Nontypeable Haemophilus influenzae (NTHi) bacteria express various molecules that contribute to their virulence. The presence of phosphocholine (PCho) on NTHi lipooligosaccharide increases adhesion to epithelial cells and is an advantage for the bacterium, enabling nasopharyngeal colonization, as measured in humans and animal models.
KEYWORDS: CRP, complement resistance, Haemophilus influenzae, IgM, lipooligosaccharide, phosphorylcholine, TEPC-15
ABSTRACT
Nontypeable Haemophilus influenzae (NTHi) bacteria express various molecules that contribute to their virulence. The presence of phosphocholine (PCho) on NTHi lipooligosaccharide increases adhesion to epithelial cells and is an advantage for the bacterium, enabling nasopharyngeal colonization, as measured in humans and animal models. However, when PCho is expressed on the lipooligosaccharide, it is also recognized by the acute-phase protein C-reactive protein (CRP) and PCho-specific antibodies, both of which are potent initiators of the classical pathway of complement activation. In this study, we show that blood isolates, which are exposed to CRP and PCho-specific antibodies in the bloodstream, have a higher survival in serum than oropharyngeal isolates, which was associated with a decreased presence of PCho. PCholow strains showed decreased IgM, CRP, and complement C3 deposition, which was associated with increased survival in human serum. Consistent with the case for the PCholow strains, removal of PCho expression by licA gene deletion decreased IgM, CRP, and complement C3 deposition, which increased survival in human serum. Complement-mediated killing of PChohigh strains was mainly dependent on binding of IgM to the bacterial surface. These data support the hypothesis that a PCholow phenotype was selected in blood during invasive disease, which increased resistance to serum killing, mainly due to lowered IgM and CRP binding to the bacterial surface.
INTRODUCTION
The introduction of the Haemophilus influenzae serotype b (Hib) vaccine has substantially decreased the number of invasive disease episodes caused by this species. (1). Other H. influenzae serotypes and nontypeable H. influenzae (NTHi) were generally associated with mild infectious diseases (2). However, since the introduction of the Hib and Streptococcus pneumoniae vaccines, the number of invasive disease cases caused by these H. influenzae groups has increased considerably (3). Furthermore, vaccination against Hib or S. pneumoniae appears to enhance NTHi nasopharyngeal colonization (4, 5), which may increase transmission to individuals who are susceptible to developing invasive disease, such as the elderly, who are most affected by invasive NTHi disease (6, 7). It is not completely understood why the elderly are particularly susceptible to invasive NTHi infections, but the patient’s immune status is thought to play an important role (3).
Besides host immune status, bacterial virulence also plays an important role in susceptibility to invasive NTHi disease (3). For instance, outer membrane protein P5 (OmpP5) was shown to bind human factor H, thereby inhibiting the alternative pathway of complement activation (8, 9). Incorporation of sialic acid into the lipooligosaccharide (LOS) increases resistance to serum killing, and mutants not able to incorporate sialic acid showed attenuated virulence in a chinchilla model for otitis media (10). We showed that NTHi isolates from patients with invasive disease incorporated galactose to heptose III (HepIII-Gal) in the LOS more frequently, which decreased binding of IgM and thereby increased resistance to serum killing (11).
Another modification of LOS is the incorporation of phosphorylcholine (PCho), which can be incorporated in the LOS as a terminal moiety on HepI or HepIII, depending on the licD allele (12). PCho is acquired from the host through the lic operon (13) and incorporated, which is controlled by phase variation, resulting in PCho-positive (PCho+) and PCho-negative (PCho−) NTHi populations. Phase-variable ON-OFF translation of genes is a stochastic process dependent on slipped-strand mispairing of tetranucleotide repeats present in the gene, which occurs with a high frequency (102 to 103 times per generation) (14) and leads to switching the coding region in or out of frame (15). PCho+ bacteria are the dominant population isolated from the nasopharynxes of humans, mice, and rats (16–18). This is explained by the fact that PCho+ NTHi strains show increased adhesion through binding of the platelet-activating factor (PAF) receptor on epithelial cells (19), decreased binding of IgG through altering the physical properties of the outer membrane (20), and increased resistance to antimicrobial peptides such as human cathelicidin LL-37 (21).
Because PCho expression is phase variable, there should also be conditions where the PCho− NTHi population has an advantage over the PCho+ NTHi population. PCho on NTHi is recognized by C-reactive protein (CRP) (17) and PCho-specific antibodies (22), which can initiate the classical complement pathway and augment serum killing (16). Therefore, in the presence of these opsonins, strains with the PCho− phenotype might have a survival advantage. Encapsulated H. influenzae serotype B strain Eagan that was constitutive lic1 phase ON, resulting in a PCho+ population, showed CRP-dependent killing in serum and reduced virulence in an infant rat model of invasive disease (23). Therefore, it is likely that switching to the PCho− phenotype is beneficial when the organism is present in blood; however, whether NTHi strains collected from blood are PCho− is thus far not known.
In this study, we compared the survival of oropharyngeal and blood NTHi isolates in pooled human serum. NTHi strains collected from blood showed higher survival in pooled human serum and were mainly PCholow (82%), whereas strains collected from the oropharynx were mainly PChohigh (65%). PCholow NTHi isolates bound less CRP and IgM, which was associated with decreased complement C3 deposition and increased serum survival.
RESULTS
Study population.
In total, 20 oropharyngeal and 22 blood NTHi isolates were included in this study. The age distributions of subjects were not significantly different (mean ages of 71.8 ± 6.6 versus 69.7 ± 7.4 years; two-tailed unpaired t test, P = 0.3500), and gender was evenly distributed (male/female, 8/12 versus 13/9; Fisher’s exact test, P = 0.3543). Oropharyngeal isolates were collected from individuals without presentation of acute disease. The clinical presentation of patients with positive blood cultures was mainly pneumonia (27%) and/or sepsis (32%). Coinfections with a virus (9%) or other bacteria in the blood (23%) were reported. A large proportion of patients with positive NTHi blood culture had malignancies (36%), chronic obstructive pulmonary disease (COPD) (27%), or cardiovascular disease (14%). All patient characteristics are presented in Table S2 in the supplemental material.
