Abstract
To cause infection, Salmonella must survive and replicate in host niches that present dramatically different environmental conditions. This requires a flexible metabolism and physiology, responsive to conditions of the local milieu. The sequence specific RNA binding protein CsrA serves as a global regulator that governs gene expression required for pathogenicity, metabolism, biofilm formation, and motility in response to nutritional conditions. Its activity is determined by two noncoding small RNAs (sRNA), CsrB and CsrC, which sequester and antagonize this protein. Here, we used ribosome profiling and RNA-seq analysis to comprehensively examine the effects of CsrA on mRNA occupancy with ribosomes, a measure of translation, transcript stability, and the steady state levels of transcripts under in vitro SPI-1 inducing conditions, to simulate growth in the intestinal lumen, and under in vitro SPI-2-inducing conditions, to simulate growth in the Salmonella containing vacuole (SCV) of the macrophage. Our findings uncovered new roles for CsrA in controlling the expression of structural and regulatory genes involved in stress responses, metabolism, and virulence systems required for infection. We observed substantial variation in the CsrA regulon under the two growth conditions. In addition, CsrB/C sRNA levels were greatly reduced under the simulated intracellular conditions and were responsive to nutritional factors that distinguish the intracellular and luminal environments. Altogether, our results reveal CsrA to be a flexible regulator, which is inferred to be intimately involved in maintaining the distinct gene expression patterns associated with growth in the intestine and the macrophage.
Introduction
Salmonella enterica serovar Typhimurium (Salmonella) is a major cause of foodborne illness and diarrheal disease worldwide [1–7]. Salmonella utilizes a variety of dedicated virulence factors, many of which are encoded within horizontally acquired genomic regions called Salmonella pathogenicity islands (SPI)[8,9]. The best characterized of these are SPI-1 and SPI-2, each of which encodes a type III secretion system (T3SS)[10–12]. The SPI-1 T3SS is required for initial invasion of the intestinal epithelium, and its expression is controlled by the transcriptional regulators HilD and HilA [13–15]. A variety of other regulators that sense diverse environmental signals converge to control the expression of HilD and thus SPI-1 [16,17]. In vitro the SPI-1 T3SS is induced by late exponential growth in rich media [18,19]. The SPI-2 T3SS is important for later stages of infection, including establishment of a modified phagosomal compartment called the Salmonella containing vacuole (SCV) and manipulation of host cells to promote intracellular growth and survival [10,12,20–23]. The two-component regulatory system (TCS) SsrA-SsrB integrates a variety of regulatory signals to control SPI-2 expression [24–29]. SPI-2 expression is induced in vitro by growth in acidic minimal media with limiting concentrations of magnesium and phosphate or in late stationary phase in rich media [18]. There is significant crosstalk between regulators of the SPIs, and models with distinct roles for SPI-1 and SPI-2 in early and later stages of infection, respectively, are likely overly simplistic [18,24]. For example, HilD regulates expression of SPI-1 but it also controls SPI-2 expression in rich media by activating the transcription of the genes encoding its master regulator SsrA-SsrB [18,30].
CsrA is a post-transcriptional regulator that controls the expression of SPI-1 and SPI-2 through direct repression of hilD translation initiation during growth in rich media [31,32]. In addition, CsrA controls the expression of genes encoding regulators of metabolism and biofilm formation in Salmonella [33,34]. CsrA binds to conserved GGA motif(s) within its RNA targets, leading to changes in their structure, transcription elongation, translation initiation, and/or RNA stability [35]. It is the core component of the carbon storage regulatory (Csr) system that is highly conserved, especially in Gammaproteobacteria [35,36]. Across multiple species, CsrA tends to repress genes expressed in the stationary phase of growth and during exposure to stress [35,37]. In E. coli and Salmonella, the activity of CsrA is controlled primarily by the levels of two inhibitory small RNAs (sRNAs), CsrB and CsrC. These sRNAs contain many high affinity CsrA binding sites that sequester CsrA from its lower affinity mRNA targets [35]. The BarA-SirA (-UvrY) TCS is a critical regulator of CsrB/C expression [36]. This TCS is stimulated by the accumulation of short chain carboxylate compounds such as formate and acetate, which are abundant in the intestinal lumen and accumulate as Salmonella approaches stationary phase in vitro [38,39]. In E. coli, the alarmone (p)ppGpp that accumulates when cells undergo amino acid starvation or other nutritional stresses also stimulates the transcription of CsrB/C [36]. The presence of preferred carbon sources such as glucose stimulates CsrB/C decay through a pathway involving unphosphorylated EIIAGlc, CsrD, and RNase E [40]. Thus, end products of metabolism and amino acid starvation stimulate CsrB/C transcription, and the abundance of preferred carbon sources lead to CsrB/C turnover, at least in closely related E. coli [36,38,40]. Together, these factors fine-tune CsrB/C expression based on the metabolic state of the cell. Control of virulence gene expression by CsrA is just one example of how nutrition and metabolism are closely linked to virulence in Salmonella [41].
Studies have shown that deletion of csrA results in diminished Salmonella invasion of epithelial cells and virulence in mouse models [32,42]. Likely, CsrA control of HilD expression is an important mediator of this effect [31], but in other species CsrA regulates a variety of genes involved in stress responses and metabolism that may also contribute [35]. Here we aimed to further explore the regulatory role of CsrA under conditions relevant for infection. Specifically, we assessed the impact of CsrA on gene expression during growth in LB, a rich medium that induces SPI-1 expression [18], and mLPM, an acidic, low phosphate and magnesium minimal medium that favors SPI-2 expression [18,43]. We used ribosome profiling and RNA-seq to measure the effect of CsrA on translation, RNA abundance, and RNA stability in both of these conditions (Fig 1). A partial csrA disruption mutant strain encoding a truncated, functionally impaired CsrA protein [44] was used for these studies rather than a csrA deletion strain, which grows very slowly and is genetically unstable [32]. This CsrA allele expresses a protein that can still bind to RNA, though with an eight-fold lower affinity [44]. Unlike the deletion mutant, the CsrA truncation mutant grows normally in both LB and mLPM (S1 Fig). The integrated results of these analyses illustrate a global regulatory role of CsrA in Salmonella metabolism, virulence, and stress responses, which appears to contribute to its success as a pathogen by helping to maintain the distinct gene expression patterns needed for growth and survival in the intestine and the macrophage.
Fig 1. Overview of experimental design.
Wild type Salmonella enterica serovar Typhimurium strain 14028s and an isogenic csrA mutant strain were grown in LB and mLPM media to mid-exponential phase. For analyses of translation, RNA abundance, and translation efficiency, cultures were treated with chloramphenicol to stall translation elongation. Samples were then quickly isolated and lysed under ribosome stabilizing conditions. Part of this lysate was treated with micrococcal nuclease, and ribosomes were isolated by ultracentrifugation through a sucrose cushion. Ribosome protected fragments (RPF) were extracted, size selected, and used to generate sequencing libraries reflecting the actively translated RNA. Another part of the lysate was used to extract total RNA. This RNA was depleted of rRNA, fragmented, and size selected. These RNA fragments were used to generate sequencing libraries reflecting steady state RNA levels. For analysis of RNA stability, cultures were treated with rifampicin to stop transcription initiation. Samples were collected at 0, 2.5, 5, 10, and 15 minutes after the addition of rifampicin and immediately stabilized with a phenol/ethanol stop solution. Total RNA was purified and depleted of rRNA. Sequencing libraries were prepared that reflect the abundance of RNA as it decays after the addition of rifampicin.
Results
CsrA is primarily a repressor of steady state RNA abundance, translation, and translation efficiency
Ribosome profiling is an RNA footprinting method that analyzes ribosomal occupancy of mRNA as a measure of apparent translation, at a genomic level by sequencing nuclease-resistant, ribosome-protected RNA fragments (RPF)[45](Fig 1). It has been used to study translation and post-transcriptional regulation in both prokaryotes and eukaryotes [45,46]. When combined with RNA-seq, ribosome profiling can be used to determine translation efficiency, a measure of post-transcriptional regulation. Here we defined translation efficiency as an interaction term between changes in RPF and RNA abundance, i.e. measuring changes in translation while controlling for changes in RNA abundance. As we expect that CsrA will indirectly affect the transcription of many genes, this will result in changes in RNA abundance and therefore translation of indirect targets. However, the translational efficiency of a gene would only be affected if there were a change in its post-transcriptional regulation. Although a change in translational efficiency indicates a changes in post-transcriptional regulation, interpreting this is not straightforward due to the intimiate link between transcription, translation, and RNA stability in bacteria. For example, repression of translation can alter the rate of mRNA decay. We used ribosome profiling and paired RNA-seq in an integrated analysis of differential translation (RPF levels), steady state RNA abundance (RNA levels), and translation efficiency (RPF and RNA levels) of protein coding genes in the wild type and csrA mutant strains grown in LB and mLPM media (Fig 1). In addition, we also separately analyzed changes in RNA abundance of protein- and non-protein coding genes. For each sample type, the four biological replicates form tight distinct clusters in a multidimensional scaling plot, suggesting that the approach is reproducible (S2 Fig). Our data replicated previous findings that growth in LB induces SPI-1 expression, whereas growth in mLPM induces SPI-2 expression [18,43] (S1 Table). In addition, CsrA generally acted as a repressor of translation, translation efficiency, and RNA abundance in both mLPM and LB (S2 Fig and S2 Table).
CsrA is a global stabilizer of RNA
As CsrA directly regulates the stability of some of its RNA targets [35], we also assessed the effect of CsrA on RNA abundance after treatment with rifampicin to arrest transcription initiation (Fig 1). RNA-seq was used to measure RNA abundance, and normalized abundances were used to calculate RNA half-lives. The RNA-seq data were normalized with scaling factors calculated by comparing absolute RNA abundances measured with qRT-PCR to RNA-seq, as described previously [47]. A multidimensional scaling plot shows the reproducibility of half-lives calculated with this approach due to tight clustering of the biological replicates (S2 Fig). The mean RNA half-life and the individual half-life distribution were significantly different for the wild type vs. csrA mutant strains in both mLPM (2.6 vs. 1.5 min) and LB media (4.5 vs. 2.6 min), where CsrA acted in both cases to globally stabilize RNAs (Wilcox rank sum test, p < 0.0005, S4 Fig). This is in agreement with recent reports in E. coli [48,49].
Integration of multiple transcriptomics analyses
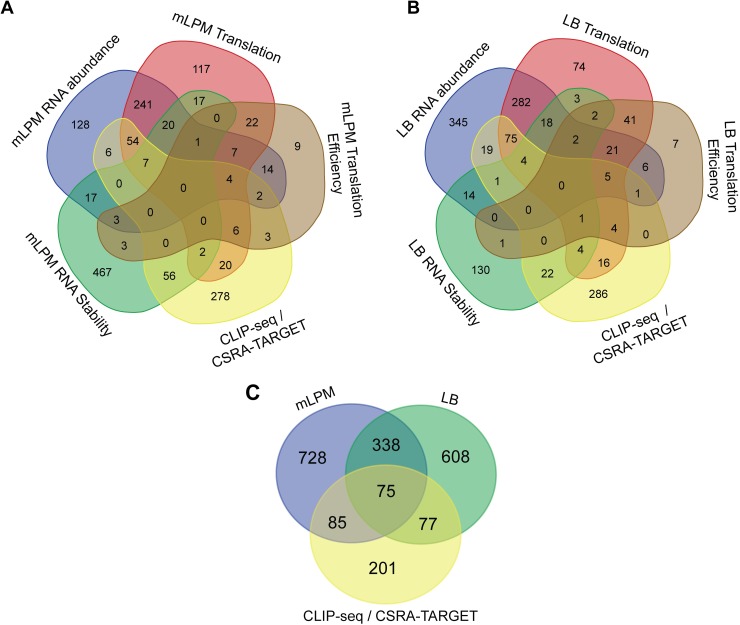
Coordinate changes in gene expression identified with multiple methods would provide strong evidence for CsrA-dependent regulation. Indeed, there was substantial overlap between the genes identified with multiple methods for both mLPM and LB (Fig 2). However, many genes with changes in their RNA stability did not have changes in their RNA abundance. A potential cause of this finding may be that there are compensatory effects on the transcription of these genes, as described previously for CsrB/C and DksA [50,51].
Fig 2. Integration of results from different transcriptomics methods.
Number of genes with changes in their expression in (A) mLPM, (B) LB, and (C) a comparison between mLPM and LB. Data for predicted direct CsrA targets was taken from Kulkarni et al. [53] (CSRA-TARGET) and Homlqvist et al. [52] (CLIP-seq).
