Abstract
Tendon injuries often require surgical intervention and even then result in poor outcomes due to scar formation and repeated failure. Biomaterial implants offer the potential to address multiple underlying concerns preventing improved tendon repair. Here, we describe modifications to the composition of an anisotropic collagen–glycosaminoglycan (CG) scaffold biomaterial, incorporating amniotic membrane (AM)-derived matrix to alter the inflammatory response and establish conditions for improved regenerative repair. We explored two methods of AM matrix incorporation to address multiple concerns associated with tendon repair. Amniotic membrane-derived matrix was incorporated directly into the scaffold microstructure during fabrication to form a C/AM composite. Alternatively, decellularized amniotic matrix was wrapped around the traditional collagen–chondroitin sulfate (C/CS) scaffold to form a core–shell composite (C/CS plus AM wrap) in a manner similar to current collagen membrane wraps used in rotator cuff and Achilles tendon surgeries to improve the mechanical strength of the repair. Human mesenchymal stem cells (MSCs) cultured within these materials were evaluated for metabolic health and immunomodulatory gene expression in response to inflammatory media challenge of interleukin 1 β and tumor necrosis factor α. The scaffolds were able to maintain MSC metabolic activity in all media conditions over the course of a 7 day culture. Expression of genes encoding for pro-inflammatory cytokines were down-regulated in AM containing scaffolds, suggesting the potential to employ AM-modified CG scaffolds for tendon-repair applications.
Keywords: Collagen, amniotic membrane, inflammation, strength, composite
Graphical Abstract
INTRODUCTION
While the human body is capable of repairing minor tendon injuries, native repair processes often result in a loss of structural integrity and mechanical strength due to the formation of poorly organized granulation tissue (scars).1 In cases of massive tissue tears or degeneration due to chronic conditions, surgical intervention is frequently required. A range of suturing techniques have been developed to facilitate tendon-to-tendon reattachment or tendon graft insertion;2 however, despite improvements in surgical techniques these large injuries also result in diminished tendon functionality and a high incidence of repeated tearing. The implantation of biomaterial scaffolds offers a promising avenue for the promotion of tendon repair and regeneration in vivo. At the most basic level, biomaterial implants can be used to mechanically stabilize the surgical reconnection. A range of commercial products have been studied in vitro3,4 and used in the clinic for rotator cuff5–7 and Achilles tendon tears.8,9 As wraps around tendon repairs, these materials provide mechanical support to the injured area, promote retention of surgically placed sutures, and provide a matrix for cellular and vascular ingrowth.10 However, they only provide a two-dimensional shell around the existing tendon and do not contain internal support of the tendon body.
The inflammatory response following injury also plays a significant role in the ultimate healing of a tissue.11 Pro-inflammatory cytokines (such as IL-1β) have been shown to induce the expression of inflammatory enzymes and matrix degradation factors in tendon cells, which leads to tendon destruction and loss of biomechanical integrity.12 Additionally, by blocking the activity of pro-inflammatory cytokine tumor necrosis factor α (TNFα), increased biomechanical strength in a rat rotator cuff model has been observed.13 Modulating this early phase of wound healing through tissue-engineering strategies (materials, cells, and soluble factors) offers the exciting potential to alter scar formation and, ultimately, improve healing outcomes. The immunomodulatory properties of the amniotic membrane (AM), the innermost layer of a placenta, have contributed to its success as a wound matrix in the treatment of a range of tissues (cornea,14–16 skin,17,18 oral mucosa,19 and others).20,21 In an injectable form, the delivery of amniotic membrane-derived proteins and cytokines has slowed the progression of osteoarthritis22 and enhanced the mechanical properties of Achilles tendons following injury repair in rats.23 In its early use as a intact membrane sheet, the AM has been suggested to reduce scar formation following digital flexor tendon repair.24 In more-recent tendon repair studies, the amniotic membrane has been used as a two-dimensional support in animal models of tendon repair,25,26 highlighting the membrane’s ability to enhance the mechanical properties of the repair without addressing its potential role in the inflammatory process.
Collagen–glycosaminoglycan (CG) scaffolds are three-dimensional extracellular matrix mimics that have been developed for a range of tissue engineering applications.27–33 Their mechanism of regeneration is based on their ability to inhibit myofibroblast recruitment to the wound site34 and, therefore, the contraction and scar formation that follows.35 To mimic the tendon’s native structural alignment, our group recently described a directional solidification method to fabricate a class of anisotropic CG scaffolds containing aligned tracks of ellipsoidal pores.36 Here, the aligned scaffold structure has been shown to promote transcriptomic stability of primary tenocytes37,38 and is sufficient to promote ROCK1 activation and pro-tenogenic differentiation of stem cells in the absence of traditional growth-factor supplements.39 To introduce inflammation modulation into these collagen scaffolds, AM-derived matrix components have been incorporated into the chemical composition of the scaffold during fabrication.40 The selective addition of AM matrix to the collagen scaffold promoted fibroblast metabolic activity and tempered pro-inflammatory gene expression in vitro in response to an inflammatory challenge in the form of IL-1β.40 However, the mode of the AM’s immunomodulatory activity is still unknown, suggesting new studies to examine the effects of its unique matrix composition,21,41 range of soluble factors,18,42 or a combination of the two.
Considering the need for tendon regeneration platforms that address concerns of mechanical strength and role of inflammatory processes in wound healing, we explore two alternate strategies for integrating AM-derived matrix into a CG scaffold to enhance the mesenchymal stem cell (MSC) response to inflammatory challenge. The first relies on the incorporation of AM matrix particles into the bulk of the collagen scaffold (C/AM) during fabrication.43 We also test an CG–AM composite formed from a conventional micro-structurally aligned collagen: chondroitin sulfate scaffold wrapped with a decellularized AM sheet to create a core– shell composite (C/CS plus AM). We examine mechanical reinforcement and immunomodulatory effect of the AM matrix when incorporated in this core–shell system and employ the MSC activity and gene expression of immunomodulatory interleukin-6 (IL-6) and interleukin-8 (IL-8) to evaluate the cellular response to an inflammatory challenge.
MATERIALS AND METHODS
Isolation and Characterization of Human Amniotic Membranes.
Amniotic-Membrane Isolation.
Human placentas were obtained following uncomplicated vaginal births (Carle Foundation Hospital, Urbana, IL). The amniotic membrane matrix components were isolated from these placentas as previously described.40,44 Briefly, the AM was mechanically separated from the placenta, washed, and decellularized via incubation in thermolysin (125 μg/mL).45 Following decellularization, the membranes were rinsed in phosphate-buffered saline (PBS) with shaking to remove cellular debris and then stored in PBS at 4 °C. The spongy layer of the AM was allowed to swell for 24–48 h before it was separated from the AM and discarded. The matrix was lyophilized in a sheet and stored in a desiccator until further use.
Characterization of Factor Release from Dried Amniotic-Membrane Sheets.
Sheets of dried amniotic membrane were cut into samples measuring 25 mm × 15 mm (n = 3). Each sheet was submerged in 2.5 mL of PBS in separate wells of an ultra-low attachment 6-well plate (Fisher, Waltham, MA). Following a 24 h incubation at 37 °C, the PBS was collected and immediately assayed for released factors using RayBiotech’s Human Cytokine Array C1000 (Norcross, GA) per the kit’s instructions. Kit membranes were imaged using Image Quant LAS 4010, and Image Studio Lite was used to acquire pixel density values from the membrane images. The values were normalized to both in-membrane negative controls and values from an assay membrane exposed only to PBS. Fold changes of each selected factor released from amniotic membrane compared with PBS controls are reported here.
Fabrication of CG Scaffolds.
Preparation of Collagen Suspension.
For C/CS scaffolds and the core of C/CS plus AM scaffolds, type I collagen from microfibrillar collagen (Collagen Matrix, Oakland, NJ) was homogenized in 0.5 M acetic acid with chondroitin sulfate from shark cartilage (Sigma-Aldrich, Saint Louis, MO). For C/AM scaffolds, the lyophilized amniotic membrane (as collected above) was homogenized into particles (Figure S1) prior to being combined with microfibrillar collagen in 0.5 M acetic acid. A collagen-to-chondroitin sulfate (C:CS) ratio of 11:1 was used, and C/ AM suspensions were made at a 5:1 w/w ratio due to the high collagen content of the AM. Each suspension was made with a total density of 0.5% w/v, stored at 4 °C, and degassed prior to use.46
Fabrication of Anisotropic Scaffolds via Freeze-Drying.