Increased complement survival for blood isolates.
To test whether there was a difference in serum killing between oropharyngeal and blood isolates, NTHi bacteria were incubated with 10% pooled normal human serum (NHS) for 60 min. Survival of NTHi isolates in 10% NHS differed greatly (range, 0.01% through 29.5%) but was significantly higher for the blood isolates than for the oropharyngeal isolates (geometric mean survival, 1.06 versus 0.32, respectively; P = 0.0370) (Fig. 1).
FIG 1.
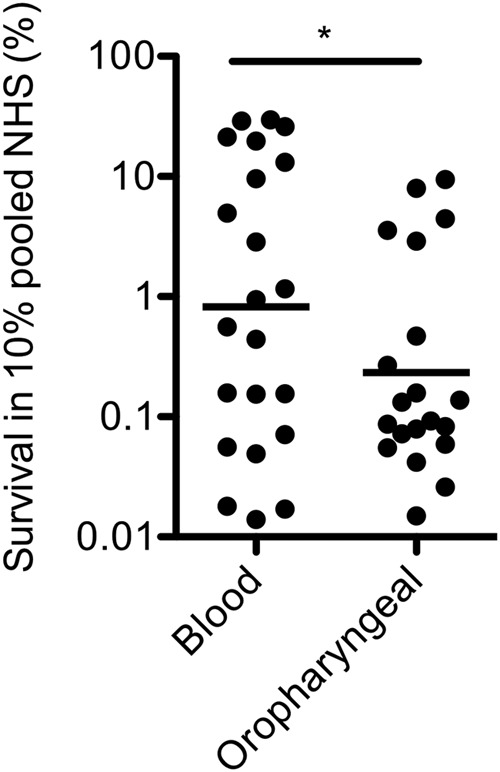
Serum survival of oropharyngeal and blood NTHi isolates. The percent survival of 22 blood and 20 oropharyngeal NTHi isolates for 1 h in 10% pooled normal human serum (NHS) is shown (n = 3). A two-tailed unpaired t test with Welch’s correction was used for statistical analysis. *, P < 0.05.
Lower PCho incorporation for blood isolates than for oropharyngeal isolates as determined by flow cytometry.
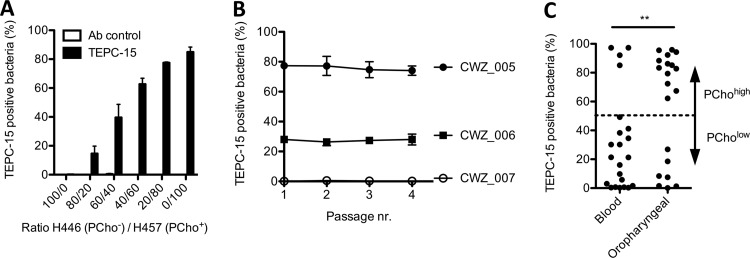
Phase-variable incorporation of PCho into the LOS of NTHi can affect serum killing. In order to determine the presence of PCho in a high-throughput manner, we have established a flow cytometry-based method to detect PCho. First, we determined the percentages of PCho-positive (PCho+) and PCho-negative (PCho−) bacteria in a mixture of H. influenzae strain H446, which is a licD mutant strain not able to incorporate PCho, and H. influenzae strain H457, which has phase-locked ON expression of PCho on HepIII (12). Flow cytometry-based detection of different mixtures of H446 and H457 corresponded with the expected PCho+ and PCho− populations (Fig. 2A; see Fig. S1 in the supplemental material).
FIG 2.
Phosphorylcholine incorporation by oropharyngeal and blood NTHi isolates as determined by flow cytometry with the TEPC-15 antibody. (A) H. influenzae strains H446 (PCho−) and H457 (PCho+) were mixed, and the presence of PCho was detected by flow cytometry with TEPC-15 (n = 3). (B) Invasive NTHi strains CWZ_005, CWZ_006, and CWZ_007 were passaged 4 times on agar plates, and the presence of PCho was detected by flow cytometry with TEPC-15 (n = 3). (C) The percentage of PCho-expressing bacteria from 22 blood and 20 oropharyngeal NTHi isolates was detected by flow cytometry with TEPC-15 (n = 3). Fisher’s exact test was used to determine significant differences in PCholow and PChohigh strain distribution between blood and oropharyngeal NTHi isolates. **, P < 0.01.
Because incorporation of PCho is phase variable and therefore might change during growth of the bacteria, we determined whether multiple passages on agar plates would alter the percentage of PCho+ bacteria. Multiple passages of three blood isolates on agar plates did not change the percentage of PCho+ bacteria (Fig. 2B). Therefore, we conclude that the percentage of PCho+ bacteria is stable under normal culture conditions.
Next, we determined the percentages of PCho+ bacteria among 22 blood and 20 oropharyngeal NTHi isolates. We found that most blood isolates (82%) were PCholow (<50% PCho+ bacteria), whereas oropharyngeal isolates were mainly PChohigh (65%) (>50% PCho+ bacteria) (Fig. 2C), which was significantly different (Fisher’s exact test, P = 0.0040).
PCholow isolates show decreased IgM, CRP, and complement C3 deposition and increased resistance to serum killing.
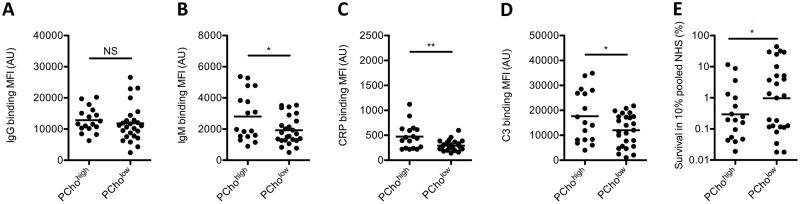
The presence of PCho in the LOS of NTHi is known to be recognized by antibodies and CRP, both of which are capable of initiating the classical pathway of complement activation (16, 17, 22). Therefore, we determined binding of IgG, IgM, and CRP to the bacterial surface of PChohigh and PCholow isolates. No significant difference in IgG binding to the bacterial surface of PChohigh and PCholow isolates was found, but significantly more IgM and CRP binding was detected on PChohigh isolates than on PCholow isolates (Fig. 3A to C).
