Abstract
Background:
Genistein (5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is the most abundant isoflavone in soybean, which has been associated with a lower risk of development of cancer and cardiovascular diseases. Of particular interest regarding cancer preventive properties of flavo-noids is their interaction with cytochrome P450 enzymes (CYPs). However, contradictory data report the effect of genistein on expression of СYPs enzymes.
Objective:
The aim of this study was to investigate the effects of genistein on cytochrome P450 (CYP) gene expression levels in human hepatocellular carcinoma (HepG2/C3A) and colon adenocarcinoma (HT29) cells.
Methods:
Real-time RT–PCR was used to examine the expression of genes families involved in xenobi-otic metabolism, such as CYP1 (CYP1A1, CYP1B1), CYP2 (CYP2E1, CYP2D6), CYP3 (CYP3A4); and of a family involved in the catabolism of the all-trans-retinoic acid (ATRA), CYP26 (CYP26A1, CYP26B1).
Results:
RT-qPCR data analysis showed that after 12 h of exposure of HepG2/C3A cells to genistein (5 and 50 µM) there was an upregulation of CYP1A1 and CYP1B1 and downregulation of CYP2D6, CYP26A1 and CYP26B1 mRNA levels. There was no change in the mRNA levels of CYP P450 genes in HT29 cells.
Conclusion:
Our results suggest that treatment with genistein in non-toxic concentrations may impact the expression level of CYPs involved in the biotransformation of xenobiotics and drug metabolizing en-zymes. Moreover, the downregulation of ATRA metabolism-related genes opens a new research path for the study of genistein as retinoic acid metabolism blocking agent for treating cancer and other patholo-gies.
Keywords: Genistein, isoflavones, cytochrome P450, All-Trans-Retinoic Acid (ATRA), xenobiotics metabolism, hepatocellular carcinoma
1. INTRODUCTION
Genistein (5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one), the most abundant isoflavone in soybean, has been found to be associated with a lower risk of chronic diseases development such as cancer and cardiovascular diseases [1]. Chemopreventive properties of genistein against cancer have emerged from epidemiological observations. The lower frequencies of breast and prostate cancer in Asian population, compared to those from Western societies have been attributed to consumption of a relatively large amount of soy products, containing isoflavones [2]. For colorectal and endometrial cancers, inverse correlation between soy products intake and tumor development has also been observed [3-5]. As reviewed by Spagnuolo et al., in vitro and in vivo studies also support that genistein has an important role in different types of cancer. It can intervene at multiple points of the carcinogenic process, altering apoptosis, cell cycle, angiogenesis and inhibiting metastasis [6].
Of particular interest regarding cancer preventive properties of flavonoids is their interaction with cytochrome P450 enzymes (CYPs). These enzymes are involved in metabolism of a wide variety of endogenous and exogenous substrates, playing a significant physiological role in the detoxification of xenobiotics, and the biosynthesis/catabolism of endogenous compounds [7, 8]. Although these enzymes have been targets historically for attempts to block early stages of tumorigenesis, emerging evidence supports that P450 enzymes may also be strategic targets in treating tumor progression, invasion, and metastasis [9].
It has been suggested that flavonoids can act (i) as inhibitors of the CYP1 family [10-15], blocking the mutagenic effects produced by the metabolism of cancerous environmental substances and also (ii) as substrates for cytochrome P450 (particularly CYP1A1 and CYP1B1) that selectively metabolize dietary flavonoids to conversion products that inhibit cancer cell proliferation [16, 17]. Furthermore, flavonoids can also affect CYP isoforms which in turn can alter drug responses including CYP3A4 and CYP2D6 which are responsible for the biotransformation of 70-80% of all drugs in clinical use [18]. Dietary compounds have been shown to interact with CYP3A4 and alter its expression and activity [19]. Therefore, because of the important role of the CYPs in the bioactivation and inactivation of carcinogens and their participation in the activation and inactivation of anticancer drugs, they play an important role both in the aetiology of cancer and as determinants of cancer therapy [20].
Other isoforms of CYPs such as CYP26A1/B1 involved in the catabolism of retinoic acid (RA), the active metabolite of vitamin A (retinol) also offers a pathway for cancer therapy. The isomer all-trans-RA (ATRA) is the biologically active isomer of RA and it was approved for use in acute promyelocytic leukemia (APL) treatment [21]. ATRA exerts inhibitory effects on cell proliferation, promotes differentiation and induces apoptosis in a variety of cancer cells [22-24]. Thus, pharmacological approaches to inhibiting of cytochrome P450-dependent ATRA-4-hydroxylase enzymes (particularly CYP26) may extend the half-life of RA and could be useful clinically in the future.
Contradictory data report the effect of genistein on the expression of СYPs enzymes. Genistein was found to increase CYP1A1 expression in prostate adenocarcinoma (LNCaP and PC-3) [25]. Wei et al. (2015) reported that genistein (5 or 25 μM) increased the mRNA levels of CYP1B1 in breast cancer cells (MCF-7) [26] while other reports showed that genistein significantly inhibited expression of CYP1B1 in Panc 1 cells at 10 μM [27]. The reasons for the different activities of genistein remain to be clarified. Furthermore, interactions between flavonoids and cytochrome P450 CYP26A1/B1, a retinoic acid (RA)-metabolizing enzyme in humans, have not yet been reported.
