Abstract
Tuberculosis transmission and progression are largely driven by social factors such as poor living conditions and poor nutrition. Increased standards of living and social approaches helped to decrease the burden of tuberculosis before the introduction of chemotherapy in the 1940s. Since then, management of tuberculosis has been largely biomedical. More funding for tuberculosis since 2000, coinciding with the Millennium Development Goals, has yielded progress in tuberculosis mortality but smaller reductions in incidence, which continues to pose a risk to sustainable development, especially in poor and susceptible populations. These at-risk populations need accelerated progress to end tuberculosis as resolved by the World Health Assembly in 2015. Effectively addressing the worldwide tuberculosis burden will need not only enhancement of biomedical approaches but also rebuilding of the social approaches of the past. To combine a biosocial approach, underpinned by social, economic, and environmental actions, with new treatments, new diagnostics, and universal health coverage, will need multisectoral coordination and action involving the health and other governmental sectors, as well as participation of the civil society, and especially the poor and susceptible populations. A biosocial approach to stopping tuberculosis will not only target morbidity and mortality from disease but would also contribute substantially to poverty alleviation and sustainable development that promises to meet the needs of the present, especially the poor, and provide them and subsequent generations an opportunity for a better future.
Introduction
Tuberculosis has been called the perfect expression of an imperfect civilisation.1 Despite scientific and social advances a high burden of tuberculosis persists worldwide, particularly affecting poor and susceptible populations.1
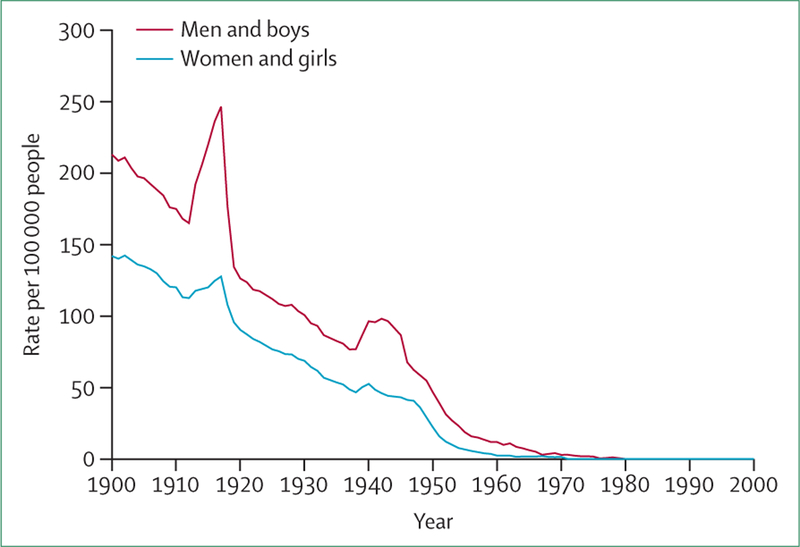
Tuberculosis transmission and progression are largely driven by social factors such as poor living conditions and poor nutrition.2 However, with the discovery of anti-tuberculous medicines in the 1940s, the approaches in fighting tuberculosis have been largely biomedical.3,4 The burden of tuberculosis declined rapidly in the early 1950s, coinciding with the use of anti-tuberculous medicines, but progress since the 1960s has been slow and uneven, with declines in many settings occurring well before the introduction of chemotherapy (figure 1), probably attributable at least in part to improved standards of living.2,5,6
Figure 1: Age-standardised tuberculosis deaths by sex, 1901–2000.
Data from England and Wales office of national statistics
In the late 1980s and early 1990s, when the HIV epidemic was emerging, funding was reduced for tuberculosis treatment programmes in many industrialised countries, and the Soviet Union was breaking up,7–9 tuberculosis incidence and mortality rose to become the sixth leading cause of death worldwide, and the eighth leading cause of disease burden worldwide in 1990.10,11 In 1993, WHO declared tuberculosis a global health emergency, and in 1994, adopted a new approach to address tuberculosis called DOTS—originally an acronym for directly observed therapy, short course, but later used to identify the entire WHO-endorsed strategy including political commitment, drug supply chain management, and monitoring and assessment in addition to treatment using standard regimens.12 However, worldwide tuberculosis incidence and mortality remained high throughout the 1990s. In 2000, the Millennium Development Goal 6: “combat HIV/AIDS, malaria and other diseases”,13 and the creation of the Global Fund to Fight AIDS, Tuberculosis and Malaria in 2002 helped to mobilise new funding to fight the tuberculosis epidemic.13 However, rates of tuberculosis incidence worldwide decreased more slowly than those for both HIV and malaria; estimates of the pace of decline in tuberculosis incidence since 2000 range from less than 1% per year to around 1.5% per year with variations by country and region.5
Tuberculosis has its roots in underdevelopment, poverty, and social exclusion.2 Slow progress in the fight against tuberculosis since the 1990s is due in part to gaps in coverage of DOTS programmes,14 but more importantly because of the failure to address the social drivers of the epidemic such as crowded living conditions among increasingly urbanised populations,15 indoor air pollution,16,17 malnutrition,18 diabetes mellitus,19 tobacco,20 alcohol,21,22 and factors such as stigma and social isolation.2,23 Despite tuberculosis having strong social determinants, efforts during the past several decades have focused almost exclusively on biomedical solutions. DOTS and the more recent WHO Stop TB Strategy largely emphasise delivery of tuberculosis services; supply interventions (eg, human resources, new diagnostics, and treatment for service provision) that are focused on test and treat strategies, and have paid insufficient heed to patient characteristics, the nature of their demands, and the broader context in which tuberculosis programmes are implemented.3,4,24
Sustainable development aims to meet “the needs of the present without compromising the ability of future generations to meet their own needs”.25,26 In practice, this statement means meeting basic needs (eg, food, shelter, and sanitation), while providing those in need with opportunities for a better quality of life through social, economic, and environmental action. Sustainable development promises to meet the needs of the poor and provide them an opportunity for a better future. The long history of tuberculosis and society’s organised response to it can be instructive; social approaches to fighting tuberculosis, such as improved nutrition and social conditions, evidently contributed to reducing the burden of tuberculosis in the pre-chemotherapy era.6
The time has come to reconsider the fight against tuberculosis as a development imperative: a response that combines social approaches from the past with enhanced biomedical approaches that not only target morbidity and mortality from disease but also contribute to poverty alleviation and sustainable development.
