Abstract
Perceptual learning is an enhancement in discriminability of similar stimuli following experience with those stimuli. Here, we examined the efficacy of adding additional active training following a standard training session, compared with additional stimulus exposure in the absence of associated task performance. Mice were trained daily in an odor-discrimination task, and then, several hours later each day, received 1 of 3 different manipulations: 1) a second active-training session, 2) non-task-related odor exposure in the home cage, or 3) no second session. For home-cage exposure, odorants were presented in small tubes that mice could sniff and investigate for a similar period of time as in the active discrimination task each day. The results demonstrate that daily home-cage exposure was equivalent to active odor training in supporting improved odor discrimination. Daily home-cage exposure to odorants that did not match those used in the active task did not improve learning, yielding outcomes similar to those obtained with no second session. Piriform cortical local field potential recordings revealed that both sampling in the active learning task and investigation in the home cage evoked similar beta band oscillatory activity. Together the results suggest that odor-discrimination learning can be significantly enhanced by addition of odor exposure outside of the active training task, potentially because of the robust activity evoked in the olfactory system by both exposure paradigms. They further suggest that odorant exposure alone could enhance or maintain odor-discrimination abilities in conditions associated with olfactory impairment, such as aging or dementia.
Keywords: odor discrimination, olfactory cortex, passive odor exposure, perceptual learning, piriform cortex, state-dependent learning
Introduction
Perceptual learning refers to long-lasting changes in perception and discriminability of sensory inputs because of experience (Gibson 1963). It has been described in all sensory systems (Fahle and Poggio 2001). Although most sensory discrimination protocols involve explicit instrumental conditioning paradigms with reward or feedback for performance, recent evidence suggests that exposure to stimuli in the absence of associated task performance (non-task-related exposure) can also enhance discrimination (Seitz and Watanabe 2005; Mandairon et al. 2006a; Escanilla et al. 2008; Wright et al. 2010; Beste and Dinse 2013; Green et al. 2016). For example, humans performing an active auditory frequency-discrimination task can improve their auditory discrimination abilities for those frequencies given sufficient training (Wright and Sabin 2007). However, combining minimal active training, that in itself is insufficient to induce perceptual learning, with non-task-related exposure to those same frequencies as background stimulation while the subjects performed an unrelated written task, induced perceptual learning (Wright et al. 2010). Thus, non-task-related exposure to the tones facilitated auditory perceptual learning. Here, we compared the efficacy of non-task-related exposure and active training in inducing perceptual learning in the olfactory system.
In olfaction, both active conditioning and stimulus exposure without associated task performance can shape the anatomy and physiology of the olfactory system, as well as modify odor discrimination. Active perceptual training can yield enhanced olfactory acuity and odor discrimination in both humans (Rabin 1988) and rodents (Gheusi et al. 2000; Fletcher and Wilson 2002; Mandairon et al. 2006a). This learning involves modifications throughout the olfactory pathway. Changes in physiology and/or anatomy induced by odor experience and learning have been demonstrated as early in the pathway as the olfactory sensory neuron input to the olfactory bulb (Kass et al. 2013), as well as within the olfactory bulb (Fletcher and Wilson 2002; Alonso et al. 2006; Mandairon et al. 2008; Fletcher 2012), within the piriform cortex (PCX) (Litaudon et al. 1997; Barnes et al. 2008; Chapuis and Wilson 2011), and in the connectivity throughout a broader network beyond the olfactory system itself (Kay and Freeman 1998; Chapuis et al. 2009; Cohen et al. 2015). The nature of these changes in circuit function can depend on the difficulty of the discrimination (Beshel et al. 2007; Chapuis and Wilson 2011), the specific task involved (Chapuis et al. 2009; Chen et al. 2011; Frederick et al. 2011; Martin and Ravel 2014; Frederick et al. 2016), and the duration of the discrimination memory (Chapuis and Wilson 2011; Saar et al. 2012). Likewise, non-task-related odor exposure can improve odor discrimination (Mandairon et al. 2006a, 2006b; Escanilla et al. 2008) as well as modify olfactory sensory neuron activity (Kass et al. 2016), olfactory bulb evoked activity (Buonviso and Chaput 2000; Fletcher and Wilson 2003; Mandairon et al. 2008, 2018; Ross and Fletcher 2018), and PCX cortical single-unit receptive fields (Wilson 2003, 2010).
Given that both non-task-related exposure and active conditioning can produce neural and behavioral changes, here we explored the relative efficacy of these 2 different forms of olfactory experience. Specifically, we were interested in 1) whether non-task-related exposure induced the same magnitude of perceptual learning as odor-reward association, 2) whether changes in active odor-discrimination performance that were induced by non-task-related odor exploration were selective to the non-task-related experienced odor, and 3) whether non-task-related odor exploration activated the PCX as strongly as odor sampling during active training. To address these questions, we quantified the value of similar temporal periods of non-task-related odor exposure and active instrumental conditioning on odor-discrimination behavior and piriform cortical activity. Mice were initially trained in an odor-discrimination task, allowing acquisition of the procedural learning component of the task. They were then transferred to a new odor pair. During this second phase, one group completed a single active training session each day; a second group completed 2 active sessions each day, separated by several hours; and a third group completed 1 active session followed by 1 non-task-related odor exposure session each day, again separated by several hours. The non-task-related exposure was provided by placing scented tubes in the home cage each day for a similar duration as the daily active training session. Animals were free to investigate the odor tubes in their home cages.
We demonstrate that, at least when provided several hours after an active-training session, non-task-related odor exposure sessions are as effective as additional active-training sessions in enhancing odor discrimination. This enhancement requires that the non-task-related odorants match those used in the active session, even when there are far fewer detectable investigations of the odors during the non-task-related exposure periods compared with the number of trials initiated during active training. Furthermore, stimulus-induced PCX local field potential (LFP) beta band oscillations are as robust during brief periods of non-task-related exposure as during odor sampling in the instrumental task. The similar olfactory system activation during both active and non-task-related odor exposure may underlie the effectiveness of non-task-related exposure to enhance odor discrimination.