The aim of the present study was to investigate the effects at low and high concentrations of genistein (5 and 50 μmol/L) on cytochrome P450 (CYP) gene expression levels in human hepatocellular carcinoma (HepG2/C3A) and colon adenocarcinoma (HT29) cells. The mRNA levels of families involved in xenobiotic metabolism, such as CYP1 (CYP1A1, CYP1B1), CYP2 (CYP2E1, CYP2D6), CYP3 (CYP3A4); and of a family involved in the catabolism of the all-trans-retinoic acid (ATRA), CYP26 (CYP26A1, CYP26B1) were evaluated by qRT PCR.
2. MATERIALS AND METHODs
2.1. Chemicals
Genistein was purchased from Sigma-Aldrich (CAS Number 446-72-0) and diluted in dimethylsulfoxide (DMSO, Acros Organics). Upon use, the solution was diluted in Dulbecco's Modified Eagle Medium (DMEM) (Gibco®, Life Technologies, USA) in experimental concentrations.
2.2. Cell Culture
Human hepatoma (HepG2/C3A) and colon adenocarcinoma (HT-29) cell lines were obtained from the Rio de Janeiro Cell Bank (RJCB - Brazil). Cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (Gibco®) and maintained in a humidified 5% CO2 incubator at 37°C. Under these conditions, the cell viability remained >90%.
2.3. Cytotoxicity Assay
Cytotoxicity of genistein in HepG2/C3A and HT-29 cells was determined by the MTT assay (3- [4, 5-dimethylthiazol-2-yl] -2, 5-diphenyltetrazolium bromide), according to Mosmann [28] with some modifications. Briefly, cells were seeded in 96 well plates, at a density of 5.103 cells/well. After 24 h of incubation, the culture medium was removed and replaced by a new DMEM without FBS, containing genistein at 0; 5; 50 and 100 μM. Positive control received 1μM doxorubicin (Doxolem, Zodiac laboratories). After 24 hours of treatment, 200 μL of MTT solution (0.5 mg/mL in DMEM) was added to each well and kept at 37°C for 4 h to form formazan crystals. After incubation, the supernatant was discarded, and formazan crystals were dissolved in 200μL of DMSO per well. The absorbance was measured in a microplate spectrophotometer (TP Reader - Thermo Plate) at 550 nm. All experiments were performed in three repetitions, and the relative cell viability (%) was expressed as a percentage relative to the untreated control cells.
2.4. Gene Expression Analysis by qRT- PCR
qRT-PCR was performed according to the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines [29]. A total of 106 cells were seeded in 10 cm2 culture tubes. After 24h of incubation, genistein (5 or 50 μM) were added and remained in culture for 12 hours. Total RNA was isolated by MagNA Pure Compact RNA Isolation kit (Roche). Concentration and purity of samples (260 and 280 nm ratio) were verified with BioDrop µlITE UV/VIS Spectrophotometer. RNA purity was considered satisfactory when the ratio of 260 and 280nm absorbance ranged between 1.9 and 2.1. The RNA integrity was assessed by electrophoresis on 1% agarose gel. cDNA synthesis was performed using reverse transcriptase MMLV (Invitrogen Life Technologies, USA) and total RNA using oligo (dT) primers (Invitrogen Life Technologies, USA) according to the protocol of the manufacturer. After the synthesis reaction, cDNA was stored under -80°C, until use in qRT-PCR assay. Real-time PCR reactions were carried out in CFX96 TOUCH thermocycler (Bio-Rad Laboratories), with SYBR Green qPCR Platinum Supermix reagent (Invitrogen). Cycling conditions were: cDNA denaturation at 50°C for 1 min, followed by 95°C for 3 minutes, 35 cycles at 95°C for 20 seconds; primer annealing at 60°C for 30 seconds; extension at 72°C for 20 seconds followed by 95°C for 10 sec. and 40°C for 1 min. Melting curve analysis was performed at the end of each reaction with the temperature ranging from 50°C to 95°C every 0.5°C, for 5 sec. The obtained data were normalized to the endogenous glyceraldehyde phosphate dehydrogenase (GAPDH) gene, which was amplified in each set of PCR experiments. Relative differences between treated and control groups were calculated according to the method described by Pfaffl (2001) [30]. All experiments were performed with three independent biological repetitions and mechanical duplicates for each sample. The primers used were: CYP1A1 [31]; CYP1B1 [31]; CYP2D6 [32]; CYP2E1 [33]; CYP3A4 [34]; CYP26A1 (designed in present study - AF005418.1*); CYP26B1 (designed in present study - AF252297.1*) and GAPDH [35]. *Design with PrimerQuest® program, IDT, http://www.idtdna.com/Scitools.
2.5. Statistical Analysis
The data from the MTT assay were subjected to analysis of variance (ANOVA), followed by Tukey post-test (p<0.05). Gene expression data obtained by qRT-PCR were normalized and analyzed according to Pffafl’s method [36]. Additionally, statistical analysis was performed by REST2009 software, using Pair Wise Fixed Reallocation Randomisation Test. The reaction efficiency was determined by LinRegPCR software version 7.5 [37].
