Abstract
Background:
Renal cell carcinoma (RCC) is one of the common malignancies in the United States. RCC incidence and mortality have been changing due to many reasons. We provide a thorough investigation of incidence and mortality trends of RCC in the US using the surveillance, epidemiology and end results (SEER) database.
Methods:
The SEER 13 registries were accessed for RCC cases diagnosed between 1992 and 2015. Incidence and mortality were calculated by demographic and tumor characteristics. We calculated annual percent changes (APC) of these rates. Rates were expressed by 100,000 person-years.
Results:
A total of 104,584 RCC cases were reviewed with 47,561 deaths. The overall incidence was 11.281 per 100,000 person-years. Incidence increased by 2.421% per year (95% CI, 2.096-2.747, p<.001) but later became stable since 2008. However, the incidence of clear-cell subtype continued to increase (1.449%; 95% CI, 0.216-2.697, P=.024). RCC overall mortality rates have been declining since 2001. However, mortality associated with distant RCC only started to decrease in 2012 with APC of −18.270% (−28.775- -6.215, P = .006)
Conclusions:
Despite an overall increase in the incidence of RCC, there has been a recent plateau in RCC incidence rates with a significant decrease in mortality.
Keywords: Renal cell carcinoma, SEER program, incidence, mortality
Micro abstract:
Renal Cell Carcinoma (RCC) incidence and mortality have been changing due to many reasons. We used SEER to review 104,584 RCC cases with 47,561 deaths diagnosed between 1992 and 2015. Despite an overall increase in the incidence of RCC, there has been a recent plateau in RCC incidence rates with a significant decrease in mortality.
1. Introduction:
Renal cell carcinoma (RCC) is ranked as the sixth and tenth most common malignancy in American males and females, respectively (1). In the United States, the estimated number of diagnosed cases in 2018 is 65,340 and the estimated number of deaths is 14,970 (2). Histologically, RCC is further classified into subtypes; the most common one is clear cell histology, followed by papillary subtype (1).
An increase in incidence may be attributed to incidental diagnosis due to increased usage of ultrasonography and computed tomography (CT) in health care settings, as well as to shifts in the prevalence of RCC risk factors such as smoking, obesity, and hypertension (3, 4). A delicate interplay between the decline in consumption of tobacco products in industrialized countries and an increased prevalence of obesity and hypertension may influence RCC incidence (5, 6).
Since the 1990s, the mortality rates of RCC is declining in western countries (7, 8). This downward shift might be partially attributed to the majority of cases being diagnosed in early stages along with the overall survival improvement of patients with advanced disease after the introduction of antiangiogenics (9).
A continuous analysis of epidemiological data is crucial in understanding the incidence and mortality trends in different populations. In this study, we aimed to use the Surveillance, Epidemiology, and End Results (SEER) cancer registry to study the trends in the incidence and mortality of RCC in the United States over the past 20 years.
2. Methodology:
2.1. Data source:
We used SEER*stat software (version 8.3.5) to access the SEER database. We used the SEER 13 registries (November 2017 submission) that includes data of patients from 1992 to 2015, and - covers about 13.4% of the US population (10, 11).
2.2. Study population:
We included RCC cases diagnosed between 1992 and 2015 and whose diagnosis did not rely only on autopsy or death certificates. For this selection, we used the following SEER variables: ‘primary site - labeled: C64.9-Kidney, NOS’, and ‘Histology recode - broad groupings: 8140-8389 adenomas and adenocarcinomas’. We reviewed the following variables within the selected cases: sex, race, age at diagnosis (or age at death in case of mortality calculation), state, stage at diagnosis (using SEER historic stage A), tumor size, and histological subtype (using ICD-O-3 histology recode). In addition, we did a subgroup analysis for clear cell RCC cases separately and reviewed the same mentioned variables in this population.
2.3. Outcomes:
We calculated incidence and incidence-based mortality rates for the RCC population and clear cell population according to the previously mentioned variables. Rates were adjusted to the 2000 US standard population and expressed by 100,000 person-years. Incidence-based mortality was calculated as the number of RCC deaths among cases diagnosed over person-time at risk among people in SEER areas (12). Rates were calculated during 1992-2015 except for chromophobe RCC cases (1992-2015 for incidence, and 1997-2015 for mortality), and collecting duct RCC cases (2001-2015). To observe the change of rates over the study period, we calculated the Annual Percentage Changes (APCs).
2.4. Statistical analysis:
Incidence and incidence-based mortality rates were calculated using SEER*stat software (11). APCs were calculated using The National Cancer Institute’s Joinpoint Regression program, version 4.5.0.1 (13). The software examined rates over time and detected significant changes in APCs, then selected the best model with the least number of joinpoints (14). P values were calculated using t-tests and were considered significant when less than 0.05. All statistical tests were two-sided.
3. Results:
3.1. Baseline characteristics:
We reviewed 104,584 patients with RCC diagnosed during 1992-2015 (Table 1). Most of these patients were males (63.7%), and whites (80%). Most tumors were smaller than 7 cm (65%) and localized at diagnosis (65.1%). The most common histological subtype was clear cell type (44.8%) with the histological type being unknown in 37.8% of the cases. During 1992-2015, 47,561 of included patients died of RCC (Table 1). Most of those patients were males (65%), whites (81.6%), and older than 65 (71.4%).
Table 1.
