Abstract
Objective
Compare the effects and costs of remotely monitored exercise-based cardiac telerehabilitation (REMOTE-CR) with centre-based programmes (CBexCR) in adults with coronary heart disease (CHD).
Methods
Participants were randomised to receive 12 weeks of telerehabilitation or centre-based rehabilitation. REMOTE-CR provided individualised exercise prescription, real-time exercise monitoring/coaching and theory-based behavioural strategies via a bespoke telerehabilitation platform; CBexCR provided individualised exercise prescription and coaching via established rehabilitation clinics. Outcomes assessed at baseline, 12 and/or 24 weeks included maximal oxygen uptake (V̇O2max, primary) modifiable cardiovascular risk factors, exercise adherence, motivation, health-related quality of life and programme delivery, hospital service utilisation and medication costs. The primary hypothesis was a non-inferior between-group difference in V̇O2max at 12 weeks (inferiority margin=−1.25 mL/kg/min); inferiority margins were not set for secondary outcomes.
Results
162 participants (mean 61±12.7 years, 86% men) were randomised. V̇O2 max was comparable in both groups at 12 weeks and REMOTE-CR was non-inferior to CBexCR (REMOTE-CR-CBexCR adjusted mean difference (AMD)=0.51 (95% CI −0.97 to 1.98) mL/kg/min, p=0.48). REMOTE-CR participants were less sedentary at 24 weeks (AMD=−61.5 (95% CI −117.8 to −5.3) min/day, p=0.03), while CBexCR participants had smaller waist (AMD=1.71 (95% CI 0.09 to 3.34) cm, p=0.04) and hip circumferences (AMD=1.16 (95% CI 0.06 to 2.27) cm, p=0.04) at 12 weeks. No other between-group differences were detected. Per capita programme delivery (NZD1130/GBP573 vs NZD3466/GBP1758) and medication costs (NZD331/GBP168 vs NZD605/GBP307, p=0.02) were lower for REMOTE-CR. Hospital service utilisation costs were not statistically significantly different (NZD3459/GBP1754 vs NZD5464/GBP2771, p=0.20).
Conclusion
REMOTE-CR is an effective, cost-efficient alternative delivery model that could—as a complement to existing services—improve overall utilisation rates by increasing reach and satisfying unique participant preferences.
Keywords: coronary artery disease, cardiac rehabilitation, ehealth/telemedicine/mobile health
Introduction
Exercise-based cardiac rehabilitation (exCR) is a critical component of the secondary prevention of coronary heart disease (CHD) that confers numerous beneficial cardiovascular and metabolic adaptations and reduces mortality.1–3 Despite these benefits, uptake and adherence to traditional centre-based programmes are low.4 To address this, participants should be offered a range of programme delivery options that meet their individual needs and preferences5; however, few alternatives exist. Innovation is needed to improve access, uptake and adherence in order to optimise the individual, clinical and economic benefits of exCR and assess sustained effects.
Technology-assisted delivery models (telerehabilitation) can overcome accessibility barriers but have commonly been limited to technologies that confine participants to fixed locations (eg, telephone, desktop computer, videoconferencing).6 Increasingly powerful mobile technologies (eg, smartphones, wearable sensors) can support more sophisticated, flexible, responsive and interactive delivery models but are not widely used in exCR. We developed a bespoke telerehabilitation platform to address this unmet opportunity. We compared the effectiveness and costs of real-time remotely monitored exercise-based cardiac telerehabilitation (termed REMOTE-CR) with traditional centre-based programmes (CBexCR) among adults with CHD. We hypothesised REMOTE-CR would be at least as effective (ie, non-inferior) and less costly than CBexCR.
Methods
Design
The effectiveness of CBexCR is well documented.3 As REMOTE-CR adapted similar intervention support to a remote delivery model, superior treatment effects were unlikely; however, if comparably effective, REMOTE-CR could improve accessibility, acceptability, adherence and delivery costs. Therefore, a two-arm parallel randomised controlled non-inferiority experimental design was chosen. The trial protocol was submitted for registration before enrolling participants (www.anzctr.org.au, ACTRN12614000843651) and has been published7; no changes were made during the trial. All participants provided written consent. Trial development and reporting were informed by Consolidated Standards of Reporting Trials statements for randomised, non-inferiority and equivalence, and eHealth trials.
Participants
Potential participants were identified via metropolitan hospitals, outpatient clinics and community-based cardiac rehabilitation seminars in Auckland and Tauranga (New Zealand). Eligible participants were clinically stable, English-speaking adults (≥18 years) with a documented diagnosis of CHD within 6 months (atherosclerosis, angina pectoris, myocardial infarction, coronary revascularisation). Participants were excluded if they had been admitted to hospital with heart disease within 6 weeks; had terminal cancer, a pacemaker or implantable cardioverter-defibrillator, or significant non-CHD exercise limitations; were contraindicated for maximal exercise testing; completed ≥150 min/week moderate to vigorous physical activity or were currently participating in supervised exCR. This cohort was comparable to previous exCR trials.6
Treatments
All participants retained access to usual care cardiac rehabilitation (eg, inpatient rehabilitation, outpatient clinics, community-based education seminars, which included aspects such as diet and psychological support); usual care service utilisation was not assessed in this trial. In addition, participants received either centre based (control) or remotely delivered (intervention) exCR. Participants completed face-to-face assessments at baseline, 12 and 24 weeks.
Intervention
REMOTE-CR comprised 12 weeks of individualised exercise prescription, exercise monitoring and coaching plus theory-based behavioural strategies to promote exercise and habitual physical activity, delivered via a bespoke telerehabilitation platform. Participants logged into the programme during available monitoring hours that aligned with the control programmes to ensure equal treatment availability. Intervention design and content development are described in detail elsewhere8 but key components are outlined herein.
