Abstract
Objective:
To evaluate the comparative effectiveness of three regional corticosteroid injections for uveitic macular edema: periocular triamcinolone acetonide (PTA), intravitreal triamcinolone acetonide (ITA), and the intravitreal dexamethasone implant (IDI).
Design:
Multicenter randomized clinical trial
Participants:
Patients with uveitic macular edema
Methods:
Patients were randomized 1:1:1 to receive one of the three therapies. Patients with bilateral macular edema were assigned the same treatment for both eyes.
Main Outcomes Measures:
The primary outcome was the proportion of baseline (PropBL) central subfield thickness (CST) at 8 weeks (CST at 8 weeks/CST at BL) assessed with optical coherence tomography (OCT) by masked readers. Secondary outcomes included ≥20% improvement and resolution of macular edema, best-corrected visual acuity (BCVA), and intraocular pressure (IOP) events over 24 weeks.
Results:
All treatment groups demonstrated improved CST during follow-up. At 8 weeks, each group had clinically meaningful reductions in CST relative to baseline (PropBL: 0.77, 0.61, and 0.54, respectively, which translates to reductions of 23%, 39%, and 46% for PTA, ITA, and IDI, respectively). Intravitreal triamcinolone acetonide (PropBL ITA/PropBL PTA, 99.87% Confidence Interval [CI]: 0.79, 0.65–0.96) and IDI (PropBL IDI/PropBL PTA, 99.87% CI: 0.69, 0.56–0.86) had larger reductions in CST than PTA (p <0.0001). Intravitreal dexamethasone implant was non-inferior to ITA at 8 weeks (PropBL IDI/PropBL ITA, 99.87%CI: 0.88, 0.71–1.08). Both ITA and IDI treatments also were superior to PTA treatment in improving and resolving uveitic macular edema. All treatment groups demonstrated BCVA improvement throughout follow-up. Both ITA and IDI groups had improvements in BCVA that was 5 letters greater than the PTA group at 8 weeks (p <0.004). The risk of having IOP ≥24 mmHg was higher in the intravitreal treatment groups compared with the periocular group (Hazard ratio [HR], 95% CI: 1.83, 0.91–3.65 and 2.52, 1.29–4.91 for ITA and IDI, respectively); however, there was no significant difference between the two intravitreal treatment groups.
Conclusions:
Intravitreal triamcinolone acetonide and the IDI were superior to PTA for treating uveitic macular edema with modest increases in the risk of IOP elevation. This risk did not differ significantly between intravitreal treatments.
Precis
In a 6-month multicenter, randomized, comparative trial, both intravitreal triamcinolone and intravitreal dexamethasone implant were superior to periocular triamcinolone for treating uveitic macular edema. Intraocular pressure elevation did not differ significantly between the intravitreal treatments.
Introduction
The uveitides collectively are a common cause of visual loss in the United States and account for ~30,000 new cases of blindness per year.1–3 The uveitides affect more patients of working age than do age-related diseases (e.g. age-related macular degeneration, cataract) and thus cause greater losses in productivity in the work force and potentially more years of vision lost.4,5
Macular edema is a common structural ocular complication of uveitis and is responsible for a substantial amount of visual impairment among these patients.4,6–9 A Dutch study in the 1990s reported that 40% of patients with intermediate uveitis, posterior uveitis, or panuveitis had macular edema, and that it was the most common cause of visual loss among all patients with uveitis, accounting for 41% of visual impairment. In the Multicenter Uveitis Steroid Treatment (MUST) Trial, macular edema was present at enrollment in 40% of eyes with uveitis with a similar frequency for patients with intermediate uveitis, posterior uveitis, and panuveitis.9–11 More recently Grajewski and colleagues12 reported on 500 consecutive uveitis patients evaluated with optical coherence tomography (OCT) and found 44% of patients with ME (25% cystoid ME, 11% diffuse ME, and 8% with subretinal fluid), suggesting that the frequency of this complication has been relatively stable over decades despite a wider availability of newer classes of medications used to treat uveitis.
Current approaches to the treatment of uveitic macular edema begin with control of the inflammation, often with systemic medications, including oral corticosteroids and immunosuppressive drugs. Despite control of inflammation with these treatments, uveitic macular edema may persist in ~50% of eyes.10,11 Uveitic macular edema which persists despite control of the uveitis typically is treated with adjunctive regional corticosteroid injections. In the MUST Trial, 62% of eyes with uveitic macular edema treated with systemic medications required regional corticosteroid injections for macular edema, typically one or two injections.10 Therefore, effective regional therapy is critical for the management of uveitic macular edema.
Regional corticosteroid injections may be delivered via a periocular or intravitreal route. Periocular injections are given adjacent but external to the eye, either inferiorly along the orbital floor or superiorly as a posterior superior sub-Tenon’s injection of triamcinolone acetonide (Kenalog®, Bristol-Myers Squibb Company, Princeton, NJ). Intravitreal injections place drug directly into the vitreous. Intravitreal corticosteroids commonly given include triamcinolone acetonide (Triesence®, Alcon Pharmaceuticals, Fort Worth, TX) and the dexamethasone sustained-release implant (Ozurdex®, Allergan Inc., Irvine, CA). Although the two periocular routes appear to have similar efficacy,13–16 there have been limited comparative trials of the periocular route versus the intravitreal route17,18 and no comparative trials of the two intravitreal corticosteroid therapies. To better understand which regional corticosteroid injection offers the best balance of efficacy and safety, we conducted a randomized comparative trial comparing periocular triamcinolone acetonide, intravitreal triamcinolone acetonide, and the intravitreal dexamethasone implant for the initial treatment of uveitic macular edema, the PeriOcular versus INTravitreal corticosteroids for the treatment of uveitic macular edema (POINT) Trial.
Methods
Study Design:
The POINT Trial was a multicenter, randomized (allocation ratio 1:1:1), parallel-treatment, comparative trial (clinicaltrial.gov identifier: NCT02374060). The specific aims of the trial were to compare the effectiveness in improving uveitic macular edema assessed by OCT (primary outcome), visual outcomes, and ocular side effects among the three treatment groups. The primary hypotheses were that: 1) intravitreal triamcinolone acetonide will have superior efficacy to periocular triamcinolone acetonide in the treatment of uveitic macular edema; 2) the intravitreal dexamethasone implant will have superior efficacy to periocular triamcinolone acetonide in the treatment of uveitic macular edema; and that 3) the intravitreal dexamethasone implant will be non-inferior to intravitreal triamcinolone acetonide in the treatment of uveitic macular edema. A secondary hypothesis was that the intravitreal dexamethasone implant would have a lower risk of intraocular pressure (IOP) elevation than intravitreal triamcinolone acetonide. Twenty-six clinical centers (23 in the United States and one center each in Australia, Canada, and the United Kingdom) participated in the trial (see Appendix 1, available at http://aaojournal.org). All patients provided written informed consent; the institutional review boards of each clinical center and the three resource centers approved this study; and the trial adhered to the principles in the Declaration of Helsinki.
Enrollment of Participants, Data Collection, and Follow-up:
Eligible patients were age 18 years or older, had non-infectious anterior, intermediate, posterior, or panuveitis (either active or inactive uveitis was permitted), and had macular edema defined as a central subfield macular thickness (CST) greater than the normal range (defined as the population normative mean CST +/− two standard deviations) for the OCT machine being used (>300 μm for Zeiss Cirrus/Topcon 3DOCT or >320 μm for Heidelberg Spectralis) without regard to the presence of cystoid spaces. If receiving systemic medications for the treatment of uveitis, patients needed to be on stable doses of oral corticosteroids (≤10 mg daily of prednisone) and immunosuppressive drugs as applicable for at least four weeks. Data collected included masked protocol refraction with best-correct visual acuity (BCVA; measured in standard letters) assessment using standardized refraction and logarithmic visual acuity charts,19 IOP measurement, slit lamp and dilated fundus examination, and OCT imaging of the macula. Participants underwent a fluorescein angiogram at baseline to evaluate for the presence of macular leakage. Optical coherence tomography images were graded in a standardized fashion at a centralized, treatment-masked image reading center. Study visits occurred at baseline and at 4, 8, 12, 20, and 24 weeks of follow-up, with closeout at the 24-week visit.
Reading Center evaluations of the OCT images were performed at all visits except the 20-week visit.
Randomization:
Patients were randomized to receive periocular triamcinolone acetonide, intravitreal triamcinolone acetonide, or intravitreal dexamethasone implant injections in an eligible eye. Patients with macular edema in both eyes were assigned to receive the same treatment in both eyes. Randomization was undertaken using permuted blocks of varying lengths to yield the expected 1:1:1 allocation ratio and stratified by the presence or absence of concomitant systemic treatment for uveitis (e.g. oral corticosteroids and/or immunosuppressive drug therapy). Randomization tables were prepared by the coordinating center and assignments were revealed via a web portal after patients were enrolled in the trial and all baseline data were collected.
Treatment:
Patients were to receive an injection of their assigned treatment (either 40 mg of triamcinolone acetonide given periocularly, 4 mg of triamcinolone acetonide given intravitreally, or the 0.7 mg dexamethasone implant given intravitreally) in each eligible eye on the day of randomization or as soon as possible thereafter, but no later than ten days. Injections were given using standardized techniques and retreatment was allowed at the 8-week visit for the periocular and intravitreal triamcinolone treatment arms and at the 12-week visit for the intravitreal dexamethasone implant treatment arm provided retreatment criteria were met (see Treatment Schema, Appendix 2, available at http://aaojournal.org). Periocular injections were given using either a periorbital floor or posterior sub Tenon’s approach (as each are reported to have similar efficacy15), based on the preference of the injecting ophthalmologist. The retreatment schedules for each treatment arm were chosen to encompass the peak efficacy of each treatment, which was expected to occur later for the intravitreal dexamethasone implant.20 Criteria for retreatment included failure to meet the definition of macular edema improvement (a ≥20% decrease in CST on OCT),21 worsening of macular edema after initial improvement, or the presence of cystoid spaces in the 1 mm central subfield in an eye with a normal central subfield thickness. For re-treatment, an eye had to have an IOP <25 mm Hg and receive ≤3 IOP-lowering medications in order to receive any injection. Eyes that demonstrated worsening of macular edema or less than a 20% improvement of the CST were permitted to change treatment at 12 weeks for the periocular or intravitreal triamcinolone acetonide arms and at 20 weeks for the intravitreal dexamethasone implant arm.
