Abstract
Strategies to refine the degradation behavior of polyester biomaterials, particularly to overcome the limitations of slow hydrolytic degradation, would broaden their utility. Herein, we examine the complexities of polyester degradation behavior, its assessment and strategies for refinement. The factors governing polyester degradation are strikingly complex. In addition to the half-life of the hydrolytically-labile bond, a series of interdependent material properties must be considered. Thus, methods used to characterize such material properties, both before and during degradation, must be carefully selected. Assessment of degradation behavior is further complicated by the variability of reported test protocols and the need for accelerated rather than real-time in vitro testing conditions. Ultimately, through better control of degradation behavior and correlation of in vitro, simulated degradation to that observed in vivo, the development of superior devices prepared with polyester biomaterials may be achieved.
Graphical Abstract
Biodegradable polymers are able to undergo chemical bond scission and accompanied physical erosion when exposed to the biological environment.1 Although the first synthetic degradable polymer was introduced in 1954,2 degradable polymers remain widely studied for ecological and biomedical applications.3 The first biodegradable synthetic suture, Dexon®, was approved by the FDA in 1969.4 Since then, applications of biodegradable polymers have expanded to include orthopedic fixation devices, vascular stents and drug delivery systems.5 A relatively more recent application involves the use of degradable polymers as scaffolds for tissue engineering.6 Several key aspects of polymer degradation behavior are necessary for biomedical device success. First, degradation by-products should not elicit a toxic response and must be suitable for renal clearance (<30 kDa).7 Additionally, the rate of degradation and the accompanied changes in material properties are critical.8 For instance, in the case of scaffolds, degradation rate should closely match the rate of neotissue ingrowth to maximize healing.4b Additionally, loss of mechanical functionality and integrity is important to consider.
While biodegradable polymers can undergo different types of degradation including photo-, thermal- and mechanical degradation, chemical degradation is particularly pertinent for polymers used in biomedical applications. As such, molecular chain scission can be initiated (1) passively by hydrolysis or (2) actively by enzyme-catalyzed hydrolysis.9 Oxidation may also occur.10 The type of degradation observed is dependent on the type of bonds comprising the polymer, typically within the backbone. While enzymatically-degradable polymers contain hydrolytically-labile bonds, these bonds are too stable under physiologic conditions and also require an enzymatic catalyst to undergo degradation.8 However, numerous bonds will undergo passive hydrolytic degradation under physiologic conditions. These include anhydride, ortho-ester, ester, urea, urethane/carbonate and amide bonds.11 Given their prevalence in biomedical applications, our discussion will hereafter be limited to synthetic, aliphatic polyesters and the material properties and processing factors that affect, as well as strategies that may be used to control, their biodegradation behavior (Figure 1).4b
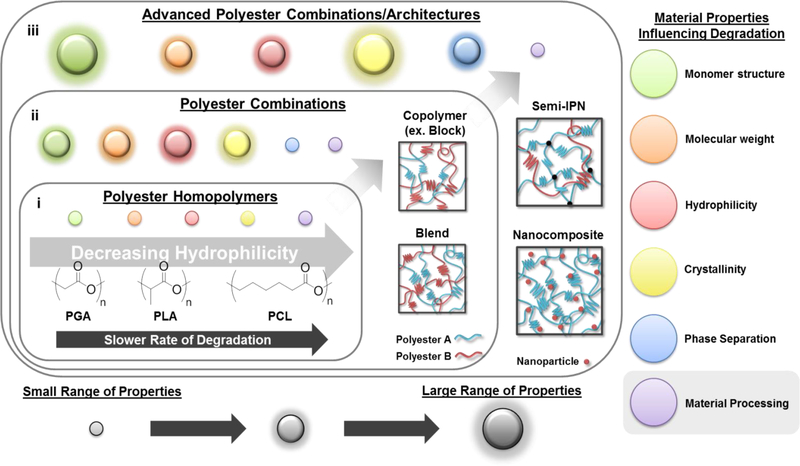
Figure 1.
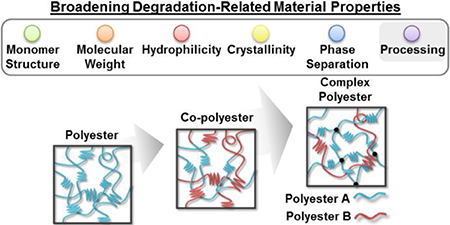
Summary of material properties that influence polyester degradation and the increasing range of each of these properties – indicated by diameter of “circle” – achieved from (i) polyester homopolymers, (ii) polyester combinations (e.g. copolymers and blends) and (iii) advanced polyester architectures (e.g. semi-IPNs and nanocomposites).