The social approach of the past
Before the introduction of chemotherapy in the 1950s, solutions for tuberculosis were largely based on improving living standards or providing infected individuals with space, clean air, sunlight, rest, and proper nutrition, typically in sanatoria, which kept infected individuals isolated from the general public.1 However, this method of treatment was expensive and largely inaccessible to poor populations that had disproportionally high rates of tuberculosis, prompting countries like Chile to introduce social approaches to managing tuberculosis (panel).
As working conditions in factories improved and public health interventions improving overall sanitation were implemented, the burden of tuberculosis began to decline in the rich and the poor.1,6 Economic prosperity enabled people to feed themselves properly and live in more spacious housing. McKeown,6 who recognised the effect of improved standards of living on tuberculosis by analysing historic death records in England and Wales, argued that an improved diet was the greatest contributor to the tuberculosis decline.
The era of biomedical interventions
After the discovery of streptomycin in 1944, various anti-tuberculosis drugs emerged in the 1950s, leading to an era of combination therapy for treating active tuberculosis. However, the risk factors for infection largely remained the same. From 1960 to 1999 the world population grew at an unprecedented rate, increasing from 3 billion to 6 billion.28 Globalisation and new technologies increased the movement and spread of people, products, and information. Urbanisation accelerated, as people moved from rural areas to crowded cities in search of employment, but found all too often cramped living quarters, low wages, and poor working conditions, and struggled to afford adequate nutrition. In low-income and middle-income countries, absence of universal health coverage meant that many individuals seeking tuberculosis care could not afford health services.29
The biomedical approach to management of tuberculosis likewise evolved over time. In 1994, WHO launched DOTS, emphasising standardised case management of tuberculosis—to replace earlier approaches that involved many different medicines for lengthy periods. DOTS-Plus followed in 1999, with the addition of culture-based diagnosis, drug susceptibility tests, and treatment with second-line drugs to DOTS to address the emerging burden of multidrug-resistant (MDR) tuberculosis.30,31 Persistent challenges in fighting tuberculosis prompted the launch of the Stop TB Strategy in 2006, which emphasised DOTS expansion, laboratory strengthening, tuberculosis with HIV, MDR tuberculosis, and the development of new methods.24,32
Although DOTS has undoubtedly contributed to the fight against tuberculosis since the 1990s, the biomedical approach has mainly emphasised supply-side interventions that rely heavily on functioning health systems. Yet tuberculosis remains deeply rooted in poverty and poor living conditions. Therein lies the difficulty: a biomedical approach to fighting tuberculosis addresses only part of the issue. A biosocial model that combines biomedical and social approaches is crucial and well overdue to win the battle against tuberculosis.
Rediscovering and enhancing the social approach
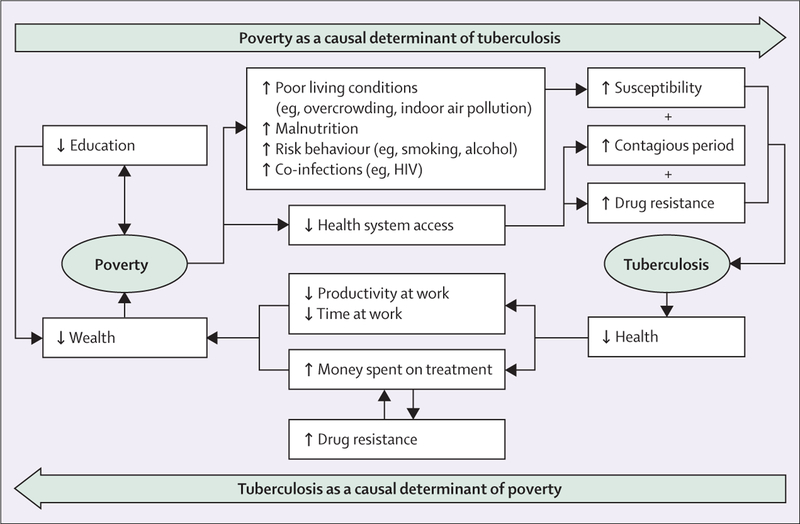
Tuberculosis is both a cause and result of poverty,23 driven by social exclusion, including malnutrition, overcrowding, and indoor air pollution.2,17 Tuberculosis risk is higher in individuals facing other illnesses such as HIV and diabetes, and those participating in behaviours that put their health at risk, such as smoking and excessive use of alcohol.2 The risk factors associated with poverty not only increase susceptibility to active tuberculosis, but are also associated with greater difficulty in accessing care in health systems because of financial and geographical constraints, and provider prejudices.30 Limited access to the health system delays diagnosis and treatment of tuberculosis, leading to longer periods of infectiousness and greater mortality risk (figure 2).
Figure 2: The cycle of poverty and tuberculosis.
Tuberculosis is a driver of poverty. The disease leads to days off work and out of pocket health expenditures. Around 60% of the financial burden of tuberculosis comes from income loss when people are on treatment, and tuberculosis patients and their families spend an average of over half their yearly income on tuberculosis treatment.30 The high cost of tuberculosis treatment makes it difficult for infected individuals to afford the full regimen of 6 or more months of therapy, impeding adherence and treatment success.31 The result is a vicious tuberculosis–poverty cycle that cannot be broken by biomedical interventions alone (figure 2).
Our analysis strongly suggests substantial association between level of development (sanitation, improved water, access to electricity, urbanicity, malnutrition, and education), poverty, health system access, and tuberculosis (table). Social interventions aimed at addressing tuberculosis must therefore address these pathways specifically and collectively in the tuberculosis–poverty cycle (figure 2). Social interventions can effectively combine with biomedical approaches that incorporate new technologies and solutions. Interventions in nutrition, urban planning and built environment, working conditions, addiction recovery, and psychological services hold much promise.
