Abstract
High serum uric acid (UA) has been reported to be associated with left ventricular (LV) dysfunction; however, the relationship between UA and plasma B-type natriuretic peptide (BNP), a sensitive biomarker of heart failure, is still unclear. This study investigated their relationship to provide an accurate assessment of high UA. The study patients consisted of 3,077 subjects who underwent cardiac catheterization because of various cardiovascular disorders. Since the explanatory factors of multiple regression analysis were mostly confounding with each other, subgroup analysis was performed by quartering the study population using the respective risk factors and by covariance structure analysis. This analysis revealed that UA was almost always well associated with a reduced LV ejection fraction (LVEF), but generally not with BNP. UA was significantly associated with BNP in lean aged females, but not in obese adolescent males, although LVEF was significantly reduced in response to a high UA in both groups. A high UA is a direct risk factor for cardiac dysfunction from the perspective of BNP; however, augmentation of BNP in response to a high UA would likely be restricted among obese adolescent males. On the other hand, the observed LV systolic dysfunction, such as LVEF, reflects a high UA on an almost constant basis.
Introduction
High serum uric acid (UA) has recently been discussed not only as a gout culprit but also as a cause of cardiovascular disorders. According to previous studies, serum UA levels predict the progression of chronic kidney disease and the development of stroke1–3. Additionally, high serum UA is associated with the presence of hypertension, diabetes, and metabolic syndrome4–8. High serum UA levels also predict an increase in morbidity and mortality in patients with heart failure3,9–13. Recently, we reported that high UA is causally associated with left ventricular (LV) systolic dysfunction and a reduced LV ejection fraction (LVEF) in patients with ischemic heart disease. Importantly, the effect of high UA on LV dysfunction is exerted not only through an atherosclerotic process in the coronary arteries (cardiac ischemia) but also directly, as represented by a possible cause-and-effect relationship14.
B-type natriuretic peptide (BNP) is a natriuretic peptide that is widely used for diagnosis and predicting prognosis in heart failure15–19. The plasma level of BNP is a highly reliable surrogate maker of heart failure. Measurement of plasma BNP is recommended as a reliable diagnostic method of heart failure in general practice and emergency medical care20,21. It is thus conceivable that plasma BNP should be potentially linked to high serum UA and associated LV dysfunction. There have been few reports of a close linkage between serum UA and plasma BNP in heart failure. Instead, there has been a negative report in which UA did not significantly add to the prognostic utility of BNP22. Although a high serum UA level is associated with heart failure, more studies are required to confirm the role of high serum UA in heart failure in consideration of the observed LV dysfunction and plasma BNP.
Statistical analysis would be of assistance in this study; however, analyses are highly intractable for precise study because plasma BNP levels are associated with many confounding factors, including ageing, gender distinction, body mass index (BMI), LV dysfunction, and renal dysfunction23,24. Additionally, serum UA is associated with the same factors14,25. To explore the precise effects of high UA on plasma BNP and the observed LV dysfunction, advanced statistical procedures are warranted.
In this study, we examined the effect of high serum UA on plasma BNP and observed LV dysfunction in patients with cardiovascular diseases who underwent cardiac catheterization in our institution. We performed a step-by-step statistical analysis to examine the possible action of high serum UA on high plasma BNP and observed LV dysfunction, taking into account possible associated factors such as age, gender distinction, BMI and renal function.
Results
Patients’ characteristics
Table 1 shows the patients’ overall and respective sub-group characteristic (gender difference) in this study.
Table 1.
Characteristics of all patients.
| Characteristics | Overall | Female | Male | P value |
|---|---|---|---|---|