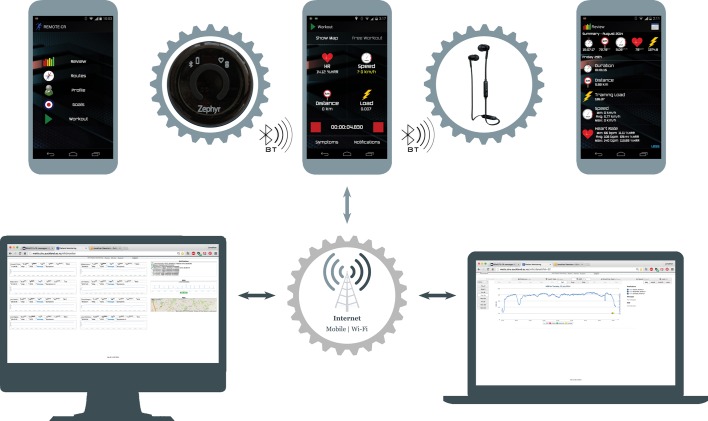
The REMOTE-CR platform comprised a smartphone and chest-worn wearable sensor (BioHarness 3, Zephyr Technology, USA), bespoke smartphone and web apps and custom middleware (figure 1). The sensor provides information on heart and respiratory rates, single lead ECG and accelerometry. Smartphones with a mobile data subscription (NZD1.50/GBP0.76 per week) were loaned to participants at no cost. Custom middleware connected smartphone and web apps and managed communication and logistic functions including data security, network connectivity and device resource usage. Authentication protocols in both apps, a secure webserver and encrypted data transmission ensured security and privacy.
Figure 1.
Remotely monitored exercise-based cardiac telerehabilitation platform schematic.
Participant-facing smartphone and exCR specialist-facing web apps were designed specifically for remote exCR delivery by the research team. App features enabled real-time remote exercise monitoring and coaching, retrospective exercise performance review, goal setting, behaviour change education and social support. The smartphone and web apps are compatible with Android (≥V.4.0) and desktop/mobile web browsers, respectively, thereby enabling participants and exCR specialists to operate from any location with an internet connection. Participants accessed REMOTE-CR via the native bespoke app only.
Behavioural intervention content was grounded in self-efficacy and self-determination theories, and the Taxonomy of Behaviour Change Techniques.9–11 Periodic smartphone app updates were deployed via Google Play to optimise stability; intervention content and core functionality remained consistent.
During exercise training, participants’ physiological (heart and respiratory rate, single lead ECG) and geopositional data were displayed in the smartphone app for self-monitoring, streamed to a web server via 3G/4G/Wi-Fi, and visualised in the web app for exCR specialist review. These processes occurred in real-time, and simultaneous monitoring of multiple participants was supported. ExCR specialists provided real-time individualised audio coaching, feedback and social support throughout (but not prior to) real-time exercise monitoring. Participants received audio communications via earphones to optimise usability and preserve the real-time context of message content. Outside of real-time interaction, participants could review all recorded exercise performance data, set individualised goals and review automated goal achievement feedback to facilitate self-monitoring. Communication was predominantly unidirectional but participants could respond if needed. Finally, participants received behaviour change education via direct messaging. Content drew on theory-based short message service interventions that improve lifestyle behaviours12 13 and was adapted for the real-time communication paradigm and larger character allowance.
Consistent with clinical exercise prescription guidelines,14 REMOTE-CR comprised three exercise sessions per week over 12 weeks and encouragement to be active ≥5 days per week. Prescribed session duration and intensity level ranged from 30 to 60 min (including warm-up and cool-down phases) and 40%–65% heart rate reserve, respectively; intensity level was adjusted to optimise physiological adaptation without inducing abnormal clinical signs or symptoms. Walking was the most accessible exercise mode but participants could choose alternatives if preferred. Exercise prescription was individualised and progressive, based on participants’ maximal aerobic exercise capacity (V̇O2max), exercise-induced signs and symptoms, age, sex, exercise tolerance and preferences. Participants could not access REMOTE-CR after their 12-week intervention.
Control
CBexCR comprised 12 weeks of supervised exercise delivered by clinical exercise physiologists in cardiac rehabilitation clinics. Exercise prescription was comparable to REMOTE-CR and studies that have established the effectiveness of CBexCR.
Outcomes
The primary (non-inferiority) outcome was the between-group difference in V̇O2max at 12 weeks. V̇O2max is an important surrogate and clinical outcome associated with reduced mortality and morbidity, and aligns with recommendations to prioritise functional capacity as a primary end point in cardiac rehabilitation research.15 16 Secondary (superiority) outcomes included fasted blood lipid (total, high-density and low-density lipoprotein cholesterol; triglyceride) and glucose concentrations, anthropometry (height, weight, body mass index (BMI), waist/hip circumference), blood pressure (systolic/diastolic), physical activity (accelerometry), exercise-related motivation (self-efficacy, intention, confidence, locus of causality), exercise adherence, adverse events (any self-reported change in health state) and health-related quality of life (HRQoL). These outcomes are comparable to studies that have established the effectiveness of CBexCR. V̇O2max, lipid and glucose concentrations were assessed at 12 weeks; remaining outcomes were assessed at 12 and 24 weeks. A within-trial cost-utility analysis—conducted from a healthcare system perspective—included programme delivery, hospital service utilisation and medication costs over 24 weeks. The usability and acceptability of the REMOTE-CR intervention were assessed among participants allocated to the intervention group; these data will be reported separately.
