Abstract
Background
Two million individuals with chronic hepatitis B (CHB) in the United States are at risk for premature death due to liver cancer and cirrhosis. CHB can be prevented by vaccination and controlled with treatment.
Methods
We created a lifetime Markov model to estimate the cost-effectiveness of strategies to prevent or treat CHB in 6 high-risk populations: foreign-born Asian/Pacific Islanders (API), Africa-born blacks (AbB), incarcerated, refugees, persons who inject drugs (PWID), and men who have sex with men (MSM). We studied 3 strategies: (a) screen for HBV infection and treat infected (“treatment only”), (b) screen for HBV susceptibility and vaccinate susceptible (“vaccination only”), and (c) screen for both and follow-up appropriately (“inclusive”). Outcomes were expressed in incremental cost-effectiveness ratios (ICERs), clinical outcomes, and new infections.
Results
Vaccination-only and treatment-only strategies had ICERs of $6000–$21 000 per quality-adjusted life-year (QALY) gained, respectively. The inclusive strategy added minimal cost with substantial clinical benefit, with the following costs per QALY gained vs no intervention: incarcerated $3203, PWID $8514, MSM $10 954, AbB $17 089, refugees $17 432, and API $18 009. Clinical complications dropped in the short/intermediate (1%–25%) and long (0.4%–16%) term. Findings were sensitive to age, discount rate, health state utility in immune or susceptible stages, progression rate to cirrhosis or inactive disease, and tenofovir cost. The probability of an inclusive program costing <$50 000 per QALY gained varied between 61% and 97% by population.
Conclusions
An inclusive strategy to screen and treat or vaccinate is cost-effective in reducing the burden of hepatitis B virus among all 6 high-risk, high-prevalence populations.
Keywords: hepatitis B, cost-effectiveness, treatment, screening, high-risk
More than 2 billion people have been infected with hepatitis B virus (HBV), and about 350 million have chronic hepatitis B (CHB) [1]. Most persons with acute HBV infection are asymptomatic, though <1% of acute infections in adults result in fulminant hepatitis, with mortality of 70%–80% [1]. The outcomes of acute HBV infection are age-dependent: <5% of adults, 25%–30% of young children, and 90% of neonates develop CHB [1]. CHB causes progressive liver damage; about 25% die prematurely from cirrhosis or hepatocellular carcinoma (HCC) [2]. HBV is referred to as a “silent killer” because infected persons can live 20–40 years before developing liver complications.
In 2013, CHB was a leading cause of mortality, with 686 000 deaths [3]. In the United States, as many as 2 million may be chronically infected with HBV, primarily foreign-born persons from high-prevalence countries and those at high behavioral risk (cohabitating with an HBV carrier or engaging in injection drug use or unprotected sex with multiple or infected partners) [4, 5]. CHB prevalence is 5%–10% in foreign-born Asian/Pacific Islander (API) and Africa-born black (AbB) populations, 1%–4% among incarcerated persons, 3.5%–20% among people who inject drugs (PWID), 1%–3% in men who have sex with men (MSM), and 6% in refugees [6–10]. Most people with CHB are unaware of their infection with HBV [4]. The Centers for Disease Control and Prevention (CDC) has identified core measures to reduce HBV burden in the United States, including improving testing, vaccination, and treatment [11].
The highly effective 3-dose hepatitis B vaccine is underutilized. Vaccination coverage is only 25% among adults >19 years [12]. Although national guidelines recommend patient screening and linkage to care and treatment of persons with CHB reduces the risk of ongoing liver damage, cirrhosis, and HCC, failure to screen results in many individuals remaining untreated [13]. HBV prevention programs in various populations have had varying levels of success [6, 14–18].
Previous US-based economic analyses have found screening programs for vaccination or treatment to be cost-effective. Vaccinating inmates costs $415 per averted infection [19]. Delivering vaccination to PWID at syringe exchange sites averts infections with cost savings [20]. In a high-prevalence population, HBV screening and treatment cost $29 230 per quality-adjusted-life-year [21]. However, no past analyses have looked broadly and consistently across populations and intervention options.
In this study, we conducted a health and economic analysis of 3 screening and follow-up strategies (screen and vaccinate; screen and treat; screen and vaccinate or treat, as appropriate) in 6 mutually exclusive high-risk, high-prevalence populations in the United States. We estimated cost, quality-adjusted life-years (QALYs), cost-effectiveness, and clinical impact.
METHODS
Overview
We constructed a decision–analytic Markov disease state model depicting the natural history of acute and chronic HBV infection and changes due to treatment or vaccination. Model assumptions and inputs are summarized below and detailed in the Supplementary Data. All input variables with >30% effect on the ICER for any population in 1-way sensitivity analysis were labeled “key” and are shown in Table 1; all other inputs are listed in Supplementary Tables 1–26.
Table 1.
