Abstract
Knowledge of Wolbachia prevalence with respect to its hosts is restricted mainly to taxonomic/phylogenetic context. In contrast, relations between infection and most host’s ecological and biological traits are poorly understood. This study aimed to elaborate on relations between bacteria and its beetle hosts in taxonomic and the ecological contexts. In particular, the goal is to verify which ecological and biological traits of beetles could cause them to be prone to be infected. Verification of Wolbachia infection status across 297 beetle taxa showed that approximately 27% of taxa are infected by supergroups A and B. Only minor support for coevolution between bacteria and its beetle hosts was observed in some genera of beetles, but in general coevolution between beetles and Wolbachia was rejected. Some traits of beetles were found to be unrelated to Wolbachia prevalence (type of range and thermal preferences); some traits were related with ambiguous effects (habitats, distribution, mobility and body size); some were substantially related (reproduction mode and trophy). The aforementioned summary does not show obvious patterns of Wolbachia prevalence and diversity in relation to host taxonomy, biology, and ecology. As both Wolbachia and Coleoptera are diverse groups, this lack of clear patterns is probably a reflection of nature, which is characterised by highly diversified and probably unstable relations.
Introduction
The intracellular α-proteobacterium Wolbachia is considered the most abundant endosymbiont of invertebrates. It has been reported as being found in arthropods and filarial nematodes around the world1,2. Taxonomy and phylogenetic relations of this bacterium has been topic of intense debate ever since Hertig’s description of Wolbachia pipientis3. The greatest problems with its classification were caused by the huge diversity of strains described on the basis of selected gene sequencing. This has resulted in the identification of sixteen phylogenetic supergroups, ten of which are found in arthropods, five in nematodes, and one in both groups4. Recently, it has been proposed that the different Wolbachia supergroups belong to several “Candidatus Wolbachia” species5; however, this approach has been criticized6.
The increased interest in Wolbachia has been motivated by the diversity of its phenotypic effects. In arthropods, it can manipulate the host reproduction through male-killing7, cytoplasmic incompatibility8, parthenogenesis induction9, and feminization of genetic males10. These effects on host reproduction and development could result in diversification of populations and consequently lead to speciation. The latter could be connected with coevolution of bacteria and its hosts; support for such phenomenon, however, is rare11. In contrast, recent studies have shown that Wolbachia is not only transmitted vertically (basically matrilineally), but could also spread horizontally using different vectors such as parasites, predators, or common food resources, e.g. host plants12–16. This process is probably common and explains the general lack of congruence between Wolbachia and host phylogenies, with some exceptions for low-taxonomic levels (e.g. within some genera). Moreover, evidence of horizontal transmission opens numerous possibilities for ecological interactions between bacteria and hosts belonging to various taxonomic units and trophic assemblages.
Wolbachia infects numerous species of insects. It is estimated that more than 65% of the species could carry members of the Wolbachia clade17. This value, however, differs greatly across examined groups. The current state of knowledge about Wolbachia infection among some taxonomic groups of nematodes and arthropods has been summarized in several studies (e.g., for: filarial nematodes (Filarioidea)1; crustaceans (Crustacea)18; spiders (Araneae)14; springtails (Collembola)19; heteropteran bugs (Heteroptera)20; wasps (Hymenoptera: Apocrita)21; and butterflies (Lepidoptera)22). Surprisingly, knowledge about infection in beetles (Coleoptera) is quite random, as has been recently summarized by Kajtoch & Kotásková23. Beetles are the most species-rich and diversified group of organisms in the world, including approximately 386,000 known species24. They can be found in most terrestrial and freshwater habitats. Members of Coleoptera belong to all major trophic guilds known in animals. Thus far, only some groups of beetles have been examined with respect to Wolbachia infection, but usually on a limited coverage of species (e.g. weevils, Curculionidae and apionids, Apionidae25,26; leaf beetles, Chrysomelidae27,28; jewel beetles, Buprestidae29, and minute moss beetles, Hydraenidae29). There are no studies that show prevalence of Wolbachia across various families of beetles, with the exception of preliminary works on insects from Panama and North America, where some random species of beetles were included resulting in bacteria detection in 10.5% of beetles from Panama and 13.5% of beetles from North America30,31. More importantly, there are also almost no studies that try to solve which biological or ecological features of the hosts could have made them prone or resistant to infection. The only exception is a preliminary work on some European weevils25, which was based only on a limited coverage of species and sampling. Systematic review of Wolbachia occurrence among beetles has shown that the approximate infection rate in this group is 38%. However, this value varies greatly in different families and genera, and it could be overestimated due to sampling biases (studies focused on groups with members known to be infected). This review rejected cospeciation between Wolbachia and Coleoptera, with only some exceptions observed for freshwater Hydraenidae29. Finally, this review has shown that, while bacteria effects on hosts are known for some taxa, almost no study has examined the effects of host biological and ecological traits on occurrence and diversity of Wolbachia. This is a large gap in knowledge, restricted not only to Coleoptera, but also to insects and all arthropods in general.
This study, by verifying Wolbachia infection status across approximately 300 beetle taxa, aimed to solve several questions related to bacteria relations with its beetle hosts. As background for this study, genetic marker data were used for understanding relationships between endosymbiotic bacteria and their beetle hosts in the phylogenetic context. This part aimed to verify whether there is coevolution between Wolbachia and its hosts from the Coleoptera (H1). This part was assessed on two levels: for several exemplary genera (H1a) and for superfamilies (H1b). As the presence or the lack of coevolution between beetles and Wolbachia does not preclude other associations between bacteria and hosts, we intended to verify the following hypotheses. First, we tested the assumption that Wolbachia infection is prevalent in particular taxonomic groups (or that some taxonomic groups of beetles are prone to infection more than others) (H2). Secondly, the presence and distribution of Wolbachia strains were analysed in the context of host (beetle) ecological affinity to verify the hypotheses that Wolbachia infection is associated with hosts representing particular ecological niches (described by some biological strategies and characteristics and ecological traits of hosts) (H3). This set of hypotheses should allow for the identification of some biological and ecological traits that make (single or together) hosts prone to Wolbachia infection. Focus was placed on verification of additional hypotheses centred around trophic affinity of hosts, i.e., i) Wolbachia infection is prevalent in beetles belonging to a particular trophic guild (H4a) and ii) Wolbachia strains are more related within members of a particular trophic guild (H4b). H4a assume unequal prevalence of Wolbachia in predators, herbivores, cambio- and xylophages, saprophages, mycetophages. H4b assume that the presence and distribution of similar (related) Wolbachia strains is correlated with host trophic affinity (e.g. that strains within herbivores are more related than strains found in herbivores and predators).
Results
297 species were selected for molecular examination. The majority of these species were analysed using two to five or more specimens; only some (mainly rare taxa) could be examined using a single representative. These beetles belonged to 37 families and 204 genera. For details about sampling design see Methods section.
General patterns of Wolbachia infection across beetle taxonomy
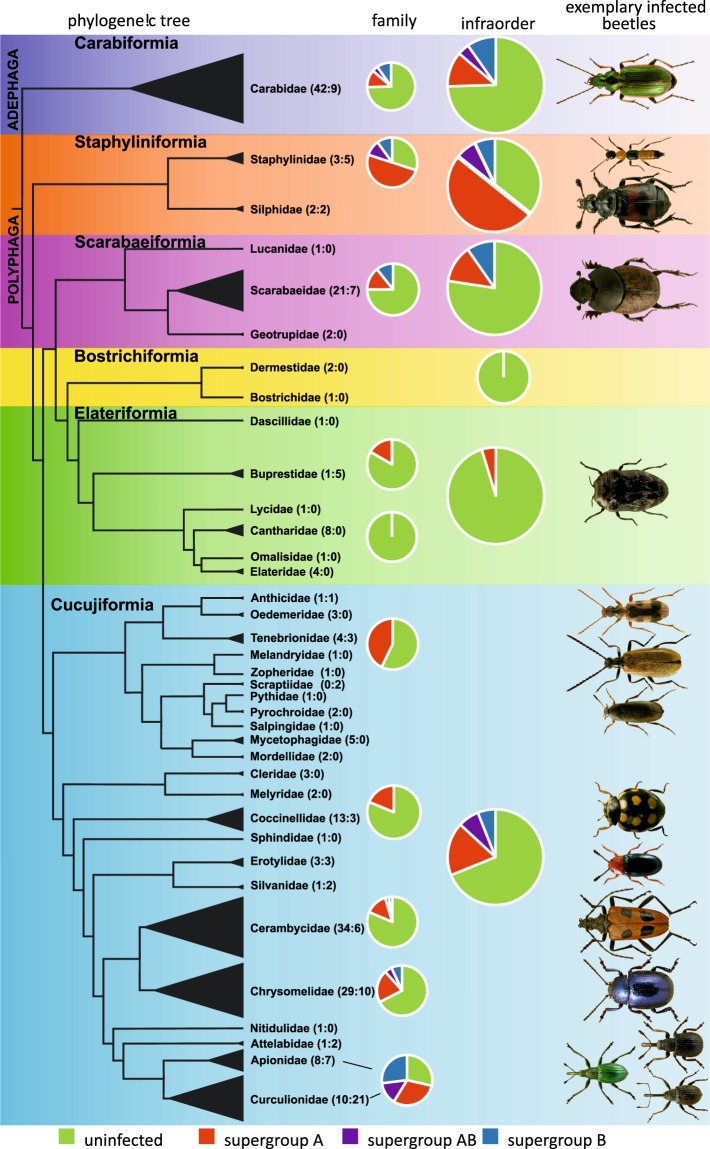
Among the 297 examined beetle species, 81 included Wolbachia, which gives an infection rate of 27.3%. For 31 taxa all genes of Multilocus sequence typing (MLST) system32 were successfully amplified and sequenced and for remaining 50 taxa only some genes could be successfully amplified and sequenced (for some, amplification succeeded but generated sequences were of poor quality despite several trials). Similar patterns of Wolbachia gene amplification and sequencing have been reported in other studies33, including studies on beetles34. These are usually caused by mismatches in priming sites of standard primers designed for this bacterium due to its great polymorphism32. The majority of species were found to be infected in all examined specimens, but one third showed infection only in some specimens. Infection rates in Polyphaga were greater (29%) than in Adephaga (19%) (Supplementary Table S1, Fig. 1). Regarding infraorders, the greatest infection rate was found in Staphyliniformia (54%), followed by Cucujiformia (31%), Scarabaeiformia (23%), Carabiformia (19%), and Elateriformia (5%). No infection was found in Bostrichiformia, but only three species from this infraorder were examined. Infection rates in particular superfamilies were highest in Curculionoidea (59%) and Staphylinoidea (54%), followed by Cucujoidea (25%), Tenebrionoidea (23%), Scarabaeoidea (23%), Chrysomeloidea (20%), Caraboidea (19%), and Buprestoidea (17%). No infection was found in examined members of Bostrichoidea, Cleroidea, Dascilloidea, and Elateroidea (all these were examined either in a single species or a few species). On the level of family, if considering only those families with more than five examined species, the most infected were: Curculionidae (68%), Staphylinidae (63%), Erotylidae (50%), Apionidae (47%), and Tenebrionidae (43%). Lower infection rates were found in Chrysomelidae (26%), Carabidae (19%), Coccinellidae (19%), and Buprestidae (17%); and no infection was found in, e.g. Cantharidae and Mycetophagidae (see details in Supplementary Table S1, Fig. 12).