Table:
Tuberculosis case notification rates and World Development Indicators of poverty and deprivation
| Country-years of data | Tuberculosis case notification coefficient | p value | |
|---|---|---|---|
| Access to sanitation (% population) | 3754 | −2.858 | 0.000 |
| Improved water source access (% population) | 3828 | −1.373 | 0.000 |
| Access to electricity (% population) | 166 | −1.993 | 0.000 |
| Urban population (% population) | 5638 | −1.611 | 0.000 |
| Malnutrition, height for age (% of children under 5 years) | 2532 | 1.630 | 0.000 |
| Primary school enrolment (% gross) | 4676 | −0.114 | 0.259 |
| Out of pocket health expenditure (% of total expenditure) | 3103 | 0.095 | 0.522 |
| DPT immunisation coverage (% children 12–23 months old) | 5214 | −0.340 | 0.000 |
| Poverty headcount, USS1–25 per day (% population) | 1063 | 0.995 | 0.000 |
| Poverty headcount, US$200 per day (% population) | 1063 | 1.233 | 0.000 |
| Income share held by highest 10% | 1074 | 1.536 | 0.006 |
Country-years of data refers to the sum of the number of countries, multiplied by the number of years of data used for each country in the analysis. The tuberculosis case notification coefficient refers to the association between the case notification rate, the dependent variable in the regression analysis, and the WDI for poverty and deprivation used in the analysis as an explanatory variable. To explore the relation between poverty indicators and tuberculosis outcome in the tuberculosis–poverty cycle (figure 2) we used WHO tuberculosis case notifications (corrected for under-reporting using case detection rates estimated from country workshops) and World Bank WDIs. The WDI variables included in our analysis were intended to capture living conditions (eg, sanitation, improved water source access, access to electricity), nutrition, education, out of pocket health expenditure, and health system access (measured by DPT immunisation). We also included the World Bank’s composite poverty indicators as a measure of income inequality. The analysis consisted of 11 separate mixed-effect regressions with random effect on country. Each regression quantified the association between the tuberculosis case notification rates with one of the WDI variables. Country random effects were included to control for dependence of reported observations over time within the same country, and calendar year was included as an additional independent variable to control for share of secular trends in tuberculosis case notification rates across countries. The regression results support the associations outlined in the tuberculosis–poverty cycle; all the regression coefficients are significant (p<0.05) with the exception of education and health-care expenditure, and all coefficients have the expected sign. Improved living conditions, health system access, and education have a negative relationship with tuberculosis case notification rates, and increased malnutrition, health expenditure, poverty, and inequality have a positive relationship with tuberculosis case notification rates.
DPT=diphtheria, pertussis, and tetanus. WDI=World Development Indicator.
Social protection for tuberculosis risk
Social protection and health interventions have been combined to effectively address infectious diseases, as well as maternal and child health.33 Social protection spending that allocates resources to elderly and susceptible populations, unemployment protection, and housing, is strongly associated with lower tuberculosis case notifications, incidence, and mortality rates. A study of 21 European countries showed between 1995 and 2012, each increase in social protection spending of US$100 per person was associated with a 1.5% decrease in the number of tuberculosis case notifications, 1.7% decrease in estimated tuberculosis incidence, 2.7% decrease in the rate of non-HIV-related tuberculosis mortality, and 3.2% decrease in the rate of all-cause mortality.34 In Peru, education, community mobilisation, psychosocial support, and poverty reduction programmes improved tuberculosis screening from 82% to 96% and treatment completion from 91% to 97%.35
Cash transfer schemes, both direct and indirect, and microfinance can help beneficiaries utilise health services and improve their health outcomes.36 Increasing socioeconomic position, food security, and health-care access—all major protective factors for tuberculosis—can reduce the burden of both poverty and tuberculosis.37,38 Direct cash transfer can increase tuberculosis treatment completion rates and decrease default rates; a study from China38 showed that individuals that received cash transfers had 8% higher treatment completion rates and 21% lower default rates compared with controls.
Enhancing nutritional value
The association between nutrition and tuberculosis is well established.18,39–42 Malnutrition and micronutrient deficiencies increase the risk of active tuberculosis, which in turn worsens malnutrition. Rapid urbanisation in low-income and middle-income countries is contributing to the nutritional transition that increases prevalence of diabetes, which amplifies tuberculosis risk and worsens outcomes in those infected with tuberculosis.29
In view of the connection between nutrition and tuberculosis, interventions such as nutritional counselling, food parcels, or high-energy oral nutritional supplements might enhance management of tuberculosis.43 Micro-nutrient supplementation (eg, vitamin A-fortified sweet potatoes) or food aid for susceptible or transient populations are population-level interventions that would help improve nutritional status and probably reduce risk of tuberculosis.41,44–46
Sustainable agricultural interventions include individual agricultural support and incentives for the growth of diversified crops that are more economically viable in the long term than cash crops (which generate money more quickly) or use of high-yield seeds that are more robust to droughts, and are additional strategies that might shape agricultural practice in low-income and middle-income countries in a way that has a positive effect on tuberculosis and other health outcomes.47
Improving the built environment
Tuberculosis is spread via aerosol droplets from a patient with active disease. Overcrowding, indoor air pollution, and poor ventilation in homes, hospitals, and public transportation assist with the spread of the infection.15,29,48 In low-income and middle-income countries, migration from rural to urban areas has led to an estimated 1 billion people living in overpopulated slum communities with poor infrastructure. From 1950 to 2014 the urban population grew from 746 million to 3.9 billion worldwide and by 2050 the UN projects that 66% of the world’s population will live in urban areas49,50 with implications for the struggle against tuberculosis. Rapid urbanisation calls for better built environment and improved housing design in urban areas,51 the development of policies controlling urbanisation, urban regeneration and slum upgrading programmes, and design of public spaces and transportation systems to reduce transmission risk at home or work, while commuting, and in public places.52 Innovative technologies that improve local exhaust, general ventilation, room filtration, and ultraviolet air disinfection can promote natural ventilation and reduce transmission risk.53 A study in rural South Africa54 suggests that the risk of tuberculosis transmission decreased from 55.4% to 9.6% by opening windows and doors to increase airflow. Improving the built environment has benefits beyond direct health effects. Better roads, for example, improve access to schools, health services, and food markets, all of which can greatly reduce the burden of tuberculosis.23
Reducing occupational risk
Factory workers and labourers working in extractive industries such as mining are populations at greater risk for contracting tuberculosis.55–57 In South Africa, the incidence of tuberculosis in miners is ten times higher than the general population and miners have 3.6 times greater odds of dying from tuberculosis compared with other workers in the region.58,59 Miners, who are in poorly paid and demanding jobs, typically work and live in congregate settings with poor ventilation and indoor air pollution. Factory workers and labourers are often migrants, spreading the infection from work to their home towns. Good employment practices (including appropriate wages, reasonable hours, health insurance, and protection from injury), decent conditions at the workplace, and living accommodations aimed at the “Declaration on Tuberculosis in the Mining Sector” by the 15 heads of state belonging to the Southern African Development Community, can greatly reduce risk of tuberculosis in working populations, especially those working in extractive industries.60
Improving mental health
Mental illness often prevents individuals from properly caring for themselves, contributing to malnutrition and a weakened immune system, and increasing the risk of developing active tuberculosis infection.61 Additionally, mental illness hampers adherence to medical care, which reduces the effectiveness of tuberculosis treatment. Mental illness carries stigma and is strongly associated with homelessness,62 making it difficult for individuals to secure employment. The relation between mental health and tuberculosis likewise works in reverse—mental illness develops or is worsened by social isolation during treatment or can emerge as a side-effect of some treatment drug regimens, all compounded by the stigma for mental illness and tuberculosis alike.61 Psychological services and housing assistance for tuberculosis patients can help improve health outcomes and provide these individuals with an opportunity to plan for their future.63 Individuals with mental health disorders are at greater risk for addictive disorders, particularly alcohol misuse.64 Alcohol use is a risk factor for the development of active tuberculosis; people who drink more than 40 g of alcohol a day are around three times more likely to develop tuberculosis compared with those who do not.65
Enhancing the biomedical approach
Although many of the technological components of the approach to preventing, diagnosing, and treating tuberculosis are decades old, there is renewed interest in developing new diagnostics, drugs, and treatment regimens.66 As potential new products are developed, it remains essential to consider whether innovations in delivery of existing interventions can greatly reduce the burden of tuberculosis.
