Abstract
Inflammation, the body’s response to harmful external agents, has long been found to be associated with depressive symptoms. The relationship between inflammation and depression is well established in the general population of people with depression, but is less so among perinatal women. Depression in the perinatal period is a common disorder, however available data do not indicate that there is a specific inflammatory picture associated with perinatal depression. We suggest that perinatal depression may be a heterogeneous construct, and that inflammation may be relevant to it in the context of other inflammatory morbidities of pregnancy. In this review we explore the available support for the hypothesis that inflammation associated with depression can represent a precipitating insult for the development of gestational diabetes, a known inflammatory morbidity of pregnancy.
Keywords: Inflammation, gestational diabetes, perinatal depression, postpartum depression, cytokine, insulin
Introduction
Inflammation is the body’s physiological response to harmful external agents. Depression is a clinically heterogeneous mental disorder that can also have far-reaching negative effects on physical health1. Despite a lack of apparent superficial similarity between these phenomena, the past two decades of research have established far-reaching connections between inflammatory pathophysiological states and the manifestations of clinical depression. The mechanisms and directions of this relationship are still under study, and many open questions remain.
Inflammation is a broad term that can cover a variety of physiological states with distinct expression profiles of the many mediators involved in proinflammatory responses. Likewise, depression is a heterogeneous disorder that may represent a final common pathway for cumulative insults at the genetic, psychosocial, endocrinological, and neurochemical levels2,3. It is possible that distinct subtypes of depression may be associated with particular inflammatory profiles, which could explain some of the conflicting or negative data obtained by some researchers in the field.
Perinatal depression is a subtype of depression that is marked by onset during pregnancy or the postpartum period. It has been suggested that perinatal depression may represent an inflammatory morbidity of pregnancy4,5. On the other hand, evidence for a unique profile of inflammatory markers in pregnancy remains mixed. One possible explanation for the heterogeneity in the literature could be hidden heterogeneity in the patient population, such that different subpopulations of perinatal women with depression may have distinct inflammatory profiles, potentially corresponding to other, comorbid inflammatory states. Gestational diabetes, which has itself been associated with a unique profile of elevated inflammatory markers, may be one such comorbidity.
In this review we will discuss the hypothesis that women with gestational diabetes represent a distinct subpopulation whose specific inflammatory profile may have unique associations with depressed mood that are not observed in the larger population of perinatal women.
Depression and inflammation in the general population
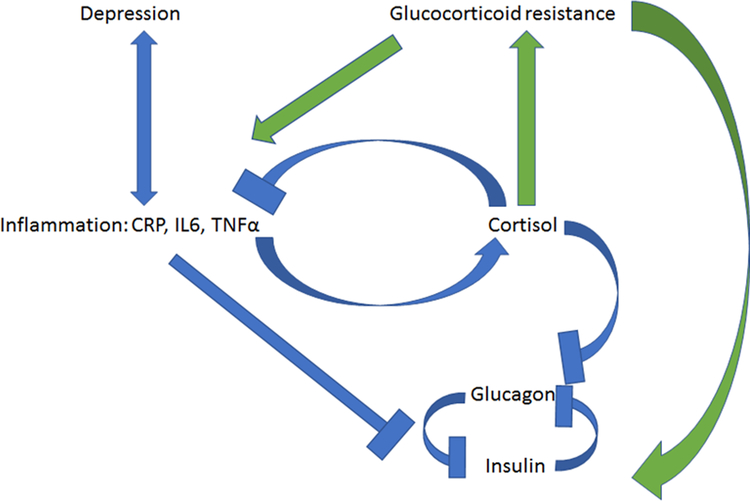
As several thorough reviews on this topic are available6–8, we will offer only a brief summary here. As a broad overview, the model asserts that depression is associated with dysfunctional signaling through the glucocorticoid receptor. This disrupts the delicate negative feedback system of the hypothalamic-pituitary-adrenal axis (HPA), resulting in HPA hyperactivity6. Proinflammatory cytokines promote this state of glucocorticoid resistance in part via direct effects on the glucocorticoid receptor, impairing its translocation to the nucleus and thus preventing glucocorticoid-induced gene transcription9. Ultimately this leads to unchecked pituitary stimulation and excess release of cortisol from the adrenal cortex10. Chronically elevated cortisol can again perpetuate the development of glucocorticoid resistance. This impairs the normal downregulatory effect of cortisol on the inflammatory response11, thus creating a mutually reinforcing feedback loop of increased inflammation and increased glucocorticoid resistance. (See Figure 1 for diagram of relationships between depression, inflammation, and metabolic impairment.)
Figure 1.
Relationships between depression, inflammation, HPA axis activity, and metabolic function. Blue indicates short-term or acute effects, green indicates chronic or homeostatic effects.
The molecular inflammatory signature most reliably associated with depression in the general population involves elevation of C-reactive protein (CRP), tumor necrosis factor alpha (TNFα), and interleukin-6 (IL6), and possibly depression of transforming growth factor beta (TGFβ)8,12,13. TNFα and IL6 are proinflammatory cytokines that are released in the acute stress response, and act directly on hypothalamic and pituitary cells to trigger the cortisol response pathway14. TNFα directly antagonizes the actions of insulin by blocking the tyrosine phosphorylation of the insulin receptor15. CRP, which is under the regulatory control of IL6, is a component of the acute stress response that identifies necrosed or pathogenic cells for destruction16. TGFβ comprises a superfamily of signaling molecules that stimulate gene expression in a wide variety of contexts17. TGFβ is a master regulator of adaptive immunity, suppressing inflammatory responses in multiple ways, including direct inhibition of Th1 and Th2 responses, upregulation of Tregs, inhibition of effector T cell function, and inhibition of B cell proliferation17.
Metabolic impairment is also strongly and bidirectionally associated with depression18, a relationship which reflects the diabetogenic effect of chronic glucocorticoid excess13. Evidence for a causal relationship can be adduced from studies demonstrating a positive benefit of insulin-sensitizing agents on mood19,20, as well as for positive effects of improved mood on metabolic control21,22.
Alterations in the expression of adipokines, inflammatory mediators associated with metabolic function, have also been documented among depressed individuals. Adiponectin, an anti-inflammatory cytokine, is associated with enhanced phosphorylation of the insulin receptor23, and exists in negative feedback balance with TNFα24. Adiponectin has been found by many groups to be lowered among depressed individuals25–27, although negative results exist also28,29, and recent meta-analyses have suggested the effect may be restricted to certain populations30 or significantly modified by other biometric factors31. While effective treatment of depression has not been definitively linked with recovery of serum adiponectin levels32, exogenous administration of adiponectin has been found to have antidepressant-like benefits in animal models33. Leptin, a negative feedback regulator of fat tissue, is reliably reduced in chronic stress levels32, although any association with depression may be complexly moderated by other factors such as body weight, metabolic function34, and depression subtype35. To date, other adipokines such as chemerin or vaspin have not been studied for potential associations with depression.
In addition to the evidence that depressive states and inflammatory states co-occur, studies of pharmacotherapy provide evidence for a bidirectional causal connection between inflammatory profiles and depressive symptoms. Antidepressant treatment is associated with reductions in the expression of the proinflammatory cytokines IL-10, TNFα, and CCL236. Conversely, antidepressant effects of anti-inflammatory medications have also been demonstrated37. Thus, the association between depressive and inflammatory symptoms appears to be integrated and bidirectional.
It has been argued that the links between depression and inflammatory responses are a key reason why alleles conferring risk for depression have been maintained in the population38. This model posits that depressive symptoms are an inextricable facet of physiological responses to infection that have been selected because they reduce infectious mortality. Under this hypothesis, biological and behavioral manifestations of major depression such as social withdrawal, anorexia, hypervigilance, elevated body temperature and hypoferremia all have specific functional roles in host defense against pathogens. This model would explain the observed functional link between depressive symptoms and inflammatory states, and also has interesting implications for the study of depression in the perinatal period, a time in which immune function is reoriented in complex and temporally variable ways.
Depression and inflammation in the perinatal period
Pregnancy requires the immune system to balance the requirement for continued defense against pathogens with the need for maternal tolerance of the immunologically foreign fetus39. Levels of pro- and anti-inflammatory cytokines differ from the nonpregnant baseline and also vary in a predictable pattern over the course of the pregnancy. Thus, studies of depression and inflammation in the nonpregnant state do not yield information that can be applied to pregnant women, because the immunological baseline in pregnancy is distinct from that in non-perinatal women, and also because it varies over the course of the pregnancy, creating distinct functional phases as required by the differing needs of the embryo and fetus over the course of gestation.
Alterations to immune function in healthy pregnancy
Healthy pregnancy requires that maintenance of immune function be balanced with the requirement for fetal tolerance. During normal pregnancy, an expanded population of regulatory T cells (Treg, CD4+ CD25+) arises to orchestrate immune responses, dampen immune reactions and support fetal tolerance40–42. The balance of T helper cells (CD4+) is globally shifted from a proinflammatory Th1 phenotype to an anti-inflammatory Th2 phenotype43. Thus, in concert with the increases in cortisol that are observed over the course of normal pregnancy44, autoimmune reactions are under tighter control and inflammatory responses are reduced.
Mor and colleagues39 have further divided pregnancy into three immunological phases with distinct inflammatory profiles: an early, proinflammatory phase which supports implantation and placentation45, a mid-pregnancy anti-inflammatory phase that supports rapid fetal growth and development, and an acute return to a more proinflammatory balance to facilitate parturition46, with a gradual return to nonpregnant immune status over the postpartum period. Periods of increased inflammatory activity, at the beginning of pregnancy and around the time of parturition and the early postpartum, tend to coincide with lower maternal well-being. Conversely, maternal well-being is generally highest mid-pregnancy, corresponding to a time of anti-inflammatory activity and dampening of immune responses. This offers an interesting parallel to the observations that depressive behaviors are linked with proinflammatory responses, and in fact function to promote effective defense against pathogens38.
