Abstract
There is growing interest from the oncology community to understand how body composition measures can be used to improve the delivery of clinical care for the 18.1 million individuals diagnosed with cancer annually. Methods that distinguish muscle from subcutaneous and visceral adipose tissue, such as computed tomography (CT), may offer new insights of important risk factors and improved prognostication of outcomes over alternative measures such as body mass index. In a meta‐analysis of 38 studies, low muscle area assessed from clinically acquired CT was observed in 27.7% of patients with cancer and associated with poorer overall survival [hazard ratio: 1.44, 95% CI: 1.32–1.56]. Therapeutic interventions such as lifestyle and pharmacotherapy that modify all aspects of body composition and reduce the incidence of poor clinical outcomes are needed in patients with cancer. In a meta‐analysis of six randomized trials, resistance training exercise increased lean body mass assessed from dual‐energy X‐ray absorptiometry [mean difference (MD): +1.07 kg, 95% CI: 0.76–1.37; P < 0.001] and walking distance [MD: +143 m, 95% CI: 70–216; P < 0.001] compared with usual care control in patients with non‐metastatic cancer. In a meta‐analysis of five randomized trials, anamorelin (a ghrelin agonist) significantly increased lean body mass [MD: +1.10 kg, 95% CI: 0.35–1.85; P = 0.004] but did not improve handgrip strength [MD: 0.52 kg, 95% CI: −0.09–1.13; P = 0.09] or overall survival compared with placebo [HR: 0.99, 95% CI: 0.85–1.14; P = 0.84] in patients with advanced or metastatic cancer. Early screening to identify individuals with occult muscle loss, combined with multimodal interventions that include lifestyle therapy with resistance exercise training and dietary supplementation combined with pharmacotherapy, may be necessary to provide a sufficient stimulus to prevent or slow the cascade of tissue wasting. Rapid, cost‐efficient, and feasible methods to quantify muscle and adipose tissue distribution are needed if body composition assessment is to be integrated into large‐scale clinical workflows. Fully automated analysis of body composition from clinically acquired imaging is one example. The study of body composition is one of the most provocative areas in oncology that offers tremendous promise to help patients with cancer live longer and healthier lives.
Keywords: Obesity paradox, Computed tomography, Cancer, Randomized trial, Cohort study, Metabolism
Introduction
There is growing interest from the oncology community to understand how body composition measures can be used to improve cancer treatment and survivorship care for the 18.1 million individuals diagnosed with cancer annually.1 Specifically, there is an emergent recognition that body mass index (BMI, weight in kilograms divided by the square of height in meters) is not adequate to identify patients who are at risk for adverse health outcomes due to poor muscle health or excess adiposity, nor does BMI accurately classify the distribution of adiposity.2 Historically, oncology has appreciated the deleterious prognostic effect of involuntary weight loss.3 Recent observational studies demonstrate that muscle and adipose tissue distribution are risk factors for clinical outcomes such as post‐operative complications, chemotherapy‐related toxicity, and overall survival in patients with cancer.4, 5 Underscoring the critical need for the evaluation of body composition in oncology, patients with cancer are often older adults who have experienced age‐related alterations in body composition that may be further exacerbated by cancer and cancer treatments.6, 7 The field is eager to identify therapeutic interventions that modify body composition and reduce the incidence of poor clinical outcomes in this population.8 Furthermore, a pre‐requisite to using body composition measures in oncology practice is to seamlessly integrate their assessment into the clinical oncology workflow.9 The purpose of this paper is to provide a concise overview of the facts and numbers that relate to the (1) epidemiology that describes the relationship between body composition and cancer prognosis, (2) evidence from clinical trials with body composition endpoints in patients with cancer, and (3) evidence describing how body composition can be integrated into oncology practice to guide patient care.
The epidemiology of body composition in cancer
Body mass index
Body mass index is often used as a proxy measure of total adiposity. Among adults, overweight is defined as a BMI of 25.0–29.9 kg/m2 and obesity as a BMI of ≥30 kg/m2. Worldwide, 1.9 billion adults are overweight; of these, 650 million are obese.10 It is estimated that 1 in 11 (9%) incident cancers diagnosed in North America and Europe is attributable to obesity.11 The International Agency for Cancer Research reviewed ≥1000 observational studies and concluded that there is convincing evidence that a high BMI is associated with an increased risk of developing 13 types of cancer.12
In contrast to cancer incidence, the association between BMI and cancer prognosis (e.g. cancer‐specific survival or overall survival) is less consistent, and for many malignancies, overweight or obesity is associated with a survival advantage (Figure 1). The association between BMI at diagnosis and prognosis may depend on cancer type, stage at diagnosis, age, sex, and type of treatments utilized. For example, in a pooled analysis of 22 randomized therapeutic treatment trials that included 11 724 patients with cancer, 67% were overweight/obese (BMI ≥25 kg/m2) at the time of enrolment (e.g. cancer diagnosis), and this was independently associated with improved overall survival in patients with several types of malignancies, including bladder [HR: 0.69; P = 0.02], gastrointestinal stromal tumours [HR: 0.73; P = 0.006], non‐small cell lung cancer [HR: 0.76; P = 0.01], and prostate cancer [HR: 0.79; P = 0.01].13 Other studies among a variety of cancer types have reported that a higher BMI is associated with improved overall survival (Table 1). The observed survival benefit associated with a higher BMI has historically been referred to as the obesity paradox14; however, it has been advised that this label be abandoned, given its inadequacy as a scientific descriptor.15
Figure 1.