Developing effective vaccines
Limitations in available tuberculosis vaccines and treatment regimens have been well characterised.67–73 The BCG vaccine was developed in the 1920s and estimates of its effectiveness have ranged from 80% protection to no benefit,74,75 with particular concerns about duration of protection and therefore subsequent population-level effects in terms of preventing active tuberculosis in adolescents and adults. Although there continues to be high interest in developing new tuberculosis vaccines, development of a novel tuberculosis vaccine with high efficacy and persistent protection remains an elusive goal.67–70
Developing new treatment approaches
For individuals with latent Mycobacterium tuberculosis infection, preventive treatment can be effective in reducing the likelihood of progression to active disease.76 Commonly recommended preventive therapy regimens have durations up to 9 months, but more recent studies have reported that shorter regimens can provide similar efficacy.69,70 For active cases of tuberculosis, standard first-line treatment regimens continue to be based on drugs that were discovered 50 to 60 years ago, and there are various challenges associated with these therapies, including long treatment durations, toxic effects, interactions with antiretroviral drugs, and drug resistance in some settings.71 Several new options are in various stages of development, with the potential to shorten regimens, yield high efficacy against MDR tuberculosis, and provide effectiveness against both latent and active tuberculosis.72,73
Improving tuberculosis detection
A rapid and accurate point-of-care diagnostic test for tuberculosis is still absent, therefore detection of tuberculosis is highly reliant on individuals accessing the health system.77 Tuberculosis prevalence surveys78 have shown that more than 50% of those with bacteriologically confirmed tuberculosis do not report the symptoms that often trigger disease investigation (eg, cough lasting 2–3 weeks). WHO reports that in 2013 more than 3 million tuberculosis cases worldwide were undiagnosed or were not notified. Case detection in many settings continues to rely largely on sputum smear microscopy, which has low sensitivity, especially in HIV-infected patients, and can cause delays in initiating treatment or loss to follow-up because immediate results are not available. However, alternative choices are becoming available, with potential to offer substantial improvements in test characteristics.79 An important advance in tuberculosis diagnosis was the introduction of the Xpert MTB/RIF test, with greater sensitivity than sputum smear microscopy, leading to 45% increase in case detection in patients infected with HIV.80,81 The experience with implementing Xpert substantiated the benefits of better case detection with new diagnostics, and highlighted the challenges in introducing new technologies in weak health systems with poor service coverage and access.82
Treatment with mobile telephone and health information technologies
Mobile telephone messaging is effectively used for managing self-management of long-term illnesses.83 SMS text messaging improves treatment adherence and success of tuberculosis treatment.84 A study done in South Africa84 reported that tuberculosis cure rates were 2.3 times higher in patients that received SMS reminders for treatment compared with another group receiving standard DOTS treatment, whereas a study in Kenya84 reported clinical attendance on scheduled days was 1.56 times higher in tuberculosis patients that received SMS reminders compared with those who did not. Electronic health records, including open source systems, mobile telephones, or personal digital assistants could be used to track and monitor patients with tuberculosis to improve health outcomes, as has been shown in Africa (Kenya, Rwanda, South Africa), Latin America (Peru), and Asia (Philippines).85,86
Combining biomedical and biosocial approaches
The enhanced social and biomedical approaches to tuberculosis care are not mutually exclusive, but can work together to address tuberculosis, especially in hard to reach and at-risk populations, and promote sustainable development. Although addressing the social determinants through a biosocial approach helps to reduce tuberculosis burden and improve outcomes, an enhanced biomedical approach could help reduce poverty and improve life chances of affected individuals and families, thereby improving their social determinants of health.
Tuberculosis places a disproportional burden on particular at-risk groups such as migrants and refugees,46 individuals living in densely populated urban areas or living in areas of conflict and crisis,15,29,45,48,52 children, and individuals with HIV/AIDS or diabetes. Understanding the context and developing context-specific solutions is important, as tuberculosis propagates through a series of local outbreaks, and the local conditions affect the success of tuberculosis programmes.7,9,87
Societal upheavals can displace large populations to new environments that lack food, adequate housing, and medical care, and increase the risk of mental illness, all of which are risk factors for tuberculosis. In the USA, the percentage of tuberculosis cases occurring in foreign-born people is over 60%.88 As a result of the Ebola crisis, several tuberculosis programmes in Liberia, Guinea, and Sierra Leone were repurposed to address Ebola, leaving new and existing patients without treatment.89
The health, social, and economic effect of tuberculosis on children is significant; almost 1 million children have tuberculosis and 10 million children have been orphaned by tuberculosis. Children with tuberculosis are often in the hospital for extended periods of time, resulting in high treatment costs or parental lost time from work.90,91 Individuals with HIV/AIDS or diabetes have suppressed immune systems that make them more susceptible to the development of active tuberculosis. A biosocial approach to tuberculosis needs to be uniquely tailored to address at-risk populations and ensure context specificity, taking into account cultural nuances and pervasive stigma.
Discussion
Sustainable development is predicated on meeting basic needs, eg, food, shelter, and sanitation, through social, economic, and environmental actions that are crucial for better quality of life and in the fight against tuberculosis.25,26 A biosocial approach that expands the biomedical model and combines it with social, economic, and environmental actions is essential for the fight against tuberculosis.