FIG 3.
IgG, IgM, CRP, and C3 opsonization and survival in serum of PChohigh and PCholow NTHi strains. (A to D) NTHi bacteria were incubated with 10% NHS (C3) or 10% HI-NHS (IgG, IgM, and CRP), and the surface binding of IgG (A), IgM (B), CRP (C), or C3 (D) was determined by flow cytometry (n = 3). (E) Bacteria were incubated with 10% NHS or 10% HI-NHS for 1 h, and survival was determined by dividing the CFU in 10% NHS by the CFU in 10% HI-NHS after 1 h of incubation (n = 3). A one-tailed unpaired t test with Welch’s correction was used for statistical analysis. *, P < 0.05; **, P < 0.01; NS, not significant.
Next, we determined complement activation by measuring complement C3 deposition on the bacterial surface and serum killing. Complement C3 deposition on the bacterial surface was increased for PChohigh isolates compared to PCholow isolates, which was in agreement with increased serum killing of PChohigh isolates (Fig. 3D and E).
Binding of IgM and CRP is associated with increased complement C3 opsonization and serum killing of NTHi.
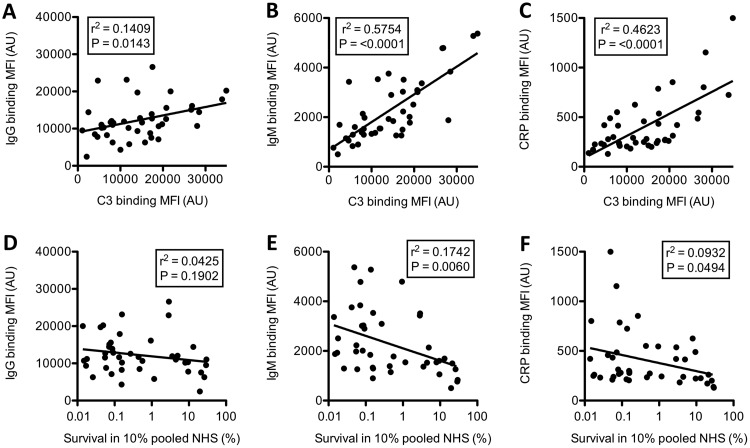
Binding of IgM and CRP, and to a lesser extent IgG, was significantly associated with complement C3 opsonization of the bacterial surface (Fig. 4A to C). An increased binding of IgM and CRP to the bacterial surface was also significantly associated with serum killing, whereas this was not the case for IgG (Fig. 4D to F). Therefore, we conclude that binding of IgM and CRP to the bacterial surface is associated with increased complement deposition and enhanced serum killing.
FIG 4.
Correlations between IgM and CRP binding to the bacterial surface with complement C3 opsonization and serum survival. Correlations between surface binding of IgG (A and D), IgM (B and E), and CRP (C and F) with surface binding of C3 (A, B, and C) or survival in 10% pooled NHS (D, E, and F) for all blood and oropharyngeal NTHi isolates are shown. Linear regression was used to determine relationships between conditions.
PCho-deficient strains show decreased binding of IgM and CRP and increased resistance to serum killing.
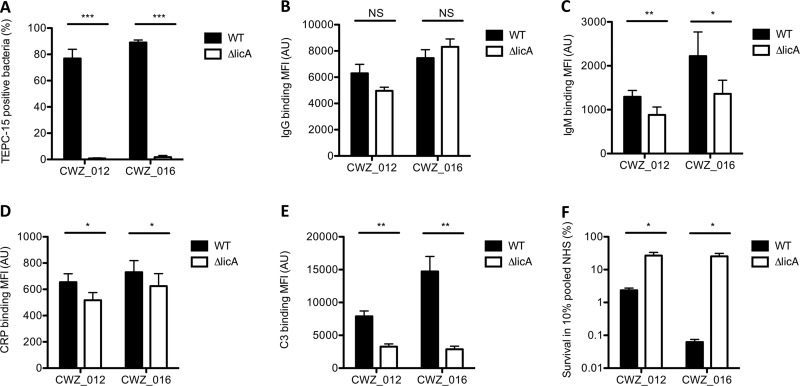
In order to determine whether binding of IgM and CRP to PCho on the bacterial surface is associated with serum killing, we deleted the licA gene from invasive NTHi strains CWZ_012 and CWZ_016, which abrogated PCho expression (Fig. 5A). In support of a direct link between the presence of PCho and binding of IgM and CRP to the bacterial surface, the CWZ_012 ΔlicA and CWZ_016 ΔlicA mutant strains showed decreased binding of IgM (Fig. 5C) and CRP (Fig. 5D) compared to the CWZ_012 and CWZ_016 strains, whereas this was not the case for IgG (Fig. 5B). Consistent with the correlations of IgM and CRP with C3 opsonization and serum killing (Fig. 4), a decreased binding of IgM and CRP decreased complement C3 deposition (Fig. 5E) and increased bacterial survival in human serum (Fig. 5F). Therefore, we conclude that binding of IgM and/or CRP to PCho is associated with increased complement activation and serum killing.
FIG 5.
IgG, IgM, CRP, and C3 opsonization and survival in serum of WT and licA mutant NTHi strains. (A to E) NTHi bacteria were incubated with TEPC-15 (PCho), 10% NHS (C3), or 10% HI-NHS (IgG, IgM, and CRP), and surface binding of TEPC-15 (A), IgG (B), IgM (C), CRP (D), or C3 (E) was determined by flow cytometry (n = 5). (F) Bacteria were incubated with 10% NHS or 10% HI-NHS for 1 h, and survival was determined by dividing the CFU in 10% NHS by the CFU in HI-NHS after 1 h of incubation (n = 4). A two-tailed paired t test was used for statistical analysis. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant.