Collagen scaffolds were fabricated as previously described.36 Briefly, collagen suspensions were added to cylindrical molds made of polytetrafluoroethylene (PTFE) sides and a copper bottom. When placed on a precooled (−10 °C) freeze-dryer shelf (VirTis, Gardiner, NY), unidirectional heat transfer through the copper bottom promotes the growth of elongated ice crystals. The suspensions were frozen at −10 °C for 2 h prior to the sublimation of the resulting ice crystals at 0 °C and 200 mTorr. Molds of 8 mm diameter and 20 mm in height were used for cell-culture experiments.
Fabrication of Wrapped, Anisotropic Collagen-Based Scaffolds via Freeze-Drying.
Scaffold-amniotic membrane core–shell constructs (C/CS + AM) were fabricated using a method previously described to integrate a high-density collagen membrane around a CG scaffold core.46 Briefly, a dry sheet of AM was cut to size and placed along the circumferential surface of the cylindrical PTFE–copper mold (8 mm diameter; 15 mm height for cell culture samples, 30 mm height for samples undergoing mechanical testing). The C/CS suspension was then pipetted into the center of the mold and allowed to hydrate the AM membrane. Subsequently, a freeze-drying cycle identical to the one described above for the C/CS and C/AM anisotropic scaffolds was used to fabricate the core–shell scaffold composites.
Cross-Linking of CG and C/CS plus AM Core–Shell Scaffolds.
Scaffolds were sterilized and dehydrothermally cross-linked under vacuum in a vacuum oven (Welch, Niles, IL) at 105 °C for 24 h.28 Sections 5 mm long were cut from the scaffold and used for all in vitro culture experiments.36 Scaffolds were hydrated in 100% ethanol overnight and washed in two changes of PBS over 36 h. Chemical cross-linking was applied to strengthen the scaffolds against contraction by seeded cells.47,48 Scaffolds were immersed in a solution of 1-ethyl-3-[3-(dimethylamino)propyl] carbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (NHS) at a molar ratio of 5:2:1 EDC/NHS/COOH. Scaffolds were cross-linked for 1.5 h under shaking at room temperature. Scaffolds were washed with PBS prior to storage in fresh PBS at 4 °C.
SEM Analysis.
The cross-sectional microstructure of dry, un-cross-linked scaffolds was visualized using scanning electron microscopy (SEM). Samples were imaged with a Philips XL30 ESEM–FEG scanning electron microscope under high vacuum. Methods for preparation and imaging of the amniotic membrane particles included in the C/AM scaffolds are found in the Supporting Information file.
Mechanical Testing of Collagen and Amniotic-Membrane Scaffold Variants.
Scaffolds from each formulation were cut to a length of 25 mm, and each end was embedded into custom 3D-printed end blocks with polydimethylsiloxane (PDMS, Fisher). Briefly, PDMS was mixed at a mass ratio of 4:1 monomer to initiator and pipetted into the hollow openings of the end blocks. The PDMS cured for 40 min in a 37 °C incubator before one scaffold end was inserted vertically into the viscous solution. This was cured overnight in the incubator before the process was repeated for the opposite end of the scaffold. Mechanical testing was completed using an Instron 5943 Mechanical Testing System with a 100 N electromechanical load frame. Dry samples were mounted by their end blocks and strained at a rate of 1 mm/min until failure. Elastic moduluswas determined from the linear region of the stress–strain curve for each sample.
Factor Release from AM-Containing Scaffolds.
The release of IL-8 from the C/CS and C/AM anisotropic scaffolds was monitored over 7 days using a sandwich ELISA kit (R&D Systems, Minneapolis, MN). A total of three dry scaffolds were placed with PBS (2 mL) in each well of a 24-well plate. Replicates (n = 3) were prepared for each time point. Following soaks of 12 h, 24 h, and 2, 3, and 7 days, the PBS was assayed for IL-8 concentration per kit instructions.
Cell Culture.
Human Mesenchymal Stem-Cell Culture.
Bone marrow-derived human mesenchymal stem cells (Lonza, Switzerland; 20 year old male) were cultured in standard culture flasks in low-glucose Dulbecco’s modified Eagle medium supplemented with 10% MSC FBS (Invitrogen, Carlsbad, CA), 1% L-glutamine (Invitrogen, Carlsbad, CA), and 1% antibiotic–antimycotic (Invitrogen, Carlsbad, CA). Media was changed every 3 days, and the cells were cultured to confluence at 37 °C and 5% CO2. Cells were used at passage 6.
Scaffold Seeding and Culture Conditions.
Hydrated, cross-linked scaffolds were soaked in growth media overnight at 37 °C before being tapped dry on a Kimwipe and placed in ultralow attachment 6-well plates (Fisher, Waltham, MA). Confluent hMSCs were trypsinized and resuspended in growth media at a concentration of approximately 78 000 cells per 20 μL in preparation for static seeding.36 Ten microliters of the cell suspension were added to one side of the scaffolds. Then the scaffolds were incubated for 15 min at 37 °C and flipped, and another 10 μL of cell suspension was added. To allow for initial cell attachment, scaffolds were placed in the incubator for 2 h before additional growth media was added. Scaffolds were incubated at 37 °C and 5% CO2 for 24 h before the media was exchanged for one of three inflammatory challenge media formulations that was then replaced every 3 days throughout the experiment: (1) control, growth media; (2) inflammatory, media supplemented with 0.1 ng/mL of the pro-inflammatory factor interleukin-1 β (IL-1β) and 1 ng/mL tumor necrosis factor α (TNFα); (3) highly inflammatory, media supplemented with 1 ng/ mL of IL-1β and 10 ng/mL TNFα.
Metabolic Activity Quantification.
A non-destructive alamarBlue assay (Invitrogen, Carlsbad, CA) was used to measure the metabolic activity of the MSCs within the collagen scaffolds.36,46 At the start of the experiment, a metabolic activity standard curve was created by culturing a known number of cells (ranging from 25 to 300% of the total cells seeded) for 2 h in a 1× alamarBlue solution supplemented with cytokines from each of the three media variants. At each experimental time point (days 1, 4, and 7), scaffolds were removed from culture, rinsed in PBS, and incubated under gentle shaking at 37°C for 2 h in a 1× alamarBlue solution containing the media variant of the primary culture. Using a fluorescent spectrophotometer, resorufin fluorescence was measured (excitation of 540 nm and emission of 590 nm). Metabolic activity was interpolated to the standard curve and reported as a percentage of the total number of seeded cells.
Gene Expression Analysis through RNA Isolation and Real-Time PCR.
RNA was isolated from scaffolds with an RNeasy Plant Mini kit (Qiagen, Valencia, CA). The scaffolds were rinsed in PBS to remove any dead or unattached cells, cut in half with a razor, and immersed in the kit’s lysis buffer for 5 min on ice.49 RNA was isolated following the kit’s instructions and total RNA was quantified using a Nanodrop. The RNA was reverse-transcribed using a QuantiTect Reverse Transcription kit (Qiagen, Valencia, CA) and a Bio-Rad S1000 thermal cycler. Real-time polymerase chain reaction (PCR) was performed with an Applied Biosystems 7900HT Fast Real-Time PCR System (Carlsbad, CA) to measure gene expression levels for IL-6 (IL6; forward: TCAATATTAGAGTCTCAACCCCCA and reverse: TTCTCTTTCGTTCCCGGTGG), IL-8 (CXCL8; forward: ACTGAGAGTGATTGAGAGTGGAC and reverse: AACCTCTGCACCCAGTTTTC), collagen I (ColI, COL1A2, forward: TGACCTCAAGATGTGCCACT and reverse: ACCAGACATGCCTCTTGTCC), collagen III (ColIII, COL3A1, forward: GCTGGCATCAAAGGACATCG and reverse: TGTTACCTCGAGGCCCTGGT), tenascin-C (TNC, TNC, forward: TTCACTGGAGCTGACTGTGG and reverse: TAGGGCAGCTCATGTCACTG), and scleraxis (SCX, SCXB, Qiagen QuantiTect Primer Assay Kit, no sequence available). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward: AAGGTGAAGGTCGGAGTCAAC and reverse: GGGGTCATTGATGGCAACAATA) was used as a housekeeping gene. The expression of IL-6 and IL-8 was evaluated at days 1, 4, and 7 post-challenge, and Col I, Col III, TNC, and SCX were only reported at day 7 for early-term tenogenesis, consistent with recent literature from our group examining MSC tenogenic differentiation in collagen scaffolds.39,50 All results were expressed as fold changes relative to expression levels of cells cultured in scaffolds at the same time point with no (pro- or anti-) inflammatory stimuli (C/CS scaffolds in control media).
