Abstract
Early diagnosis improves survival of females with breast cancer. Mammographic screening improves early diagnosis of breast cancer. And yet, there appears to be room for improvement. Major shortcomings of mammographic screening are overdiagnosis of prognostically unimportant cancer, as well as underdiagnosis of cancers that are indeed relevant. Failure to detect biologically relevant breast cancer with mammographic screening is driven not only by host-related factors, i.e. breast tissue density, but also by tumour-related factors: Biologically relevant cancers may exhibit imaging features that render them indistinguishable from normal or benign breast tissue on mammography. These cancers will then progress to become the advanced-stage interval cancers observed in females undergoing mammographic screening. Since breast cancer continues to represent a major cause of cancer death in females, the search for improved breast cancer screening method continues. Abbreviated breast MRI has been proposed for this purpose because it will greatly reduce the cost associated with this method, due to a greatly reduced magnet time (down to 3 min), but especially also due to a greatly abridged image interpretation time, i.e. radiologist reading time. This commentary reviews the current situation and presents the EA1141 trial designed to investigate the utility of abbreviated breast MRI for screening average-risk females with dense breast tissue.
Background: The clinical need for improved breast cancer screening methods
Breast cancer continues to represent one of the major causes of cancer death in the female population.1 Improved understanding of the biologic heterogeneity of breast cancer and of its molecular determinants has led to considerably refined treatment strategies that are targeted to specific genomic tumour features, and that are better tailored to the individual patient’s need for treatment. Introduction of population-wide mammographic screening programmes have improved early diagnosis of breast cancer. Both, improved breast cancer treatment efficacy, as well as improved early diagnosis, do contribute to the reduced breast cancer mortality observed in western countries over the past decade.2–4
Mammography is the mainstay of breast cancer screening; it is broadly available, with established quality assurance, and has been tested within prospective randomized trials. Based on long-term follow up of females participating in mammographic screening trials conducted in the last century, there is compelling evidence that mammography, through early diagnosis of breast cancer, will improve survival.1,5
Major points of criticisms risen against mammographic screening relate to overdiagnosis of biologically irrelevant, and underdiagnosis of biologically important cancer.6,7 The latter is evidenced by the fact that, in spite of long-standing mammographic screening programmes, breast cancer continues to be a major cause of cancer death. This, in turn, will be due to incomplete participation rates in mammographic screening programmes, as well as high interval cancer rates—i.e. a high rate of females in whom early diagnosis by mammographic screening fails.
Failure to detect biologically relevant breast cancer with mammographic screening is driven not only by host-related factors, i.e. the individual female’s mammographic breast density, but also by tumour-related factors, i.e. the individual cancer’s genotype and thus its imaging phenotype: Biologically relevant cancers may exhibit imaging features that renders them indistinguishable from normal or benign breast tissue on mammography. These cancers will then progress to become the advanced-stage interval cancers observed in females undergoing mammographic screening.8
Breast ultrasound, or automated whole-breast ultrasound, has been shown to improve cancer detection rate in females with dense breast tissue. However, it is associated with long examination or image reviewing times, and a low PPV. So although ultrasound per se is an inexpensive test, it is associated with significant direct and downstream costs. Moreover, additional cancer detection rate is moderate, ranging from 3.5 to 4.4 per 1000.9
Digital breast tomosynthesis has been proposed to improve breast cancer detection in females with dense breasts; however, the additional cancer detection rate is modest, with an average 1.2 per 1000. Moreover, and more importantly, although several large-scale tomosynthesis screening trials have been published over the past years, none of these trials report on the biologic profiles of cancers detected through tomosynthesis, or investigated its clinical impact, i.e. regarding its efficacy in reducing interval cancer rates, or shift of cancer stage distribution.10
Breast MRI has repeatedly been shown to represent the most sensitive imaging method for diagnosing breast cancer regardless of stage (DCIS or invasive) or type, and regardless of radiographic breast density. The major advantage of MRI, however, is the fact that it improves detection of biologically relevant cancers, i.e. cancers with active angiogenic activity. Although it has so far been mainly recommended for screening females at high risk of breast cancer, there is increasing evidence to suggest that it appears to be equally useful to improve cancer detection rate in females at average risk. The additional cancer detection rate afforded by supplemental MRI screening in average risk females was unexpectedly high, with 15.5 per 1000.11 Most importantly, the interval cancer rate of females undergoing MRI screening was reduced to zero. This was similar to findings previously reported for females at increased risk of breast cancer.12 The more or less complete absence of interval cancers observed with MRI screening of females at average or even at increased risk of breast cancer suggests that interval cancers do not, or not mostly, develop in between screening rounds, but are present, yet missed by current screening methods, i.e. mammography. In other words: screening MRI detects cancers which, if MRI had not been done, would have progressed to become the more advanced interval cancers observed in females participating in usual mammographic screening. Since interval cancers are known to be associated with an adverse biologic profile,10,13 this observation is another piece of evidence for the fact that mammographic breast cancer detection fails especially in many biologically important cancers. This is the reverse side of a well-known phenomenon called “length time bias”, i.e. the observation that mammographic detectability of a cancer is associated with improved prognosis of a breast cancer. Breast MRI, by way of contrast, preferentially detects cancers with adverse biologic profile due to their increased perfusion.14
Overdiagnosis is a major concern with mammographic screening. Additional cancers found by supplemental imaging. The low (absent) interval cancer rate associated with breast MRI screening indicates that the additional cancers found by supplemental MRI will not, or not for the majority, constitute overdiagnosis.