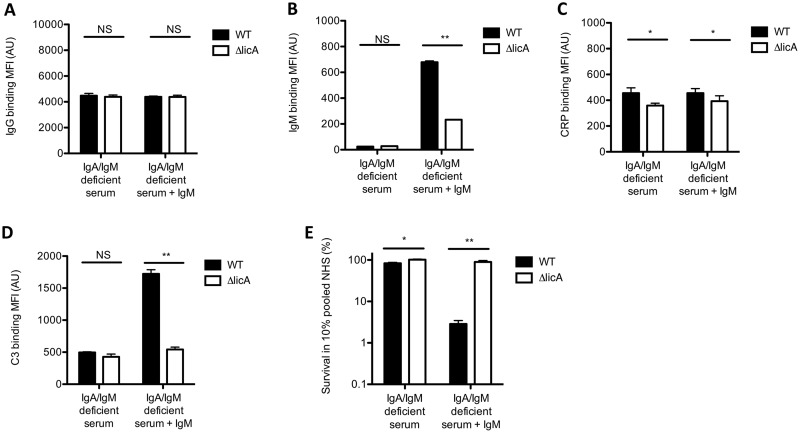
Binding of IgM to PChohigh strain contributes to serum killing.
Binding of IgM and CRP to PChohigh strains increases complement-mediated serum killing (Fig. 5F). To determine the contribution of IgM to complement activation and serum killing, we performed experiments with serum from an agammaglobulinemia patient, which lacks IgA and IgM, with and without supplementing purified serum IgM. Binding of IgG to the bacterial surface of the CWZ_016 wild-type (WT) and ΔlicA mutant strains was not different in the absence or presence of IgM (Fig. 6A). Binding of IgM to the CWZ_016 WT and ΔlicA mutant strains was absent in IgA/IgM-deficient serum but was clearly detected with IgM supplementation and was significantly higher for the PChohigh CWZ_016 WT strain than for the CWZ_016 ΔlicA mutant strain lacking PCho (Fig. 6B). A higher CRP binding to the CWZ_016 WT strain than to the CWZ_016ΔlicA mutant strain was observed with and without IgM supplementation (Fig. 6C). Binding of C3 to the bacterial surface of the CWZ_016 WT and ΔlicA mutant strains was not different in IgA/IgM-deficient serum, but supplementation of IgM increased C3 binding significantly more for the CWZ_016 WT strain than for the CWZ_016 ΔlicA mutant strain (Fig. 6D). Survival of the CWZ_016 WT and ΔlicA mutant strains in IgA/IgM-deficient serum was high, at 83% and 99%, respectively (Fig. 6E). In accordance with a clear difference in C3 binding to the bacterial surface in the presence of IgM, supplementation of serum IgM decreased survival of the CWZ_016 WT strain to 3%, whereas this was 90% for the CWZ_016 ΔlicA mutant strain. These results show that binding of IgM, in addition to CRP, increases complement-mediated killing of the PChohigh CWZ_016 WT strain significantly more than that of the PCho-deficient CWZ_016 ΔlicA mutant strain.
FIG 6.
IgG, IgM, CRP, and C3 opsonization and survival of CWZ_016 and the CWZ_016 licA mutant in IgA/IgM-deficient serum with and without IgM supplementation. (A to D) NTHi bacteria were incubated with 10% IgA/IgM-deficient serum supplemented with or without 10 µg/ml IgM (C3) or with 10% HI-IgA/IgM-deficient serum supplemented with or without 10 µg/ml IgM (IgG, IgM, and CRP), and surface binding of IgG (A), IgM (B), CRP (C), or C3 (D) was determined by flow cytometry (n = 3). (E) Bacteria were incubated with 10% HI-IgA/IgM-deficient serum, 10% IgA/IgM-deficient serum, or 10% IgA/IgM-deficient serum supplemented with 10 µg/ml IgM for 1 h, and survival was determined by dividing the CFU in 10% IgA/IgM-deficient serum with or without IgM by the CFU in 10% HI-IgA/IgM-deficient serum after 1 h of incubation (n = 4). A two-tailed paired t test was used for statistical analysis. *, P < 0.05; **, P < 0.01; NS, not significant.
DISCUSSION
We studied whether PCho expression, as determined by flow cytometry, was different on oropharyngeal and blood isolates. We are the first to show that NTHi isolates collected from blood are mainly PCholow. We also found that most oropharyngeal isolates (65%) are PChohigh, which is in accordance with a study where nasopharyngeal isolates were collected from children with otitis media with effusion, in which 63% of NTHi isolates were PCho+ (24). In an earlier study, 13 out of 14 specimens (93%) collected from respiratory tract secretions were predicted to be licA phase ON (17), which is higher than found in our study. This discrepancy might be due to detecting phase variation in comparison to actual PCho expression on the bacterial surface in our study.
The PCho+ phenotype has advantages for NTHi during colonization of the upper respiratory tract, as it enhances adhesion to epithelial cells through binding of the PAF receptor (19) and increases resistance to killing by the antimicrobial peptide LL-37 (21). PCho+ NTHi strains were associated with the development of otitis media in a chinchilla model (25), and the PCho+ phenotype was correlated with increased persistence in children with otitis media (24), indicating that PCho plays a role in otitis media and persistence of the colonization of the upper respiratory tract.
The fact that PCho is phase-variably expressed strongly suggests that PCho exposure also has disadvantages for NTHi. For instance, PCho is recognized by CRP (17) and PCho-specific antibodies (22), which can initiate the classical pathway of complement activation and augment serum killing (16). This is in line with our results showing that oropharyngeal isolates, which are mainly PChohigh, are more vulnerable to serum killing than blood isolates, which include mainly PCholow variants (Fig. 1). Previous studies have shown that nasopharyngeal isolates are more susceptible to serum killing than isolates collected from lungs or middle ear fluid, but the presence of PCho was not determined in those studies (26, 27). The increased survival of blood isolates compared to oropharyngeal isolates is in contrast to a previous study where no difference in complement resistance between blood and nasopharyngeal isolates was observed (28). A possible explanation might be differences in patient populations, such as age and immune status. In addition, nasopharyngeal isolates in this previous study were collected from patients suffering from upper respiratory tract infection (25), whereas we have used oropharyngeal isolates from individuals without presentation of acute disease.