Aliphatic polyesters biodegrade via hydrolytically-labile ester bonds.4b A notable aspect of polyester degradation is the acidic by-products of the hydrolytic breakdown. This has raised concern of an inflammatory response during the degradation of implantable devices.12 Towards minimizing this pH reduction, Agrawal et al. reported the incorporation of basic salts within biodegradable polyester specimens.13 Acidic by-products have also been shown to have an “autocatalytic” effect on degradation.14 In nonporous materials, where the acidic by-products are presumed to be somewhat trapped within bulk, a hollow core is created within the specimen, which may be undesirable.15 Thus, the introduction of porosity facilitates by-product removal and can reduce this effect.16
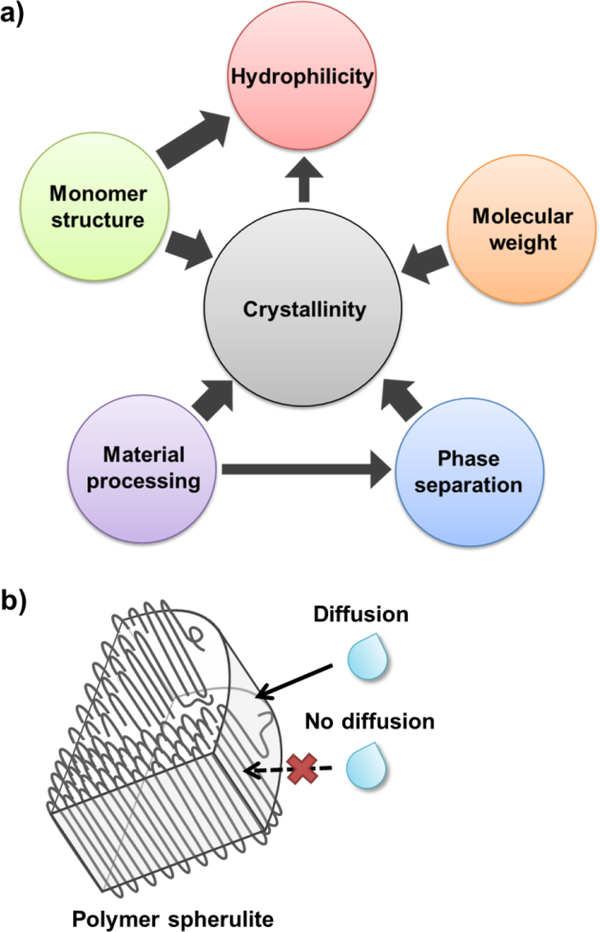
Material properties as well as processing can influence polyester degradation behavior,17 including monomer (“building block”) structure, molecular weight,18 hydrophilicity,19 crystallinity,20 phase microstructure,21 and material processing (e.g. annealing procedures, specimen dimensions, etc.) (Figure 1).22 Crystallinity, if present, plays a significant role given its ability to limit water diffusion, hence restrict access to hydrolytically-labile bonds (Figure 2). The proximity of the glass transition temperature (Tg) to physiological temperature can also contribute to polyester degradation behavior.23
Figure 2.
a) Material properties known to influence polyester hydrolytic degradation are interrelated. When present, crystallinity may be a primary contributor. b) Water diffusion is more restricted within the crystalline lamellae of semi-crystalline spherulites versus within the amorphous tie chain regions.
Early studies focused on homo-polyesters possessing different degradation rates resulting from their material properties (Figure 1, i). The first reported synthetic, degradable polymer, poly(glycolic acid) (PGA), is known for its relatively rapid degradation rate. However, its use is limited by its insolubility in common solvents and, hence, its difficulty in processing.8, 24 This led to the subsequent development of poly(lactic acid) (PLA), which can achieve a wide range of physical properties based on the chirality of the monomer units.25 Poly(ε-caprolactone) (PCL) exhibits relatively slow degradation rates.26 Poly(dioxanone) (PDS) exhibits relatively rapid degradation rates, similar to PGA.24 For poly(propylene fumarate) (PPF), cross-linking via its unsaturated bonds within the backbone has been shown to be useful to decrease its degradation rate.27 Nonetheless, due to the simplicity of their structures, the range of material properties achieved by homo-polyesters is somewhat narrow.
Polyesters have also been utilized in a variety of combinations to tune degradation rates and to achieve other physical properties (Figure 1, ii). Such combinations expand the range of materials properties possible and so provide the opportunity to tailor degradation behavior. Perhaps the most common strategy is the copolymerization of two or more monomers with different distributions of the monomeric units to produce random, alternating or block copolymers.28 The typical hydrolytic degradation rate trends of thermoplastic copolymers relative to parent homopolymers are well known, especially for those based on glycolide and lactide monomers.7a Copolymerization and the specific monomer distribution particularly influence crystallinity, notably significant to degradation. In one example, stereocomplex crystallization of isotactic D- and L-lactyl “stereoblock” segments was shown to reduce degradation rates when combined as poly(L-lactic acid) (PLLA)-b-poly(D-lactic acid) (PDLA) copolymers.29 Rahaman et al. evaluated multi- and diblock copolymers of PLLA and atactic poly(D,L-lactic acid) (PDLLA), finding that hydrolytic degradation decreased with an increase in average “stereoblock” length.30 Additionally, Wang et al. showed that the degradation rate of PPF-co-PCL copolymers could be controlled not only by PCL content, but also by cross-linking.31 Physical blending of homo-polyesters is another approach used to tailor degradation behavior. While blending is a relatively simple and flexible strategy, the immiscibility of polymers can limit certain combinations.25, 32 Methods to increase the miscibility of blends include the addition of low molecular weight copolymer (e.g. random or di-block) “compatibilizers” comprised of blend components, as well as introducing cross-linking.33,34 More recently, copolymers have been prepared containing ester linkages and another type of hydrolytically-labile bond.8 For instance, PDS is a poly(ester ether), and a salicylic acid-derived poly(anhydride esters) was reported by Erdmann et al.35
The physical erosion that accompanies hydrolytic degradation of polyesters and other biodegradable polymers is classified as either (1) bulk erosion or (2) surface erosion. Bulk erosion entails mass loss throughout the material, whereas surface erosion is limited to the specimen surface, proceeding via an erosion front.36 Aliphatic polyesters are summarily reported to exhibit bulk erosion. This type of erosion is associated with specimen cracking, even at low mass loss, which can severely compromise mechanical functionality. Whether surface or bulk erosion occurs was traditionally correlated simply to the hydrolysis half-life of the degradable bond.9, 36 However, many factors are now known to contribute. Overall, two kinetic processes have an impact: (1) hydrolytic degradation via chain scission and (2) diffusion of water into the bulk. Specimen dimensions play a role in degradation, as reported by von Burkersroda et al. who developed an erosion model to predict and explain whether surface or bulk erosion will dominate.37 The model quantifies an ‘erosion number,’ ε, based on the comparative rates of bond degradation and water diffusion via the equation:
where L is the half-thickness of the material, λ (the hydrolytic rate constant) is related to the polymer bond half-life, Deff is the effective diffusion coefficient, Mn is the number average molecular weight, NA is Avogadro’s number, N is the degree of polymerization and ρ is the polymer density. If bond hydrolysis occurs more rapidly than water diffusion, surface erosion is expected to occur (i.e. ε > 1). Conversely, if the rate of water diffusion exceeds the rate of bond hydrolysis, bulk erosion would occur (i.e. ε < 1). This erosion model more accurately reflects the comprehensive contributions of the materials parameters, in addition to bond chemistry. For instance, the group also showed that a critical L dimension (Lcritical) could be determined, above which surface erosion will dominate.