| Number (%) or Mean ± SD, Median [interquartile range] | ||||
| Numbers of patients | 3,077 (100.0) | 542 (17.6) | 2,535 (82.4) | |
| Age (years old) | 65.7 ± 11.9 | 69.6 ± 13.0 | 64.8 ± 11.4 | <0.001 |
| BMI (kg/m2) | 24.4 ± 4.0 | 22.5 ± 4.5 | 24.8 ± 3.7 | <0.001 |
| Current smoker | 651 (21.2) | 56 (1.8) (10.3†) | 595 (19.3) (23.5†) | <0.001 |
| Family history of IHD | 743 (24.1) | 131 (4.3) (24.2†) | 615 (19.9) (24.1†) | 0.985 |
| Laboratory findings | ||||
| Hb (g/dL) | 13.4 ± 1.9 | 12.2 ± 1.7 | 13.7 ± 1.8 | <0.001 |
| Creatinine (mg/dL) | 0.92 ± 0.47 | 0.76 ± 0.35 | 0.96 ± 0.49 | <0.001 |
| eGFR (mL/min/1.73 m2) | 68.5 ± 19.6 | 65.6 ± 20.8 | 69.1 ± 19.3 | <0.001 |
| UA (mg/dL) | 6.1 ± 1.5 | 5.5 ± 1.7 | 6.2 ± 1.5 | <0.001 |
| 6.1 [5.1, 7.0] | 5.2 [4.4, 6.5] | 6.2 [5.3, 7.1] | ||
| FBS (mg/dL) | 121.3 ± 42.4 | 114.1 ± 32.9 | 122.9 ± 44.1 | <0.001 |
| HbA1c (%) | 6.3 ± 1.0 | 6.1 ± 0.9 | 6.3 ± 1.0 | 0.002 |
| TG (mg/dL) | 123.4 ± 94.1 | 105.0 ± 52.5 | 127.4 ± 100.3 | <0.001 |
| HDL-C (mg/dL) | 51.7 ± 15.3 | 58.8 ± 16.6 | 50.2 ± 14.5 | <0.001 |
| LDL-C (mg/dL) | 101.6 ± 30.4 | 107.8 ± 32.0 | 100.2 ± 29.9 | <0.001 |
| LDL-C/HDL-C | 2.1 ± 0.8 | 2.0 ± 0.8 | 2.1 ± 0.8 | <0.001 |
| CRP (mg/dL) | 0.46 ± 1.36 | 0.48 ± 1.47 | 0.46 ± 1.34 | 0.722 |
| BNP (ng/L) | 122.5 ± 261.1 | 181.9 ± 351.9 | 109.7 ± 235.2 | <0.001 |
| 43.2 [16.7, 123.3] | 70.0 [26.3, 192.6] | 38.6 [15.2, 111.0] | ||
| Left ventricular hemodynamic parameter | ||||
| LVEF (%) | 56.9 ± 11.8 | 59.0 ± 11.7 | 56.4 ± 11.8 | <0.001 |
| Underlying cardiovascular disease | ||||
| Ischemic heart disease | 2591 (84.2) | 375 (12.2) (69.2†) | 2216 (72.0) (87.4†) | <0.001 |
| Acute coronary syndrome | 444 (14.4) | 69 (2.2) (12.7†) | 375 (12.2) (14.8†) | 0.215 |
| Angina pectoris | 2154 (70.0) | 287 (9.3) (53.0†) | 1867 (60.7) (73.6†) | <0.001 |
| Cardiomyopathy | 236 (7.7) | 62 (2.0) (11.4†) | 174 (5.7) (6.9†) | <0.001 |
| Valvular disease | 220 (7.1) | 77 (2.5) (14.2†) | 143 (4.6) (5.6†) | <0.001 |
| Arrhythmia | 416 (13.5) | 80 (2.7) (14.8†) | 336 (10.9) (13.3†) | 0.352 |
| Atrial fibrillation | 190 (6.2) | 38 (1.3) (7.0†) | 152 (4.9) (6.0†) | 0.327 |
| Other than AF | 226 (7.3) | 42 (1.4) (7.7†) | 184 (6.0) (7.3†) | 0.691 |
| Other clinical diagnosis | ||||
| Hypertension | 2279 (74.1) | 373 (12.1) (68.8†) | 1906 (61.9) (75.2†) | 0.002 |
| Type-2 diabetes mellitus | 1212 (39.4) | 175 (5.7) (32.3†) | 1037 (33.7) (40.9†) | <0.001 |
| Dyslipidemia | 2221 (72.2) | 368 (12.0) (67.9†) | 1853 (60.2) (73.1†) | 0.014 |
| Medication | ||||
| Antiplatelet agents | 2153 (70.0) | 299 (9.7) (55.2†) | 1854 (60.3) (73.1†) | <0.001 |
| Anticoagulant agents | 407 (13.2) | 79 (2.6) (14.6†) | 328 (10.7) (12.9†) | 0.307 |
| ACE inhibitors | 597 (19.4) | 82 (2.7) (15.1†) | 515 (16.7) (20.3†) | 0.006 |
| ARBs | 1201 (39.0) | 202 (6.6) (37.3†) | 999 (32.5) (39.4†) | 0.354 |
| Beta blockers | 1235 (40.1) | 187 (6.1) (34.5†) | 1048 (34.1) (41.3†) | 0.003 |
| Calcium channel blockers | 1626 (52.8) | 270 (8.8) (49.8†) | 1356 (44.1) (53.5†) | 0.120 |
| Diuretics | 633 (20.6) | 144 (4.7) (26.6†) | 489 (15.9) (19.3†) | <0.001 |
| Anti-hyperuricemia | 501 (16.3) | 35 (1.1) (6.5†) | 466 (15.1) (18.4†) | <0.001 |
| Statins | 1794 (58.3) | 291 (9.5) (53.7†) | 1503 (48.8) (59.3†) | 0.016 |
| Other than statins | 349 (11.3) | 65 (2.1) (12.0†) | 284 (9.2) (11.2†) | 0.599 |
| Oral antidiabetic agents | 839 (27.3) | 120 (3.9) (22.1†) | 719 (23.4) (28.4†) | 0.003 |
| Insulin | 276 (9.0) | 50 (1.6) (9.2†) | 236 (7.3) (8.9†) | 0.819 |
Hb, hemoglobin; UA, uric acid; FBS, fasting blood sugar; HbA1c, hemoglobin A1c; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ACE, angiotensin-converting enzyme; ARBs, angiotensin II type I-receptor blockers; BMI, body mass index; BNP, B-type natriuretic peptide; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; IHD, ischemic heart disease; and LVEF, left ventricular ejection fraction. †, the percentage of each sub-group of patients. The significant difference comparisons between female group and male group were described using P value.
Single regression analysis among serum UA, plasma BNP and LVEF
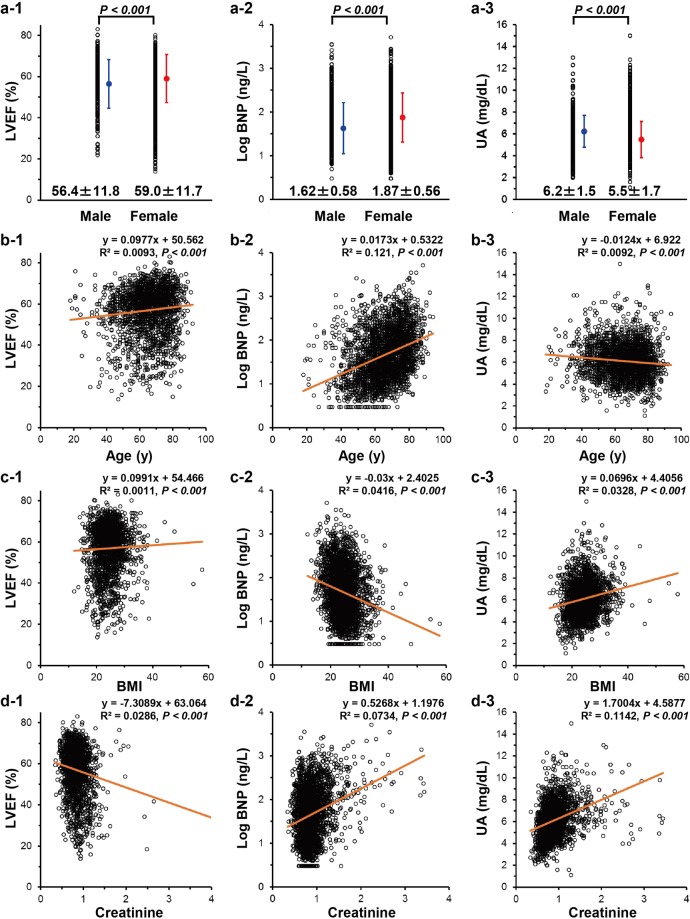
Figure 1 shows the single regression analysis between two values among UA, plasma BNP, and LVEF. There was a significant correlation in the respective graphs (P < 0.001). There appears to be a close linkage among serum UA, plasma BNP and LVEF.
Figure 1.
