Abstract
Pituitary adenylate cyclase activating polypeptide (PACAP, Adcyap1) activation of PAC1 receptors (Adcyap1r1) significantly increases excitability of guinea pig cardiac neurons. This modulation of excitability is mediated in part by plasma membrane G protein-dependent activation of adenylyl cyclase and downstream signaling cascades, as well as by endosomal signaling mechanisms. PACAP/PAC1 receptor-mediated activation of plasma membrane adenylyl cyclase (AC) and the resulting increase in cellular cAMP enhances a hyperpolarization-induced nonselective cationic current Ih, which contributes to the PACAP-induced increase in cardiac neuron excitability. Further, PACAP-mediated AC/cAMP/PKA downstream signaling also appears to enhance cardiac neuron IT to facilitate the excitatory responses. PACAP activation of PAC1 receptors rapidly stimulates receptor internalization, and reducing ambient temperature or treatments with the clathrin inhibitor Pitstop2 or the dynamin I/II inhibitor dynasore to block endocytic events can suppress PACAP-enhanced neuronal excitability. Thus, endocytosis inhibitors essentially eliminate PACAP-enhanced excitability suggesting that endosomal platforms represent a primary signaling mechanism. Endosomal signaling is associated canonically with ERK activation and in accord, PACAP enhanced cardiac neuron excitability is reduced by MEK inhibitor pretreatments. PACAP activation of MEK/ERK signaling can enhance currents through voltage-dependent Nav1.7 channels. Hence, PACAP-induced PAC1 receptor internalization/endosomal signaling, recruitment of MEK/ERK signaling and modulation of Nav1.7 are implicated as key mechanisms contributing to the PACAP enhanced neuronal excitability. PACAP/PAC1 receptor-mediated endosomal ERK signaling in central circuits can play key roles in development of chronic pain and anxiety-related responses; thus, PAC1 endosomal signaling likely participates in a variety of homeostatic responses within neuronal circuits in the CNS.
Keywords: Cardiac neuron, PACAP, PAC1 receptor internalization/endosomal signalling, MAPK modulation of Nav1.7
Introduction
Pituitary adenylate cyclase activating polypeptide (PACAP) peptides (Adcyap1) are trophic and intercellular signaling molecules that are widely distributed within neural and endocrine tissues (Arimura, 1998; Vaudry et al. 2009). PACAP effects play critical roles in central stress challenges, regulation of sensory and autonomic function, cognitive learning and protection from injury paradigms (Legradi et al. 2007; Tompkins et al. 2007; Hammack et al. 2009; Vaudry et al. 2009; Hill et al. 2011; Ressler et al. 2011; Cho et al.2012; Stroth et al. 2012; Hammack and May 2014). PACAP is highly expressed in the central nervous system (CNS) in the hypothalamus, hippocampus and related allocortex, limbic system as well as in the peripheral nervous system (PNS) within sensory and autonomic pathways. The actions of PACAP are mediated through several seven transmembrane G-protein coupled receptor (GPCR) subtypes including the PACAP-selective PAC1 receptor (Adcyap1r1) and PACAP/VIP VPAC receptors (Vipr1 and Vipr2; also VPAC1 and VPAC2, respectively) (Harmar and Lutz 1994; Arimura, 1998; Braas and May 1999; Vaudry et al. 2009). There are multiple PAC1 receptor isoforms from alternative splicing; most notably, the absence or presence of Hip and/or Hop cassette inserts into the third cytoplasmic loop have the potential of modulating intracellular signal generation. Accordingly, the PAC1 receptor variants may be null (neither Hip nor Hop), Hip, Hop or HipHop inserts. The PAC1null and PAC1Hop receptor variants predominate in the CNS and PNS. Cellular actions of the PACAP selective PAC1 receptor are mediated commonly by membrane delimited recruitment of Gαs and Gαq/11 leading to adenylyl cyclase (AC) and for phospholipase C (PLC) activation, respectively (Deutsch and Sun 1992; Spengler et al. 1993; Pisegna and Wank 1996; Braas and May 1999). However, additional intracellular signaling cascades, such as mitogen- activated protein kinase (MAPK) and Akt (also protein kinase B) (Pisegna and Wank 1996; Barrie et al. 1997; Bouschet et al. 2003; May et al. 2010) can be recruited, especially following PACAP-induced PAC1 receptor internalization and endosomal signaling (May et al. 2010; May et al. 2014).
We have demonstrated that PACAP is present in parasympathetic cholinergic preganglionic nerve terminals innervating guinea pig cardiac ganglia neurons (Braas et al.1998; Calupca et al. 2000), and that neurally-released or exogenous PACAP application depolarizes and increases cardiac neuron excitability via activation of the selective PAC1 receptor (Adcyap1r1) (Braas et al. 1998; Tompkins et al. 2006, 2007; Hoover et al. 2009). The cardiac neurons express predominantly the PAC1null receptor variant, represent a readily accessible neuronal system compared to CNS nuclei for experimental manipulation, and have well characterized electrophysiological properties (Edwards et al.1995). Thus, cardiac ganglia neurons provide an excellent neuronal system to elucidate PACAP/PAC1 receptor-mediated recruitment of second messengers and modulation of ionic conductances that potentially contribute to the regulation of neuronal excitability.