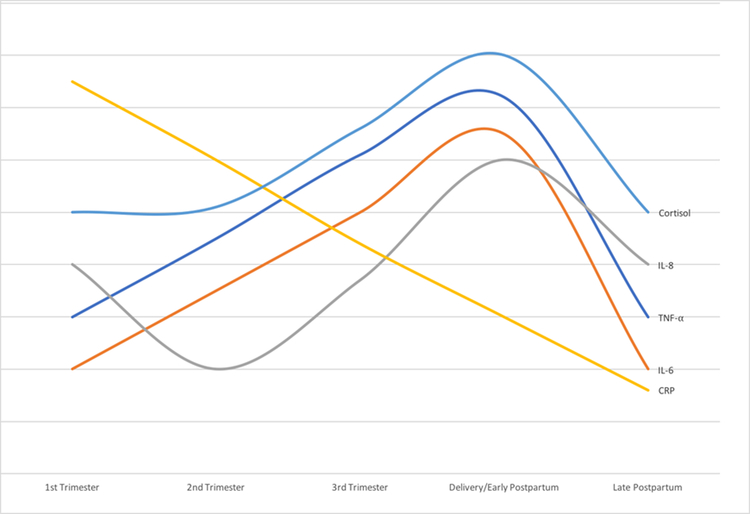
The observed changes in circulating inflammatory markers during pregnancy also reflect these functional shifts. Several different patterns have been observed. One common pattern is a monotonic increase over the course of the pregnancy, with a maximum observed around delivery. This pattern has been observed for both TNFα, a pro-inflammatory, Th1-associated cytokine, and IL-6, a Th2-associated cytokine with both pro-and anti-inflammatory properties47–49. A similar pattern has been observed for the counter-regulatory receptor molecules TNF RII and IL-1 Ra, which promote the expansion of Tregs and dampen inflammatory responses50. A complementary pattern is monotonic reduction over the pregnancy. The inflammatory acute-phase reactant CRP follows this pattern49,50, albeit with an acute peak in the first 1 to 3 days after delivery51. Other patterns include a nadir in the second trimester with a maximum around delivery, as observed for the pro-inflammatory, neutrophil-recruiting cytokine IL-849,52 , and the Th2-promoting cytokine MCP-150,53. The adipokine chemerin, a proinflammatory chemoattractant that recruits immune cells to sites of injury, also rises over the course of pregnancy, while the anti-inflammatory adipokine adiponectin does not show obvious changes throughout most of the pregnancy54 but exhibits a sharp rise in the postpartum period55. (See Figure 2 for a schematic diagram of the trajectories of selected pro- and anti-inflammatory cytokines over the course of pregnancy and the postpartum period.)
Figure 2.
Schematic diagram showing trajectories of selected cytokines over the course of pregnancy and the postpartum period44,49,50.
Thus, cytokine expression in pregnancy follows complex temporal patterns that support the distinct requirements of each phase of pregnancy, delivery, and the puerperium, and any investigation of changes in immune function must be assessed against this dynamic background.
Changes in cytokine expression in perinatal depression
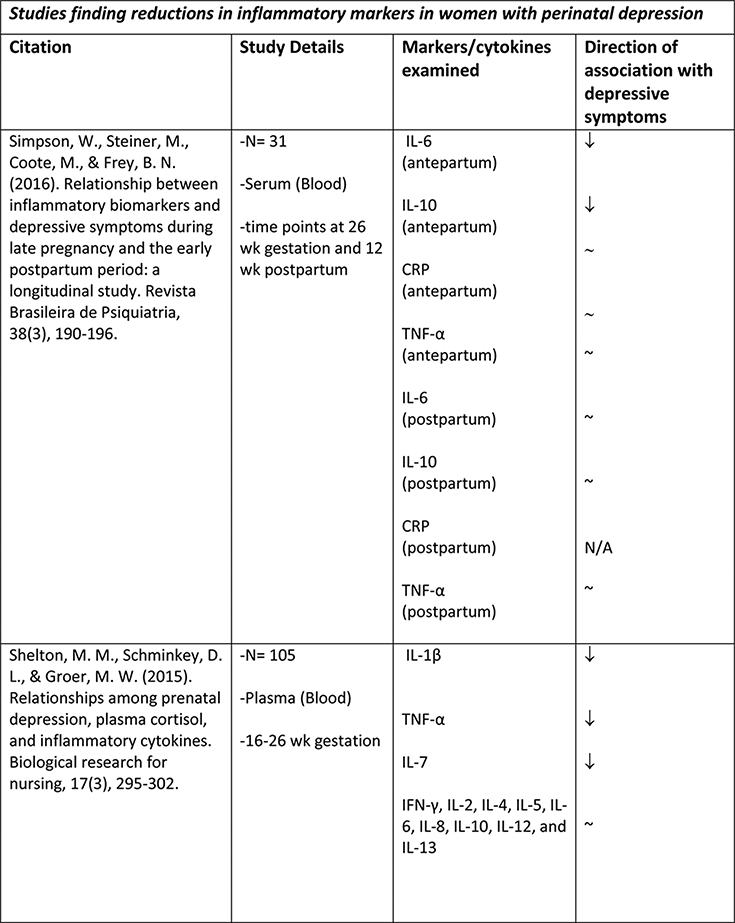
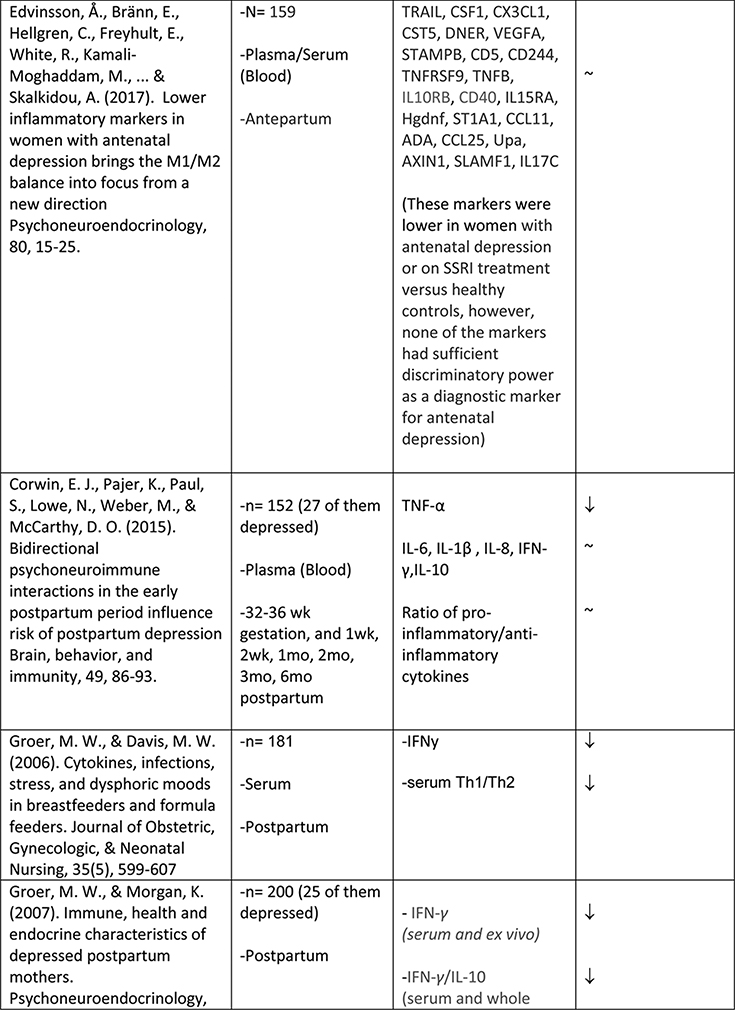
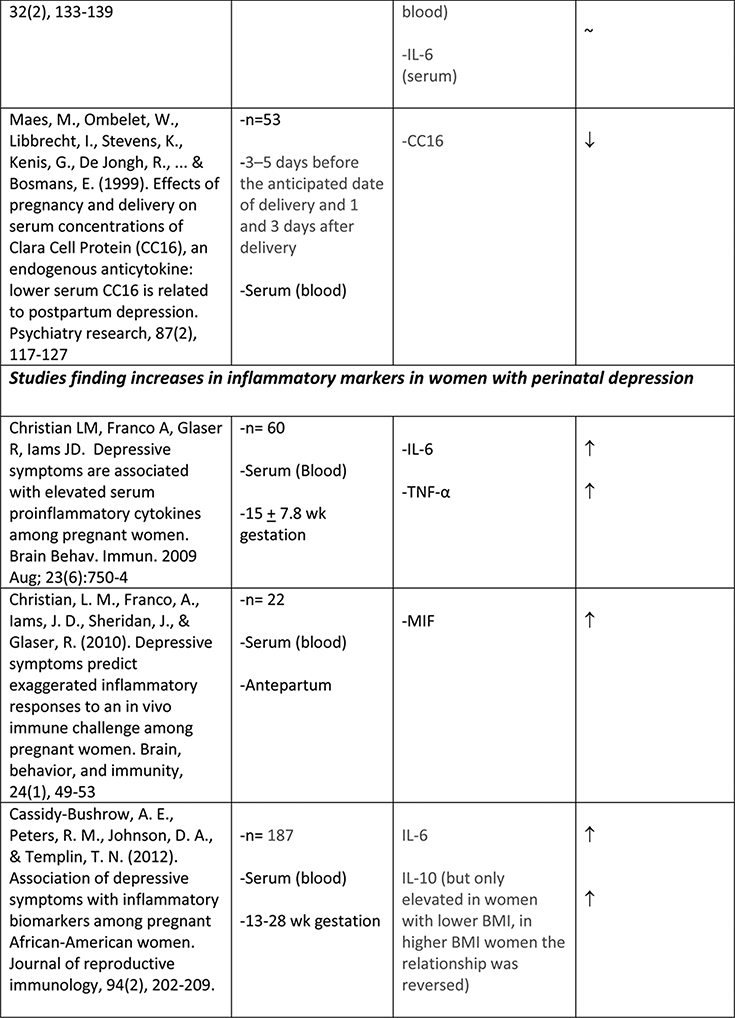
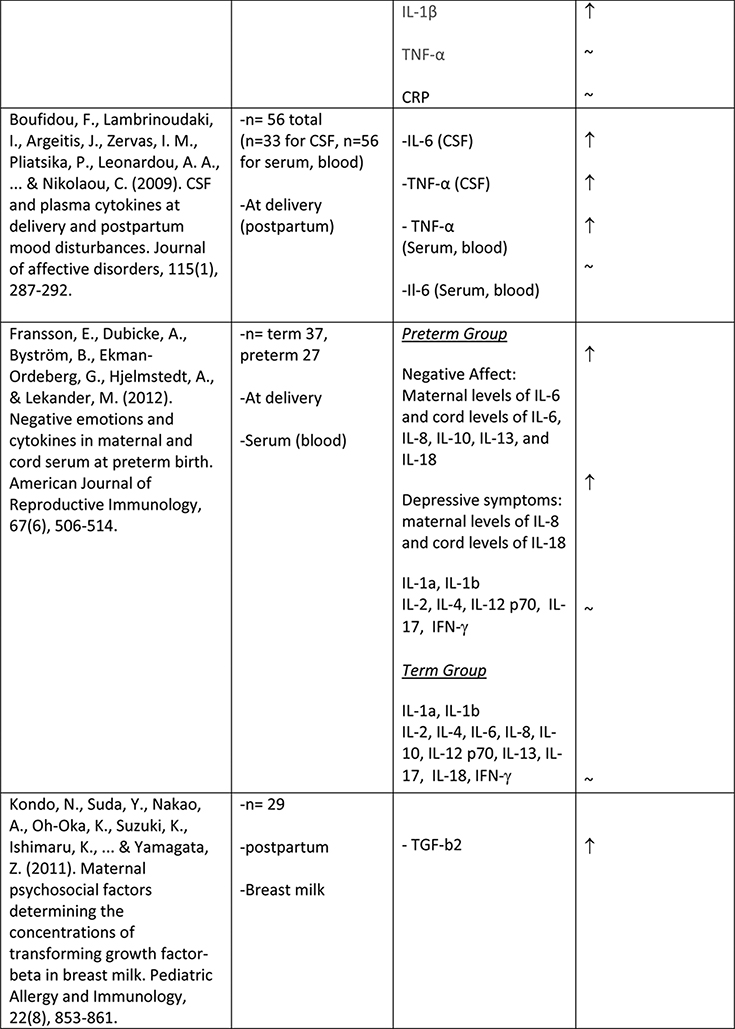
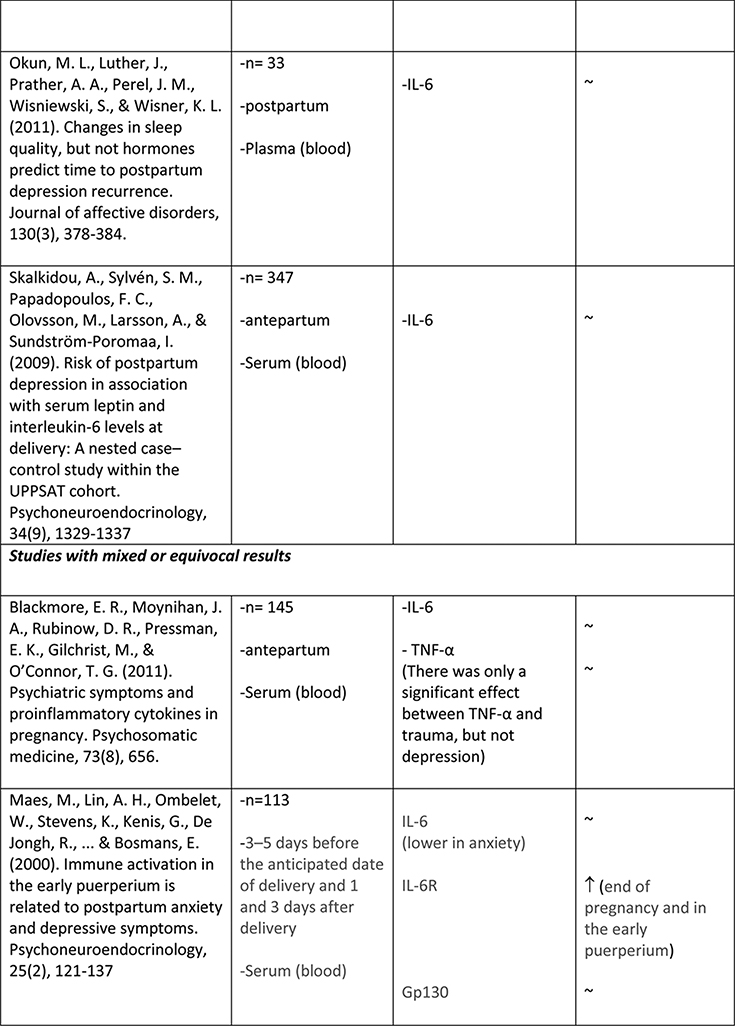
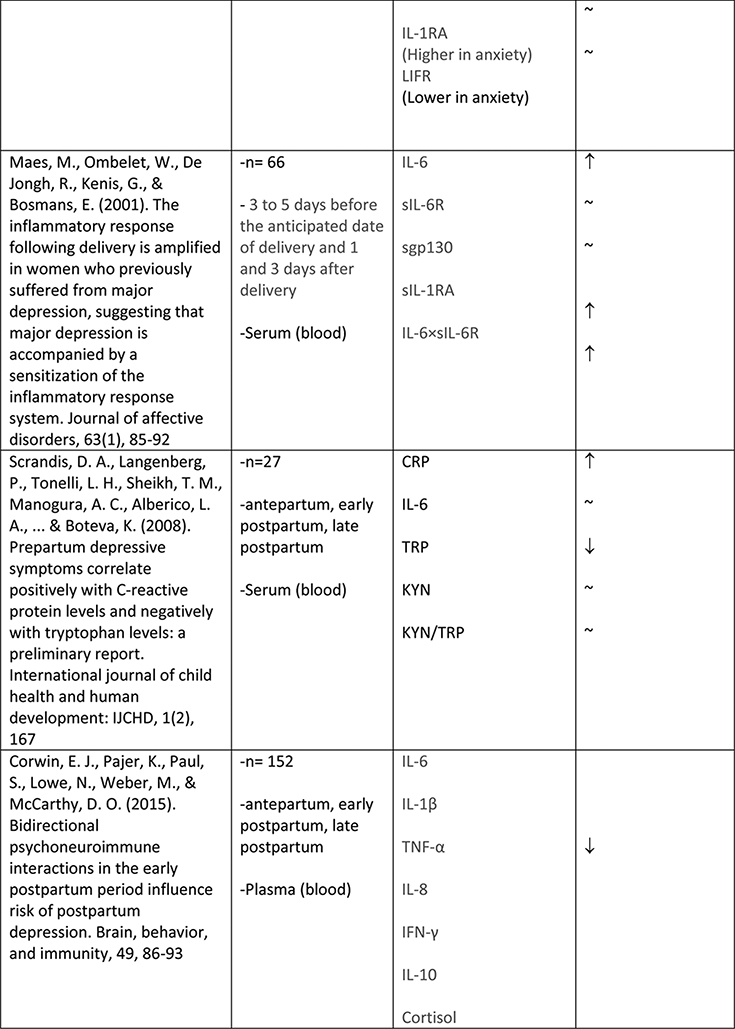
The available studies of changes in cytokine expression with perinatal depression have yielded a complicated mixture of results (Table 1).
Table 1.
Summary of changes in pro- and anti-inflammatory cytokine expression in perinatal depression.