Material and methods
Subjects
Subjects (n = 94) were B6SJLF1/J adult male (61%) and female (39%) mice from Jackson Laboratory. Animals were singly housed in polypropylene cages. All handling, housing, and experimental procedures were approved by, and performed in accordance with, the Nathan Kline Institutional Animal Care and Use Committee guidelines at Nathan S. Kline Institute as well as National Institutes of Health guidelines for the proper treatment of animals. Food was available ad lib and water was available ad lib on weekends. On training days, 5 days/week, access to water was restricted to that consumed during the training and an additional 140–180 µL of water following training. Body weight and grooming state were monitored throughout the training regime to ensure health status.
Behavioral training
All animals were trained on a Go-Left, Go-Right odor-discrimination task, denoted the “active task”. For each session, mice were placed in a computer-controlled operant chamber (Voluntis, Inc) with 3 infrared-monitored nose ports in the rear wall (exterior: 7′′ × 6′′ × 5′′; interior: 4.75′′ × 4.75′′ × 4.25′′). The center port was connected to a multichannel olfactometer that delivered odorants on entry of the mouse’s nose into the port. The 2 other ports were reward ports that delivered a water droplet (approximately 20 µL) depending on which of two odors was presented (left port for odor A, right port for odor B). For each trial, mice were required to hold in the center port for 0.4 before exiting to choose a reward port. The mice had a maximum of 3 s to make a choice. All trials were self-initiated. Trials for which the animal either did not hold for a sufficient time in the center, sample port, or failed to make a choice in the allotted time were not included in the analysis of correct trail proportion.
Procedure
Initial active training for all mice was with a vanilla–peppermint odor pair for 30 min per session. After successfully mastering this discrimination (>80% correct), the mice were switched to a new odor pair, marking the onset of the collection of the data presented here.
In Experiment 1, animals were randomly divided into 3 groups: 1) a single active-training session per day, 2) 2 active-training sessions per day with 4–5 h between sessions, or 3) a single active-training session per day and 20 min of non-task-related exposure at least 4 h later. Each active-training session was terminated after 30 min or 60 valid trials (whichever came first). Non-task-related exposure was performed by placing 10 µL of each of the 2 odorants used in the active-training task on separate quarter pieces of Kimwipe in separate microfuge tubes drilled with holes for odor release. The two tubes were placed in the home cages, in diagonally opposite corners, to allow the mice to freely explore and sniff them. During non-task-related exposure all items except bedding were removed from the cages to prevent odor contamination, and the cages were physically isolated from the cages of the other groups to avoid incidental odor exposure between cages. During the non-task-related exposure the animals were not required to perform specific behaviors. They were exposed to the odors when they investigated the tubes and potentially at other times during the exposure period as well.
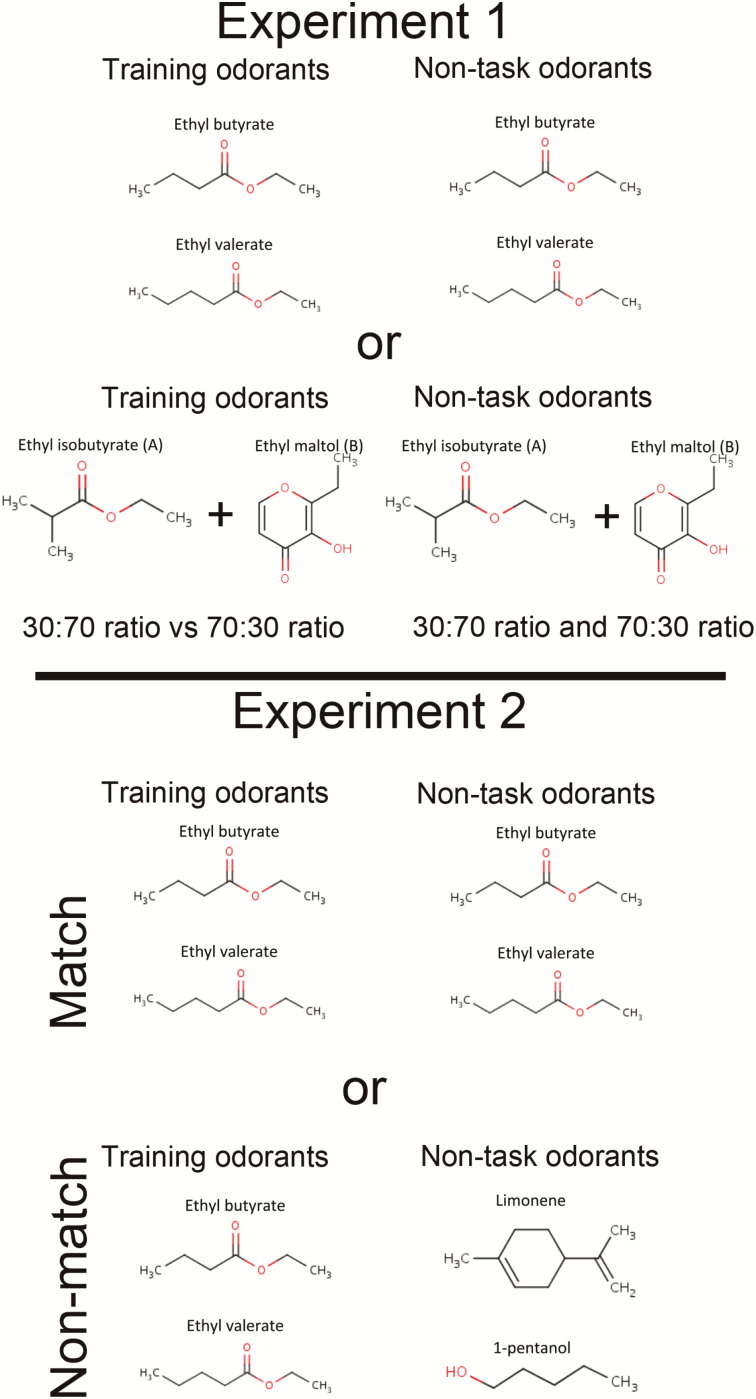
Two different sets of odorants were used in different animals (Figure 1), with the same 2 odorants used during both the active-training task and the non-task-related exposure. For some animals, the odorants were ethyl valerate (vapor pressure = 4.74 mm Hg) and ethyl butyrate (14.0 mm Hg) (Figure 1, top row). For other animals the odorants were 2 different ratios of a binary mixture of ethyl isobutyrate (40 mm Hg) and ethyl maltol (0.00022 mm Hg) (Figure 1, second row). Mixture 1 was a 30:70 ratio of ethyl isobutyrate and ethyl maltol, which has been demonstrated to be perceived configurally (Le Berre et al. 2008). Mixture 2 was a 70:30 ratio of ethyl isobutyrate and ethyl maltol, which has been demonstrated to be perceived elementally (Le Berre et al. 2008). To create the mixtures, component stock solutions were made with 100 mg of ethyl maltol diluted in 10 mL of 100% ethanol, and 100.5 mg of ethyl isobutyrate diluted in 10 mL of 100% ethanol. The 30:70 ratio was then created by mixing 7.5 µL ethyl isobutyrate stock and 17.5 µL ethyl maltol stock in 25 mL dH2O. Likewise, the 70:30 ratio was created by mixing 37.5 µL of ethyl isobutyrate stock and 17.5 µL of ethyl maltol stock in 25 mL H2O. Mice learned to discriminate both the esters and the binary mixtures at the same rates (data not shown), and thus the data from the 2 odorant sets are combined.