PACAP increases cardiac neuron excitability
The PACAP-induced increase in cardiac neuron excitability is evident from the shift in firing pattern elicited by long depolarizing current steps as shown in Figure 1 A1, B1. Quantification of the increased excitability is determined by plotting the number of action potentials generated by 1 second depolarizing current steps of increasing magnitude (Figure 1C). Shifts in the slope of the excitability curve indicate increases or decreases in neuronal excitability. Both plasma membrane delimited (Gαs and Gαq/11) and endosomal signaling mechanisms can potentially contribute to the PACAP enhanced excitability of the guinea pig cardiac neurons. Results from a number of studies indicate that recruitment of Gαq/11 for PLC activation following activation of the cardiac neuron PAC1 receptor does not play any role in the PACAP-induced increase in cardiac neuron excitability (Parsons et al. 2008). In contrast, a PACAP/PAC1 receptor-mediated activation of Gαs/adenylyl cyclase and the subsequent increase in intracellular cAMP stimulates a hyperpolarization-induced nonselective cationic current Ih, as evidenced by an enhanced rectification or “sag” in the voltage change produced by hyperpolarizing current steps. This enhancement of Ih could be a component of the PACAP-induced increase in cardiac neuron excitability (Figure 1 A2, B2) (Merriam et al. 2004; Tompkins et al. 2009). Concurrently, PACAP activation of the nickel-sensitive, low voltage-activated calcium current IT may also participate in the PACAP-induced increase in excitability (Tompkins et al. 2015). This enhancement of IT is evident as an enhanced post-hyperpolarization-induced rebound depolarization, which is a signature characteristic of T-type calcium channels. (Talavera and Nilius 2006; Iftinca and Zamponi 2008; Simms and Zamboni 2014). In the example shown, the post-hyperpolarization-induced depolarization in a control cell was sufficient to elicit one action potential whereas PACAP-treated neurons were capable of generating multiple action potentials under the same recording protocol (Figure 1 A1, A2). Protein kinase A (PKA) phosphorylation of T-type channel α subunits has been shown to enhance IT (Talavera and Nilius 2006; Chemin et al. 2007; Iftinca and Zamponi 2008; Simms and Zamboni 2014). Thus, PACAP/PAC1 receptor activation of adenylyl cyclase/cAMP and downstream PKA-mediated phosphorylation of T-type channels to enhance cardiac neuron IT could be contributory to PACAP regulation of cardiac neuron excitability. Other PACAP-mediated ionic mechanisms have been suggested to regulate neuronal function. In dissociated cultured hippocampal neurons, for example, a PACAP-induced decrease in the voltage-dependent potassium current IA, flowing through KV4.2 subunits, has been proposed to contribute to a PACAP-enhanced excitability (Gupte et al. 2016). Although cardiac neurons express Kv4.2 transcripts, the IA blocker 4-aminopyridine did not replicate PACAP effects on cardiac neurons (Tompkins et al. 2016). From these observations, a decrease in membrane Kv4.2 subunit expression does not appear to impact PACAP function on cardiac neuron excitability (Tompkins et al. 2016).
Figure 1.
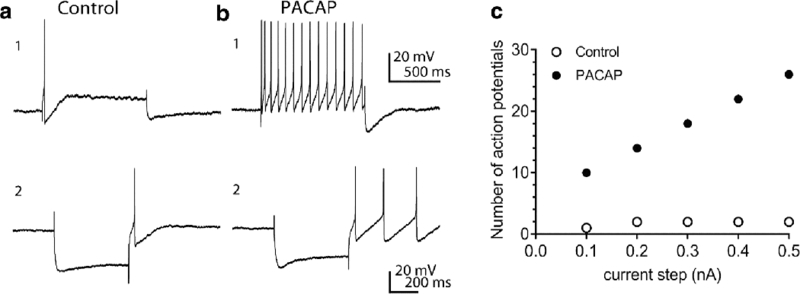
PACAP can enhance excitability, rectification and a hyperpolarization-induced rebound depolarization in guinea pig cardiac neurons. Panels A1, B1 illustrate the 20 nM PACAP-induced shift from phasic to multiple action potential generation. Prior to PACAP application, a 1 second, 0.2 nA depolarizing constant current pulse elicited 1 action potential. During exposure to PACAP, the number of action potentials generated by the same depolarizing current pulse increased markedly. Panels A2, B2 show that PACAP also increased the rectification in the hyperpolarization elicited by a 500 second constant current pulse and likewise enhanced the hyperpolarization-induced rebound depolarization. C. Excitability curve showing the PACAP enhancement of action potentials generated by 1 second depolarizing current steps of increasing intensity. Open circles: number of action potentials generated prior to PACAP; closed circles: number of action potentials elicited during exposure to PACAP. Reprinted with permission from the American Journal of Physiology Cell Physiology (Tompkins et al, 2016).
The ability for PACAP to stimulate cardiac neuron excitability through enhancement of Ih and IT currents is further substantiated by treatments with cesium to block Ih, or with nickel to block IT. Both treatments significantly, but only partially, suppress the PACAP-induced increase in excitability. These results in total suggested other PACAP/PAC1 receptor signaling processes are important in the regulation of neuronal function (Parsons et al. 2016), and as described below, environmental conditions or drugs that suppress PAC1 receptor endocytosis eliminated PACAP-enhanced neuronal excitability to implicate endosomal mechanisms.