The aforementioned model highlights the complexity and interdependency of material properties that contribute to the degradation behavior of polyesters. While the inherent hydrolytic rate constant, λ, is significant, it should not vary between polyesters. This leaves differences in numerous material properties to contribute to degradation via water diffusion (i.e. Deff), most notably crystallinity. For example, Bergsma et al. analyzed the in vivo degradation of PLLA and a PLA-based copolymer, poly(96%L-, 4%D-lactic acid) (PLA96).38 The reduction in crystallinity (from 64.5% to 28.0%) for the copolymer, PLA96 implants resulted in more-rapid degradation rates (particle size of ~3.65 mm2 versus ~0.28 mm2 after 16 weeks). The impact of molecular weight and porosity on degradation has also been noted, for example by Braunecker et al. for PGA.39 In the study, decreased molecular weight generally increased degradation rate, as did an increase pore size and porosity. Finally, polyester material processing can also have a significant effect on degradation. For instance, a study by Ginde et al. found that heat treatment of PGA pellets and fibers altered molecular chain organization, and thus degradation rates.40
Evaluation and characterization of key material properties known to impact degradation behavior is essential – both prior to and during different stages of degradation. Initially, once monomer structure and molecular weight have been confirmed, differential scanning calorimetry (DSC) can assess thermal properties such as transition temperatures (i.e. Tg, melt transition temperature [Tm] and crystallization temperature [Tc]) as well as percent crystallinity. Dynamic mechanical thermal analysis (DMTA) can also provide information about thermal transitions.41 Semi-crystalline spherulite morphologies can be visualized with polarized optical microscopy (POM). Phase separation is often visualized with scanning electron microscopy (SEM) or atomic force microscopy (AFM).21 Peponi et al. utilized small angle X-ray scattering (SAXS) to discern micro- and nanoscale structures, providing another indication of phase separation.42 Lastly, hydrophilicity can be determined either at the surface (e.g. via contact angle) or as it relates to water diffusion quantified via water uptake.4a
In addition to those mentioned above, a variety of characterization techniques can determine additional material properties during degradation.14a First, material appearances may be observed, visually and with SEM. Key observations include surface versus bulk erosion behavior and early signs of fracture or cracking. When utilizing SEM, consideration should be given to the sample drying method. Vacuum-drying has been shown to collapse degradation-induced pores, which can lead to misinterpretation.43 Further, freeze-drying is also known to alter a material’s internal structure. As a result, low temperature SEM (cryo-SEM) has been recently proposed to provide representative characterization.5d Next, changes to molecular weight may be quantified via determining inherent viscosity and via gel permeation chromatography (GPC).33, 44 Molecular weight values obtained via GPC (i.e. Mn and Mw) can be further utilized to estimate degradation rate constant (k) values and to evaluate changes to the dispersity index (PDI).30, 44 Additionally, Gaona et al. determined evolving material composition with high performance liquid chromatography (HPLC).21 Such techniques, including thermogravimetric analysis (TGA),45 can be useful for multicomponent polymer systems to monitor composition and the temporal changes thereof. Changes to transition temperatures and crystallinity can provide a mechanistic understanding of degradation.18, 22 In addition, the evolution of water uptake into the material, determined gravimetrically, can indicate degradation.5d Lastly, Cohn et al. evaluated the dynamic mechanical properties during degradation,34 which could be paramount. In particular, Daniels et al. notes the need for assessing mechanical properties and for standardizing test methods and reporting thereof.46
The manner by which degradation studies are conducted is of tremendous importance. In vitro degradation studies are used to provide insight into degradation rates and erosion behavior in vivo. This generally involves directly monitoring the temporal mass loss in an aqueous solution or utilization of an indirect method (e.g. swelling and release).47 Typically, specimens are exposed to phosphate-buffered saline (PBS) closely maintained at pH = 7.4 ± 0.2 and physiologic temperatures (37 ± 1 °C). However, this does not fully replicate environmental factors that a biomaterial will be exposed to (i.e. physical forces, inflammation).21, 46 Additional testing parameters for in vitro degradation have been outlined in ASTM F1635 for surgical implants. Key features of the standard include: (1) a solution-to-specimen mass ratio of greater than 30:1 to provide adequate buffer capacity, (2) a sealable container to prevent solution loss by evaporation, (3) a minimum number of specimens (N) of N = 3 per time period, (4) packaged and sterilized specimens consistent with that of the final device and (5) removal of the dried and weighed specimens from a mass loss study. Nonetheless, the standard was only recently established (i.e. original edition approved in 1995), and limitations exist. Primarily, slow degradation (e.g. over the course of 3+ years for some polyesters)22, 48 hinders the practically of such methods. As a result, polyester degradation testing is often done under “accelerated” conditions.49
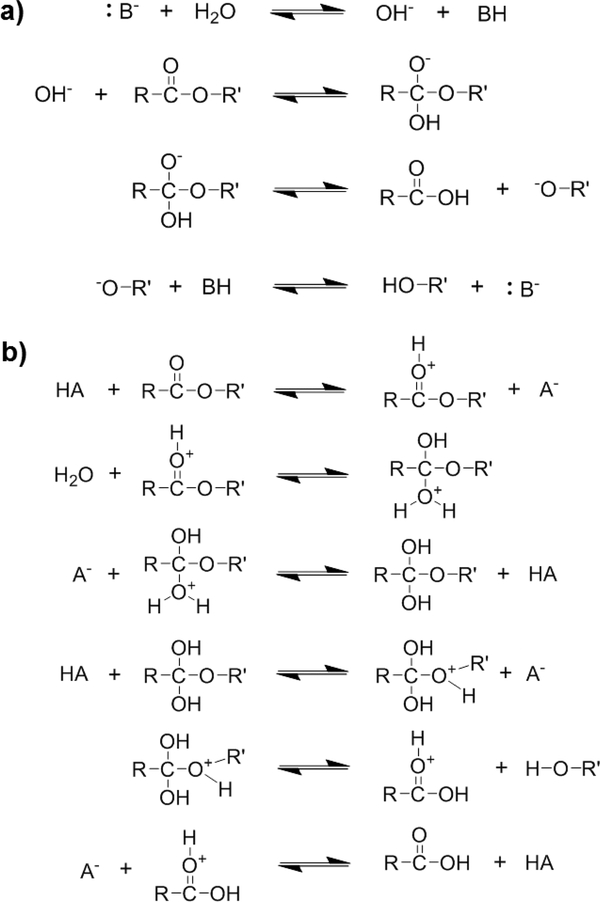