Figure 1.
Odorants used in the main experiments reported here. Unless otherwise noted, odorants were used at 100 ppm based on vapor pressure. In Experiment 1, animals were trained in the active-training task with the same set of odorants as were used for the non-task-related exposure in the home cage. Some animals were trained with ethyl esters and some with binary mixtures varying in concentration ratio. Results from the 2 odorant sets were combined for analyses.
All animals received 17–20 days of training. Animals that failed to achieve above 60% correct during the course of training were not included in the final analyses (30 of 55 reached criterion across all groups). The reported data are based on n = 11 in the 1 active-session group (11 of 16 reached criterion), n = 11 in the 2 active-sessions group (11 of 20 reached criterion), and n = 8 in the 1-session plus non-task-related-exposure group (8 of 19 reached criterion).
In Experiment 2, animals were randomly divided into 3 groups: Each received a single active-training session per day and 20 min of non-task-related exposure 4–6 h later with 1) the same odors as used in the active-training task, 2) different odors, or 3) no odors (Figures 1 and 2A). The active-training and non-task-related exposure sessions were performed as described for Experiment 1. For all groups, the active-training odorants were ethyl-valerate and ethyl-butyrate. For the Same-Odors Group, the non-task-related odors were the same 2 odors as used in active training: ethyl-valerate and ethyl-butyrate (Figure 1, third row). For the Different-Odors Group the non-task-related odors differed from the active odors: limonene (1.98 mm Hg) and 1-pentanol (2.2 mm Hg) (Figure 1, bottom row). For the No-Odors Group, the odor tubes were empty (plain Kimwipes). All animals received 20 days of training. Animals that failed to achieve above 60% correct during the course of training were not included in the final analyses (23 of 35 reached criterion across all groups). The reported data are based on n = 8 in the Same-Odors group (8 of 15 reached criterion), n = 9 in the Different-Odors group (9 of 11 reached criterion), and n = 6 in the No-Odors group (6 of 9 reached criterion).
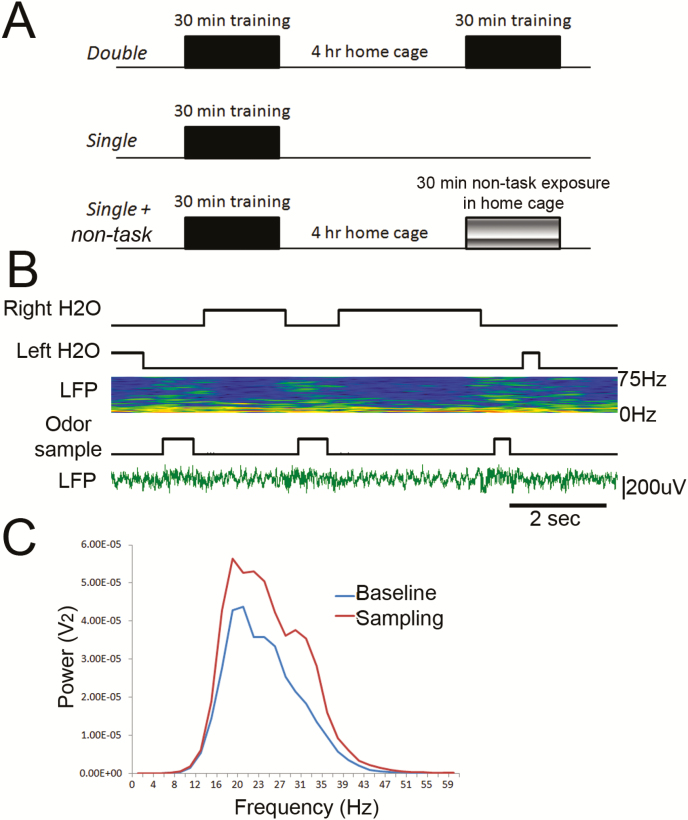
Figure 2.
(A) Depiction of active-training sessions and non-task-related exposure for Experiment 1. The odorants used for active training and non-task-related exposure were the same. A similar design was used for Experiment 2, though whether the non-task-related odorants matched the active-training odorants varied by group (see text). (B) A subset of animals was implanted for telemetered LFP recordings from the anterior PCX. An example of a recording showing elevated beta and gamma band activity during sampling in the odor-discrimination task, along with event markers for odor sampling and water port entries. The pseudocolor spectrogram shows high power in reds and yellows and low power in blues. (C) Example of beta band filtered LFP power averaged over odorant sampling during the odor-discrimination task (rust line) compared with beta power during task disengagement (baseline) in the operant chamber (blue line).
Behavior directed toward the odorants in the active-training and non-task-related sessions was quantified during Experiment 2 over the first 10 days of training. The temporal structure of self-initiated trials during the active-training sessions and of self-initiated investigation of the odorant tubes during the non-task-related sessions was quantified by counting the number of events (trials or nose contacts to the tubes) per minute for animals in each group.
