Abstract
Perfluoroalkyl acids (PFAAs) are a class of organic contaminants notable for their extreme persistence. The unique chemical properties of these compounds make them difficult to remove from water using most standard water treatment techniques. To gain insight into the possibility of remediating contaminated groundwater by in situ chemical oxidation with heat-activated persulfate, PFAA removal and the generation of transformation products were evaluated under laboratory conditions. Solution pH had a strong influence on the removal of perfluorooctanoic acid (PFOA), resulting in its transformation into shorter-chain perfluorocarboxylic acids at pH values below 3. The presence of chloride and aquifer sediments decreased the efficiency of the process by less than 25% under conditions likely to be encountered in drinking water aquifers. Perfluorooctane sulfonate (PFOS) was not transformed by heat-activated persulfate under any of the conditions tested. Despite challenges related to the need to manipulate aquifer pH, the possible generation of undesirable short-chain perfluorinated carboxylic acids (PFCAs) and chlorate, and metals mobilization, heat-activated persulfate may be a useful treatment technology for sites contaminated with perfluorinated carboxylic acids and fluorotelomer-based compounds, including those used in current-generation aqueous film-forming foams.
Keywords: PFOA, PFOS, PFAS, persulfate, in situ chemical oxidation, remediation
1. INTRODUCTION
Perfluoroalkyl acids (PFAAs) are a family of organofluorine surfactants that contain a fully fluorinated carbon chain attached to an acid moiety. The high strength of the carbon-fluorine bonds enhances the stability of PFAAs, and the perfluoroalkyl chain and the polar acid group give them excellent surfactant properties.1-3 PFAAs comprise a subgroup of the poly- and perfluoroalkyl substances (PFAS) – a large and diverse class of organofluorine compounds that share in common the perfluoroalkyl functional group.4 Due to their unique chemical properties, PFAAs have been used for decades in numerous industrial and manufacturing processes.5 Among the PFAAs, perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkane sulfonic acids (PFSAs) have been produced in the greatest quantities (e.g., approximately 3200-7300 tons of PFCAs were produced globally between 1951 and 20046). The eight-carbon acids, perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), as well as polyfluorinated compounds containing a perfluorooctyl group, have been particularly important in commerce.6
Some of the same chemical properties that make PFAAs commercially useful are problematic when the compounds are released to the environment. PFSAs with six or more carbons and PFCAs with seven or more perfluorinated carbons are considered to be persistent, bioaccumulative, and toxic.7 Shorter-chain PFAAs are less bioaccumulative in animals, but appear to share certain of the persistent and toxic characteristics of their long-chain homologues. Both PFCAs and PFSAs are strong acids that exist mainly in anionic forms at environmentally relevant pH values. Their negative charge contributes to their relatively high water solubilities, which makes them mobile in the aquatic environment.2, 8, 9 Recognition of their toxicity, stability, and long-range transport potential led to a phase out of long-chain PFAA production in North America and Europe starting in 2000. Nevertheless, these compounds continue to be detected in water, soil and biota. In particular, the presence of PFAAs in drinking water supplies at concentrations ranging from approximately 1 to 1,000 ng/L has become a topic of concern.10, 11 For example, in 2016 the U.S. EPA issued a Lifetime Health Advisory of 70 ng/L for PFOA, PFOS, or the sum of PFOA and PFOS.12 A number of U.S. states including New Jersey and Minnesota have issued their own lower science-based drinking water guidelines for PFOA, PFOS, and other PFAAs.13
PFAAs enter water supplies through various pathways, including emissions from manufacturing facilities,14, 15 land application of biosolids,16 landfill leachate,17 and the use of aqueous film-forming foams (AFFF).2, 18 Proximity to PFAA point sources has been linked to increased frequency of detection of PFAAs in drinking water,10 and the concentration of PFAAs in drinking water has been correlated with serum levels of PFAAs in humans.19 Results from epidemiological studies suggest associations between serum PFAA levels and adverse health outcomes,20 including kidney and testicular cancer.
As concerns about contamination increase, there is a growing need for technologies to remediate PFAA-contaminated groundwater.21 PFAAs are resistant to biodegradation and hydroxyl radical-based advanced oxidation processes,22 but several researchers have reported that persulfate (S2O82−)-based treatments degrade PFCAs. In this process, sulfate radical (SO4•−), produced by photolysis of S2O82− by ultraviolet light 23, 24 or thermolysis,25-29 reacts with PFOA to form shorter-chain PFCAs. The proposed mechanism of PFOA decomposition involves sequential cleavage of −CF2 units, with formation of shorter-chain PFCAs and liberation of F− and CO2.22, 23, 30 A simplified reaction can be used to represent the multi-step mechanism responsible for the transformation of PFOA to perfluoroheptanoic acid (PFHpA):
| (1) |
The subsequent steps (i.e., PFHpA oxidation by SO4•− to produce perfluorohexanoic acid), follow an analogous mechanism.
Reports of PFOS degradation by persulfate are inconsistent. One research group employed S2O82− concentrations of up to 84 mM and activation temperatures up to 60°C, but observed no PFOS defluorination,27 whereas another research team reported up to 22.5% defluorination of PFOS using 18.5 mM of heat-activated S2O82−.31
The majority of studies on S2O82− treatment of PFAAs have been performed without pH control, and in the absence of inorganic solutes, organic matter, and surfaces that are typically present in the aquatic environment. Reactions between SO4•− and solutes could lower the concentration of SO4•−, which would reduce the efficiency of PFAA removal. The few studies that considered the effects of matrix components indicated that the presence of Cl− 24 and soil27 reduced the efficiency of PFOA transformation by S2O82− because added impurities scavenged of SO4•−.