Outcomes and Masking:
The primary outcome was the relative change in CST as measured by OCT at the 8-week visit based upon the RC assessment, which standardized the measurements across all OCT machines. The relative change was quantified as the proportion of baseline (PropBL), i.e. the primary outcome was the CST at 8 weeks divided by the CST at baseline. The time point of 8 weeks was chosen for assessment of the primary outcome because it encompasses the window for maximum benefit for all treatment arms. Secondary outcomes related to efficacy included the proportion of baseline in CST at other time points and mean change in BCVA over the entire 24 weeks of follow-up. The proportion of eyes with the following macular edema characteristics also were calculated over follow-up: “improvement”, ≥20% reduction in macular thickness (or normalization of macular thickness even if there is <20% reduction), and “resolution”, defined as normalization of the macular thickness to less than 2 standard deviations above normative mean for the standardized OCT assessment (i.e. < 260μm). The need for further injections, both assigned and non-assigned, was evaluated. Safety outcomes focused primarily on the risk of IOP elevation to the ≥24 mm Hg and ≥30 mm Hg thresholds, to ≥10 mm Hg from baseline during follow-up, and the use of IOP-reducing medication. Need for glaucoma or cataract surgery, severe vision loss (≥15 standard letters), hypotony (IOP <6 mm Hg), and immediate complications from injections were evaluated.
Visual acuity examiners and members of the Reading Center that graded the OCT images were masked to study treatment and were trained and certified to perform these evaluations. Participants, treating clinicians, and coordinators were not masked.
Sample Size Determination:
Central subfield retinal thickness was modeled on the log scale in order to adjust for skewness in the data and to provide estimates of the proportion of baseline. A Bonferroni correction was applied to the type I error for the primary outcome to account for the multiplicity of the pairwise comparisons between the three treatment groups, i.e. a type I error of 0.01667 was allocated for each comparison. The sample size was selected to provide a minimum of 90% power for the superiority hypotheses (intravitreal triamcinolone injection and dexamethasone implant superior to periocular injection) and 80% power for the non-inferiority hypothesis (intravitreal dexamethasone implant non-inferior to intravitreal triamcinolone injection). We assumed that the standard deviation of the log transformation of retinal thickness was 0.33, 25% of individuals would have bilateral macular edema with a correlation between eyes of 0.40, and 10% losses to follow-up. The sample size required for the non-inferiority hypothesis dictated the final sample size for the trial.
For the first two superiority hypotheses and based on the assumptions above, a sample size of 267 (89 per arm) provided 98% power to detect a greater reduction in retinal thickness from 25% for periocular injection to 40% for intravitreal injection or dexamethasone implant on the log scale, i.e., difference in log change in retinal thickness of log(0.6) vs. log(0.75), with a two-sided type I error rate of 0.01667. For the third hypothesis, a non-inferiority margin of 10% was selected based on previous research indicating that the threshold for reproducibility of OCT evaluation of retinal thickness is 10% and that a 20% change is associated with meaningful changes in visual acuity.21 Based upon these assumptions, a sample size of 89 independent eyes per treatment group provided 80% power to demonstrate non-inferiority of the intravitreal dexamethasone implant as compared to intravitreal triamcinolone injection with a one-sided type I error rate of 0.01667. We would reject the null hypothesis of inferiority if the upper bound of the 96.7% confidence interval of the difference in log retinal change from baseline at 8 weeks for the dexamethasone implant et minus intravitreal triamcinolone acetonide was less than 0.15 (log[0.7] - log[0.6]) or equivalently if the ratio of the proportion of baseline for the two groups was less than 1.16 (0.7/0.6). A test of superiority would be performed for those visits that demonstrated non-inferiority.
A single interim analysis was scheduled to occur at the first regularly scheduled Data Safety and Monitoring Committee (DSMC) meeting after at least 50% of the information had been collected. An O’Brien-Fleming spending function was used to determine the type I error threshold, 0.00132, corresponding to actual data collection level of 60%, for the interim analysis. This threshold translates to a two-sided 99.87% confidence interval. In order to be conservative, the evaluation of the non-inferiority hypothesis was based upon this two-sided 99.87% confidence interval (i.e. a type I error rate of 0.00066 for the one-sided test).
Statistical Analysis:
Analyses were conducted “as randomized” and included all available data from all eyes that were identified as being eligible at the time of randomization regardless of treatment actually received. Longitudinal analyses of continuous outcomes were performed using mixed effects models with a saturated mean structure (including indicators for time, treatment group, and treatment time interactions). An unstructured covariance structure was used to account for the longitudinal, within eye correlation and a random intercept was used to account for between eye correlations in patients with bilateral disease. Due to skewness in the data, retinal thickness (the primary outcome) was measured on the log scale; hence, the interpretation of the parameters is on the relative scale: proportion of baseline for within-treatment measurements and the ratio of proportion of baseline for between-treatment measurements. The percent reduction was calculated as 1 minus the proportion of baseline times 100. The proportion of eyes with ≥20% improvement retinal thickness, resolution of macular edema, and use of IOP reducing medication was modeled using generalized estimating equations with a saturated means model and an unstructured correlation to model the longitudinal within- eye correlation. The bootstrap was used to account for the correlation between eyes of the same patient. Cox proportional hazards models with a random intercept to account for between-eye correlation were used to assess the time from randomization to ocular events, e.g. IOP elevation ≥24 mm Hg or BCVA decrease of 15 or more letters.
The MUST Research Group Statistical Analysis Committee (see Credit Roster, Appendix 1, available at http://aaojournal.org) conducted all analyses for the POINT trial. Robust standard errors were computed for all models. The type I error threshold for the primary hypotheses was 0.00132, the threshold established for the interim analysis after accounting for a Bonferroni correction for pairwise comparisons. All other confidence intervals and p-values are reported using a two-sided type I error rate of 0.05 and are not adjusted for multiple comparisons. Statistical analyses were performed using SAS (SAS/STAT User’s Guide, Version 9.2; SAS, Inc., Cary NC) and R (The R Project for Statistical Computing, Version 3.3.1, available at: http://www.r-project.org).
Results
Characteristics at Enrollment:
Between June 2015 and July 2017, 192 patients (235 eyes with uveitic macular edema) were enrolled. In July 2017, enrollment was halted after a DSMC review concluded that the stopping criteria for all three of the primary hypotheses (superiority of both intravitreal therapies vs periocular therapy and non-inferiority of the intravitreal dexamethasone implant vs intravitreal triamcinolone at 8 weeks) had been met at the pre-planned interim analysis. Since no safety concerns were identified, patients that were already enrolled continued follow-up until the 24-week visit as originally planned in order to obtain additional data for comparisons at 12-, 20-, and 24-weeks. Baseline demographic and clinical characteristics at the time of randomization are summarized by treatment group (Table 1). The characteristics were distributed similarly across the three treatment groups with the exception of the presence of active uveitis and baseline BCVA. Patients in the intravitreal triamcinolone group were more likely to have active uveitis (83% of eyes) than the intravitreal dexamethasone implant group (72% of eyes) or the periocular triamcinolone group (65% of eyes). Eyes assigned to the periocular triamcinolone group had slightly better BCVA at baseline compared to the other treatment groups numerically, although the differences were neither statistically nor clinically significant. Fluorescein angiographic leakage was similar among the three groups: 78%, 78%, and 82% of eyes had leakage involving the entire central macular subfield in the periocular triamcinolone, intravitreal triamcinolone, and intravitreal dexamethasone implant groups, respectively.
Table 1.
Demographic and clinical characteristics at randomization by treatment group.
| Assigned Treatment | |||
|---|---|---|---|
| Characteristic | Periocular | Intravitreal | Dexamethasone |
| Person-level (Randomized individuals) | 65 | 63 | 64 |
| Age at enrollment (years), Median (Range) | 55 (22, 87) | 56 (18, 86) | 55 (19, 85) |
| Male, N (%) | 26 (40%) | 23 (37%) | 24 (38%) |
| Race/Ethnicity, N (%) | |||
| White | 46 (71%) | 37 (59%) | 39 (61%) |
| Hispanic | 1 (2%) | 3 (5%) | 5 (8%) |
| Black | 11 (17%) | 19 (30%) | 17 (27%) |
| Other | 7 (11%) | 4 (6%) | 3 (5%) |
| Smoking, N (%) | |||
| Current | 9 (14%) | 13 (21%) | 9 (14%) |
| Former | 15 (23%) | 13 (21%) | 20 (31%) |
| Never | 41 (63%) | 37 (59%) | 35 (55%) |
| Bilateral uveitis, N (%) | 49(75%) | 51 (81%) | 47 (73%) |
| Bilateral macular edema, N (%) | 9 (14%) | 19 (30%) | 15 (23%) |
| On systemic medications*, N (%) | 24 (37%) | 23 (37%) | 23 (36%) |
| Primary uveitis diagnosis†, N (%) | |||
| Anterior | 11 (17%) | 8 (13%) | 10 (16%) |
| Anterior/Intermediate | 13 (20%) | 7 (11%) | 14 (22%) |
| Intermediate | 17 (26%) | 14 (22%) | 15 (23%) |
| Posterior | 13 (20%) | 15 (24%) | 9 (14%) |
| Panuveitis | 11 (17%) | 19 (30%) | 16 (25%) |
| Any uveitis associated systemic disease, N (%) | 14 (22%) | 15 (24%) | 12 (19%) |
| Duration of uveitis (years), Median (Range) | 3.5 (0.0, 24.4) | 3.5 (0.0, 36.1) | 4.5 (0.0, 30.2) |
| Eye-level (Eligible eyes) | 74 | 82 | 79 |
| Intraocular pressure (mm Hg): Median (Range) | 14 (6, 22) | 14 (7, 21) | 13 (6, 20) |
| Cup to disc ratio: Median (Range) | 0.2 (0.0, 0.8) | 0.3 (0.0, 0.8) | 0.3 (0.0, 0.8) |
| VA (standard letters): Median (Range) | 68 (25, 91) | 63 (13, 88) | 64 (23, 86) |
| Snellen equivalent | 20/44 | 20/55 | 20/53 |
| VA worse than 20/40, N (%) | 43 (58%) | 60 (73%) | 50 (63%) |
| Active uveitis, N (%) | 48 (65%) | 68 (83%) | 57 (72%) |
| Aphakic or pseudophakic, N (%) | 36 (49%) | 47 (57%) | 46 (58%) |
| Glaucoma, N (%) | 5 (7%) | 11 (13%) | 8 (10%) |
| Prior treatment, N (%) | |||
| Periocular | 36 (49%) | 31 (38%) | 37 (47%) |
| Intravitreal | 22 (30%) | 22 (27%) | 24 (30%) |
| Ozurdex | 8 (11%) | 11 (13%) | 13 (16%) |
| Retisert | 6 (8%) | 4 (5%) | 6 (8%) |
| IOP lowering medications | 16 (22%) | 16 (20%) | 19 (24%) |
| IOP lowering surgery | 9 (12%) | 5 (6%) | 12 (15%) |
| OCT characteristics | |||
| Retinal thickness at the center subfield, Median (Range) | 438 (278, 922) | 485 (236, 824) | 449 (243, 1300) |
| Cystoid spaces, N (%) | 70 (95%) | 76 (95%) | 77 (97%) |
| Subretinal fluid, N (%) | 29 (39%) | 41 (51%) | 28 (35%) |
| FA leakage central subfield, N (%) | |||
| None (No ML) | 2 (3%) | 2 (3%) | 3 (4%) |
| Partial (0 < ML < 0.44 DA) | 14 (19%) | 16 (20%) | 11 (14%) |
| Complete (0.44 DA) | 57 (78%) | 62 (78%) | 62 (82%) |
Baseline form data does not confirm systemic therapy for two patients in systemic therapy strata.