Figure 1.
Approximate prevalence of Wolbachia infection in selected families and infraorders of beetles. Presented are shares of uninfected species (green), infected species by only supergroup (A) (red), only supergroup (B) (blue) and by both supergroups (violet). Wolbachia prevalence is presented on the background of simplified phylogenetic tree of beetle families considered in the study (reconstructed on the basis of mitochondrial trees topologies from Bocak et al.71 and McKenna et al.72). Exemplary infected beetle hosts presented to the right (photographs are reprinted from ICONOGRAPHIA COLEOPTERORUM POLONIAE under a CC BY license, with permission (© Copyright by Prof. Lech Borowiec, Wrocław 2007–2018, Department of Biodiversity and Evolutionary Taxonomy, University of Wrocław, Poland)).
All infected beetles harboured either strains belonging to supergroup A or B. Supergroup A was found in 64 species (21.5% prevalence); supergroup B in 34 species (11.4% prevalence). Supergroup A was more abundant in Polyphaga (2-times) and nearly the same frequency as supergroup B in Adephaga (1.1-times) (Supplementary Table S1, Fig. 1). Also, in the levels of infraorders and superfamilies, supergroup A prevailed over supergroup B. Supergroup A was prevalent in most families, especially in Cerambycidae (3.5-times) and Staphylinidae (3-times), whereas supergroup B was more abundant only in Apionidae (1.4-times). In some families the share of supergroup A and B was the same (Anthicidae) or very similar (Carabidae, Curculionidae, Scarabaeidae) (Supplementary Table S1).
Infection rates were significantly different only on the level of families (Table 1, Supplementary Fig. S1A). Also, the share of both supergroups differed significantly only between families. On the levels of superfamilies and suborders, these differences were nearly significant (Table 1, Supplementary Fig. S1A).
Table 1.
Summary of Kruskal-Wallis analysis of variance (ANOVA) calculated for Wolbachia (infected vs. uninfected beetle hosts and for hosts infected by supergroup A vs. supergroup B) in respect to major levels of beetles taxonomy.
| groups | uninfected vs infected | supergroups A vs B | ||||
|---|---|---|---|---|---|---|
| ANOVA | H-test | df | p-value | H-test | df | p-value |
| Taxonomy | ||||||
| family | 65.34 | 36 | 0.002 | 23.68 | 14 | 0.050 |
| superfamily | 8.34 | 11 | 0.682 | 19.29 | 11 | 0.056 |
| infraorder | 0.38 | 5 | 0.996 | 10.31 | 5 | 0.067 |
| suborder | 0.07 | 1 | 0.793 | 3.71 | 1 | 0.054 |
In bold: significant tests (p < 0.05).
Wolbachia diversity and co-evolution
Among the 81 infected beetle species, 64 (79%) were found to harbour Wolbachia supergroup A and 34 (42%) were found to harbour supergroup B, including 16 species (20%) with strains belonging to both supergroups. The majority of beetle hosts (individuals) were infected by a single strain, but 11 were infected by two or more strains.
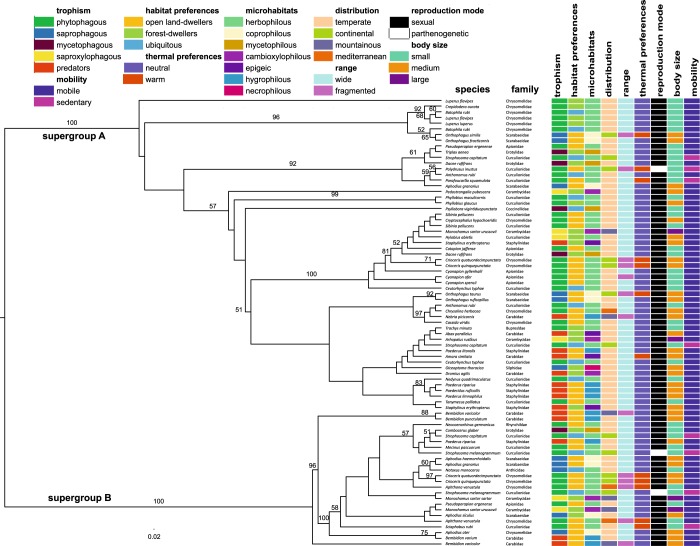
Phylogenetic trees of the cell division protein gene (ftsZ) showed that Wolbachia is highly diversified in beetle hosts (Fig. 2, Supplementary Fig. S2). Both datasets DS1 and DS2 (see Material and Methods for details) confirmed that strains belonging to supergroup A were prevalent over those from supergroup B in beetle hosts. Moreover, the diversity of strains from supergroup A found in beetles (in this study as well as if including hosts from other studies, summarized in Kajtoch & Kotásková23) is much greater than those belonging to supergroup B.
Figure 2.
Wolbachia phylogenetic tree (reconstructed on the basis of ftsZ sequences obtained from infected beetle hosts) with reference to selected ecological and biological traits of the hosts.
Unique Wolbachia strains were found in the majority of beetles. In contrast, similar or the same strains were found only rarely, in some closely related species (Fig. 2, Supplementary Fig. S2). Most of these exemplary similarities have already been described in previous works on particular genera (e.g. Crioceris leaf beetles35, Cyanapion apionids36; Polydrusus and Eusomus weevils37,38; Strophosoma weevils39; Monochamus longhorn beetles40; Bembidion ground beetles and Paederus/Paederidus rove beetles41). Analyses in Procrustes Approach to Cophylogeny (PACo) confirmed coevolution of Wolbachia with only Monochamus longhorn beetles (P = 0.0364) and Bembidion ground beetles (P = 0.0299) (Supplementary Fig. S3). For other groups, cospeciation events were rejected (Crioceris leaf beetles, P = 0.7998; Cyanapion apionids, P = 0.3599; Aphodius dung beetles, P = 0.9685; Paederus/Paederidus rove beetles, P = 0.1051) (Supplementary Fig. S3). There were no significant differences in Wolbachia strain genetic distances within and between superfamilies of beetle hosts (Supplementary Table S2, U = 43.00, Z = −1.592, P = 0.111).
General patterns of Wolbachia infection across beetle traits
Regarding trophic guilds, Wolbachia was most abundant within phytophagous species (38.6%), followed by saprophagous (34.3%), mycetophagous (23.5%), and less abundant in cambioxylophagous (18.5%) and predators (17.6%) (Supplementary Table S1). Wolbachia was most abundant among open land-dwellers (32.8%) and less numerous in forest-dwellers (23.0%) and ubiquitous species (22.2%). Considering microhabitats, infection was most prevalent in necrophilous species (40.0%), hygrophilous (36.0%), coprophilous (35.0%), and herbophilous (33.6%); less infected species were found in mycetophilous (26.7%), cambioxylophilous (16.4%), and epigeic (13.2%). The lowest infection rate was found in Mediterranean species (11.8%). Similar rates were found within continental (32.0%), temperate (27.8%), and mountainous (27.3%) species. Wolbachia had the same prevalence in species with fragmented ranges (27.1%) and widespread ranges (27.7%). There are also no obvious differences with respect to thermal preferences, as 30.6% species of neutral temperature preferences were infected and 26.7% species of warm preferences were infected. In contrast, a great difference was found between bisexual species (26.3% infected) and parthenogenetic species (80.0% infected, but only five species in this group could be examined). There were also differences in infection rate with respect to body size of the hosts: the most frequently infected hosts were found to be small beetles (40.0%), next medium-sized (21.7%), and finally large taxa (13.0%). Flightless beetles were much more often infected (43.5%) than mobile taxa (25.8%) (Supplementary Table S1).
Across species traits of beetles in almost all states, the more abundant was supergroup A (Supplementary Table S1). Differences were particularly noticeable, for example, in cambioxylophagous (10-times more supergroup A strains than these from supergroup B), mycetophagous (3.0-times), forest-dwellers (4.6-times), epigeic species (4.0-times), necrophilous (all infected by supergroup A), widespread species (2.8-times), or medium size species (2.8-times). The same share of supergroup A and B was found in Mediterranean taxa and large species, and nearly the same share in phytophagous, open land-dwellers, coprophilous, and hygrophilous species (Supplementary Table S1).