In assessing polyester degradation under accelerated conditions, considerations must be made due to the catalytic effect of elevated media temperature and pH on hydrolysis.37 In a basic environment (pH >> 7.4), hydroxide ions attack the ester carbonyl, while in an acidic environment (pH << 7.4), the carbonyl group is protonated, making it susceptible to nucleophilic attack by water (Figure 3).48b Different degradation rates are observed in basic versus acidic media. For instance, Sailema-Palate et al. studied the degradation of PCL at both highly acidic (pH = 1) and highly basic (pH = 13) conditions.7d The PCL specimens degraded more rapidly but exhibited less water uptake at pH = 13 versus at pH = 1. Absent at a pH = 13, PCL samples at pH = 1 underwent a substantial induction period (~300 hr) prior to exhibiting steady degradation. A more rapid degradation in highly basic media versus highly acidic media was also observed for PDLA.50 Moreover, in highly basic media, PCL degraded more slowly than PDLA, attributed in part to the lower electrophilicity of PCL’s carbonyl carbon atoms, and hence lower susceptibility to attack by hydroxide ions. The opposite was true in highly acidic media, with PCL degrading faster than PDLA. This was here attributed to the higher nucleophilicity of PCL’s carbonyl oxygen atoms and susceptibility to attack by hydrogen ions. In another example, Lam et al. similarly observed distinct differences in PCL-based scaffold degradation between the use of basic and physiologic (pH = 7.4) solutions.48a Surface erosion and minimal changes in molecular weight with mass loss were observed under basic conditions. In contrast, physiological conditions produced bulk erosion and large reductions to molecular weight. Surface erosion was also observed for solid PCL specimens at pH = 13.7d The previously discussed ‘erosion number’ (ε) supports these experimental results, where catalyzing hydrolysis (i.e. high pH) increases the λ-based numerator leading to a greater ε value, consistent with surface erosion.37 While pH accelerated in vitro degradation testing is useful, such studies point to its limitations.
Figure 3.
Mechanism for a) base-catalyzed and b) acid-catalyzed hydrolysis of polyesters.
Due to the limitations of in vitro biodegradation tests, in vivo degradation behavior of polyester materials remains the most compelling for assessing utility of biomedical devices. In vivo degradation is most commonly observed in subcutaneous tissue pockets, often with simultaneous biocompatibility tests.51 Rodent animal models have been most prevalent for such studies, and time points evaluated depend on the material, ranging from weeks to 3 or more years.52 Degradation is then ultimately evaluated in the most relevant physiological environment to the device’s application. For instance, Lam et al. analyzed the hydrolytic degradation of a PCL-based scaffold first in subcutaneous and in intramuscular tissue prior to analysis in their calvarial defect model.51 Hedberg et al. similarly assessed degradation of PPF/PDLA composite scaffolds upon implantation into the segmental defects of rabbit radii.53 To overcome slow degradation rates, Bergsma et al. utilized a pre-degradation technique to reduce the extensive time for total resorption of PLA particles (~5.6 years).54 After initial in vitro degradation in distilled water at 100 °C for 30 hours, the pre-degraded implants were able to be assessed in vivo within 3 to 80 weeks. Upon surgical explantation, implant appearance changes and mass loss can be determined.55 Other properties of interest have included crystallinity (via DSC),48a molecular weight (via GPC),51, 56 composition (via NMR),57 swelling,55 and mechanical properties.14b Imaging techniques including SEM, transmission electron microscopy (TEM), computed tomography (μ-CT) and light microscopy can be also used,51, 54, 58 and histology provides the most extensive evaluation of the host response. Non-invasive in vivo degradation imaging is a new means for evaluating in vivo degradation and has the potential for provide interesting insights. Kim et al. developed a method based on ultrasound elasticity imaging (UEI) that utilized phase-sensitive speckle tracking as a non-invasive means to quantify scaffold degradation and tissue formation.40 Wang et al. recently conjugated Rhodamine B to a polyester-based hydrogel to facilitate fluorescence tracking of degradation in vivo.59 Such methods that allow continuous monitoring of the same sample can significantly reduce the deviation in results, in addition to substantially reducing animal use, thus overcoming significant challenges in in vivo degradation testing. Labeling techniques can also allow for further analysis of the distribution, accumulation and ultimate excretion of degradation by-products.52b
While copolymers, as well as traditional blends, expanded the range of material properties and thus degradation behavior, more complex systems or “architectures” for the combination of polyesters may be useful (Figure 1, iii). For instance, we recently reported a semi-interpenetrating network (semi-IPN) of thermoplastic PLLA within cross-linked PCL-DA.60 This design concept resulted in drastically-accelerated degradation rates versus cross-linked PCL-DA controls and analogous PCL/PLLA blends. Second, new polyester chemistries also have the potential to expand degradation behavior. For instance, towards developing polyesters that exhibit surface erosion, Xu et al. recently reported a library of hydrophobic polyesters prepared by varying monomer chemistries.49 While surface erosion was observed, it should be noted that the highly basic conditions (pH = 10) that were employed may have contributed to this observation, a phenomena previously mentioned.37 In a final example, the incorporation of common inorganic fillers can elicit local changes in pH. The effect on pH (i.e. lower or raise) and subsequent specimen degradation will depend many factors, including the choice of filler. Chouzouri et al. observed a pH increase from 7.4 to ~9.7 corresponding with ~42% mass loss for a PLA/Bioglass 45S5 composite over 42 days, while the analogous PLA specimen saw negligible changes to pH or to mass loss.61 Thus, polyester nanocomposites may achieve tunable degradation behavior,62 potentially achieving surface erosion.
