Abstract
Acquired sensorineural hearing loss (SNHL), including age-related hearing loss (ARHL), noise-induced hearing loss (NIHL), drug-induced hearing loss (DIHL) and sudden sensorineural hearing loss (SSHL), is one of the most common sensory deficits in humans. Several studies have reported that antioxidant gene glutathione s-transferase M1 and T1 (GST M1 and T1) polymorphisms have a close relationship with the susceptibility to acquired SNHL, but other articles have reported opposite results. This meta-analysis aims to identify whether an association exists between GST M1 and T1 polymorphisms and the susceptibility to acquired SNHL. Seventeen independent studies containing 1749 cases and 2018 controls were included. According to the I2 value of the heterogeneity test, random-effects model was selected to calculate the pooled odds ratios (ORs) with their 95% confidence intervals (95% CIs) and p values. The pooled ORs (95% CI, p-value) of GST M1 and T1 were 1.186(0.955–1.473, p = 0.122) and 1.107(0.841–1.458, p = 1.467), respectively. In addition, subgroup analyses according to the type of SNHL and ethnicity showed no relationship between GST M1 and T1 polymorphisms and the susceptibility to acquired SNHL. Our results suggest that no significant relationship was found between GST M1 and T1 polymorphisms and the susceptibility to acquired SNHL.
Introduction
Acquired sensorineural hearing loss (SNHL), including age-related hearing loss (ARHL), noise-induced hearing loss (NIHL), drug-induced hearing loss (DIHL) and sudden sensorineural hearing loss (SSHL), is one of the most common sensory deficits in humans in modern society1. Approximately 360 million people worldwide suffer from this health problem2. People with acquired SNHL exhibit decreased hearing sensitivity and a decline in speech intelligibility, which can lead to serious difficulties in an individual’s communication and social interactions and consequently reduce life expectancy3,4. Despite the high prevalence and serious effects of acquired SNHL, few therapeutic methods have been found to be clinically effective5.
The susceptibility to acquired SNHL among individuals is diverse. Some individuals are more susceptible to acquired SNHL, while others are not. Several studies have suggested that this individual difference in susceptibility to acquired SNHL is mostly due to the different genetic backgrounds of individuals, especially genetic polymorphisms that affect the expression of some functional proteins or enzymes6–10. Therefore, exploring these genetic differences and then exploiting them may enable the development of individual prevention strategies for SNHL.
Oxidative stress has been proven to be the most important molecular mechanism in the pathogenesis of acquired SNHL11–14. Researchers have successfully alleviated several kinds of SNHL with the application of antioxidants in animal experiments11,13–15. Glutathione s-transferase (GST) encodes a system of antioxidative enzymes that have been demonstrated to play an important role in antioxidative protection in cochlear cells16–18. Among the GST subclasses, GST T1 and M1 are genetically deleted (null genotype) in a high percentage in humans. Approximately 30–50% of individuals have a null genotype for GST M1, depending on their race19, and 25–40% carry the null genotype of GST T120. Rabinnowitz et al. once suggested that individuals with the null genotypes of GST M1 or GST T1 are more susceptible to oxidative stress damage and are possibly more susceptible to NIHL21.
Many studies have attempted to correlate mutant genotypes of GST to the susceptibility to acquired SNHL. Some have demonstrated a close relationship between GST M1 or T1 polymorphisms and the susceptibility to SNHL6,22–25, and others have reported conflicting results8,10,16,26–34. Meta-analysis is an effective way to address this type of contradiction. Therefore, we performed this meta-analysis to identify whether a close association exists between GST M1 and T1 polymorphisms and the susceptibility to acquired SNHL and whether GST M1 and T1 polymorphisms can serve as predictive factors for the susceptibility to acquired SNHL.