Biomedical approaches alone have not achieved substantial decreases in tuberculosis burden. Although expanding universal health coverage will improve access to essential health services and protect from catastrophic health expenditures, it alone will not be sufficient to stop tuberculosis, as social determinants greatly affect the burden of tuberculosis, the fight against tuberculosis and the tuberculosis–poverty cycle. Evidence from Vietnam, Morocco, Pakistan, Sri Lanka, Myanmar, and India suggest that the benefits of improved diagnostics and treatment is offset by susceptibility to tuberculosis in at-risk populations.92–94
Although the thrust of this Series paper is on biosocial approaches, we also argue for enhancing biomedical approaches by strengthening health systems and scaling up innovations in tuberculosis detection and treatment. Combining biomedical and biosocial approaches will allow cooperation in detection and treatment of tuberculosis, and address comorbidities (such as HIV and diabetes) and risks through social protection and improvements in nutrition, the built environment, occupational safety, and mental health.
Tuberculosis both causes and results from weak or failing health, education, and economic systems and development. Tuberculosis takes a heavy toll, not only on health and social services, but also on entire regions participating in the global economy, as tuberculosis predominantly affects the most economically active age group. An ailing workforce reduces productivity, lowers revenue, and weakens economies. By trapping people in a cycle of poverty and disease, tuberculosis slows national development and reduces competitiveness.95 Combined biomedical and biosocial approaches are essential to mitigate the adverse effects of tuberculosis on individuals, households, and economies.
Implementing a biosocial approach to stop tuberculosis will be challenging, as the risk factors for tuberculosis will rise in the future. The number of people with diabetes is projected to rise from 171 million in 2000 to 366 million in 2030,96 an estimated 70% of the world population could be living in urban areas by 2050,97 the number of people living in slum dwellings will more than double to 2 billion in 2030 from 924 million in 2001, and worldwide migration will increase, with adverse risks for development.98
A proposed biosocial approach will align the tuberculosis response with sustainable development goals, extending the responsibility for national tuberculosis strategies beyond the health sector. Successful implementation of a biosocial model will need shared vision and collaboration across government sectors, professional groups, and civil society to mount integrated multisectoral action. Leadership and political commitment at the highest level of government is essential to include ministries of finance, development, housing, labour, education, and health to comprehensively address the social, environmental, nutritional, and occupational risk factors for tuberculosis and monitor progress.60 Just as food security is often used to assess the success of the nutritional effect of poverty reduction strategies,99 tuberculosis indicators could be used to monitor the effect of poverty reduction, urban planning, and development strategies on health. Accountability for the tuberculosis response across sectors could be further amplified through targets that relate to sectoral contributions to the tuberculosis response, such as the proportion of well aerated houses in public housing projects.
International institutions, such as the WHO, the Global Fund to Fight AIDS, Tuberculosis and Malaria, the Stop TB Partnership, UNAIDS, UNICEF, and the World Bank, have an important part to play, as they have done with the HIV response, to support country-led initiatives aimed at introducing the biosocial approach.
In addition to government and international organisations, the private sector, non-governmental organisations, and civil society will be instrumental in targeting social, economic, and environmental tuberculosis risks. For instance, companies employing workers who face high tuberculosis risks either because of the nature of the occupation or socioeconomic characteristics (eg, low wage or seasonal workers),23,55–57 can contribute to biosocial approach through targeted prevention programmes. Similarly, labour unions and professional organisations, for example those in the mining, health-care, and building industries, could contribute to the fight against tuberculosis—for example, by making sure their members are aware of approaches that reduce tuberculosis transmission risks through better design and building of public housing, schools, social spaces, and hospitals.
Introduction and scaling up of innovations for tuberculosis prevention, detection, and treatment, although crucial, might be hindered because of low rewards for innovators and commercial investors,100 inability of governments to invest in new health technologies while coverage of other cost-effective health interventions are low, and the difficulty for patients (who are typically the economically worst-off populations), to pay for novel tuberculosis interventions. Innovative approaches are needed to motivate the private and public sectors to invest in tuberculosis related research and development. Innovative financing that has been used to fund product development partnerships, provide advance market commitments, establish patent pools for new medicines, and encourage rapid uptake of new diagnostics, treatments, and care models to address health priority interventions would be instructive to create incentives for innovations to address tuberculosis.101 However, an important lesson from large-scale implementation of Xpert in South Africa, is that technological innovations can fail to achieve their potential effect because of health system barriers to implementation, such as access to services, paucity of human resources, or inappropriate infrastructure.81
We have to learn from the past to create effective solutions for the future. Before the introduction of chemotherapy, the burden of tuberculosis was already decreasing as a result of social interventions and rising standards of living. Although biomedical interventions have helped to augment this decline, the improvements have been slow, and tuberculosis remains a major challenge worldwide. Stopping tuberculosis will need a renewed focus on multisectoral action to forge a biosocial response. The sustainable development agenda provides the opportunity to harness social, economic, and environmental actions to stop tuberculosis that has proven so difficult to defeat with biomedical approaches alone.
Key messages.
Tuberculosis has its roots in underdevelopment, poverty, and social exclusion, but worldwide efforts to address tuberculosis during the past several decades have emphasised biomedical solutions.
Progress against tuberculosis has been slow because of gaps in coverage of tuberculosis programmes and unmitigated risk factors for tuberculosis transmission and progression in low-income and middle-income countries, including overcrowding, indoor air pollution, malnutrition, diabetes mellitus, and tobacco and alcohol use.
Social solutions for fighting tuberculosis, such as improved nutrition and improved housing conditions, were evidently major drivers of reductions in the burden of tuberculosis in the pre-chemotherapy era.
The fight against tuberculosis should strengthen current biomedical solutions through new treatments, diagnostics, and service delivery models—and introduce approaches to combat the social drivers of the epidemic.
Stopping tuberculosis needs a biosocial solution—one that integrates the social and biomedical approaches for sustainable development.
Panel: Social reforms in 1934 for improving health and well being of workers and reducing the burden of tuberculosis in Chile.
During the 1930s, Chile had the highest tuberculosis mortality burden of any country globally. Chile—fully aware of the social determinants for tuberculosis—devised a 14-point social reform to address the epidemic by reducing the country’s underlying social inequalities and improving the living standards of the growing working class.27
This social reform shifted the focus of the tuberculosis response from the individual patient (eg, sanatoria and surgical interventions) to the larger social context. Despite initial successes in decreasing deaths from tuberculosis in Chile through the 1940s, social reform was quickly replaced by biomedical solutions after the introduction of chemotherapy in the 1950s.27
Chile’s 14-point social reform to address the tuberculosis epidemic:
Increase wages.