The categories represent the location of the primary diagnosis and are mutually exclusive.
N = number; % = percent; IOP = intraocular pressure; VA = visual acuity; OCT = optical coherence tomography; ML = macular leakage; DA = disk area.
In the periocular triamcinolone acetonide, intravitreal triamcinolone acetonide, and intravitreal dexamethasone implant groups, 73 (99%), 79 (96%), and 78 (99%) eyes respectively, received their assigned treatment at baseline (Figure 1, Table 2). Losses to follow-up were low (≤5%) among all treatment groups. 185 patients (227 eyes with macular edema) completed the 8-week visit (74, 77, and 76 eyes in the periocular, intravitreal triamcinolone, and intravitreal dexamethasone implant groups, respectively). Sixty-three patients (97%) in the periocular group, 62 patients (98%) in the intravitreal triamcinolone group, and 61 patients (95%) in the intravitreal dexamethasone implant group completed the 24-week visit.
Figure 1.

CONSORT diagram of the POINT trial.
table 2.
Assigned and non-assigned treatments for uveitic macular edema by treatment group.
| Assigned treatment | |||
|---|---|---|---|
| Number of Eyes with | Periocular (N=74 eyes) |
Intravitreal (N=82 eyes) |
Dexamethasone (N=79 eyes) |
| Assigned Treatments Received* | |||
| 1st injection | 73 | 79 | 78 |
| 2nd injection | 36 | 38 | 44 |
| 3rd injection | 4 | 8 | 3 |
| 4th injection | 1 | 2 | 0 |
| 5th injection | 0 | 1 | 0 |
| Non-Assigned Treatments Received† | |||
| Periocular | |||
| 1st injection | n/a | 0 | 4 |
| Intravitreal | |||
| 1st injection | 24 | n/a | 0 |
| 2nd injection | 3 | n/a | 0 |
| Dexamethasone | |||
| 1st injection | 6 | 6 | n/a |
All assigned treatments were received according to the protocol schedule except for 2 eyes (1 periocular and 1 intravitreal) that received their second injection at the week 4 visit.
All non-assignment treatment were received after the week 8 assessment (the primary outcome) except for 2 eyes that were assigned to receive periocular injections but received a dexamethasone pellet at the week 4 visit.
N = number; n/a = not applicable.
Administration of assigned and non-assigned treatments are summarized in Table 2. Overall, in the periocular treatment group, 36 eyes received second injections (one at week 4) with four eyes receiving a total of three injections and one eye receiving four injections. In the intravitreal triamcinolone group, 38 eyes received second injections (one at week 4) with eight, two, and one eyes going on to receive a 3rd, 4th, or 5th injection, respectively. In the intravitreal dexamethasone implant group, 44 eyes had repeat injections of intravitreal dexamethasone implants with three eyes receiving a 3rd injection. Retreatments with non-assigned treatments (i.e. treatment crossovers) were most common in the periocular triamcinolone group with 30 eyes receiving one of the intravitreal treatments as compared to six eyes assigned to intravitreal triamcinolone receiving intravitreal dexamethasone implants and four eyes assigned to dexamethasone implant receiving periocular triamcinolone injections. All of the non-assigned treatments were received after the week 8 assessment except for two eyes in which the intravitreal dexamethasone implant was placed in eyes assigned to receive periocular injections at the week 4 visit.
Relative Change in Central Subfield Thickness:
Overall, the CST improved compared to baseline at all follow-up visits for all treatment groups (p <0.0001, Table 3, Figure 2, Figure 3 available at http://aaojournal.org). At the 8-week visit, all three treatment groups demonstrated significant reductions in the CST relative to baseline (PropBL: 0.77, 0.61, 0.54 for periocular triamcinolone acetonide (PTA), intravitreal triamcinolone acetonide (ITA), and intravitreal dexamethasone implant (IDI), respectively, which translates to percent reductions of 23%, 39%, 46%). Both intravitreal triamcinolone (PropBL ITA/PropBL PTA, 99.87% Confidence Interval [CI]: 0.79, 0.65–0.96) and the dexamethasone implant (PropBL IDI/PropBL PTA, 99.87% CI: 0.69, 0.56–0.86) were superior to periocular triamcinolone (p <0.0001, ratios <1 indicate larger reductions for the intravitreal treatments). These patterns were evident at the 4-week visit but were attenuated at later visits.
table 3.
Proportion of baseline retinal thickness at each visit by treatment group. Values less than one indicate a decrease in retinal thickness with lower values indicating greater decreases.
| Proportion of Baseline within Each Treatment Group* | ||||||
| Estimate (99.87%Confidence interval)† P-value | ||||||
| Periocular | Intravitreal | Dexamethasone | ||||
| Week 4 | 0.78 (0.69, 0.89) | <0.0001 | 0.58 (0.50, 0.66) | <0.0001 | 0.56 (0.48, 0.65) | <0.0001 |
| Week 8 | 0.77 (0.67, 0.89) | <0.0001 | 0.61 (0.53, 0.70) | <0.0001 | 0.54 (0.46, 0.63) | <0.0001 |
| Week 12 | 0.75 (0.64, 0.86) | <0.0001 | 0.61 (0.54, 0.69) | <0.0001 | 0.63 (0.53, 0.75) | <0.0001 |
| Week 24 | 0.68 (0.59, 0.79) | <0.0001 | 0.64 (0.56, 0.74) | <0.0001 | 0.61 (0.52, 0.71) | <0.0001 |
| Ratio of the Proportion of Baseline between Treatment Groups | ||||||
| Estimate (99.87%Confidence interval)† P-value | ||||||
| Superiority Hypotheses | Non-inferiority Hypothesis‡ | |||||
| Intravitreal/ Periocular |
Dexamethasone/ Periocular |
Dexamethasone/ Intravitreal |
||||
| Week 4 | 0.73 (0.61, 0.88) | <0.0001 | 0.71 (0.59, 0.86) | <0.0001 | 0.97 (0.80, 1.18) | n/a |
| Week 8 | 0.79 (0.65, 0.96) | <0.0001 | 0.69 (0.56, 0.86) | <0.0001 | 0.88 (0.71, 1.08) | n/a |
| Week 12 | 0.82 (0.67, 0.98) | 0.0003 | 0.84 (0.67, 1.06) | 0.012 | 1.03 (0.84, 1.27) | n/a |
| Week 24 | 0.95 (0.77, 1.16) | 0.35 | 0.89 (0.72, 1.10) | 0.07 | 0.94 (0.77, 1.16) | n/a |
The percent decline from baseline is 100*(1-proportion of baseline). For example, there is a 23% decrease for periocular, a 39% decrease for intravitreal, and a 46% decrease for dexamethasone at week 8.
The two-sided type I error threshold was 0.00132 since recruitment was halted after the single pre-planned interim analysis.
Non-inferiority is evaluated by comparing the upper limit of the 99.87% confidence interval with the pre-defined non-inferiority margin as opposed to p-values, which translates to a one-sided test with a type I error rate of 0.00066. The non-inferiority margin for the comparison between dexamethasone and intravitreal treatment was 1.16, i.e. dexamethasone is considered non-inferior if the upper boundary of the 99.87% confidence interval is less than 1.16.
Figure 2.
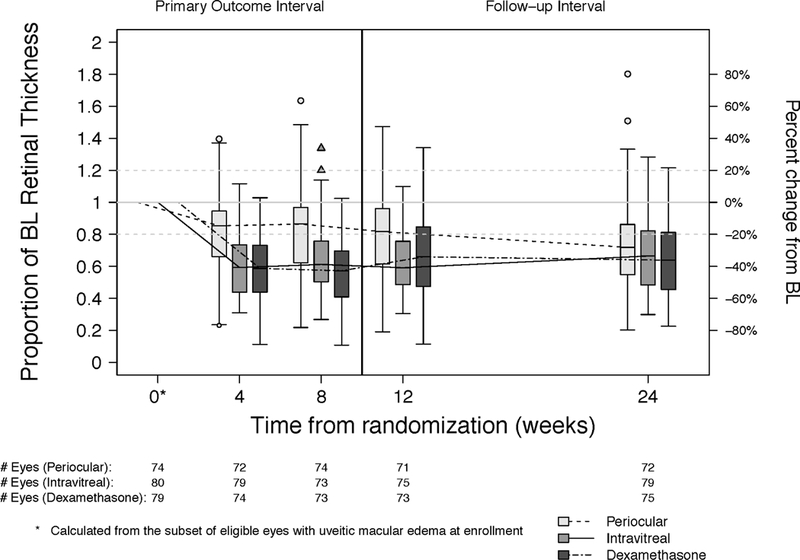
Change in retinal thickness at the central subfield measured by optical coherence tomography at each visit by treatment group.
Figure 3.
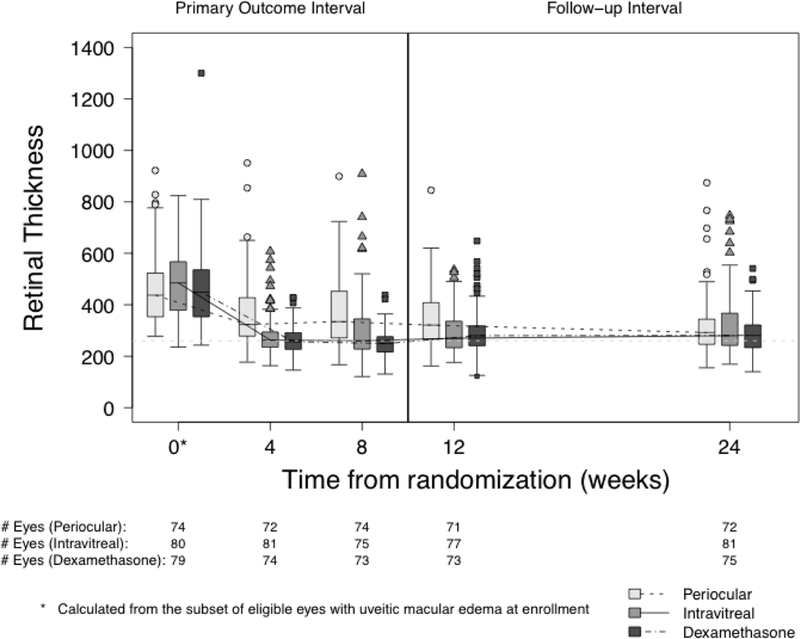
Retinal thickness at the central subfield measured by optical coherence tomography at each visit by treatment group.