Renal Cell Carcinoma ‘RCC’ Incidence and incidence-based mortality rates (1992-2015)
| characteristic | Incidence | Incidence-based mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| RCC | Clear cell type | RCC | Clear cell type | |||||
| Cases, No (%)a |
Rate (95% CI)b | Cases, No (%)a |
Rate (95% CI)b | Deaths ,No (%)a |
Rate (95% CI)b | Deaths, No (%)a |
Rate (95% CI)b | |
| Overall | 104,584 (100) | 11.281 (11.212-11.350) | 46,818 (100) | 5.020 (4.974-5.066) | 47,561 (100) | 5.256 (5.209-5.304) | 15,561 (100) | 1.715 (1.688-1.742) |
| Sex | ||||||||
| Male | 66,624 (63.7) | 15.795 (15.673-15.917) | 29,221 (62.4) | 6.789 (6.710-6.868) | 30,943 (65) | 8.107 (8.015-8.199) | 10,126 (65) | 2.606 (2.554-2.658) |
| Female | 37,960 (36.3) | 7.562 (7.486-7.638) | 17,597 (37.6) | 3.524 (3.472-3.576) | 16,618 (34.9) | 3.190 (3.141-3.239) | 5,435 (35) | 1.054 (1.026-1.082) |
| Race | ||||||||
| White | 83,687 (80) | 11.548 (11.469-11.627) | 38,722 (82.7) | 5.331 (5.278-5.384) | 38,816 (81.6) | 5.373 (5.319-5.426) | 13,115 (84.3) | 1.816 (1.784-1.847) |
| Black | 11,820 (11.3) | 13.899 (13.643-14.159) | 3,307 (7) | 3.805 (3.673-3.940) | 5,237 (11) | 6.922 (6.731-7.118) | 1,161 (7.5) | 1.504 (1.416-1.595) |
| American Indian/ Alaska native | 1,406 (1.3) | 13.419 (12.682-14.197) | 638 (1.4) | 5.723 (5.262-6.222) | 651 (1.4) | 7.772 (7.149-8.439) | 184 (1.2) | 2.171 (1.851-2.537) |
| Asian or Pacific islander | 7,074 (6.8) | 6.628 (6.473-6.786) | 3,829 (8.2) | 3.550 (3.438-3.666) | 2,804 (5.9) | 2.825 (2.721-2.933) | 1,083 (7) | 1.084 (1.020-1.152) |
| Age at diagnosis, y | ||||||||
| <65 | 54,451 (52) | 6.304 (6.251-6.357) | 26,631 (56.9) | 3.085 (3.048-3.123) | 13,598 (28.6) | 1.550 (1.524-1.576) | 4,882 (31.4) | 0.548 (0.532-0.564) |
| >65 | 50.133 (48) | 45.686 (45.286-46.089) | 20,187 (43.1) | 18.392 (18.138-18.648) | 33,963 (71.4) | 30.876 (30.548-31.207) | 10,739 (69) | 9.780 (9.595-9.967) |
| State | ||||||||
| Alaska | 340 (0.32) | 20.475 (18.225-22.95) | 156 (0.33) | 8.708 (7.332-10.351) | 160 (0.33) | 12.603 (10.614-14.902) | 44 (0.3) | 3.393 (2.410-4.699) |
| California | 36,515 (35) | 10.089 (9.986-10.194) | 16,680 (35.6) | 4.571 (4.502-4.642) | 16,507 (34.7) | 4.720 (4.648-4.793) | 5,391 (34.6) | 1.536 (1.495-1.578) |
| Connecticut | 10,968 (10.5) | 11.886 (11.664-12.112) | 4,145 (8,9) | 4.501 (4.364-4.641) | 4,829 (10) | 5.075 (4.932-5.221) | 1,312 (8) | 1.383 (1.309-1.461) |
| Georgia | 6,970 (6.7) | 11.095 (10.828-11.367) | 2,105 (4.5) | 3.307 (3.163-3.456) | 2,895 (6) | 5.223 (5.029-5.423) | 593 (3.8) | 1.055 (0.969-1.146) |
| Hawaii | 3,208 (3) | 9.668 (9.335-10.011) | 1,782 (3.8) | 5.358 (5.111-5.615) | 1,326 (2.8) | 3.947 (3.737-4.167) | 535 (3.4) | 1.585 (1.453-1.727) |
| Iowa | 10,680 (10,2) | 13.348 (13.094-13.606) | 5,712 (12.2) | 7.260 (7.072-7.453) | 5,403 (11.4) | 6.320 (6.152-6.493) | 2,257 (14.5) | 2.677 (2.566-2.791) |
| Michigan | 13,743 (13.1) | 13.817 (13.587-14.051) | 6,345 (13.6) | 6.371 (6.215-6.530) | 6,552 (13.8) | 6.665 (6.504-6.829) | 2,404 (15.4) | 2.437 (2.340-2.536) |
| New Mexico | 5,129 (4.9) | 10.947 (10.647-11.254) | 1,816 (3.9) | 3.850 (3.674-4.033) | 2,484 (5.2) | 5.455 (5.241-5.676) | 568 (3.7) | 1.236 (1.136-1.343) |
| Utah | 4,364 (4.1) | 9.684 (9.396-9.978) | 2,576 (5.5) | 5.653 (5.435-5.878) | 1,868 (3.9) | 4.402 (4.203-4.608) | 931 (6) | 2.176 (2.038-2.322) |
| Washington | 12,667 (12.1) | 12.354 (12.138-12.573) | 5,492 (11.7) | 5.288 (5.148-5.431) | 5,537 (11.6) | 5.626 (5.478-5.777) | 1,526 (9.8) | 1.539 (1.462-1.619) |
| Stage at diagnosisc | ||||||||
| Localized | 68,094 (65.1) | 7.323 (7.268-7.379) | 32,983 (70.4) | 3.532 (3.494-3.570) | 21,313 (44.8) | 2.376 (2.344-2.408) | 7,744 (49.8) | 0.861 (0.842-0.881) |
| Regional | 16,480 (15.8) | 1.785 (1.758-1.813) | 8,183 (17.8) | 0.883 (0.863-0.902) | 8,921 (18.8) | 0.989 (0.969-1.010) | 3,423 (22) | 0.379 (0.366-0.392) |
| Distant | 16,513 (15.8) | 1.785 (1.758-1.812) | 5,217 (11.1) | 0.557 (0.542-0.573) | 14,641 (30.1) | 1.592 (1.566-1.618) | 4,153 (26.7) | 0.447 (0.434-0.461) |
| Tumor size, cm | ||||||||
| < 7 | 67,936 (65) | 7.328 (7.273-7.34) | 32,846 (70.1) | 3.526 (3.488-3.564) | 23,824 (50) | 2.655 (2.621-2.689) | 8,403 (54) | 0.935 (0.915-0.955) |
| 7 - 10 | 16,265 (15.6) | 1.751 (1.724-1.778) | 7,608 (16.3) | 0.814 (0.796-0.833) | 9,706 (20.4) | 0.997 (0.976-1.017) | 3,349 (21.5) | 0.367 (0.354-0.379) |
| > 10 | 12,839 (12.3) | 1.375 (1.351-1.399) | 4,983 (10.6) | 0.530 (0.515-0.545) | 8,554 (1818) | 0.930 (0.910-0.950) | 2,859 (18.4) | 0.309 (0.297-0.320) |
| Histological subtype | ||||||||
| Clear cell | 46,818 (44.8) | 5.020 (4.974-5.066) | 15,414 (32.4) | 1.698 (1.672-1.726) | ||||
| Papillary | 8,730 (8.3) | 0.934 (0.915-0.954) | 2,219 (4.7) | 0.245 (0.235-0.255) | ||||
| Chromophobed | 4,127 (3.9) | 0.443 (0.430-0.457) | 664 (1.4) | 0.074 (0.068-0.080) | ||||
| Collecting ducte | 209 (0.2) | 0.022 (0.020-0.026) | 140 (0.3) | 0.015 (0.013-0.018) | ||||
| Others | 5,145 (4.9) | 0.554 (0.539-0.570) | 2,683 (5.6) | 0.294 (0.283-0.305) | ||||
| Unknown | 39,555 (37.8) | 4.307 (4.264-4.350) | 26,441 (55.6) | 2.929 (2.894-2.965) | ||||
Cases included first primary tumors that matched the selection criteria, were microscopically confirmed, and were not identified only from autopsy records or death certificates.