Key Input Variables; Includes All Inputs With >30% Effect on the Incremental Cost-effectiveness Ratio for Any Population in 1-Way Sensitivity Analysis (Full Listing of Inputs in the Supplementary Data)
| Variables | Base Case (Range) | References |
| General inputs | ||
| Age of cohort, y | 30 (20–60) | Selected |
| Discount rate for costs and QALYs, % | 0.03 (0.01–0.05) | [22] |
| Cost inputs | ||
| Monthly cost of tenofovir 300 mg daily, US$ | 798 (499–998) | [38] |
| Annual cost of managing HBeAg-, active CHB, noncirrhotic, US$ | 1293 (647–3880) | [50] |
| Annual cost of managing HBeAg+, active CHB, noncirrhotic, US$ | 1293 (647–3880) | [50] |
| Total cost per person of running the people who inject drugs screening program at syringe service programs, US$ | 97 (73–291) | [18, 51] |
| Intervention inputs | ||
| Discontinuation rate of treatment-naïve in year 2 with tenofovir, % | 0.035 (0.0175–0.0525) | [30] |
| Incarcerated persons accepting treatment with universal screening, % | 0.75 (0.5–1) | Assumption |
| Proportion of incarcerated accepting vaccination and getting first dose, % | 0.7 (0.4–1) | [52, 53] |
| Proportion of incarcerated getting second vaccination dose, % | 0.65 (0.49–0.81) | Assumption |
| Proportion of people who inject drugs getting second vaccination dose, % | 0.53 (0.4–0.67) | [51] |
| People who inject drugs linked to care after screening at syringe service programs, % | 0.086 (0.06–0.4) | [54] |
| People who inject drugs referred to care after screening at syringe service programs, % | 0.75 (0.56–0.95) | Assumption |
| Natural history inputs | ||
| Probability of going from HBeAg-, active CHB, noncirrhotic to HBeAg-, active CHB, cirrhotic, % | 0.046 (0.005–0.15) | [55, 56] |
| Probability of going from HBeAg-, active CHB, noncirrhotic to HBeAg-, inactive CHB, noncirrhotic, % | 0.016 (0–0.11) | [56] |
| Probability of going from HBeAg-, active CHB, cirrhotic to HBeAg-, inactive CHB, cirrhotic, % | 0.016 (0–0.11) | [56] |
| Probability of going from acute hepatitis, adult, asymptomatic to HBeAg+, active CHB, no cirrhosis, % | 0.05 (0.01–0.1) | [55, 57, 58] |
| Probability of going from acute hepatitis, adult, symptomatic to HBeAg+, active CHB, no cirrhosis, % | 0.05 (0.01–0.1) | [55, 57, 58] |
| AHB to CHB transition probability for people who inject drugs, % | 0.1 (0.05–0.15) | [27] |
| Prevalence/incidence inputs | ||
| Annual incidence for developing acute hepatitis B in incarcerated persons, % | 0.0231 (0.0082–0.038) | [52] |
| Prevalence of active CHB in incarcerated persons, % | 0.014 (0.003–0.031) | [8] |
| Susceptibility to HBV in incarcerated persons, % | 0.53 (0.4–0.66) | [52, 53] |
| Prevalence of active CHB in people who inject drugs (general population), % | 0.118 (0.035–0.2) | [10] |
| Proportion of susceptible people who inject drugs, % | 0.44 (0.33–0.55) | [51] |
| Utility inputs | ||
| Utility in immune state | 0.99 (0.98–1) | [55] |
| Utility in susceptible state | 0.99 (0.98–1) | [55] |
Abbreviations: AHB, acute hepatitis B; CHB, chronic hepatitis B; HBeAg, hepatitis B e antigen (+ or -); HBV, hepatitis B virus; QALY, quality-adjusted life-year.
The model starts with a 30-year-old cohort (age range, 20–60 years), depicts progression of HBV from initial exposure and infection to acute and chronic infection, through immune phases, and to advanced liver disease. We examined no intervention and 3 intervention strategies: (1) screen for HBV infection only and treat those with active CHB (“treatment only”), (2) screen for HBV susceptibility only and vaccinate susceptible persons (“vaccination only”), and (3) screen for both HBV infection and/or immunity and treat or vaccinate appropriately (“inclusive”). We portrayed societal costs of intervention and CHB medical management. Costs and QALYs are discounted at 3% per year [22]. We conducted sensitivity analyses. We used TreeAge Pro 2016 (Williamstown, MA) and Excel 2016 (Redmond, WA).
Natural History of Hepatitis B
To establish a baseline of cost and clinical outcomes of hepatitis B without treatment or vaccination, we modeled a no intervention (no screening, vaccination, or treatment) group. The model portrays progression through immune-tolerant, inactive, immune-active, cirrhosis, and mortality (Supplementary Figures 1–5, Supplementary Table 1). Immune-tolerant is characterized by high HBV DNA, e-antigen-positive (HBeAg+), and no discernible liver damage (normal alanine aminotransferase [ALT]) [23]. Immune active has high DNA levels, HBeAg-positive or -negative, with elevated ALT [23]. Immune-inactive has low or undetectable DNA levels and is HBeAg-, with normal ALT [23]. These distinct phases are a reasonable representation of a natural history that includes some indeterminate, heterogeneous states. Annual probabilities for initial infection and progression to acute and chronic phases were collected from the literature, including quantitative synthesis, as needed (Supplementary Table 2) [24, 25]. We assumed that CHB progression probabilities from immune-active HBeAg+ and HBeAg- are equivalent as the literature does not clearly distinguish their transition probabilities. We used +/- 25% of base case to portray uncertainty for inputs lacking formal confidence intervals.
As Sub-Saharan Africa-born black individuals have higher incidence of HCC at a younger age than the general CHB population, we increased annual incidence of HCC by 1.5 (uncertainty range, 1–2.5) [26]. PWID may have higher risk of CHB after initial exposure than general population; thus, we simulated this risk for PWID at 10% (range, 5%–15%), compared with 5% for other populations [27].
We incorporate the prevalence of active CHB (Supplementary Table 3) and susceptibility to infection (Supplementary Table 4) for each population.
Intervention
Strategies for Screening and Care
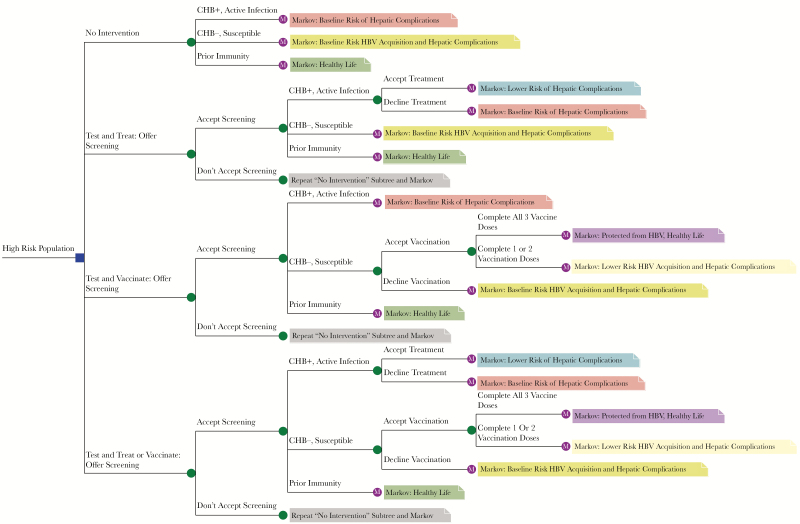
The goal of screening is to clarify disease status and to refer appropriately to treatment or vaccination [15]. Interventions are described above and are represented in Figure 1. We model population-specific screening programs (details in Supplementary Tables 5–8).
Figure 1.