The dexamethasone implant was non-inferior to intravitreal triamcinolone at the 8-week visit (PropBL IDI/PropBL ITA, 99.87% CI: 0.88, 0.71–1.08). Since non-inferiority was established, a test for superiority was performed (p=0.035). However, the difference did not achieve statistical significance at the interim analysis threshold (0.00132), as demonstrated by the fact that the 99.87% CI included 1, or at the original Bonferroni-adjusted threshold (0.0167). At all other visits, the differences between the two therapies was smaller; however, non-inferiority was only established at week 24 without evidence of superiority (p=0.39).
Additional Macular Edema Outcomes:
Intravitreal triamcinolone and the intravitreal dexamethasone implant were superior to periocular triamcinolone for improvement of the uveitic macular edema at all follow-up time points except at the 24- week visit (Figure 4a and Table 4 available at http://aaojournal.org). Similarly, both intravitreal treatment groups had higher proportions of eyes with resolution of uveitic macular edema when compared to the periocular treatment group at each follow-up visit through the 8-week visit (Figure 4b and Table 4 available at http://aaojournal.org). There were no significant differences in the proportion of eyes with improvement in or resolution of macular edema for the dexamethasone implant group versus the intravitreal triamcinolone group.
Figure 4.
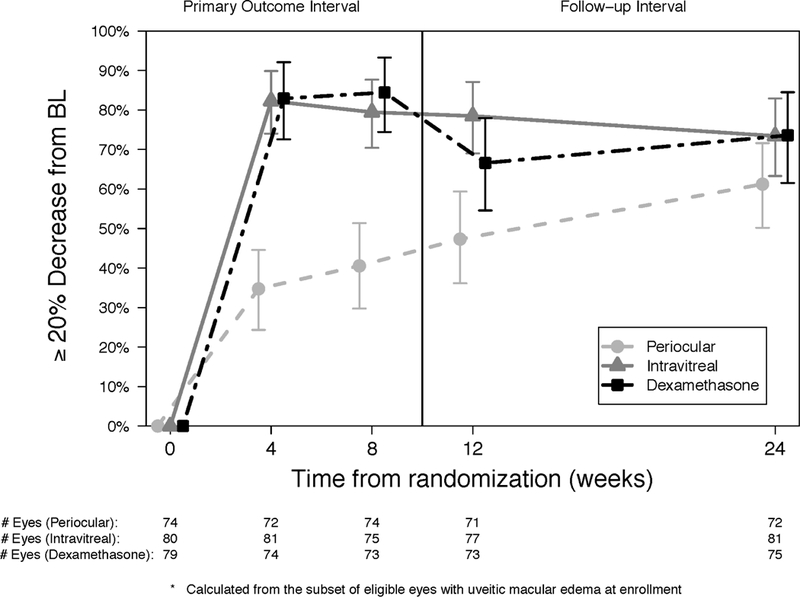
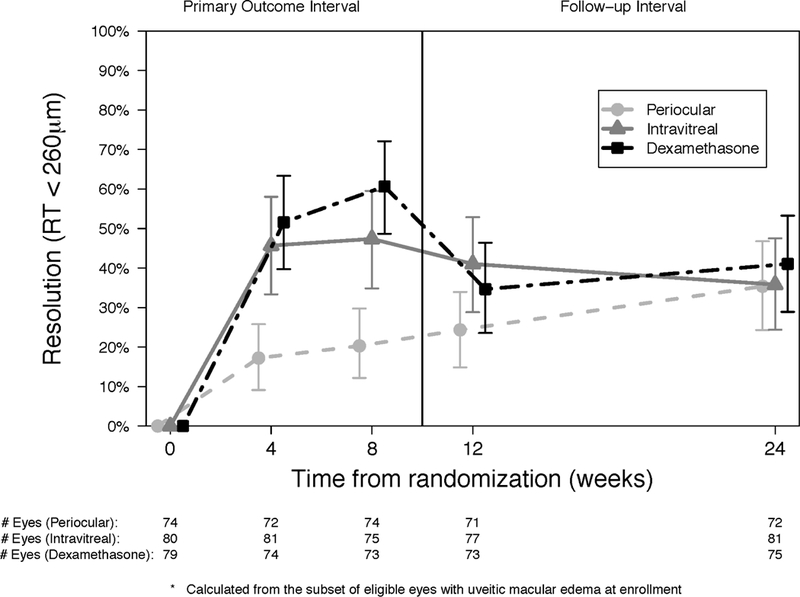
Proportion of eyes with improvement (a) and resolution (b) in macular edema at each visit for periocular triamcinolone (dashed line, circle), intravitreal triamcinolone (solid line, triangle) and dexamethasone implant (staggered line, square).
Table 4.
Improvement and resolution of macular edema at each visit by treatment group.
| Periocular | Intravitreal | Dexamethasone | Intravitreal - Periocular | Dexamethasone - Periocular | Dexamethasone - Intravitreal | |
|---|---|---|---|---|---|---|
| Proportion (95% Confidence Interval) | Difference in Proportion (95% Confidence Interval) P-value |
|||||
|
20% Improvement |
||||||
| 4 weeks | 0.35 (0.24, 0.45) | 0.82 (0.74, 0.90) | 0.83 (0.72, 0.93) | 0.48 (0.34, 0.61) <0.0001 | 0.48 (0.33, 0.62) <0.0001 | 0.01 (−0.13, 0.13) 0.92 |
| 8 weeks | 0.41 (0.29, 0.52) | 0.79 (0.70, 0.88) | 0.84 (0.74, 0.94) | 0.39 (0.24, 0.53) <0.0001 | 0.44 (0.29, 0.59) <0.0001 | 0.05 (−0.09, 0.19) 0.45 |
| 12 weeks | 0.47 (0.36, 0.60) | 0.78 (0.69, 0.88) | 0.67 (0.54, 0.78) | 0.31 (0.16, 0.46) <0.0001 | 0.19 (0.03, 0.36) 0.020 | −0.12 (−0.27, 0.04) 0.11 |
| 24 weeks | 0.61 (0.50, 0.72) | 0.73 (0.63, 0.83) | 0.74 (0.61, 0.85) | 0.12 (−0.03, 0.27) 0.10 | 0.12 (−0.03, 0.28) 0.11 | 0.002 (−0.16, 0.16) 0.98 |
| Resolution | ||||||
| 4 weeks | 0.17 (0.09, 0.26) | 0.46 (0.33, 0.59) | 0.52 (0.39, 0.64) | 0.28 (0.12, 0.44) 0.0002 | 0.34 (0.19, 0.49) <0.0001 | 0.06 (−0.11, 0.23) 0.50 |
| 8 weeks | 0.20 (0.12, 0.30) | 0.47 (0.34, 0.60) | 0.61 (0.48, 0.73) | 0.27 (0.11, 0.43) 0.0005 | 0.40 (0.25, 0.56) <0.0001 | 0.13 (−0.04, 0.30) 0.12 |
| 12 weeks | 0.24 (0.14, 0.34) | 0.41 (0.28, 0.53) | 0.35 (0.23, 0.47) | 0.17 (0.009, 0.32) 0.034 | 0.10 (−0.05, 0.26) 0.18 | −0.06 (−0.23, 0.11) 0.45 |
| 24 weeks | 0.35 (0.24, 0.47) | 0.36 (0.24, 0.48) | 0.41 (0.28, 0.54) | 0.004 (−0.16, 0.17) 0.96 | 0.06 (−0.11, 0.23) 0.51 | 0.05 (−0.12, 0.22) 0.54 |
Changes in Visual Acuity:
Best-corrected visual acuity improved in all treatment groups throughout follow-up (Table 5, top, Figure 5 available at http://aaojournal.org). Both intravitreal treatment groups had statistically significantly greater improvements (approximately 4 to 7 letters better) in BCVA from baseline relative to the periocular treatment group during the initial treatment period (4- and 8-weeks) and at the end of the follow-up period (5 letters at 24-weeks, Table 5, bottom). The difference in BCVA improvement between the intravitreal treatment groups was not clinically or statistically significantly different at any point during follow-up.
Table 5.
Change in best-corrected visual acuity (based on standard letters) at each visit by treatment group.
| Change from Baseline within Treatment Group* | ||||||
| Estimate (95% Confidence interval) P-value | ||||||
| Periocular | Intravitreal | Dexamethasone | ||||
| Week 4 | 2.78 (0.20, 5.36) | 0.034 | 10.14 (7.59, 12.69) | <0.0001 | 7.28 (4.45, 10.10) | <0.0001 |
| Week 8 | 4.37 (1.86, 6.89) | 0.0007 | 9.70 (7.26, 12.13) | <0.0001 | 9.53 (7.01, 12.05) | <0.0001 |
| Week 12 | 4.67 (2.14, 7.21) | 0.0003 | 10.43 (7.67, 13.18) | <0.0001 | 7.24 (4.34, 10.14) | <0.0001 |
| Week 20 | 5.17 (1.89, 8.46) | 0.002 | 9.88 (6.72, 13.04) | <0.0001 | 7.67 (5.23, 10.11) | <0.0001 |
| Week 24 | 4.07 (0.64, 7.51) | 0.020 | 9.60 (6.87, 12.34) | <0.0001 | 9.21 (6.62, 11.80) | <0.0001 |
| Difference in Change from Baseline between Treatment Groups* | ||||||
| Estimate (95% Confidence interval) P-value | ||||||
| Intravitreal - Periocular | Dexamethasone - Periocular | Dexamethasone Intravitreal | ||||
| Week 4 | 7.36 (3.74, 10.98) | <0.0001 | 4.49 (0.67, 8.31) | 0.021 | −2.86 (−6.66, 0.94) | 0.14 |
| Week 8 | 5.32 (1.82, 8.82) | 0.003 | 5.16 (1.60, 8.72) | 0.004 | ࢤ0.16 (−3.67, 3.34) | 0.93 |
| Week 12 | 5.75 (2.01, 9.49) | 0.003 | 2.57 (−1.28, 6.42) | 0.19 | ࢤ3.19 (−7.19, 0.81) | 0.12 |
| Week 20 | 4.71 (0.16, 9.26) | 0.042 | 2.50 (−1.59, 6.58) | 0.23 | ࢤ2.21 (−6.20, 1.78) | 0.28 |
| Week 24 | 5.53 (1.14, 9.92) | 0.013 | 5.14 (0.84, 9.44) | 0.019 | ࢤ0.40 (−4.16, 3.37) | 0.84 |
An improvement of 15 letters (3 lines) corresponds to halving of the visual angle.
