Abstract
Objective:
To evaluate relationships among obesity in pregnancy and plasma levels of tryptophan (TRP) and kynurenine (KYN), inflammatory markers, and depressed mood.
Methods:
Pregnant women (N = 374) were enrolled, and data were collected at a mean gestation of 20 weeks in this cross-sectional study. Plasma was analyzed for TRP, KYN, neopterin, and nitrite levels. Women completed demographic and mood scales.
Results:
There was a statistically significant inverse correlation between body mass index (BMI) and TRP and positive correlations between BMI and KYN and the kynurenine/tryptophan (KYN/TRP) ratio. Neopterin was correlated with KYN/TRP, suggesting that the indoleamine 2,3-dioxygenase-1 (IDO-1) enzyme was activated. The correlations of neopterin and nitrite with BMI were too small to be clinically meaningful but may provide mechanistic insight. There was a correlation between depressed mood and nitrite levels. Depressed mood was also associated with lower TRP levels. When the sample was divided into pregnant women with or without obesity, TRP was significantly lower and the KYN/TRP ratio was significantly higher in the women with obesity.
Conclusion:
The pro-inflammatory state of obesity in pregnancy may drive activation of IDO-1, resulting in diversion of TRP away from serotonin and melatonin production and toward KYN metabolites. This alteration could contribute to depression, impaired sleep, increased production of excitotoxic neurotransmitters, and reinforcement of a pro-inflammatory state in pregnancy.
Keywords: BMI, pregnancy, tryptophan, kynurenine, inflammation
Obesity during pregnancy is a well-known risk factor for multiple maternal and fetal complications. Metabolic, neuroendocrine, microbiome, and immune pathways are thought to be affected by obesity during pregnancy. Defining obesity during pregnancy is somewhat problematic, but one guideline is a body mass index (BMI) of 30 kg/m2 or greater at the start of pregnancy. Approximately 18% of women in the United States exceed this limit (Chu, Callaghan, Bish, & D’Angelo, 2009). Recommendations are that women at or above this BMI gain no more than 7 kg during their entire pregnancy (Davies et al., 2010). The greatest risk for obesity in pregnancy is the presence of prepregnancy obesity.
One effect of obesity, both in general and in pregnancy, is a chronic inflammatory state. An inflammatory in utero environment in obese mothers contributes to adverse pregnancy outcomes such as gestational diabetes, hypertension, preeclampsia, and complications during labor (Rowlands, Graves, de Jersey, McIntyre, & Callaway, 2010). In addition, the offspring of obese mothers are more likely to be obese and to have metabolic syndrome, neural tube defects, and cognitive deficits (van der Burg et al., 2016). In fact, overweight as well as obesity is found to increase risk for neurodevelopmental deficits in the offspring. Fetal over nutrition may occur in obese women and may be associated with inflammatory signaling in both mother and fetus (Lawlor, 2013). Researchers have found high levels of pro-inflammatory cytokines (interleukin 1-β, interleukin-6 and -10 [IL-6 and IL-10], monocyte chemoattractant protein 1, interferon-γ [IFN-γ], and tumor necrosis factor-α [TNF-α]) in placental tissue and serum in association with obesity in pregnancy in humans and rodents (Madan et al., 2009; Roberts et al., 2011). Others have noted that activation of p-38-MAP Kinase (MAPK) and Signal transducer and activator of transcription 3 (STAT3) pro-inflammatory pathways were elevated in obesity (van der Burg et al., 2016).
Of course, obesity is not the only cause of inflammation during pregnancy. Infectious diseases, stress, micronutrient deficiencies, low socioeconomic status, and even paternal factors may also play a role (van der Burg et al., 2016). While maternal obesity may affect the fetus through inflammation-mediated pathophysiological mechanisms, other pathways are potentially important as well, including the maternal gut microbiome (Soderborg, Borengasser, Barbour, & Friedman, 2016), direct excitotoxic and oxidative stress effects (Kaindl, Favrais, & Gressens, 2009), or circulatory and coagulation effects on the fetal brain (Leviton & Dammann, 2004).