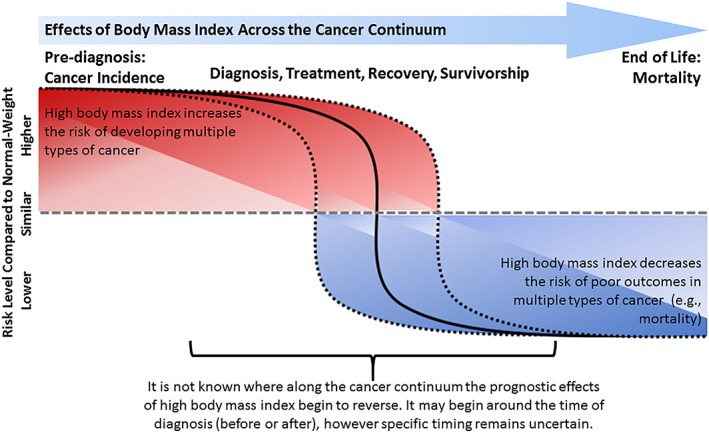
The effects of body mass index across the cancer continuum. Higher body mass index before diagnosis increases the risk of developing multiple cancers and higher body mass index at or after diagnosis lowers the risk of dying from multiple cancers. The interval along the cancer continuum where the higher body mass index begins to switch from a deleterious risk factor to an advantageous risk factor, and why, is not yet known.
Table 1.
Association between body mass index (BMI) at cancer diagnosis and overall survival
| Cancer site or type | Relative risk of the overweight/obese BMI category evaluated vs. normal BMI (95% CI) |
|---|---|
| Myeloid leukemia94 | 0.47 (0.26–0.82) |
| Non‐metastatic colorectal65 | 0.52 (0.35–0.77) |
| Metastatic melanomaa, 29 | 0.72 (0.57–0.91) |
| Lymphoma95 | 0.76 (0.67–0.86) |
| Gastric96 | 0.76 (0.59–0.99) |
| Renal97 | 0.84 (0.73–0.95) |
| Metastatic colorectal28 | ≈0.90 (≈0.85–0.95) |
Among patients treated with targeted therapy
There are several proposed explanations for the observation that higher BMI is associated with improved overall and cancer‐specific survival in patients with cancer.16, 17, 18 Some of these explanations relate to methodological concerns of study design and statistical analysis such as selection biases, unmeasured or residual confounding, and illness‐related weight loss. However, when these methodological concerns are empirically tested, many are not substantiated and the previously observed associations persist.17, 18, 19, 20, 21, 22 Other explanations involve BMI being too crude a measure to be useful at the individual patient level.23 BMI does not differentiate lean mass from adipose mass, nor does it describe regional adipose tissue deposition (e.g. visceral vs subcutaneous).24 Compared with bioelectrical impedance analysis and dual‐energy X‐ray absorptiometry to diagnose excess adiposity, BMI has poor sensitivity (36–49%) which results in high misclassification rates,25 and this limitation is worsened in older adults, particularly among males (32–38% sensitivity).26 This observation may explain, in part, why many studies have observed a statistical interaction between BMI and sex, such that a high BMI is associated with improved overall survival among male but not female patients with cancer. Such an interaction has been observed in non‐metastatic colon cancer (P interaction = 0.012),27 metastatic colorectal cancer (P interaction < 0.001),28 and metastatic melanoma (P interaction = 0.01).29 Moreover, in a pooled analysis of solid and hematologic malignancies, sex‐stratified analysis demonstrated that a BMI ≥25 kg/m2 was associated with improved overall survival in males [HR: 0.82; P = 0.003] but not in females [HR: 1.04; P = 0.86].13 The totality of these data suggests that BMI alone is insufficient and that more accurate measures of muscle and adipose tissue distribution may improve prognostication of outcomes.30, 31
Body composition
At the time of diagnosis, patients with cancer often complete radiologic measures such as computed tomography or magnetic resonance imaging to characterize the primary tumour and identify distant metastatic foci. Computed tomography and magnetic resonance imaging are gold‐standard techniques to quantify body composition,32 and the strengths and weaknesses of these modalities in oncology have been reviewed.33 A single abdominal cross‐sectional computed tomography image at the third lumbar vertebra provides an accurate estimate of whole‐body skeletal muscle (R 2 = 0.855; P < 0.001) and adipose tissue (R 2 = 0.927; P < 0.001) volumes.34 Existing clinically acquired images from patients with cancer have provided a rich source of data for investigators to quantify measures of body composition and their prognostic importance in patients with cancer.