Decrease the length of the average working day
Eliminate overtime
Regulate the working conditions of night workers
Construct sound, affordable housing for workers
Improve unsafe working environments in factories
Enact legislation to protect worker’s health and provide protection for those that are sick or injured
Make unemployment insurance compulsory
Carry out anti-alcohol campaigns
Carry out anti-venereal disease campaigns
Protect abandoned infants and children
Promote sports. Construct stadiums, parks, and gardens
Clean up all public places in which there are regular assemblages of people: theatres, churches, etc
Reform Law 4054 (a law that addressed illness, disability, ageing, and death)
Acknowledgments
We acknowledge Janice Hu for her contributions to draft revisions.
Footnotes
Declaration of interests
The authors declare no conflicts of interest. Role of funding: TB was supported by the Wellcome Trust and the US National Institutes of Health (1P01AG041710–01). KFO, JAS, and RA have no external funding sources to disclose in relation to this Series paper. The funders had no roles in the conception, preparation, review, approval, or submission of this manuscript.
References
- 1.Dormandy T The white death. New York: New York University Press, 2000. [Google Scholar]
- 2.Dye C, Lönnroth K, Jaramillo E, Williams BG, Raviglione M. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ 2009; 87: 683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. The five elements of DOTS. http://www.who.int/tb/dots/whatisdots/en/ (accessed June 3, 2015).
- 4.World Health Organization. The Stop TB Strategy. http://www.who.int/tb/strategy/stop_tb_strategy/en/ (accessed June 3, 2015).
- 5.Murray CJL, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 1005–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeown T, Record RG. Reasons for the Decline of Mortality in England and Wales during the Nineteenth Century. Popil Stud 1962; 16: 94–122. [Google Scholar]
- 7.Atun RA, Samyshkin YA, Drobniewski F, et al. Barriers to sustainable tuberculosis control in the Russian Federation health system. Bull World Health Organ 2005; 83: 217–23. [PMC free article] [PubMed] [Google Scholar]
- 8.Atun RA, Samyshkin Y, Drobniewski F, et al. Costs and outcomes of tuberculosis control in the Russian Federation: retrospective cohort analysis. Health Policy Plan 2006; 21: 353–64. [DOI] [PubMed] [Google Scholar]
- 9.Coker RJ, Atun RA, McKee M. Health-care system frailties and public health control of communicable disease on the European Union’s new eastern border. Lancet 2004; 363: 1389–92. [DOI] [PubMed] [Google Scholar]
- 10.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2197–223. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO declares tuberculosis a global emergency. 1993. [Google Scholar]
- 13.United Nations. The Millennium Development Goals Report 2012. http://www.un.org/millenniumgoals/pdf/MDG%20Report%202012.pdf (accessed June 3, 2015). [Google Scholar]
- 14.Dye C, Watt CJ, Bleed DM, Williams BG. What is the limit to case detection under the DOTS strategy for tuberculosis control? Tuberculosis (Edinb) 2003; 83: 35–43. [DOI] [PubMed] [Google Scholar]
- 15.Lienhardt C From exposure to disease: the role of environmental factors in susceptibility to and development of tuberculosis. Epidemiol Rev 2001; 23: 288–301. [DOI] [PubMed] [Google Scholar]
- 16.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med 2007; 4: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin HH, Suk CW, Lo HL, Huang RY, Enarson DA, Chiang CY. Indoor air pollution from solid fuel and tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2014; 18: 613–21. [DOI] [PubMed] [Google Scholar]
- 18.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004; 8: 286–98. [PubMed] [Google Scholar]
- 19.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 2009; 9: 737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slama K, Chiang CY, Enarson DA, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis 2007; 11: 1049–61. [PubMed] [Google Scholar]
- 21.Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health 2009; 9: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coker R, McKee M, Atun R, et al. Risk factors for pulmonary tuberculosis in Russia: case-control study. BMJ 2006; 332: 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JD. The social determinants of tuberculosis: from evidence to action. Am J Public Health 2011; 101: 654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Stop TB Partnership: Operational Strategy 2013–2015. http://www.stoptb.org/assets/documents/about/OperationalStrategy2013-2015.pdf (accessed June 3, 2015). [Google Scholar]
- 25.United Nations. Our Common Future: The World Commission on Environment and Development. 1987. http://www.un-documents.net/ocf-02.htm (accessed June 3, 2015). [Google Scholar]
- 26.United Nations. Report of the United Nations Conference on Sustainable Development; Rio de Janeiro, Brazil; 20–22 June 2012. A/Conf.216/16. United Nations; New York, 2012. http://www.uncsd2012.org/content/documents/814UNCSD%20REPORT%20final%20revs.pdf (accessed July 23, 2015). [Google Scholar]
- 27.Cox HS, Morrow M, Deutschmann PW. Long term effi cacy of DOTS regimens for tuberculosis: systematic review. BMJ 2008; 336: 484–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam D How the world survived the population bomb: lessons from 50 years of extraordinary demographic history. Demography 2011; 48: 1231–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMichael AJ. The urban environment and health in a world of increasing globalization: issues for developing countries. Bull World Health Organ 2000; 78: 1117–26. [PMC free article] [PubMed] [Google Scholar]
- 30.Tanimura T, Jaramillo E, Weil D, Raviglione M, Lönnroth K. Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J 2014; 43: 1763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dheda K, Gumbo T, Gandhi NR, et al. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med 2014; 2: 321–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. The Global Plan to Stop TB, 2011–2015. http://www.stoptb.org/assets/documents/global/plan/tb_globalplantostoptb2011-2015.pdf (accessed June 3, 2015). [Google Scholar]
- 33.de Andrade LOM, Pellegrini Filho A, Solar O, et al. Social determinants of health, universal health coverage, and sustainable development: case studies from Latin American countries. Lancet 2015; 385: 1343–51. [DOI] [PubMed] [Google Scholar]
- 34.Reeves A, Basu S, McKee M, Stuckler D, Sandgren A, Semenza J. Social protection and tuberculosis control in 21 European countries, 1995–2012: a cross-national statistical modelling analysis. Lancet Infect Dis 2014; 14: 1105–12. [DOI] [PubMed] [Google Scholar]