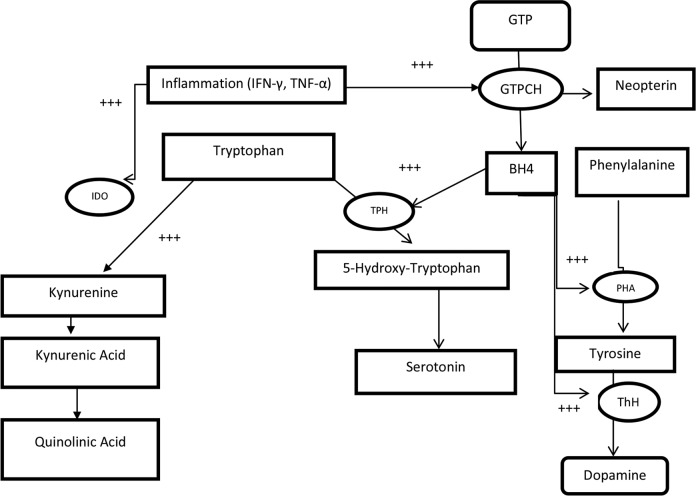
The tryptophan (TRP) catabolite pathway is one important biochemical pathway that is affected by inflammation but that researchers have not yet explored extensively in studies of obesity in pregnancy. TRP is an essential amino acid that, when not used in protein synthesis, is catalyzed by several enzymes, including hydrolases and dioxygenases. The latter includes tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase-1 and -2 (IDO-1 and IDO-2). TDO, the most prevalent of the dioxygenases under healthy conditions, is mainly found in the liver and is stimulated by cortisol (Mangge et al., 2014). IDO-1 is produced by macrophages and dendritic cells and in high amounts by the placenta (Yamazaki, Kuroiwa, Takikawa, & Kido, 1985). The major activator of IDO-1 is IFN-γ, but other inflammogens such as IL-6, TNF-α, lipopolysaccharides, and oxidative stress can also activate IDO-1. This activation results in the diversion of TRP into the kynurenine (KYN) pathway and away from serotonin and melatonin production (Maes, Leonard, Myint, Kubera, & Verkerk, 2011) and is associated with an increased ratio of KYN to TRP and with neopterin production (Neurauter et al., 2008). Neopterin is a biomarker of T helper-1 immune and inflammatory responses, as it is released from IFN-γ -stimulated macrophages (Jones et al., 2015). The kynurenine/tryptophan (KYN/TRP) ratio is a proxy measure of IDO activation (Darcy et al., 2011), and a correlation between neopterin and the KYN/TRP ratio further suggests IDO-1 activation (Murr, Widner, Wirleitner, & Fuchs, 2002). KYN is an intermediate molecule. Its downstream catabolites kynurenic acid (KA) and quinolinic acid (QA) can have diverse pathophysiological excitotoxic, oxidative, and inflammatory effects as well as immunoregulating effects (Pérez-De La Cruz, Königsberg, & Santamaría, 2007). Figure 1 depicts the multiple interactions among the pathways and metabolites that are affected by inflammation. Inflammation acts on the IDO-1 enzyme to generate the KYN catabolites as well as on the guanosine triphosphate (GTP) cyclohydrolase enzyme to generate neopterin from GTP. The accumulation of neopterin decreases the availability of tetrahydrobiopterin (BH4), a cofactor for amino acid hydrolases. If these enzymes are less active due to decreased BH4, decreased production of the neurotransmitters serotonin, melatonin, and dopamine results and inhibition of nitric acid synthase (NOS), which generates nitric oxide (NO), occurs. If IDO-1 is activated, KYN metabolites can increase NOS but decreased formation of BH4 opposes this action, and the result is a shift from NO synthesis to superoxide anion production (G. F. Oxenkrug, 2011). In either case, damaging free-radical stress may result.
Figure 1.
Inflammation affects multiple pathways. Serotonin can be enzymatically activated by tryptophan hydroxylase to form 5-hydroxytryptophan,N-methyl-D-aspartate which is then converted into serotonin BH4, a cofactor for this and other amino acid hydoxylases (phenylalanine, tyrosine), has GTP as its source through the GTP cyclohydrolase enzymatic reaction. These amino acids are substrates for the formation of dopamine, melatonin, and other catecholamines. In the other pathway, tryptophan is enzymatically catabolized to kynurenine, which forms kynurenic acid and then QA. QA is able to bind to the receptors, thus acquiring neurotransmitter properties. The enzymes TDO and IDO catalyze this reaction, but TDO is largely active only in the liver, while IDO reactivity is immune cell bound. These reactions occur in macrophages and other immune cells, so this interaction of amino acid substrates and immune cells is of note. Of particular importance is the role of the inflammatory cytokines IFN-γ and TNF-α, which stimulate BH4 production and IDO activation. +++ = pathways affected by inflammation; BH4 = tetrahydrobiopterin; GTP = guanosine triphosphate; GTPCH = guanosine triphosphate cyclohydrolase; IDO = indoleamine 2,3-dioxygenase; IFN-γ = interferon-γ; PHA = phenylalanine hydrolase; ThH = tyrosine hydrolase; TNF-α = tumor necrosis factor-α; TPH = tryptophan hydrolase; QA = quinolinic acid.
Individuals with obesity have lower levels of TRP and an increased KYN/TRP ratio in adipose tissue, indicating IDO-1 activation (Favennec et al., 2015; Wolowczuk et al., 2012). IDO-1 activity mostly takes place in immune cells. However, in pregnancy, a major additional large source of IDO-1 is the placenta (Davies et al., 2010; Yamazaki et al., 1985). The placental form of IDO-1 may prevent TRP from serving as a protein synthesis substrate in immune cells, particularly T lymphocytes (Munn et al., 1998). Authors have posited that this function represents an important adaptive mechanism that protects the fetal allograft from attack by cytotoxic T cells (Mellor & Munn, 1999). Newer evidence suggests that IDO-1 is also involved in trophoblast proliferation and migration (Zong et al., 2016). The action of KYN metabolites can suppress allogeneic T cells, a mechanism that may be active in pregnancy. KYN may participate in the generation of T regulatory cells, which play a major role in maternal–fetal tolerance (Mezrich et al., 2010). Researchers have observed decreasing TRP and increasing KYN/TRP ratios (an index of TRP degradation and IDO-1 activity) across pregnancy, along with evidence of a role for inflammation in these effects (K. Schröcksnadel et al., 2003). However, the interrelationships among obesity in pregnancy, inflammation, and TRP biochemistry have not been studied.