To help interpret the changes in gene expression, we also made use of the results from previous studies aimed at identifying direct binding targets of CsrA [52,53]. A recent study used CLIP-seq to identify CsrA RNA binding partners in Salmonella Typhimurium strain SL1344 during stationary phase growth in LB media [52]. Another study used the consensus CsrA binding site determined using systematic evolution of ligands by exponential enrichment (SELEX) to identify potential CsrA binding sites in silico in the Salmonella Typhimurium strain LT2 genome [53]. We used these data to enrich our interpretation of changes in gene expression (S3 Table). However, as the CLIP-seq study was conducted under different growth conditions it may not identify genes relevant under our conditions and vice versa. In addition, few of the in silico predicted CsrA targets have been verified in vitro or in vivo. An additional caveat is that as transcription, translation, and RNA stability are often intimately linked in bacteria [54], translational efficiency may be limited in its sensitivity. Thus, when a gene is associated with a signal in the CLIP-seq analysis, which we refer to as a CLIP-seq peak, and changes in translation but not with changes in translation efficiency, we still consider this to be suggestive of post-transcriptional regulation. Similar analyses conducted in E. coli also suggest that this is a reasonable hypothesis [48].
Condition-specific effects of CsrA
Examination of the overlap between genes regulated by CsrA in mLPM and LB indicated that CsrA regulated many genes under only one condition (Fig 2C). Closer analysis of the log2 transformed fold change effects of CsrA in mLPM versus LB and their associated Spearman’s correlations highlighted the condition specific effects for translation (ρ = 0.34), RNA abundance (ρ = 0.20), translation efficiency (ρ = 0.11), and RNA stability (ρ = 0.23) (S5 Fig). These correlation coefficients suggested there is not a strong relationship between the effects of CsrA in these two conditions. We also formally analyzed these changes in gene expression by testing the statistical significance of the interaction between strain and media for ribosome profiling and RNA abundance and found that many genes were differentially regulated by CsrA between the two media (S4 Table). Together, these results suggest that there are strong condition-specific effects of CsrA on gene expression.
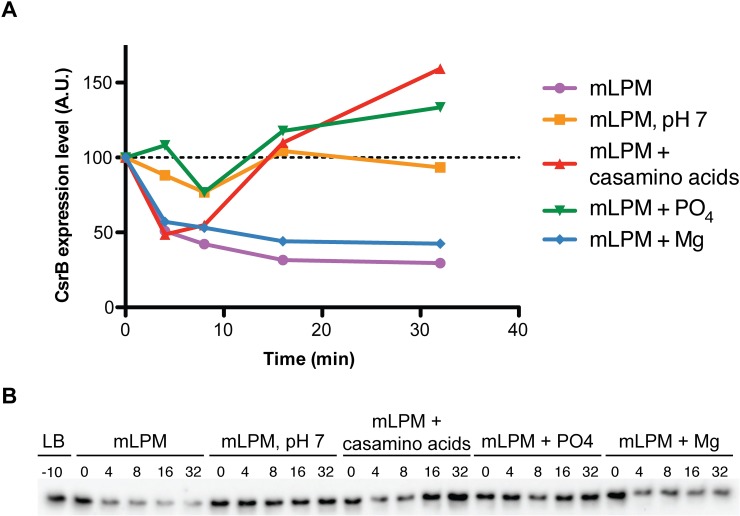
There may be multiple explanations for the condition-specific effects. Transcription from alternative promoters under the different growth conditions may present alternative sites for CsrA-dependent regulation. CsrA regulation may also require coregulators that are expressed in only one condition. Conditionally regulated genes might be indirectly controlled by CsrA, leading to different effects on expression depending on condition specific cues or expression of regulators that are required to mediate CsrA effects. In fact, predicted and experimentally determined direct CsrA targets were significantly enriched (1.7 fold, two-sided Chi-square test, p < 0.0001) in genes that are expressed in both conditions (Fig 2C). This supports the idea that indirect regulation by CsrA is a major contributor to condition-specific effects. Finally, there may be differences in the expression or activity of Csr system components between LB and mLPM media. There was no apparent change in CsrA, BarA, or SirA expression, but CsrB and CsrC levels were substantially decreased, 8.1- and 3.3-fold, respectively, in mLPM relative to LB (S1 Table). This is consistent with the previously observed decrease in CsrB/C levels during intracellular growth in macrophages versus growth in LB [55]. We confirmed that exposure to mLPM leads to a sharp decrease in CsrB levels relative to mid-exponential growth in LB (Fig 3A and 3B and S6 Fig). In addition, the acidic pH, carbon source, and the low level of phosphate in mLPM contributed to this change in CsrB levels (Fig 3A and 3B and S6 Fig). This is consistent with a large transcriptomic study that found that mild acid shock leads to a decrease in CsrB and CsrC levels in LB medium [56]. We would predict that such a sharp decrease in the levels of CsrB/C should greatly increase the activity of CsrA in mLPM compared to LB. However, overall we did not observe a global increase in the magnitude of CsrA-dependent effect on gene expression in mLPM (S5 Fig and S2 Table). This suggests that differences in CsrA-dependent regulation between the two media cannot be explained due to altered CsrB/C levels alone, and that there may be other regulators of CsrA in mLPM.
Fig 3. Exposure to mLPM medium reduces CsrB levels.
(A) Levels of CsrB were analyzed during mid-exponential growth in LB medium and at several time points after transfer to several variations of mLPM medium. CsrB levels were quantified from the northern blot in (B) and displayed as arbitrary units (A.U.) after normalized to the 16s rRNA levels. Data from an additional experiment are presented in S6 Fig, showing that this response is reproducible.
Core and condition-specific roles of CsrA identified by functional enrichment
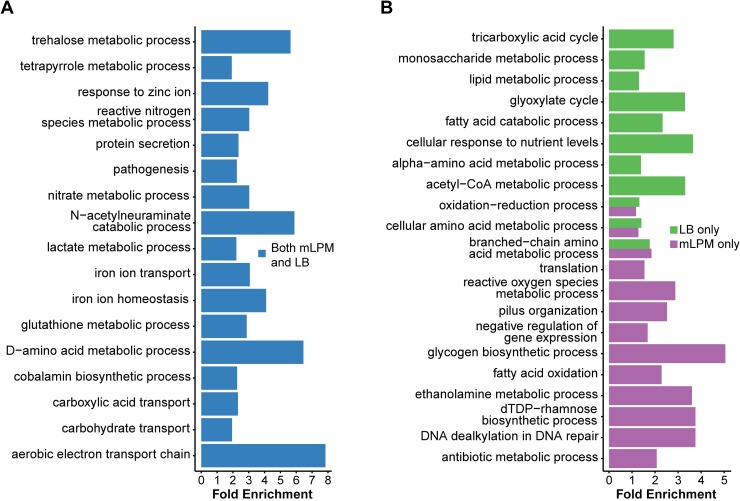
To identify the functional roles of genes regulated by CsrA, we used enrichment analysis with Gene Ontology (GO) term annotations. Due to the strong condition specific effects, we analyzed genes regulated by CsrA in both mLPM and LB, those just in mLPM, and those just in LB (S6 Table and summarized in Fig 4). Several enriched functional categories include known regulatory roles of CsrA in Salmonella, such as in pathogenesis, type III secretion, and the use of ethanolamine as an electron donor [32,34,57](Fig 4). In addition, several functional roles of CsrA described in E. coli were also identified, such as regulation of the glycogen metabolism, the tricarboxylic acid cycle, and fatty acid metabolism [48,58–60]. Although the genes regulated by CsrA differed between mLPM and LB, there were some shared functional roles, such as amino acid metabolism (Fig 4B). On the other hand, several functions were only enriched in one condition, including the tricarboxylic acid cycle in LB and pilus expression in mLPM (Fig 4B). Several of these enriched GO terms are relevant to Salmonella infection, some of which will be explored in more detail in the following sections.
Fig 4. GO terms significantly enriched in CsrA regulated genes.
Fold enrichment of GO terms in genes regulated by CsrA in both mLPM and LB (A) and in either LB or mLPM (B). Significance was determined with a Fisher’s exact test with TopGO [152]. Categories shown twice in panel B are indicative of larger lists of non-overlapping genes in mLPM and/or LB vs. genes in common. The list of significant terms (S6 Table) was shortened for visualization with REViGO [153].
CsrA regulates genes encoded in Salmonella pathogenicity islands and their regulators
Initial studies using a csrA deletion strain with unmapped suppressor mutation(s) found that both deletion and overexpression of csrA led to decreased HilD and SPI-1 expression in Salmonella [32,34]. On the other hand, the BarA-SirA TCS has been shown to activate SPI-1 and SPI-2 expression through its activation of CsrB/C, which relieves direct CsrA-mediated repression of HilD [31,36]. The later study demonstrating that CsrA directly represses HilD translational initiation illustrates the only known molecular mechanism by which CsrA affects SPI-1 expression. We found that expression of SPI-1 encoded genes increased in the csrA disruption mutant in both LB and mLPM, with less pronounced effects on SPI-2 gene expression (S2 Table), which is consistent with the previous mechanistic results. In addition, we also observed that CsrA decreased the translational efficiency of HilD in LB and mLPM (S2 Table). It is unclear why our data do not agree with the initial studies showing that deletion of csrA decreases SPI-1 expression. Perhaps this reflects a difference between reduced CsrA activity in our mutant vs. elimination of all CsrA activity by the deletion. But growth differences genetic instability of the csrA deletion, or effects of the unknown suppressor(s) of this defect might also account for the difference.
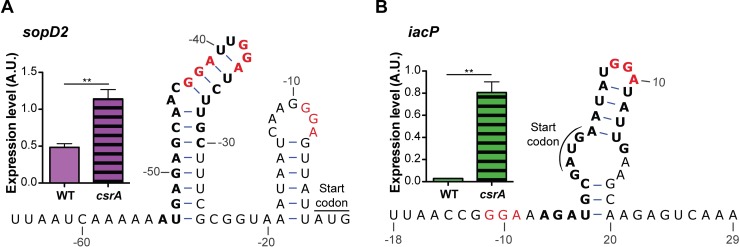
Recent studies have indicated that CsrA interacts in vivo with several mRNAs that encode T3SS effectors and genes encoded by SPI-1 and SPI-2 [52]. SopD2 is a SPI-2 secreted effector that is involved in disrupting host regulation of endocytic trafficking and promotes SCV stability [61,62]. Holmqvist et al. identified a CsrA CLIP-seq peak upstream of sopd2 and found a sopD2’-‘gfp translation fusion was sensitive to changes in CsrB and CsrC expression [52]. Furthermore, mutation of the GGA motif within an apparent CsrA binding site in the 5’-UTR of the sopD2’-‘gfp fusion to CCU eliminated the effects of CsrB/C on its expression [52]. Consistent with these findings, we found that CsrA repressed the RNA abundance and translation of sopD2 at its native locus in mLPM and LB (S2 Table), and we confirmed the effect on sopD2 mRNA abundance with qRT-PCR (Fig 5A). Closer analysis of the 5’-UTR also showed a possible lower affinity CsrA binding site, containing a GGA motif at -9 to -7 relative to the translation initiation codon and overlapping the Shine Dalgarno sequence (SD) of sopD2 (Fig 5A). This arrangement may allow a CsrA protein dimer to directly block 30S ribosome access to initiate translation by bridging from the upstream high affinity site to the SD, as shown previously for glgC mRNA [63].
Fig 5. CsrA regulates genes encoded in Salmonella pathogenicity islands.
RNA sequence and mFold [154] predicted structure of 5’-untranslated and early coding regions and qRT-PCR analysis of steady state mRNA levels of (A) sopD2 and (B) iacP. GGA motifs are shown in red and CLIP-seq peaks from Holmqvist et al. [52] are shown in bold. RNA levels were assessed at mid-exponential growth in LB (green) or mLPM (purple) and normalized to the 16S rRNA levels. Error bars show SEM of three biological replicates. Statistical significance was determined with a two-sided student’s t-test. Asterisks denote statistical significance (p<0.05: *, p<0.005: **, p<0.0005: ***).
CsrA also repressed the RNA abundance, translation, and translation efficiency of the SPI-1 encoded gene, iacP in LB (S2 Table). IacP is an acyl carrier protein that can acylate SPI-1 effectors, enhancing SopB, SopA, and SopD secretion and translocation [64,65]. An iacP mutant is attenuated for infection in mouse models of infection and exhibits decreased invasion of non-phagocytic cells [64]. A CsrA CLIP-seq peak was found overlapping the start of iacP [52](Fig 5B). A possible high affinity CsrA binding site lies within the CLIP-seq peak just within the coding region of iacP, and another GGA motif located within the SD sequence lies just upstream of the identified CLIP-seq peak in the 5’-UTR of iacP (Fig 5B). Plausibly, CsrA binding to these two GGA motifs blocks ribosome access to the SD. Indeed, the translation efficiency of iacP is enhanced in the csrA mutant (S2 Table). Although the level of iacP RNA was below the level of reliable detection, qRT-PCR was able to detect its expression and showed that CsrA represses accumulation of iacP mRNA (S2 Table, Fig 5B) in LB. These data suggest that CsrA has multiple inputs controlling genes located in SPI-1 (hilD, iacP) and genes of chromosomally encoded effectors secreted by the SPI-2 T3SS (sopD2). Such multi-layered regulation appears to be common for other complex phenotypes regulated by CsrA, such as biofilm formation [35,37].