Simplified depiction of the hepatitis B virus screening model. A high-risk population enters the model and receives either no intervention, screening for infection and appropriate treatment, screening for susceptibility and appropriate vaccination, or screening for both infection and susceptibility with appropriate treatment or vaccination. Patients then progress through the model based on patient status and administration of treatment or vaccination. Abbreviations: CHB, chronic hepatitis B; CHB+, patient has chronic hepatitis B; CHB-, patient does not have hepatitis B; HBV, hepatitis B virus.
Course of Hepatitis B With Treatment
The goal of treatment is to transition from immune-active to immune-inactive, which has lower clinical progression rates. Therapeutic success is defined as achieving undetectable serum HBV DNA, normal ALT, and conversion from HBeAg+ to HBeAg-, which post-treatment has a higher transition to the inactive state [23, 28]. The ideal outcome of hepatitis B surface antigen (HBsAg) clearance (functional cure) is rare with therapy [28].
Entecavir and tenofovir are current mainstays of therapy owing to high resistance barriers. Efficacy data (annual transition from immune-active to -inactive and loss of HBsAg) in both treatment-naïve and lamivudine-experienced patients were collected from clinical trials, postmarket studies, and other literature; per the data, higher first-year response to therapy was modeled (Supplementary Tables 9 and 10) [25, 29–32]. Treatment duration was modeled per guidelines in which noncirrhotic HBeAg+ patients continue treatment for 1 year after seroconversion (anti-HBe), whereas all others continue treatment until surface antigen loss (functional cure) [23].
Antiviral resistance leads to treatment failure, as seen in clinical trials and postmarket studies [23]. We portrayed annual risk of resistance, based on treatment-naïve or -experienced status (Supplementary Table 11), increasing annually for first 5 years and then stable [25, 32]. Resistance was not observed for tenofovir. Details of retreatment, HBV DNA suppression, and reductions in risk of advanced liver disease can be found in Supplementary Tables 12–14.
Treatment outcomes for PWID may be compromised by variable adherence and failure to be retained in care [33–35]. We estimated treatment discontinuation in PWID to be double that of the general CHB population. Patients who terminated treatment before disease control experienced a natural history of hepatitis B (Supplementary Table 9); if patients were retreated after discontinuation, they experienced treatment-related outcomes (Supplementary Table 12).
HBV Infection With Vaccination
The model simulates population-specific annual incidence of acute HBV infection among susceptible persons in each of 6 high-risk population groups, determined from published literature (Supplementary Tables 15) [36]. Given limited data, we used API rates for AbB and refugee populations.
Patients who achieve immunity through vaccination have no risk of HBV infection. We assumed full lifelong immunity from 3 vaccine doses and lower immunity for incomplete vaccination (Supplementary Table 16) [13]. Patients who did not complete the vaccination series experienced the natural history of hepatitis B according to dose-based efficacy, listed in Supplementary Table 16.
Model Inputs
Health-Related Quality of Life
We assigned health state utility for HBV states and treatment adverse events based on published empirical studies and economic evaluations (Supplementary Tables 17 and 18) [25].
Costs of CHB Management and Interventions
The costs of managing CHB were obtained from published studies (Supplementary Table 19) [24, 37]. For noncirrhotic immune-active disease, this is $1293 per year. We assumed that the costs of managing patients in the inactive phase and in HBsAg clearance (ie, functional cure) were half and one-quarter of costs of those in active disease, respectively. We modeled initial diagnostic tests (Supplementary Table 20) and ongoing monitoring test costs (Supplementary Table 21). For noncirrhotic/cirrhotic states (both immune-active/inactive) and functional cure states, we used uncertainty intervals of 50%–300% to represent wide variation in the literature.
Cost of Screening/Linkage to Care
The model estimates total cost for specified screening/linkage to care programs from published studies (Supplementary Table 22). This includes recruitment, personnel, and supplies to conduct screening. For unavailable program-specific costs, we used costs from similar programs [18].
Cost of Treatment/Vaccine
Base case costs for antiviral treatments (Supplementary Table 23) and vaccine (Supplementary Table 24) were set at 80% of wholesale acquisition cost from Red Book Online, because most payers receive discounts from the listed cost [38, 39]. Treatment-related adverse event costs were calculated from published costs of similar complications (Supplementary Table 25) [29–31].
Model Outputs
For each strategy and population, we estimate (a) net cost, incorporating intervention and medical costs; (b) quality-adjusted life years; (c) across strategies, incremental net cost per QALY gained (ICER); and (d) percentage of averted clinical outcomes (acute and fulminant hepatitis, acute liver death, new CHB) in the short and intermediate term (<6 months after HBV exposure) and long term (>6 months, including cirrhosis, liver failure, HCC, death).
We present below for tenofovir treatment. Entecavir, alternative screening and linkage programs, and breakdown of screening, treatment, and health care costs are in the Supplementary Data.
Model Validation
We validated natural history and treatment models by comparing clinical outcomes with published benchmarks (details in the Supplementary Data). The infection component was compared with incidence of active CHB (Supplementary Figure 6; Supplementary Table 26). The natural history model was validated for transition from immune-tolerant to immune-active and 3 clinical outcomes (cirrhosis, liver failure, HCC) using targets from a large longitudinal study (Supplementary Tables 27 and 28, Supplementary Figures 7 and 8) [40]. For treatment, outcomes were compared with observational and clinical trial data on cirrhosis, liver failure, HCC, and mortality (Supplementary Table 29, Supplementary Figure 9) [23].
Sensitivity Analyses
We conducted scenario analyses to estimate the efficacy of a hypothetical inclusive program that operates with high adherence: 90% have been screened for HBV infection and/or immunity; 100% have been referred to treatment or vaccination as appropriate; 90% have been linked to care; 80% have completed the hepatitis B vaccine series; and 90% of CHB patients initiate treatment.
Uncertainty in the model was tested using 1-way and multiway (probabilistic) sensitivity analyses on all input variables. In the main text, we present 1-way analyses for inputs that had a >30% effect on the ICER (additional analyses in the Supplementary Data). For probabilistic analyses, we conducted 10 000 simulations in which all variables were simultaneously varied, yeilding acceptability curves (details in the Supplementary Data).
Institutional Review Board
A review by the institutional review board was not required because cost-effectiveness analysis using publicly available data is not human subjects research.
RESULTS
Cost, QALYs, Cost-effectiveness
Table 2 shows the results of all strategies (vaccination only, treatment only, and inclusive) specific to the population. All 3 interventions had ICERs below $26 000/QALY gained compared with less intensive alternatives (additional results in Supplementary Tables 30–36).