Results
Literature search and characteristics of the included studies
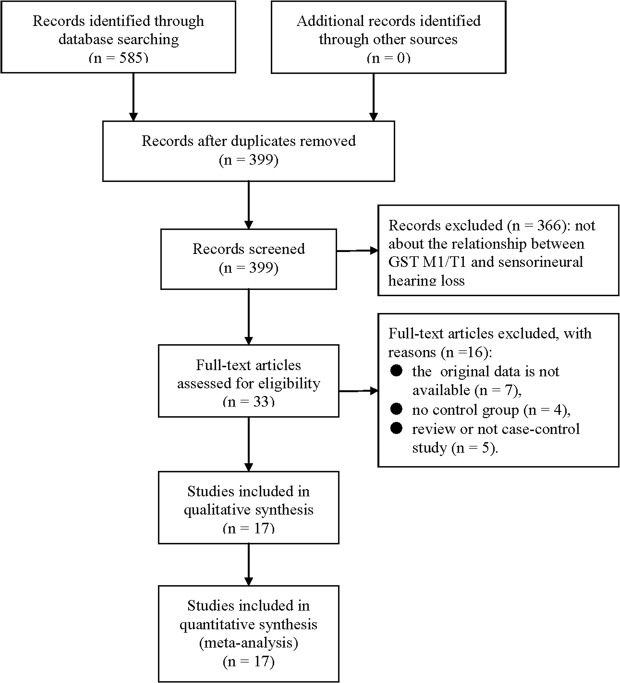
The literature selection process is shown in Fig. 1. Through the search in the databases, 585 potentially relevant records were identified, 399 of which were retained after duplicates were removed. After screening the records, 366 records were excluded because they did not discuss the relationship between GST M1 and T1 polymorphisms and acquired SNHL. The remaining 33 articles were assessed for eligibility via full-text screening. Of these, 16 studies were excluded for various reasons, such as unavailable original data, no control groups, reviews or non-original articles. Finally, 17 independent studies were included in the meta-analysis. Therefore, a total of 1749 cases with acquired SNHL and 2018 controls were included. Table 1 summarizes the basic information of the 17 included eligible studies.
Figure 1.
Flow diagram of the study selection process.
Table 1.
Characteristics of included studies.
| First author | Year | Country | Ethnicity | Genotype method | NOS score | Hearing loss type | Case | Control | GST T1 | GST M1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||||||||||||
| WT | null | WT | null | WT | null | WT | null | |||||||||
| Manche | 2016 | India | Indian | Multiplex-PCR | 8 | ARHL | 220 | 270 | 119 | 101 | 202 | 68 | 121 | 99 | 201 | 69 |
| Zhu | 2011 | China | Chinese | Multiplex-PCR | 8 | ARHL | 110 | 114 | 61 | 49 | 58 | 56 | 39 | 71 | 33 | 81 |
| Bared | 2010 | USA | Mixed | Multiplex-PCR | 8 | ARHL | 55 | 79 | 21 | 31 | 52 | 27 | 16 | 36 | 41 | 38 |
| Ates | 2005 | Turkey | Turkish | Real-time PCR | 8 | ARHL | 68 | 69 | 54 | 14 | 53 | 16 | 36 | 32 | 40 | 29 |
| Shen | 2012 | China | Chinese | PCR-RFLP | 8 | NIHL | 444 | 445 | 215 | 229 | 210 | 235 | 198 | 246 | 253 | 192 |
| Abreu-Silva | 2011 | Brazil | Brazilian | PCR-RFLP | 8 | NIHL | 151 | 104 | 40 | 111 | 22 | 82 | 115 | 36 | 77 | 27 |
| Liu | 2006 | China | Chinese | PCR | 8 | NIHL | 123 | 123 | 58 | 60 | 48 | 66 | 38 | 85 | 54 | 69 |
| Yang | 2005 | China | Chinese | Multiplex-PCR | 8 | NIHL | 93 | 101 | 31 | 62 | 47 | 57 | 37 | 56 | 35 | 66 |
| Carlsson | 2005 | Sweden | Swedish | PCR-RFLP | 8 | NIHL | 103 | 112 | 90 | 13 | 104 | 8 | 50 | 53 | 59 | 53 |
| Choeyprasert | 2013 | Thailand | German | Multiplex-PCR | 8 | DIHL | 55 | 13 | 38 | 17 | 4 | 9 | 24 | 31 | 5 | 8 |