In addition to its effects on T3SS effectors, CsrA controlled the expression of other regulators of Salmonella virulence. Predictably, CsrA repressed the expression of several transcriptional regulators under the control of HilD, which control SPI-1 and SPI-2 expression (hilA, hilC, ssrA, ssrB, invF, and rtsA) (S2 Table)[2,31]. CsrA repressed spvR translation 4-fold in mLPM, but levels of this mRNA were insufficient for analysis in LB (S2 Table). The virulence plasmid-encoded spvR gene is induced during growth in acidic minimal media and SpvR activates the expression of the SpvB and SpvC effector proteins [66,67]. These effectors aid non-typhoidal strains in mounting systemic infections and rely on the SPI-2 encoded T3SS for secretion. Consequently, spvR deletion strains are defective for virulence in mouse models of infection [42,66]. SlyA is a transcriptional regulator that coordinates with PhoP to induce the expression of SPI-2, thus contributing to intracellular survival and destruction of M cells [68–70]. CsrA repressed slyA translation 1.8-fold in LB but not in mLPM (S2 Table). SlyA is induced by growth in macrophages and in the stationary phase of growth in an rpoS-independent manner [71]. SlyA activates pagC and sseA expression in a PhoP-dependent manner [68,72,73], and only in LB was their translation repressed by CsrA 3.5- and 4.0-fold, respectively (S2 Table). This suggests that regulation of slyA by CsrA affects the expression of its downstream regulatory targets. Thus, in addition to the effects of CsrA on hilD expression, CsrA controlled the expression of regulators of other aspects of Salmonella virulence including repressing the activator of plasmid-encoded effectors spvR in mLPM and repressing the regulator of SPI-2 encoded effectors, slyA in LB. These effects may also contribute to the defects in virulence of a csrA mutant [32,34,42].
CsrA represses genes required for resistance to stresses encountered during infection
During the course of infection, Salmonella encounters a variety of environmental stressors that it must survive in order to mount a successful infection [1,2,74]. The systems used to sense and respond to in vivo environmental conditions are important for Salmonella survival and growth within the host. Our functional enrichment analysis revealed several stress response pathways as enriched in CsrA regulated genes (Fig 4). RpoS (σs) is involved in the activation of many of the genes required for stress resistance in Salmonella and is expressed during both early and late stages of infection [75,76]. CsrA repressed translation of rpoS 2.4-fold in LB but had no effect in mLPM (S2 Table). Thus, in LB CsrA may mediate some of its effects on stress responses through the master regulator of stress resistance, RpoS.
Before reaching the intestine, Salmonella must survive exposure to the acidic gastric contents of the stomach, which triggers the acid tolerance response (ATR)[77–79]. In addition, during intracellular growth Salmonella resides within the SCV, an acidic endosomal compartment [77–80]. In LB, CsrA repressed adiAC (S2 Table), which maintains intracellular pH by decreasing the concentration of intracellular protons via decarboxylation and exchange of arginine/agmatine. Previous work has shown that exogenous induction of adiAC is sufficient to protect Salmonella strains deficient of regulators of the ATR from killing by acidic pH [81]. CsrA also repressed expression of cysB, which encodes a LysR-family transcriptional regulator of cysteine biosynthesis that in E. coli is critical for expression of adiA and participates in the acid shock response [82,83]. In mLPM, CsrA repressed translation of yciG, which encodes a protein of unknown function that contributes strongly to Salmonella survival under acidic conditions [84]. CsrA also repressed translation of another acid-inducible gene in mLPM, cfa (S2 Table), which encodes cyclopropane fatty acid synthase [84]. By promoting cyclopropanation of unsaturated moieties of membrane fatty acids, this enzyme makes them more resistant to oxidation or chemical damage [85]. This plays a major role in E. coli survival during acid shock, especially during growth in minimal media when the amino acid decarboxylase systems are not functional [86]. Thus, CsrA repressed systems for the ATR in both mLPM and LB, which may facilitate ATR inducibility under conditions that increase CsrB/C levels.
Salmonella experiences changes in solute concentration as it encounters different host compartments, and must respond appropriately to osmotic stress during the course of infection [87]. Low moisture and high solute concentrations are also important conditions for food preservation, and mechanisms used to survive these conditions are relevant for foodborne Salmonella outbreaks [88]. Upon exposure to high solute concentrations, Salmonella attempts to maintain its turgor pressure by adjusting its intracellular solute concentration by inducing K+ pumps and either the transport or biosynthesis of osmoprotectants such as proline, glycine betaine, and trehalose [89]. Upon continuing stress, Salmonella then adjusts its outer membrane protein composition by activating expression of the smaller pore size OmpC over OmpF [88]. CsrA repressed the translation of proP 2.7-fold in mLPM (S2 Table), which encodes a permease that imports L-proline and glycine betaine, which is required for long term survival of Salmonella in low moisture environments [90]. In addition, CsrA repressed the translation of otsBA 3.0- and 2.2-fold in mLPM and LB, respectively (S2 Table). These genes encode enzymes required for the biosynthesis of trehalose and allow enhanced growth of E. coli during osmotic stress and at low temperatures [91,92]. CsrA also repressed translation of osmY 5.1- and 2.3-fold in mLPM and LB, respectively, which gives E. coli enhanced resistance to osmotic stress in rich media [93] (S2 Table). CsrA activated expression of both ompF and ompC, which encode outer membrane porins (S2 Table), and Holmqvist et al. identified a CLIP-seq peak in the 5’-UTR of ompC [52]. Overall, CsrA repressed a variety of genes required for osmotic stress resistance in Salmonella in both mLPM and LB.
Control of free iron in the intestinal lumen and blood is an important strategy used by the host to limit growth of pathogens, which is sometimes referred to as nutritional immunity [94]. Indeed, supplementation of iron in the diet has been shown to increase the prevalence and virulence of Salmonella in the intestine [95,96]. Due to limiting iron levels in the intestine, Salmonella must outcompete the native microbiota for access to this iron [97]. To do so, Salmonella uses siderophores, including enterobactin and its glycosylated derivative, salmochelin [98]. Salmonella can also scavenge iron that has been chelated by siderophores produced by other organisms including ferrioxamines and ferrichrome [99]. Our transcriptomics data revealed that in mLPM CsrA activated genes involved in enterobactin biosynthesis (entCEAB and entF), the outer membrane receptor for enterobactin (fepA), some genes involved salmochelin biosynthesis and transport (iroBCD), and the outer membrane receptor for ferrioxamines (foxA) (S2 Table). In LB, CsrA activated other genes involved in iron transport, including those involved in ferrichrome and ferrioxamine transport (fhuACDB), and ferrous iron transport (feoABC) (S2 Table). One possible mediator of these effects is fur, the master regulator of iron homeostasis [100,101]. However, CsrA did not regulate the expression of fur in mLPM or LB (S2 Table). These results suggest that CsrA plays a role in modulating the ability of Salmonella to acquire iron in the intestine and within host cells.
In addition to its effects on genes involved in iron acquisition, CsrA repressed translation of genes encoding the iron storage proteins ftnB (13- and 5.6-fold), dps (5.1- and 2.6-fold), and bfr (2.7-fold and not significant) in mLPM and LB, respectively (S2 Table). A CsrA CLIP-seq peak was associated with bfr (S2 Table)[52]. Bfr is the major iron storage protein in Salmonella, while FtnB and Dps contribute very little to intracellular iron levels [102]. FtnB contributes to the repair of oxidatively damaged iron sulfur clusters, while Dps protects DNA from oxidative damage [102,103]. Mutants of ftnB or dps have diminished Salmonella virulence in a mouse model of infection, whereas mutation of bfr has little or no effect on virulence [102]. Thus, repression of ftnB and dps by CsrA may be more closely related to repression of the oxidative stress response than to iron storage.
Other genes involved in oxidative stress resistance were also regulated by CsrA. It repressed translation of the catalases katE and katN 4.4- and 4.5-fold in mLPM only, respectively (S2 Table). Additional qRT-PCR experiments found that CsrA repressed accumulation of katE mRNA in both mLPM and LB (Fig 6A). CsrA also repressed translation of the superoxide dismutases sodB and sodC2 in mLPM 2.2- and 2.5-fold, respectively (S2 Table). CsrA repressed translation of btuE 2-fold in mLPM (S2 Table), which encodes a peroxidase that can use thioredoxin or glutathione as a reductant [104]. CsrA also repressed genes involved in the glutaredoxin, thioredoxin, and glutathione systems in mLPM, including the glutaredoxin 3 (grxC), thioredoxin 2 (trxC), and three glutathione S-transferases (yfcG, yqjG, and STM14_5130) (S2 Table). These systems help maintain the redox status of the cell and protect against oxidative stress [105,106]. In LB, CsrA repressed the expression of the arginine permease argT (Fig 6A and S2 Table), which allows Salmonella to deplete arginine from its environment [107]. Arginine is a substrate for host nitric oxide synthases, so expression of argT helps Salmonella decrease the production of toxic nitric oxide [108]. In LB, CsrA also repressed expression of slyA, rpoS, and soxS, regulators that contribute to resistance to oxidative stress in Salmonella [71,109,110]. To determine if CsrA impacts the resistance to oxidative stress in Salmonella, we compared the susceptibility of wild type and csrA mutant strains to H2O2 in both LB and mLPM medium. Mutation of csrA resulted in a sharp increase in resistance to H2O2 in LB at 2 hr of exposure (Fig 6B), which is consistent with de-repression of genes involved in oxidative stress resistance. Growth in mLPM resulted in greater resistance to H2O2, and surprisingly, CsrA did not affect H2O2 sensitivity in mLPM (Fig 6B and S7 Fig). However, Salmonella encodes multiple redundant systems that can detoxify H2O2 and several must be deleted before a change in sensitivity is observed [111]. Often exposure to stresses can result in cross resistance to other stress. For example, pre-exposure to carbon limitation results in enhanced H2O2 resistance, which is mediated partly by RpoS. Thus, it is perhaps not surprising that the Salmonella is more resistant to oxidative stress in mLPM media, which is limiting for multiple critical nutrients. In contrast, the stress resistant phenotype may be apparent in LB because CsrA represses the expression of multiple regulators (slyA, soxS, and rpoS) that induce expression of genes required for oxidative stress resistance under this condtion.
Fig 6. CsrA regulates oxidative stress responses.
(A) qRT-PCR analysis of steady state argT and katE mRNA levels during mid-exponential growth in LB (green) or mLPM (purple) normalized to the 16S rRNA levels. Error bars show SEM of three biological replicates. Statistical significance was determined with a two-sided student’s t-test. Asterisks denote statistical significance (p<0.05: *, p<0.005: **, p<0.0005: ***). (B) Survival of wild type and csrA mutant strains upon exposure to H2O2. Strains in mid-exponential phase of growth in mLPM or LB were exposed to 20 μM or 5 μM of H2O2, respectively, for the indicated times. Washed and 10-fold serially diluted samples were plated and grown overnight on LB agar plates before imaging. Data from a biological replicate analyzed in the same experimented is presented in S7 Fig. Altogether, this experiment was repeated three times with similar results.
CsrA regulates metabolism important for establishing infection
One important contributor to success of Salmonella is its nutritional flexibility, including the ability to utilize many different carbon sources, electron donors, and electron acceptors [108]. CsrA regulated a variety of genes and regulators of alternative carbon source utilization (S2 Table). For example, in mLPM CsrA activated the translation of transcriptional regulators of genes involved in galactose (galS), rhamnose (rhaSR) and fucose (fucR) utilization (S2 Table). In LB, CsrA activated the translation of genes for fucose (fucR), 1,2-propanediol (pocR), and threonine/serine (tdcA) metabolism (S2 Table). With the exception of GalS, these regulators are all activators whose expression is required for induction of structural genes needed for catabolism of these nutrients. Thus, CsrA may have a role in the ability of Salmonella to sense and utilize these nutrients, of which galactose is important for growth in the SCV [55] while fucose and 1,2-propanediol are particularly important in the intestine [41].