Statistical Analysis.
One-way analysis of variance (ANOVA) followed by Tukey-HSD post-hoc test was performed on the mechanical analysis data as well as the gene expression data at each time point. The metabolic activity was analyzed using a two-way, repeated-measures ANOVA followed by Tukey-HSD post-hoc test. A p value of <0.05 was used for significance. All analyses were based on a minimum of n = 4 scaffolds unless otherwise noted. Error is reported as the standard error of the mean in the figures.
RESULTS
Soluble Factors Released from Amniotic Membrane.
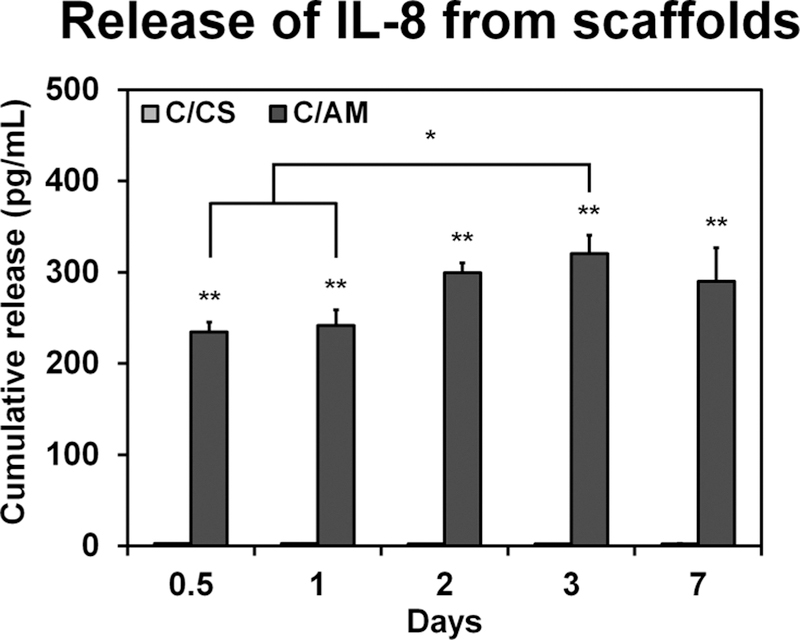
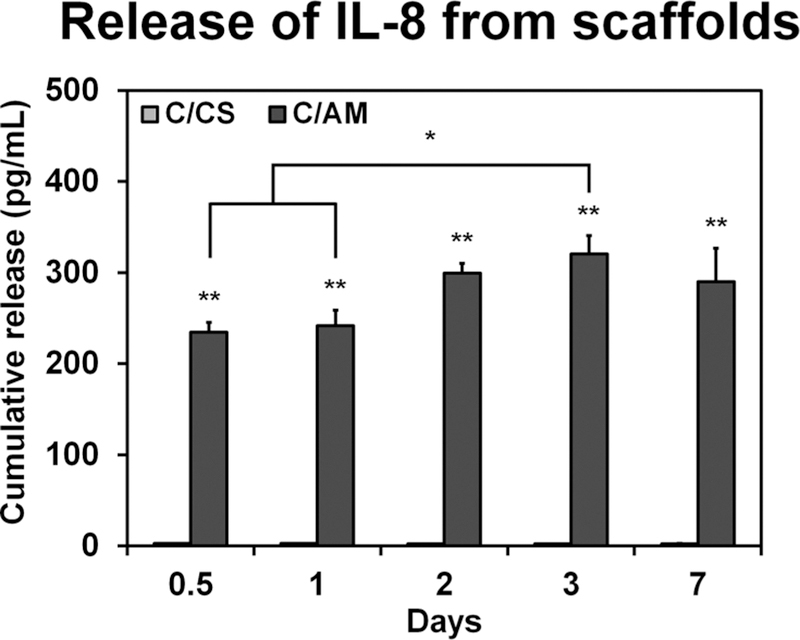
The factors released from sheets of amniotic membrane were semiquantitatively analyzed using a cytokine array. The fold change in spot intensity of factors of interest, those active in the wound healing cascade compared to a blank control are presented in Table 1. The release of pro-proliferative factors such as PDGF and VEGF is low. There is heightened release of anti-inflammatory factors (IL-10, IL-1RA, and TIMP-1/2) and large increases in the release of factors associated with pro-inflammatory processes (IL-1β, IL-8, and TNF-α). We subsequently monitored the release kinetics of a single factor of interest (IL-8) from the decellularized C/AM versus control C/CS scaffolds over time via an ELISA assay (Figure 1). While conventional C/CS scaffolds showed no IL-8 release, C/AM scaffolds released a significantly (p < 0.0001) larger amount of cytokine (approximately 250 pg/mL). Furthermore, the cumulative release of IL-8 increased with time until day 3, which showed significantly greater IL-8 release compared with the results from 12 and 24 h (p < 0.05).
Table 1.
Factors Released from Sheets of Amniotic Membrane and Assessed with a Cytokine Arraya
| Factor | Role | Folo Change | Key |
|---|---|---|---|
| Platelet-derived growth factor (PDGF-BB) | Immunomodulatory: stimulates chemotaxis and ECM production | <2 | |
| Vascular endothelial growth factor (VEGF) | Proliferative: angiogenic | <5 | |
| Interleukin 10 (IL-IO) | Anti-inflammatory, anti-fibrotic | <10 | |
| Interleukin I receptor antagonist (IL-IRA) | Anti-inflammatory: blocks activity of 1L-1 | >10 | |
| Tissue inhibitor of metalloproteinase 1 (TIMP-1) | Anti-inflammatory: inhibits MMP induced degradation | ||
| Tissue inhibitor of metalloproteinase 2 (TIMP-2) | Anti-inflammatory: inhibits MMP induced degradation | ||
| Basic fibroblast growth factor (bFGF) | Proliferative: enhances fibroblast proliferation, angiogensis | ||
| Interferon gamma (IFNγ) | Immunomodulatory: activates macrophages | ||
| Interleukin 1 alpha (IL-lα) | Pro-inflammatory | ||
| Interleukin 1 beta (IL-lβ) | Pro-inflammatory | ||
| Interleukin 8 (IL-8) | Pro-inflammatory: enhances cell migration | ||
| Transforming growth factor beta 1 (TGF-β) | Immunomodulatory: angiogenic | ||
| I ‘umor necrosis factor alpha (TNF-α) | Pro-inflammatory | ||
| Tumor necrosis factor beta (TNF-β) | Pro-inflammatory |
Select factors are shown here, normalized to a blank control, and reported as fold changes.
Figure 1.
Cumulative release of IL-8 from un-cross-linked C/CS and C/AM scaffolds into PBS over the course of a week. Single asterisks denote statistical significance (p < 0.05) between the groups indicated. Double asterisks indicate statistical significance (p < 0.05) from the CS scaffolds at corresponding time points.
Cell Metabolic Activity under Pro-Inflammatory Media Conditions.
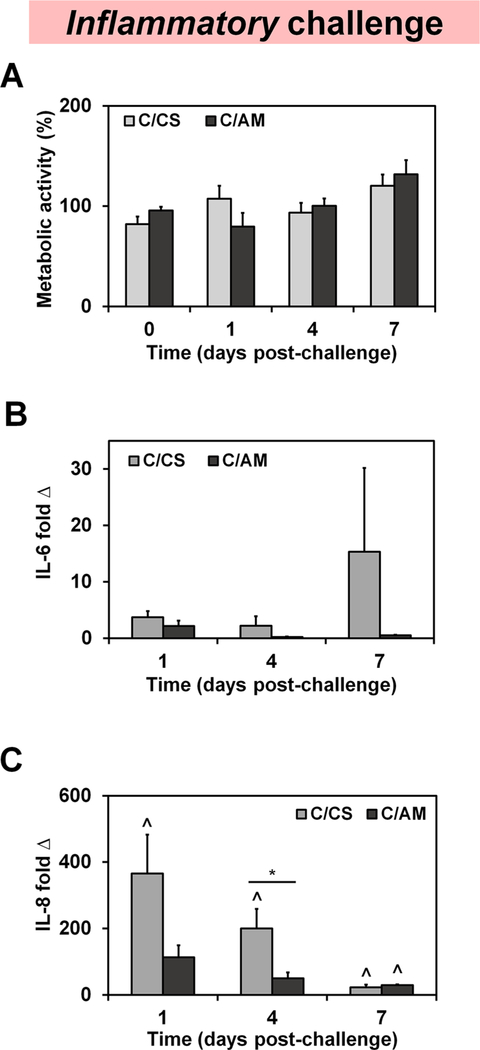
We subsequently tracked the metabolic health of human MSCs within each scaffold variant, comparing the metabolic health prior to the pro-inflammatory challenge (day 0) with that observed at subsequent time points in response to inflammatory challenge (days 1, 4, and 7) (Figures 2A and5A). While increases in metabolic health per construct over time suggests cell expansion, there were no statistically significant differences in the metabolic activity of cells within these scaffold groups over the course of the entire 7 day experiment, regardless of whether the scaffolds were in control media (Figure S2), inflammatory media (Figure 2A), or highly inflammatory media (Figure 5A).