To determine the potential for using heat-activated persulfate to treat groundwater contaminated with PFAAs, batch experiments were performed under controlled conditions designed to represent those encountered during in situ chemical oxidation (ISCO) treatment. The effects of pH, chloride concentration and aquifer solids were examined using PFOA and PFOS as representative PFAAs. The results of this research can be used to identify conditions under which heat-activated persulfate is likely to be an effective remedial strategy for PFAA-contaminated aquifers.
2. MATERIALS AND METHODS
2.1. Materials
A list of the full names and abbreviations for PFAAs measured in this study is provided in Table S1. Analytical standards and isotopically labeled PFAA reference standards were obtained from Wellington Laboratories. Reagent grade PFOS (40% in H2O) and PFOA (96% purity) were purchased from Sigma Aldrich. HPLC-grade water and LC-MS-grade methanol were obtained from Fisher Scientific. All other chemicals and solvents were of the highest possible purity and were purchased from Fisher Scientific or Sigma Aldrich. Solutions were prepared in ultrapure water (18 MΩ) from a Millipore system.
Uncontaminated aquifer sediment was prepared and characterized as described elsewhere.32 Characterization data for the sediment are reported in Table S2.
2.2. Persulfate Oxidation Experiments
Batch oxidation experiments were performed in sealed 15- or 50-mL polypropylene or polystyrene centrifuge tubes containing 10 or 40 mL of solution. Concentrated aqueous stocks of PFOA or PFOS were added to ultrapure water to obtain the desired nominal concentration. Most experiments were performed with an initial PFOA or PFOS concentration of 0.5 μM, which is within the range that these compounds are typically detected at contaminated sites. An initial PFOA concentration of 5 μM was used in one experiment to facilitate measurement of fluoride generation. In some experiments, small aliquots of 1.0 M NaCl were added to achieve initial chloride concentrations ranging from 1 to 500 mM. Sediment slurry experiments were performed as described above, with the addition of 20 g/L dried aquifer solids. An initial S2O82− concentration of 50 mM was achieved by addition of aliquots of 500 mM Na2S2O8.
For experiments at fixed pH values, solutions were buffered at pH 8, 6, and 3 with 50 mM borate, phosphate or H2SO4, respectively. Under the conditions studied (e.g., 85°C), persulfate decomposition occurs rapidly according to this overall reaction33:
| (2) |
For field applications of this remedial technology, lower temperatures are typically used (e.g., 40-60°C). Under these conditions, the rate at which persulfate is activated decreases, but the dominant reactions (i.e., persulfate conversion to sulfate radical and subsequent reactions of persulfate with PFCAs and other solutes) remain the same. The acid produced in reaction 2 caused a decrease in pH, requiring addition of aliquots of 1N NaOH at 30-minute intervals to maintain near constant pH conditions during the experiments. The maximum temporary decrease in pH observed during a buffered experiment was 0.4 units. As an additional measure to limit the extent of pH change in the buffered experiments, smaller aliquots of persulfate were added (i.e., 10 mM increments instead of 50 mM). In experiments without an added pH buffer, decomposition of 50 mM S2O82− decreased pH values to 1.3 when all of the S2O82− decomposed.
All experiments were performed in an 85°C water bath, as described in detail elsewhere.32 Briefly, reactors were placed in the hot-water bath after addition of the persulfate and were removed from the bath momentarily to withdraw samples at pre-determined time points. Most experiments used a 50 mM initial concentration of S2O82− and were sampled for 7.5 hours. In a subset of experiments, additional persulfate aliquots were added after the first 7.5-hour period to simulate the effect of additional treatment.
Control experiments without persulfate were performed to assess PFAA losses unrelated to S2O82− treatment, including controls in which samples were acidified to pH values of 1 or 2 with H2SO4. Most experiments were carried out in duplicate or triplicate, and the results presented represent averages plus or minus one standard deviation. Experiments examining the effect of chloride used a single reactor for each initial Cl− concentration.
All samples were diluted in a combination of HPLC-grade water and methanol prior to analysis, as described in detail elsewhere.32 Samples from sediment slurry experiments were separated by centrifugation, and the sediments were subjected to additional solvent extraction to quantify the concentration of PFAAs sorbed to aquifer solids, as described previously.32 Samples for F− analysis were stored at 4°C prior to analysis, and were typically analyzed within two days. Samples for dissolved organic carbon (DOC) determination were filtered sequentially through 0.7 μm and 0.2 μm syringe filters, diluted five times in 500 mM borate buffer, and stored at 4°C for less than eight hours prior to measurement. Samples were analyzed for persulfate and pH immediately after collection.
2.3. Analytical Methods
PFAAs were quantified by LC-MS/MS operating in the negative electrospray ionization mode, using procedures and equipment described previously.32, 34 A list of ion transitions monitored and MS parameters is provided in Table S3. Ultra-short-chain PFAAs,35 including two- and three-carbon PFCAs and PFSAs, were not included in the analytical method.
Established methods were used for determination of all other analytes.32 Persulfate was quantified by the KI colorimetric method.36 Fluoride, chloride, and chlorate were measured using a Dionex ICS-1100 ion chromatograph with a mobile phase of 0.8 mM NaHCO3 and 4.5 mM Na2CO3. Standard addition was used for quantification of fluoride. DOC was measured using a Shimadzu TOC-V CSH, and soil organic matter was quantified by loss-on-ignition.37
3. RESULTS AND DISCUSSION
3.1. Unbuffered sediment free experiments
In unbuffered water at 85°C, 99.5% of the added persulfate decomposed after 6 hr. Decomposition of 50 mM S2O82− followed pseudo-first order kinetics with a rate constant of 2.3 × 10−4 s−1 ± 0.02 × 10−4 s−1, which agreed with results from previous studies38 (Figure S1).