Table 2.
Base Case Results for Screening and Linkage to Care Strategies, by Population
| Strategy, by Populationa | Cost, USDb | Incremental Cost, USDc | QALYs | Incremental QALYsc | ICER, USD/QALYd |
| Asian and Pacific Islanders | |||||
| No intervention | 3902 | – | 23.780 | – | – |
| Vaccination only | 4001 | 99 | 23.787 | 0.007 | 13 397 |
| Treatment only | 5360 | 1359 | 23.853 | 0.066 | Extended dominated |
| Inclusive | 5361 | 1 | 23.861 | 0.007 | 18 378 |
| Africa-born black population | |||||
| No intervention | 4928 | – | 23.551 | – | – |
| Vaccination only | 5024 | 96 | 23.559 | 0.009 | 11 086 |
| Inclusive | 6676 | 1652 | 23.653 | 0.094 | 17 645 |
| Treatment only | 6739 | 63 | 23.646 | –0.007 | Dominated |
| Incarcerated persons | |||||
| Vaccination only | 999 | – | 24.415 | – | – |
| No intervention | 1105 | 106 | 24.365 | –0.050 | Dominated |
| Inclusive | 1321 | 322 | 24.432 | 0.017 | 18 922 |
| Treatment only | 1446 | 125 | 24.382 | –0.050 | Dominated |
| Refugee population | |||||
| No intervention | 3183 | – | 23.934 | – | – |
| Vaccination only | 3278 | 95 | 23.944 | 0.010 | 9453 |
| Inclusive | 4716 | 1438 | 24.021 | 0.078 | 18 465 |
| Treatment only | 4746 | 29 | 24.011 | –0.010 | Dominated |
| People who inject drugs | |||||
| No intervention | 6924 | – | 23.070 | – | – |
| Vaccination only | 6974 | 50 | 23.078 | 0.008 | 6438 |
| Inclusive | 6999 | 24 | 23.079 | 0.001 | 25 551 |
| Treatment only | 7016 | 18 | 23.071 | –0.008 | Dominated |
| Men who have sex with men | |||||
| No intervention | 1354 | – | 24.325 | – | – |
| Vaccination only | 1361 | 8 | 24.336 | 0.011 | 695 |
| Inclusive | 1626 | 264 | 24.349 | 0.014 | 19 052 |
| Treatment only | 1637 | 11 | 24.338 | –0.011 | Dominated |
Abbreviations: ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life-years; USD, US dollar.
aStrategies are listed in order of total cost per patient for a given population.
bTotal health care costs are per person.
cIncremental health care costs and QALYs are per strategy compared with the preceeding intervention.
dIncremental ratios are the difference between costs divided by the QALYs of an intervention compared with the preceeding intervention.
The screen and vaccinate strategy had ICERs of <$14 000/QALY gained, or were dominant (less expensive and more effective), compared with no intervention. The costs per QALY gained were API $13 397, AbB $11 086, refugee $9453, PWID $6438, and MSM $695. In the incarcerated, vaccination dominates no intervention by being less costly (–$106) and more effective (0.05 QALYs).
Screen and treat (with tenofovir-based regimens) added cost and QALYs compared with screen and vaccinate, with ICERs of $17 000–$26 000 per QALY gained. For example, in API, screen/treat compared with screen/vaccinate increases cost by $1359 and QALYs by 0.066, but was extended dominated by the inclusive strategy.
The inclusive strategy yielded both health gains and savings compared with screen and treat only in most populations. Only API did not yield savings, with an incremental cost of $1 (QALY gain, 0.007). In AbB, which has a higher HCC baseline risk, the inclusive strategy had lower cost than treatment by $63 and higher QALYs by 0.007.
As expanding from a narrower strategy to inclusive saves money or has a highly favorable ICER, we compared it directly to no intervention. The costs per QALY gained by group were API $18 009, AbB $17 089, incarcerated $3203, refugee $17 432, PWID $8514, and MSM $10 954.
Clinical Outcomes
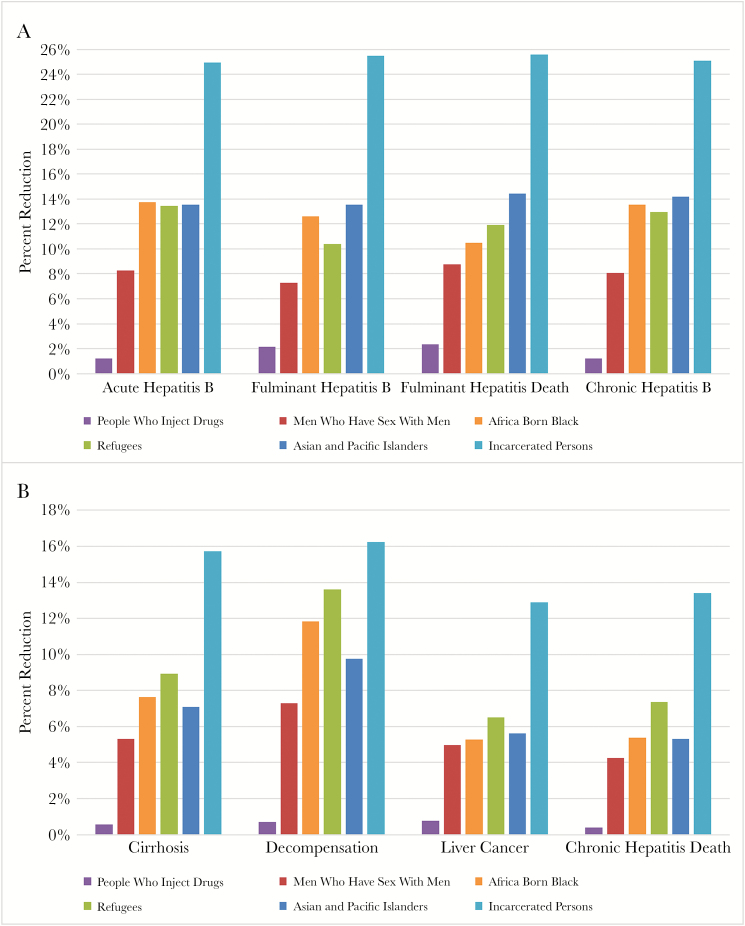
An inclusive program lowered all clinical outcomes by amounts that varied by risk group. Compared with no intervention, inclusive reduced new acute HBV cases for PWID (1%), MSM (8%), AbP (14%), refugee (13%), API (14%), and incarcerated (25%) (Figure 2A). These percentages reflect the proportion susceptible (low in PWID), vaccine completion (very low in PWID), and HBV incidence rates. This approach similarly reduced the intermediate clinical outcomes of acute HBV infections, fulminant hepatitis B, and liver failure (Figure 2A). Long-term clinical outcomes due to HBV (cirrhosis, liver failure, HCC, death) were reduced in the inclusive strategy compared with no intervention (Figure 2B). Incarcerated persons had the highest reduction in all clinical outcomes, whereas PWID experienced the smallest.