Low abdominal muscle cross‐sectional area measured using computed tomography at the time of diagnosis in patients with cancer is associated with poor prognosis. In a meta‐analysis of 38 studies that included 7843 patients with solid tumours, low muscle cross‐sectional area was observed in 27.7% of patients with cancer and associated with poorer overall survival [HR: 1.44, 95% CI: 1.32–1.56, P < 0.001], cancer‐specific survival [HR: 1.93, 95% CI: 1.38–2.70, P < 0.001], and disease‐free survival [HR: 1.16, 95% CI: 1.00–1.30, P = 0.014].5 The deleterious effects of low muscle area on overall survival were similar between non‐metastatic [HR: 1.54, 95% CI: 1.31–1.79, P < 0.001] and metastatic disease [HR: 1.37, 95% CI: 1.21–1.56, P < 0.001], and consistent across various tumour types. This observation has been confirmed in two large cohort studies of 3241 females with breast cancer and 3262 males and females with colorectal cancer, where low abdominal muscle cross‐sectional area was observed in 34–42% of patients, and this was independently associated with a 27–41% higher risk of overall mortality.35, 36 Multiple meta‐analyses have now summarized the prognostic importance of low muscle area in a variety of cancer sites, such as colorectal [HR: 1.63, 95% CI: 1.24–2.14; P < 0.01],37 gastric [HR: 1.70, 95% CI: 1.45–1.99; P < 0.01],38 esophageal [HR: 1.70, 95% CI: 1.33–2.17; P < 0.001],39 and hepatocellular carcinoma [HR: 1.95, 95% CI: 1.60–2.37; P < 0.001].40 In addition to low muscle area, low muscle radiodensity (indicative of intramyocellular lipid)41 is associated with poorer overall survival in patients with colorectal cancer [HR: 1.61, 95% CI: 1.36–1.90],42 non‐small cell lung cancer [HR: 1.19, 95% CI: 1.07–1.33],43 B‐cell lymphoma [HR: 2.52, 1.40–4.54],44 and endometrial cancer [HR: 2.03, 95% CI: 1.09–3.78].45 Muscle cross‐sectional area and radiodensity may each be independent prognostic factors for overall and cancer‐specific mortality. For example, among 1924 patients with stage I–III colorectal cancer, the deterioration of muscle area [HR: 2.15, 95% CI: 1.59–2.92; P < 0.001] and muscle radiodensity [HR: 1.61, 95% CI: 1.20–2.15; P = 0.002] were independently prognostic of all‐cause and cancer‐specific mortality.46 There are also emerging data that excess visceral adiposity may be associated with overall survival; however, these data are mixed, with higher visceral adiposity associated with poorer overall survival in colorectal and pancreatic cancer, but improved overall survival in renal cell carcinoma.47
The use of body composition quantified with clinical imaging is, however, not without limitation. One of the principal methodological challenges is disentangling the physiological, prognostic, and statistical interactions between muscle and adiposity. Patients with higher BMI have more muscle mass on the absolute (kg) scale, but less muscle mass on the relative (%) scale, compared with those with a lower BMI.48 It is not yet established if absolute muscle mass or the relative proportion of muscle mass compared with total adiposity is a superior predictor of outcome in patients with cancer. To address this issue, some studies have statistically adjusted for the complementary body composition tissue. For example, low muscle cross‐sectional area was associated with poorer overall survival in patients with breast cancer [HR: 1.30, 95% CI: 1.10–1.54, P < 0.001], and after adjustment for total adiposity, the magnitude of the association was strengthened [HR: 1.41, 95% CI: 1.18–1.69].35 Another approach is to model the joint effects of muscle and adiposity using phenotype methods. For example, the combined presence of both low muscle cross‐sectional area and high total adiposity was associated with poorer overall survival in patients with colorectal cancer [HR: 1.40, 95% CI: 1.03–1.90] compared with those with adequate muscle and low adiposity.36 There is emerging interest in the co‐occurrence of low muscle and excess adiposity, known as sarcopenic obesity,49 and additionally osteoporosis known as osteo‐sarcopenic obesity.50 Many studies to date have not accounted for the potential opposing or joint prognostic effects that may exist between muscle and adipose tissue.
The need for randomized clinical trials to modify body composition in cancer
A robust pipeline of randomized clinical trials will advance this research area.8, 51 It is unknown if body composition is causally related to the occurrence of clinical events, such as post‐operative complications, chemotherapy‐related toxicities, disease recurrence or progression, and overall survival in patients with cancer. Randomized clinical trials are necessary to determine if the effects of body composition on clinical endpoints are both causal, and, more importantly, reversible through intervention. The physiological mechanisms that link body composition with clinical events are multi‐factorial and include metabolic alterations such as inflammation, oxidative stress, myostatin activation, and insulin resistance which promote a catabolic state, and are worsened with physical inactivity and nutritional deficiency.52, 53 Herein, we describe lifestyle and pharmacotherapies that have been examined in patients with cancer for the purposes of manipulating body mass or body composition and proposed recommendations for the next generation of randomized clinical trials.