PACAP induces PAC1 receptor internalization in cell line cultures
Human embryonic kidney cells that stably express a human PAC1 receptor tethered to EGFP at the C-terminus (HEK PAC1R-EGFP receptor cells) have been used to establish conditions and pharmacological treatments that blunt PACAP-induced internalization of the PAC1 receptor (May et al. 2010; Merriam et al. 2013). The HEK PAC1null-EGFP and PAC1Hop1-EGFP receptor cells yielded comparable results but the studies described used predominantly cultures of the latter. Under control conditions at 37°C, the receptor remains primarily in the cell membrane, whereas within a few minutes of exposure to nanomolar concentrations of PACAP at 37°C, there is extensive PAC1R-EGFP endocytosis into intracellular vesicles, with a concomitant reduction in cell surface fluorescence. In contrast, culture PACAP exposures of similar duration at 24°C resulted in scant receptor translocation into intracellular vesicles and no loss of membrane fluorescence, indicating that receptor internalization is suppressed at room temperature. Effects similar to reduced temperature were noted when the PACAP-stimulated HEK PAC1R-EGFP cells were pretreated with Pitstop2, a clathrin inhibitor (von Kleist et al. 2011), or dynasore, a dynamin I/II inhibitor (Maca et al. 2006), both of which are established blockers of endocytosis. These observations were notable as internalized receptors are now well appreciated not to represent solely desensitization mechanisms, but as alternative signaling platforms that would allow the dynamic targeting of secondary messengers to intracellular sites with high temporal and spatial resolution (Calebiro et al. 2009; Ferrandon et al. 2009; Calebiro et al. 2010, Irannejad et al. 2013; Vilardaga et al. 2014). Internalized endosomal GPCRs have been best studied with respect to β-arrestin and MAPK/extracellular signal-regulated kinase (ERK) signaling although recent work also has suggested that endosomes can also drive cAMP generation (Calebiro et al. 2009; Ferrandon et al. 2009; Calebiro et al. 2010, Irannejad et al. 2013; Vilardaga et al. 2014). In agreement, our preliminary studies have shown that the internalized PAC1R-EGFP vesicles are rapidly co-localized (in minutes) with β-arrestin immunoreactivity and the early endosomal marker Rab5. Only at later time points (approximately 30 min) are the receptor endosomes co-localized with late endosomal marker Rab7a to suggest vesicular trafficking to lysosomal compartments for potential degradation (data not shown).
PACAP/PAC1 receptor internalization participates in neuronal excitability mechanisms
Having established that ambient temperature or treatments with Pitstop2 or dynasore can suppress the PACAP-induced PAC1 receptor internalization in the stably expressing cell lines, similar conditions/treatments were used to determine the role of PAC1 receptor endocytosis in supporting PACAP-induced increase in cardiac neuron excitability. Reducing the bath temperature from 33°C to 22–24°C essentially eliminated the PACAP enhanced excitability (Figure 2) (Merriam et al. 2013). When cardiac ganglia preparations were maintained at near physiological temperatures (33 – 35 °C), PACAP consistently increased cardiac neuron excitability in ~90% of cells tested (Figure 2 A1). In contrast, when cardiac ganglia preparations were maintained at room temperature (21 – 25°C), PACAP increases excitability in only 13% of cells tested (Figure 2 A2); the averaged excitability curve in PACAP for cells maintained at room temperature was not different from the averaged excitability curve generated in untreated control cells (Figure 2C). Furthermore, cardiac neuron exposure at room temperature to 1 mM barium to block the M-current still dramatically enhanced action potential generation by depolarizing steps, indicating the neurons remained excitable even at lower temperatures (Figure 2 A3).
Figure 2.