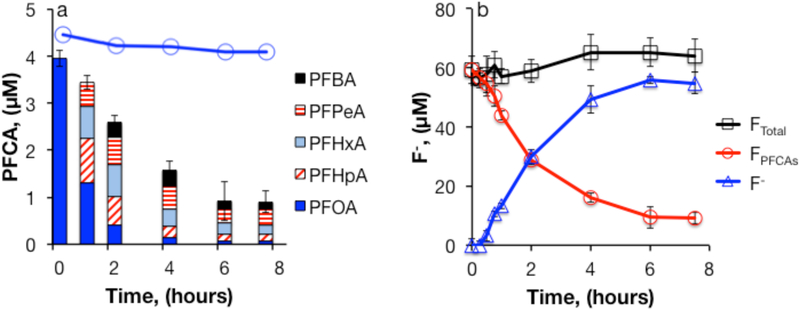
In the absence of buffer, sulfate radicals generated through S2O82− decomposition reacted with PFOA, yielding 98% loss of PFOA and production of shorter-chain PFCAs as transformation products (Figure 1a). Data were consistent with the sequential –(CF2) cleavage mechanism (reaction 1), with PFHpA reaching its maximum concentration first, followed by PFHxA, perfluoropentanoic acid (PFPeA), and perfluorobutyric acid (PFBA), as in other studies.24-27 At the end of the experiment, C4 to C7 PFCAs accounted for approximately 24% of the PFOA loss. A heated control containing PFOA without persulfate indicated that PFOA loss due to other processes (e.g., heating or sorption) was negligible. This conclusion was supported by the observed increase in F− (Figure 1b). A mass balance on fluorine was determined by summing the fluorine contained in measured PFCAs and F− (Eqns. 3 and 4).
Figure 1.
Heat-activated persulfate treatment of PFOA in unbuffered water: (a) PFCAs; (b) Fluorine mass balance. [S2O82−]0 = 50 mM, [PFOA]0 = 5 μM, T = 85°C. The line with open circles in panel (a) represents PFOA in persulfate-free controls (n=1).
| (3) |
| (4) |
The F− mass balance ranged from 95 - 110% in experiments with an initial PFOA concentration of 5 μM. Fluoride was detected in experiments conducted with 0.5 μM PFOA, but the mass balance was not reproducible due to the difficulty of accurately quantifying lower F− concentrations in a high-salt matrix.
3.2. Effect of pH
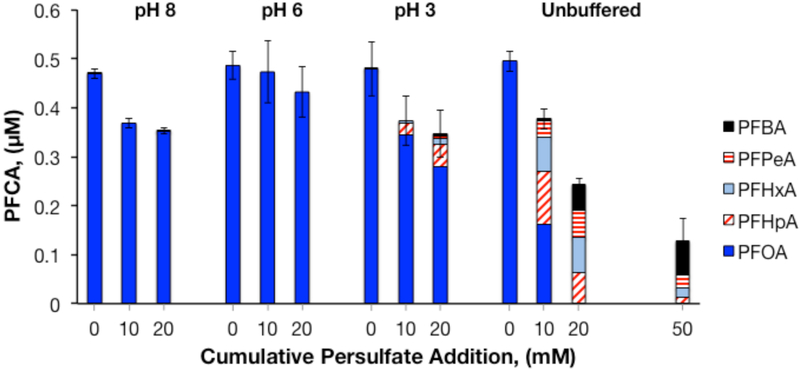
Solution pH had a strong effect on the transformation of PFOA to shorter-chain PFCAs by heat-activated persulfate (Figure 2). In experiments buffered at pH 6 and pH 8, no shorter-chain PFCAs were detected. In experiments conducted at pH 8, the small observed decrease in the concentration of PFOA may have been due to sorption losses to the reactor walls. At pH 3, treatment with 10 mM S2O82− resulted in conversion of 7% of PFOA to PFHpA. Treatment with 20 mM S2O82− at pH 3 increased PFOA conversion to 20% and resulted in formation of PFHpA, PFHxA, PFPeA, and PFBA. PFOA transformation was greatest in reactors without pH control. In these reactors, PFOA conversion was 57% and the pH was approximately 2.0 after decomposition of the first 10 mM aliquot of S2O82−, and PFOA conversion was 100% and pH was approximately 1.8 after decomposition of 20 mM S2O82−. The pH dropped to 1.3 after decomposition of 50 mM S2O82−. Experiments conducted at initial pH values of 1.0 and 2.0 resulted in even higher rates of PFOA loss (Figure S2).
Figure 2.
Effect of pH on heat-activated persulfate treatment of PFOA in ultrapure water. [PFOA]0 = 0.5 μM, T = 85°C, reactors were sampled after decomposition of S2O82−.
Previous reports of PFOA decomposition by heat-activated persulfate have indicated conflicting results with respect to the effect of pH on PFOA removal. Among these studies, two research groups did not control pH,25, 27 two adjusted the initial pH but allowed it to decrease throughout the reactions,26, 28 and one performed experiments at fixed pH values of 1, 8.2, and 13.29 Of the two groups that adjusted initial pH (but did not hold pH at a fixed value throughout the reaction) one reported that the extent of PFOA transformation increased with increasing initial pH (at initial pH values ranging from 2.5 to 9.2),26 while the other observed the opposite trend (at initial pH values ranging from 2.5 to 11).28 The study in which greater PFOA removal occurred at high pH26 employed HCl to acidify reactors that started out at pH values below 7.1. Under these conditions it is possible that scavenging of SO4•− by Cl−, as discussed below, explains the observed trend. In the study in which fixed pH conditions were employed, loss of PFOA and production of shorter-chain PFCAs was observed at pH 1, but not at pH 8.2 or 13.29 The same study also indicated that PFOA transformation to shorter-chain PFCAs did not occur until sequential addition of S2O82− caused the pH to drop to approximately 3.
Possible explanations for the observed relationship between PFOA removal and pH include: 1) a reduction in reaction efficiency as SO4•− is converted into HO• by reaction with OH−; 2) increased S2O82− decomposition kinetics at low pH; 3) an increase in reaction efficiency due to increasing concentrations of protonated PFOA or a related intermediate at low pH; and, 4) an increase in reaction efficiency due to protonation of SO4•−.