Rates were calculated as number of cases per 100,000 person-years and age adjusted to the 2000 US standard population.
using SEER historic stage A
chromophobe RCC cases diagnosed during 1992-2014, and deaths during 1997-2014
collecting duct RCC cases diagnosed during 2001-2014, and deaths during 2001-2014
3.2. Incidence rates and trends over time:
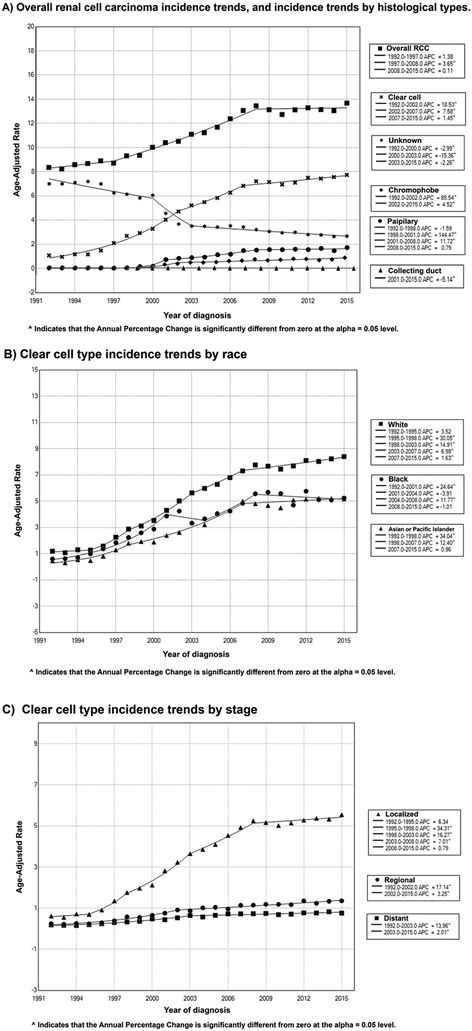
The overall RCC incidence during the study period was 11.281 per 100,000 person-years [95% CI, 11.212-11.350]). Incidence of RCC was highest among males (15.795 [95% CI, 15.673-15.917]), blacks (13.899 [95% CI, 13.643-14.159]), and people older than 65 years (45.686 [95% CI, 45.286-46.089]). When looking at the geographical differences, incidence was highest in Alaska (20.475 [95% CI, 18.225-22.95]) and lowest in Hawaii (9.668 [95% CI, 9.335-10.011]) compared to the other states included in the registries. Among histological subtypes, the incidence of clear cell type was the highest (5.020 [95% CI, 4.974-5.066) (Table 1, Supplementary Table 1, Supplementary Table 2).
Over the study period, RCC incidence rates increased at 2.421% per year (95% CI, 2.096-2.747, p<.001). However, this increase in incidence plateaued over the last 7 years of the study period (2008 to 2015) (APC of 0.111%, 95% CI, [−0.483, 0.708], P = .699). Furthermore, this overall increase was reflected over most of the various study subgroups including Whites (APC of 2.444, 95% CI [2.107, 2.783], P= <0.001) and Blacks (APC of 2.579, 95 % CI [2.069, 3.091], P=<0.001). This incremental incidence in RCC, however, was mostly notable in the localized and regional diseases rather than distant RCC where the overall incidence was stable (APC of - 0.240%, 95% CI [−0.551,0.072], P = .125), (Figure 1, Table 2, Supplementary Table 3).
Figure 1.
Trends in annual renal cell carcinoma incidence (1992-2015).
Table 2.