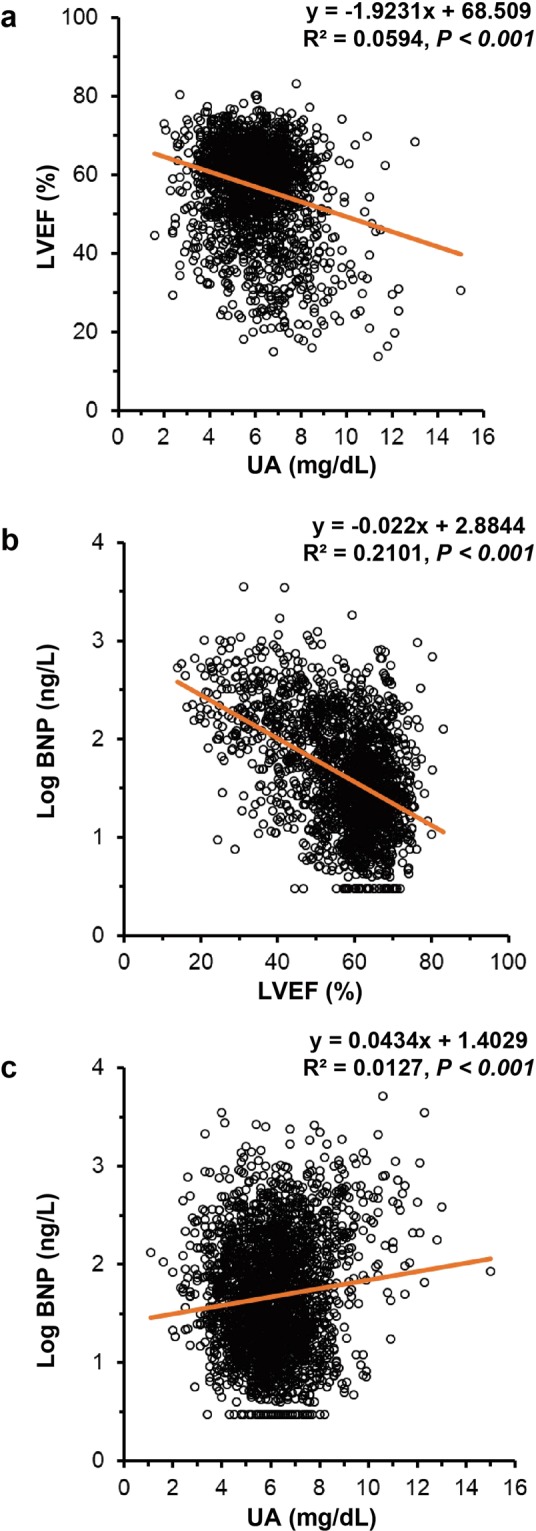
Single regression analysis among serum UA, plasma BNP and LVEF Scatter plots and a simple regression line (orange straight line) with the regression equation demonstrate the correlation between two values among UA, plasma BNP, and LVEF. BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; UA, uric acid.
Single regression analysis among serum UA, plasma BNP, LVEF and other risk factors
Several potential risk factors, which were listed as candidates based on previous information, were included in the analysis: gender, age, BMI and creatinine (Cr). To examine a possible interruption of a possible linkage of serum UA to plasma BNP and LVEF by these surrounding factors, a single regression analysis was performed (also see Table 2), and the respective effects of age, BMI or Cr on serum UA or plasma BNP are presented in Fig. 2(a-1–d-3). As a result, female gender was positively associated with LVEF (a-1) and Log BNP (a-2) but negatively associated with serum UA (a-3; P < 0.001). Age was positively associated with LVEF (b-1) and Log BNP (b-2) but negatively associated with high UA (b-3; P < 0.001). BMI was negatively associated with Log BNP (c-2) but positively associated with high UA (c-3) and LVEF (c-1; P < 0.001). Additionally, Cr was positively associated with Log BNP (d-2) and serum UA (d-3) but negatively associated with LVEF (d-1; P < 0.001). These analyses suggested that surrounding factors such as ageing, BMI and Cr would seemingly modulate the authentic relationship among serum UA, plasma BNP and LVEF.
Table 2.
Results of Pearson’s product-moment correlation coefficient analysis of hemodynamic parameters.
| Age | BMI | Creatinine | UA | Log BNP | LVEF | |
|---|---|---|---|---|---|---|
| Age | — | −0.245 | 0.122 | −0.094 | 0.348 | 0.097 |
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | ||
| BMI | −0.245 | — | 0.058 | 0.178 | −0.200 | 0.034 |
| P < 0.001 | P = 0.002 | P < 0.001 | P < 0.001 | P = 0.136 | ||
| Creatinine | 0.122 | 0.058 | — | 0.248 | 0.261 | −0.169 |
| P < 0.001 | P = 0.002 | P < 0.001 | P < 0.001 | P < 0.001 | ||
| UA | −0.094 | 0.178 | 0.248 | — | 0.112 | −0.244 |
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | ||
| Log BNP | 0.348 | −0.200 | 0.261 | 0.112 | — | −0.458 |
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | ||
| LVEF | 0.097 | 0.034 | −0.169 | −0.244 | −0.458 | — |
| P < 0.001 | P = 0.136 | P < 0.001 | P < 0.001 | P < 0.001 |
BMI, body mass index; BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; and UA, uric acid.
Figure 2.
Single regression analysis among serum UA, plasma BNP, LVEF and other risk factors Scatter plots and a simple regression line (orange straight line) with the regression equation demonstrate the correlation between two values among UA, plasma BNP, LVEF, gender, age, BMI, and creatinine. The means of each value are marked in blue (male) or red (female) plots in each panel, with standard deviations (same colored lines). Statistical significance was indicated as the P value with the horizontal bar in the upper part of each scatter plot in the top three panels. BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; UA, uric acid.
Multivariate analysis for determination of the risk factors of LVEF or BNP
Next, to examine the contribution of serum UA and the surrounding factors to LVEF, we adopted a multiple linear regression analysis, as shown in Table 3. As a result, the analysis revealed that age, UA, Cr and BMI represented risks for a low LVEF (P < 0.001), except for male gender. Next, multiple linear regression analysis was adapted for the determination of Log BNP, as shown in Table 3. As a result, the analysis revealed that ageing, female gender, high Cr, low BMI and low LVEF represented causes for a high Log BNP (P < 0.001); however, UA did not affect the Log BNP. These results suggested that high UA clearly represented a direct risk for a reduction in LVEF, even under the influence of surrounding factors. On the other hand, the association between high UA and plasma BNP was low or unrecognizable.
Table 3.