Lifestyle therapy for body composition management
Lifestyle therapy, including exercise and dietary modification, are efficacious interventions to influence body composition in patients with cancer. The primary modality to quantify body composition in randomized controlled trials has been dual‐energy X‐ray absorptiometry. In a meta‐analysis of six randomized controlled trials that included patients with early stage breast and prostate cancer, progressive resistance training exercise increased lean body mass [mean difference (MD): +1.07 kg, 95% CI: 0.76–1.37, P < 0.001] and decreased body fat [MD: −2.08%, 95% CI: −3.46 to −0.70, P = 0.003] compared with usual care control during an average of 18 weeks.54 Resistance training exercise also improved functional outcomes including muscle strength of the lower [MD: +14.6 kg, 6.3–22.8, P < 0.001] and upper [MD: +6.9, 95% CI: 4.8–9.0, P < 0.001] extremities, and walking distance [MD: +143 m, 95% CI: 70–216, P < 0.001]. Similar magnitude of benefit has been summarized in meta‐analyses for patients with breast and prostate cancer.55, 56 In a meta‐analysis of 22 randomized control trials among healthy adults, the combination of resistance exercise plus protein supplementation increased lean body mass vs. resistance exercise plus placebo [MD: +0.69 kg, 95% CI: 0.47–0.91, P < 0.001].57 An ongoing randomized trial is examining the efficacy of resistance exercise and protein supplementation to improve lean mass and reduce chemotherapy‐related dose‐limiting toxicities in patients with colon cancer (ClinicalTrials.gov Identifier: NCT03291951). Aerobic exercise significantly reduces visceral adiposity in patients with colon cancer [MD: −2.7 cm2 per 60 min of aerobic exercise; P < 0.001] while preserving lean mass over 6 months.58 Additional benefits of exercise for patients with cancer have been described.59, 60 There are limited data testing the effects of specific dietary interventions on body composition in patients with cancer; many studies have focused on weight loss using caloric restriction.61, 62 Randomized controlled trials have demonstrated that caloric restriction reduces body mass [MD: −5.8%, 95% CI: −3.8 to −7.8; P < 0.001] and fat mass [MD: −3.2 ± 0.7 kg, P < 0.001] in patients with cancer; however, these changes are also accompanied by declines in lean body mass [MD: −1.7 ± 0.4 kg, P < 0.001].63 A randomized phase III trial is evaluating the effects of purposeful weight loss on distant disease‐free survival among 3136 overweight and obese women with breast cancer.64 Until definitive evidence emerges, the benefits of weight loss for overweight and obese patients with cancer remain contested.65
Pharmacotherapy for body composition management
Pharmacotherapy may offer benefit for the management of body composition in patients with advanced or metastatic cancer.66 Therapies that utilize or target ghrelin,67, 68 androgen receptors,69 interleukin‐1α,70, 71 β receptor blockade,72 testosterone,73 and myostatin74 have been evaluated in randomized clinical trials. Among therapies with phase III data, none have received regulatory approval for clinical use. Anamorelin, a ghrelin receptor agonist, has extensive clinical data from randomized controlled trials evaluating therapeutic efficacy in patients with inoperable stage III or IV non‐small‐cell lung cancer and cachexia (defined as ≥5% weight loss within 6 months or BMI <20 kg/m2).68 In a meta‐analysis of five randomized controlled trials, anamorelin significantly increased lean muscle mass compared with placebo [MD: +1.10 kg, 95% CI: 0.35–1.85; P = 0.004]. However, handgrip strength was not improved [MD: 0.52 kg, 95% CI: −0.09 − 1.13; P = 0.09], and overall survival did not differ between randomized groups [HR: 0.99, 95% CI: 0.85–1.14; P = 0.84].75 The most common treatment‐related adverse events were hyperglycemia and gastrointestinal disorders. Despite the success of these therapies to increase lean body mass, their inability to influence functional measures has led to failure of regulatory approval. Many studies to date have investigated pharmacotherapy in patients with advanced or metastatic cancer with established cachexia. The risk to benefit ratio of pharmacotherapy, including weight loss agents in patients with early stage cancer who have not yet developed cachexia, has not been comprehensively evaluated.
The future of randomized clinical trials
If body composition has a causal effect on the incidence of clinical events such as overall survival in patients with cancer, multimodal interventions may be necessary to provide a sufficient therapeutic stimulus to impact disease progression. Early screening to identify individuals with occult muscle loss, combined with lifestyle therapy including resistance exercise training and dietary supplementation (e.g. protein), and pharmacotherapy may be necessary to provide a sufficient stimulus to prevent or slow the cascade of tissue wasting (Figure 2).76 To date, most trials of lifestyle therapy have focused on patients with early stage breast and prostate cancer, whereas trials of pharmacotherapy have focused on patients with advanced or metastatic lung and gastrointestinal cancer who have overt cachexia. Early intervention to prevent the deterioration of body composition may be more effective than efforts to improve body composition in patients with established cachexia. For example, resistance exercise among patients with early‐stage breast cancer attenuated the rate of decline of appendicular lean mass [−0.01 vs −0.08 kg/m2; P = 0.041],77 and the deterioration of physical functioning (relative risk: 0.49, 95% CI: 0.25–0.96; P = 0.04) over 12 months.78 Furthermore, lifestyle therapy and pharmacotherapy may have complementary effects, that when used together have the potential to increase lean mass, increase muscle strength, and obviate functional and clinical decline. An example of a multimodal intervention that is being tested within an ongoing phase III trial includes non‐steroidal anti‐inflammatory medication, eicosapentaenoic acid, resistance and aerobic exercise, and dietary counselling with oral nutritional supplements to prevent weight loss and the deterioration of body composition in patients with advanced or metastatic cancer.79 Studies to date have focused on the importance of muscle; however, greater attention to the prognostic effects of excess adiposity and the development of interventions with the potential to simultaneously increase muscle and reduce adiposity may be of critical importance. Continued efforts to investigate the efficacy of multimodal interventions are urgently needed to advance this area.
Figure 2.
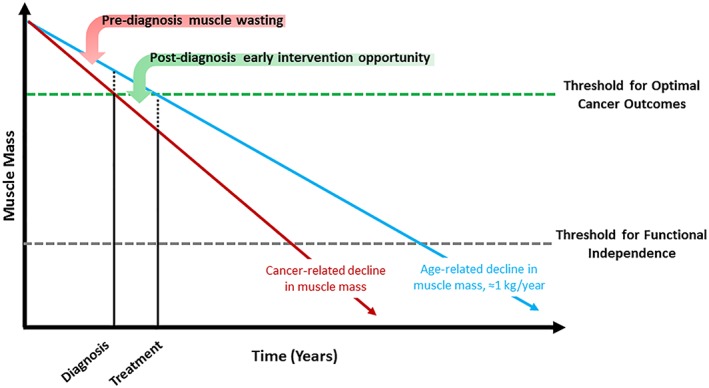
Schematic underscoring the hypothesized importance of early multimodal intervention to preserve muscle mass in patients with cancer. Relative to healthy adults, patients with cancer may experience pre‐diagnosis muscle wasting, and after diagnosis, this muscle wasting may be accelerated from cancer treatments. Early identification and multimodal intervention may help to retard the rate of decline in muscle mass and thereby prevent patients from falling below the critical threshold of muscle mass that is necessary for optimal cancer outcomes.