We had the opportunity to study these relationships in data collected for a larger parent study. We hypothesized that there would be associations among obesity, inflammatory markers, and TRP catabolites in pregnant women. We also explored whether maternal depressive mood might be related to these catabolites and inflammatory markers due to the potential effects of IDO-1 activation on neurotransmitters. A new hypothesis about the development of depression in relation to inflammatory states suggests that serotonin reduction leading to depression is an outcome of IDO-1 activation (Chaves Filho et al., 2018) In an earlier study in which Postolache, a coauthor of the present article, participated, researchers reported relationships between prenatal depression and lower TRP levels and higher C-reactive protein levels (Scrandis et al., 2008). Other studies have also shown a relationship between prenatal depression and lower TRP levels (Field et al., 2008; Kiryanova, Meunier, Vecchiarelli, Hill, & Dyck, 2016), so we felt this relationship was worth exploring in the secondary data since a mood measure was available.
The purpose of the present study, therefore, was to describe relationships among TRP/KYN as a proxy for IDO-1 activation, neopterin and nitrite as proxies for inflammation, and BMI and depressed mood in healthy pregnant women in the early second trimester.
Method
The present study was cross-sectional in nature and was a secondary analysis of data collected in a parent study of the relationships between postpartum thyroiditis and immunity and mood (M. Groer & Jevitt, 2014; M. E. Groer, Jevitt, & Ji, 2015;).
Sample
Pregnant women were recruited from several prenatal practice sites in Tampa, FL, during the early second trimester. Measures taken included plasma levels of thyroid peroxidase (TPO) autoantibodies and a panel of immune and metabolic markers. Exclusion criteria for entry into the study were the following: drug/alcohol abuse, autoimmune disease, previous thyroid disease, medications that could affect immune function, BMI < 18 kg/m2, in vitro fertilization for current pregnancy, and twinning. All women received a US$10 stipend for participating in the parent study. The majority of women did not have these autoantibodies (the healthy control group), and these were the participants whom we included in the current substudy. Participants signed informed consent forms, and the university institutional review board approved both the parent study and this substudy.
Measures
Descriptive data
Demographic data were obtained using an investigator-developed instrument and from the medical record. BMIs were calculated from the prepregnancy weight and the 16- to 20-week prenatal visit weight recorded on the office scale along with patient-reported heights. Women were classified based on their prepregnancy BMI as normal weight (BMI of 25 kg/m2 or less), overweight (BMI range of 25.1–29.9 kg/m2), or obese (BMI of 30 kg/m2 or higher).
Depressed mood
At their study visit, women were administered the Profile of Mood States (POMS) instrument, which has a depression/dejection subscale (POMS-D) that we used in the present analysis (McNair, Lorr, & Droppleman, 1992). The POMS provides a multidimensional assessment of mood to measure how the respondent has felt over the previous week. It has not been used extensively with pregnant women. POMS scores have good internal consistency, .87 to .92, and test–retest reliabilities ranging from .65 to .74 in adult populations (Cruess et al., 2000). The POMS-D subscale uses a Likert-type scale of 0–4 for each of 15 items and has a range of 0–60, with higher scores indicating more depressed mood. While not diagnostic, it is a screening tool that can be used for referral. Participants with a POMS-D score over 20 were referred to health-care providers for further evaluation of depression.
Plasma measures
The blood sample was collected in heparinized tubes by venipuncture in the morning hours and brought to the laboratory within 3 hr of collection for processing. The whole blood was centrifuged at 10,000 × g at 4°C for 20 min, and the plasma was aliquotted into sterile Eppendorf tubes. Plasma aliquots were sent to Dr. Fuch’s laboratory in Innsbruck, Austria, where they were analyzed for TRP (µmol/L) and KYN (µmol/L) by high-performance liquid chromatography (HPLC). The KYN/TRP ratio was calculated (expressed as micromole KYN/millimole TRP). TRP and KYN analyses were performed using HPLC on reversed phase. Serum samples were diluted with potassium phosphate buffer containing l-nitro-tyrosine as an internal standard and were deproteinized with trichloroacetic acid. After injection of 100 μl of trichloroacetic acid, separation was performed on a reversed-phase C18 column (LiChroCART®, Merck/Millipore Sigma, Darmstadt, Germany) using sodium acetate buffer (15 mmol/L, pH 4.0) with 3% acetonitrile and a flow rate of 0.9 ml/min at a temperature of 25°C and a Varian ProStar liquid chromatography system with autosampler Model 400 (Varian, Palo Alto, CA). KYN was monitored by its ultraviolet light-absorption at 360 nm (UV-detector SPD-6A, Shimadzu, Japan) and TRP by detection of its natural fluorescence at 285 nm excitation and 365 nm emission wavelengths (ProStar fluorescence-detector Model 360, Varian). Peaks were identified by comparing peak retention time to an albumin-based calibrator containing 10 μmol/L l-KYN and 100 μmol/L l-TRP prepared like plasma samples. To estimate IDO-1 activity, KYN/TRP was calculated. Neopterin was measured by enzyme linked immunoassay (BRAHMS, Hennigsdorf, Germany) according to the manufacturer’s instructions, with a detection limit of 2 nmol/L. Nitrite level was determined by the Griess method (Giustarini, Dalle-Donne, Colombo, Milzani, & Rossi, 2004).
Statistics and Power
The data were examined for normality and transformed by natural logarithm, if necessary. Descriptive statistics were calculated and Pearson correlations were performed on all variables except for nitrite and neopterin levels, which could not be transformed to normality. On these variables, we calculated Kendall-Tau correlations.