Figure 2.
Human mesenchymal stem-cell activity. (A) Metabolic activity in each scaffold variant was completed using alamarBlue in 3D culture media supplemented with 0.1 ng/mL of the pro-inflammatory factor interleukin-1 β (IL-1β) and 1 ng/mL tumor necrosis factor α (TNFα) (inflammatory group). The expression of pro-inflammatory cytokines (B) IL-6 and (C) IL-8 was also evaluated in this inflammatory media condition. Expressed as fold change internally normalized to GAPDH and externally normalized to the expression of cells cultured in CS scaffolds in control media (non-inflammatory control group). C/CS, collagen–chondroitin sulfate scaffolds; C/AM, collagen–amniotic membrane scaffolds. Asterisks denote statistically significant (p < 0.05) differences between groups, while the upward caret indicates a significant difference (p < 0.05) between the group indicated and the expression in corresponding non-inflammatory control group.
Figure 5.
Comparison of cellular response of MSCs in C/CS and C/ AM scaffolds to those cultured in C/CS plus AM scaffolds in highly inflammatory media conditions (1 ng/mL of IL-1β and 10 ng/mL TNFα). (A) Metabolic activity assayed using alamarBlue. Gene expression is reported as fold change internally normalized to GAPDH and externally normalized to the expression of cells in the non-inflammatory control for both (B) IL-6 and (C) IL-8. Asterisks denote statistically significant (p < 0.05) differences between groups, while upward carets indicate a significant difference (p < 0.05) between the group indicated and the expression in the corresponding control.
Gene Expression of MSCs Cultured in 3D Scaffolds under Pro-Inflammatory Conditions.
We then examined the expression of genes encoding for pro-inflammatory cytokines IL-6 and IL-8 as a function of scaffold environment in inflammatory media (Figure 2B,C). Following inflammatory challenge with IL-1β and TNFα, IL-6 expression is elevated in C/CS scaffolds but not significantly greater than that of the control or the expression in scaffolds containing amniotic membrane (Figure 2B). Conversely, in response to inflammatory challenge, IL-8 expression is significantly (p < 0.05) higher in C/CS scaffolds (compared to the control media) at all time points (days 1, 4, and 7). Interestingly, MSCs cultured in C/ AM scaffolds show significantly lower expression of IL-8 than those in challenged CS scaffolds at day 4 of the experiment. Furthermore, IL-8 expression at days 1 and 4 for MSCs challenged with inflammatory media in the C/AM scaffolds was no different than IL-8 expression levels for MSCs in CS scaffolds in control media (Figure 2C).
Structural and Mechanical Performance of the CG–AM Core–Shell Composite.
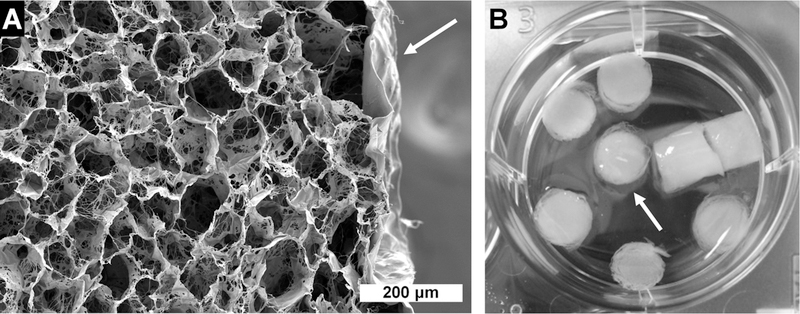
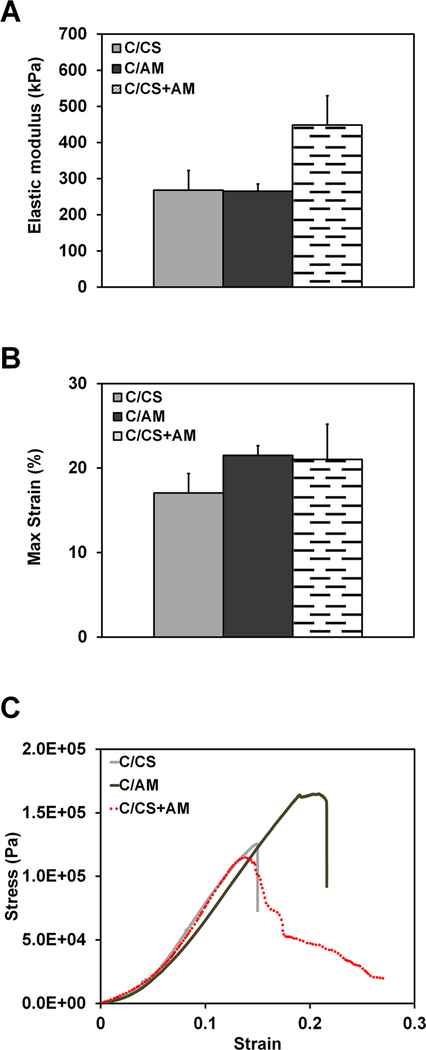
SEM images of dry, core–shell C/CS plus AM composites show that there is consistent union between the scaffold core and the amniotic membrane (indicated with an arrow) without evidence of delamination, confirming that the membrane is well-integrated with the bulk of the material (Figure 3A). A similar microstructure is seen in the C/CS and C/AM scaffolds (Figure S3). Following hydration, the AM shell swells (arrow) but remains in contact with the core scaffold (Figure 3B). Tensile mechanical testing (representative curves in Figure 4C) revealed a single layer of AM wrap around the CG scaffold was insufficient to significantly increase the elastic modulus (Figure 4A) of the core–shell composite as compared with C/CS and C/AM scaffolds alone. All scaffolds performed as low-density open cell foams, with maximum strain at failure occurring at less than 25% applied strain (Figure 4B,C).
Figure 3.
Images of amniotic membrane-wrapped scaffolds. (A) Scanning electron microscopy of core–shell scaffolds with a collagen–chondroitin sulfate core and amniotic membrane shell (white arrow). The scale bar is 200 μm. (B) Macro image showing hydrated scaffolds in the well of a 6-well plate; the amniotic membrane indicated by a white arrow.
Figure 4.
Mechanics of dry, three-dimensional scaffolds. (A) Elastic modulus. (B) Maximum strain. (C) Representative stress–strain curves for each scaffold type. C/CS, collagen–chondroitin sulfate scaffolds; C/AM, collagen–amniotic membrane scaffolds; C/CS +AM: core–shell scaffolds with a collagen–chondroitin sulfate core and amniotic membrane shell.
Cellular Response of MSCs Cultured in Core–Shell Amnion Scaffolds.