So although breast MRI appears to be a useful screening test for females regardless of their personal risk, it is currently only used in a minority of high risk females. The many reservations against a more widespread use of MRI for screening include its high cost, its limited availability and its false positive rate. However, the false positive rate, or PPV3 (positive predictive value) of MRI screening of recent trial results, has been shown to be comparable to that observed for screening mammography.
The concept of abbreviated breast MRI
To reduce cost associated with image acquisition and image interpretation of screening breast MRI, abbreviated protocols have been proposed. The method was introduced by our group in 2014, when we published results of a prospective clinical study on the use of abbreviated breast MRI in 443 females at average or mildly increased risk who underwent 606 MRI screening rounds.15 Key components of the proposed method was (a) to reduce the women’s magnet time by acquiring only one pre-contrast and one post-contrast T1 weighted image set, and (b) to use maximum intensity projections (MIP) to fuse the first post-contrast subtracted images into one single high-contrast image. Concept was to use these MIP images for a very fast overview to check for presence or absence of significant enhancement, and then to use the individual subtracted images for further categorization of possible enhancement seen on the MIP image. Further categorization of enhancement was achieved by assessing its configuration, morphology, margins and internal architecture. Since all of these features are best evaluated in the early post-contrast phase, working without late post-contrast images was not considered problematic. Although no complete dynamic series was acquired, the MIPs and the respective first post-contrast subtracted images provide implicit kinetic information because they are obtained immediately after contrast injection, such that all enhancement seen on these images corresponds to fast enhancement. In difficult cases, further classification of enhancement was achieved based on the review of the respective non-subtracted T1 weighted pre- and post-contrast source images because they provide useful information on breast architecture at the site of the lesion.
With the abbreviated protocol, radiologists reading time was below 3 s for interpretation of a MIP, i.e. deciding upon presence or absence of significant enhancement, and below 30 s for the interpretation of the complete study in cases where there was significant enhancement on the MIPs. These values are competitive with batch reading of screening mammograms, and are substantially shorter than the time it takes to review tomosynthesis images.
Since in a screening situation, the vast majority of participants will not harbour breast cancer, and the respective imaging studies will be normal, the time it takes to establish absence of breast cancer will be the single most important metric for cost associated with screening. The specific advantage of abbreviated breast MRI is that establishing absence of breast cancer—in other words: reviewing a negative MIP image—is a simple task that is done within time which is counted in seconds. This is explainable by the fact that MIPs are high-contrast images, whereas with mammographic or tomosynthesis screening, radiologists need to meticulously search low-contrast images for subtle signs of breast cancer.
With the abbreviated protocol, we were able to achieve the same cancer detection rate of 18.2 per 1000, and the same sensitivity as that achieved with the full multiparametric protocol (Figure 1). Although our expectation was that using less information for lesion classification would result in reduced specificity and PPV, the results after interpretation of the abbreviated protocol were similar or even (not statistically significantly) better than those achieved after reviewing the full multiparametric protocol.
Figure 1.