| Jurajda | 2012 | Czech | Czechs | PCR | 7 | DIHL | 12 | 26 | 10 | 2 | 20 | 6 | 4 | 8 | 10 | 16 |
| Palodetto | 2010 | Brazil | Brazilian | Multiplex-PCR | 5 | DIHL | 10 | 20 | 8 | 2 | 13 | 7 | 6 | 4 | 11 | 9 |
| Barahmani | 2009 | USA | Mixed | Multiplex-PCR | 7 | DIHL | 19 | 15 | 13 | 6 | 10 | 5 | 11 | 8 | 9 | 6 |
| Oldenburg | 2007 | Norway | Norwegians | Multiplex-PCR | 7 | DIHL | 89 | 84 | 75 | 14 | 70 | 14 | 49 | 40 | 45 | 39 |
| Peters | 2000 | Germany | Thai | PCR | 8 | DIHL | 19 | 20 | 12 | 8 | 16 | 3 | 11 | 9 | 8 | 11 |
| Um | 2011 | Korea | Korean | Multiplex-PCR | 6 | SSHL | 98 | 343 | 51 | 47 | 173 | 170 | 40 | 58 | 138 | 205 |
| Cadoni | 2006 | Italy | Italian | Multiplex-PCR | 8 | SSHL | 80 | 80 | 60 | 20 | 62 | 18 | 41 | 39 | 36 | 44 |
NOS: Newcastle-Ottawa Scale. GST: glutathione s-transferase. ARHL: age-related hearing loss. NIHL: noise-induced hearing loss. DIHL: drug-induced hearing loss. SSHL: sudden sensorineural hearing loss. WT: wild type. PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphis.
The relationship between GST M1 and T1 polymorphisms and the susceptibility to acquired sensorineural hearing loss
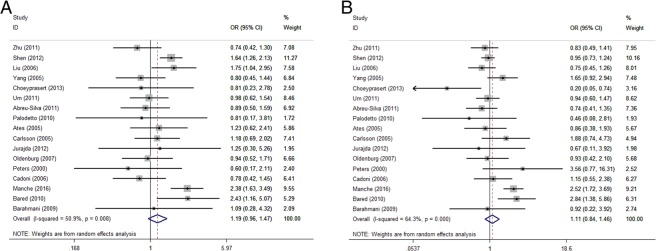
The I2 values for GST M1 and T1 were 50.9% and 64.3%, respectively, which are shown in Fig. 2. Both I2 values were ≥30%, so we used a random-effects model to calculate the pooled odds ratios (ORs) and 95% confidence intervals (95% CIs). The pooled ORs (95% CI, p-value) of GST M1 and T1 were 1.186(0.955–1.473, 0.122) and 1.107(0.841–1.458, 1.467), respectively.
Figure 2.
Forest plot presenting the association between GST M1 (A) and T1 (B) polymorphisms and the susceptibility to acquired SNHL.
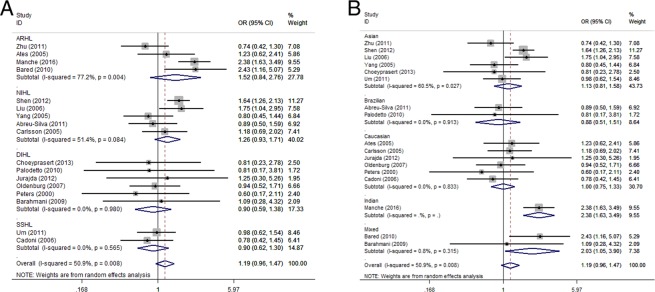
To address heterogeneity, we performed a subgroup analysis according to the acquired SNHL type and ethnicity. For GST M1, no heterogeneity was observed in the DIHL and the SSHL subgroups. However, relatively strong heterogeneity was observed in the ARHL and NIHL subgroups (77.2% for the ARHL subgroup and 51.4% for the NIHL subgroup, Fig. 3A). For the subgroup analysis according to ethnicity, no heterogeneity was observed in the Caucasian subgroup, while intermediate heterogeneity was observed in the Asian subgroup (49.7%, Fig. 3B). In addition, no statistically significant relationship between GST M1 polymorphisms and the susceptibility to acquired SNHL was found in any of the acquired SNHL type and ethnicity subgroups.