A number of studies have addressed those inflammatory mediators that had previously been associated with depression in the general population, including CRP, TNFα, IL-1, and IL-656. However, results from women in pregnancy and the postpartum period have been less clear-cut than those in the general population of people with depression.
Albacar and colleagues57 found no difference in CRP concentrations between 87 women with and 966 without depression at 8 weeks postpartum. Cassidy-Bushrow and colleagues58 similarly did not find any association between CRP concentrations and depressive symptoms in a sample of 187 pregnant women who were between 13–28 weeks gestation. Of two smaller studies, one with an N of 2759 found an increase in CRP with depressive symptoms at both 35–38 weeks gestation and at 1–5 days postpartum, though not at 5–6 weeks postpartum; while another with an N of 3160 found no association of CRP with depressive symptoms, either at 26+ weeks gestation or at 12 weeks postpartum. Given the disparate timeframes within which these studies were conducted, it is difficult to draw any solid conclusions; however taken together they suggest that CRP elevation is not a consistent feature of perinatal depression.
A larger number of studies have examined IL-6 concentrations with respect to perinatal depression, though again with a mixed set of results. Several available studies did produce an association of IL-6 concentration with depressive symptoms or with negative affect, including Maes et al. (N=113, postpartum)61, Christian et al. (N=60, mean 15 weeks gestation)62, Cassidy-Bushrow et al. (N=187, 13–28 weeks gestation)58, Boufidou et al. (N=33, postpartum, observed in CSF but not serum)63, and Fransson et al. (at delivery, observed only for preterm group, N=27)64. However the majority of studies, including most of the larger ones, found no association (Groer et al., 4–6 weeks postpartum, N=20065; Blackmore et al., 18 and 32 weeks gestation66, N=145; Okun et al., postpartum, N=3367; Skalkidou et al., at delivery, N=34768; Corwin et al., 3rd trimester and multiple postpartum timepoints, N = 15269; Simpson et al., 26+ weeks gestation and 12 weeks postpartum, N=3160; Shelton et al., 16–26 weeks gestation, N=10570). Simpson et al. (N=31)60 found that lower IL-6 in pregnancy was predictive of postpartum but not concurrent depressive symptoms. Taken together, these studies do not suggest any consistent association of IL-6 concentration with depressive symptoms, either during pregnancy or the postpartum period.