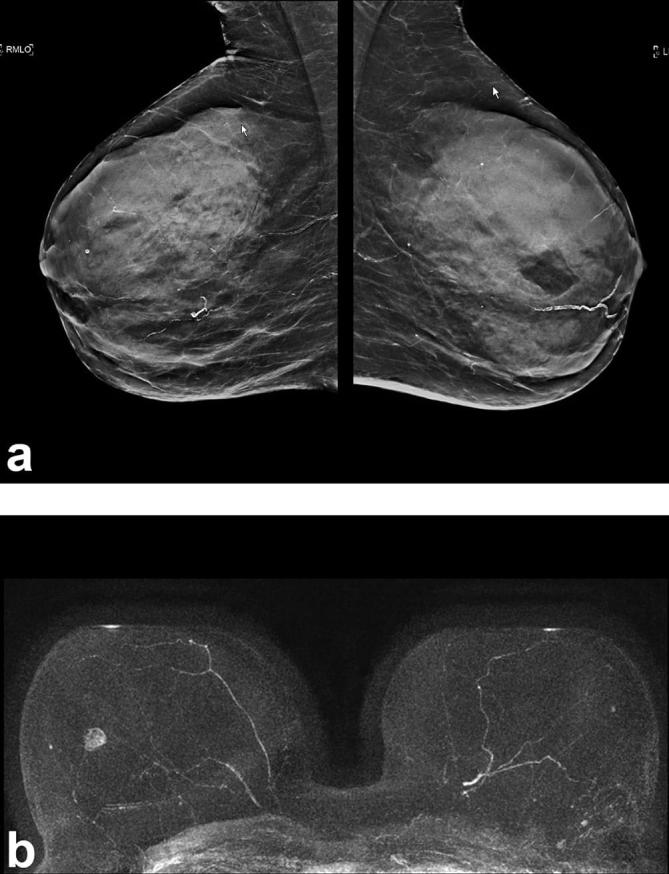
(a) C-view of a breast tomosynthesis study, MLO view, of a 55-year-old female at average risk with dense breast tissue, interpreted as negative (BIRADS2). (b) The corresponding MIP image of the abbreviated breast MRI study is positive for significant enhancement which corresponded to a small invasive breast cancer. MIP, maximum intensity projections; MLO, mediolateral oblique.
Meanwhile, several groups have replicated our results by using abbreviated breast MRI protocols. All studies are concordant in that a comparable diagnostic accuracy is achieved.16–27
The promising and concordant results of several single-centre studies on the utility of abbreviated breast MRI, together with the urge to find adequate supplemental breast cancer screening methods in view of the breast density legislation in the USA, have prompted the proposal of a multicentre study that investigates the utility of a low-cost, abbreviated breast MRI as a supplemental screening test for females with dense breast tissue.
The EA1141 trial
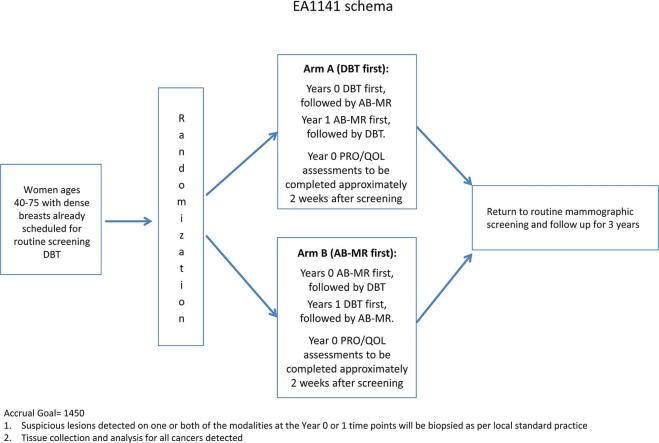
The so-called EA1141 study entitled “Comparison of Abbreviated Breast MRI and Digital Breast Tomosynthesis in Breast Cancer Screening in Women with Dense Breasts”28 is designed as a prospective multicentre diagnostic accuracy study and sponsored by ECOG/ACRIN. Asymptomatic females aged 40–75 with dense breast tissue, defined as mammographic density categories C and D, and without breast cancer-related risk factors, will undergo digital breast tomosynthesis and abbreviated breast MRI in randomized order for 2 consecutive years; females will then return to regular mammographic screening and followed for another 3 years (Figure 2). Metric will be the cancer detection rate achieved by digital breast tomosynthesis vs abbreviated breast MRI in the same females in the first (prevalence) and second (incidence) screening round. Secondary objectives are to compare the PPVs and—and most importantly—the type of cancers found by the two competing imaging methods. The latter will be done by investigating the differences of the biological detection profiles of tomosynthesis vs AB-MRI. The biological importance will be interrogated by genomic profiling studies of screen detected cancers (PAM50 for invasive disease and DCIS score for intraductal cancer). Since breast MRI has the reputation of being a strain to females, the study will also investigate patient-reported quality of life, as well as their willingness to return for repeat AB-MRI or tomosynthesis after the first study screening round.