In order to understand why PChohigh strains are more susceptible to serum killing, we determined the binding of CRP, IgG, and IgM to the bacterial surface, because these proteins are able to initiate the classical pathway of complement activation. We expected a lowered IgG binding to PChohigh NTHi isolates because PChohigh bacteria were previously shown to evade IgG binding (20), but this was not detected in our experiments (Fig. 3A). This can be explained by the fact that the lower IgG binding to PCho+ bacteria was detected with serum in which CRP was depleted using a p-aminophenyl phosphoryl choline gel column, which also depletes PCho-specific antibodies, including PCho-specific IgG and IgM (22). In serum where PCho-specific IgG antibodies are depleted, PCho+ bacteria bind less IgG (22). However, when normal human serum is used, as in our experiments, the difference in IgG binding between PChohigh and PCholow isolates might be eliminated by the presence of PCho-specific IgG antibodies.
In addition to PCho-specific IgG, human serum also contains PCho-specific IgM (29), which is a B1-lineage natural antibody that is also known to bind apoptotic cells and oxidized low-density lipoprotein (LDL) (30, 31). Levels of PCho-specific IgM differs greatly among individuals, and a low level of anti-PCho IgM is associated with a higher risk for cardiovascular disease (32, 33) and systemic lupus erythematosus (SLE) (34). In mice, PCho-specific IgM antibodies were shown to protect against S. pneumoniae infections (35). Whether differences in these anti-PCho-specific IgM antibodies might affect the magnitude of classical complement pathway activation in patients during infections with PCho-expressing bacteria is unknown.
The presence of PCho appeared to result in increased complement activation through binding of PCho-specific IgM and CRP, resulting in serum killing. In order to confirm this, we removed PCho from the bacterial surface by generating strains with mutations in the licA gene, which codes for the protein required for incorporation of PCho into the LOS (36), in two PChohigh strains. Removal of PCho on PChohigh NTHi strains resulted in decreased binding of IgM and CRP, which resulted in lowered complement activation and increased survival in human serum. This result was consistent with a previous study in which deletion of the licA gene in a PChohigh NTHi strain abrogated CRP binding and increased serum survival (37). Binding of PCho-specific IgM in the presence of CRP appears to play a major role in complement-mediated killing of PChohigh NTHi strains because survival of PChohigh strain CWZ_016 in IgA/IgM-deficient serum was 83%, which decreased to only 3% when the strain was supplemented with serum IgM.
The lowered expression of PCho on blood NTHi isolates can be explained by the presence of CRP and PCho-specific antibodies, both of which are capable of initiating the classical pathway of complement activation. However, bacteria in the blood are very unlikely to be transmitted, and therefore, switching to a PCholow phenotype would not lead to an evolutionary advantage for NTHi. It is therefore likely that selection for the PCholow phenotype happens in the upper respiratory tract during colonization. Out of the 20 oropharyngeal strains included in this study, 7 (35%) were PCholow. The selection of PCholow during colonization might be affected by fluctuating levels of CRP and PCho-specific antibodies, although there are no data about the levels of CRP or PCho-specific antibodies in the upper respiratory tract.
PCho is considered a vaccine candidate for NTHi but also for S. pneumoniae, which is a bacterial species that incorporates PCho into the cell wall teichoic acid and lipoteichoic acid (38). However, since PCho is phase variable and we have shown that the majority of blood isolates are mostly PCholow, this vaccine would not protect against NTHi bacteremia.
In conclusion, we found that blood NTHi isolates have a higher survival in serum, which was associated with a decreased presence of PCho. The presence of PCho increased IgM and CRP binding to the bacterial surface and increased complement C3 deposition and serum killing. These data show that NTHi isolates from blood are selected for a PCholow phenotype, likely due to the presence of PCho-specific antibodies and CRP in human serum.
MATERIALS AND METHODS
NTHi isolate collection.
Oropharyngeal NTHi isolates were collected from participants at baseline in the influenza-like-illness-2 (ILI-2) study (39). The study was approved by the acknowledged ethical committee METC Noord Holland (http://www.trialregister.nl; NTR3386). Oropharyngeal samples were obtained with a sterile swab with a flocked nylon tip and stored separately in 1 ml modified liquid Amies transport medium (eSwab). Samples were transported at room temperature to the laboratory and processed within 8 h after sampling. For pathogen isolation, a single colony was picked. Subsequent passaging was performed with several colonies pooled and passaged together. Discrimination between Haemophilus haemolyticus and H. influenzae was done by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (40). NTHi strains from positive oropharyngeal swabs at baseline from persons ≥55 years of age were included in this study. Oropharyngeal NTHi strains were collected between October 2012 and February 2013. Invasive NTHi strains collected between August 2009 and August 2017 from patients aged ≥55 years were obtained from two clinical microbiology laboratories in Nijmegen, the Netherlands. For pathogen isolation, a single colony was picked. Subsequent passaging was performed with several colonies pooled and passaged together. Gene-specific primers (see Table S1 in the supplemental material) were used to confirm the identity of all NTHi isolates. The bacterial strains used in this study are listed in Table S1.
Culture conditions.
NTHi strains were passaged overnight at 37°C and 5% CO2 on brain heart infusion (BHI) agar plates (BD Biosciences) supplemented with 1 µg/ml hemin (Sigma-Aldrich) and 2 µg/ml β-NAD (Merck) (supplemented BHI [sBHI]), followed by growth with shaking at 225 rpm in liquid sBHI medium at 37°C to an optical density at 620 nm (OD620) of 0.5 to 1.0. Bacterial stocks were stored in sBHI plus 16% glycerol at –80°C. Bacterial counts were determined by plating serial dilutions in phosphate-buffered saline (PBS) on sBHI plates.
Genotyping.