For the equal variance t test using TRP levels, the sample sizes of the groups (243 women without obesity [120 of normal weight and 123 who were overweight] and 131 women with obesity) provided 90% power to reject the null hypothesis of equal means between the groups with a mean difference of 5 points and a standard deviation of 15.
Results
Demographics
Participants for this substudy comprised 374 pregnant women from the parent study who were TPO-negative and had complete data. The mean week of pregnancy was 19.8 ± 2.8 (range = 15.2–25.6) and the mean age was 27.9 ± 5 years. The racial/ethnic distribution was 45.6% Caucasian, 23.2% African American, 7.5% other races, with 23.7% declaring themselves to be of Hispanic origin. The majority of the women had a high school (38.5%) or college (41.9%) education. Incomes ranged from less than US$25,000/year (16%) to over US$70,000/year (40%). Most of the participants were married (71%). Nearly half (47.2%) were primiparous. The mean score on the POMS-D scale was 5.52 ± 7.9, with a range of 0–49. We used a score of 20 as a cutoff for referral to a health-care provider for further assessment for depression, and 23 women met this criterion.
BMI
The mean prepregnancy BMI for the entire sample was 27.5 ± 12 kg/m2 and the mean BMI at the prenatal visit was 28.9 ± 6.5 kg/m2. Of the entire sample, 25% (n = 92) had a prepregnancy BMI in the obesity range (≥30 kg/m2; mean BMI of 36.8 ± 5.7) and 28% (n = 104) were overweight (BMI of 24.9 to < 30 kg/m2; mean BMI of 27.1 ± 1.3 kg/m2). The women who entered pregnancy with BMIs in the obese range had a mean pregnancy BMI of 36.1 ± 5.46 kg/m2, with a range of 36.1–60 kg/m2, while women who were not obese prior to pregnancy had a mean pregnancy BMI of 26.1 ± 3.4 kg/m2, with a range of 18.9–29.9 kg/m2. Those who entered pregnancy with BMIs in the obese range had gained a mean weight of 11.1 lbs, with a range of −20 to 54 lbs, by early second trimester. Of the 104 women (38%) who were in the overweight range at prepregnancy, 39 had a BMI of 30 kg/m2 or higher by 20 weeks of pregnancy. None of the women who had a BMI in the normal weight range at prepregnancy had a BMI in the obese range at the pregnancy measurement point, with a mean weight gain in this group of 10.8 lbs and a range of −19 to 34 lbs.
Metabolites
Mean levels of TRP were 62.6 ± 15.2 µmol/L, KYN 1.9 ± .75 µmol/L, neopterin 5.9 ± 2.0 nmol/L, and nitrite 19.3 ± 13.9 µmol/L. The mean KYN/TRP ratio was 31.2 ± 11.7. We analyzed data in two ways: first, we analyzed pregnancy BMI and metabolites in only those women who entered pregnancy at BMI of 30 kg/m2 or higher, and second, we analyzed these variables for all women who had a BMI of 30 kg/m2 or higher at the pregnancy measurement, which included an additional 39 women who all were overweight at prepregnancy (≥25 kg/m2) and had gained sufficient weight to move into the obesity category during pregnancy. These two approaches did not result in different statistical results, so we report the latter analyses, which included 131 women with BMIs of 30 kg/m2 or higher at the measurement time of 20 weeks’ gestation. Table 1 provides the correlations (r values) between pregnancy BMI and the metabolites. African American women had a higher mean pregnancy BMI (31 kg/m2) than Caucasian women (28.5 kg/m2; t = 2.6, p = .01). They also had lower TRP levels (t = 2.7, p = .07), but none of the other biomarkers differed by race. Therefore, we calculated a partial correlation for the BMI and TRP relationship, controlling for race. We conducted Pearson correlations for all variables except for nitrite levels and neopterin, as these could not be transformed adequately. For these latter variables, we used the Kendall-Tau nonparametric correlation.
Table 1.
Correlations Between Demographic and Metabolic Variables (r) in Women With Obesity at the Early Second Trimester of Pregnancy.
| BMI | TRP | KYN | KYN/TRP | Neopterin | |
|---|---|---|---|---|---|
| TRP | −.142** | ||||
| KYN | .117* | .41*** | |||
| KYN/TRP | .219*** | −.212*** | .797*** | ||
| Neopterin | .077* | .057 | .268*** | .236*** | |
| Nitrite | .098** | .009 | −.042 | −.041 | −.002 |
Note. N = 131. BMI = body mass index; KYN = kynurenine; TRP = tryptophan.
*p ≤ .05. **p ≤.01. ***p ≤ .001.
In order to discern whether these correlations were actually related to obesity (BMI > 30 kg/m2), we conducted an independent samples t test to compare TRP, KYN, and the KYN/TRP ratio for those women whose BMIs were below (n = 43) and above (n = 131) 30 kg/m2 at 20 weeks’ gestation (Table 2). TRP and the KYN/TRP ratio differed significantly by group, as did nitrite levels, which were significantly higher in women with obesity. Neopterin levels were not statistically higher (p = .07) in women with obesity compared to women without.
Table 2.