At high pH values, SO4•− reacts with OH− to form HO• (Eqn. 5)39
| (5) |
Under sufficiently alkaline conditions, the conversion from SO4•− to HO• is nearly instantaneous.34 HO• does not react at an appreciable rate with PFOA,40 so conversion of SO4•− to HO• at higher pH could explain the lack of PFOA loss observed at increased pH. The relative importance of the reaction in Eqn. 5 can be approximated by accounting for all of the known sinks for SO4•−: 41
| (6)24 |
where fPFOA is the fraction of SO4•− reacting with PFOA, kPFOA is the second order rate constant for the reaction of SO4•− and PFOA, and k5, k6, and k7 are the second order reaction rate constants for the reactions in Eqns. 5 – 7, respectively.
| (7)24 |
| (8)24 |
| (9) |
Calculation of fPFOA as a function of pH indicates that the estimated fraction of SO4•− reacting with PFOA remains constant at low-to-circumneutral pH and only begins to decrease when pH increases above 8 (Figure S3). On the basis of this analysis, conversion of SO4•− to HO• does not explain the observed pH dependence.
Another potential explanation for the pH dependence of PFOA transformation is that the decomposition rate of S2O82− increases as pH decreases. The kinetics of S2O82− thermolysis in deionized water conform to the following rate law:
| (10) |
where kN, kA, and kB are equal to 0.81 h−1, 13 h−1, and 0.27 h−1, at T = 85°C, respectively.38
The rate of persulfate decay exhibits little pH-dependence between pH 2 and 8. Below pH 2, the contribution of acid catalysis becomes noticeable.29 The 2% increase of overall S2O82− decomposition rate that is predicted as pH decreases from 8 to 3 is unlikely to account for the significant variation in PFOA transformation observed.
Another potential explanation for the increased PFOA loss observed at low pH values is that a protonated species that has a pKa value below 3 is involved in the transformation reaction. For instance, protonated PFOA may react more readily with SO4•− than its anionic form because of reduced electrostatic repulsion. The pKa of PFOA is uncertain, with estimates ranging from −0.542 to 3.8.43 If the protonated form is the only species reacting at a significant rate, the observed rate of reaction between SO4•− and PFOA would increase by a factor of 10 for every unit decrease in pH as the pH approaches the pKa. Evidence for the protonation of PFOA at low pH was observed in persulfate-free control reactors conducted at different pH values. PFOA loss in controls increased as initial pH decreased from 3 to 1, presumably due to volatilization of protonated PFOA at the elevated temperatures employed.29
A related explanation is that SO4•− was protonated at low pH, or that an acid-catalyzed reaction was involved in the transformation of PFOA. The pKa of SO4•− has not been reported,39 but it is expected to be less than 2.44 It has been hypothesized that the neutral H+SO4•− ion pair could have a higher rate constant with anions than the SO4•− anion alone.45 The uncertainty of the pKa values for PFOA and SO4•− make it difficult to differentiate between these two possible explanations. Additional research is needed to determine which of the three most likely explanations (i.e., protonation of PFOA, acid catalysis or protonation of SO4•−) explain the observed effect of pH on PFOA transformation.
A parallel set of experiments was conducted using PFOS instead of PFOA. No loss of PFOS was observed in experiments conducted with initial S2O82− concentrations as high as 50 mM (Figure S4). This observation agrees with data reported in a recent study27 but is at odds with the results of a study in which PFOS was transformed by heat-activated persulfate to produce shorter-chain PFCAs.31 It is difficult to reconcile the results of the study in which PFOS degradation was reported for several reasons. First, the temperature at which persulfate activation was performed was not reported. Second, the researchers also reported loss of 1.45% of the PFOS by defluorination after 12 hours of treatment with S2O82− at room temperature—an unexpected finding given the slow rate of persulfate thermolysis under these conditions. Finally, the measurements of PFOS and other PFAAs were made without internal standards, which makes it difficult to determine if instrument sensitivity was affected by changes in the matrix that occurred as the persulfate decomposed.
3.3. Effect of Chloride
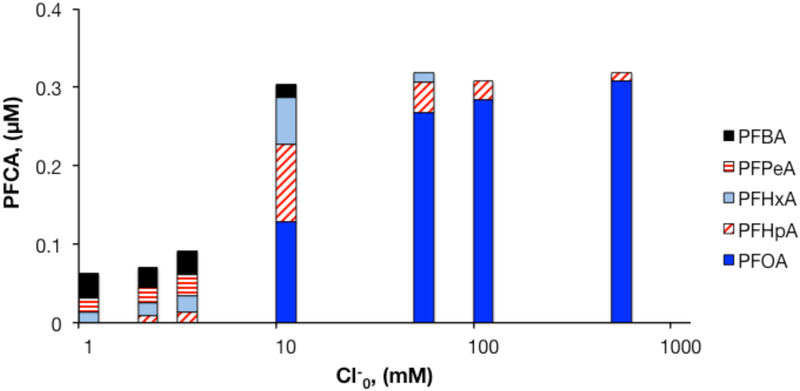
The presence of chloride slowed the transformation of PFOA by heat-activated persulfate under acidic conditions (Figure 3). In experiments with 50 mM S2O82− and varying initial concentrations of Cl− from concentrations typical of freshwater (i.e., 1 mM) up to conditions encountered in saline groundwater (i.e., 700 mM), persulfate disappeared at the same rate as observed in chloride-free experiments. Furthermore, the pH change observed in these experiments was comparable to that observed in the chloride-free system, with final pH values ranging from 1.4 to 1.7.
Figure 3.
Effect of initial Cl− concentration on PFCA concentrations after 7.5 h of heat-activated persulfate treatment of PFOA in water. [S2O82−]0 = 50 mM, [PFOA]0 = 0.5 μM, unbuffered, T = 85°C.