Figure 2.
Percent reduction in short/intermediate-term and long-term clinical outcomes with screen and treat or vaccinate strategy compared with no intervention for all study populations. Percentages for reduction in cases were calculated per 1 million patients vaccinated or treated using 1 million Monte Carlo simulations. The figures show, for every 1 million patients for each study population, the percentage of short/intermediate-term (A) and long-term (B) clinical outcomes that could be reduced by employing a program that either treats or vaccinates patients compared with no intervention.
Sensitivity Analyses
Scenario Analysis
The broad increase in adherence to screening and intervention led to an increase in both costs and QALYs, with more favorable ICERs (Supplementary Tables 37 and 38). Short- and intermediate-term adverse clinical events decreased by 44%–48% (Supplementary Figure 10) and long-term clinical complications by 11%–30% (Supplementary Figure 11).
One-Way Analyses
One-way sensitivity analyses for the inclusive strategy compared with no intervention indicated that the input uncertainties with the highest impact on ICER for most populations were age, discount rate, tenofovir cost, health state utility in immune or susceptible states, and rate of progression of disease from HBeAg- to cirrhosis or inactive (Figure 3). For example, in API populations, compared with the 30-year-old base case with ICER of $18 009/QALY, a 20-year old cohort results in a lower (more cost-effective) ICER of $16 418/QALY gained, whereas an older 60-year-old cohort has a higher (less cost-effective) ICER of $32 067/QALY gained.
Figure 3.
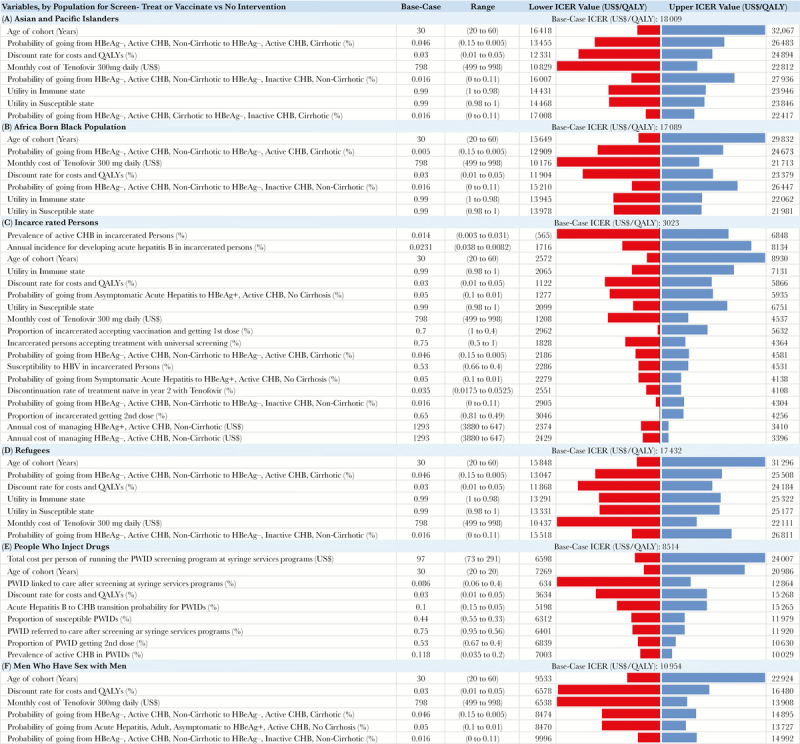
Sensitivity analysis for the inclusive strategy compared with no intervention for each population. The figure shows variables with >30% effect on the incremental cost-effectiveness ratio (ICER) for each population. Red bars indicate the lower ICER for the value listed first in the range; blue bars indicate an increase for the second value in the range. Cost-effectiveness of the inclusive strategy compared with no intervention was sensitive to multiple inputs. For example, a high annual transition from noncirrhotic to cirrhotic states decreased the ICERs across populations. The decrease is because, as the transition to cirrhosis (worse health state) increases, the clinical value of treating to prevent progression also increases. Conversely, a higher annual transition to inactive CHB (better health state) from immune active disease decreases treatment value, as patients would naturally move into less damaging CHB phases. However, even at the extreme upper and lower limits of uncertainty ranges, the ICERs remained very cost-effective for all populations at a cost of less $50 000 per QALY gained. Abbreviations: AHB, acute hepatitis B; CHB, chronic hepatitis B; HBeAg, hepatitis B e antigen (+ or -); ICER, incremental cost-effectiveness ratio; PWID, people who inject drugs; QALY, quality-adjusted life-year.
Another input substantially affecting results for most populations was annual transition from noncirrhotic, immune-active HBeAg- to other phases. In API, faster transition from noncirrhotic to cirrhotic HBeAg- state decreased the ICER to $13 455/QALY gained; slower transition increased the ICER to $26 483. Similar patterns were seen in other populations. Annual transitions from HBeAg+ had a much smaller effect on ICERs. More detailed tornado diagrams are available in the Supplementary Data (Supplementary Figures 12–17; for results not shown, data available from authors).
The cost-effectiveness results for the incarcerated population had a high sensitivity to prevalence and incidence inputs (Figure 3); although ICERs for other populations were also sensitive, the uncertainty was less pronounced (Supplementary Table 39). Across all uncertainty ranges for all populations, the ICER of the inclusive strategy compared with no intervention remained <$50 000/QALY or highly cost-effective.
