Abstract
Objective
To assess the benefits and harms of pregabalin in the management of neuropathic pain.
Design
Rapid review and meta-analysis of phase III, randomised, placebo-controlled trials.
Participants
Adults aged 18 years and above with neuropathic pain defined according to the International Association for the Study of Pain criteria.
Interventions
Pregabalin or placebo.
Primary and secondary outcome measures
Our primary outcomes were pain (as measured using validated scales) and adverse events. Our secondary outcomes were sleep disturbance, quality of life, Patient Global Impression of Change, Clinician Global Impression scale, anxiety and depression scores, overall discontinuations and discontinuations because of adverse events.
Results
We included 28 trials comprising 6087 participants. The neuropathic pain conditions studied were diabetic peripheral neuropathy, postherpetic neuralgia, herpes zoster, sciatica (radicular pain), poststroke pain and spinal cord injury-related pain. Patients who took pregabalin reported significant reductions in pain (numerical rating scale (NRS)) compared with placebo (standardised mean difference (SMD) −0.49 (95% CI −0.66 to −0.32, p<0.00001), very low quality evidence). Pregabalin significantly reduced sleep interference scores (NRS) compared with placebo (SMD −0.38 (95% CI −0.50 to −0.26, p<0.00001), moderate quality evidence. Pregabalin significantly increased the risk of adverse events compared with placebo (RR 1.33 (95% CI 1.23 to 1.44, p<0.00001, low quality evidence)). The risks of experiencing weight gain, somnolence, dizziness, peripheral oedema, fatigue, visual disturbances, ataxia, non-peripheral oedema, vertigo and euphoria were significantly increased with pregabalin. Pregabalin was significantly more likely than placebo to lead to discontinuation of the drug because of adverse events (RR 1.91 (95% CI 1.54 to 2.37, p<0.00001), low quality evidence).
Conclusion
Pregabalin has beneficial effects on some symptoms of neuropathic pain. However, its use significantly increases the risk of a number of adverse events and discontinuation due to adverse events. The quality of the evidence from journal publications is low.
Keywords: Pregabalin, benefits, harms, systematic review, meta-analysis
Strengths and limitations of this study.
We used the Cochrane criteria to assess the risk of bias.
This is the first review that rates the quality of the evidence for each outcome assessed.
The review may be prone to sampling bias, and we may have missed potentially eligible studies.
We did not assess the extent to which different doses of pregabalin influenced the outcomes.
Introduction
Pregabalin is a gabapentinoid licenced for treatment of neurological disorders. It is one of the earlier drugs approved by the US Food and Drug Administration (2004) for the treatment of painful diabetic neuropathy and postherpetic neuralgia (PHN).1 Pregabalin is thought to exert its analgesic action through antagonistic activity at the voltage gated Ca2+ channels where it binds to the alpha-2-delta subunit.1 2
Prescriptions of pregabalin (and gabapentin) have markedly increased over the last few years. In the USA, prescriptions for pregabalin rose from 39 million in 2012 to 64 million in 2016 (annual prescription costs increased from approximately $2 billion to $4.4 billion over the same period). 3 In the UK, pregabalin use increased 350% over a 5-year period between 2008 and 2013.4 In England alone, there were over 6.2 million prescriptions of pregabalin across GP practices in 2017 costing about $440 million.5
Pregabalin is recommended as first-line pharmacological agent for management of neuropathic pain.6 There is, however, some evidence of increased mortality attributed to pregabalin in the UK,7 and this has led some authors to caution clinicians about the risk of harms when prescribing.8 The risks are thought to be particularly acute for patients who use heroin and those who misuse gabapentinoids. Indeed, the UK government is soon to classify the drug as a class C controlled substance because of its abuse potential and increased reports of deaths attributed to its use.9 Practising clinicians have also recently called for the evidence for the effectiveness of pregabalin to be re-examined in the light of its potential to cause harms.3 4
Rapid reviews use accelerated methods to identify and synthesise the evidence from the literature in order to meet the needs of target audiences including policy makers, healthcare professionals and patient groups.10 The objective of this rapid review was therefore to evaluate the evidence for benefits and harms of pregabalin in the treatment of neuropathic pain in adults, using evidence from published randomised clinical trials (RCTs).
Methods
We conducted electronic searches in the following databases: MEDLINE, Embase and Cochrane Central Register of Controlled Trials (CENTRAL). We searched each database from inception until January 2018. No language restrictions were imposed (see online supplementary appendix 1 for a full search strategy). We also hand searched the bibliography of eligible studies (see online supplementary appendix 2 for the full protocol).
bmjopen-2018-023600supp001.pdf (89.7KB, pdf)
bmjopen-2018-023600supp002.pdf (234.8KB, pdf)
We included phase III, double-blinded, placebo-controlled RCTs (efficacy studies) assessing the effects of pregabalin on neuropathic pain in adults aged 18 years and above. We included studies based on the definition of the International Association for the Study of Pain definition.11 These included trials on diabetic neuropathy, HIV-related neuropathy, lumbar radiculopathy, PHN and chronic postsurgical pain. We included RCTs irrespective of study size and duration of intervention. If we included RCTs with a cross-over design, we used data from the first phase of the study. We excluded phase IV trials because they are typically unblinded. We also excluded studies that combined pregabalin with other types of pain intervention because the effects of such interventions would not be exclusively due to the actions of pregabalin; however, cointerventions used as rescue medication were allowed. Trials that randomised participants based on response to pregabalin therapy in the run-in phase were also excluded. Our main outcomes were pain (as measured using validated scales because such scales enhance the credibility of the measured outcomes12) and adverse events. Our secondary outcomes were sleep disturbance, quality of life (QOL), Patient Global Impression of Change (PGIC), Clinician Global Impression (CGI) scale, anxiety and depression, overall discontinuations and discontinuations because of adverse events.
The risk of bias for each included study was rated using the Cochrane criteria.13 Two reviewers (IJO and ETT) independently screened abstracts and determined study eligibility. Disagreements were resolved through discussion. Three reviewers (IJO (8 studies), ETT (8 studies) and JL (10 studies)) independently extracted data according to predefined criteria into customised Excel spreadsheets. The extracted data were independently verified by two reviewers (ETT and IJO). Any disagreements were resolved through discussion. For each included study, we extracted data on study ID, settings, populations, interventions, outcomes and results.
Using the random effects model (Mantel-Haenszel) of the standard meta-analysis software (RevMan V.5.3),14 we computed standardised mean differences (SMDs) and 95% CIs for continuous outcomes and risk ratios with 95% CI for binary outcomes. We used preintervention to postintervention changes to assess intervention effects between pregabalin and placebo. Where studies reported data on change from baseline but did not report SD, we imputed SDs (five studies) based on the SD of other studies included in the meta-analysis.15 We used a value of p=0.05 as our threshold for statistical significance. We assessed heterogeneity using the I2 statistic: values of 25%, 50% and 75% judged mild, moderate and substantial heterogeneity, respectively. We investigated heterogeneity using subgroup (based on central or peripheral neuropathic pain) and sensitivity (based on study quality and/or duration) analyses. We used a funnel plot to assess publication bias.
One reviewer (ETT) entered the data on benefits on RevMan, and these were independently verified by a second reviewer (IJO). One reviewer (IJO) entered the data on harms onto RevMan, and these were independently verified by a second reviewer (ETT). Using the GRADEpro software (V.3.6),16 we rated the overall quality of the body of evidence for each outcome using the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE)17 criteria, which examines the following domains: study design, risk of bias, inconsistency, indirectness and imprecision.
Patient public involvement
Because this was a rapid review, we did not enlist the services of patient representatives in this research.