Figure 2.
Flow chart of the EA1141 study. AB-MR, abbreviated breast MRI. DBT, digital breast tomosynthesis. PRO, patient reported outcomes; QOL, quality of life.
Assuming that inadequate information will be available on up to 6% of cases, we expect that a sample size of 1450 females will provide 90% power to compare the diagnostic yield regarding invasive cancer detection rates of tomosynthesis vs AB-MRI.
The study was initiated in November 2016; it is open for participation of international sites and will include both academic as well as community level radiologists. At the time this article was written, a total of 30 sites were open for accrual. Quality assurance is achieved through an in-depth accreditation process that also involves a test of reader expertise in interpreting breast MRI studies. Moreover, interpretation guidelines have been set up and are used throughout the EA1141 consortium.
Regarding pulse sequence protocol, this trial uses a relatively liberal definition of “abbreviated” breast MRI in that the protocol requires an acquisition time of less than 10 min. Instead of imposing a specific pulse sequence to be used, this trial leaves it up to the individual site to use their respective known pulse sequence, and sets a corridor of acquisition parameters regarding contrast and spatial resolution.
This also includes the time of centre of k-space sampling for the post-contrast acquisition k-space centre passage. Technique in terms of contrast and A comparable image quality is achieved. Every site is able to use its established technology as long as specific. The concept to fuse subtracted images and generate MIPs, as well as investigating radiologist reading time is not addressed in this trial.
While in this trial, abbreviated MRI will be used as a supplemental screening tool (supplemental to digital breast tomosynthesis), future studies will investigate the utility of abbreviated breast MRI as a stand-alone breast cancer screening method. In view of the limited or absent cancer yield of mammography in females undergoing MRI for screening observed in high risk13,29–31 or average risk females15 a prospective study investigating the use of abbreviated MRI as a stand-alone screening method is currently in preparation.
Investigating the use of MRI alone as a screening method will also address another important issue: Overdiagnosis of breast cancer by mammographic screening is driven by detection of low- or intermediate grade DCIS, usually through detection of microcalcifications. MRI is an imaging method that is unable to detect microcalcifications, and, as such, may remain negative in females with low-grade DCIS. Therefore, using MRI alone as an imaging method can be expected to help avoid diagnosis of such low-grade disease. Therefore, there is a reason to assume that screening by MRI alone could be useful not only to avoid underdiagnosis of biologically important cancer, but also to avoid overdiagnosis of unimportant disease—quite similar to the way multiparametric MRI of the prostate is used to improve detection of significant prostate cancer, and help avoid detection of insignificant disease.32
Conclusion
In spite of decades of mammographic screening, breast cancer continues to represent a major medical and socio-economic challenge. Mammography is a good test with proven outcomes for breast cancer screening, but has well-established limitations. Based on current evidence, these limitations are only marginally addressed with improved radiographic imaging such as digital mammography or digital breast tomosynthesis. Aim of contemporary breast cancer screening is not to simply increase the number of cancers detected—but to improve and ensure early detection of biologically important breast cancer. If combined with dedicated MRI systems that are optimized for breast imaging, and that ensure fast patient throughput, abbreviated breast MRI could be a viable alternative for population-wide screening.