Results of the multiple regression analysis to identify clinical factors influencing the LVEF and logarithmic BNP levels (in upper and bottom tables, respectively).
| Dependent variable: LVEF (R2 = 0.089) | Regression coefficient [95% CI] | Standardized regression coefficient | P value | VIF |
|---|---|---|---|---|
| Age | 0.117 [0.071, 0.163] | 0.116 | <0.001 | 1.133 |
| Gender | −0.444 [−1.846, 0.957] | −0.015 | 0.534 | 1.156 |
| UA | −1.719 [−2.082, −1.356] | −0.218 | <0.001 | 1.160 |
| Creatinine | −5.419 [−7.408, −3.431] | −0.125 | <0.001 | 1.161 |
| BMI | 0.380 [0.245, 0.515] | 0.130 | <0.001 | 1.156 |
| Constant | 55.315 [50.080, 60.550] | — | <0.001 | — |
| Dependent variable: Log BNP (R2 = 0.401) | Regression coefficient [95% CI] | Standardized regression coefficient | P value | VIF |
| Age | 0.016 [0.014, 0.017] | 0.320 | <0.001 | 1.148 |
| Gender | −0.263 [−0.318, −0.209] | −0.180 | <0.001 | 1.156 |
| UA | 0.011 [−0.003, 0.026] | 0.030 | 0.122 | 1.210 |
| Creatinine | 0.234 [0.156, 0.312] | 0.113 | <0.001 | 1.178 |
| BMI | −0.013 [−0.019, −0.008] | −0.095 | <0.001 | 1.173 |
| LVEF | −0.023 [−0.025, −0.021] | −0.476 | <0.001 | 1.101 |
| Constant | 2.198 [1.972, 2.424] | — | <0.001 | - |
R2 adjusted coefficient of determination; BMI, body mass index; BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; UA, uric acid; CI, confidence interval; VIF, variance inflation factor.
Subgroup analysis using covariance structure analysis
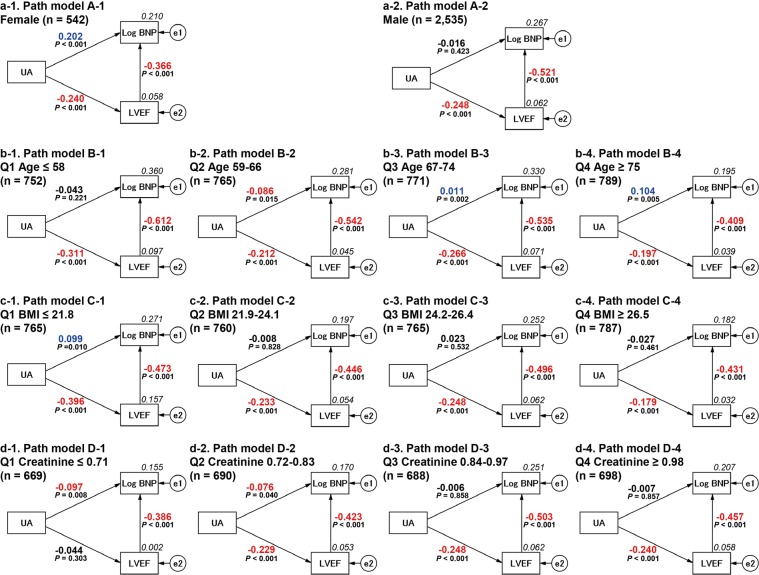
These respective multivariate analyses would still be inadequate for correctly interpreting the risk factors because the factors are expected to confound each other (Fig. 3). We thought that the authentic relationship of high UA with plasma BNP and LVEF was still unclear. To examine the more precise relationship among serum UA, plasma BNP and LVEF, we adopted a covariance structure analysis in the respective subgroups; the subgroups were designed by gender difference and by quartile groups of ageing, BMI and Cr. The covariance structure analysis was planned to construct a simultaneous comparison of the effect of serum UA on plasma BNP and LVEF in one equation model. As a matter of logic, the theoretical path model was proposed by positioning the serum UA level for the determination of both LVEF and Log BNP with a possible causal link from LVEF to Log BNP.
Figure 3.
Path models from A-1 to D-4 were dependent on the gender difference, ageing, BMI, and creatinine (the groups quartered by each value) The path has a coefficient showing the standardized coefficient of a regressing independent variable on a dependent variable of the relevant path (UA to Log BNP; UA to LVEF; LVEF to Log BNP). These variables are standardized regression coefficients (direct effect) with P values if statistical significance was present, squared multiple correlations [on the upper right side of rectangles (in italics)] and correlations among exogenous variables. The positive regression coefficients are indicated as blue-colored values, and the negative regression coefficients are indicated as red-colored values. Uncolored values denote P values less than 0.05. BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; UA, uric acid; e, extraneous variable.
Results of path models A-1 and A-2 depending on the gender difference
Path models A-1 and A-2 in Fig. 3(a-1,a-2) and Supplementary Table S1 show the results for gender difference. In the female group (path model A-1), the analysis revealed that UA was significantly linked to LVEF and Log BNP (P < 0.001). Additionally, there was a significant linkage from LVEF to Log BNP (P < 0.001). On the other hand, in the male group (path model A-2), the analysis revealed that UA was significantly linked to LVEF (P < 0.001) but not to Log BNP (P = 0.423), although there was a significant linkage from LVEF to Log BNP (P < 0.001). These results showed that high UA had a negative impact on LVEF in different genders, but it had a positive impact on Log BNP only in females.
Results of path models B-1, B-2, B-3 and B-4 depending on ageing
Path model B-1, B-2, B-3 and B-4 in Fig. 3(b-1,b-2,b-3 and b-4) and Supplementary Table S2 show the results for ageing. The analysis in path model B-1 showed that UA was significantly linked to LVEF (P < 0.001) but not to Log BNP (P = 0.221). Path model B-2 showed that serum UA showed a significant link to LVEF (P < 0.001) and an inverse link to Log BNP (not a positive link) (P = 0.015). Path model B-3 showed that serum UA had a significant link to LVEF (P < 0.001) and Log BNP (P = 0.002). Additionally, path model B-4 showed that serum UA had a significant link to LVEF (P < 0.001) and Log BNP (P = 0.005). These results showed that high UA had a significant impact on LVEF at any age and on Log BNP only in the elderly population (over 67 years).
Results of path models C-1, C-2, C-3 and C-4 depending on BMI
Path models C-1, C-2, C-3 and C-4 in Fig. 3(c-1,c-2,c-3 and c-4) and Supplementary Table S3 show the results for BMI. The analysis in path model C-1 revealed that serum UA was significantly linked to LVEF (P < 0.001) and Log BNP (P = 0.010). Path model C-2 showed that serum UA showed a significant link to LVEF (P < 0.001) but not to Log BNP (P = 0.828). Path model C-3 showed that serum UA had a significant link to LVEF (P < 0.001) but not to Log BNP (P = 0.532). Additionally, path model C-4 showed that serum UA had a significant link to LVEF (P < 0.001) but not to Log BNP (P = 0.461). These results showed that high UA had a significant impact on LVEF at any stage of BMI, but it had an effect on Log BNP only in the lean body mass population (BMI ≤ 21.8).