Genomic DNA was isolated from a 1-ml culture at an OD620 between 0.5 and 1.0 with the DNeasy kit (Qiagen) according to the manufacturer’s instructions. PCR was performed using standard Phusion DNA polymerase (New England Biolabs) with 0.5 µM each primer and 1 µl of genomic DNA in a 25-µl reaction mixture. The annealing temperature was 60°C, and cycles were repeated 39 times. The pepN gene was amplified as a DNA positive control (41). H. haemolyticus isolates were excluded based on the absence of either the iga or fucK gene as described previously (42, 43). The presence of the capsule gene bexA was determined as described previously (44). The presence of licA was determined as described previously (45). The primers used in this study are listed in Table S1.
Generation of NTHi licA mutants.
Flanking regions (∼1,000 bp) and the kanamycin cassette of the R2866 ΔlicA galE mutant were PCR amplified with primers R2866_1107_L1 and R2866_1107_R1 and purified with the Qiagen PCR purification kit (Qiagen). The PCR product was used to transform M-IV competent NTHi as described previously (46). The absence of the licA gene and presence of the kanamycin cassette were determined by PCR with primers R2866_1107_L1 and R2866_1107_C (licA gene) and R2866_1107_L1 and HBKanR3 (kanamycin cassette). Strains CWZ_012 and CWZ_16 were selected because of their high PCho expression and successful transformation. The primers used in this study are listed in Table S1.
Serum killing assay.
Experiments were conducted with pooled normal human serum (NHS) obtained from Immucor (catalog number PHS-N100, lot 2148U). The presence of IgG and IgM antibodies recognizing NTHi was determined by Western blot analysis on whole protein lysates and purified lipooligosaccharide samples from various clinical NTHi isolates (47). The level of complement resistance of the NTHi isolates was determined with 10% NHS because this serum percentage allowed efficient killing of most NTHi isolates, as previously reported (47). Serum from an agammaglobulinemia patient on IgG replacement therapy was used as IgA/IgM-deficient serum (48). Purified IgM from human serum (Sigma-Aldrich, I8260) was washed with PBS on a Amicon Ultra-0.5 centrifugal filter unit column (Millipore) to remove the preservative sodium azide and suspended into PBS at a concentration of 1 mg/ml. NTHi was grown in supplemented BHI medium to an OD620 of ∼0.5, washed once with PBS, diluted to an OD620 of 0.1 in PBS, and diluted 10,000-fold in Hanks balanced salt solution (HBSS) plus Ca2+/Mg2+ plus 0.1% gelatin (HBSS3+) to obtain a concentration of ∼200,000 CFU/ml. Fifty microliters of bacteria was mixed with 50 µl of 20% NHS, 20% heat-inactivated (HI) NHS, 10% IgA/IgM-deficient serum, 10% IgA/IgM-deficient serum supplemented with 10 µg/ml IgM, or 10% HI-IgA/IgM-deficient serum in HBSS3+ and incubated for 1 h at 37°C. Samples were diluted 10- and 100-fold with PBS, and three droplets of 20 μl of the undiluted and 10- and 100-fold-diluted bacteria was plated on sBHI plates and grown overnight at 37°C and 5% CO2. Survival was determined by dividing the CFU in 10% NHS by the CFU in HI-NHS after a 1-h incubation. Survival of all NTHi strains was determined in three independent experiments, and the averages from the three experiments are reported.
Flow cytometry.
NTHi was grown in supplemented BHI medium to an OD620 of ∼0.5, washed once with PBS and diluted to an OD620 of 0.1 in HBSS3+. Fifty microliters of bacteria was mixed with 50 µl of 20% NHS, 10% IgA/IgM-deficient serum supplemented with or without 10 µg/ml IgM (C3), 20% HI-NHS, or 10% IgA/IgM-deficient serum supplemented with or without 10 µg/ml IgM (IgG, IgM, and C-reactive protein [CRP]) or 2 µg/ml TEPC-15 (a monoclonal antibody that recognizes PCho) (Sigma-Aldrich) diluted in HBSS3+ and incubated for 30 min at 37°C. Bacteria were pelleted by centrifugation at 3,200 × g and fixed for 20 min in 2% paraformaldehyde in PBS at room temperature. Bacteria were washed with PBS, and all antibody incubations were performed in PBS plus 2% bovine serum albumin (BSA). Surface-bound complement C3 was detected with 1:500-diluted fluorescein isothiocyanate (FITC)-labeled polyclonal goat anti-human C3 (MP Biomedicals). Surface-bound IgG or IgM was detected with 1:100-diluted FITC-labeled polyclonal goat anti-human IgG (Sigma-Aldrich) or FITC-labeled polyclonal goat anti-human IgM (Sigma-Aldrich), respectively. Surface-bound CRP was detected with 1:100-diluted polyclonal goat anti-human CRP (Acris Antibodies), followed by staining with 1:200-diluted FITC-labeled polyclonal donkey anti-goat IgG (Thermo Fisher). Surface-bound TEPC-15 was detected by using 1:100-diluted FITC-labeled anti-mouse IgA antibody (Sigma-Aldrich). For an antibody control experiment, the TEPC-15 antibody was omitted and bacteria were stained only with FITC-labeled anti-mouse IgA antibody. Surface binding of C3, IgG, IgM, CRP, and TEPC-15 was determined by flow cytometry using a FACS LSR II instrument (BD Biosciences) and expressed as mean fluorescence intensity (MFI) in arbitrary units (AU). Data were analyzed by using FlowJo version 10.4.1. IgG, IgM, TEPC-15, C3, and CRP binding to all NTHi strains was determined in three independent experiments, and the averages from the three experiments are reported.
Statistical analysis.
Statistical analyses were performed with GraphPad Prism version 5.03 for Windows (GraphPad Software, Inc.). Differences were considered significant at a P value of <0.05. The specific statistical tests that were used for the various experiments are specified in Results or in the figure legends.
Supplementary Material
ACKNOWLEDGMENT
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00604-18.