Figure 5.
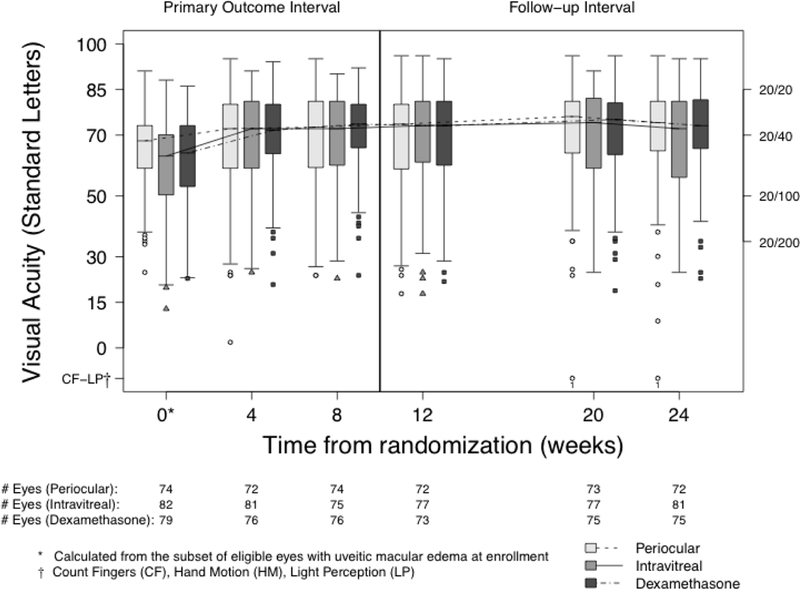
Best-corrected visual acuity at each visit by treatment group.
Intraocular Pressure over Follow-up Time:
Intraocular pressures events are summarized as absolute thresholds (≥24 mm Hg [Figure 6a], ≥30 mm Hg, or <6 mm Hg) and as a ≥10 mm Hg change from baseline (Table 6 available at http://aaojournal.org). Compared to periocular triamcinolone, the risk of having an IOP ≥24 mm Hg (Hazard ratio [HR], 95%CI: 2.52, 1.29,−4.91) or an IOP ≥10 mm Hg from baseline (HR, 95%CI: 2.85, 1.30–6.28) was higher for the intravitreal dexamethasone implant. Compared to periocular triamcinolone, the risk of having an IOP ≥24 mm Hg (HR, 95%CI: 1.83, 0.91– 3.65) or an IOP elevation ≥ 10 mm Hg from baseline (HR, 95%CI: 1.92, 0.86–4.29) was higher for intravitreal triamcinolone. The risks of having an IOP ≥ 24 mm Hg or an IOP elevation from baseline of ≥ 10 mm Hg and did not differ significantly between the intravitreal dexamethasone implant vs intravitreal triamcinolone groups. The number of eyes with IOP elevation ≥ 30 mm Hg was low in all three groups (4, 5, and 3 for the periocular triamcinolone, intravitreal triamcinolone, and dexamethasone implant, respectively), with no significant differences in the risk among the groups. There were no significant differences in the use of IOP medications between the three treatment groups at any time (p ≥0.14, Figure 6b). The proportion of eyes treated with IOP medications increased steadily throughout follow-up from 22% at randomization to 32% at 8-weeks (Change from BL [Δ], 95% Cl: 11%, 6%−16%) and 39% at 24-weeks (Δ, 95% Cl: 17%, ll%−24%).
Figure 6.
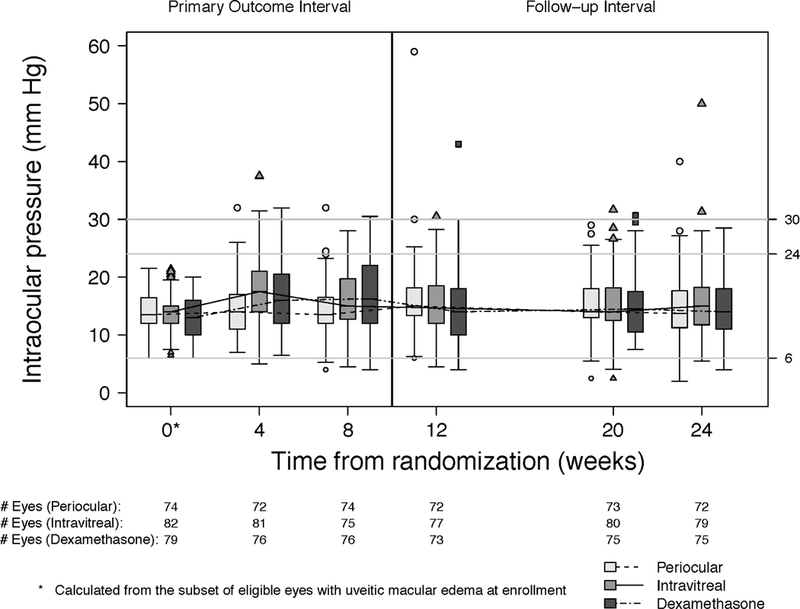
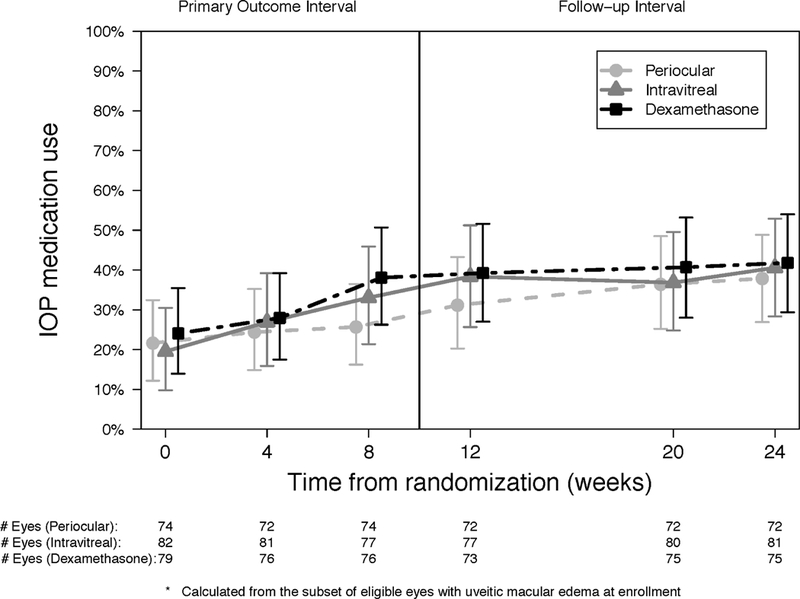
Intraocular pressure (IOP) (a) and use of IOP medication (b) at each visit for periocular triamcinolone (dashed line, circle), intravitreal triamcinolone (solid line, triangle) and dexamethasone implant (staggered line, square).
Table 6.
Time to first ocular adverse event and comparisons among treatment groups.
| Ocular event during follow up | Treatment | N Events/ N at risk | Cumulative % at 24 weeks (95% CI) | Treatment Comparison |
Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| ≥ 10mmHg increase in IOP from baseline | Periocular | 9 / 74 | 14 (5,22) | Intravitreal/ Periocular |
1.92 (0.86,4.29) | 0.11 |
| Intravitreal | 18 / 81 | 26 (13,38) | Dexamethasone/ Periocular |
2.85 (1.30,6.28) | 0.009 | |
| Dexamethasone | 24 / 78 | 39 (20,53) | Dexamethasone/ Intravitreal |
1.43 (0.72,2.81) | 0.30 | |
| IOP ≥ 24 mm Hg | Periocular | 13 / 74 | 20 (9,29) | Intravitreal/ Periocular |
1.83 (0.91,3.65) | 0.09 |
| Intravitreal | 24 / 81 | 30 (17,40) | Dexamethasone/ Periocular |
2.52 (1.29,4.91) | 0.007 | |
| Dexamethasone | 29 / 78 | 41 (26,53) | Dexamethasone/ Intravitreal |
1.32 (0.72,2.43) | 0.37 | |
| IOP ≥ 30 mm Hg | Periocular | 4 / 74 | 6 (0,12) | Intravitreal/ Periocular |
1.07 (0.28,4.01) | 0.92 |
| Intravitreal | 5 / 81 | 6 (1,12) | Dexamethasone/ Periocular |
0.71 (0.16,3.11) | 0.65 | |
| Dexamethasone | 3 / 78 | 4 (0,8) | Dexamethasone/ Intravitreal |
0.64 (0.16,2.59) | 0.53 | |
| Hypotony (IOP < 6 mm Hg) | Periocular | 1 / 71 | 12 (0,30) | Intravitreal/ Periocular |
Not estimable | |
| Intravitreal | 5 / 81 | 6 (1,12) | Dexamethasone/ Periocular |
6.17 (0.97,39.38) | 0.05 | |
| Dexamethasone | 5 / 77 | 12 (0,24) | Dexamethasone/ Intravitreal |
1.08 (0.31,3.71) | 0.90 | |
| Initiating IOP lowering medication |
Periocular | 14 / 58 | 32 (13,46) | Intravitreal/ Periocular |
1.38 (0.68,2.80) | 0.37 |
| Intravitreal | 20 / 65 | 43 (22,59) | Dexamethasone/ Periocular |
1.58 (0.76,3.29) | 0.22 | |
| Dexamethasone | 20 / 60 | 34 (18,48) | Dexamethasone/ Intravitreal |
1.12 (0.54,2.29) | 0.76 | |
| VA decrease of 15 or more standard letters | Periocular | 8 / 74 | 11 (4,18) | Intravitreal/ Periocular |
0.33 (0.09,1.24) | 0.10 |
| Intravitreal | 3 / 81 | 10 (0,22) | Dexamethasone/ Periocular |
0.46 (0.14,1.50) | 0.20 | |
| Dexamethasone | 4 / 78 | 5 (0,10) | Dexamethasone/ Intravitreal |
1.45 (0.34,6.26) | 0.62 |
IC = confidence interval; IOP = intraocular pressure; VA = visual acuity; IOP = intraocular pressure.
Other Ocular Complications:
One eye from a patient in the periocular treatment group received a new diagnosis of glaucoma (1/69 eyes without glaucoma at baseline, 1%). There were no IOP-lowering surgeries and no cataract surgeries during the study. Transient hypotony and loss of BCVA ≥15 letters were infrequent for all treatment groups (Table 6, available at http://aaojournal.org).
Immediate complications after injections also were low for all treatment groups. Four eyes in the intravitreal triamcinolone group (4/79, 5%) required anterior chamber paracentesis as a result of IOP elevation immediately after the injection. Two eyes (1/78 [1%] in the dexamethasone implant group and 1/73 [1%] in the periocular group) had a vitreous hemorrhage. The vitreous hemorrhage in the eye assigned to the periocular group occurred at the week 12 visit after receiving an intravitreal triamcinolone injection. Another eye in the periocular group had a retinal tear (1/73, 1%), which was a result of a scleral breach and subretinal deposition of corticosteroid. There were no cases of retinal detachment and one case of sterile endophthalmitis in the periocular group.