Trends in renal cell carcinoma Incidence Rates (1992-2015)
| Overall (1992-2015) |
Trend | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||||||
| APCa
(95% CI) |
P valueb |
year | APCa
(95% CI) |
P valueb |
year | APCa
(95% CI) |
P valueb |
year | APCa
(95% CI) |
P valueb |
year | APCa
(95% CI) |
P valueb |
|
| Overall | 2.421 (2.096-2.747) | <.001 | 1992-1997 | 1.378 (−0.079-2.855) | .062 | 1997-2008 | 3.650 (3.221-4.082) | <.001 | 2008-2015 | 0.111 (−0.483-0.708) | .699 | |||
| Sex | ||||||||||||||
| Male | 2.373 (2.077-2.671) | <.001 | 1992-1997 | 1.153 (−0.745-3.088) | .218 | 1997-2008 | 3.494 (2.935-4.055) | <.001 | 2008-2015 | 0.390 (−0.371-1.157) | .294 | |||
| Female | 2.280 (1.859-2.703) | <.001 | 1992-2008 | 3.267 (2.826-3.711) | <.001 | 2008-2015 | −0.330 (−1.522-0.876) | .572 | ||||||
| Race | ||||||||||||||
| White | 2.444 (2.107-2.783) | <.001 | 1992-1997 | 1.395 (−0.446-3.271) | .129 | 1997-2007 | 3.893 (3.234-4.556) | <.001 | 2007-2015 | 0.378 (−0.266-1.025) | .232 | |||
| Black | 2.579 (2.069-3.091) | <.001 | 1992-2009 | 3.442 (2.705-4.184) | <.001 | 2009-2015 | −0.306 (−2.797-2.249) | .502 | ||||||
| American Indian/Alaska Native | 0.747 (−0.194-1.696) | .114 | 1992-2012 | 1.460 (0.263-2.672) | .019 | 2012-2015 | −7.661 (−20.944-7.854) | .296 | ||||||
| Asian or Pacific Islander | 3.017 (2.592-3.444) | <.001 | 1992-2004 | 2.723 (2.073-3.377) | <.001 | 2004-2007 | 9.723 (1.568-18.532) | .022 | 2007-2010 | −2.03 (−8.598-5.007) | .534 | 2010-2015 | 2.210 (0.794-3.646) | .005 |
| Age at diagnosis, y | ||||||||||||||
| <65 | 2.564 (2.266-2.863) | <.001 | 1992-1997 | 1.324 (−0.954-3.655) | .233 | 1997-2008 | 3.709 (3.056-4.367) | <.001 | 2008-2013 | −0.11 (−2.214-2.035) | .911 | 2013-2015 | 4.756 (−1.807-11.757) | .145 |
| >65 | 2.287 (1.892-2.683) | <.001 | 1992-2003 | 2.546 (1.995-3.100) | <.001 | 2003-2007 | 5.549 (1.979-9.244) | .004 | 2007-2015 | −0.442 (−1.103-0.223) | .177 | |||
| Stage at diagnosisc | ||||||||||||||
| Localized | 3.812 (3.258-4.369) | <.001 | 1992-1997 | 2.778 (0.558-5.047) | .017 | 1997-2007 | 6.169 (5.439-6.905) | <.001 | 2007-2015 | 0.560 (−0.082-1.206) | .083 | |||
| Regional | 0.711 (0.441-0.982) | <.001 | 1992-2015 | 0.711 (0.441-0.982) | <.001 | |||||||||
| Distant | −0.240 (−0.551-0.072) | .125 | 1992-1998 | 1.879 (−0.483-4.296) | .113 | 1998-2015 | −0.640 (−1.070--.0208) | .006 | ||||||
| Tumor size, cm | ||||||||||||||
| < 7 | 4.174 (3.632-4.719) | <.001 | 1992-1997 | 2.491 (0.518-4.503) | .016 | 1997-2007 | 6.622 (5.970-7.278) | <.001 | 2007-2015 | 1.016 (0.456-1.580) | .001 | |||
| 7-10 | 0.658 (0.223-1.094) | .005 | 1992-2004 | 2.080 (1.106-3.065) | <.001 | 2004-2015 | −0.706 (−1.639-0.236) | .133 | ||||||
| > 10 | 0.222 (−0.071-0.515) | .130 | 1992-2015 | 0.222 (−0.071-0.515) | .130 | |||||||||
| Histological subtype | ||||||||||||||
| Clear cell | 7.193 (5.464-8.950) | <.001 | 1992-2002 | 18.526 (16.195-20.904) | <.001 | 2002-2007 | 7.575 (3.067-12.281) | .002 | 2007-2015 | 1.449 (0.216-2.697) | .024 | |||
| Papillary | 9.052 (5.451-12.776) | <.001 | 1992-1998 | −1.591 (−15.20-14.213) | .820 | 1998-2001 | 144.469 (55.289-284.864) | .001 | 2001-2008 | 11.724 (8.507-15.035) | <.001 | 2008-2015 | 0.789 (−0.981-2.592) | .355 |
| Chromophobe | 7.846 (4.298-11.515) | <.001 | 1992-2002 | 85.539 (60.374-114.652) | <.001 | 2002-2015 | 4.517 (2.846-6.214) | <.001 | ||||||
| Collecting ductd | −5.141 (−8.755--1.384) | .012 | 2001-2015 | −5.141 (−8.755--1.384) | .012 | |||||||||
| Others | 2.598 (0.323-4.923) | .027 | 1992-1999 | 8.751 (4.092-13.617) | .001 | 1999-2002 | 32.430 (6.001-65.448) | .017 | 2002-2015 | −5.239 (−7.470--2.955) | <.001 | 2010-2015 | 0.774 (−3.371-5.097) | .698 |
| Unknown | −5.085 (−5.708--4.458) | <.001 | 1992-2000 | −2.992 (−4.050--1.923) | <.001 | 2000-2003 | −15.358 (−24.990--4.488) | .010 | 2003-2015 | −2.262 (−3.008--1.509) | <.001 | |||
Annual Percentage Changes, calculated using Joinpoint regression software
Two-sided P value was calculated using t test to determine the significance of APC change
Using SEER historic stage A
collecting duct RCC cases diagnosed during 2001-2015
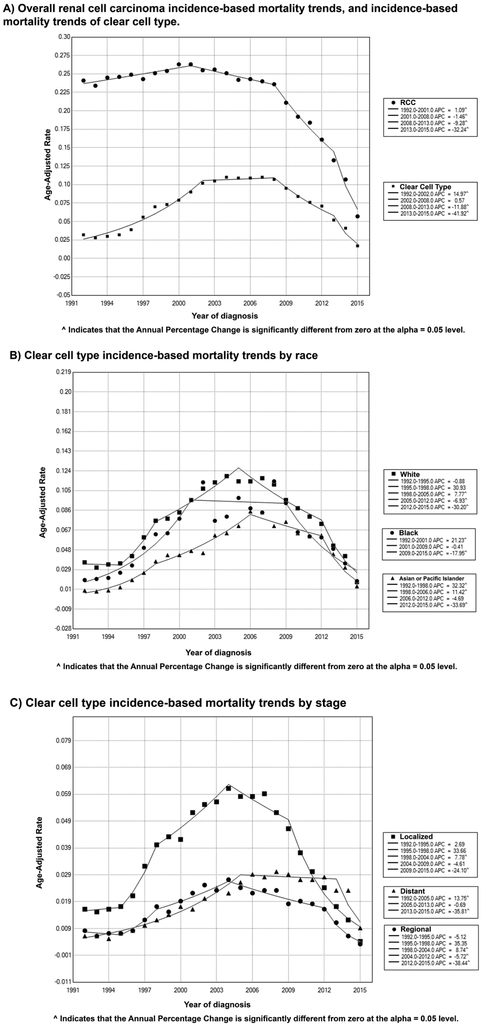
3.3. Incidence-based mortality rates and trends over time:
Overall incidence-based mortality rates of RCC during the study period was 5.256 (95%CI, [5.209,5.304]) per 100,000 person-years. Incidence-based mortality rates were highest among males (8.107 [95% CI, 8.015, 8.199]), American Indians/Alaska natives (7.772 [95% CI, 7.149, 8.439]), and patients older than 65 years (30.876 [95% CI, 30.548,31.207]). Incidence-based mortality was highest in Alaska (12.603 [95% CI, 10.614, 14.902]), and lowest in Hawaii (3.947 [95% CI, 3.737, 4.167]) when compared to other states included in the registries (Table 1, Supplementary Table 1, Supplementary Table 2).