Figure 3.
Subgroup analysis of the association between GST M1 and the susceptibility to acquired SNHL according to the acquired SNHL types (A) and ethnicity (B).
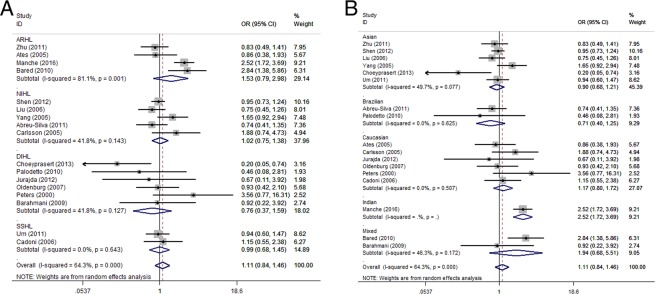
As exhibited in Fig. 4, the subgroup analysis according to the type of acquired SNHL and ethnicity of GST T1 showed similar results to those of GST M1.
Figure 4.
Subgroup analysis of the association between GST T1 and the susceptibility to acquired SNHL according to the acquired SNHL types (A) and ethnicity (B).
Sensitivity analysis
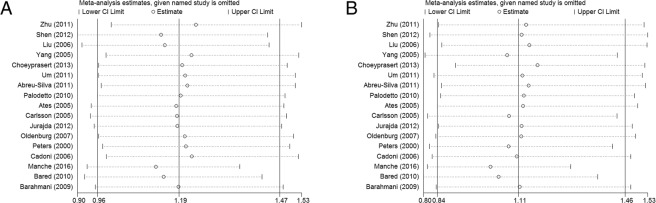
The included studies were removed one by one to investigate whether the study removed was the source of heterogeneity. Figure 5 shows that there was no significant difference in the pooled effect size when any of the studies were excluded. This result of the sensitivity analysis demonstrates that the pooled effect size of this meta-analysis was stable.
Figure 5.
Sensitivity analysis of the pooled effect size on the association between GST M1 (A) and T1 (B) polymorphisms and the susceptibility to acquired SNHL.
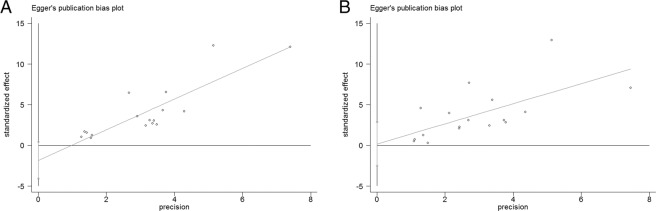
Publication bias
The risk of publication bias was analyzed by Egger’s test. The results are shown in Fig. 6. The p-values (95% CI) of GST M1 and T1 were 0.102(−4.071, 0.410) and 0.887(−2.504, 2.870), respectively. Both p values were >0.05, and the 95% CIs contained 0. Therefore, no publication bias was considered.
Figure 6.
Publication bias analyses (Egger’s test) for the pooled effect size.