3. RESULTS
3.1. Cytotoxicity Assay
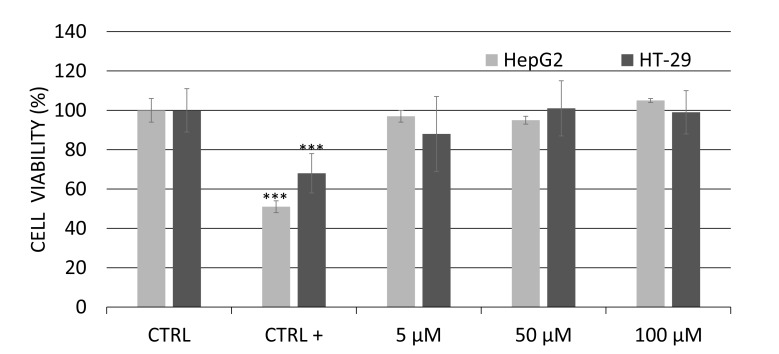
The effect of genistein (5 to 100μM) on cell viability after 24 hours of treatment was analyzed by MTT assay (Fig. 1). There was no cell viability decrease after exposure of HepG2C3a or HT29 cells to genistein. Based on these results, two non-toxic concentrations of genistein were chosen for the qRT-PCR analysis: “low” (5 μM) and “high” (50 μM) doses. All of the genistein concentrations were based on their positive biological effects related to previous studies. The concentration of 5 µM was chosen based on serum level achievable by diet or dietary supplementation in humans [38] and a concentration of 50 µM based on plausible intestinal concentration reached in the gut lumen after ingestion of a normal diet [39].
Fig. (1).
Viability (MTT assay) of human hepatoma (HEPG2/C3A) and colon adenocarcinoma cells (HT-29) between treated cells in Genistein medium (5 to 100 µM) and untreated cells in control medium (CTRL -) after 24 h exposure. Doxorubicin containing media was used as positive control (CTRL +). Data were expressed as mean ± standard deviation of 3 independent experiments with ten repetitions each. Asterisks (*) indicate statistically significant differences between the treated genistein groups and the control without genistein group.
3.1.1. Xenobiotic Metabolism Genes
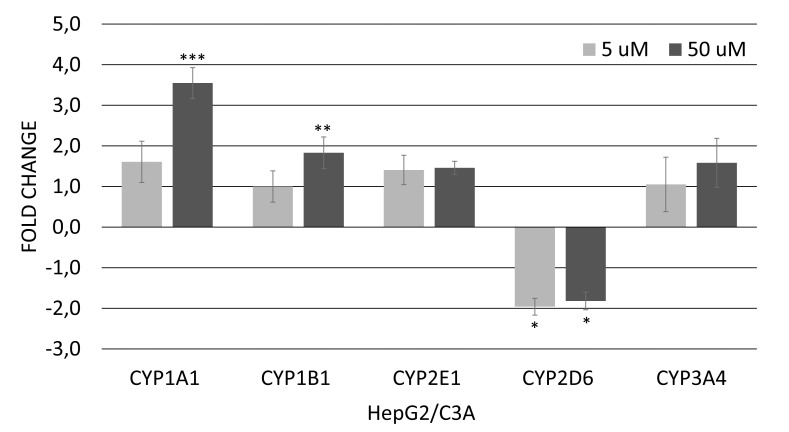
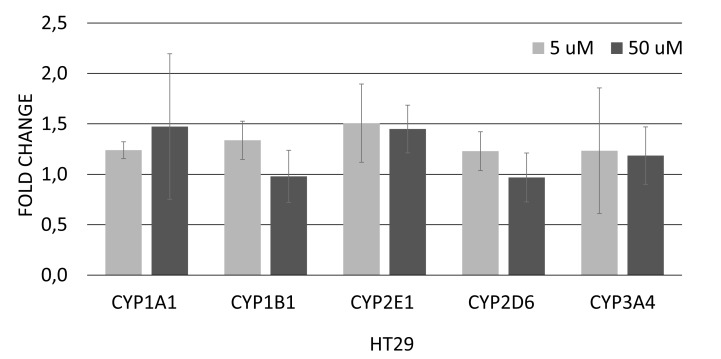
In HepG2/C3A cells, the result obtained by real-time PCR demonstrates that treatment with 5 and 50 μM of genistein caused a significant decrease of 2.1 and 1.8-fold in CYP2D6 mRNA expression, respectively (Fig. 2A). In addition, the expression of CYP1A1 and CYP1B1 was upregulated after treatment with 50 μM genistein (3,8- and 1,9-fold, respectively) (Fig. 2A). In HT-29 cells, genistein at 5 or 50 µM did not alter the expression of genes involved in the xenobiotic metabolism (Fig. 2B).
Fig. (2.