Comparison of Metabolite Levels Between Women in the Early Second Trimester of Pregnancy With Body Mass Indexes (BMIs) Lower and Higher than 30 kg/m2 Independent t Tests.
| Measure | BMI < 30 kg/m2 (n = 43) | BMI > 30 kg/m2 (n = 131) |
|---|---|---|
| TRP** (µmol/L) | 64.7 ± 15.8 | 59.2 ± 13.5 |
| KYN (µmol/L) | 1.89 ± .76 | 1.92 ± .75 |
| KYN/TRP** | 29.75 ± 11.4 | 33.82 ± 11.9 |
| Neopterin* (nmol/L) | 5.7 ± 1.98 | 6.1 ± 2.17 |
| Nitrite* (µmol/L) | 17.83 ± 11.7 | 21.59 ± 16.8 |
Note. KYN = kynurenine; TRP = tryptophan.
*p ≤ .05; **p ≤ .01; ***p ≤ .001.
TRP was significantly lower in women with POMS-D scores >20 (mean TRP level = 56.8 µmol/L) compared to women with lower POMS-D scores (mean TRP level = 63.2 µmol/L; t = −2.5, df = 381, p = .017). POMS-D scores were not correlated with any of the other metabolites except for nitrite levels (r = .10, p = .04).
Discussion
The enzymatic conversion of TRP to KYN is normally carried out by hepatic TDO. The IDO-1 pathway becomes important in pregnancy through high expression of placental IDO-1 and in immune-related conditions such as chronic inflammation or infections (Badawy, 2015). Obesity in pregnancy is an inflammatory condition in which this latter pathway could become influential. The induction of IDO-1 by IFN-γ in inflammatory conditions including pregnancy ranges from 20- to 4,000-fold above normal, so TRP metabolism is largely regulated by IDO-1 in these situations (Yamazaki et al., 1985).
The findings of the present study support the hypothesis that TRP depletion and activation of IDO-1 is greater in pregnant women with obesity compared to those with BMIs lower than 30 kg/m2. This difference may be driven by the inflammatory state associated with obesity (Gregor & Hotamisligil, 2011). Although we did not measure IDO-1 activation directly in the present study, the KYN/TRP ratio and the correlation of neopterin with both this ratio and KYN levels are indicators of activation. Previous research has reported correlations of KYN and KYN/TRP with neopterin in patients with diabetes (Oxenkrug, Ratner, and Summergrad, 2013) as well as many other clinical conditions such as systemic lupus erythematosus, HIV-1 infection, and colorectal cancer (Schröcksnadel, Wirleitner, Winkler, & Fuchs, 2006). In a recent study, researchers found that both neopterin and the KYN/TRP ratio at 18 weeks of pregnancy were associated with prepregnancy BMI (Bjorke-Monsen et al., 2016), which supports the current study findings. Nevertheless, contributions of TDO activity may have been important along with IDO-1 activation in the present study, as we did not observe statistically significant elevations in levels of plasma neopterin or KYN. KYN is, however, an intermediate metabolite, and measurement of downstream metabolites would be needed to fully support our hypotheses.
Nitrite levels were slightly, but significantly, higher in women with obesity in the present study. One might expect these levels to be lower since activation of IDO-1 is associated with a reduction in BH4, which is a required cofactor for the NOS enzyme, which generates NO and is the source of nitrite. In a recent report relying on a small sample of pregnant women, researchers found that nitrite levels did not differ between overweight and normal weight women (Hernandez-Trejo et al., 2017). The nitrite difference we observed could potentially be related to factors other than inflammation or NOS, such as diet or renal function. Nitric acid is a free radical that antioxidants protect against, and its elevation may indicate inadequate antioxidant status. If excessive nitrite production is present, it may signal nitrosative stress, which results in damaging free radical production. We also found that women with obesity in the present study had higher, but not significantly so, neopterin levels. These findings suggest that future studies should include an array of inflammatory markers.
In normal pregnancies, placental IDO-1 is expressed at very high levels, plasma levels of TRP decrease, and the KYN/TRP ratio, a surrogate for IDO-1 activity, increases (Blaschitz et al., 2011; Schröcksnadel, Baier-Bitterlich, Dapunt, Wachter, & Fuchs, 1996). However, free TRP is more bioavailable during pregnancy, and its nutritional value to the fetus, as an essential amino acid, must be balanced against its potential role in immunosuppression (Badawy, 2015). The immunosuppressive effect involves reducing the availability of TRP through placental IDO to “starve” immune cells that would normally recognize fetal cells as foreign. The additional IDO-1 activation we observed by proxy in the present study in women with obesity may be deleterious for both infant and mother. Production of excessive levels of KYN catabolites such as KA and QA may cause neurochemical changes in the fetal brain that increase the risk for diseases such as schizophrenia later in life (Pershing et al., 2015). Complications of pregnancy potentially associated with obesity-related excessive KYN metabolites include disrupted sleep (Cain & Louis, 2016), diabetes (Agha-Jaffar, Oliver, Johnston, & Robinson, 2016), preeclampsia (Persson, Cnattingius, Wikström, & Johansson, 2016), and recurrent spontaneous abortions (Zong et al., 2016).
Our findings only partially supported our hypothesis that depressed mood was related to inflammatory mediators and IDO-1 activation in pregnant women with obesity. Women with very high POMS-D scores (>20) did have significantly lower TRP levels in the present study, as authors have previously reported (Field et al., 2008; Kiryanova et al., 2016; Scrandis et al., 2008). We also found a positive association between nitrite levels and POMS-D scores. Nitrite is a relatively stable end product of NO and plays a role in the immune process and metabolic regulation (Bogdan, 2015; Murr et al., 2002). Nitrite level is a surrogate marker of NOS activation. Our finding is thus in line with hypotheses regarding an association of inflammation with depression, although the POMS-D is only a screening measure of depressive mood. Future studies should include measures of clinical prenatal depression.