We subsequently compared the response of MSCs to highly inflammatory challenge media when cultured in conventional C/CS scaffolds, C/AM scaffolds that incorporate the AM matrix into the scaffold structure, or core–shell composite composed of a C/CS scaffold core and an AM membrane shell (C/CS plus AM). Consistent with the Cell Metabolic Activity under Pro-Inflammatory Media Conditions section results for C/CS and C/AM scaffolds in inflammatory media, MSCs did not exhibit significant difference in metabolic activity under highly inflammatory media challenge (Figure 5A). Examining IL-6 and IL-8 gene expression levels under highly inflammatory challenge, MSCs in C/CS plus AM scaffolds showed a trend of higher expression at day 1 but then a drop in expression at later time points (days 4 and 7; Figure 5B,C). Furthermore, while IL-6 expression was increased in MSCs in the C:CS scaffolds compared to control media after 4 days of inflammatory challenge, IL-6 expression levels were significantly reduced in MSCs in C/AM scaffolds and C/CS plus AM wrap composites (p < 0.05, compared to C/CS scaffolds) (Figure 5B). By day 7, only the MSCs in the C/CS plus AM scaffolds show a significant decrease in IL-6 expression compared with those cultured in CS scaffolds in highly inflammatory media (Figure 5B). While MSCs in the C/CS plus AM wrap composite and the C/AM scaffold showed reduced IL-8 expression compared with MSCs in the C/CS scaffolds at day 4, the effect was only significant in the C/CS plus AM composites at day 7 (p < 0.05; Figure 5C).
Tenogenic Phenotype of MSCs Cultured in Core–Shell Amnion Scaffolds.
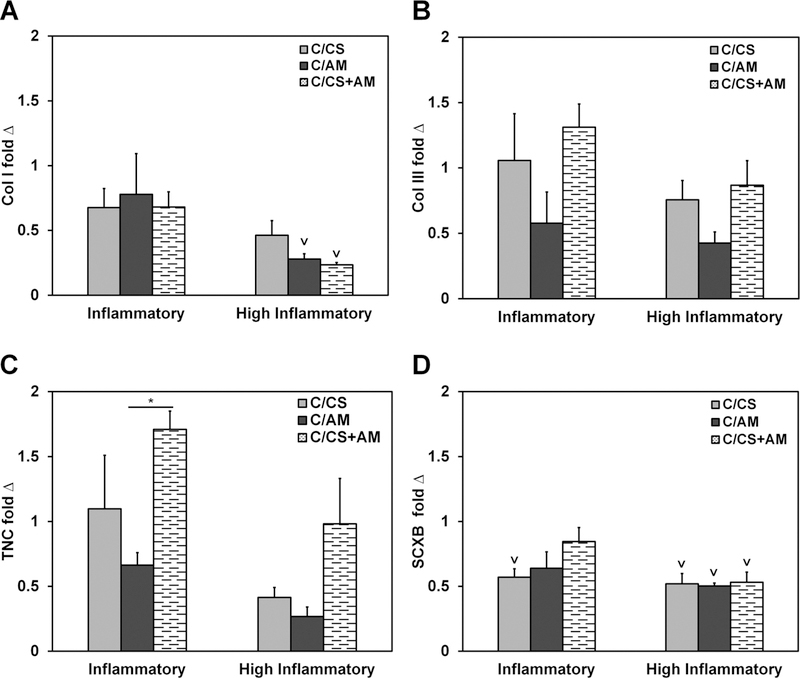
Due to recent evidence that the aligned nature of these collagen-based scaffolds supports tenogenic differentiation of mesenchymal stem cells,39,50 the expression of key tenogenic genes was evaluated; here, under inflammatory challenge. The expression of collagen type I (Col I) under inflammatory conditions was not significantly different across all scaffold types and control conditions (Figure 6A). However, MSCs in the scaffolds containing amniotic membrane matrix (C/AM and C/CS plus AM) under the highly inflammatory challenge showed significantly (p < 0.05) lower expression of Col I compared with cells in C/ CS scaffolds receiving no inflammatory challenge (control) (Figure 6A). There were no significant differences in collagen type III (Col III) expression (Figure 6B). The C/CS plus AM scaffolds supported increased expression of the tendon marker tenascin-C (TNC) over C/AM scaffolds in inflammatory media conditions (Figure 6C). While MSC expression of scleraxis (SCXB) in both C/AM and C/CS plus AM scaffolds in inflammatory media was no different from the aligned C/CS scaffolds cultured without inflammatory media, C/CS scaffolds under inflammatory conditions were not able to sustain the expression of this marker of tenogenic differentiation (Figure 6D).
Figure 6.
Tenogenic gene expression of MSCs cultured in C/CS, C/AM, or C/CS plus AM scaffolds under inflammatory or highly inflammatory media conditions. Gene expression is reported as fold change internally normalized to GAPDH and externally normalized to the expression of cells in the non-inflammatory control. Asterisks denote statistically significant (p < 0.05) differences between groups, while the downward caret indicates a significant difference (p < 0.05) between the group indicated and the expression in the corresponding control.
DISCUSSION
In the treatment of massive tendon tears, biomaterials provide an exciting opportunity for improved healing over native healing cascades and surgical reattachment techniques. Current technologies often do not address inflammatory and repair-mediated signaling cascades, which can lead to scar formation, decreased mechanical properties, and high rates of repeated failure.51 Particularly important is the understanding that the material implant is likely to experience the body’s native inflammatory response to the initial injury. Extended inflammation can have detrimental effects on healing outcomes.11 Facilitating the transition from the inflammatory phase of healing to one of proliferation and remodeling is a strategy that may have advantages in preventing scar formation.52–55
Our efforts were inspired by two orthogonal technologies that have demonstrated promise regarding promotion of regenerative healing and the potential to alter host inflammatory response. First, collagen–glycosaminoglycan (CG) scaffolds are biodegradable, bioactive, three-dimensional materials that mimic the composition and microstructure of the extracellular matrix. Studied for an array of applications,27–33 they promote regeneration by preventing myofibroblast-initiated contraction and subsequent scar tissue formation.34,35 A microstructurally aligned (anisotropic) CG scaffold variant has recently been introduced as a platform for tendon-repair applications.36–38,56,57 The second technology, human amniotic membrane, has also been suggested to promote regenerative healing but is typically employed as a two-dimensional decellularized sheet to temper the inflammatory response at the injury site.17,21 Together, these materials offer both a tendon-mimicking structure and a source of immunomodulatory matrix and soluble molecules to alter cellular response in tendon regeneration applications. In the body, MSCs travel to sites of injury and inflammation as part of the tissue repair and regeneration process. Their complex immunomodulatory role in wound healing has been extensively studied, and while it is not completely clear how MSCs regulate the body’s immune response, their response to the local environment of soluble factors can direct macrophage phenotype.58
This work demonstrates that amniotic membrane matrix can be incorporated into the bulk of a collagen scaffold but also as a membrane wrap around the scaffold to form a core–shell composite. We employ a directional solidification approach to create an anisotropic pore architecture within the scaffold phase to mimic the anisotropic characteristics of native tendon. Previous studies using these anisotropic CG scaffolds have shown that scaffold architecture is sufficient to maintain a tenocyte phenotype of primary equine tenocytes37,38 and to enhance Smad2/3 expression and subsequent MSC differentiation toward a tenogenic phenotype.38,56,57 Our alternative incorporation of AM matrix as a membrane wrap to form a core–shell composite design is inspired by modern surgical techniques that use commercially available tendon wraps.3,4 We previously showed that multiple layers of a high-density collagen membrane could be stably incorporated around a CG scaffold core to tune the mechanical competence of the resultant core–shell composite for tendon applications.46,59 In the current study, the amniotic membrane did not significantly improve the scaffold strength. This could be due to potential points of weakness introduced by the physical manipulation of the membrane during isolation, drying, and scaffold fabrication or delamination of the AM during testing as a result of incomplete integration with the core scaffold. This could be improved by increasing the number of AM layers used for wrapping and the time allowed for the collagen suspension to soak into the AM prior to lyophilization. While, for this study, we only used a single layer of AM, this alone provides the ability to incorporate a larger amount of AM matrix per scaffold (3.4 times in mass) than in the traditional C/AM scaffold format.
The amniotic membrane has been shown to promote anti-inflammatory, scarless wound-healing processes in vivo.21 This tissue is biologically active and contains a host of growth factors and cytokines that play essential roles in tissue development in utero and as a wound dressing.60 In this study, the growth factor content of the amniotic membrane was evaluated with a cytokine array. While it was expected that the amniotic membrane would release anti-inflammatory factors such as IL-10, it was a surprise to see the extent to which pro-inflammatory signals were also released from the membrane (Table 1). We speculate that these factors may be important in priming the wound healing cascade and, in combination with anti-inflammatory signals, may expedite the transition to pro-healing processes. Further work characterizing the AM’s growth factor/cytokine release kinetics as well as inflammatory responses in vivo needs to be completed to confirm this. When the release of pro-inflammatory IL-8 from AM-containing scaffolds was quantified, we report cytokine concentrations on the order of 300 pg/mL, suggesting that the release of biomolecules from the AM content of the scaffolds may contribute to the immunomodulatory responses observed for both the C/AM and C/CS plus AM scaffold variants.