At Cl− concentrations ranging from 1 to 3 mM, PFOA was completely removed. Detectable transformation products (i.e., shorter-chain PFCAs) accounted for 13 to 19% of initial PFOA, indicating a similar degree of mineralization to results obtained under Cl−-free conditions. PFOA removal decreased at initial Cl− concentrations above 10 mM, with PFOA transformation accounting for just 6% and 3% of initial PFOA at initial Cl− concentrations of 100 and 500 mM, respectively. Pseudo-first order decay constants for PFOA were calculated from data collected during the first hour of the experiment. A plot of initial Cl− concentration versus the observed rate constants for PFOA loss demonstrates an inverse relationship between the amount of chloride present in solution and PFOA removal rates (Figure S5). These results indicate that the presence of Cl− decreases the efficiency of PFOA treatment, likely due to scavenging of sulfate radicals by Cl−. The relationship observed between initial Cl− concentration and extent of PFOA removal is consistent with an analysis of the relevant reactions. SO4•− reacts with Cl− at rates that are approximately three orders of magnitude higher than with PFOA:
| (11)24 |
The fate of Cl• is pH-dependent.46 At pH ≥ 5, Cl• is converted to HO• with regeneration of Cl−. At pH < 5, Cl• is oxidized to ClO3− through sequential reactions with Cl−. During this process, some of the Cl• or other intermediates may react with other contaminants if they are present at high concentrations. Under the acidic pH conditions that occur in the absence of buffer, chloride outcompeted PFOA for SO4•− and PFOA could not be transformed until all of the chloride had been converted to ClO3−. This finding is consistent with a study in which PFOA transformation occurred only after complete conversion of Cl− to ClO3− when SO4•− was produced by photolysis of persulfate.24 Under typical conditions encountered in groundwater47 (i.e., 1 mM Cl−), scavenging of SO4•− by Cl− is expected to have a negligible effect on the efficiency of treatment due to the high concentration of S2O82− used for in situ chemical oxidation. However, in brackish groundwater or groundwater contaminated with chloride, S2O82− consumption by chloride could increase the cost of PFCA remediation. In either case, precautions should be taken to monitor ClO3− concentrations, because ClO3− is associated with toxicity to the thyroid and other organs. Heath-activated persulfate treatment of typical groundwater containing a 1 mM concentration of Cl− could generate ClO3− concentrations well in excess of the World Health Organization’s 700 μg/L provisional guideline.48
3.4. Effect of Aquifer Sediments
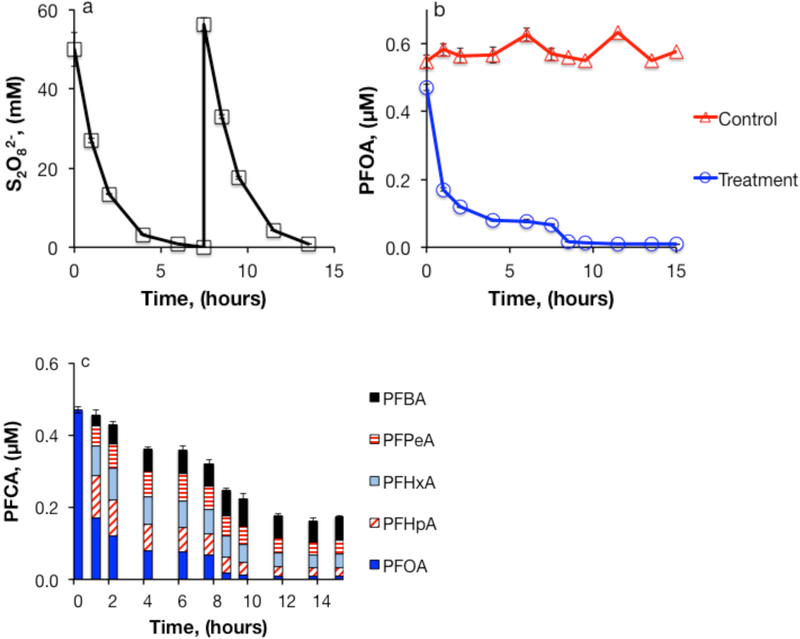
Persulfate decomposition in the presence of aquifer sediments was approximately 17% slower than the rate observed in the sediment free systems (Figure S1). The slight decrease in S2O82− decomposition rate may have been attributable to the higher starting pH in the slurry experiments caused by buffering by the sediments. Similar to the experiments in unbuffered water, 99.5% of the added persulfate was decomposed after 6 hours in the presence of sediments. Acid production from decomposition of the first aliquot of persulfate caused the pH to drop from 5.4 to 1.4 during the first 7.5 hours of the experiment. The second 50 mM aliquot of persulfate did not result in further pH decrease.
The presence of aquifer sediments decreased the efficiency of heat-activated S2O82− treatment of PFOA (Figure 4a-c). In reactors containing aquifer sediments, treatment with 50 mM S2O82− resulted in loss of 86% of the PFOA, approximately 60% of which could be accounted for by C4-C7 homologues. Treatment with a second 50 mM aliquot of S2O82− increased overall PFOA loss to 98%. At the end of the experiment, 34% of total PFOA loss was accounted for by shorter-chain PFCAs, indicating partial mineralization of PFOA. Methanol extraction of the sediments resulted in recovery of only 3 to 10% of the total PFCAs, so sorption to sediments was not a major mechanism of PFOA loss under these conditions. Because the rate of S2O82− loss and change in pH during the experiment were similar to those observed in the absence of sediments, the decrease in PFCA treatment efficiency observed in the slurry reactors was likely due to scavenging of SO4•− by inorganic anions (e.g., Cl−, metals), organic matter, or surfaces. This explanation is supported by measurements of Cl− and TOC concentrations, with concentrations decreasing rapidly at the beginning of the experiment (Figures S6 and S7). Cl− was converted into ClO3− within two hours (Figure S6). Another slurry experiment conducted in surficial soil with a higher organic matter content (0.96%) than the aquifer sediment resulted in an even greater loss of PFOA treatment efficiency (Figure S8).