IL-1α and IL-1β were examined by four groups. Cassidy-Bushrow et al. (13–28 weeks gestation, N=187)58 found a positive association of IL-1β with depressive symptoms. Shelton et al. (16–26 weeks gestation, N=105)70 found an inverse association of IL-1β with depressive symptoms. Corwin et al. (3rd trimester and multiple postpartum timepoints, N=152)69 found no association of IL-1β with depressive symptoms. Fransson et al. (at delivery, N=64)64 found no association of IL-1α in cord serum with maternal depressive symptoms. (This group attempted to measure both IL-1α and IL-1β in maternal serum at delivery as well but found both below the limit of detection of their assay.)
Similarly mixed results were obtained among the seven studies that examined levels of TNFα in relation to depressive symptoms. Two studies reported a positive association (N=60, mean 15wk gestation62; and N=56, postpartum63). Two studies reported a negative association (N=105, 16–26 weeks gestation70;and N=152, significant at all six postpartum timepoints measured69). Three studies reported no association (N=31, 26+ wk gestation and postpartum60; N=18, 13–28 weeks gestation58; N=145, 18 and 32 weeks gestation66).
Regarding adipokines in perinatal depression, lower adiponectin levels have been associated with postpartum depression by at least one group71; however a second group that followed women throughout pregnancy and the postpartum period did not observe this association55. While the first of these two studies71 also examined leptin levels and found no concurrent relationship with postpartum depression, others have reported that reduced serum leptin after delivery predicts the later development of postpartum depression72.
In general the heterogeneous results in the current literature suggest either that there is no reliable relationship between immune activation and perinatal depression, or that there is a complex relationship that either is variable over the course of the pregnancy, or else is affected by unmeasured factors in the available datasets, precluding the discovery of reliable associations.
Variability in immune activation over pregnancy could possibly explain the disparate results obtained by different investigators. Most of the available studies included only a single timepoint, which often covered a range of gestational ages, and thus their results may not be directly comparable to each other. However even investigators examining overlapping gestational ages have produced conflicting results, as for the opposite sign of the association between IL-1 β and depressive symptoms found by Cassidy-Bushrow et al.58 and Shelton et al.70, who examined populations of similar gestational age.
Unmeasured covariates are also a plausible explanation for these heterogeneous results. Obesity has been raised as one such potential interacting factor58, especially given the complex associations of increased body weight with immune activation, trauma, depression, and diabetes; but many others are possible. External stressors and trauma histories are other factors that have been associated with increased inflammatory biomarkers, and indeed some of the studies discussed above that did not find significant associations between depressive symptoms and cytokine markers of inflammation, did find such associations between the cytokine markers and measures of stress or trauma66.
In general, it is likely that perinatal depression, like non-perinatal depression, is a pathophysiologically heterogeneous disorder. This is an idea that has garnered increasing support on the basis of observable clinical characteristics73,74: a class analysis of phenotypic heterogeneity in women with postpartum depression identified three distinct subtypes that differed in timing of onset, severity, and comorbidities73. Thus if there is a relationship between perinatal depressive symptoms and inflammatory status, the specific profile of inflammatory markers might differ among different subsets of women with depression. Such a mixed picture could explain the highly variable results obtained by different investigators in this area.
If this is true, one way to disentangle the picture could be to explore depressive symptoms in subsets of pregnant women with existing proinflammatory disruptions, controlling for phase of pregnancy. For example, gestational diabetes, pre-eclampsia, preterm birth, and history of early life adversity are all conditions that have been associated both with proinflammatory physiological states and with increased risk for depression in pregnancy4. Addressing the question of inflammatory contributions to depression in the context of women who all have a similar identified proinflammatory condition may permit the disentanglement of pathophysiologically distinct subgroups.
To explore whether such an approach could be effective, the following portion of this review will focus on the subgroup of women with gestational diabetes. We will discuss the profile of inflammatory markers associated with gestational diabetes and cover literature relevant to the nexus of gestational diabetes, inflammation, and perinatal depression.
Inflammatory Mediators: Parallels Between Gestational Diabetes and Perinatal Depression
Gestational Diabetes and Perinatal Depression
Gestational diabetes mellitus (GDM) and depression are both common disorders in pregnancy. Many cross-sectional analyses have found significant degrees of comorbidity between the two conditions, particularly when glucose tolerance is examined as a scale variable75,76. However, other cross-sectional studies have suggested that depression in pregnancy is associated with pre-existing diabetes rather than with diabetes of gestational onset77,78. Thus this observation could represent the known association between diabetes and depression in the general population, rather than any specific association of perinatal depression with GDM.
More recently, longitudinal studies examining the effects of depression prior to pregnancy or present in early pregnancy have found that it is associated with greater rates of onset of GDM79,80. Racial disparities may also play a role, with Hispanic populations less likely than Caucasian populations to display this association79,81. Conversely, the available studies examining GDM as a risk factor for postpartum depression have either not found an association75,82 or else found that when a relationship is present, it is explained by adjustment for medical comorbidities83,84. Gestational weight gain and Caesarean delivery, both complications of GDM, were particularly strongly associated with postpartum depressive symptoms83, suggesting that increases in body weight may be an important mediator in those cases where GDM does predate depressive symptoms.
Taken as a whole, there is somewhat more evidence for a forward causation, where pre-existing depression predisposes women to develop GDM, rather than the reverse. In those cases where GDM does precede depression, excessive maternal weight gain and Caesarean delivery may be important mediating factors.
This suggests that, if inflammatory processes are indeed involved in this cascade, the mechanism could be such that the early development of depressed mood, in concert with a proinflammatory physiological state , contributes to impaired insulin secretion and leads to gestational diabetes.
Inflammatory Activity in Gestational Diabetes
Reductions in the sensitivity to insulin are a feature of healthy pregnancy85. Unlike general adult-onset diabetes, which is associated initially with increases in peripheral resistance to insulin, GDM is associated with a failure of insulin secretion to compensate for this reduced sensitivity, resulting in impaired ability to metabolize ingested sugars85.
GDM is accompanied by a disruption to the normal pregnancy balance of pro- and anti-inflammatory mediators86,87. T cell subsets expressing markers of activation are increased in women with GDM compared to healthy controls, while T cells expressing CTLA-4, a downregulator of immune response that is constitutively expressed in Tregs, are reduced88,89. Shifts in subpopulations of Tregs suggest a reduction in the suppressive capacity of the Treg pool in GDM90. These findings indicate a deficiency in the processes that downregulate immune activation in order to support maternal-fetal tolerance. Conversely however, the Toll-like receptors TLR2 and TLR4, which promote activation of inflammatory mediators, are increased in peripheral blood mononuclear cells of women with GDM91,92.
Some of the inflammatory mediators implicated in depression among the general population are also elevated in women with GDM. TNFα is perhaps the most extensively studied cytokine in this context, with most studies finding a positive association with GDM93–96, and for reviews see 86,87. While some others have reported no changes in TNFα with GDM91,97,98, overall the literature suggests a fairly reliable association of elevations in TNFα with GDM87. CRP elevation in GDM is a consistent finding also98–102. IL-6 has also been found elevated in GDM by a number of investigators97,99,103–106. While some others have failed to find this association107–109, the magnitude of the difference is not large in any case, suggesting it could be easily missed in smaller study cohorts, and it has been suggested that the negative results in some studies could be due to methodological differences87. TGFβ has not been as well studied in this context: one group examined plasma of women with or without GDM and detected no difference in TGFβ levels (N=28)110, while another found elevated levels of TGFβ mRNA in the placentas of women with GDM (N=60)111.
Another set of cytokines of particular interest in the context of both pregnancy and diabetes is the adipokines, including adiponectin, leptin, chemerin, and vaspin112,113
GDM is associated with lower plasma levels of adiponectin94–97,114–116. Interestingly, a study of mRNA expression in placenta found increases in adiponectin mRNA in placentas from women with GDM111. This could possibly suggest a compensatory response by the fetus to an impaired maternal metabolic environment, although the methods section of this paper does not detail whether the placental tissue obtained was of fetal or maternal origin.
Leptin is increased in GDM96,117,118. Vaspin has also been documented to be increased in GDM by most authors who have studied this119–121, though a report of no association also exists122. Results of investigations into the association between serum chemerin and gestational diabetes have been mixed, with the majority of studies finding no effect123–126, while two studies reported an elevation118,127, and at least one small study described a reduction in chemerin among women with GDM128. Many of the above authors reported that obesity was a stronger determinant than GDM of serum chemerin.
Available evidence suggests that the increases in certain inflammatory markers predate frank GDM; for example, higher CRP99 (Maged et al. 2014) and lower adiponectin112,113,129,130 in early pregnancy predict the later development of GDM. This order of events supports the model suggested above, i.e. that depression is associated with an inflammatory picture that then interacts with other risk factors to increase the likelihood of developing metabolic impairment and gestational diabetes.
Comparison of Inflammatory Profiles of Gestational Diabetes and Perinatal Depression
As outlined above, there is noticeable overlap in the profiles of inflammatory markers that characterize GDM and those that characterize depression in the general population. Gestational diabetes is particularly associated with elevations in TNFα, CRP, and IL-6, as well as lower levels of adiponectin. TNFα, CRP, and IL-6 are also the markers that are most reliably elevated in studies of non-perinatal depression. Several studies have found adiponectin to be reduced in depression in the general population as well, although not as reliably as in gestational diabetes.
As discussed, a clear pattern of inflammatory markers reliably associated with perinatal depression has not emerged; nonetheless, the overlap between the inflammatory profile of GDM with that of depression more generally suggests that this could be an important area for future research. The implication that depression may precede GDM, derived from epidemiological work, is consistent with the hypothesis that depression occurs in association with a proinflammatory milieu, characterized by elevations in TNFα, IL-6, and CRP, which then precipitates impairments in insulin secretion and, ultimately, the development of GDM.