Discussion
Oxidative stress is the most important and common molecular mechanism of acquired SNHL11–14. However, difficulties remain with the effective clinical application of antioxidants11,13,15,35. Therefore, identifying susceptibility factors of individuals in terms of the oxidative stress-related genetic background or gene polymorphisms may represent a new concept. GST M1 and T1 have been found to be important antioxidant enzymes in the human body, and they are associated with several kinds of oxidative stress-related diseases, including acquired SNHL6,22–25,36. However, in previous studies on the association of GST M1 and T1 polymorphisms with the susceptibility to acquired SNHL, the results are inconsistent or even contradictory. There may be at least two reasons for this inconsistency: (1) most of the previous studies were single-center studies with small sample sizes; and (2) most of the previous studies focused on the associations of GST M1 and T1 polymorphisms with only one type of acquired SNHL and neglected the fact that oxidative stress is the most important and common molecular mechanism of acquired SNHL. Based on the above theoretical basis, this meta-analysis included more studies on oxidative stress-related acquired SNHL types and larger sample sizes to further identify the association of GST M1 and T1 polymorphisms with the susceptibility to acquired SNHL. According to our results, neither the collective nor the subgroup analyses suggested an association between GST M1 and T1 polymorphisms and susceptibility to acquired SNHL.
A meta-analysis regarding the association between GST M1 and T1 polymorphisms and NIHL was performed by Zhou et al. in 2014. The study concluded that GSTM1polymorphisms, but not GST T1 polymorphisms, are related to noise-induced hearing loss. There are at least two major differences between Zhou’s study and our study. (1) Zhou et al. evaluated the association of GST M1 and T1 polymorphisms only with NIHL. Five studies were included in their meta-analysis, with a total of (GST M1 and T1) 914/909 cases with NIHL and 885/876 controls. However, the purpose of our study was to identify the possible relationship between GST M1 and T1 polymorphisms and acquired SNHL, which includes three other kinds of SNHL in addition to NIHL, with a total of 1749 cases and 2018 controls included in our meta-analysis. (2) Zhou et al. concluded that GST M1 polymorphisms are related to NIHL, but in our study, we found opposite results, although the included studies and samples in the NIHL subgroup in our study were the same as those included in their study. The different results can be attributed to the different models used in the two studies. In Zhou’s study, the fixed-effects model was applied to calculate the pooled effect size even though significant heterogeneity (51%) was observed among the studies. Such a method is worth discussing.
Heterogeneity is a major problem that affects the reliability of the pooled effect size in meta-analysis. In our meta-analysis, heterogeneity was observed for both GSTM1 and GST T1. The results of the subgroup analysis according to the type of acquired SNHL and ethnicity showed that heterogeneity was much smaller in some subgroups, but it was strong in other groups, suggesting that some other factors besides the acquired SNHL type and ethnicity served as sources of heterogeneity. We also performed a meta-regression analysis (Supplementary Table S1) and found that the various sample sizes, different publication date and diverse Newcastle-Ottawa scale (NOS) scores in the included studies were not major source of heterogeneity either. Other factors that may influence heterogeneity are listed as follows: (1) the diagnostic criteria of hearing loss in each study are not completely consistent; (2) the matching methods of cases and controls in different studies are diverse; (3) diverse methods of GST M1 and T1genotype detection were used; (4) the quality of each study (NOS score) was not completely consistent; and (5) different age ranges were involved in the included samples in each study.
There are at least two limitations in this meta-analysis. (1) Several studies that possibly met the inclusion criteria did not include primary data, so the ORs with their 95% CIs could not be calculated. We attempted to contact the authors for more information, but we received no response. The results may be influenced by these missing studies. (2) Although 17 independent studies containing a total of 1749 cases with acquired SNHL and 2018 controls were included in this meta-analysis, the sample size is still limited, especially in the process of subgroup analysis. For the DIHL subgroup, most studies contained only 10–20 samples, and for the SSHL subgroup, only 2 articles met the inclusion criteria.
To our knowledge, this is the first meta-analysis to focus on the association of GST M1 and T1 polymorphisms with the susceptibility to acquired SNHL. The results of our meta-analysis suggested that GST M1 and T1 polymorphisms may not serve as susceptibility factors for acquired SNHL. Considering the limitations of our meta-analysis, further prospective studies with large sample size and additional studies (e.g. effect of this polymorphism on gene expression, haplotype analysis for GST polymorphism etc.) are needed to validate study findings.