According to univariate models, differences between uninfected and infected beetles were best explained by trophism, microhabitats, reproduction mode, and body size (Table 2, Supplementary Fig. S1B). These traits have significant Wald statistics (with exception of microhabitats), lowest Akaike Information Criterion (AIC), and highest Nagelkerke pseudo R2 values (Table 2). Any univariate models comparing infection by supergroups A and B have significant Wald statistics; all have similar AIC and R2 values (Table 2, Supplementary Fig. S1B, Supplementary Fig. S1B).
Table 2.
Performance of univariate models representing analysed traits of Wolbachia hosts (beetles).
| trait | d.f. | Wald | p-value | AIC | R2 |
|---|---|---|---|---|---|
| unifected vs infected | |||||
| Intercept | 1 | 57.3 | 0.000 | 350.7 | — |
| trophism | 4 | 13.5 | 0.009 | 344.7 | 0.046 |
| habitat preferences | 2 | 3.7 | 0.154 | 350.9 | 0.013 |
| microhabitats | 6 | 11.7 | 0.068 | 349.5 | 0.043 |
| distribution | 3 | 2.2 | 0.534 | 354.0 | 0.009 |
| range | 1 | 0.2 | 0.628 | 352.5 | 0.001 |
| thermal preferences | 1 | 0.0 | 0.936 | 352.7 | 0.000 |
| reproduction mode | 1 | 4.6 | 0.032 | 346.5 | 0.021 |
| body size | 2 | 12.7 | 0.002 | 341.6 | 0.043 |
| mobility | 1 | 3.2 | 0.073 | 349.6 | 0.010 |
| supergroup A vs supergroup B | |||||
| Intercept | 1 | 9.6 | 0.002 | 126.4 | — |
| trophism | 4 | 4.0 | 0.406 | 129.0 | 0.054 |
| habitat preferences | 2 | 4.6 | 0.101 | 125.2 | 0.052 |
| microhabitats | 6 | 4.4 | 0.617 | 130.3 | 0.080 |
| distribution | 3 | 0.4 | 0.947 | 132.0 | 0.004 |
| range | 1 | 0.0 | 0.951 | 128.4 | 0.000 |
| thermal preferences | 1 | 0.4 | 0.515 | 127.9 | 0.005 |
| reproduction mode | 1 | 0.1 | 0.773 | 128.3 | 0.001 |
| body size | 2 | 2.2 | 0.339 | 128.2 | 0.022 |
| mobility | 1 | 0.0 | 0.862 | 128.4 | 0.000 |
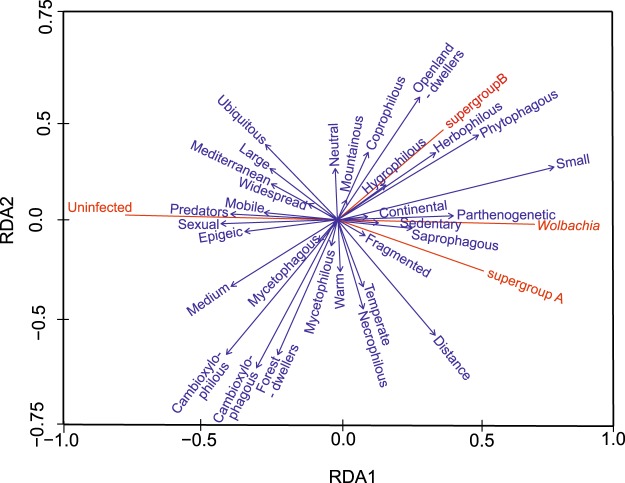
Redundancy analysis (RDA) showed that general infection by Wolbachia in beetles is associated mostly with parthenogenesis, limited mobility, continental distribution, and small body size, whereas uninfected species are mostly bisexual, mobile, predatorial, and epigeic (Fig. 3). Specifically, infection by supergroup A was associated with fragmented range but not with large body size and Mediterranean distribution. Supergroup B was mostly associated with hydrophilous and herbophilous/phytophagous species but not with cambioxylophagous/philous species (Fig. 3).
Figure 3.
Relationships between the Wolbachia infection status in examined beetle hosts and explanatory variables (ecological and biological traits of the hosts) revealed by redundancy analysis (RDA).
Multivariate analyses showed that occurrence of infected beetles is best explained by a model that includes trophism and reproduction mode. Slightly “worse” models also included range/climate and genetic distance (Table 3). Summarized Akaike weights were the highest for reproduction mode, distance, and trophism (Supplementary Table S3). Differences in prevalence of supergroups were best explained by a model that included habitat and other similarly fitted models including trophism and genetic distance (Table 3). Habitat and distance also had highest Akaike weights (Supplementary Table S3).
Table 3.
Set of competing generalized linear models with binomial error distribution and logit-link function explaining the Wolbachia infection status of examined beetle hosts or explaining differences in infection between two supergoups (A and B) of infected hosts on the basis of predictors described in the ‘Methods’ section.
| No | Model | k | AIC | Δ | w |
|---|---|---|---|---|---|
| infected vs uninfected | |||||
| INTERCEPT | 0 | 350.7 | 8.0 | 0.001 | |
| 1 | Dist + Trophism + Reprod | 3 | 342.7 | 0.0 | 0.032 |
| 2 | Dist + Range/Climate + Trophism + Reprod | 4 | 342.7 | 0.0 | 0.032 |
| 3 | Trophism + Reprod | 2 | 342.9 | 0.2 | 0.029 |
| 4 | Range/Climate + Trophism + Reprod | 3 | 343.4 | 0.7 | 0.023 |
| 5 | Dist + Habitat + Reprod | 3 | 343.5 | 0.8 | 0.022 |
| 6 | Dist + Reprod | 2 | 343.6 | 0.9 | 0.021 |
| 7 | Dist + Range/Climate + Habitat + Reprod | 4 | 343.8 | 1.1 | 0.019 |
| 8 | Dist + Range/Climate + Habitat + Trophism + Reprod | 5 | 343.9 | 1.2 | 0.018 |
| 9 | Trophism + Body + Reprod | 3 | 343.9 | 1.2 | 0.017 |
| 10 | Dist + Trophism + Body + Reprod | 4 | 344.1 | 1.4 | 0.016 |
| 11 | Dist + Trophism | 2 | 344.1 | 1.4 | 0.016 |
| 12 | Dist + Body + Reprod | 3 | 344.1 | 1.4 | 0.016 |
| 13 | Dist + Range/Climate + Trophism + Reprod + Mobility | 5 | 344.1 | 1.4 | 0.016 |
| 14 | Dist + Range/Climate + Trophism + Body + Reprod | 5 | 344.2 | 1.5 | 0.015 |
| 15 | Range/Climate + Trophism + Body + Reprod | 4 | 344.4 | 1.7 | 0.014 |
| 16 | Dist + Trophism + Reprod + Mobility | 4 | 344.4 | 1.7 | 0.013 |
| 17 | Dist + Habitat + Trophism + Reprod | 4 | 344.5 | 1.8 | 0.013 |
| 18 | Trophism + Reprod + Mobility | 3 | 344.5 | 1.8 | 0.013 |
| 19 | Range/Climate + Trophism + Reprod + Mobility | 4 | 344.6 | 1.9 | 0.012 |
| 20 | Dist + Habitat + Body + Reprod | 4 | 344.6 | 1.9 | 0.012 |
| 21 | Dist + Body + Reprod + Mobility | 4 | 344.6 | 1.9 | 0.012 |
| supergroup A vs supergroup B | |||||
| INTERCEPT | 126.4 | 3.4 | 0.011 | ||
| 1 | Dist + Trophism | 2 | 123.0 | 0.0 | 0.062 |
| 2 | Dist + Habitat | 2 | 123.1 | 0.1 | 0.059 |
| 3 | Habitat | 1 | 123.7 | 0.6 | 0.045 |
| 4 | Dist + Habitat + Trophism | 3 | 123.9 | 0.9 | 0.039 |
| 5 | Dist + Range/Climate + Habitat | 3 | 124.3 | 1.3 | 0.032 |
| 6 | Dist + Habitat + Body | 3 | 124.5 | 1.5 | 0.030 |
| 7 | Dist + Habitat + Mobility | 3 | 124.5 | 1.5 | 0.029 |
| 8 | Dist + Trophism + Mobility | 3 | 124.6 | 1.6 | 0.028 |
| 9 | Range/Climate + Habitat | 2 | 124.7 | 1.7 | 0.026 |
| 10 | Dist + Habitat + Reprod | 3 | 124.9 | 1.9 | 0.024 |
| 11 | Dist + Range/Climate + Trophism | 3 | 124.9 | 1.9 | 0.024 |
| 12 | Dist + Body + Trophism | 3 | 125.0 | 2.0 | 0.023 |
| 13 | Dist + Trophism + Reprod | 3 | 125.0 | 2.0 | 0.023 |
Only best performed models (with Δ < 2.0) are presented. The number of predictors (k), the Akaike information criterion score (AIC), the difference between the given model and the most parsimonious model (Δ) and Akaike weight (w) are listed for each model. The models with highest (w) are marked in bold; and the best fitted model according to recommendation of Arnold (2010) are marked in italic bold.
There were no significant differences of Wolbachia strains genetic distances within and between trophic guilds of beetle hosts (Supplementary Table S2, U = 19.00, Z = −0.674, P = 0.501).
Discussion
Surprisingly, among numerous studies on Wolbachia infection prevalence and interactions with its hosts1,2 no study treats with significance host ecological and biological traits. This study brings the first comprehensive analyses showing which host traits might or might not cause some species prone to harbour Wolbachia infection. This analysis was executed on the example of beetles – the most diverse order of insects and arthropods, members of which exhibit the majority of ecological and biological traits known for animals. There is extensive knowledge about Wolbachia infection in this group (summary in Kajtoch & Kotásková23); however, previous data omitted aspects related with general impact of ecological and biological traits on bacteria occurrence. This study substantially improve current state of knowledge about Wolbachia infection in Coleoptera as it extend number of inspected species of about 60% (from c. 530 to c. 830 species) and number of infected species of about 40% (from 204 to 271 species)23.