Discussion
Although there is a substantial body of literature reporting the efficacy of periocular triamcinolone, intravitreal triamcinolone, and the intravitreal dexamethasone implant in the treatment of uveitic macular edema, there have been no previous head-to- head comparisons of these three commonly used therapies. Because uveitic macular edema represents a significant cause of ocular morbidity and remains a leading cause of visual impairment, having comparative data from a randomized trial with an objective and masked primary outcome measure is essential to understanding which regional corticosteroid offers the optimal balance of efficacy and safety in the management of these patients.
In the POINT Trial, eyes in all three treatment arms had improvements in macular edema on OCT throughout the follow-up period and the results in each group were comparable to previous publications investigating the effectiveness of periocular triamcinolone13–18,22–24, intravitreal triamcinolone13,17,18,25–31, and the dexamethasone implant20,32–36 individually. At the 8-week visit, both intravitreal treatment groups had significantly greater improvements in CST on OCT from baseline when compared with the periocular treatment group. In addition, treatment with the intravitreal dexamethasone implant met criteria for non-inferiority compared to treatment with intravitreal triamcinolone acetonide at the 8-week visit. Both intravitreal treatments had significantly higher proportions of eyes with ≥20% improvement and resolution of uveitic macular edema when compared to the periocular group starting at the 4-week visit and continuing until the 12-week visit. The difference was attenuated at 24 weeks likely due to the fact that approximately one-third of eyes in the periocular group receiving intravitreal corticosteroid therapy during follow-up. These findings suggest that both intravitreal triamcinolone and the dexamethasone implant are superior to periocular triamcinolone in both speed of improvement/resolution and sustainability of effect. Our primary outcome was an anatomical one, reduction in macular thickness on OCT, as decreases in macular thickness among eyes with uveitic macular edema have been demonstrated to reflect visual acuity improvement. Our definition of improvement in macular edema, a 20% reduction in macular thickness, was chosen as previous work showed it optimized the correlation with improvement in BCVA.21 In the MUST Trial, both resolution of macular edema and improvement in macular edema were associated with a 10-letter (2-line) improvement in BCVA, whereas failure to improve was not, and worsening thickness was associated with a decline in BCVA.10,21
All treatment groups demonstrated improvement in BCVA compared to baseline BCVA. At the 8-week primary outcome visit, eyes in both intravitreal groups had significantly greater visual improvement from the baseline examination compared to eyes in the periocular group. However, the estimates of the differences may be inflated since numerically the periocular group had slightly better BCVA at baseline and therefore and less potential for improvement due to this ceiling effect; however the differences were neither statistically nor clinically significant. There was no significant difference in the improvement in BCVA from baseline between intravitreal triamcinolone and dexamethasone implant groups at any follow-up visit. Taken together, the retinal thickness and BCVA data suggest that although all three regional corticosteroids are effective in treating uveitic macular edema, treatment via the intravitreal approach is superior to that of the periocular approach regardless of the type of corticosteroid utilized.
There was a suggestion of superiority of the intravitreal dexamethasone implant compared to intravitreal triamcinolone at the 8-week visit (PropBL IDI/PropBL ITA: 88, p=0.035). The results, however, were not statistically significant at 8 weeks due to the strict significance criterion imposed by adjustments made for multiple comparisons and stopping early (0.00132) and were not significant at any other visit including the 12- week visit. Nonetheless, there are biologically plausible reasons for this observation. The dexamethasone implant is designed to release an initial burst of dexamethasone followed by a lower steady state release as compared to a single initial burst for intravitreal triamcinolone, which might account for the differences observed at 8 weeks. As such, the dexamethasone implant might perform better earlier on (e.g. the 4- and 8- week visits vs the 12-week visit).37 The relative decrease in performance for the intravitreal dexamethasone implant at the 12-week visit compared to the intravitreal triamcinolone group was unexpected as preliminary data suggested that the peak effect for the intravitreal dexamethasone implant would occur later than that of intravitreal triamcinolone. Therefore, participants in the intravitreal triamcinolone group were allowed second injections at 8 weeks, four weeks earlier than in the dexamethasone implant group. These data suggest that the lifespan of the intravitreal dexamethasone implant is shorter than originally thought and that earlier retreatment may be necessary. Nevertheless, the dexamethasone implant appears at least as good as intravitreal triamcinolone (i.e. “non-inferior”), and there is a suggestion that it might be slightly better at 8 weeks.
Side effects of intravitreal corticosteroid therapies tended to be higher than with periocular corticosteroid treatment, although the risks were not significantly different between the intravitreal corticosteroid groups. The risks were comparable to the reported literature for intravitreal triamcinolone,25,26,29,38–43 but higher than those reported in the HURON Trial for the dexamethasone implant20. Our eligibility criteria were broad in terms of ocular hypertension and glaucoma, which likely allowed for patients at greater risk for IOP elevation to participate in POINT relative to HURON (which excluded patients with a history of IOP elevation due to corticosteroids). Regardless of the inclusion of higher risk patients, the development of severe IOP elevations was uncommon in our study. There were no eyes that required glaucoma surgery and only one eye developed newly diagnosed glaucoma. Other adverse events such as hypotony and BCVA decrease of ≥15 letters were low for all treatment groups. These data support the safety of all three types of regional corticosteroid therapies.
The strengths of this study are that it provides a randomized comparative trial of the three most commonly used regional corticosteroid therapies for treating uveitic macular edema. Eyes with any anatomic type of uveitis (active or inactive), and with macular edema both with and without subretinal fluid or cysts, angiographic leakage, and epiretinal membrane were eligible as were patients with a history of glaucoma (as long as it was controlled), which allow for broad generalization of our results. The primary outcome was objective and measured in a masked fashion. Furthermore, there were very few non-assigned treatments prior to the primary outcome visit and very few losses to follow-up or missing data. The study was stopped early at a pre-planned interim analysis, which could have affected our results by overestimating the effect of treatment; however, the magnitude of any potential overestimation appears to be small in randomized trials with defined, statistically based stopping criteria as in the POINT Trial.44
Nonetheless, there are several limitations. Due to differing pharmacokinetics of the three treatments, the protocol allowed second injections at different time points and changes of treatment from periocular triamcinolone to intravitreal triamcinolone and from intravitreal triamcinolone to the dexamethasone implant. Because 41% of eyes assigned to periocular treatment received intravitreal treatment and 7% of eyes assigned to intravitreal triamcinolone received the dexamethasone implant, interpretation of the comparative results beyond the 8-week, primary outcome visit becomes more difficult, and convergence of success rates (as was observed) might be expected. The trial evaluated the three shorter-acting (vs the fluocinolone acetonide implant) regional corticosteroid injections in use at the time of its inception. Corticosteroid implants in development for uveitis (e.g. fluocinolone acetonide implants, such as Medidur™, pSivida Corporation, Watertown, MA and Iluvien®, Alimera Sciences, Alpharetta, GA) were not included due to the need to limit the number of treatments included in the trial and the lack of sufficient data on the safety and efficacy of these emerging therapies for uveitic macular edema. Additional research will be needed to evaluate their relative effectiveness compared to the treatments investigated in the POINT Trial. Noncorticosteroid regional therapies, such as anti-VEGF antibodies and intravitreal methotrexate, used for treating macular edema were not included in this study, but are being evaluated in another MUST Research Group randomized clinical trial, the Macular Edema Ranibizumab v. Intravitreal anti-inflammatory Therapy (MERIT) Trial. The study duration was relatively short (24 weeks), which limited the ability to detect adverse events requiring time to develop (e.g. incident cataract, cataract progression, incident glaucoma) and to evaluate the rates of recurrence of uveitic macular edema in each treatment group. In addition, the trial was stopped early which limits the power to address secondary hypotheses (e.g. superiority of the intravitreal dexamethasone implant and subgroup analyses). Nevertheless, the POINT Trial provides evidence to guide the utilization of the most commonly used regional corticosteroid injections for uveitic macular edema.
In summary, both intravitreal triamcinolone acetonide and the dexamethasone implant were superior to periocular triamcinolone acetonide for the treatment of uveitic macular edema with modestly greater rates of mild IOP elevation. These data suggest that intravitreal therapy may be the preferred initial therapy for uveitic macular edema. Intravitreal dexamethasone implant was judged non-inferior to intravitreal triamcinolone at the 8-week visit, with the potential to be better, although it did not have a lower risk of IOP elevation than intravitreal triamcinolone as was originally expected.
Supplementary Material
Acknowledgments
Financial Support: This study is a multicenter clinic trial supported by collaborative agreements from the National Eye Institute, National Institutes of Health (Dr. Jabs U10EY024526, Dr. Holbrook U10EY024527, Dr. Altaweel U10EY024531). Allergan (Irvine, CA) donated a limited supply of dexamethasone implants for participants who could not obtain them otherwise. A representative of the National Eye Institute participated in the oversight of the study and review of the manuscript but not directly in the study design, data collection, analysis and interpretation of the data, or in the preparation of this manuscript.
Appendix 1. Credit Roster for MUST Research Group
Duke Eye Center, Durham, NC: Glenn J. Jaffe, MD (Director); Christina F. Bunn; LaToya Greene; Dilraj Grewal, MD; Sean Grout; Michael Hughes; Ryan Imperio; Michael P. Kelly; Brian Lutman; Paola Perez.
Emory University, Atlanta, GA: Steven Yeh, MD (Director); Jannah Dobbs; Alcides Fernandes, MD; Deborah Gibbs, COMT, CCRC; Donna Leef, MMSc, COMT; Kelly Mazur; Ghazala O’Keefe, MD; Rhonda Waldron, BS, MMSc.
Johns Hopkins University, Baltimore, MD: Jennifer E Thorne, MD, PhD (Director); Olukemi Adeyemo; Joseph Belz; Meghan Berkenstock, MD; Jeff Boring, COA; Bryn Burkholder, MD; Dennis Cain; Eric Crowell, MD, MPH; Russ Distler; David Emmert; Janis R. Graul; Charles Mark Herring; Pat Hines; Dana Hornbeak, MD; Irfan Khan, MD; Jacquelyn McDonald; Kelly Miller, COA; Robert Moore; Antonia Nwankwo, COA; Chinwenwa Okeagu, MD; Ashvini Reddy, MD; Terry L. Reed, COA; Nicholas Rhoton; Amde S. Shifera, MD, PhD.