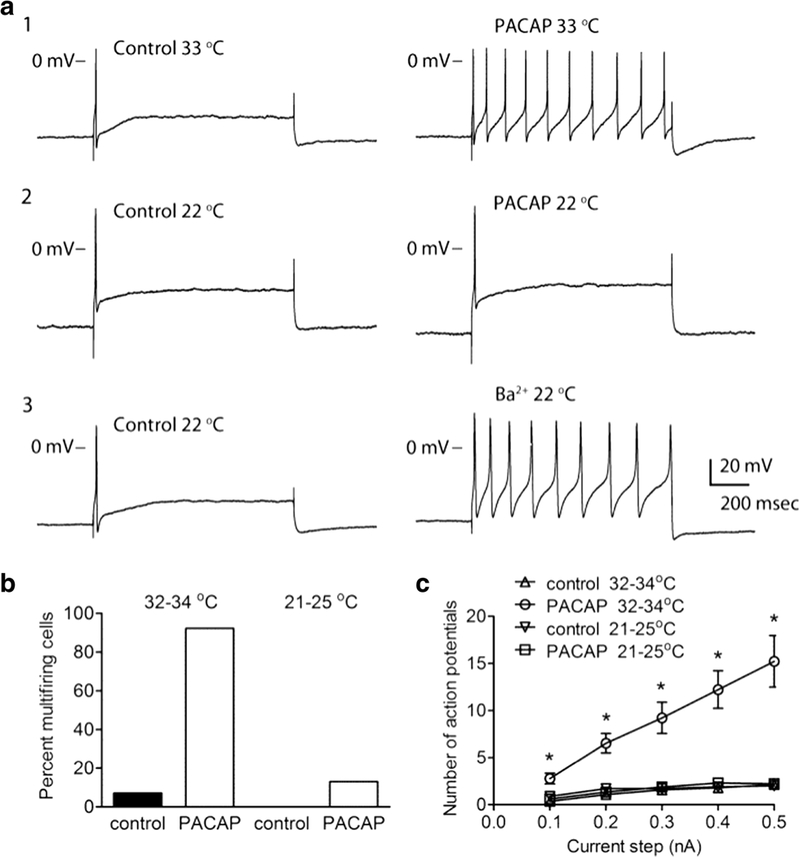
PACAP-induced increase in excitability is temperature-sensitive. A: Recordings from different cells showed that 20 nM PACAP increased excitability when the bath solution was 33°C (A1), but not when the bath temperature was 22°C (A2). The recording in A3 demonstrated that the addition of 1 mM BaCl2 (Ba2+) increased excitability at 22°C. In all three recordings, the cells exhibited a phasic firing pattern prior to the addition of PACAP (A1, 2) or barium (A3). The firing pattern shifted to multiple firing in A1 and A3, but not in A2. The amplitude of the 1 sec depolarizing current pulse was 0.3 nA in each experiment. B: The percentage of cells exhibiting multiple firing in 20 nM PACAP was significantly greater when the temperature was 32 – 34°C (n = 13 cells) than when the bath temperature was 21 – 25°C (n = 23 cells; Fisher’s exact test, Ρ < 0.0001; Also, at 32 – 34°C: control (n = 28) vs. PACAP (n = 13), significantly different, Ρ < 0.0001; at 21 – 25°C: control (n = 10) vs PACAP (n = 23), not significantly different, Ρ = 0.5363). C: Averaged excitability curves generated in the cells maintained at either 33 – 34°C or 21 – 25°C prior to and during exposure to 20 nM PACAP. The number of action potentials generated at each current step was significantly greater (P< 0.003, indicated by asterisks) at the warmer temperature in the presence of PACAP (n = 13) when compared to untreated control at warm temperature (n = 28), and to PACAP at room temperature (n = 23). The number of action potentials generated at each current step was not different between untreated control (n=10) and PACAP at room temperature. Reprinted with permission from the Journal of Neuroscience (Merriam et al, 2013).
Pretreatment with Pitstop2 or dynasore also eliminated the PACAP-induced increase in excitability (Figure 3). In the presence of either Pitstop2 or dynasore, less than 10% of the cardiac neurons exhibited a PACAP-induced increase in excitability, whereas 90% of the cells treated with PACAP alone significantly increased excitability (Figure 2A,C) (Merriam et al. 2013). Furthermore, in the Pitstop2- or dynasore-pretreated cells, the excitability curve in PACAP was not different from the excitability curve noted for control cells not exposed to PACAP (Figure 2 B, D) (Merriam et al. 2013). PACAP increased cardiac neuron excitability in preparations pretreated with the inactive Pitstop analogue, indicating a specific action of Pitstop2 (May and Parsons 2016). The suppression of the PACAP effect on excitability by Pitstop2 or dynasore was not due to a nonspecific blunting of neuronal excitability as treatment with either barium or bethanechol to block the voltage-dependent M-current still markedly increased action potential generation by depolarizing current steps (Merriam et al. 2013). Interestingly, the addition of Pistop2 or dynasore to the cardiac ganglia preparation after the PACAP-induced increase in excitability had fully developed, was able to progressively reverse the effect (Merriam et al. 2013). The reversal of the PACAP-induced increase in excitability by the endocytosis inhibitors was not accompanied by any change in action potential properties, effective membrane resistance or rectification in the current step-induced hyperpolarization (Merriam et al. 2013). These results suggest that continued PAC1 receptor endocytosis and endosomal signaling is necessary to sustain PACAP-induced excitability.
Figure 3.