Mesenchymal stem and stromal cells are multipotent progenitor cells,61 found primarily in bone marrow or adipose tissue,62 which offer favorable characteristics in the form of self-renewal61 and capacity to differentiate toward specialized cells of the musculoskeletal system, including tenocytes.63 While they can be added exogenously to a tissue-engineering scaffolds, MSCs often also accumulate at sites of injury, making it essential to evaluate MSC activity in response to inflammatory challenge.64,65 We first evaluated the bioactivity of MSCs within our C/CS versus C/AM scaffold variants using an alamarBlue metabolic activity assay. MSCs showed similar responses in terms of overall metabolic activity in each scaffold variant (C/CS, C/AM, and C/CS plus AM) under all media condition: control (Figure S2), inflammatory (Figure 2A), and highly inflammatory (Figure 5A). Metabolic activity was normalized to the activity of the population of MSCs initially seeded into the scaffolds. Thus, while constructs showed metabolic activities initially less than 100% at experiment Day 0 (1 day post-seeding when inflammatory media was first added), this is consistent with previous findings in CG scaffolds. Not all cells seeded into the scaffold will attach, and cell attachment is directly related to scaffold microstructure.32 The uniformity in initial MSC metabolic activity between groups is consistent with our previous finding that the incorporation of AM matrix into the scaffold does not alter scaffold pore structure.43 Most importantly, MSC metabolic activity increased over time, showing all AM-functionalized scaffold variants support the cellular activity of the MSCs. Even though high levels of IL-1β have been previously shown to negatively affect MSC proliferation,66 there were no significant differences associated with inflammatory media challenge in any scaffold variant. Because age and sex are factors in MSC behavior, future studies would benefit from testing MSCs from multiple donors.
Several MSC secreted factors have been identified as key immunomodulators in the wound-healing cascade.58 Of these, this manuscript discusses the gene expression for pro-inflammatory cytokines IL-6 and IL-8. When MSCs are applied therapeutically to a cutaneous wound site, decreased inflammation and improved healing is correlated with decreases in the overall secretion of IL-6.67 The same is seen in the case of MSC treatment for corneal injuries.68 IL-6 can also promote the migration of immune cells and fibroblasts to the wound site and is correlated with the stimulation of wound healing.69 Similarly, the presence of IL-8 in normal wound healing environments leads to keratinocyte migration in vitro70,71 and enhanced wound healing in mice,72,73 although excessive inflammation and IL-8 production can lead to delayed wound closure.74 Studies have shown that by introducing MSCs to a myocardial injury, the overall expression of IL-8 is reduced along with the extent of inflammation, leading to improved cardiac function.75 In the current work, the expression levels of IL-6 and IL-8 in MSCs were moderately down-regulated (Figures 2 and 5) when the cells were cultured in collagen scaffolds containing amniotic membrane matrix (either C/AM or C/CS plus AM scaffolds). General trends in the study of tenogenic markers here show an inhibition of tenogenesis in MSCs as a result of pro-inflammatory media conditions. AM-containing scaffolds only perform marginally better than C/CS scaffolds compared with their controls. A study that combines anisotropic C/AM or C/ CS plus AM scaffolds with mechanical strain stimuli, which we have previously shown to enhance MSC tenogenesis in C/CS scaffolds,50 would be informative in evaluating what factors are primed to make an impact in pro-inflammatory conditions. In both immunomodulatory genes and the genes associated with tenogenic function, protein expression to confirm the MSC response at later time points will be a critical consideration moving forward.
Future work considering MSC culture in conjunction with inflammatory cells found in early tendinopathy (e.g., macrophages and T cells)76 will also be critical for investigating the downstream effects of scaffold-induced immunomodulation. Additionally, in the scope of tendon regeneration, there are additional factors that should be considered in future works such as the mediation of IL-17A on tissue remodeling77 and the activation of inflammatory pathways particular to tendon disease.78 It is unclear if the modulation of IL-6 and IL-8 expression will have positive or negative implications on long-term kinetics of macrophage activity, matrix remodeling, and improved healing, all of which are the subject of ongoing efforts in our laboratory. However, recent results in the literature demonstrated aligned substrates can induce more predominant M2-like responses in the absence of cytokines.79 As a result, AM-modified CG scaffolds, which exhibit both aligned matrix elements along with biomolecular features of the AM, may be particularly beneficial for modulation of macrophage phenotype. Ongoing efforts are also investigating the role amniotic membrane scaffolds play in cell recruitment, macrophage response, and early metrics of wound healing in vivo.
CONCLUSIONS
The amniotic membrane serves as a powerful source of immunomodulatory material. While it has been conventionally used as a two-dimensional membrane or as a micronized powder for tissue regeneration or altered healing applications, the potential benefits developing fully three-dimensional biomaterials based on AM matrix remains underexplored. In this manuscript, AM matrix was incorporated into 3D collagen biomaterial under development of tendon repair applications via two means: bulk incorporation to form a porous collagen– AM scaffold or as a membrane wrap surrounding a collagen– glycosaminoglycan scaffold to form a core–shell composite. We examined the metabolic activity and immunomodulatory response of human mesenchymal stem cells within these scaffolds in response to an inflammatory media challenge. Expression profiles of immunomodulatory genes IL-6 and IL-8 suggest that while MSCs exhibit a response to media challenge, MSCs within AM-functionalized collagen scaffolds show a varied response in early stages after inflammatory challenge. These results, along with the potential to utilize the amniotic membrane as a wrap to increase the mechanical performance of the resultant composite while also maintaining the beneficial immunomodulatory effect of the AM matrix, suggest a robust path for incorporating amniotic membrane matrix to create three-dimensional biomaterials to enhance tendon regeneration potential.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Dr. Sandra McMasters (SCS, UIUC) for culture media preparation, Cate Wallace (Beckman Institute, UIUC) for assistance with SEM imaging, the IGB Core Facilities for assistance with real-time PCR, and Aidan Gilchrist for assistance with mechanical testing. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award no. R03 AR062811, as well as the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award no. R01 DK099528. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomaterials.8b01154.