A). Relative expression of genes involved in regulation of xenobiotic metabolism in HepG2/C3A cells after 12 hours of exposure to genistein (5 or 50 µM). Values are the mean ± standard deviation of three independent experiments. Asterisks (*) indicate statistically significant differences (p<0.05); (**) indicate statistically significant differences (p<0.01) and (***) indicate statistically significant differences (p<0.001) between the treated genistein groups and the control without genistein group, according to the REST software 2009. Normalized data with GAPDH.
3.2. ATRA Metabolism Genes
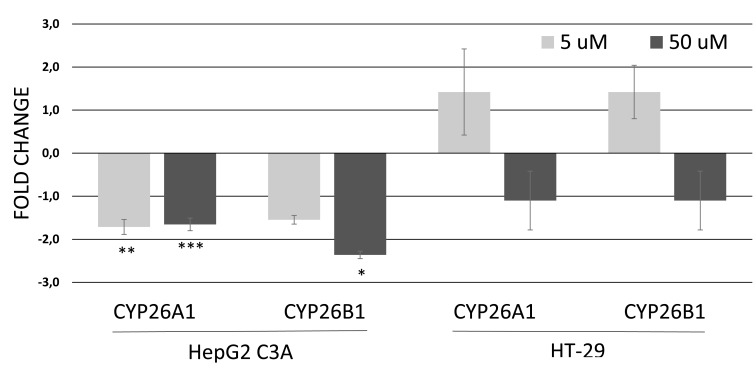
In HepG2/C3A cells, the mRNA levels of CYP26A1 were downregulated after exposure to 5 µM genistein (1.8-fold) and to 50 μM genistein (1.6-fold). The concentration of 50 µM genistein also caused a downregulation (2.3-fold) of CYP26B1 gene in this cell. No significant changes in mRNA levels of CYP26A1 or CYP26B1 were observed after exposure of HT-29 cells to genistein (Fig. 3).
Fig. (3).
Relative expression of genes involved in ATRA metabolism in HepG2/C3A cells and HT-29 after 12 hours of exposure to genistein at 5 or 50 µM. Values are the mean ± standard deviation of three independent experiments. Asterisks (*) indicate statistically significant differences (p<0.05); (**) indicate statistically significant differences (p<0.01) and (***) indicate statistically significant differences (p<0.005) between the treated genistein groups and the control without genistein group, according to the REST software 2009. Normalized data with GAPDH.
4. DISCUSSION
In the present study, we investigated the effects of genistein (5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) on mRNA expression of cytochrome P450 enzymes involved in xenobiotic metabolism (CYP1, 2 and 3 family), as well as of ATRA (CYP26A1/B1) metabolism in hepatocellular carcinoma (HepG2/C3A) and colon adenocarcinoma (HT-29). Basal expression of CYPs isoforms was detected of all tested cell lines. There was no toxicity in both HepG2/C3A and HT-29 cell lines after exposure to genistein (5 to 100 μM). Based on the MTT assay, low and high concentrations of genistein (5 μM and 50 μM, respectively) were chosen for subsequent gene expression evaluation.
Exposure of HepG2/C3A cells to genistein resulted in upregulation of CYP1 family members, CYP1A1 and CYP1B1, and downregulation of CYP2D6, CYP26A1 and CYP26B1. In HT-29 cells, genistein did not alter the expression of evaluated genes. Differences in CYP450 expression response to genistein could be explained by numerous factors including genetic profile possibly related to the tumoral origin of the cell lines.
In this study, both of the tested concentrations of genistein caused an upregulation of CYP1A1 and CYP1B1 in HepG2/C3A cells. Consistent with our results, previous studies showed that genistein upregulates CYP1A1 basal expression in prostate cancer cells (LNCaP and PC-3) and Wistar rats [25, 40] and CYP1B1 in breast cancer cells (MCF-7) at 25 µM [41]. The induction of CYP1 is associated with the activation of many carcinogens and numerous compounds that exert their genotoxic and carcinogenic effects only after metabolic activation. Wei et al, showed that genistein (5 and 25 µM) causes a synergistic effect on the CYP1B1 mRNA level induced by the environmental carcinogen 7,12-dimethylbenz[a]anthracene (DMBA), suggesting a potential role of this flavonoid on the chemical carcinogenesis [26]. In addition, CYP1B1 is commonly overexpressed in a wide range of human cancers [42] and has been shown to interact with several anticancer drugs including the taxanes [43] may affecting anticancer drug resistance [44]. Therefore, genistein-mediated induction of CYP1A1/B1 mRNA may potentially result in an increase in the toxicity and carcinogenicity of procarcinogens as well as induction of CYP1B1 may represent a mechanism of resistance, influencing the clinical outcome of chemotherapy.
However, a contrast with the consensus that inducible CYP1A1 is deleterious to the human organism has recently been demonstrated in vivo with the generation of CYP1A1 (- / -) knockout mice. Mice with the absence of the CYP1A1 gene died within 30 days of treatment with benzo(a)pyrene (B[a]P) 125 mg/kg/day, while CYP1A1 (+/-) mice survived without obvious signs of toxicity [45]. These data showed for the first time that this enzyme is more important for the detoxification of B[a]P in the liver and intestine than for the metabolic activation of cancer products. Therefore, it is difficult to explain chemopreventive influence of genistein in the metabolism of procarcinogens and development of carcinogenesis. However, understanding these mechanisms of enzyme induction and inhibition is extremely important to give appropriate multiple drug therapy. Further investigations are needed to elucidate the role of genistein in carcinogenesis through the regulation of CYP1A1/B1 gene expression.