The present study had a number of limitations. It was a cross-sectional study, so it only provides a single measure in time. We did not follow participants through their pregnancies. Thus, we were not able to exclude women with later complications such as preeclampsia from our analysis, but their data may have differed from those of women without future complications at our measurement time. The parent study provided sufficient data to address our hypotheses, but it was, nonetheless, limited. Better measures of inflammation such as inflammatory cytokines, particularly interferon-γ, would have improved our ability to interpret these data. We did not have measures of all the TRP catabolites that are potentially involved in the pathways depicted in Figure 1. We also did not have measures of a number of additional variables that could have affected TRP levels, including diet, medications, ingestion of alcoholic and common hot drinks, use of illicit drugs, exercise, and mild stressors (Badawy, 2010). Severe malnutrition is also likely to decrease brain levels of TRP and increase levels of KYN (Honorio de Melo Martimiano et al., 2017). We explored the potential relationship between activation of IDO-1 and depression using a measure of depressive mood that has not been well validated in pregnancy. The mood we measured could have been a transitory state. As it is a reduction in serotonin that is hypothesized to mediate this relationship, it would be ideal to explore the association between levels of this neurotransmitter and IDO-1 activation. However, because serotonin does not cross the blood–brain barrier, it is not currently possible to accurately measure it. A better measure of depression, though, would improve future research.
Conclusion
Multiple studies have noted the many deleterious effects associated with obesity in pregnancy. Women entering pregnancy at or above a BMI of 30 kg/m2 are essentially assured of continued obesity throughout pregnancy, and our data in the present study suggest that overweight women have an increased risk of becoming obese in pregnancy and undergoing associated metabolic changes. Since our measures were cross-sectional only, we do not know the ultimate outcome of these women’s weight gain during pregnancy; it is clear, however, that they were exceeding the recommended weight gain for women with obesity at the time of measurement, and it is unlikely that their weight-gain trajectory would have changed markedly. Our findings in the present study add to the literature on the pathophysiological effects of obesity in pregnancy on mothers and fetuses and the complex mechanisms underlying these effects.
Footnotes
Author Contribution: Maureen Groer and Allyson Duffy contributed to conception, design and acquisition; critically drafted the manuscript; revised the manuscript; and gave final approval, agree to be accountable for all aspects of work ensuring integrity and accuracy. Dietmar Fuchs contributed to acquisition, critically drafted the manuscript, revised the manuscript, and gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy. Amy D’Agata contributed to interpretation, critically revised the manuscript, and gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy. Adetola Louis-Jacques contributed to analysis and interpretation, critically revised the manuscript, and gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy. Teodore Postolache contributed to conception, design, analysis, and interpretation, critically revised the manuscript gave final approval, agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH (R01NR05000).
References
- Agha-Jaffar R., Oliver N., Johnston D., Robinson S. (2016). Gestational diabetes mellitus: Does an effective prevention strategy exist? Nature Reviews Endocrinology, 12, 533–546. [DOI] [PubMed] [Google Scholar]
- Badawy A. A. (2010). Plasma free tryptophan revisited: What you need to know and do before measuring it. Journal of Psychopharmacology, 24, 809–815. doi:10.1177/0269881108098965 [DOI] [PubMed] [Google Scholar]
- Badawy A. A. (2015). Tryptophan metabolism, disposition and utilization in pregnancy. Bioscience Reports, 35, e00261 doi:10.1042/bsr20150197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorke-Monsen A. L., Ulvik A., Nilsen R. M., Midttun O., Roth C., Magnus P.…Ueland P. M. (2016). Impact of pre-pregnancy BMI on B vitamin and inflammatory status in early pregnancy: An observational cohort study. Nutrients, 8, 776 doi:10.3390/nu8120776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschitz A., Gauster M., Fuchs D., Lang I., Maschke P., Ulrich D.…Sedlmayr P. (2011). Vascular endothelial expression of indoleamine 2,3-dioxygenase 1 forms a positive gradient towards the feto-maternal interface. PLoS One, 6, e21774 doi:10.1371/journal.pone.0021774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C. (2015). Nitric oxide synthase in innate and adaptive immunity: An update. Trends in Immunology, 36, 161–178. [DOI] [PubMed] [Google Scholar]
- Cain M. A., Louis J. M. (2016). Sleep disordered breathing and adverse pregnancy outcomes. Clinics in Laboratory Medicine, 36, 435–446. [DOI] [PubMed] [Google Scholar]
- Chaves Filho A. J. M., Lima C. N. C., Vasconcelos S. M. M., de Lucena D. F., Maes M., Macedo D. (2018). IDO chronic immune activation and tryptophan metabolic pathway: A potential pathophysiological link between depression and obesity. Progress in Neuropsychopharmacology and Biological Psychiatry, 80(Pt C), 234–249. doi 10.1016/j.pnpbp.2017.04.035. [DOI] [PubMed] [Google Scholar]