Results
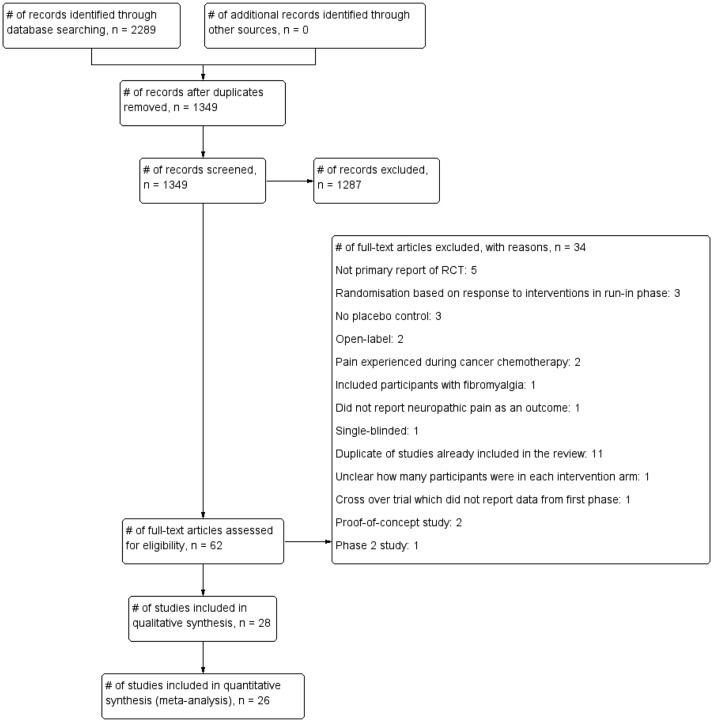
Our searches identified 1349 non-duplicate citations, out of which 62 articles were considered eligible (figure 1). We excluded 34 articles that did not fit our inclusion criteria (see online supplementary appendix 3 for list of excluded studies and the reasons for exclusion). In total, we included 28 studies18–45 comprising 6087 participants (table 1). The intervention duration was between 3 weeks and 20 weeks (median 8 weeks), and all the trials were industry funded.
Figure 1.
Flow chart showing the process for inclusion of RCTs assessing the effects of pregabalin in the management of neuropathic pain. RCTs, randomised clinical trials.
Table 1.
Main characteristics of RCTs assessing the effects of PGB in the management of central and peripheral neuropathic pain
| Study ID | Design | Sample size | Duration | Setting | Population | Duration of neuropathic pain | Outcome measures | Interventions | ||
| PGB | PLA | Cointerventions | ||||||||
| Arezzo et al 18 | Parallel group | PGB 82; PLA 85. | 13 weeks | 23 centres; USA. | Men or women with T1DM or T2DM. | ≥3 months | Primary: mean pain score (MPS); proportion of responders; adverse events ≥3%; secondary: sleep interference (11 point NRS), Present Pain Intensity (PPI) index; SF-MPQ VAS; CGIC; PGIC. | 600 mg/day fixed. | Not described. | Aspirin (up to 325 mg/day for cardiac and stroke prophylaxis), acetaminophen (up to 4 g/day), SSRIs, and benzodiazepines such as lorazepam (dosed at bedtime with stable (>30 days) regimen for sleep problems) were allowed. |
| Cardenas et al 19 | Parallel group | PGB 112; PLA 108. | 16 weeks | 60 centres; Chile, China, Columbia, Czech Republic, Hong Kong, India, Japan, Philippines, Russia and USA. | Patients aged ≥18 years with C2-T12 complete/incomplete SCI. | ≥12 months | Primary: duration-adjusted average change in pain (DAAC); secondary: change in MPS (from baseline to endpoint); percentage of patients with ≥30% reduction in MPS at endpoint; PGIC scores at endpoint; change in mean pain-related sleep interference score; change from baseline in mean pain at each study week; change from baseline in pain-related sleep interference scores at each week; Medical Outcomes Study-Sleep Scale (MOS-SS); HADS scores (at baseline and endpoint). | 150–600 mg/day flexible phase followed by maintenance phase. | Matching grey capsule. | NSAIDs, cyclo-oxygenase-2 inhibitors (COX-2) and acetaminophen (≤1.5 g/day in Japan, ≤4 g/day in all other countries) were permitted as rescue therapy. Antidepressants were permitted if the patient was on a stable dose within 30 days before the first visit. |
| Dworkin et al 20 | Parallel group | PGB 89; PLA 84. | 8 weeks | 29 centres; USA. | Men or women ≥18 years old with PHN. | ≥3 months | Primary: pain reduction in last 24 hours; safety and adverse events; secondary: SF-MPQ at baseline, weeks 1, 3, 5 and 8; daily sleep interference score; MOS-SS; SF-36; PGIC; CGIC. | 300 mg/day, 600 mg/day fixed. | Identical in appearance; administered one capsule three times daily. | Permitted medications included narcotic and non-narcotic analgesics, acetaminophen (not to exceed 4 g/day), NSAIDs, aspirin and antidepressants, including SSRIs (provided that dosing had been stable for at least 30 days before baseline). |
| Freynhagen et al 21 |
Parallel group | PGB 273; PLA 65. | 12 weeks | 60 centres; 9 European countries that were not specified. | Men or women ≥18 years old with primary diagnosis of painful DPN or PHN. | ≥3 months PHN, ≥6 months DPN. | Primary: MPS; adverse events; secondary: daily sleep interference diary; MOS-SS; PGIC. | 150–600 mg/day flexible. 300 mg/day, 600 mg/day fixed. | Matching capsules; matching twice daily dosing schedule. | SSRIs for treatment of depression, aspirin for myocardial infarction and stroke prophylaxis, short-acting benzodiazepines for insomnia and paracetamol as rescue medication were allowable medications during the study period. |
| Guan et al 22 | Parallel group | PGB 206; PLA 102. | 8 weeks | 11 centres; China | Males or females 18–75 years with primary diagnosis of painful DPN or PHN. | ≥3 months PHN, ≥1 year, <5 years DPN. | Primary: MPS (DPRS) during preceding 24 hours; DAAC score; secondary: daily sleep interference scale; SF-MPQ; PGIC; CGIC; safety and adverse events. | 150–600 mg/day flexible. | Flexible dose PLA in matching capsules; doses titrated using same regimen. | NSAIDs and SSRIs allowed to be continued on stable dose. |
| Holbech et al 23 | Cross-over | PGB 18; PLA 19. | 5 weeks | e centres; Denmark. | Males or females 20–85 years with polyneuropathy due to DPN. | ≥6 months | Primary: total pain intensity on NRS; adverse events; secondary: pain-related sleep disturbances; pain relief on six-point verbal scale; other: specific pain symptoms on the NRS; number of paracetamol tablets used as escape medication; SF-36 (health-related QoL); Major Depression Inventory; QST tests. | 150 mg/day, 300 mg/day fixed. | Matched PLAs of identical appearance to the two trial drugs were dosed similarly using double-dummy technique. | Up to 6 tablets of 500 mg paracetamol could be used daily as escape medication. |
| Huffman et al 24 | Cross-over | PGB 101; PLA 102. | 6 weeks | 36 centres; USA (25), Sweden (4), South Africa (4) and Czech Republic (3). | Men or women ≥18 years old with painful DPN and with pain on walking. | Not described. | Primary: NRS; DPN pain on walking (NRS); secondary: 30%, 50% responders; Brief Pain Inventory-Short Form (BPI-sf); daytime total activity counts per day; steps per day; Walk 12 questionnaire; Norfolk Quality of Life-Diabetic Neuropathy (Norfolk QOL-DN) Total Quality of Life Score; EuroQoL-5 Dimensions (EQ-5D); Mean Sleep Interference Rating Score; HADS. | 150–300 mg/day fixed. | Matching PLA also administered in three divided doses. | Not described. |
| Kanodia and Singhal25 | Parallel group | PGB 23; PLA 22. | 4 weeks | 1 centre; India. | Patients with acute HZ presenting within 72 hours of onset. | <3 days. | Primary: pain on linear VAS; adverse events. | 150 mg/day fixed. | Not described. | Oral acyclovir 800 mg was given five times per day for 7 days. |
| Kim et al 26 | Parallel group | PGB 110; PLA 109. | 12 weeks | 32 centres; Asia-Pacific. | Males or females ≥18 years with diagnosis of central poststroke pain. | ≥3 months | Primary: mean pain score; secondary: daily Sleep Interference Scale (DSIS); weekly MPS; proportion of 30%, 50% responders; Quantitative Assessment of Neuropathic Pain; Neuropathic Pain Symptom Inventory; weekly mean sleep interference scores; MOS-SS; HADS; SF-MPQ VAS – Part B; EQ-5D; PGIC; CGIC; safety and tolerability. | 300 or 600 mg/day dose adjustment followed by fixed maintenance phase. | Matching PLA. | Stable medications for pain or insomnia if used normally >30 days before screening. |
| Krcevski Skvarc and Kamenik27 | Parallel group | PGB 14; PLA 15. | 3 weeks | 1 centre; Slovenia. | Men or women 30–80 years with HZ pain. | Primary: assessment of pain severity (11-point Likert scale); secondary: patients’ ratings of the severity of allodynia, hyperalgesia, and burning, prickling and tingling sensations; rating of quality of sleep and physical activity; consumption of analgesics; occurrence of adverse events; SHN; PHN. | 150 or 300 mg/day fixed. | PLA also administered twice daily. | Oxycodone, naproxen and/or tramadol, morphine and diclofenac. | |
| Lesser et al 28 | Parallel group | PGB 240; PLA 97. | 5 weeks | 45 centres; USA. | Men or women ≥18 years old who were diagnosed with diabetes mellitus (type 1 or 2) and had distal symmetric sensorimotor polyneuropathy. | 1–5 years | Primary: pain (11-point NRS); secondary: daily sleep interference diary; SF-MPQ; CGIC; PGIC; SF-36; Profile of Mood States (POMS); safety outcomes. | 75, 300, 600 mg/day fixed. | PLA administered three times daily. | Acetaminophen and SSRIs permitted. |