Methods
Search strategy
A comprehensive literature search was performed in the following databases: (1) PubMed; (2) Web of Science; (3) EMBASE; (4) OVID; (5) CNKI Chinese database and (6) Wanfang Chinese database. The MeSH and free terms were all included in our search terms, which are listed as follows: “Glutathione s-transferase”, “Glutathione transferase”, “hearing impairment”, “hearing loss”, “ototoxicity” and “deafness”. Our search logic in the PubMed database is listed as follows: “((((“hearing”[MeSH Terms] OR “hearing”[All Fields]) OR (“ear, inner”[MeSH Terms] OR (“ear”[All Fields] AND “inner”[All Fields]) OR “inner ear”[All Fields] OR “cochlea”[All Fields] OR “cochlea”[MeSH Terms])) OR ototoxicity[All Fields]) OR (“audiology”[MeSH Terms] OR “audiology”[All Fields])) AND (((“glutathione transferase”[MeSH Terms] OR (“glutathione”[All Fields] AND “transferase”[All Fields]) OR “glutathione transferase”[All Fields] OR “glutathione s transferase”[All Fields]) OR (“Glutathione Transferase”[Mesh] AND “Glutathione S-Transferase pi”[Mesh] AND “glutathione S-transferase T1”[Supplementary Concept] AND “glutathione S-transferase M1”[Supplementary Concept])) OR (“glutathione transferase”[MeSH Terms] OR (“glutathione”[All Fields] AND “transferase”[All Fields]) OR “glutathione transferase”[All Fields])) AND “humans”[MeSH Terms]”.
All studies that we searched were published before November 20th, 2018. We also manually checked all articles listed in the reference lists of the retrieved literature.
Inclusion criteria
Studies that met the following criteria were included: (1) independent studies investigating the relationship between GST M1 and T1 polymorphisms and the susceptibility to acquired SNHL and (2) studies including sufficient and definite original data (the genotype frequencies of GST M1 and T1 in the case and control groups) that could be used to calculate the OR with its 95% CI of each genotype. When duplicate publications were found, the data in the latest publication were used.
Data extraction and Quality assessment
The data in the included studies were extracted by two investigators independently using the same “Data Extraction Form”. The information extracted from the included studies is listed as follows: first author’s name, publication year, country of origin, ethnicity, genotype detection methods, the type of SNHL, and the number of cases and controls. The quality of each included study was evaluated using the Newcastle-Ottawa scale (NOS). The studies with an NOS score ≥7 were considered high-quality studies. All disagreements in the process of study selection, data extraction and quality assessment were discussed and resolved by consensus.
Meta-analysis
The association of GST M1 and T1 polymorphisms with acquired SNHL susceptibility was evaluated by the pooled OR and 95% CI.Statistical heterogeneity among the studies was measured with the I2 test. For a value of I2 < 30% and p > 0.1, a fixed-effects model was used to calculate the pooled ORs; otherwise, a random-effects model was used for a value of I2 ≥ 30%.Woolf’s method was applied to estimate the 95% CIs. We considered that there was statistical significance when the overall 95% CI did not include 1 and the p-value transformed from the Z score was less than 0.05. In addition, a subgroup analysis was performed according to the type of acquired SNHL and ethnicity. Sensitivity analysis was used to evaluate the stability of the pooled effect size. Publication bias was assessed by Egger’s test. Publication bias was considered for a p-value < 0.05 or if the 95% CI did not contain 0. All statistical analyses were performed using the Stata 13.1 software.
Supplementary information
Results of meta-regression considering sample size, publication year and NOS scores of the included studies.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81470697 and No. 81771002).
Author Contributions
Designed the study: Hongjun Xiao and Shimin Zong. Searched databases and collected full-text papers: Shimin Zong and Yexiao Guan. Extracted and analyzed the data: Tianyi Liu, Xue Zeng and Yexiao Guan. Statistical analyses: Pan Luo, Yanji Qu and Xue Zeng. Wrote the manuscript: Shimin Zong, Fangmin Wan, Pei Chen and Yexiao Guan. All authors reviewed the manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shimin Zong, Xue Zeng and Yexiao Guan contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37386-w.