V̇O2max was measured with an online metabolic cart during individualised treadmill cardiopulmonary exercise tests, conducted by blinded exercise physiologists in accordance with clinical guidelines.14 Baseline tests were initiated at 0% gradient and a self-selected velocity, thereafter gradient increased by 1%/min until volitional fatigue or clinical indications for test termination. Baseline protocols were replicated at 12-week follow-up; however, modifications were permitted after surpassing the baseline duration if required to elicit V̇O2max.
Blood lipid and glucose concentrations were measured with point of care analysers; participants were asked to fast for ≥3 hours prior to assessments. Height and weight were measured using a calibrated stadiometer and electronic scale, respectively, and BMI was calculated. Waist and hip circumference measurement followed standard anthropometric protocols. Blood pressure was measured with a calibrated automated sphygmomanometer (T9P, Omron Healthcare, Netherlands) after ≥5 min of seated rest. These outcomes were measured prior to exercise testing.
Physical activity was assessed objectively using the Actrigraph (GT1M, ActiGraph Corp, USA) uniaxial accelerometer, worn at the waist, over seven consecutive days prior to assessments. Data were processed according to accepted procedures17 and mean daily sedentary, light, moderate and vigorous activity durations were calculated. Valid measurement required >10 hours wear time on ≥3 weekdays plus one weekend day; non-wear time was defined as >60 min of consecutive zero values.
Exercise-related task and barrier self-efficacy, intentions, confidence and locus of causality were assessed using valid, reliable questionnaires.18–20 Exercise adherence was calculated as the completion of prescribed exercise sessions (maximum=36). Self-reported adverse events were collected at study assessments using a custom questionnaire. HRQoL was assessed using the EuroQol 5-dimension (EQ-5D).21
Pathway analyses identified programme delivery resource items and cost data were derived from project records. Programmes were assumed to operate in steady state, therefore, REMOTE-CR development costs were excluded. Sensitivity analyses assessed the impact of varying two REMOTE-CR costs: wearable sensor annuitising rate and efficiency of exCR specialist time use. Costs are expressed in 2014 New Zealand dollars with indicative Pound Sterling equivalents (assuming NZD1=GBP0.5072). No discounting was necessary for the 24 week analysis time horizon.
Hospital service and medication utilisation were extracted from the New Zealand Ministry of Health National Minimum Dataset and Pharmaceuticals Collection, respectively. Hospital service data included the nature, type, duration, diagnoses (coded using ICD-10-AM-v6) and geographic location of admissions and emergency department visits. ICD codes were converted to Australian refined diagnosis-related group codes (AR-DRG; also used in New Zealand). Cost weights were sourced from the New Zealand Ministry of Health to derive hospitalisation unit costs using the Australian Consortium for Classification Development mapping table.22 23 Costs were adjusted for admission duration; if a single diagnosis included multiple AR-DRG codes (eg, multiple disease severity levels), the average cluster cost was used. Medication utilisation costs included Government and patient components.
HRQoL utility weights were derived from EQ-5D scores following accepted methods.21 If HRQoL differed between groups, quality-adjusted life year (QALY) gains were calculated as
and the incremental cost-effectiveness ratio (ICER) was calculated as
Sample size
ExCR typically elicits 10%–15% improvements in V̇O2max.3 Assuming a 2.4±2.7 ml/kg/min improvement for CBexCR24 and accounting for 10% loss-to-follow-up, we estimated 162 participants (81 per group) would provide 80% power (one-sided α=0.025) to demonstrate REMOTE-CR V̇O2max would be no more than 1.25 mL/kg/min lower than CBexCR. This inferiority margin is clinically significant and associated with lower cardiovascular mortality.16
Randomisation and blinding
Participants were randomised (1:1) to receive REMOTE-CR (intervention) or CBexCR (control) using a computer-generated sequence—created by a blinded statistician—that included variable blocking (n=2/4) and stratification (sex/study site). Treatment allocation was concealed until completion of baseline assessment in sequentially numbered, sealed, opaque envelopes. Participants could not be blinded to treatment allocation but staff performing V̇O2max testing at 12 weeks were blinded to treatment allocation.
Statistical analysis
Analyses were conducted using SAS V.9.4 and R V.3.3 (R Foundation for Statistical Computing, Austria). Baseline characteristics and outcomes were summarised descriptively, continuous variables as mean±SD and categorical variables as frequencies and percentages. Treatment evaluations were performed on the principle of intention-to-treat. Multiple imputations were applied to missing primary (but not secondary) outcome data using the Markov chain Monte Carlo method assuming the data were multivariate normally distributed and the missing data were missing at random. In line with recommendations for non-inferiority trials,25 a prespecified sensitivity analysis was conducted on observed primary outcome data to estimate sensitivity to attrition. Statistical tests were one sided at α=0.025 for the primary non-inferiority outcome and two sided at α=0.05 for secondary outcomes. Between-group treatment effects were evaluated using analysis of covariance adjusted for the baseline outcome, age and sex. The primary hypothesis was assessed using the non-inferiority CI method.25 Random effects mixed models were used on secondary outcomes measured repeatedly at 12/24 weeks. Estimates of treatment effects are reported as mean between-group differences with 95% CI and probability values. Unpaired t-tests were conducted to evaluate between-group differences in hospital service and medication utilisation costs.