| Liu et al 29 | Parallel group | PGB 112; PLA 110. | 8 weeks | 22 centres; China. | Male and female ethnically Chinese patients aged ≥18, diagnosed with PHN. | Symptoms persisting ≥3 months after the healing of HZ lesions. | Primary: mean score of Daily Pain Rating Score; secondary: change from baseline on pain VAS; change from baseline on PPI of the SF-MPQ; 30% pain responders at endpoint; change from baseline in weekly MPS; change from baseline in sleep interference score (11-point NRS); CGIC; PGIC; MOS-SS; adverse events. | 150 mg/day, 300 mg/day fixed. | Matched PLA capsules on the same dosing schedule. | Concomitant use of medications permitted except antidepressants, epileptics, analgesics or corticosteroids, skeletal muscle relaxants, mexelitine and dextromethorphan as well as electrotherapy, transcutaneous electrical nerve stimulation, acupuncture and neurosurgical therapy. |
| Mathieson et al 30 | Parallel group | PGB 108; PLA 101. | 8 weeks | Number not specified; Australia. | Patients with sciatica. | ≥1 week, <1 year. | Primary: average leg pain intensity score over the course of previous 24 hours as assessed at 8 weeks and 52 weeks; secondary: extent of disability (Roland Disability Questionnaire for sciatica); back pain intensity; global perceived effect; quality of life as measured on Short-Form Health Survey 12; adverse events. | 150–600 mg/day flexible. | Matching PLA capsules were packaged in white, opaque, sealed containers at a central pharmacy. | Concomitant therapies included physical therapies as well as other analgesic medications (except for adjuvant analgesic agents), which would ideally be prescribed in accordance with the WHO pain ladder. Trial clinicians were asked not to prescribe certain medicines (antiepileptic medications, SSRIs, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, topical lidocaine and benzodiazepines) or to schedule interventional procedures. |
| Moon et al 31 | Parallel group | PGB 162; PLA 78. | 10 weeks | Multicentre (number not specified); Korea. | Korean patients aged 18 years with neuropathic pain (DPN, PHN or post-traumatic neuropathic pain). | Mean duration of pain PGB patients: 3 years, PLA patients: 3.2 years. | Primary: endpoint mean DPRS score, Secondary: weekly mean DPRS score, DAAC of adjusted mean DPRS from baseline to endpoint, proportion of responders (whose scores reduced by 30% or 50%), DSIS, Euro Quality of Life assessment (EQ-5D): utility and VAS score; MOS-SS; HADS; PGIC; CGIC; Tolerability evaluation of adverse events and vital signs | 150–600 mg/day flexible. | Matching PLA capsules provided by Pfizer. | Most patients were taking drug therapy at baseline, and the majority (83.8%) remained on concomitant drug therapy during the study, including one-third who received tricyclic antidepressants. |
| Rauck et al 32 | Parallel group | PGB 56; PLA 112. | 20 weeks | 85 centres; USA. | Men or women ≥18 years old who were diagnosed with diabetes mellitus (type 1 or 2) and had pain attributed to DPN, defined as painful distal symmetric sensorimotor polyneuropathy. | ≥6 months, <5 years. | Primary: change from baseline in pain intensity score (11 point PI-NRS); secondary: change from baseline in mean 24 hours average pain intensity score, daytime average pain intensity score, night-time average pain intensity score, current pain intensity score, daytime worst pain intensity score, night-time worst pain intensity score, sleep interference score and rescue analgesia consumption (mg); Neuropathic Pain Scale; SF-MPQ; pre-50-foot and post-50-foot (15 m) walk pain scores; PGIC; CGIC; proportion of subjects achieving various levels of reduction in the 24 hours average pain intensity score; time to onset of sustained improvement in the 24 hours average pain intensity score; POMS; SF-36 health-related quality of life questionnaire; safety assessments. | 300 mg/day fixed. | Matching PLA in blister card. | Acetaminophen, up to 3 g/day was allowed as rescue medication for pain throughout the trial but was not allowed within 24 hours of any site visit for assessments. |
| Richter et al 33 | Parallel group | PGB 161; PLA 85. | 6 weeks | Multicentre; not specified. | Patients with diabetes and painful distal symmetrical sensorimotor polyneuropathy. | 1–5 years | Primary: pain; adverse events; secondary: pain characteristics (SF-MPQ and PPI); sleep interference (11-point NRS: 0–10); health status (SF-36); psychological state (POMS); global improvement (PGIC and CGIC). | 150 mg/day and 600 mg/day fixed. | Matching dose and schedule. | Aspirin (for prophylaxis of myocardial infarction and transient ischaemic attacks), acetaminophen (3 g/day) and stable doses of serotonin reuptake inhibitors were allowed. |
| Rosenstock et al 34 | Parallel group | PGB 76; PLA 70. | 8 weeks | 25 centres | Men or women ≥18 years old with type 1 or 2 diabetes mellitus who reported symmetrical painful symptoms in distal extremities for a period of 1–5 years prior to study. | 1–5 years | Primary: endpoint mean score; secondary: SF-MPQ – sensory, affective and total score; daily sleep interference score; PGIC; CGIC; SF-36; POMS; safety. | 300 mg/day fixed. | Lactose USP, one capsule three times daily. | Acetaminophen (up to 4 g/day), aspirin (up to 325 mg/day for myocardial infarction or transient ischaemic attack prophylaxis), and serotonin reuptake inhibitors provided no dose changes occurred within 30 days prior to randomisation or during the study). |
| Sabatowski et al 35 | Parallel group | PGB 157; PLA 81. | 8 weeks | 53 centres; Europe and Australia. | Men or women ≥18 years old with PHN. | ≥6 months | Primary: endpoint mean score; secondary: mean sleep interference scores, PGIC, CGIC, SF-36 health survey, Zung Self-Rating Depression Scale, VAS of the SF-MPQ, adverse events. | 150 mg/day, 300 mg/day fixed. | Identical in appearance. | Patients allowed to continue acetaminophen (up to 3 g/day), NSAIDs, opioid or non-opioid analgesics or antidepressants. |
| Satoh et al 36 | Parallel group | PGB 179; PLA 90. | 13 weeks **intervention period. | 62 centres; Japan. | Men or women ≥18 years old with DPN. | ≥1 year. | Primary: change from baseline in mean weekly pain score at week 13 using a 11-point NRS; secondary: weekly MPS, responder rates, SF-MPQ score, weekly mean sleep interference scores using 11-point NRS; MOS-Sleep Scale, SF-36, PGIC, CGIC, safety: adverse events. | 300 mg/day, 600 mg/day fixed. | Not described, same schedule. | Not described. |
| Shabbir et al 37 | Parallel group | PGB 70; PLA 70. | 6 weeks | 2 centres; Mayo Hospital and Services Hospital, Lahore. | Men or women ≥18 years old with DPN. | ≥6 months. | Primary: reduction in pain (measured with NRS); responders who experienced 50% or more reduction in baseline pain score on NRS. | 150–600 mg/day flexible. | Not described. | Not described. |
| Siddall et al 38 | Parallel group | PGB 70; PLA 67. | 12 weeks | 8 centres; Australia. | Patients with central neuropathic pain in spinal cord injury. | Persisted continuously for at least 3 months or with relapses and remission for at least 6 months. | Primary: endpoint MPS, Sleep-interference scores, SF-MPQ Total, sensory and affective scores, from which VAS and PPI score was derived. MOS-SS and HADS, PGIC; tolerability and safety. | 150–600 mg/day flexible. | PLA also administered twice daily. | 70% of patients taking other medications too: opiates, tricyclics, AEDs, NSAIDs/COX-2, Benzos, SSRI/SSNI and muscle relaxants. |