Massachusetts Eye and Ear Infirmary, Boston, MA: George Papaliodis, MD; Steven Bennett; Sarah Brett; Lisa Dennehy; Erica D’Souza; Matthew DiRocco; Ellen Fitzgerald; John Head; John Hensel; Mansab Jafri; Anneliese Koleber; Tuyen Nguyen; Milka Nova; Abdulrahman Rageh; John Romano; Lucia Sobrin, MD; Lynn Sullivan; Megan Thompson; Kathleen Warren; Iris Wen.
Mayo Clinic, Rochester, MD: Wendy Smith, MD (Director); Mario Ayers; Jean Burrington; Shannon Goddard; Daniel Hamiel; Anita Hermanson; Shannon Howard; Zbigniew Krason; Jessica Morgan; Joan Overend; Heidi Rubin; Jane Sultze; Jamie Tesmer.
McGill University, Montreal, Quebec: Jean Deschenes, MD (Director); Carolina Ararat; Isabelle Delpech; Lianne Marcon; Karin Marianna Oliver, MD; Patricia Sorya; Emiko Vervroegen-Inoue; Debbie Williams.
Moorfields Eye Hospital, London, UK: Susan Lightman, MD, PhD (Director); Ahmed Al- Janabi, MD; Julitiana Baltinas; Rose Gilbert, MBBS, MRCOphth; Dimitrios Ladas MD; Rebecca Shields; Iazha Talat, MD; Anastasia Tasiopoulou MD; Oren Tomkins-Netzer; Sophia Zagora, MD.
National Eye Institute, Bethesda, MD: H. Nida Sen, MD, MHSc (Director); Mike Arango; Matt Atkinson; Faith Chen, BSN, RN; Denise Cunningham, CRA; Darryl Hayes, COA; Dessie Koutsandreas, BS, COA; Rob Mays; Leanne Reuter, COA; Patti Sherry, MSHS, BSN, CCRP.
New York Eye & Ear Infirmary, New York, NY: Paul A. Latkany, MD (Director); Maya Ben-Zeev; Corinne Coonan; Andrea Honda, MD; Monica Lorenzo-Latkany, MD; Robert Masini; Azinda Morrow.
Northwestern University: Debra A. Goldstein, MD (Director); Maritza Barragan, AS; Andrea Birnbaum, MD, PhD; Natalia Brooks; Gemma De la Rosa, BHS, COT; Anjum Koreishi, MD; Sharnia Lashley, MS; Cason Moore; Mia Quintana, AS; Carmen Ramirez; Nicole Seddon, BS; Evica Simjanowski, CRA; Mariner Skelly; Catherine Thuruthumaly, MD; Mei Zhou.
Ophthalmic Consultants of Boston, Boston, MA: Lana Rifkin, MD (Director); Joanne Berry; Amanda Campbell; Dennis Donovan; Marissa M. Giel; Margaret Graham; Mike Jones; Jennifer Kruger.
Retina Consultants of Houston: Rosa Y. Kim, MD (Director); Belinda Almanza; Matthew S. Benz, MD; David M. Brown, MD; Eric Chen, MD; Marie Chin; Cassandra Cone; Richard H. Fish, MD; Christopher Henry, MD; Eric Kegley, BA, CRA, COA; Nubia Landaverde; James C. Major, Jr., MD; Chelsey Moore; Beau Richter; Amy C. Schefler, MD; Laura Shawver; Robert Smith; Veronica A. Sneed; Cary Stoever; Tien P. Wong, MD; Charles C. Wykoff, MD.
Royal Victorian Eye & Ear Hospital, East Melbourne, Australia: Richard J. Stawell, MD (Principal Investigator);Thuy Chau; Xavier Fagan, MD; Rathika Kandasamy, MD; Lyndell Lim, MD; Ming-Lee Lin, MD; Cecilia Ling, MD; Dai Ni Ong, MD; Sutha Sanmugasundram; Robyn Troutbeck, MD; Ehud Zamir, MD.
Rush University Medical Center, Chicago, IL: Pauline T. Merrill, MD (Director); Elizabeth Atchison, MD; Carla Edwards, PhD, MPH; Mathew MacCumber,MD, PhD; Blanca Ornelas; Loreen Pappas; Len Richine; Brenda Rodriguez; Kisung Woo; John C. Zeyer, MD.
University of California at Los Angeles, Los Angeles, CA: Gary N. Holland, MD (Director); Robert D. Almanzor, COA; Meghan Berkenstock, MD; Jose Castellanos, COT; Orly Catz; Daniel Cordova; Lauren Eash; Martin Garcia, BS; Jean Pierre Hubschman, MD; Colin A. McCannel, MD; Ellen Pascual, BS; Pradeep S. Prasad, MD.
University of California at San Francisco, San Francisco, CA: Nisha Acharya, MD (Director); Thuy Doan, MD, PhD; John Gonzales, MD; Betty Hom; Mary Lew, BA; Sarah Lopez; Maya Rao, MPH; Jay Stewart, MD; Lina Zhong.
University of Iowa, Iowa City, IA: James Folk, MD (Director); Brice Critser, CRA; Maria C. Davis Hochstedler, BA; Lacy Flanagan, BA; Ian C. Han, MD; Mitchell J. Martin, BS; Christine A. Sinkey, BSN; Nasreen Syed, MD; Barbara K. Taylor, BBA; Toni Venckus, BA; Jean A. Walshire, AASc.
University of Miami, Miami, FL: Janet L. Davis, MD (Director); Jim Crowell; Giselle De Oliveira; Marcella Hicky; Carmen Huerta; Jennifer Johnson; Michael Kicak; Jaclyn Kovach, MD; Christiaan Lopez Miro; Daisy Monteagudo; Jim Oramas; Sira Padron; Stephen Schwartz, MD, MBA; Brandon Sparling; Richard R. Stanfield; Richard Vorperian.
University of Michigan, Ann Arbor, MI: Susan G. Elner, MD (Director); Pamela Campbell, COT, CCRP; Sonya Cosby; Timothy Costello; Linda Fournier, COT; Lindsay M. Godsey, MS, COA, CCRP; Linda Goings, BFA, CRA; Dena Harris; Moella Hesselgrave, COA; Munira Hussain, MS; K. Thiran Jayasundera, MD; Mathew Lawrence; Katherine Morehead; Robert Prusak, BA, CRA; Laura Rozek, COA; Anjali R. Shah, MD; Timothy J. Steffens, CRA; Laura Trebesh, CRA.
University of Pennsylvania, Philadelphia, PA: Nirali Bhatt, MD (Director); Mauricio Alvarez; James Berger; Dominique Caggiano; Judy Chen; Cheryl Devine; Joan C. DuPont; Andre Estanislau; Sheri Grand-Drossner, MSW; Matthew Henderson; Meghan Karlik; Sara Morales; Janice Petner; Tyrone Quarterman; Collin Russell; Adrienne Saludades.
University of Pittsburgh Medical Center, Pittsburgh, PA: Andrew Eller, MD (Director); Natalie L. Anthony; Tyler M. Banash; Shane Belin, BS; Rhonda J. Dahlstrom, MA; Michael J. DeRosa, BS, BA; Thomas Friberg, MD; Denise S. Gallagher, MD; Chad Indermill, BS; Joseph N. Martel, MD; Caterina M. Massimino; Kathyryn L. Mentzer; Sharon A. Murajda- Jumba, BS; Tina L. Niggl; Jessica S. Toro; Melessa Salay, MPH; Amy M. Smith; Kristy Truman, COA; Gary L. Vagstad; Evan L. Waxman, MD.
University of South Florida, Tampa, FL: Peter R. Pavan, MD (Director); Ken Albritton; Swetangi Bhaleeya, MD; Sharon Charton; JoAnn Leto; Brian Madow, MD; Paula Russian; Scott E. Pautler, MD; Wyatt Saxon; Susan Scherouse.
University of Utah, Salt Lake City, UT: Albert Vitale, MD (Director); Susan Allman; Paul S. Bernstein, MD, PhD; Melissa Chandler; Kelliann Farnsworth; Katie J. Farnsworth, BS James Gilman, CRA; Deborah Harrison, MS, CCRC; Elizabeth Nuttall; Akbar Shakoor, MD; Becky Weeks, CRA; Kimberley Wegner, BS, CRC.
University of Washington, Seattle, WA: Russell Van Gelder, MD, PhD (Director); Ron Jones; James Leslie; Thellea Leveque, MD, MPH; Ian Luttrell; Frances Moses; Juli Pettingill, COMT; Kathryn Pepple, MD, PhD.
Washington University, St. Louis, MO: Humeyra Karacal, MD (Director); Eve Adcock; Patricia Arnold; Sarah Gardner; John Greer, COT; Erika Hoehn; Charla Meyer; Greg Rathert; P. Kumar Rao, MD.
Wills Eye Hospital, Mid Atlantic Retina, Philadelphia, PA: James P. Dunn, MD (Director); Suely Bascope; Hannah Benfield; Christina Centinaro; Michele Formoso, MD; Sunir Garg, MD; Elaine Gonzales; Lisa Grande; Omesh Gupta, MD, Allen Ho, MD; Jason Hsu, MD; Brianna Kenney; Zafiirah Khodabukus; Rachel Magner; Sonia Mehta, MD; Michelle Millard; Lila Ortiz; Carl Park, MD; Marc Spirn, MD.
Resource Centers
Chairman’s Office: Mount Sinai School of Medicine, New York, NY: Douglas A. Jabs, MD, MBA (Chair); John H. Kempen, MD, PhD; Karen Pascual, MBA; Jill S. Slutsky-Sanon, MPA.
Coordinating Center, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD: Janet T. Holbrook, PhD, MPH (Director); Debra Amend-Libercci; Alyce Burke, MPH; Cathleen Ewing; David S. Friedman, MD, PhD; Andrea Lears, BS; Jill Meinert; Vinnette Morrison, BS; Deborah Nowakowski; Nancy Prusakowski, MS; Dave M. Shade, JD; Jacqueline Smith, AA; Akrit Sodhi, MD, PhD; Elizabeth A. Sugar, PhD; V. Ben Supan; Mark Van Natta, MHS.
Data and Safety Monitoring Committee:Voting members: Alan G. Palestine, MD (Chair); William E. Barlow, PhD; Alice T. Lyon, MD; David C. Musch, PhD, MPH; Lee S. Simon, MD.
Non-voting members: Michael M. Altaweel, MD; Sangetta Bhargava, PhD; Donald Everett, PhD; Janet T. Holbrook, PhD, MPH; Douglas A. Jabs, MD, MBA. Former member: Kim Overby, MD, MBE
Executive Committee: Douglas A. Jabs, MD, MBA (Chair); Michael M. Altaweel, MD; Sangeeta Bhargava, PhD; Donald Everett, PhD; Janet T. Holbrook, PhD, MPH; John H. Kempen, MD, PhD; Elizabeth E. Sugar, PhD.