Over the study period, RCC incidence-based mortality rates decreased by −2.159% per year (95% CI, −3.342, −0.962, P < .001). The incidence-based mortality rates increased from 1992 and peaked in 2001, when it started to decrease significantly until 2015. This recent decrease in mortality became more pronounced since 2013 (APC of −32.242, 95% CI [−38.991, −24.745], P < .001). This trend was noted in most subgroups including males (APC of −2.110, 95% CI [−3.322, −0.883], P < .001) females (APC of −2.725, 95% CI [−4.099, −1.331], P < .001), Whites (APC of - 2.505, 95% CI [−3.691, −1.304], P < .001), and Blacks (APC of −1.738, 95% CI [−3.205, −0.250], P < .001). (Figure 2, Table 3, Supplementary Table 4).
Figure 2.
Trends in annual renal cell carcinoma incidence-based mortality (1992-2015).
Table 3.
Trends in renal cell carcinoma Incidence-based mortality Rates (1992-2015)
| Overall (1992-2015) |
Trend | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||||||
| APCa
(95% CI) |
P valueb |
year | APCa
(95% CI) |
P valueb |
year | APCa
(95% CI) |
P valueb |
year | APCa
(95% CI) |
P valueb |
year | APCa
(95% CI) |
P valueb |
|
| Overall | −2.159 (−3.342- -0.962) | .001 | 1992-2001 | 1.092 (0.631-1.555) | <.001 | 2001-2008 | −1.465 (−2.297- -0.625) | .002 | 2008-2013 | −9.282 (−11.013- -7.517) | <.001 | 2013-2015 | −32.242 (−38.991- -24.745) | <.001 |
| Sex | ||||||||||||||
| Male | −2.110 (−3.322- -0.883) | .002 | 1992-2001 | 1.251 (0.732-1.772) | <.001 | 2001-2008 | −1.329 (−2.235- -0.415) | .008 | 2008-2013 | −9.167 (−10.923- -7.376) | <.001 | 2013-2015 | −32.913 (−39.353- -25.789) | <.001 |
| Female | −2.725 (−4.099- -1.331) | .001 | 1992-2006 | 0.143 (−0.343-0.632) | .541 | 2006-2013 | −7.965 (−9.782- -6.112) | <.001 | 2013-2015 | −38.369 (−48.328- -26.492) | <.001 | |||
| Race | ||||||||||||||
| White | −2.505 (−3.691- -1.304) | <.001 | 1992-2001 | 0.850 (0.216-1.488) | .012 | 2001-2008 | −1.949 (−3.117- -0.767) | .004 | 2008-2013 | −9.666 (−11.985- -7.286) | <.001 | 2013-2015 | −33.833 (−42.509- -23.847) | <.001 |
| Black | −1.738 (−3.205- -0.250) | .024 | 1992-/2009 | 0.718 (−0.217-1.661) | .125 | 2009-2015 | −17.216 (−22.175- -11.942) | <.001 | ||||||
| American Indian/Alaska Native | −0.702 (−2.536-1.166) | .441 | 1992-2010 | 1.375 (−0.516-3.301) | .145 | 2010-2015 | −19.902 (−33.518- -3.498) | .022 | ||||||
| Asian or Pacific Islander | 0.586 (−0.854-2.046) | .410 | 1992-2007 | 3.387 (2.793-3.984) | <.001 | 2007-2013 | −4.61 (−7.216- -1.948) | .002 | 2013-2015 | −36.2 (−47.467- -22.736) | <.001 | |||
| Age at death, y | ||||||||||||||
| <65 | −1.606 (−3.225-0.039) | .055 | 1992-2006 | 2.015 (1.363-2.671) | <.001 | 2006-2013 | −5.619 (−7.870- -3.312) | <.001 | 2013-2015 | −37.753 (−46.951- -26.961) | <.001 | |||
| >65 | −2.704 (−3.927- -1.466) | <.001 | 1992-2001 | 0.627 (0.099-1.158) | .024 | 2001-2008 | −2.007 (−2.979- -1.026) | .001 | 2008-2013 | −10.183 (−12.149- -8.172) | <.001 | 2013-2015 | −33.729 (−40.709- -25.926) | <.001 |
| Stage at diagnosisd | ||||||||||||||
| Localized | −3.225 (−5.042- -1.373) | .002 | 1992-2001 | 1.745 (0.893-2.604) | .001 | 2001-2008 | −2.115 (−3.695- -0.509) | .014 | 2008-2013 | −16.099 (−18.729- -13.383) | <.001 | 2013-2015 | −42.356 (−52.584- -29.921) | <.001 |
| Regional | −3.465 (−4.749- -2.164) | <.001 | 1992-2004 | −0.629 (−1.241- -0.013) | .046 | 2004-2012 | −6.111 (−7.848- -4.341) | <.001 | 2012-2015 | −36.292 (−44.120- -27.368) | <.001 | |||
| Distant | 0.046 (−0.856-0.957) | .917 | 1992-2012 | 1.162 (0.553-1.774) | .001 | 2012-2015 | −18.270 (−28.775- -6.215) | .006 | ||||||
| Tumor size, cm | ||||||||||||||
| < 7 | −2.054 (−3.580- -0.505) | .012 | 1992-2001 | 1.967 (1.070-2.873) | <.001 | 2001-2008 | −0.698 (−2.218-0.846) | .345 | 2008-2012 | −11.099 (−15.408- -6.570) | <.001 | 2012-2015 | −28.267 (−34.748- -21.144) | <.001 |
| 7-10 | −2.105 (−3.550- -0.640) | .007 | 1992-2004 | 1.587 (0.464-2.723) | .008 | 2004-2013 | −5.553 (−7.912- -3.134) | < | 2013-2015 | −39.736 (−57.670- -14.204) | .008 | |||
| > 10 | −1.358 (−2.458- -0.246) | .019 | 1992-2002 | 1.574 (0.616-2.541) | .003 | 2002-2013 | −2.427 (−3.375- -1.470) | <.001 | 2013-2015 | −34.826 (−45.828- -21.588) | <.001 | |||
Annual Percentage Changes, calculated using Joinpoint regression software
Two-sided P value was calculated using t test to determine the significance of APC change
Using SEER historic stage A
4. Discussion:
Our study evaluates the trends of incidence and mortality rates of RCC in the United States utilizing a single comprehensive registry system (SEER database) for over two decades. We found that there had been an initial overall increase in incidence and mortality rates of RCC. However, over the last decade, there has been a plateau in the incidence of RCC accompanied by a significant improvement in mortality.