Probabilistic Analyses
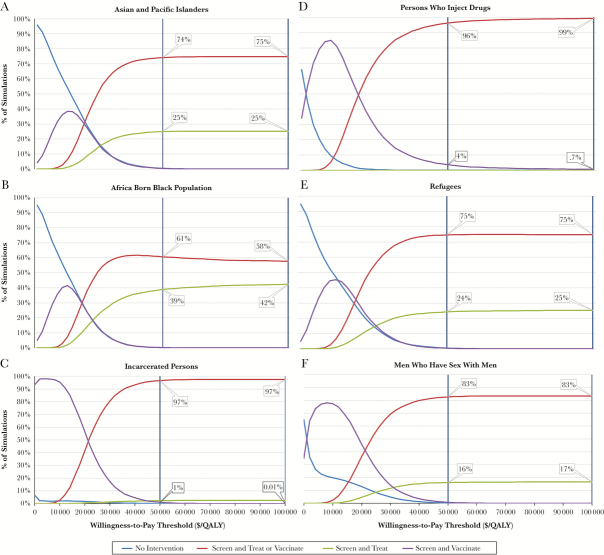
Multivariable analyses, where we compared all strategies, found that the inclusive strategy was cost-effective in 61%–97% of simulations at a willingness-to-pay (WTP) threshold of $50 000/QALY and was preferred in most simulations for all populations (Figure 4).
Figure 4.
Probabilistic sensitivity analyses of all intervention strategies for each population. The x-axis lists willingness-to-pay threshold in $/quality-adjusted life-year (QALY), whereas the y-axis shows the percentage of simulations in which a given strategy is cost-effective. For example, for the Asian and Pacific Islander population, at $50 000/QALY, the inclusive program to treat or screen was cost-effective 74% of the time, and screen and treat was cost-effective 25% of the time. Abbreviation: QALY, quality-adjusted life-year.
DISCUSSION
Our cost-effectiveness analysis found that inclusive HBV screen and vaccinate or treat strategies cost $3000 to $18 000 dollars per QALY gained, compared with no intervention. Although isolated screen for immunity and vaccinate or screen for infection and treat strategies are also cost-effective ($6000 to $21 000 per QALY gained), the broadening to an inclusive approach was incrementally very cost-effective or even cost-saving. These findings were qualitatively consistent across the modeled populations and a range of input values.
The cost-effectiveness of primary and secondary prevention of HBV reflects the severity of the disease and the effectiveness of available vaccination and treatment strategies. The highly cost-effective strategy of using both population-based vaccination and treatment is a result of efficiencies in combining the approaches. Although the screening for vaccination and treatment is slightly different, doing both within the same program adds relatively little cost.
To our knowledge, this is the first cost-effectiveness analysis to compare inclusive screen and treat or vaccinate with the isolated screen and vaccinate or screen and treat among multiple high-risk populations. Our findings are consistent with previous studies showing cost-effectiveness and savings for screening programs in individual high-prevalence populations [19, 20, 24, 41]. However, Hutton et al. assumed very little benefit from vaccination in the Asian and Pacific Islander population (except ring vaccination), such that the “inclusive” strategy appeared very cost-ineffective compared with screen and treat [36].
Of the populations we modeled, incarcerated persons had the lowest ICER and the highest reduction in HBV-associated outcomes. This is explained in large part by our portrayal of no prison release during intervention, allowing continuous linkage to care and adherence. In reality, those who leave correctional facilities may face difficulties gaining access to affordable care, and thus have lower intervention completion. However, when we varied these adherence assumptions in multivariable analysis, the strategy to screen and treat or vaccinate was still preferred 97% of the time.
In multivariable analyses, all populations except AbP experienced the inclusive strategy to be cost-effective at least 75% of the time, whereas in AbP it was 58%. The decrease in favorability of the inclusive strategy in AbP is largely explained by higher risk of developing HCC from any state, including in inactive and functional cure states [26]. This higher risk of HCC may negate some benefits of therapy (ie, QALYs), even with succesfull treatment, relative to CHB patients in other populations.
Our model also showed a relatively large decrease in HBV-associated clinical outcomes among refugees. Although declining, the United States has, in recent years, received about 70 000 refugees annually, often from intermediate– to high–HBV prevalence countries (about 20 000 from African nations and 17 000 from East Asia) [42]. Further, refugees may arrive from countries that have underdeveloped health care, with low likelihood of vaccination, CHB diagnosis, or treatment, leading to more opportunity for intervention [43]. If left untreated, Africa-born black refugees have higher risk of developing HCC than other CHB populations, and thus greater potential reduction in risk [26]. As such, it appears appropriate to treat and vaccinate refugees through targeted approaches.
We found that PWID would benefit from an inclusive intervention as well, albeit with the smallest anticipated reduction in HBV-associated outcomes. This is because in the populations we examined, PWID have the least social stability, higher chance of concurrent mental health issues, and lower health literacy [35]. These factors lead to lower linkage to care, vaccination completion, and treatment adherence. Despite these challenges, research shows syringe service programs (SSPs) to be an effective venue to screen and deliver care [44, 45]. In our model, an inclusive program implemented at SSPs increased QALYs compared with vaccination-only and dominated treatment–only programs. Multivariable analysis found that despite the uncertainty associated with screening and linking PWID to care, treating/vaccinating through an SSP is cost-effective up to 95% of the time at $50 000/QALY.
Our model has several limitations. First, we did not portray co-infections with HIV, hepatitis C, or other diseases (nonalcoholic steatohepatitis [NASH], alcoholic liver disease, etc.), which may alter the hepatitis B disease course. Second, we modeled new infections in adults based on current estimates of incidence; we did not portray dynamic interactions between populations, nor pediatric infections, and thus did not capture potential prevention benefits of treatment. However, a recent National Academy of Sciences (NAS) report concluded that there is limited evidence to suggest that treatment as prevention is a viable strategy for controlling hepatitis B in adults [13, 46]. Third, our model does not distinguish between hepatitis B genotypes or take into account sex differences, which may affect disease course. Fourth, screening and linkage data were unavailable for some populations, requiring adaptation of data from other populations. Fifth, we did not analyze the budget impact of the proposed screening and linkage to care programs. Finally, we were unable to examine 2 new interventions for HBV because they were approved too recently. A 2-dose HBV vaccine is now available, which could increase adherence [47]. Tenofovir alafenamide may have lower side effects with efficacy similar to the modeled therapy (tenofovir disoproxil fumarate) [48]. Further, a new generic version of modeled tenofovir was not commercially available at the time of this analysis. All of these could lead to more attractive ICERs for all 3 strategies examined in this study.