Ultimately, there is a need for improved standardization for evaluating the degradation behavior of polyesters. This would allow researchers to readily compare results of new systems to those reported in the literature, helping to promote the accuracy of the conclusions being made. This can begin with heightened awareness of and implementation of protocols listed in ASTM F1635 for non-accelerated in vitro testing. However, standard protocols for assessing degradation under accelerated conditions are also important. Although challenging, the standard would need to establish methods that account for influences of hydrolysis catalysis. Inclusion of controls that are well-characterized under both accelerated and non-accelerated testing conditions can be highly useful to isolate material versus environmental effects. Finally, approaches to more-theoretically model polymer degradation may reduce the dependence on experimentally measured degradation behavior.63 Ultimately, new polyester designs, as well as, superior methods to measure degradation behavior will lead to biomedical devices and applications with enhanced predictability and efficacy in vivo.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Ottenbrite R; Albertsson A; Scott G, Discussion on Degradation Terminology In Biodegradable Polymers and Plastics, The Royal Society of Chemistry: London, 1992; pp 73–92. [Google Scholar]
- 2.Li S; Vert M, Biodegradation of Aliphatic Polyesters In Degradable Polymers, Springer: 2002; pp 71–131. [Google Scholar]
- 3.Ikada Y; Tsuji H, Biodegradable Polyesters for Medical and Ecological Applications. Macromol. Rapid Commun 2000, 21, 117–132. [Google Scholar]
- 4. (a).Gilding DK; Reed AM, Biodegradable Polymers for Use in Surgery—Polyglycolic/Poly(Actic Acid) Homo- and Copolymers: 1. Polymer 1979, 20 (12), 1459–1464 [Google Scholar]; (b) Nair LS; Laurencin CT, Biodegradable Polymers as Biomaterials. Prog. Polym. Sci 2007, 32 (8), 762–798. [Google Scholar]
- 5. (a).Barrows T, Degradable Implant Materials: A Review of Synthetic Absorbable Polymers and Their Applications. Clin. Mater 1986, 1 (4), 233–257 [Google Scholar]; (b) Kulkarni R; Pani K; Neuman C; Leonard F, Polylactic Acid for Surgical Implants. Arch. Surg 1966, 93 (5), 839–843 [DOI] [PubMed] [Google Scholar]; (c) Brem H; Mahaley MS Jr; Vick NA; Black KL; Schold SC Jr; Burger PC; Friedman AH; Ciric IS; Eller TW; Cozzens JW, Interstitial Chemotherapy with Drug Polymer Implants for the Treatment of Recurrent Gliomas. J. Neurosurg 1991, 74 (3), 441–446 [DOI] [PubMed] [Google Scholar]; (d) Gu B; Sun X; Papadimitrakopoulos F; Burgess DJ, Seeing Is Believing PLGA Microsphere Degradation Revealed in PLGA Microsphere/PVA Hydrogel Composites. J. Control. Release 2016, 228, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. (a).Bonassar LJ; Vacanti CA, Tissue Engineering: The First Decade and Beyond. J. Cell. Biochem 1998, 72, 297–303 [DOI] [PubMed] [Google Scholar]; (b) Kim B-S; Baez CE; Atala A, Biomaterials for Tissue Engineering. World J. Urol 2000, 18 (1), 2–9. [DOI] [PubMed] [Google Scholar]
- 7. (a).Middleton JC; Tipton AJ, Synthetic Biodegradable Polymers as Orthopedic Devices. Biomaterials 2000, 21 (23), 2335–2346 [DOI] [PubMed] [Google Scholar]; (b) Healy JM; Lewis SD; Kurz M; Boomer RM; Thompson KM; Wilson C; McCauley TG, Pharmacokinetics and Biodistribution of Novel Aptamer Compositions. Pharm. Res 2004, 21 (12), 2234–2246 [DOI] [PubMed] [Google Scholar]; (c) Petersen H; Merdan T; Kunath K; Fischer D; Kissel T, Poly(Ethylenimine-co-L-Lactamide-co-Succinamide): A Biodegradable Polyethylenimine Derivative with an Advantageous pH-Dependent Hydrolytic Degradation for Gene Delivery. Bioconjug. Chem 2002, 13 (4), 812–821 [DOI] [PubMed] [Google Scholar]; (d) Sailema-Palate GP; Vidaurre A; Campillo-Fernández AJ; Castilla-Cortázar I, A Comparative Study on Poly(e-Caprolactone) Film Degradation at Extreme pH Values. Polym. Degrad. Stab 2016, 130, 118–125. [Google Scholar]
- 8.Ulery BD; Nair LS; Laurencin CT, Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys 2011, 49 (12), 832–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Göpferich A, Mechanisms of Polymer Degradation and Erosion. Biomaterials 1996, 17 (2), 103–114. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadi-Rovshandeh J; Mohammadikhah R; Asadi-Malekshah R, Thermal-Oxidative Degradation of PGA, PLLA, and Random Binary PLLA-PGA Copolymers. J. Pet. Sci. Technol 2016, 6 (2), 66–83. [Google Scholar]