The changes in the incidence rate may be attributed to incidental diagnosis and/or changes in the prevalence of RCC risk factors. Recently, there has been a significant increase in the use of advanced abdominal imaging in the evaluation of unrelated abdominal symptoms (15, 16). For example, a recent study found that the frequent use of CT scans is associated with increased risk of undergoing a nephrectomy (17). CT scans have a better sensitivity in detecting a renal mass than an ultrasonography. However, imaging studies cannot reliably distinguish benign vs malignant features of solid renal masses prompting more intensive workup for all solid tumors regardless of the size (18-21).
In addition to an increase in the use of abdominal imaging, the prevalence of the risk factors affecting RCC has been shifting over the years. Changing prevalence of environmental factors affecting RCC constitutes a real change in the incidence rather than a mere increase in detection. Multiple environmental factors have been implied as risk factors for RCC such as smoking, occupational exposure to cadmium and asbestos, phenacetin-containing analgesics, as well as chemotherapeutic agents used for childhood malignancies. In addition to chemical exposure, numerous medical conditions can increase the risk of RCC, namely, obesity, hypertension, diabetes mellitus, and dialysis (22-26). Smoking increases the risk of RCC as well as the risk of lymph node involvement and distant metastasis on presentation. This increased risk is evident in both current and previous smokers (5, 27, 28). However, smoking has been trending down in the US over the past 5 decades which correlates with the trends of RCC significantly (28). In contrast to the decreasing prevalence of smoking in the US, the prevalence of medical conditions that increase the risk of RCC has been on the rise over the past decades. Since 1960, the prevalence of obesity has increased three-fold and diabetes mellitus has increased seven-fold (29-31). Obesity is associated with a higher risk of developing RCC. Paradoxically, it is also associated with lower stage at diagnosis as well as longer survival (32, 33). Those changes in lifestyle factors in the United States, declining smoking and inclining obesity and diabetes, have significantly affected the incidence rates as well as mortality of the developed RCC.
Management of patients with RCC is multi-disciplinary consisting of surgical resection, radiotherapy, and systemic therapy. For a patient with a limited localized disease, surgical resection is the treatment of choice which can be either radical or partial nephrectomy. Partial nephrectomy can be done laparoscopically, is less invasive, and can be used to resect multiple smaller tumors while preserving renal parenchymal tissue that is utilized in patients who have impaired renal function, bilateral disease, or solitary kidney (34). Radical nephrectomy is more commonly used and is more appropriate for lesions with regional invasion (35). Multiple studies have assessed the survival of patient undergoing partial vs. radical nephrectomy and found that partial nephrectomy is associated with a better overall survival as well as cancer specific survival. However, populations with specific cancer stages, T1b and T2, were the populations demonstrating better survival outcomes following partial nephrectomy (36-38).
The introduction of vascular endothelial growth factor (VEGF) inhibitors (also known as antiangiogenics) and checkpoint inhibitors for advanced cases of RCC has significantly impacted the survival of these patients (39-42). The Food and Drug Administration (FDA) approval of sorafenib and sunitinib in 2005 and 2006, respectively, followed by the approval of more antiangiogenic therapies have been a pivotal point in advanced RCC treatment. Following the approval of the first VEGF inhibitors, mTOR (mammalian Target Of Rapamycin) inhibitors were also developed and used as monotherapies or in combination in the treatment of advanced clear cell RCC. The activity of the newer targeted therapies have been investigated over the last decade with multiple clinical trials concluding that the VEGF-TKI and mTOR inhibitors were associated with improved overall survival as well as progression disease survival (43). The decreasing mortality trends seen starting 2007 and continuing until 2015 is associated with the introduction of such therapies for RCC treatment (44). In addition, mortality rates of cases with distant metastasis have decreased significantly during that time period. This further solidifies the evidence that introduction of VEGF inhibitors for patients with RCC has significantly affected the survival and mortality. Other studies have also reported trends similar to our results with a decline in mortality following introduction of VEGF inhibitors (45, 46).
This study has certain limitations. We did not perform mortality over incidence (MOI) analysis. While the MOI analysis could account for the variation in incidence over time, a recent study demonstrated a correlation between changes in survival rates and MOI changes overtime, showing that survival measures alone can be used as rough estimation of progress in clinical care of cancer (47).