Estimated prevalence of Wolbachia among beetles in this study – approximately 27% – is lower than most calculations for various groups of insects, which are estimated in a wide range between 20% and 70%17,20,22. It is also lower than an estimate based on systematic review across literature about Wolbachia infection in beetles (approximately 38%23). However, most other estimates were based on non-random sampling, i.e. they were calculated on studies of particular taxonomic group of insects, which were usually selected on the basis of previous knowledge about Wolbachia occurrence. Consequently, these studies were focused on groups with a relatively high share of infected taxa. This especially concerned beetles for which the majority of studies were done for weevils (Curculionidae). This group was found to have an exceptionally high Wolbachia prevalence (approximately 40%, according to Lachowska et al.25 and 68% for Curculionidae or 47% for Apionidae in this study). A much lower infection rate in Coleoptera was suggested by geographically-focused studies in which Wolbachia was found in only 10.5% of beetles from Panama and 13.5% of beetles from North America30,31. These values, which were probably underestimated due to less effective Wolbachia detection in early studies, showed that in totally random sampling Wolbachia found to be less frequent than in taxonomy-focused studies (e.g. for some particular families).
This study supported a statement that Wolbachia is not associated with its hosts’ phylogeny. Among six genera tested in details, only in two cases significant support was found for cospeciation events. Monochamus longhorn beetles probably gave false positive results, as only two out of six examined taxa were infected with Wolbachia and these two were closest relatives (presumed subspecies)40. Only in the case of Bembidion ground beetles analyses demonstrate cospeciation between various hosts and bacterial strains (more details in Kolasa et al.,41). This lack of congruence between beetles and Wolbachia phylogeny is frequently reported in studies with only minor exceptions concerning aquatic species like Hydraenidae29. Aquatic insects are generally more prone to be infected with Wolbachia than terrestrial insects42,43. Also, a comparison of genetic distances among Wolbachia strains found in beetles show that there are no significant differences between strains found within members of the same superfamily and these from different superfamilies. That general lack of coevolution between beetle hosts and Wolbachia should be interpreted in light of the latest conception that Wolbachia is not tightly linked with its hosts, but exists as invasion dynamics resulting from horizontal transfers and extinctions44. These gains and loses of bacteria explain its global distribution and suggest that Wolbachia is in an epidemiological equilibrium44. Horizontal transmission of Wolbachia has become a major topic of numerous studies and has resulted in increasing evidence that Wolbachia can spread not only vertically (maternally) but frequently change its hosts using direct or indirect contacts (such as predators, parasitoids, host plants, and just common habitat28,35,37,45). The role of horizontal transfer for spread and diversity of endosymbiotic bacteria is probably unsatisfactory studied and deserves more attention, especially in the light of the recent review by Chrostek et al.46. Horizontal transfer has been reported in 13% of examined beetle species, but was exceptionally frequent among leaf-eaters such as Curculionidae and Chrysomelidae23. Horizontal transmission could also mimic cospeciation if occurring between closely related species sharing the same habitat35,37. In such cases, similar Wolbachia strains could be present in related species, not because of inheritance of bacteria from common ancestor, but because of bacteria transfer between species, e.g. feeding on the same host plant.
If coevolution is not responsible for Wolbachia prevalence among its hosts, here in the example of beetles, what other factors could be responsible? Horizontal transmission is probably an underestimated phenomenon46. It can explain widespread occurrence of this bacterium, including presence of similar strains in unrelated hosts. But horizontal transmission could either be random or depend on some ecological and/or biological traits of infected and uninfected species. Surprisingly, there are almost no studies that consider if some traits could make species to be more prone or resistant to Wolbachia infection. There are numerous studies which deal with some aspects of hosts’ biological relations with Wolbachia, particularly those related with the impact of bacteria on host reproduction. This is why Wolbachia is mainly known from its effects including cytoplasmic incompatibility, thelytokous parthenogenesis, feminization of genetic males, male-killing, increased mating success of infected males via sperm competition, and the host’s complete dependence on bacteria for egg production (for reviews see9,47). Indeed, among the ecological/biological traits of beetles, reproduction mode was among those that had the greatest impact on Wolbachia prevalence. This effect could somehow be biased by unequal sampling in this study, as there are only approximately 2% of parthenogenetic species (and almost all of them were infected). This sampling simply reflects reality because parthenogenesis among European beetles is known almost exclusively from several species of weevils (in general, only approximately 0.15% of beetle species worldwide from 20 families are parthenogenetic48). Reproduction is one of the traits for which it is the hardest to solve if the Wolbachia is abundant in parthenogens as it induced parthenogenesis, or because this bacteria benefits from spreading across parthenogenetic hosts49. This problem has been raised in several studies focused on parthenogenetic weevils (e.g.34,37,39,50).
Multiple analyses considering relations between infection and ecological or biological traits of infected and uninfected beetles showed that there is no general pattern that clearly explains this problem. Both infected and uninfected taxa of beetles share various combinations of traits related to their biology and ecology. This could suggest that there are no clear factors that cause some species to be Wolbachia infected or not. It seems congruent with the latest finding that Wolbachia exists in equilibrium among arthropods, i.e., it spreads frequently among species (mostly via horizontal transmission, rarely via inheritance from common ancestors), and is also lost in some taxa/populations44. However, some results suggest (Tables 2 and 3, Fig. 3, Supplementary Fig. S1B) that particular states of some traits are significantly more often present in infected than in uninfected beetles. It is interesting that all traits related with geography (if consider separately), such as their distribution, range and thermal preferences, showed almost no relation with infection status (Table 2, Fig. 3, Supplementary Fig. S1B). On the other hand, in multivariate analyses was the component “Range/Climate” (which was calculated on correlated geographic traits) included in one of models that explained Wolbachia occurrence (Table 3 and Supplementary Table S3). Apparently, distribution, range and thermal preferences separately have no visible effect on the occurrence of Wolbachia, but the resultant of these traits (here component “Range/Climate”) is more powerful in explanation of Wolbachia presence. This is not surprising, as this bacterium is known all over the world – everywhere where potential hosts occur51. Moreover, identification of the same or similar strains from hosts in distant parts of the world has proved that Wolbachia is widespread and that there are no barriers preventing its distribution as a bacterial taxa. Although there could be some limitations in geographic distribution if consider particular strains. Mobility of beetles showed ambiguous results. This trait was nearly significant in a comparison of infected and uninfected beetles, but was not important in multivariate analyses (Table 3 and Supplementary Table S3). Wolbachia was 1.8-times more often found in flightless species than those of high mobility. This could be explained in light of the finding reported above that geography has also ambiguous important meaning for infection when assuming that mobile species should be at least similarly infected as the less mobile if not more so (due to higher chances of meeting infected species). The high prevalence of Wolbachia among species with limited mobility could be associated with horizontal transfer in local scale, e.g. within the same habitat or even host plant in a particular area. This is especially noticeable in the case of steppic weevils and leaf beetles, in which many stenotopic and flightless species sharing similar strains35,37. Another trait that clearly differentiates infected and uninfected species is body size (Tables 2 and 3, Fig. 3, Supplementary Fig. S1B). Smaller species were 3-times more often infected than the largest ones and 2-times more often infected than medium-size taxa. This pattern is quite unexpected, as there was no other report suggesting such relation. It is hard to find reasonable explanations for this observation. It is rather not related to lower efficiency of PCR on templates from smaller specimens, which should contain less genetic material of bacteria as the smaller species found to be even more frequently infected than the bigger ones. We cannot rule out the possibility that detection of Wolbachia was depended on dilution effect of bacterial DNA localized specifically in a tissue (e.g. gonads)52–54. However, more simple explanation could be that observed effect was related to a generally lower number of large beetle taxa, which are present in central-east European coleopterofauna and could be collected for this study. Therefore, this hypothesis should be verified on species from tropics, where many more large species exist. Another, even more probable explanation is that small species tend to live in high densities, whereas large species have less numerous populations and some are also territorial. This difference in densities could explain the higher prevalence of bacteria in abundant species which are also often of small size. The most important finding of this study is proof that trophic affinity (as well as habitats and microhabitats, which are correlated with trophy) is probably the most important explanation for Wolbachia occurrence in beetles. The most frequently infected were found to be phytophagous species (38.6%), followed by saprophagous (34.3%). The lowest rates of infection among beetles were in mycetophagous (23.5%) and cambioxylophagous (18.5%), with the lowest rates of Wolbachia prevalence in predators (17.6%). This pattern is generally congruent with results of previous studies on beetles23 as the highest rates of infection among the species were reported from leaf-eating beetles such as weevils and leaf beetles25,27,28,36,55. Surprisingly, Wolbachia occurrence among saprophagous has been almost overlooked, as few studies have done with, for example, Tenebrionidae56,57. Also, there are no reports about Wolbachia occurrence among mycetophagous beetles, which indicates how large the “white spots” are in studies on Wolbachia in beetles. Regarding cambioxylophagous, almost all previous knowledge on this group was based on a single study on Buprestidae29 and single studies on Cerambycidae (only a few members of the genus Monochamus were tested40,58). The same concern for predatory species of terrestrial beetles exists, as there are only single studies on ground beetles59, rove beetles60 and ladybird beetles61,62. Interestingly, predatory beetles from aquatic environments (e.g. Dytiscidae, Hydraenidae) are more frequently infected29 than terrestrial beetles. This pattern could be associated with the higher densities of aquatic predatory beetles compared to terrestrial predatory beetles, as even riverine (not aquatic) ground and rove beetles have been found to have a high prevalence of bacteria41. The lowest prevalence of Wolbachia among predators is an unexpected result, as this group should have numerous occasions to gain infection via feeding on infected species. Especially that some possibility has been provided by studies that detected DNA from the bacterium in uninfected predators that fed on infected prey63–65. Direct foraging is probably not an efficient way for Wolbachia transmission. A possible explanation is that the predator-prey route cannot transfer Wolbachia because the symbionts may not be able to survive in the predators’ digestive tracts18.