Results of path models D-1, D-2, D-3 and D-4 depending on Cr
Path models D-1, D-2, D-3 and D-4 in Fig. 3(d-1,d-2,d-3 and d-4) and Supplementary Table S4 show the results for Cr. The analysis of path model D-1 revealed that serum UA was not significantly associated with LVEF (P = 0.303) and was significantly associated with Log BNP (not a positive link) (P = 0.008). Path model D-3 showed that serum UA was significantly associated with LVEF (P < 0.001) but not with Log BNP (P = 0.858). Additionally, path model D-4 showed that serum UA was significantly associated with LVEF (P < 0.001) but not with Log BNP (P = 0.857).
Summary of the results of the covariance structure analysis and another single regression analysis among the discriminative groups
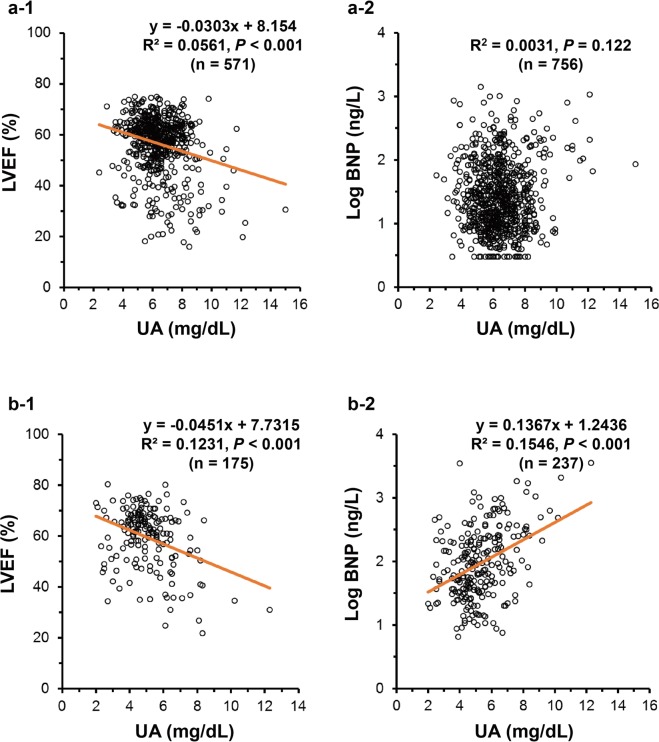
Summarizing the results of the covariance structure analysis among all path models (A, B, C and D in Fig. 3) revealed that high serum UA was almost always associated with reduced LVEF, except in one model (path model D-1 in Fig. 3). On the other hand, the authentic association between serum UA and plasma BNP was recognized only among several subgroups: the female gender group (path model A-1 in Fig. 3), the aged groups (path models B-3 and B-4 in Fig. 3) and the lean body mass group (path model C-1 in Fig. 3). When taken together, the results showed that the association of high serum UA and high plasma BNP might be clarified more sharply among lean aged females but was not observed among obese, adolescent males. In the final analysis, a single regression analysis was performed in respective population groups with cut-off values of 67 years in age and 24.2 in BMI, as shown in Fig. 4. As a consequence, there was indeed a close correlation between serum UA and LVEF (b-1 in Fig. 4) and between serum UA and Log BNP (b-2 in Fig. 4, P < 0.001) among lean aged females. On the other hand, there was a close linkage between serum UA and LVEF (a-1 in Fig. 4, P < 0.001) but never between serum UA and Log BNP (a-2 in Fig. 4, P = 0.122) among obese, adolescent males.
Figure 4.
Single regression analysis between UA and LVEF and between UA and Log BNP among obese, adolescent males, and among lean, aged females These upper and lower panels demonstrate a single regression analysis between UA and LVEF, and UA and Log BNP among each group, divided into obese, adolescent males (upper panels: age <67 years and BMI ≥ 24.2 kg/m2) and lean, aged females (lower panels: age ≥67 years and BMI < 24.2 kg/m2). Scatter plots and a simple regression line (orange straight line) with the regression equation demonstrate the correlation between the two values among UA, plasma BNP, and LVEF. BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; UA, uric acid.
Discussion
This study was planned to revalidate the harmful effects of high serum UA on LV function from the perspective of plasma BNP, which is regarded as a sensitive biomarker of LV dysfunction. Thus, the precise relationship among serum UA, plasma BNP and LVEF was examined in a large number of patients with cardiovascular diseases. First, we performed a multivariate analysis, which showed that high serum UA was a significant cause of reduced LVEF but did not affect plasma BNP. This result represents a curious consequence because serum UA, LVEF and plasma BNP were closely linked in the single regression analysis. We then hypothesized that some surrounding factors mediate high serum UA and high plasma BNP, blunting their authentic relationship. By reference to the single regression analysis including surrounding factors, we proposed hierarchical equation models in the respective subgroups. Importantly, the models clearly demonstrated that 1) high serum UA was sharply associated with reduced LVEF and that 2) plasma BNP authentically responded to high serum UA, but only under the appropriate conditions, such as among lean aged females but never among obese, adolescent males.
As observed above, we reaffirmed that high serum UA is a risk factor for cardiac dysfunction, which can be evaluated not only by LVEF but also by plasma BNP, even under some limitations. Although the molecular mechanisms underlying the unfavorable effects of high serum UA on the heart have not been precisely examined, potential mechanisms are as follows. The UA level is elevated with subsequent upregulation of the xanthine oxidase (XO) enzymatic pathway, which accounts for the production of reactive oxygen species (ROS) via nicotinamide adenine dinucleotide phosphate (NADPH)26–28. High XO-induced ROS production would be significant throughout the whole body, but the precise mechanism in the local heart has not been well clarified. However, it has recently been reported that cardiac function is reduced in accordance with energy deficiency, which is linked to possible cardiac UA production and XO activity29–31. If XO enzymatic activity is sufficiently upregulated for a local increase in oxidative stress in the heart, BNP production may also be activated under such circumstances32. As outlined above, high serum UA, high plasma BNP and low LVEF would be mutually related in heart failure by oxidative stress, which is probably activated not only throughout the whole body but also locally in the heart.