Finally, LFPs (Figure 2B) were recorded in the PCX of a subset of animals (n 7) used in Experiment 2. These animals were originally trained with 1 active-training session and 1 matching non-task-related session per day, and now continued that training for an additional 10 days with odorants to which they had not been previously exposed (limonene and 1-pentanol). As a control for the prior odor training, an additional 4 naive mice were also recorded from during their first exposure to non-task-related odor in the home cage. Odorant/investigation-evoked activity was assessed with fast Fourier transforms (FFTs) of LFP oscillations relative to baseline (a period of at least 30 s without trial or investigation initiation) (Figure 2C). FFTs were initiated at the onsets of active-training trials, and of periods of non-task-related investigation, and extended over 500 ms. Although the mice undergoing training were still performing under criterion at the end of the 10 daily sessions, this period included the most active investigation of the odorant tubes in the home cage and thus allowed the most reliable measure of LFP activity in both conditions. At least 4 days prior to the recorded conditioning sessions, mice were implanted with a telemetry device (model ETA-F10; DSI) connected to a stainless-steel electrode (125 µm) implanted in the anterior PCX. The operant chamber and home cages were placed on top of the telemetry receiver during sessions. Data were acquired at 1 kHz and analyzed with FFT (2 Hz bins) in Spike 2 software (CED, Inc).
All data were analyzed with ANOVA and then with Fisher’s post hoc tests where appropriate. Statistical significance was set at P < 0.05. Normality of the data distributions was confirmed with D’Agostino–Pearson omnibus normality tests using Prism software.
Results
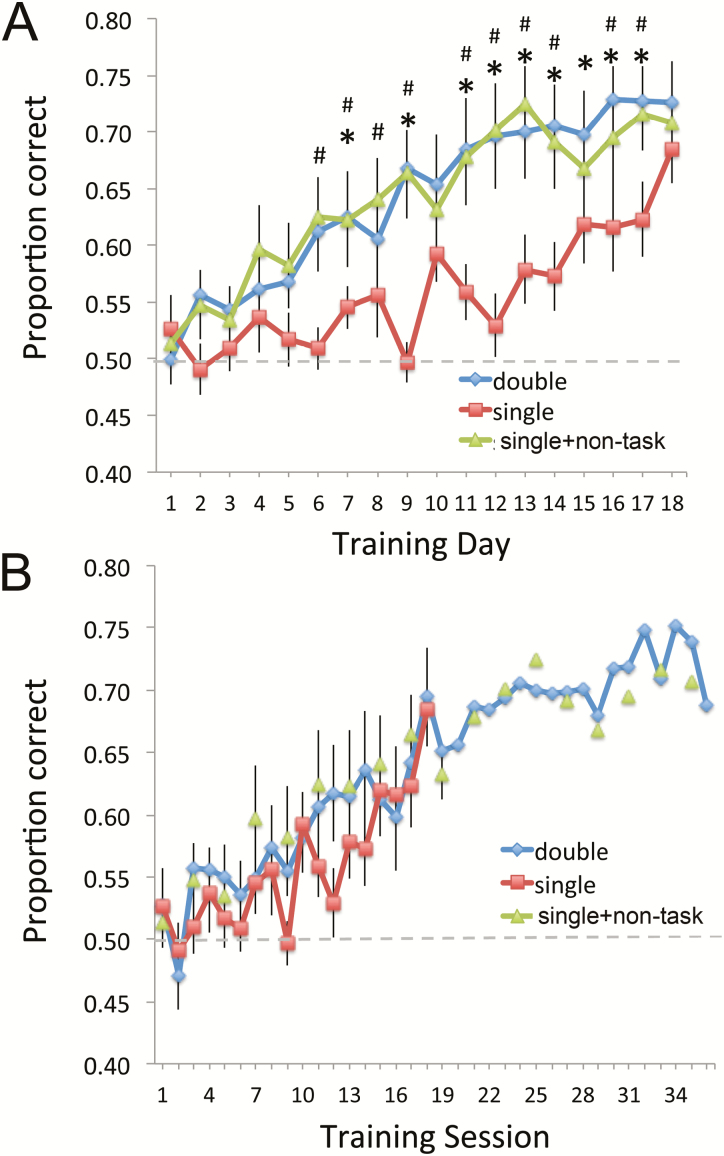
As expected, in Experiment 1, mice trained with 2 active-training sessions per day (ethyl valerate vs. ethyl butyrate or 70:30 vs. 30:70 ratio of ethyl isobutyrate + ethyl maltol) learned significantly faster than mice that received a single active-training session per day (Figure 3). Importantly, however, mice that were trained with a single active-training session per day combined with a non-task-related-exposure session learned comparably to the 2 active-training session mice. A 3 × 20 (group × day) repeated measures ANOVA revealed a significant main effect of day (F(19,380) = 14.22, P < 0.0001), no main effect of group (F(2,380) = 1.85, P = 0.18), and a significant group × day interaction (F(38,380) = 1.93, P = 0.0011). Fisher’s post hoc tests revealed both the 2 active-training session and single active-training session with non-task-related exposure groups performed significantly better than the single active-training session group, though the single-session group did ultimately reach the same level of proficiency (Figure 3A).
Figure 3.
(A) Mean behavioral performance (±standard error of the mean) in the odor-discrimination task over days of training in 3 groups in Experiment 1. Mice trained with 2 active-training sessions per day (diamonds) or with 1 active-training session combined with non-task-related exposure to those same odorants in the home cage (triangles) learned significantly faster than mice trained with 1 active-training session per day (squares). Asterisks signify significant differences between double active-training sessions per day compared with single active-training sessions per day; #s signify significant differences between single active training plus non-task-related exposure compared with single active training alone. (B) The same data plotted by individual session, rather than by day. Mice in the single active-training session plus non-task-related exposure showed the same magnitude of improvement from session to session as the animals receiving 2 active-training sessions per day. The animals trained with 1 active-training session per day also improved at the same rate per session, though had fewer sessions. This figure is reproduced in color in the online version of the issue.
The data shown in Figure 3A are plotted by day, showing the mean performance for each day regardless of the number of training sessions per day. Replotting the data by session, with non-task-related sessions counted as a session but with no accuracy measure, shows that individual sessions in all groups, including non-task-related exposure sessions, were relatively equal in their contribution to performance acquisition (Figure 3B). For example, regressions of each group’s session by session performance had nearly identical slopes (increment in performance per session; 2 active training, slope = 0.006; single active plus non-task-related session, slope = 0.006; single active training, slope = 0.008). This corresponds to a mean 0.6% improvement per session—active or non-task-related—in both the 2 active-training group and the single active-training plus non-task-related exposure session group. Thus, non-task-related home-cage exposure with odorants used in an active-training session facilitates active training odor learning to the same extent as a daily second active-training session.