| Simpson et al 39 | Parallel group | PGB 151; PLA 151. | 14 weeks | 44 centres; USA and Puerto Rico. | Men or women ≥18 years old with painful HIV-DSP. | ≥3 months | Primary: change from baseline in mean NPRS score; secondary: change in sleep interference scores; MOS-SS; PGIC; Pain- modified Brief Pain Inventory; Gracely Pain Scale; safety: adverse events. | 150–600 mg/day flexible. | PLA also administered twice daily. | Neurotoxic antiretroviral (ARV) drugs known to cause sensory neuropathy clinically similar to HIV-DSP must have been on stable doses for ≥3 months before screening. Doses of other pain medications had to be stable for ≥1 month before treatment and throughout the study. |
| Simpson et al 40 | Parallel group | PGB 183; PLA 194. | 16 weeks | 45 centres; South Africa, USA, India, Columbia, Thailand, Peru, Puerto Rico and Poland. | Men and women ≥18 years of age with HIV neuropathy. | ≥3 months | Primary: change in pain scores (NRS); secondary: PGIC/CGIC; Brief Pain Symptom Inventory short form (BPI-sf); MOS-SS; pain-related sleep interference and overall sleep disturbance (NRS-Sleep scale); safety. | 150–600 mg/day flexible. | Matching PLA delivered through system for randomisation and drug dispensing. | NSAIDs, if taken at stable dose for ≥4 weeks before study, antidepressants without efficacy for neuropathic pain if taken at stable dose for ≥30 days before study (SSRIs, bupropion and trazodone), non-benzodiazepine hypnotics no more than once/week for sleep disturbance if clinically essential, rescue therapy of oral acetaminophen (max 3 g/day), low dose (≤650 mg/day) aspirin and stable ARV treatment >8 weeks before study. |
| Stacey et al 41 | Parallel group | PGB 179; PLA 90. | 4 weeks | 42 centres; USA, Germany, Italy, Spain and UK. | Men or women ≥18 years old with PHN. | ≥3 months. | Primary: pain reduction; time to onset of meaningful pain relief; secondary: daily sleep interference score; PGIC; VAS of the SF-MPQ; VAS anxiety; VAS allodynia; safety evaluation. | 150–600 mg/day flexible dose; 300 mg/day fixed dose. | PLA also administered twice daily. | Concomitant pain treatments permitted given that it must be stable for at least 30 days. |
| Tölle et al 42 | Parallel group | PGB 299; PLA 96. | 12 weeks | 58 centres; Germany, Hungary, Poland, UK, Australia, and South Africa. | Men or women ≥18 years old with painful symmetrical sensorimotor polyneuropathy due to diabetes. | ≥1 year. | Primary: pain reduction (according to 11-point NRS) from baseline; treatment responders; secondary: PGIC; CGIC; EuroQoL Health Utilities Index; daily pain-related sleep interference scores; EQ-5D (VAS); safety evaluation. | 150, 300, 300/600 mg/d fixed. | PLA also administered twice daily. | SSRIs for depression or anxiety given in a stable dose for >30 days. |
| van Seventer et al 43 | Parallel group | PGB 275; PLA 93. | 13 weeks | 76 centres. | Men or women ≥18 years old with PHN. | >3 months | Primary: endpoint MPS; patients with ≥50% and ≥30% reduction in pain score from baseline; weekly MPS; secondary: endpoint mean sleep interference scores, weekly mean sleep interference scores and PGIC. | 150, 300, 600 mg/day fixed. | PLA also administered twice daily. | Non-narcotic analgesics, for example, noramidopyrine and paracetamol, and stable regimens of opioids, anti-inflammatories and antidepressants. |
| van Seventer et al 44 | Parallel group | PGB 127; PLA 127. | 8 weeks | 44 centres; Belgium, Canada, Denmark, Finland, Italy, Netherlands, Portugal, Romania, Sweden, Switzerland and UK. | Men or women aged 18–80 years with post-traumatic peripheral neuropathic pain. | ≥3 months | Primary: endpoint MP; secondary: rating of extent to which pain interfered with sleep; MOS-SS; HADS; mBPI-sf; PGIC; tolerability and safety assessment. | 150–600 mg/day flexible. | PLA also administered twice daily. | NSAIDs, COX-2 inhibitors, opioid and non-opioid analgesics, anti-epileptic drugs, antidepressant medications, other concomitant medications if they had been stable for at least 1 month before the study and would remain stable throughout the study |
| Vranken et al 45 | Parallel group | PGB 20; PLA 20. | 4 weeks | 1 centre; the Netherlands. | Men and women ≥18 years old with central neuropathic pain. | ≥6 months | Primary: pain intensity score (VAS); mean endpoint pain score; Pain Disability Index; EQ-5D; Medical Outcomes Short-Form Health Survey Questionnaire 36 (SF-36); safety. | 150–600 mg/day flexible. | Flexible dose PLA (1–4 capsules per day); matching capsules; on same dosing schedule. | Adjuvant analgesics. |
CGIC, Clinician Global Impression of Change; DAAC, duration-adjusted average change; DPN, diabetic peripheral neuropathy; HADS, Hospital Anxiety and Depression Scale; NRS, numerical rating scale; NSAIDs, non-steroidal anti-inflammatory drugs; HZ, herpes zoster; PGB, pregabalin; PGIC, Patient Global Impression of Change; PHN, postherpetic neuralgia; PLA, placebo; SF-MPQ PPI, Short-Form McGill Pain Questionnaire personal pain intensity; SF-MPQ VAS, Short-Form McGill Pain Questionnaire visual assessment scale; SSRIs, selective serotonin reuptake inhibitors; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; VAS, visual assessment scale.
bmjopen-2018-023600supp003.pdf (38.3KB, pdf)
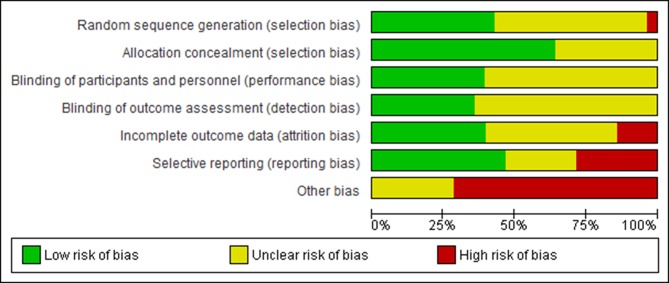
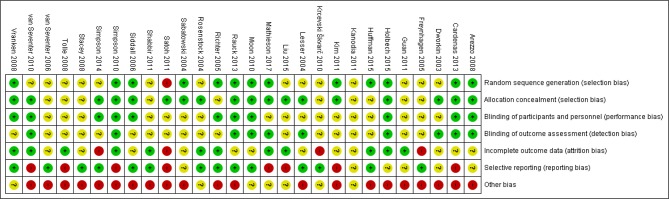
Twenty-three studies examined the effectiveness of pregabalin in treatment of peripheral neuropathic pain including diabetic peripheral neuropathy (DPN), PHN and herpes zoster (table 1). Five studies examined the effectiveness of pregabalin for treating central neuropathic pain including sciatica (radicular pain), poststroke pain and spinal cord injury-related pain. Twenty-five studies were conducted in two or more centres. Outcome measures for pain included numerical rating scale (NRS), visual assessment scale (VAS), Short-Form McGill Pain Questionnaire visual assessment scale (SF-MPQ VAS) and SF-MPQ personal pain intensity (SF-MPQ PPI) index (see table 1 for full characteristics of included studies). The overall risk of bias in the included studies was moderate to high (figures 2 and 3). This was mainly due to inadequate reporting of blinding procedures, selective outcome reporting and financial conflicts of interest among study authors (see online supplementary appendix 4 for the risk of bias judgements).
Figure 2.
Graphical representation of the risk of bias in RCTs assessing the effects of pregabalin in the management of neuropathic pain.
Figure 3.
Risk of bias summary for RCTs assessing the effects of pregabalin in the management of neuropathic pain.
bmjopen-2018-023600supp004.pdf (342.1KB, pdf)
Pain
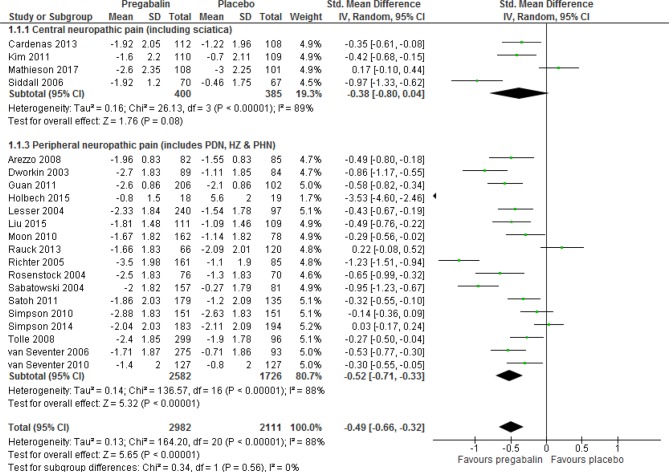
Twenty-one studies provided adequate data on pain using the NRS or variants of it to allow meta-analysis. Meta-analysis showed a significant reduction in pain scores with pregabalin compared with placebo (SMD −0.49 (95% CI −0.66 to −0.32, p<0.00001, I2=88%; figure 4)). Visual inspection of a funnel plot showed that the studies were almost symmetrically distributed around the mean difference for all trials (online supplementary figure S1); trim and fill analyses showed that the subsequent addition of studies with smaller sample sizes did not change the direction of effect. The effect was significant for peripheral neuropathic pain (p<0.00001), but not for central neuropathic pain (p=0.08; online supplementary appendix table 1). The overall quality of the evidence was very low (Summary of Findings (SoF) table 2). Sensitivity analyses revealed similar direction of effects (online supplementary appendix table 2). Four studies that measured pain using NRS did not provide adequate data for meta-analysis; three of these reported significant reductions in pain scores favouring pregabalin over placebo, while one reported no significant difference between groups (see online supplementary appendix table 3).