Such a hypothesis would imply that the proinflammatory milieu precedes the GDM, which is generally in agreement with the results from those studies that have used a longitudinal design to examine the time course of elevation in inflammatory markers and the onset of GDM99,112,113,129. This model is also supported by in vitro work showing that the precipitaton of an inflammatory response by introduction of a viral dsRNA analogue impairs insulin-mediated glucose uptake in tissues cultured from pregnant women131. Thus, while no study has yet demonstrated a direct causal progression from depression-associated inflammation to gestational diabetes, it has been shown that depression and elevation of inflammatory markers generally precede the development of GDM.
Inflammation, Depression, and GDM: Potential Molecular Mechanisms of Cytokine Action
It is important to note that, while proinflammatory cytokine activity has been shown to contribute to glucocorticoid resistance via direct effects on the glucocorticoid receptor9, the mechanism of GDM is not mainly related to pathological levels of insulin resistance, but rather to a failure of the appropriate compensatory insulin response to the physiological increase in insulin resistance that is present in normal pregnancy. Control of insulin sensitivity in pregnancy is mediated by factors such as estrogen, progesterone, and human placental lactogen (hPL)132. Thus it is not clear whether increased inflammatory activity should precipitate GDM by mechanisms analogous to those involved in its pro-diabetic action in the general population.
At least one study has addressed this concern directly: McLachlan et al.133 explored associations between proinflammatory cytokines, insulin sensitivity, and insulin secretion in 40 pregnant women with and without GDM. This group found that TNFα levels were inversely related to insulin secretion in pregnancy, supporting the hypothesis that TNFα could indeed have a direct influence on the development of GDM. However, none of the studied factors (TNFα, adiponectin, leptin, and CRP) correlated with insulin sensitivity in pregnancy. TNFα, adiponectin, and leptin all had associations with insulin sensitivity in the same group of women when retested at 4 months postpartum. This suggests that while the mechanisms of diabetogenesis may differ from the nonpregnant to the pregnant state, TNFα may facilitate diabetogenesis in both contexts, albeit by distinct mechanisms.
As to the question of whether the observed associations are causal in nature, it has been established that impairments in insulin response can result from an inflammatory stressor, via an in vitro study of the effects of a viral dsRNA analogue on insulin-mediated glucose uptake in skeletal muscle cultured from pregnant women131. It was not clear from this work whether the inflammatory cascade mediated the relationship between the stressor and the metabolic impairment or whether these were parallel pathways. Furthermore, any potential contribution of depression to inflammation could not be examined in this model. Nonetheless, given the established bidirectional associations between inflammatory responses and depression in the general population, it is reasonable to speculate that a rise in depression-associated inflammatory factors could have metabolic effects that parallel those of the viral analogue.
Thus, existing work suggests that inflammatory pathways indeed contribute to metabolic impairment in pregnancy, and that TNFα, a factor that is reliably increased among the general population of people with depression, may be a crucial mediator in this process. While available mechanistic evidence for a link with depression is scant, epidemiological evidence suggests that depression could provide the initial inflammatory insult that predisposes to the development of GDM.
Conclusions
Activation of inflammatory pathways features in many physiological processes, both those that are healthy, including host defense as well as embryo implantation and parturition, and those that are pathological, including depression as well as inflammatory morbidities of pregnancy such as preterm birth and gestational diabetes. In many of these contexts, the adaptive and pathological facets of inflammation are two faces of the same coin, and are not readily disentangled.
While inflammation and depression in the general population are clearly related, questions remain about the relationships of these two processes in the context of pregnancy and the postpartum period, where inflammatory processes take on new and important roles that are crucial for the healthy development of the fetus and safe delivery of the newborn; and at the same time, risk for depression is increased. The small collection of studies to date that have examined inflammatory markers in pregnancy in relation to depressive symptoms have yielded disparate and inconsistent results.
We suggest that if perinatal depression has a relationship to inflammation, this relationship may be complex and could be moderated either by the specific phase of pregnancy and its corresponding inflammatory profile, or by the presence of inflammatory comorbidities such as gestational diabetes, trauma history, pre-eclampsia, or preterm birth, each of which might present a distinct profile of immune activation and, potentially, a correspondingly distinct relationship with the expression of depressive phenotypes.
Our examination of the available evidence has disclosed a general correspondence between the most commonly elevated inflammatory mediators in depression and those typically associated with gestational diabetes, including CRP, TNFα, and IL-6. Furthermore, epidemiological evidence indicates that depression and inflammation may precede gestational diabetes, suggesting a pathway where pre-existing depression is associated with a proinflammatory milieu that raises risk for metabolic impairment in pregnancy.
While these findings are intriguing, they remain circumstantial, as to date no study has explored the causal relationship between markers of inflammation and depressive symptoms specifically with respect to the development of gestational diabetes. We suggest that separating out populations of women with distinct inflammatory comorbidities may be key to disentangling the complex relationship between immune function and depressive symptoms in the perinatal period.
Recommendations for Future Study
At present, none of the available studies of inflammatory markers in perinatal depression have attempted to separate out distinct populations of women with inflammatory comorbidities. We suggest that study designs that take this into account may prove more fruitful in dissecting the contributions of inflammatory cascades to the development of depression in the perinatal period.
Thus, studies which consider women with perinatal depression en bloc, as most of the studies conducted to this point have done, may continue to yield inconclusive results. We suggest instead that inflammatory markers in perinatal depression should be studied with attention to both the metabolic and psychiatric context. This would permit investigators to establish whether depression severity is related to the metabolic dysfunction and inflammatory activity of GDM. Similar approaches examining women with other inflammatory morbidities of pregnancy, including pre-eclampsia, trauma history, and preterm birth, would also be exceedingly informative.
In order to establish whether depression can act as the precipitating insult for the chain of events leading from inflammation to metabolic impairment, longitudinal clinical studies would be needed. An observation that elevation of depressive symptoms and inflammatory cytokine activity in pre-pregnancy or early pregnancy is associated with the later development of GDM would offer support for the hypotheses advanced here.
From a mechanistic standpoint, studies are needed that explore whether TNFα, IL-6, and CRP can produce impairments in insulin secretion such as are present in GDM. This could be done with in vitro study designs that examine insulin secretion after exogenous application of inflammatory cytokines in cultured cells.
A clinical population that is of particular interest with respect to these questions is women with PCOS and/or diabetes who are treated with metformin during pregnancy134. Metformin is an effective treatment for pre-existing as well as gestational diabetes135 and has been documented to reduce serum concentrations of inflammatory markers including CRP136, yet to our knowledge no research examining the effect of metformin in pregnancy on maternal mental health exists. A comparison of mental health outcomes and inflammatory profiles in women using metformin as compared to insulin would be a very useful addition to the existing literature.
We hope that future research in the suggested directions will contribute to our ability to disentangle the complex relationships between inflammatory processes and psychiatric dysfunction in the perinatal period.
References
- 1.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, & Ustun B (2007). Depression, chronic diseases, and decrements in health: results from the World Health Surveys. The Lancet, 370(9590), 851–858. [DOI] [PubMed] [Google Scholar]
- 2.Chen LS, Eaton WW, Gallo JJ, & Nestadt G (2000). Understanding the heterogeneity of depression through the triad of symptoms, course and risk factors: a longitudinal, population-based study. Journal of affective disorders, 59(1), 1–11. [DOI] [PubMed] [Google Scholar]
- 3.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, … & Schatzberg AF (2017). Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature medicine, 23(1), 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborne LM, & Monk C (2013). Perinatal depression—the fourth inflammatory morbidity of pregnancy?: theory and literature review. Psychoneuroendocrinology, 38(10), 1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leff-Gelman P, Mancilla-Herrera I, Flores-Ramos M, Cruz-Fuentes C, Reyes-Grajeda JP, del Pilar García-Cuétara M, … & Pulido-Ascencio DE (2016). The immune system and the role of inflammation in perinatal depression. Neuroscience bulletin, 32(4), 398–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pariante CM (2017). Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. European Neuropsychopharmacology 27(6):554. [DOI] [PubMed] [Google Scholar]