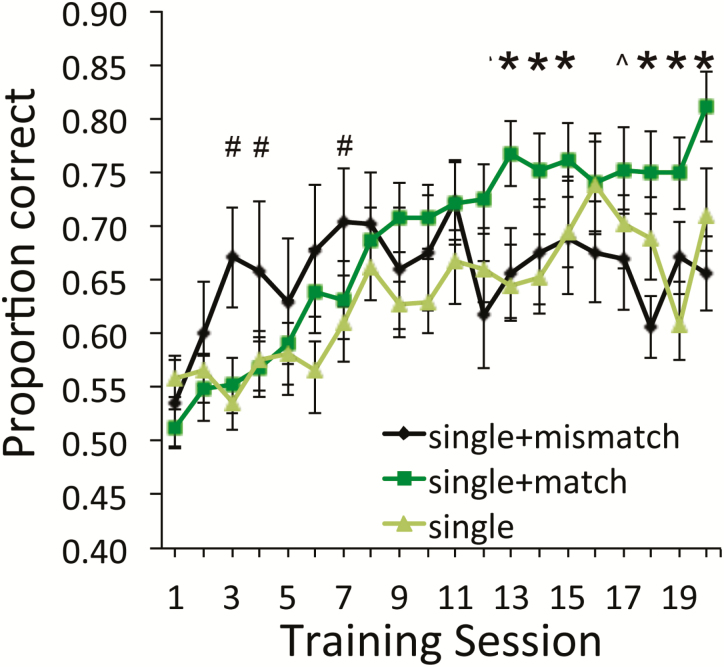
The results of Experiment 2, as shown in Figure 4, suggest that the benefits of non-task-related odorant exposure on active training (ethyl-valerate vs. ethyl-butyrate) require that the non-task-related odorants match the active-training odorants. Animals trained with the same odorants during active training and non-task-related exposure learned faster than animals receiving either a single active-training session per day with no non-task-related exposure or a single active-training session per day and non-task-related exposure to odorants that differed (limonene and 1-pentanol) from those in the active-training session. A repeated measures ANOVA (3 × 20; group × day) showed a main effect of day (F(20,540) = 10.18, P < 0.0001), no main effect of group (F(2,540) = 1.16, P = 0.33), and a significant group × day interaction (F(40,540) = 2.31, P < 0.0001). Fisher’s post hoc tests revealed that although the active-training plus non-task-related odorant-mismatch group had an initial rapid rate of learning, in later sessions, the odorant-matched active-training plus non-task-related exposure-trained animals performed significantly better than both other groups. Furthermore, animals trained with mismatched odorants during active training and non-task-related exposure performed no differently than single active-training session per day trained animals.
Figure 4.
Mean behavioral performance [±standard error of the mean (SEM)] in the active-training task over days of training in the 3 groups in Experiment 2. Mice trained with a single active-training session plus non-task-related exposure to the same odorants (squares) attained significantly better performance than mice trained with a single active-training session and exposed to mismatching odorants (diamonds) or to no non-task-related odorants at all (triangles). #s signify single plus mismatching odorants different from both other groups; asterisks signify single plus matching non-task-related odorants different from both other groups; ^ signifies single plus matching non-task-related odorants different from single plus mismatch only (post hoc Fisher’s tests, P < 0.05). Error bars are ± SEM. This figure is reproduced in color in the online version of the issue.
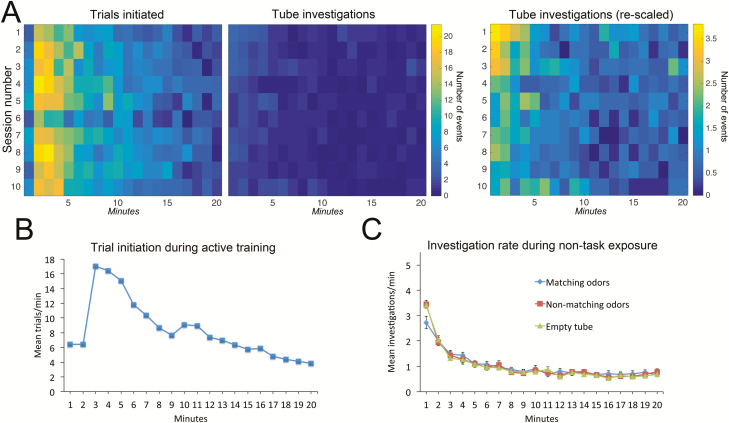
We also compared the number of direct odorant exposures within daily sessions between the active-training and non-task-related exposure in Experiment 2 (Figure 5). Direct odorant exposure (number of exposures per minute within a session) was far greater during active training than during non-task-related home-cage exposure (Figure 5A). In both cases, trials initiated and investigation events were highest during the first 5 min and then rapidly declined, though mice initiated more than 5 times the number of active-training trials within the operant sessions than direct investigation of the scented tubes during the non-task-related home-cage exposure sessions (Figure 5B and C). Furthermore, there was no main effect of group on non-task-related home-cage odor investigation among animals exposed to odorants that matched the active-training session, odorants that did not match the active-training session, or tubes that were not intentionally scented (data from Experiment 2: group × time repeated measures ANOVA; main effect of group F(2,513) = 0.03, P = 0.97; main effect of time within a session F(19,513) = 125.7, P < 0.0001). Although there was a significant group × time interaction (F(38,513) = 1.69, P = 0.007), this was due to mice investigating the matching odorants significantly less than either the mismatching odorants or the unscented tubes during the first minute of non-task-related home-cage exposure. The mean duration of an odor investigation event in the home cage did not significantly vary between groups, with an overall mean of 3.03 ± 0.18 s/investigation (data not shown). This suggests that relatively limited non-task-related odorant exposure is sufficient to facilitate odor learning.
Figure 5.
Mice had far fewer direct exposures to the non-task-related presented odorants than odorant exposure during the active-training task. (A) Pseudocolor plots of mean trials per minute (active-training) (left panel) and investigations per minute (non-task-related home-cage exposure) (right panels) across 10 days of training (session number). In both paradigms, odorant exposures occurred primarily within the first 5 min of a session. (B) Mean number of trials per minute in the active-training task (same data as in pseudocolor plot). (C) Mean number of investigations of non-task-related odor tubes in the home cage for mice exposed to the same odorants as in the active-training task (blue diamonds), mice exposed to odorants mismatched from the active-training odorants (rust squares) and mice exposed to unscented tubes (green triangles).