Bridging the gap to understand how body composition can be integrated into patient care
Despite the absence of randomized clinical trials, opportunities exist to integrate body composition measures into oncology care to guide clinical decision making. Despite interest from radiologists to quantify body composition,80 several barriers exist to seamless integration into clinical workflows. Automated and semi‐automated methods to quantify body composition using clinical imaging, such as computed tomography and magnetic resonance imaging, have been developed.81, 82 Diagnostic imaging is already available clinically on large numbers of patients, but it is not standard of care in all cancers and the validity of these methods has not been extensively studied. Other modalities to quantify body composition that can be implemented within clinical settings are also being explored. Ultrasound is a valid, safe, and portable method to quantify various muscle parameters such as volume, cross‐sectional area, and thickness. Several studies have demonstrated that ultrasound measures of muscle correlate well with lean body mass assessed using dual‐energy X‐ray absorptiometry among older adults (R 2 = 0.929–0.955).83 Non‐imaging methods, such as bioelectrical impedance analysis and creatine (methyl‐d3) dilution, have also been evaluated. Bioelectrical impedance analysis and the related reactance and resistance measures are associated with overall survival in patients with cancer; however, these measures are limited by high inter‐patient variability that is influenced by hydration status.84 Creatine dilution, measured by the enrichment of urinary D3‐creatinine 3–5 days after ingestion, is correlated with total muscle mass quantified using magnetic resonance imaging (r = 0.868).85 Any method used to screen for low muscle or excess adiposity in oncology practice will need to be predictive of important cancer outcomes and easily implemented within existing clinical workflows (e.g. automated or rapid assessments with standardized risk thresholds).
Body composition can also be used as a prognostic biomarker to identify patients who are most likely to experience adverse events and toxicities from cancer‐directed therapy. For example, low muscle mass is associated with an increased risk of major post‐operative surgical complications [HR: 1.40, 95% CI: 1.20–1.64, P < 0.001],86 and chemotherapy‐related toxicity in patients with non‐metastatic colorectal [odds ratio: 2.34, 95% CI: 1.04–5.24, P = 0.03],87 and metastatic breast cancer [57 vs 18%, P = 0.02].88 Chemotherapy dosing currently utilizes body surface area, which does not account for the distribution of lean and adipose tissue throughout the body.89 For as long as clinical trials continue to use body surface area, it is likely that chemotherapy dosing in clinical practice will continue to be guided by this measure; however, some studies have begun to explore the use of body composition to guide chemotherapy dosing in the setting of advanced cancer (ClinicalTrials.gov Identifier: NCT01624051).90, 91 Until clinical trial data emerge, oncologists may use measures of body composition to identify patients who may benefit from preventive interventions (e.g. pegfilgrastim prophylaxis for febrile neutropenia).92
Conclusions
The study of body composition is one of the most provocative areas in oncology. There is growing observational evidence that measures of body composition obtained from clinically acquired imaging are associated with numerous outcomes in patients with cancer. Randomized clinical trials that test multimodal interventions including early identification, lifestyle therapy, and pharmacotherapy may offer the largest potential for clinical benefit. The emergence of automated techniques to quantify body composition will allow for rapid and early intervention of high‐risk patients. Transdisciplinary teams of investigators that span basic, clinical, and population sciences will accelerate the discovery of therapeutics and their translation into clinical practice. The emerging opportunities to integrate body composition measures into oncology offers tremendous promise to help patients with cancer live longer and healthier lives.
Conflict of interest
None declared.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.93 The research reported in this publication was supported by the National Cancer Institute (K99‐CA218603; R25‐CA092203; R01‐CA175011; and K01‐CA226155).
Brown, J. C. , Cespedes Feliciano, E. M. , and Caan, B. J. (2018) The evolution of body composition in oncology—epidemiology, clinical trials, and the future of patient care: facts and numbers. Journal of Cachexia, Sarcopenia and Muscle, 9: 1200–1208. 10.1002/jcsm.12379.