Figure 4.
Effect of pregabalin on pain scores in patients with neuropathic pain.
Table 2.
Effect of pregabalin on NRS scores in patients with neuropathic pain
|
Patient or population: patients with neuropathic pain Settings: Intervention: effect of pregabalin on pain | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
No. of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Effect of pregabalin in pain | |||||
| MPS | The MPS in the intervention groups was 0.49 SD lower (0.66 to 0.32 lower). |
5093 (21 studies). |
⊕⊝⊝⊝ Very low†‡ | SMD −0.49 (−0.66 to −0.32). | ||
| MPS – central neuropathic pain (including sciatica (radicular pain)) | The mean MPS – central neuropathic pain (including sciatica) in the intervention groups was 0.38 SD lower (0.8 lower to 0.04 higher). |
785 (four studies). |
⊕⊝⊝⊝ Very low‡§¶ | SMD −0.38 (−0.8 to 0.04). | ||
| MPS – peripheral neuropathic pain (includes PDN, HZ and PHN) | The mean MPS – peripheral neuropathic pain (includes PDN, HZ and PHN) in the intervention groups was 0.52 SD lower (0.71–0.33 lower). |
4308 (17 studies). |
⊕⊝⊝⊝ Very low† ‡ | SMD −0.52 (−0.71 to −0.33). | ||
GRADE Working Group grades of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate.
*The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
†Inconsistency in allocation concealment and blinding, selective reporting, authors had financial ties to industry sponsor.
‡Substantial heterogeneity.
§Industry-sponsored selective reporting.
¶Wide CI.
HZ, herpes zoster; GRADE, Grading of Recommendation, Assessment, Development, and Evaluation; MPS, mean pain score; NRS, numerical rating scale; PDN, painful diabetic neuropathy; PHN, postherpetic neuralgia; SMD, standard mean deviation.
bmjopen-2018-023600supp005.pdf (359.3KB, pdf)
bmjopen-2018-023600supp006.pdf (23.3KB, pdf)
bmjopen-2018-023600supp007.pdf (9.9KB, pdf)
bmjopen-2018-023600supp008.pdf (234KB, pdf)
Three studies measured pain using the VAS, and all showed significant reduction in pain scores favouring pregabalin over placebo (online supplementary appendix table 3). Nine studies measured pain using SF-MPQ VAS, and all reported significant reduction in pain scores favouring pregabalin over placebo. Four studies measured pain using SF-MPQ PPI index, and all reported significant reduction in pain scores favouring pregabalin over placebo.
Adverse events
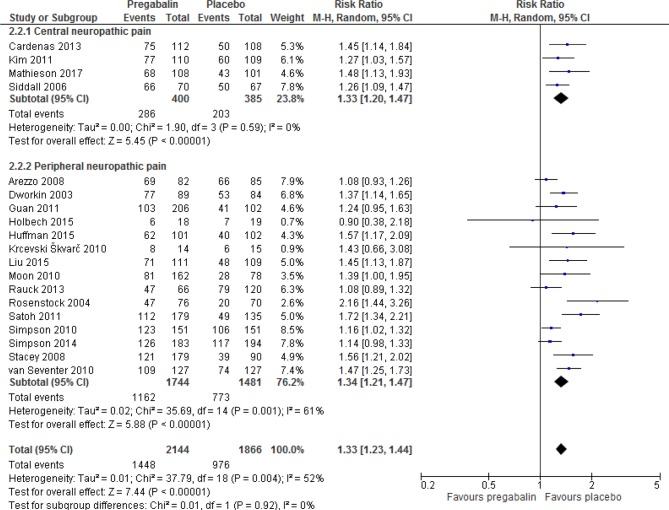
Figure 5 shows that pregabalin was significantly more likely to cause adverse events compared with placebo (RR 1.33 (95% CI 1.23 to 1.44, p<0.00001, I2=52%). This translates into an absolute effect of 145 (95% CI 101 to 194) more adverse events per 1000 treated. The overall quality of the evidence was low (SoF table 3). Sensitivity analyses revealed similar direction of effects (online supplementary appendix table 2). The risk of experiencing individual adverse events of weight gain, somnolence, dizziness, peripheral oedema, fatigue, visual disturbances, ataxia, non-peripheral oedema, dry mouth, vertigo and euphoria were significantly increased with pregabalin compared with placebo (see online supplementary appendix table 1 and supplementary figures S2 to 12). Pregabalin was also significantly more likely to cause discontinuation because of adverse events (RR 1.91, 95% CI 1.54 to 2.37, p<0.00001, I2=0%); the quality of the evidence was low (SoF table 3; online supplementary appendix table 1; and online supplementary figure S13). Sensitivity analyses by study duration revealed similar direction of effects, but there was no significant difference with higher quality studies (online supplementary appendix table 2).
Figure 5.
Effect of pregabalin on the risk of adverse events in patients with neuropathic pain.
Table 3.
Effect of pregabalin on adverse events in patients with neuropathic pain
|
Patient or population: patients with neuropathic pain Settings: Intervention: effect of pregabalin on adverse events | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
No. of participants (studies) |
Quality of the evidence (GRADE) |
Number needed to harm (NNH) | |
| Assumed risk | Corresponding risk | |||||
| Control | Effect of pregabalin on adverse events | |||||
| Adverse events | Study population |
RR 1.33
(1.23 to 1.44) |
4010 (19 studies) |
⊕⊕⊝⊝ Low†‡ | 6 (5–9) | |
| 523 per 1000 |
696 per 1000
(643–753) |
|||||
| Moderate | ||||||
| 440 per 1000 |
585 per 1000
(541–634) |
|||||
| Discontinuations because of adverse events | Study population |
RR 1.91
(1.54 to 2.37) |
5426 (24 studies) |
⊕⊕⊝⊝ Low†§ | 22 (15–37) | |
| 51 per 1000 |
98 per 1000
(79–121) |
|||||
| Moderate | ||||||
| 47 per 1000 |
90 per 1000
(72–111) |
|||||
| Serious adverse events | Study population |
RR 0.9
(0.66–1.24) |
4272 (16 studies) |
⊕⊕⊕⊝ Moderate† | 289 (−121 to 85) | |
| 35 per 1000 |
31 per 1000
(23–43) |
|||||
| Moderate | ||||||
| 20 per 1000 |
18 per 1000
(13–25) |
|||||
GRADE Working Group grades of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate.
*The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
†Selective reporting, authors had financial ties to industry sponsor.
‡Moderate heterogeneity.
§Wide CI.
GRADE, Grading of Recommendation, Assessment, Development, and Evaluation.
There was no significant difference in the risk of serious adverse events (RR 0.9; 95% CI 0.66 to 1.24, p=0.50, I2=0%; SoF table 3; online supplementary appendix table 1; and online supplementary figure S14); the quality of the evidence was moderate. Sensitivity analyses showed a significant effect in favour on pregabalin with three higher quality studies, but there was no difference based on study duration (online supplementary appendix table 2). In total, six deaths were reported across four trials, five in pregabalin group and one in placebo (RR 0.86, 95% CI 0.18 to 4.06, p=0.85, I2=0%).
Sleep disturbance
Twenty-one studies measured sleep interference using the NRS sleep interference scale or variants of it. Pregabalin significantly reduced sleep interference scores compared with placebo (SMD −0.38, 95% CI −0.50 to −0.26, p<0.00001, I2=32%); the quality of the evidence was moderate (SoF table 4; online supplementary appendix table 1; and online supplementary figure S15). Fourteen studies reported sleep interference outcome measures with the NRS scale but did not provide adequate data for statistical pooling; 12 of these reported significant reductions in sleep interference scores favouring pregabalin over placebo, while two studies reported no significant difference between groups (online supplementary appendix table 3). Seven studies measured sleep outcomes using the Medical Outcomes Study Sleep Scale (MOS-Sleep). We could not pool results from these studies because of insufficient data. All the studies reported significant improvements in sleep scores in favour of pregabalin over placebo (online supplementary appendix table 3).