Results
Participant characteristics
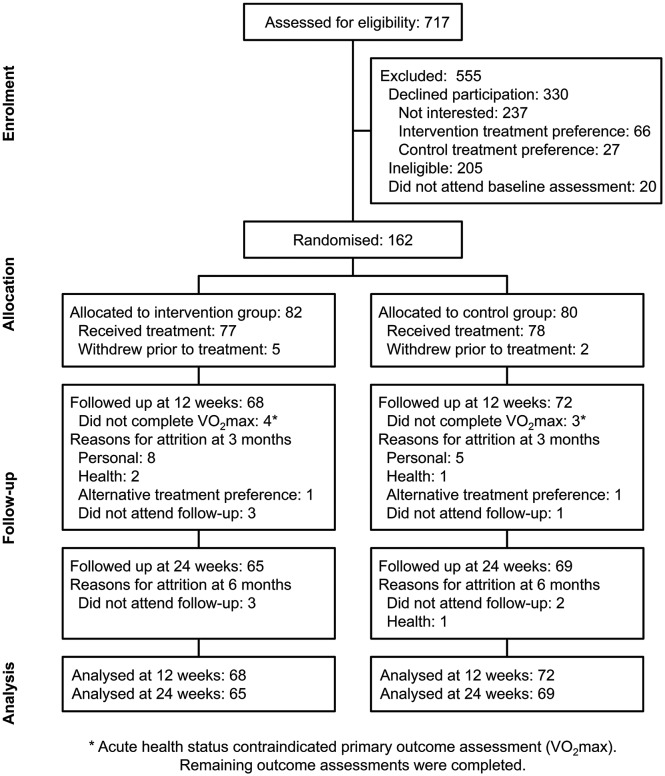
Recruitment took place between August 2014 and January 2016 (figure 2). Participants were predominantly male, of New Zealand European ethnicity, mean age 61 years and approximately 50% earned above the median national household income (table 1). Most participants had previous diagnoses of myocardial infarction and/or angina pectoris; almost two-thirds had undergone angioplasty, and one-quarter had undergone coronary artery bypass graft surgery. Most participants were prescribed multiple medications. Baseline demographic and clinical characteristics were balanced between groups.
Figure 2.
Trial flowchart.
Table 1.
Baseline demographic and clinical characteristics
| REMOTE-CR (n=82) | Centre-based (n=80) | |
| Age (years, mean±SD) | 61.0±13.2 | 61.5±12.2 |
| Sex (n/%) | ||
| Male | 69/84.2 | 70/87.5 |
| Female | 13/15.9 | 10/12.5 |
| Ethnicity (n/%)* | ||
| NZ European | 61/74.4 | 61/76.3 |
| NZ Maori | 2/2.4 | 5/6.3 |
| Pacific | 4/4.9 | 0/0.0 |
| Asian | 4/4.9 | 9/11.3 |
| Other | 13/15.9 | 7/8.8 |
| Household income (n/%)† | ||
| <NZD70 000 | 32/39.0 | 32/40.0 |
| ≥NZD70 000 | 38/46.3 | 45/56.3 |
| Don’t know/refuse to answer | 12/14.6 | 3/3.8 |
| Medical history (n/%) | ||
| Hypertension | 53/64.6 | 49/61.3 |
| Diabetes | 15/18.3 | 14/17.5 |
| Hypercholesterolaemia | 63/76.8 | 70/87.5 |
| Angina pectoris | 33/40.2 | 35/43.8 |
| Myocardial infarction | 61/74.4 | 60/75.0 |
| Angioplasty | 54/65.9 | 51/63.8 |
| CABG | 17/20.7 | 22/27.5 |
| Never smoked | 46/56.1 | 41/51.3 |
| Ex-smoker | 36/43.9 | 38/47.5 |
| Current smoker | 0/0.0 | 1/1.2 |
| Medications (n/%) | ||
| Beta blocker | 54/65.9 | 52/65.0 |
| Calcium channel blocker | 13/15.9 | 12/15.0 |
| ACE inhibitor | 48/58.5 | 44/55.0 |
| Aspirin | 75/91.5 | 79/98.8 |
| Anticoagulant | 59/72.0 | 59/73.8 |
| Statin | 78/95.1 | 74/92.5 |
Anticoagulants are a class of drugs with different mechanisms of action that share the common goal of preventing intravascular blood clots and limiting the growth of existing thrombi (eg, warfarin).
*Participants could identify with multiple ethnicities.
†Categorised above/below median NZ household income (≈NZD70 000/GBP35 504).30
CABG, coronary artery bypass graft; NZ, New Zealand; NZD, New Zealand Dollar; REMOTE-CR, remotely monitored exercise-based cardiac telerehabilitation.
Treatment effects
Baseline, 12-week and 24-week outcomes are summarised in table 2, and between-group effects are reported in table 3. As hypothesised, V̇O2max was comparable in both groups at 12 weeks and the 95% CI indicated REMOTE-CR was non-inferior to CBexCR. A sensitivity analysis of complete cases supported this finding (adjusted mean difference=0.46 (95% CI −0.92 to 1.84) mL/kg/min, p=0.51), suggesting it was not sensitive to attrition.
Table 2.