- 11.Li S, Hydrolytic Degradation Characteristics of Aliphatic Polyesters Derived from Lactic and Glycolic Acids. J. Biomed. Mater. Res 1999, 48 (3), 342–353. [DOI] [PubMed] [Google Scholar]
- 12. (a).Ratcliffe JH; Hunneyball IM; Smith A; Wilson CG; Davis SS, Preparation and Evaluation of Biodegradable Polymeric Systems for the Intra-Articular Delivery of Drugs. J. Pharm. Pharmacol 1984, 36 (7), 431–436 [DOI] [PubMed] [Google Scholar]; (b) Bergsma EJ; Rozema FR; Bos RRM; Bruijn WCD, Foreign Body Reactions to Resorbable Poly(L-Lactide) Bone Plates and Screws Used for the Fixation of Unstable Zygomatic Fractures. J. Oral Maxillofac. Surg 1993, 51 (6), 666–670. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal CM; Athanasiou KA, Technique to Control pH in Vicinity of Biodegrading PLA-PGA Implants. J. Biomed. Mater. Res 1997, 38 (2), 105–114. [DOI] [PubMed] [Google Scholar]
- 14. (a).Pitt CG; Chasalow F; Hibionada Y; Klimas D; Schindler A, Aliphatic Polyesters. I. The Degradation of Poly (ϵ‐Caprolactone) in vivo. J. Appl. Polym. Sci 1981, 26 (11), 3779–3787 [Google Scholar]; (b) Pitt GG; Gratzl MM; Kimmel GL; Surles J; Sohindler A, Aliphatic Polyesters II. The Degradation of Poly (DL-Lactide), Poly (e-Caprolactone), and Their Copolymers in vivo. Biomaterials 1981, 2 (4), 215–220. [DOI] [PubMed] [Google Scholar]
- 15.Thompson DE; Agrawal CM; Athanasiou K, The Effects of Dynamic Compressive Loading on Biodegradable Implants of 50–50% Polylactic Acid–Polyglycolic Acid. Tissue Eng. 1996, 2 (1), 61–74. [DOI] [PubMed] [Google Scholar]
- 16.Athanasiou K; Schmitz J; Agrawal C, The Effects of Porosity on in Vitro Degradation of Polylactic Acid–Polyglycolic Acid Implants Used in Repair of Articular Cartilage. Tissue Eng. 1998, 4 (1), 53–63. [Google Scholar]
- 17.Schmitt EA; Flanagan D; Linhardt RJ, Importance of Distinct Water Environments in the Hydrolysis of Poly (DL-Lactide-co-Glycolide). Macromolecules 1994, 27 (3), 743–748. [Google Scholar]
- 18.Saha SK; Tsuji H, Effects of Molecular Weight and Small Amounts of D-Lactide Units on Hydrolytic Degradation of Poly(L-Lactic Acid)s. Polym. Degrad. Stab. 2006, 91 (8), 1665–1673. [Google Scholar]
- 19.Makadia HK; Siegel SJ, Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3 (3), 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuji H; Mizuno A; Ikada Y, Properties and Morphology of Poly(L-Lactide). III. Effects of Initial Crystallinity on Long-Term in vitro Hydrolysis of High Molecular Weight Poly(L-Lactide) Film in Phosphate-Buffered Solution. J. Appl. Polym. Sci 2000, 77, 1452–1464. [Google Scholar]
- 21.Gaona LA; Gómez Ribelles JL; Perilla JE; Lebourg M, Hydrolytic Degradation of PLLA/PCL Microporous Membranes Prepared by Freeze Extraction. Polym. Degrad. Stab 2012, 97 (9), 1621–1632. [Google Scholar]
- 22.Tsuji H; Ikada Y, Properties and Morphology of Poly(L-Lactide) 4. Effects of Structural Parameters on Long-Term Hydrolysis of Poly(L-Lactide) in Phosphate-Buffered Solution. Polym. Degrad. Stab 2000, 67 (1), 179–189. [Google Scholar]
- 23.Reed AM; Gilding DK, Biodegradable Polymers for Use in Surgery — Poly(Glycolic)/Poly(Lactic Acid) Homo and Copolymers: 2. In vitro Degradation. Polymer 1981, 22 (4), 494–498. [Google Scholar]
- 24.Maurus PB; Kaeding CC, Bioabsorbable Implant Material Review. Oper. Tech. Sports Med 2004, 12 (3), 158–160. [Google Scholar]
- 25.Ferri JM; Fenollar O; Jorda-Vilaplana A; García-Sanoguera D; Balart R, Effect of Miscibility on Mechanical and Thermal Properties of Poly(Lactic Acid)/ Polycaprolactone Blends. Polym. Int 2016, 65 (4), 453–463. [Google Scholar]
- 26.Saini P; Arora M; Kumar MNVR, Poly(Lactic Acid) Blends in Biomedical Applications. Adv. Drug Deliv. Rev 2016, 107 (Supplement C), 47–59. [DOI] [PubMed] [Google Scholar]
- 27.He S; Timmer MD; Yaszemski MJ; Yasko AW; Engel PS; Mikos AG, Synthesis of Biodegradable Poly(Propylene Fumarate) Networks with Poly(Propylene Fumarate)–Diacrylate Macromers as Crosslinking Agents and Characterization of Their Degradation Products. Polymer 2001, 42 (3), 1251–1260. [Google Scholar]