References
- 1. Brown JC, Meyerhardt JA. Obesity and energy balance in GI cancer. J Clin Oncol 2016;34:4217–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strulov Shachar S, Williams GR. The obesity paradox in cancer‐moving beyond BMI. Cancer Epidemiol Biomarkers Prev 2017;26:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin L, Watanabe S, Fainsinger R, Lau F, Ghosh S, Quan H et al. Prognostic factors in patients with advanced cancer: use of the patient‐generated subjective global assessment in survival prediction. J Clin Oncol 2010;28:4376–4383. [DOI] [PubMed] [Google Scholar]
- 4. Hopkins JJ. Sawyer MB. A review of body composition and pharmacokinetics in oncology. Expert Rev Clin Pharmacol 2017;10:947–956. [DOI] [PubMed] [Google Scholar]
- 5. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 6. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. AACR 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Demark‐Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 2001;19:2381–2389. [DOI] [PubMed] [Google Scholar]
- 8. Fearon K, Argiles JM, Baracos VE, Bernabei R, Coats A, Crawford J et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle 2015;6:272–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andreoli A, Garaci F, Cafarelli FP, Guglielmi G. Body composition in clinical practice. Eur J Radiol 2016;85:1461–1468. [DOI] [PubMed] [Google Scholar]
- 10. Collaboration NRF . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19· 2 million participants. The Lancet 2016;387:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnold M, Leitzmann M, Freisling H, Bray F, Romieu I, Renehan A et al. Obesity and cancer: an update of the global impact. Cancer Epidemiol 2016;41:8–15. [DOI] [PubMed] [Google Scholar]
- 12. Lauby‐Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenlee H, Unger JM, LeBlanc M, Ramsey S, Hershman DL. Association between body mass index and cancer survival in a pooled analysis of 22 clinical trials. AACR 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lainscak M, von Haehling S, Doehner W, Anker SD. The obesity paradox in chronic disease: facts and numbers. J Cachexia Sarcopenia Muscle 2012;3:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flegal KM, Ioannidis JP. The obesity paradox: a misleading term that should be abandoned. Obesity 2018;26:629–630. [DOI] [PubMed] [Google Scholar]
- 16. Caan BJ, Cespedes Feliciano EM, Kroenke CH. The importance of body composition in explaining the overweight paradox in cancer‐counterpoint. Cancer Res 2018;78:1906–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cespedes Feliciano EM, Kroenke CH, Caan BJ. The obesity paradox in cancer: how important is muscle? Annu Rev Nutr 2018;38:357–379. [DOI] [PubMed] [Google Scholar]
- 18. Cespedes Feliciano EM, Prentice RL, Aragaki AK, Neuhouser ML, Banack HR, Kroenke CH et al. Methodological considerations for disentangling a risk factor's influence on disease incidence versus postdiagnosis survival: The example of obesity and breast and colorectal cancer mortality in the W omen's Health Initiative. Int J Cancer 2017;141:2281–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flegal KM, Kit BK, Graubard BI. Body mass index categories in observational studies of weight and risk of death. Am J Epidemiol 2014;180:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flegal KM, Graubard BI, Williamson DF, Cooper RS. Reverse causation and illness‐related weight loss in observational studies of body weight and mortality. Am J Epidemiol 2011;173:1–9. [DOI] [PubMed] [Google Scholar]
- 21. Flegal KM, Graubard BI, Williamson DF, Gail MH. Impact of smoking and preexisting illness on estimates of the fractions of deaths associated with underweight, overweight, and obesity in the US population. Am J Epidemiol 2007;166:975–982. [DOI] [PubMed] [Google Scholar]
- 22. Flegal KM, Graubard BI, Yi SW. Comparative effects of the restriction method in two large observational studies of body mass index and mortality among adults. Eur J Clin Invest 2017;47:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rothman KJ. BMI‐related errors in the measurement of obesity. Int J Obes (Lond) 2008;32:S56–S59. [DOI] [PubMed] [Google Scholar]
- 24. Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist‐stature ratio in adults. Am J Clin Nutr 2009;89:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romero‐Corral A, Somers VK, Sierra‐Johnson J, Thomas RJ, Collazo‐Clavell ML, Korinek J et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez‐Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999‐2004. Int J Obes (Lond) 2016;40:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sinicrope FA, Foster NR, Yothers G, Benson A, Seitz JF, Labianca R et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer 2013;119:1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Renfro LA, Loupakis F, Adams RA, Seymour MT, Heinemann V, Schmoll HJ et al. Body mass index is prognostic in metastatic colorectal cancer: pooled analysis of patients from first‐line clinical trials in the ARCAD database. J Clin Oncol 2016;34:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR et al. Association of body‐mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 2018;19:310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prado CM, Gonzalez MC, Heymsfield SB. Body composition phenotypes and obesity paradox. Curr Opin Clin Nutr Metab Care 2015;18:535–551. [DOI] [PubMed] [Google Scholar]
- 31. Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr 2014;38:940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heymsfield SB, Wang Z, Baumgartner RN, Ross R. Human body composition: advances in models and methods. Annu Rev Nutr 1997;17:527–558. [DOI] [PubMed] [Google Scholar]
- 33. Di Sebastiano KM, Mourtzakis M. A critical evaluation of body composition modalities used to assess adipose and skeletal muscle tissue in cancer. Appl Physiol Nutr Metab 2012;37:811–821. [DOI] [PubMed] [Google Scholar]