Our analysis lays the groundwork for estimating the impact of scaled-up programming. We found that to achieve a 45% reduction in HBV-associated burden, 90% of persons with CHB would have to be linked to care and 80% of susceptible persons would have to complete the vaccination. Such a program may be feasible for some populations and in some localities, such as in prisons or in an integrated health care system [49]. It will be more challenging among PWID. Although our model showed a relatively large decrease in HBV-associated clinical outcomes among many risk groups, HBV scale-up strategies are needed to achieve the NAS-articulated target of 50% reduction in mortality and morbidity by the year 2030 [46].
CONCLUSIONS
This analysis showed that an inclusive screen and treat or vaccination strategy was cost-effective in reducing the burden of hepatitis B virus among high-risk, high-prevalence populations.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Authors’ contributions. H.S.C., M.P., A.H., and J.G.K. conceived and designed the study. H.S.C. led the model design, data collection, analyses, and drafted the manuscript. M.P., A.H., and P.V. provided expert guidance on the subject matter, including input parameters, reviewed the findings, and edited the manuscript. D.M. serverd as the project manager, conducted literature reviews, and reviewed and edited the manuscript. J.G.K. supervised all aspects of the project and manuscript.
Financial support. This work was supported by the Centers for Disease Control and Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (NEEMA, # 5U38PS004649).
Role of the funding source. The Centers for Disease Control and Prevention provided input to the model design and input values, reviewed outputs, and collaborated on the manuscript.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. M.P. has received honoraria from Abbott, Gilead, Genentech, J&J, Merck and Roche; M.P.’s spouse is an employee of gRED, a subsidiary of Roche. P.V. chairs the Data Safety Monitoring Board for Merck. The other authors have no conflicts to declare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet 2014; 384:2053–63. [DOI] [PubMed] [Google Scholar]
- 2. Mast EE, Margolis HS, Fiore AE, et al. ; Advisory Committee on Immunization Practices (ACIP) A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005; 54:1–31. [PubMed] [Google Scholar]
- 3. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wasley A, Kruszon-Moran D, Kuhnert W, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis 2010; 202:192–201. [DOI] [PubMed] [Google Scholar]
- 5. Gish RG, Gadano AC. Chronic hepatitis B: current epidemiology in the Americas and implications for management. J Viral Hepat 2006; 13:787–98. [DOI] [PubMed] [Google Scholar]
- 6. Bailey MB, Shiau R, Zola J, et al. San Francisco hep B free: a grassroots community coalition to prevent hepatitis B and liver cancer. J Community Health 2011; 36:538–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris AM, Schoenbachler BT, Ramirez G, Vellozzi C, Beckett GA. Testing and linking foreign-born people with chronic hepatitis B virus infection to care at nine U.S. programs, 2012–2014. Public Health Rep 2016; 131:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamarulzaman A, Reid SE, Schwitters A, et al. Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners. Lancet 2016; 388:1115–26. [DOI] [PubMed] [Google Scholar]
- 9. Linde AC, Sweet KA, Nelson K, Mamo B, Chute SM. Impact of the Hepatitis Testing and Linkage to Care (HepTLC) initiative on linkage to care for Minnesota refugees with hepatitis B, 2012–2014. Public Health Rep 2016; 131:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011; 378:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Updated Action Plan for the Prevention, Care, & Treatment of Viral Hepatitis. Atlanta, Georgia: US Department of Health and Human Services; 2015. [Google Scholar]
- 12. Williams WW, Lu PJ, O’Halloran A, et al. ; Centers for Disease Control and Prevention (CDC) Surveillance of vaccination coverage among adult populations - United States, 2014. MMWR Surveill Summ 2016; 65:1–36. [DOI] [PubMed] [Google Scholar]
- 13. Buckley GJ, Strom BL, eds. Eliminating the Public Health Problem of Hepatitis B and C in the United States: Phase One Report. Washington, DC:National Academies of Sciences, Engineering, and Medicine; 2016. [PubMed] [Google Scholar]
- 14. Ward JW. Strategies for expanding access to HBV and HCV testing and care in the United States: the CDC hepatitis testing and linkage to care initiative, 2012–2014. Public Health Rep 2016; 131:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chandrasekar E, Kaur R, Song S, Kim KE. A comparison of effectiveness of hepatitis B screening and linkage to care among foreign-born populations in clinical and nonclinical settings. J Multidiscip Healthc 2015; 8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perumalswami PV, Factor SH, Kapelusznik L, et al. Hepatitis Outreach Network: a practical strategy for hepatitis screening with linkage to care in foreign-born communities. J Hepatol 2013; 58:890–7. [DOI] [PubMed] [Google Scholar]
- 17. Hsu L, Bowlus CL, Stewart SL, et al. Electronic messages increase hepatitis B screening in at-risk Asian American patients: a randomized, controlled trial. Dig Dis Sci 2013; 58:807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rein DB, Lesesne SB, Smith BD, Weinbaum CM. Models of community-based hepatitis B surface antigen screening programs in the U.S. and their estimated outcomes and costs. Public Health Rep 2011; 126:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pisu M, Meltzer MI, Lyerla R. Cost-effectiveness of hepatitis B vaccination of prison inmates. Vaccine 2002; 21:312–21. [DOI] [PubMed] [Google Scholar]
- 20. Hu Y, Grau LE, Scott G, et al. Economic evaluation of delivering hepatitis B vaccine to injection drug users. Am J Prev Med 2008; 35:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eckman MH, Kaiser TE, Sherman KE. The cost-effectiveness of screening for chronic hepatitis B infection in the United States. Clin Infect Dis 2011; 52:1294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA 1996; 276:1253–8. [PubMed] [Google Scholar]
- 23. Terrault NA, Bzowej NH, Chang KM, et al. ; American Association for the Study of Liver Diseases AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016; 63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SY, Billah K, Lieu TA, Weinstein MC. Cost effectiveness of hepatitis B vaccination at HIV counseling and testing sites. Am J Prev Med 2006; 30:498–506. [DOI] [PubMed] [Google Scholar]
- 25. Dakin H, Bentley A, Dusheiko G. Cost-utility analysis of tenofovir disoproxil fumarate in the treatment of chronic hepatitis B. Value Health 2010; 13:922–33. [DOI] [PubMed] [Google Scholar]