Additional details on the methods for AM particle sizing; figures showing particle size distribution of amniotic membrane post-homogenization, human mesenchymal stem-cell metabolic activity in 3D scaffolds cultured in non-inflammatory control media, and cross-sectional SEM images of C/CS and C/AM scaffolds (PDF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Liu Y; Ramanath HS; Wang D-A Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol 2008, 26 (4), 201–209. [DOI] [PubMed] [Google Scholar]
- (2).Mall NA; Tanaka MJ; Choi LS; Paletta GA Jr. Factors affecting rotator cuff healing. J. Bone Joint Surg Am 2014, 96 (9), 778–788. [DOI] [PubMed] [Google Scholar]
- (3).Derwin KA; Baker AR; Spragg RK; Leigh DR; Iannotti JP Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J. Bone Joint Surg Am 2006, 88 (12), 2665–2672. [DOI] [PubMed] [Google Scholar]
- (4).Smith RD; Carr A; Dakin SG; Snelling SJ; Yapp C; Hakimi O The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur. Cell Mater 2016, 31, 107–118. [DOI] [PubMed] [Google Scholar]
- (5).Bond JL; Dopirak RM; Higgins J; Burns J; Snyder SJ Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy 2008, 24 (4), 403–409. [DOI] [PubMed] [Google Scholar]
- (6).Wong I; Burns J; Snyder S Arthroscopic GraftJacket repair of rotator cuff tears. J. Shoulder Elbow Surg 2010, 19 (2), 104–109. [DOI] [PubMed] [Google Scholar]
- (7).Barber FA; Burns JP; Deutsch A; Labbe MR; Litchfield RB A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 2012, 28 (1), 8–15. [DOI] [PubMed] [Google Scholar]
- (8).Lee DK Achilles tendon repair with acellular tissue graft augmentation in neglected ruptures. J. Foot Ankle Surg 2007, 46 (6), 451–455. [DOI] [PubMed] [Google Scholar]
- (9).Lee DK A preliminary study on the effects of acellular tissue graft augmentation in acute Achilles tendon ruptures. J. Foot Ankle Surg 2008, 47 (1), 8–12. [DOI] [PubMed] [Google Scholar]
- (10).Song L; Olsen RE; Spalazzi JP; Davisson T Biomechanical evaluation of acellular collagen matrix augmented Achilles tendon repair in sheep. J. Foot Ankle Surg 2010, 49 (5), 438–441. [DOI] [PubMed] [Google Scholar]
- (11).Eming SA; Krieg T; Davidson JM Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol 2007, 127 (3), 514–525. [DOI] [PubMed] [Google Scholar]
- (12).Tsuzaki M; Guyton G; Garrett W; Archambault JM; Herzog W; Almekinders L; Bynum D; Yang X; Banes AJ IL-1 beta induces COX2, MMP-1, −3 and −13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J. Orthop. Res 2003, 21 (2), 256–264. [DOI] [PubMed] [Google Scholar]
- (13).Gulotta LV; Kovacevic D; Cordasco F; Rodeo SA Evaluation of tumor necrosis factor alpha blockade on early tendon-to-bone healing in a rat rotator cuff repair model. Arthroscopy 2011, 27 (10), 1351–1357. [DOI] [PubMed] [Google Scholar]
- (14).Tseng SCG; Prabhasawat P; Barton K; Gray T; Meller D Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch. Ophthalmol 1998, 116 (4), 431–41. [DOI] [PubMed] [Google Scholar]
- (15).Azuara-Blanco A; Pillai CT; Dua HS Amniotic membrane transplantation for ocular surface reconstruction. Br. J. Ophthalmol 1999, 83 (4), 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chen HJ; Pires RT; Tseng SC Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br. J. Ophthalmol 2000, 84 (8), 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kim H; Son D; Choi TH; Jung S; Kwon S; Kim J; Han K Evaluation of an amniotic membrane-collagen dermal substitute in the management of full-thickness skin defects in a pig. Arch Plast Surg 2013, 40 (1), 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Koob TJ; Rennert R; Zabek N; Massee M; Lim JJ; Temenoff JS; Li WW; Gurtner G Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. International wound journal 2013, 10 (5), 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Samandari MH; Yaghmaei M; Ejlali M; Moshref M; Saffar AS Use of amnion as a graft material in vestibuloplasty: a preliminary report. Oral Surg Oral Med. Oral Pathol Oral Radiol Endod 2004, 97 (5), 574–578. [DOI] [PubMed] [Google Scholar]
- (20).Fairbairn NG; Randolph MA; Redmond RW The clinical applications of human amnion in plastic surgery. J. Plast Reconstr Aesthet Surg 2014, 67 (5), 662–675. [DOI] [PubMed] [Google Scholar]
- (21).Niknejad H; Peirovi H; Jorjani M; Ahmadiani A; Ghanavi J; Seifalian AM Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cell Mater 2008, 7, 88–99. [DOI] [PubMed] [Google Scholar]
- (22).Willett NJ; Thote T; Lin AS; Moran S; Raji Y; Sridaran S; Stevens HY; Guldberg RE Intra-articular injection of micronized dehydrated human amnion/chorion membrane attenuates osteoarthritis development. Arthritis Res. Ther 2014, 16 (1), R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kueckelhaus M; Philip J; Kamel RA; Canseco JA; Hackl F; Kiwanuka E; Kim MJ; Wilkie R; Caterson EJ; Junker JP; Eriksson E Sustained release of amnion-derived cellular cytokine solution facilitates achilles tendon healing in rats. Eplasty 2014, 14, e29. [PMC free article] [PubMed] [Google Scholar]
- (24).Pinkerton MC Amnioplastin for adherent digital flexor tendons. Lancet 1942, 239, 70–72. [Google Scholar]
- (25).Ozboluk S; Ozkan Y; Ozturk A; Gul N; Ozdemir RM; Yanik K The effects of human amniotic membrane and periosteal autograft on tendon healing: experimental study in rabbits. J. Hand Surg Eur 2010, 35 (4), 262–268. [DOI] [PubMed] [Google Scholar]
- (26).Dogramaci Y; Duman IG Reinforcement of the Flexor Tendon Repair Using Human Amniotic MembraneA Biomechanical Evaluation Using the Modified Kessler Method of Tendon Repair. J. Am. Podiatr Med. Assoc 2016, 106 (5), 319–322. [DOI] [PubMed] [Google Scholar]
- (27).Farrell E; O’Brien FJ; Doyle P; Fischer J; Yannas I; Harley BA; O’Connell B; Prendergast PJ; Campbell VA A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng 2006, 12 (3), 459–468. [DOI] [PubMed] [Google Scholar]
- (28).Yannas IV; Lee E; Orgill DP; Skrabut EM; Murphy GF Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. U. S. A 1989, 86 (3), 933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Harley BA; Freyman TM; Wong MQ; Gibson LJ A new technique for calculating individual dermal fibroblast contractile forces generated within collagen-GAG scaffolds. Biophys. J 2007, 93 (8), 2911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Harley BA; Kim HD; Zaman MH; Yannas IV; Lauffenburger DA; Gibson LJ Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys. J 2008, 95 (8), 4013–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Harley BA; Spilker MH; Wu JW; Asano K; Hsu HP; Spector M; Yannas IV Optimal degradation rate for collagen chambers used for regeneration of peripheral nerves over long gaps. Cells Tissues Organs 2004, 176, 153–165. [DOI] [PubMed] [Google Scholar]
- (32).O’Brien FJ; Harley BA; Yannas IV; Gibson LJ The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26 (4), 433–41. [DOI] [PubMed] [Google Scholar]
- (33).Torres DS; Freyman TM; Yannas IV; Spector M Tendon cell contraction of collagen-GAG matrices in vitro: effect of cross-linking. Biomaterials 2000, 21 (15), 1607. [DOI] [PubMed] [Google Scholar]
- (34).Murphy GF; Orgill DP; Yannas IV Partial dermal regeneration is induced by biodegradable collagen-glycosaminoglycan grafts. Lab Invest 1990, 62 (3), 305–13. [PubMed] [Google Scholar]
- (35).Yannas IV Emerging rules for inducing organ regeneration. Biomaterials 2013, 34 (2), 321–330. [DOI] [PubMed] [Google Scholar]
- (36).Caliari SR; Harley BAC The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 2011, 32 (23), 5330–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Caliari SR; Weisgerber DW; Ramirez MA; Kelkhoff DO; Harley BAC The influence of collagen-glycosaminoglycan scaffold relative density and microstructural anisotropy on tenocyte bioactivity and transcriptomic stability. J. Mech Behav Biomed Mater 2012, 11, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Grier WK; Iyoha EM; Harley BAC The influence of pore size and stiffness on tenocyte bioactivity and transcriptomic stability in collagen-GAG scaffolds. J. Mech Behav Biomed Mater 2017, 65, 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Caliari SR; Harley BAC Structural and biochemical modification of a collagen scaffold to selectively enhance MSC tenogenic, chondrogenic, and osteogenic differentiation. Adv. Healthcare Mater 2014, 3 (7), 1086–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Hortensius RA; Ebens JH; Harley BA Immunomodulatory effects of amniotic membrane matrix incorporated into collagen scaffolds. J. Biomed. Mater. Res., Part A 2016, 104, 1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Cooper LJ; Kinoshita S; German M; Koizumi N; Nakamura T; Fullwood NJ An investigation into the composition of amniotic membrane used for ocular surface reconstruction. Cornea 2005, 24 (6), 722–729. [DOI] [PubMed] [Google Scholar]