- 28.Noshay A; McGrath JE, Block Copolymers: Overview and Critical Survey. Elsevier: 2013. [Google Scholar]
- 29.Okada Y; Jamshidi K; Tsuji H; Hyon S-H, Stereocomplex Formation Between Enantomeric Poly(Lactides). Macromolecules 1987, 20, 904. [Google Scholar]
- 30.Rahaman H; Tsuji H, Hydrolytic Degradation Behavior of Stereo Multiblock and Diblock Poly(Lactic Acid)s: Effects of Block Lengths. Polym. Degrad. Stab 2013, 98 (3), 709–719. [Google Scholar]
- 31.Wang S; Kempen DHR; de Ruiter GCW; Cai L; Spinner RJ; Windebank AJ; Yaszemski MJ; Lu L, Molecularly Engineered Biodegradable Polymer Networks with a Wide Range of Stiffness for Bone and Peripheral Nerve Regeneration. Adv. Funct. Mater 2015, 25 (18), 2715–2724. [Google Scholar]
- 32.Tsuji H; Ikada Y, Blends of Aliphatic Polyesters. II. Hydrolysis of Solution-Cast Blends from Poly(L-Lactide) and Poly(e-Caprolactone) in Phosphate-Buffered Solution. J. Appl. Polym. Sci 1998, 67 (3), 405–415. [Google Scholar]
- 33.Choi N-S; Kim C-H; Cho KY; Park J-K, Morphology and Hydrolysis of PCL-PLLA Blends Compatibilized with P(LLA-co-ϵCL) or P(LLA-b-ϵCL). J. Appl. Polym. Sci 2002, 86 (8), 1892–1898. [Google Scholar]
- 34.Cohn D; Hotovely Salomon A, Designing Biodegradable Multiblock PCL/PLA Thermoplastic Elastomers. Biomaterials 2005, 26 (15), 2297–2305. [DOI] [PubMed] [Google Scholar]
- 35.Erdmann L; Uhrich KE, Synthesis and Degradation Characteristics of Salicylic Acid-Derived Poly(Anhydride-Esters). Biomaterials 2000, 21 (19), 1941–1946. [DOI] [PubMed] [Google Scholar]
- 36.Tamada JA; Langer R, Erosion Kinetics of Hydrolytically Degradable Polymers. Proc. Natl. Acad. Sci. U.S.A 1993, 90 (2), 552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Burkersroda F; Schedl L; Göpferich A, Why Degradable Polymers Undergo Surface Erosion or Bulk Erosion. Biomaterials 2002, 23 (21), 4221–4231. [DOI] [PubMed] [Google Scholar]
- 38.Bergsma JE; Rozema FR; Bos RRM; van Rozendaal AWM; De Jong WH; Teppema JS; Joziasse CAP, Biocompatibility and Degradation Mechanisms of Predegraded and Non-Predegraded Poly(Lactide) Implants: An Animal Study. J. Mater. Sci. Mater. Med 1995, 6 (12), 715–724. [Google Scholar]
- 39.Braunecker J; Baba M; Milroy GE; Cameron RE, The Effects of Molecular Weight and Porosity on the Degradation and Drug Release from Polyglycolide. Int. J. Pharm 2004, 282 (1), 19–34. [DOI] [PubMed] [Google Scholar]
- 40.Ginde RM; Gupta RK, In Vitro Chemical Degradation of Poly(Glycolic Acid) Pellets and Fibers. J. Appl. Polym. Sci 1987, 33 (7), 2411–2429. [Google Scholar]
- 41.Dell’Erba R; Groeninckx G; Maglio G; Malinconico M; Migliozzi A, Immiscible Polymer Blends of Semicrystalline Biocompatible Components: Thermal Properties and Phase Morphology Analysis of PLLA/PCL Blends. Polymer 2001, 42 (18), 7831–7840. [Google Scholar]
- 42.Peponi L; Navarro-Baena I; Báez JE; Kenny JM; Marcos-Fernández A, Effect of the Molecular Weight on the Crystallinity of PCL-b-PLLA Di-Block Copolymers. Polymer 2012, 53 (21), 4561–4568. [Google Scholar]
- 43.Shah SS; Cha Y; Pitt CG, Poly (Glycolic Acid-co-DL-Lactic Acid): Diffusion or Degradation Controlled Drug Delivery? J. Control. Release 1992, 18 (3), 261–270. [Google Scholar]
- 44.Lu L; Garcia CA; Mikos AG, In vitro Degradation of Thin Poly(DL-Lactic-co-Glycolic Acid) Films. J. Biomed. Mater. Res 1999, 46 (2), 236–244. [DOI] [PubMed] [Google Scholar]
- 45.Guarino V; Causa F; Taddei P; di Foggia M; Ciapetti G; Martini D; Fagnano C; Baldini N; Ambrosio L, Polylactic Acid Fibre-Reinforced Polycaprolactone Scaffolds for Bone Tissue Engineering. Biomaterials 2008, 29 (27), 3662–3670. [DOI] [PubMed] [Google Scholar]
- 46.Daniels A; Chang MK; Andriano KP; Heller J, Mechanical Properties of Biodegradable Polymers and Composites Proposed for Internal Fixation of Bone. J. Appl. Biomater 1990, 1 (1), 57–78. [DOI] [PubMed] [Google Scholar]
- 47.van de Wetering P; Metters AT; Schoenmakers RG; Hubbell JA, Poly(Ethylene Glycol) Hydrogels Formed by Conjugate Addition with Controllable Swelling, Degradation, and Release of Pharmaceutically Active Proteins. J. Control. Release 2005, 102 (3), 619–627. [DOI] [PubMed] [Google Scholar]