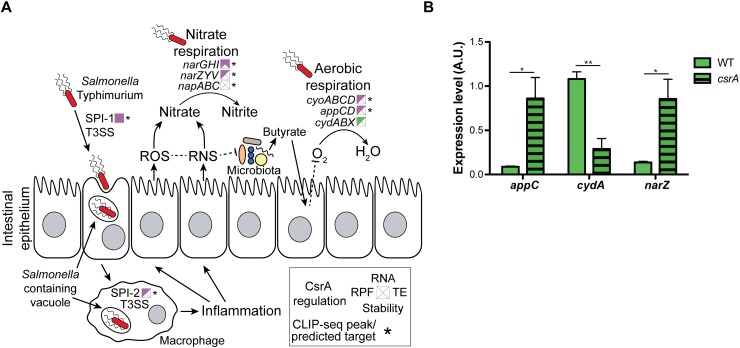
CsrA modulates the expression of genes encoding terminal reductases for both aerobic and anaerobic respiration (Fig 7A and S2 Table). CsrA repressed translation of cytochrome oxidase bo (cyoABCD) an average of 2.3-fold in LB (S2 Table), and 3 CLIP-seq peaks were associated with this gene (S3 Table). In addition, CsrA repressed cytochrome oxidase bd-II (appCD) an average of 2.4- and 5.6-fold in LB and mLPM, respectively (S2 Table), confirmed with qRT-PCR in LB (Fig 7B). Cytochrome oxidase bo is expressed during aerobic growth when oxygen is abundant [112]. Recently, appCD (cyxAB) was found to contribute to post-antibiotic expansion of Salmonella and enhanced shedding in non-antibiotic treated mice [113]. After antibiotic treatment or Salmonella infection, depletion of butyrate producing Clostridia leads to an increase in the partial pressure of oxygen at the epithelium, which Salmonella exploits via appCD-mediated aerobic respiration [113]. On the other hand, CsrA activated translation of cydABX 3.5-fold in LB (S2 Table). Additional qRT-PCR experiments confirmed that CsrA activated cydA expression in LB (Fig 7B). This gene encodes cytochrome oxidase bd-I that plays an important role in low oxygen environments, such as the 5–10% oxygen found in tissues, due to its high affinity for oxygen [114]. Expression of cytochrome oxidase bd-I has also been shown to contribute significantly to the anti-nitrosative defenses of Salmonella during infection in addition to its contribution to respiration [114].
Fig 7. CsrA regulates metabolism important for establishing infection in the intestine.
(A) Summary of the effect of CsrA on the expression of genes involved growth in the intestine. Squares represent the effect of CsrA on translation (RPF, left), RNA abundance (RNA, top), RNA stability (stability, bottom), and translational efficiency (TE, right). Purple indicates repression by CsrA, and green indicates activation. Asterisks show gene(s) that are associated with CLIP-seq peak(s) and/or were predicted to express CsrA-binding transcripts [52,53]. Genes of SPI-1 or SPI-2 are represented as a single entity although the specific genes vary in response to CsrA effects and as direct CsrA targets. S2 Table contains a full description of the observed effects. (B) Levels of steady state appC (cyxA), cydA and narZ mRNA levels during mid-exponential growth in LB normalized to the 16S rRNA levels measured with qRT-PCR and shown in arbitrary units (A.U.). Error bars show SEM of three biological replicates. Statistical significance was determined with a two-sided student’s t-test. Asterisks denote statistical significance (p<0.05: *, p<0.005: **, p<0.0005: ***).
In addition to its effects on the expression of cytochrome oxidases, in LB CsrA regulated genes involved with anaerobic respiration relevant for Salmonella growth in the intestine (Fig 7A and S2 Table). CsrA repressed translation of nitrate reductase A (narGHI) and nitrate reductase Z (narZYV). The latter effects were supported by qRT-PCR examination of narZ transcript levels, and this gene was also identified as a predicted CsrA target (Fig 7B and S2 Table)[53]. Nitrate is produced in the intestine from the reaction of NO and O2- produced by host enzymes as part of inflammation triggered by Salmonella infection [115]. CsrA also repressed expression of genes encoding formate dehydrogenase (fdnGHI) an average of 2.5-fold (S2 Table), which is active under anaerobic conditions and is involved in nitrate respiration [116]. High levels of formate in the intestine would provide ample substrate for this enzyme [39] and would decrease CsrA activity by inducing CsrB/C transcription [38,39]. CsrA also activated L-lactate dehydrogenase (lldD) 5.3-fold (S2 Table). L-lactate dehydrogenase contributes to aerobic metabolism, but it also can contribute to anaerobic nitrate respiration in E. coli [116,117]. Overall, our data suggest that CsrA has multiple regulatory inputs into expression of genes required for aerobic and anaerobic metabolism associated with its intestinal and intracellular niches.
Discussion
To our knowledge, this is the first study to examine the global effects of CsrA on gene expression under more than one growth condition. As a result, we found that the growth condition is a strong determinant of CsrA-dependent regulation. There may be multiple explanations for the observed condition-specific effects. First, differences in CsrA activity between the two conditions may contribute. CsrA may also require coregulators expressed in only one condition. In addition, transcription from alternative promoters under the two conditions may present alternative sites for CsrA-dependent regulation. On the other hand, conditionally regulated genes may be indirectly responsive to CsrA via regulator(s) that are subject to the direct effects of CsrA as well as differentially affected by the two growth conditions. Regardless of the regulatory mechanisms, it is difficult to predict the effect of CsrA on gene expression under new growth conditions based on the wealth of data reported here. Nevertheless, our findings reveal that CsrA acts as a truly flexible global regulator of gene expression.
Our data also add to the increasing evidence that CsrA globally stabilizes RNA, while it paradoxically globally represses RNA abundance in both E. coli and Salmonella (Fig 2)[49]. On the other hand, few examples of RNA stabilization by CsrA have been studied in detail [35]. One recent study proposed that the overall stabilizing effect of CsrA was a consequence of its role as a repressor of RNA abundance because low abundance transcripts are fundamentally more stable than high abundance transcripts [49,118]. However, the present study and another in E. coli have shown that CsrA stabilizes many transcripts without significantly altering their abundance (S2 Table)[49]. In addition, this model relies on the hypothesis that enhanced RNA stability is a fundamental attribute of low RNA abundance due to a decreased probability of interaction with nucleases [118]. However, other studies in E. coli have concluded that mRNA stability is not predictive of abundance [119] and findings in other organisms have supported the opposite conclusion, that highly abundant RNAs are often more stable [47]. Existing models of the determinants of mRNA stability hold that differences in RNA stability are primarily a consequence of changes in the accessibility of nuclease cleavage sites and the expression of RNases [120]. Thus, the model that CsrA stabilizes transcripts as a consequence of its repression of RNA abundance is not clearly supported by existing evidence. CsrA may have an underappreciated global role in stabilizing RNA via direct RNA binding or may have indirect effect(s) via direct or indirect roles in the expression of other RNA binding proteins, nucleases or factors that globally influence RNA turnover. We did not find changes in expression of any RNases or components of the degradosome, which are responsible for bulk RNA turnover (S2 Table). CsrA in E. coli represses hfq and pnp expression [121,122], but we did not find effects on the expression of these genes in Salmonella (S2 Table). Without question, the role of CsrA in bulk RNA stabilization is worthy of further exploration.
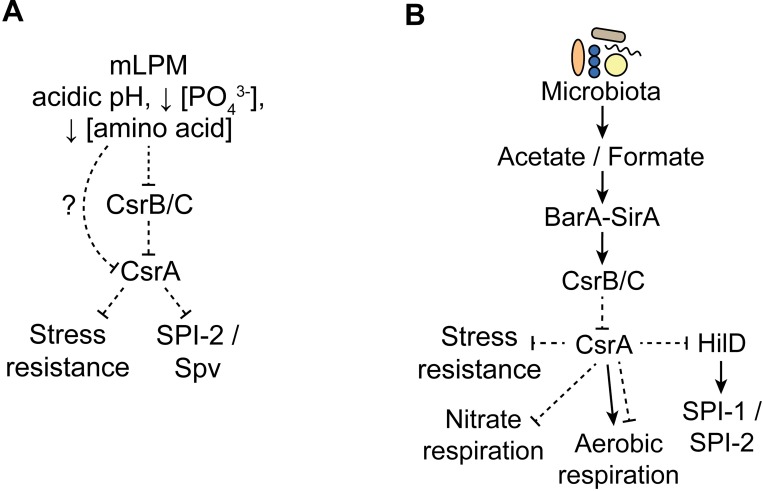
Growth in mLPM minimal medium resulted in decreased CsrB/C levels (Fig 3 and S1 Table). We were able to identify some of the cues that contribute to this difference, including phosphate limitation, acidic pH, and carbon source (Figs 3 and 8A). Studies in E. coli showed that growth in a nutrient poor medium can result in increased CsrB/C expression, while addition of tryptone or casamino acids caused a decrease in CsrB/C expression [123]. However, our data show that growth of Salmonella in a different nutrient poor medium (mLPM) resulted in a strong decrease in CsrB/C levels compared to LB, which supplies amino acids and oligopeptides (S1 Table). Furthermore, the addition of casamino acids to mLPM minimal medium led to an increase in CsrB levels in Salmonella (Fig 3). Thus, the conclusion that nutrient poor growth conditions inevitably lead to higher expression of CsrB/C while amino acids cause decreased CsrB/C expression appears to be incorrect or overly simplistic in Salmonella. Due to the importance of CsrB/C sRNAs on CsrA activity, identifying additional cues and signals that cause their levels to increase in mLPM in response to the addition of phosphorus, amino acids or increased pH is worthy of additional investigation.
Fig 8. Models of Csr system in mLPM and LB.
Dotted lines represent repression and solid lines activation. These arrows represent both direct and indirect effects, as we are unable to differentiate these without further study.
To derive information about genes that are regulated by CsrA during infection, we use findings observed here in vitro to develop hypotheses about what may be occurring in the intestine or at systemic sites, in which Salmonella is typically located intracellularly. These hypotheses gain some validity from the observation that growth in LB induces the expression of SPI-1, which is important for Salmonella to inflame the intestine [31,124], and mLPM induces SPI-2, which is important for Salmonella survival at systemic sites and within host cells. We are most likely to observe regulation by CsrA for infection-relevant genes that are highly transcribed in vitro, such as SPI-1, SPI-2, and those involved in central metabolism. We are less likely to observe regulation of infection-relevant genes that are poorly transcribed in vitro, such as those encoding nutrient acquisition systems, for which the nutrients are not present in the growth media used here.
Generally, the magnitude of CsrA-dependent effects on gene expression in mLPM medium was less pronounced than in LB (S3 Fig and S4 Table). This is in spite of no apparent change in CsrA expression and a pronounced decrease in CsrB/C levels in mLPM (Fig 3 and S1 Table). Together this suggests that there might be other repressors of CsrA activity functioning in mLPM medium. The 5’-UTR of the fimAICDHF transcript is known to act as a CsrA titrating factor in Salmonella [125]. However, we did not detect significant expression of this transcript in our study (S1 Table). In E. coli, the two sRNAs McaS and GadY have been proposed to act as CsrA titration factors [126,127]. In addition, recent publications identified a chaperone protein in enteropathogenic E. coli and several sRNAs in both E. coli and Salmonella that interact with CsrA in vivo [48,52,128]. It is unclear if these sRNAs or other CsrA binding factors are responsible for the reduced CsrA activity in mLPM. Despite its seemingly diminished activity in mLPM, CsrA still regulated the expression of many genes involved in stress resistance and virulence (S2 Table, Fig 8A).
The effects of CsrA on gene expression in LB revealed that CsrA regulates a myriad of genes that support competition and survival in the intestine. Consistent with previous work, CsrA repressed expression of hilD, which is required for induction of SPI-1 (Fig 8B). There are 17 other potential CsrA binding sites within SPI-1 transcripts, revealed by CLIP-seq, and the majority of the island is strongly repressed by CsrA (S2 Table). Repression of SPI-1 genes was most readily observed in LB due to higher expression but was also observed in mLPM. The T3SS encoded by SPI-1 provokes inflammation of the host, which involves the production of reactive oxygen species that generate oxidized compounds such as tetrathionate and nitrate, which Salmonella uses as electron acceptors for respiration and to establish a privileged nutritional niche in a location that is otherwise colonized by native microbiota [57,124,129,130]. CsrA repressed genes involved in anaerobic nitrate respiration and low oxygen aerobic respiration (S2 Table, Fig 8B). CsrA also repressed stress response genes related to its survival in the gut, including osmotic, acid and oxidative stresses (S2 Table, Fig 8). Conceivably, CsrA-mediated repression of hilD and other genes required for proliferation in the gut is relieved when Salmonella encounters formate and acetate, rich in the intestinal lumen, which activate CsrB/C transcription through the BarA-SirA TCS (Fig 8B).
CsrA also regulated many genes involved in the metabolism of specific substrates in the gut (S2 Table). We caution that these substrates were not added to the media, so we are presumably observing the effects of CsrA on basal level expression. It is plausible that CsrA effects on specific genes might be altered in the presence of the substrates. Salmonella can respire compounds in the intestine such as 1,2-propanediol, from the microbiota [131]; ethanolamine, from damaged cells [57]; glucarate and galactarate, from Nos2-mediated oxidation of glucose and galactose [132]; L-lactate, from altered host metabolism [133], and fructose-asparagine, from the diet [134–136]. CsrA activated cbi genes, for cyanocobalamin synthesis, required for metabolism of ethanolamine and 1,2-propanediol, as well as genes for metabolism of glucarate, galactarate, L-lactate, and fructose-asparagine in LB (S2 Table). In contrast, CsrA repressed eut (ethanolamine utilization) genes in mLPM, but not LB. Thus, the Csr system may represent a physiological switch, responsive to intestinal cues, which governs the expression of virulence, metabolic, and stress response genes important for Salmonella survival in the gastrointestinal tract.