It is interesting to not only find traits related to infection of Wolbachia but also to identify those traits that differentiate hosts infected by various supergroups of this bacteria. Beetles are known to be infected by three supergroups. Yet supergroup F has thus far only been found in three taxa: Agrilus araxenus and Lamprodila mirifica (both Buprestidae29) and Rhinocyllus conicus (Curculionidae66). Among the Coleoptera examined in this study, no species harboured supergroup F, a fact that additionally supports the rare presence of this supergroup among beetle hosts. Previous data has shown that in the four groups of beetles with the highest numbers of examined and infected species, the distributions of supergroups has varied: it was equal in Buprestidae29; A overdominated B in Hydraenidae and Chrysomelidae28,29,67 and B overdominated A in Curculionoidea (true weevils with apionids)25,34,45. This study shows that supergroup A overdominated B in a majority of families. The only exceptions was Apionidae, with prevailed over supergroup B, whereas in Carabidae and Scarabaeidae both supergroups infected a similar number of taxa. All these data suggest that diversity of Wolbachia across beetle families is not random, as supergroup A overdominates B with rare exceptions in some families.
Conclusions
In summary, data above concerning Wolbachia prevalence among Coleoptera demonstrate that there is only minor support for coevolution between bacteria and the beetle hosts. Cospeciation only occurred in some genera, usually between closely related species (H1a confirmed in some cases). However, this effect could be mimicked in some cases by horizontal transmission of bacteria between taxa sharing the same environment. In general, coevolution between beetles and Wolbachia has not played a substantial role in the spread and diversification of both groups (H1b rejected). Moreover, significant differences in Wolbachia prevalence among particular taxonomic groups of beetles can be observed on the family level, but not on higher taxonomic ranks (H2 confirmed for families, rejected for superfamilies, infraorders and suborders).
Regarding the biological and ecological traits of beetles, some of them were found to be unrelated to Wolbachia prevalence (e.g. thermal preferences and range), with ambiguous effect on bacteria occurrence (such as mobility and body size) and some traits found to be substantially related with infection (reproduction mode and trophy). In summary, some states of particular ecological and biological traits of beetles could be used as good explanatory factors for Wolbachia prevalence. However, in general, there are no obvious combinations of these traits that clearly allow for the differentiation of uninfected and infected beetles (as well as those infected by various supergroups) (H3 rejected). Reproduction mode, a trait that explains infection, has been already described as related to Wolbachia. The best explanatory trait of Wolbachia prevalence has been found to be trophic affinity of beetles (H4a confirmed). However, the diversity of Wolbachia was unrelated with affinity to particular trophic guild by its hosts (H4b rejected). It needs to be stated that abovementioned conclusions are based on observational data (rather simple information about presence or absence of the bacteria in examined species) and are mostly correlative analyses, what does not prove a direct causal link. Causes and mechanisms of relations among hosts traits and the infection by Wolbachia (or any other endosymbiotic bacteria; see Duron et al.68) need to be investigated in future studies focused on some exemplary taxa and the best with use of more sophisticated methods (like next-generation sequencing technologies). There are simultaneous research to the study presented here, which elaborate infection by other endosymbiotic bacteria (namely Rickettsia, Spiroplasma, and Cardinium) on the same set of beetles (Kolasa et al., in press), as well as characterize the whole microbiome of selected beetle species on large number of specimens (Kolasa et al., unpublished).
This summary does not demonstrate obvious patterns of Wolbachia prevalence and diversity in relation to host taxonomy, biology, and ecology. As both Wolbachia and Coleoptera are diverse groups (there are numerous supergroups of bacteria raised to the level of species and thousands taxa of beetles that could be characterized by a wide spectrum of traits), such result should not be unexpected. This lack of clear patterns is most probably a reflection of nature, which is characterised by highly diversified and probably unstable relations (if one considers Wolbachia prevalence to be constant gains and losses, which explains its global distribution and suggests an epidemiological equilibrium between bacteria and its potential hosts).
Materials and Methods
Sampling design
Beetle species were collected during several field trips organized across central, eastern, and southeastern Europe from 2014 to 2017. The majority of species were collected from various sites in Poland, Slovakia, Romania, and Bulgaria; some taxa were also taken from Czechia, Germany, Austria, Hungary, Croatia, Greece, Ukraine, and Belarus (192 sites in total, Supplementary Table S4, Supplementary File 1). Special attention was given sampling in two highly diverse areas in central Europe: Białowieża Forest (mainly for forest-dwellers, particularly cambioxylophagous species) and the Carpathians (for species from most types of environments, particularly rivers, valleys, forests, and grasslands). Beetles were searched for in fields using numerous standard entomological techniques (using sweep-net, sieve, light-capturing, traps for scavengers, searching in dead wood, mushrooms, etc.) by experienced entomologists – specialists in various groups of beetles. Specimens were immediately preserved in 96% ethanol, then deposited in laboratories in −20°C. The specimens were morphologically identified by expert taxonomists using appropriate keys and handbooks. The nomenclature adopted in this study follows that of the Catalogue of Palaearctic Coleoptera (see Supplementary File 2 for references).
Finally, more than 400 species were collected and preserved, most of them from a few or several localities. These species were selected on the basis on three major features: i) to cover a wide spectrum of taxonomic groups of beetles known from central, eastern, and south-eastern Europe; ii) to cover all trophic guilds with a relatively large number of taxa, iii) to include in analyses two to three or more specimens per species, each from different localities if possible. Finally, after examining the available collection of beetles, 297 species were selected for molecular examination (Supplementary Table S5).
Species traits
Several biological and ecological traits were identified from selected beetle species for further analyses. These traits were selected on the basis of general available knowledge about beetles biology and ecology, taken from various studies (see Supplementary File 2 for references) and based on expert knowledge of specialists involved in this study.
To avoid confusing nomenclature in the paper, groups of ecological/biological features are called “traits” (e.g. trophic guild) while different types of traits are called as “states” (e.g. predators). Beetles (based on the biology and ecology of imaginal stages) were assigned to states of nine selected traits: i) trophic guilds, ii) habitat types, iii) microhabitats, iv) distribution, v) ranges, vi) thermal preferences, vii) reproduction modes, viii) body size and ix) mobility. Details about these traits and characteristics of rules according to which species were assigned to particular states are presented in Table 4.
Table 4.
Characterization of biological and ecological traits and their states used for beetle species assignment for purposes of this study.
| Trait | State | Description | No of assigned species |
|---|---|---|---|
| trophic guild | predators | species which kill and feed on other animals | 91 |
| herbivores | species which feed on living (green) plant tissues | 101 | |
| mycetophages | species which feed on living mushrooms | 17 | |
| saprophages | species which feed on dead organisms (except dead wood) | 35 | |
| cambioxylophages | species which feed on wood and phloem | 53 | |
| habitat type | forest-dwellers | species which live only in forested habitats | 112 |
| open land-dwellers | species which live only in grassland habitats | 131 | |
| ubiquitous | species which could live in all types of terrestrial habitats | 54 | |
| microhabitats | coprophilous | species which inhabit animal feces | 20 |
| epigeic | species which inhabit soil surface (free living) | 38 | |
| mycetophilous | species which inhabit mushrooms | 15 | |
| herbophilous | species which inhabit living plants | 128 | |
| hygrophilous | species which inhabit wet habitats (but not aquatic) | 25 | |
| necrophilous | species which inhabit carcases | 5 | |
| cambioxylophilous | species which inhabit inside trees | 66 | |
| distribution | continental | species of xeric habitats in Pontic and Pannonian regions (mostly steppes) | 25 |
| mediterranean | species of dry habitats in Mediterranean basin (the Balkans) | 17 | |
| mountainous | species of high mountain zones (the Sudetes, the Carpathians and the Balkan Mts.) | 11 | |
| temperate | species widespread in central Europe except above listed areas | 244 | |
| range | fragmented | species with fragmented and isolated populations | 36 |
| widespread | species with continuous ranges | 261 | |
| thermal preferences | neutral | species which wide tolerance to temperatures | 250 |
| warm | species which prefer only warm temperatures | 47 | |
| reproduction mode | bisexual | species which reproduce only bisexually | 292 |
| parthenogenetic | species which parthenogenetic reproduction (at least in central Europe) | 5 | |
| body size | large | species larger than 20 mm length | 23 |
| medium | species of 10–20 mm length | 174 | |
| small | species smaller than 10 mm length | 100 | |
| mobility | mobile | species winged and capable to fly | 274 |
| sedentary | species apterous (flightless) | 23 |
Laboratory works
Before DNA extraction, all specimens were cleaned using ethanol and distilled water in order to reduce the risk of external contamination. DNA was extracted from the whole insect body (for beetles up to approximately10 mm length; only from abdomen for larger specimens) using the Nucleospin Tissue kit (Macherey-Nagel), following the manufacturer’s instructions. The mitochondrial cytochrome oxidase subunit I gene (cox1) was amplified using polymerase chain reaction (PCR) with the following primers: LCO-1490/HCO-2198 or LepF1/LepR1 or, in case of failure of the previous two, C1-J-2183/TL2-N-301469,70. The concentration of reagents used for the amplification of cox1 marker and the cycling profile for PCR followed36. Cox1 was amplified first as the control for PCR efficiency and second as the DNA marker for genetic distance calculations. We were not able to sequence the same cox1 fragment for all species (this problem is known from barcoding projects on beetles, see e.g.71; therefore, some species have cox1 fragments from the 3′ or 5′ site of this gene). Moreover, we downloaded cox1 from GenBank for several species (see Supplementary Table S4). Different sets of cox1 sequences presented no problems, as this study did not intend to do phylogenetic analyses on all the beetles (this topic has already been described in several papers, e.g.71,72). Homologous cox1 were available for coevolutionary analyses (tree reconstructions and distance calculations) for selected genera (see below).