Descriptive outcome measures for the REMOTE-CR (intervention) and centre-based (control) programmes
| REMOTE-CR (mean±SD) | Centre-based (mean±SD) | |||||
| Baseline | Week 12 | Week 24 | Baseline | Week 12 | Week 24 | |
| V̇O2max (mL/kg/min) | 27.22±7.91 | 30.52±9.63 | – | 27.70±6.77 | 29.39±6.75 | – |
| Physical activity (min/day)* | ||||||
| Sedentary | 596.8±120.1 | 596.4±110.9 | 595.9±94.9 | 591.0±124.2 | 604.2±143.8 | 659.6±181.9 |
| Light | 256.7±67 | 258.3±65.2 | 253.9±51.4 | 260.5±65.6 | 287.0±74.2 | 270.5±68.3 |
| Moderate | 24.6±20.6 | 25.3±17.6 | 23.0±15.8 | 24.3±16.3 | 30.6±18.4 | 23.3±16.0 |
| Vigorous | 0.4±1.3 | 1.4±3.9 | 1.3±4.3 | 0.3±1.8 | 0.8±3.3 | 1.2±4.0 |
| Body composition | ||||||
| Body mass (kg) | 85.83±15.83 | 85.73±14.59 | 86.49±15.35 | 83.06±14.96 | 82.08±14.2 | 82.94±14.73 |
| BMI (kg/m2) | 29.09±4.59 | 29.03±4.32 | 29.13±4.50 | 27.94±3.49 | 27.58±3.34 | 27.90±3.44 |
| Waist (cm) | 103.30±10.97 | 103.20±10.82 | 102.90±11.10 | 101.50±9.99 | 99.60±10.11 | 101.10±10.11 |
| Hip (cm) | 106.00±8.59 | 106.00±8.22 | 105.80±8.19 | 103.60±6.68 | 102.30±6.34 | 103.70±6.83 |
| Blood pressure (mm Hg) | ||||||
| Systolic | 139.00±17.38 | 135.60±16.66 | 135.4±18.17 | 134.40±17.00 | 130.50±15.14 | 132.70±17.42 |
| Diastolic | 80.90±10.84 | 78.04±9.04 | 79.18±10.50 | 79.83±10.17 | 78.11±10.83 | 77.76±10.79 |
| Blood lipids (mmol/L) | ||||||
| Total-C | 3.39±0.79 | 3.62±0.98 | – | 3.45±0.79 | 3.55±0.92 | – |
| LDL-C | 1.80±0.69 | 1.95±0.97 | – | 1.68±0.58 | 1.71±0.59 | – |
| HDL-C | 1.05±0.37 | 1.15±0.40 | – | 1.09±0.37 | 1.13±0.37 | – |
| Triglyceride | 1.46±0.89 | 1.48±0.81 | – | 1.68±1.08 | 1.66±1.11 | – |
| Blood glucose (mmol/L) | 5.44±1.9 | 5.87±1.61 | – | 5.49±1.51 | 6.07±2.35 | – |
| HRQoL | ||||||
| EQ-5D index | 0.91±0.10 | 0.90±0.13 | 0.89±0.13 | 0.91±0.10 | 0.93±0.09 | 0.92±0.09 |
| Health state (%) | 68.95±18.56 | 77.24±14.23 | 78.84±16.19 | 71.16±19.26 | 81.21±12.42 | 81.28±13.76 |
| Exercise motivation | ||||||
| Task self-efficacy (%) | 63.44±21.90 | 79.48±18.26 | 72.45±21.64 | 70.56±15.51 | 80.09±14.04 | 78.14±17.79 |
| Barrier self-efficacy (%) | 75.52±18.79 | 75.06±20.04 | 71.05±22.79 | 80.72±15.45 | 81.09±15.37 | 77.54±19.3 |
| Confidence to adhere (%) | 91.92±14.58 | 91.50±13.87 | 83.42±22.19 | 83.42±22.19 | 89.65±15.65 | 88.79±18.41 |
| Intention† | 6.73±0.52 | 6.53±0.98 | 6.25±1.01 | 6.81±0.71 | 6.60±0.83 | 6.53±0.91 |
| Locus of causality† | 4.13±1.64 | 4.08±1.76 | 4.23±1.69 | 4.38±1.73 | 4.31±1.58 | 4.30±1.53 |
| Exercise adherence (n/36) | – | 21±13 | – | – | 23±11 | – |
*Objective accelerometry.
†Seven-point Likert scale.
BMI, body mass index; EQ-5D, EuroQol 5-dimension; HDL-C, high-density lipoprotein cholesterol; HRQoL, health-related quality of life; LDL-C, low-density lipoprotein cholesterol; REMOTE-CR, remotely monitored exercise-based cardiac telerehabilitation; total-C, total cholesterol; V̇O2max, maximal aerobic exercise capacity.
Table 3.
Between-group treatment effects
| Adjusted mean difference (95% CI)* | ||
| Week 12 | Week 24 | |
| V̇O2max (mL/kg/min) | 0.51 (−0.97 to 1.98) | – |
| Physical activity (min/day)† | ||
| Sedentary | −32.8 (−88.0 to 22.4) | −61.5 (−117.8 to −5.3)‡ |
| Light | −21.4 (−46.3 to 3.5) | −7.1 (−32.6 to 18.5) |
| Moderate | −2.7 (−9.2 to 3.8) | −1.7 (−8.4 to 4.9) |
| Vigorous | 0.4 (−0.7 to 1.5) | 0.0 (−1.1 to 1.1) |
| Body composition | ||
| Body mass (kg) | 0.42 (−0.74 to 1.58) | 0.12 (−1.08 to 1.32) |
| BMI (kg/m2) | 0.16 (−0.29 to 0.60) | −0.05 (−0.52 to 0.42) |
| Waist (cm) | 1.71 (0.09 to 3.34)§ | 0.17 (−1.52 to 1.86) |
| Hip (cm) | 1.16 (0.06 to 2.27)§ | −0.10 (−1.24 to 1.05) |
| Blood pressure (mm Hg) | ||
| Systolic | 3.21 (−1.89 to 8.32) | 1.06 (−4.24 to 6.37) |
| Diastolic | −1.00 (−3.88 to 1.87) | 0.04 (−2.96 to 3.03) |
| Blood lipids (mmol/L) | ||
| Total-C | 0.10 (−0.09 to 0.29) | – |
| LDL-C | 0.19 (−0.04 to 0.43) | – |
| HDL-C | 0.04 (−0.03 to 0.11) | – |
| Triglyceride | −0.06 (−0.34 to 0.22) | – |
| Blood glucose (mmol/L) | −0.21 (−0.85 to 0.42) | – |
| HRQoL | ||
| EQ-5D index | −0.03 (−0.06 to 0.01) | −0.03 (−0.06 to 0.01) |
| Health state (%) | −3.12 (−7.04 to 0.79) | −0.94 (−4.96 to 3.08) |
| Exercise motivation | ||
| Task self-efficacy (%) | 1.24 (−3.91 to 6.38) | −4.62 (−9.89 to 0.65) |
| Barrier self-efficacy (%) | −4.07 (−10.19 to 2.04) | −3.84 (−10.10 to 2.41) |
| Confidence to adhere (%) | 3.28 (−2.54 to 9.10) | −4.19 (−10.19 to 1.8) |
| Intention¶ | −0.05 (−0.36 to 0.26) | −0.27 (−0.58 to 0.05) |
| Locus of causality¶ | −0.05 (−0.47 to 0.36) | 0.18 (−0.24 to 0.60) |
| Exercise adherence (n/36) | −1.97 (−5.74 to 1.81) | – |
Statistically significant between-group differences.