Table 4.
Effect of pregabalin on sleep scores in patients with neuropathic pain
|
Patient or population: patients with neuropathic pain Settings: Intervention: effect of pregabalin on sleep | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
No. of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Effect of pregabalin on sleep | |||||
| Sleep interference | The mean sleep interference in the intervention groups was 0.38 SD lower (0.5–0.26 lower). |
1641 (seven studies). |
⊕⊕⊕⊝ Moderate† | SMD −0.38 (−0.5 to −0.26). | ||
GRADE Working Group grades of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate.
*The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
†Selective reporting, authors had financial ties to industry sponsor.
GRADE, Grading of Recommendation, Assessment, Development, and Evaluation; SMD, standardised mean difference.
Quality of life
Four studies assessed QOL using EuroQoL-5 dimensions scores or variants of it. Two of these reported significant improvements with pregabalin compared with placebo, while the other two reported no significant differences between groups (online supplementary appendix table 3).
Patient Global Impression of Change
Thirteen studies reported this outcome. Ten studies reported significant improvements in PGIC scores with pregabalin compared with placebo, while three studies found no significant differences between groups (online supplementary appendix table 3). We could not pool results from these studies because insufficient data were published.
Clinician Global Impression of Change
Six studies reported this outcome; four of these reported significant improvements with pregabalin compared with placebo, while two found no significant differences between groups (online supplementary appendix table 3).
Anxiety and depression scores
Four studies were pooled for this outcome. There was no significant difference in HADS-Anxiety scores between groups (SMD −0.12, 95% CI −0.29 to 0.04, p=0.14, I2=44%) the quality of the evidence was moderate (SoF table 5; online supplementary figure S16). There was also no significant difference in HADS-Depression scores between groups (SMD −0.06, 95% CI −0.26 to 0.13, p=0.54, I2=60%) the quality of the evidence was low (SoF table 5; online supplementary appendix table 1 and online supplementary figure S17). One study41 that did not provide sufficient data for statistical pooling reported significant improvement in the HADS-Anxiety scores in favour of pregabalin, but no significant difference in HADS-depression scores between groups (online supplementary appendix table 1). One study40 measured anxiety using the VAS anxiety scale and reported significant improvements in QOL scores with fixed-dose and flexible-dose pregabalin compared with placebo (p=0.03 and p=0.02, respectively).
Table 5.
Effect of pregabalin on anxiety and depression scores in patients with neuropathic pain
|
Patient or population: patients with neuropathic pain Settings: Intervention: effect of pregabalin on anxiety and depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
No. of participants (studies) |
Quality of the evidence (GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Effect of pregabalin on anxiety and depression | |||||
| HADS-Anxiety | The mean HADS-Anxiety in the intervention groups was 0.12 SD lower (0.29 lower to 0.04 higher). |
1041 (four studies). |
⊕⊕⊕⊝ Moderate* | SMD −0.12 (−0.29 to 0.04). | ||
| HADS-Depression | The mean HADS-Depression in the intervention groups was 0.06 SD lower (0.26 lower to 0.13 higher). |
1041 (four studies). |
⊕⊕⊝⊝ Low 1 2 | SMD −0.06 (−0.26 to 0.13). | ||
GRADE Working Group grades of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate.
*The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
†Selective reporting, authors had financial ties to industry sponsor.
‡Moderate heterogeneity.
GRADE, Grading of Recommendation, Assessment, Development, and Evaluation; HADS, Hospital Anxiety and Depression Scale; SMD, standardised mean difference
Overall discontinuations
In total, there were 1203 drop-outs (approximately 20%) in the 28 trials (n=5972) that reported the data (online supplementary appendix table 1). There was no significant difference in overall discontinuation rates between groups (RR 1.09 (95% CI 0.93 to 1.28, p=0.29, I2=51%)).
Discussion
Summary of the evidence
The evidence from published RCTs suggests that pregabalin reduces pain in patients with neuropathic pain. The effect is statistically significant in peripheral neuropathic pain, but not with central neuropathic pain. Pregabalin significantly increases the risk of adverse events including weight gain, somnolence, dizziness, dry mouth, peripheral oedema, fatigue, visual disturbances, ataxia, non-peripheral oedema, vertigo and euphoria. Pregabalin significantly reduces sleep interference scores compared with placebo. There was insufficient evidence to assess an effect on QOL. The evidence for PGIC and CGIC scores was mixed among studies that reported these outcomes, and there were no significant effects on HADS anxiety and depression scores compared with placebo. There were five deaths in the pregabalin arms and one in the placebo but insufficient power to detect an overall effect.
Comparison with the existing literature
We have identified several published reviews assessing the effectiveness of pregabalin the management of neuropathic pain, and our results are partly consistent with these. Zhang et al 46 and Wang et al 47 showed that pregabalin was more efficacious than placebo for treatment of DPN-associated pain and PHN-associated pain respectively; however, the two reviews did not base their results on changes from baseline between groups. Semel et al 48 and Freeman et al 49 also concluded that pregabalin was more effective than placebo for neuropathic pain; however, both reviews did not account for the quality of the included primary studies. Finnerup et al 50 concluded that there was modest evidence supporting the use of pregabalin for treatment of neuropathic pain; although the authors used GRADE criteria to assess the strength of recommendation, they did not report the quality of the evidence. In an overview of Cochrane reviews, Wiffen et al 51 concluded that there was clinical trial evidence supporting the use of pregabalin for treatment of some aspects of neuropathic pan; however, the authors did not rate the quality of the evidence for the outcomes reported.
Two reviews52 53 that examined the safety profile of pregabalin concluded that pregabalin use was significantly more associated with adverse events than placebo; however, both reviews did not rate the quality of the evidence for the outcomes reported.
Comparison with existing guidelines
We identified several guidelines that recommend the use of pregabalin for treatment of neuropathic pain, and some of their specifications are consistent with our results. For instance, the European Federation of Neurological Societies guideline54 based on data from comparative studies recommended pregabalin as first-line treatment for neuropathic pain; however, the guidance assessed only the level, but not the quality, of the evidence, and also notes that there are too few large-scale comparative studies to make definite conclusions about the benefits and harms. Similarly, the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine and the American Academy of Physical Medicine and Rehabilitation guidance55 recommend pregabalin as first-line treatment based on levels (and not quality) of the evidence; however, they guidance recommends that clinical trials of longer duration should be conducted. The Canadian Pain Society guidance56 recommends pregabalin as first-line treatment for neuropathic pain but acknowledges that paucity of longer duration trials limit the conclusions that can be drawn about its benefits and harms on the long term.
Strengths and limitations
This rapid review has limitations due to its streamlined methods and search strategy. First, the rapid review methodology employed could have introduced selective outcome reporting bias; nevertheless, most of the outcomes reported in this review have been listed as outcomes of interest to be considered when designing trials of neuropathic pain interventions.57 There is a risk that our review may be prone to sampling bias and that we may have missed potentially eligible studies, which could have been identified by searching clinical trials registries and grey literature. However, we comprehensively searched the literature and used standard criteria to assess the risk of bias and rate the quality of the evidence. It has also been reported that generally the conclusions of rapid reviews and full reviews do not greatly differ,10 and enhanced rapid reviews where data are independently checked by a second reviewer could help policy makers with quicker access to the evidence base.58 This review therefore provides the most up-to-date comprehensive summary of the available literature, as it accounts for study quality and reports clinically meaningful patient outcomes. We did not assess the extent to which different doses of pregabalin influenced the outcomes assessed; in addition, the benefits and harms of pregabalin were not analysed according to specific neuropathic pain conditions; only two subgroups (central and peripheral neuropathic pain) were assessed.
Implications for research
The quality of the included studies examining efficacy of pregabalin for pain was rated as low or very low according to the GRADE framework. This highlights the need for larger, robust, high-quality clinical trials to be conducted, with particular attention paid to minimising selective reporting of outcomes. Concerns about selective reporting could be mitigated if drug manufacturers enabled access to clinical study reports (CSRs), especially as industry-sponsored trials are likely to skew reports in favour of benefits over harms.59 60 This would allow for a more comprehensive assessment of the benefits and harms of pregabalin. Of note, all the included trials were industry sponsored, and an overwhelming majority of the authors of the include studies had financial ties to the pharmaceutical industry. Of note, the results of the only published charity-funded phase IV placebo-controlled trial that assessed the effectiveness of pregabalin in management of neuropathic (radicular) pain contrast our meta-analysis results; there was no significant difference in pain scores between groups.61 Independent and publicly funded trials assessing the benefits and harms of pregabalin should be conducted. Only a few studies assessed the effect of pregabalin in improving QOL, anxiety and depression and CGIC. Future trials should further assess the role of pregabalin for these outcomes. Studies investigating the type of neuropathic pain pregabalin relieves (eg, stimulus-dependent pain such as hyperalgesia or allodynia) or spontaneous pain could be an area of consideration for future research.