References
- 1.Hesse G. Inner Ear Hearing Loss. Laryngorhinootologie. 2016;95:383–391. doi: 10.1055/s-0042-105216. [DOI] [PubMed] [Google Scholar]
- 2.Olusanya BO. “The right stuff”: the global burden of disease. PLoS Med. 2007;4:e84. doi: 10.1371/journal.pmed.0040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvill S. Sensory impairments, intellectual disability and psychiatry. J Intellect Disabil Res. 2001;45:467–483. doi: 10.1046/j.1365-2788.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 4.Mohr PE, et al. The societal costs of severe to profound hearing loss in the United States. Int J Technol Assess Health Care. 2000;16:1120–1135. doi: 10.1017/S0266462300103162. [DOI] [PubMed] [Google Scholar]
- 5.Kelly KM, Lalwani AK. On the Distant Horizon–Medical Therapy for Sensorineural Hearing Loss. Otolaryngol Clin North Am. 2015;48:1149–1165. doi: 10.1016/j.otc.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Manche SK, Jangala M, Putta P, Koralla RM, Akka J. Association of oxidative stress gene polymorphisms with presbycusis. Gene. 2016;593:277–283. doi: 10.1016/j.gene.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Kitoh R, et al. SOD1 gene polymorphisms in sudden sensorineural hearing loss. Acta Otolaryngol. 2016;136:465–469. doi: 10.3109/00016489.2015.1116047. [DOI] [PubMed] [Google Scholar]
- 8.Um JY, et al. Steroid combination therapy and detoxification enzyme gene polymorphisms in sudden sensorineural hearing loss patients. Otol Neurotol. 2011;32:872–876. doi: 10.1097/MAO.0b013e31821341ac. [DOI] [PubMed] [Google Scholar]
- 9.Oldenburg J, Fossa SD, Ikdahl T. Genetic variants associated with cisplatin-induced ototoxicity. Pharmacogenomics. 2008;9:1521–1530. doi: 10.2217/14622416.9.10.1521. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson PI, et al. The influence of genetic variation in oxidative stress genes on human noise susceptibility. Hear Res. 2005;202:87–96. doi: 10.1016/j.heares.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Tavanai E, Mohammadkhani G. Role of antioxidants in prevention of age-related hearing loss: a review of literature. Eur Arch Otorhinolaryngol. 2017;274:1821–1834. doi: 10.1007/s00405-016-4378-6. [DOI] [PubMed] [Google Scholar]
- 12.Jiang M, Karasawa T, Steyger PS. Aminoglycoside-Induced Cochleotoxicity: A Review. Front Cell Neurosci. 2017;11:308. doi: 10.3389/fncel.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gul F, et al. A comprehensive study of oxidative stress in sudden hearing loss. Eur Arch Otorhinolaryngol. 2017;274:1301–1308. doi: 10.1007/s00405-016-4301-1. [DOI] [PubMed] [Google Scholar]
- 14.Karasawa T, Steyger PS. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett. 2015;237:219–227. doi: 10.1016/j.toxlet.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sha SH, Schacht J. Emerging therapeutic interventions against noise-induced hearing loss. Expert Opin Investig Drugs. 2017;26:85–96. doi: 10.1080/13543784.2017.1269171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cadoni G, et al. Glutathione s-transferase gene polymorphisms in Italian patients with sudden sensorineural hearing loss. Otol Neurotol. 2006;27:1166–1169. doi: 10.1097/01.mao.0000226303.59198.ce. [DOI] [PubMed] [Google Scholar]
- 17.Takumi Y, et al. Various glutathione S-transferase isoforms in the rat cochlea. Neuroreport. 2001;12:1513–1516. doi: 10.1097/00001756-200105250-00042. [DOI] [PubMed] [Google Scholar]
- 18.Touliatos JS, Neitzel L, Whitworth C, Rybak LP, Malafa M. Effect of cisplatin on the expression of glutathione-S-transferase in the cochlea of the rat. Eur Arch Otorhinolaryngol. 2000;257:6–9. doi: 10.1007/PL00007509. [DOI] [PubMed] [Google Scholar]