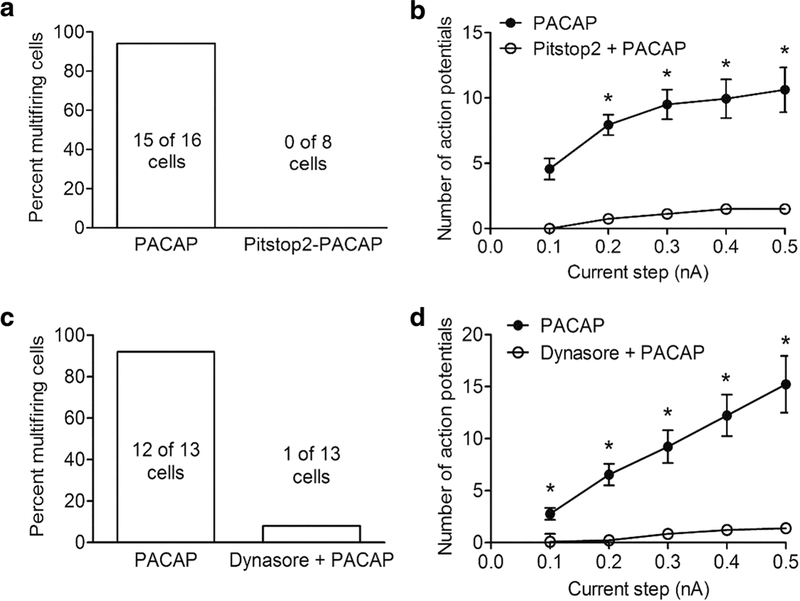
Pretreatment with Pitstop2 or dynasore suppresses the PACAP-induced increase in excitability. A: The percentage of cells exhibiting multiple firing when exposed to PACAP alone (n = 16) was significantly greater than that exposed to PACAP after pretreatment with 15 μM Pitstop2 (n = 8; Fisher’s exact test, Ρ < 0.0001). B: Averaged excitability curves show that Pitstop2 greatly suppressed the PACAP-induced increase in excitability. Asterisks indicate the number of action potentials generated at each current step was significantly greater in PACAP (n = 16) than in PACAP and Pitstop2 (n = 8; unpaired t-test, Ρ < 0.0001 for steps 0.2 nA – 0.5 nA). C: The percentage of cells exhibiting multiple firing when exposed to PACAP alone (n = 13) was significantly greater than when exposed to PACAP after pretreatment with 20 μM dynasore (n = 13; Fisher’s exact test, Ρ < 0.0001). D: Averaged excitability curves show that dynasore greatly suppressed the PACAP-induced increase in excitability. Asterisks indicate the number of action potentials generated at each current step was significantly greater in PACAP (n = 13) than in PACAP and dynasore (n = 13; unpaired t-test, Ρ <0.0005). Reprinted with permission from the Journal of Neuroscience (Merriam et al, 2013).
PAC1 receptor endosomal ERK signaling contributes to heightened excitability
In aggregate from the studies described above, the conditions that suppress PACAP-induced PAC1 receptor internalization clearly also blunt enhanced excitability. Subsequent studies were initiated to identify what intracellular signaling cascade(s) recruited through endosomal signaling can potentially contribute to the PACAP modulation of excitability. The inhibitor and more recent endosomal marker co-localization studies using the HEK PAC1R-EGFP cells indicated that PAC1 internalization/endosomal signaling contributes to the recruitment of MAPK kinase (MEK)/ERK signaling (May et al. 2010; May et al. 2014; Missig et al. 2017). The MEK/ERK signaling cascade has critical roles in synaptic plasticity and regulation of neuronal excitability (Sweatt 2004). Prior studies have shown that ERK activation can modulate activity of excitable cells through phosphorylation of different voltage-dependent ion channels (Toledo-Aral et al. 1995; Fitzgerald and Dolphin 1997; Hu et al. 2003; Stamboulian et al. 2010). Consequently, several studies were initiated to investigate whether PACAP stimulates MEK/ERK signaling in guinea pig cardiac neurons and whether this mechanism contributes to the PACAP-induced modulation of neuronal excitability. From immunostaining and confocal analysis of phosphorylated ERK (pERK) immunoreactivity, PACAP was shown to significantly increase pERK levels in guinea pig cardiac neurons. This effect of PACAP was mediated solely through PAC1 receptor activation and blunted by reducing temperature and treatment with Pitstop2 (Clason et al. 2016). Thus, a significant component of the increase in pERK generation appears dependent on PAC1 receptor internalization and endosomal signaling. In subsequent experiments, pretreatment of cardiac ganglia whole mounts with the MEK inhibitor PD98059, significantly suppressed the PACAP-induced increase in neuronal excitability (Tompkins et al. 2016). However, exposure to the MEK inhibitor did not alter either the PACAP enhancement of Ih, seen as rectification in hyperpolarizing voltage steps, or the hyperpolarization-induced rebound depolarization due to activation of IT. However, guinea pig cardiac neurons express the voltage-dependent sodium channel Nav1.7, an established target of MEK/ERK signaling. This suggested that PACAP/PAC1 receptor coordinated the neuronal responses in part via Nav1.7 sodium channels through endosomal MEK/ERK signaling, and in support of this hypothesis, pretreatments with a selective Nav1.7 blocker (McCormack et al. 2013) suppressed the PACAP-induced increase in excitability (Tompkins et al. 2016). Thus, PACAP-induced PAC1 receptor internalization/endosomal signaling recruitment of MEK/ERK signaling and modulation of Nav1.7 appear to be key mechanisms contributing to the PACAP enhanced neuronal excitability.
Most recent studies have focused on elucidating co-factors supporting the PACAP-induced PAC1 receptor endocytosis. Mechanisms mediating the internalization of many GPCRs have been investigated extensively (Luttrell et al. 1999; Jalink and Moolenaar 2010; Scita and Di Fiore 2010; Delom and Fessart 2011; McMahon and Boucrot 2011; Magalhaes et al 2012; Di Fiore and von Zastrow 2016). Following GPCR activation, G protein-coupled receptor kinases (GRKs) phosphorylate GPCRs to foster receptor dissociation from its G protein partners and initiate recruitment of other effector and accessory proteins, including β-arrestins and Src family kinases, which in turn recruit clathrin for receptor endocytosis via clathrin-coated pits (Luttrell et al. 1999; Wang et al. 2006; Reinecke and Caplain 2014). Src kinases also mediate phosphorylation of the GTPase dynamin II, a protein responsible for the scission of clathrin-coated vesicles (Auciello et al. 2013; Reinecke and Caplain 2014). Given that activation of Src family kinases can play a number of critical roles supporting endocytosis of other GPCRs, some recent studies have investigated whether recruitment of Src family kinases is also critical to the PACAP-induced PAC1 internalization/endosomal signaling. Using the HEK PAC1R-EGFP cells, treatment with Src family kinase inhibitors blunted PACAP-induced PAC1 receptor endocytosis and pERK generation (Tompkins et al. 2018). Furthermore, Src family kinase inhibitors also decreased the PACAP-induced activation of cardiac neuron MEK/ERK signaling and significantly decreased PACAP-enhanced cardiac neuron excitability (Tompkins et al. 2018). Together these studies demonstrate recruitment of Src family kinases supports the PACAP-induced PAC1 receptor internalization, pERK generation and increased neuronal excitability.