- 35.Rocha C, Montoya R, Zevallos K, et al. The Innovative Socio-economic Interventions Against Tuberculosis (ISIAT) project: an operational assessment. Int J Tuberc Lung Dis 2011; 15 (suppl 2): S50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atun R, de Andrade LO, Almeida G, et al. Health-system reform and universal health coverage in Latin America. Lancet 2015; 385: 1230–47. [DOI] [PubMed] [Google Scholar]
- 37.Boccia D, Hargreaves J, Lönnroth K, et al. Cash transfer and microfinance interventions for tuberculosis control: review of the impact evidence and policy implications. Int J Tuberc Lung Dis 2011; 15 (suppl 2): S37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei X, Zou G, Yin J, et al. Providing financial incentives to rural-to-urban tuberculosis migrants in Shanghai: an intervention study. Infect Dis Poverty 2012; 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramakrishnan CV, Rajendran K, Jacob PG, Fox W, Radhakrishna S. The role of diet in the treatment of pulmonary tuberculosis. An evaluation in a controlled chemotherapy study in home and sanatorium patients in South India. Bull World Health Organ 1961; 25: 339–59. [PMC free article] [PubMed] [Google Scholar]
- 40.Coker RJ, Dimitrova B, Drobniewski F, et al. Health system frailties in tuberculosis service provision in Russia: an analysis through the lens of formal nutritional support. Public Health 2005; 119: 837–43. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet 2000; 355: 618–21. [DOI] [PubMed] [Google Scholar]
- 42.Podewils LJ, Holtz T, Riekstina V, et al. Impact of malnutrition on clinical presentation, clinical course, and mortality in MDR-TB patients. Epidemiol Infect 2011; 139: 113–20. [DOI] [PubMed] [Google Scholar]
- 43.Sinclair D, Abba K, Grobler K, Sudarsanam T. Nutritional supplements for people being treated for active tuberculosis. The Cochrane Collaboration, 2011. http://fr.cmamforum.org/Pool/Resources/Nutr-supplements-and-active-TB-Cochrane-review-2011.pdf (accessed June 3, 2015). [DOI] [PubMed] [Google Scholar]
- 44.Shah R USAID Frontiers in Development. USAID, 2012. http://www.usaid.gov/sites/default/files/documents/1868/USAID_eBook.pdf (accessed June 3, 2015). [Google Scholar]
- 45.Rodger AJ, Toole M, Lalnuntluangi B, Muana V, Deutschmann P. DOTS-based tuberculosis treatment and control during civil conflict and an HIV epidemic, Churachandpur District, India. Bull World Health Organ 2002; 80: 451–56. [PMC free article] [PubMed] [Google Scholar]
- 46.Minetti A, Camelique O, Hsa Thaw K, et al. Tuberculosis treatment in a refugee and migrant population: 20 years of experience on the Thai-Burmese border. Int J Tuberc Lung Dis 2010; 14: 1589–95. [PubMed] [Google Scholar]
- 47.Masters WA, Webb P, Griffiths JK, Deckelbaum RJ. Agriculture, nutrition, and health in global development: typology and metrics for integrated interventions and research. Ann N Y Acad Sci 2014; 1331: 258–69. [DOI] [PubMed] [Google Scholar]
- 48.Hasan R Drug resistant tuberculosis: Challenges of urbanization. Int J Mycobacteriology 2014; 3: 79–81. [DOI] [PubMed] [Google Scholar]
- 49.Cordaid. UN-Habitat: number of slum dwellers grows to 863 million. 2014. https://www.cordaid.org/en/news/un-habitat-number-slum-dwellers-grows-863-million/ (accessed June 3, 2015). [Google Scholar]
- 50.United Nations Department of Economic and Social Affairs. World Urbanization Prospects, 2014 Revision. 2014. http://esa.un.org/unpd/wup/Highlights/WUP2014-Highlights.pdf (accessed June 3, 2015). [Google Scholar]
- 51.WagstaffK. Architecture latest tool in fight against malaria, tuberculosis in slums. The Utopianist, 2011. http://utopianist.com/2011/04/non-profit-hopes-to-fight-malaria-tuberculosis-in-slums-with-architecture/ (accessed June 3, 2015). [Google Scholar]
- 52.Northridge ME, Sclar E. A joint urban planning and public health framework: contributions to health impact assessment. Am J Public Health 2003; 93: 118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nardell EA. Fans, filters, or rays? Pros and cons of the current environmental tuberculosis control technologies. Infect Control Hosp Epidemiol 1993; 14: 681–85. [DOI] [PubMed] [Google Scholar]
- 54.Lygizos M, Shenoi SV, Brooks RP, et al. Natural ventilation reduces high TB transmission risk in traditional homes in rural KwaZulu-Natal, South Africa. BMC Infect Dis 2013; 13: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zafar Ullah AN, Huque R, Husain A, Akter S, Akter H, Newell JN. Tuberculosis in the workplace: developing partnerships with the garment industries in Bangladesh. Int J Tuberc Lung Dis 2012; 16: 1637–42. [DOI] [PubMed] [Google Scholar]
- 56.Hanifa Y, Grant AD, Lewis J, Corbett EL, Fielding K, Churchyard G. Prevalence of latent tuberculosis infection among gold miners in South Africa. Int J Tuberc Lung Dis 2009; 13: 39–46. [PubMed] [Google Scholar]
- 57.Stuckler D, Basu S, McKee M, Lurie M. Mining and risk of tuberculosis in sub-Saharan Africa. Am J Public Health 2011; 101: 524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reid PJ, Sluis-Cremer GK. Mortality of white South African gold miners. Occup Environ Med 1996; 53: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Government of South Africa. Tuberculosis Strategic Plan for South Africa, 2007–2011. http://www.tbonline.info/archive/document/7/ (accessed June 3, 2015). [Google Scholar]
- 60.Southern African Development Community. Declaration on Tuberculosis in the Mining Sector. 2012. http://www.stoptb.org/assets/documents/news/Declaration%20on%20Tuberculosis%20in%20the%20Mining%20Sector2012English.pdf (accessed July 23, 2015). [Google Scholar]
- 61.Doherty AM, Kelly J, McDonald C, O’Dywer AM, Keane J, Cooney J. A review of the interplay between tuberculosis and mental health. Gen Hosp Psychiatry 2013; 35: 398–406. [DOI] [PubMed] [Google Scholar]
- 62.Fischer PJ, Breakey WR. Homelessness and mental health: an overview. Int J Ment Health 1985; 14: 6–41. [Google Scholar]
- 63.Pachi A, Bratis D, Moussas G, Tselebis A. Psychiatric morbidity and other factors affecting treatment adherence in pulmonary tuberculosis patients. Tuberc Res Treat 2013; 2013: e489865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990; 264: 2511–18. [PubMed] [Google Scholar]
- 65.Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis—a systematic review. BMC Public Health 2008; 8: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yasinskaya Y, Plikaytis B, Sizemore C, Sacks L. Advancing the development of diagnostic tests and biomarkers for tuberculosis. Int J Tuberc Lung Dis 2011; 15: 985–87. [DOI] [PubMed] [Google Scholar]