EyeKor: Nathan Diers; Anne Goulding; Jeremy Lawn
Statistical Analysis Committee: Elizabeth A. Sugar, PhD (Chair); Alyce E. Burke, MPH; Lea T. Drye, PhD; Janet T. Holbrook, PhD, MPH; Mark L. Van Natta, MHS.
University of Wisconsin Fundus Photograph Reading Center, Madison, WI: Michael M. Altaweel, MD (Director); Amitha Domalpally, MD; Daniel Lawrence, PhD; Susan Reed, BS; Pam Vargo; Hugh Wabers.
Visual Function Quality Assurance Committee: Robert D. Almanzor, COA (Chair); Deborah Gibbs, COMT, CCRC; Mary Lew, COT; Nancy Prusakowski, MS; Jennifer E. Thorne, MD, PhD (Advisor).
Footnotes
Conflicts of Interest: Jennifer E. Thorne, MD, PhD, Grant support from Allergan, Inc., Consultant for AbbVie, Inc., Gilead, Santen; Janet T. Holbrook, PhD, Data Safety Monitoring Board member for Gilead; Albert T. Vitale, MD, Consultant for AbbVie, Inc., ACIONT; Nisha R. Acharya, MD, MBA, AbbVie Inc., Santen; John H. Kempen, MD, PhD, Consultant for Gilead, Santen; Elizabeth A. Sugar, PhD, Alyce Burke, MPH, Michael M. Altaweel, MD, and Douglas A. Jabs, MD, MBA, none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier: NCT02374060
References
- 1.Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234–235. [DOI] [PubMed] [Google Scholar]
- 2.ten Doesschate J Causes of blindness in The Netherlands. Doc Ophthalmol. 1982;52:279–285. [DOI] [PubMed] [Google Scholar]
- 3.Tomkins-Netzer O, Talat L, Bar A et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology. 2014;121:2387–2392. [DOI] [PubMed] [Google Scholar]
- 4.Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88:1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorne JE, Skup M, Tundia N, et al. Direct and indirect resource use, healthcare costs and work force absence in patients with non-infectious intermediate, posterior or panuveitis. Acta Ophthalmol. 2016;94:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okhravi N, Lightman S. Cystoid macular edema in uveitis. Ocul Immunol Inffamm. 2003;11:29–38. [DOI] [PubMed] [Google Scholar]
- 7.Lardenoye CW, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006;113(8):1446–1449. [DOI] [PubMed] [Google Scholar]
- 8.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Sugar EA. The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. Am J Ophthalmol. 2010;149:550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomkins-Netzer O, Lightman S, Drye L, et al. Outcome of treatment of uveitic macular edema. The Multicenter Uveitis Steroid Treatment Trial 2-Year Results. Ophthalmology 2015;122(11):2351–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempen JH, Altaweel MM, Holbrook JT, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118:1916–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grajewski RS, Boelke AC, Adler W, et al. Spectral-domain optical coherence tomography findings of the macula in 500 consecutive patients with uveitis. Eye (Lond). 2016;30: 1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roesel M, Gutfleisch M, Heinz C, Heimes B, Zurek-Imhoff B, Heiligenhaus A. Intravitreal and orbital floor triamcinolone acetonide injections in noninfectious uveitis: a comparative study. Ophthalmic Research. 2009;42:81–86. [DOI] [PubMed] [Google Scholar]
- 14.Thach AB, Dugel PU, Flindall RJ, Sipperley JO, Sneed SR. A comparison of retrobulbar versus sub-Tenon’s corticosteroid therapy for cystoid macular edema refractory to topical medications. Ophthalmology. 1997;104:2003–2008. [DOI] [PubMed] [Google Scholar]
- 15.Ferrante P, Ramsey A, Bunce C, Lightman S. Clinical trial to compare efficacy and side-effects of injection of posterior sub-Tenon triamcinolone versus orbital floor methylprednisolone in the management of posterior uveitis. Clin Experiment Ophthalmol. 2004;32:563–568. [DOI] [PubMed] [Google Scholar]
- 16.Venkatesh P, Kumar CS, Abbas Z, Garg S. Comparison of the efficacy and safety of different methods of posterior subtenon injection. Ocul Immunol Inflamm. 2008;16:217–223. [DOI] [PubMed] [Google Scholar]
- 17.Choudhry S, Ghosh S. Intravitreal and posterior subtenon triamcinolone acetonide in idiopathic bilateral uveitic macular oedema. Clin Experiment Ophthalmol. 2007;35:713–718. [DOI] [PubMed] [Google Scholar]
- 18.Roesel M, Gutfleisch M, Heinz C, Heimes B, Zurek-Imhoff B, Heiligenhaus A. Intravitreal and orbital floor triamcinolone acetonide injections in noninfectious uveitis: a comparative study. Ophthalmic Research. 2009;42:81–86. [DOI] [PubMed] [Google Scholar]
- 19.Ferris FL 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 20.Lowder C, Belfort R Jr., Lightman S, Foster CS, Robinson MR, Schiffman RM, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):545–553. [DOI] [PubMed] [Google Scholar]
- 21.Sugar EA, Jabs DA, Altaweel MM, Lightman S, Acharya N, Vitale AT, et al. Identifying a clinically meaningful threshold for change in uveitic macular edema evaluated by optical coherence tomography. Am J Ophthalmol. 2011; 152(6): 1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jennings T, Rusin MM, Tessler HH, Cunha-Vaz JG. Posterior sub-Tenon’s injections of corticosteroids in uveitis patients with cystoid macular edema. Jpn J Ophthalmol. 1988;32(4):385–391. [PubMed] [Google Scholar]
- 23.Helm CJ, Holland GN. The effects of posterior subtenon injection of triamcinolone acetonide in patients with intermediate uveitis. Am J Ophthalmol. 1995;120(1):55–64. [DOI] [PubMed] [Google Scholar]
- 24.Leder HA, Jabs DA, Galor A, Dunn JP, Thorne JE. Periocular triamcinolone acetonide injections for cystoid macular edema complicating noninfectious uveitis. Am J Ophthalmol. 2011;152:441–448. [DOI] [PubMed] [Google Scholar]
- 25.Dong Z, Namba K, Kitaichi N, Goda C, Kitamura M, Ohno S. Efficacy and complications of intravitreal injection of triamcinolone acetonide for refractory cystoid macular edema associated with intraocular inflammation. Jpn J Ophthalmol. 2008;52(5):374–379. [DOI] [PubMed] [Google Scholar]
- 26.Young S, Larkin G, Branley M, Lightman S. Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Experiment Ophthalmol. 2001;29(1):2–6. [DOI] [PubMed] [Google Scholar]
- 27.Antcliff RJ, Spalton DJ, Stanford MR, Graham EM, ffytche TJ, Marshall J. Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology. 2001;108(4):765–772. [DOI] [PubMed] [Google Scholar]
- 28.Hogewind BFT, Zijlstra C, Klevering BJ, Hoyng CB. Intravitreal triamcinolone for the treatment of refractory macular edema in idiopathic intermediate or posterior uveitis. Eur J Ophthalmol. 2008;18:429–434. [DOI] [PubMed] [Google Scholar]
- 29.Androudi S, Letko E, Meniconi M, Papadaki T, Ahmed M, Foster CS. Safety and efficacy of intravitreal triamcinolone acetonide for uveitic macular edema. Ocul Immunol Inflamm. 2005;13(2–3):205–12. [DOI] [PubMed] [Google Scholar]
- 30.Sallam A, Taylor SR, Habot-Wilner Z, Elgohary M, Do HH, McCluskey P, et al. Repeat intravitreal triamcinolone acetonide injections in uveitic macular oedema. Acta Ophthalmol. 2012;90(4):e323–325. [DOI] [PubMed] [Google Scholar]
- 31.Habot-Wilner Z, Sallam A, Pacheco PA, Do HH, McCluskey P, Lightman S. Intravitreal triamcinolone acetonide as adjunctive treatment with systemic therapy for uveitic macular edema. Eur J Ophthalmol. 2011;21 Suppl 6:S56–61. [DOI] [PubMed] [Google Scholar]
- 32.Kuppermann BD, Blumenkranz MS, Haller JA, Williams GA, Weinberg DV, Chou C, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125(3):309–317. [DOI] [PubMed] [Google Scholar]
- 33.Williams GA, Haller JA, Kuppermann BD, Blumenkranz MS, Weinberg DV, Chou C, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am J Ophthalmol. 2009; 147(6): 1048–1054 [DOI] [PubMed] [Google Scholar]
- 34.Herrero-Vanrell R, Cardillo JA, Kuppermann BD. Clinical applications of the sustained-release dexamethasone implant for treatment of macular edema. Clin Ophthalmol. 2011;5:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saraiya NV, Goldstein DA. Dexamethasone for ocular inflammation. Expert Opin Pharmacother. 2011;12(7):1127–1131. [DOI] [PubMed] [Google Scholar]
- 36.Miserocchi E, Modorati G, Pastore MR, Bandello F. Dexamethasone intravitreal implant: an effective adjunctive treatment for recalcitrant noninfectious uveitis. Ophthalmologica. 2012;228(4): 229–233. [DOI] [PubMed] [Google Scholar]
- 37.Chang-Lin JE, Attar M, Achearnpong AA, et ta. Pharmacokinetics and pharmacodynamics of sustained-release dexamethasone intravitreal implant. IOVS 2011; 52:80–6. [DOI] [PubMed] [Google Scholar]
- 38.Hirano Y, Ito T, Nozaki M, Yasukawa T, Sakurai E, Yoshida M, et al. Intraocular pressure elevation following triamcinolone acetonide administration as related to administration routes. Jpn J Ophthalmol. 2009;53(5):519–522. [DOI] [PubMed] [Google Scholar]
- 39.Jonas JB, Degenring RF, Kreissig I, Akkoyun I, Kamppeter BA. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology. 2005;112(4):593–598. [DOI] [PubMed] [Google Scholar]
- 40.Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138(5):740–743. [DOI] [PubMed] [Google Scholar]
- 41.Gillies MC, Simpson JM, Billson FA, Luo W, Penfold P, Chua W, et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004;122(3):336–340. [DOI] [PubMed] [Google Scholar]
- 42.Roth DB, Verma V, Realini T, Prenner JL, Feuer WJ, Fechtner RD. Long-term incidence and timing of intraocular hypertension after intravitreal triamcinolone acetonide injection. Ophthalmology. 2009;116(3):455–460. [DOI] [PubMed] [Google Scholar]
- 43.Jonas JB, Schlichtenbrede F. Visual acuity and intraocular pressure after high-dose intravitreal triamcinolone acetonide in selected ocular diseases. Eye (Lond). 2008;22(7): 869–873. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Rosner GL, Goodman SN. Quantifying over-estimation in early stopped clinical trials and the “freezing effect” on subsequent research. Clin Trials 2016;13(6):621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