- 7.Zorn JV, Schür RR, Boks MP, Kahn RS, Joëls M, & Vinkers CH (2016). Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology. [DOI] [PubMed] [Google Scholar]
- 8.Furtado M, & Katzman MA (2015). Examining the role of neuroinflammation in major depression. Psychiatry research, 229(1), 27–36. [DOI] [PubMed] [Google Scholar]
- 9.Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, and Miller AH The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999; 140: 4359–4366 [DOI] [PubMed] [Google Scholar]
- 10.McKay MS and Zakzanis KK The impact of treatment on HPA axis activity in unipolar major depression. Journal of Psychiatric Research, 44 (3) (2010), pp. 183–192 [DOI] [PubMed] [Google Scholar]
- 11.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, & Turner RB (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences, 109(16), 5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, & Lanctôt KL (2010). A meta-analysis of cytokines in major depression. Biological psychiatry, 67(5), 446–457. [DOI] [PubMed] [Google Scholar]
- 13.Stuart MJ, & Baune BT (2012). Depression and type 2 diabetes: inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neuroscience & Biobehavioral Reviews, 36(1), 658–676. [DOI] [PubMed] [Google Scholar]
- 14.Chrousos GP (1995). The hypothalamic–pituitary–adrenal axis and immune-mediated inflammation. New England Journal of Medicine, 332(20), 1351–1363. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS, Murray DL, Choy LN, & Spiegelman BM (1994). Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proceedings of the National Academy of Sciences, 91(11), 4854–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black S, Kushner I, & Samols D (2004). C-reactive protein. Journal of Biological Chemistry, 279(47), 48487–48490. [DOI] [PubMed] [Google Scholar]
- 17.Kelly A, Houston SA, Sherwood E, Casulli J, & Travis MA (2017). Chapter Four-Regulation of Innate and Adaptive Immunity by TGFβ. Advances in Immunology, 134, 137–233. [DOI] [PubMed] [Google Scholar]
- 18.Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, & Hu FB (2012). Bidirectional association between depression and metabolic syndrome. Diabetes care, 35(5), 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashani L, Omidvar T, Farazmand B, Modabbernia A, Ramzanzadeh F, Tehraninejad ES, … & Akhondzadeh S (2013). Does pioglitazone improve depression through insulin-sensitization? Results of a randomized double-blind metformin-controlled trial in patients with polycystic ovarian syndrome and comorbid depression. Psychoneuroendocrinology, 38(6), 767–776. [DOI] [PubMed] [Google Scholar]
- 20.Lin KW, Wroolie TE, Robakis T, & Rasgon NL (2015). Adjuvant pioglitazone for unremitted depression: Clinical correlates of treatment response. Psychiatry research, 230(3), 846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrahamian H, Hofmann P, Prager R, & Toplak H (2009). Diabetes mellitus and co-morbid depression: treatment with milnacipran results in significant improvement of both diseases (results from the Austrian MDDM study group). Neuropsychiatric disease and treatment, 5, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lustman PJ, Williams MM, Sayuk GS, Nix BD, & Clouse RE (2007). Factors influencing glycemic control in type 2 diabetes during acute-and maintenance-phase treatment of major depressive disorder with bupropion. Diabetes Care, 30(3), 459–466. [DOI] [PubMed] [Google Scholar]
- 23.Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, … & Tataranni PA (2002). Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes, 51(6), 1884–1888. [DOI] [PubMed] [Google Scholar]
- 24.Ruan H, & Lodish HF (2003). Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-α. Cytokine & growth factor reviews, 14(5), 447–455. [DOI] [PubMed] [Google Scholar]
- 25.Leo R, Di Lorenzo G, Tesauro M, Cola C, Fortuna E, Zanasi M, … & Romeo F (2006). Decreased plasma adiponectin concentration in major depression. Neuroscience letters, 407(3), 211–213. [DOI] [PubMed] [Google Scholar]
- 26.Lehto SM, Huotari A, Niskanen L, Tolmunen T, Koivumaa‐Honkanen H, Honkalampi K, … & Hintikka J (2010). Serum adiponectin and resistin levels in major depressive disorder. Acta Psychiatrica Scandinavica, 121(3), 209–215. [DOI] [PubMed] [Google Scholar]
- 27.Zeugmann S, Quante A, Heuser I, Schwarzer R, & Anghelescu I (2010). Inflammatory biomarkers in 70 depressed inpatients with and without the metabolic syndrome. The Journal of clinical psychiatry, 71(8), 1007–1016. [DOI] [PubMed] [Google Scholar]
- 28.Mamalakis G, Kiriakakis M, Tsibinos G, Hatzis C, Flouri S, Mantzoros C, & Kafatos A (2006). Depression and serum adiponectin and adipose omega-3 and omega-6 fatty acids in adolescents. Pharmacology Biochemistry and Behavior, 85(2), 474–479. [DOI] [PubMed] [Google Scholar]
- 29.Pan A, Ye X, Franco OH, Li H, Yu Z, Wang J, … & Lin X (2008). The association of depressive symptoms with inflammatory factors and adipokines in middle-aged and older Chinese. PLoS One, 3(1), e1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Dong X, & Chen J (2015). Adiponectin and depression: A meta‑analysis. Biomedical reports, 3(1), 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carvalho AF, Rocha DQ, McIntyre RS, Mesquita LM, Köhler CA, Hyphantis TN, … & Berk M (2014). Adipokines as emerging depression biomarkers: a systematic review and meta-analysis. Journal of psychiatric research, 59, 28–37. [DOI] [PubMed] [Google Scholar]
- 32.Taylor VH, & MacQueen GM (2010). The role of adipokines in understanding the associations between obesity and depression. Journal of obesity, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Guo M, Zhang D, Cheng SY, Liu M, Ding J, … & Lu XY (2012). Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proceedings of the National Academy of Sciences, 109(30), 12248–12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haleem DJ, Sheikh S, Fawad A, & Haleem MA (2017). Fasting leptin and glucose in normal weight, over weight and obese men and women diabetes patients with and without clinical depression. Metabolic Brain Disease, 32(3), 757–764. [DOI] [PubMed] [Google Scholar]
- 35.Milaneschi Y, Lamers F, Bot M, Drent ML, & Penninx BW (2017). Leptin dysregulation is specifically associated with major depression with atypical features: evidence for a mechanism connecting obesity and depression. Biological psychiatry, 81(9), 807–814. [DOI] [PubMed] [Google Scholar]
- 36.Köhler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, …& Herrmann N (2017). Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Molecular Neurobiology, 1–12. [DOI] [PubMed] [Google Scholar]
- 37.Hannestad J, DellaGioia N, & Bloch M (2011). The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology, 36(12), 2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raison CL, & Miller AH (2013). The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Molecular psychiatry, 18(1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mor G, & Cardenas I (2010). The immune system in pregnancy: a unique complexity. American journal of reproductive immunology, 63(6), 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. [DOI] [PubMed] [Google Scholar]
- 41.Schlossberger V, Schober L, Rehnitz J, et al. The success of assisted reproduction technologies in relation to composition of the total regulatory T cell (Treg) pool and different Treg subsets. Hum Reprod. 2013;28:3062–73. [DOI] [PubMed] [Google Scholar]
- 42.Ruocco MG, Chaouat G, Florez L, Bensussan A, & Klatzmann D (2014). Regulatory T-cells in pregnancy: historical perspective, state of the art, and burning questions. Frontiers in immunology, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito S, Nakashima A, Shima T, & Ito M (2010). Th1/Th2/Th17 and regulatory T‐cell paradigm in pregnancy. American journal of reproductive immunology, 63(6), 601–610. [DOI] [PubMed] [Google Scholar]
- 44.Allolio B, Hoffmann J, Linton EA, Winkelmann W, Kusche M, & Schulte HM (1990). Diurnal salivary cortisol patterns during pregnancy and after delivery: relationship to plasma corticotrophin‐releasing‐hormone. Clinical endocrinology, 33(2), 279–289. [DOI] [PubMed] [Google Scholar]
- 45.Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, & Wagner GP (2017). Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proceedings of the National Academy of Sciences, 201701129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norman JE, Bollapragada S, Yuan M, & Nelson SM (2007). Inflammatory pathways in the mechanism of parturition. BMC pregnancy and childbirth, 7(1), S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greci LS, Gilson GJ, Nevils B, Izquierdo LA, Qualls CR, & Curet LB (1998). Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. American journal of obstetrics and gynecology, 179(1), 172–178. [DOI] [PubMed] [Google Scholar]
- 48.Keski‐Nisula L, Hirvonen MR, Roponen M, Heinonen S, & Pekkanen J (2004). Spontaneous and stimulated interleukin‐6 and tumor necrosis factor‐alpha production at delivery and three months after birth. European cytokine network, 15(1), 67–72. [PubMed] [Google Scholar]
- 49.Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine. 2014;70:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friis CM, Paasche Roland MC, Godang K, et al. Adiposity-related inflammation: effects of pregnancy. Obesity 2013;21:E124–30. [DOI] [PubMed] [Google Scholar]
- 51.Kääpä P, & Koistinen E (1993). Maternal and neonatal C-reactive protein after interventions during delivery. Acta obstetricia et gynecologica Scandinavica, 72(7), 543–546. [DOI] [PubMed] [Google Scholar]
- 52.Poston L (2000). Interleukin 8 expression in human myometrium: changes in relation to labor onset and with gestational age. American journal of reproductive immunology, 43(5), 272–277. [DOI] [PubMed] [Google Scholar]
- 53.Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, … & Adashi EY (2005). Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta, 26(8), 661–671. [DOI] [PubMed] [Google Scholar]
- 54.Garces MF, Sanchez E, Ruíz-Parra AI, Rubio-Romero JA, Angel-Müller E, Suarez MA, … & Caminos JE (2013). Serum chemerin levels during normal human pregnancy. Peptides, 42, 138–143. [DOI] [PubMed] [Google Scholar]