*REMOTE-CR (intervention)-centre-based (control), adjusted for the baseline outcome, age and sex.
†Objective accelerometry.
‡Seven-point Likert scale.
§p=0.03,
¶p=0.04).
BMI, body mass index; EQ-5D, EuroQol 5-dimension; HDL-C, high-density lipoprotein cholesterol; HRQoL, health-related quality of life; LDL-C, low-density lipoprotein cholesterol; total-C, total cholesterol; V̇O2max, maximal aerobic capacity.
Small between-group differences in waist and hip circumferences favoured CBexCR at 12 but not 24 weeks, while a small difference in sedentary time favoured REMOTE-CR at 24 weeks. Remaining secondary outcomes were comparable in both groups.
Economic evaluation
REMOTE-CR programme delivery costs were substantially lower than CBexCR (table 4, see online supplementary data for itemised costs). ExCR specialist salary accounted for the majority of REMOTE-CR costs (48.18%); wearable sensors (11.06%), office lease (10.71%) and smartphones (5.61%) were also key cost drivers. Exercise equipment (37.67%) and facility lease/utilities (31.83%) were the largest cost drivers for CBexCR, while the equivalent exCR specialist salary represented only 16.09% of total delivery costs. Sensitivity analyses indicate REMOTE-CR delivery costs could be reduced to NZD811/GBP411 per capita if exCR specialist time was utilised at full efficiency (ie, participant capacity not limited by trial recruitment rate; NZD-272/GBP-138) and wearable sensors were annuitised over 5 years (NZD-47/GBP-24).
Table 4.
Per capita costs for REMOTE-CR (intervention) and centre-based (control) treatments
| REMOTE-CR (NZD (GBP)) |
Centre-based (NZD (GBP)) |
Difference (NZD (GBP))* |
|
| Intervention | 1130 (573) | 3466 (1758) | −2336 (−1185) |
| Hospital services | 3459 (1754) | 5464 (2771) | −2005 (−1017) |
| Medications | 331 (168) | 605 (307) | −274 (−139)† |
| Total | 4920 (2495) | 9535 (4836) | −4615 (−2341) |
*REMOTE-CR—centre-based.
†p=0.02.
REMOTE-CR, remotely monitored exercise-based cardiac telerehabilitation.
heartjnl-2018-313189supp001.pdf (49.9KB, pdf)
Hospital service utilisation costs were not statistically significantly different between groups, but medication costs were significantly lower for REMOTE-CR (table 4). HRQoL (and, therefore, QALY gain) did not differ between groups over 24 weeks (table 3), and thus, no incremental cost-effectiveness ratio was calculated.
Adverse events
Self-reported adverse events were higher in the REMOTE-CR group during treatment, but comparable during post-treatment follow-up (see online supplementary data). Most events were mild (21/50) or moderate (25/50) severity and unrelated (42/50) or possibly related (4/50) to treatments. Events that were probably or definitely related to treatments included soft tissue injuries and a broken ankle.
Discussion
Our trial is the first to compare the effectiveness and costs of real-time remote exercise monitoring and coaching to traditional face-to-face exCR. Consistent with a recent meta-analysis of cardiac telerehabilitation,6 the effects of REMOTE-CR on functional, risk factor, and behavioural outcomes were at least as favourable as CBexCR immediately postintervention and at longer-term follow-up. Moreover, REMOTE-CR realised a 70% reduction in programme delivery costs, and very few adverse events were attributed to participation in the programme. Building on recent research,26 27 our findings highlight the potential for REMOTE-CR to augment existing exCR services by providing a complementary alternative option that is effective, cost-efficient, safe and satisfies unique patient preferences that traditional programmes have difficulty addressing.
REMOTE-CR extended our previous research that demonstrated SMS-based exCR improves physical activity but not maximal cardiorespiratory fitness.12 REMOTE-CR provided responsive, individualised exercise monitoring and coaching that was similar to centre-based programmes, in order to improve adherence to prescribed intensity levels. REMOTE-CR also provided more frequent patient-to-exercise specialist interaction than has been typical of previous telerehabilitation interventions that incorporated telephone, email or web-based communication. This enhanced interpersonal interaction may have beneficial effects that extend beyond exercise-induced adaptations such as enhancing enjoyment, engagement, self-efficacy (ie, verbal persuasion) and self-determined motivation (ie, relatedness).