Salmonella-mediated gastroenteritis is typically self-limiting. However, some serovars including Typhi and Paratyphi A, B, or C, can cause a systemic infection of humans called Typhoid or enteric fever. While these serovars cannot infect mice, systemic infection is often modeled using serovar Typhimurium in a mouse model with defective macrophages. In this model, Typhimurium penetrates the intestinal barrier, often using the M-cells of the Peyer’s patches as the portal of entry [137]. The M-cells are destroyed and Salmonella is taken up by macrophages where it resides within a vacuole called the SCV, or Salmonella containing vacuole [77,138]. This vacuole is acidified, which is required for Salmonella to express SPI-2 [80,139]. The T3SS encoded by SPI-2 then secretes effectors that modify host cell trafficking in ways that prevent Salmonella destruction and allow growth [12,140]. Transcription profiling of Salmonella that is residing within host cells demonstrates that numerous virulence genes are activated in addition to a variety of metabolic pathways and nutrient acquisition systems [141]. It is known that CsrA regulates SPI-2 [31], which is required for intracellular survival, and consistent with this, eight CsrA binding sites within SPI-2 mRNAs were identified by CLIP-seq (S3 Table) [52]. Additionally, CsrA weakly activates SPI-2 gene expression in mLPM and represses their expression in LB (S2 Table).
While the details of the trafficking alterations that are induced by SPI-2 and the nutrient composition of the SCV are under intense investigation, it is clear that Salmonella metabolism within these compartments is complex, involving over 31 nutrients that are used with considerable plasticity [142]. Bumann and coworkers used a combination of approaches to model Salmonella metabolism in the spleen of infected mice. They observed that Salmonella uses a diversity of nutrients in this situation including glycerol, fatty acids, N-acetylglucosamine, glucose, lactate, and arginine. Except for glycerol, these nutrients are not present in our minimal mLPM, but we may observe CsrA-dependent regulation of basal levels of transcription. Indeed, glpF, encoding the glycerol uptake facilitator protein, was activated by CsrA in mLPM, and there are CsrA binding sites identified by CLIP-seq on both glpF and glpK, encoding the glycerol kinase (S2 Table). The fatty acid transporter fadL has a CsrA binding site identified by CLIP-seq, but we did not observe regulation by CsrA, possibly due to a lack of fatty acids in the media. Similarly, CLIP-seq identified a CsrA binding site on the nagA gene encoding N-acetylglucosamine-6-phosphate deacetylase, and on the artI gene, encoding a component of an arginine transporter, although their regulation by CsrA was not observed under the conditions used here. It is important to remember that the population of Salmonella in the spleen is likely to be heterogeneous with some bacteria being extracellular, and those that are intracellular being present in different intracellular environments. These fine details of Salmonella metabolism and its regulation by CsrA await further detailed studies.
Materials and methods
Bacterial strains and culture media
The csrA mutant strain was constructed from a wild type Salmonella enterica subsp. enterica serovar Typhimurium str. 14028s parental strain (ATCC). The last 30 base pairs of the csrA coding region were replaced with a chloramphenicol resistance marked using the lambda red recombinase method, which was subsequently removed with flippase [143]. This csrA mutant replicates the truncated form of CsrA encoded by the csrA::kan transposon mutant allele used widely in E. coli, and is genetically stable, unlike csrA deletion strains [32,35,60]. In addition, the growth of this csrA truncation mutant was the same as its isogenic parental strain under the conditions of this study (S1 Fig). Bacterial cultures were grown at 37°C with shaking at 250 rpm. Cultures were grown overnight in LB medium (1% NaCl, 1% tryptone, and 0.5% yeast extract) then subcultured into LB and grown to mid-exponential phase. These exponentially growing cultures were used as inocula for all experiments. For experiments in LB, cultures were grown from an initial OD600 of 0.01 and collected at mid-exponential phase (OD600 0.5). For experiments in mLPM (5 mM KCl, 7.5 mM (NH4)2SO4, 0.5 mM K2SO4, 0.3% (vol/vol) glycerol, 0.0001% thiamine, 0.5 μM ferric citrate, 8 μM MgCl2, 337 μM PO43-, and 80 mM MES, pH 5.8)[43], cultures were grown from an initial OD600 of 0.1 and collected at mid-exponential phase (OD600 0.3) except for experiments measuring CsrB levels in response to mLPM exposure.
Ribosome profiling and paired RNA-seq analysis
Ribosome profiling was carried out as previously described [48,144,145]. Briefly, ribosome protected fragments were purified from ribosomes isolated by ultracentrifugation of micrococcal nuclease treated cell lysate through a sucrose cushion. Total RNA was isolated in parallel, depleted of rRNA, and fragmented with alkaline hydrolysis. RPF and RNA fragments of 25–40 bases were then size selected with denaturing PAGE. Sequencing libraries were prepared as described previously using primers in S5 Table [48,144]. Libraries were sequenced by the Genomic Services Laboratory at HudsonAlpha with 50SE Hiseq 2500 (Illumina). Raw sequencing reads were demultiplexed, trimmed, depleted of rRNA sequences, and mapped to the Salmonella enterica serovar Typhimurium strain 14028s genome and plasmid sequences (NC_016856.1 and NC_016855.1) with bowtie [146]. Read counts per gene were calculated with htseq-count [147]. Genes with less than an average of 10 counts per gene in all samples were eliminated. Differential expression analysis was carried out with limma voom with quality weights [148]. Analysis of RNA abundance (RNAcsrA–RNAWT), translation (RPFcsrA–RPFWT), and translation efficiency ([RPFcsrA–RPFWT]–[RNAcsrA–RNAWT]) was combined into a single model of expression for protein coding genes only, allowing us to analyze the contribution of each to the gene’s overall expression. Multiple testing correction was conducted at the gene level using a nested F test, which weighs comparisons with multiple significant contrast more heavily. Comparisons were considered significant when the log2 fold change was greater than ±0.8 and the F test was significant. All genes including protein coding and noncoding genes were analyzed together for differences in RNA abundances (RNAcsrA–RNAWT). These comparisons were considered significant when the log2 fold change was greater than ±0.8 and the false discovery rate was less than 0.05.
RNA stability analysis
RNA stability was analyzed as described previously with some modifications [48]. At the mid-exponential phase, cultures were treated with 500 μg ml-1 of rifampicin to stop transcription initiation. Samples were collected after 0, 2.5, 5, 10, and 15 minutes and added directly to a stop solution (5% water saturated phenol in ethanol). RNA was purified with hot phenol chloroform extraction followed by ethanol precipitation. Genomic DNA contamination was removed by treatment with Turbo DNase (ThermoFisher), and RNA was purified with the RNeasy kit (Qiagen). Samples were sent to BGI America for rRNA depletion, sequencing library preparation, and sequencing with 100PE Hiseq 2000 (Illumina). Raw sequencing reads were demultiplexed, trimmed, depleted of rRNA sequences, and mapped to the Salmonella enterica serovar Typhimurium strain 14028s genome and plasmid sequences (NC_016856.1 and NC_016855.1) with bowtie2 [149]. Scaling factors were calculated for each sample at each time point by comparing RNA abundance calculated with qRT-PCR to RNA-seq, as described previously [47]. Normalization factors were calculated for each sample using the geometric average of scaling factors calculated from 11 genes (STM14_0169, STM14_0523, STM14_0577, STM14_0882, STM14_1056, STM14_1594, STM14_2331, STM14_2356, STM14_2422, STM14_2491, and STM14_3077). These genes were chosen to reflect a variety of gene lengths, positions in operons, and RNA stabilities. qRT-PCR RNA decay curves were collected from 4 independent biological replicates. RNA stability was modeled with linear regression on a semi-log plot, and RNA half-lives were calculated with the equation , where k is the slope of the linear regression. Genes with initial read counts less than 75 and R2 values less than 0.85 were not analyzed further. Genes with significant changes in their average RNA half-life were identified with two-tailed student’s t-test. Effects of CsrA on stability were considered significant if the log2 fold change was greater than ±0.8 and the false discovery rate less than 0.05.
qRT-PCR
Total RNA was stabilized upon collection by addition to the stop solution then purified with hot phenol chloroform extraction and ethanol precipitation. Turbo DNase (ThermoFisher) was used to degrade genomic DNA, and RNA was purified from these reactions using the RNeasy kit (Qiagen). cDNA was synthesized with Superscript IV (ThermoFisher) using random hexamers as primers. Quantitative reverse transcriptase PCR (qRT-PCR) was conducted with the iTaq Universal SYBR Green Supermix (Bio-Rad) using an iQ5 iCycler real time PCR system (Bio-Rad) for detection. qRT-PCR primer sequences are listed in S5 Table. Reactions of 10 μl consisted of 20 ng of cDNA or DNA standard, 300 nM of each primer, and 1x iTaq universal SYBR Green reaction mix. These reactions were incubated for 1 min of initial denaturation at 95°C, and then 45 cycles of 10 sec of denaturation at 95°C and 20 sec of annealing, extension, and imaging at 60°C. Melt curve analysis was used to verify the specificity of amplifications with the parameters: 95°C for 1 min, 55°C for 1 min, and increasing the temperature 0.5°C/10 sec until reaching 95°C. RNA quantities were determined relative to a standard curve of PCR products using iQ5 analysis software (Bio-Rad) and normalized to 16s rRNA levels.
Measurement of CsrB levels after transfer to mLPM medium
The wild type strain was grown to mid-exponential phase of growth in LB (OD600 = 0.5), washed with phosphate buffered saline (PBS), and added to variants of mLPM medium (OD600 = 0.3). mLPM was adjusted with NaOH to pH 7.0 (mLPM, pH 7). The glycerol in mLPM was replaced with 0.3% casamino acids (mLPM + casamino acids). mLPM with supplemented with 1000x higher concentration of MgCl2 (final concentration 8 mM, mLPM + Mg) or PO43- (final concentration 337 mM, mLPM + PO4). Samples were collected at several time points after the transfer and added to a stop solution. RNA was extracted with hot phenol chloroform extraction and ethanol precipitation. Total RNA (0.25 μg) was separated by denaturing PAGE and blotted onto a positively charged nylon membrane (Roche) with a Mini Trans-Blot Cell (Bio-Rad) according to the manufacturer's instructions. Northern blotting and quantification were carried out as previously described [150].
Gene Ontology (GO) term enrichment analysis
Genes expressed during growth in our conditions were annotated with GO terms using BLAST2GO software [151]. Enriched GO terms were identified with topGO [152] with Fisher’s exact tests. Only terms associated with more than 3 genes were tested for enrichment. Lists of significant terms were shortened with REViGO [153] to aid visualization. The full lists of enriched terms are presented in S6 Table.
Oxidative stress assay
Wild type and csrA mutant strains were grown in the appropriate media to mid-exponential phase. Hydrogen peroxide was added at the indicated concentrations, and samples were collected before and 0.5, 1, and 2 hours after H2O2 addition. Samples were washed three times with PBS, serially diluted, and spotted onto LB media. Plates were incubated for 18 hours at 37°C before imaging.
Supporting information
Differential gene expression analysis comparing the effect of growth media (mLPM versus LB) separately for each strain (wild type and csrA mutant). There is a key to the right of the table.
(XLSX)
Differential gene expression analysis comparing the effect of the csrA mutation separately for each growth media (mLPM and LB). There is a key to the right of the table.
(XLSX)
(XLSX)
Differential gene expression analysis testing the interaction between the csrA mutation and growth media in a single test. As this analysis is testing the significance of an interaction term, care should be taken in interpreting the biological meaning of a significant result in this comparison. There is a key to the right of the table.
(XLSX)
(XLSX)
There is a key to the right of the table.
(XLSX)
Strains growing exponentially in LB media were subcultured into (A) LB or (B) mLPM media, and growth was measured by optical density at 600 nm.
(TIF)
Multidimensional scaling (MDS) plot of (A) ribosome profiling and RNA-seq normalized counts and (B) RNA half-lives.
(TIF)
Volcano plots showing log2 transformed fold change versus log odds comparing wild type and csrA mutant strains for translation in (A) mLPM and (B) LB, RNA abundance of protein coding genes in (C) mLPM and (D) LB, translation efficiency in (E) mLPM and (F) LB, and RNA abundance of protein coding and non-protein coding genes in (G) mLPM and (H) LB. Significant comparisons are shown in black.
(TIF)
(A) Plot of mean RNA half-life in wild type and csrA mutant strains in LB and mLPM. The distributions are significantly difference (Wilcox rank sum test, p < 0.0005: ***). Half-lives of individual genes in (B) mLPM and (C) LB with significant differences shown in black.
(TIF)
Plots showing log2 transformed fold change between wild type and csrA mutant strains in mLPM versus LB for (A) translation, (B) RNA abundance of protein coding genes, (C) translation efficiency, and (D) RNA stability.
(TIF)
Wild type Salmonella was grown to mid-exponential phase in LB media, collected and washed in phosphate buffered saline, and added to mLPM medium. Samples were collected at various time points and RNA immediately stabilized in a phenol ethanol stop solution. (A) 16S rRNA normalized quantification of CsrB levels derived from (B) northern blot data. These data are an independent experimental replication of the data shown in Fig 3.