Conclusion
In addition to membrane delimited G protein-dependent signaling, GPCRs undergo a sequence of steps leading to receptor internalization and endosomal signaling (Calebiro et al. 2009; Ferrandon et al. 2009; Calebiro et al. 2010, Irannejad et al. 2013; Vilardaga et al. 2014). GPCR endocytosis had initially been associated with receptor desensitization and recycling pathways, but is now recognized to represent a mechanism supporting sustained second messenger generation via signaling endosomes (Calebiro et al. 2009; Ferrandon et al. 2009; Calebiro et al. 2010, Irannejad et al. 2013; Vilardaga et al. 2014). These internalization events not only could regulate cell surface receptor density, but also could provide alternative signaling platforms that can diversify signaling events to regulate neuronal function. Furthermore, cumulative results from our studies provide support for the hypothesis that PAC1 receptor internalization/endosomal signaling is a critical mechanism supporting the PACAP modulation of neuronal excitability.
The structural motifs important for PAC1 receptor internalization and endosomal signaling have not been completely established. As the cytoplasmic loop and C-terminal segments of the GPCRs have critical roles in G protein/β-arrestin docking for signaling, the amino acid sequences in these regions are likely to have roles in PAC1 receptor internalization and downstream ERK activation. In accord, truncation of the distal C-terminus of the PAC1 receptor attenuated internalization 50 – 60% (Lyu et al. 2000). Interestingly, both the null and Hop receptor variants undergo endocytosis in HEK cells, suggesting that the different cassettes may not be determinants of internalization initiation. However, the duration of PAC1Hop1 receptor-mediated ERK signaling is extended compared to that for PAC1null receptors, suggesting that the third cytoplasmic loop segment may regulate how long the receptor is engaged with β-arrestin for long term ERK activation (data not shown).
The present review has focused on PAC1 receptor internalization endosomal signaling in the regulation of cardiac neuron excitability. Parasympathetic PAC1 receptor signaling at cardiac neurons can facilitate bradycardia, but how these endosomal-mediated responses can be applied for cardiovascular therapeutics is currently unclear. However, from the diversity of PACAP functions in many physiological systems, PAC1 receptor endosomal signaling may offer alternative approaches to treat disorders. Other studies, for example, have demonstrated that PACAP/PAC1 receptor-mediated endosomal ERK signaling in central circuits plays key roles in development of chronic pain and anxiety-related psychopathologies. As both behavioral responses can be attenuated by inhibitors of endocytosis processes (Missig et al., 2017), PAC1 receptor endosomal mechanisms may represent novel means of intervention for maladaptive chronic pain and stress-related responses for potential therapeutics. As PACAP participates in many CNS circuits and physiological systems, the modulation PAC1 receptor endosomal may have broad applications in homeostatic maintenance.
Acknowledgements:
This work was supported in part by National Institutes of Health (NIH) grant National Institute of General Medical Sciences (NIGMS) P30 GM103498 / National Center for Research Resources (NCRR) P30 RR032135 (RLP) and Office of the Director grant S10 OD017969–01 (RLP).
Footnotes
The authors declare that they have no conflict of interest.