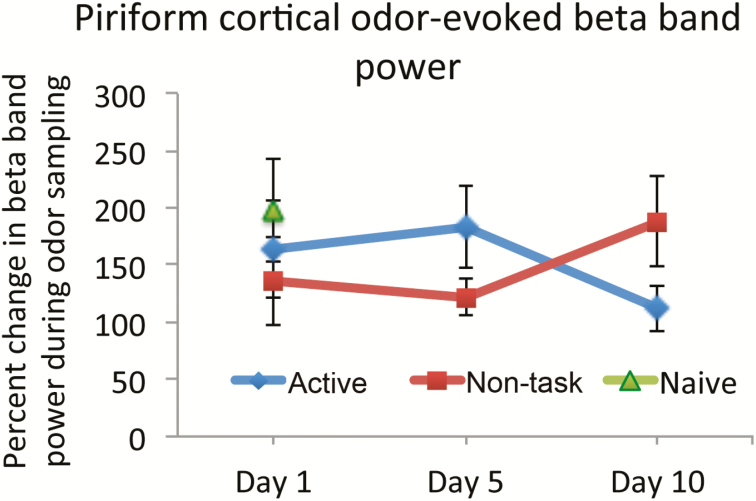
Finally, we examined whether PCX LFP activity differed between home-cage non-task-related odorant exposure and active training. Did active training evoke a unique LFP response that was not detected during non-task-related odor exposure while in the home cage? Animals previously trained using the single active-training session plus non-task-related exposure protocol with one set of matching odorants were implanted for LFP telemetry, allowed to recover, and then trained using the same protocol but with a new set of odorants. Odorant exposure in both paradigms evoked beta band (15–35 Hz) oscillations in the PCX as previously reported (Lowry and Kay 2007; Kay and Beshel 2010). Although non-task-related exposure sessions resulted in fewer direct odorant exposures than active-training sessions (Figure 5), both types of exposure induced similar PCX beta band responses over the first 10 days of training (Figure 6). Given that animals occasionally had strong recording artifacts, data were only analyzed from days 1, 5, and 10 on which all animals had useable data. A 2 × 3 (condition × day) ANOVA revealed no significant effect of exposure condition (active training vs. non-task-related exposure) on stimulus-evoked beta band power [main effect of condition F(1,41) = 0.023, P = 0.88] or of day [F(2,41) = 0.003, P = 0.99]. A separate group of 4 naive mice were recorded from only during their first exposures to non-task-related odor in the home cage and were not otherwise trained. Naive mice showed similar beta band activity during non-task-related odor exposure as the other two groups (Figure 6). This suggests that individual non-task-related odorant investigation events, even in otherwise naive mice, evoke PCX activity similar to that evoked by active training trials, potentially allowing circuit plasticity necessary for perceptual memory to occur in either condition.
Figure 6.
Stimulus-evoked PCX LFP beta band power during odor sampling in the active-training task (active) (diamonds) and during tube investigation in the non-task-related home-cage exposure (non-task-related) (squares). Data from a separate group of naive mice that were not previously trained and were only exposed to the odors in their home cage is shown at Day 1 (triangle). Beta power is expressed as percent change from power during disengagement from the active-training task or during time not investigating the odorant tubes in the home cage. There was no significant difference in mean stimulus-evoked beta band power across days or stimulation paradigm. Error bars are ± SEM. This figure is reproduced in color in the online version of the issue.
Discussion
The present results extend previous work to show that, several hours after a daily active-training session, non-task-related exposure to odorants for the same duration as the active training is equivalent to a second active-training session per day. Both active training and non-task-related home-cage exposure generated a mean 0.6% improvement in performance per session (Figure 3B). This performance enhancement requires that the non-task-related exposed odorants match those used in the active-training task. This robust effect on perceptual learning occurred even though mice had far fewer investigative bouts per session with the odorant in the non-task-related sessions than in the active-training sessions, with the number of these bouts decreasing over time within each session. Importantly, activation of the PCX during either non-task-related exposure investigation or active-training trials was equivalent, as assessed with PCX LFP beta oscillations. This suggests that both odor sampling during active training and odor sampling in the home cage, even in naive animals, evoke cortical activity known to be important for odor memory (Kay et al. 2009). Together, the results suggest that olfactory perceptual learning in an odor-discrimination task can be enhanced by additional, non-task-related odorant exposure to the same extent as induced by additional active training.
Performance improvement in an odor-discrimination task involves at least 3 basic forms of learning. First, the animal must learn the procedures of the task; for example, that a trial begins with an odor-sampling behavior and that one odor signals to go to the right water-reward port and the other signals to go to the left water-reward port. Second, the animal must learn which odor is associated with which reward port. Finally (not necessarily in this order), the animal must learn to discriminate between the 2 odors, that is, perceptual learning. In the experiments here, animals were pretrained to learn the active-training task, then switched to a new odor pair for data collection. Given that non-task-related odor exposure in the home cage would not provide information about which reward port was associated with a specific odor, we hypothesize that the benefits of home-cage odor exposure are entirely related to olfactory perceptual learning. This non-task-related benefit could derive both from attention to, and investigation of, the odor tube, and from purely passive exposure when the animal is not investigating the odor tube (Mandairon et al. 2008; Wright et al. 2010).
Olfactory perceptual learning induced by active training has been associated with neural plasticity throughout the olfactory pathway, including changes in olfactory sensory neuron input to the olfactory bulb (Kass et al. 2013) as well as changes in odorant coding in both the olfactory bulb (Fletcher and Wilson 2002; Mandairon et al. 2018) and PCX (Calu et al. 2007; Chapuis and Wilson 2011; Wilson and Sullivan 2011). The present demonstration that non-task-related home-cage odor exposure following an active training session the same day is as beneficial to perceptual learning as an additional active-training session raises the possibility that the improvements from these 2 different experiences arise from similar neural plasticity. For example, the results here indicate that home-cage odorant exposure, even in naive mice, evokes levels of beta oscillations in the PCX that are similar to those evoked by odor sampling in the active-training task. Beta band LFP oscillations are a robust signature of learning-associated neural activity in the olfactory system following active training and can reflect bottom-up or top-down interactions between the olfactory bulb and PCX (Neville and Haberly 2003; Kay et al. 2009; Kay and Beshel 2010; Martin and Ravel 2014).Therefore, in addition to reported changes in the structure and function of the olfactory bulb following non-task-related/passive odor exposure (Buonviso and Chaput 2000; Fletcher and Wilson 2003; Mandairon et al. 2008; Mandairon et al. 2018; Ross and Fletcher 2018), the plasticity in the PCX induced by home-cage odorant exposure here may be just as effective in the development of experience-dependent odor-object coding as that induced during active training (Wilson and Sullivan 2011). Future work will help isolate the importance of non-task-related exposure-induced changes in different regions of the olfactory system to supporting perceptual learning, and whether the same mechanisms are involved in perceptual learning driven by active training.