- 19.Board PG. Biochemical genetics of glutathione-S-transferase in man. Am J Hum Genet. 1981;33:36–43. [PMC free article] [PubMed] [Google Scholar]
- 20.Pemble S, et al. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J. 1994;300(Pt 1):271–276. doi: 10.1042/bj3000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabinowitz PM, et al. Antioxidant status and hearing function in noise-exposed workers. Hear Res. 2002;173:164–171. doi: 10.1016/S0378-5955(02)00350-7. [DOI] [PubMed] [Google Scholar]
- 22.Choeyprasert W, et al. Cisplatin-induced ototoxicity in pediatric solid tumors: the role of glutathione S-transferases and megalin genetic polymorphisms. J Pediatr Hematol Oncol. 2013;35:e138–143. doi: 10.1097/MPH.0b013e3182707fc5. [DOI] [PubMed] [Google Scholar]
- 23.Shen H, et al. Genetic variation in GSTM1 is associated with susceptibility to noise-induced hearing loss in a Chinese population. J Occup Environ Med. 2012;54:1157–1162. doi: 10.1097/JOM.0b013e31825902ce. [DOI] [PubMed] [Google Scholar]
- 24.Bared A, et al. Antioxidant enzymes, presbycusis, and ethnic variability. Otolaryngol Head Neck Surg. 2010;143:263–268. doi: 10.1016/j.otohns.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Yimin YZ, Yongmei X, Qihua X, Xiaoli L, Lvwu X. The relationship between GSTM1 and GSTT1 genetic polymorphisms and susceptibility to noise-induced hearing loss. zhong guo zhi ye yi xue. 2006;33:343–354. [Google Scholar]
- 26.Jurajda M, et al. Genetic background of cisplatin induced ototoxicity. Klin Onkol. 2012;25:184–187. [PubMed] [Google Scholar]
- 27.YuHua Z, Pu D, HuiJun Y, SuoQiang Z. Association analysis of GSTT1 and GSTM1 genes with hereditary susceptibility to age-related hearing loss among Chinese. Chinese Journal of Otology. 2011;9:12–16. [Google Scholar]
- 28.Abreu-Silva RS, et al. The search of a genetic basis for noise-induced hearing loss (NIHL) Ann Hum Biol. 2011;38:210–218. doi: 10.3109/03014460.2010.513774. [DOI] [PubMed] [Google Scholar]
- 29.Palodetto B, Postal M, Grignoli CR, Sartorato EL, Oliveira CA. Influence of glutathione s-transferase on the ototoxicity caused by aminoglycosides. Braz J Otorhinolaryngol. 2010;76:306–309. doi: 10.1590/S1808-86942010000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barahmani N, et al. Glutathione S-transferase M1 and T1 polymorphisms may predict adverse effects after therapy in children with medulloblastoma. Neuro Oncol. 2009;11:292–300. doi: 10.1215/15228517-2008-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25:708–714. doi: 10.1200/JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- 32.Yang M, Tan H, Zheng JR, Jiang CZ. Relationship between GSTM1 and GSTT1 gene polymorphisms and noise induced hearing loss in Chinese workers. Wei Sheng Yan Jiu. 2005;34:647–649. [PubMed] [Google Scholar]
- 33.Ates NA, et al. Glutathione S-transferase gene polymorphisms in presbycusis. Otol Neurotol. 2005;26:392–397. doi: 10.1097/01.mao.0000169774.23668.f1. [DOI] [PubMed] [Google Scholar]
- 34.Peters U, et al. Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Drugs. 2000;11:639–643. doi: 10.1097/00001813-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg. 2007;15:364–369. doi: 10.1097/MOO.0b013e3282eee452. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, et al. Role of Glutathione-S-transferases in neurological problems. Expert Opin Ther Pat. 2017;27:299–309. doi: 10.1080/13543776.2017.1254192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of meta-regression considering sample size, publication year and NOS scores of the included studies.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).