The possible reasons or mechanisms for blunting the close linkage between serum UA and plasma BNP under a given set of conditions are as follows. First, we briefly describe the possible reasons for the beclouded linkage between serum UA and plasma BNP from the perspective of obesity. The molecular mechanisms have just started to be examined; however, it has recently been reported that natriuretic peptide reduces body mass by improving the biological function of adipocytes and accelerating fat burning33. On the other hand, free fatty acid suppresses the production of BNP34,35. These pathological actions would lead to a close linkage between low fat content and high plasma BNP or between high fat content and low plasma BNP. However, it is quite natural that high body mass would be substantially linked to high UA, suggesting that adipose tissue or FFA has different effects on serum UA (raising) and plasma BNP (lowering). Thus, the authentic correlation between serum UA and plasma BNP becomes less visible in obesity.
Second, it is well accepted that serum UA is higher in youth because of increased metabolic activity, high physical activity and a high level of food intake without a relationship to heart failure status. Additionally, the plasma BNP level would generally be low in the young population compared with the aged population24. Ageing then likely has different effects on serum UA (raising) and plasma BNP (lowering); thus, the authentic correlation between serum UA and plasma BNP becomes less apparent in the adolescent population.
Third, the strong linkage was absolute between serum UA and plasma BNP in females; however, the linkage disappeared in males. It is difficult to proceed with a discussion of the precise mechanism(s). Sex steroid hormones may be associated with serum UA36–38 and with plasma BNP via a wide variety of mechanisms39–41.
Finally, a close linkage between serum UA and plasma BNP was hardly observed in any stage of renal dysfunction. The reasons or mechanisms are unknown; however, other risk factors including ageing, male gender and obesity, which are highly correlated with renal dysfunction, would have substantially affected the values of UA and BNP in diverse ways. Importantly, the close correlation between serum UA and reduced LVEF was mostly recognizable in advanced stages of renal dysfunction in this study.
The direct effect of high serum UA on cardiac damage would be definite based on the results of the current and our previous study14. However, this study clearly demonstrated that the adverse effect of high serum UA on heart failure status would likely be underestimated only by measuring plasma BNP, which must be dealt with carefully in clinical practice. It is important to search for factors or clinical parameters that are strongly associated with serum UA. For example, oxidative stress markers may be conceivable. Additionally, the direct measurement of XO activity in the circulation enters the picture. On the other hand, the harmful effects of high serum UA are almost always demonstrated by the observed cardiac function such as the LVEF. In a further study, we should investigate whether high serum UA is more likely linked to patients with heart failure with an reduced ejection fraction (HFrEF) or to those with heart failure with a preserved ejection fraction (HFpEF).
Limitations of this study
This was a cross-sectional study, and regarding the underlying diseases of the patients in this study, most had ischemic heart disease (84.2%). Further analysis is warranted to determine the adverse effect of high UA prospectively in a large number and various kinds of patients with heart failure. Additionally, in the present study, we did not directly examine the biological interaction between BNP and UA. Therefore, there may be a limitation to determining whether UA affects BNP or whether BNP affects UA as a definitive conclusion. A precise biological analysis should be conducted in the future.
Conclusion
High serum UA is a direct risk factor for cardiac dysfunction from the perspective of plasma BNP; however, augmentation of plasma BNP in response to high serum UA would likely be restricted among obese adolescent males. On the other hand, the observed LV systolic dysfunction such as LVEF sharply reflects high serum UA on an almost constant basis.
Methods
Study patients
The study patients consisted of 3,077 subjects with any cause who were consecutively admitted to our institutions from 2012 to 2016. All patients underwent cardiac catheterization for an evaluation of respective cardiac disorders. We excluded patients receiving hemodialysis because cardiac function was largely changed by artificial volume control. The ethics committee of The Jikei University School of Medicine approved the study protocol (24-355[7121]), and we complied with our institution’s routine ethical regulations. Informed consent was obtained from each patient, and all clinical investigations were conducted in accordance with the principles expressed in the Declaration of Helsinki. According to our routine ethical regulations, we posted a notice about the study design and contact information at a public location in our institution.
Underlying cardiac diseases and their definitions
Baseline diseases, hypertension, diabetes mellitus, and dyslipidemia were defined as previously described23,24. The definitions of the diseases were as follows: In brief, ischemic heart disease (IHD) was diagnosed by symptoms, electrocardiography results, blood sampling, and the morphology of the coronary arteries. The patients with IHD included those with clinically stable IHD. Organic stenosis was defined as ≥75% occlusion of the coronary arteries on coronary angiography. The patients with coronary spastic angina were included in the IHD group if the disease activity was stable, and a provocation test was planned during hospitalization. Valvular diseases included heart failure caused by moderate valvular disease and patients who were scheduled for surgery. Arrhythmia included the need for catheter ablation, an implantable cardioverter-defibrillator, cardiac resynchronization therapy, and patients with a pacemaker or syncope. Cardiomyopathy was defined when patients were diagnosed before admission and underwent treatment or if they were diagnosed after admission (excluding cases of ischemic cardiomyopathy). Infectious heart disease included pericarditis, myocarditis, and infectious endocarditis. BMI was calculated by the square of the height divided by the weight on admission.
Cardiac catheterization
LVEF was obtained from the left ventriculography (LVG) trace during the end-systolic and end-diastolic phases. The contrast LVG images were acquired at a frame rate of 30 frames per second in the right anterior oblique 30-degree projection. The LVEF was calculated from single-plane cineangiograms by means of the area-length formula using a semi-automated trace method with QAngio XA version 7.1 (Medis Medical Imaging Systems B.V., Leiden, The Netherlands).
Blood sampling and measurement of plasma BNP and other levels
We collected data for plasma BNP and other levels during cardiac catheterization. Whole blood (5 mL) was collected in tubes containing sodium ethylenediaminetetraacetic acid (EDTA) (1 mg/mL blood). Plasma BNP was measured with a rapid enzyme-linked immunosorbent assay (non-extracted) using an antibody against human BNP (Shionogi Co. Ltd., Tokyo, Japan). Serum biochemical analyses, including UA and Cr, were performed in a central laboratory in our hospital during the study.
Statistical analysis
Continuous variables are expressed as the means ± standard deviation (SD) or medians with the interquartile range [IQR]. Categorical variables are expressed as the percentages of overall and/or each sub-group and were compared using a chi-square test as appropriate. Comparisons between two data sets of continuous variables were performed using Pearson’s product-moment correlation coefficient analysis, and significant differences between them were analyzed using a t-test and/or the Mann–Whitney U test where appropriate. After confirming the normality of distribution of the BNP variable by using a formal test, to achieve a normal distribution, the BNP value was log-transformed to (Log) BNP before conducting the analysis. Multiple regression analysis was performed when multiple values were compared. All statistical analyses were performed using SPSS Statistics version 23.0 (SPSS Inc., Chicago, IL, USA).