- 34. Shen W, Punyanitya M, Wang Z, Gallagher D, St‐Onge MP, Albu J et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol (1985) 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 35. Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 2018;4:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E et al. Explaining the obesity paradox: the association between body composition and colorectal cancer survival. Cancer Epidemiol Biomarkers Prev 2017;26:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X et al. Can sarcopenia be a predictor of prognosis for patients with non‐metastatic colorectal cancer? A systematic review and meta‐analysis. Int J Colorectal Dis 2018;33:1419–1427. [DOI] [PubMed] [Google Scholar]
- 38. Yang Z, Zhou X, Ma B, Xing Y, Jiang X, Wang Z. Predictive value of preoperative sarcopenia in patients with gastric cancer: a meta‐analysis and systematic review. J Gastrointest Surg 2018;1–13. [DOI] [PubMed] [Google Scholar]
- 39. Boshier PR, Heneghan R, Markar SR, Baracos VE, Low DE. Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta‐analysis. Dis Esophagus 2018;31. [DOI] [PubMed] [Google Scholar]
- 40. Chang K‐V, Chen J‐D, Wu W‐T, Huang K‐C, Hsu C‐T, Han D‐S. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta‐analysis. Liver cancer 2018;7:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 2001;90:2157–2165. [DOI] [PubMed] [Google Scholar]
- 42. Kroenke CH, Prado CM, Meyerhardt JA, Weltzien EK, Xiao J, Cespedes Feliciano EM et al. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 2018;124:3008–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sjøblom B, Grønberg BH, Wentzel‐Larsen T, Baracos VE, Hjermstad MJ, Aass N et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non‐small cell lung cancer. Clin Nutr 2016;35:1386–1393. [DOI] [PubMed] [Google Scholar]
- 44. Chu MP, Lieffers J, Ghosh S, Belch A, Chua NS, Fontaine A et al. Skeletal muscle density is an independent predictor of diffuse large B‐cell lymphoma outcomes treated with rituximab‐based chemoimmunotherapy. J Cachexia Sarcopenia Muscle 2017;8:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Paula NS, Rodrigues CS, Chaves GV. Comparison of the prognostic value of different skeletal muscle radiodensity parameters in endometrial cancer. Eur J Clin Nutr 2018. [DOI] [PubMed] [Google Scholar]
- 46. Brown JC, Caan BJ, Meyerhardt JA, Weltzien E, Xiao J, Cespedes Feliciano EM et al. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I–III colorectal cancer: a population‐based cohort study (C‐SCANS). J Cachexia Sarcopenia Muscle 2018;9:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xiao J, Mazurak VC, Olobatuyi TA, Caan BJ, Prado CM. Visceral adiposity and cancer survival: a review of imaging studies. Eur J Cancer Care (Engl) 2018;27:e12611. [DOI] [PubMed] [Google Scholar]
- 48. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985) 2000;89:81–88. [DOI] [PubMed] [Google Scholar]
- 49. Carneiro IP, Mazurak VC, Prado CM. Clinical implications of sarcopenic obesity in cancer. Curr Oncol Rep 2016;18:62. [DOI] [PubMed] [Google Scholar]
- 50. Ormsbee MJ, Prado CM, Ilich JZ, Purcell S, Siervo M, Folsom A et al. Osteosarcopenic obesity: the role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle 2014;5:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dunne RF, Mustian KM, Garcia JM, Dale W, Hayward R, Roussel B, et al. Research priorities in cancer cachexia: The University of Rochester Cancer Center NCI Community Oncology Research Program Research Base Symposium on Cancer Cachexia and Sarcopenia. Curr Opin Support Palliat Care 2017;11:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 2015;14:58–74. [DOI] [PubMed] [Google Scholar]
- 53. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–99. [DOI] [PubMed] [Google Scholar]
- 54. Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta‐analysis. Med Sci Sports Exerc 2013;45:2080–2090. [DOI] [PubMed] [Google Scholar]
- 55. Keilani M, Hasenoehrl T, Baumann L, Ristl R, Schwarz M, Marhold M et al. Effects of resistance exercise in prostate cancer patients: a meta‐analysis. Support Care Cancer 2017;25:2953–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu G, Zhang X, Wang Y, Xiong H, Zhao Y, Sun F. Effects of exercise intervention in breast cancer survivors: a meta‐analysis of 33 randomized controlled trails. Onco Targets Ther 2016;9:2153–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance‐type exercise training: a meta‐analysis. Am J Clin Nutr 2012;96:1454–1464. [DOI] [PubMed] [Google Scholar]
- 58. Brown JC, Zemel BS, Troxel AB, Rickels MR, Damjanov N, Ky B et al. Dose‐response effects of aerobic exercise on body composition among colon cancer survivors: a randomised controlled trial. Br J Cancer 2017;117:1614–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ballard‐Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 2012;104:815–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer 2008;8:205–211. [DOI] [PubMed] [Google Scholar]
- 61. Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A et al. Randomized trial of a telephone‐based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol 2014;32:2231–2239. [DOI] [PubMed] [Google Scholar]
- 62. Rock CL, Flatt SW, Byers TE, Colditz GA, Demark‐Wahnefried W, Ganz PA et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol 2015;33:3169–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brown JC, Yung RL, Gobbie‐Hurder A, Shockro L, O'Connor K, Campbell N et al. Randomized trial of a clinic‐based weight loss intervention in cancer survivors. J Cancer Surviv 2018;12:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ligibel JA, Barry WT, Alfano C, Hershman DL, Irwin M, Neuhouser M et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer 2017;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML et al. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol 2016;2:1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Morley JE, von Haehling S, Anker SD. Are we closer to having drugs to treat muscle wasting disease? J Cachexia Sarcopenia Muscle 2014;5:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo‐controlled, double‐blind trials. Lancet Oncol 2015;16:108–116. [DOI] [PubMed] [Google Scholar]
- 68. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol 2016;17:519–531. [DOI] [PubMed] [Google Scholar]
- 69. Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, Hancock ML et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double‐blind, randomised controlled phase 2 trial. Lancet Oncol 2013;14:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hickish T, Andre T, Wyrwicz L, Saunders M, Sarosiek T, Kocsis J et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Oncol 2017;18:192–201. [DOI] [PubMed] [Google Scholar]