The plasma concentration of pharmaceutical drugs might be affected by the induction of CYP by flavonoids, resulting in loss of therapeutic effect or overdose [46]. The isoforms CYP3A4 and CYP2D6 are two particularly important because they are responsible for the metabolism of approximately 50% and 25%, respectively, of the currently known therapeutic drugs [47]. Our results showed an inhibitory effect of genistein on CYP2D6 expression in HepG2C3A cells, suggesting the existence of metabolic interactions between genistein and CYP2D6 substrates. CYP2D6 plays a crucial role in the metabolic activation of cytotoxic prodrugs such tamoxifen widely used to treat breast cancer [20]. When CYP2D6 inhibitors are coadministered with tamoxifen, the enzymatic activity is decreased causing tamoxifen accumulation in plasma and leading to severe clinical consequences [48]. Data on metabolic interaction between genistein and tamoxifen is contradicting. It appears that the regulation of CYPs by herbal products is complex, depending on the herb type, their administration dose and rout, the target organ, and species [47]. Genistein reverses the effect of tamoxifen on cell growth and cell cycle arrest in T47D breast cancer cells [49]; and blocked the antiproliferative effect of tamoxifen on human breast cancer (MCF-7) cells implanted in athymic mice [50]. In contrast, administration of genistein potentiated the anti-proliferative effect of tamoxifen in dysplastic breast cell lines (MCF-10th, MCF-ANeoT, MCF-T63B) and malignant cell lines (MCF-7, MDA-231 and MDA-435) [51, 52].
Other clinically relevant cytochrome P450 isoforms are CYP26A1/B1 involved in the metabolism of all-trans-retinoic acid (ATRA or retinol) [53]. ATRA is the most active metabolite of retinoic acid, and exerts inhibitory effects on cell proliferation, induces cell cycle arrest and apoptosis in a variety of cancer cells [22, 23, 54, 55]. In addition, retinoic acid receptors are not mutated in cancer cells, so ATRA could potentially exert its anticancer effects in many malignancies [24, 55]. In a randomized phase II trial, patients with advanced non-small-cell lung cancer (NSCLC) that received chemotherapy coadministered with ATRA, presented improved disease endpoints with an acceptable toxicity profile [56]. ATRA is already being used to treat acute promyelocytic leukaemia (APL), with remission rates of around 80–90% [57]. However, the therapeutic use of retinoic acid isomers is limited due to side effects such as teratogenicity and acquired resistance to treatment [58]. Thereafter, a new related strategy consists in modulating or increasing the levels of endogenous ATRA through the inhibition of cytochrome P450-dependent ATRA-4-hydroxylase enzymes (particularly CYP26) responsible for its metabolism of ATRA [53]. Our data showed that genistein inhibited the basal expression of CYP26A1/B1 in human hepatocellular carcinoma cells (HepG2/C3A). The significance of these findings is that endogenous RA concentrations will be increased in the presence of the genistein, thus, potentiating the activity of endogenous RA in a cell-type specific manner. This is one of the first report demonstrating a natural product as CYP26 inhibitor. Based on the growing evidence supporting the physiological and clinical importance of genistein and the observations presented herein, we hypothesize that downregulation CYP26A1/B1 by genistein could be of clinical importance and requires further investigation.
Conclusion
Our results suggest that in vitro effect of genistein on CYP450 mRNA is cell line-dependent. Genistein impacts CYP1A1, CYP1B1, CYP2D6 and CYP26A1/B1 expression in HepG2/C3A but not in HT-29 cell line. The results provide insights into the interactions of genistein with xenobiotic and drug-metabolizing enzymes. In addition, the significant decrease in CYP26A1/B1 expression, enzymes involved in the degradation of ATRA, opens a new path for genistein study as retinoic acid metabolism blocking agent.
Fig. (2B).
Relative expression of genes involved in regulation of xenobiotic metabolism in HT-29 cells after 12 hours of exposure to genistein (5 or 50 µM). Values are the mean ± standard deviation of three independent experiments. Asterisks (*) indicate statistically significant differences between the treated genistein groups and the control without genistein group, according to the REST software 2009. Normalized data with GAPDH.
Acknowledgements
Declared none.
AUTHORS’ CONTRIBUTION
Sandra Sandra Regina Lepri and Mário Sérgio Mantovani conceived the project and experimental design. Sandra Regina Lepri, Daniele Sartori, Simone Cristine Semprebon and Adrivanio Baranoski performed the experiments and analyzed the data. Sandra Regina Lepri was involved in the reporting of the study. Giuliana Coatti and Simone Cristine Semprebon revised the manuscript. Simone Cristine Semprebon participated in the formatting and journal preparation. All authors have read and approved the final manuscript.
Funding Information
This work was supported by CNPq (National Council of Scientific and Technological Development - process n. 304776/2013-0); CAPES (Coordination for the Improvement of Superior Level - PROAP 23038007030/2014) and Araucaria Foundation for the Support of scientific and Technological Development of Parana—Brazil process n. 167/2014.