- 48. (a).Lam CX; Savalani MM; Teoh S-H; Hutmacher DW, Dynamics of in vitro Polymer Degradation of Polycaprolactone-Based Scaffolds: Accelerated Versus Simulated Physiological Conditions. Biomed. Mater 2008, 3 (3), 034108. [DOI] [PubMed] [Google Scholar]; (b) Pickett JE; Coyle DJ, Hydrolysis Kinetics of Condensation Polymers under Humidity Aging Conditions. Polym. Degrad. Stab 2013, 98 (7), 1311–1320. [Google Scholar]
- 49.Xu X-J; Sy JC; Prasad Shastri V, Towards Developing Surface Eroding Poly(a-Hydroxy Acids). Biomaterials 2006, 27 (15), 3021–3030. [DOI] [PubMed] [Google Scholar]
- 50.Jung JH; Ree M; Kim H, Acid- and Base Catalyzed Hydrolyses of Aliphatic Polycarbonates and Polyesters. Catalysis Today 2006, 115, 283–287. [Google Scholar]
- 51.Lam CXF; Hutmacher DW; Schantz J-T; Woodruff MA; Teoh SH, Evaluation of Polycaprolactone Scaffold Degradation for 6 Months in vitro and in vivo. J. Biomed. Mater. Res. A 2009, 90A (3), 906–919. [DOI] [PubMed] [Google Scholar]
- 52. (a).Kim K; Jeong CG; Hollister SJ, Non-Invasive Monitoring of Tissue Scaffold Degradation Using Ultrasound Elasticity Imaging. Acta Biomater. 2008, 4 (4), 783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sun H; Mei L; Song C; Cui X; Wang P, The in vivo Degradation, Absorption and Excretion of PCL-Based Implant. Biomaterials 2006, 27 (9), 1735–1740. [DOI] [PubMed] [Google Scholar]
- 53.Hedberg EL; Kroese-Deutman HC; Shih CK; Crowther RS; Carney DH; Mikos AG; Jansen JA, In vivo Degradation of Porous Poly(Propylene Fumarate)/Poly(DL-Lactic-co-Glycolic Acid) Composite Scaffolds. Biomaterials 2005, 26 (22), 4616–4623. [DOI] [PubMed] [Google Scholar]
- 54.Bergsma JE; Rozema FR; Bos RRM; Boering G; de Bruijn WC; Pennings AJ, In vivo Degradation and Biocompatibility Study of in vitro Pre-Degraded As-Polymerized Polylactide Particles. Biomaterials 1995, 16 (4), 267–274. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y; Kim YM; Langer R, In vivo Degradation Characteristics of Poly(Glycerol Sebacate). J. Biomed. Mater. Res. A 2003, 66A (1), 192–197. [DOI] [PubMed] [Google Scholar]
- 56. (a).Lu L; Peter SJ; D. Lyman M; Lai H-L; Leite SM; Tamada JA; Uyama S; Vacanti JP; Robert L; Mikos AG, In vitro and in vivo Degradation of Porous Poly(DL-Lactic-co-Glycolic Acid) Foams. Biomaterials 2000, 21 (18), 1837–1845 [DOI] [PubMed] [Google Scholar]; (b) Bölgen N; Menceloğlu YZ; Acatay K; Vargel İ; Pişkin E, In vitro and in vivo Degradation of Non-Woven Materials Made of Poly(e-Caprolactone) Nanofibers Prepared by Electrospinning under Different Conditions. J. Biomater. Sci. Polym. Ed 2005, 16 (12), 1537–1555. [DOI] [PubMed] [Google Scholar]
- 57.Therin M; Christel P; Li S; Garreau H; Vert M, In vivo Degradation of Massive Poly(a-Hydroxy Acids): Validation of in vitro Findings. Biomaterials 1992, 13 (9), 594–600. [DOI] [PubMed] [Google Scholar]
- 58. (a).Jeong SI; Kim B-S; Kang SW; Kwon JH; Lee YM; Kim SH; Kim YH, In Vivo Biocompatibilty and Degradation Behavior of Elastic Poly(L-Lactide-co-e-Caprolactone) Scaffolds. Biomaterials 2004, 25 (28), 5939–5946 [DOI] [PubMed] [Google Scholar]; (b) Vergnol G; Ginsac N; Rivory P; Meille S; Chenal J-M; Balvay S; Chevalier J; Hartmann DJ, In vitro and in vivo Evaluation of a Polylactic Acid-Bioactive Glass Composite for Bone Fixation Devices. J. Biomed. Mater. Res. B Appl. Biomater 2016, 104 (1), 180–191. [DOI] [PubMed] [Google Scholar]
- 59.Wang W; Liu J; Li C; Zhang J; Liu J; Dong A; Kong D, Real-Time and Non-Invasive Fluorescence Tracking of in vivo Degradation of the Thermosensitive PEGlyated Polyester Hydrogel. J. Mater. Chem. B 2014, 2 (26), 4185–4192. [DOI] [PubMed] [Google Scholar]
- 60.Woodard LN; Page VM; Kmetz KT; Grunlan MA, PCL-PLLA Semi-IPN Shape Memory Polymers (SMPs): Degradation and Mechanical Properties. Macromol. Rapid Commun 2016, 37 (23), 1972–1977. [DOI] [PubMed] [Google Scholar]
- 61.Chouzouri G; Xanthos M, Degradation of Aliphatic Polyesters in the Presence of Inorganic Fillers. J. Plast. Film Sheeting 2007, 23 (1), 19–36. [Google Scholar]
- 62.Bikiaris DN, Nanocomposites of Aliphatic Polyesters: An Overview of the Effect of Different Nanofillers on Enzymatic Hydrolysis and Biodegradation of Polyesters. Polym. Degrad. Stab 2013, 98 (9), 1908–1928. [Google Scholar]
- 63.Ford Versypt AN; Pack DW; Braatz RD, Mathematical Modeling of Drug Delivery from Autocatalytically Degradable PLGA Microspheres — A Review. J. Control. Release 2013, 165 (1), 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]