- 71. Hong DS, Hui D, Bruera E, Janku F, Naing A, Falchook GS et al. MABp1, a first‐in‐class true human antibody targeting interleukin‐1alpha in refractory cancers: an open‐label, phase 1 dose‐escalation and expansion study. Lancet Oncol 2014;15:656–666. [DOI] [PubMed] [Google Scholar]
- 72. Stewart Coats AJ, Ho GF, Prabhash K, von Haehling S, Tilson J, Brown R et al. Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non‐small cell lung cancer or colorectal cancer: a randomized, double‐blind, placebo‐controlled, international multicentre phase II study (the ACT‐ONE trial). J Cachexia Sarcopenia Muscle 2016;7:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wright TJ, Dillon EL, Durham WJ, Chamberlain A, Randolph KM, Danesi C et al. A randomized trial of adjunct testosterone for cancer‐related muscle loss in men and women. J Cachexia Sarcopenia Muscle 2018;9:482–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Golan T, Geva R, Richards D, Madhusudan S, Lin B6, Wang HT et al. LY2495655, an antimyostatin antibody, in pancreatic cancer: a randomized, phase 2 trial. J Cachexia Sarcopenia Muscle 2018;9:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nishie K, Yamamoto S, Nagata C, Koizumi T, Hanaoka M. Anamorelin for advanced non‐small‐cell lung cancer with cachexia: systematic review and meta‐analysis. Lung Cancer 2017;112:25–34. [DOI] [PubMed] [Google Scholar]
- 76. Argiles JM, Lopez‐Soriano FJ, Stemmler B, Busquets S. Novel targeted therapies for cancer cachexia. Biochem J 2017;474:2663–2678. [DOI] [PubMed] [Google Scholar]
- 77. Brown JC, Schmitz KH. Weight lifting and appendicular skeletal muscle mass among breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat 2015;151:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brown JC, Schmitz KH. Weight lifting and physical function among survivors of breast cancer: a post hoc analysis of a randomized controlled trial. J Clin Oncol 2015;33:2184–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Solheim TS, Laird BJA, Balstad TR, Bye A, Stene G, Baracos V et al. Cancer cachexia: rationale for the MENAC (Multimodal‐Exercise, Nutrition and Anti‐inflammatory medication for Cachexia) trial. BMJ Support Palliat Care 2018;8:258–265. [DOI] [PubMed] [Google Scholar]
- 80. Murray TE, Williams D, Lee MJ. Osteoporosis, obesity, and sarcopenia on abdominal CT: a review of epidemiology, diagnostic criteria, and management strategies for the reporting radiologist. Abdom Radiol (NY) 2017;42:2376–2386. [DOI] [PubMed] [Google Scholar]
- 81. Popuri K, Cobzas D, Esfandiari N, Baracos V, Jägersand M. Body composition assessment in axial CT images using FEM‐based automatic segmentation of skeletal muscle. IEEE Trans Med Imaging 2016;35:512–520. [DOI] [PubMed] [Google Scholar]
- 82. Takahashi N, Sugimoto M, Psutka SP, Chen B, Moynagh MR, Carter RE. Validation study of a new semi‐automated software program for CT body composition analysis. Abdom Radiol (NY) 2017;42:2369–2375. [DOI] [PubMed] [Google Scholar]
- 83. Nijholt W, Scafoglieri A, Jager‐Wittenaar H, Hobbelen JSM, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle 2017;8:702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Grundmann O, Yoon SL, Williams JJ. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients‐‐a comprehensive review. Eur J Clin Nutr 2015;69:1290–1297. [DOI] [PubMed] [Google Scholar]
- 85. Clark RV, Walker AC, O'Connor‐Semmes RL, Leonard MS, Miller RR, Stimpson SA et al. Total body skeletal muscle mass: estimation by creatine (methyl‐d3) dilution in humans. J Appl Physiol (1985) 2014; 116(12): 1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta‐analysis. Ann Surg 2018;268:58–69. [DOI] [PubMed] [Google Scholar]
- 87. Cespedes Feliciano EM, Lee VS, Prado CM, Meyerhardt JA, Alexeeff S, Kroenke CH1 et al. Muscle mass at the time of diagnosis of nonmetastatic colon cancer and early discontinuation of chemotherapy, delays, and dose reductions on adjuvant FOLFOX: The C‐SCANS study. Cancer 2017;123:4868–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane based chemotherapy. Clin Cancer Res 2016; clincanres 0940.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Griggs JJ, Mangu PB, Temin S, Lyman GH. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Oncol Pract 2012;8:e59–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Crosby V, D'Souza C, Bristow C, Proffitt A, Hussain A, Potter V et al. Can body composition be used to optimize the dose of platinum chemotherapy in lung cancer? A feasibility study. Support Care Cancer 2017;25:1257–1261. [DOI] [PubMed] [Google Scholar]
- 91. Sjoblom B, Benth JS, Gronberg BH, Baracos VE, Sawyer MB, Fløtten Ø et al. Drug dose per kilogram lean body mass predicts hematologic toxicity from carboplatin‐doublet chemotherapy in advanced non‐small‐cell lung cancer. Clin Lung Cancer 2017;18:e129–e136. [DOI] [PubMed] [Google Scholar]
- 92. Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas‐Figueroa LJ, Wiens BL et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double‐blind, placebo‐controlled phase III study. J Clin Oncol 2005;23:1178–1184. [DOI] [PubMed] [Google Scholar]
- 93. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brunner AM, Sadrzadeh H, Feng Y, Drapkin BJ, Ballen KK, Attar EC et al. Association between baseline body mass index and overall survival among patients over age 60 with acute myeloid leukemia. Am J Hematol 2013;88:642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Navarro WH, Loberiza FR Jr, Bajorunaite R, van Besien K, Vose JM, Lazarus HM et al. Effect of body mass index on mortality of patients with lymphoma undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 2006;12:541–551. [DOI] [PubMed] [Google Scholar]
- 96. Chen HN, Chen XZ, Zhang WH, Yang K, Chen XL, Zhang B et al. The impact of body mass index on the surgical outcomes of patients with gastric cancer: a 10‐year, single‐institution cohort study. Medicine (Baltimore) 2015;94:e1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Albiges L, Hakimi AA, Xie W, McKay RR, Simantov R, Lin X et al. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J Clin Oncol 2016;34:3655–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]