REFERENCES
- 1.Adams WG, Deaver KA, Cochi SL, Plikaytis BD, Zell ER, Broome CV, Wenger JD. 1993. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA 269:221–226. doi: 10.1001/jama.1993.03500020055031. [DOI] [PubMed] [Google Scholar]
- 2.Van Eldere J, Slack MP, Ladhani S, Cripps AW. 2014. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis 14:1281–1292. doi: 10.1016/S1473-3099(14)70734-0. [DOI] [PubMed] [Google Scholar]
- 3.Langereis JD, de Jonge MI. 2015. Invasive disease caused by nontypeable Haemophilus influenzae. Emerg Infect Dis 21:1711–1718. doi: 10.3201/eid2110.150004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biesbroek G, Wang X, Keijser BJF, Eijkemans RMJ, Trzciński K, Rots NY, Veenhoven RH, Sanders EAM, Bogaert D. 2014. Seven-valent pneumococcal conjugate vaccine and nasopharyngeal microbiota in healthy children. Emerg Infect Dis 20:201–210. doi: 10.3201/eid2002.131220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spijkerman J, Prevaes SM, van Gils EJ, Veenhoven RH, Bruin JP, Bogaert D, Wijmenga-Monsuur AJ, van den Dobbelsteen GP, Sanders EA. 2012. Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS One 7:e39730. doi: 10.1371/journal.pone.0039730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puig C, Grau I, Marti S, Tubau F, Calatayud L, Pallares R, Linares J, Ardanuy C. 2014. Clinical and molecular epidemiology of Haemophilus influenzae causing invasive disease in adult patients. PLoS One 9:e112711. doi: 10.1371/journal.pone.0112711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Wessel K, Rodenburg GD, Veenhoven RH, Spanjaard L, van der Ende A, Sanders EA. 2011. Nontypeable Haemophilus influenzae invasive disease in The Netherlands: a retrospective surveillance study 2001-2008. Clin Infect Dis 53:e1–e7. doi: 10.1093/cid/cir268. [DOI] [PubMed] [Google Scholar]
- 8.Langereis JD, de Jonge MI, Weiser JN. 2014. Binding of human factor H to outer membrane protein P5 of non-typeable Haemophilus influenzae contributes to complement resistance. Mol Microbiol 94:89–106. doi: 10.1111/mmi.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosadini CV, Ram S, Akerley BJ. 2014. Outer membrane protein P5 is required for resistance of nontypeable Haemophilus influenzae to both the classical and alternative complement pathways. Infect Immun 82:640–649. doi: 10.1128/IAI.01224-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueira MA, Ram S, Goldstein R, Hood DW, Moxon ER, Pelton SI. 2007. Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect Immun 75:325–333. doi: 10.1128/IAI.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langereis JD, Weiser JN. 2014. Shielding of a lipooligosaccharide IgM epitope allows evasion of neutrophil-mediated killing of an invasive strain of nontypeable Haemophilus influenzae. mBio 5:e01478-14. doi: 10.1128/mBio.01478-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lysenko E, Richards JC, Cox AD, Stewart A, Martin A, Kapoor M, Weiser JN. 2000. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein-mediated killing. Mol Microbiol 35:234–245. doi: 10.1046/j.1365-2958.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiser JN, Lindberg AA, Manning EJ, Hansen EJ, Moxon ER. 1989. Identification of a chromosomal locus for expression of lipopolysaccharide epitopes in Haemophilus influenzae. Infect Immun 57:3045–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bolle X, Bayliss CD, Field D, van de Ven T, Saunders NJ, Hood DW, Moxon ER. 2000. The length of a tetranucleotide repeat tract in Haemophilus influenzae determines the phase variation rate of a gene with homology to type III DNA methyltransferases. Mol Microbiol 35:211–222. doi: 10.1046/j.1365-2958.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 15.Weiser JN, Love JM, Moxon ER. 1989. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell 59:657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 16.Weiser JN, Pan N. 1998. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol Microbiol 30:767–775. doi: 10.1046/j.1365-2958.1998.01108.x. [DOI] [PubMed] [Google Scholar]
- 17.Weiser JN, Pan N, McGowan KL, Musher D, Martin A, Richards J. 1998. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med 187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole J, Foster E, Chaloner K, Hunt J, Jennings MP, Bair T, Knudtson K, Christensen E, Munson RS Jr, Winokur PL, Apicella MA. 2013. Analysis of nontypeable haemophilus influenzae phase-variable genes during experimental human nasopharyngeal colonization. J Infect Dis 208:720–727. doi: 10.1093/infdis/jit240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swords WE, Buscher BA, Ver Steeg Ii K, Preston A, Nichols WA, Weiser JN, Gibson BW, Apicella MA. 2000. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol Microbiol 37:13–27. doi: 10.1046/j.1365-2958.2000.01952.x. [DOI] [PubMed] [Google Scholar]
- 20.Clark SE, Snow J, Li J, Zola TA, Weiser JN. 2012. Phosphorylcholine allows for evasion of bactericidal antibody by Haemophilus influenzae. PLoS Pathog 8:e1002521. doi: 10.1371/journal.ppat.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lysenko ES, Gould J, Bals R, Wilson JM, Weiser JN. 2000. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect Immun 68:1664–1671. doi: 10.1128/IAI.68.3.1664-1671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldenberg HB, McCool TL, Weiser JN. 2004. Cross-reactivity of human immunoglobulin G2 recognizing phosphorylcholine and evidence for protection against major bacterial pathogens of the human respiratory tract. J Infect Dis 190:1254–1263. doi: 10.1086/424517. [DOI] [PubMed] [Google Scholar]
- 23.Humphries HE, High NJ. 2002. The role of licA phase variation in the pathogenesis of invasive disease by Haemophilus influenzae type b. FEMS Immunol Med Microbiol 34:221–230. doi: 10.1111/j.1574-695X.2002.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 24.Fujita K, Hirano T, Kodama S, Suzuki M. 2009. Prognostic impact of phosphorylcholine expression in nontypeable Haemophilus influenzae in otitis media with effusion. Acta Otolaryngol 129:832–838. doi: 10.1080/00016480802468195. [DOI] [PubMed] [Google Scholar]