Sources of bias and variations exist due to the retrospective and descriptive nature of our study. In addition, SEER database does not capture the environmental exposure or individual lifestyle habits and comorbidities, thus negating the documentation of a direct association between individual exposure to the incidence and mortality of RCC and only allowing speculation over the association with the observed trends. In addition to that, SEER database was limited in tumor histology with a large number of cases described as unknown histology. Moreover, data on tumor size and stage were only available on certain years leading to a potential bias in the analysis and the results. In addition, the SEER database misses clinically important data as well as temporal follow up of patients (48). While the SEER database is not sensitive enough to compare outcomes conditioned on treatment or comparative effectiveness research, it certainly covers around 10-30% of the US population based on the registry. On top of that, SEER database is one of the best epidemiological tools and databases currently available to study incidence and mortality trends.
5. Conclusions:
In patients residing in the United States with a diagnosis of RCC from 1992-2015, overall incidence and mortality rates have increased. However, recent years have shown that the incidence rates have stabilized, and the mortality rates have decreased. The changes seen in the incidence trends may be attributed to increasing detection in addition to social changes in the prevalence of modifiable risk factors. The decreasing mortality trends can be correlated to multiple factors including the improvement in overall survival and management of advanced disease with the introduction of antiangiogenics and the impact of these therapeutic agents has on RCC survival.
Supplementary Material
Clinical practice points:
Renal Cell Carcinoma (RCC) incidence and mortality have been changing due to many reasons. Since the 1990s, the mortality rates of RCC is declining in western countries. A continuous analysis of epidemiological data is crucial in understanding the incidence and mortality trends in different populations. We used SEER to review 104,584 RCC cases with 47,561 deaths diagnosed between 1992 and 2015. In patients residing in the United States with a diagnosis of RCC from 1992-2015, overall incidence and mortality rates have increased. However, recent years have shown that the incidence rates have stabilized, and the mortality rates have decreased. The decreasing mortality can be correlated to the improvement in overall survival and management of advanced disease with the introduction of antiangiogenics.
Acknowledgments
Sources of Funding:
This work was supported by the NCI R01CA224917 (T.H.H.) and the Department of Defense W81XWH-17-1-0546 (T.H.H.). Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of authors:
All authors participated in designing the concept of the paper. AS and MA conducted all data analyses and had full access to the database. All authors have contributed to data interpretation and writing the paper. All authors have revised and agreed to the content of the paper. MS and TH supervised the whole project scientifically and had final responsibility for the decision to submit for publication. AS Managed and coordinated the research activity planning and execution.
Conflicts of Interest/Disclosure Statements:
All authors declare that they have no conflict of interest.
References:
- 1.Cheng L, Zhang S, T MacLennan G, Lopez-Beltran A, Montironi R. Molecular and cytogenetic insights into the pathogenesis, classification, differential diagnosis, and prognosis of renal epithelial neoplasms 2009. 10–29 p. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nature reviews Urology. 2010;7(5):245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabaria R, Klaassen Z, Terris MK. Renal cell carcinoma: links and risks. International journal of nephrology and renovascular disease. 2016;9:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cumberbatch MG, Rota M, Catto JW, La Vecchia C. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. European urology. 2016;70(3):458–66. [DOI] [PubMed] [Google Scholar]
- 6.Wilson KM, Cho E. Obesity and Kidney Cancer. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2016;208:81–93. [DOI] [PubMed] [Google Scholar]
- 7.Bosetti C, Bertuccio P, Chatenoud L, Negri E, La Vecchia C, Levi F. Trends in mortality from urologic cancers in Europe, 1970–2008. European urology. 2011;60(1):1–15. [DOI] [PubMed] [Google Scholar]
- 8.Levi F, Ferlay J, Galeone C, Lucchini F, Negri E, Boyle P, et al. The changing pattern of kidney cancer incidence and mortality in Europe. BJU international. 2008;101(8):949–58. [DOI] [PubMed] [Google Scholar]
- 9.Gu L, Ma X, Li H, Chen L, Xie Y, Li X, et al. Comparison of oncologic outcomes between partial and radical nephrectomy for localized renal cell carcinoma: A systematic review and meta-analysis. Surg Oncol. 2016;25(4):385–93. [DOI] [PubMed] [Google Scholar]
- 10.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2017 Sub (1992–2015) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission
- 11.Surveillance Research Program, National Cancer Institute SEER*Stat software (www.seer.cancer.gov/seerstat) version 8.3.5.