That the median duration of intervention was 9 weeks suggests that the intermediate to longer term benefits of pregabalin for neuropathic pain are unproven. Indeed, in real-life clinical care, it has been reported that the initial benefits seen with use of the drug in patients with neuropathic pain were no longer apparent after 6–12 months of therapy.62 Therefore, RCTs that are adequately powered and with longer durations of interventions are desirable. The finding of five deaths among 891 participants on pregabalin, versus one death among 320 participants on placebo, is somewhat concerning. Given the low frequency of this outcome (coupled with the short trial durations), RCTs are unlikely to be informative; we suggest pharmacoepidemiological studies in routinely collected electronic health records and spontaneous reporting databases to assess the impact of pregabalin on mortality.
Implications for clinical practice
Very low-to-moderate quality evidence suggests that pregabalin improves some symptoms of neuropathic pain. However, it significantly increases the risk of adverse events including somnolence, oedema, visual disturbances, ataxia, vertigo and euphoria. Pregabalin also increases the risk of drug discontinuation because of adverse events. Clinicians should be cautious about prescribing pregabalin and should consider whether its benefits outweigh potential harms in individual patients.
Conclusions
The evidence from RCTs in journal publications suggests that pregabalin has beneficial effects on some symptoms of neuropathic pain. However, its use significantly increases the risk of adverse events and discontinuation due to adverse events. The quality of the evidence from journal publications is overall low, and the duration of trials is short. Greater transparency in the reporting of outcomes is advocated; independent and publicly funded trials assessing the effects of pregabalin in neuropathic pain should be encouraged. Allowing researchers access to full CSRs of pregabalin trials should be a priority for drug companies and regulators.
Supplementary Material
Footnotes
Patient consent for publication: Not required.
Contributors: IJO was involved with devising the review methods, conducting electronic searches, screening of abstracts, data extraction, data analysis and interpretation and codrafting of the review. ETT was involved with devising the review methods, screening of abstracts, data extraction, data analysis and interpretation and codrafting of the review. JJL was involved with data extraction, data analysis and interpretation and codrafting of the review. BG and CJH were involved with devising the review methods, data analysis and interpretation and codrafting of the review.
Funding: IJO, BG and CJH are part of the Evidence Synthesis Working Group. The Evidence Synthesis Working Group is funded by the National Institute for Health Research School for Primary Care Research (NIHR SPCR) (ProjectNumber 390). JJL is supported by an NIHR In Practice Fellowship.
Disclaimer: The views expressed are those of the author(s) and not necessarily those of the NIHR, the NHS or the Department of Health.
Competing interests: CJH has received expenses and fees for his media work. He has received expenses from the WHO and FDA and holds grant funding from the NIHR, the NIHR School of Primary Care Research, The Wellcome Trust and the WHO. He has received financial remuneration from an asbestos case. He has also received income from the publication of a series of toolkit books published by Blackwell’s. On occasion, he receives expenses for teaching EBM and is also paid for his general practitioner work in National Health Service out of hours. CEBM jointly runs the EvidenceLive Conference with the BMJ and the Overdiagnosis Conference with some international partners that are based on a non-profit making model. BG receives funding from the Laura and John Arnold Foundation and reports personal fees from intermittent additional personal income from speaking and writing for lay audiences on problems in science and medicine including regulatory shortcomings.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1. Verma V, Singh N, Singh Jaggi A. Pregabalin in neuropathic pain: evidences and possible mechanisms. Curr Neuropharmacol 2014;12:44–56. 10.2174/1570159X1201140117162802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 2007;73:137–50. 10.1016/j.eplepsyres.2006.09.008 [DOI] [PubMed] [Google Scholar]
- 3. Goodman CW, Brett AS. Gabapentin and Pregabalin for pain - is increased prescribing a cause for concern? N Engl J Med 2017;377:411–4. 10.1056/NEJMp1704633 [DOI] [PubMed] [Google Scholar]
- 4. Spence D. Bad medicine: gabapentin and pregabalin. BMJ 2013;347:f6747 10.1136/bmj.f6747 [DOI] [PubMed] [Google Scholar]
- 5. OpenPrescribing.net. High-level prescribing trends for Pregabalin (BNF code 0408010AE) across all GP practices in NHS England. 2010. https://openprescribing.net/chemical/0408010AE/ (accessed 8th Mar 2018).
- 6. Wang Y, Yang H, Shen C, et al. . Morphine and pregabalin in the treatment of neuropathic pain. Exp Ther Med 2017;13:1393–7. 10.3892/etm.2017.4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lyndon A, Audrey S, Wells C, et al. . Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction 2017;112:1580–9. 10.1111/add.13843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017;47:310–3. 10.4997/JRCPE.2017.402 [DOI] [PubMed] [Google Scholar]
- 9. Iacobucci G. UK government to reclassify pregabalin and gabapentin after rise in deaths. BMJ 2017;358:j4441 10.1136/bmj.j4441 [DOI] [PubMed] [Google Scholar]
- 10. Ganann R, Ciliska D, Thomas H. Expediting systematic reviews: methods and implications of rapid reviews. Implement Sci 2010;5:56 10.1186/1748-5908-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. International Association for the Study of Pain. What is neuropathic pain? https://s3.amazonaws.com/rdcms-iasp/files/production/public/AM/Images/GYAP/What%20is%20Neuropathic%20Pain.pdf (accessed 19th Jan 2018).
- 12. Sullivan GM. A primer on the validity of assessment instruments. J Grad Med Educ 2011;3:119–20. 10.4300/JGME-D-11-00075.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JP, Altman DG, Gøtzsche PC, et al. . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Review Manager (RevMan) (Computer program). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011. [Google Scholar]
- 15. Furukawa TA, Barbui C, Cipriani A, et al. . Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006;59:7–10. 10.1016/j.jclinepi.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 16. GRADEpro. Computer program on www.gradepro.org. Version 3.6: McMaster University, 2014. [Google Scholar]
- 17. Atkins D, Best D, Briss PA, et al. . Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arezzo JC, Rosenstock J, Lamoreaux L, et al. . Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol 2008;8:33 10.1186/1471-2377-8-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cardenas DD, Nieshoff EC, Suda K, et al. . A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology 2013;80:533–9. 10.1212/WNL.0b013e318281546b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dworkin RH, Corbin AE, Young JP, et al. . Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2003;60:1274–83. 10.1212/01.WNL.0000055433.55136.55 [DOI] [PubMed] [Google Scholar]
- 21. Freynhagen R, Strojek K, Griesing T, et al. . Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 2005;115:254–63. 10.1016/j.pain.2005.02.032 [DOI] [PubMed] [Google Scholar]
- 22. Guan Y, Ding X, Cheng Y, et al. . Efficacy of pregabalin for peripheral neuropathic pain: results of an 8-week, flexible-dose, double-blind, placebo-controlled study conducted in China. Clin Ther 2011;33:159–66. 10.1016/j.clinthera.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 23. Holbech JV, Bach FW, Finnerup NB, et al. . Imipramine and pregabalin combination for painful polyneuropathy: a randomized controlled trial. Pain 2015;156:958–66. 10.1097/j.pain.0000000000000143 [DOI] [PubMed] [Google Scholar]
- 24. Huffman CL, Goldenberg JN, Weintraub J, et al. . Efficacy and safety of once-daily controlled-release pregabalin for the treatment of patients with postherpetic neuralgia: a double-blind, enriched enrollment randomized withdrawal, placebo-controlled trial. Clin J Pain 2017;33:569–78. 10.1097/AJP.0000000000000445 [DOI] [PubMed] [Google Scholar]