REMOTE-CR was substantially cheaper to deliver than CBexCR and sensitivity analyses identified opportunities for further cost efficiencies if the programme was operating at full participant capacity, rather than being limited by trial recruitment rate. Furthermore, cost savings would likely be compounded in large-scale implementation scenarios as REMOTE-CR does not require duplication of centre-based facility and equipment expenses in order to increase programme capacity and reach. Telerehabilitation specialists could be distributed across numerous geographic locations if desired; alternatively, a large-scale programme could be coordinated from a centralised location in order to maximise cost efficiencies. The advantages of distributing exCR specialist expertise across many geographic areas could benefit all exCR participants; however, the impact is likely to be greatest for individuals who have limited access to centre-based programmes—particularly those in regional and rural areas where accessibility barriers are exacerbated.28
Medication costs were lower in the REMOTE-CR group; however, our data cannot determine whether this reflected reduced medication requirements or lower adherence to prescribed medications.
Strengths and limitations
The REMOTE-CR platform is among the first to capitalise on advances in wearable sensor and mobile communication technologies to deliver responsive, tailored and immersive exCR support. Real-time remote exercise monitoring and coaching, in particular, is unique in this population. The non-inferiority randomised controlled trial design was adequately powered to mitigate bias and provide valuable inferences about how REMOTE-CR compared with gold-standard centre-based programmes. The primary outcome aligns with recommendations to prioritise functional capacity as a principal end point for CVD therapies15 and our findings can inform clinical practice, including decisions about whether to implement telerehabilitation as a complementary option alongside existing centre-based services. However, our findings do no indicate how REMOTE-CR would compare to more traditional home-based CR programmes, which typically comprise hard copy education resources and landline telephone support.
In line with recommendations, we evaluated patient-centred and usability outcomes29 (reported in a separate paper) and extended follow-up beyond the treatment period to demonstrate the short-term sustainability of effects. Finally, our economic evaluation addresses an urgent need for evidence about the cost-effectiveness of telerehabilitation.
Our findings are limited by unexpectedly high attrition—13.6% at 12 weeks and 17.3% at 24 weeks. In addition to common participation barriers (eg, travel, work/family commitments), the burden of maximal exercise testing may have contributed to attrition. Furthermore, 30% of individuals who declined trial participation were unwilling to undergo randomisation due to strong preferences for REMOTE-CR (n=66) or CBexCR (n=27) programmes; latent preferences among participants who consented to randomisation may also have increased attrition. These preferences reinforce our assertion that optimising exCR utilisation will require multiple delivery models to enable individuals to identify interventions that best meet their needs.7 8 Our sample was demographically comparable to previous exCR cohorts but effects of treatment preferences on population sampling may limit the generalisability of our findings. The change in V̇O2max for the CBexCR group was smaller than expected. Both programmes adhered to the same clinical exercise prescription guidelines but we were not able to quantify CBexCR participants’ performance to verify equivalent training loads. Self-reported outcomes were subject to recall and social desirability biases. Finally, participants could not be blinded to treatment allocation; however, blinded outcome assessment and objective primary outcome measurement may mitigate this potential bias.
Conclusions
REMOTE-CR is an effective, cost-efficient alternative exCR delivery model that closes the gap between home-based and centre-based programmes by making responsive, individualised intervention support available to individuals in almost any location. As a complement to existing services, interventions like REMOTE-CR could enhance overall exCR utilisation by increasing reach and satisfying unique participant preferences.
Key messages.
What is already known about this topic?
Exercise-based cardiac rehabilitation confers numerous benefits but accessibility barriers contribute to persistent low utilisation of traditional centre-based services.
Telerehabilitation can overcome accessibility barriers but previous interventions have been unable to deliver gold-standard exercise coaching and supervision.
What does this study add?
The remotely monitored exercise-based cardiac telerehabilitation (REMOTE-CR) programme emulated centre-based supervision and coaching, was comparably effective, and offered considerable programme delivery cost savings.
How might this impact on clinical practice?
As a complement to existing rehabilitation services, the REMOTE-CR programme could increase overall utilisation rates by increasing reach, overcoming accessibility barriers and satisfying unique participant preferences.
Acknowledgments
We thank Dr Stacey Reading, Brendon Roxburgh and staff at the University of Auckland Cardiac Rehabilitation Clinic, as well as staff at The Centre for Health, for assistance with exercise testing and provision of the centre-based treatment. We also thank Melissa Rawstorn and Hannah Lowe for coordinating recruitment and data collection at the Auckland and Tauranga study sites.
Footnotes
Contributors: RM conceived the study and is the guarantor. RM, JCR, RW, YJ and NG designed the study. RM and JCR implemented the trial at the Auckland site and drafted the manuscript. RM, JCR, IW and AM developed the REMOTE-CR platform. RAHS and JB provided clinical oversight during study design and conduct. AR implemented the trial at the Tauranga site. YJ wrote the statistical analysis plan and conducted primary and secondary analyses. LG and MM conducted the economic analyses. All authors revised the manuscript, approved the final version and agreed to be accountable for all aspects of the work.
Funding: This work was supported by the Auckland Medical Research Foundation (1113020). AMRF had no input into the trial design, conduct, analysis, reporting or decision to submit this manuscript for publication.
Competing interests: RM was supported by the New Zealand Health Research Council (Sir Charles Hercus health research fellowship). MM is supported by the Australian National Health and Medical Research Council (Centre for Research Excellence, 1041020). We declare no further competing interests.
Patient consent: Not required.
Ethics approval: University of Auckland Human Participants Ethics Committee (011021).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Requests for deidentified individual participant data or study documents will be considered where the proposed use aligns with public good purposes, does not conflict with other requests or planned use by the trial steering committee, and the requestor is willing to sign a data access agreement.