It should be emphasized that although described here as non-task-related stimulation, the odorant exposure in the home cage led to exploration of the scented tubes and other odor-oriented behaviors consistent with attention directed toward the stimuli. Attention has been hypothesized to play an important role in perceptual learning and in plasticity at multiple levels of the sensory pathway, in part because of the effects of attention-related neuromodulation of stimulus salience (Ahissar and Hochstein 1993; Treue 2003; Roelfsema et al. 2010; Mukai et al. 2011; Byers and Serences 2012; Rokem and Silver 2013; Kang et al. 2014b; Szpiro and Carrasco 2015; Carlson et al. 2018; Glennon et al. 2018). For example, modulating attention through task demands (Mukai et al. 2011), or directly enhancing noradrenergic (Glennon et al. 2018) or cholinergic (Kang et al. 2014a, 2014b) activity, normally associated with attention or stimulus saliency can improve perceptual learning. However, auditory stimuli presented as background during performance on an unrelated task, and thus presumably not entraining attention as in active training, can also facilitate perceptual learning (Wright et al. 2010). Further work is required to identify the role of attention toward the odorants in driving olfactory perceptual learning.
Finally, olfactory perceptual abilities that are commonly lost during aging and dementia (Doty et al. 1984; Murphy 1999), among other pathologies (Doty 2017), have been shown to improve with odor training (Haehner et al. 2013; Pekala et al. 2016; Leon and Woo 2018). The ability of non-task-related exposure to sensory stimuli to facilitate perceptual learning could provide an excellent method to accelerate rehabilitation in olfaction and potentially in other sensory modalities as well (Wright et al. 2010; Beste and Dinse 2013; Wright et al. 2015).
Funding
This work was supported by the National Institutes of Health [grant number R01-DC003906 to D.A.W.]. B.A.W. was sponsored by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) TNT program under the auspices of Dr Doug Weber and Tristan McClure-Begley through the Space and Naval Warfare Systems Center, Pacific Grant/Contract No. N66001-17-2-4011. Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO).
Conflicts of interest
None.
Acknowledgments
The authors thank Chelsea Schoen and Mirielle Lopez-Guzman for assistance with behavioral assays.
References
- Ahissar M, Hochstein S. 1993. Attentional control of early perceptual learning. Proc Natl Acad Sci U S A. 90:5718–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Viollet C, Gabellec MM, Meas-Yedid V, Olivo-Marin JC, Lledo PM. 2006. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci. 26:10508–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DC, Hofacer RD, Zaman AR, Rennaker RL, Wilson DA. 2008. Olfactory perceptual stability and discrimination. Nat Neurosci. 11:1378–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beshel J, Kopell N, Kay LM. 2007. Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci. 27:8358–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Dinse HR. 2013. Learning without training. Curr Biol. 23:R489–R499. [DOI] [PubMed] [Google Scholar]
- Buonviso N, Chaput M. 2000. Olfactory experience decreases responsiveness of the olfactory bulb in the adult rat. Neuroscience. 95:325–332. [DOI] [PubMed] [Google Scholar]
- Byers A, Serences JT. 2012. Exploring the relationship between perceptual learning and top-down attentional control. Vision Res. 74:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Roesch MR, Stalnaker TA, Schoenbaum G. 2007. Associative encoding in posterior piriform cortex during odor discrimination and reversal learning. Cereb Cortex. 17:1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson KS, Gadziola MA, Dauster ES, Wesson DW. 2018. Selective attention controls olfactory decisions and the neural encoding of odors. Curr Biol. 28:2195–2205.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis J, Garcia S, Messaoudi B, Thevenet M, Ferreira G, Gervais R, Ravel N. 2009. The way an odor is experienced during aversive conditioning determines the extent of the network recruited during retrieval: a multisite electrophysiological study in rats. J Neurosci. 29:10287–10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis J, Wilson DA. 2011. Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nat Neurosci. 15:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Barnes DC, Wilson DA. 2011. Generalized vs. stimulus-specific learned fear differentially modifies stimulus encoding in primary sensory cortex of awake rats. J Neurophysiol. 106:3136–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y, Wilson DA, Barkai E. 2015. Differential modifications of synaptic weights during odor rule learning: dynamics of interaction between the piriform cortex with lower and higher brain areas. Cereb Cortex. 25:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. 2017. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 16:478–488. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. 1984. Smell identification ability: changes with age. Science. 226:1441–1443. [DOI] [PubMed] [Google Scholar]
- Escanilla O, Mandairon N, Linster C. 2008. Odor-reward learning and enrichment have similar effects on odor perception. Physiol Behav. 94:621–626. [DOI] [PubMed] [Google Scholar]
- Fahle M, Poggio TE. 2001. Perceptual learning. Cambridge (MA): MIT Press. [Google Scholar]
- Fletcher ML. 2012. Olfactory aversive conditioning alters olfactory bulb mitral/tufted cell glomerular odor responses. Front Syst Neurosci. 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. 2002. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 22:RC201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. 2003. Olfactory bulb mitral-tufted cell plasticity: odorant-specific tuning reflects previous odorant exposure. J Neurosci. 23:6946–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DE, Brown A, Brim E, Mehta N, Vujovic M, Kay LM. 2016. Gamma and beta oscillations define a sequence of neurocognitive modes present in odor processing. J Neurosci. 36:7750–7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DE, Rojas-Líbano D, Scott M, Kay LM. 2011. Rat behavior in go/no-go and two-alternative choice odor discrimination: differences and similarities. Behav Neurosci. 125:588–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. 2000. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 97:1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EJ. 1963. Perceptual learning. Annu Rev Psychol. 14:29–56. [DOI] [PubMed] [Google Scholar]