- 55.Rebelo F, Farias DR, Struchiner CJ, & Kac G (2016). Plasma adiponectin and depressive symptoms during pregnancy and the postpartum period: A prospective cohort study. Journal of affective disorders, 194, 171–179. [DOI] [PubMed] [Google Scholar]
- 56.Howren MB, Lamkin DM, & Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic medicine, 71(2), 171–186. [DOI] [PubMed] [Google Scholar]
- 57.Albacar G, Sans T, Martín-Santos R, García-Esteve L, Guillamat R, Sanjuan J, … & Gaviria A (2010). Thyroid function 48h after delivery as a marker for subsequent postpartum depression. Psychoneuroendocrinology, 35(5), 738–742. [DOI] [PubMed] [Google Scholar]
- 58.Cassidy-Bushrow AE, Peters RM, Johnson DA, & Templin TN (2012). Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. Journal of reproductive immunology, 94(2), 202–209. [DOI] [PubMed] [Google Scholar]
- 59.Scrandis DA, Langenberg P, Tonelli LH, Sheikh TM, Manogura AC, Alberico LA, … & Boteva K (2008). Prepartum depressive symptoms correlate positively with C-reactive protein levels and negatively with tryptophan levels: a preliminary report. International journal of child health and human development: IJCHD, 1(2), 167. [PMC free article] [PubMed] [Google Scholar]
- 60.Simpson W, Steiner M, Coote M, & Frey BN (2016). Relationship between inflammatory biomarkers and depressive symptoms during late pregnancy and the early postpartum period: a longitudinal study. Revista Brasileira de Psiquiatria, 38(3), 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maes M, Lin AH, Ombelet W, Stevens K, Kenis G, De Jongh R, … & Bosmans E (2000). Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology, 25(2), 121–137 [DOI] [PubMed] [Google Scholar]
- 62.Christian LM, Franco A, Glaser R, Iams JD. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav. Immun 2009. August; 23(6):750–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boufidou F, Lambrinoudaki I, Argeitis J, Zervas IM, Pliatsika P, Leonardou AA, … & Nikolaou C (2009). CSF and plasma cytokines at delivery and postpartum mood disturbances. Journal of affective disorders, 115(1), 287–292. [DOI] [PubMed] [Google Scholar]
- 64.Fransson E, Dubicke A, Byström B, Ekman‐Ordeberg G, Hjelmstedt A, & Lekander M (2012). Negative emotions and cytokines in maternal and cord serum at preterm birth. American Journal of Reproductive Immunology, 67(6), 506–514. [DOI] [PubMed] [Google Scholar]
- 65.Groer MW, & Morgan K (2007). Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology, 32(2), 133–139 [DOI] [PubMed] [Google Scholar]
- 66.Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, & O’Connor TG (2011). Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosomatic medicine, 73(8), 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okun ML, Luther J, Prather AA, Perel JM, Wisniewski S, & Wisner KL (2011). Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. Journal of affective disorders, 130(3), 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skalkidou A, Sylvén SM, Papadopoulos FC, Olovsson M, Larsson A, & Sundström-Poromaa I (2009). Risk of postpartum depression in association with serum leptin and interleukin-6 levels at delivery: A nested case–control study within the UPPSAT cohort. Psychoneuroendocrinology, 34(9), 1329–1337 [DOI] [PubMed] [Google Scholar]
- 69.Corwin EJ, Pajer K, Paul S, Lowe N, Weber M, & McCarthy DO (2015). Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain, behavior, and immunity, 49, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shelton MM, Schminkey DL, & Groer MW (2015). Relationships among prenatal depression, plasma cortisol, and inflammatory cytokines. Biological research for nursing, 17(3), 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yildiz G, Senturk MB, Yildiz P, Cakmak Y, Budak MS, & Cakar E (2017). Serum serotonin, leptin, and adiponectin changes in women with postpartum depression: controlled study. Archives of gynecology and obstetrics, 295(4), 853–858. [DOI] [PubMed] [Google Scholar]
- 72.Chen C, Gao J, Zhang J, Jia L, Yu T, & Zheng Y (2016). Serum leptin level measured 48 h after delivery is associated with development of postpartum depressive symptoms: a 3-month follow-up study. Archives of women’s mental health, 19(6), 1001–1008. [DOI] [PubMed] [Google Scholar]
- 73.Consortium PACT (2015). Heterogeneity of postpartum depression: a latent class analysis. The Lancet Psychiatry, 2(1), 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, … & Apter G (2017). Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an International Consortium. The Lancet Psychiatry, 4(6), 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang T, Rifas-Shiman SL, Ertel KA, Rich-Edwards J, Kleinman K, Gillman MW, … James-Todd T (2015). Pregnancy Hyperglycaemia and Risk of Prenatal and Postpartum Depressive Symptoms. Paediatric & Perinatal Epidemiology, 29(4), 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gezginç K, Sahingöz M, Uguz F, & Yazıcı F (2013). Is depression associated with glucose tolerance abnormality in pregnant women? A cross-sectonal study. Archives of Psychiatric Nursing, 27(5), 219–22 [DOI] [PubMed] [Google Scholar]
- 77.Katon JG, Russo J, Gavin AR, Melville JL, & Katon WJ (2011). Diabetes and depression in pregnancy: is there an association? Journal of Women’s Health (2002), 20, 983–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langer N, & Langer O (2000). Comparison of Pregnancy Mood Profiles in Gestational Diabetes and Preexisting Diabetes. The Diabetes Educator, 26(4), 667–672. [DOI] [PubMed] [Google Scholar]
- 79.Bowers K, Laughon SK, Kim S, Mumford SL, Brite J, Kiely M, & Zhang C (2013). The association between a medical history of depression and gestational diabetes in a large multi-ethnic cohort in the United States. Paediatric and Perinatal Epidemiology, 27, 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morrison C, McCook JG, & Bailey BA (2015). First trimester depression scores predict development of gestational diabetes mellitus in pregnant rural Appalachian women. J Psychosom Obstet Gynaecol, 1–5. [DOI] [PubMed] [Google Scholar]
- 81.Ertel KA, Silveira M, Pekow P, Braun B, Manson JE, Solomon CG, … Chasan-Taber L (2014). Prenatal depressive symptoms and abnormalities of glucose tolerance during pregnancy among Hispanic women. Archives of Women’s Mental Health, 17, 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller ES, Peri MR, & Gossett DR (2016). The association between diabetes and postpartum depression. Archives of Women’s Mental Health, 19(1), 183–186. [DOI] [PubMed] [Google Scholar]
- 83.Nicklas JM, Miller LJ, Zera CA, Davis RB, Levkoff SE, & Seely EW (2013). Factors associated with depressive symptoms in the early postpartum period among women with recent gestational diabetes mellitus. Maternal and child health journal, 17(9), 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walmer R, Huynh J, Wenger J, Ankers E, Mantha AB, Ecker J, … & Bentley‐Lewis R (2015). Mental health disorders subsequent to gestational diabetes mellitus differ by race/ethnicity. Depression and anxiety, 32(10), 774–782. [DOI] [PubMed] [Google Scholar]
- 85.Buchanan TA, Metzger BE, Freinkel N, & Bergman RN (1990). Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. American journal of obstetrics and gynecology, 162(4), 1008–1014. [DOI] [PubMed] [Google Scholar]
- 86.Lekva T, Norwitz ER, Aukrust P, & Ueland T (2016). Impact of systemic inflammation on the progression of gestational diabetes mellitus. Current diabetes reports, 16(4), 1–11. [DOI] [PubMed] [Google Scholar]
- 87.Gomes CP, Torloni MR, Gueuvoghlanian‐Silva BY, Alexandre SM, Mattar R, & Daher S (2013). Cytokine levels in gestational diabetes mellitus: a systematic review of the literature. American Journal of Reproductive Immunology, 69(6), 545–557. [DOI] [PubMed] [Google Scholar]
- 88.Mahmoud F, Abul H, Omu A, & Haines D (2005). Lymphocyte Sub‐populations in Gestational Diabetes. American Journal of Reproductive Immunology, 53(1), 21–29. [DOI] [PubMed] [Google Scholar]
- 89.Pendeloski KPT, Mattar R, Torloni MR, Gomes CP, Alexandre SM, & Daher S (2015). Immunoregulatory molecules in patients with gestational diabetes mellitus. Endocrine, 50(1), 99–109. [DOI] [PubMed] [Google Scholar]
- 90.Schober L, Radnai D, Spratte J, et al. The role of regulatory T cell (Treg) subsets in gestational diabetes mellitus. Clin Exp Immunol. 2014;177:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie BG, Jin S, & Zhu WJ (2014). Expression of toll‑like receptor 4 in maternal monocytes of patients with gestational diabetes mellitus. Experimental and therapeutic medicine, 7(1), 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuzmicki M, Telejko B, Wawrusiewicz-Kurylonek N, Lipinska D, Pliszka J, Wilk J, … & Gorska M (2013). The expression of genes involved in NF-κB activation in peripheral blood mononuclear cells of patients with gestational diabetes. European journal of endocrinology, 168(3), 419–427. [DOI] [PubMed] [Google Scholar]
- 93.Winkler G, Cseh K, Baranyi É, Melczer Z, Speer G, Hajós P, … & Karádi I (2002). Tumor necrosis factor system in insulin resistance in gestational diabetes. Diabetes research and clinical practice, 56(2), 93–99. ety, 32(10), 774–782. [DOI] [PubMed] [Google Scholar]
- 94.Kinalski M, Telejko B, Kuźmicki M, Krętowski A, & Kinalska I (2005). Tumor necrosis factor alpha system and plasma adiponectin concentration in women with gestational diabetes. Hormone and Metabolic Research, 37(07), 450–454. [DOI] [PubMed] [Google Scholar]
- 95.Altinova AE, Toruner F, Bozkurt N, Bukan N, Karakoc A, Yetkin I, … & Arslan M (2007). Circulating concentrations of adiponectin and tumor necrosis factor-α in gestational diabetes mellitus. Gynecological Endocrinology, 23(3), 161–165. [DOI] [PubMed] [Google Scholar]
- 96.Gao XL, Yang HX, & Zhao Y (2008). Variations of tumor necrosis factor-alpha, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chinese medical journal, 121(8), 701–705. [PubMed] [Google Scholar]
- 97.Ategbo JM, Grissa O, Yessoufou A, Hichami A, Dramane KL, Moutairou K, … & Khan NA (2006). Modulation of adipokines and cytokines in gestational diabetes and macrosomia. The Journal of Clinical Endocrinology & Metabolism, 91(10), 4137–4143. [DOI] [PubMed] [Google Scholar]
- 98.Salmi AA, Zaki NM, Zakaria R, Nor Aliza AG, & Rasool AH (2012). Arterial stiffness, inflammatory and pro-atherogenic markers in gestational diabetes mellitus. Vasa, 41(2), 96–104. [DOI] [PubMed] [Google Scholar]