Figure 4.
Heat-activated persulfate treatment of PFOA in aquifer sediment slurry. (a) persulfate, (b) PFOA loss compared to persulfate-free control, and (c) PFCAs. 200 g/L aquifer sediments, [S2O82−]0 = 50 mM × 2, [PFOA]0 = 0.5 μM, unbuffered, T = 85°C.
3.5. Opportunities and Limitations for in situ Remediation Treatment of PFAAs
In situ chemical oxidation by thermal activation of persulfate could prove useful at sites where PFCAs are the only type of PFAS present. It could also be useful at sites where fluorotelomer-based AFFF was used because PFASs in these formulations are readily converted into PFCAs by hydroxyl radical18 or sulfate radical.29 When PFCAs and PFSAs are present as co-contaminants, heat-activated persulfate could be employed in a treatment-train approach to reduce the contaminant mass in source zones as a complement to pump-and-treat remediation.
Heat-activated persulfate treatment could also be useful for mobilizing sorbed cationic and zwitterionic polyfluorinated compounds. Positively charged PFAS species were major components of several AFFF formulations.18 These cationic and zwitterionic species are suspected to comprise an important portion of total PFAS in contaminant source zones at AFFF-impacted sites because of their affinity for surfaces.49 If ISCO treatment results in oxidation of sorbed PFAS species, PFAAs would be released into the dissolved phase. Alternatively, persulfate ISCO could mobilize sorbed PFAA-precursors through cation exchange. Low-pH persulfate treatment will increase the concentration of H+ and polyvalent cations, such as Ca2+, in groundwater, both of which could displace cationic or zwitterionic polyfluorinated compounds from sorption sites. After entering the dissolved phase, these PFAS would react with oxidants or could be removed by pump-and-treat systems. Few studies to date have investigated the affect of ISCO treatment on subsurface mobility of PFASs. One research group observed decreased PFAA transport in column studies with persulfate treatment at room temperature,50 but further research is needed to assess the impact of persulfate treatment on transport of polyfluorinated compounds under heat-activation conditions.
Site geochemistry is an important factor affecting the efficacy of heat-activated persulfate for in situ treatment for PFAAs and other PFAS. For instance, scavenging of SO4•− by chloride or organic matter will reduce the efficacy of the treatment. Also, the high buffering capacity of carbonate-rich aquifers will make it difficult to lower groundwater pH to values necessary for successful treatment. Conversely, returning the pH of groundwater to circumneutral after pH persulfate treatment at low pH will be more difficult in poorly buffered aquifers. In such cases, permeable reactive barriers containing material capable of neutralizing acidity (e.g., CaCO3(s)) could be installed downstream of the ISCO treatment zone to neutralize acidity. Irrespective of the means of neutralization, most cationic metals mobilized under acidic conditions should precipitate or adsorb after acidity is neutralized.
Another limitation of this technology is the potential for production of hazardous byproducts. Unless persulfate treatment results in complete mineralization of PFAAs, the treatment process will generate short-chain PFCAs. This is undesirable because these compounds are generally more mobile in the subsurface51 and less easily removed by sorptive technologies52 than long-chain PFCAs. Another potentially hazardous byproduct of oxidative PFAA treatment is HF. HF is a weak acid (pKa of 3.2). If heat-activated persulfate is employed to treat PFCAs at low pH, a substantial portion of the F− generated will be present in the protonated form. This is unlikely to pose substantial risks if AFFF in the source zone has been diluted, but the potential for exposure of workers to HF should be considered prior to initiating heat-activated persulfate treatment of PFAAs, especially if the source zone contains very high concentrations of PFASs. As mentioned previously, heat-activated persulfate also may generate concentrations of ClO3− at concentrations that could pose health risks to consumers of groundwater.
4. CONCLUSIONS
Under acidic conditions (pH ≤ 3), heat-activated persulfate treatment resulted in transformation of PFOA into shorter-chain PFCAs, some of which were eventually mineralized. The presence of both Cl− and aquifer solids decreased the efficiency of PFOA treatment. Persulfate did not transform PFOS. Despite these limitations, the lack of other proven treatment options suggests that further investigation of heat-activated persulfate as an in situ treatment for PFCAs is warranted.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the U.S. National Institute for Environmental Health Sciences (NIEHS) Superfund Research Program (Grant P42 ES004705) and the Superfund Research Center at University of California, Berkeley. Additional support came from the Strategic Environmental Research and Development Program (SERDP ER2128). We thank our colleagues Erika Houtz, Shan Yi, and Katie Harding for insightful discussions and help in the laboratory. We also thank Dr. Christopher Higgins for helpful comments during the preparation of this manuscript.