References
- 1.Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380: 1778–86. [DOI] [PubMed] [Google Scholar]
- 2.Njor SH, Schwartz W, Blichert-Toft M, Lynge E. Decline in breast cancer mortality: how much is attributable to screening? J Med Screen 2015; 22: 20–7. [DOI] [PubMed] [Google Scholar]
- 3.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005; 353: 1784–92. [DOI] [PubMed] [Google Scholar]
- 4.Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst 2014; 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers ER, Moorman P, Gierisch JM, Havrilesky LJ, Grimm LJ, Ghate S, et al. Benefits and harms of breast cancer screening: a Systematic review. JAMA 2015; 314: 1615–34. [DOI] [PubMed] [Google Scholar]
- 6.Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 2016; 375: 1438–47. [DOI] [PubMed] [Google Scholar]
- 7.Berg WA. Current status of supplemental screening in dense breasts. J Clin Oncol 2016; 34: 1840–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksson L, Czene K, Rosenberg LU, Törnberg S, Humphreys K, Hall P. Mammographic density and survival in interval breast cancers. Breast Cancer Res 2013; 15: R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast Cancer. JAMA 2008; 299: 2151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, et al. Breast-cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311: 2499–507. [DOI] [PubMed] [Google Scholar]
- 11.Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology 2017; 283: 361–70. [DOI] [PubMed] [Google Scholar]
- 12.Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, König R, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol 2010; 28: 1450–7. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Holm J, Bergh J, Eriksson M, Darabi H, Lindström LS, et al. Breast-cancer genetic risk profile is differentially associated with interval and screen-detected breast cancers. Ann Oncol 2015; 26: 517–22. [DOI] [PubMed] [Google Scholar]
- 14.Sung JS, Stamler S, Brooks J, Kaplan J, Huang T, Dershaw DD, et al. Breast cancers detected at screening MR imaging and mammography in patients at high risk: method of detection reflects tumor histopathologic results. Radiology 2016; 280: 716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol 2014; 32: 2304–10. [DOI] [PubMed] [Google Scholar]
- 16.Petrillo A, Fusco R, Sansone M, Cerbone M, Filice S, Porto A, et al. Abbreviated breast dynamic contrast-enhanced MR imaging for lesion detection and characterization: the experience of an Italian oncologic center. Breast Cancer Res Treat 2017; 164: 401–10. [DOI] [PubMed] [Google Scholar]
- 17.Romeo V, Cuocolo R, Liuzzi R. Preliminary results of a simplified breast MRI Protocol to characterize breast lesions: comparison with a Full Diagnostic Protocol and a review of the Current literature. Acad Radiol Jun 2017; Jun 2017; pii: S1076-6332(17)30202–7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Panigrahi B, Mullen L, Falomo E, Panigrahi B, Harvey S. An Abbreviated protocol for High-risk Screening breast Magnetic resonance imaging: impact on performance metrics and BI-RADS assessment. Acad Radiol Jun 2017; pii: S1076-6332(17)30180–0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Machida Y, Shimauchi A, Kanemaki Y, Igarashi T, Harada M, Fukuma E. Feasibility and potential limitations of abbreviated breast MRI: an observer study using an enriched cohort. Breast Cancer 2017; 24: 411–9. [DOI] [PubMed] [Google Scholar]
- 20.Chen SQ, Huang M, Shen YY, Liu CL, Xu CX, Cx X. Abbreviated MRI protocols for detecting breast cancer in women with dense breasts. Korean J Radiol 2017; 18: 470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat 2017; 162: 283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen SQ, Huang M, Shen YY, Liu CL, Xu CX. Application of abbreviated protocol of magnetic resonance imaging for breast cancer screening in dense breast tissue. Acad Radiol 2017; 24: 316–20. [DOI] [PubMed] [Google Scholar]
- 23.Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L. An abbreviated protocol for high-risk screening breast MRI saves time and resources. J Am Coll Radiol 2016; 13(11S): R74–R80. [DOI] [PubMed] [Google Scholar]
- 24.Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Abbreviated combined MR protocol: a new faster strategy for characterizing breast lesions. Clin Breast Cancer 2016; 16: 207–11. [DOI] [PubMed] [Google Scholar]
- 25.Heacock L, Melsaether AN, Heller SL, Gao Y, Pysarenko KM, Babb JS, et al. Evaluation of a known breast cancer using an abbreviated breast MRI protocol: Correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol 2016; 85: 815–23. [DOI] [PubMed] [Google Scholar]
- 26.Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS. Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol 2015; 22: 1157–62. [DOI] [PubMed] [Google Scholar]
- 27.Mango VL, Morris EA, David Dershaw D, Abramson A, Fry C, Moskowitz CS, et al. Abbreviated protocol for breast MRI: are multiple sequences needed for cancer detection? Eur J Radiol 2015; 84: 65–70. [DOI] [PubMed] [Google Scholar]
- 28.ECOG-ACRIN Cancer Research Group. EA1141—Educational materials. 2017. Available from: http://ecog-acrin.org/clinical-trials/ea1141-educational-materials [10 July 2017]
- 29.Sardanelli F, Podo F, Santoro F, Manoukian S, Bergonzi S, Trecate G, et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the High Breast Cancer Risk Italian 1 Study): final results. Invest Radiol 2011; 46: 94–105. [DOI] [PubMed] [Google Scholar]
- 30.Riedl CC, Luft N, Bernhart C, Weber M, Bernathova M, Tea MK, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol 2015; 33: 1128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Zelst JCM, Mus RDM, Woldringh G, Rutten MJCM, Bult P, Vreemann S. Surveillance of women with the BRCA 1 or BRCA 2 mutation by using biannual automated breast US, MR imaging, and mammography. Radiology 2017; 13: 161218. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–22. [DOI] [PubMed] [Google Scholar]