- 99.Maged AM, Moety GA, Mostafa WA, et al. Comparative study between different biomarkers for early prediction of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2014;27:1108–12. [DOI] [PubMed] [Google Scholar]
- 100.Kuzmicki M, Telejko B, Zonenberg A, et al. Circulating pro- and anti-inflammatory cytokines in Polish women with gestational diabetes. Horm Metab Res. 2008;40:556–60. [DOI] [PubMed] [Google Scholar]
- 101.Wolf M, Sandler L, Hsu K, Vossen-Smirnakis K, Ecker JL, & Thadhani R (2003). First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes care, 26(3), 819–824. [DOI] [PubMed] [Google Scholar]
- 102.Ozgu-Erdinc AS, Yilmaz S, Yeral MI, Seckin KD, Erkaya S, & Danisman AN (2015). Prediction of gestational diabetes mellitus in the first trimester: comparison of C-reactive protein, fasting plasma glucose, insulin and insulin sensitivity indices. The Journal of Maternal-Fetal & Neonatal Medicine, 28(16), 1957–1962. [DOI] [PubMed] [Google Scholar]
- 103.Kuzmicki M, Telejko B, Szamatowicz J, Zonenberg A, Nikolajuk A, Kretowski A, & Gorska M (2009). High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecological endocrinology, 25(4), 258–263. [DOI] [PubMed] [Google Scholar]
- 104.Morisset AS, Dube MC, Cote JA, Robitaille J, Weisnagel S, & Tchernof A (2011). Circulating interleukin‐6 concentrations during and after gestational diabetes mellitus. Acta obstetricia et gynecologica Scandinavica, 90(5), 524–530. [DOI] [PubMed] [Google Scholar]
- 105.Hassiakos D, Eleftheriades M, Papastefanou I, Lambrinoudaki I, Kappou D, Lavranos D, … & Chrousos G (2016). Increased maternal serum interleukin-6 concentrations at 11 to 14 weeks of gestation in low risk pregnancies complicated with gestational diabetes mellitus: development of a prediction model. Hormone and Metabolic Research, 48(01), 35–41. [DOI] [PubMed] [Google Scholar]
- 106.Nergiz S, Altınkaya ÖS, Küçük M, Yüksel H, Sezer SD, Kurt Ömürlü İ, & Odabaşı AR (2014). Circulating galanin and IL-6 concentrations in gestational diabetes mellitus. Gynecological Endocrinology, 30(3), 236–240. [DOI] [PubMed] [Google Scholar]
- 107.Georgiou HM, Lappas M, Georgiou GM, Marita A, Bryant VJ, Hiscock R, Permezel M, Khalil Z, Rice GE: Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol 2008; 45:157–165. [DOI] [PubMed] [Google Scholar]
- 108.Abdel Gader AG, Khashoggi TY, Habib F, Awadallah SB: Haemostatic and cytokine changes in gestational diabetes mellitus. Gynecol Endocrinol 2011; 27:356–360. [DOI] [PubMed] [Google Scholar]
- 109.Gueuvoghlanian-Silva BY, Torloni MR, Mattar R, de Oliveira LS, Scomparini FB, Nakamura MU, Daher S: Profile of inflammatory mediators in gestational diabetes mellitus: phenotype and genotype. Am J Reprod Immunol 2012; 67:241–250. [DOI] [PubMed] [Google Scholar]
- 110.Lygnos MC, Pappa KI, Papadaki HA, Relakis C, Koumantakis E, Anagnou NP, & Eliopoulos GD (2006). Changes in maternal plasma levels of VEGF, bFGF, TGF-β1, ET-1 and sKL during uncomplicated pregnancy, hypertensive pregnancy and gestational diabetes. In Vivo, 20(1), 157–163. [PubMed] [Google Scholar]
- 111.Mrizak I, Grissa O, Henault B, Fekih M, Bouslema A, Boumaiza I, … & Khan NA (2014). Placental infiltration of inflammatory markers in gestational diabetic women. Gen Physiol Biophys, 33, 169–176. [DOI] [PubMed] [Google Scholar]
- 112.Abell SK, Court D, Boyle JA, et al. Inflammatory and other biomarkers: role in pathophysiology and prediction of gestational diabetes mellitus. Int J Mol Sci. 2015;16:13442–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bao W, Baecker A, Song Y, et al. Adipokine levels during the first or early second trimester of pregnancy and subsequent risk of gestational diabetes mellitus: a systematic review. Metabolism. 2015;64:756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, & Drevon CA (2004). Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta obstetricia et gynecologica Scandinavica, 83(4), 341–347. [DOI] [PubMed] [Google Scholar]
- 115.Worda C, Leipold H, Gruber C, Kautzky-Willer A, Knöfler M, & Bancher-Todesca D (2004). Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. American journal of obstetrics and gynecology, 191(6), 2120–2124. [DOI] [PubMed] [Google Scholar]
- 116.Vitoratos N, Valsamakis G, Mastorakos G, Boutsiadis A, Salakos N, Kouskouni E, & Creatsas G (2008). Pre-and early post-partum adiponectin and interleukin-1beta levels in women with and without gestational diabetes. Hormones (Athens), 7(3), 230–6. [DOI] [PubMed] [Google Scholar]
- 117.Kautzky-Willer A, Pacini G, Tura A, Bieglmayer C, Schneider B, Ludvik B, … & Waldhäusl W (2001). Increased plasma leptin in gestational diabetes. Diabetologia, 44(2), 164–172. [DOI] [PubMed] [Google Scholar]
- 118.Fatima SS, Alam F, Chaudhry B, & Khan TA (2017). Elevated levels of chemerin, leptin, and interleukin-18 in gestational diabetes mellitus. The Journal of Maternal-Fetal & Neonatal Medicine, 30(9), 1023–1028. [DOI] [PubMed] [Google Scholar]
- 119.Mm WQ, Fan J, Khor S, Song M, Hong W, & Dai X (2014). Serum vaspin levels and vaspin mRNA expression in subcutaneous adipose tissue in women with gestational diabetes mellitus. European Journal of Obstetrics & Gynecology and Reproductive Biology, 182, 98–101. [DOI] [PubMed] [Google Scholar]
- 120.Jia X, Wang S, Ma N, Li X, Guo L, Liu X, … & Lu Q (2015). Comparative analysis of vaspin in pregnant women with and without gestational diabetes mellitus and healthy non-pregnant women. Endocrine, 48(2), 533–540. [DOI] [PubMed] [Google Scholar]
- 121.Tang Y, Qiao P, Qu X, Bao Y, Li Y, Liao Y, & Ying H (2017). Comparison of serum vaspin levels and vaspin expression in adipose tissue and smooth muscle tissue in pregnant women with and without gestational diabetes. Clinical Endocrinology. [DOI] [PubMed] [Google Scholar]
- 122.Stepan H, Kralisch S, Klostermann K, Schrey S, Reisenbüchler C, Verlohren M, … & Kratzsch J (2010). Preliminary report: circulating levels of the adipokine vaspin in gestational diabetes mellitus and preeclampsia. Metabolism, 59(7), 1054–1056. [DOI] [PubMed] [Google Scholar]
- 123.Görkem Ü, Küçükler FK, Toğrul C, & Güngör T (2016). Are adipokines associated with gestational diabetes mellitus?. Journal of the Turkish German Gynecological Association, 17(4), 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barker G, Lim R, Rice GE, & Lappas M (2012). Increased chemerin concentrations in fetuses of obese mothers and correlation with maternal insulin sensitivity. The Journal of Maternal-Fetal & Neonatal Medicine, 25(11), 2274–2280. [DOI] [PubMed] [Google Scholar]
- 125.Pfau D, Stepan H, Kratzsch J, Verlohren M, Verlohren HJ, Drynda K, … & Fasshauer M (2010). Circulating levels of the adipokine chemerin in gestational diabetes mellitus. Hormone research in paediatrics, 74(1), 56–61. [DOI] [PubMed] [Google Scholar]
- 126.Poppel MN, Zeck W, Ulrich D, Schest EC, Hirschmugl B, Lang U, … & Desoye G (2014). Cord blood chemerin: differential effects of gestational diabetes mellitus and maternal obesity. Clinical endocrinology, 80(1), 65–72. [DOI] [PubMed] [Google Scholar]
- 127.Pan BL, & Ma RM (2016). Correlation of serum omentin-1 and chemerin with gestational diabetes mellitus. Nan fang yi ke da xue xue bao= Journal of Southern Medical University, 36(9), 1231–1236. [PubMed] [Google Scholar]
- 128.Hare KJ, Bonde L, Svare JA, Randeva HS, Asmar M, Larsen S, … & Knop FK (2014). Decreased plasma chemerin levels in women with gestational diabetes mellitus. Diabetic Medicine, 31(8), 936–940. [DOI] [PubMed] [Google Scholar]
- 129.Lacroix M, Battista MC, Doyon M, Ménard J, Ardilouze JL, Perron P, & Hivert MF (2013). Lower adiponectin levels at first trimester of pregnancy are associated with increased insulin resistance and higher risk of developing gestational diabetes mellitus. Diabetes care, 36(6), 1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thagaard IN, Krebs L, Holm JC, Lange T, Larsen T, & Christiansen M (2017). Adiponectin and leptin as first trimester markers for gestational diabetes mellitus: a cohort study. Clinical Chemistry and Laboratory Medicine (CCLM). [DOI] [PubMed] [Google Scholar]
- 131.Lappas M (2015). Double stranded viral RNA induces inflammation and insulin resistance in skeletal muscle from pregnant women in vitro. Metabolism, 64(5), 642–653. [DOI] [PubMed] [Google Scholar]
- 132.Ryan EA, & Enns L (1988). Role of gestational hormones in the induction of insulin resistance. The Journal of Clinical Endocrinology & Metabolism, 67(2), 341–347. [DOI] [PubMed] [Google Scholar]
- 133.McLachlan KA, O’neal D, Jenkins A, & Alford FP (2006). Do adiponectin, TNFα, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes/metabolism research and reviews, 22(2), 131–138. [DOI] [PubMed] [Google Scholar]
- 134.Lindsay RS, & Loeken MR (2017). Metformin use in pregnancy: promises and uncertainties. Diabetologia, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hickman MA, McBride R, Boggess KA, & Strauss R (2013). Metformin compared with insulin in the treatment of pregnant women with overt diabetes: a randomized controlled trial. American journal of perinatology, 30(06), 483–490. [DOI] [PubMed] [Google Scholar]
- 136.Diamanti-Kandarakis E, Paterakis T, Alexandraki K, Piperi C, Aessopos A, Katsikis I, … & Panidis D (2006). Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Human Reproduction, 21(6), 1426–1431. [DOI] [PubMed] [Google Scholar]
References of Interest
- Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, & Wagner GP (2017). Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proceedings of the National Academy of Sciences, 201701129.This work provides molecular evidence that ancient inflammatory signals were repurposed to facilitate the process of embryo implantation in mammals.
- Consortium PACT (2015). Heterogeneity of postpartum depression: a latent class analysis. The Lancet Psychiatry, 2(1), 59–67.This study analyzes the clinical characteristics of a large number of women with postpartum depression, identifying three subtypes with distinct patterns of onset, timing, and severity.
- Raison CL, & Miller AH (2013). The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Molecular psychiatry, 18(1), 15.This review summarizes evidence for a bidirectional, functional linkage between the physiology of inflammation and the symptoms of depression.