References
- 1. Heran BS, Chen JM, Ebrahim S, et al. . Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2011;7:CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buckley JP, Furze G, Doherty P, et al. . BACPR scientific statement: British standards and core components for cardiovascular disease prevention and rehabilitation. Heart 2013;99:1069–71. 10.1136/heartjnl-2012-303460 [DOI] [PubMed] [Google Scholar]
- 3. Lavie CJ, Milani RV. Cardiac rehabilitation and exercise training in secondary coronary heart disease prevention. Prog Cardiovasc Dis 2011;53:397–403. 10.1016/j.pcad.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 4. Walters DL, Aroney CN, Chew DP, et al. . Variations in the application of cardiac care in Australia. Med J Aust 2008;188:218–23. [DOI] [PubMed] [Google Scholar]
- 5. Dalal HM, Doherty P, Taylor RS. Cardiac rehabilitation. BMJ 2015;351:h5000 10.1136/bmj.h5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rawstorn JC, Gant N, Direito A, et al. . Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart 2016;102:1183–92. 10.1136/heartjnl-2015-308966 [DOI] [PubMed] [Google Scholar]
- 7. Maddison R, Rawstorn JC, Rolleston A, et al. . The remote exercise monitoring trial for exercise-based cardiac rehabilitation (REMOTE-CR): a randomised controlled trial protocol. BMC Public Health 2014;14:1236 10.1186/1471-2458-14-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rawstorn JC, Gant N, Meads A, et al. . Remotely delivered exercise-based cardiac rehabilitation: design and content development of a novel mhealth platform. JMIR Mhealth Uhealth 2016;4:e57 10.2196/mhealth.5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michie S, Richardson M, Johnston M, et al. . The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81–95. 10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- 10. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191–215. 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- 11. Deci E, Ryan R. Intrinsic motivation & self-determination in human behaviour. New York: Plenum, 1985. [Google Scholar]
- 12. Maddison R, Pfaeffli L, Whittaker R, et al. . A mobile phone intervention increases physical activity in people with cardiovascular disease: Results from the HEART randomized controlled trial. Eur J Prev Cardiol 2015;22:701–9. 10.1177/2047487314535076 [DOI] [PubMed] [Google Scholar]
- 13. Pfaeffli Dale L, Whittaker R, Jiang Y, et al. . Text message and internet support for coronary heart disease self-management: results from the text4heart randomized controlled trial. J Med Internet Res 2015;17:e237 10.2196/jmir.4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. : Thompson WR, Gordon NF, Pescatello LS, ACSM’s Guidelines for exercise testing and prescription. 8th edn Philadelphia: Lippincott Williams & Wilkins, 2010. [Google Scholar]
- 15. Forman DE, Arena R, Boxer R, et al. . Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017;135:e894–e918. 10.1161/CIR.0000000000000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin BJ, Arena R, Haykowsky M, et al. . Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc 2013;88:455–63. 10.1016/j.mayocp.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 17. Mâsse LC, Fuemmeler BF, Anderson CB, et al. . Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc 2005;37(11 Suppl):S544–54. 10.1249/01.mss.0000185674.09066.8a [DOI] [PubMed] [Google Scholar]
- 18. Ajzen I. Constructing a TPB questionnaire: conceptual and methodological considerations. 2002. http://socgeo.ruhosting.nl/html/files/spatbeh/tpb.measurement.pdf (accessed Dec 2007).
- 19. Markland D. Self-determination moderates the effects of perceived competence on intrinsic motivation in an exercise setting. Journal of Sport and Exercise Psychology 1999;21:351–61. 10.1123/jsep.21.4.351 [DOI] [Google Scholar]
- 20. McAuley E, Mihalko SL. Measuring exercise-related self-efficacy : Duda JL, Advances in sport and exercise psychology measurement. Morgantown, WV: Fitness Information Technology, 1998:371–90. [Google Scholar]
- 21. EuroQol Research Foundation. EQ-5D-3L User Guide: Basic information on how to use the EQ-5D-3L instrument V5.1. 2015.
- 22. Australian Consortium for Classification Development. AR-DRG V9.0. 2016.
- 23. New Zealand Ministry of Health. WIESNZ14 cost weights. 2014. https://www.health.govt.nz/nz-health-statistics/data-references/weighted-inlier-equivalent-separations/wiesnz14-cost-weights
- 24. Lucini D, Milani RV, Costantino G, et al. . Effects of cardiac rehabilitation and exercise training on autonomic regulation in patients with coronary artery disease. Am Heart J 2002;143:977–83. 10.1067/mhj.2002.123117 [DOI] [PubMed] [Google Scholar]
- 25. Piaggio G, Elbourne DR, Pocock SJ, et al. . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594–604. 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 26. Frederix I, Solmi F, Piepoli MF, et al. . Cardiac telerehabilitation: a novel cost-efficient care delivery strategy that can induce long-term health benefits. Eur J Prev Cardiol 2017;24:1708–17. 10.1177/2047487317732274 [DOI] [PubMed] [Google Scholar]
- 27. Kraal JJ, Van den Akker-Van Marle ME, Abu-Hanna A, et al. . Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: results of the FIT@Home study. Eur J Prev Cardiol 2017;24:1260–73. 10.1177/2047487317710803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blair J, Corrigall H, Angus NJ, et al. . Home versus hospital-based cardiac rehabilitation: a systematic review. Rural Remote Health 2011;11:1532. [PubMed] [Google Scholar]
- 29. Beatty AL, Fukuoka Y, Whooley MA. Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. J Am Heart Assoc 2013;2:e000568 10.1161/JAHA.113.000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Statistics New Zealand. New Zealand Income Survey: June 2015 quarter. 2015. http://www.stats.govt.nz/browse_for_stats/income-and-work/Income/nz-income-survey-info-releases.aspx (accessed 24 Dec 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2018-313189supp001.pdf (49.9KB, pdf)