- 26. Kew MC. Epidemiology of hepatocellular carcinoma in sub-Saharan Africa. Ann Hepatol 2013; 12:173–82. [PubMed] [Google Scholar]
- 27. Houdt RV, Bruisten SM, Speksnijder AGCL, Prins M. Unexpectedly high proportion of drug users and men having sex with men who develop chronic hepatitis B infection. J Hepatol 2012; 57:529–33. [DOI] [PubMed] [Google Scholar]
- 28. Martin P, Lau DT, Nguyen MH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2015 update. Clin Gastroenterol Hepatol 2015; 13:2071–87 e16. [DOI] [PubMed] [Google Scholar]
- 29. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–55. [DOI] [PubMed] [Google Scholar]
- 30. Buti M, Fung S, Gane E, et al. Long-term clinical outcomes in cirrhotic chronic hepatitis B patients treated with tenofovir disoproxil fumarate for up to 5 years. Hepatol Int 2015; 9:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang TT, Lai CL, Kew Yoon S, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2010; 51:422–30. [DOI] [PubMed] [Google Scholar]
- 32. Buti M, Tsai N, Petersen J, et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci 2015; 60:1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feelemyer J, Des Jarlais D, Arasteh K, Uusküla A. Adherence to antiretroviral medications among persons who inject drugs in transitional, low and middle income countries: an international systematic review. AIDS Behav 2015; 19:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rich ZC, Chu C, Mao J, et al. Facilitators of HCV treatment adherence among people who inject drugs: a systematic qualitative review and implications for scale up of direct acting antivirals. BMC Public Health 2016; 16:994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mravčík V, Strada L, Stolfa J, et al. Factors associated with uptake, adherence, and efficacy of hepatitis C treatment in people who inject drugs: a literature review. Patient Prefer Adherence 2013; 7:1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hutton DW, Tan D, So SK, Brandeau ML. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med 2007; 147:460–9. [DOI] [PubMed] [Google Scholar]
- 37. Chahal HS, Marseille EA, Tice JA, et al. Cost-effectiveness of early treatment of hepatitis C virus genotype 1 by stage of liver fibrosis in a US treatment-naive population. JAMA Intern Med 2016; 176:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Red Book Online. Micromedex (University of California, San Francisco library), Greenwood Village, CO: Truven Health Analytics. http://www.micromedexsolutions.com . Accessed February 16, 2016.
- 39. Congressional Budget Office. Prices for Brand-Name Drugs Under Selected Federal Programs. Washington, DC: Congressional Budget Office, The Congress of the United States; 2005. [Google Scholar]
- 40. Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol 2011; 26:628–38. [DOI] [PubMed] [Google Scholar]
- 41. Miriti MK, Billah K, Weinbaum C, et al. Economic benefits of hepatitis B vaccination at sexually transmitted disease clinics in the U.S. Public Health Rep 2008; 123:504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.US Department of State, Bureau of Population, Refugees, and Migration. Cumulative Summary of Refugee Admissions as of December 31, 2015. https://2009-2017.state.gov/j/prm/releases/statistics/251288.htm . Accessed January 10, 2016.
- 43. Pottie K, Greenaway C, Feightner J, et al. ; coauthors of the Canadian Collaboration for Immigrant and Refugee Health Evidence-based clinical guidelines for immigrants and refugees. CMAJ 2011; 183:E824–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alanko Blomé M, Björkman P, Flamholc L, et al. Vaccination against hepatitis B virus among people who inject drugs - a 20 year experience from a Swedish needle exchange program. Vaccine 2017; 35:84–90. [DOI] [PubMed] [Google Scholar]
- 45. Treloar C, Rance J, Yates K, Mao L. Trust and people who inject drugs: the perspectives of clients and staff of needle syringe programs. Int J Drug Policy 2016; 27:138–45. [DOI] [PubMed] [Google Scholar]
- 46. National Academies of Sciences, Engineering, and Medicine. Buckley GJ, Strom BL, eds. Eliminating the Public Health Problem of Hepatitis B and C in the United States: Phase Two Report. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 47. US Food and Drug Administration. Prescribing information for Heplisav-B (Hepatitis B Vaccine (Recombinant), Adjuvanted) 2017. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf Accessed January 15, 2018.
- 48. US Food and Drug Administration. Prescribing information for vemlidy (tenofovir alafenamide) 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208464s000lbl.pdf Accessed July 29, 2017.
- 49. Jonas MC, Rodriguez CV, Redd J, et al. Streamlining screening to treatment: the hepatitis C cascade of care at Kaiser Permanente mid-Atlantic states. Clin Infect Dis 2016; 62:1290–6. [DOI] [PubMed] [Google Scholar]
- 50. Lee TA, Veenstra DL, Iloeje UH, Sullivan SD. Cost of chronic hepatitis B infection in the United States. J Clin Gastroenterol 2004; 38:S144–7. [DOI] [PubMed] [Google Scholar]
- 51. Heimer R, Grau LE, Singer M, et al. Hepatitis B virus prevalence and vaccination rates among hispanic injection drug users participating in a vaccination campaign. J Drug Issues 2008; 38:335–50. [Google Scholar]
- 52. Weinbaum C, Lyerla R, Margolis HS; Centers for Disease Control and Prevention Prevention and control of infections with hepatitis viruses in correctional settings. Centers for Disease Control and Prevention. MMWR Recomm Rep 2003; 52:1–36; quiz CE1–4. [PubMed] [Google Scholar]
- 53. Weinbaum CM, Sabin KM, Santibanez SS. Hepatitis B, hepatitis C, and HIV in correctional populations: a review of epidemiology and prevention. AIDS 2005; 19:S41–6. [DOI] [PubMed] [Google Scholar]
- 54. Akyar E, Seneca KH, Akyar S, et al. Linkage to care for suburban heroin users with hepatitis C virus infection, New Jersey, USA. Emerg Infect Dis 2016; 22:907–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rossi C, Schwartzman K, Oxlade O, et al. Hepatitis B screening and vaccination strategies for newly arrived adult Canadian immigrants and refugees: a cost-effectiveness analysis. PLoS One 2013; 8:e78548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Toy M, Hutton DW, So SK. Cost-effectiveness and cost thresholds of generic and brand drugs in a National Chronic Hepatitis B treatment program in China. PLoS One 2015; 10:e0139876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–39. [DOI] [PubMed] [Google Scholar]
- 58. Shepard CW, Simard EP, Finelli L, et al. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006; 28:112–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.