The presence of Wolbachia in particular beetles was first screened using two sets of primers amplifying Wolbachia surface protein (wsp) and cell division protein (ftsZ) (primers and PCR conditions follow https://pubmlst.org/Wolbachia/). Two controls were used in this step: negative (samples with distilled water instead of DNA isolates) and positive (DNA isolates from Polydrusus inustus weevil, which is known to be infected in its entire range38). Next, positive samples were amplified with other genes of Multilocus sequence typing (MLST) system32, that is: aspartyl/glutamyl-tRNA(Gln) amidotransferase, subunit B (gatB); cytochrome c oxidase, subunit I (coxA); conserved hypothetical protein (hcpA); and fructose-bisphosphate aldolase (fbpA). In case of multiple infection by different Wolbachia supergroups, specific primers were used according to MLST protocols (https://pubmlst.org/wolbachia/info/amp_seq_double.shtml). Cloning of PCR products prior to sequencing was not adopted due to a large number of examined species, and lack of obvious signs of the presence of multiple strains from the same supergroup within DNA isolates from particular individuals (based on examination of chromatograms). Although multiple infections are not so rare in insects (see e.g.73–76), the majority of such examples concern the presence of different strains within species or populations, but rarely within particular individuals. Moreover, even in this study, some multiple-infected beetles were overlooked, this should not have consequences for analyses and conclusions, as this study did not aim to examine the overall diversity of Wolbachia in particular hosts. After DNA purification (Exo-BAP Kit; EURx, Poland), the PCR fragments (all – cox1 and Wolbachia genes) were sequenced using a BigDye Terminator v.3.1. Cycle Sequencing Kit (Applied Biosystems) and performed with an ABI 3100 Automated Capillary DNA Sequencer. All newly generated sequences (both from beetles and bacteria) were submitted to GenBank (see Supplementary Table S4) for accession numbers.
Co-evolutionary analyses
On the level of genus, six genera were selected, as within these groups those species that were infected and uninfected by Wolbachia were identified; moreover, infected species harbour various bacteria stains. Moreover, selected genera belong to different beetle families and various trophic guilds. Among selected genera were Cyanapion apionid weevils (phytophagous, six species); Crioceris leaf beetles (phytophagous, five species); Monochamus longhorn beetles (cambioxylophagous, six species); Aphodius dung beetles (saprophagous, ten species); Bembidion ground beetles (predators, six species); and Paederus/Paederidus rove beetles (predators, six species). For this part of analysis, data newly generated in this study as well as sequences obtained from previous published works on beetle-Wolbachia associations were used35,36,40,41. For all these groups, phylogenetic trees were reconstructed for both the cox1 of beetles and the selected MLST genes of Wolbachia from infected hosts. Maximum likelihood trees of cox1 were reconstructed using IQ-TREE web server http://www.iqtree.org/77 under the following settings: auto selection of substation model; ultrafast bootstrap approximation (UFBoot)78 with 10,000 iterations; maximum correlation coefficient = 0.99; single branch test with use of the approximate Likelihood-Ratio Test (SH-aLRT)79; and other default options. Trees of MLST were inferred with ClonalFrame 1.2, software that infers clonal relationships from MLST data and incorporates recombination events80. Three independent runs were performed with 500,000 generations each and a burn-in of 20%. The convergence of runs was assessed using methods of Gelman and Rubin81 implemented in ClonalFrame. All post-burn-in trees were used to build a majority-rule consensus tree and infer posterior probabilities from clade frequencies. Finally, both trees were used for visualization of relations between phylogeny of the hosts (beetles) and bacteria (Wolbachia). To directly test for congruence between Wolbachia and beetle trees, the Procrustean Approach to Cophylogeny (PACo) software was used82. This method provides test statistics to assess whether phylogenetic positions of corresponding hosts (beetles) and symbionts (Wolbachia) are independent of one another. This is achieved via randomization of host-symbiont associations. This test requires distance matrices of hosts and symbionts, so genetic distances were calculated on the MLST dataset for Wolbachia strains and the cox1 gene for beetles (for each genera separately) using the “dist.dna” function of the R package and the TN93 model83. PACo was performed within the R statistical environment84, using both types of distance matrices and 100,000 permutations each.
Diversity of Wolbachia strains was compared within and between superfamilies of examined beetles based on genetic distances (adopting Kimura-two parameters as the substitution model85 calculated for wsp sequences). Wsp was selected for this part of analyses because it is more polymorphic than any MLST gene and because in this study the largest dataset of strains from examined beetles was collected for this gene. The Mann-Whitney U test was used to assess the difference in distances within and between Wolbachia strains found in superfamilies of beetles. Additionally, differences in prevalence of Wolbachia in particular taxonomic levels of its beetle hosts were estimated using Kruskal-Wallis one-way analysis of variance (ANOVA) for each level separately.
Wolbachia diversity
For a brief estimation of Wolbachia diversity, the maximum likelihood phylogenetic trees were reconstructed using IQ-TREE under conditions described above for two datasets: DS1, which includes all newly obtained ftsZ sequences of Wolbachia generated from beetles in this study, and DS2, which includes the aforementioned sequences plus all other ftsZ sequences of Wolbachia found in beetle hosts. Data was taken from the review of Kajtoch & Kotásková23. Among MLST genes, only the ftsZ sequences were chosen for these analyses because this gene has the largest database of sequences (e.g. NCBI GenBank, Wolbachia MLST) available from various hosts belonging to Coleoptera.
Wolbachia-host traits statistics
Box plots visualized differences in Wolbachia prevalence in groups of hosts assigned to particular traits (also with respect to infection by two supergroups). Moreover, performance of univariate models (each including single trait) was assessed using Wald statistics, Akaike Information Criterion (AIC), and Nagelkerke pseudo R2. Redundancy analysis (RDA) was used for the visualization of relations among infection status (uninfected, infected, as well as infected by both supergroups) and ecological and biological traits of the beetles.
Next, correlations among traits were assessed (Spearman rank correlation). Numerous states of particular traits were found to be significantly correlated. This especially concerned trophic guilds and microhabitats (e.g. mycetophagous with mycetophilous, cambioxylophagous with cambioxylophilous, phytophagous with herbophilous, etc.). This also concerned states of habitat traits (forest-dwellers and open land-dwellers) and the majority of states from traits: range, distribution, and thermal preferences. To avoid using these correlated variables in multivariate analyses, some of them were omitted (microhabitats due to high correlation with trophic guilds). Other correlated variables were grouped into components using Principal Component Analysis (PCA) (Habitats, which include all states of this trait, PC1 = 56.3%; Range/Climate, which include most states from range, distribution, and thermal preferences traits, PC1 = 56.9%). Finally, uncorrelated traits, the aforementioned components of correlated states and cox1 distances among beetle species (adopting Kimura-two parameters as the substitution model85) were used as explanatory variables in multivariate analyses. Generalized Linear Models (GLMs) with binomial distribution were constructed for two datasets: GLM1 – for all beetle species (297) with Wolbachia infection as the explained variable (infected vs uninfected); and GLM2 – only for infected beetles (81) with occurrence of supergroups as the explained variable (supergroup A vs supergroup B). Performance of multivariate models was estimated using AIC, delta (∆), and Akaike weight (w). Following Arnold86, we treated parameters which did not improve the AIC of a model by more than 2.0 as uninformative.
Finally, diversity of Wolbachia strains was compared within and between trophic guilds of examined beetles based on genetic distances (adopting Kimura-two parameters as the substitution model85 calculated for wsp sequences (the same as above for superfamilies). Difference in distances within and between Wolbachia strains found in trophic guilds of beetles was assessed using the Mann-Whitney U test.
Statistical analyses were performed in the R statistical environment84 or with STASTISTICA v.1187 and PAST 3.20 software88.
Ethical note
This study complied with European, Polish, Czechian, Slovakian, Austrian, Hungarian, Romanian, Bulgarian, Croatian, Greek, Ukrainian and Belarusian regulations regarding the collection of invertebrates (beetles). No of collected and examined species of beetles is protected under European or national laws (there are no regulations of sampling of not-protected beetles in countries where sampling was executed).
Supplementary information
Acknowledgements
This research was funded by the grant DEC-2013/11/D/NZ8/00583 (Kajtoch Ł., National Science Centre, Poland). The authors thank all people who contributed specimens of beetles for this study (Marek Wanat, Nela Kotásková, Radosław Plewa) and who identified specimens of the species for some groups (Marcin Józefczyk and Łukasz Minkina). We are grateful to Michał Żmihorski and Piotr Skórka for their helpful advices related to statistical analyses. We kindly thank Prof. Lech Borowiec for providing the pictures of beetles from his ICONOGRAPHIA COLEOPTERORUM POLONIAE (© Copyright by Prof. Lech Borowiec, Wrocław 2007–2018, Department of Biodiversity and Evolutionary Taxonomy, University of Wrocław, Poland), which were used for preparing some of the graphics.
Author Contributions
Ł.K. & D.K. designed the project, Ł.K. obtained funds, D.K., J.M.G., R.Ś., M.A.M., M.H., Ł.K. sampled beetles, M.K. & Ł.K. performed the laboratory and analytical works, R.Ś., M.K. & Ł.K. designed illustrations. All the authors contributed to writing the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the paper and the supplementary information files.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38155-5.