- (42).Wolbank S; Hildner F; Redl H; van Griensven M; Gabriel C; Hennerbichler S Impact of human amniotic membrane preparation on release of angiogenic factors. J. Tissue Eng. Regener. Med 2009, 3 (8), 651–4. [DOI] [PubMed] [Google Scholar]
- (43).Hortensius RA; Ebens JH; Harley BAC Immunomodulatory effects of amniotic membrane matrix incorporated into collagen scaffolds. J. Biomed. Mater. Res., Part A 2016, 104 (6), 1332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Miki T; Marongiu F; Dorko K; Ellis EC; Strom SC Isolation of amniotic epithelial stem cells. Current protocols in stem cell biology 2010, 1 DOI: 10.1002/9780470151808.sc01e03s12. [DOI] [PubMed] [Google Scholar]
- (45).Hopkinson A; Shanmuganathan VA; Gray T; Yeung AM; Lowe J; James DK; Dua HS Optimization of amniotic membrane (AM) denuding for tissue engineering. Tissue Eng., Part C 2008, 14 (4), 371–81. [DOI] [PubMed] [Google Scholar]
- (46).Caliari SR; Ramirez MA; Harley BAC The development of collagen-GAG scaffold-membrane composites for tendon tissue engineering. Biomaterials 2011, 32 (34), 8990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Olde Damink LHH; Dijkstra PJ; Van Luyn MJA; Van Wachem PB; Nieuwenhuis P; Feijen J Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 1996, 17 (8), 765–73. [DOI] [PubMed] [Google Scholar]
- (48).Harley BA; Leung JH; Silva EC; Gibson LJ Mechanical characterization of collagen-glycosaminoglycan scaffolds. Acta Biomater 2007, 3 (4), 463–74. [DOI] [PubMed] [Google Scholar]
- (49).Caliari SR; Harley BA Composite growth factor supplementation strategies to enhance tenocyte bioactivity in aligned collagen-GAG scaffolds. Tissue Eng., Part A 2013, 19 (9–10), 1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Grier WK; Moy AS; Harley BA Cyclic tensile strain enhances human mesenchymal stem cell Smad 2/3 activation and tenogenic differentiation in anisotropic collagen-glycosaminoglycan scaffolds. Eur. Cell Mater 2017, 33, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Galatz LM; Sandell LJ; Rothermich SY; Das R; Mastny A; Havlioglu N; Silva MJ; Thomopoulos S Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J. Orthop. Res 2006, 24 (3), 541–50. [DOI] [PubMed] [Google Scholar]
- (52).Witherel CE; Graney PL; Freytes DO; Weingarten MS; Spiller KL Response of human macrophages to wound matrices in vitro. Wound Repair Regen 2016, 24 (3), 514–24. [DOI] [PubMed] [Google Scholar]
- (53).Garash R; Bajpai A; Marcinkiewicz BM; Spiller KL Drug delivery strategies to control macrophages for tissue repair and regeneration. Exp. Biol. Med. (London, U. K.) 2016, 241 (10), 1054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Spiller KL; Freytes DO; Vunjak-Novakovic G Macrophages modulate engineered human tissues for enhanced vascularization and healing. Ann. Biomed. Eng 2015, 43 (3), 616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Spiller KL; Nassiri S; Witherel CE; Anfang RR; Ng J; Nakazawa KR; Yu T; Vunjak-Novakovic G Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Grier WK; Moy AS; Harley BAC Cyclic tensile strain enhances human mesenchymal stem cell Smad2/3 activation and tenogenic differentiation in anisotropic collagen-glycosaminoglycan scaffolds. Eur. Cell Mater 2017, 33, 227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Caliari SR; Harley BA Structural and biochemical modification of a collagen scaffold to selectively enhance MSC tenogenic, chondrogenic, and osteogenic differentiation. Adv. Healthcare Mater 2014, 3 (7), 1086–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Ulivi V; Tasso R; Cancedda R; Descalzi F Mesenchymal stem cell paracrine activity is modulated by platelet lysate: induction of an inflammatory response and secretion of factors maintaining macrophages in a proinflammatory phenotype. Stem Cells Dev 2014, 23 (16), 1858–69. [DOI] [PubMed] [Google Scholar]
- (59).Caliari SR; Mozdzen LC; Armitage O; Oyen ML; Harley BAC Periodically perforated core–shell collagen biomaterials balance cell infiltration, bioactivity, and mechanical properties. J. Biomed. Mater. Res., Part A 2014, 102 (4), 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Koob TJ; Lim JJ; Massee M; Zabek N; Denoziere G Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J. Biomed. Mater. Res., Part B 2014, 102 (6), 1353–62. [DOI] [PubMed] [Google Scholar]
- (61).Carrillo-Galvez AB; Cobo M; Cuevas-Ocana S; Gutierrez-Guerrero A; Sanchez-Gilabert A; Bongarzone P; Garcia-Perez A; Munoz P; Benabdellah K; Toscano MG; Martin F; Anderson P Mesenchymal stromal cells express GARP/LRRC32 on their surface: effects on their biology and immunomodulatory capacity. Stem Cells 2015, 33 (1), 183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Uccelli A; Moretta L; Pistoia V Mesenchymal stem cells in health and disease. Nat. Rev. Immunol 2008, 8 (9), 726–36. [DOI] [PubMed] [Google Scholar]
- (63).Ryan JM; Barry F; Murphy JM; Mahon BP Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol 2007, 149 (2), 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Nakajima H; Uchida K; Guerrero AR; Watanabe S; Sugita D; Takeura N; Yoshida A; Long G; Wright KT; Johnson WE; Baba H Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J. Neurotrauma 2012, 29 (8), 1614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Manning CN; Martel C; Sakiyama-Elbert SE; Silva MJ; Shah S; Gelberman RH; Thomopoulos S Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Res. Ther 2015, 6, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Lacey DC; Simmons PJ; Graves SE; Hamilton JA Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage 2009, 17 (6), 735–42. [DOI] [PubMed] [Google Scholar]
- (67).Liu L; Yu Y; Hou Y; Chai J; Duan H; Chu W; Zhang H; Hu Q; Du J Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One 2014, 9 (2), e88348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Yun YI; Park SY; Lee HJ; Ko JH; Kim MK; Wee WR; Reger RL; Gregory CA; Choi H; Fulcher SF; Prockop DJ; Oh JY Comparison of the anti-inflammatory effects of induced pluripotent stem cell–derived and bone marrow–derived mesenchymal stromal cells in a murine model of corneal injury. Cytotherapy 2017, 19 (1), 28–35. [DOI] [PubMed] [Google Scholar]
- (69).Yew T-L; Hung Y-T; Li H-Y; Chen H-W; Chen L-L; Tsai K-S; Chiou S-H; Chao K-C; Huang T-F; Chen H-L; Hung S-C Enhancement of Wound Healing by Human Multipotent Stromal Cell Conditioned Medium: The Paracrine Factors and p38 MAPK Activation. Cell Transplantation 2011, 20 (5), 693–706. [DOI] [PubMed] [Google Scholar]
- (70).Walter MN; Wright KT; Fuller HR; MacNeil S; Johnson WE Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp. Cell Res 2010, 316 (7), 1271–81. [DOI] [PubMed] [Google Scholar]
- (71).Jiang WG; Sanders AJ; Ruge F; Harding KG Influence of interleukin-8 (IL-8) and IL-8 receptors on the migration of human keratinocytes, the role of PLC-gamma and potential clinical implications. Exp. Ther. Med 2012, 3 (2), 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Chen L; Xu Y; Zhao J; Zhang Z; Yang R; Xie J; Liu X; Qi S Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One 2014, 9 (4), e96161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Rennekampff HO; Hansbrough JF; Kiessig V; Dore C; Sticherling M; Schroder JM Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J. Surg. Res 2000, 93 (1), 41–54. [DOI] [PubMed] [Google Scholar]
- (74).Iocono JA; Colleran KR; Remick DG; Gillespie BW; Ehrlich HP; Garner WL Interleukin-8 levels and activity in delayed-healing human thermal wounds. Wound Repair Regen 2000, 8 (3), 216–225. [DOI] [PubMed] [Google Scholar]
- (75).Gao S; Zhao Z; Wu R; Zeng Y; Zhang Z; Miao J; Yuan Z Bone marrow mesenchymal stem cell transplantation improves radiation-induced heart injury through DNA damage repair in rat model. Radiat. Environ. Biophys 2017, 56, 63. [DOI] [PubMed] [Google Scholar]
- (76).Millar NL; Hueber AJ; Reilly JH; Xu Y; Fazzi UG; Murrell GA; McInnes IB Inflammation is present in early human tendinopathy. Am. J. Sports Med 2010, 38 (10), 2085–91. [DOI] [PubMed] [Google Scholar]
- (77).Millar NL; Akbar M; Campbell AL; Reilly JH; Kerr SC; McLean M; Frleta-Gilchrist M; Fazzi UG; Leach WJ; Rooney BP; Crowe LA; Murrell GA; McInnes IB IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci. Rep 2016, 6, 27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Dakin SG; Martinez FO; Yapp C; Wells G; Oppermann U; Dean BJ; Smith RD; Wheway K; Watkins B; Roche L; Carr AJ Inflammation activation and resolution in human tendon disease. Sci. Transl. Med 2015, 7 (311), 311ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).McWhorter FY; Wang T; Nguyen P; Chung T; Liu WF Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (43), 17253–17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.