- Chu S. Y., Callaghan W. M., Bish C. L., D’Angelo D. (2009). Gestational weight gain by body mass index among US women delivering live births, 2004–2005: Fueling future obesity. American Journal of Obstetrics and Gynecology, 200, 271e1–7. [DOI] [PubMed] [Google Scholar]
- Cruess S., Antoni M., Cruess D., Fletcher M. A., Ironson G., Kumar M.…Schneiderman N. (2000). Reductions in herpes simplex virus Type 2 antibody titers after cognitive behavioral stress management and relationships with neuroendocrine function, relaxation skills, and social support in HIV-positive men. Psychosomatic Medicine, 62, 828–837. [DOI] [PubMed] [Google Scholar]
- Darcy C. J., Davis J. S., Woodberry T., McNeil Y. R., Stephens D. P., Yeo T. W., Anstey N. M. (2011). An observational cohort study of the kynurenine to tryptophan ratio in sepsis: Association with impaired immune and microvascular function. PLoS One, 6, e21185 doi:10.1371/journal.pone.0021185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. A. L., Maxwell C., McLeod L., Gagnon R., Basso M., Bos H.…Wilson K. (2010). Obesity in pregnancy: No. 239, February 2010. International Journal of Gynecology & Obstetrics, 110, 167–173. [DOI] [PubMed] [Google Scholar]
- Favennec M., Hennart B., Caiazzo R., Leloire A., Yengo L., Verbanck M.…Bessede A. (2015). The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity, 23, 2066–2074. [DOI] [PubMed] [Google Scholar]
- Field T., Diego M., Hernandez-Reif M., Figueiredo B., Deeds O., Ascencio A.…Kuhn C. (2008). Prenatal serotonin and neonatal outcome: Brief report. Infant Behavior & Development, 31, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustarini D., Dalle-Donne I., Colombo R., Milzani A., Rossi R. (2004). Adaptation of the Griess reaction for detection of nitrite in human plasma. Free Radical Research, 38, 1235–1240. [DOI] [PubMed] [Google Scholar]
- Gregor M. F., Hotamisligil G. S. (2011). Inflammatory mechanisms in obesity. Annual Review of Immunology, 29, 415–445. doi:10.1146/annurev-immunol-031210-101322 [DOI] [PubMed] [Google Scholar]
- Groer M., Jevitt C. (2014). Symptoms and signs associated with postpartum thyroiditis. Journal of Thyroid Research, 2014, 531969 doi:10.1155/2014/531969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer M. E., Jevitt C., Ji M. (2015). Immune changes and dysphoric moods across the postpartum. American Journal of Reproductive Immunology, 73, 193–198. doi:10.1111/aji.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Trejo M., Montoya-Estrada A., Torres-Ramos Y., Espejel-Nunez A., Guzman-Grenfell A., Morales-Hernandez R.…Laresgoiti-Servitje E. (2017). Oxidative stress biomarkers and their relationship with cytokine concentrations in overweight/obese pregnant women and their neonates. BMC Immunology, 18, 3 doi:10.1186/s12865-016-0184-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honorio de Melo Martimiano P., de Sa Braga Oliveira A., Ferchaud-Roucher V., Croyal M., Aguesse A., Grit I.…Bolanos-Jimenez F. (2017). Maternal protein restriction during gestation and lactation in the rat results in increased brain levels of kynurenine and kynurenic acid in their adult offspring. Journal of Neurochemistry, 140, 68–81. doi:10.1111/jnc.13874 [DOI] [PubMed] [Google Scholar]
- Jones S. P., Franco N. F., Varney B., Sundaram G., Brown D. A., de Bie J.…Brew B. J. (2015). Expression of the kynurenine pathway in human peripheral blood mononuclear cells: Implications for inflammatory and neurodegenerative disease. PLoS One, 10, e0131389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaindl A. M., Favrais G., Gressens P. (2009). Molecular mechanisms involved in injury to the preterm brain. Journal of Child Neurology, 24, 1112–1118. doi:10.1177/0883073809337920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryanova V., Meunier S. J., Vecchiarelli H. A., Hill M. N., Dyck R. H. (2016). Effects of maternal stress and perinatal fluoxetine exposure on behavioral outcomes of adult male offspring. Neuroscience, 320, 281–296. [DOI] [PubMed] [Google Scholar]
- Lawlor D. A. (2013). The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition—An old hypothesis with new importance? International Journal of Epidemiology, 42, 7–29. doi:10.1093/ije/dys209 [DOI] [PubMed] [Google Scholar]
- Leviton A., Dammann O. (2004). Coagulation, inflammation, and the risk of neonatal white matter damage. Pediatric Research, 55, 541–545. doi:10.1203/01.pdr.0000121197.24154.82 [DOI] [PubMed] [Google Scholar]
- Madan J. C., Davis J. M., Craig W. Y., Collins M., Allan W., Quinn R., Dammann O. (2009). Maternal obesity and markers of inflammation in pregnancy. Cytokine, 47, 61–64. [DOI] [PubMed] [Google Scholar]
- Maes M., Leonard B. E., Myint A. M., Kubera M., Verkerk R. (2011). The new ‘5-HT’ hypothesis of depression: Cell-mediated immune activation induces indoleamine 2, 3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35, 702–721. [DOI] [PubMed] [Google Scholar]