References
- 1.Taylor MJ, Hoerauf A. Wolbachia Bacteria of Filarial Nematodes. Parasitol. Today. 1999;15:437–442. doi: 10.1016/S0169-4758(99)01533-1. [DOI] [PubMed] [Google Scholar]
- 2.Werren JH, Windsor DM. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc. R. Soc. Lond. B Biol. Sci. 2000;267:1277–1285. doi: 10.1098/rspb.2000.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hertig M. The Rickettsia, Wolbachia pipientis (gen. et sp.n.) and Associated Inclusions of the Mosquito, Culex pipiens. Parasitology. 1936;28:453–486. doi: 10.1017/S0031182000022666. [DOI] [Google Scholar]
- 4.Augustinos AA, et al. Detection and characterization of Wolbachia infections in natural populations of aphids: is the hidden diversity fully unraveled? PloS One. 2011;6:e28695. doi: 10.1371/journal.pone.0028695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramirez-Puebla, S. T. et al. Species in Wolbachia? Proposal for the designation of ‘Candidatus Wolbachia bourtzisii’, ‘Candidatus Wolbachia onchocercicola’, ‘Candidatus Wolbachia blaxteri’, ‘Candidatus Wolbachia brugii’, ‘Candidatus Wolbachia taylori’, ‘Candidatus Wolbachia collembolicola’ and ‘Candidatus Wolbachia multihospitum’ for the different species within Wolbachia supergroups. Syst. Appl. Microbiol.38, 390–399 (2015). [DOI] [PubMed]
- 6.Lindsey, A. R., Bordenstein, S. R., Newton, I. L. & Rasgon, J. L. Wolbachia pipientis should not be split into multiple species: A response to Ramírez-Puebla et al., “Species in Wolbachia? Proposal for the designation of ‘Candidatus Wolbachia bourtzisii’, ‘Candidatus Wolbachia onchocercicola’, ‘Candidatus Wolbachia blaxteri’, ‘Candidatus Wolbachia brugii’, ‘Candidatus Wolbachia taylori’, ‘Candidatus Wolbachia collembolicola’ and ‘Candidatus Wolbachia multihospitum’ for the different species within Wolbachia supergroups”. Syst. Appl. Microbiol.39, 220–222 (2016). [DOI] [PMC free article] [PubMed]
- 7.Jiggins FM, Bentley JK, Majerus ME, Hurst GD. How many species are infected with Wolbachia? Cryptic sex ratio distorters revealed to be common by intensive sampling. Proc. R. Soc. Lond. B Biol. Sci. 2001;268:1123–1126. doi: 10.1098/rspb.2001.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poinsot D, Charlat S, Mercot H. On the mechanism of Wolbachia-induced cytoplasmic incompatibility: Confronting the models with the facts. Bioessays. 2003;25:259–265. doi: 10.1002/bies.10234. [DOI] [PubMed] [Google Scholar]
- 9.Stouthamer R, Breeuwer JA, Hurst GD. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Hiroki M, Kato Y, Kamito T, Miura K. Feminization of genetic males by a symbiotic bacterium in a butterfly, Eurema hecabe (Lepidoptera: Pieridae) Naturwissenschaften. 2002;89:167–170. doi: 10.1007/s00114-002-0303-5. [DOI] [PubMed] [Google Scholar]
- 11.Charlat S, Hurst GDD, Merçot H. Evolutionary consequences of Wolbachia infections. Trends Genet. 2003;19:217–223. doi: 10.1016/S0168-9525(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 12.Vavre F, Fleury F, Lepetit D, Fouillet P, Boulétreau M. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol. Biol. Evol. 1999;16:1711–1723. doi: 10.1093/oxfordjournals.molbev.a026084. [DOI] [PubMed] [Google Scholar]
- 13.Sintupachee S, Milne JR, Poonchaisri S, Baimai V, Kittayapong P. Closely Related Wolbachia Strains within the Pumpkin Arthropod Community and the Potential for Horizontal Transmission via the Plant. Microb. Ecol. 2006;51:294–301. doi: 10.1007/s00248-006-9036-x. [DOI] [PubMed] [Google Scholar]
- 14.Yun Y, Peng Y, Liu FX, Lei C. Wolbachia screening in spiders and assessment of horizontal transmission between predator and prey. Neotrop. Entomol. 2011;40:164–169. [PubMed] [Google Scholar]
- 15.Caspi-Fluger A, et al. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc. R. Soc. Lond. B Biol. Sci. 2012;279:1791–1796. doi: 10.1098/rspb.2011.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S-J, et al. Plantmediated horizontal transmission of Wolbachia between whiteflies. Isme J. 2016;11:1019. doi: 10.1038/ismej.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia? – a statistical analysis of current data. FEMS Microbiol. Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cordaux R, Bouchon D, Grève P. The impact of endosymbionts on the evolution of host sex-determination mechanisms. Trends Genet. 2011;27:332–341. doi: 10.1016/j.tig.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Czarnetzki AB, Tebbe CC. Detection and phylogenetic analysis of Wolbachia in Collembola. Environ. Microbiol. 2018;6:35–44. doi: 10.1046/j.1462-2920.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi Y, Fukatsu T. Diversity of Wolbachia Endosymbionts in Heteropteran Bugs. Appl. Environ. Microbiol. 2003;69:6082–6090. doi: 10.1128/AEM.69.10.6082-6090.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoemaker DD, et al. The distribution of Wolbachia in fig wasps: correlations with host phylogeny, ecology and population structure. Proc. R. Soc. Lond. B Biol. Sci. 2002;269:2257–2267. doi: 10.1098/rspb.2002.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tagami Y, Miura K. Distribution and prevalence of Wolbachia in Japanese populations of Lepidoptera. Insect Mol. Biol. 2004;13:359–364. doi: 10.1111/j.0962-1075.2004.00492.x. [DOI] [PubMed] [Google Scholar]
- 23.Kajtoch Ł, Kotásková N. Current state of knowledge on Wolbachia infection among Coleoptera: a systematic review. Peer J. 2018;6:e4471. doi: 10.7717/peerj.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ślipiński, S. A., Leschen, R. A. B. & Lawrence, J. F. Order Coleoptera Linnaeus, 1758. In: Zhang, Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 203–208 (2011). [DOI] [PubMed]
- 25.Lachowska D, Kajtoch Ł, Knutelski S. Occurrence of Wolbachia in central European weevils: correlations with host systematics, ecology, and biology. Entomol. Exp. Appl. 2010;135:105–118. doi: 10.1111/j.1570-7458.2010.00974.x. [DOI] [Google Scholar]
- 26.Kawasaki Y, Ito M, Miura K, Kajimura H. Superinfection of five Wolbachia in the alnus ambrosia beetle, Xylosandrus germanus (Blandford) (Coleoptera: Curuculionidae) Bull. Entomol. Res. 2010;100:231–239. doi: 10.1017/S000748530999023X. [DOI] [PubMed] [Google Scholar]
- 27.Clark TL, Meinke LJ, Skoda SR, Foster JE. Occurrence of Wolbachia in Selected Diabroticite (Coleoptera: Chrysomelidae) Beetles. Ann. Entomol. Soc. Am. 2001;94:877–885. doi: 10.1603/0013-8746(2001)094[0877:OOWISD]2.0.CO;2. [DOI] [Google Scholar]
- 28.Jäckel R, Mora D, Dobler S. Evidence for selective sweeps by Wolbachia infections: phylogeny of Altica leaf beetles and their reproductive parasites. Mol. Ecol. 2013;22:4241–4255. doi: 10.1111/mec.12389. [DOI] [PubMed] [Google Scholar]
- 29.Sontowski R, Bernhard D, Bleidorn C, Schlegel M, Gerth M. Wolbachia distribution in selected beetle taxa characterized by PCR screens and MLST data. Ecol. Evol. 2015;5:4345–4353. doi: 10.1002/ece3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werren JH, Windsor D, Guo LR. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. Lond. B Biol. Sci. 1995;262:197–204. doi: 10.1098/rspb.1995.0196. [DOI] [Google Scholar]
- 32.Baldo L, et al. Multilocus Sequence Typing System for the Endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roehrdanz RL, Wichmann SS. Wolbachia Multilocus Sequence Typing of Singly Infected and Multiply Infected Populations of Northern Corn Rootworm (Coleoptera: Chrysomelidae) Ann. Entomol. Soc. Am. 2014;107:832–841. doi: 10.1603/AN14006. [DOI] [Google Scholar]
- 34.Rodriguero MS, Confalonieri VA, Guedes JVC, Lanteri AA. Wolbachia infection in the tribe Naupactini (Coleoptera, Curculionidae): association between thelytokous parthenogenesis and infection status. Insect Mol. Biol. 2010;19:631–640. doi: 10.1111/j.1365-2583.2010.01018.x. [DOI] [PubMed] [Google Scholar]
- 35.Kolasa M, et al. Preliminary evidence of the horizontal transmission of Wolbachia between Crioceris leaf-beetles (Coleoptera: Chrysomelidae) and their Asparagus host plants. Eur. J. Entomol. 2017;114:446–454. doi: 10.14411/eje.2017.057. [DOI] [Google Scholar]
- 36.Kajtoch Ł, Montagna M, Wanat M. Species delimitation within the Bothryorrhynchapion weevils: Multiple evidence from genetics, morphology and ecological associations. Mol. Phylogenet. Evol. 2018;120:354–363. doi: 10.1016/j.ympev.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Mazur MA, et al. Selective sweep of Wolbachia and parthenogenetic host genomes–the example of the weevil Eusomus ovulum. Insect Mol. Biol. 2016;25:701–711. doi: 10.1111/imb.12255. [DOI] [PubMed] [Google Scholar]
- 38.Kajtoch Ł, Korotyaev B. & Lachowska-Cierlik, D. Genetic distinctness of parthenogenetic forms of European Polydrusus weevils of the subgenus. Scythodrusus. Insect Sci. 2012;19:183–194. doi: 10.1111/j.1744-7917.2011.01448.x. [DOI] [Google Scholar]
- 39.Kotásková N, Kolasa M, Kajtoch Ł. Contrasting patterns of molecular diversity and Wolbachia infection in bisexual and parthenogenetic Strophosoma weevils (Coleoptera: Curculionidae) Entomol. Sci. 2018;21:385–395. doi: 10.1111/ens.12317. [DOI] [Google Scholar]
- 40.Plewa R, et al. Morphology, genetics and Wolbachia endosymbionts support distinctiveness of Monochamus sartor sartor and M. s. urussovii (Coleoptera: Cerambycidae). Arthropod Syst. Phylogeny. 2018;76:123–135. [Google Scholar]