References
- Arimura A (1998) Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jap J Physiol. 48:301–331. [DOI] [PubMed] [Google Scholar]
- Auciello G, Cunningham DL, Tatar T, Heath JK, Rappoport JZ (2013) Regulation of fibroblast growth factor receptor signalling and trafficking by Src and Eps8. J Cell Science 126: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrie AP, Clohessy AM, Buensuceso CS, Rogers MV, Allen JM (1997) Pituitary adenylyl cyclase-activating peptide stimulates extracellular signal-regulated kinase 1 or 2 (ERK1/2) activity in a Ras-independent, mitogen-activated protein Kinase/ERK kinase 1 or 2-dependent manner in PC12 cells. J Biol Chem. 272: 19666–19671. [DOI] [PubMed] [Google Scholar]
- Bouschet T, Perez V, Fernandez C, Bockaert J, Eychene A, Journot L (2003) Stimulation of the ERK pathway by GTP-loaded Rap1 requires the concomitant activation of Ras, protein kinase C, and protein kinase A in neuronal cells. J Biol Chem. 278: 4778–4785. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V (1999) Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC1 receptor isoform activation of specific intracellular signaling pathways. J Biol Chem 274: 27702–27710. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL (1998) Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci 18: 9766–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ (2009) Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 7: e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Persani L, Lohse MJ (2010) Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci 31: 221–228. [DOI] [PubMed] [Google Scholar]
- Calupca MA, Vizzard MA, Parsons RL (2000) Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J Comp Neurol 423: 26–39. [PubMed] [Google Scholar]
- Chemin J, Mezghrani A, Bidaud I, Dupasquier S, Marger F, Barrère C, Nargeot J, Lory P (2007). Temperature-dependent modulation of CaV3 T-type calcium channels by protein kinases C and A in mammalian cells. J Biol Chem. 282: 32710–32718. [DOI] [PubMed] [Google Scholar]
- Cho J-H, Zushida K, Shumyatsky GP, Carlezon WA, Meloni EG, Bolshakov VY (2012). Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. J Neurosci 32: 14165–141177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clason TA, Girard BM, May V, Parsons RL (2016). Activation of MEK/ERK signaling by PACAP in guinea pig cardiac neurons. J Mol Neurosci 59: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delom F, Fessart D (2011) Role of phosphorylation in the control of clathrin-mediated internalization of GPCR. Int J Cell Biol 2011: 246954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch PJ, Sun Y (1992) The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J Biol Chem. 267: 5108–5113. [PubMed] [Google Scholar]
- Di Fiore PP, von Zastrow M (2016). Endocytosis, signaling and beyond. Cold Spring Harb Perspect Biol 6: a016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GDS, Klemm MF, Steele PA (1995) Different types of ganglion cell in the cardiac plexus of guinea-pigs. J Physiol 486: 453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP (2009). Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 5: 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EM, Dolphin AC (1997) Regulation of rat neuronal voltage-dependent calcium channels by endogenous p21-ras. Eur J Neurosci 9: 1252–1261. [DOI] [PubMed] [Google Scholar]
- Gupte RP, Kadunganattil S, Shepard AJ, Merrill R, Planer W, Bruchas MR, Strack S, Mphapatra DP (2016) Convergent phosphomodulation of the major neuronal dendritic potassium channel KV4.2 by pituitary adenylate cyclase-activating polypeptide. Neuropharm 101: 291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Chung J, Rhoades KM, Schutz KC, Falls WA, Braas KM, May V (2009) Chronic stress increases pituitary adenylate cyclase-activating polypeptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST); roles for PACAP in anxiety-like behavior. Psychoneuroendrocrinology 34: 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, May V (2014) Pituitary adenylate cyclase activating polypeptide in stress-related disorders: Data convergence from animal and human studies. Biological Psychiatry 78(3):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar T, Lutz E (1994) Multiple receptors for PACAP and VIP. Trends Pharmacol. Sci. 15:97–99. [DOI] [PubMed] [Google Scholar]
- Hill J, Chan S-A, Kuri B, Smith C (2011) Pituitary adenylate cyclase-activating peptide (PACAP) recruits low voltage-activated T-type calcium influx under acute sympathetic stimulation in mouse adrenal chromaffin cells. J Biol Chem 286: 42459–42469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DB, Tompkins JD, Parsons RL (2009) Differential activation of guinea pig intrinsic cardiac neurons by the PAC1 agonists maxadilan and pituitary adenylate cyclase-activating polypeptide 27 (PACAP27). J Pharmacol Exp Ther 331: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Glauner KS, Gereau RW 4th (2003) ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J Neurophysiol 90: 1671–1679. [DOI] [PubMed] [Google Scholar]
- Iftinca MC, Zamponi GW (2008) Regulation of neuronal T-type calcium channels. Trends in Pharmacol Sci. 30:32–40. [DOI] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SG, Sunahara RK, El-Samad H, Huang B, von Zastrow M (2013) Conformational biosensors reveal GPCR signalling from endosomes. Nature 495: 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K, Moolenaar WH (2010). G protein-coupled receptors: the inside story. Bioessays 32: 13–16. [DOI] [PubMed] [Google Scholar]
- Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM (2007). Microinjection of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdale of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast. 2007: 79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ (1999). Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283: 655–661. [DOI] [PubMed] [Google Scholar]
- Lyu RM, Germano PM, Choi JK, Le SV, Pisegna JR (2000) Identification of an essential amino acid motif within the C terminus of the pituitary adenylate cyclase-activating polypeptide type I receptor that is critical for signal transduction but not for receptor internalization. J Biol Chem 275: 36134–36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maca E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10: 839–850. [DOI] [PubMed] [Google Scholar]