(TIF)
Strains in mid-exponential phase of growth in mLPM or LB were exposed to 2.5, 5, 10 or 20 μM of H2O2 for 0, 30, 60, and 120 minutes. Washed and 10-fold serially diluted samples were plated and grown overnight on LB agar before imaging.
(TIF)
Acknowledgments
We would like to thank Jörg Vogel and Erik Holmqvist for sharing their CLIP-seq data with us before publication of their manuscript in the EMBO Journal.
Data Availability
All data are available within the paper, supporting information, or uploaded to the NCBI GEO repository. Sequencing data can be accessed in GEO under the accession numbers GSE107834 and GSE107835.
Funding Statement
This work was supported by the National Institutes of Health [R01AI097116 to T.R. and B.M.M.A., R01GM059969 to T.R.]; and the National Science Foundation Graduate Research Fellowship Program [DGE-1315138 to A.H.P.]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fàbrega A, Vila J. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev. American Society for Microbiology; 2013;26: 308–41. 10.1128/CMR.00066-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erhardt M, Dersch P. Regulatory principles governing Salmonella and Yersinia virulence. Frontiers in Microbiology. 2015. 10.3389/fmicb.2015.00949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLOS Med. 2015;12: e1001921 10.1371/journal.pmed.1001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pires SM, Fischer-Walker CL, Lanata CF, Devleesschauwer B, Hall AJ, Kirk MD, et al. Aetiology-Specific Estimates of the Global and Regional Incidence and Mortality of Diarrhoeal Diseases Commonly Transmitted through Food. PLoS One. 2015;10: e0142927 10.1371/journal.pone.0142927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382: 209–22. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 6.Scallan E, Mahon BE, Hoekstra RM, Griffin PM. Estimates of illnesses, hospitalizations and deaths caused by major bacterial enteric pathogens in young children in the United States. Pediatr Infect Dis J. 2013;32: 217–21. 10.1097/INF.0b013e31827ca763 [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann S, Batz MB, Morris JG. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot. 2012;75: 1292–302. 10.4315/0362-028X.JFP-11-417 [DOI] [PubMed] [Google Scholar]
- 8.Marcus SL, Brumell JH, Pfeifer CG, Finlay BB. Salmonella pathogenicity islands: big virulence in small packages. Microbes Infect. 2000;2: 145–56. [DOI] [PubMed] [Google Scholar]
- 9.Hensel M. Evolution of pathogenicity islands of Salmonella enterica. Int J Med Microbiol. 2004;294: 95–102. 10.1016/j.ijmm.2004.06.025 [DOI] [PubMed] [Google Scholar]
- 10.Srikanth C V., Mercado-Lubo R, Hallstrom K, McCormick BA. Salmonella effector proteins and host-cell responses. Cellular and Molecular Life Sciences. 2011. pp. 3687–3697. 10.1007/s00018-011-0841-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galán JE, Lara-Tejero M, Marlovits TC, Wagner S. Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annu Rev Microbiol. 2014;68: 415–38. 10.1146/annurev-micro-092412-155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings E, Thurston TLM, Holden DW. Salmonella SPI-2 Type III Secretion System Effectors: Molecular Mechanisms And Physiological Consequences. Cell Host Microbe. 2017;22: 217–231. 10.1016/j.chom.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 13.Lee CA, Jones BD, Falkow S. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci U S A. 1992;89: 1847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akbar S, Schechter LM, Lostroh CP, Lee CA. AraC/XylS family members, HilD and HilC, directly activate virulence gene expression independently of HilA in Salmonella typhimurium. Mol Microbiol. 2003;47: 715–28. [DOI] [PubMed] [Google Scholar]
- 15.Galán JE, Curtiss R. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989;86: 6383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas RL, Lostroh CP, DiRusso CC, Spector MP, Wanner BL, Lee CA. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J Bacteriol. 2000;182: 1872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellermeier JR, Slauch JM. Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol. 2007;10: 24–9. 10.1016/j.mib.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 18.Bustamante VH, Martínez LC, Santana FJ, Knodler LA, Steele-Mortimer O, Puente JL, et al. HilD-mediated transcriptional cross-talk between SPI-1 and SPI-2. Proc Natl Acad Sci U S A. 2008;105: 14591–6. 10.1073/pnas.0801205105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajaj V, Hwang C, Lee CA. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18: 715–27. 10.1111/j.1365-2958.1995.mmi_18040715.x [DOI] [PubMed] [Google Scholar]
- 20.Shea JE, Hensel M, Gleeson C, Holden DW. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1996;93: 2593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knuff K, Finlay BB. What the SIF Is Happening-The Role of Intracellular Salmonella-Induced Filaments. Front Cell Infect Microbiol. 2017;7: 335 10.3389/fcimb.2017.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6: 53–66. 10.1038/nrmicro1788 [DOI] [PubMed] [Google Scholar]
- 23.LaRock DL, Chaudhary A, Miller SI. Salmonellae interactions with host processes. Nat Rev Microbiol. 2015;13: 191–205. 10.1038/nrmicro3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Morales D, Banda MM, Chau NYE, Salgado H, Martínez-Flores I, Ibarra JA, et al. The transcriptional regulator SsrB is involved in a molecular switch controlling virulence lifestyles of Salmonella. PLoS Pathog. 2017;13: e1006497 10.1371/journal.ppat.1006497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai SK, Winardhi RS, Periasamy S, Dykas MM, Jie Y, Kenney LJ. The horizontally-acquired response regulator SsrB drives a Salmonella lifestyle switch by relieving biofilm silencing. Elife. 2016;5 10.7554/eLife.10747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown NF, Rogers LD, Sanderson KL, Gouw JW, Hartland EL, Foster LJ. A horizontally acquired transcription factor coordinates Salmonella adaptations to host microenvironments. MBio. 2014;5: e01727–14. 10.1128/mBio.01727-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Hensel M. Systematic analysis of the SsrAB virulon of Salmonella enterica. Infect Immun. 2010;78: 49–58. 10.1128/IAI.00931-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fass E, Groisman EA. Control of Salmonella pathogenicity island-2 gene expression. Current Opinion in Microbiology. 2009. pp. 199–204. 10.1016/j.mib.2009.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walthers D, Carroll RK, Navarre WW, Libby SJ, Fang FC, Kenney LJ. The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol Microbiol. 2007;65: 477–93. 10.1111/j.1365-2958.2007.05800.x [DOI] [PubMed] [Google Scholar]
- 30.Martínez LC, Banda MM, Fernández-Mora M, Santana FJ, Bustamante VH. HilD induces expression of Salmonella pathogenicity island 2 genes by displacing the global negative regulator H-NS from ssrAB. J Bacteriol. 2014;196: 3746–55. 10.1128/JB.01799-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez LC, Yakhnin H, Camacho MI, Georgellis D, Babitzke P, Puente JL, et al. Integration of a complex regulatory cascade involving the SirA/BarA and Csr global regulatory systems that controls expression of the Salmonella SPI-1 and SPI-2 virulence regulons through HilD. Mol Microbiol. 2011;80: 1637–56. 10.1111/j.1365-2958.2011.07674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altier C, Suyemoto M, Lawhon SD. Regulation of Salmonella enterica serovar Typhimurium invasion genes by csrA. Infect Immun. 2000;68: 6790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonas K, Edwards AN, Ahmad I, Romeo T, Römling U, Melefors O. Complex regulatory network encompassing the Csr, c-di-GMP and motility systems of Salmonella Typhimurium. Environ Microbiol. 2010;12: 524–40. 10.1111/j.1462-2920.2009.02097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawhon SD, Frye JG, Suyemoto M, Porwollik S, McClelland M, Altier C. Global regulation by CsrA in Salmonella typhimurium. Mol Microbiol. 2003;48: 1633–45. [DOI] [PubMed] [Google Scholar]
- 35.Vakulskas CA, Potts AH, Babitzke P, Ahmer BMM, Romeo T, Paul B, et al. Regulation of Bacterial Virulence by Csr (Rsm) Systems. Microbiol Mol Biol Rev. Am Soc Microbiol; 2015;79: 193–224. 10.1128/MMBR.00052-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zere TR, Vakulskas CA, Leng Y, Pannuri A, Potts AH, Dias R, et al. Genomic Targets and Features of BarA-UvrY (-SirA) Signal Transduction Systems. PLoS One. 2015;10: e0145035 10.1371/journal.pone.0145035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romeo T, Vakulskas CA, Babitzke P. Post-transcriptional regulation on a global scale: form and function of Csr/Rsm systems. Environ Microbiol. 2013;15: 313–24. 10.1111/j.1462-2920.2012.02794.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chavez RG, Alvarez AF, Romeo T, Georgellis D. The physiological stimulus for the BarA sensor kinase. J Bacteriol. 2010;192: 2009–12. 10.1128/JB.01685-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Y, Suyemoto M, Garner CD, Cicconi KM, Altier C. Formate acts as a diffusible signal to Induce Salmonella invasion. J Bacteriol. 2008;190 10.1128/JB.00205-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leng Y, Vakulskas CA, Zere TR, Pickering BS, Watnick PI, Babitzke P, et al. Regulation of CsrB/C sRNA decay by EIIA(Glc) of the phosphoenolpyruvate: carbohydrate phosphotransferase system. Mol Microbiol. 2015;99: 627–639. 10.1111/mmi.13259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivera-Chávez F, Bäumler AJ. The Pyromaniac Inside You: Salmonella Metabolism in the Host Gut. Annu Rev Microbiol. 2015;69: 31–48. 10.1146/annurev-micro-091014-104108 [DOI] [PubMed] [Google Scholar]
- 42.Yoon H, McDermott JE, Porwollik S, McClelland M, Heffron F. Coordinated regulation of virulence during systemic infection of Salmonella enterica serovar Typhimurium. PLoS Pathog. 2009;5: e1000306 10.1371/journal.ppat.1000306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niemann GS, Brown RN, Gustin JK, Stufkens A, Shaikh-Kidwai AS, Li J, et al. Discovery of novel secreted virulence factors from Salmonella enterica serovar Typhimurium by proteomic analysis of culture supernatants. Infect Immun. 2011;79: 33–43. 10.1128/IAI.00771-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yakhnin A V, Baker CS, Vakulskas CA, Yakhnin H, Berezin I, Romeo T, et al. CsrA activates flhDC expression by protecting flhDC mRNA from RNase E-mediated cleavage. Mol Microbiol. 2013;87: 851–66. 10.1111/mmi.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ingolia NT. Ribosome profiling: new views of translation, from single codons to genome scale. Nat Rev Genet. 2014; 1–9. 10.1038/nrg3645 [DOI] [PubMed] [Google Scholar]
- 46.Subramaniam AR, Deloughery A, Bradshaw N, Chen Y, O’Shea E, Losick R, et al. A serine sensor for multicellularity in a bacterium. Elife. 2013;2: e01501 10.7554/eLife.01501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kristoffersen SM, Haase C, Weil MR, Passalacqua KD, Niazi F, Hutchison SK, et al. Global mRNA decay analysis at single nucleotide resolution reveals segmental and positional degradation patterns in a Gram-positive bacterium. Genome Biol. 2012;13: R30 10.1186/gb-2012-13-4-r30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potts AH, Vakulskas CA, Pannuri A, Yakhnin H, Babitzke P, Romeo T. Global role of the bacterial post-transcriptional regulator CsrA revealed by integrated transcriptomics. Nat Commun. 2017;8: 1596 10.1038/s41467-017-01613-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esquerré T, Bouvier M, Turlan C, Carpousis AJ, Girbal L, Cocaign-Bousquet M. The Csr system regulates genome-wide mRNA stability and transcription and thus gene expression in Escherichia coli. Sci Rep. 2016;6: 25057 10.1038/srep25057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki K, Babitzke P, Kushner SR, Romeo T. Identification of a novel regulatory protein (CsrD) that targets the global regulatory RNAs CsrB and CsrC for degradation by RNase E. Genes Dev. 2006;20: 2605–17. 10.1101/gad.1461606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards AN, Patterson-Fortin LM, Vakulskas CA, Mercante JW, Potrykus K, Vinella D, et al. Circuitry linking the Csr and stringent response global regulatory systems. Mol Microbiol. 2011;80: 1561–80. 10.1111/j.1365-2958.2011.07663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmqvist E, Wright PR, Li L, Bischler T, Barquist L, Reinhardt R, et al. Global RNA recognition patterns of post-transcriptional regulators Hfq and CsrA revealed by UV crosslinking in vivo. EMBO J. 2016;35: 991–1011. 10.15252/embj.201593360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kulkarni PR, Jia T, Kuehne SA, Kerkering TM, Morris ER, Searle MS, et al. A sequence-based approach for prediction of CsrA/RsmA targets in bacteria with experimental validation in Pseudomonas aeruginosa. Nucleic Acids Res. 2014;42: 6811–25. 10.1093/nar/gku309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deana A, Belasco JG. Lost in translation: the influence of ribosomes on bacterial mRNA decay. Genes Dev. 2005;19: 2526–2533. 10.1101/gad.1348805 [DOI] [PubMed] [Google Scholar]