Path analysis based on covariance structure analysis was used to investigate the relationship among clinical factors in this study population and, in particular, to identify probable causal effects in heart failure. Path analysis was performed using IBM SPSS AMOS version 23 (Amos Development Corporation, Meadville, PA, USA). The obtained structural equation models were tested and confirmed at the significance level of P values < 0.05. The implementation procedures for the covariance structure analysis have been described previously14,42–47. The paths between the variables were drawn from the independent to the dependent variables with directional arrows that were used for every regression model (i.e., arrowhead at one end only). A two-way arrow between two variables indicated a correlation between these variables. For every regression, the total variance in the dependent variable was theorized to be caused either by the independent variables in the model or by extraneous variables (e). Each path had a coefficient that indicated the standardized coefficient of the regressing independent variable on the dependent variable of the relevant path. The indirect effect was determined by multiplying the path coefficients of the intervening variables. The causality model defined certain hierarchical regression models that compare clinical factors in LVEF and plasma BNP. The corresponding author had full access to all the data in the study and takes responsibility for both its integrity and the data analysis.
Supplementary information
Acknowledgements
We appreciate all of the trial physicians and nurses at all of the participating hospitals for their important contributions to this study. We are grateful to Ms. Kumiko Nishiyama for assisting with the data. We thank Nature Research Editing Service (https://authorservices.springernature.com) for the English language editing.
Author Contributions
Y.O., M.K. and M.Y. conceived and designed this study, Y.O., M.K., K.M., K.O. Y.I. S.M. T.T.T.N. and T.O. acquired the data, Y.O., M.K., K.M. K.O. and M.Y. analyzed and interpreted the data. Y.O., M.K. and M.Y. wrote the main manuscript text. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Data Availability
All relevant or analyzed data during this study are included in this published article (and its Supplementary Table file).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37053-0.
References
- 1.Kang DH, et al. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002;13:2888–2897. doi: 10.1097/01.ASN.0000034910.58454.FD. [DOI] [PubMed] [Google Scholar]
- 2.Uchida S, et al. Targeting Uric Acid and the Inhibition of Progression to End-Stage Renal Disease–A Propensity Score Analysis. PLoS One. 2015;10:e0145506. doi: 10.1371/journal.pone.0145506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feig D. I., Kang D. H., Johnson R. J. Uric acid and cardiovascular risk. N. Engl. J. Med. 359, 1811–1821, 10.1056/NEJMra0800885 (2008), Erratum in: N. Engl. J. Med. 362, 2235, 10.1056/NEJMx100028 (2010). [DOI] [PMC free article] [PubMed]
- 4.Kuriyama S, et al. Serum uric acid and the incidence of CKD and hypertension. Clin. Exp. Nephrol. 2015;19:1127–1134. doi: 10.1007/s10157-015-1120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forman JP, et al. Association between sodium intake and change in uric acid, urine albumin excretion, and the risk of developing hypertension. Circulation. 2012;125:3108–3116. doi: 10.1161/CIRCULATIONAHA.112.096115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cicero AF, et al. Brisighella Heart Study Group. High serum uric acid is associated to poorly controlled blood pressure and higher arterial stiffness in hypertensive subjects. Eur J Intern Med. 2017;37:38–42. doi: 10.1016/j.ejim.2016.07.026.. [DOI] [PubMed] [Google Scholar]
- 7.Kodama S, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32:1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicero AFG et al. Brisighella Heart Study Group. LDL-oxidation, serum uric acid, kidney function and pulse-wave velocity: Data from the Brisighella Heart Study cohort. Int J Cardiol. 261, 204–208, 10.1016/j.ijcard.2018.03.077. Epub 2018 Mar17 (2018). [DOI] [PubMed]
- 9.Hare J. M., Johnson R. J. Uric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation 2003 107, 1951–1953, 10.1161/01.CIR.0000066420.36123.35 (2003). [DOI] [PubMed]
- 10.Sakai H, et al. Serum level of uric acid, partly secreted from the failing heart, is a prognostic marker in patients with congestive heart failure. Circ. J. 2006;70:1006–1011. doi: 10.1253/circj.70.1006. [DOI] [PubMed] [Google Scholar]
- 11.Volterrani M, Iellamo F, Sposato B, Romeo F. Uric acid lowering therapy in cardiovascular diseases. Int. J. Cardiol. 2016;213:20–22. doi: 10.1016/j.ijcard.2015.08.088. [DOI] [PubMed] [Google Scholar]
- 12.Doehner W, et al. Uric acid and xanthine oxidase in heart failure - Emerging data and therapeutic implications. Int. J. Cardiol. 2016;213:15–19. doi: 10.1016/j.ijcard.2015.08.089. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki H, et al. The prognostic impact of uric acid in patients with severely decompensated acute heart failure. J. Cardiol. 2016;68:384–391. doi: 10.1016/j.jjcc.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y, et al. Close linkage between serum uric acid and cardiac dysfunction in patients with ischemic heart disease according to covariance structure analysis. Sci. Rep. 2017;7:2519. doi: 10.1038/s41598-017-02707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 16.Mukoyama M, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J. Clin. Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasue H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/circ.90.1.8025996. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S, et al. Plasma level of B-type natriuretic peptide as a prognostic marker after acute myocardial infarction: a long-term follow-up analysis. Circulation. 2004;110:1387–1391. doi: 10.1161/01.CIR.0000141295.60857.30. [DOI] [PubMed] [Google Scholar]
- 19.Daniels LB, et al. Prognosis of stage A or B heart failure patients with elevated B-type natriuretic peptide levels. J. Card. Fail. 2010;16:93–98. doi: 10.1016/j.cardfail.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Kelder JC, et al. Quantifying the added value of BNP in suspected heart failure in general practice: an individual patient data meta-analysis. Heart. 2011;97:959–963. doi: 10.1136/hrt.2010.220426. [DOI] [PubMed] [Google Scholar]
- 21.Nakata K, et al. The Optimal Cut-off Value of Plasma BNP to Differentiate Heart Failure in the Emergency Department in Japanese Patients with Dyspnea. Intern. Med. 2015;54:2975–2980. doi: 10.2169/internalmedicine.54.4786. [DOI] [PubMed] [Google Scholar]