References
- 1.Kissa E, Fluorinated surfactants: synthesis, properties, applications. M. Dekker; New York: 1994. [Google Scholar]
- 2.Moody CA; Field JA, Perfluorinated Surfactants and the Environmental Implications of Their Use in Fire-Fighting Foams. Environmental Science & Technology 2000, 34 (18), 3864–3870. [Google Scholar]
- 3.Key BD; Howell RD; Criddle CS, Fluorinated organics in the biosphere. Environmental Science & Technology 1997, 31 (9), 2445–2454. [Google Scholar]
- 4.Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; de Voogt P; Jensen AA; Kannan K; Mabury SA; van Leeuwen SP, Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integrated environmental assessment and management 2011, 7 (4), 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kissa E, Fluorinated surfactants and repellents. CRC Press: 2001. [Google Scholar]
- 6.Prevedouros K; Cousins IT; Buck RC; Korzeniowski SH, Sources, fate and transport of perfluorocarboxylates. Environmental Science & Technology 2006, 40 (1), 32–44. [DOI] [PubMed] [Google Scholar]
- 7.Scheringer M; Trier X; Cousins IT; de Voogt P; Fletcher T; Wang Z; Webster TF, Helsingør Statement on poly-and perfluorinated alkyl substances (PFASs). Chemosphere 2014, 114, 337–339. [DOI] [PubMed] [Google Scholar]
- 8.Shinoda K; Hato M; Hayashi T, Physicochemical properties of aqueous solutions of fluorinated surfactants. The Journal of Physical Chemistry 1972, 76 (6), 909–914. [Google Scholar]
- 9.Giesy JP; Kannan K, Peer reviewed: perfluorochemical surfactants in the environment. Environmental science & technology 2002, 36 (7), 146A–152A. [DOI] [PubMed] [Google Scholar]
- 10.Hu XC; Andrews DQ; Lindstrom AB; Bruton TA; Schaider LA; Grandjean P; Lohmann R; Carignan CC; Blum A; Balan SA, Detection of Poly- and Perfluoroalkyl Substances (PFASs) in US Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environmental Science & Technology Letters 2016, 3(10) 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao F; Simcik MF; Halbach TR; Gulliver JS, Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a US metropolitan area: Migration and implications for human exposure. Water Research 2015, 72, 64–74. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Environmental Protection Agency. Drinking Water Health Advisories for PFOA and PFOS. 2016. https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos . Accessed 19 Apr 2017.
- 13.Post GB; Gleason JA.; Cooper KR, Key scientific issues in developing drinking water guidelines for perfluoroalkyl acids: Contaminants of emerging concern. PLoS Biol 2017, 15 (12), e2002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis KL; Aucoin MD; Larsen BS; Kaiser MA; Hartten AS, Transport of ammonium perfluorooctanoate in environmental media near a fluoropolymer manufacturing facility. Chemosphere 2007, 67 (10), 2011–2019. [DOI] [PubMed] [Google Scholar]
- 15.Hansen K-J; Johnson H; Eldridge J; Butenhoff J; Dick L, Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environmental Science & Technology 2002, 36 (8), 1681–1685. [DOI] [PubMed] [Google Scholar]
- 16.Lindstrom AB; Strynar MJ; Delinsky AD; Nakayama SF; McMillan L; Libelo EL; Neill M; Thomas L, Application of WWTP biosolids and resulting perfluorinated compound contamination of surface and well water in Decatur, Alabama, USA. Environmental science & technology 2011, 45 (19), 8015–8021. [DOI] [PubMed] [Google Scholar]
- 17.Huset CA; Barlaz MA; Barofsky DF; Field JA, Quantitative determination of fluorochemicals in municipal landfill leachates. Chemosphere 2011, 82 (10), 1380–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houtz EF; Higgins CP; Field JA; Sedlak DL, Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environmental science & technology 2013, 47 (15), 8187–8195. [DOI] [PubMed] [Google Scholar]
- 19.Hurley S; Houtz EF; Goldberg D; Wang M; Park J; Nelson DO; Reynolds P; Bernstein L; Anton-Culver H; Horn-Ross P, Preliminary Associations between the Detection of Perfluoroalkyl Acids (PFAAs) in Drinking Water and Serum Concentrations in a Sample of California Women. Environmental Science & Technology Letters 2016. [Google Scholar]
- 20.C8 Science Panel: C8 Probable Link Reports. http://www.c8sciencepanel.org/prob_link.html (accessed Oct. 3).
- 21.Kucharzyk KH; Darlington R; Benotti M; Deeb R; Hawley E, Novel treatment technologies for PFAS compounds: A critical review. Journal of Environmental Management 2017, 204, 757–764. [DOI] [PubMed] [Google Scholar]
- 22.Vecitis C; Park H; Cheng J; Mader B; Hoffmann M, Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Frontiers of Environmental Science & Engineering in China 2009, 3 (2), 129–151. [Google Scholar]
- 23.Hori H; Yamamoto A; Hayakawa E; Taniyasu S; Yamashita N; Kutsuna S; Kiatagawa H; Arakawa R, Efficient Decomposition of Environmentally Persistent Perfluorocarboxylic Acids by Use of Persulfate as a Photochemical Oxidant. Environmental Science & Technology 2005, 39 (7), 2383–2388. [DOI] [PubMed] [Google Scholar]
- 24.Qian Y; Guo X; Zhang Y; Peng Y; Sun P; Huang C-H; Niu J; Zhou X; Crittenden JC, Perfluorooctanoic Acid Degradation Using UV–Persulfate Process: Modeling of the Degradation and Chlorate Formation. Environmental science & technology 2015, 50 (2), 772–781. [DOI] [PubMed] [Google Scholar]
- 25.Hori H; Nagaoka Y; Murayama M; Kutsuna S, Efficient decomposition of perfluorocarboxylic acids and alternative fluorochemical surfactants in hot water. Environmental Science & Technology 2008, 42 (19), 7438–7443. [DOI] [PubMed] [Google Scholar]
- 26.Liu CS; Higgins CP; Wang F; Shih K, Effect of temperature on oxidative transformation of perfluorooctanoic acid (PFOA) by persulfate activation in water. Separation and Purification Technology 2012, 91, 46–51. [Google Scholar]