- 55.Srikumar S, Kröger C, Hébrard M, Colgan A, Owen S V., Sivasankaran SK, et al. RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Salmonella Typhimurium. PLoS Pathog. 2015;11: e1005262 10.1371/journal.ppat.1005262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kröger C, Colgan A, Srikumar S, Händler K, Sivasankaran SK, Hammarlöf DL, et al. An infection-relevant transcriptomic compendium for Salmonella enterica serovar Typhimurium. Cell Host Microbe. 2013;14: 683–695. 10.1016/j.chom.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 57.Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, et al. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A. 2011;108: 17480–5. 10.1073/pnas.1107857108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKee AE, Rutherford BJ, Chivian DC, Baidoo EK, Juminaga D, Kuo D, et al. Manipulation of the carbon storage regulator system for metabolite remodeling and biofuel production in Escherichia coli. Microb Cell Fact. 2012;11: 79 10.1186/1475-2859-11-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Revelles O, Millard P, Nougayrède J-P, Dobrindt U, Oswald E, Létisse F, et al. The carbon storage regulator (Csr) system exerts a nutrient-specific control over central metabolism in Escherichia coli strain Nissle 1917. PLoS One. 2013;8: e66386 10.1371/journal.pone.0066386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romeo T, Gong M, Liu MY, Brun-Zinkernagel AM. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J Bacteriol. 1993;175: 4744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brumell JH, Kujat-Choy S, Brown NF, Vallance BA, Knodler LA, Finlay BB. SopD2 is a Novel Type III Secreted Effector of Salmonella typhimurium That Targets Late Endocytic Compartments Upon Delivery Into Host Cells. Traffic. 2003;4: 36–48. 10.1034/j.1600-0854.2003.40106.x [DOI] [PubMed] [Google Scholar]
- 62.D’Costa VM, Braun V, Landekic M, Shi R, Proteau A, McDonald L, et al. Salmonella Disrupts Host Endocytic Trafficking by SopD2-Mediated Inhibition of Rab7. Cell Rep. 2015;12: 1508–1518. 10.1016/j.celrep.2015.07.063 [DOI] [PubMed] [Google Scholar]
- 63.Mercante J, Edwards AN, Dubey AK, Babitzke P, Romeo T. Molecular geometry of CsrA (RsmA) binding to RNA and its implications for regulated expression. J Mol Biol. 2009;392: 511–28. 10.1016/j.jmb.2009.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim JS, Eom JS, Jang JI, Kim HG, Seo DW, Bang IS, et al. Role of Salmonella pathogenicity island 1 protein iacP in Salmonella enterica serovar Typhimurium pathogenesis. Infect Immun. 2011;79: 1440–1450. 10.1128/IAI.01231-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Viala JP, Prima VV, Puppo RR, Agrebi R, Canestrari MJMJ, Lignon S, et al. Acylation of the Type 3 Secretion System Translocon Using a Dedicated Acyl Carrier Protein. PLoS Gen. 2017;13: e1006556 10.1371/journal.pgen.1006556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guiney DG, Fierer J. The Role of the spv Genes in Salmonella Pathogenesis. Front Microbiol. Frontiers; 2011;2: 129 10.3389/fmicb.2011.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson JA, Gulig PA. Regulation of the spvR gene of the Salmonella typhimurium virulence plasmid during exponential-phase growth in intracellular salts medium and at stationary phase in L broth. Microbiology. 1998;144: 1823–1833. 10.1099/00221287-144-7-1823 [DOI] [PubMed] [Google Scholar]
- 68.Navarre WW, Halsey TA, Walthers D, Frye J, McClelland M, Potter JL, et al. Co-regulation of Salmonella enterica genes required for virulence and resistance to antimicrobial peptides by SlyA and PhoP/PhoQ. Mol Microbiol. Blackwell Publishing Ltd; 2005;56: 492–508. 10.1111/j.1365-2958.2005.04553.x [DOI] [PubMed] [Google Scholar]
- 69.Linehan SA, Rytkönen A, Yu XJ, Liu M, Holden DW. SlyA regulates function of Salmonella pathogenicity island 2 (SPI-2) and expression of SPI-2-associated genes. Infect Immun. 2005;73: 4354–4362. 10.1128/IAI.73.7.4354-4362.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daniels JJD, Autenrieth IB, Ludwig A, Goebel W. The gene slyA of Salmonella typhimurium is required for destruction of M cells and intracellular survival but not for invasion or colonization of the murine small intestine. Infect Immun. 1996;64: 5075–5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchmeier N, Bossie S, Chen CY, Fang FC, Guiney DG, Libby SJ. SlyA, a transcriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect Immun. 1997;65: 3725–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao G, Weatherspoon N, Kong W, Curtiss R, Shi Y. A dual-signal regulatory circuit activates transcription of a set of divergent operons in Salmonella Typhimurium. Proc Natl Acad Sci. 2008;105: 20924–20929. 10.1073/pnas.0807071106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christian Perez J, Latifi T, Groisman EA. Overcoming H-NS-mediated transcriptional silencing of horizontally acquired genes by the PhoP and SlyA proteins in Salmonella enterica. J Biol Chem. 2008;283: 10773–10783. 10.1074/jbc.M709843200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fang FC, Frawley ER, Tapscott T V-TA. Bacterial Stress Responses during Host Infection. Cell Host and Microbe. 2016. pp. 133–143. 10.1016/j.chom.2016.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen CYI, Eckmann L, Libby SJ, Fang FC, Okamoto S, Kagnoff MF, et al. Expression of Salmonella typhimurium rpoS and rpoS-dependent genes in the intracellular environment of eukaryotic cells. Infect Immun.1996;64: 4739–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nickerson CA, Curtiss R. Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect Immun. 1997;65: 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steele-Mortimer O. The Salmonella-containing vacuole: moving with the times. Curr Opin Microbiol. 2008;11: 38–45. 10.1016/j.mib.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rathman M, Sjaastad MD, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64: 2765–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Álvarez-Ordóñez A, Begley M, Prieto M, Messens W, López M, Bernardo A, et al. Salmonella spp. survival strategies within the host gastrointestinal tract. Microbiology. 2011. pp. 3268–3281. 10.1099/mic.0.050351-0 [DOI] [PubMed] [Google Scholar]
- 80.Yu X-J, McGourty K, Liu M, Unsworth KE, Holden DW. pH sensing by intracellular Salmonella induces effector translocation. Science. 2010;328: 1040–3. 10.1126/science.1189000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brenneman KE, Willingham C, Kong W, Iii C, Roland L. Low-pH Rescue of Acid-Sensitive Salmonella enterica Serovar Typhi Strains by a Rhamnose-Regulated Arginine Decarboxylase System. J Bacteriol. 2013;195: 3062–3072. 10.1128/JB.00104-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rowbury RJ. Regulatory components, including integration host factor, CysB and H-NS, that influence pH responses in Escherichia coli. Lett Appl Microbiol. 1997;24: 319–328. 10.1046/j.1472-765X.1997.00065.x [DOI] [PubMed] [Google Scholar]
- 83.Shi X, Bennett GN. Effects of rpoA and cysB mutations on acid induction of biodegradative arginine decarboxylase in Escherichia coli. J Bacteriol. 1994;176: 7017–7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan D, Pati NB, Ojha UK, Padhi C, Ray S, Jaiswal S, et al. Global transcriptome and mutagenic analyses of the acid tolerance response of Salmonella enterica serovar Typhimurium. Appl Environ Microbiol. 2015;81: 8054–8065. 10.1128/AEM.02172-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grogan DW, Cronan JE. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol Mol Biol Rev. 1997;61: 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang YY, Cronan JE. Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol Microbiol. 1999;33: 249–259. doi: 10411742 [DOI] [PubMed] [Google Scholar]
- 87.Wood JM, Rosenthal AZ, Gralla J, Greie J-C, Booth IR, Altendorf K. Osmotic Stress. EcoSal Plus. 2009;3 10.1128/ecosalplus.5.4.5 [DOI] [PubMed] [Google Scholar]
- 88.Finn S, Condell O, McClure P, Amézquita A, Fanning S. Mechanisms of survival, responses and sources of Salmonella in low-moisture environments. Front Microbiol. Frontiers; 2013;4: 331 10.3389/fmicb.2013.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Balaji B, O'Connor K, Lucas JR, Anderson JM, Csonka LN. Timing of induction of osmotically controlled genes in Salmonella enterica serovar Typhimurium, determined with quantitative real-time reverse transcription-PCR. Appl Environ Microbiol. 2005;71: 8273–8283. 10.1128/AEM.71.12.8273-8283.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Finn S, Händler K, Condell O, Colgan A, Cooney S, McClure P, et al. ProP is required for the survival of desiccated Salmonella enterica serovar Typhimurium cells on a stainless steel surface. Appl Environ Microbiol. 2013;79: 4376–4384. 10.1128/AEM.00515-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Purvis JE, Yomano LP, Ingram LO. Enhanced trehalose production improves growth of Escherichia coli under osmotic stress. Appl Environ Microbiol. 2005;71: 3761–3769. 10.1128/AEM.71.7.3761-3769.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kandror O, DeLeon A, Goldberg AL. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc Natl Acad Sci U S A. 2002;99: 9727–32. 10.1073/pnas.142314099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yim HH, Villarejo M. osmY, a new hyperosmotically inducible gene, encodes a periplasmic protein in Escherichia coli. J Bacteriol. 1992;174: 3637–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen–host interface. Nat Rev Microbiol. Nature Publishing Group; 2012;10: 525–537. 10.1038/nrmicro2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jaeggi T, Kortman GAM, Moretti D, Chassard C, Holding P, Dostal A, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015;64: 731–742. 10.1136/gutjnl-2014-307720 [DOI] [PubMed] [Google Scholar]
- 96.Kortman GAM, Boleij A, Swinkels DW, Tjalsma H. Iron availability increases the pathogenic potential of Salmonella Typhimurium and other enteric pathogens at the intestinal epithelial interface. PLoS One. 2012;7: e29968 10.1371/journal.pone.0029968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, et al. Probiotic bacteria reduce Salmonella Typhimurium intestinal colonization by competing for iron. Cell Host Microbe. 2013;14: 26–37. 10.1016/j.chom.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith KD. Iron metabolism at the host pathogen interface: Lipocalin 2 and the pathogen-associated iroA gene cluster. Int J Biochem Cell Biol. 2007. pp. 1776–1780. 10.1016/j.biocel.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kingsley R, Rabsch W, Stephens P, Roberts M, Reissbrodt R, Williams PH. Iron supplying systems of Salmonella in diagnostics, epidemiology and infection. FEMS Immunol and Med Microbiol. 1995. pp. 257–264. 10.1016/0928-8244(95)00031-2 [DOI] [PubMed] [Google Scholar]
- 100.Troxell B, Fink R, Porwollik S, McClelland M, Hassan H. The Fur regulon in anaerobically grown Salmonella enterica sv. Typhimurium: identification of new Fur targets. BMC Microbiol. 2011;11: 236 10.1186/1471-2180-11-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McHugh JP, Rodríguez-Quinoñes F, Abdul-Tehrani H, Svistunenko DA, Poole RK, Cooper CE, et al. Global iron-dependent gene regulation in Escherichia coli. A new mechanism for iron homeostasis. J Biol Chem. 2003;278: 29478–86. 10.1074/jbc.M303381200 [DOI] [PubMed] [Google Scholar]
- 102.Velayudhan J, Castor M, Richardson A, Main-Hester KL, Fang FC. The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron-sulphur cluster repair and virulence. Mol Microbiol. 2007;63: 1495–507. 10.1111/j.1365-2958.2007.05600.x [DOI] [PubMed] [Google Scholar]
- 103.Chiancone E, Ceci P. The multifaceted capacity of Dps proteins to combat bacterial stress conditions: Detoxification of iron and hydrogen peroxide and DNA binding. Biochimica et Biophysica Acta. 2010. pp. 798–805. 10.1016/j.bbagen.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 104.Arenas FA, Díaz WA, Leal CA, Pérez-Donoso JM, Imlay JA, Vásquez CC. The Escherichia coli btuE gene, encodes a glutathione peroxidase that is induced under oxidative stress conditions. Biochem Biophys Res Commun. 2010;398: 690–694. 10.1016/j.bbrc.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeller T, Klug G. Thioredoxins in bacteria: Functions in oxidative stress response and regulation of thioredoxin genes. Naturwissenschaften. 2006. pp. 259–266. 10.1007/s00114-006-0106-1 [DOI] [PubMed] [Google Scholar]
- 106.Allocati N, Federici L, Masulli M, Di Ilio C. Glutathione transferases in bacteria. FEBS J. 2009. pp. 58–75. 10.1111/j.1742-4658.2008.06743.x [DOI] [PubMed] [Google Scholar]