- Glennon E, Carcea I, Martins ARO, Multani J, Shehu I, Svirsky MA, Froemke RC. 2018. Locus coeruleus activation accelerates perceptual learning. Brain Res. doi: 10.1016/j.brainres.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DB, Ohlemacher J, Rosen MJ. 2016. Benefits of stimulus exposure: developmental learning independent of task performance. Front Neurosci. 10:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehner A, Tosch C, Wolz M, Klingelhoefer L, Fauser M, Storch A, Reichmann H, Hummel T. 2013. Olfactory training in patients with Parkinson’s disease. PLoS One. 8:e61680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JI, Groleau M, Dotigny F, Giguère H, Vaucher E. 2014a. Visual training paired with electrical stimulation of the basal forebrain improves orientation-selective visual acuity in the rat. Brain Struct Funct. 219:1493–1507. [DOI] [PubMed] [Google Scholar]
- Kang JI, Huppé-Gourgues F, Vaucher E. 2014b. Boosting visual cortex function and plasticity with acetylcholine to enhance visual perception. Front Syst Neurosci. 8:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MD, Guang SA, Moberly AH, McGann JP. 2016. Changes in olfactory sensory neuron physiology and olfactory perceptual learning after odorant exposure in adult mice. Chem Senses. 41:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MD, Rosenthal MC, Pottackal J, McGann JP. 2013. Fear learning enhances neural responses to threat-predictive sensory stimuli. Science. 342:1389–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Beshel J. 2010. A beta oscillation network in the rat olfactory system during a 2-alternative choice odor discrimination task. J Neurophysiol. 104:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Brea J, Martin C, Rojas-Líbano D, Kopell N. 2009. Olfactory oscillations: the what, how and what for. Trends Neurosci. 32:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Freeman WJ. 1998. Bidirectional processing in the olfactory-limbic axis during olfactory behavior. Behav Neurosci. 112:541–553. [DOI] [PubMed] [Google Scholar]
- Le Berre E, Thomas-Danguin T, Béno N, Coureaud G, Etiévant P, Prescott J. 2008. Perceptual processing strategy and exposure influence the perception of odor mixtures. Chem Senses. 33:193–199. [DOI] [PubMed] [Google Scholar]
- Leon M, Woo C. 2018. Environmental enrichment and successful aging. Front Behav Neurosci. 12:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litaudon P, Mouly AM, Sullivan R, Gervais R, Cattarelli M. 1997. Learning-induced changes in rat piriform cortex activity mapped using multisite recording with voltage sensitive dye. Eur J Neurosci. 9:1593–1602. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Kay LM. 2007. Chemical factors determine olfactory system beta oscillations in waking rats. J Neurophysiol. 98:394–404. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Didier A, Linster C. 2008. Odor enrichment increases interneurons responsiveness in spatially defined regions of the olfactory bulb correlated with perception. Neurobiol Learn Mem. 90:178–184. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Kuczewski N, Kermen F, Forest J, Midroit M, Richard M, Thevenet M, Sacquet J, Linster C, Didier A. 2018. Opposite regulation of inhibition by adult-born granule cells during implicit versus explicit olfactory learning. Elife. 7 doi: 10.7554/eLife.34976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. 2006a. Enrichment to odors improves olfactory discrimination in adult rats. Behav Neurosci. 120:173–179. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Linster C. 2006b. Olfactory enrichment improves the recognition of individual components in mixtures. Physiol Behav. 89:379–384. [DOI] [PubMed] [Google Scholar]
- Martin C, Ravel N. 2014. Beta and gamma oscillatory activities associated with olfactory memory tasks: different rhythms for different functional networks? Front Behav Neurosci. 8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai I, Bahadur K, Kesavabhotla K, Ungerleider LG. 2011. Exogenous and endogenous attention during perceptual learning differentially affect post-training target thresholds. J Vis. 11 doi: 10.1167/11.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. 1999. Loss of olfactory function in dementing disease. Physiol Behav. 66:177–182. [DOI] [PubMed] [Google Scholar]
- Neville KR, Haberly LB. 2003. Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. J Neurophysiol. 90:3921–3930. [DOI] [PubMed] [Google Scholar]
- Pekala K, Chandra RK, Turner JH. 2016. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 6:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin MD. 1988. Experience facilitates olfactory quality discrimination. Percept Psychophys. 44:532–540. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, van Ooyen A, Watanabe T. 2010. Perceptual learning rules based on reinforcers and attention. Trends Cogn Sci. 14:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokem A, Silver MA. 2013. The benefits of cholinergic enhancement during perceptual learning are long-lasting. Front Comput Neurosci. 7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JM, Fletcher ML. 2018. Learning-dependent and -independent enhancement of mitral/tufted cell glomerular odor responses following olfactory fear conditioning in awake mice. J Neurosci. 38:4623–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Reuveni I, Barkai E. 2012. Mechanisms underlying rule learning-induced enhancement of excitatory and inhibitory synaptic transmission. J Neurophysiol. 107:1222–1229. [DOI] [PubMed] [Google Scholar]
- Seitz A, Watanabe T. 2005. A unified model for perceptual learning. Trends Cogn Sci. 9:329–334. [DOI] [PubMed] [Google Scholar]
- Szpiro SF, Carrasco M. 2015. Exogenous attention enables perceptual learning. Psychol Sci. 26:1854–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S. 2003. Visual attention: the where, what, how and why of saliency. Curr Opin Neurobiol. 13:428–432. [DOI] [PubMed] [Google Scholar]
- Wilson DA. 2003. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol. 90:65–72. [DOI] [PubMed] [Google Scholar]
- Wilson DA. 2010. Single-unit activity in piriform cortex during slow-wave state is shaped by recent odor experience. J Neurosci. 30:1760–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. 2011. Cortical processing of odor objects. Neuron. 72:506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BA, Baese-Berk MM, Marrone N, Bradlow AR. 2015. Enhancing speech learning by combining task practice with periods of stimulus exposure without practice. J Acoust Soc Am. 138:928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BA, Sabin AT. 2007. Perceptual learning: how much daily training is enough? Exp Brain Res. 180:727–736. [DOI] [PubMed] [Google Scholar]
- Wright BA, Sabin AT, Zhang Y, Marrone N, Fitzgerald MB. 2010. Enhancing perceptual learning by combining practice with periods of additional sensory stimulation. J Neurosci. 30:12868–12877. [DOI] [PMC free article] [PubMed] [Google Scholar]