- 12.Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: an application to female breast cancer. Journal of clinical epidemiology. 1994;47(12):1451–61. [DOI] [PubMed] [Google Scholar]
- 13.Joinpoint Regression Program, Version 4.5.0.1. June, 2017; Statistical Research and Applications Branch, National Cancer Institute. https://surveillance.cancer.gov/joinpoint/. Last accessed on 26/7/2017. [Google Scholar]
- 14.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in medicine. 2000;19(3):335–51. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Kang G, Moon SB. Increasing utilization of abdominal CT in the Emergency Department of a secondary care center: does it produce better outcomes in caring for pediatric surgical patients? Annals of surgical treatment and research. 2014;87(5):239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maehara CK, Silverman SG, Lacson R, Khorasani R. Journal club: Renal masses detected at abdominal CT: radiologists' adherence to guidelines regarding management recommendations and communication of critical results. AJR American journal of roentgenology. 2014;203(4):828–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welch HG, Skinner JS, Schroeck FR, Zhou W, Black WC. Regional Variation of Computed Tomographic Imaging in the United States and the Risk of Nephrectomy. JAMA internal medicine. 2018;178(2):221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverman SG, Israel GM, Trinh QD. Incompletely characterized incidental renal masses: emerging data support conservative management. Radiology. 2015;275(1):28–42. [DOI] [PubMed] [Google Scholar]
- 19.Oon SF, Foley RW, Quinn D, Quinlan DM, Gibney RG. Contrast-enhanced ultrasound of the kidney: a single-institution experience. Irish journal of medical science. 2017. [DOI] [PubMed] [Google Scholar]
- 20.Siddaiah M, Krishna S, McInnes MDF, Quon JS, Shabana WM, Papadatos D, et al. Is Ultrasound Useful for Further Evaluation of Homogeneously Hyperattenuating Renal Lesions Detected on CT? American Journal of Roentgenology. 2017;209(3):604–10. [DOI] [PubMed] [Google Scholar]
- 21.Schoots IG, Zaccai K, Hunink MG, Verhagen P. Bosniak Classification for Complex Renal Cysts Reevaluated: A Systematic Review. The Journal of urology. 2017;198(1):12–21. [DOI] [PubMed] [Google Scholar]
- 22.Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. The Journal of urology. 2017;198(3):520–9. [DOI] [PubMed] [Google Scholar]
- 23.Yu MC, Mack TM, Hanisch R, Cicioni C, Henderson BE. Cigarette smoking, obesity, diuretic use, and coffee consumption as risk factors for renal cell carcinoma. Journal of the National Cancer Institute. 1986;77(2):351–6. [PubMed] [Google Scholar]
- 24.Argani P, Lae M, Ballard ET, Amin M, Manivel C, Hutchinson B, et al. Translocation carcinomas of the kidney after chemotherapy in childhood. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(10):1529–34. [DOI] [PubMed] [Google Scholar]
- 25.Zucchetto A, Dal Maso L, Tavani A, Montella M, Ramazzotti V, Talamini R, et al. History of treated hypertension and diabetes mellitus and risk of renal cell cancer. Annals of Oncology. 2007;18(3):596–600. [DOI] [PubMed] [Google Scholar]
- 26.Cho E, Curhan G, Hankinson SE, Kantoff P, Atkins MB, Stampfer M, et al. Prospective evaluation of analgesic use and risk of renal cell cancer. Archives of internal medicine. 2011;171(16):1487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsivian M, Moreira DM, Caso JR, Mouraviev V, Polascik TJ. Cigarette smoking is associated with advanced renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(15):2027–31. [DOI] [PubMed] [Google Scholar]
- 28.CDC - Trends in Current Cigarette Smoking - Smoking & Tobacco Use [Internet]. Smoking and Tobacco Use. 2018. [cited 7 January 2018]. Available from: https://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/index.htm.
- 29.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama. 2016;315(21):2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adult Obesity Facts ∣ Overweight & Obesity ∣ CDC [Internet]. Cdc.gov. 2018. [cited 7 January 2018]. Available from: https://www.cdc.gov/obesity/data/adult.html.
- 31.Diabetes [Internet]. 2018. [cited 7 January 2018]. Available from: https://www.cdc.gov/diabetes/statistics/slides/long_term_trends.pdf.
- 32.Choi Y, Park B, Jeong BC, Seo SI, Jeon SS, Choi HY, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. International journal of cancer. 2013;132(3):625–34. [DOI] [PubMed] [Google Scholar]
- 33.Albiges L, Hakimi AA, Xie W, McKay RR, Simantov R, Lin X, et al. Body Mass Index and Metastatic Renal Cell Carcinoma: Clinical and Biological Correlations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, Hilvano-Cabungcal AM, et al. Systematic review of oncological outcomes following surgical management of localised renal cancer. European urology. 2012;61(5):972–93. [DOI] [PubMed] [Google Scholar]
- 35.Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. European urology. 2011;59(4):543–52. [DOI] [PubMed] [Google Scholar]
- 36.Roos FC, Steffens S, Junker K, Janssen M, Becker F, Wegener G, et al. Survival advantage of partial over radical nephrectomy in patients presenting with localized renal cell carcinoma. BMC Cancer. 2014;14(1):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M, Zhao Z, Duan X, Deng T, Cai C, Wu W, et al. Partial versus radical nephrectomy for T1b-2N0M0 renal tumors: A propensity score matching study based on the SEER database. PloS one. 2018;13(2):e0193530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shum CF, Bahler CD, Sundaram CP. Matched Comparison Between Partial Nephrectomy and Radical Nephrectomy for T2 N0 M0 Tumors, a Study Based on the National Cancer Database. Journal of endourology. 2017;31(8):800–5. [DOI] [PubMed] [Google Scholar]
- 39.Tannir N, Hammers H, Amin A. First-line vascular endothelial growth factor targeted therapy in renal cell carcinoma: priming the tumor microenvironment for immunotherapy. Current medical research and opinion. 2018;34(5):825–31. [DOI] [PubMed] [Google Scholar]
- 40.Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(6):591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangoni AA, Kichenadasse G, Rowland A, Sorich MJ. Predictors of anti-VEGF drug-induced hypertension using different hypertension criteria: a secondary analysis of the COMPARZ study. Therapeutic advances in medical oncology. 2018;10:1758834018755090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cella D, Grunwald V, Nathan P, Doan J, Dastani H, Taylor F, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. The Lancet Oncology. 2016;17(7):994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanian P, Haas NB. Recent advances in localized RCC: A focus on VEGF and immuno-oncology therapies. Urologic oncology. 2018;36(1):23–30. [DOI] [PubMed] [Google Scholar]
- 44.Choueiri TK, Motzer RJ. Systemic Therapy for Metastatic Renal-Cell Carcinoma. The New England journal of medicine. 2017;376(4):354–66. [DOI] [PubMed] [Google Scholar]
- 45.Li P, Wong YN, Armstrong K, Haas N, Subedi P, Davis-Cerone M, et al. Survival among patients with advanced renal cell carcinoma in the pretargeted versus targeted therapy eras. Cancer medicine. 2016;5(2):169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal SK, Ghate SR, Li N, Swallow E, Peeples M, Zichlin ML, et al. Real-World Survival Outcomes and Prognostic Factors Among Patients Receiving First Targeted Therapy for Advanced Renal Cell Carcinoma: A SEER-Medicare Database Analysis. Clinical genitourinary cancer. 2017;15(4):e573–e82. [DOI] [PubMed] [Google Scholar]
- 47.Maruvka YE, Tang M, Michor F. On the validity of using increases in 5-year survival rates to measure success in the fight against cancer. PLoS One. 2014;9(7):e83100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smaldone MC, Egleston B, Hollingsworth JM, Hollenbeck BK, Miller DC, Morgan TM, et al. Understanding Treatment Disconnect and Mortality Trends in Renal Cell Carcinoma Using Tumor Registry Data. Med Care. 2017;55(4):398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.