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
No Animals/Humans were used for studies that are the basis of this research.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Birt D.F., Hendrich S., Wang W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 2.Park O.J., Surh Y.J. Chemopreventive potential of epigallocatechin gallate and genistein: Evidence from epidemiological and laboratory studies. Toxicol. Lett. 2004;150:43–56. doi: 10.1016/j.toxlet.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Zhang G.Q., Chen J.L., Liu Q., Zhang Y., Zeng H., Zhao Y. Soy intake is associated with lower endometrial cancer risk: A systematic review and meta-analysis of observational studies. Medicine (Baltimore) 2015;94:e2281. doi: 10.1097/MD.0000000000002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan L., Spitznagel E.L. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am. J. Clin. Nutr. 2009;89:1155–1163. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- 5.Zhu B., Sun Y., Qi L., Zhong R., Miao X. Dietary legume consumption reduces risk of colorectal cancer: Evidence from a meta-analysis of cohort studies. Sci. Rep. 2015;5:8797. doi: 10.1038/srep08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spagnuolo C., Russo G.L., Orhan I.E., Habtemariam S., Daglia M., Sureda A., Nabavi S.F., Devi K.P., Loizzo M.R., Tundis R., Nabavi S.M. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015;6:408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Androutsopoulos V.P., Papakyriakou A., Vourloumis D., Tsatsakis A.M., Spandidos D.A. Dietary flavonoids in cancer therapy and prevention: Substrates and inhibitors of cytochrome P450 CYP1 enzymes. Pharmacol. Ther. 2010;126:9–20. doi: 10.1016/j.pharmthera.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Liu J., Sridhar J., Foroozesh M. Cytochrome P450 family 1 inhibitors and structure-activity relationships. Molecules. 2013;18:14470–14495. doi: 10.3390/molecules181214470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson H.I., Njar V.C., Yu Z., Castro D.J., Gonzalez F.J., Williams D.E., Huang Y., Kong A.N., Doloff J.C., Ma J., Waxman D.J., Scott E.E. Targeting drug-metabolizing enzymes for effective chemoprevention and chemotherapy. Drug Metab. Dispos. 2010;38:539–544. doi: 10.1124/dmd.109.031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan H.Y., Leung L.K. A potential protective mechanism of soya isoflavones against 7,12-dimethylbenz[a]anthracene tumour initiation. Br. J. Nutr. 2003;90:457–465. doi: 10.1079/bjn2003913. [DOI] [PubMed] [Google Scholar]
- 11.Wen X., Walle U.K., Walle T. 5,7-Dimethoxyflavone downregulates CYP1A1 expression and benzo[a]pyrene-induced DNA binding in Hep G2 cells. Carcinogenesis. 2005;26:803–809. doi: 10.1093/carcin/bgi015. [DOI] [PubMed] [Google Scholar]
- 12.Puppala D., Gairola C.G., Swanson H.I. Identification of kaempferol as an inhibitor of cigarette smoke-induced activation of the aryl hydrocarbon receptor and cell transformation. Carcinogenesis. 2007;28:639–647. doi: 10.1093/carcin/bgl169. [DOI] [PubMed] [Google Scholar]
- 13.Berge G., Ovrebo S., Eilertsen E., Haugen A., Mollerup S. Analysis of resveratrol as a lung cancer chemopreventive agent in A/J mice exposed to benzo[a]pyrene. Br. J. Cancer. 2004;91:1380–1383. doi: 10.1038/sj.bjc.6602125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan H.Y., Wang H., Leung L.K. The red clover (Trifolium pratense) isoflavone biochanin A modulates the biotransformation pathways of 7,12-dimethylbenz[a]anthracene. Br. J. Nutr. 2003;90:87–92. doi: 10.1079/bjn2003868. [DOI] [PubMed] [Google Scholar]
- 15.Ho P.C., Saville D.J., Wanwimolruk S. Inhibition of human CYP3A4 activity by grapefruit flavonoids, furanocoumarins and related compounds. J. Pharm. Pharm. Sci. 2001;4:217–227. [PubMed] [Google Scholar]
- 16.Androutsopoulos V.P., Papakyriakou A., Vourloumis D., Spandidos D.A. Comparative CYP1A1 and CYP1B1 substrate and inhibitor profile of dietary flavonoids. Bioorg. Med. Chem. 2011;19:2842–2849. doi: 10.1016/j.bmc.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Arroo R.R.J., Beresford K., Bhambra A.S., Boarder M., Budriesi R., Cheng Z., Micucci M., Ruparelia K.C., Surichan S., Androutsopoulos V.P. Phytoestrogens as natural prodrugs in cancer prevention: towards a mechanistic model. Phytochem. Rev. 2014;13:853–866. [Google Scholar]