- 67.Weiner J 3rd, Kaufmann SHE. Recent advances towards tuberculosis control: vaccines and biomarkers. J Intern Med 2014; 275: 467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.da Costa C, Walker B, Bonavia A. Tuberculosis vaccines—state of the art, and novel approaches to vaccine development. Int J Infect Dis 2015; 32: 5–12. [DOI] [PubMed] [Google Scholar]
- 69.Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med 2011; 365: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villarino ME, Scott NA, Weis SE, et al. , and the International Maternal Pediatric and Adolescents AIDS Clinical Trials Group, and the Tuberculosis Trials Consortium. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr 2015; 169: 247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zumla AI, Gillespie SH, Hoelscher M, et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis 2014; 14: 327–40. [DOI] [PubMed] [Google Scholar]
- 72.Zumla A, Nahid P, Cole ST. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov 2013; 12: 388–404. [DOI] [PubMed] [Google Scholar]
- 73.Lienhardt C, Vernon A, Raviglione MC. New drugs and new regimens for the treatment of tuberculosis: review of the drug development pipeline and implications for national programmes. Curr Opin Pulm Med 2010; 16: 186–93. [DOI] [PubMed] [Google Scholar]
- 74.Kernodle DS. Decrease in the effectiveness of Bacille Calmette-Guérin vaccine against pulmonary tuberculosis: a consequence of increased immune suppression by microbial antioxidants, not overattenuation. Clin Infect Dis 2010; 51: 177–84. [DOI] [PubMed] [Google Scholar]
- 75.Abubakar I, Pimpin L, Ariti C, et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol Assess 2013; 17: 1–372, v–vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rangaka MX, Cavalcante SC, Marais BJ, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet 2015; published online Oct 26. 10.1016/S0140-6736(15)00323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McNerney R, Maeurer M, Abubakar I, et al. Tuberculosis diagnostics and biomarkers: needs, challenges, recent advances, and opportunities. J Infect Dis 2012; 205 (suppl 2): S147–58. [DOI] [PubMed] [Google Scholar]
- 78.World Health Organization. Systematic screening for active tuberculosis; Principles and reccomendations. 2013. http://www.who.int/tb/publications/Final_TB_Screening_guidelines.pdf (accessed June 3, 2015). [PubMed] [Google Scholar]
- 79.Pai M, Schito M. Tuberculosis diagnostics in 2015: landscape, priorities, needs, and prospects. J Infect Dis 2015; 211 (suppl 2): S21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010; 363: 1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lawn SD, Kerkhoff AD, Vogt M, Ghebrekristos Y, Whitelaw A, Wood R. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis 2012; 54: 1071–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Creswell J, Codlin AJ, Andre E, et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis 2014; 14: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Jongh T, Gurol-Urganci I, Vlasta Vodopivec-Jamsek V, Car J, Atun R. Mobile phone messaging telemedicine for facilitating self management of long-term illnesses. Cochrane Database Syst Rev 2012; 12: CD007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nglazi MD, Bekker LG, Wood R, Hussey GD, Wiysonge CS. Mobile phone text messaging for promoting adherence to anti-tuberculosis treatment: a systematic review. BMC Infect Dis 2013; 13: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fraser HS, Allen C, Bailey C, Douglas G, Shin S, Blaya J. Information systems for patient follow-up and chronic management of HIV and tuberculosis: a life-saving technology in resource-poor areas. J Med Internet Res 2007; 9: e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fraser H, Choi SS, Galipot M, Jazayeri D, Mangubat N. Successful transfer of a Web-based TB medical record from Peru to the Philippines. AMIA Annu Symp Proc 2006; 2006: 924. [PMC free article] [PubMed] [Google Scholar]
- 87.Atun R, Olynik I. Resistance to implementing policy change: the case of Ukraine. Bull World Health Organ 2008; 86: 147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.CDC. Executive Commentary—Reported Tuberculosis in the United States. 2011. http://www.cdc.gov/tb/statistics/reports/2011/executivecommentary.htm (accessed June 3, 2015). [Google Scholar]
- 89.Edelstein M, Angelides P, Heymann DL. Ebola: the challenging road to recovery. Lancet 2015; 385: 2234–35. [DOI] [PubMed] [Google Scholar]
- 90.Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet 2014; 383: 1572–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.World Health Organization. No more crying, no more dying: towards zero TB deaths in children. 2012. http://www.who.int/tb/ChildhoodTB_report_singles.pdf (accessed July 22, 2015). [Google Scholar]
- 92.Walley JD, Khan MA, Newell JN, Khan MH. Effectiveness of the direct observation component of DOTS for tuberculosis: a randomised controlled trial in Pakistan. Lancet 2001; 357: 664–69. [DOI] [PubMed] [Google Scholar]
- 93.Vree M, Bui DD, Dinh NS, Nguyen VC, Borgdorff MW, Cobelens FG. Tuberculosis trends, Vietnam. Emerg Infect Dis 2007; 13: 796–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dye C, Ottmani S, Laasri L, Bencheikh N. The decline of tuberculosis epidemics under chemotherapy: a case study in Morocco. Int J Tuberc Lung Dis 2007; 11: 1225–31. [PubMed] [Google Scholar]
- 95.World Health Organization. Tuberculosis and sustainable development. WHO/CDS/STB/2000.4. http://apps.who.int/iris/bitstream/10665/66239/1/WHO_CDS_STB_2000.4.pdf?ua=1 (accessed July 22, 2015). [Google Scholar]
- 96.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–53. [DOI] [PubMed] [Google Scholar]
- 97.UN-Habitat. State of the World’s Cities 2012/2013, Prosperity of Cities. 2013. https://sustainabledevelopment.un.org/content/documents/745habitat.pdf (accessed July 23, 2015). [Google Scholar]
- 98.UN-Habitat. The challenge of slums—global report on human settlements. 2003. http://www.unhabitat.org/pmss/getElectronicVersion.aspx?nr=1156&alt=1%E2%80%8E (accessed July 23, 2015). [Google Scholar]
- 99.McMichael P, Schneider M. Food security politics and the Millennium Development Goals. Third World Q 2011; 32: 119–39. [DOI] [PubMed] [Google Scholar]
- 100.Trouiller P, Olliaro P, Torreele E, Orbinski J, Laing R, Ford N. Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet 2002; 359: 2188–94. [DOI] [PubMed] [Google Scholar]
- 101.Atun R, Knaul FM, Akachi Y, Frenk J. Innovative financing for health: what is truly innovative? Lancet 2012; 380: 2044–49. [DOI] [PubMed] [Google Scholar]