- 25.Tong HH, Blue LE, James MA, Chen YP, DeMaria TF. 2000. Evaluation of phase variation of nontypeable Haemophilus influenzae lipooligosaccharide during nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect Immun 68:4593–4597. doi: 10.1128/IAI.68.8.4593-4597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langereis JD, Stol K, Schweda EK, Twelkmeyer B, Bootsma HJ, de Vries SP, Burghout P, Diavatopoulos DA, Hermans PW. 2012. Modified lipooligosaccharide structure protects nontypeable Haemophilus influenzae from IgM-mediated complement killing in experimental otitis media. mBio 3:e00079-12. doi: 10.1128/mBio.00079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura S, Shchepetov M, Dalia AB, Clark SE, Murphy TF, Sethi S, Gilsdorf JR, Smith AL, Weiser JN. 2011. Molecular basis of increased serum resistance among pulmonary isolates of non-typeable Haemophilus influenzae. PLoS Pathog 7:e1001247. doi: 10.1371/journal.ppat.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallstrom T, Resman F, Ristovski M, Riesbeck K. 2010. Binding of complement regulators to invasive nontypeable Haemophilus influenzae isolates is not increased compared to nasopharyngeal isolates, but serum resistance is linked to disease severity. J Clin Microbiol 48:921–927. doi: 10.1128/JCM.01654-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown M, Schiffman G, Rittenberg MB. 1984. Subpopulations of antibodies to phosphocholine in human serum. J Immunol 132:1323–1328. [PubMed] [Google Scholar]
- 30.Ehrenstein MR, Notley CA. 2010. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 31.Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. 2000. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest 105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Faire U, Su J, Hua X, Frostegård A, Halldin M, Hellenius M-L, Wikström M, Dahlbom I, Grönlund H, Frostegård J. 2010. Low levels of IgM antibodies to phosphorylcholine predict cardiovascular disease in 60-year old men: effects on uptake of oxidized LDL in macrophages as a potential mechanism. J Autoimmun 34:73–79. doi: 10.1016/j.jaut.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Su J, Georgiades A, Wu R, Thulin T, de Faire U, Frostegård J. 2006. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis 188:160–166. doi: 10.1016/j.atherosclerosis.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Su J, Hua X, Concha H, Svenungsson E, Cederholm A, Frostegard J. 2008. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology (Oxford) 47:1144–1150. doi: 10.1093/rheumatology/ken120. [DOI] [PubMed] [Google Scholar]
- 35.Briles DE, Forman C, Hudak S, Claflin JL. 1982. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med 156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiser JN, Shchepetov M, Chong ST. 1997. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect Immun 65:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puig C, Marti S, Hermans PW, de Jonge MI, Ardanuy C, Linares J, Langereis JD. 2014. Incorporation of phosphorylcholine into the lipooligosaccharide of nontypeable Haemophilus influenzae does not correlate with the level of biofilm formation in vitro. Infect Immun 82:1591–1599. doi: 10.1128/IAI.01445-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark SE, Weiser JN. 2013. Microbial modulation of host immunity with the small molecule phosphorylcholine. Infect Immun 81:392–401. doi: 10.1128/IAI.01168-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Beek J, Veenhoven RH, Bruin JP, van Boxtel RAJ, de Lange MMA, Meijer A, Sanders EAM, Rots NY, Luytjes W. 2017. Influenza-like illness incidence is not reduced by influenza vaccination in a cohort of older adults, despite effectively reducing laboratory-confirmed influenza virus infections. J Infect Dis 216:415–424. doi: 10.1093/infdis/jix268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruin JP, Kostrzewa M, van der Ende A, Badoux P, Jansen R, Boers SA, Diederen BM. 2014. Identification of Haemophilus influenzae and Haemophilus haemolyticus by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Eur J Clin Microbiol Infect Dis 33:279–284. doi: 10.1007/s10096-013-1958-x. [DOI] [PubMed] [Google Scholar]
- 41.Ecevit IZ, McCrea KW, Pettigrew MM, Sen A, Marrs CF, Gilsdorf JR. 2004. Prevalence of the hifBC, hmw1A, hmw2A, hmwC, and hia Genes in Haemophilus influenzae isolates. J Clin Microbiol 42:3065–3072. doi: 10.1128/JCM.42.7.3065-3072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meats E, Feil EJ, Stringer S, Cody AJ, Goldstein R, Kroll JS, Popovic T, Spratt BG. 2003. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol 41:1623–1636. doi: 10.1128/JCM.41.4.1623-1636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitovski S, Dunkin KT, Howard AJ, Sayers JR. 2002. Nontypeable Haemophilus influenzae in carriage and disease: a difference in IgA1 protease activity levels. JAMA 287:1699–1705. doi: 10.1001/jama.287.13.1699. [DOI] [PubMed] [Google Scholar]
- 44.Falla TJ, Crook DW, Brophy LN, Maskell D, Kroll JS, Moxon ER. 1994. PCR for capsular typing of Haemophilus influenzae. J Clin Microbiol 32:2382–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark SE, Eichelberger KR, Weiser JN. 2013. Evasion of killing by human antibody and complement through multiple variations in the surface oligosaccharide of Haemophilus influenzae. Mol Microbiol 88:603–618. doi: 10.1111/mmi.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herriott RM, Meyer EY, Vogt M, Modan M. 1970. Defined medium for growth of Haemophilus influenzae. J Bacteriol 101:513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langereis JD, van Dongen TM, Stol K, Venekamp RP, Schilder AG, Hermans PW. 2013. Resistance to complement-mediated killing and IgM binding to non-typeable Haemophilus influenzae is not altered when ascending from the nasopharynx to the middle ears in children with otitis media. Med Microbiol Immunol 202:407–415. doi: 10.1007/s00430-013-0302-5. [DOI] [PubMed] [Google Scholar]
- 48.Langereis JD, Henriet SS, Kuipers S, Weemaes CMR, van der Burg M, de Jonge MI, van der Flier M. 2018. IgM augments complement bactericidal activity with serum from a patient with a novel CD79a mutation. J Clin Immunol 38:185–192. doi: 10.1007/s10875-017-0474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.