- 18.Zanger U.M., Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Basheer L., Kerem Z. Interactions between CYP3A4 and Dietary Polyphenols. Oxid. Med. Cell. Longev. 2015;2015:1–15. doi: 10.1155/2015/854015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Antona C., Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 21.Tang X.H., Gudas L.J. Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 22.Tabata C., Tabata R., Hirayama N., Yasumitsu A., Yamada S., Murakami A., Iida S., Tamura K., Terada T., Kuribayashi K., Fukuoka K., Nakano T. All-trans-retinoic acid inhibits tumour growth of malignant pleural mesothelioma in mice. Eur. Respir. J. 2009;34:1159–1167. doi: 10.1183/09031936.00195708. [DOI] [PubMed] [Google Scholar]
- 23.Jianglong H., Hongbo W., Guili C., Zongheng Z., Weiping G., Tufeng C., Yong H., Fucheng Z. The effect of all-trans retinoic acid (ATRA) on the expression of Vascular Endothelial Growth Factor (VEGF) and VEGF receptors of human colon cancer LoVo cell line. Afr. J. Biotechnol. 2011;10:12326–12332. [Google Scholar]
- 24.Schenk T., Stengel S., Zelent A. Unlocking the potential of retinoic acid in anticancer therapy. Br. J. Cancer. 2014;111:2039–2045. doi: 10.1038/bjc.2014.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith S., Sepkovic D., Bradlow H.L., Auborn K.J. 3,3′-Diindolylmethane and genistein decrease the adverse effects of estrogen in LNCaP and PC-3 prostate cancer cells. J. Nutr. 2008;138:2379–2385. doi: 10.3945/jn.108.090993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Y.K., Gamra I., Davenport A., Lester R., Zhao L., Wei Y. Genistein induces cytochrome P450 1B1 gene expression and cell proliferation in human breast cancer MCF-7 Cells. J. Environ. Pathol. Toxicol. Oncol. 2015;34:153–159. doi: 10.1615/jenvironpatholtoxicoloncol.2015013315. [DOI] [PubMed] [Google Scholar]
- 27.Bai J., Sata N., Nagai H., Wada T., Yoshida K., Mano H., Sata F., Kishi R. Genistein-induced changes in gene expression in Panc 1 cells at physiological concentrations of genistein. Pancreas. 2004;29:93–98. doi: 10.1097/00006676-200408000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 29.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., Vandesompele J., Wittwer C.T. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsunawa M., Amano Y., Endo K., Uno S., Sakaki T., Yamada S., Makishima M. The aryl hydrocarbon receptor activator benzo[a]pyrene enhances vitamin D3 catabolism in macrophages. Toxicol. Sci. 2009;109:50–58. doi: 10.1093/toxsci/kfp044. [DOI] [PubMed] [Google Scholar]
- 32.Matsunaga N.I.M., Kusunose N., Kakimoto K., Hamamura K., Hanada Y., Toi A., Yoshiyama Y., Sato F., Fujimoto K., Koyanagi S., Ohdo S. Time-dependent interaction between differentiated embryo chondrocyte-2 and CCAAT/enhancer-binding protein α underlies the circadian expression of CYP2D6 in serum-shocked HepG2 cells. Mol. Pharmacol. 2012;81:739–747. doi: 10.1124/mol.111.076406. [DOI] [PubMed] [Google Scholar]
- 33.Haufroid V., Toubeau F., Clippe A., Buysschaert M., Gala J.L., Lison D. Real-time quantification of cytochrome P4502E1 mRNA in human peripheral blood lymphocytes by reverse transcription-PCR: method and practical application. Clin. Chem. 2001;47:1126–1129. [PubMed] [Google Scholar]
- 34.Niemira M., Dastych J., Mazerska Z. Pregnane X receptor dependent up-regulation of CYP2C9 and CYP3A4 in tumor cells by antitumor acridine agents, C-1748 and C-1305, selectively diminished under hypoxia. Biochem. Pharmacol. 2013;86:231–241. doi: 10.1016/j.bcp.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Sugaya S., Nakanishi H., Tanzawa H., Sugita K., Kita K., Suzuki N. Down-regulation of SMT3A gene expression in association with DNA synthesis induction after X-ray irradiation in nevoid basal cell carcinoma syndrome (NBCCS) cells. Mutat. Res. 2005;578:327–332. doi: 10.1016/j.mrfmmm.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 36.Pfaffl M.W., Horgan G.W., Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramakers C., Ruijter J.M., Deprez R.H., Moorman A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 38.Klein C.B., King A.A. Genistein genotoxicity: Critical considerations of in vitro exposure dose. Toxicol. Appl. Pharmacol. 2007;224:1–11. doi: 10.1016/j.taap.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 39.Sergent T., Garsou S., Schaut A., De Saeger S., Pussemier L., Van Peteghem C., Larondelle Y., Schneider Y.J. Differential modulation of ochratoxin A absorption across Caco-2 cells by dietary polyphenols, used at realistic intestinal concentrations. Toxicol. Lett. 2005;159:60–70. doi: 10.1016/j.toxlet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Perepechaeva M.L., Grishanova A.Y. In vivo effects of genistein, herbimycin a and geldanamycin on rat hepatic cytochrome P4501A. J. Biophys. Chem. 2012;3:334–340. [Google Scholar]