- Mangge H., Stelzer I., Reininghaus E. Z., Weghuber D., Postolache T. T., Fuchs D. (2014). Disturbed tryptophan metabolism in cardiovascular disease. Current Medicinal Chemistry, 21, 1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D., Lorr M., Droppleman L. (1992). Profile of mood states manual. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Mellor A. L., Munn D. H. (1999). Tryptophan catabolism and T-cell tolerance: Immunosuppression by starvation? Immunology Today, 20, 469–473. [DOI] [PubMed] [Google Scholar]
- Mezrich J. D., Fechner J. H., Zhang X., Johnson B. P., Burlingham W. J., Bradfield C. A. (2010). An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. Journal of Immunology, 185, 3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn D. H., Zhou M., Attwood J. T., Bondarev I., Conway S. J., Marshall B.…Mellor A. L. (1998). Prevention of allogeneic fetal rejection by tryptophan catabolism. Science, 281, 1191–1193. [DOI] [PubMed] [Google Scholar]
- Murr C., Widner B., Wirleitner B., Fuchs D. (2002). Neopterin as a marker for immune system activation. Current Drug Metabolism, 3, 175–187. [DOI] [PubMed] [Google Scholar]
- Neurauter G., Schrocksnadel K., Scholl-Burgi S., Sperner-Unterweger B., Schubert C., Ledochowski M., Fuchs D. (2008). Chronic immune stimulation correlates with reduced phenylalanine turnover. Current Drug Metabolism, 9, 622–627. [DOI] [PubMed] [Google Scholar]
- Oxenkrug G., Ratner R., Summergrad P. (2013). Kynurenines and vitamin B6: Link between diabetes and depression. Journal of Bioinformatics and Diabetes, 1 doi:10.14302/issn.2374-9431.jbd-13-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug G. F. (2011). Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: Implications for aging and aging-associated psychiatric and medical disorders. Journal of Neural Transmission, 118, 75–85. doi:10.1007/s00702-010-0475-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-De La Cruz V., Königsberg M., Santamaría A. (2007). Kynurenine pathway and disease: An overview. CNS Neurological Disorders and Drug Targets, 6, 398–410. [DOI] [PubMed] [Google Scholar]
- Pershing M. L., Bortz D. M., Pocivavsek A., Fredericks P. J., Jorgensen C. V., Vunck S. A.…Bruno J. P. (2015). Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: Implications for schizophrenia. Neuropharmacology, 90, 33–41. doi:10.1016/j.neuropharm.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson M., Cnattingius S., Wikström A-K., Johansson S. (2016). Maternal overweight and obesity and risk of pre-eclampsia in women with Type 1 diabetes or Type 2 diabetes. Diabetologia, 59, 2099–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K. A., Riley S. C., Reynolds R. M., Barr S., Evans M., Statham A.…Denison F. C. (2011). Placental structure and inflammation in pregnancies associated with obesity. Placenta, 32, 247–254. [DOI] [PubMed] [Google Scholar]
- Rowlands I., Graves N., de Jersey S., McIntyre H. D., Callaway L. (2010). Obesity in pregnancy: Outcomes and economics. Seminars in Fetal and Neonatal Medicine, 15, 94–99. doi:10.1016/j.siny.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Schröcksnadel H., Baier-Bitterlich G., Dapunt O., Wachter H., Fuchs D. (1996). Decreased plasma tryptophan in pregnancy. Obstetrics & Gynecology, 88, 47–50. [DOI] [PubMed] [Google Scholar]
- Schröcksnadel K., Widner B., Neurauter G., Fuchs D., Schröcksnadel H., Bergant A. (2003). Tryptophan degradation during and after gestation In Graziella A., Costa C. V. L., Ragazzi E., Steinhart H., Laresio L. (Eds.), Developments in tryptophan and serotonin metabolism (pp. 77–83). New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- Schröcksnadel K., Wirleitner B., Winkler C., Fuchs D. (2006). Monitoring tryptophan metabolism in chronic immune activation. Clinica Chimica Acta, 364, 82–90. [DOI] [PubMed] [Google Scholar]
- Scrandis D. A., Langenberg P., Tonelli L. H., Sheikh T. M., Manogura A. C., Alberico L. A.…Hasday J. D. (2008). Prepartum depressive symptoms correlate positively with C-reactive protein levels and negatively with tryptophan levels: A preliminary report. International Journal of Child Health and Human Development, 1, 167. [PMC free article] [PubMed] [Google Scholar]
- Soderborg T. K., Borengasser S. J., Barbour L. A., Friedman J. E. (2016). Microbial transmission from mothers with obesity or diabetes to infants: An innovative opportunity to interrupt a vicious cycle. Diabetologia, 59, 895–906. doi:10.1007/s00125-016-3880-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burg J. W., Sen S., Chomitz V. R., Seidell J. C., Leviton A., Dammann O. (2016). The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatric Research, 79, 3–12. doi:10.1038/pr.2015.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolowczuk I., Hennart B., Leloire A., Bessede A., Soichot M., Taront S.…Guillemin G. J. (2012). Tryptophan metabolism activation by indoleamine 2, 3-dioxygenase in adipose tissue of obese women: An attempt to maintain immune homeostasis and vascular tone. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 303, R135–R143. [DOI] [PubMed] [Google Scholar]
- Yamazaki F., Kuroiwa T., Takikawa O., Kido R. (1985). Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochemical Journal, 230, 635–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong S., Li C., Luo C., Zhao X., Liu C., Wang K.…Bao S. (2016). Dysregulated expression of IDO may cause unexplained recurrent spontaneous abortion through suppression of trophoblast cell proliferation and migration. Scientific Reports, 6 19916 doi:10.1038/srep19916 [DOI] [PMC free article] [PubMed] [Google Scholar]