- 27.Park S; Lee LS; Medina VF; Zull A; Waisner S, Heat-activated persulfate oxidation of PFOA, 6: 2 fluorotelomer sulfonate, and PFOS under conditions suitable for in-situ groundwater remediation. Chemosphere 2016, 145, 376–383. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y-C; Lo S-L; Kuo J; Lin Y-L, Persulfate oxidation of perfluorooctanoic acid under the temperatures of 20–40 C. Chemical engineering journal 2012, 198, 27–32. [Google Scholar]
- 29.Sun B; Ma J; Sedlak DL, Chemisorption of Perfluorooctanoic Acid on Powdered Activated Carbon Initiated by Persulfate in Aqueous Solution. Environmental Science & Technology 2016, 50 (14), 7618–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hori H; Hayakawa E; Einaga H; Kutsuna S; Koike K; Ibusuki T; Kiatagawa H; Arakawa R, Decomposition of environmentally persistent perfluorooctanoic acid in water by photochemical approaches. Environmental science & technology 2004, 38 (22), 6118–6124. [DOI] [PubMed] [Google Scholar]
- 31.Yang S; Cheng J; Sun J; Hu Y; Liang X, Defluorination of aqueous perfluorooctanesulfonate by activated persulfate oxidation. PloS one 2013, 8 (10), e74877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruton TA; Sedlak DL, Treatment of aqueous film-forming foam by heat-activated persulfate under conditions representative of in situ chemical oxidation. Environmental Science & Technology 2017, 51 (23), 13878–13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H; Bruton TA; Doyle FM; Sedlak DL, In Situ Chemical Oxidation of Contaminated Groundwater by Persulfate: Decomposition by Fe(III)- and Mn(IV)-Containing Oxides and Aquifer Materials. Environmental Science & Technology 2014, 48 (17), 10330–10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houtz EF; Sedlak DL, Oxidative conversion as a means of detecting precursors to perfluoroalkyl acids in urban runoff. Environmental science & technology 2012, 46 (17), 9342–9349. [DOI] [PubMed] [Google Scholar]
- 35.Barzen-Hanson KA; Field JA, Discovery and Implications of C2 and C3 Perfluoroalkyl Sulfonates in Aqueous Film-Forming Foams and Groundwater. Environmental Science & Technology Letters 2015, 2 (4), 95–99. [Google Scholar]
- 36.Liang CJ; Huang CF; Mohanty N; Kurakalva RM, A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73 (9), 1540–1543. [DOI] [PubMed] [Google Scholar]
- 37.Schulte E; Kaufmann C; Peter J, The influence of sample size and heating time on soil weight loss–on–ignition. Communications in Soil Science & Plant Analysis 1991, 22 (1–2), 159–168. [Google Scholar]
- 38.Johnson RL; Tratnyek PG; Johnson RO, Persulfate Persistence under Thermal Activation Conditions. Environmental Science & Technology 2008, 42 (24), 9350–9356. [DOI] [PubMed] [Google Scholar]
- 39.Neta P; Huie RE; Ross AB, Rate constants for reactions of inorganic radicals in aqueous solution. American Chemical Society: 1988. [Google Scholar]
- 40.Plumlee MH; McNeill K; Reinhard M, Indirect photolysis of perfluorochemicals: hydroxyl radical-initiated oxidation of N-ethyl perfluorooctane sulfonamido acetate (N-EtFOSAA) and other perfluoroalkanesulfonamides. Environmental science & technology 2009, 43 (10), 3662–3668. [DOI] [PubMed] [Google Scholar]
- 41.Schwarzenbach RP; Gschwend PM; Imboden DM, Environmental organic chemistry. John Wiley & Sons: 2005. [Google Scholar]
- 42.Goss K-U, The p K a values of PFOA and other highly fluorinated carboxylic acids. Environmental science & technology 2007, 42 (2), 456–458. [DOI] [PubMed] [Google Scholar]
- 43.Burns DC; Ellis DA; Li H; McMurdo CJ; Webster E, Experimental p K a determination for perfluorooctanoic acid (PFOA) and the potential impact of p K a concentration dependence on laboratory-measured partitioning phenomena and environmental modeling. Environmental science & technology 2008, 42 (24), 9283–9288. [DOI] [PubMed] [Google Scholar]
- 44.Jiang P-Y; Katsumura Y; Nagaishi R; Domae M; Ishikawa K; Ishigure K; Yoshida Y, Pulse radiolysis study of concentrated sulfuric acid solutions. Formation mechanism, yield and reactivity of sulfate radicals. Journal of the Chemical Society, Faraday Transactions 1992, 88 (12), 1653–1658. [Google Scholar]
- 45.Bao Z-C; Barker JR, Temperature and ionic strength effects on some reactions involving sulfate radical [SO4-(aq)]. The Journal of Physical Chemistry 1996, 100 (23), 9780–9787. [Google Scholar]
- 46.Lutze HV; Kerlin N; Schmidt TC, Sulfate radical-based water treatment in presence of chloride: Formation of chlorate, inter-conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate. Water research 2015, 72, 349–360. [DOI] [PubMed] [Google Scholar]
- 47.Snoeyink VL; Jenkins D, Water chemistry. Wiley: 1980. [Google Scholar]
- 48.World Health Organization. Chlorite and Chlorate in Drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality. 2005. [Google Scholar]
- 49.Backe WJ; Day TC; Field JA, Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from US military bases by nonaqueous large-volume injection HPLC-MS/MS. Environmental science & technology 2013, 47 (10), 5226–5234. [DOI] [PubMed] [Google Scholar]
- 50.McKenzie ER; Siegrist RL; McCray JE; Higgins CP, Effects of chemical oxidants on perfluoroalkyl acid transport in one-dimensional porous media columns. Environmental science & technology 2015, 49 (3), 1681–1689. [DOI] [PubMed] [Google Scholar]
- 51.Guelfo JL; Higgins CP, Subsurface transport potential of perfluoroalkyl acids at aqueous film-forming foam (AFFF)-impacted sites. Environmental science & technology 2013, 47 (9), 4164–4171. [DOI] [PubMed] [Google Scholar]
- 52.Appleman TD; Higgins CP; Quinones O; Vanderford BJ; Kolstad C; Zeigler-Holady JC; Dickenson ER, Treatment of poly-and perfluoroalkyl substances in US full-scale water treatment systems. Water research 2014, 51, 246–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.