- 41.Lavigne J.A., Takahashi Y., Chandramouli G.V., Liu H., Perkins S.N., Hursting S.D., Wang T.T. Concentration-dependent effects of genistein on global gene expression in MCF-7 breast cancer cells: an oligo microarray study. Breast Cancer Res. Treat. 2008;110:85–98. doi: 10.1007/s10549-007-9705-6. [DOI] [PubMed] [Google Scholar]
- 42.Arroo R.R.J., Androutsopoulos V., Surichan A.P.E.S., Wilsher N., Potter G.A. Phytoestrogens as natural prodrugs in cancer prevention: A novel concept. Phytochem. Rev. 2008;7:431–443. [Google Scholar]
- 43.McFadyen M.C., Melvin W.T., Murray G.I. Cytochrome P450 enzymes: novel options for cancer therapeutics. Mol. Cancer Ther. 2004;3:363–371. [PubMed] [Google Scholar]
- 44.McFadyen M.C., McLeod H.L., Jackson F.C., Melvin W.T., Doehmer J., Murray G.I. Cytochrome P450 CYP1B1 protein expression: A novel mechanism of anticancer drug resistance. Biochem. Pharmacol. 2001;62:207–212. doi: 10.1016/s0006-2952(01)00643-8. [DOI] [PubMed] [Google Scholar]
- 45.Uno S., Dalton T.P., Derkenne S., Curran C.P., Miller M.L., Shertzer H.G., Nebert D.W. Oral exposure to benzopyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol. Pharmacol. 2004;65:1225–1237. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- 46.Kyselova Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip. Toxicol. 2011;4:173–183. doi: 10.2478/v10102-011-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou S., Gao Y., Jiang W., Huang M., Xu A., Paxton J.W. Interactions of herbs with cytochrome P450. Drug Metab. Rev. 2003;35:35–98. doi: 10.1081/dmr-120018248. [DOI] [PubMed] [Google Scholar]
- 48.Brauch H., Murdter T.E., Eichelbaum M., Schwab M. Pharmacogenomics of tamoxifen therapy. Clin. Chem. 2009;55:1770–1782. doi: 10.1373/clinchem.2008.121756. [DOI] [PubMed] [Google Scholar]
- 49.Jones J.L., Daley B.J., Enderson B.L., Zhou J.R., Karlstad M.D. Genistein inhibits tamoxifen effects on cell proliferation and cell cycle arrest in T47D breast cancer cells. Am. Surg. 2002;68:575–577. [PubMed] [Google Scholar]
- 50.Ju Y.H., Doerge D.R., Allred K.F., Allred C.D., Helferich W.G. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002;62:2474–2477. [PubMed] [Google Scholar]
- 51.Tanos V., Brzezinski A., Drize O., Strauss N., Peretz T. Synergistic inhibitory effects of genistein and tamoxifen on human dysplastic and malignant epithelial breast cells in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002;102:188–194. doi: 10.1016/s0301-2115(01)00582-6. [DOI] [PubMed] [Google Scholar]
- 52.Mai Z., Blackburn G.L., Zhou J.R. Soy phytochemicals synergistically enhance the preventive effect of tamoxifen on the growth of estrogen-dependent human breast carcinoma in mice. Carcinogenesis. 2007;28:1217–1223. doi: 10.1093/carcin/bgm004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Njar V.C., Gediya L., Purushottamachar P., Chopra P., Vasaitis T.S., Khandelwal A., Mehta J., Huynh C., Belosay A., Patel J. Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases. Bioorg. Med. Chem. 2006;14:4323–4340. doi: 10.1016/j.bmc.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 54.Niles R.M. Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutat. Res. 2004;555:81–96. doi: 10.1016/j.mrfmmm.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Osanai M., Petkovich M. Expression of the retinoic acid-metabolizing enzyme CYP26A1 limits programmed cell death. Mol. Pharmacol. 2005;67:1808–1817. doi: 10.1124/mol.104.005769. [DOI] [PubMed] [Google Scholar]
- 56.Arrieta O., Gonzalez-De la Rosa C.H., Arechaga-Ocampo E., Villanueva-Rodriguez G., Ceron-Lizarraga T.L., Martinez-Barrera L., Vazquez-Manriquez M.E., Rios-Trejo M.A., Alvarez-Avitia M.A., Hernandez-Pedro N., Rojas-Marin C., De la Garza J. Randomized phase II trial of All-trans-retinoic acid with chemotherapy based on paclitaxel and cisplatin as first-line treatment in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28:3463–3471. doi: 10.1200/JCO.2009.26.6452. [DOI] [PubMed] [Google Scholar]
- 57.Das S., Guha I., Chatterjee A., Banerji A. Anti-cancer Potential of All-trans Retinoic Acid (ATRA): A review. Proc. Zool. Soc. 2013;66:1–7. [Google Scholar]
- 58.Nelson C.H., Buttrick B.R., Isoherranen N. Therapeutic potential of the inhibition of the retinoic acid hydroxylases CYP26A1 and CYP26B1 by xenobiotics. Curr. Top. Med. Chem. 2013;13:1402–1428. doi: 10.2174/1568026611313120004. [DOI] [PMC free article] [PubMed] [Google Scholar]