- 41.Kolasa M, Kubisz D, Mazur MA, Ścibior R, Kajtoch L. Wolbachia prevalence and diversity in selected riverine predatory beetles (Bembidiini and Paederini) Bull. Insectology. 2018;71:193–200. [Google Scholar]
- 42.Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. Lond. B Biol. Sci. 2015;282:20150249. doi: 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sazama, E. J., Bosch, M. J., Shouldis, C. S., Ouellette, S. P. & Wesner, J. S. Incidence of Wolbachia in aquatic insects. Ecol. Evol. 7, 1165–1169 (2017). [DOI] [PMC free article] [PubMed]
- 44.Bailly-Bechet M, et al. How Long Does Wolbachia Remain on Board? Mol. Biol. Evol. 2017;34:1183–1193. doi: 10.1093/molbev/msx073. [DOI] [PubMed] [Google Scholar]
- 45.Kawasaki Y, Schuler H, Stauffer C, Lakatos F, Kajimura H. Wolbachia endosymbionts in haplodiploid and diploid scolytine beetles (Coleoptera: Curculionidae: Scolytinae) Environ. Microbiol. Rep. 2016;8:680–688. doi: 10.1111/1758-2229.12425. [DOI] [PubMed] [Google Scholar]
- 46.Chrostek E, Pelz-Stelinski K, Hurst GDD, Hughes GL. Horizontal Transmission of Intracellular Insect Symbionts via Plants . Front. Microbiol. 2017;8:2237. doi: 10.3389/fmicb.2017.02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Werren JH. Biology of Wolbachia. Annu. Rev. Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 48.Gokhman, V. E. & Kuznetsova, V. G. Parthenogenesis in Hexapoda: holometabolous insects. J. Zool. Syst. Evol. Res. 56, 23–34
- 49.Huigens ME, Stouthamer R. Parthenogenesis associated with Wolbachia. Insect. Symbiosis. 2003;1:247–266. [Google Scholar]
- 50.Rodriguero MS, Lanteri AA, Confalonieri VA. Mito-nuclear genetic comparison in a Wolbachia infected weevil: insights on reproductive mode, infection age and evolutionary forces shaping genetic variation. BMC Evol. Biol. 2010;10:340. doi: 10.1186/1471-2148-10-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PloS One. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arthofer W, et al. Hidden Wolbachia diversity in field populations of the European cherry fruit fly, Rhagoletis cerasi (Diptera, Tephritidae) Mol. Ecol. 2009;18:3816–3830. doi: 10.1111/j.1365-294X.2009.04321.x. [DOI] [PubMed] [Google Scholar]
- 53.Hughes GL, et al. Variable Infection Frequency and High Diversity of Multiple Strains of Wolbachia pipientis in Perkinsiella Planthoppers. Appl. Environ. Microbiol. 2011;77:2165. doi: 10.1128/AEM.02878-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mee PT, Weeks AR, Walker PJ, Hoffmann AA, Duchemin J-B. Detection of Low-Level Cardinium and Wolbachia infections in Culicoides. Appl. Environ. Microbiol. 2015;81:6177. doi: 10.1128/AEM.01239-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montagna M, et al. A new strain of Wolbachia in an Alpine population of the viviparous Oreina cacaliae (Coleoptera: Chrysomelidae) Environ. Entomol. 2014;43:913–922. doi: 10.1603/EN13228. [DOI] [PubMed] [Google Scholar]
- 56.Fialho RF, Stevens L. Wolbachia Infections in the Flour Beetle Tribolium confusum: Evidence for a Common Incompatibility Type across Strains. J. Invertebr. Pathol. 1996;67:195–197. doi: 10.1006/jipa.1996.0032. [DOI] [Google Scholar]
- 57.Ming Q-L, Shen J-F, Cheng C, Liu C-M, Feng Z-J. Wolbachia Infection Dynamics in Tribolium confusum (Coleoptera: Tenebrionidae) and Their Effects on Host Mating Behavior and Reproduction. J. Econ. Entomol. 2015;108:1408–1415. doi: 10.1093/jee/tov053. [DOI] [PubMed] [Google Scholar]
- 58.Aikawa T, et al. Longicorn beetle that vectors pinewood nematode carries many Wolbachia genes on an autosome. Proc. R. Soc. Lond. B Biol. Sci. 2009;276:3791–3798. doi: 10.1098/rspb.2009.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heintzman PD, Elias SA, Moore K, Paszkiewicz K, Barnes I. Characterizing DNA preservation in degraded specimens of Amara alpina (Carabidae: Coleoptera) Mol. Ecol. Resour. 2014;14:606–615. doi: 10.1111/1755-0998.12205. [DOI] [PubMed] [Google Scholar]
- 60.Bili M, et al. Bacterial Community Diversity Harboured by Interacting Species. PLOS ONE. 2016;11:e0155392. doi: 10.1371/journal.pone.0155392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Majerus MEN, Hinrich J, Schulenburg GVD, Zakharov IA. Multiple causes of male-killing in a single sample of the two-spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae) from Moscow. Heredity. 2000;84:605–609. doi: 10.1046/j.1365-2540.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- 62.Goryacheva II, Blekhman AV, Andrianov BV, Gorelova TV, Zakharov IA. Genotypic diversity of Wolbachia pipientis in native and invasive Harmonia axyridis Pall., 1773 (Coleoptera, Coccinellidae) populations. Russ. J. Genet. 2015;51:731–736. doi: 10.1134/S1022795415080037. [DOI] [PubMed] [Google Scholar]
- 63.Johanowicz DL, Hoy MA. Wolbachia in a Predator–Prey System: 16S Ribosomal Dna Analysis of Two Phytoseiids (Acari: Phytoseiidae) and Their Prey (Acari: Tetranychidae) Ann. Entomol. Soc. Am. 1996;89:435–441. doi: 10.1093/aesa/89.3.435. [DOI] [Google Scholar]
- 64.Huigens ME, de Almeida RP, Boons PaH, Luck RF, Stouthamer R. Natural interspecific and intraspecific horizontal transfer of parthenogenesis–inducing Wolbachia in Trichogramma wasps. Proc. R. Soc. Lond. B Biol. Sci. 2004;271:509–515. doi: 10.1098/rspb.2003.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clec’h WL, et al. Cannibalism and Predation as Paths for Horizontal Passage of Wolbachia between Terrestrial Isopods. PLOS ONE. 2013;8:e60232. doi: 10.1371/journal.pone.0060232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campbell BC, Bragg TS, Turner CE. Phylogeny of symbiotic bacteria of four weevil species (coleoptera: curculionidae) based on analysis of 16S ribosomal DNA. Insect Biochem. Mol. Biol. 1992;22:415–421. doi: 10.1016/0965-1748(92)90136-3. [DOI] [Google Scholar]
- 67.Kondo NI, et al. Wolbachia Infections in World Populations of Bean Beetles (Coleoptera: Chrysomelidae: Bruchinae) Infesting Cultivated and Wild Legumes. Zoolog. Sci. 2011;28:501–508. doi: 10.2108/zsj.28.501. [DOI] [PubMed] [Google Scholar]
- 68.Duron O, et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008;6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 70.Hebert PD, Ratnasingham S, de Waard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. B Biol. Sci. 2003;270:S96–S99. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bocak L, et al. Building the Coleoptera tree-of-life for >8000 species: composition of public DNA data and fit with Linnaean classification. Syst. Entomol. 2014;39:97–110. doi: 10.1111/syen.12037. [DOI] [Google Scholar]
- 72.Mckenna DD, et al. The beetle tree of life reveals that Coleoptera survived end-Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Syst. Entomol. 2015;40:835–880. doi: 10.1111/syen.12132. [DOI] [Google Scholar]
- 73.Malloch G, Fenton B, Butcher RDJ. Molecular evidence for multiple infections of a new subgroup of Wolbachia in the European raspberry beetle Byturus tomentosus. Mol. Ecol. 2000;9:77–90. doi: 10.1046/j.1365-294x.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 74.Reuter M, Keller L. High Levels of Multiple Wolbachia Infection and Recombination in the Ant Formica exsecta. Mol. Biol. Evol. 2003;20:748–753. doi: 10.1093/molbev/msg082. [DOI] [PubMed] [Google Scholar]
- 75.Hiroki M, Tagami Y, Miura K, Kato Y. Multiple infection with Wolbachia inducing different reproductive manipulations in the butterfly Eurema hecabe. Proc. Biol. Sci. 2004;271:1751–1755. doi: 10.1098/rspb.2004.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arai, H. et al. Multiple Infection and Reproductive Manipulations of Wolbachia in Homona magnanima (Lepidoptera: Tortricidae). Microb. Ecol (2018). [DOI] [PubMed]
- 77.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minh BQ, Nguyen MAT, von Haeseler A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013;30:1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guindon S, et al. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 80.Didelot X, Falush D. Inference of Bacterial Microevolution Using Multilocus Sequence Data. Genetics. 2006;175:1251–1266. doi: 10.1534/genetics.106.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gelman A, Rubin DB. Inference from Iterative Simulation Using Multiple Sequences. Stat. Sci. 1992;7:457–472. doi: 10.1214/ss/1177011136. [DOI] [Google Scholar]
- 82.Balbuena JA, Míguez-Lozano R, Blasco-Costa I. PACo: A Novel Procrustes Application to Cophylogenetic Analysis. PLOS ONE. 2013;8:e61048. doi: 10.1371/journal.pone.0061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paradis E, Claude J, Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 84.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing (2017).
- 85.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 86.Arnold TW. Uninformative Parameters and Model Selection Using Akaike’s Information Criterion. J. Wildl. Manag. 2010;74:1175–1178. doi: 10.1111/j.1937-2817.2010.tb01236.x. [DOI] [Google Scholar]
- 87.Statsoft, I. N. C. STATISTICA (2016).
- 88.Hammer, O. PAST: Paleontological Statistics Software Package for Education and Data Analysis Palaeontologia Electronica. Httppalaeo-Electron. Accessed1 (2001).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the paper and the supplementary information files.