- Magalhaes A, Dunn H, Ferguson S (2012) Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol 165: 1717–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Buttolph TR, Girard BM, Clason TA, Parsons RL (2014) PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am J Physiol Cell Physiol 306: C1068–C1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Lutz E, MacKenzie C, Schutz KC, Dozark K, Braas KM (2010) Pituitary adenylate cyclase-activating polypeptide (PACAP)/PACAP1HOP1receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase gamma and vesicle endocytosis for neuronal survival. J Biol Chem 285: 9749–9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Parsons RL (2016) G Protein-coupled receptor endosomal signaling and regulation of neuronal excitability and stress responses: Signaling options and lessons from the PAC1 receptor. J Cell Physiol 232:698–706. [DOI] [PubMed] [Google Scholar]
- McCormack K, Santos S, Chapman ML, Krafte DS, Marron BE, West CW, Krambis MJ, Antonio BM, Zellmer SG, Printzenhoff D, Padilla KM, Lin Z, Wagoner PK, Swain NA, Stupple PA, de Groot M, Butt RP, Castle NA (2013) Voltage sensor interaction site for selective small molecule inhibitors of voltage-gated sodium channels. PNAS 110: E2724–E2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT and Boucrot E (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 12: 517–533. [DOI] [PubMed] [Google Scholar]
- Merriam LA, Baran CN, Girard BM, Hardwick JC, May V, Parsons RL (2013) Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. J Neurosci. 33: 4614–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam LA, Barstow KL, Parsons RL (2004) Pituitary adenylate cyclase-activating polypeptide enhances the hyperpolarization-activated nonselective cationic conductance, Ih, in dissociated guinea pig intracardiac neurons. Regul Pept 123: 123–133. [DOI] [PubMed] [Google Scholar]
- Missig G, Mei L, Vizzard MA, Braas KM, Waschek JA, Ressler KJ, Hammack SE, May V (2017) Parabrachial Pituitary Adenylate Cyclase-Activating Polypeptide Activation of Amygdala Endosomal Extracellular Signal-Regulated Kinase Signaling Regulates the Emotional Component of Pain. Biol Psychiatry 81: 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RL, Tompkins JD, Hardwick JC, Merriam LA, Girard BM, May V (2016) Multiple mechanisms contribute to the PAC1 modulation of parasympathetic cardiac neuron excitability. In: Pituitary Adenylate Cyclase Activating Polypeptide – PACAP (Reglodi D, Tamas A, ed) New York, Springer Nature, 205–225. [Google Scholar]
- Parsons RL, Tompkins JD, Merriam LA (2008). Source and action of pituitary adenylate cyclase-activating polypeptide in guinea pig intrinsic cardiac ganglia. Tzu Chi Med J 20: 11–18 [Google Scholar]
- Pisegna JR, Wank SA (1996) Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J Biol Chem 271: 17267–17274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke J, Caplain S (2014) Endocytosis and the Src family of non-receptor tyrosine kinases. Biomol. Concepts 5: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Javovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V (2011) Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470: 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G, Di Fiore PP (2010) The endocytic matrix. Nature 463: 464–473. [DOI] [PubMed] [Google Scholar]
- Simms BA and Zamponi GW (2014) Neuronal voltage-gated calcium channels: Structure, function and dysfunction. Neuron 82: 24–45. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L (1993) Differential signal transduction by five splice variants of the PACAP receptor. Nature 365: 170–175. [DOI] [PubMed] [Google Scholar]
- Stamboulian S, Choi J-S, Ahn H-S, Chang Y-W, Tyrrell L, Black JA, Waxman SC, Dib-Hajj SD (2010) ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel NaV1.7 and alters its gating properties. J Neurosci 30: 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, Eiden LE (2012) PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice. Endocrinology 154: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiolo 14:311–317. [DOI] [PubMed] [Google Scholar]
- Talavera K, Nilius B (2006) Biophysics and structure-function relationships of T-type Ca2+ channels. Cell Calcium 40: 97–114. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Brehm P, Halegoua S, Mandel G (1995) A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron 14: 607–611. [DOI] [PubMed] [Google Scholar]
- Tompkins JD, Ardell JL, Hoover DB, Parsons RL (2007) Neurally released pituitary adenylate cyclase-activating polypeptide enhances guinea pig intrinsic cardiac neurone excitability. J Physiol 582: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Clason TA, Buttolph TR, Girard BM, Linden AK, Hardwick JC, Merriam LA, May V, Parsons RL (2018) Src family inhibitors blunt the PACAP-induced PAC1 receptor endocytosis, phosphorylation of ERK and increase in cardiac neuron excitability. Am J Physiol Cell Physiol. 314: C233–C241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Clason TA, Hardwick JC, Girard BM, Merriam LA, May V, Parsons RL (2016) Activation of MEK/ERK signalling contributes to the PACAP-induced increase in guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol 311: C643–C651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Hardwick JC, Locknar SA, Merriam LA, Parsons RL (2006) Ca2+ influx, but not Ca2+ release from internal stores, is required for the PACAP-induced increase in excitability in guinea pig intracardiac neurons. J Neurophysiol 95: 2134–2142. [DOI] [PubMed] [Google Scholar]
- Tompkins JD, Lawrence YT, Parsons RL (2009) Enhancement of Ih, but not inhibition of IM, is a key mechanism underlying the PACAP-induced increase in excitability of guinea pig intrinsic cardiac neurons. Am J Physiol Regul Integr Comp Physiol 297: R52–R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Merriam LA, Girard BM, May V, Parsons RL (2015) Nickel suppresses the PACAP-induced increase in guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol. 308: C857–C866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BKC, Hashimoto H, Galas L, Vaudry H (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61: 283–357. [DOI] [PubMed] [Google Scholar]
- Vilardaga J-P, Jean-Alphonse FG, Gardella T (2014) Endosomal generation of cAMP in GPCR signalling. Nat Chem Biol. 10: 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Kräusslich HG, Shupliakov O, Robinson PJ, McCluskey A, Haucke V (2011) Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146: 471–484. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lu R, Zhao J, Limbird LE (2006) Arrestin serves as a molecular switch, linking endogenous α2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J Biol Chem 281: 25948–25955. [DOI] [PubMed] [Google Scholar]