- 22.Henry-Okafor Q, et al. Relationship between Uric Acid Levels and Diagnostic and Prognostic Outcomes in Acute Heart Failure. Open Biomark. J. 2012;5:9–15. doi: 10.2174/1875318301205010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakane T, et al. Contribution of extracardiac factors to the inconsistency between plasma B-type natriuretic peptide levels and the severity of pulmonary congestion on chest X-rays in the diagnosis of heart failure. Intern. Med. 2012;51:239–248. doi: 10.2169/internalmedicine.51.6206. [DOI] [PubMed] [Google Scholar]
- 24.Kawai M, et al. Determination of the B-type natriuretic peptide level as a criterion for abnormalities in Japanese individuals in routine clinical practice: the J-ABS Multi-Center Study (Japan Abnormal BNP Standard) Intern. Med. 2013;52:171–177. doi: 10.2169/internalmedicine.52.8704. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, et al. The interaction effects of risk factors for hypertension in adults: a cross-sectional survey in Guilin, China. BMC Cardiovasc. Disord. 2016;16:183. doi: 10.1186/s12872-016-0358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viel EC, et al. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H281–H288. doi: 10.1152/ajpheart.00304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura T, et al. Effects of topiroxostat and febuxostat on urinary albumin excretion and plasma xanthine oxidoreductase activity in db/db mice. Eur. J. Pharmacol. 2016;780:224–231. doi: 10.1016/j.ejphar.2016.03.055. [DOI] [PubMed] [Google Scholar]
- 28.Yisireyili M, et al. Xanthine oxidase inhibition by febuxostat attenuates stress-induced hyperuricemia, glucose dysmetabolism, and prothrombotic state in mice. Sci. Rep. 2017;7:1266. doi: 10.1038/s41598-017-01366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch GA, Bottomley PA, Gerstenblith G, Weiss RG. Allopurinol acutely increases adenosine triphospate energy delivery in failing human hearts. J. Am. Coll. Cardiol. 2012;59:802–808. doi: 10.1016/j.jacc.2011.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opie LH. Allopurinol for heart failure: novel mechanisms. J. Am. Coll. Cardiol. 2012;59:809–812. doi: 10.1016/j.jacc.2011.09.072. [DOI] [PubMed] [Google Scholar]
- 31.Bottomley PA, et al. Metabolic rates of ATP transfer through creatine kinase (CK Flux) predict clinical heart failure events and death. Sci. Transl. Med. 2013;5:215re3. doi: 10.1126/scitranslmed.3007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mushtaq S, et al. N-Acetyl Cysteine Inhibits Endothelin-1-Induced ROS Dependent Cardiac Hypertrophy through Superoxide Dismutase Regulation. Cell. J. 2015;17:355–360. doi: 10.22074/cellj.2016.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura H, et al. The thermogenic actions of natriuretic peptide in brown adipocytes: The direct measurement of the intracellular temperature using a fluorescent thermoprobe. Sci. Rep. 2017;7:12978. doi: 10.1038/s41598-017-13563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuno Y, et al. Cardiac production of B-type natriuretic peptide is inversely related to the plasma level of free fatty acids in obese individuals - possible involvement of the insulin resistance. Endocr. J. 2013;60:87–95. doi: 10.1507/endocrj.EJ12-0239. [DOI] [PubMed] [Google Scholar]
- 35.Zois NE, et al. Natriuretic peptides in cardiometabolic regulation and disease. Nat. Rev. Cardiol. 2014;11:403–412. doi: 10.1038/nrcardio.2014.64. [DOI] [PubMed] [Google Scholar]
- 36.Nagasawa Y, et al. Serum Uric Acid Level Predicts Progression of IgA Nephropathy in Females but Not in Males. PLoS One. 2016;11:e0160828. doi: 10.1371/journal.pone.0160828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurahashi H, et al. Testosterone replacement elevates the serum uric acid levels in patients with female to male gender identity disorder. Endocr. J. 2013;60:1321–1327. doi: 10.1507/endocrj.EJ13-0203. [DOI] [PubMed] [Google Scholar]
- 38.Yahyaoui R et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J. Clin. Endocrinol. Metab. 93, 2230-2233, 10.1210/jc.2007-2467. PMID: 18349066 (2008). [DOI] [PubMed]
- 39.Chang AY, et al. Associations among androgens, estrogens, and natriuretic peptides in young women: observations from the Dallas Heart Study. J. Am. Coll. Cardiol. 2007;49:109–116. doi: 10.1016/j.jacc.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 40.El-Gehani F, Tena-Sempere M, Ruskoaho H, Huhtaniemi I. Natriuretic peptides stimulate steroidogenesis in the fetal rat testis. Biol. Reprod. 2001;65:595–600. doi: 10.1095/biolreprod65.2.595. [DOI] [PubMed] [Google Scholar]
- 41.Khurana ML, Pandey KN. Receptor-mediated stimulatory effect of atrial natriuretic factor, brain natriuretic peptide, and C-type natriuretic peptide on testosterone production in purified mouse Leydig cells: activation of cholesterol side-chain cleavage enzyme. Endocrinology. 1993;133:2141–2149. doi: 10.1210/endo.133.5.8404664. [DOI] [PubMed] [Google Scholar]
- 42.Kinoshita K, et al. Potent influence of obesity on suppression of plasma B-type natriuretic peptide levels in patients with acute heart failure: An approach using covariance structure analysis. Int. J. Cardiol. 2016;215:283–290. doi: 10.1016/j.ijcard.2016.04.111. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa K, et al. Parallel comparison of risk factors between progression of organic stenosis in the coronary arteries and onset of acute coronary syndrome by covariance structure analysis. PLoS One. 2017;12:e0173898. doi: 10.1371/journal.pone.0173898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito S, et al. Possible increase in insulin resistance and concealed glucose-coupled potassium-lowering mechanisms during acute coronary syndrome documented by covariance structure analysis. PLoS One. 2017;12:e0176435. doi: 10.1371/journal.pone.0176435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsutsumi J, et al. Manifold implications of obesity in ischemic heart disease among Japanese patients according to covariance structure analysis: Low reactivity B-type natriuretic peptide as an intervening risk factor. PLoS One. 2017;12:e0177327. doi: 10.1371/journal.pone.0177327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida J, et al. Associations between Left Ventricular Cavity Size and Cardiac Function and Overload Determined by Natriuretic Peptide Levels and a Covariance StructureAnalysis. Sci. Rep. 2017;7:2037. doi: 10.1038/s41598-017-02247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukumoto R, et al. Conflicting relationship between age-dependent disorders, valvular heart disease and coronary artery disease by covariance structure analysis: Possible contribution of natriuretic peptide. PLoS One. 2017;12:e0181206. doi: 10.1371/journal.pone.0181206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant or analyzed data during this study are included in this published article (and its Supplementary Table file).