgp activity in drug-resistant cancer cells: a structural and functional study of antitubulin furan metotica compounds, Mol. Cancer Ther 11 (5) (2012) 1103–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].El-Sahwi K, et al. , In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma, Br. J. Cancer 102 (1) (2010) 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Santin AD, et al. , Effects of cytokines combined with high-dose gamma irradiation on the expression of major histocompatibility complex molecules and intercellular adhesion molecule-1 in human ovarian cancers, Int. J. Cancer 65 (5) (1996) 688–694. [DOI] [PubMed] [Google Scholar]
- [20].Lau DHM, et al. , Multifactorial mechanisms associated with broad cross-resistance of ovarian carcinoma cells selected by cyanomorpholino doxorubicin, Cancer Res 51 (19) (1991) 5181. [PubMed] [Google Scholar]
- [21].Rankin EB, et al. , AXL is an essential factor and therapeutic target for metastatic ovarian cancer, Cancer Res 70 (19) (2010) 7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pinzón-Daza ML, et al. , Oxidative stress promotes doxorubicin-induced Pgp and BCRP expression in colon cancer cells under hypoxic conditions, J. Cell. Biochem 118 (7) (2017) 1868–1878. [DOI] [PubMed] [Google Scholar]
- [23].Rankin EB, et al. , Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET, Proc. Natl. Acad. Sci 111 (37) (2014) 13373–13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heinrich MC, et al. , Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor, Blood 96 (3) (2000) 925. [PubMed] [Google Scholar]
- [25].Palisoul ML, et al. , Inhibition of the receptor tyrosine kinase AXL restores paclitaxel chemosensitivity in uterine serous cancer, Mol. Cancer Ther 16 (12) (2017) 2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Divine LM, et al. , AXL modulates extracellular matrix protein expression and is essential for invasion and metastasis in endometrial cancer, Oncotarget 7 (47) (2016) 77291–77305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huang Z, et al. , Bifidenone: Structure-activity Relationship and Advanced Preclinical Candidate, 2018. (Submitted). [DOI] [PubMed]
- [28].Liu J, Matulonis UA, New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances, Clin. Cancer Res 20 (20) (2014) 5150–5156. [DOI] [PubMed] [Google Scholar]
- [29].Boruta DM, et al. , Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review, Gynecol. Oncol 115 (1) (2009) 142–153. [DOI] [PubMed] [Google Scholar]
- [30].Lu KH, Daniels M, Endometrial and ovarian cancer in women with Lynch syndrome: update in screening and prevention, Familial Cancer 12 (2) (2013) 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Berton-Rigaud D, et al. , Gynecologic Cancer InterGroup (GCIG) consensus review for uterine and ovarian carcinosarcoma, Int. J. Gynecol. Cancer 24 (9) (2014) S55–S60. [DOI] [PubMed] [Google Scholar]
- [32].Xiaoxin W, Qinghui W, Wei L, Recent advances in heterocyclic tubulin inhibitors targeting the colchicine binding site, Anti Cancer Agents Med. Chem 16 (10) (2016) 1325–1338. [DOI] [PubMed] [Google Scholar]
- [33].Dong M, et al. , Novel natural product- and privileged scaffold-based tubulin inhibitors targeting the colchicine binding site, Molecules 21 (10) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang Z, et al. , Novel tubulin polymerization inhibitors overcome multidrug resistance and reduce melanoma lung metastasis, Pharm. Res 29 (11) (2012) 3040–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bai Z, et al. , BZML, a novel colchicine binding site inhibitor, overcomes multidrug resistance in A549/Taxol cells by inhibiting P-gp function and inducing mitotic catastrophe, Cancer Lett 402 (2017) 81–92. [DOI] [PubMed] [Google Scholar]
- [36].Gangjee A, et al. , Synthesis and discovery of water soluble microtubule targeting agents that bind to the colchicine site on tubulin and circumvent Pgp mediated resistance, J. Med. Chem 53 (22) (2010) 8116–8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Keating AK, et al. , Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity, Mol. Cancer Ther 9 (5) (2010) 1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Elkabets M, et al. , AXL mediates resistance to PI3Kα inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas, Cancer Cell 27 (4) (2015) 533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Linger RMA, et al. , TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer, Adv. Cancer Res 100 (2008) 35–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Byers LA, et al. , An epithelial-mesenchymal transition (EMT) gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance, Clin. Cancer Res 19 (1) (2013) 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang Z, et al. , Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer, Nat. Genet 44 (8) (2012) 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wilson C, et al. , AXL inhibition sensitizes mesenchymal cancer cells to antimitotic drugs, Cancer Res 74 (20) (2014) 5878. [DOI] [PubMed] [Google Scholar]
- [43].Gjerdrum C, et al. , Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival, Proc. Natl. Acad. Sci 107 (3) (2010) 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Paccez JD, et al. , Inactivation of GSK3β and activation of NF-κB pathway via Axl represents an important mediator of tumorigenesis in esophageal squamous cell carcinoma, Mol. Biol. Cell 26 (5) (2015) 821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sun W, Fujimoto J, Tamaya T, Coexpression of Gas6/Axl in human ovarian cancers, Oncology 66 (6) (2004) 450–457. [DOI] [PubMed] [Google Scholar]
- [46].Antony J, et al. , The GAS6-AXL signaling network is a mesenchymal (Mes) molecular subtype-specific therapeutic target for ovarian cancer, Sci. Signal 9 (448) (2016) ra97. [DOI] [PubMed] [Google Scholar]
- [47].Kanlikilicer P, et al. , Therapeutic targeting of AXL receptor tyrosine kinase inhibits tumor growth and intraperitoneal metastasis in ovarian cancer models, Mol. Ther.-Nucleic Acids 9 (2017) 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.