- 25. Kanodia SK, Singhal KC. A study on efficacy of Pregabalin in acute Herpetic Neuralgia. Ann Neurosci 2011;18:148–50. 10.5214/ans.0972.7531.1118405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JS, Bashford G, Murphy TK, et al. . Safety and efficacy of pregabalin in patients with central post-stroke pain. Pain 2011;152:1018–23. 10.1016/j.pain.2010.12.023 [DOI] [PubMed] [Google Scholar]
- 27. Krcevski Skvarc N, Kamenik M. Effects of pregabalin on acute herpetic pain and postherpetic neuralgia incidence. Wien Klin Wochenschr 2010;122(Suppl 2):49–53. 10.1007/s00508-010-1345-x [DOI] [PubMed] [Google Scholar]
- 28. Lesser H, Sharma U, LaMoreaux L, et al. . Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology 2004;63:2104–10. 10.1212/01.WNL.0000145767.36287.A1 [DOI] [PubMed] [Google Scholar]
- 29. Liu Q, Chen H, Xi L, et al. . A randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of pregabalin for postherpetic neuralgia in a population of Chinese patients. Pain Pract 2017;17:62–9. 10.1111/papr.12413 [DOI] [PubMed] [Google Scholar]
- 30. Mathieson S, Maher CG, McLachlan AJ, et al. . Trial of pregabalin for acute and chronic sciatica. N Engl J Med 2017;376:1111–20. 10.1056/NEJMoa1614292 [DOI] [PubMed] [Google Scholar]
- 31. Moon DE, Lee DI, Lee SC, et al. . Efficacy and tolerability of pregabalin using a flexible, optimized dose schedule in Korean patients with peripheral neuropathic pain: a 10-week, randomized, double-blind, placebo-controlled, multicenter study. Clin Ther 2010;32:2370–85. 10.1016/j.clinthera.2011.01.014 [DOI] [PubMed] [Google Scholar]
- 32. Rauck R, Makumi CW, Schwartz S, et al. . A randomized, controlled trial of gabapentin enacarbil in subjects with neuropathic pain associated with diabetic peripheral neuropathy. Pain Pract 2013;13:485–96. 10.1111/papr.12014 [DOI] [PubMed] [Google Scholar]
- 33. Richter RW, Portenoy R, Sharma U, et al. . Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 2005;6:253–60. 10.1016/j.jpain.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 34. Rosenstock J, Tuchman M, LaMoreaux L, et al. . Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 2004;110:628–38. 10.1016/j.pain.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 35. Sabatowski R, Gálvez R, Cherry DA, et al. . Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain 2004;109:26–35. 10.1016/j.pain.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 36. Satoh J, Yagihashi S, Baba M, et al. . Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med 2011;28:109–16. 10.1111/j.1464-5491.2010.03152.x [DOI] [PubMed] [Google Scholar]
- 37. Shabbir B, Shafi F, Mahboob F. Amitriptyline Vs Pregabalin in Painful Diabetic Neuropathy A Randomised Placebo-Based Study. P J M H S 2011;5:745–7. [Google Scholar]
- 38. Siddall PJ, Cousins MJ, Otte A, et al. . Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology 2006;67:1792–800. 10.1212/01.wnl.0000244422.45278.ff [DOI] [PubMed] [Google Scholar]
- 39. Simpson DM, Schifitto G, Clifford DB, et al. . Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial. Neurology 2010;74:413–20. 10.1212/WNL.0b013e3181ccc6ef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simpson DM, Rice AS, Emir B, et al. . A randomized, double-blind, placebo-controlled trial and open-label extension study to evaluate the efficacy and safety of pregabalin in the treatment of neuropathic pain associated with human immunodeficiency virus neuropathy. Pain 2014;155:1943–54. 10.1016/j.pain.2014.05.027 [DOI] [PubMed] [Google Scholar]
- 41. Stacey BR, Barrett JA, Whalen E, et al. . Pregabalin for postherpetic neuralgia: placebo-controlled trial of fixed and flexible dosing regimens on allodynia and time to onset of pain relief. J Pain 2008;9:1006–17. 10.1016/j.jpain.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 42. Tölle T, Freynhagen R, Versavel M, et al. . Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain 2008;12:203–13. 10.1016/j.ejpain.2007.05.003 [DOI] [PubMed] [Google Scholar]
- 43. van Seventer R, Feister HA, Young JP, et al. . Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin 2006;22:375–84. 10.1185/030079906X80404 [DOI] [PubMed] [Google Scholar]
- 44. van Seventer R, Bach FW, Toth CC, et al. . Pregabalin in the treatment of post-traumatic peripheral neuropathic pain: a randomized double-blind trial. Eur J Neurol 2010;17:1082–9. 10.1111/j.1468-1331.2010.02979.x [DOI] [PubMed] [Google Scholar]
- 45. Vranken JH, Dijkgraaf MG, Kruis MR, et al. . Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain 2008;136(1-2):150–7. 10.1016/j.pain.2007.06.033 [DOI] [PubMed] [Google Scholar]
- 46. Zhang SS, Wu Z, Zhang LC, et al. . Efficacy and safety of pregabalin for treating painful diabetic peripheral neuropathy: a meta-analysis. Acta Anaesthesiol Scand 2015;59:147–59. 10.1111/aas.12420 [DOI] [PubMed] [Google Scholar]
- 47. Wang SL, Wang H, Nie HY, et al. . The efficacy of pregabalin for acute pain control in herpetic neuralgia patients: A meta-analysis. Medicine 2017;96:e9167 10.1097/MD.0000000000009167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Semel D, Murphy TK, Zlateva G, et al. . Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract 2010;11:85 10.1186/1471-2296-11-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care 2008;31:1448–54. 10.2337/dc07-2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Finnerup NB, Attal N, Haroutounian S, et al. . Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wiffen PJ, Derry S, Moore RA, et al. . Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev 2013;11:CD010567 10.1002/14651858.CD010567.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zaccara G, Gangemi P, Perucca P, et al. . The adverse event profile of pregabalin: a systematic review and meta-analysis of randomized controlled trials. Epilepsia 2011;52:826–36. 10.1111/j.1528-1167.2010.02966.x [DOI] [PubMed] [Google Scholar]
- 53. Freynhagen R, Serpell M, Emir B, et al. . A comprehensive drug safety evaluation of pregabalin in peripheral neuropathic pain. Pain Pract 2015;15:47–57. 10.1111/papr.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Attal N, Cruccu G, Baron R, et al. . EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 2010;17:1113–e88. 10.1111/j.1468-1331.2010.02999.x [DOI] [PubMed] [Google Scholar]
- 55. Bril V, England J, Franklin GM, et al. . Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2011;76:1758–65. 10.1212/WNL.0b013e3182166ebe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moulin D, Boulanger A, Clark AJ, et al. . Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag 2014;19:328–35. Nov-Dec 10.1155/2014/754693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gilron I. Methodological issues associated with clinical trials in neuropathic pain. Expert Rev Clin Pharmacol 2016;9:1399–402. 10.1080/17512433.2016.1240029 [DOI] [PubMed] [Google Scholar]
- 58. Taylor-Phillips S, Geppert J, Stinton C, et al. . Comparison of a full systematic review versus rapid review approaches to assess a newborn screening test for tyrosinemia type 1. Res Synth Methods 2017;8:475–84. 10.1002/jrsm.1255 [DOI] [PubMed] [Google Scholar]
- 59. Flacco ME, Manzoli L, Boccia S, et al. . Head-to-head randomized trials are mostly industry sponsored and almost always favor the industry sponsor. J Clin Epidemiol 2015;68:811–20. 10.1016/j.jclinepi.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 60. Lundh A, Lexchin J, Mintzes B, et al. . Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017;2:MR000033 10.1002/14651858.MR000033.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Malik KM, Nelson AM, Avram MJ, et al. . Efficacy of Pregabalin in the Treatment of Radicular Pain: Results of a Controlled Trial. Anesth Pain Med 2015;5:e28110 10.5812/aapm.28110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. NHS Gloucestershire. Guidance for Review of Patients taking Pregabalin for Neuropathic Pain. Available at http://www.gloshospitals.nhs.uk/SharePoint19/Chronic%20and%20Acute%20Pain%20Services%20Web%20Documents/Pregabalin%20review%20(neuropathic%20pain)%20mg%204%20(2).pdf (Last accessed 1st Mar 2018).
- 63. Watt A, Cameron A, Sturm L, et al. . Rapid versus full systematic reviews: validity in clinical practice? ANZ J Surg 2008;78:1037–40. 10.1111/j.1445-2197.2008.04730.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023600supp001.pdf (89.7KB, pdf)
bmjopen-2018-023600supp002.pdf (234.8KB, pdf)
bmjopen-2018-023600supp003.pdf (38.3KB, pdf)
bmjopen-2018-023600supp004.pdf (342.1KB, pdf)
bmjopen-2018-023600supp005.pdf (359.3KB, pdf)
bmjopen-2018-023600supp006.pdf (23.3KB, pdf)
bmjopen-2018-023600supp007.pdf (9.9KB, pdf)
bmjopen-2018-023600supp008.pdf (234KB, pdf)