- 107.Das P, Lahiri A, Lahiri A, Sen M, Iyer N, Kapoor N, et al. Cationic Amino Acid Transporters and Salmonella Typhimurium ArgT Collectively Regulate Arginine Availability towards Intracellular Salmonella Growth. PLoS One. 2010;5: e15466 10.1371/journal.pone.0015466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dandekar T, Fieselmann A, Fischer E, Popp J, Hensel M, Noster J. Salmonella-how a metabolic generalist adopts an intracellular lifestyle during infection. Front Cell Infect Microbiol. 2015;4: 191 10.3389/fcimb.2014.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pomposiello PJ, Demple B. Identification of SoxS-regulated genes in Salmonella enterica serovar Typhimurium. J Bacteriol. 2000;182: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fang FC, Libby SJ, Buchmeier NA, Loewen PC, Switala J, Harwood J, et al. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci. 1992;89: 11978–11982. 10.1073/pnas.89.24.11978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hébrard M, Viala JPM, Méresse S, Barras F, Aussel L. Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J Bacteriol. 2009;191: 4605–4614. 10.1128/JB.00144-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Borisov VB, Verkhovsky MI. Oxygen as Acceptor. EcoSal Plus. 2015;6 10.1128/ecosalplus.ESP-0012-2015 [DOI] [PubMed] [Google Scholar]
- 113.Rivera-Chavez F, Zhang LLF, Faber F, Lopez CCA, Byndloss MXM, Olsan EEE, et al. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe. 2016;19: 443–454. 10.1016/j.chom.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jones-Carson J, Husain M, Liu L, Orlicky DJ V-TA. Cytochrome bd-dependent bioenergetics and antinitrosative defenses in Salmonella pathogenesis. MBio. 2016;7: e02052–16. 10.1128/mBio.02052-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rivera-Chávez F, Lopez CA, Zhang LF, García-Pastor L, Chávez-Arroyo A, Lokken KL, et al. Energy Taxis toward Host-Derived Nitrate Supports a Salmonella Pathogenicity Island 1-Independent Mechanism of Invasion. MBio. 2016;7: e00960–16. 10.1128/mBio.00960-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dünnwald P, Unden G. The Aerobic and Anaerobic Respiratory Chain of Escherichia coli and Salmonella enterica: Enzymes and Energetics. EcoSal Plus. 2013;1 10.1128/ecosalplus.3.2.2 [DOI] [PubMed] [Google Scholar]
- 117.Iuchi S, Aristarkhov A, Dong JM, Taylor JS, Lin ECC. Effects of Nitrate Respiration on Expression of the Arc- Controlled Operons Encoding Succinate Dehydrogenase and Flavin-Linked L-Lactate Dehydrogenase. J Bacteriol. 1994;176: 1695–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Esquerré T, Moisan A, Chiapello H, Arike L, Vilu R, Gaspin C, et al. Genome-wide investigation of mRNA lifetime determinants in Escherichia coli cells cultured at different growth rates. BMC Genomics. 2015;16: 275 10.1186/s12864-015-1482-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bernstein JA, Khodursky AB, Lin P-H, Lin-Chao S, Cohen SN. Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays. Proc Natl Acad Sci U S A. 2002;99: 9697–702. 10.1073/pnas.112318199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deutscher MP. Degradation of RNA in bacteria: Comparison of mRNA and stable RNA. Nucleic Acids Res. 2006;34: 659–666. 10.1093/nar/gkj472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baker CS, Eöry LA, Yakhnin H, Mercante J, Romeo T, Babitzke P. CsrA inhibits translation initiation of Escherichia coli hfq by binding to a single site overlapping the Shine-Dalgarno sequence. J Bacteriol. 2007;189: 5472–81. 10.1128/JB.00529-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Park H, Yakhnin H, Connolly M, Romeo T, Babitzke P. CsrA participates in a PNPase autoregulatory mechanism by selectively repressing translation of pnp transcripts that have been previously processed by RNase III and PNPase. J Bacteriol. 2015;197: 3751–3759. 10.1128/JB.00721-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jonas K, Melefors Ö. The Escherichia coli CsrB and CsrC small RNAs are strongly induced during growth in nutrient-poor medium. FEMS Microbiol Lett. 2009;297: 80–86. 10.1111/j.1574-6968.2009.01661.x [DOI] [PubMed] [Google Scholar]
- 124.Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, et al. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5: 2177–89. 10.1371/journal.pbio.0050244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sterzenbach T, Nguyen KT, Nuccio S-P, Winter MG, Vakulskas CA, Clegg S, et al. A novel CsrA titration mechanism regulates fimbrial gene expression in Salmonella typhimurium. EMBO J. 2013;32: 2872–83. 10.1038/emboj.2013.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Parker A, Cureoglu S, De Lay N, Majdalani N, Gottesman S. Alternative pathways for Escherichia coli biofilm formation revealed by sRNA overproduction. Mol Microbiol. 2017;105: 309–325. 10.1111/mmi.13702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jørgensen MG, Thomason MK, Havelund J, Valentin-Hansen P, Storz G. Dual function of the McaS small RNA in controlling biofilm formation. Genes Dev. 2013;27: 1132–1145. 10.1101/gad.214734.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Katsowich N, Elbaz N, Pal RR, Mills E, Kobi S, Kahan T, et al. Host cell attachment elicits posttranscriptional regulation in infecting enteropathogenic bacteria. Science (80-). 2017;355: 735–739. 10.1126/science.aah4886 [DOI] [PubMed] [Google Scholar]
- 129.Lopez CA, Rivera-Chavez F, Byndloss MX, Baumler AJ. The periplasmic nitrate reductase NapABC supports luminal growth of Salmonella enterica serovar Typhimurium during colitis. Infect Immun. 2015;83: 3470–3478. 10.1128/IAI.00351-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lopez CA, Winter SE, Rivera-Chávez F, Xavier MN, Poon V, Nuccio S-P, et al. Phage-mediated acquisition of a type III secreted effector protein boosts growth of salmonella by nitrate respiration. MBio. 2012;3: e00143-12-e00143-12. 10.1128/mBio.00143-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Faber F, Thiennimitr P, Spiga L, Byndloss MX, Litvak Y, Lawhon S, et al. Respiration of Microbiota-Derived 1,2-propanediol drives Salmonella expansion during colitis. PLoS Pathog. 2017;13: e1006129 10.1371/journal.ppat.1006129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Faber F, Tran L, Byndloss MX, Lopez CA, Velazquez EM, Kerrinnes T, et al. Host-mediated sugar oxidation promotes post-antibiotic pathogen expansion. Nature. 2016;534: 697–699. 10.1038/nature18597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gillis CC, Hughes ER, Spiga L, Winter MG, Zhu W, Furtado de Carvalho T, et al. Dysbiosis-associated change in host metabolism generates lactate to support Salmonella growth. Cell Host Microbe. 2017; 10.1016/j.chom.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu J, Sabag-Daigle A, Borton MA, Kop LFM, Szkoda BE, Deatherage Kaiser BL, et al. Salmonella-mediated inflammation eliminates competitors for fructose-asparagine in the gut. Infect Immun. 2018;86: e00945–17. 10.1128/IAI.00945-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu J, Sabag-Daigle A, Metz TO, Deatherage Kaiser BL, Gopalan V, Behrman EJ, et al. Measurement of fructose-asparagine concentrations in human and animal Foods. J Agric Food Chem. 2018;66: 212–217. 10.1021/acs.jafc.7b04237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ali MM, Newsom DL, González JF, Sabag-Daigle A, Stahl C, Steidley B, et al. Fructose-asparagine is a primary nutrient during growth of Salmonella in the inflamed intestine. PLoS Pathog. 2014;10: e1004209 10.1371/journal.ppat.1004209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.LaRock DL, Chaudhary A, Miller SI. Salmonellae interactions with host processes. Nat Rev Microbiol. 2015;13: 191–205. 10.1038/nrmicro3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chakraborty S, Mizusaki H, Kenney LJ. A FRET-Based DNA biosensor tracks OmpR-Dependent acidification of Salmonella during macrophage infection. PLoS Biol. 2015;13: e1002116 10.1371/journal.pbio.1002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Abrahams GL, Hensel M. Manipulating cellular transport and immune responses: dynamic interactions between intracellular Salmonella enterica and its host cells. Cell Microbiol. 2006;8: 728–37. 10.1111/j.1462-5822.2006.00706.x [DOI] [PubMed] [Google Scholar]
- 141.Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JCD. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol. 2003;47: 103–18. [DOI] [PubMed] [Google Scholar]
- 142.Steeb B, Claudi B, Burton NA, Tienz P, Schmidt A, Farhan H, et al. Parallel exploitation of diverse host nutrients enhances Salmonella virulence. PLoS Pathog. 2013;9: e1003301 10.1371/journal.ppat.1003301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Datsenko K a, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97: 6640–5. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Becker AH, Oh E, Weissman JS, Kramer G, Bukau B. Selective ribosome profiling as a tool for studying the interaction of chaperones and targeting factors with nascent polypeptide chains and ribosomes. Nat Protoc. 2013;8: 2212–39. 10.1038/nprot.2013.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ingolia NT, Brar G a, Rouskin S, McGeachy AM, Weissman JS. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat Protoc. 2012;7: 1534–50. 10.1038/nprot.2012.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10: R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anders S, Pyl PT, Huber W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31: 166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu R, Holik AZ, Su S, Jansz N, Chen K, Leong HS, et al. Why weight? Modelling sample and observational level variability improves power in RNA-seq analyses. Nucleic Acids Res. 2015;43: e97 10.1093/nar/gkv412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9: 357–9. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vakulskas CA, Leng Y, Abe H, Amaki T, Okayama A, Babitzke P, et al. Antagonistic control of the turnover pathway for the global regulatory sRNA CsrB by the CsrA and CsrD proteins. Nucleic Acids Res. 2016;44: 7896–7910. 10.1093/nar/gkw484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ RM, Talón M, Dopazo J CA. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36: 3420–3435. 10.1093/nar/gkn176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alexa A, Rahnenführer J, Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics. Oxford University Press; 2006;22: 1600–1607. 10.1093/bioinformatics/btl140 [DOI] [PubMed] [Google Scholar]
- 153.Supek F, Bošnjak M, Škunca N, Šmuc T. Revigo summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6: e21800 10.1371/journal.pone.0021800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31: 3406–3415. 10.1093/nar/gkg595 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differential gene expression analysis comparing the effect of growth media (mLPM versus LB) separately for each strain (wild type and csrA mutant). There is a key to the right of the table.
(XLSX)
Differential gene expression analysis comparing the effect of the csrA mutation separately for each growth media (mLPM and LB). There is a key to the right of the table.
(XLSX)
(XLSX)
Differential gene expression analysis testing the interaction between the csrA mutation and growth media in a single test. As this analysis is testing the significance of an interaction term, care should be taken in interpreting the biological meaning of a significant result in this comparison. There is a key to the right of the table.
(XLSX)
(XLSX)
There is a key to the right of the table.
(XLSX)
Strains growing exponentially in LB media were subcultured into (A) LB or (B) mLPM media, and growth was measured by optical density at 600 nm.
(TIF)
Multidimensional scaling (MDS) plot of (A) ribosome profiling and RNA-seq normalized counts and (B) RNA half-lives.
(TIF)
Volcano plots showing log2 transformed fold change versus log odds comparing wild type and csrA mutant strains for translation in (A) mLPM and (B) LB, RNA abundance of protein coding genes in (C) mLPM and (D) LB, translation efficiency in (E) mLPM and (F) LB, and RNA abundance of protein coding and non-protein coding genes in (G) mLPM and (H) LB. Significant comparisons are shown in black.
(TIF)
(A) Plot of mean RNA half-life in wild type and csrA mutant strains in LB and mLPM. The distributions are significantly difference (Wilcox rank sum test, p < 0.0005: ***). Half-lives of individual genes in (B) mLPM and (C) LB with significant differences shown in black.
(TIF)
Plots showing log2 transformed fold change between wild type and csrA mutant strains in mLPM versus LB for (A) translation, (B) RNA abundance of protein coding genes, (C) translation efficiency, and (D) RNA stability.
(TIF)
Wild type Salmonella was grown to mid-exponential phase in LB media, collected and washed in phosphate buffered saline, and added to mLPM medium. Samples were collected at various time points and RNA immediately stabilized in a phenol ethanol stop solution. (A) 16S rRNA normalized quantification of CsrB levels derived from (B) northern blot data. These data are an independent experimental replication of the data shown in Fig 3.
(TIF)
Strains in mid-exponential phase of growth in mLPM or LB were exposed to 2.5, 5, 10 or 20 μM of H2O2 for 0, 30, 60, and 120 minutes. Washed and 10-fold serially diluted samples were plated and grown overnight on LB agar before imaging.
(TIF)
Data Availability Statement
All data are available within the paper, supporting information, or uploaded to the NCBI GEO repository. Sequencing data can be accessed in GEO under the accession numbers GSE107834 and GSE107835.